-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Mystery Unraveled: Essentiality of RNase III in Is Caused by Resident Prophages
article has not abstract
Published in the journal: . PLoS Genet 8(12): e32767. doi:10.1371/journal.pgen.1003199
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003199Summary
article has not abstract
One of the key issues in our understanding of life is the study of the essential set of genes and proteins that make up a living cell and a living organism. Genome-wide studies have identified between 270 and 650 essential genes in bacteria and about 900 essential genes in yeast [1]. Once the essential gene set is known, questions are raised as to why these genes are essential and which important functions the encoded proteins fulfil. The issue is complicated by the fact that essential functions may be carried out by pairs of homologous genes that functionally replace each other and by convergent metabolic functions of non-homologous proteins [2], [3].
Comparative analyses of the essential gene sets of different bacteria have revealed a significant set of genes that is essential in all bacteria studied so far. These genes can be referred to as obligatory essential genes. In contrast, facultative essential genes are essential in one organism but may be non-essential or even absent in other organisms, related or unrelated. It is obvious that the obligatory essential genes encode proteins that fulfil the most important housekeeping functions, such as the processing of the genetic information. Indeed, many proteins involved in DNA replication, transcription and translation are conserved and essential in all bacteria.
Essential RNases
Several RNases are essential in many bacteria. Most prominently, RNase E, the paradigm of a key enzyme for bacterial RNA degradation, is essential in Escherichia coli. RNase E organizes a protein complex, the RNA degradosome, but none of the other degradosome components nor the corresponding scaffold region of RNase E are essential. The reason for the essentiality of this protein has remained enigmatic [4]. In Bacillus subtilis, five RNases are essential. Two of them, RNase P and RNase Z, are required for the maturation of tRNA [5]. For the remaining essential RNases, III, J1 and Y, the reason for the essentiality is not so obvious. RNase Y was first identified as a potential interaction partner of the essential glycolytic enzymes enolase and phosphofructokinase. Recently, this RNase was proposed to be the functional equivalent of E. coli RNase E [6], [7]. Recent transcriptome studies failed to provide a clear explanation for the essential nature of the endoribonuclease Y and the exoribonuclease J1; however, several essential genes, among them those encoding aminoacyl-tRNA synthetases, enzymes of cytochrome c biogenesis, and the essential subunit of pyruvate dehydrogenase, are less expressed if RNase Y is limiting [8]–[11].
A Protective Function for RNase III in B. subtilis
Interestingly, RNases III and Y are essential in B. subtilis, whereas they are non-essential in other related Gram-positive bacteria such as Staphylococcus aureus and Streptococcus pyogenes [11]–[14]. As discussed above, this facultative essentiality suggests that these RNases are required for the protection of the cell against toxic molecules or for other specific functions in the context of the B. subtilis cell. For RNase III, the existence of suppressor mutations allowing the deletion of the rnc gene was reported, suggesting that RNase III has a protective function [15]. In this issue of PLOS Genetics, Durand et al. [16] identify this essential function of RNase III. In their previous transcriptome analysis, the authors observed that depletion of RNase III resulted in the accumulation of toxin-encoding mRNAs [10]. Based on this observation, they have now performed a series of elegant genetic experiments to demonstrate that RNase III is indeed required for the degradation of the mRNAs of two toxin genes, txpA and yonT. These toxin genes are parts of a cryptic prophage, the skin element, and of the prophage SPβ, respectively. Once the txpA and yonT toxin genes are expressed, they can harm their own cell since the two mRNAs have the strongest ribosomal binding sites found in B. subtilis, suggesting that they are very efficiently translated to toxin protein [17]. The expression of these type I toxin/antitoxin systems is controlled by base-pairing with the specific antisense RNAs ratA and anti-yonT that form hybrids with the txpA and yonT toxin mRNAs, respectively. Biochemical experiments presented by Durand et al. [16] show that these base-paired RNA hybrids are subject to degradation by the activity of the double strand–specific endonuclease RNase III.
In the case of txpA, which has been studied down to the molecular details by Durand et al. [16], there is a 15-fold excess of the ratA RNA as compared to the txpA mRNA. This strong excess ensures that the txpA mRNA is always bound by the ratA RNA and thus targeted for degradation. Indeed, the absence of the ratA RNA results in a substantial stabilization of the txpA mRNA. This accumulation of txpA mRNA can only be tolerated if the mRNA cannot be translated due to a mutation of the start codon. The double-stranded txpA mRNA–ratA RNA hybrid molecule is cleaved in vitro by RNase III at multiple sites, resulting in the inactivation of the txpA message (see Figure 1). In addition, RNase Y was found to have a major cleavage site at a single-stranded region of the ratA RNA that is immediately adjacent to the double-stranded part of the ratA RNA. In consequence, cleavage of the ratA RNA by RNase Y results in a trimming of the end of the double-stranded RNA molecule, and in a destabilization of the ratA RNA, both in vivo and in vitro. It is tempting to speculate that the trimming of the txpA mRNA–ratA RNA hybrid molecule facilitates recognition of and/or access to the complex by RNase III (see Figure 1). Indeed, the absence of RNase Y results in a duplication of the txpA mRNA half-life from 1.1 to 2.4 minutes. It should, however, be noted that the depletion of RNase III increases the half-life of the txpA mRNA to more than 20 minutes. Thus, the major role of RNase Y might be the fine-tuning of the ratA–txpA RNA ratio. Similar to the txpA/ratA system, RNase III cleaves the hybrids between yonT/as-yonT and bsrG/as-bsrG, resulting in the degradation of the toxin mRNAs [16], [18]. Interestingly, the bsrG/as-bsrG duplex is cleaved by RNase III downstream of the toxin open reading frame, leaving the question of how RNase III might affect the control of toxin synthesis [18].
Fig. 1. Degradation of a phage-encoded toxin mRNA in B. subtilis. 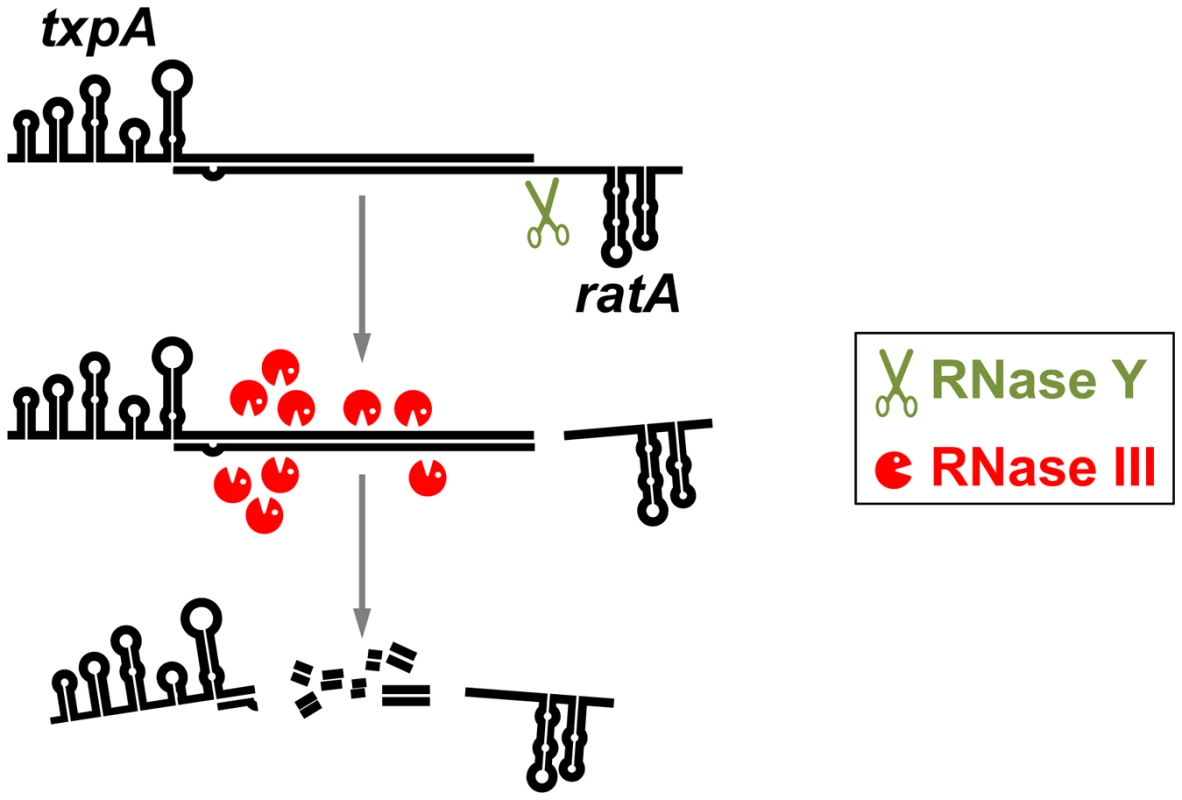
The toxin-encoding mRNA txpA is degraded by the combined action of the antisense RNA ratA and the double strand–specific endonuclease RNase III. The txpA–ratA RNA hybrid may be destabilized due to prior processing by the essential RNase Y. The current study by Durand et al. [16] explains why RNase III is essential in B. subtilis, whereas it is dispensable in most other bacteria. It is tempting to speculate that this facultative essentiality of RNase III (as well as of other RNases such as RNase Y) is directly coupled to the presence of toxin systems in the genomes where the RNases are essential. However, due to the experimental investigation of only few laboratory strains and the genomic variability of bacteria with non-essential RNases III and Y (especially with respect to the presence of prophages), no clear statement about such a correlation is possible at the moment. From an evolutionary point of view, a next interesting question would address the reason for the persistence of several prophages and sequences derived from prophage in the B. subtilis genome even though these sequences do not provide any obvious selective advantage to the cell.
Zdroje
1. JuhasM, EberlL, GlassJI (2011) Essence of life: essential genes of minimal genomes. Trends Cell Biol 21 : 562–568.
2. ThomaidesHB, DavisonEJ, BurstonL, JohnsonH, BrownDR, et al. (2007) Essential functions encoded by gene pairs. J Bacteriol 189 : 591–602.
3. AzumaY, OtaM (2009) An evaluation of minimal cellular functions to sustain a bacterial cell. BMC Systems Biol 3 : 111.
4. CarpousisAJ (2007) The RNA degradosome of Escherichia coli: an mRNA-degrading machine assembled on RNase E. Annu Rev Microbiol 61 : 71–87.
5. HartmannRK, GössringerM, SpäthB, FischerS, MarchfelderA (2009) The making of tRNAs and more – Rnase P and tRNase Z. Prog Mol Biol Transl Sci 85 : 319–368.
6. CommichauFM, RotheFM, HerzbergC, WagnerE, HellwigD, et al. (2009) Novel activities of glycolytic enzymes in Bacillus subtilis: interactions with essential proteins involved in mRNA processing. Mol Cell Proteomics 8 : 1350–1360.
7. Lehnik-HabrinkM, NewmanJ, RotheFM, SolovyovaAS, RodriguesC, et al. (2011) RNase Y in Bacillus subtilis: a natively disordered protein that is the functional equivalent of RNase E from Escherichia coli. J Bacteriol 193 : 5431–5441.
8. MäderU, ZigL, KretschmerJ, HomuthG, PutzerH (2008) mRNA processing by RNases J1 and J2 affects Bacillus subtilis gene expression on a global scale. Mol Microbiol 70 : 183–196.
9. Lehnik-HabrinkM, SchafferM, MäderU, DiethmaierC, HerzbergC, StülkeJ (2011) RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol Microbiol 81 : 1459–1473.
10. DurandS, GiletL, BessièresP, NicolasP, CondonC (2012) Three essential ribonucleases – RNase Y, J1 and III – control the abundance of a majority of Bacillus subtilis mRNAs. PLoS Genet 8: e1002520 doi:10.1371/journal.pgen.1002520.
11. Lehnik-HabrinkM, MäderU, LewisRJ, StülkeJ (2012) RNA degradation in Bacillus subtilis: an interplay of essential endo - and exoribonucleases. Mol Microbiol 84 : 1005–1017.
12. MarincolaG, SchäferT, BehlerJ, BernhardtJ, OhlsenK, et al. (2012) RNase Y of Staphylococcus aureus and its role in the activation of virulence genes. Mol Microbiol 85 : 817–832.
13. DeltchevaE, ChylinskiK, SharmaCM, GonzalesK, ChaoY, et al. (2011) CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471 : 602–607.
14. ChevalierC, HuntzingerE, FechterP, BoissetS, VandeneschF, et al. (2008) Staphylococcus aureus endoribonuclease III purification and properties. Methods Enzymol 447 : 309–327.
15. HerskovitzMA, BechhoferD (2000) Endoribonuclease RNase III is essential in Bacillus subtilis. Mol Microbiol 38 : 1027–1033.
16. DurandS, GiletL, CondonC (2012) The essential function of B. subtilis RNase III is to silence foreign toxin genes. PLoS Genet 8: e1003181 doi:10.1371/journal.pgen.1003181.
17. Daou-ChaboR, MathyN, BénardL, CondonC (2009) Ribosomes initiating translation of the hbs mRNA protect it from 5′-to-3′ exoribinucleolytic degradation by RNase J1. Mol Microbiol 71 : 1538–1550.
18. JahnN, PreisH, WiedemannC, BrantlS (2012) BsrG/SR4 from Bacillus subtilis – the first temperature-dependent type I toxin-antitoxin system. Mol Microbiol 83 : 579–598.
Štítky
Genetika Reprodukční medicína
Článek Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction DiseaseČlánek Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination SchemesČlánek Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 12- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- A Mystery Unraveled: Essentiality of RNase III in Is Caused by Resident Prophages
- Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction Disease
- Insertion/Deletion Polymorphisms in the Promoter Are a Risk Factor for Bladder Exstrophy Epispadias Complex
- Mi2β Is Required for γ-Globin Gene Silencing: Temporal Assembly of a GATA-1-FOG-1-Mi2 Repressor Complex in β-YAC Transgenic Mice
- Dissection of a Quantitative Trait Locus for PR Interval Duration Identifies as a Novel Modulator of Cardiac Conduction
- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Construction of a Global Pain Systems Network Highlights Phospholipid Signaling as a Regulator of Heat Nociception
- Genes Contributing to Pain Sensitivity in the Normal Population: An Exome Sequencing Study
- Identification of , a Locus Controlling Dominant Resistance to Autoimmune Orchitis, as Kinesin Family Member 1C
- ATX1-Generated H3K4me3 Is Required for Efficient Elongation of Transcription, Not Initiation, at ATX1-Regulated Genes
- Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome
- Identification of Novel Type 2 Diabetes Candidate Genes Involved in the Crosstalk between the Mitochondrial and the Insulin Signaling Systems
- The Genetic Architecture of Adaptations to High Altitude in Ethiopia
- Population Genomics of the Endosymbiont in
- Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination Schemes
- Testicular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals
- A Yeast GSK-3 Kinase Mck1 Promotes Cdc6 Degradation to Inhibit DNA Re-Replication
- Genetic Adaptation Associated with Genome-Doubling in Autotetraploid
- The Essential Function of RNase III Is to Silence Foreign Toxin Genes
- Long-Range Regulatory Polymorphisms Affecting a GABA Receptor Constitute a Quantitative Trait Locus (QTL) for Social Behavior in
- A New Isolation with Migration Model along Complete Genomes Infers Very Different Divergence Processes among Closely Related Great Ape Species
- Chromosome Fragile Sites in Harbor Matrix Attachment Regions That May Be Associated with Ancestral Chromosome Rearrangement Events
- Genome-Wide Association Study Implicates Testis-Sperm Specific as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions
- A Mechanism of Gene Amplification Driven by Small DNA Fragments
- Base Damage within Single-Strand DNA Underlies Hypermutability Induced by a Ubiquitous Environmental Agent
- Integrative Analysis of a Cross-Loci Regulation Network Identifies as a Gene Regulating Insulin Secretion from Pancreatic Islets
- Reciprocal Signaling between the Ectoderm and a Mesendodermal Left-Right Organizer Directs Left-Right Determination in the Sea Urchin Embryo
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Genome-Wide Fine-Scale Recombination Rate Variation in
- Deciphering the Transcriptional-Regulatory Network of Flocculation in
- On Lung Function and Interactions Using Genome-Wide Data
- Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function
- The Effective Population Size of Malaria Mosquitoes: Large Impact of Vector Control
- Recessive Mutations in Implicate β-III Spectrin in Both Cognitive and Motor Development
- An Excess of Gene Expression Divergence on the X Chromosome in Embryos: Implications for the Faster-X Hypothesis
- Reduced Life- and Healthspan in Mice Carrying a Mono-Allelic MVA Mutation
- Natural Variation at the MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in
- Histone Deacetylase HDA6 Is Functionally Associated with AS1 in Repression of Genes in
- A Framework for the Establishment of a Cnidarian Gene Regulatory Network for “Endomesoderm” Specification: The Inputs of ß-Catenin/TCF Signaling
- A Polycomb Group Protein Is Retained at Specific Sites on Chromatin in Mitosis
- Diapause Formation and Downregulation of Insulin-Like Signaling via DAF-16/FOXO Delays Axonal Degeneration and Neuronal Loss
- Genes That Act Downstream of Sensory Neurons to Influence Longevity, Dauer Formation, and Pathogen Responses in
- A Genome-Wide RNAi Screen Reveals MAP Kinase Phosphatases as Key ERK Pathway Regulators during Embryonic Stem Cell Differentiation
- Recurrent Targeted Genes of Hepatitis B Virus in the Liver Cancer Genomes Identified by a Next-Generation Sequencing–Based Approach
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
- Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
- Systems Genetic Analysis of Osteoblast-Lineage Cells
- Population Genomics of Inversion Polymorphisms in
- Spreading of Heterochromatin Is Limited to Specific Families of Maize Retrotransposons
- DNA Topoisomerases Maintain Promoters in a State Competent for Transcriptional Activation in
- A Histone Deacetylase Adjusts Transcription Kinetics at Coding Sequences during Morphogenesis
- Approaching the Functional Annotation of Fungal Virulence Factors Using Cross-Species Genetic Interaction Profiling
- Evidence for the Robustness of Protein Complexes to Inter-Species Hybridization
- Systematic Identification of Rhythmic Genes Reveals as a New Element in the Circadian Clockwork
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



