-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSystematic Identification of Rhythmic Genes Reveals as a New Element in the Circadian Clockwork
A wide variety of biochemical, physiological, and molecular processes are known to have daily rhythms driven by an endogenous circadian clock. While extensive research has greatly improved our understanding of the molecular mechanisms that constitute the circadian clock, the links between this clock and dependent processes have remained elusive. To address this gap in our knowledge, we have used RNA sequencing (RNA–seq) and DNA microarrays to systematically identify clock-controlled genes in the zebrafish pineal gland. In addition to a comprehensive view of the expression pattern of known clock components within this master clock tissue, this approach has revealed novel potential elements of the circadian timing system. We have implicated one rhythmically expressed gene, camk1gb, in connecting the clock with downstream physiology of the pineal gland. Remarkably, knockdown of camk1gb disrupts locomotor activity in the whole larva, even though it is predominantly expressed within the pineal gland. Therefore, it appears that camk1gb plays a role in linking the pineal master clock with the periphery.
Published in the journal: . PLoS Genet 8(12): e32767. doi:10.1371/journal.pgen.1003116
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003116Summary
A wide variety of biochemical, physiological, and molecular processes are known to have daily rhythms driven by an endogenous circadian clock. While extensive research has greatly improved our understanding of the molecular mechanisms that constitute the circadian clock, the links between this clock and dependent processes have remained elusive. To address this gap in our knowledge, we have used RNA sequencing (RNA–seq) and DNA microarrays to systematically identify clock-controlled genes in the zebrafish pineal gland. In addition to a comprehensive view of the expression pattern of known clock components within this master clock tissue, this approach has revealed novel potential elements of the circadian timing system. We have implicated one rhythmically expressed gene, camk1gb, in connecting the clock with downstream physiology of the pineal gland. Remarkably, knockdown of camk1gb disrupts locomotor activity in the whole larva, even though it is predominantly expressed within the pineal gland. Therefore, it appears that camk1gb plays a role in linking the pineal master clock with the periphery.
Introduction
All organisms demonstrate a wide variety of physiological, biochemical and behavioral daily rhythms that are driven by intrinsic oscillators, known as circadian clocks. These oscillators work in harmony with the 24 hours periodic changes in environmental conditions. The maintenance and synchronization of the circadian oscillator constitute an adaptive advantage that is evident from the high evolutionary conservation of the circadian system [1].
The current dogma regarding the mechanism of a circadian oscillator is based on positive and negative transcriptional-translational feedback loops with a time period of ∼24 hours. According to this model, in vertebrates, a positive transcription complex, the CLOCK:BMAL heterodimer, activates transcription of the negative clock components, period (per) and cryptochrome (cry) genes, by binding to E-box elements in their promoters. Negative feedback is achieved by PER:CRY heterodimers that enter the nucleus and suppress their own transcription by physically associating with the CLOCK:BMAL heterodimers, thus closing the feedback loop. Transduction of circadian information from this core oscillator is accomplished by the rhythmic activation of clock-controlled output genes, which in turn regulate downstream processes [2]. Several output genes contribute to the accuracy and stability of the oscillator. These encode transcriptional regulators, which constitute accessory loops that feedback to the core loops, or post-translational modifiers of core clock proteins. This system regulates diverse biochemical pathways which are thought to ultimately lead to the wide variety of physiological and behavioral daily rhythms [2]. In recent years, several factors which receive information from the core clock and schedule various output pathways have been revealed [3]–[5]. It is likely that these factors do not account for all core clock regulated processes. Accordingly, the quest for additional mediators is ongoing.
As is the case for many other non-mammalian vertebrates, the zebrafish pineal gland is considered to function as a master circadian clock organ; it is photoreceptive and houses a self-sustained autonomous clock that drives the daily rhythm in the synthesis of melatonin, an important endocrine element of the vertebrate circadian system [6], [7]. In addition to this hormonal output, neurons of the pineal gland project to brain targets [8]. Through these neuronal and hormonal signals, the pineal gland is thought to convey information regarding the circadian cycle to physiological and behavioral processes [9]. Therefore, this tissue has been extensively studied with the intention of elucidating the molecular components of the core clock [10]–[13]. However, the exact pathways which link the core molecular oscillator within the pineal gland to rhythmic physiological and behavioral processes of the entire organism remain largely unknown.
DNA microarray technology is a powerful tool, extensively used to identify circadian changes in the abundance of transcripts (i.e., circadian genes) throughout the animal kingdom. Using this approach in various tissues including the pineal gland, it has been demonstrated that the circadian clock controls groups of genes linked to a large number of molecular and cellular functions [14]–[17]. Surprisingly, different studies show only a moderate level of overlap among the genes identified as circadian in the same tissue from different species and sometimes even in the same species [18], [19]. These discrepancies could be explained by true biological differences or by the use of different experimental procedures and data analysis methods [18]. However, these discrepancies can also be partially attributed to the inherent limitations of DNA microarray technology, for example cross-hybridization of probe sets [20]. Improvement of circadian profiling is now feasible using next-generation sequencing technology to perform RNA-seq. This method is superior because it provides an unbiased measurement of the entire transcriptome without being restricted to only a subset of genes interrogated by the probe sets on a microarray chip [21]. However, methods to minimize errors and biases generated by RNA-seq are still being developed [22].
Here, we have systematically identified circadian genes in the zebrafish pineal gland, employing both DNA microarrays and RNA-seq; these findings were subsequently confirmed using independent quantitative assays. As described below, this strategy has resulted in the identification of a new element in the circadian timing system that possibly links the core clock with rhythmic locomotor activity in the zebrafish.
Results
Systematic identification of circadian genes in the pineal gland
Aiming at identifying circadian genes, we extracted RNA through two daily cycles from pineal glands of zebrafish previously adapted to 24 hours light dark cycles and then transferred to constant darkness during sampling (Figure 1 and Methods). This procedure was repeated twice with different sets of fish. The mRNA from the first experiment was quantified using Affymetrix DNA microarrays whereas the mRNA from the second experiment was quantified using RNA-seq (Methods). The data obtained from the DNA microarrays and RNA-seq analysis was subjected to Fourier analysis (Methods and Levy et al. [23]). Demanding 90% true-positives rate, the DNA microarrays and RNA-seq analysis resulted in 112 circadian probe-sets and 309 circadian genes, respectively (Tables S1 and S2). Altogether, 82 out of the 112 probe-sets identified by the DNA microarray method reliably represent zebrafish mRNAs from GenBank, 66 of which are well-annotated NCBI mRNA reference sequences collection (RefSeq) genes (Table S1). In the analysis of the RNA-seq, only sequencing reads that were aligned to genomic locations of RefSeq genes were used (Methods).
Fig. 1. A step-by-step flow-chart of the systematic identification of pineal gland circadian genes. 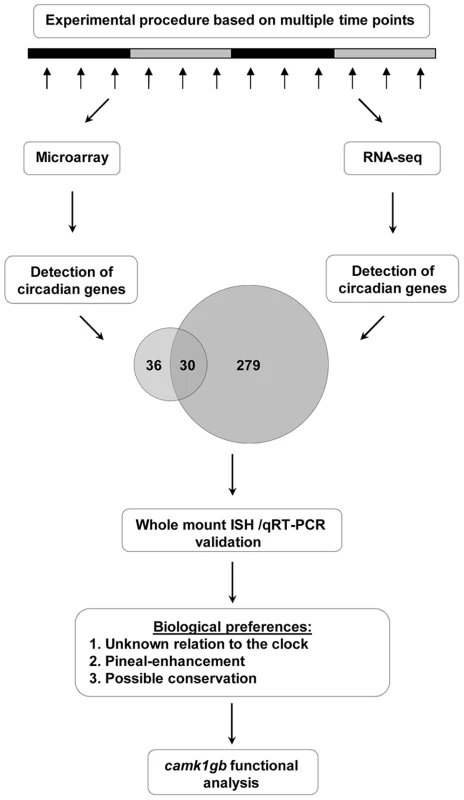
This procedure ultimately led to the detection of a new player, camk1gb, within the clock system. The larger number of circadian genes identified using RNA-seq is due in part to the greater number of genes measured; only about half of the RefSeq genes are represented on the DNA microarray (7634 out of 14263 RefSeq genes). In addition, RNA-seq has a higher detection power due to better accuracy in expression measurement [24]: out of the 309 RefSeq genes identified using RNA-seq, 180 were represented on the DNA microarray but only 30 of them (17%) were identified as circadian. In contrast, about half (30 out of 66) of the RefSeq genes detected as circadian using DNA microarrays were also identified as being circadian using RNA-seq, demonstrating better accuracy of the RNA-seq and overall reasonable agreement between the two methods. Notably, the 309 circadian genes are enriched with pineal-enhanced genes, i.e. genes with higher expression in the pineal gland compared to other tissues (3 out of the 29 pineal-enhanced genes identified in [25], P-value<0.05, binomial cumulative distribution) and with genes which were previously reported to have notable expression in the pineal gland (28 out of the 485 genes mentioned in the ZFIN database [26], P-value<10−3, binomial cumulative distribution).
A comprehensive view of the expression pattern of known clock components
Nearly all (15 out of 16) of the known zebrafish core clock genes were identified as circadian in the RNA-seq analysis (Figure 2 and Table S3). The only exception was per2 which is known to be light-induced in the pineal gland and not circadian under constant darkness [27]. Notably, the RNA-seq analysis is in agreement with the reported phases of 14 core clock genes (Table S3). Similarly, most of the genes (12 out of 14) that are considered to form accessory loops of the molecular circadian oscillator were identified as circadian and their phases are in agreement with previous experimental data (Table S4). In accordance, functional annotation analysis using DAVID [28] reveals the pathway ‘Circadian rhythms’ as significantly enriched (Benjamini-Hochberg adjusted P-value<1e-17) within the identified circadian genes (Table S5 and Methods). As only a portion of the zebrafish genes are represented on the Affymetrix DNA microarray it is reasonable that the list of circadian genes revealed by RNA-seq is larger. Nevertheless, 8 known clock genes (3 core clock and 5 accessory loops-related) are included within the 82 circadian genes identified in the DNA microarray experiment (Tables S3 and S4), thereby providing evidence that other results generated by this analysis are reliable. Importantly, the extensively studied pineal gland clock-controlled gene, aanat2 (arylalkylamine N-acetyltransferase) [29], was identified using both the DNA microarray and RNA-seq analyses. Accordingly, it is clear that these methods provide an informative view of circadian changes in the abundance of transcripts in the zebrafish pineal gland.
Fig. 2. Circadian profiles of known core clock genes which were identified in the zebrafish pineal gland by the RNA–seq analysis. 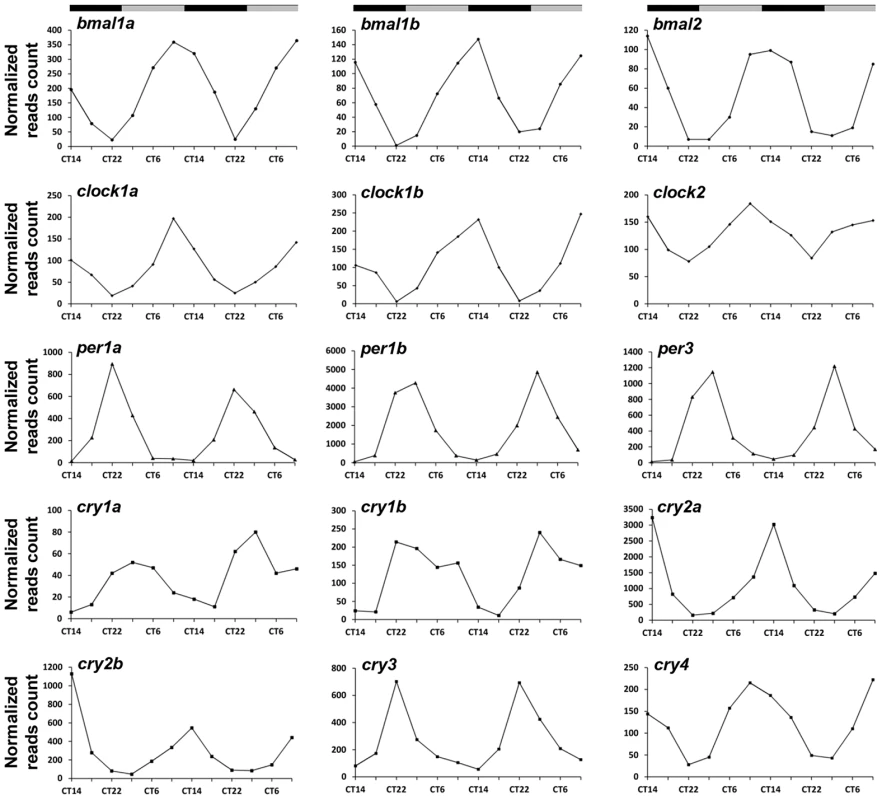
CT = circadian time. Gray and black bars represent subjective day and subjective night, respectively. Selecting potential candidates that relay circadian timing information
We aimed to characterize new regulators of the pineal master clock or new mediators relaying circadian information to downstream processes. The genes detected using RNA-seq and DNA microarray analysis can serve as a basis for this quest. Thirty genes that were identified using both these two independent methods were considered for further functional analysis (Table 1). Notably, about one third of these genes (9 out of 30) were previously reported as core clock or clock-controlled genes (Table 1). In addition, qRT-PCR and quantitative whole mount in situ hybridization (ISH) were performed on selected genes as a validation procedure (Methods); as expected, nearly all (8 out of 9) of the tested genes were indeed validated as circadian, showing similar phases to those identified by the DNA microarray and RNA-seq data (Figure S1, Table 1 and Methods). The use of the two independent genome-wide methods, the re-discovery of previously reported core clock genes and the validation procedure, confirmed that the concise list (Table 1) represent bona fide circadian genes.
Tab. 1. Genes detected as circadian using both DNA microarray and RNA–seq. 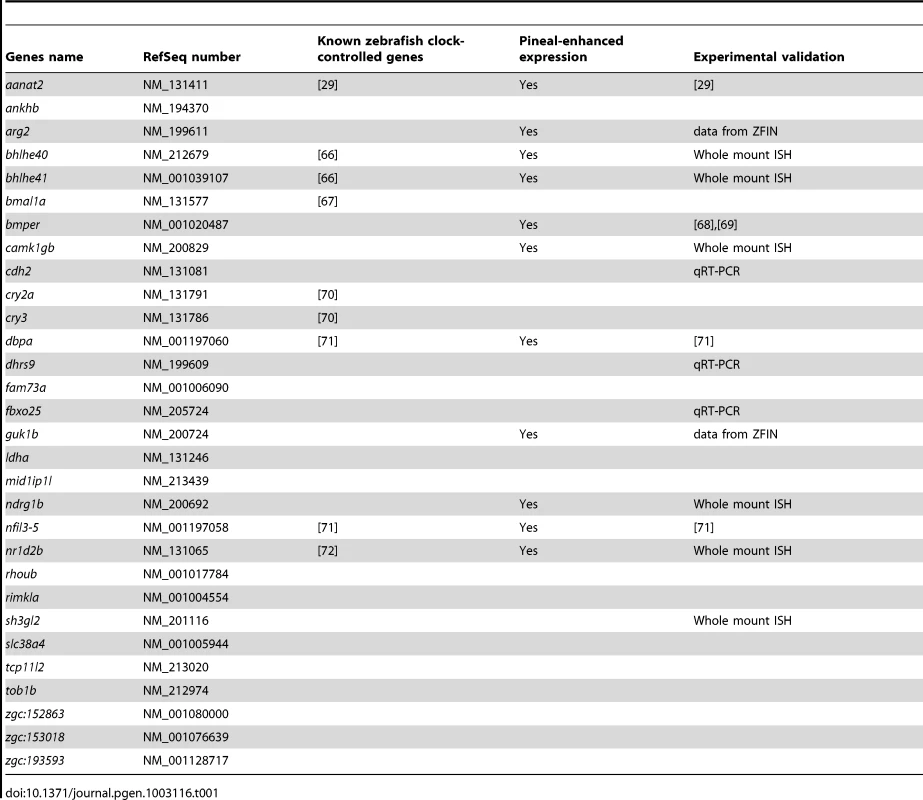
For further functional analysis we focused on genes that were not previously connected to the core clock or the core clock accessory loops (Tables S3 and S4). Studying pineal-enhanced genes can aid in elucidating the role of the master clock in coordinating downstream circadian rhythms. We thus selected genes from the concise list based on their expression pattern, focusing only on those showing enhanced expression in the pineal gland. This was determined using whole mount ISH in larvae (Table 1 and Figure S1). Of the previously unreported genes from the concise list, 5 fulfilled the above requirements: camk1gb, guk1b, arg2, bmper and ndrg1b (Table 1). Of these, we chose to focus on camk1gb (calcium/calmodulin-dependent protein kinase IGb). The mammalian Camk1g is a member of a larger family of calcium/calmodulin-dependent protein kinases, the CaMKI family. Like other members of this family, Camk1g requires both calcium/calmodulin and phosphorylation by CaMKK for its full activation [30]. camk1gb is one of two zebrafish homologs of the mammalian Camk1g. Our pineal gland RNA-seq data shows that the expression levels of the other paralog, camk1ga, are similar to those of camk1gb. Yet, it was not found to be expressed in a circadian manner (Table S2). Interestingly, like camk1gb, the mammalian Camk1g was reported to exhibit enhanced and circadian expression in the rat pineal gland [16], [31], suggesting a conserved role in the pineal clock or in other aspects of pineal function.
camk1gb spatiotemporal expression
Whole mount ISH of larvae clearly reveals enhanced pineal expression of camk1gb (Figure 3a and Figure S2). Importantly, the circadian expression pattern in the pineal gland of larvae, characterized by an expression peak at CT6, is similar to the profile in the pineal gland of adults: Pearson's correlations of 0.89 and 0.77 to the DNA microarrays and RNA-seq profiles, respectively (Figure 3b). This similarity may suggest that the temporal profile of camk1gb has a functional significance starting from early life stages until adulthood.
Fig. 3. camk1gb spatio-temporal expression. 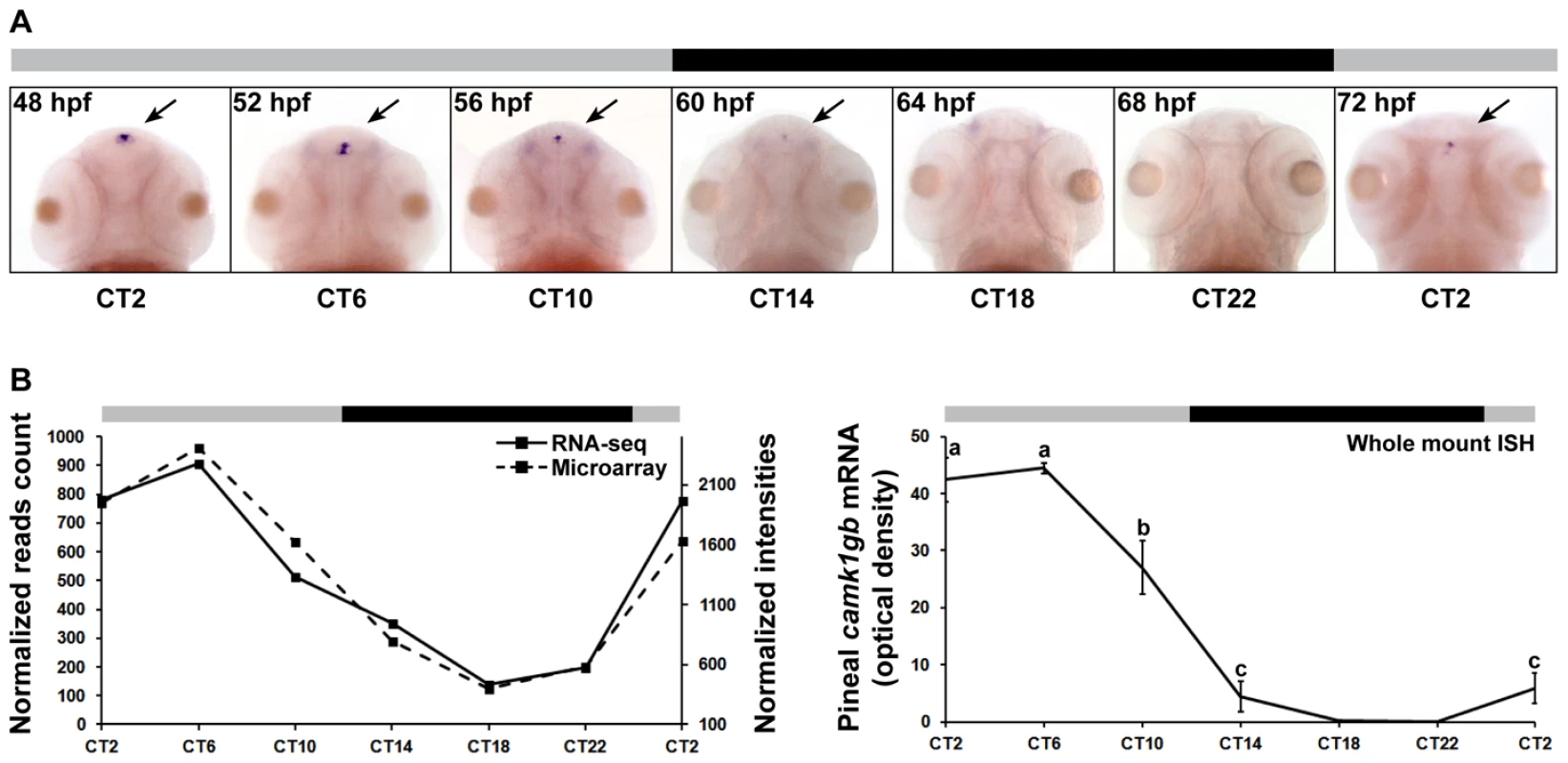
A) Rhythmic expression of camk1gb exclusively in the pineal glands (indicated by black arrows) of 48–72 hpf embryos as detected by whole mount ISH under constant darkness. B) Rhythmic expression of camk1gb in the zebrafish embryo (right) is correlated with the RNA-seq (solid line, left vertical axis) and the microarray data (dashed line, right vertical axis) from the adult (left). Correlation coefficients between the whole mount ISH results and the data obtained by microarrays and RNA-seq were determined by Pearson correlation (r = 0.89 and 0.77, respectively). For whole mount ISH, statistical differences in mRNA levels were determined by one-way ANOVA followed by a Tukey test (P-value<0.05). Error bars represent SE (n = 10–15). CT = circadian time. Gray and black bars represent subjective day and subjective night, respectively. camk1gb knockdown disrupts circadian larval locomotor activity
We examined the effect of camk1gb on clock-regulated zebrafish behavior. Zebrafish larvae exhibit robust circadian locomotor activity with highest activity during the subjective day [32], [33]. To determine whether camk1gb is required for normal circadian locomotor activity, embryos were injected with either control morpholino or camk1gb morpholino (Methods). camk1gb morpholino treatment results in the inclusion of an intron within the mRNA coding sequence and the consequent introduction of a premature stop codon (Figure S3 and Text S1). Strikingly, camk1gb knockdown significantly disrupted the circadian activity pattern (Figure 4a and Methods). This experiment was repeated 4 times using a total of 75 larvae injected with camk1gb morpholino and 75 larvae injected with control morpholino (Methods). The disrupted circadian activity pattern is also evident when analyzing individual larvae using Fourier analysis (Figure 4b, Figure 4c, Figure 4d and Methods). Only 8/75 of the camk1gb knockdown larvae have shown a 24 h-period signal that surpasses the median signal for the 75 control larvae (Figure 4d and Methods). Furthermore, we tracked locomotor activity levels at abrupt light to dark transitions [34]; both control and camk1gb knockdown groups showed similar levels of locomotor activity, indicating that camk1gb knockdown does not impair larval movement abilities (Figure S4 and Methods). Lastly, a rescue experiment, in which camk1gb mRNA was co-injected along with the camk1gb morpholino, restored normal circadian activity thereby demonstrating the specificity of the injected morpholino (Figure 4a and Methods). The success of the rescue experiment is remarkable given that the injected mRNA is likely to restore the levels of camk1gb but less likely to restore its rhythmic expression. Therefore, it seems that sufficient expression levels of camk1gb are necessary for proper circadian rhythms of locomotor activity. Alternatively, it is possible that posttranscriptional regulation may restore the rhythmic expression pattern of the protein, thereby contributing to the success of the rescue experiment.
Fig. 4. The effect of camk1gb knockdown on larval locomotor activity rhythms. 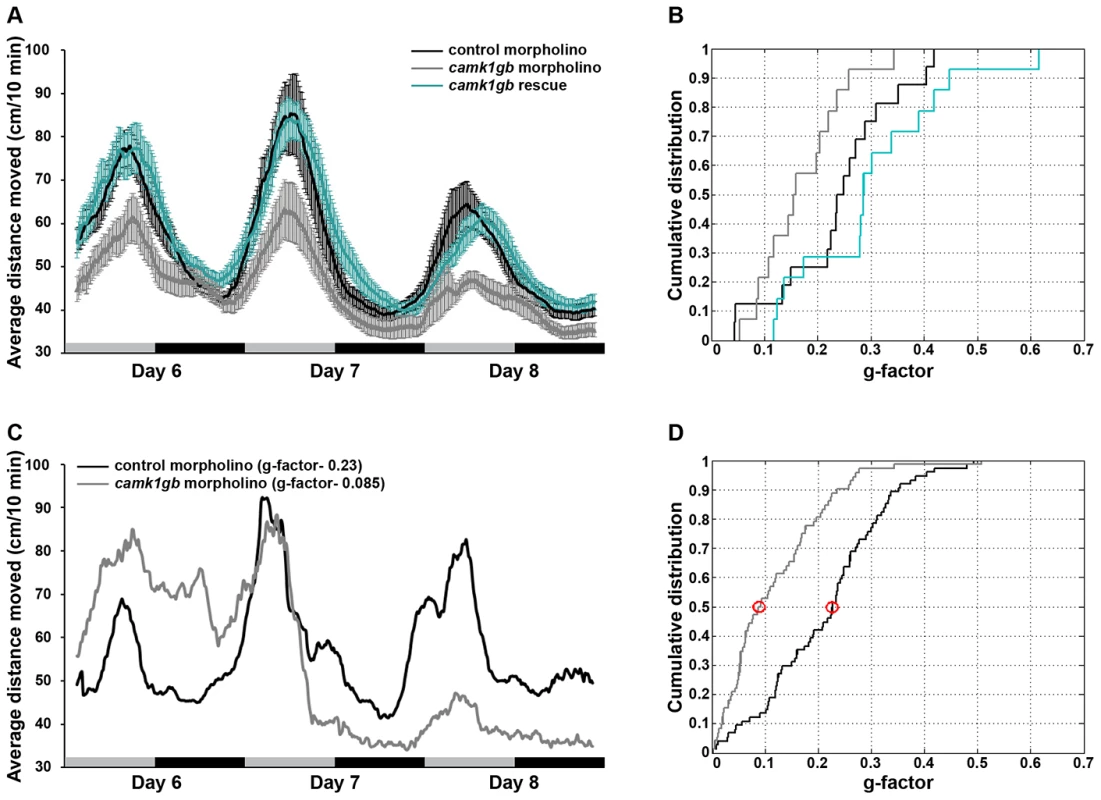
A) Average locomotor activity of 6–8 dpf larvae injected with either control morpholino (black trendline), camk1gb morpholino (gray trendline) or co-injected camk1gb morpholino together with camk1gb mRNA (green trendline), under constant dim-light. Activity was measured as the average distance moved for time units of 10 min and smoothed using moving average. Error bars represent SE (n = 16). Black and gray horizontal boxes represent subjective night and day, respectively. B) Cumulative distribution of g-factor values for each group. Significant differences in the g-factor distribution were revealed between the control morpholino and camk1gb morpholino treated groups as well as between the rescue and camk1gb morpholino injected groups (Kolmogorov-Smirnov test, P-value<0.05 and <0.01, respectively). C) Representative activity profiles of control morpholino (black curve) and camk1gb morpholino (gray curve) injected larvae which correspond to the median g-factor of each group. The median g-factor values are marked by red circles in (D). D) Significant differences in the cumulative distribution of g-factor values between control morpholino (black curve) and camk1gb morpholino (gray curve) injected groups (n = 75), generated from the analysis of four similar experiments (Kolmogorov-Smirnov test, P-value<10−5). camk1gb is required for the proper rhythmic transcription of aanat2
The AANAT enzyme drives the rhythmic production of melatonin [35]. Zebrafish pineal aanat2 transcription exhibits a robust circadian rhythm that begins at 2 days post-fertilization [29], [36]. The transcription of aanat2 is tightly regulated by the core molecular oscillator [29] as well as other transcription factors [37]. Importantly, camk1gb knockdown significantly reduced (Student's t-test, Bonferroni corrected P-value<0.05) the amplitude of the aanat2 expression rhythm by half as detected by whole mount ISH (Figure 5a, Figure 5b and Methods). camk1gb knockdown did not affect normal pineal gland development as indicated by whole mount ISH for otx5 [13] (Figure 5c). These results demonstrate that camk1gb is not necessary for aanat2 to be transcribed but is involved in the physiological regulation of the rhythmic transcription of pineal aanat2.
Fig. 5. Effect of camk1gb knockdown on pineal aanat2 and otx5 mRNA rhythms. 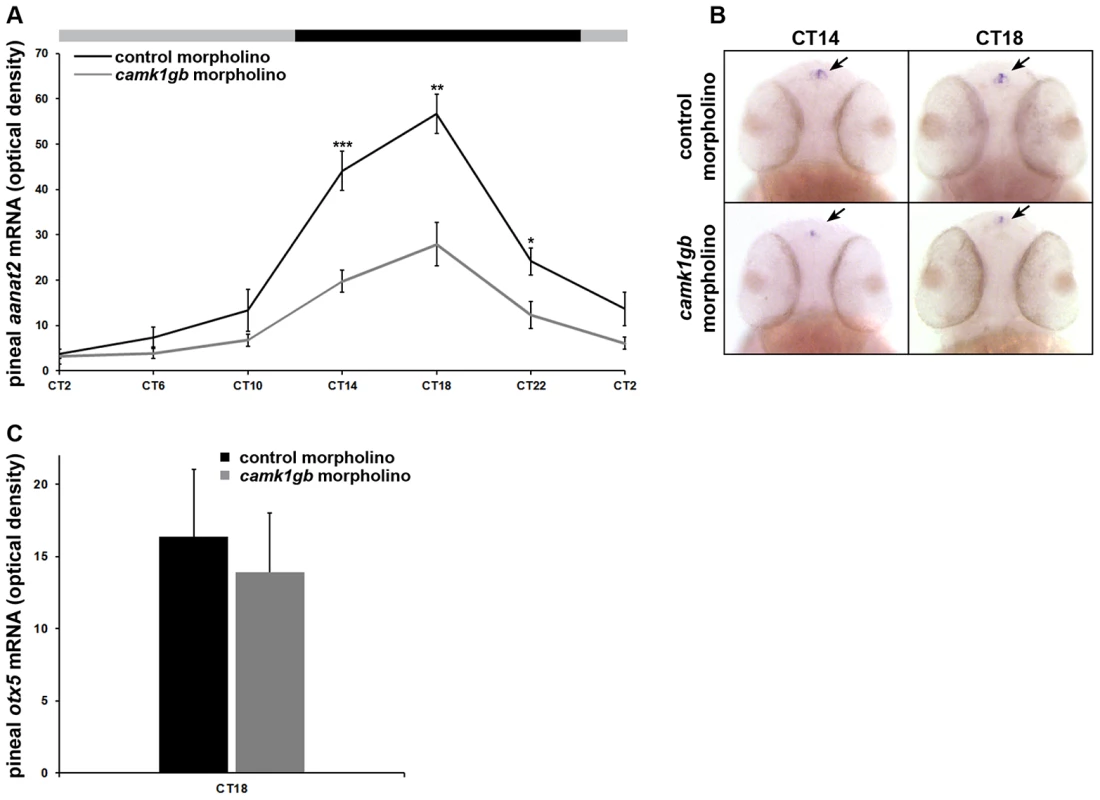
Zebrafish embryos injected with either control morpholino (black line) or camk1gb morpholino (gray line) were subjected to DD during their third day of development and pineal aanat2 and otx5 mRNA levels were determined by whole mount ISH. A) Embryos were sampled at 4-hr intervals for aanat2. Statistical differences in aanat2 mRNA levels between the control morpholino and camk1gb morpholino injected embryos were determined by two-tailed t-test with Bonferroni correction (* P-value<0.05, ** P-value<0.01, *** P-value<0.001). B) Whole-mount ISH for aanat2 in the heads (dorsal views) of representative larvae from each group, at CT14 and CT18. Arrows indicate pineal aanat2 mRNA expression. C) otx5 expression at CT18. Error bars represent SE (n = 10–15). CT = circadian time. camk1gb knockdown effects are not due to core clock disruption
The disruption of the circadian locomotor activity and the reduction in aanat2 rhythmic expression may suggest that camk1gb is a previously unrecognized regulator of the core clock. This notion is in line with findings showing that distant CaMK family members can modulate core clock genes [38]–[41]. We reasoned that if indeed camk1gb is important for normal core clock function, the effect of its knockdown will be manifested in the expression patterns of additional circadian genes. Hence, we tested the effect of camk1gb knockdown on the expression levels of 3 additional pineal-enhanced clock-controlled genes (sh3gl2, opn1lw1 and ndrg1b) and 2 pineal-enhanced core clock accessory loops genes (dec1 and dec2) using whole mount ISH at the peak and the nadir of their rhythm (Methods). However, the expression pattern of the tested genes was not significantly affected by camk1gb knockdown (Figure S5). Accordingly, over-expression of camk1gb in the zebrafish cell line, Pac-2, did not disrupt the core clock as indicated by examining the promoter activity of the core clock marker gene, per1b (Figure S6 and Methods). Thus, camk1gb knockdown affects circadian locomotor activity and aanat2 expression levels without affecting the core clock.
Discussion
In this study we set out to identify new molecular elements that affect the circadian timing system, either directly through the core clock or indirectly by relaying timing information from the core clock to downstream processes. It was previously demonstrated that identification of circadian genes using DNA microarrays can lead to the discovery of previously unrecognized clock components [42]. We reasoned that by using RNA-seq a significant improvement in the number of the detected circadian genes can be achieved, with better detection reliability. However, this new technique introduces non-trivial problems into data analysis and can also bring about biases in genes' quantification [43]–[46]. These hurdles can be overcome by integrating several experimental procedures and employing rigorous and stringent data analysis. Therefore, circadian genes were systematically identified using two independent high-throughput methods, RNA-seq and DNA microarray analyses, followed by computational analysis and extensive in vivo validations.
In this study we focused on the zebrafish pineal gland for two main reasons: 1) most of the molecular clock components are likely to be functional in this autonomous clock tissue. Indeed, the expression of nearly all the core clock genes was found to be circadian in this tissue (Figure 2). 2) By focusing on pineal genes the link between a master clock and peripheral tissues may be elucidated. Indeed, we demonstrated that camk1gb, which is pineal-enhanced, controls circadian downstream processes within the pineal gland and in the entire animal (i.e. locomotor activity).
The link between camk1gb and circadian locomotor activity is intriguing, especially since camk1gb is a pineal-enhanced gene. Although the possibility that camk1gb affects circadian locomotor activity through its low expression in structures outside the pineal gland cannot be ruled out, the enhanced and circadian expression of this gene in the pineal gland (Figure 3) suggests otherwise. At least two possible mechanisms might explain how camk1gb relays circadian timing information from the pineal gland. One is by regulating melatonin secretion: camk1gb knockdown caused a 50% reduction in the night-time expression of aanat2, the key enzyme in melatonin production [35]. Melatonin administration has a profound effect on locomotor activity rhythms in many organisms including zebrafish [47], [48]. Therefore, it is tempting to speculate that disruption of melatonin levels leads to the observed alteration in circadian locomotor activity. However, the role of endogenous melatonin rhythms in the maintenance of normal locomotor activity rhythms in zebrafish is still not fully understood and therefore warrants further investigation. A second possible mechanism is by modulating neuronal innervations between the pineal gland and deeper brain regions [8]. Mammalian Camk1g is known to coordinate neuronal morphogenesis. This CaMKI isoform is a membrane-anchored protein, abundant in neurons, which mediates dendritic and axonal outgrowth of neurons in culture [49], [50]. In teleost fish, the majority of pineal photoreceptor cells form contacts with postsynaptic neurons which send processes to the brain. A fraction of the pineal photoreceptor cells possess long axons that project directly to the brain [9]. Interestingly, we find that camk1gb is indeed expressed within photoreceptors (Figure S7 and Methods). Taken together, these observations point to the possibility that camk1gb is required for the transmission of circadian timing information from the central clock in the pineal gland to the brain.
We provide evidence that camk1gb regulates the transcription of aanat2. Naturally, understanding this regulatory mechanism is of interest. As is the case for most members of the CaMKI family, it was previously demonstrated in vitro that the transcription factor cAMP-response element-binding protein (CREB) is one of the phosphorylation targets of the mammalian homolog, Camk1g [30]. It was also reported that Camk1g regulates CREB-mediated transcription [51]. Interestingly, phosphorylated CREB regulates the transcription of Aanat in mammals [52], [53], and possibly in other vertebrates [37]. Therefore, it is possible that camk1gb modulates the levels of phosphorylated CREB which in turn affects the transcription levels of aanat2. We note however that the camk1gb expression peak is in mid-day whereas aanat2 peaks in mid-night (Figure 3 and Figure 5). A reasonable explanation may be an expected time lag between camk1gb transcription and the appearance of its translated product. An alternative mechanism involves an indirect effect in which camk1gb regulates the pineal core clock and thereby controls aanat2 transcription. However, we found no evidence that camk1gb knockdown affects the core clock mechanism (Figures S5 and S6). Nevertheless, based on findings showing that distant members of the CaMK family can modulate core clock genes [38]–[41], further examination of this possibility in tissues other than the pineal gland is justified.
A comprehensive view of the pineal gland circadian transcriptome allows a dissection of functions that are clock-related inside the pineal gland. As expected, genes that belong to the ‘Circadian rhythms’ pathway are significantly enriched within the pineal gland circadian transcriptome (Table S5 and Methods). The ‘Glycolysis’ and ‘Pyruvate metabolism’ processes are also significantly enriched (Table S5), including 4 circadian enzymes out of the 10 required for glycolysis (Table S6a). Recent studies have revealed a close link between the core clock and metabolism that is mediated by REV-ERB transcription factors [3], [4]. In particular, rev-erb couples glycolysis/gluconeogenesis with the core clock in the mouse liver [54]. Our findings suggest that links between glycolysis and the core clock are not restricted to the liver but may be present in other tissues. Another interesting function which was found to be enriched is ‘Oxidation reduction’ (Table S5). Twenty-five circadian genes belong to this pathway including catalase and 7 different cytochromes P-450 (Table S6b). Several genes in the ‘Oxidation reduction’ pathway were shown to be clock-regulated [55]. In zebrafish cells, catalase has been implicated in the light-dependent transcription of clock genes [56]. Our data suggest that the link between this pathway and the clock system may be more general and includes both central and peripheral clock tissues.
We have constructed a database that contains many interesting candidates for future investigation in the context of either regulating the core clock or in linking of the core clock to downstream pathways. We have focused on camk1gb and showed that this gene is rhythmically expressed in the pineal gland and affects daily rhythms of behavior. In mammals, several genes which connect the master clock to downstream circadian locomotor activity have been discovered [57]. They all share in common the following characteristics: 1. Rhythmic expression in the master clock (which is the suprachiasmatic nucleus in mammals). 2. Alterations in their levels disrupt circadian locomotor activity. Since camk1gb shares these properties and regulates aanat2, and therefore possibly melatonin, we suggest that this gene serves to connect the master clock with circadian locomotor activity in zebrafish.
For over a decade, zebrafish seemed to represent an ideal vertebrate model for the quest to identify and characterize novel clock components [10]. However, with the exception of one study [58], no novel clock components have been identified to date using this model. Instead, the zebrafish has been used to further characterize clock components that were previously identified in mammals. Here, we have demonstrated that the design and analysis of systematic high-throughput experiments based on zebrafish can lead to the discovery of new clock elements.
Methods
Ethics statement
All procedures were approved by the Tel Aviv University Animal Care Committee and conducted in accordance with the council for experiments on animal subjects, Ministry of Health, Israel.
DNA microarrays and RNA–seq experimental design
The experimental procedure for the DNA microarrays experiment was performed as follows. Adult (0.5–1.5 years old) transgenic zebrafish, Tg(aanat2:EGFP)Y8, which express enhanced green fluorescent protein (EGFP) in the pineal gland under the control of the aanat2 regulatory regions, were used [29]. Fish were raised under 12-hr light∶12-hr dark (LD) cycles, in a temperature controlled room, and transferred to constant darkness (DD) for tissue collection. Fish were anesthetized in 1.5 mM Tricane (Sigma), sacrificed by decapitation, and pineal glands were removed under a fluorescent dissecting microscope. Starting from circadian time (CT) 14, pineal glands were collected at 4-hr intervals for 48 hours (12 time points identified as CT 14, 18, 22, 2, 6, 10, 14b, 18b, 22b, 2b, 6b and 10b). Pools of 12 (DNA microarray) or 20 (RNA-Seq) pineal glands were prepared at each time-point and total RNA was extracted using the RNeasy Lipid Tissue Mini Kit (QIAGEN), according to the manufacturer's instructions.
DNA microarrays
Labeled RNA preparation and hybridization to DNA microarrays were performed according to the Affymetrix manual with the two-cycle target labeling protocol (http://www.affymetrix.com/support/downloads/manuals/expression_analysis_technical_manual_.pdf). A total of 12 Affymetrix DNA microarrays were hybridized with RNA-pools of pineal glands from 12 time points throughout two daily cycles. Each DNA microarray was normalized using Affymetrix GeneChip Operating Software (GCOS). The entire DNA microarray dataset, logarithmically transformed, was normalized using quantile normalization to guarantee that the distribution of probe intensities was the same in all the chips [59]. The microarray data was deposited to the Gene Expression Omnibus (GEO), under accession GSE41696.
RNA–seq
Illumina TruSeq protocol was used to prepare libraries from RNA samples. Overall, 12 libraries (12 time points) were run on 2 lanes of Illumina HiSeq2000 machine using the multiplexing strategy of the TruSeq protocol (Institute of Applied Genomics). On average, ∼30 million paired-end reads were obtained for each library. The reads were 2×100 base pairs for 8 time points (CTs 22, 2, 6, 10, 18b, 2b, 6b, 10b) and 2×50 base pairs for the remaining time points (CTs 14, 18, 14b and 22b). TopHat [60] was used for aligning the reads against the zebrafish genome allowing only uniquely aligned reads and up to two mismatches per read. On average, 56% of the reads had unique alignment to the zebrafish genome. Reads aligned to the protein coding regions of known RefSeq genes were used. A custom script written in Perl was used to parse the output of TopHat, which is given in Sequence Alignment/Map (SAM) format (http://samtools.sourceforge.net/), and to convert it into raw number of reads aligned to each position in each RefSeq gene. The RefSeq genes information was obtained from the Table Browser of the UCSC genome browser (genome.ucsc.edu/) using the zebrafish Jul. 2010 (Zv9/danRer7) assembly. To avoid PCR duplicates, only paired-end reads that have unique start position in the genome in both pairs were used [61].
The quality of the sequencing libraries was assessed as described in Levin et al. [61] and the data was normalized using Quantile normalization (Text S1). We made sure that the normalization scheme properly corrects for different RNA levels and other technical differences between samples (Text S1). The sequencing data was deposited to the Sequence Read Archive (SRA), under accession SRA054264.
Fourier analysis
The time-dependent signal was converted into a frequency-dependent signal using the Fast Fourier Transform (FFT). The extent to which the original signal is circadian was quantified by the ratio (‘g-factor’) of the power (squared amplitude) of the frequency which corresponds to 24 hr period to the sum of powers of all frequencies [23]. The higher the g-factor, the higher is the confidence that the transcript is circadian. We note that changing the definition of the g-factor by adding the powers of higher harmonics of the 24 hr period to the numerator, gave similar results compared to the use of the definition above. To determine the true-positive rate for a list of transcripts constructed using a given g-factor cut-off, permutation analysis was conducted as follows:
-
The time-dependent signals of each transcript were randomly shuffled.
-
FFT was performed and g-factor was calculated for each transcript.
-
The cumulative histogram of the g-factor values (ranging from 0 to 1) was calculated, resulting in the number of transcripts whose calculated g-factor is larger than a given value (‘random detection function’).
-
Steps (1)–(3) were repeated a thousand times and the averaged random detection function was calculated. This function estimates the number of false-positive detections of circadian genes for any given cutoff value of the g-factor.
-
Steps (2)–(3) were performed on the original data set. The resulting function, providing the number of transcripts exhibiting a g-factor larger than a given value in the real data, was termed the ‘detection function’.
-
For any given choice of cutoff for the g-factor, the difference between the detection function and the average random detection function estimates the number of true-positives in the list of transcripts constructed with this cutoff.
Finally, using the number of transcripts detected for a given g-factor (step 5) and the number of true-positives for a given g-factor (step 6), the true-positive rate as a function of the number of transcripts detected was calculated and further used to identify circadian genes with high accuracy (Figure S8). The procedure described here was implemented using in-house MATLAB (The Mathworks, Inc.) script.
Gene ontology analysis
The 308 circadian RefSeq genes identified using the RNA-seq were analyzed to find over-represented molecular functions (Table S2), using the DAVID bioinformatics tools [28] and focusing on over-represented gene ontology (GO) categories and KEGG pathways [62], [63]. The DAVID's default zebrafish genes background was used. All the significantly enriched (Benjamini-Hochberg adjusted P-value<0.05) GO categories and KEGG pathways are presented in Table S5.
Validation of the DNA microarrays and RNA–seq data
The temporal expression pattern of candidate genes was determined by whole mount ISH in zebrafish larvae or by quantitative RT-PCR in the adult pineal gland as previously described [12], [27] (Figure S1 and Text S1).
Morpholino design and knockdown experiments
Morpholino experiments were conducted as previously described [11] (Text S1).
Rescue experiments
Rescue experiments were conducted by co-injection of approximately 2 nl volume of camk1gb morpholino (1 mM) and in vitro transcribed camk1gb mRNA (100 ng/µl). The camk1gb protein-coding sequence was PCR-amplified with a KAPA HIFI PCR kit (KAPA Biosystems) using the same set of primers that was used for ISH experiments. The PCR products were cloned into a pCS2+ vector, linearized with NotI restriction enzyme and transcribed using the SP6 mMessage mMachine kit (Ambion), according to the manufacturer's instructions, to generate capped camk1gb mRNA.
Locomotor activity experiments
Embryos were microinjected with either control morpholino, camk1gb morpholino or co-injected with camk1gb morpholino and in vitro transcribed camk1gb mRNA and kept under LD conditions for 3 days. On the fourth day post-fertilization, embryos were placed in 48-well plates in the observation chamber of the DanioVision Tracking System (Noldus Information Technology) and exposed, for acclimation, to two days under 12-hr light (3400 lux)∶12-hr dim light (40 lux) regime followed by 3 days of constant dim light. Live video tracking and analysis was conducted using the Ethovision 8.0 software (Noldus Information Technology). Activity was measured at days 6–8 post fertilization, as the distance moved by a larva in 10 min time bins (Figure 4). The activity record of each individual was subjected to Fourier analysis, and scored with a g-factor (see Methods section ‘Fourier analysis’). Significant differences in the g-factor distributions between the control and camk1gb morpholino treated groups (n = 75; combining 4 different experiments) as well as between the rescue and camk1gb morpholino treated groups (n = 16) were determined by the Kolmogorov-Smirnov test (Figure 4b and Figure 4d). The percent of larvae which are considered circadian depends on the g-factor used as the detection criteria, but for all values of g-factor cutoff tested (ranging between 0.05 and 0.3, Figure 4d), significantly more larvae are considered circadian in the control group.
To determine whether camk1gb knockdown impairs larval movement abilities, locomotor activity levels were tracked under abrupt light to dark transitions [34]. On day 6 post fertilization, control morpholino and camk1gb morpholino injected larvae (n = 24) were subjected to 3 dark flashes of 5 sec each during the light phase [34]. Activity was measured as the distance moved by each larva during the dark flash. No statistical difference was observed between the activity of the control morpholino and camk1gb morpholino injected groups (Student's t-test, P-value>0.2; Figure S4), indicating that the camk1gb morpholino does not impair larval movement abilities.
Transfection of camk1gb into the Pac-2 cell line
Transient co-transfection of the Pac-2 cell line with camk1gb and per1b:luciferase constructs was performed as previously described [64] (Figure S6 and Text S1).
Double ISH of camk1gb and aanat2:EGFP
For double fluorescence ISH we followed the protocol of Machluf and Levkowitz [65] (Text S1).
Supporting Information
Zdroje
1. PandaS, HogeneschJB, KaySA (2002) Circadian rhythms from flies to human. Nature 417 : 329–335.
2. KoCH, TakahashiJS (2006) Molecular components of the mammalian circadian clock. Hum Mol Genet 15: R271–277.
3. ChoH, ZhaoX, HatoriM, YuRT, BarishGD, et al. (2012) Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485 : 123–127.
4. SoltLA, WangY, BanerjeeS, HughesT, KojetinDJ, et al. (2012) Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485 : 62–68.
5. GachonF, OlelaFF, SchaadO, DescombesP, SchiblerU (2006) The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metabolism 4 : 25–36.
6. CahillGM (1996) Circadian regulation of melatonin production in cultured zebrafish pineal and retina. Brain Res 708 : 177–181.
7. FalcónJ, BesseauL, FuentèsM, SauzetS, MagnanouE, et al. (2009) Structural and functional evolution of the pineal melatonin system in vertebrates. Ann N Y Acad Sci 1163 : 101–111.
8. YáñezJ, BuschJ, AnadónR, MeisslH (2009) Pineal projections in the zebrafish (Danio rerio): overlap with retinal and cerebellar projections. Neuroscience 164 : 1712–1720.
9. Ekström P, Meissl H (2010) Pineal photoreception and temporal physiology in fish. In: Kulczykowska E, Popek W, Kapoor B, editors. Biological clock in fish. Science Publishers. pp. 35–70.
10. VatineG, ValloneD, GothilfY, FoulkesNS (2011) It's time to swim! Zebrafish and the circadian clock. FEBS Letters 585 : 1485–1494.
11. ZivL, GothilfY (2006) Circadian time-keeping during early stages of development. Proc Natl Acad Sci USA 103 : 4146–4151.
12. VatineG, ValloneD, AppelbaumL, MracekP, Ben-MosheZ, et al. (2009) Light directs zebrafish period2 expression via conserved D and E boxes. PLoS Biol 7: e1000223 doi: 10.1371/journal.pbio.1000223.
13. GamseJT, ShenYC, ThisseC, ThisseB, RaymondPA, et al. (2001) Otx5 regulates genes that show circadian expression in the zebrafish pineal complex. Nature Genetics 30 : 117–121.
14. DuffieldGE (2003) DNA microarray analyses of circadian timing: the genomic basis of biological time. Journal of Neuroendocrinology 15 : 991–1002.
15. BaileyMJ, BeremandPD, HammerR, Bell-PedersenD, ThomasTL, et al. (2003) Transcriptional profiling of the chick pineal gland, a photoreceptive circadian oscillator and pacemaker. Mol Endocrinol 17 : 2084–2095.
16. BaileyMJ, CoonSL, CarterDA, HumphriesA, KimJS, et al. (2009) Night/day changes in pineal expression of >600 genes: central role of adrenergic/cAMP signaling. J Biol Chem 284 : 7606–7622.
17. ToyamaR, ChenX, JhawarN, AamarE, EpsteinJ, et al. (2009) Transcriptome analysis of the zebrafish pineal gland. Dev Dyn 238 : 1813–1826.
18. KeeganKP, PradhanS, WangJP, AlladaR (2007) Meta-analysis of drosophila circadian microarray studies identifies a novel set of rhythmically expressed genes. PLoS Comput Biol 3: e208 doi:10.1371/journal.pcbi.0030208.
19. RovsingL, ClokieS, BustosDM, RohdeK, CoonSL, et al. (2011) Crx broadly modulates the pineal transcriptome. J Neurochem 119 : 262–274.
20. DraghiciS, KhatriP, EklundAC, SzallasiZ (2006) Reliability and reproducibility issues in DNA microarray measurements. Trends in Genetics 22 : 101–109.
21. MarioniJC, MasonCE, ManeSM, StephensM, GiladY (2008) RNA-Seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res 18 : 1509–1517.
22. HaydenEC (2012) RNA studies under fire. Nature 484 : 428.
23. LevyO, KaniewskaP, AlonS, EisenbergE, Karako-LampertS, et al. (2011) Complex diel cycles of gene expression in coral-algal symbiosis. Science 331 : 175–175.
24. 't HoenPAC, AriyurekY, ThygesenHH, VreugdenhilE, VossenRHAM, et al. (2008) Deep sequencing-based expression analysis shows major advances in robustness, resolution and inter-lab portability over five microarray platforms. Nucl Acids Res 36: e141–e141.
25. AlonS, EisenbergE, Jacob-HirschJ, RechaviG, VatineG, et al. (2009) A new cis-acting regulatory element driving gene expression in the zebrafish pineal gland. Bioinformatics 25 : 559–562.
26. BradfordY, ConlinT, DunnN, FashenaD, FrazerK, et al. (2011) ZFIN: enhancements and updates to the zebrafish model organism database. Nucleic Acids Res 39: D822–829.
27. ZivL, LevkovitzS, ToyamaR, FalconJ, GothilfY (2005) Functional development of the zebrafish pineal gland: light‐induced expression of period2 is required for onset of the circadian clock. Journal of Neuroendocrinology 17 : 314–320.
28. HuangDW, ShermanBT, LempickiRA (2008) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols 4 : 44–57.
29. GothilfY, CoonSL, ToyamaR, ChitnisA, NamboodiriMA, et al. (1999) Zebrafish serotonin N-acetyltransferase-2: marker for development of pineal photoreceptors and circadian clock function. Endocrinology 140 : 4895–4903.
30. Takemoto-KimuraS, TeraiH, TakamotoM, OhmaeS, KikumuraS, et al. (2003) Molecular cloning and characterization of CLICK-III/CaMKIgamma, a novel membrane-anchored neuronal Ca2+/calmodulin-dependent protein kinase (CaMK). J Biol Chem 278 : 18597–18605.
31. NishimuraH, SakagamiH, UezuA, FukunagaK, WatanabeM, et al. (2003) Cloning, characterization and expression of two alternatively splicing isoforms of Ca2+/calmodulin‐dependent protein kinase Iγ in the rat brain. Journal of Neurochemistry 85 : 1216–1227.
32. CahillGM (2007) Automated video image analysis of larval zebrafish locomotor rhythms. Methods Mol Biol 362 : 83–94.
33. HurdMW, CahillGM (2002) Entraining signals initiate behavioral circadian rhythmicity in larval zebrafish. J Biol Rhythms 17 : 307–314.
34. BurgessHA, GranatoM (2007) Modulation of locomotor activity in larval zebrafish during light adaptation. J Exp Biol 210 : 2526–2539.
35. KleinDC (2007) Arylalkylamine N-acetyltransferase: “the Timezyme.”. J Biol Chem 282 : 4233–4237.
36. BégayV, FalcónJ, CahillGM, KleinDC, CoonSL (1998) Transcripts encoding two melatonin synthesis enzymes in the teleost pineal organ: circadian regulation in pike and zebrafish, but not in trout. Endocrinology 139 : 905–912.
37. FalcÓn J, Besseau L, Boeuf G (2006) Molecular and cellular regulation of pineal organ responses. Sensory Systems Neuroscience. Academic Press. pp. 243–306.
38. YangY, ChengP, ZhiG, LiuY (2001) Identification of a calcium/calmodulin-dependent protein kinase that phosphorylates the Neurospora circadian clock protein FREQUENCY. J Biol Chem 276 : 41064–41072.
39. WeberF, HungH-C, MaurerC, KaySA (2006) Second messenger and Ras/MAPK signalling pathways regulate CLOCK/CYCLE-dependent transcription. J Neurochem 98 : 248–257.
40. AgostinoPV, FerreyraGA, MuradAD, WatanabeY, GolombekDA (2004) Diurnal, circadian and photic regulation of calcium/calmodulin-dependent kinase II and neuronal nitric oxide synthase in the hamster suprachiasmatic nuclei. Neurochem Int 44 : 617–625.
41. FukushimaT, ShimazoeT, ShibataS, WatanabeA, OnoM, et al. (1997) The involvement of calmodulin and Ca2+/calmodulin-dependent protein kinase II in the circadian rhythms controlled by the suprachiasmatic nucleus. Neurosci Lett 227 : 45–48.
42. DohertyCJ, KaySA (2010) Circadian control of global gene expression patterns. Annu Rev Genet 44 : 419–444.
43. HansenKD, BrennerSE, DudoitS (2010) Biases in Illumina transcriptome sequencing caused by random hexamer priming. Nucl Acids Res 38: e131.
44. CheungMS, DownTA, LatorreI, AhringerJ (2011) Systematic bias in high-throughput sequencing data and its correction by BEADS. Nucl Acids Res 39: e103.
45. SchwartzS, OrenR, AstG (2011) Detection and removal of biases in the analysis of next-generation sequencing reads. PLoS ONE 6: e16685 doi:10.1371/journal.pone.0016685.
46. RobertsA, TrapnellC, DonagheyJ, RinnJL, PachterL (2011) Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biology 12: R22.
47. FullerPM, GooleyJJ, SaperCB (2006) Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms 21 : 482–493.
48. ZhdanovaIV (2006) Sleep in Zebrafish. Zebrafish 3 : 215–226.
49. DavareMA, FortinDA, SaneyoshiT, NygaardS, KaechS, et al. (2009) Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons. J Neurosci 29 : 9794–9808.
50. Takemoto-KimuraS, Ageta-IshiharaN, NonakaM, Adachi-MorishimaA, ManoT, et al. (2007) Regulation of dendritogenesis via a lipid-raft-associated Ca2+/calmodulin-dependent protein kinase CLICK-III/CaMKIgamma. Neuron 54 : 755–770.
51. WaymanGA, ImpeyS, MarksD, SaneyoshiT, GrantWF, et al. (2006) Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50 : 897–909.
52. RoseboomPH, CoonSL, BalerR, McCuneSK, WellerJL, et al. (1996) Melatonin synthesis: analysis of the more than 150-fold nocturnal increase in serotonin N-acetyltransferase messenger ribonucleic acid in the rat pineal gland. Endocrinology 137 : 3033–3045.
53. BalerR, CovingtonS, KleinDC (1997) The rat arylalkylamine N-acetyltransferase gene promoter. cAMP activation via a cAMP-responsive element-CCAAT complex. J Biol Chem 272 : 6979–6985.
54. YinL, WuN, CurtinJC, QatananiM, SzwergoldNR, et al. (2007) Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 318 : 1786–1789.
55. FroyO (2009) Cytochrome P450 and the biological clock in mammals. Curr Drug Metab 10 : 104–115.
56. HirayamaJ, ChoS, Sassone-CorsiP (2007) Circadian control by the reduction/oxidation pathway: catalase represses light-dependent clock gene expression in the zebrafish. Proc Natl Acad Sci USA 104 : 15747–15752.
57. DibnerC, SchiblerU, AlbrechtU (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72 : 517–549.
58. DeBruyneJ, HurdMW, GutiérrezL, KanekoM, TanY, et al. (2004) Isolation and phenogenetics of a novel circadian rhythm mutant in zebrafish. J Neurogenet 18 : 403–428.
59. BolstadBM, IrizarryRA, AstrandM, SpeedTP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19 : 185–193.
60. TrapnellC, PachterL, SalzbergSL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25 : 1105–1111.
61. LevinJZ, YassourM, AdiconisX, NusbaumC, ThompsonDA, et al. (2010) Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nat Methods 7 : 709–715.
62. AshburnerM, BallCA, BlakeJA, BotsteinD, ButlerH, et al. (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25 : 25–29.
63. KanehisaM, GotoS (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28 : 27–30.
64. CavallariN, FrigatoE, ValloneD, FröhlichN, Lopez-OlmedaJF, et al. (2011) A blind circadian clock in cavefish reveals that opsins mediate peripheral clock photoreception. PLoS Biol 9: e1001142 doi:10.1371/journal.pbio.1001142.
65. Machluf Y, Levkowitz G (2011) Visualization of mRNA expression in the zebrafish embryo. In: Gerst JE, editor. RNA detection and visualization. Methods in Molecular Biology. Humana Press. pp. 83–102.
66. AbeT, IshikawaT, MasudaT, MizusawaK, TsukamotoT, et al. (2006) Molecular analysis of Dec1 and Dec2 in the peripheral circadian clock of zebrafish photosensitive cells. Biochem Biophys Res Commun 351 : 1072–1077.
67. CermakianN, WhitmoreD, FoulkesNS, Sassone-CorsiP (2000) Asynchronous oscillations of two zebrafish CLOCK partners reveal differential clock control and function. Proc Natl Acad Sci USA 97 : 4339–4344.
68. MoserM, YuQ, BodeC, XiongJW, PattersonC (2007) BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish. Journal of Molecular and Cellular Cardiology 43 : 243–253.
69. RentzschF, ZhangJ, KramerC, SebaldW, HammerschmidtM (2006) Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development 133 : 801–811.
70. KobayashiY, IshikawaT, HirayamaJ, DaiyasuH, KanaiS, et al. (2000) Molecular analysis of zebrafish photolyase/cryptochrome family: two types of cryptochromes present in zebrafish. Genes Cells 5 : 725–738.
71. Ben-MosheZ, VatineG, AlonS, TovinA, MracekP, et al. (2010) Multiple PAR and E4BP4 bZIP transcription factors in zebrafish: diverse spatial and temporal expression patterns. Chronobiol Int 27 : 1509–1531.
72. AmaralIPG, JohnstonIA (2012) Circadian expression of clock and putative clock-controlled genes in skeletal muscle of the zebrafish. Am J Physiol Regul Integr Comp Physiol 302: R193–R206.
Štítky
Genetika Reprodukční medicína
Článek Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction DiseaseČlánek Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination SchemesČlánek Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 12- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- A Mystery Unraveled: Essentiality of RNase III in Is Caused by Resident Prophages
- Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction Disease
- Insertion/Deletion Polymorphisms in the Promoter Are a Risk Factor for Bladder Exstrophy Epispadias Complex
- Mi2β Is Required for γ-Globin Gene Silencing: Temporal Assembly of a GATA-1-FOG-1-Mi2 Repressor Complex in β-YAC Transgenic Mice
- Dissection of a Quantitative Trait Locus for PR Interval Duration Identifies as a Novel Modulator of Cardiac Conduction
- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Construction of a Global Pain Systems Network Highlights Phospholipid Signaling as a Regulator of Heat Nociception
- Genes Contributing to Pain Sensitivity in the Normal Population: An Exome Sequencing Study
- Identification of , a Locus Controlling Dominant Resistance to Autoimmune Orchitis, as Kinesin Family Member 1C
- ATX1-Generated H3K4me3 Is Required for Efficient Elongation of Transcription, Not Initiation, at ATX1-Regulated Genes
- Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome
- Identification of Novel Type 2 Diabetes Candidate Genes Involved in the Crosstalk between the Mitochondrial and the Insulin Signaling Systems
- The Genetic Architecture of Adaptations to High Altitude in Ethiopia
- Population Genomics of the Endosymbiont in
- Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination Schemes
- Testicular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals
- A Yeast GSK-3 Kinase Mck1 Promotes Cdc6 Degradation to Inhibit DNA Re-Replication
- Genetic Adaptation Associated with Genome-Doubling in Autotetraploid
- The Essential Function of RNase III Is to Silence Foreign Toxin Genes
- Long-Range Regulatory Polymorphisms Affecting a GABA Receptor Constitute a Quantitative Trait Locus (QTL) for Social Behavior in
- A New Isolation with Migration Model along Complete Genomes Infers Very Different Divergence Processes among Closely Related Great Ape Species
- Chromosome Fragile Sites in Harbor Matrix Attachment Regions That May Be Associated with Ancestral Chromosome Rearrangement Events
- Genome-Wide Association Study Implicates Testis-Sperm Specific as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions
- A Mechanism of Gene Amplification Driven by Small DNA Fragments
- Base Damage within Single-Strand DNA Underlies Hypermutability Induced by a Ubiquitous Environmental Agent
- Integrative Analysis of a Cross-Loci Regulation Network Identifies as a Gene Regulating Insulin Secretion from Pancreatic Islets
- Reciprocal Signaling between the Ectoderm and a Mesendodermal Left-Right Organizer Directs Left-Right Determination in the Sea Urchin Embryo
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Genome-Wide Fine-Scale Recombination Rate Variation in
- Deciphering the Transcriptional-Regulatory Network of Flocculation in
- On Lung Function and Interactions Using Genome-Wide Data
- Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function
- The Effective Population Size of Malaria Mosquitoes: Large Impact of Vector Control
- Recessive Mutations in Implicate β-III Spectrin in Both Cognitive and Motor Development
- An Excess of Gene Expression Divergence on the X Chromosome in Embryos: Implications for the Faster-X Hypothesis
- Reduced Life- and Healthspan in Mice Carrying a Mono-Allelic MVA Mutation
- Natural Variation at the MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in
- Histone Deacetylase HDA6 Is Functionally Associated with AS1 in Repression of Genes in
- A Framework for the Establishment of a Cnidarian Gene Regulatory Network for “Endomesoderm” Specification: The Inputs of ß-Catenin/TCF Signaling
- A Polycomb Group Protein Is Retained at Specific Sites on Chromatin in Mitosis
- Diapause Formation and Downregulation of Insulin-Like Signaling via DAF-16/FOXO Delays Axonal Degeneration and Neuronal Loss
- Genes That Act Downstream of Sensory Neurons to Influence Longevity, Dauer Formation, and Pathogen Responses in
- A Genome-Wide RNAi Screen Reveals MAP Kinase Phosphatases as Key ERK Pathway Regulators during Embryonic Stem Cell Differentiation
- Recurrent Targeted Genes of Hepatitis B Virus in the Liver Cancer Genomes Identified by a Next-Generation Sequencing–Based Approach
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
- Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
- Systems Genetic Analysis of Osteoblast-Lineage Cells
- Population Genomics of Inversion Polymorphisms in
- Spreading of Heterochromatin Is Limited to Specific Families of Maize Retrotransposons
- DNA Topoisomerases Maintain Promoters in a State Competent for Transcriptional Activation in
- A Histone Deacetylase Adjusts Transcription Kinetics at Coding Sequences during Morphogenesis
- Approaching the Functional Annotation of Fungal Virulence Factors Using Cross-Species Genetic Interaction Profiling
- Evidence for the Robustness of Protein Complexes to Inter-Species Hybridization
- Systematic Identification of Rhythmic Genes Reveals as a New Element in the Circadian Clockwork
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



