-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genome-Wide Association Study Implicates Testis-Sperm Specific as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions
Impaired acrosomal reaction (IAR) of sperm causes male subfertility in humans and animals. Despite compelling evidence about the genetic control over acrosome biogenesis and function, the genomics of IAR is as yet poorly understood, providing no molecular tools for diagnostics. Here we conducted Equine SNP50 Beadchip genotyping and GWAS using 7 IAR–affected and 37 control Thoroughbred stallions. A significant (P<6.75E-08) genotype–phenotype association was found in horse chromosome 13 in FK506 binding protein 6 (FKBP6). The gene belongs to the immunophilins FKBP family known to be involved in meiosis, calcium homeostasis, clathrin-coated vesicles, and membrane fusions. Direct sequencing of FKBP6 exons in cases and controls identified SNPs g.11040315G>A and g.11040379C>A (p.166H>N) in exon 4 that were significantly associated with the IAR phenotype both in the GWAS cohort (n = 44) and in a large multi-breed cohort of 265 horses. All IAR stallions were homozygous for the A-alleles, while this genotype was found only in 2% of controls. The equine FKBP6 was exclusively expressed in testis and sperm and had 5 different transcripts, of which 4 were novel. The expression of this gene in AC/AG heterozygous controls was monoallelic, and we observed a tendency for FKBP6 up-regulation in IAR stallions compared to controls. Because exon 4 SNPs had no effect on the protein structure, it is likely that FKBP6 relates to the IAR phenotype via regulatory or modifying functions. In conclusion, FKBP6 was considered a susceptibility gene of incomplete penetrance for IAR in stallions and a candidate gene for male subfertility in mammals. FKBP6 genotyping is recommended for the detection of IAR–susceptible individuals among potential breeding stallions. Successful use of sperm as a source of DNA and RNA propagates non-invasive sample procurement for fertility genomics in animals and humans.
Published in the journal: . PLoS Genet 8(12): e32767. doi:10.1371/journal.pgen.1003139
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003139Summary
Impaired acrosomal reaction (IAR) of sperm causes male subfertility in humans and animals. Despite compelling evidence about the genetic control over acrosome biogenesis and function, the genomics of IAR is as yet poorly understood, providing no molecular tools for diagnostics. Here we conducted Equine SNP50 Beadchip genotyping and GWAS using 7 IAR–affected and 37 control Thoroughbred stallions. A significant (P<6.75E-08) genotype–phenotype association was found in horse chromosome 13 in FK506 binding protein 6 (FKBP6). The gene belongs to the immunophilins FKBP family known to be involved in meiosis, calcium homeostasis, clathrin-coated vesicles, and membrane fusions. Direct sequencing of FKBP6 exons in cases and controls identified SNPs g.11040315G>A and g.11040379C>A (p.166H>N) in exon 4 that were significantly associated with the IAR phenotype both in the GWAS cohort (n = 44) and in a large multi-breed cohort of 265 horses. All IAR stallions were homozygous for the A-alleles, while this genotype was found only in 2% of controls. The equine FKBP6 was exclusively expressed in testis and sperm and had 5 different transcripts, of which 4 were novel. The expression of this gene in AC/AG heterozygous controls was monoallelic, and we observed a tendency for FKBP6 up-regulation in IAR stallions compared to controls. Because exon 4 SNPs had no effect on the protein structure, it is likely that FKBP6 relates to the IAR phenotype via regulatory or modifying functions. In conclusion, FKBP6 was considered a susceptibility gene of incomplete penetrance for IAR in stallions and a candidate gene for male subfertility in mammals. FKBP6 genotyping is recommended for the detection of IAR–susceptible individuals among potential breeding stallions. Successful use of sperm as a source of DNA and RNA propagates non-invasive sample procurement for fertility genomics in animals and humans.
Introduction
Acrosome is a large, membrane bound secretory vesicle in the head of mammalian sperm which releases its contents when sperm contacts the egg for fertilization. The process is known as regulated acrosomal exocytosis or acrosomal reaction (AR) and is triggered by egg's zona pellucida proteins which induce an influx of calcium in to the sperm. Acrosomal exocytosis facilitates the passage of the sperm through the zona and is obligatory for natural fertilization [1], [2], [3], [4], [5]. The AR is a one-time event during which the acrosome releases all its contents and loses structural integrity [2]. Consequently, any kind of acrosome dysfunction, i.e., premature AR, reduced release of the acrosomal contents or the absence of AR, contribute to male subfertility because such sperm are incapable of fertilization [1], [6].
The success of AR is determined by a number of events such as acrosome biogenesis, sperm capacitation, sperm-oocyte recognition, sperm-zona binding, and elicitation of signaling pathways. Each of these and the AR are regulated by multiple proteins and protein complexes [1], [2], [5], [7], [8], the best known of which is SNARE – a group of proteins that controls membrane fusion and exocytosis in humans and mice, both in the sperm acrosome [7], [9] and neuronal synapses [10].
Current knowledge about the genetic regulation of AR is limited. Knockout and mutant rodent models have revealed 32 genotypes and 26 genes that are associated with impaired AR (IAR) phenotype (MGI: http://www.informatics.jax.org/). The AR involves genes for neurotransmitter receptors [11], [12], calcium channels [13], [14], [15], membrane fusions [16] and vesicle exocytosis [9], [17]. Mouse triple knockout models for five sperm motility and sperm-egg adhesion genes (Tnp2, H1.1, H1t, Smcp) suggest that AR might be the result of synergistic interaction of several genes [18]. The few mutations associated with IAR have been found only in mouse [11], [12], while the molecular causes of IAR in humans and other non-rodent mammals remain as yet poorly understood. At the same time, it has been proposed that about 20–25% of cases of unexplained male subfertility in humans can be attributed to defects in sperm functions responsible for fertilization - the AR and sperm's ability to bind and penetrate the zona [7], [19], [20], [21], [22], [23]. The prevalence of these conditions in horses is not known but the incidence of unexplained subfertility in breeding stallions mimics that of humans [24], [25]. Recently, a few such cases in Thoroughbreds were associated with IAR [25].
Stallion fertility is one of the key components of the economy of the equine industry. Despite this, to date, with the exception of a few chromosome aberrations [26] and Y chromosome deletions [27], no genetic mutations have been associated with reproductive disorders in stallions. This is likely because the genetic regulation of male fertility and reproduction in mammals is thought to involve 10–20% of the genes in the genome [28], [29], [30], [31] which complicates the candidate gene approach for mutation discovery and requires the use of pan-genomic platforms, such as SNP chips that facilitate hypothesis free, genome-wide discovery of associated genes or genomic regions.
Here we report about a Genome Wide Association Study (GWAS) that resulted in the mapping, identification and functional analysis of a susceptibility locus for impaired acrosome reaction in stallions.
Results
Relatedness and stratification within the study population
Analysis of five generation pedigrees in the 44 horses used for SNP genotyping showed moderate to low degree of relatedness among the seven affected Thoroughbred stallions (Table 1). Six animals (HS03, HS29, HS30, HS32, HS33, HS34) formed one closely related group, with relatedness coefficient (r) values between 11 and 30%, and shared common ancestors in paternal, maternal or in both parental lineages. The percentage of genes shared by common descent was the highest (r = 30.6%) between stallions HS30 and HS34 (Table 1). One of the affected stallions (HS31) clearly originated from a different Thoroughbred lineage with r<8% to other animals. In contrast to the moderate relatedness values, inbreeding coefficients for the seven stallions were relatively low and ranged from 2.2% to 4.3% implying low genetic relationships among the parents of the affected individuals. A detailed, five-generation pedigree for the 7 cases and 37 control male Thoroughbreds is presented in Figure S1.
Tab. 1. Inbreeding coefficients (diagonal boxes) and genetic relationship coefficients of and among the seven affected stallions calculated from i) pedigree data in bold (upper triangle of the matrix) and from ii) SNP genotyping data in normal font (lower triangle of the matrix). 
Stallion code names correspond to the codes in the 5-generation pedigree (Figure S1). Similarly, calculation of relationships from genome wide SNP genotypes demonstrated a greater extent of genome sharing among the 7 cases, with estimates of genome sharing identity by descent (IBD) between case-case pairs (mean 0.126; standard deviation, sd 0.085) higher than estimates of genome sharing IBD between control-control pairs (mean 0.026; sd 0.049), or across the entire cohort (mean 0.032; sd 0.05). Relationship coefficients for the 7 cases were similar between the SNP and pedigree data (Table 1), with HS31 being less related to the other 6 cases. Separation of HS31 from other cases, as well as, relative clustering of cases when compared to controls was also visualized on multidimensional scaling (MDS) plots (Figure S2). Empirical P-values for the group differences in IBS sharing demonstrate that on average, cases were more related to other cases (P = 3.99E-05) than controls were related to other controls (P = 0.006), however, overall case-case pairs were not significantly (P = 0.5) more related than case-control pairs (Table S1).
Genome-wide SNP association study (GWAS)
The standard chi-square association test revealed high (P = 6.75E-08) to moderate (P = 9.26E-05) association with the IAR phenotype for 12 SNPs on ECA13 and moderate association (7.98E-05>P>4.23E-06) for 12 SNPs on ECA4 (Figure 1A). In addition, moderate associations with one or two SNPs were identified on five other chromosomes, ECA7, ECA15, ECA18, ECA22, and ECA23. The most significant 36 SNPs by the basic association test and their significance values by different regression analyses are presented in Table S2. After data correction by 10,000 t-max permutations, a single SNP on ECA13 was significant with a t-max permuted P<0.05 (Figure 1B). Because the small number of available cases precluded segregation analysis to determine the mode of inheritance, in addition to the chi-square allelic test of association, logistic regression models where marker genotypes were coded as additive, dominant or recessive were also tested. Association with ECA13 was evident in all three logistic regression tests (Figure S3), though the values remained below the significance threshold which was log10P>5 or log10 EMP2>1. 5.
Fig. 1. GWAS plots for the IAR phenotype displaying results for the 52,583 SNPs that passed quality control. 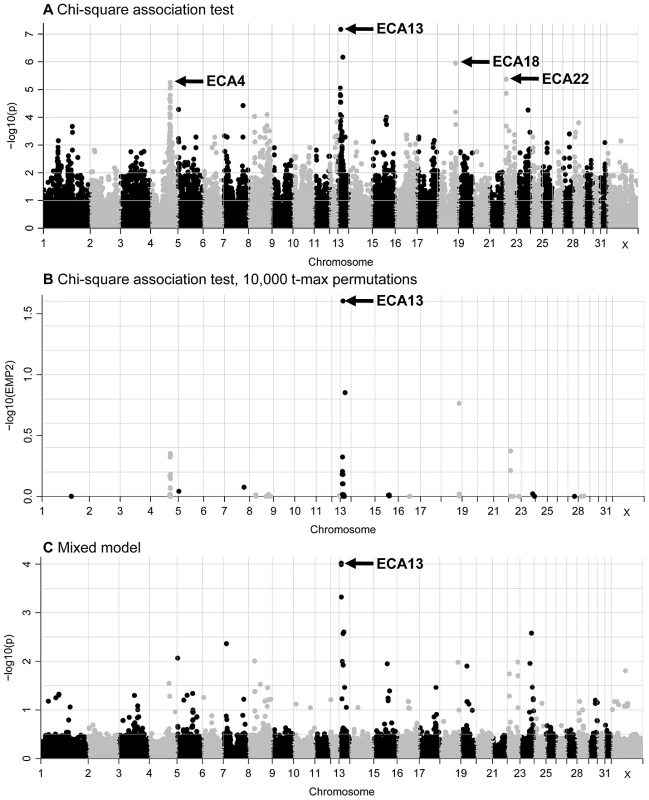
(A) standard chi-square based association test without permutations; (B) standard association test after 10,000 t-max permutations, and (C) mixed-model test. The SNPs are plotted according to their position on each chromosome (x-axis) and association with the IAR phenotype (y-axis). Significance is given as the −log10 of the uncorrected (A and C) or corrected (B) P-value. The genome-wide significance threshold is log10P>5 or log10 EMP2>1. 5. The genomic inflation factor (lambda = 1.31) in the chi-square analysis suggested that relatedness in the sample cohort was leading to inflation of the test statistic. Therefore, a mixed model analysis was conducted to account for relatedness and correct for inflation of the test statistic. Seven SNPs on ECA13 showed moderate evidence of association to IAR in the mixed model (1.0E-04>P>9.54E-05; Figure 1C and Table S2). No suggestive associations on other chromosomes were detected.
In summary, association of the IAR phenotype to ECA13 was evident from all genome-wide studies. Next, statistical tests with the 721 ECA13 SNPs present on the SNP50 Beadchip showed that the most significant SNPs and their P-values varied between analyses (Figure S4 and Tables S2, S3). Yet, 7 SNPs (Figure 2; SNPs 1–3, 5, 6, 8, 10) had consistently strong to moderate association (4.7E-04>P>6.75E-08) with IAR by chi-square and mixed-model tests demarcating a 3.9 Mb association region in ECA13p.
Fig. 2. Schematic of the IAR–associated region in ECA13p. 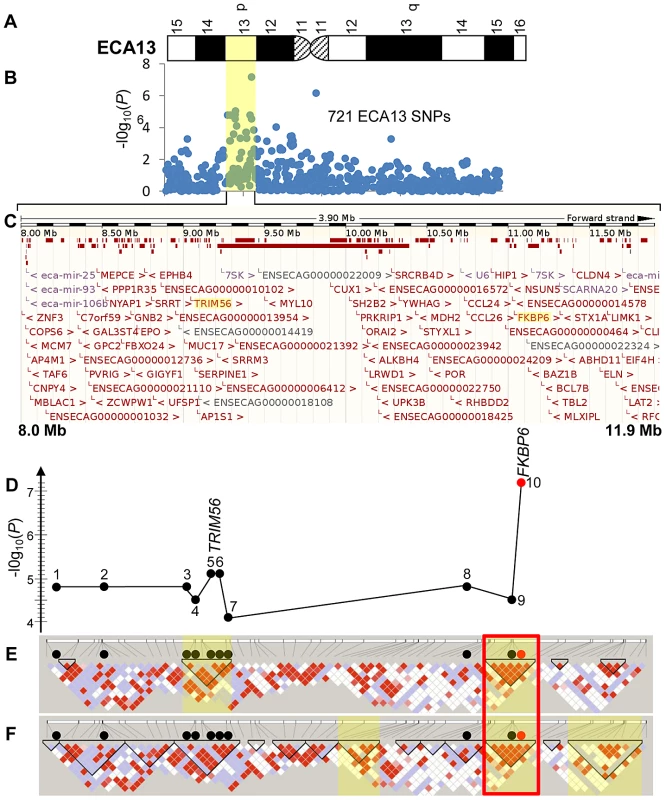
(A) A G-banded ideogram of ECA13 (ISCNH, 1997) showing the cytogenetic location of the IAR associated region; (B) Statistical significance values for 721 ECA13 SNPs analyzed for genome wide association using chi-square; yellow shade highlights the IAR-associated ∼3.9 Mb region; (C) Sequence map of chr13:8,000,000–11,900,000 showing all ENSMBL annotated genes in the region; TRIM56 and FKBP6 are highlighted. (D) A graph showing chi-square test −log10(P) values for 10 significant SNPs: 1–8027172, 2–8382955, 3–8977804, 4–8987922, 5–9034435, 6–9034502, 7–9183989, 8–10894213, 9–11043916 (black circles), and 10–11044175 (red circle); the seven SNPs above the grey line showed moderate significance also in mixed model; (E) Confidence interval LD blocks; (F) Solid spine LD blocks; blocks with significant permuted P-values (P<0.0005) for association with IAR are highlighted yellow; red diamonds represent D′ values equal to 1, lower values of D′ are presented in blue and white; the ∼306 kb highly associated haplotype in both LD analyses is in a red box. Haplotypes and linkage disequilibrium (LD)
Eightly-six (86) SNPs from Equine SNP50 Beadchip, representing an ECA13 region 8,023,293–11,902,171, were computationally phased and a single 3.31 Mb long haplotype from SNP 13 : 8,023,293 to SNP 13 : 11,334,980 was present in all IAR cases (Figure S5). Five affected stallions (HS03, HS29, HS30, HS33 and HS34) were homozygous while two stallions (HS31 and HS32) were heterozygous for the haplotype. The frequency of this extended haplotype was 0.86 in cases compared to 0.18 in controls.
Using the confidence interval (CI) method (Table S4), 5 haplotype blocks were defined in the region. Two of these blocks contained haplotypes that were strongly associated with IAR (Figure 2E shaded regions; Table S4). When haplotypes were defined using the solid spine (SS) of LD method, 14 haplotype blocks were identified of which three contained strongly associated haplotypes (Figure 2F shaded, Table S5). Notably, a ∼306 kb block at ECA13 : 11,028,316–11,334,980 bp with a single highly associated haplotype (P = 2.49E-08) was identical in the two LD analyses (Figure 2E, 2F; red box).
Identification of FKBP6 as a candidate gene
The 3.9 Mb highly associated region in ECA13 is extremely gene rich with 105 protein coding genes (27 genes per Mb), 4 RNA genes, and 6 pseudogenes (Figure 2B; Ensembl: http://www.ensembl.org/index.html). The region harbors over 2,126 SNPs in the Broad Institute database (http://www.broadinstitute.org/ftp/distribution/horse_snp_release/v2/) which makes re-genotyping unpractical. Therefore, for candidate gene discovery, we determined the closest genes in a 100 kb window around the best SNPs (Figure 2, Table S2) and the genes in haplotype blocks (Tables S6, S7) using BioMart (http://www.biomart.org/). Two genes, FKBP6 and TRIM56, were selected for further analysis because intron 4–5 of FKBP6 contained the only SNP (13 : 11,044,175) that retained significant association after permutations, and TRIM56 was located ∼12 kb from moderately associated SNPs 13 : 9,034,435 and 13 : 9,034,502 (Figure 2). Functionally, FKBP6 encodes a member of FK506 binding protein family and has been associated with male infertility in humans [32], [33], [34] and mice [33], while TRIM56 belongs to tripartite motif-containing gene family, members of which, like TRIM36 (alias HAPRIN), are involved in acrosome reaction of mammalian sperm [9], [17].
Individual PCR amplification and Sanger sequencing of the 3 exons of TRIM56 and the 7 exons of FKBP6 (Ensembl; http://www.ensembl.org/index.html) in all cases (n = 7) and selected Thoroughbred (TB) controls (n = 7) revealed two SNPs in TRIM56, and four SNPs in FKBP6, of which two were non-synonymous (Table 2). Polymorphisms in TRIM56 were not significantly associated with IAR and the gene was rejected as a candidate locus, whereas association with IAR was found with FKBP6 exon 4 and the gene was subjected for further analysis.
Tab. 2. Polymorphisms identified in the sequences of FKBP6 and TRIM56. 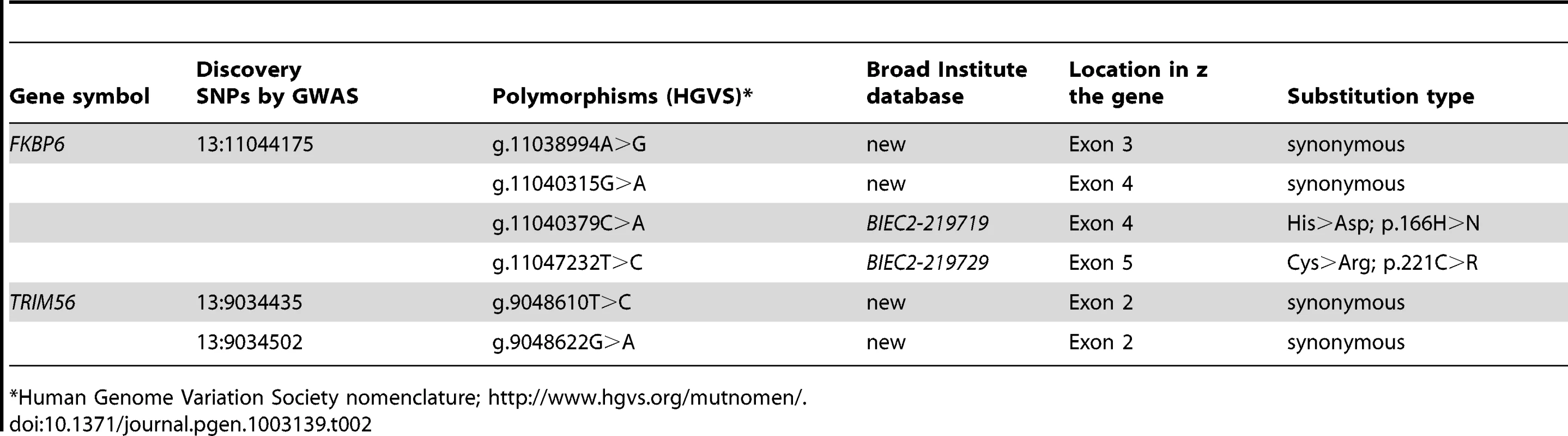
Human Genome Variation Society nomenclature; http://www.hgvs.org/mutnomen/. Case-control genotyping and association tests in the entire GWAS discovery cohort (n = 44) showed highly significant association of the IAR phenotype with FKBP6 SNP g.11040315G>A (P = 1.51E-05), and significant association with SNP g.11040379C>A (p.166H>N; P = 0.004) (Table 3). This was further validated by sequencing exon 4 in a larger, multi-breed cohort (n = 265) of male horses with known (n = 94) and unknown (n = 171) reproductive phenotypes (Table S8). Homozygosity for the minor A-allele at g.11040315G>A was 100% in IAR affected stallions (n = 7), 0% in stallions with normal AR (n = 5), and only 9% in other fertile stallions (n = 82; Table S9). Likewise, all IAR stallions were homozygous for the major A-allele at g.11040379C>A; however this genotype was common in both stallions with normal AR (60%) and other fertile stallions (39%). While the IAR stallions were homozygous at both SNPs, this haplotype was rare (12%) across all horses tested (Table S9). The distribution of genotypes in the IAR cases and controls for the g.11040315G>A and g.11040379C>A alleles suggested a potential mode of inheritance for IAR, although this conclusion could not be unequivocally proven with this data. Therefore, tests of association between these alleles were computed using both genotypic and recessive coding. To test for association across g.11040315 and g.11040379, the combination of genotypes at both loci were used (Table 3). Fisher's exact tests were utilized due to the low number of counts in some cells of the contingency table. Overall, statistical analysis demonstrated highly significant associations with each SNP independently as well as the AAAA haplotype across both loci (Table 3; Table S9), whereas differences in allele frequencies among phenotypic groups were more pronounced for the synonymous SNP g.11040315G>A than for the non-synonymous change g.11040379C>A (p.166H>N). In summary, these findings suggested FKBP6 as a susceptibility locus for IAR in stallions.
Tab. 3. Association analysis of FKBP6 exon 4 and the IAR phenotype in three different study cohorts using Fisher's exact test over genotypic and recessive coding. 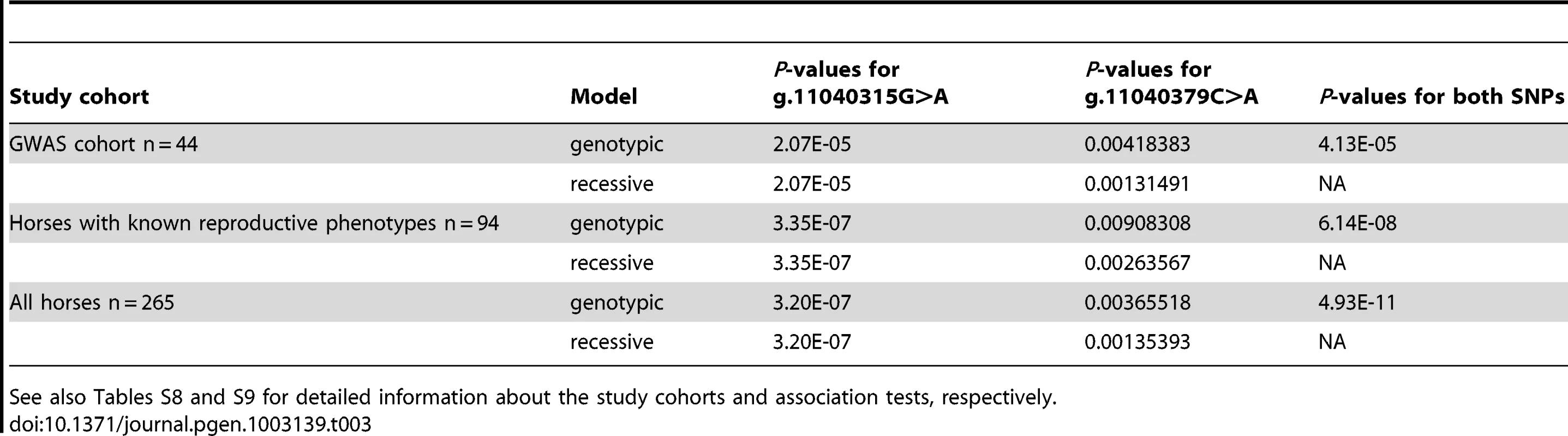
See also Tables S8 and S9 for detailed information about the study cohorts and association tests, respectively. Analysis of FKBP6 expression
Transcriptional profiling of equine FKBP6 by RT-PCR in a panel of 10 adult equine body tissues revealed male limited expression in testis and sperm, including the sperm of IAR stallions (Figure 3A). Quantitative RT-PCR (qRT-PCR) with exon 4 primers showed low to moderate up-regulation of FKBP6 in the sperm of IAR individuals compared to the sperm of normal stallions (Figure 3B), though the observed differences did not meet the 5% significance level (P = 0.08). Further studies with a larger number of samples are needed to determine whether there is a significant connection between the IAR phenotype and FKBP6 up-regulation.
Fig. 3. Expression analysis of equine FKBP6. 
(A) Reverse transcriptase (RT) PCR with FKBP6 exons 5–6 spanning primers in a panel of 10 adult equine tissues; (B) Quantitative RT PCR showing moderate up-regulation of FKBP6 in the sperm of IAR stallions. Next, we compared the expression of all seven FKBP6 exons in testis, normal sperm and IAR sperm, and investigated the presence of splice variants. We observed that equine FKBP6 has 5 splice variants – two in testis, two in sperm and one common for both (Figure 4; Figure S6). Of these, only one - the largest testis variant comprised of exons 1–7 - has been reported in Ensembl (http://www.ensembl.org/index.html), while transcripts omitting exon1, exon3, or comprised of exons 2 and 7 only, are novel findings and improve the current annotation of this equine gene. Notably, while minor differences in FKBP6 transcripts (regarding exon 1) were seen between testis and sperm, no differences were observed between the transcripts in the sperm of normal and IAR stallions. We concluded that the sequence polymorphism that was strongly associated with the IAR phenotype shared no relationship with splicing.
Fig. 4. Equine FKBP6 organization and splicing. 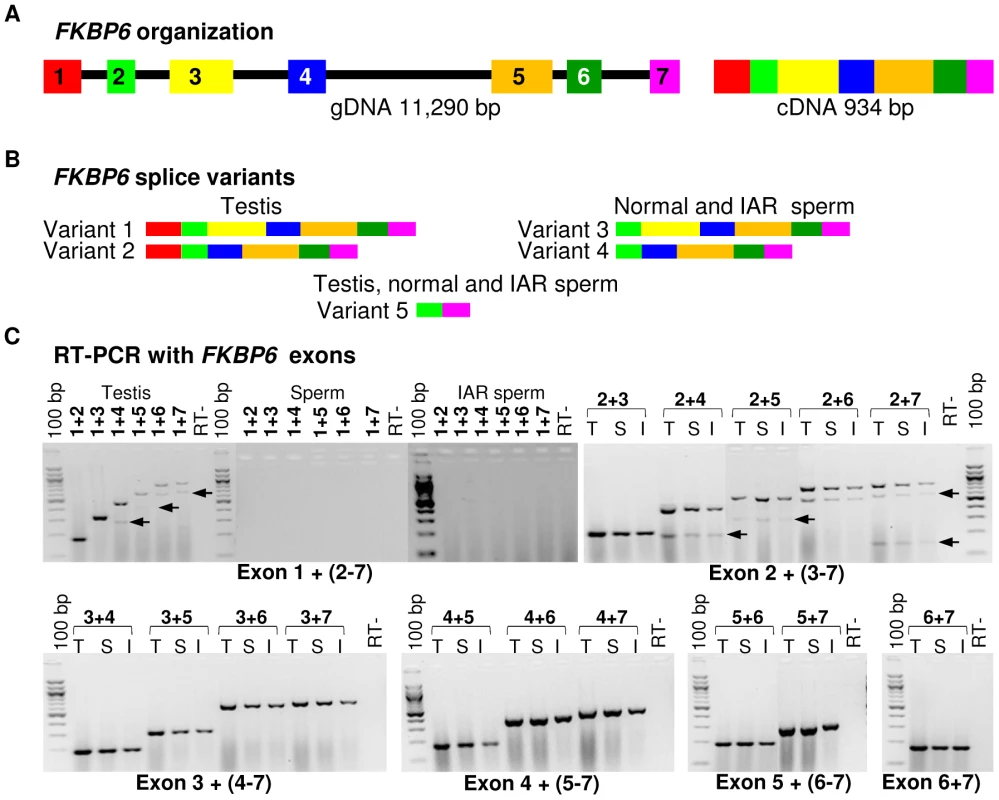
(A) FKBP6 genomic (left) and cDNA (right) according to ENSEMBL (http://www.ensembl.org/index.html); (B) FKBP6 splice variants in stallion testis and sperm according to this study; (C) RT-PCR with combinations of FKBP6 exon primers to determine alternative splicing in testis (T), normal sperm (S) and IAR sperm (I). Arrows show PCR products for alternative splice variants. Finally, we investigated possible allelic bias of FKBP6 expression by direct cDNA sequencing from testis and sperm of one IAR stallion (HS03, the only one with available testis and sperm RNA) and control horses (n = 8) with heterozygous AGAC genotype for the exon 4 SNPs. As expected, cDNA of the IAR stallion was homozygous for the A-alleles. Remarkably, cDNA from heterozygous control animals showed only the G-allele at site g.11040315G>A, and the C-allele at site g.11040379C>A (Figure 5, Figure S7), suggesting monoallelic expression of the gene. However, because of the small sample size it is not clear at this point whether the expression of FKBP6 is predominantly or exclusively monoallelic, and whether the allelic exclusion is random or always biased towards the non-A alleles. Due to the unavailability of parental genotypes, it also remains unclear whether FKBP6 is a subject of genomic imprinting.
Fig. 5. Monoallelic expression of equine FKBP6. 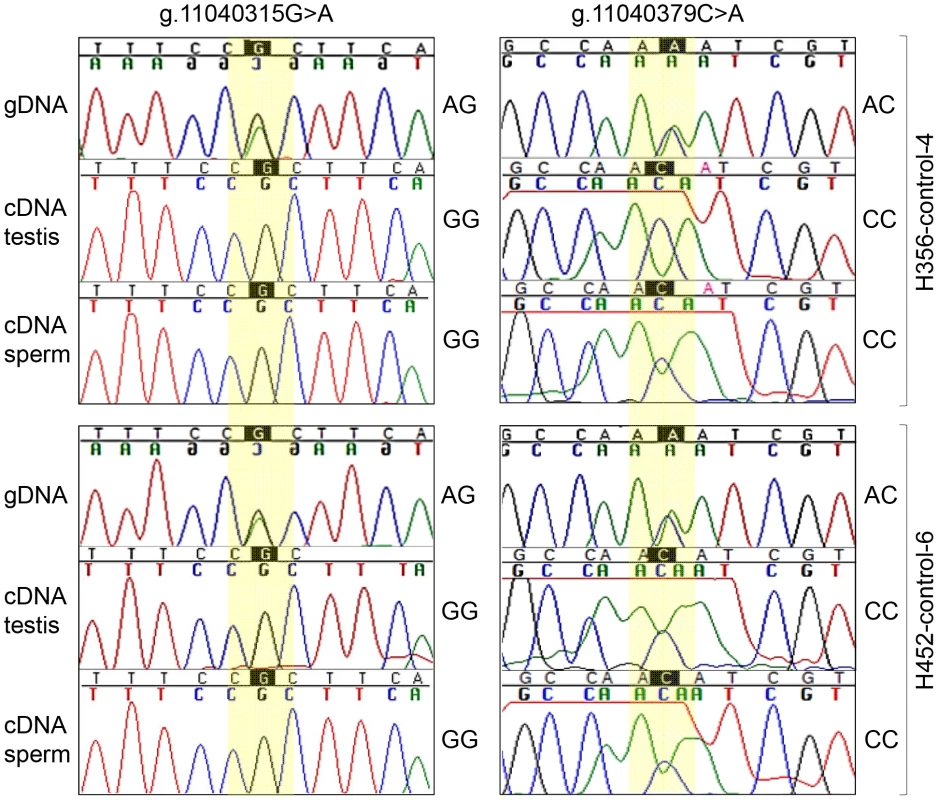
gDNA and cDNA sequences from testis and sperm of two heterozygous control stallions. As an additional observation, the sequence reads of the cDNA, but not the gDNA, flanking g.11040379C>A consistently showed a ‘noisy’ pattern in all individuals regardless of the genotype (Figure 5, Figure S7) suggesting the presence of post-transcriptional mRNA/cDNA modifications at this site.
In silico analysis of the equine FKBP6 protein
The FKBP6 encodes for the FKBP6 protein (alias FKBP36) which is comprised of an N-terminal PPIase domain of the FKBP type encoded by exons 1–3, and a C-terminal domain containing three tetratrico peptide repeat (TPR) motifs, encoded by exons 4–7 (Ensembl: http://www.ensembl.org/index.html; Figure S8). Thus, SNP g.11040379C>A in exon 4, changing histidine to asparagine, is expected to affect the SMART SM00028 domain of TPR-repeat and Superfamily SSF48452 domain of TPR-like repeats (Figure S8). These domains are critical for trans-membrane protein-protein interactions and the formation of multiprotein complexes [35]. However, in silico analysis of the effect p.166H>N in exon 4 did not reveal any changes in the protein structure or the configuration of the trans-membrane segments.
Discussion
Here we report about a successful application of the Equine SNP50 BeadChip [36] and GWAS for the discovery of a susceptibility gene for impaired acrosome reaction in stallions. The utility of this high density equine SNP genotyping platform has been previously validated for association mapping and phylogeny studies in horses and equids [36], for the discovery of a few single gene mutations [37], [38] and for the identification of genomic regions associated with complex traits and genetic disorders, such as osteochondrosis [39], [40], [41], neurological disorders [42], disease resistance [43], and athletic performance [44], [45]. However, to our best knowledge, this is the first application of the SNP50 genotyping platform for the discovery of genes associated with stallion subfertility. It is also among the very few GWAS for reproduction-related disorders in other mammals: previously, GWAS has been used for genetic analysis of impaired sperm functions in humans [46], [47] and pigs [48].
The key factor for detecting significant phenotype-genotype association in the small discovery population (n = 7) in this study was probably the affected horses being related Thoroughbreds. This horse breed originates from a small number of founders, has been a relatively closed population for the past 300 years [49], and is characterized by low genetic diversity, high inbreeding and the highest LD among horse breeds [36]. The latter facilitates GWAS which exploit pair-wise correlations between loci on a common genetic background not interrupted by recombination events [50]. Therefore, a much smaller number of genetic markers (or individuals) allowed effective capturing of information on the variants which were not genotyped. The downside of long LDs is that the associated regions are typically large making the discovery of causative genes/mutations difficult. Indeed, the associated region for IAR spanned about 3.9 Mb in ECA13p (Figure 2) and harbored over 100 genes and 2000 additional SNPs. The search for candidate genes was narrowed down to FKBP6 because it was located in the region of most significant association with IAR both by GWAS and LD analyses (Figure 2). The involvement of FKBP6 in male infertility in mice [33], [51] and humans [52] further strengthened the hypothesis.
Genomic organization, expression profile and domain structure of FK506-binding protein 6 (FKBP6) were first described in humans in connection with Williams syndrome [53]. The gene belongs to the immunophilins FKBP family of which all members feature two domains: an N-terminal prolyl isomerase/FK506-binding domain (a PPIase domain of the FKBP type) and a C-terminal protein-protein interaction domain containing three tetratrico peptide repeat (TPR) motifs [53], [54], [55]. The FKBP6 (alias FKBP36) protein is a component of synaptonemal complex and plays a critical role in male meiosis due to which FKBP6 knockout male mice and rats are sterile with azoospermia, whereas females are reproductively normal [33], [51]. The role of FKBP6 in human male fertility is more enigmatic. There are reports showing that FKBP6 mutations do not cause azoospermia [32], [55], and those suggesting that FKBP6 single nucleotide transitions play a modifying role in the susceptibility to idiopathic spermatogenic impairment [52]. At the same time, the expression of FKBP6 in humans [32], rodents [33] and horses (this study) is limited to males and occurs predominantly or exclusively in testis – an indication of the involvement in spermatogenesis (meiosis) and maybe in sperm functions. The latter is supported by this study where we showed for the first time the presence of FKBP6 transcripts in ejaculated stallion sperm, suggesting that the functions of this gene reach beyond meiotic prophase. Indeed, FKBP6 is thought to mediate the assembly of multiprotein complexes associated with clathrin-coated vesicles [54], such as acrosome or the synaptic vesicles. Further, through the C-terminal TPR domains, FKBP6 acts like a co-chaperone forming complexes with Hsp72, Hsp90, and GAPDH; GAPDH, in turn, promotes membrane-membrane fusion processes [56], e.g., the acrosome reaction [2]. Since FKBP6 acts as GAPDH inhibitor [56], we theorize that FKBP6 transcriptional up-regulation, a tendency observed in the IAR stallions in this study (Figure 3), will negatively affect membrane fusion and acrosome reaction.
The FK506 binding proteins interact through protein-protein complexes with extracellular calcium-sensing receptors, such as ryanodine receptors (RyR), and are involved in maintenance of intracellular Ca2+ homeostasis and the control of membrane excitability in neuronal synapses [57]. If so, it is tempting to speculate that via interactions with calcium channels, FKBP6 could regulate acrosome exocytosis. In parallel to co-chaperone activity, complexes of FKBPs and Hsp90 facilitate interaction with steroid hormone receptors, thus potentially modifying the activity, stability and subcellular localization of the androgen receptor [34], [35], [53], [56].
Despite the evidence of the involvement of FK506 binding proteins in multiple biological processes, including spermatogenesis and fertilization, the current knowledge about FKBP6 is inadequate to properly interpret its role in sperm functions or understand the molecular link between FKBP6 sequence polymorphism and IAR phenotype in this study. However, association of IAR with FKBP6 exon 4 AAAA-genotype (Table 3; Table S9) and FKBP6 monoallelic expression (Figure 5), inspire some ideas.
For example, it has been shown that post-translational protein modifications are encoded in the mRNA, so that even synonymous codons may provide subtly different information for the speed of translation and the nature of post-translational modifications [58], [59], [60]. It is therefore tempting to theorize that the SNPs g.11040315G>A and g.11040379C>A (p.166H>N) may affect transcription and the protein via chromatin-loops, transcription factor recruitment, or mRNA folding, whereas the A-allele might be a modifying factor. Special status of the A-allele is further emphasized by the monoallelic transcription of FKBP6 and the preference for non-A-allele in heterozygotes (Figure 5, Figure S7). Our findings are consistent with the studies in humans where the expression of FKBP6 is also allele-specific [32], and SNPs in the gene are thought to have a modifying effect on impaired spermatogenesis [52]. Despite this, causative relationship between the FKBP6 and reproductive phenotypes in horses or humans need further understanding.
Subtle differences in the expression of two alleles have been detected in numerous non-imprinted human [61], [62], [63], [64] and murine [65] genes. In a few cases, allele-specific expression in low penetrance regulatory loci has been associated with genetic predisposition to disease [62]. Though the underlying mechanisms of allele-specific expression remain largely unknown, interaction of epigenetic factors with genomic variants in cis (within a gene) and trans (non-coding RNA genes, other regulatory elements) has been proposed. It is thought that some of the SNPs associated with disease phenotype by GWAS, might indicate the trans-regulators [62] giving another potential meaning to the FKBP6 polymorphism.
Comparison of equine FKBP6 DNA and protein sequences with human and other mammalian orthologs (http://www.ensembl.org/index.html) shows extensive sequence conservation (pairwise identity between horse and other species >92%; data not shown) but provides no clues to understand the genotype-phenotype association in this study: the polymorphic sites in horse exon 4 are monomorphic for the A-allele in human, mouse, cattle, pig and dog; also, FKBP6 in other mammals comprises 8–9 exons compared to the 7 exons in horses.
Even though our findings clearly point at FKBP6 as a susceptibility gene for IAR in stallions, its penetrance is incomplete, as revealed by genotyping and association analysis of 265 male horses from multiple breeds (Table 3, Tables S8, S9) showing that AA-genotype at the highly associated g.11040315G>A site was present in 9% of fertile stallions and 12% of other controls, and the AAAA-genotype was found in 2% of control stallions of known and unknown fertility. From another hand, incomplete penetrance, is in good agreement with the quantitative nature of IAR as demonstrated by Brinsko and colleagues (2007) showing that the failure of the AR in affected stallions is not complete and affects about 97% of the sperm, while the remaining 3% can perform normal AR. This could have led to phenotyping errors in the follow-up cohort where known fertility for stallions was quantified as at least one living offspring, whereas conception rates for each stallion were unavailable. Thus, it is conceivable that some of these stallions with the IAR susceptibility genotypes could have IAR, and only have living offspring as the result of multiple breeding attempts or assisted reproduction practices.
It is also plausible that either both sequence variants play a role in penetrance of the trait, or perhaps more likely, that the genotype is tagging an underlying functional variant. The results showing that the non-synonymous SNP (p.166H>N) causes no apparent change in protein structure and is less significantly associated with the IAR phenotype than the synonymous SNP, further support the possibility that FKBP6 relates to the IAR phenotype via regulatory or modifying pathways. Besides, given the complexity of acrosome biogenesis and fertilization, it has been proposed that acrosome reaction is regulated by synergistic interactions of several sperm motility and sperm-egg adhesion genes [18] of which FKBP6 might be one. This agrees with our initial uncorrected GWAS results showing moderate association of IAR to several horse chromosomes (Figure 1, Figure S3, Table S2). Among these, a ∼3.5 Mb region from 75 to 78.5 Mb on ECA4q might be of interest for future studies: though extremely gene poor, it contains CADPS2 (Table S2) which encodes a SNARE-complex binding protein and is involved in vesicle exocytosis in the nervous system [66].
Male infertility/subfertility is a recognized concern in humans [67] and livestock species [68], whereas considerable proportion (∼25%) of unexplained male subfertility is caused by defects in sperm functions responsible for fertilization, including the acrosome reaction [20], [22], [24]. Implication of FKBP6 as a susceptibility gene for IAR in stallions in this study, and evolutionary conservation of the gene across diverged mammalian species, suggest it as a new candidate gene for unexplained male subfertility for mammals, including humans. Importantly, because mouse and human fertilization are different [19] and human and horse sperm share more common features [1], molecular studies of fertility in stallions might provide valuable alternative to the rodent models in the search of molecular causes of infertility in man.
In summary, previous studies in humans [32], [47], [52] and the present results strongly suggest the involvement of FKBP6 in various aspects of male fertility, molecular mechanisms of which remain yet to be fully explored. Nevertheless, testing prospective breeding stallions for FKBP6 polymorphism is recommended to identify animals that might require specialized acrosome reaction analysis. This, in turn, may reveal more stallions with IAR, thus increasing the study cohort for future research. Breed-wise statistics of FKBP6 genotype frequencies (Table 4) indicated that the likelihood finding the IAR susceptibility genotype AAAA is the highest in Thoroughbreds (7%) and the lowest in Quarter Horses (0%), while the 3% estimate for other breeds remains tentative because each of the 21 breeds studied was represented by only 1 to 8 individuals.
Tab. 4. FKBP6 exon 4 genotype frequencies across horse breeds: Thoroughbred, TB (n = 145); Quarter Horse, QH (n = 56); other breeds (21 breeds; n = 64; 1 to 8 individuals each). 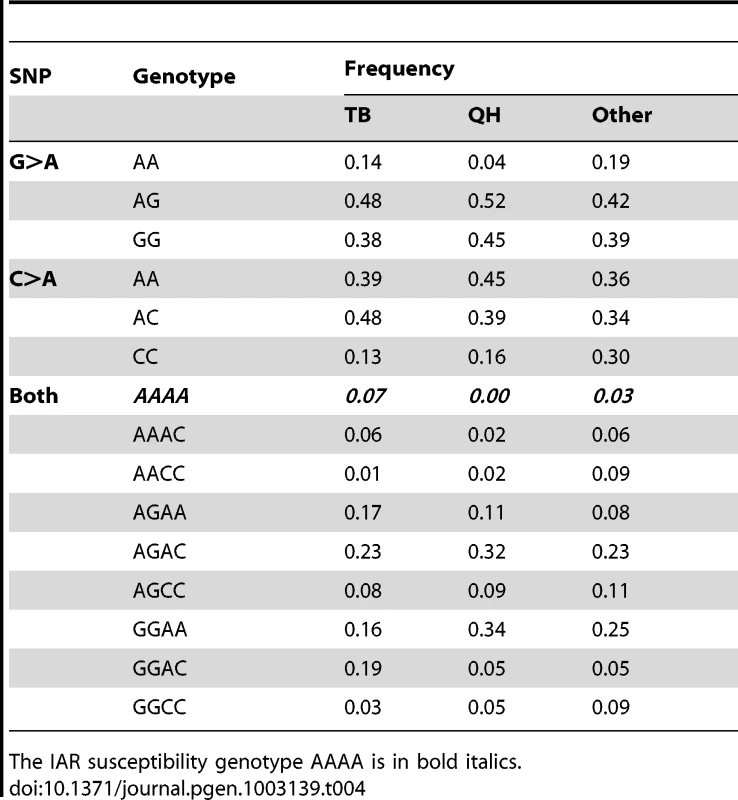
The IAR susceptibility genotype AAAA is in bold italics. Last but not least, the study is one of the first examples of successful use of ejaculated sperm as the source of DNA and RNA for GWAS and gene expression analysis, thus propagating non-invasive sample procurement for fertility genomics in animals and humans.
Materials and Methods
Ethics statement
Procurement of stallion semen, peripheral blood and equine tissues was performed according to the United States Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training and were approved by the Clinical Research Review Committee (CRRCs #08-19; #08-33; #09-32; #09-47) and Animal Use Protocol #2009-115 at Texas A&M University.
Horses and phenotypes
The study cohort for genome wide association comprised of 44 male Thoroughbreds (breed purity confirmed by studbook data over 5 generations) - 7 cases and 37 normal male controls. The cases were initially identified due to unexplained subfertility (0–15% per cycle pregnancy rate) and diagnosed with IAR by incubating sperm with the calcium ionophore A23187 [25]. Acrosome reaction rate of the sperm in cases was 2.9% compared to 96% of sperm in normal stallions. Other sperm parameters, such as percentages of motile sperm and progressively motile sperm, as well as sperm morphologic characteristics, including percentages of abnormal heads and abnormal acrosomes, were in the normal range in the IAR stallions [25]. The control male horses (n = 37) were chosen from our Thoroughbred samples repository (n>200) so that they were not closely related (within a single generation) to the cases.
Follow-up genotyping for discovered variants in exons 4 and 5 of FKBP6 was performed in a cohort of 263 horses from 20 known breeds or of mixed or unknown breed origin and 2 domestic donkeys. This cohort included 82 fertile male horses (including 37 controls for GWAS) that were known to be fertile defined as at least one viable offspring, 5 horses with known normal acrosomal reactions from Brinsko et al. 2007, 7 stallions with IAR, and 171 normal male individuals with unknown fertility status (Table S8). The majority of these horses were Thoroughbreds (n = 145) or Quarter Horses (n = 56) and originated from cytogenetic service lab DNA repositories (n>1000). Samples were coded numerically for confidentiality.
Samples, DNA and RNA extraction
Snap frozen sperm pellets [25] were the only samples available for the IAR stallions, except stallion HS03 (Table 1), from whom testis and somatic tissues were procured by necropsy because the animal was euthanized due to health reasons. Control samples were selected from our equine DNA repository and were originally isolated from peripheral blood using standard protocols [69]. Samples for gene expression analysis came from our established equine tissue panels [70], and from our sperm/testis collections procured from reproductively normal stallions [71]. All tissue samples were stored in RNAlater (Ambion) at −80°C until use. Stallion sperm DNA was extracted as described by with the difference that the sperm were pre-processed with DTT solution (final conc. 8 µM) at 56°C for 1 h. Total RNA was extracted from sperm according to our detailed protocol [71] and from tissues using RNeasy Minikit (Qiagen). Sperm RNA was linearly amplified using using RampUp RNA Amplification Kit (Genisphere).
Pedigree analysis
Data for 5-generation pedigrees of the 7 affected and 37 control Thoroughbreds was retrieved from Pedigree Online Thoroughbred Database (https://www.pedigreeonline.com/ped/renew.php). Multiple Trait Derivative Free Restricted Maximum Likelihood software, MTDFREML [72] was used to construct a pedigree and calculate inbreeding and genetic relationship coefficients among the seven affected (IAR) stallions using methods developed by Wright [73]. A 5-generation pedigree was drawn and pedigree-based statistics calculated in PEDIGRAPH software [74] for the 44 Thoroughbreds used for GWAS. Pedigree-based kinship and inbreeding coefficients were used to construct a relationship matrix for mixed model analyses (below).
SNP genotyping, quality assurance, and detection of population stratification
The SNP genotyping and GWAS encompassed 44 male Thoroughbreds – 7 cases and 37 controls. The Equine SNP50 Beadchip [36] assay was performed at Neogen-GeneSeek, according to the manufacturer's standard procedures using 1 µg of genomic DNA (gDNA) per sample. The equine SNP chip comprised of 54,602 SNPs distributed on the 31 equine autosomes and X chromosome with a 43.2 kb average distance between markers.
Across the 44 samples the mean genotyping call rate was 97.8% (range 96.3% to 98.1%) and no animals were excluded from analysis. Genotypes were filtered using the PLINK toolset v1.06 [75]. A total of 41,521 SNPs passed quality control after excluding those with genotyping rates below 90% (n = 1922), deviating from Hardy-Weinberg equilibrium in controls (P<0.001; n = 154), with minor allele frequency<0.05 (n = 11,224), or having a differential case/control missingness (P<0.01; n = 38); 257 SNPs occurred in more than one category; 41,521 SNPs were used in all subsequent analyses.
In addition to pedigree analysis, the PLINK toolset was used to quantify population stratification based on pair-wise identity-by-state (IBS) distances from SNP genotypes [75]. Genetic distance (D) was calculated as D = 1−[(IBS2+0.5*IBS1)/N], where IBS2 and IBS1 are the number of loci that share either 2 or 1 alleles identical by state (IBS), respectively, and N is the number of loci. Metric multidimensional scaling (MDS) analysis of pair-wise genetic distances (6 dimensions) was used to identify the relationships between animals with PLINK (–mds-plot 6). Differences in IBS pair-wise distances within and between case and control cohorts were evaluated and empirical P-values were computed after 10,000 case/control label swapping permutations in PLINK. Co-ancestry coefficients were estimated from SNP data using PLINK by estimation of the proportion of the genome identical-by-decent (IBD) from P(IBD = 2)+0.5*P(IBD = 1), where P(IBD = 1) and P(IBD = 2) are the probabilities 1 or 2 alleles being IBD. SNP genotyping data are available at www.animalgenome.org/repository/pub/UMN2012.1105/.
Association analyses
Unstructured genome-wide association tests were performed in PLINK using a basic case-control chi-square test as well as logistic modeling for additive, dominant, and recessive allelic effects. For all analyses, genomic inflation factor (GIF, lambda) was calculated and quantile-quantile plots (QQ plots) were generated to detect inflation of test statistics due to population stratification. Basic association and logistic regression analyses in PLINK were followed by 10,000 t-max permutations to correct for multiple testing.
To control for population structure and relatedness within the sample cohort, known pedigree relationships were accounted for using a logistic mixed model [76], which included a relationship matrix using inbreeding and kinship coefficients constructed from available 5 generation pedigree information using Pedigreemm (http://cran.r-project.org/web/packages/pedigreemm/pedigreemm.pdf) and SNPMatrix (http://bioconductor.org/packages/2.6/bioc/manuals/snpMatrix/man/snpMatrix.pdf) in the R environment (R Development Core Team, 2009). For GWAS, uncorrected P-values<5×10−7 and permutation P-values≤0.05 were considered as strong evidence of association [77]. Plots of GWAS results were generated in R (Figure 1, Figure S3).
Haplotype analysis
Eighty six (86) SNPs from the region of interest were computationally phased using fastPHASE [78]. The extent of conserved haplotypes within cases was determined by visual inspection (Figure S5). Haplotype blocks across the entire cohort (cases and controls) were defined in Haploview v 4.1 [79] using both the confidence interval and solid spine of linkage disequilibrium (LD) methods [80]. For the confidence interval method, blocks were created when 90% of informative comparisons were in strong LD (i.e., had confidence interval minimum between 0.7 and 0.98), markers with MAF<0.01 were excluded; D′>0.8 was required to extend haplotype blocks using the strong spine of LD. For haplotype blocks called by both methods, haplotypic associations were computed using the chi-square statistic in Haploview. Correction for multiple testing was achieved by using 10,000 label swapping permutations within haplotypes in blocks to generate permuted P-values. The BioMart software suite (www.biomart.org) was used to query Ensembl, to identify genes within the haplotype boundaries from the Equus caballus (EquCab2) genome assembly.
Candidate gene sequencing, mutation discovery, and association estimates
Equine genes from the significantly associated region and their human orthologs were identified and analyzed for functional relevance using the Ensemble (http://www.ensembl.org/index.html) and the UCSC genome browsers (http://genome.ucsc.edu/). Two candidate genes, TRIM56 and FKBP6 were selected for sequencing. Primers to amplify all exons and exon-intron boundaries were designed using horse WG sequence assembly EquCab2 (http://www.ensembl.org/Equus_caballus/index.html) and Primer 3 software (http://frodo.wi.mit.edu/primer3/input.htm), and are listed in Table S10. The initial PCR reactions were carried out on the gDNA of cases (n = 7) and controls; the products were sequenced using BigDye (Applied Biosystems) chemistry and the reactions were resolved on an ABI-3730 capillary sequencer (Applied Biosystems). Sequences were analyzed for mutations in Sequencher V 4.7 software (GeneCodes Co).
Genotypic and recessive chi-square and Fisher's exact tests of association were performed in discovered SNPs suing the GWAS cohort to identify SNPs associated with IAR phenotype. Two statistically significant SNPs in FKBP6 exon 4 were selected for further genotyping, first in the entire GWAS cohort (n = 44) and next, in a large cohort of 265 samples (Table S8). Genotypes were determined by direct Sanger sequencing of PCR products. Additionally, SNaPShot primers were designed (Table S10) and tested for efficiency on selected samples. Association between phenotype and SNP genotype were tested using chi-square and Fisher's exact tests. Genotypes were coded as genotypic or recessive (0, 1) for the analysis. Genotypic tests were also performed using the combined 2-locus genotypes for both exon 4 SNPs (Table 3, Table S9).
FKBP6 expression analysis
Primers for reverse transcriptase (RT) PCR were designed for all FKBP6 exons (Table S10). In order to determine FKBP6 expression profile, RT-PCR reactions were carried out with primers for exon 4 and intron spanning primers for exons 5 and 6 on RNA isolated from 10 equine tissues, viz., brain, heart, kidney, lung, liver, skeletal muscle, ovary, spleen and testis. Comparison of FKBP6 expression between cases and controls was carried out by quantitative real time (qRT) PCR with primers for exons 4 (Table S10). The qRT-PCR assays were carried out on a LightCycler 480 (Roche Diagnostics), and the results were analyzed with LightCycler 480 Software v1.5. Next, multiple primer combinations (Table S11) were used for RT-PCR on sperm and testis of cases (n = 4) and controls (n = 2) to identify FKBP6 splice variants. The quality of testis and sperm RNA was checked by RT-PCR with intron-spanning primers for sperm - and testis-expressed genes SPA17 (our unpublished data) and PRM2 (Figure S9), and with a sperm-negative gene PTPRC [71]. Amplifications were performed in 15 µL reactions using Superscript III One-Step RT-PCR System and Platinum Taq DNA polymerase (Invitrogen Corporation) as described previously [71]. Finally, RNA from the sperm and/or testis of control horses (n = 8) and one case (HS03) was subjected to direct cDNA sequencing to determine allelic expression of FKBP6 (Table S12). The controls were (AGAC) heterozygous and the case (AAAA) homozygous for the two exon 4 SNPs. The cDNA was synthesized from sperm/testis RNA using SuperScript VILO cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA). Synthesis of cDNA was performed in two-step qRT-PCR by incubating at 25°C for 10 min, 42°C for 60 min and 85°C for 5 min. Synthesized cDNA was purified by Qiagen MinElute PCR purification kit according to manufacturers' protocol, and used as template to amplify FKBP6 exon 4 by PCR. The PCR products were sequenced by BigDye chemistry and the sequences were analyzed in Sequencher 4.7 (Gene Codes) software.
FKBP6 protein analysis
The effect of DNA sequence polymorphism on FKBP6 amino acid sequence and protein functions was studied using DAS (Dense Alignment Surface method) algorithm to identify clear start and end configurations of transmembrane segments in both wild and mutated FKBP6 amino acid sequence (http://www.sbc.su.se/~miklos/DAS/maindas.html). Initially, subcellular localization sites of FKBP6 amino acid sequences were identified by WoLF PSORT software (http://wolfpsort.org/). These protein predictions were based on both known sorting signal motifs and amino acid content. Finally, conserved domains were detected using BLASTp (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Supporting Information
Zdroje
1. VarnerDD, JohnsonL (2007) From a Sperm's Eye View - Revisiting Our Perception of This Intriguing Cell. AAEP Proceedings 53 : 104–177.
2. MayorgaLS, TomesCN, BelmonteSA (2007) Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life 59 : 286–292.
3. ZhaoL, BurkinHR, ShiX, LiL, ReimK, et al. (2007) Complexin I is required for mammalian sperm acrosomal exocytosis. Dev Biol 309 : 236–244.
4. ZhaoL, ReimK, MillerDJ (2008) Complexin-I-deficient sperm are subfertile due to a defect in zona pellucida penetration. Reproduction 136 : 323–334.
5. ChavezJC, de BlasGA, de la Vega-BeltranJL, NishigakiT, ChirinosM, et al. (2011) The opening of maitotoxin-sensitive calcium channels induces the acrosome reaction in human spermatozoa: differences from the zona pellucida. Asian J Androl 13 : 159–165.
6. LeβigJ, ReibetanzU, ArnholdJ, GlanderHJ (2008) Destabilization of acrosome and elastase influence mediate the release of secretory phospholipase A2 from human spermatozoa. Asian J Androl 10 : 829–836.
7. De BlasGA, RoggeroCM, TomesCN, MayorgaLS (2005) Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol 3: e323 doi:10.1371/journal.pbio.0030323.
8. ChirinosM, CarinoC, Gonzalez-GonzalezME, ArreolaE, RevelesR, et al. (2011) Characterization of human sperm binding to homologous recombinant zona pellucida proteins. Reprod Sci 18 : 876–885.
9. KitamuraK, TanakaH, NishimuneY (2005) The RING-finger protein haprin: domains and function in the acrosome reaction. Curr Protein Pept Sci 6 : 567–574.
10. SudhofTC, RizoJ (2011) Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol 3.
11. MeizelS, SonJH (2005) Studies of sperm from mutant mice suggesting that two neurotransmitter receptors are important to the zona pellucida-initiated acrosome reaction. Mol Reprod Dev 72 : 250–258.
12. SatoY, SonJH, TuckerRP, MeizelS (2000) The zona pellucida-initiated acrosome reaction: defect due to mutations in the sperm glycine receptor/Cl(-) channel. Dev Biol 227 : 211–218.
13. FukamiK, NakaoK, InoueT, KataokaY, KurokawaM, et al. (2001) Requirement of phospholipase Cdelta4 for the zona pellucida-induced acrosome reaction. Science 292 : 920–923.
14. JinJL, O'DohertyAM, WangS, ZhengH, SandersKM, et al. (2005) Catsper3 and catsper4 encode two cation channel-like proteins exclusively expressed in the testis. Biol Reprod 73 : 1235–1242.
15. GibbsGM, OrtaG, ReddyT, KoppersAJ, Martinez-LopezP, et al. (2011) Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function. Proceedings of the National Academy of Sciences of the United States of America 108 : 7034–7039.
16. TanigawaM, MiyamotoK, KobayashiS, SatoM, AkutsuH, et al. (2008) Possible involvement of CD81 in acrosome reaction of sperm in mice. Mol Reprod Dev 75 : 150–155.
17. KitamuraK, NishimuraH, NishimuneY, TanakaH (2005) Identification of human HAPRIN potentially involved in the acrosome reaction. J Androl 26 : 511–518.
18. NayerniaK, DrabentB, MeinhardtA, AdhamIM, SchwandtI, et al. (2005) Triple knockouts reveal gene interactions affecting fertility of male mice. Mol Reprod Dev 70 : 406–416.
19. BarrattCLR, PublicoverSJ (2001) Interaction between sperm and zona pellucida in male fertility. Lancet 358 : 1660–1662.
20. Conner SJ, Lefievre L, Kirkman-Brown J, Machado-Oliveira GSM, Michelangeli F, et al.. (2007) Physiological and proteomic approaches to understanding human sperm function. In: Carrell DT, editor. The Genetics of Male Infertility. Totowa, New Jersey: Humana Press. pp. 77–97.
21. CalvoL, Dennison-LagosL, BanksSM, SherinsRJ (1994) Characterization and frequency distribution of sperm acrosome reaction among normal and infertile men. Hum Reprod 9 : 1875–1879.
22. Liu deY, LiuML, GarrettC, BakerHW (2007) Comparison of the frequency of defective sperm-zona pellucida (ZP) binding and the ZP-induced acrosome reaction between subfertile men with normal and abnormal semen. Hum Reprod 22 : 1878–1884.
23. Liu deY, BakerHW (2007) [Assessment of human sperm function and clinical management of male infertility]. Zhonghua Nan Ke Xue 13 : 99–109.
24. VarnerDD, BlanchardTL, BrinskoSP, LoveCC, TaylorTS, et al. (2000) Techniques for evaluating selected reproductive disorders of stallions. Anim Reprod Sci 60–61 : 493–509.
25. BrinskoSP, LoveCC, BauerJE, MacphersonML, VarnerDD (2007) Cholesterol-to-phospholipid ratio in whole sperm and seminal plasma from fertile stallions and stallions with unexplained subfertility. Anim Reprod Sci 99 : 65–71.
26. Durkin K, Raudsepp T, Chowdhary BP (2011) Cytogenetic Evaluation of the Stallion. In: A.OMcKinnon ELS, W.EVaala and D.DVarner, editor. Equine Reproduction: Wiley Blackwell. pp. 1462–1468.
27. RaudseppT, DurkinK, LearT, DasPJ, AvilaF, et al. (2010) Molecular heterogeneity of XY sex reversal in the horse. Anim Genet Suppl 2 : 41–52.
28. MatzukMM, LambDJ (2002) Genetic dissection of mammalian fertility pathways. Nat Cell Biol 4 Suppl: s41–49.
29. MatzukMM, LambDJ (2008) The biology of infertility: research advances and clinical challenges. Nat Med 14 : 1197–1213.
30. Carrell DT (2007) The genetics of male infertility in the era of genomics. In: Carrell DT, editor. The Genetics of Male Infertility. Totowa, New Jersey: Humana press. pp. 3–27.
31. NazRK, CatalanoB (2010) Gene knockouts that affect female fertility: novel targets for contraception. Front Biosci (Schol Ed) 2 : 1092–1112.
32. MiyamatoT, SatoH, YogevL, KleimanS, NamikiM, et al. (2006) Is a genetic defect in Fkbp6 a common cause of azoospermia in humans? Cell Mol Biol Lett 11 : 557–569.
33. CrackowerMA, KolasNK, NoguchiJ, SaraoR, KikuchiK, et al. (2003) Essential role of Fkbp6 in male fertility and homologous chromosome pairing in meiosis. Science 300 : 1291–1295.
34. SunnotelO, HiripiL, LaganK, McDaidJR, De LeonJM, et al. (2010) Alterations in the steroid hormone receptor co-chaperone FKBPL are associated with male infertility: a case-control study. Reprod Biol Endocrin 8 : 22.
35. AllanRK, RatajczakT (2011) Versatile TPR domains accommodate different modes of target protein recognition and function. Cell Stress Chaperon 16 : 353–367.
36. McCueME, BannaschDL, PetersenJL, GurrJ, BaileyE, et al. (2012) A high density SNP array for the domestic horse and extant Perissodactyla: utility for association mapping, genetic diversity, and phylogeny studies. PLoS Genet 8: e1002451 doi:10.1371/journal.pgen.1002451.
37. BrooksSA, GabreskiN, MillerD, BrisbinA, BrownHE, et al. (2010) Whole-genome SNP association in the horse: identification of a deletion in myosin Va responsible for Lavender Foal Syndrome. PLoS Genet 6: e1000909 doi:10.1371/journal.pgen.1000909.
38. Fox-ClipshamLY, CarterSD, GoodheadI, HallN, KnottenbeltDC, et al. (2011) Identification of a mutation associated with fatal Foal Immunodeficiency Syndrome in the Fell and Dales pony. PLoS Genet 7: e1002133 doi:10.1371/journal.pgen.1002133.
39. LykkjenS, DolvikNI, McCueME, RendahlAK, MickelsonJR, et al. (2010) Genome-wide association analysis of osteochondrosis of the tibiotarsal joint in Norwegian Standardbred trotters. Anim Genet 41 Suppl 2 : 111–120.
40. TeyssedreS, DupuisMC, GuerinG, SchiblerL, DenoixJM, et al. (2012) Genome-wide association studies for osteochondrosis in French Trotter horses. J Anim Sci 90 : 45–53.
41. CorbinLJ, BlottSC, SwinburneJE, SibbonsC, Fox-ClipshamLY, et al. (2012) A genome-wide association study of osteochondritis dissecans in the Thoroughbred. Mammalian Genome 23 : 294–303.
42. DupuisMC, ZhangZ, DruetT, DenoixJM, CharlierC, et al. (2011) Results of a haplotype-based GWAS for recurrent laryngeal neuropathy in the horse. Mammalian Genome 22 : 613–620.
43. GoYY, BaileyE, CookDG, ColemanSJ, MacleodJN, et al. (2011) Genome-wide association study among four horse breeds identifies a common haplotype associated with in vitro CD3+ T cell susceptibility/resistance to equine arteritis virus infection. J Virol 85 : 13174–13184.
44. HillEW, GuJ, EiversSS, FonsecaRG, McGivneyBA, et al. (2010) A sequence polymorphism in MSTN predicts sprinting ability and racing stamina in thoroughbred horses. PLoS ONE 5: e8645 doi:10.1371/journal.pone.0008645.
45. HillEW, McGivneyBA, GuJ, WhistonR, MachughDE (2010) A genome-wide SNP-association study confirms a sequence variant (g.66493737C>T) in the equine myostatin (MSTN) gene as the most powerful predictor of optimum racing distance for Thoroughbred racehorses. BMC Genomics 11 : 552.
46. AstonKI, CarrellDT (2009) Genome-wide study of single-nucleotide polymorphisms associated with azoospermia and severe oligozoospermia. J Androl 30 : 711–725.
47. AstonKI, KrauszC, LafaceI, Ruiz-CastaneE, CarrellDT (2010) Evaluation of 172 candidate polymorphisms for association with oligozoospermia or azoospermia in a large cohort of men of European descent. Hum Reprod 25 : 1383–1397.
48. SironenA, UimariP, NagyS, PakuS, AnderssonM, et al. (2010) Knobbed acrosome defect is associated with a region containing the genes STK17b and HECW2 on porcine chromosome 15. BMC Genomics 11 : 699–706.
49. CunninghamEP, DooleyJJ, SplanRK, BradleyDG (2001) Microsatellite diversity, pedigree relatedness and the contributions of founder lineages to thoroughbred horses. Anim Genet 32 : 360–364.
50. McCarthyMI, AbecasisGR, CardonLR, GoldsteinDB, LittleJ, et al. (2008) Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nature Reviews Genetics 9 : 356–369.
51. NoguchiJ, OzawaM, NakaiM, SomfaiT, KikuchiK, et al. (2008) Affected homologous chromosome pairing and phosphorylation of testis specific histone, H2AX, in male meiosis under FKBP6 deficiency. Journal Reprod Devel 54 : 203–207.
52. ZhangW, ZhangS, XiaoC, YangY, ZhoucunA (2007) Mutation screening of the FKBP6 gene and its association study with spermatogenic impairment in idiopathic infertile men. Reproduction 133 : 511–516.
53. MengX, LuX, MorrisCA, KeatingMT (1998) A novel human gene FKBP6 is deleted in Williams syndrome. Genomics 52 : 130–137.
54. JarczowskiF, FischerG, EdlichF (2008) FKBP36 forms complexes with clathrin and Hsp72 in spermatocytes. Biochemistry 47 : 6946–6952.
55. WesterveldGH, ReppingS, LombardiMP, van der VeenF (2005) Mutations in the chromosome pairing gene FKBP6 are not a common cause of non-obstructive azoospermia. Molecular Human Reproduction 11 : 673–675.
56. JarczowskiF, JahreisG, ErdmannF, SchierhornA, FischerG, et al. (2009) FKBP36 is an inherent multifunctional glyceraldehyde-3-phosphate dehydrogenase inhibitor. The Journal of Biological Chemistry 284 : 766–773.
57. ChattopadhayaS, HarikishoreA, YoonHS (2011) Role of FK506 binding proteins in neurodegenerative disorders. Current Medicinal Chemistry 18 : 5380–5397.
58. ZhangF, SahaS, ShabalinaSA, KashinaA (2010) Differential arginylation of actin isoforms is regulated by coding sequence-dependent degradation. Science 329 : 1534–1537.
59. BakerM (2010) Biochemistry. Hidden code in the protein code. Nature Methods 7 : 874.
60. VisserM, KayserM, PalstraRJ (2012) HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Research 22 : 446–455.
61. GimelbrantA, HutchinsonJN, ThompsonBR, ChessA (2007) Widespread monoallelic expression on human autosomes. Science 318 : 1136–1140.
62. de la ChapelleA (2009) Genetic predisposition to human disease: allele-specific expression and low-penetrance regulatory loci. Oncogene 28 : 3345–3348.
63. DuttaD, EnsmingerAW, ZuckerJP, ChessA (2009) Asynchronous replication and autosome-pair non-equivalence in human embryonic stem cells. PLoS ONE 4: e4970 doi:10.1371/journal.pone.0004970.
64. SongMY, KimHE, KimS, ChoiIH, LeeJK (2012) SNP-based large-scale identification of allele-specific gene expression in human B cells. Gene 493 : 211–218.
65. LiSM, ValoZ, WangJ, GaoH, BowersCW, et al. (2012) Transcriptome-wide survey of mouse CNS-derived cells reveals monoallelic expression within novel gene families. PLoS ONE 7: e31751 doi:10.1371/journal.pone.0031751.
66. KhodthongC, KabachinskiG, JamesDJ, MartinTF (2011) Munc13 homology domain-1 in CAPS/UNC31 mediates SNARE binding required for priming vesicle exocytosis. Cell Metab 14 : 254–263.
67. SamplaskiMK, AgarwalA, SharmaR, SabaneghE (2010) New generation of diagnostic tests for infertility: review of specialized semen tests. Int J Urol 17 : 839–847.
68. OgorevcJ, DovcP, KunejT (2010) Comparative Genomics Approach to Identify Candidate Genetic Loci for Male Fertility. Reprod Domest Anim 46 : 229–239.
69. Birren B, Green ED, Klapholz S, Myers RM, Roskams J (1997) Genome Analysis. Analyzing DNA. Laboratory Manual. New York: Cold Spring Harbor Laboratory press. 675 p.
70. PariaN, RaudseppT, Pearks WilkersonAJ, O'BrienPC, Ferguson-SmithMA, et al. (2011) A gene catalogue of the euchromatic male-specific region of the horse Y chromosome: comparison with human and other mammals. PLoS ONE 6: e21374 doi:10.1371/journal.pone.0021374.
71. DasPJ, PariaN, Gustafson-SeaburyA, VishnoiM, ChakiSP, et al. (2010) Total RNA isolation from stallion sperm and testis biopsies. Theriogenology 74 : 1099–1106, 1106e1091–1092.
72. Boldman KG, Kriese LA, Van Vleck LD, Kachman SD (1993) A Manual for Use of MTDFREML. A set of programs to obtain estimates of variance and covariances [Draft]. US Department of Agriculture, Agricultural Research Service.
73. WrightS, McPheeHC (1925) An approximate method of calculating coefficients of inbreeding and relationship from livestock pedigrees. J Agr Res 31 : 377.
74. GarbeJR, DaY (2003) A software tool for the graphical visualization of large and complex populations. Acta Genetica Sinica 30 : 1193–1195.
75. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics 81 : 559–575.
76. YuJ, PressoirG, BriggsWH, Vroh BiI, YamasakiM, et al. (2006) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nature Genetics 38 : 203–208.
77. HirschhornJN, DalyMJ (2005) Genome-wide association studies for common diseases and complex traits. Nature Reviews Genetics 6 : 95–108.
78. ScheetP, StephensM (2006) A fast and flexible statistical model for large-scale population genotype data: applications to inferring missing genotypes and haplotypic phase. American Journal of Human Genetics 78 : 629–644.
79. BarrettJC, FryB, MallerJ, DalyMJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 : 263–265.
80. GabrielSB, SchaffnerSF, NguyenH, MooreJM, RoyJ, et al. (2002) The structure of haplotype blocks in the human genome. Science 296 : 2225–2229.
Štítky
Genetika Reprodukční medicína
Článek Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction DiseaseČlánek Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination SchemesČlánek Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 12- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- A Mystery Unraveled: Essentiality of RNase III in Is Caused by Resident Prophages
- Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction Disease
- Insertion/Deletion Polymorphisms in the Promoter Are a Risk Factor for Bladder Exstrophy Epispadias Complex
- Mi2β Is Required for γ-Globin Gene Silencing: Temporal Assembly of a GATA-1-FOG-1-Mi2 Repressor Complex in β-YAC Transgenic Mice
- Dissection of a Quantitative Trait Locus for PR Interval Duration Identifies as a Novel Modulator of Cardiac Conduction
- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Construction of a Global Pain Systems Network Highlights Phospholipid Signaling as a Regulator of Heat Nociception
- Genes Contributing to Pain Sensitivity in the Normal Population: An Exome Sequencing Study
- Identification of , a Locus Controlling Dominant Resistance to Autoimmune Orchitis, as Kinesin Family Member 1C
- ATX1-Generated H3K4me3 Is Required for Efficient Elongation of Transcription, Not Initiation, at ATX1-Regulated Genes
- Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome
- Identification of Novel Type 2 Diabetes Candidate Genes Involved in the Crosstalk between the Mitochondrial and the Insulin Signaling Systems
- The Genetic Architecture of Adaptations to High Altitude in Ethiopia
- Population Genomics of the Endosymbiont in
- Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination Schemes
- Testicular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals
- A Yeast GSK-3 Kinase Mck1 Promotes Cdc6 Degradation to Inhibit DNA Re-Replication
- Genetic Adaptation Associated with Genome-Doubling in Autotetraploid
- The Essential Function of RNase III Is to Silence Foreign Toxin Genes
- Long-Range Regulatory Polymorphisms Affecting a GABA Receptor Constitute a Quantitative Trait Locus (QTL) for Social Behavior in
- A New Isolation with Migration Model along Complete Genomes Infers Very Different Divergence Processes among Closely Related Great Ape Species
- Chromosome Fragile Sites in Harbor Matrix Attachment Regions That May Be Associated with Ancestral Chromosome Rearrangement Events
- Genome-Wide Association Study Implicates Testis-Sperm Specific as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions
- A Mechanism of Gene Amplification Driven by Small DNA Fragments
- Base Damage within Single-Strand DNA Underlies Hypermutability Induced by a Ubiquitous Environmental Agent
- Integrative Analysis of a Cross-Loci Regulation Network Identifies as a Gene Regulating Insulin Secretion from Pancreatic Islets
- Reciprocal Signaling between the Ectoderm and a Mesendodermal Left-Right Organizer Directs Left-Right Determination in the Sea Urchin Embryo
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Genome-Wide Fine-Scale Recombination Rate Variation in
- Deciphering the Transcriptional-Regulatory Network of Flocculation in
- On Lung Function and Interactions Using Genome-Wide Data
- Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function
- The Effective Population Size of Malaria Mosquitoes: Large Impact of Vector Control
- Recessive Mutations in Implicate β-III Spectrin in Both Cognitive and Motor Development
- An Excess of Gene Expression Divergence on the X Chromosome in Embryos: Implications for the Faster-X Hypothesis
- Reduced Life- and Healthspan in Mice Carrying a Mono-Allelic MVA Mutation
- Natural Variation at the MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in
- Histone Deacetylase HDA6 Is Functionally Associated with AS1 in Repression of Genes in
- A Framework for the Establishment of a Cnidarian Gene Regulatory Network for “Endomesoderm” Specification: The Inputs of ß-Catenin/TCF Signaling
- A Polycomb Group Protein Is Retained at Specific Sites on Chromatin in Mitosis
- Diapause Formation and Downregulation of Insulin-Like Signaling via DAF-16/FOXO Delays Axonal Degeneration and Neuronal Loss
- Genes That Act Downstream of Sensory Neurons to Influence Longevity, Dauer Formation, and Pathogen Responses in
- A Genome-Wide RNAi Screen Reveals MAP Kinase Phosphatases as Key ERK Pathway Regulators during Embryonic Stem Cell Differentiation
- Recurrent Targeted Genes of Hepatitis B Virus in the Liver Cancer Genomes Identified by a Next-Generation Sequencing–Based Approach
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
- Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
- Systems Genetic Analysis of Osteoblast-Lineage Cells
- Population Genomics of Inversion Polymorphisms in
- Spreading of Heterochromatin Is Limited to Specific Families of Maize Retrotransposons
- DNA Topoisomerases Maintain Promoters in a State Competent for Transcriptional Activation in
- A Histone Deacetylase Adjusts Transcription Kinetics at Coding Sequences during Morphogenesis
- Approaching the Functional Annotation of Fungal Virulence Factors Using Cross-Species Genetic Interaction Profiling
- Evidence for the Robustness of Protein Complexes to Inter-Species Hybridization
- Systematic Identification of Rhythmic Genes Reveals as a New Element in the Circadian Clockwork
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



