-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Wing Patterns in the Mist
article has not abstract
Published in the journal: . PLoS Genet 6(2): e32767. doi:10.1371/journal.pgen.1000822
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1000822Summary
article has not abstract
The aesthetic appeal of butterfly wing patterns has been costly to their status as a tool of fundamental scientific inquiry. Thus, while mimetic convergence in wing patterns between edible “Batesian” mimics and distasteful models, or between different distasteful “Müllerian” mimics (species that cooperate to educate predators) has long been the subject of genetic analysis [1] and field experiments [2], most biology text books confine mimicry to sections on striking adaptations without applying these examples to broader topics of evolution. Meanwhile, the study of color patterns in animals, often tucked into the same sections of texts, is undergoing a revolution in this age of evo-devo and genomics [3]. Among insect models for studying color pattern, the genus Heliconius is gaining the attention of an ever-widening audience ([4]–[6]; Figure 1).
Fig. 1. Three natural hybrid zones between parapatric populations of different Heliconius species. 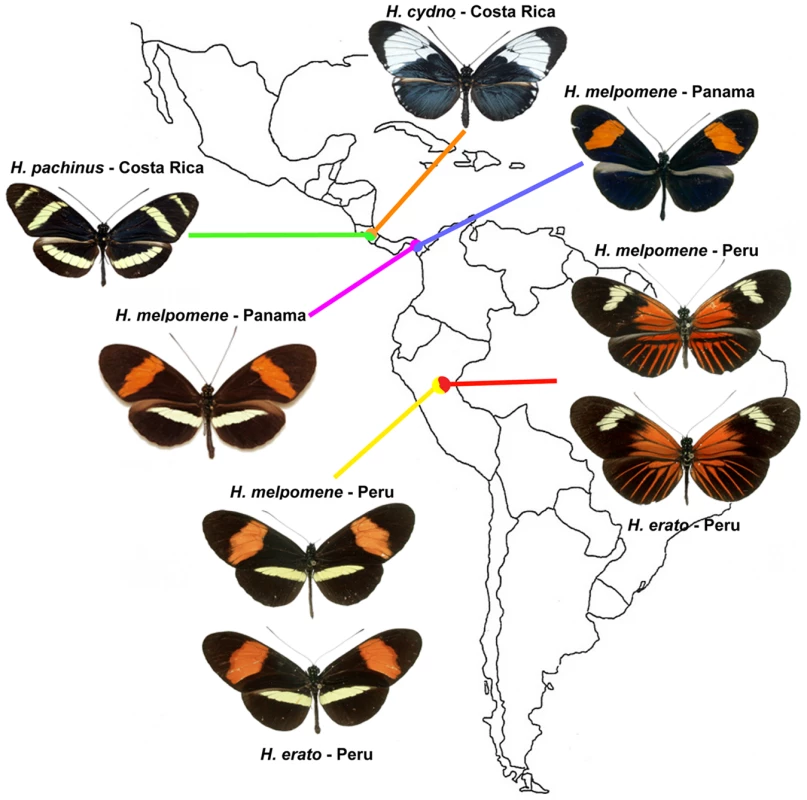
Only a subset of the phenotypic diversity and geographical distribution of the erato, melpomene, and cydno clades is represented. Müllerian mimicry is here illustrated by the convergence in wing patterns of H. melpomene and H. erato on both sides of the Peruvian Andes (yellow and red). H. pachinus and H. cydno are sister species that occasionally hybridize. Heliconius: Taxonomic Hotspot
Early in the 20th century, Oxford's pre-eminent evolutionist and student of insect color patterns, Edward B. Poulton, urged Harry Eltringham to study taxonomic relationships of a spectacularly colorful, mimetic, and diverse set of specimens pouring into European museums from field collectors across the Neotropics. Eltringham [7] distinguished Heliconius erato and Heliconius melpomene groupings and noted repeated mimetic convergence between them. However, within those groupings, he failed to distinguish species, races, and hybrids. In the mid 1950s, William Beebe and associates initiated studies of life history, behavior, systematics, and genetics of Heliconius at Simla in Trinidad. There, Michael Emsley elucidated biogeographic details of the system [8]. It soon became clear that many rare “taxa” described as species by museum workers were in fact recombinants occurring in narrow hybrid zones between two distinct mimetic races. In these zones Müllerian partners erato and melpomene each generate similar arrays of hybrid phenotypes, many of which would be sufficiently distinct to warrant separate species status when viewed out of context.
Genetics of Parallel Mimetic Radiations
In the 1960s, genetic studies of H. erato and H. melpomene at Simla established the framework of classification of pattern loci in general use today [9]. In 1979, Turner [10] reported a strong discrepancy in levels of differentiation in color pattern versus allozyme loci across the geographical range of erato and melpomene. Thus, if viewed only through the lens of structural genes not manifest in the visible phenotype, few of the many races described for these species would be delimited. Later research in Peru on selection and gene flow in parallel interracial hybrid zones by James Mallet [11] set the stage for work on genomic hot spots described in this issue [12],[13].
Several teams have been busy in recent years trying to relate underlying allelic variation in color pattern observed in laboratory crosses and in natural hybrid zones to changes occurring in the genome. Classic genetic mapping previously showed that these adaptive polymorphisms in four different radiations were linked to homologous intervals [14]–[16]. In particular, the B/D locus, which controls the presence/absence of red patterns, and the Yb/Cr locus, which controls the presence/absence of a yellow bar, respectively map to homologous linkage groups between the co-mimics H. melpomene and H. erato, although co-mimetic phenotypes evolved independently. In other words, convergent evolution in wing patterning between species involved the same genetic intervals, and, since synteny between distantly related Lepidoptera is conserved [17], by extension, likely many of the same genes. This ignited a push to narrow the search to actual genes or nucleotide changes responsible for parallel wing pattern shifts, to illuminate genetic and developmental mechanisms responsible for generating spectacular and adaptive morphological diversity. Are cis-regulatory or trans-regulatory changes responsible for these polymorphisms [18],[19]? Do similar phenotypes reflect identical nucleotide changes, or independent functional changes in homologous genes or developmental pathways? The current work appears to be on a path that will help resolve questions about genotype phenotype connections.
Hybrid Zones Uncover the Smoking Guns of Selection
The Heliconius system forms a unique replicated natural experiment to study the genetics of adaptive traits: allowing comparison between parapatric races of different phenotypes, between geographically distant races of similar phenotypes, and finally, between different species (co-mimics) across parallel inter-racial hybrid zones. The papers in this issue exploit this system by seeking signatures of selection across previously identified genetic intervals B/D and Yb/Cr in hybrid zones where populations of different phenotypes are admixed. Indeed, in these species mimicry ring structure on both sides of a hybrid zone imposes a strong positive frequent-dependent selection favoring common wing patterns [2],[11]. This is expected to result in a peak of population differentiation at causative genetic loci, because pattern alleles from race A that introgress into race B should be quickly eliminated according to their altered visual effects on pattern (and vice-versa). Accordingly, both Baxter et al. [13] and Counterman et al. [12] found peaks of population differentiation within H. melpomene and H. erato wing pattern loci, whereas unlinked regions of the genome showed no deviation from neutrality. Also, both studies form a consistent set of observations at a finer genomic scale by looking for haplotypes statistically associated with a certain phenotype. They found a rapid decay in linkage disequilibrium in these species, yet they did not identify completely fixed differences that would pinpoint wing pattern genes with confidence. However, both studies implicated a kinesin-motor gene (kinesin) as a B/D candidate gene, since it was close to a hotspot of genotype-by-phenotype association and also showed a higher expression level correlating with red pattern phenotypes. Similarly, both studies identified a “parallel” peak of genotype-to-phenotype association between polymorphism in a Leucine-Rich Repeat gene (LRR) and the Yb/Cr phenotypes. Finally, although the two studies are somewhat complementary in their design, they do not always converge in their results. For instance, while Baxter et al. sampled three geographically distant pairs of admixing populations, Counterman et al. focused their geographical sampling on a narrow area with a sharp transition in wing phenotypes where numerous generations of recombination have had the opportunity to break down variation around causative switch genes. In this latter study (and to a lesser extent in Baxter et al.), several hotspots of pattern association were observed in addition to kinesin and LRR. This raises the possibility that loci involved in pattern variation in each zone of a wing consist of several functional sites, whether they are coding or regulatory changes. While puzzling at first sight, this observation is consistent with the notion that these loci are supergenes with multiple wing patterning effects [14],[15], with the observation in Drosophila that tightly linked mutations of small effect participate in shaping an allele of major effect [20] and with a “Window/Shutter” model for interpreting variation in Heliconius wing patches and bands [21].
The Best Model Organism for Integrative Biology?
Understanding the evolution of diversity will surely involve better integration of ecology, behavior, population genetics, and developmental biology, leading to new models of species diversification that incorporate well-characterized selective environments, adaptive peaks, and how networks of genes determine important phenotypes. Heliconius butterflies are clearly emerging as a premier model system for such integrative research (e.g., [22]). The studies reported here represent major steps forward in more respects than could be abstracted. But, to be honest, the genes that underlie Heliconius wing patterns still seem like a rainforest under a shroud of fog. Only a few canopy trees are visible as we fly over. It should be exciting when the clouds lift.
Zdroje
1. ClarkeCA
SheppardPM
1963 Interactions between major genes and polygenes in the determination of the mimetic patterns of Papilio dardanus. Evolution 17 404 413
2. KapanDD
2001 Three-butterfly system provides a field test of müllerian mimicry. Nature 409 338 340
3. ProtasME
PatelN
2008 Evolution of Coloration Patterns. Ann Rev Cell Dev Biol 24 425 46
4. ParchemRJ
PerryMW
PatelNH
2007 Patterns on the Insect wing. Curr Opin Genet Dev 17 300 308
5. JoronM
JigginsCD
PapanicolaouA
McMillanWO
2006 Heliconius wing patterns: an evo-devo model for understanding phenotypic diversity. Heredity 97 157 67
6. PapaR
MartinA
ReedRD
2008 Genomic hotspots of adaptation in butterfly wing pattern evolution. Curr Opin Genet Dev 18 559 64
7. EltringhamH
1916 On specific and mimetic relationships in the genus Heliconius. Trans Ent Soc Lond 1916 101 155
8. EmsleyMG
1965 Speciation in Heliconius (Lep., Nymphalidae): morphology and geographical distribution. Zoologica 50 191 254
9. SheppardPM
TurnerJRG
BrownKS
BensonWW
SingerMC
1985 Genetics and the evolution of Müllerian mimicry in Heliconius butterflies. Philos Trans R Soc Lond B Biol Sci 308 433 610
10. TurnerJRG
1979 Contrasting modes of evolution in the same genome: allozymes and adaptive changes in Heliconius. Proc Natl Acad Sci USA 76 1924 1928
11. MalletJ
1989 The genetics of warning colour in Peruvian hybrid zones of Heliconius erato and H. melpomene. Proc R Soc Lond B Biol Sci 236 163 185
12. CountermanBA
Araujo-PerezF
HinesHM
BaxterSW
MorrisonCM
2010 Genomic hotspots for adaptation: the population genetics of Müllerian mimicry in Heliconius erato. PLoS Genet 6(2) e1000796 doi:10.1371/journal.pgen.1000796
13. BaxterSW
NadeauN
MarojaL
WilkinsonP
BrianA
2010 Genomic hotspots for adaptation: the population genetics of Müllerian mimicry in the Heliconius melpomene clade. PLoS Genet 6(2) e1000794 doi:10.1371/journal.pgen.1000794
14. BaxterSW
PapaR
ChamberlainN
HumphraySJ
JoronM
2008 Convergent evolution in the genetic basis of Müllerian mimicry in Heliconius butterflies. Genetics 180 1567 77
15. JoronM
PapaR
BeltránM
ChamberlainN
MavárezJ
2006 A conserved supergene locus controls colour pattern diversity in Heliconius butterflies. PLoS Biol 4(10) e303 doi:10.1371/journal.pbio.0040303
16. KronforstMR
KapanDD
GilbertLE
2006 Parallel genetic architecture of parallel adaptive radiations in mimetic Heliconius butterflies. Genetics 174 535 539
17. PringleB
BaxterSW
WebsterCL
PapanicolaouA
LeeSF
2007 Synteny and chromosome evolution in the Lepidoptera: Evidence from mapping in Heliconius melpomene. Genetics 177 417 426
18. HoekstraHE
CoyneJA
2007 The locus of evolution: evo devo and the genetics of adaptation. Evolution 61 995 1016
19. SternDL
OrgogozoV
2008 The loci of evolution: how predictable is genetic evolution? Evolution 62 2155 77
20. McGregorAP
OrgogozoV
DelonI
ZanetJ
SrinivasanDG
2007 Morphological evolution through multiple cis-regulatory mutations at a single gene. Nature 448 587 90
21. GilbertLE
2003 Adaptive novelty through introgression in Heliconius wing patterns: evidence for a shared genetic “tool box” from synthetic hybrid zones and a theory of diversification.
BoggsCL
WattWB
EhrlichPR
Ecology and evolution taking flight: Butterflies as model systems Chicago University of Chicago Press 281 318
22. ChamberlainNL
HillR
GilbertL
KapanD
KronforstM
2009 Polymorphic butterfly reveals the missing link in ecological speciation. Science 326 847 850
Štítky
Genetika Reprodukční medicína
Článek Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the GenomeČlánek Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in MiceČlánek Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 2- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Scale of Population Structure in
- Allelic Exchange of Pheromones and Their Receptors Reprograms Sexual Identity in
- Genetic and Functional Dissection of and in Age-Related Macular Degeneration
- A Single Nucleotide Polymorphism within the Acetyl-Coenzyme A Carboxylase Beta Gene Is Associated with Proteinuria in Patients with Type 2 Diabetes
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Cdk2 Is Required for p53-Independent G/M Checkpoint Control
- Uncoupling of Satellite DNA and Centromeric Function in the Genus
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in the Clade
- Use of DNA–Damaging Agents and RNA Pooling to Assess Expression Profiles Associated with and Mutation Status in Familial Breast Cancer Patients
- Cheating by Exploitation of Developmental Prestalk Patterning in
- Replication and Active Demethylation Represent Partially Overlapping Mechanisms for Erasure of H3K4me3 in Budding Yeast
- Cdk1 Targets Srs2 to Complete Synthesis-Dependent Strand Annealing and to Promote Recombinational Repair
- A Genome-Wide Association Study Identifies Susceptibility Variants for Type 2 Diabetes in Han Chinese
- Genome-Wide Identification of Susceptibility Alleles for Viral Infections through a Population Genetics Approach
- Transcriptional Rewiring of the Sex Determining Gene Duplicate by Transposable Elements
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in
- Proteasome Nuclear Activity Affects Chromosome Stability by Controlling the Turnover of Mms22, a Protein Important for DNA Repair
- Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in Mice
- Structure, Function, and Evolution of the spp. Genome
- Human and Non-Human Primate Genomes Share Hotspots of Positive Selection
- A Kinase-Independent Role for the Rad3-Rad26 Complex in Recruitment of Tel1 to Telomeres in Fission Yeast
- Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' Disease
- Molecular Evolution and Functional Characterization of Insulin-Like Peptides
- Molecular Poltergeists: Mitochondrial DNA Copies () in Sequenced Nuclear Genomes
- Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD Tags
- Wing Patterns in the Mist
- DNA Binding of Centromere Protein C (CENPC) Is Stabilized by Single-Stranded RNA
- Genome-Wide Association Study Reveals Multiple Loci Associated with Primary Tooth Development during Infancy
- Mutations in , Encoding an Equilibrative Nucleoside Transporter ENT3, Cause a Familial Histiocytosis Syndrome (Faisalabad Histiocytosis) and Familial Rosai-Dorfman Disease
- Genome-Wide Identification of Binding Sites Defines Distinct Functions for PHA-4/FOXA in Development and Environmental Response
- Ku Regulates the Non-Homologous End Joining Pathway Choice of DNA Double-Strand Break Repair in Human Somatic Cells
- Nucleoporins and Transcription: New Connections, New Questions
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Nucleoporins and Transcription: New Connections, New Questions
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



