-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMolecular Poltergeists: Mitochondrial DNA Copies () in Sequenced Nuclear Genomes
The natural transfer of DNA from mitochondria to the nucleus generates nuclear copies of mitochondrial DNA (numts) and is an ongoing evolutionary process, as genome sequences attest. In humans, five different numts cause genetic disease and a dozen human loci are polymorphic for the presence of numts, underscoring the rapid rate at which mitochondrial sequences reach the nucleus over evolutionary time. In the laboratory and in nature, numts enter the nuclear DNA via non-homolgous end joining (NHEJ) at double-strand breaks (DSBs). The frequency of numt insertions among 85 sequenced eukaryotic genomes reveal that numt content is strongly correlated with genome size, suggesting that the numt insertion rate might be limited by DSB frequency. Polymorphic numts in humans link maternally inherited mitochondrial genotypes to nuclear DNA haplotypes during the past, offering new opportunities to associate nuclear markers with mitochondrial markers back in time.
Published in the journal: . PLoS Genet 6(2): e32767. doi:10.1371/journal.pgen.1000834
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1000834Summary
The natural transfer of DNA from mitochondria to the nucleus generates nuclear copies of mitochondrial DNA (numts) and is an ongoing evolutionary process, as genome sequences attest. In humans, five different numts cause genetic disease and a dozen human loci are polymorphic for the presence of numts, underscoring the rapid rate at which mitochondrial sequences reach the nucleus over evolutionary time. In the laboratory and in nature, numts enter the nuclear DNA via non-homolgous end joining (NHEJ) at double-strand breaks (DSBs). The frequency of numt insertions among 85 sequenced eukaryotic genomes reveal that numt content is strongly correlated with genome size, suggesting that the numt insertion rate might be limited by DSB frequency. Polymorphic numts in humans link maternally inherited mitochondrial genotypes to nuclear DNA haplotypes during the past, offering new opportunities to associate nuclear markers with mitochondrial markers back in time.
Introduction
Endosymbiosis is germane to eukaryote evolution, and gene transfers from organelles to the nucleus were an important mechanism of genetic variation that helped to forge the prokaryote-to-eukaryote transition [1]–[3]. Though DNA can be experimentally relocated from organelles to the nucleus in the laboratory [4],[5], the more far-reaching experiment is the one ongoing in nature over evolutionary time. All genome sequences from eukaryotes that have DNA in their mitochondria (for exceptions see [6]) harbour evidence for the ongoing process of organelle-to-nuclear DNA transfer in the form of nuclear copies of mitochondrial and, in the case of plants, chloroplast DNA [7]. Genome sequences from those eukaryotes that have lost their mitochondrial DNA altogether still harbour evidence for gene transfers from the mitochondrion during the early phases of eukaryote history [3],[6],[8].
The story of gene wanderings, from organelles to the nucleus during recent evolutionary time, started with the report of a gene sequence that was present in both the nuclear and the mitochondrial genome in Neurospora [6],[9]. That set the stage for a deluge of other examples for Òpromiscuous DNAÓ [10]. The term numts (pronounced “new-mights”), for nuclear sequence of mitochondrial origin, was coined [11] to designate such DNA, which was often discovered inadvertently in the search for bona fide mtDNA (Box 1). Since that time, numt population polymorphism [12],[13] and numt variation among human siblings has been found [14]. In the case of photosynthetic species, the corresponding sequences are called nupts (nuclear copies of plastid DNA, pronounced “new-peats”). With the recent eruption of eukaryotic genome data, it is opportune to take a look at the prevalence and properties of numts in sequenced eukaryotic genomes.
Box 1. Numts Cause Confusion
Due to their sequence similarity to mitochondrial DNA, numts are responsible for many instances of misidentification, both in mitochondrial disease studies and phylogenetic reconstruction.
Mitochondrial Disease Confusions
Numts are common in humans. As a result, numt variation is continuously mis-reported as mitochondrial mutations in patients [82],[83]. At least one numt (5,842 bp numt on chromosome 1) was erroneously implicated in causing diseases, such as low sperm motility [84] and cystic fibrosis (see details in [82]). Even the HapMap data first classified this numt as mitochondrial variation [85]. If you have this variant in your genome, there is no cause for concern because it is not mitochondrial variation, it is a nuclear pseudogene.
DNA Barcoding and Phylogenetic Confusion
Mitochondrial DNA is commonly used as a marker for molecular systematics, phylogeny and for species diagnosis (“DNA barcoding”). The DNA barcoding technique for animals aims to identify organisms by using a short fragment of mitochondrial cytochrome c oxidase I (COI) gene [86],[87]. Numts are a major challenge in using mitochondria for these purposes [88],[89]. It was suggested that because of numts, the barcoding approach is unreliable, at least in primates [90]. Recently, DNA barcoding among arthropods was found to overestimate the number of species when numts are coamplified [91], showing that numts introduce serious ambiguity into the DNA barcoding paradigm as arthropods are one the major phyla studied in taxonomy.
Ancient DNA That Isn't Ancient
The report that 80-million-year-old dinosaur bones harboured DNA [92] made quite a splash in its time, appearing a year after the filming of Jurassic Park. But it did not take long to uncover the real source of dinosaur bone DNA; it was a mtDNA pseuodgene in the human nuclear genome [93],[94], now called a numt. Newer findings even implicate numts in reports of horizontal gene transfer among plants [95].
The Human Genome—Visible, Ongoing Numt Transfer
Sequenced eukaryotic genomes can be readily scanned for numts using standard data-mining tools. Attempts to identify numts solely with computer methods started with partial genome sequences of plants and yeast [15],[16] followed by scanning of the full genomes of human, fruitfly, Plasmodium, and Caenorhabditis [17],[18]. Various studies focused on the identification of numts specifically in the human genome [18]–[20]. The number of human numts was reported with values ranging from 286 to 612 depending on the search parameters and depending on how closely related were combined hits into a single numt contig. Later calculations based on numts from both human and chimpanzee suggested an intermediate number of 452 numts [21]. Some of the human numts stem from independent insertion events from the mitochondrion, whereas others are the results of tandem duplications [19] or subsequent segmental duplications. Older numts appear in more copies than recent ones [22].
The largest human numt covers 90% (14,654 bp) of the human mitochondrial genome [18]. Comparisons involving primate mitochondrial sequences allow one to approximately date the timing of insertion for long numts [22],[23] (Figure 1A). Such dating is based on the observation that the mean evolutionary rate in primate mitochondrial genomes is about ten times higher than that in the nuclear genome [24]–[26]. Therefore numts inserted into the nucleus decelerate their evolutionary rate and become “molecular fossils” resembling ancestral mitochondrial fragments [27],[28]. With the possible exception of an event involving either rapid post-insertion duplication [22] or rapid insertion per se [23] during the time corresponding to the Platyrrhini–Catarrhini divergence, numt insertion appears to have been more or less continuous over time in the lineages leading to the human genome [18],[22],[23].
Fig. 1. Dating numt insertion. 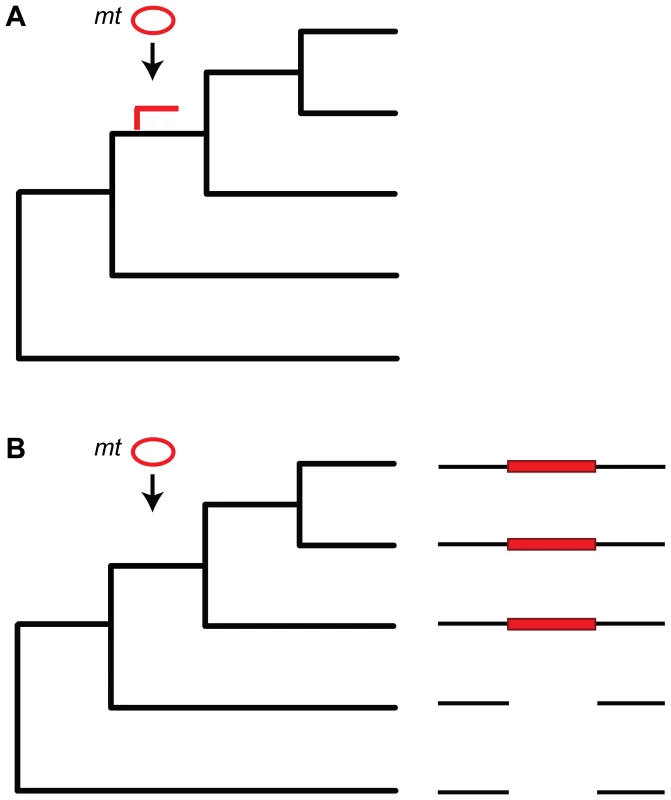
(A) Dating numt insertion based on a mitochondrial phylogenetic tree (black branches). An arrow indicates time of insertion and the numt branch is shown in red. The methodology can be used only in species where the mitochondrial rate of evolution is lower than the nuclear rate of evolution (e.g., mammals but not plants) and when the numts are long enough (>1 kb) to carry enough evolutionary signal. (B) Dating numt insertion based on patterns of presence and absence on a phylogeny. Few nuclear genomes and their genome alignment are used to identify numt insertions. Species that share the descendant from the common ancestor where the transfer occurred include the numts (red rectangle) whereas this numt is missing in the others. Phylogenetic and PCR amplification studies in humans suggest that the rate of numt insertion is ∼5.1–5.6×10−6 per germ cell per generation, or that every two human haploid genomes should be polymorphic for at least two numt loci [23],[29],[30]. Ricchetti et al. [30] used a PCR analysis with primers from both the nuclear flanking regions and the numt sequence to identify recent numt insertions that appear only in the human genome but not in the chimpanzee genome. Based on whole genome alignments, more than 80% of the numts in the human and chimpanzee genomes were found to be orthologous in that they are present at the same loci in the two species [21], but non-orthologous numts stemming from recent numt insertions, deletions, and tandem duplications were also identified. Current estimates have it that there are 40 and 68 species-specific insertions in the human and chimpanzee lineages, respectively [31].
Eight loci that are polymorphic for numts have been reported in humans so far [12],[14],[30] using PCR-based approaches. We have uncovered four additional polymorphic numts by searching the human dbSNP database for numts that appear in the reference human genome and are missing in the variation data. Overall, about a third of human-specific numts (12/40) are variable (Figure 2). Ten out of the 12 polymorphic numts appear in genes or in predicted genes [30]. With the increasing availability of structural variation data in populations, the number of loci polymorphic for numts is predicted to increase, and it should be possible to identify variable more numts that are missing in the reference genome(s) but appear in the variation data.
Fig. 2. Human polymorphic numts and numts that cause diseases. 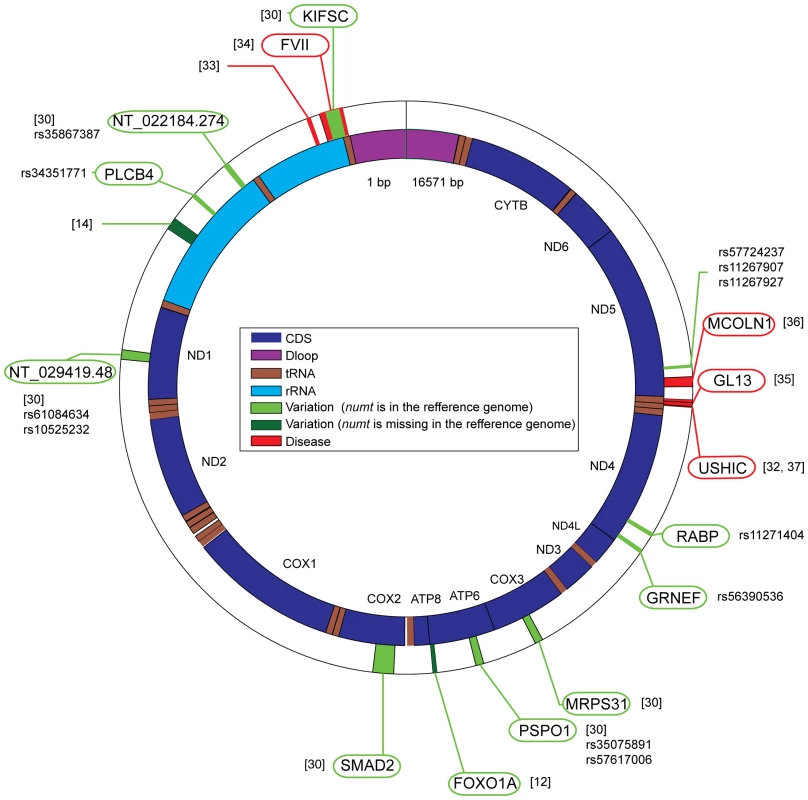
Human mitochondrial DNA (NC_001807) is shown in the inner circle, and numt insertions are shown in the outer circle. Polymorphic numts are shown in light green (numts exist in the reference genome) or dark green (numts are missing from the reference genome). Numts causing disease are shown in red. In each case, the reference and the SNP accession numbers (if available) are given. When a numt is inserted within gene, the gene name is indicated (green and red ellipses for polymorphic numts and for numts causing disease, respectively). Numts and Diseases
Integration of numts not only appears as neutral polymorphism but, more rarely, is also associated with human diseases [32]; five cases are currently known (Figure 2). One involved a 41-bp mtDNA insertion at the breakpoint junction of a reciprocal translocation between chromosome 9 and 11 [33], the remaining cases involve insertion of mtDNA into genes. A splice site mutation in the human gene for plasma factor VII that causes severe plasma factor VII deficiency (bleeding disease) results from a 251-bp numt insertion [34]. A rare case of Pallister-Hall syndrome in which a 72-bp numt insertion into exon 14 of the GLI3 gene causes a premature stop codon, is associated with Chernobyl [35]. A case of mucolipidosis IV in which a 93-bp segment was inserted into exon 2 of MCOLN1, eliminated proper splicing of the gene [36]. As the last known example, a 36-bp insertion in exon 9 of the USH1C gene associated with Usher syndrome type IC [37] is a numt [32]. As in other cases of numt insertions, the mitochondrial genome remains intact in the afflicted individuals.
More Genomes, More Numts
Beyond humans, the whole genome repertoire of numts has been estimated in various species including yeasts [38], rodents [39], plants [40], and honeybees [41],[42]. Numts show not only different frequencies in different genomes, but also different size distributions [43],[44]. Numts are abundant in plants, where the longest numt known so far, a 620-kb partially duplicated insertion of the 367-kb mtDNA of Arabidopsis thaliana, was reported [45].
The honeybee genome is currently the record-holder for numt frequency among metazoans so far [41],, although their numts are relatively short. Since the last genome-wide survey encompassing 13 nuclear genomes [44], 72 new eukaryotic genome sequences have become available for study. Table 1 summarizes the numt repertoire in 85 fully sequenced genomes including 20 fungi, 11 protists, 7 plants, and 47 animals, for which both nuclear and mitochondrial genomes are available, reporting the number of BLAST nucleotides that were found in the genome (BLASTN of entire mitochondria against the genome using e-score of 0.0001). Some mitochondrial genomes (those of plants, for example), contain repetitive sequences, such that a single nuclear fragment can be found by BLAST to match multiple mitochondria pieces, a source of differences between tabulations in earlier reports. Each nuclear nucleotide appearing in Table 1 is unique and is counted only once even if the corresponding numt matches multiple mtDNA regions.
Tab. 1. Blast analysis of 85 mitochondria against their nuclear genomes (BlastN, e-score = 0.0001). 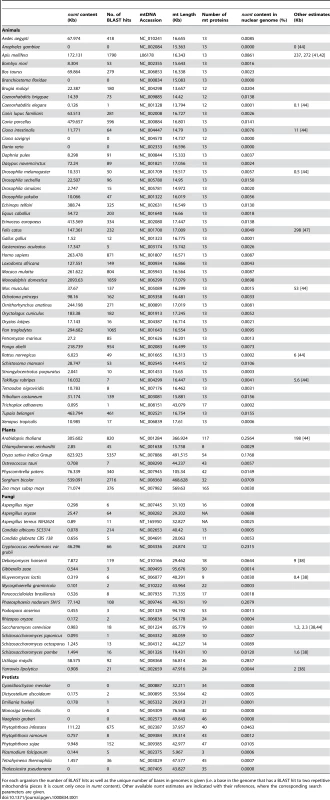
For each organism the number of BLAST hits as well as the unique number of bases in genomes is given (i.e. a base in the genome that has a BLAST hit to two repetitive mitochondria pieces it is count only once in numt content). Other available numt estimates are indicated with their references, where the corresponding search parameters are given. Numts are common in all groups that were examined. The numt content of these genomes varies from no detectable numts in eight species to more than 500 kb in three genomes. As noted by Richly and Leister [44] the fraction of the nuclear genome represented by numts is usually less than 0.1%, with the higher proportions of numts appearing in plants and yeast [15],, two groups that each include a few genomes consisting to >0.1% out of numts. At first sight, 0.1% might not seem like much, but numt sequences are constantly becoming undetectable through mutation and deletion, such that 0.1% represents a steady state level of recently incorporated and detectable numts at any given point in time.
For organisms that have only one mitochondrion, such as Cyanidioschyzon, the absence of numts makes sense, because if an organelle must lyse in order for DNA to escape to the nucleus, then more than one organelle per cell (one for gene transfer and one for healthy progeny) would be required for the DNA to escape [46]. The absence of numts in the present releases of several animal genomes, from insects to vertebrates, is an exception in that regard, but annotations can change over time. The highest total numt content was found in the opossum Monodelphis domestica, whose genome sequence contains over 2000 kb of numt nucleotides. However, most opossum numts do not map to known chromosome arms, and some fraction of these may turn out to be true mitochondrial sequences. In plants, the highest numt content appears in Oryza sativa Indica group with more than 800 kb of numts. Among fungi, the highest numt content appears in Phaeosphaeria nodorum with 77 kb, and in protists the highest numt content so far appears in Phytophthora infestans with 111 kb.
The number of numts one detects can change with search strategy, genome version and level of genome completion. For example, when calculated in 2009, the genome of Arabidopsis has 54% more total numt length (305.6 kb) than it did five years ago (198 kb) [44], in part because some numts were initially removed during the annotation process [46]. Similarly, the numt content in the Drosophila melanogaster genome has grown from 0.5 kb in 2004 to a current value of 10.3 kb (Table 1), corresponding to a roughly 20-fold increase. These differences are due to changes in the curation of the available genome sequence data. For example, the current version of the D. melanogaster genome includes 4.7 Mb of heterochromatic sequence that was previously unavailable. By contrast, in the cat genome, not all of the numts reported by Lopez et al. (1994) [11] are identified using the standard parameters, and a careful analysis of numts [47] suggests that the genome might include as much as double the number of numts identified here. Other available assessments of numt content in genomes are shown in Table 1.
The data from 85 genomes reveal a strong correlation between genome size and total numt content (Spearman non-parametric rho = 0.67, P = 2.77×10−12). Bensasson et al. [17],[43] suggested that such a correlation might exist for metazoans because genomes with more non-coding DNA will have more numts (see below). Early searches detected no such correlations [44], probably owing to the small sample size. A fresh look at the data reveals the predicted correlation, which however seems to explain mainly the differences between small and big genomes (Figure 3), as it disappears when considering only genomes smaller than 200 Mb. No correlations appear between numt content and mitochondrial genome size, even when numt content is normalized by the nuclear genome size. Three different processes can thus contribute to the differences in numts between species—the frequency of mitochondrial transfer, the amount of chromosomal integration, and the dynamics of post-insertion processes, such as duplications and deletions affecting all DNA as part of bulk genome evolution.
Fig. 3. Numt content is correlated to genome size. 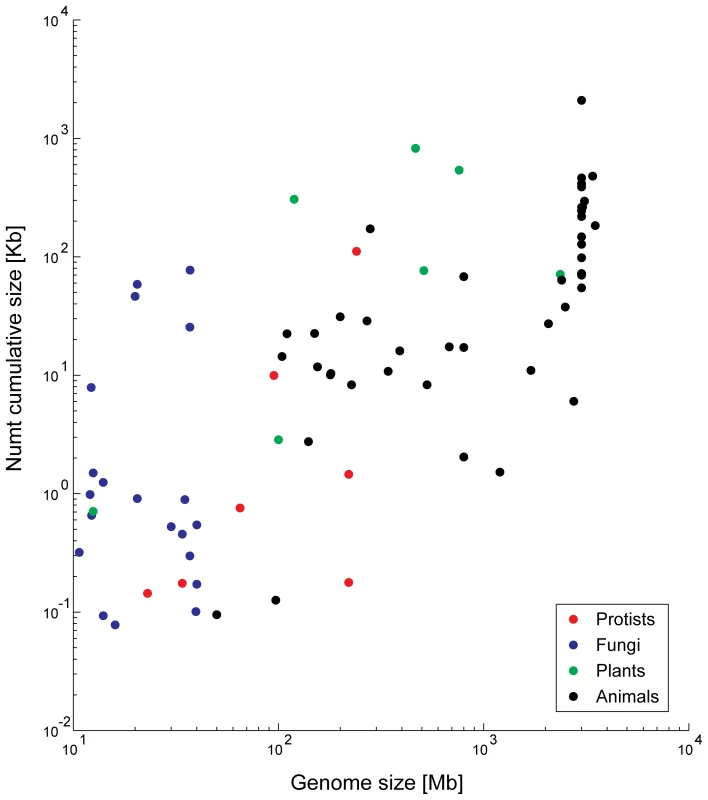
A log–log scale graph showing the dependency between numt content in genomes and genome size. Information regarding genome size is from http://www.ncbi.nlm.nih.gov/genomes/leuks.cgi. Mechanism of Numt Insertions
For numts to persist in nuclear genomes, mitochondrial DNA must first physically reach the nucleus, then it must integrate into the nuclear chromosome, with intragenomic dynamics of amplification, mutation, or deletion following. Work so far has focused on the escape of DNA from the mitochondria and on the integration of mtDNA within the nucleus but not on its physical entrance into the nucleus (the notion that nuclear chromosomes should actively pluck mtDNA from the organelle seems unlikely enough to exclude). The current picture is summarized in Figure 4, but we are still far from understanding the full details.
Fig. 4. Mechanism of numt insertion. 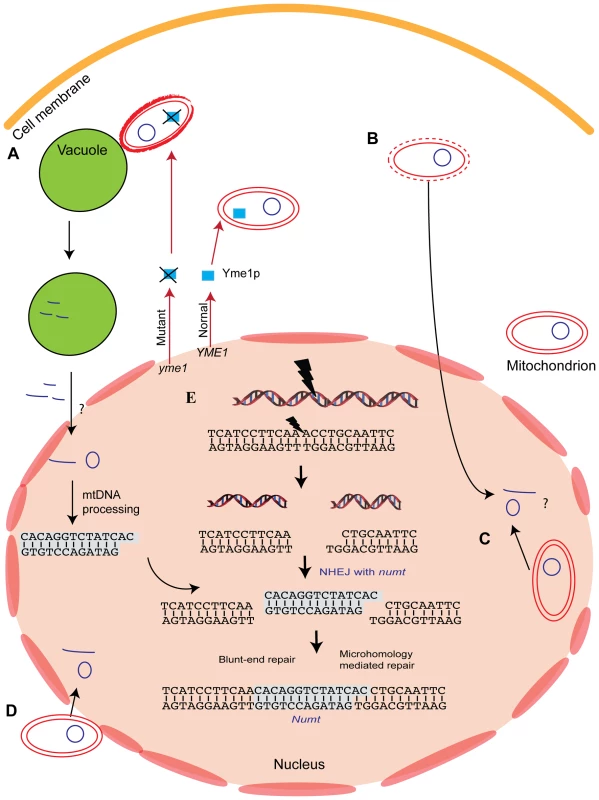
Mitochondrial DNA has been suggested to get into the nucleus via a few different pathways. (A) The most supported pathway so far involve the degradation of abnormal mitochondria [53]. Several yme (yeast mitochondrial escape) strains show high level of DNA escape to the nucleus. yme1 mutant cause the inactivation of YMe1p protein, a mitochondrial-localized ATP-dependent metallo-protease leading to high escape rate of mtDNA to the nucleus. Mitochondria of yme1 strain are taken up for degradation by the vacuole more frequently than the wild-type strain. Other pathways to get mitochondrial DNA into the nucleus were suggested including: (B) lysis of mitochondrial compartment, (C) encapsulation of mitochondrial DNA inside the nucleus, (D) direct physical association between the mitochondria and the nucleus and membrane fusions. (E) Mitochondrial DNA that enters the nucleus can integrate into nuclear chromosomes. mtDNA integrated into the chromosome during the repair of DSBs in a mechanism known as non-homologous end-joining (NHEJ). The insertion involves two DSB repair events. Each can be repaired with or without the involvement of short microhomology. In microhomology-mediated NHEJ, base-pair complements are available between the numt and the chromosome ends, similar to the sticky ends created by restriction enzymes. Export from the Mitochondria
Thorsness and Fox [48] utilized an assay to measure the rate of mtDNA escape to the nucleus in S. cerevisiae. Their assay was based on engineering the URA3 gene, which is involved in uracil biosynthesis, from the nuclear genome to a plasmid that is maintained in the mitochondrion. During the propagation of such yeast strains carrying a nuclear ura3 mutation, plasmid DNA that escapes from the mitochondrion to the nucleus complements the uracil biosynthetic defect, restoring growth in the absence of uracil, an easily scored phenotype. The rate of DNA transfer from the mitochondria to the nucleus was estimated as 2×10−5 per cell per generation [48]. Since the URA3 gene carrying its own promoter was located on a plasmid, that experimental system only measured relocation of mtDNA into the nucleus and did not measure integration of the plasmid or mtDNA into the chromosome. In addition, it only measured the transport of the entire URA3 gene, while shorter or other mitochondrial fragments went undetected. In a different experimental setup, mtDNA fragments joined to linear DNAs to form circular DNA plasmids. The integration frequency was suggested to be as high as 10−3 to 10−4, or that 1 in every 1,000–10,000 yeast cells might contain a new mitochondrial insertion [49]. The escape event was found to be intracellular, that is, lysis of cells in culture with mtDNA uptake by neighboring cells is not involved [50].
Increased rates of yeast mtDNA escape are observed in different conditions, including in cells that have been frozen and thawed, in cells that were grown in non-optimal temperature, and, when environment favors fermentation, as primary energy source. In addition, mutations in at least 12 nuclear loci called the yme (yeast mitochondrial escape) mutations, lead to an elevated rate of mtDNA escape to the nucleus [51],[52]. Some of the yme mutants have protein products that are mitochondrion-associated, and it has been suggested that perturbation in mitochondrial functions due to the alteration of gene products affect mitochondrial integrity, leading to mtDNA escape. In the case of the yme1 strain, abnormal mitochondria are targeted for degradation by the vacuole, and this degradation increases mtDNA escape to the nucleus [53] in a process known as mitophagy [54],[55]. Cytological investigations have suggested several other pathways in diverse species (reviewed in [50]) including a lysis of the mitochondrial compartment, direct physical association between mitochondrial, and nuclear membranes [56], membrane fusions, and encapsulation of mitochondrial compartments inside the nucleus [57]. It was also suggested that the frequency of mitochondrial DNA transfer into the cytoplasm might change with the number of mitochondria within the germ-line [58], although experimental tests of this idea are so far lacking.
Integration into the Nuclear Chromosome
The appearance of large mitochondrial segments within nuclear genomes including large fragments of non-coding regions [18],[20],[59] and no preference for transcribed over non-transcribed regions indicate that bulk organelle DNA, not transcripts or cDNAs, is integrated into nuclear chromosomes [60]. This is consistent with the observations from genetically engineered organelle-to-nucleus gene transfer experiments [4].
Based on numt integration sites, Blanchard and Schmidt [16] proposed that numts are inserted into double-strand breaks (DSBs) by the non-homologous end joining (NHEJ) machinery. This was later borne out in an important study on yeast under conditions where homologous recombination was not possible [5]. Later analyses were consistent with the involvement of NHEJ in numt integration [30] in humans.
At the mechanistic level, there is a junction with chromosomal DNA to one side and mitochondrial DNA on the other at each end of a numt, and these junctions reflect the repair events at each end of the original chromosomal break (Figure 4). Numts can be integrated to chromosome ends with short microhomology of 1–7 bp, a NHEJ sub-mechanism known as microhomology-mediated repair. Insertion of numt can also occur without microhomology—a process known as blunt-end repair. It is possible to follow the details of numt insertion through NHEJ by analyzing the integration sites of recent numt insertions in primates. Comprehensive analysis of 90 recent numt insertions in human and chimpanzee suggest that 35% of the fusion points involve microhomology of at least 2 bp, thus, it appears that repair involving microhomology plays some role in numt integration but is not totally required [61].
Throughout the evolutionary history of human and chimpanzee, more than half of the DSBR events that involve numts do not show deletions. When deletions appear, they are very small [61]. This is surprising as the NHEJ mechanism underlying DSBR is inherently mutagenic; NHEJ repair events of similar break configurations without filler DNA (extrachromosomal DNA, i.e., numts) always involve small deletions and even in NHEJ reaction with filler DNA the frequency of deletions is significantly bigger (e.g., [62],[63] and referenced in [61]). This difference indicates that numts provide the end-joining machinery with a tool to seal breaks without the necessity to process the nuclear DNA further using a nuclease. Providing the repair system with numts as an alternative to nuclease activity might be important in cases where the structure of the DSB is chemically complex. Repairing complex DSBs without numts may require significant nuclease processing of chromosomal DNA, yielding a long stretch of single-strand DNA, which would potentially put the genome at risk for big deletions or translocations. It is thus possible that sealing DSBs with numts might abolish the risk of more deleterious DSBR [61]. There is a price tag for numt-mediated DSBR, though—an insertion. But this is a small price to pay for healing complex DSBs in non-coding regions. Numts are usually short; therefore their insertion might be less deleterious than the effects of exposed single-strand DNA. While the amount of numts in the genomes is too small to suggest that numts are significant in maintaining genome integrity by themselves, no other class of DNA fragments has yet been found that is captured into DSBs in a similarly healing role.
Despite its utility for mending DSBs in a manner that avoids deletions, mitochondrial DNA is not maintained during evolution as a spare parts warehouse for nuclear chromosomes. Instead it is, like chloroplast DNA, maintained because the membrane-associated electron transport functions of bioenergetic organelles demand that organelles have the capacity to immediately respond to redox imbalance at the level of individual organelles [64],[65]. Yet, when we consider the early phases of mitochondrial origins, the flux of DNA from the endosymbiont is generally thought to have had two major consequences for the evolution of eukaryotic chromosomes: it was a rich source of genetic novelties, on the one hand (for example eubacterial operational genes [66]), and a source of constructively disruptive forces on the other (for example introns [67]). As a third consequence, pieces of endosymbiont DNA might have been involved in DSB repair of the archaebacterial chromosomes of the host [68] right from the beginning as well.
Post-Insertion Processes within the Nuclear Genome
Numts sometimes show a more complex pattern than a single mitochondria piece, and can include non-continuous pieces of mitochondrial DNA that can appear in different orientations [5],[19],[20]. In plants, such complex patterns of numts are very common and can involve shared clusters with nupts [29],[40]. It has been suggested that these complex patterns are the result of concatenation prior to insertion rather than the result of multiple numt or nupt insertions at insertional hotspots [69]. If they are, contrary to expectation, insertional (or DSBR) hotspots after all, they should turn out to be more polymorphic than other sites for numts and/or nupts in “1,000 genome”–type surveys; this will be something to look for as those data becomes available.
Processes that occur after numt insertion, such as duplications or deletions of numts, can also contribute to numt diversity, but there the fate of numts just follows that of the genome as a whole. As a perhaps mundane aspect of genomic fate, numts and nupts are rapidly methylated in higher plants and thus rapidly undergo C-to-T transitions [59]. The same process probably also occurs in animals, but is more difficult to detect because of the paucity of CpG sites in animal mtDNA [70]. Numts have no self-replicating mechanism or transposition mechanism; therefore, numt duplication is expected to occur in tandem or to involve larger segmental duplication at rates representative for the rest of the genome [23].
In domestic cats, a 7.9-kb mtDNA segment is repeated in 38–76 tandem copies on chromosome D2 [11]. While these repeats were originally suggested as being duplicated pre-insertion, their copy number variability may also result from post-insertion recombination. Additional tandem repeats of 47 bp–long numts appear 18 times on human chromosome 12 [19],[21]. Evidence for numt duplications that are not in proximity to other numts is present in many genomes [22],[23],[71] and probably happens as part of segmental duplication [23]. However, duplications of recent human-specific numts as part of segmental duplication seem to be rare. Four human numts showed overlap with segmental duplications. In these cases, numts were found in only one of the copies while missing from the others, clearly demonstrating that the numts were inserted subsequent to the duplication events [61].
Deletion of numts from genomes has not been studied in the same amount of detail as has insertion. However, a recent report in plants shows that nupts that are engineered into the genome from transformed plastids are subject to severe instability due to rapid loss [72]. In humans, phylogenetic analyses suggest that the oldest numt was inserted 58 million years ago [23]. That suggests that older numts have been deleted from the genome, but at the same time, finding similarly ancient numts using human mitochondria becomes difficult because of the continuous erosion of phylogentic signal through mutation and the high mutation rate of animal mitochondrial DNA. Similar to recent insertions (Figure 1B) and cases in which the presence–absence pattern of numts does not agree with the phylogenetic tree (lineage sorting or reversal) [31], it should be possible to detect recent numt losses using a multiple genome alignment when an outgroup is present.
Correlation between Numt Content and Genome Size
Barring a role for differential mtDNA escape into the nucleus as a limiting factor in lineage-specific numt frequency (at least in species where multiple copies of mitochondria exist), the finding that numt content is strongly correlated with genome size points to the participation of two mechanistically independent processes: integration into the nuclear chromosome and post-insertional processes.
Integration now appears to implicate DSBs. DSBs can arise spontaneously during growth or can be induced by external stimuli such as radiation. Reactive oxygen species (ROS) arising in the mitochondria can also cause nuclear DNA damage [73],[74]. In yeast, it was suggested that increasing the amount of DNA, from diploid to tetraploid, is accompanied by a proportional increase in the fraction of spontaneous DSBs in cells [75]. If this trend is universal (which is a big if), then larger genomes will experience more DSBs. Since numts are captured in DSBs, then numts would be predicted to appear more often in bigger genomes than in smaller ones (but at a roughly constant per Mb rate). If true, then numts should be more common in genomic regions that are prone to DSBs. For example, transcription itself can increase DSBs and genome instability [76]. The enrichments of numts in introns versus intergenic regions [30],[42] indicates that an open chromosome is conducive to insertion and thus is consistent with this idea. A further prediction is that numt frequency should be higher in regions known to be associated with genome instability as in fragile sites, cells that undergo radiation, and in cancer cells.
Another possible explanation for the correlation between genome size and numt content is the previously detected negative correlation between DNA loss and genome size [77],[78]. Larger genomes tend to lose less DNA than smaller ones, as was shown for Drosophila and Laupala, which vary 11-fold in their DNA content [77]. A negative correlation also exists between genome size and repetitive DNA content [79]. Correspondingly, inaccurate DSB repair after a break-induction in Arabidopsis involves large deletions while DSBR of the tobacco genome, which is 20-fold larger, is associated with insertions [80]. Bensasson et al. [17],[43] suggested that numts might show similar patterns; animal genomes with more non-coding nuclear DNA would be expected to have more numts, while ones with less non-coding DNA will tend to lose them. In other words, this mechanism simply entails a genome-wide tendency to lose DNA in small genomes, such that the numt frequency would be independent of DSB frequency, in which case numt frequency might be expected to correlate with noncoding DNA amount.
Numts and New Horizons
Over longer evolutionary timeframes, with DNA continuously being transferred from organelles to the nucleus, one might wonder why any DNA has remained in the organelles at all. The reasons for this have to do with the essential bioenergetic function of the organelle [64], namely generating a protonmotive force across the inner mitochondrial membrane with the help of redox chemistry within the inner mitochondiral membrane; the organelle has to have a decisive say in maintaining redox balance throughout the respiratory chain, and this requires retention and regulation of a few genes within the organelle [65]. Indeed, only when organelles fully relinquish their membrane-associated electron transport chains do they fully relinquish their DNA [81].
Over more recent evolutionary timeframes, one finding stands out, namely that about one third (12 out of 40) of those numts that were inserted specifically in the human lineage are polymorphic for the presence versus absence of the insertion among human populations (Figure 2). Of course, when the 1,000 genome data for humans becomes available, the number of loci polymorphic for numts can be expected to increase.
Future challenges will include gaining a fuller understanding of post-insertion processes at the population genetic level. For example, do numts segregate in populations at frequencies that are consistent with neutral, deleterious, or beneficial effects? While there are good reasons to assume neutrality [23], the disease-related phenotypes of several numts, as well as the potentially beneficial role that numts play in DSBR, indicate that the spectrum of numt mutational effects may be broad. More studies on polymorphism for numts in human genomes should provide incisive clues. With the sequencing of 1,000 human genomes—and 1,000 Drosophila, 1,000 Arabidopsis, and many more after that—the data to test many ideas about the evolutionary dynamics of numts are not far away.
A particularly interesting aspect is that numts can tell us about the history of the species and which populations or subspecies must have had historically overlapping biogeographic distributions. Neanderthal's numts and a scan for Neanderthal mtDNA in a broad sample of human nuclear genome sequences might be an interesting undertaking. An additional fascinating aspect especially in humans, is that polymorphic numts potentially provide much more information than just another segregating marker [31], because they can link a given maternally inherited mitochondrial genotype with nuclear DNA polymorphism. The nuclear haplotypes flanking a particular numt insertion can tell us which nuclear genotypes and which mitochondrial haplotypes coexisted within the same germline at the particular point in time during which the numt was inserted. As such, they offer the opportunity, so far unexplored, to associate nuclear markers with mitochondrial markers back in time and thus to tie mitochondrial with nuclear genome evolution. While recombination within the nuclear genome might put a limit on the detectablility of such associations for numts inserted during the early phases of human evolution, this could still potentially represent a rich source of information about human history and admixture to be gleaned from the 1,000 human genome data, and similar endeavours, when it becomes available.
Zdroje
1. GouldSB
WallerRF
McFaddenGI
2008 Plastid evolution. Annu Rev Plant Biol 59 491 517
2. KleineT
MaierUG
LeisterD
2009 DNA transfer from organelles to the nucleus: the idiosyncratic genetics of endosymbiosis. Annu Rev Plant Biol 60 115 138
3. TimmisJN
AyliffeMA
HuangCY
MartinW
2004 Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet 5 123 135
4. HuangCY
AyliffeMA
TimmisJN
2003 Direct measurement of the transfer rate of chloroplast DNA into the nucleus. Nature 422 72 76
5. RicchettiM
FairheadC
DujonB
1999 Mitochondrial DNA repairs double-strand breaks in yeast chromosomes. Nature 402 96 100
6. van der GiezenM
2009 Hydrogenosomes and mitosomes: conservation and evolution of functions. J Eukaryot Microbiol 56 221 231
7. LeisterD
2005 Origin, evolution and genetic effects of nuclear insertions of organelle DNA. Trends Genet 21 655 663
8. TovarJ
Leon-AvilaG
SanchezLB
SutakR
TachezyJ
2003 Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 426 172 176
9. van den BoogaartP
SamalloJ
AgsteribbeE
1982 Similar genes for a mitochondrial ATPase subunit in the nuclear and mitochondrial genomes of Neurospora crassa. Nature 298 187 189
10. EllisJ
1982 Promiscuous DNA–chloroplast genes inside plant mitochondria. Nature 299 678 679
11. LopezJV
YuhkiN
MasudaR
ModiW
O'BrienSJ
1994 Numt, a recent transfer and tandem amplification of mitochondrial DNA to the nuclear genome of the domestic cat. J Mol Evol 39 174 190
12. GiampieriC
CenturelliM
BonafeM
OlivieriF
CardelliM
2004 A novel mitochondrial DNA-like sequence insertion polymorphism in Intron I of the FOXO1A gene. Gene 327 215 219
13. WilliamsST
KnowltonN
2001 Mitochondrial pseudogenes are pervasive and often insidious in the snapping shrimp genus Alpheus. Mol Biol Evol 18 1484 1493
14. YuanJD
ShiJX
MengGX
AnLG
HuGX
1999 Nuclear pseudogenes of mitochondrial DNA as a variable part of the human genome. Cell Res 9 281 290
15. BlanchardJL
SchmidtGW
1995 Pervasive migration of organellar DNA to the nucleus in plants. J Mol Evol 41 397 406
16. BlanchardJL
SchmidtGW
1996 Mitochondrial DNA migration events in yeast and humans: integration by a common end-joining mechanism and alternative perspectives on nucleotide substitution patterns. Mol Biol Evol 13 893
17. BensassonD
ZhangD
HartlDL
HewittGM
2001 Mitochondrial pseudogenes: evolution's misplaced witnesses. Trends Ecol Evol 16 314 321
18. MourierT
HansenAJ
WillerslevE
ArctanderP
2001 The Human Genome Project reveals a continuous transfer of large mitochondrial fragments to the nucleus. Mol Biol Evol 18 1833 1837
19. TourmenY
BarisO
DessenP
JacquesC
MalthieryY
2002 Structure and chromosomal distribution of human mitochondrial pseudogenes. Genomics 80 71 77
20. WoischnikM
MoraesCT
2002 Pattern of organization of human mitochondrial pseudogenes in the nuclear genome. Genome Res 12 885 893
21. Hazkani-CovoE
GraurD
2007 A comparative analysis of numt evolution in human and chimpanzee. Mol Biol Evol 24 13 18
22. Hazkani-CovoE
SorekR
GraurD
2003 Evolutionary dynamics of large numts in the human genome: rarity of independent insertions and abundance of post-insertion duplications. J Mol Evol 56 169 174
23. BensassonD
FeldmanMW
PetrovDA
2003 Rates of DNA duplication and mitochondrial DNA insertion in the human genome. J Mol Evol 57 343 354
24. BrownWM
GeorgeMJr
WilsonAC
1979 Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci USA 76 1967 1971
25. BrownWM
PragerEM
WangA
WilsonAC
1982 Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol 18 225 239
26. Haag-LiautardC
CoffeyN
HouleD
LynchM
CharlesworthB
2008 Direct estimation of the mitochondrial DNA mutation rate in Drosophila melanogaster. PLoS Biol 6 e204
27. PernaNT
KocherTD
1996 Mitochondrial DNA: molecular fossils in the nucleus. Curr Biol 6 128 129
28. ZhangDX
HewittGM
1996 Nuclear integrations: Challenges for mitochondrial DNA markers. Trends Ecol Evol 11 247 251
29. LeisterD
2005 Origin, evolution and genetic effects of nuclear insertions of organelle DNA. Trends Genet 21 655 663
30. RicchettiM
TekaiaF
DujonB
2004 Continued colonization of the human genome by mitochondrial DNA. PLoS Biol 2 E273
31. Hazkani-CovoE
2009 Mitochondrial insertions into primate nuclear genomes suggest the use of numts as a tool for phylogeny. Mol Biol Evol 26 2175 2179
32. ChenJM
ChuzhanovaN
StensonPD
FerecC
CooperDN
2005 Meta-analysis of gross insertions causing human genetic disease: novel mutational mechanisms and the role of replication slippage. Hum Mutat 25 207 221
33. Willett-BrozickJE
SavulSA
RicheyLE
BaysalBE
2001 Germ line insertion of mtDNA at the breakpoint junction of a reciprocal constitutional translocation. Hum Genet 109 216 223
34. BorensztajnK
ChafaO
Alhenc-GelasM
SalhaS
ReghisA
2002 Characterization of two novel splice site mutations in human factor VII gene causing severe plasma factor VII deficiency and bleeding diathesis. Br J Haematol 117 168 171
35. TurnerC
KilloranC
ThomasNS
RosenbergM
ChuzhanovaNA
2003 Human genetic disease caused by de novo mitochondrial-nuclear DNA transfer. Hum Genet 112 303 309
36. GoldinE
StahlS
CooneyAM
KaneskiCR
GuptaS
2004 Transfer of a mitochondrial DNA fragment to MCOLN1 causes an inherited case of mucolipidosis IV. Hum Mutat 24 460 465
37. AhmedZM
SmithTN
RiazuddinS
MakishimaT
GhoshM
2002 Nonsyndromic recessive deafness DFNB18 and Usher syndrome type IC are allelic mutations of USHIC. Hum Genet 110 527 531
38. SacerdotC
CasaregolaS
LafontaineI
TekaiaF
DujonB
2008 Promiscuous DNA in the nuclear genomes of hemiascomycetous yeasts. FEMS Yeast Res 8 846 857
39. TriantDA
DeWoodyJA
2008 Molecular analyses of mitochondrial pseudogenes within the nuclear genome of arvicoline rodents. Genetica 132 21 33
40. NoutsosC
RichlyE
LeisterD
2005 Generation and evolutionary fate of insertions of organelle DNA in the nuclear genomes of flowering plants. Genome Res 15 616 628
41. PamiloP
ViljakainenL
VihavainenA
2007 Exceptionally high density of NUMTs in the honeybee genome. Mol Biol Evol 24 1340 1346
42. BehuraSK
2007 Analysis of nuclear copies of mitochondrial sequences in honeybee (Apis mellifera) genome. Mol Biol Evol 24 1492 1505
43. BensassonD
PetrovDA
ZhangDX
HartlDL
HewittGM
2001 Genomic gigantism: DNA loss is slow in mountain grasshoppers. Mol Biol Evol 18 246 253
44. RichlyE
LeisterD
2004 NUMTs in sequenced eukaryotic genomes. Mol Biol Evol 21 1081 1084
45. StuparRM
LillyJW
TownCD
ChengZ
KaulS
2001 Complex mtDNA constitutes an approximate 620-kb insertion on Arabidopsis thaliana chromosome 2: implication of potential sequencing errors caused by large-unit repeats. Proc Natl Acad Sci USA 98 5099 5103
46. MartinW
2003 Gene transfer from organelles to the nucleus: frequent and in big chunks. Proc Natl Acad Sci USA 100 8612 8614
47. AntunesA
PontiusJ
RamosMJ
O'BrienSJ
JohnsonWE
2007 Mitochondrial introgressions into the nuclear genome of the domestic cat. J Hered 98 414 420
48. ThorsnessPE
FoxTD
1990 Escape of DNA from mitochondria to the nucleus in Saccharomyces cerevisiae. Nature 346 376 379
49. SchiestlRH
DominskaM
PetesTD
1993 Transformation of Saccharomyces cerevisiae with nonhomologous DNA: illegitimate integration of transforming DNA into yeast chromosomes and in vivo ligation of transforming DNA to mitochondrial DNA sequences. Mol Cell Biol 13 2697 2705
50. ThorsnessPE
WeberER
1996 Escape and migration of nucleic acids between chloroplasts, mitochondria, and the nucleus. Int Rev Cytol 165 207 234
51. ShaferKS
HanekampT
WhiteKH
ThorsnessPE
1999 Mechanisms of mitochondrial DNA escape to the nucleus in the yeast Saccharomyces cerevisiae. Curr Genet 36 183 194
52. ParkS
HanekampT
ThorsnessMK
ThorsnessPE
2006 Yme2p is a mediator of nucleoid structure and number in mitochondria of the yeast Saccharomyces cerevisiae. Curr Genet 50 173 182
53. CampbellCL
ThorsnessPE
1998 Escape of mitochondrial DNA to the nucleus in yme1 yeast is mediated by vacuolar-dependent turnover of abnormal mitochondrial compartments. J Cell Sci 111 2455 2464
54. PriaultM
SalinB
SchaefferJ
ValletteFM
di RagoJP
2005 Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ 12 1613 1621
55. AbeliovichH
2007 Mitophagy: the life-or-death dichotomy includes yeast. Autophagy 3 275 277
56. MotaM
1963 Electron microscope study of relationship between nucleus and mitochondria in Chlorophytum capense (L.) Kuntze. Cytologia (Tokyo) 28 409 416
57. JensenH
EngedalH
SaetersdalTS
1976 Ultrastructure of mitochondria-containing nuclei in human myocardial cells. Virchows Archiv B Cell Pathology Zell-pathologie 21 1 12
58. ListerDL
BatemanJM
PurtonS
HoweCJ
2003 DNA transfer from chloroplast to nucleus is much rarer in Chlamydomonas than in tobacco. Gene 316 33 38
59. HuangCY
GrunheitN
AhmadinejadN
TimmisJN
MartinW
2005 Mutational decay and age of chloroplast and mitochondrial genomes transferred recently to angiosperm nuclear chromosomes. Plant Physiol 138 1723 1733
60. HenzeK
MartinW
2001 How do mitochondrial genes get into the nucleus? Trends Genet 17 383 387
61. Hazkani-CovoE
CovoS
2008 Numt-mediated double-strand break repair mitigates deletions during primate genome evolution. PLoS Genet 4 e1000237
62. LinY
WaldmanAS
2001 Capture of DNA sequences at double-strand breaks in mammalian chromosomes. Genetics 158 1665 1674
63. RamadanK
MagaG
ShevelevIV
VillaniG
BlancoL
2003 Human DNA polymerase lambda possesses terminal deoxyribonucleotidyl transferase activity and can elongate RNA primers: implications for novel functions. J Mol Biol 328 63 72
64. AllenJF
1993 Control of gene expression by redox potential and the requirement for chloroplast and mitochondrial genomes. J Theor Biol 165 609 631
65. PuthiyaveetilS
KavanaghTA
CainP
SullivanJA
NewellCA
2008 The ancestral symbiont sensor kinase CSK links photosynthesis with gene expression in chloroplasts. Proc Natl Acad Sci USA 105 10061 10066
66. LakeJA
2007 Disappearing act. Nature 446 983
67. MartinW
KooninEV
2006 Introns and the origin of nucleus-cytosol compartmentalization. Nature 440 41 45
68. CoxCJ
FosterPG
HirtRP
HarrisSR
EmbleyTM
2008 The archaebacterial origin of eukaryotes. Proc Natl Acad Sci USA 105 20356 20361
69. RichlyE
LeisterD
2004 NUPTs in sequenced eukaryotes and their genomic organization in relation to NUMTs. Mol Biol Evol 21 1972 1980
70. KellerI
BensassonD
NicholsRA
2007 Transition-transversion bias is not universal: a counter example from grasshopper pseudogenes. PLoS Genet 3 e22
71. TriantDA
DeWoodyJA
2007 Extensive mitochondrial DNA transfer in a rapidly evolving rodent has been mediated by independent insertion events and by duplications. Gene 401 61 70
72. SheppardAE
TimmisJN
2009 Instability of plastid DNA in the nuclear genome. PLoS Genet 5 e1000323
73. KaranjawalaZE
MurphyN
HintonDR
HsiehCL
LieberMR
2002 Oxygen metabolism causes chromosome breaks and is associated with the neuronal apoptosis observed in DNA double-strand break repair mutants. Curr Biol 12 397 402
74. KarthikeyanG
ResnickMA
2005 Impact of mitochondria on nuclear genome stability. DNA Repair (Amst) 4 141 148
75. StorchovaZ
BrenemanA
CandeJ
DunnJ
BurbankK
2006 Genome-wide genetic analysis of polyploidy in yeast. Nature 443 541 547
76. AguileraA
2002 The connection between transcription and genomic instability. EMBO J 21 195 201
77. PetrovDA
SangsterTA
JohnstonJS
HartlDL
ShawKL
2000 Evidence for DNA loss as a determinant of genome size. Science 287 1060 1062
78. PetrovDA
HartlDL
1998 High rate of DNA loss in the Drosophila melanogaster and Drosophila virilis species groups. Mol Biol Evol 15 293 302
79. KidwellMG
2002 Transposable elements and the evolution of genome size in eukaryotes. Genetica 115 49 63
80. KirikA
SalomonS
PuchtaH
2000 Species-specific double-strand break repair and genome evolution in plants. EMBO J 19 5562 5566
81. AllenJF
2003 The function of genomes in bioenergetic organelles. Philos Trans R Soc Lond B Biol Sci 358 19 37
82. YaoYG
KongQP
SalasA
BandeltHJ
2008 Pseudomitochondrial genome haunts disease studies. J Med Genet 45 769 772
83. WallaceDC
StugardC
MurdockD
SchurrT
BrownMD
1997 Ancient mtDNA sequences in the human nuclear genome: a potential source of errors in identifying pathogenic mutations. Proc Natl Acad Sci USA 94 14900 14905
84. ThangarajK
JoshiMB
ReddyAG
RasalkarAA
SinghL
2003 Sperm mitochondrial mutations as a cause of low sperm motility. J Androl 24 388 392
85. BiswasNK
DeyB
MajumderPP
2007 Using HapMap data: a cautionary note. Eur J Hum Genet 15 246 249
86. BlaxterML
2004 The promise of a DNA taxonomy. Philos Trans R Soc Lond B Biol Sci 359 669 679
87. LorenzJG
JacksonWE
BeckJC
HannerR
2005 The problems and promise of DNA barcodes for species diagnosis of primate biomaterials. Philos Trans R Soc Lond B Biol Sci 360 1869 1877
88. SorensonMD
QuinnTW
1998 Numts: a challenge for avian systematics and population biology. Auk 115 214 221
89. van der KuylAC
KuikenCL
DekkerJT
PerizoniusWR
GoudsmitJ
1995 Nuclear counterparts of the cytoplasmic mitochondrial 12S rRNA gene: a problem of ancient DNA and molecular phylogenies. J Mol Evol 40 652 657
90. ThalmannO
HeblerJ
PoinarHN
PaaboS
VigilantL
2004 Unreliable mtDNA data due to nuclear insertions: a cautionary tale from analysis of humans and other great apes. Mol Ecol 13 321 335
91. SongH
BuhayJE
WhitingMF
CrandallKA
2008 Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc Natl Acad Sci USA 105 13486 13491
92. WoodwardSR
WeyandNJ
BunnellM
1994 DNA sequence from Cretaceous period bone fragments. Science 266 1229 1232
93. ColluraRV
StewartCB
1995 Insertions and duplications of mtDNA in the nuclear genomes of Old World monkeys and hominoids. Nature 378 485 489
94. ZischlerH
HossM
HandtO
von HaeselerA
van der KuylAC
1995 Detecting dinosaur DNA. Science 268 1192 1193
95. GoremykinVV
SalaminiF
VelascoR
ViolaR
2009 Mitochondrial DNA of Vitis vinifera and the issue of rampant horizontal gene transfer. Mol Biol Evol 26 99 110
Štítky
Genetika Reprodukční medicína
Článek Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the GenomeČlánek Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in MiceČlánek Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' DiseaseČlánek Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD TagsČlánek Wing Patterns in the Mist
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Scale of Population Structure in
- Allelic Exchange of Pheromones and Their Receptors Reprograms Sexual Identity in
- Genetic and Functional Dissection of and in Age-Related Macular Degeneration
- A Single Nucleotide Polymorphism within the Acetyl-Coenzyme A Carboxylase Beta Gene Is Associated with Proteinuria in Patients with Type 2 Diabetes
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Cdk2 Is Required for p53-Independent G/M Checkpoint Control
- Uncoupling of Satellite DNA and Centromeric Function in the Genus
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in the Clade
- Use of DNA–Damaging Agents and RNA Pooling to Assess Expression Profiles Associated with and Mutation Status in Familial Breast Cancer Patients
- Cheating by Exploitation of Developmental Prestalk Patterning in
- Replication and Active Demethylation Represent Partially Overlapping Mechanisms for Erasure of H3K4me3 in Budding Yeast
- Cdk1 Targets Srs2 to Complete Synthesis-Dependent Strand Annealing and to Promote Recombinational Repair
- A Genome-Wide Association Study Identifies Susceptibility Variants for Type 2 Diabetes in Han Chinese
- Genome-Wide Identification of Susceptibility Alleles for Viral Infections through a Population Genetics Approach
- Transcriptional Rewiring of the Sex Determining Gene Duplicate by Transposable Elements
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in
- Proteasome Nuclear Activity Affects Chromosome Stability by Controlling the Turnover of Mms22, a Protein Important for DNA Repair
- Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in Mice
- Structure, Function, and Evolution of the spp. Genome
- Human and Non-Human Primate Genomes Share Hotspots of Positive Selection
- A Kinase-Independent Role for the Rad3-Rad26 Complex in Recruitment of Tel1 to Telomeres in Fission Yeast
- Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' Disease
- Molecular Evolution and Functional Characterization of Insulin-Like Peptides
- Molecular Poltergeists: Mitochondrial DNA Copies () in Sequenced Nuclear Genomes
- Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD Tags
- Wing Patterns in the Mist
- DNA Binding of Centromere Protein C (CENPC) Is Stabilized by Single-Stranded RNA
- Genome-Wide Association Study Reveals Multiple Loci Associated with Primary Tooth Development during Infancy
- Mutations in , Encoding an Equilibrative Nucleoside Transporter ENT3, Cause a Familial Histiocytosis Syndrome (Faisalabad Histiocytosis) and Familial Rosai-Dorfman Disease
- Genome-Wide Identification of Binding Sites Defines Distinct Functions for PHA-4/FOXA in Development and Environmental Response
- Ku Regulates the Non-Homologous End Joining Pathway Choice of DNA Double-Strand Break Repair in Human Somatic Cells
- Nucleoporins and Transcription: New Connections, New Questions
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Nucleoporins and Transcription: New Connections, New Questions
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



