-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDNA Binding of Centromere Protein C (CENPC) Is Stabilized by Single-Stranded RNA
Centromeres are the attachment points between the genome and the cytoskeleton:
centromeres bind to kinetochores, which in turn bind to spindles and move chromosomes. Paradoxically, the DNA sequence of centromeres has little or no role in perpetuating kinetochores. As such they are striking examples of genetic information being transmitted in a manner that is independent of DNA sequence (epigenetically). It has been found that RNA transcribed from centromeres remains bound within the kinetochore region, and this local population of RNA is thought to be part of the epigenetic marking system. Here we carried out a genetic and biochemical study of maize CENPC, a key inner kinetochore protein. We show that DNA binding is conferred by a localized region 122 amino acids long, and that the DNA-binding reaction is exquisitely sensitive to single-stranded RNA. Long, single-stranded nucleic acids strongly promote the binding of CENPC to DNA, and the types of RNAs that stabilize DNA binding match in size and character the RNAs present on kinetochores in vivo. Removal or replacement of the binding module with HIV integrase binding domain causes a partial delocalization of CENPC in vivo. The data suggest that centromeric RNA helps to recruit CENPC to the inner kinetochore by altering its DNA binding characteristics.
Published in the journal: . PLoS Genet 6(2): e32767. doi:10.1371/journal.pgen.1000835
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000835Summary
Centromeres are the attachment points between the genome and the cytoskeleton:
centromeres bind to kinetochores, which in turn bind to spindles and move chromosomes. Paradoxically, the DNA sequence of centromeres has little or no role in perpetuating kinetochores. As such they are striking examples of genetic information being transmitted in a manner that is independent of DNA sequence (epigenetically). It has been found that RNA transcribed from centromeres remains bound within the kinetochore region, and this local population of RNA is thought to be part of the epigenetic marking system. Here we carried out a genetic and biochemical study of maize CENPC, a key inner kinetochore protein. We show that DNA binding is conferred by a localized region 122 amino acids long, and that the DNA-binding reaction is exquisitely sensitive to single-stranded RNA. Long, single-stranded nucleic acids strongly promote the binding of CENPC to DNA, and the types of RNAs that stabilize DNA binding match in size and character the RNAs present on kinetochores in vivo. Removal or replacement of the binding module with HIV integrase binding domain causes a partial delocalization of CENPC in vivo. The data suggest that centromeric RNA helps to recruit CENPC to the inner kinetochore by altering its DNA binding characteristics.Introduction
Centromeres are important features of the genome that connect chromosomes to spindles. The connection occurs through a large multifunctional kinetochore complex that binds DNA, binds microtubules, and regulates the timing of anaphase [1]–[4]. Most plant and animal genomes contain diagnostic repeats that can be used to identify centromere boundaries, but unlike in fungi [5],[6], these sequences are not always necessary for organizing a functional kinetochore [7]–[9]. How kinetochores are accurately replicated is unknown and generally described as epigenetic–meant in the broadest sense that it is not easily classified as genetic.
Functional centromere domains are marked by a histone H3 variant known as Centromeric Histone H3 (CENH3) that has received intensive scrutiny as an important epigenetic identifier of centromeres [5]. In the absence of CENH3, all other kinetochore proteins fail to localize and chromosomes cannot move on the spindle [10]–[13]. CENH3 is assembled relatively late in the cell cycle, as late as anaphase-G1 [14]–[16], by specialized CENH3 nucleosome assembly factors such as Mis16 and Mis18 [17]. In addition several proteins that require CENH3 for localization also serve to target new CENH3 [18]–[20]. One such protein is Centromere protein C (CENPC), a DNA binding protein that has a key role in centromere recognition and maintenance [21]. Drosophila CENPC is required to target CENH3, but CENH3 is also required to target CENPC [20],[22]. These data suggest that kinetochore replication is a self reinforcing process whereby key inner kinetochore proteins such as CENPC work in concert with CENH3 to replicate the content and position of centromeres.
In species such as maize, the available data suggest that centromeric DNA does not function to recruit kinetochores until it is combined with specific epigenetic marks. Maize centromere repeats are under-methylated [23] and transcribed to produce stable RNAs that remain tightly bound to chromatin [24]. The centromeric RNAs are 40–200 nt in length, transcribed from both strands, and maintained in the single stranded state. It was proposed that RNA may serve as a structural template to help recruit kinetochore proteins [24]–[26]. A recent study revealed that human centromeres contain similar RNAs and established that RNAse treatment delocalizes CENPC from mature kinetochores [27]. More recent data show that suppression of transcription over a single LINE element in a human neocentromere impairs the formation of a kinetochore complex [28]. These data support the view that RNA facilitates or stabilizes the association of kinetochore proteins with centromeric DNA and implicate CENPC as a primary target of this activity.
CENPC is a highly divergent protein defined by a short 23 amino acids motif. Outside the defining motif lies the DNA binding region(s), which in animals appear to be distributed in several broad domains [29]–[31]. In plants, there is no evidence that CENPC binds to centromeric DNA beyond the presumption that CENPC is functionally conserved. In this regard we were encouraged by the sequence analysis of Talbert and colleagues [32] who found that a small region of CENPC has been repeatedly duplicated in the grasses. The authors suggested that the exon 9–12 duplicated region may bind to DNA. Here we use a combination of in vitro and in vivo studies to show that maize CENPC has both DNA-binding and RNA-binding capacities, that the DNA/RNA-binding domain is localized to the exon duplication region, and that the binding domain is required for efficient centromere localization. We further show that RNA directly facilitates the binding of CENPC to DNA in vitro, providing a biochemical mechanism for the involvement of RNA in centromere specification. We argue that CENPC and RNA are a part of the template that directs CENH3 to newly replicated centromere DNA.
Results
CENPC binds to DNA
In animals, CENPC is a non-specific DNA binding protein in vitro [29]–[31]. As a first step towards understanding the biochemical properties of maize CENPC, we used a standard gel shift assay to test whether the full-length protein binds DNA. We used double stranded CentC DNA as the binding substrate. CentC is the primary tandem repeat in maize centromeres [33] and a suspected binding substrate for CENPC, although this interaction has not been shown directly. Bacterially expressed CENPC was mixed with 33P-labeled full-length CentC DNA (156 bp) and the products resolved by non-denaturing PAGE. Consistent with expectations, the data show that the mobility of CentC is shifted upwards in the presence of CENPC, indicating that DNA and protein are associated in a complex that slows migration on gels (Figure 1). As controls we used bovine serum albumin and maize NDC80, another kinetochore protein [34]. There was no gel shift with either control protein (data not shown).
Fig. 1. Maize CENPC binds to DNA. 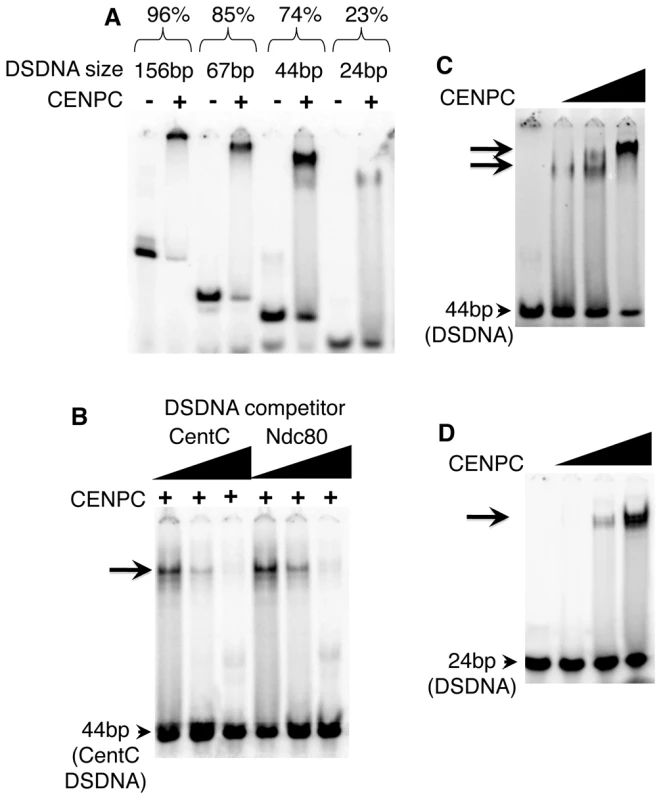
(A) Purified full CENPC and different sized CentC DSDNA binding substrates (indicated at top). The binding efficiency as % binding is shown (bound DNA/total). (B) Competition experiments. Lanes were loaded with the mixture of full CENPC protein, 44 bp CentC DSDNA, and either unlabeled 44 bp CentC or 44 bp Ndc80 DSDNA. (C) Increasing quantities of CENPC cause a supershift on a 44 bp substrate. (D) Increasing quantities of CENPC do not cause a supershift on the 24 bp substrate. The triangles represent the amount of protein or DNA added, and arrows indicate the shifted bands. CentC fragments of different lengths were incubated with CENPC to identify the optimal binding substrate. As shown in Figure 1A, CENPC complexes form with increasing efficiency as DNA length increases: 23.1% (shifted) with a 24 bp fragment, 73.7% with a 44 bp fragment, 85.3% with a 67 bp fragment, and 95.9% with a 156 bp fragment. Since the 67 bp and 156 bp fragments produced complexes that were too large to enter the polyacrylamide matrix, we opted for a 44 bp probe in subsequent assays.
The effects of CENPC concentration on DNA binding was investigated using 44 bp and 24 bp molecules. When the amount of CENPC was increased, a second, supershifted band was observed on the 44 bp template (Figure 1C), but not the smaller 24 bp DNA template (Figure 1D). These data suggest that larger DNA strands (44 bp) can accommodate two forms of CENPC-DNA complex. The stoichiometry could be skewed in either of two ways: Either there is more than one CENPC protein per DNA molecule on the supershifted band (e.g. two CENPC/one DNA), or each CENPC binds to more than one DNA (e.g. one CENPC/two DNA). The first option seems more likely since the shift occurs as more CENPC is added. Further, if CENPC could bind to a second (or third etc) DNA molecule at high concentrations, we would expect the same type of supershift with a 24 bp DNA fragment.
DNA binding is not sequence-specific
Binding specificity can be determined by competition experiments in which unlabeled DNA (‘challenger’) is added as a competitor to a mix of protein and labeled (‘defender’) DNA. If the DNA-protein binding is sequence-specific, the defender DNA will not be competed away by the challenger DNA [35]. Here, three sequences were used as competitors. A repetitive knob repeat found on chromosome arms [36], a centromere repeat from sorghum [37], and a fragment of the single-copy Ndc80 gene [34], were all efficient competitors for CENPC binding (Ndc80 is shown in Figure 1B). In no case did CENPC appear to bind with higher affinity to CentC than other molecules. While it remains possible that CENPC has minor binding preferences in vitro, our results suggest that the differences (if any) cannot be reliably detected by the gel shift assay. These results reinforce the interpretation that CENPC targeting to centromeres is DNA sequence-independent.
Maize CENPC binds both DNA and RNA at exons 9–12
To determine the DNA binding sites on maize CENPC empirically, fourteen subdomains of CENPC were tested for their capacity to shift DNA on gels (examples in Figure 2A–2C). The amount of protein required to confer a quantifiable shift was used as a measure of binding affinity. The data reveal that full-length CENPC has the highest DNA binding and that partial proteins bind DNA much less efficiently. By comparing the locations of the subdomains we inferred that the major DNA binding region maps to an area between exons 9 and 12 (Figure 2A). To confirm this interpretation, we prepared a construct that is identical to full length CENPC, but deleted for the entire 122 amino acid region containing exons 9–12 (Δexon 9–12). Gel shift results reveal that Δexon 9–12 has no detectable DNA binding activity (Figure 2B).
Fig. 2. DNA and RNA binding localize to exon 9–12. 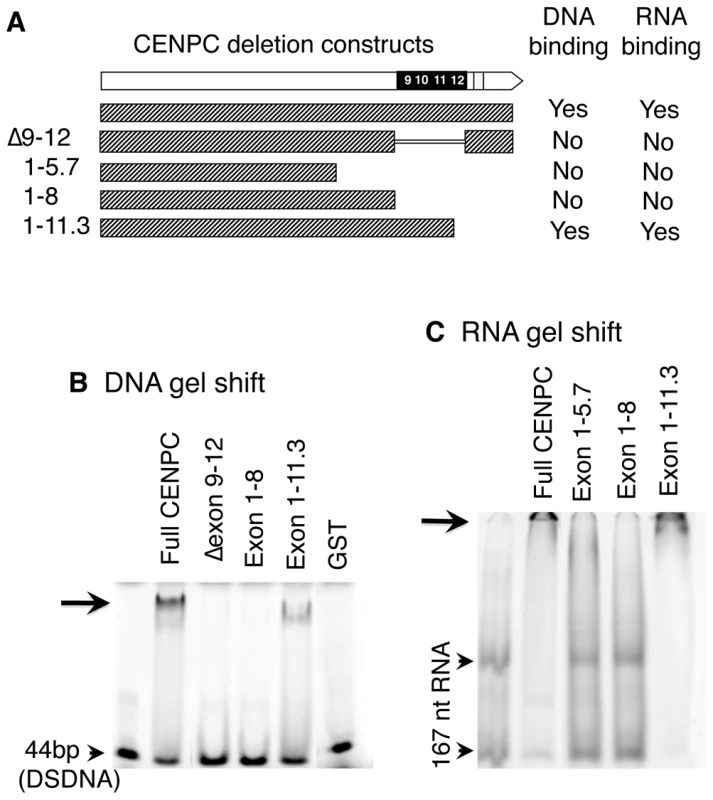
(A) Schematic representation of CENPC constructs used. (B) DNA binding. Radiolabeled 44 bp DSDNA was incubated with equal amounts of the different CENPC fragments. A shifted band (arrow) was seen for full CENPC and Exon 1–11.3, but not for Δ Exon 9–12, Exon 1–5.7 or Exon 1–8. As a negative control, GST (Glutathione S-transferase) was tested and no band shift was seen. (C) RNA binding. Radiolabeled 167 nt RNA was incubated with equal amounts of different CENPC fragments. Free RNA appears as two bands as expected for a long RNA with double stranded character (arrowheads). A shifted band, which is too large to enter the gel matrix (but visible, see arrow), was seen for full CENPC and Exon 1–11.3, but not for Exon 1–5.7 or Exon 1–8. Since centromere/kinetochores are rich in RNA [24], CentC RNA was also used in gel shift assays. As shown in Figure 2C, CENPC is an RNA binding protein. Analysis of several constructs suggests that the RNA binding is conferred by the same exon 9–12 region that binds DNA. RNA transcribed from either strand of synthetic sequences containing a 134 bp sorghum centromeric repeat [37], the 156 bp CentC repeat, and the non-centromeric maize 180 bp knob repeat [36] were roughly equivalent in their affinity for CENPC. We also tested whether CENPC can bind small single stranded 24 nt RNAs homologous to CentC (SSRNA). The results show that CENPC does bind to the small RNA (Figure 3A), though with much lower efficiency than to longer SSRNA or to double-stranded DNA (Figure 2B and 2C).
Fig. 3. SSRNA causes a supershift of the CENPC/DNA complex. 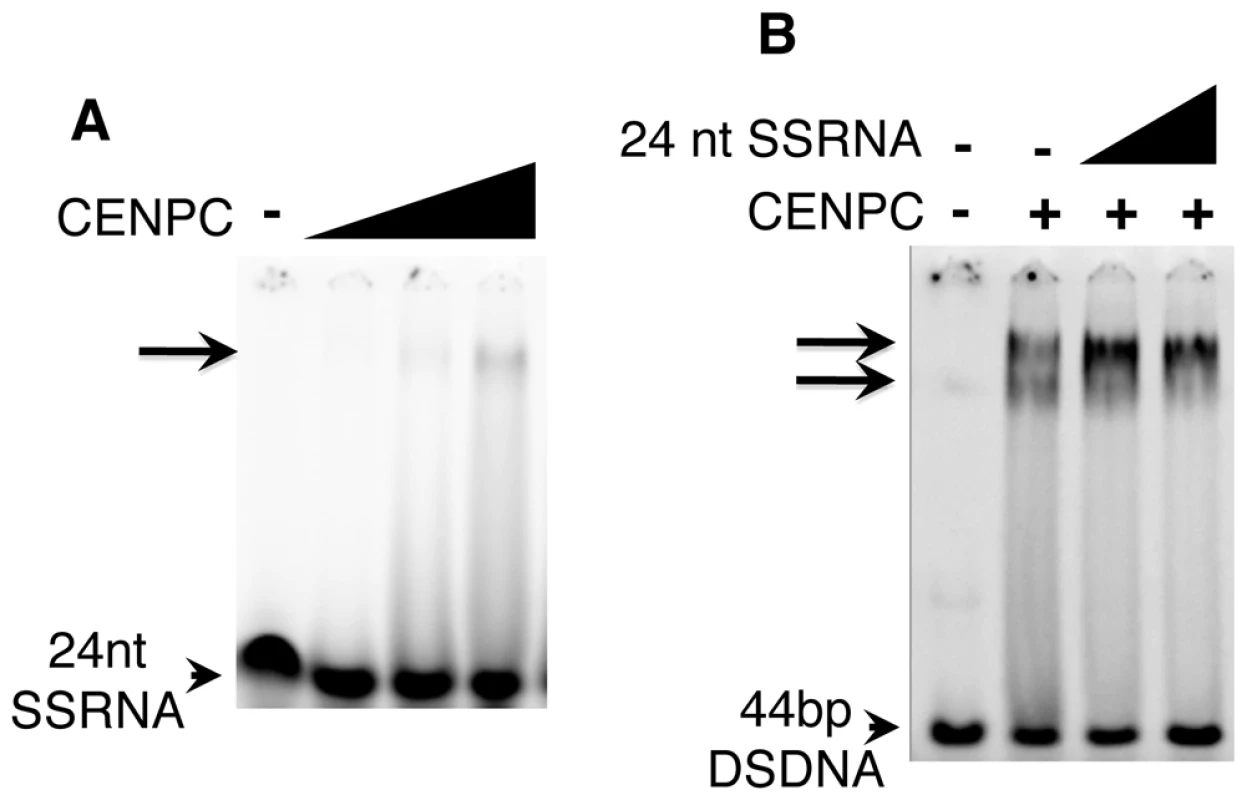
(A) 24 nt SSRNA binds weakly to CENPC. A faint shifted band is seen when CENPC is added at high concentrations. (B) Increasing amounts (triangles) of unlabeled 24 nt SSRNA cause the formation of a supershifted band similar to what is observed when excess CENPC protein is added (Figure 1C). RNA promotes DNA binding by CENPC
The synthesized 24 nt SSRNA was used in competition assays with double stranded CentC DNA (Figure 3B). We found that CENPC DNA binding is not affected by adding excessive small RNA competitor (unlike DSDNA challengers). Instead, small RNA promotes the formation of a larger supershifted product (Figure 3B). The mobility of the RNA-supershifted band is similar or identical to the band observed when more CENPC is added (Figure 1C), which we argue represents two or more CENPC proteins on a single DNA substrate. CENPC without exon 9–12 (Δexon 9–12) was also tested for RNA-stabilized DNA binding and the results suggest that RNA has no effect on other domains (data not shown).
Remarkably, the DNA binding region alone does not bind DNA efficiently. However, when small RNA is added concurrently, a clear and strongly shifted band appears (Figure 4A). When a 4,000-fold molar excess of 24 nt SSRNA (relative to DNA) was added to the reaction, the effect was indistinguishable from the effect when small amounts were added. To further study this effect, we prepared an exon 9–10 peptide (Figure 4B) as well as a single exon 12 peptide (Figure 4C), and showed both of the smaller polypeptides also require SSRNA to bind DNA effectively. These data demonstrate that single stranded RNA does not compete with DNA binding but instead has a clear positive effect on DNA binding, perhaps by resolving a folding defect in the expressed protein.
Fig. 4. The purified Exon 9–12 domain requires RNA to bind DNA in vitro. 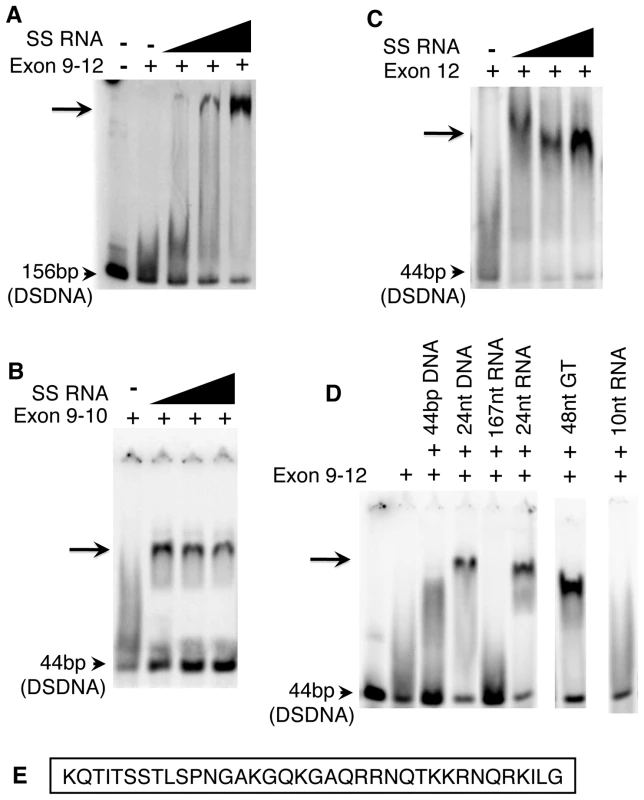
(A) The DNA binding domain of CENPC alone does not bind to DNA, however a shifted band becomes evident as increasing amounts of SSRNA are added. The same is true for Exon 9–10 (B) and exon 12 alone (C). (D) Only single-stranded RNA or DNA stabilizes Exon 9–12 for DSDNA binding. Gel shift is observed in the presence of 24 nt SSDNA, 24 nt SSRNA, and 48 nt poly-GT (SSDNA). However, no shift is observed for 44 bp DSDNA, 167 nt RNA (with double stranded character) or 10 nt RNA. (E) Sequence of CENPC exon 12. Other single-stranded nucleic acids stabilize the CENPC–DNA interaction
The majority of centromere-associated CentC and CRM transcripts are single-stranded and larger than 40 nt [24]. To test whether RNAs of this type are effective CENPC stabilizers, we tested a variety of single - and double-stranded nucleic acids in the gel-shift assay. We found that both 24 nt SSRNA and SSDNA increase the association of CENPC and DNA (Figure 4D), suggesting that single-strandedness is the key stabilizing feature. However, very short single-stranded RNA (10 nt) had no effect on the CENPC-DNA interaction (Figure 4D). These data suggest that that the stabilization event required oligomers with a minimum length, and that multiple very small RNAs cannot compensate for a proportionally longer RNA.
Tests of longer molecules are confounded by the fact that single-stranded nucleic acids tend to form hairpin secondary structures based on partial homology. Long 167 nt transcripts containing CentC and double stranded small molecules (Figure 4D) did not stabilize the CENPC-DNA complex but instead competed in the binding reaction. Therefore, we used a long (48 nt) DNA polydinucleotide with a repeating GTGT motif that cannot form a double-stranded state. The GTGT polynucleotide stabilized the CENPC-DNA binding reaction efficiently, similar to small RNA (Figure 4D). Thus, long single-stranded nucleic acids similar to those present in vivo [24] are effective stabilizers of CENPC binding in vitro.
We performed the same tests on the well-studied HIV Integrase DNA-binding domain (IntBD) [38], which binds DNA non-specifically similar to CENPC. IntBD is also similar in size to the DNA binding modules in maize CENPC (51 amino acids as compared to 61 amino acids for either exons 9–10 or exons 11–12). The IntBD region was synthesized in vitro and used in DNA gel shift assays. The data show that IntBD binds strongly to DNA without the need for RNA (Figure S1). When RNA is added to the IntBD-DNA mixture, there was no observable effect. These data suggest that stabilization by RNA is a unique feature of the CENPC DNA-binding domain.
The exons 9–12 domain is necessary in vivo for accurate CENPC targeting
We tested the function of the CENPC binding domain in vivo using two assays, transient and stable. Transient transformation was used to provide a large sample size while stable transformation was used for more detailed assessments of tissue specificity and heritability. Transient assays were conducted by biolistic transformation of embryogenic callus surface cells. Three constructs were tested: the full length CENPC gene, a gene with exon 9–12 deleted (delCENPC), and a construct with the natural exon 9–12 replaced with HIV IntBD (IntCENPC, Figure 5A). The genes were constitutively expressed under control of the 35S promoter and CENPC was tagged by YFP at the N-terminus (our preliminary studies revealed that YFP at the CENPC C-terminus impairs kinetochore localization). Assays from transient transformation revealed that deletion of the DNA/RNA binding domain (delCENPC) reduced centromere localization to 56% (n = 39 cells) while substitution of exon 9–12 with IntBD decreased centromere localization to 72% (n = 32 cells; Figure 5A, 5B, and 5E). These data show that exon 9–12 is necessary for efficient centromere targeting in tissue-cultured interphase cells.
Fig. 5. Removal or replacement of Exon 9–12 delocalizes CENPC in vivo. 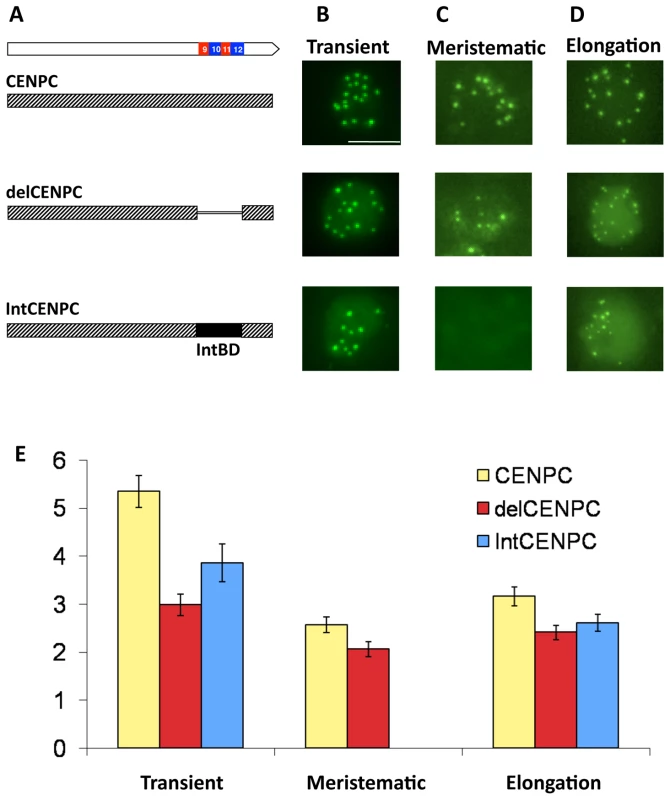
(A) Schematic representation of the YFP–CENPC constructs used in maize transformation. (B) Transient expression of YFP–CENPC in cultured cells. The green YFP spots represent kinetochore localization, as determined from fixed cells (Figure 6). (C) Fluorescence in root tips of stably transformed plants. (D) Fluorescence in the elongation zone of stably transformed plants. Images are projections showing all YFP signal (green) from single nuclei. (E) Quantification of the data in (B–D). The Y axis represents the ratio of YFP signal in kinetochores to YFP signal in the nucleoplasm. There are significant differences between full CENPC and delCENPC and IntCENPC in all three types of tissue (P<0.05, bars show SEM). The same constructs were then introduced into whole plants by Agrobacterium-mediated transformation [39]. Fixed cells were used to confirm that the transformed YFP–CENPC protein localizes to kinetochores at all stages of the cell cycle (Figure 6). The data show that the number of YFP-positive spots in interphase is twenty or fewer (usually 15–20; some kinetochores stick together), and that in prophase the kinetochore spots are paired and limited to primary constrictions, consistent with prior observations [34]. Live-cell assays were then used to quantify the efficiency of localization (by comparing kinetochore to nucleoplasm fluorescence). Two cell types were assayed: root tips, which are rich in actively dividing cells, and elongation zone cells, which are older and undergo few divisions. Deletion of the binding domain (delCENPC) reduced centromere localization to ∼80% of full CENPC in both tissue types (Figure 5C–5E, and Figure S2). In contrast, replacement of exon 9–12 with IntBD abolished kinetochore localization in root tips (Figure 5C, Figure S2). Nevertheless kinetochore localization of IntCENPC recovered in elongation zones and accumulated to ∼80% of the full-length CENPC control (similar to delCENPC; Figure 5D and 5E, and Figure S2). These data suggest that DNA/RNA binding increases the affinity of CENPC for intact centromeres, and that the DNA/RNA binding region is most important during cell division.
Fig. 6. YFP–CENPC localizes to kinetochores. 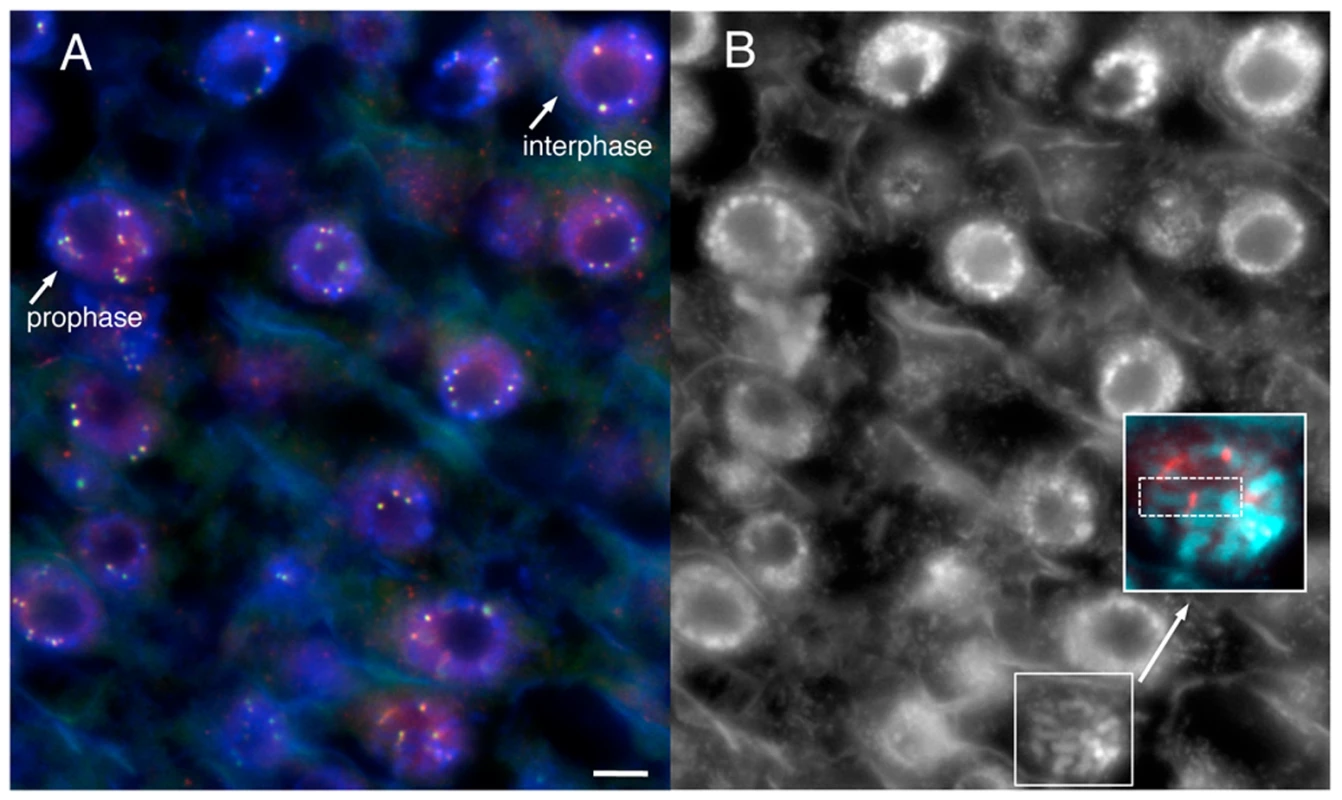
(A) A cryostat section from a root tip, showing cells in various stages of the cell cycle. YFP–CENPC is labeled by anti–YFP antisera (red), while YFP itself is shown in green. The red and green signals overlay to produce a yellow color. DNA (DAPI) is shown in blue. Cells in interphase and prophase are noted and differentiated by chromatin staining. Kinetochores on prophase chromosomes are noted with arrows to show the paired spots on replicated chromatids. (B) A black and white version of the DNA stain in (A). An early prophase cell is enlarged in the panel to highlight a single chromosome, with anti–YFP staining (red) lying in the primary constriction. Each kinetochore is noted with an arrow. Bar = 5 µm. Native CentC transcripts are predominantly 75 nt and transcribed from one strand
In order to better understand the nature of centromeric transcripts in vivo, we subjected total maize RNA to a careful analysis. The intent of these experiments was to identify the full suite of centromeric RNAs in maize. Our prior work had focused only on those RNAs associated with chromatin, and did not reveal an siRNA-sized class [24]. Total cellular RNA was assayed by a standard northern protocol (Fure 7A and 7B). These data show that the majority of CentC RNAs are discretely-sized, but much longer than micro or siRNAs (compare to miR166 at 22 nt; Figure 7A). At higher resolution it is clear that the major form is 75 nt. Most of the transcripts originated from the ‘forward’ strand of CentC (as defined by AY530283.1; Figure 7A), although both strands are abundant in CENH3-associated chromatin [24]. Other longer transcripts(s) are also present, as well as a 40 nt band seen previously [24], and the predicted siRNA-sized bands (Figure 7B). Centromeric RNAs of similar size have also been observed in other species [40]–[43].
Fig. 7. Chromatin-associated CentC transcripts are predominantly 75 nt and do not include siRNAs. 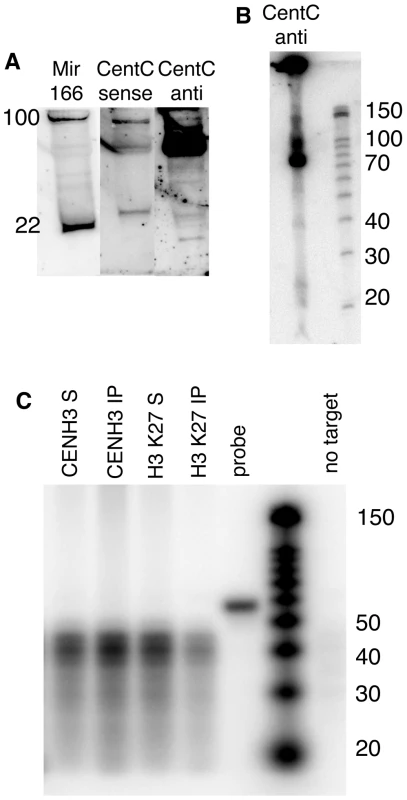
(A) Northern blot of total maize RNA. DNase-treated total RNA (enriched for ≤200 bp) was separated by PAGE and blotted to a membrane. Radiolabeled RNA probes specific to microRNA166 (miR166) [55] and CentC forward (GenBank AY530283.1) and reverse strands were hybridized in succession. The mature form of miR166 is 22 nt while its precursors are ∼100 nt and seen near the top of the gel. Molecular weights were estimated by ethidium bromide staining of 22 and 28 nt RNA oligonucleotides (not shown). (B) Higher resolution RNA blot showing the size classes of CentC forward transcripts. Molecular markers are shown on the right. (C) Analysis of RNA associated with CENH3 and H3-containing chromatin. RNA recovered from ChIP experiments (3.34 µg) was subjected to RNase protection (RPA) using the CentC forward probe (52 nt). The upper resolution of detectable sizes is 44 nt (8 nt less than the probe size). The fraction associated with immune complex is labeled ‘IP’ and the non-associated fraction is labeled ‘S’. Yeast RNA was used to demonstrate complete digestion in the absence of target DNA, and is labeled ‘no target’. Native CentC transcripts are not exclusively present at maize centromere cores
Prior research established that long single stranded transcripts are associated with maize centromeric chromatin [24], but did not address the question of whether similar transcripts can be associated with pericentromeric (or other) regions. Therefore we carried out ChIP experiments with antisera to H3K27me1, an abundant form of histone H3 in pericentromeric areas [44], and compared it to CENH3 ChIP. An RNase protection assay (RPA) was used to gain a sensitive measurement of the CentC RNA associated with ChIP samples (Figure 7C). The data reveal that long CentC RNAs are associated with CENH3 chromatin, while siRNAs are not, despite the fact that siRNAs can be observed in total RNA preparations (Figure 7A and 7B). We also observed detectable (though lesser) quantities of CentC RNAs associated with H3K27me1-containing nucleosomes. This result was confirmed in a second experiment using a real time PCR as the assay (see Materials and Methods). Therefore we do not suppose that kinetochores are unique in retaining RNA on chromatin, but rather that centromeric RNAs are more abundant within kinetochore domains.
Discussion
This study was designed to identify the DNA binding characteristics of CENPC, which in plants is presumed to be the primary protein that binds to the surface of centromeric DNA. Our intent was to test the idea that CENPC is a principle factor in conferring heritability to centromeres. Prior data indicated that single-stranded RNA is abundant in maize centromeric nucleosome purifications [24], that maize CENPC contains an adaptively evolving exon duplication domain [32], that in animals single-stranded RNA is required to maintain CENPC at kinetochores [27], and that transcription of a LINE retroelement is required for kinetochore maintenance over a human neocentromere [28]. Here we provide data that suggest an underlying mechanism for these observations–that centromeric RNA provides a means for CENPC to effectively bind DNA.
In animals the DNA binding region of CENPC is poorly defined and shows no homology to CENPC homologs in non-mammalian species. The lack of homology outside of a 23 aa acid region (of unknown function) has been cast as evidence that the protein is under selection to adapt to DNA sequence change [32]. The argument is perhaps strongest in the grasses, where a small exon 9–12 region has been repeatedly duplicated as if under diversifying selection [32],[45]. Maize CENPC exon 9–12 is rich in arginine and lysine similar to other DNA binding regions [46],[47]. Our study was initiated in part to test the hypothesis that exon 9–12 is indeed the primary DNA binding region.
A comprehensive truncation/deletion analysis confirmed that nearly all DNA and RNA binding activity in maize CENPC lies within the exon 9–12 domain (Figure 2A–2C). The binding reaction lacks sequence specificity, to the extent that any double stranded nucleic acid competes with the native CentC repeat in gel shift assays. Single-stranded molecules, in contrast, do not compete but instead strongly promote DNA binding. The addition of RNA causes full CENPC to bind as a supershifted product that we associate with multiple CENPC proteins per DNA substrate (Figure 3B). The RNA effect is much more dramatic with the purified DNA binding module alone, which cannot bind DNA efficiently unless single-stranded nucleic acids are present (Figure 4A). These data may suggest that the DNA binding module is naturally unstructured [48], perhaps in a way that blocks the protein from folding or dimerizing properly.
Further studies revealed that the stabilizing molecules must be single-stranded and larger than 10 nt (Figure 4D), that excessive SSRNA does not compete with a DNA-CENPC complex, and that RNA stabilizes a single 36 amino acid-binding module that is probably too small to accommodate the binding of both DNA and RNA (Figure 4C). These data suggest that RNA binds transiently to CENPC and alters CENPC structure to facilitate DNA binding, similar to the function of a protein chaperone. RNA-stabilized DNA binding may be a unique feature of CENPC, since our assays show that another DNA-binding domain (HIV IntDB) lacks this property (Figure S1).
Our in vitro observations correspond well to the observation that maize centromeric chromatin is rich in 40 to 200 nt single-stranded RNAs [24]. We have shown here (Figure 7C) and previously [24] that long SSRNAs are preferentially associated with CENH3 domains. The most abundant forms are discretely sized at 75 nt and 40 nt (Figure 7A and 7B), while siRNAs, which are detectable in total RNA extracts (Figure 7A and 7B), are not associated with CENH3 chromatin (Figure 7C). Notably, several groups have found centromeric RNAs of similar size, suggesting the possibility of a processing system distinct to centromeres [24], [41]–[43],[49].
Prior data from human cells strongly suggest that RNA-facilitated DNA binding is a conserved feature of CENPC. Human CENPC contains a nucleolus-localizing sequence (NoLS) that is essential for SSRNA binding [27], and the same RNA binding region is necessary for CENPC centromere localization [27]. The authors argue that human CENPC assembles as an RNA-containing pre-kinetochore complex in the nucleolus before being incorporated into centromeres. Although we do not see CENPC nucleolar localization in maize, it is likely that RNA serves to stabilize a CENPC protein complex in both human and maize. In a second report, a human neocentromere (mardel 10) was shown to contain a single actively transcribing LINE retrotransposon at the core of the CENH3 domain. The authors showed that LINE transcripts are incorporated into CENH3 chromatin and necessary for kinetochore replication in dividing cells [28].
A model for RNA facilitated exon duplication binding in centromeric assembly of CENPC
Our observation that the delCENPC and IntCENPC constructs, which lack the natural DNA binding domain of maize CENPC, localize to kinetochores with 80% efficiency (Figure 5) suggests that initial targeting of CENPC occurs independently of DNA binding. From these data we infer that the role of the DNA binding domain is to reinforce and/or stabilize accurate localization once it occurs. The fact that single stranded RNA has a strong positive effect on DNA binding suggests that centromeric RNA serves as an epigenetic mark that mediates this final and most stable state of assembly. Therefore we propose that CENPC is first recruited to kinetochores by protein-protein contacts and then converted to a functional DNA binding protein by centromeric RNA (Figure 8). We emphasize that our interpretation relies heavily on in vitro experiments, and that our in vivo data are complicated by the presence of wild type CENPC in the transformed lines, which may have influenced the recruitment of introduced YFP-tagged proteins. Although our data show that DNA binding has a role in maize CENPC function, our experiments cannot be used to quantify how important DNA binding is; nor do our data establish with certainty that RNA facilitates DNA binding in vivo. Nevertheless our data provide the first plausible mechanistic explanation for a previously unexplained phenomenon.
Fig. 8. A model for how RNA facilitates the CENPC-DNA interaction. 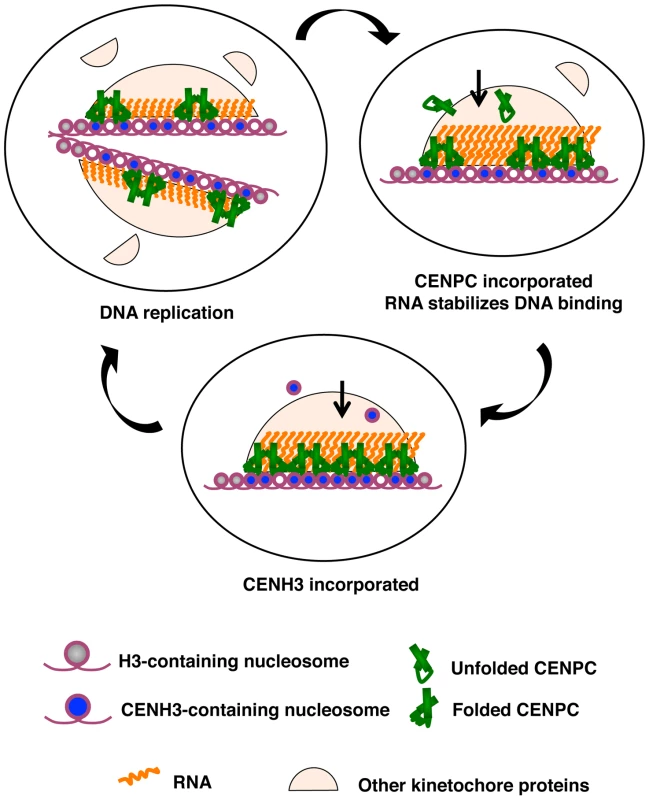
Three stages of kinetochore replication are shown in a cycle that broadly represents the cell cycle. DNA replication splits kinetochores and distributes the resident kinetochore proteins to sister chromatids. CENPC recruitment is continuous. Protein–protein contacts bring CENPC to the kinetochore while resident RNA stabilizes CENPC for DNA binding. CENH3 assembly is a discrete event that occurs after S phase, as early as G2 [14] or as late as anaphase or G1 [15],[16]. CENPC and CENH3 assembly are separated in time such that one can guide the other. The role of RNA in CENPC function may be similar in principle to the well-understood roX1 and roX2 RNAs of Drosophila, which function to up-regulate genes on the male X chromosome [50]. roX RNAs bind to a small complex of MSL (male-specific lethal) proteins and change their binding specificity so that they interact with many sites along the X chromosome [50]. Without roX RNAs, the MSL complex loses its specificity and dosage compensation is lost. Similarly, most of the centromeric RNA in maize probably functions in trans; that is, it is unlikely that all RNA associated with centromeric DNA is encoded by the DNA directly beneath it. However it is also very probable that each centromere has the capacity to transcribe its own large population of RNA. The transcriptionally competent state of each centromere [23] can provide a renewable, effective cis-acting source of RNA for stabilizing CENPC at the inner kinetochore.
In the larger view, the mode of centromere replication may revolve around that fact that two proteins can serve as place markers, CENH3 and CENPC, and that they have different temporal patterns of incorporation. CENH3 is assembled in a defined period (G2 in plants, G1 in animals), while CENPC is incorporated throughout the cell cycle [16]. If, as in Drosophila, the two proteins can target each other to the kinetochore [20],[22], then at least one of the place markers will be present on DNA at all times (Figure 8). While CENH3 appears to be a defining feature in all eukaryotes, any number of other kinetochore proteins could serve as the second marker and target CENH3 or its assembly factors. CENPC is currently the best candidate in plants, but it is conceivable that other known or unknown kinetochore proteins, such as the functional equivalent of mammalian CENP-I or CENP-H may have similar roles [16],[18],[19].
Materials and Methods
Preparation of recombinant CENPC protein and its variants
Full CENPC (1–701 aa), Δexon 9–12 (1–502 aa+625–701 aa), exon1–5.7 (1–399 aa), exon1–8 (1–502 aa), and exon1–11.3 (1–601 aa) were amplified from the CIMMH01 plasmid (GenBank AF129857) [51]. To generate Δexon 9–12, a 1∼1506 bp fragment at the 5′-end and an 1873∼2106 bp fragment at the 3′-end of the maize Cenpc gene were first amplified separately. The two amplicons were then joined together by overlapping ends in a secondary PCR. The same strategy was used to generate IntCENPC except that an amplified DNA-binding domain of HIV integrase (GenBank AAC83550, amino acids 220–270) [38] was added as a third template for secondary PCR.
All constructs were inserted into the pET28a vector (Novagen) and transformed into Rosetta Blue (DE3) competent cells (Novagen). Recombinant CENPC subdomains were expressed as histidine-fused proteins and purified according to the manufacturers protocols. The expressed proteins were verified by size using His-tag staining (Invitrogen) and western blotting. The peptide for exon 12 of maize CENPC was synthesized by Sigma-Genosys and the peptides for CENPC exon 9&10 and the DNA-binding domain of HIV integrase were synthesized by Abgent.
Preparation of DNA probes and competitors
A 156 bp CentC monomer identical to GenBank AF078922 was synthesized by annealing two long primers and cloned into the pCR4 vector (Invitrogen). The 67 bp CentC probe was generated using primers GGTTCCGGTGGCAAAAACTCGTGC and GCACGTCACCCATTCTGAAAACGG. Shorter single-stranded DNA sequences were synthesized as oligonucleotides, and if needed, annealed with a complementary oligonucleotide to form duplexes. These were the 44 bp CentC probe AATGGGTGACGTGCGGCAACGAAATTGCGCGAAACCACCCCAAA, the 24 bp CentC probes CCGTTTTCAGAATGGGTGACGTGC, and a 44 bp fragment of the maize Ndc80 cDNA. For gel shift assays, DNA was end-labeled with 33P-ATP using T4 polynucleotide kinase (Invitrogen).
Preparation of RNA probes and competitors
CentC RNA was synthesized from a construct containing the SP6 promoter upstream of a sequence identical to GenBank AF078922. The 167 nt RNA was transcribed in vitro using Sp6 RNA polymerase and a Riboprobe kit (Promega). To label RNA, 33P-labeled UTP was added to the reaction. The 24 nt RNA rCrCrGrUrUrUrUrCrArGrArArUrGrGrGrUrGrArCrGrUrGrC and 10 nt RNA rCrCrGrUrUrUrUrCrArG molecules were synthesized by Integrated DNA Technologies and end-labeled with 33P-ATP using T4 polynucleotide kinase (Invitrogen).
Electrophoretic mobility shift assays
Radiolabelled DNA or RNA probes were incubated with CENPC or CENPC derivatives on ice for 20 min in a 20 µl solution of 10 mM Tris (pH 7.5), 50 mM NaCl, 0.5 mM dithiothreitol, 0.5 mM EDTA, 1 mM MgCl2 and 4% glycerol. For competition experiments, unlabeled DNA of different sequence was added in excess. The reaction mixtures were separated on 5% polyacrylamide gels and detected using a PhosphorImager.
Generation of YFP–tagged CENPC constructs
CENPC, delCENPC, and intCENPC sequences were cloned into the pENTR/D-TOPO vector (Invitrogen). These were then recombined into the pEarleyGate 104 vector [52] using LR clonase (Invitrogen) to form N-terminal YFP fusions. Recombinant plasmids were transformed into Agrobacterium strain C58C1 for maize transformation.
Plant transformation and analysis of YFP expression in vivo
Agrobacterium-mediated transformation of maize (hybrid line HiII) was performed by the Plant Transformation Facility at Iowa State University [39]. Transgenic plantlets were grown to maturity in the UGA Plant Biology greenhouse and crossed to inbred B73. Progeny were grown at 30°C and root tips observed in vivo.
The YFP–CENPC, YFP-delCENPC, and YFP-IntCENPC plasmids were also used for particle bombardment of maize HiII callus. Transient transformation was performed with plasmid DNA-coated gold particles using a PDS1000 system (Bio-Rad). The bombarded callus was cultured in dark for 18 h prior to observation.
Immunolocalization
Root tips of transgenic plantlets were fixed in PHEMS buffer as previously described [53] and sections 10 µm in thickness were prepared on a cryostat (−20°C). Tissue sections transferred on polylysine slides were incubated with rabbit anti-YFP antibodies (1∶50) and then rhodamine-conjugated goat anti-rabbit antibodies (1∶100; Jackson Immunoresearch, West Grove, PA). The DNA was stained with 0.1 ug/ml 4,6-diamidino-2-phenylindole (DAPI).
Localization data were captured as 3D data sets by an Intelligent Imaging Innovations (Denver) Everest Digital Microscope Workstation and further analyzed by SlideBook 4.0 (Intelligent Imaging Innovations) and SoftWoRx (Applied Precision, Issaquah WA) software packages.
Image capture, processing, and intensity analysis
Three-dimensional image sets of YFP localization were acquired using an Intelligent Imaging Innovations (Denver, CO, USA) Everest Digital Microscope Workstation. For the transient expression assays of YFP–CENPC, YFP-delCENPC and YFP-intCENPC, 60 cells, 39 cells, and 32 cells were sampled respectively. For the stable transgenic lines, all levels of assay were performed in triplicate. For each construct, progeny from three different transformants were analyzed. For each transformant, three different progeny were assayed. For each progeny, three meristematic areas and three elongation areas, each containing 20∼50 cells per area were assayed.
Image analysis was carried out using SlideBook 4.0 software. Maximum projection was obtained from each 3D stack and used for signal measurement. For each cell, kinetochores and nucleoplasm were masked and the sum intensities and volumes recorded. Background, calculated from four random areas, was subtracted from the intensity data. Kinetochore localization was calculated as the mean intensity of kinetochore staining divided by the mean intensity of nucleoplasm staining.
Native centromere RNA analysis
Total RNA from young maize ears was isolated and enriched for RNA smaller than 200 nt using the mirVana miRNA Isolation Kit (Ambion), then treated with the TURBO DNA-free kit (Ambion). The quantity of RNA recovered was calculated using a NanoDrop (ThermoScientific). Samples were separated on 12% or 15% TRIS-UREA polyacrylamide gels and blotted to BrightStar-Plus charged membranes (Ambion) using 0.5X TBE in a semidry blotter. Radiolabeled probes were generated using the mirVana miRNA Probe Construction Kit from Ambion, a T7 RNA polymerase based procedure that incorporates 32P-rCTPs internally at every cytosine. Template sequences were as follows, where lower case letters are T7 primer regions: CentC ‘forward’ TTTGGGGTGGTTTCGCGCAATTTCGTTGCCGCACGTCACCCATTcctgtctc, CentC ‘reverse’ AATGGGTGACGTGCGGCAACGAAATTGCGCGAAACCACCCCAAAcctgtctc, and mir166 TCGGACCAGGCTTCATTCCCCcctgtctc. Full-length probes were gel purified and measured by scintillation.
Northern blot hybridization was conducted using ULTRAhyb buffer (Ambion). Prehybridization was at 68° C for ≥1 hour, and hybridization was at 42° C for ≥12 hours, using a probe concentration of 106 cpm/mL. Washes were at 42° C in 2X SSC and 0.1% SDS for 15 minutes. Similar results were obtained with additional forward and reverse probes that targeted other regions of CentC.
Chromatin immunoprecipitation was carried out as previously described [24]. H3K27me1 antibodies (Upstate 07-448) were used at a dilution of 1∶250. RNase protection assays on DNase treated samples were conducted using the RPA III kit (Ambion) with the CentC ‘forward’ probe. A PhosphorImager and ImageQuant software (Amersham Biosciences) were used to capture and quantify images.
Quantitative RTPCR was performed by standard methods. DNase I treated ChIP samples were reverse-transcribed using random hexamers and Superscript III enzyme (Invitrogen). Each sample was subsequently assayed using primer CentC-F1 GAAATGGTTCCGGTGGCAA and CentC-R1 TGGTTTCGCGCAATTTCGTT, or Zm5S-F1 GATGCGATCATACCAGCACTA and Zm5S-R1 GAATGCAACACGAGGACTT (to 5S ribosomal RNA). Relative fold enrichment (RFE) was calculated by the 2−ΔΔCt method [54] using the 5S ribosomal RNA sequence as a control. Reactions were averaged from triplicate wells and normalized to controls lacking reverse transcriptase.
Supporting Information
Zdroje
1. YuHG
HiattEN
DaweRK
2000 The plant kinetochore. Trends Plant Sci 5 543 547
2. Kline-SmithSL
SandallS
DesaiA
2005 Kinetochore-spindle microtubule interactions during mitosis. Curr Opin Cell Biol 17 35 46
3. KotwaliwaleC
BigginsS
2006 Microtubule capture: a concerted effort. Cell 127 1105 1108
4. FukagawaT
2008 The kinetochore and spindle checkpoint in vertebrate cells. Front Biosci 13 2705 2713
5. HenikoffS
AhmadK
MalikHS
2001 The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293 1098 1102
6. McAinshAD
TytellJD
SorgerPK
2003 Structure, function, and regulation of budding yeast kinetochores. Annu Rev Cell Dev Biol 19 519 539
7. JinW
MeloJR
NagakiK
TalbertPB
HenikoffS
2004 Maize centromeres: organization and functional adaptation in the genetic background of oat. Plant Cell 16 571 581
8. DaweRK
2005 Centromere renewal and replacement in the plant kingdom. Proc Natl Acad Sci U S A 102 11573 11574
9. CheesemanIM
DesaiA
2008 Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol 9 33 46
10. HowmanEV
FowlerKJ
NewsonAJ
RedwardS
MacDonaldAC
2000 Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc Natl Acad Sci U S A 97 1148 1153
11. CollinsKA
CastilloAR
TatsutaniSY
BigginsS
2005 De novo kinetochore assembly requires the centromeric histone H3 variant. Mol Biol Cell 16 5649 5660
12. RegnierV
VagnarelliP
FukagawaT
ZerjalT
BurnsE
2005 CENP-A is required for accurate chromosome segregation and sustained kinetochore association of BubR1. Mol Cell Biol 25 3967 3981
13. BlowerMD
DaigleT
KaufmanT
KarpenGH
2006 Drosophila CENP-A mutations cause a BubR1-dependent early mitotic delay without normal localization of kinetochore components. PLoS Genet 2 e110 doi:10.1371/journal.pgen.0020110
14. LermontovaI
SchubertV
FuchsJ
KlatteS
MacasJ
2006 Loading of Arabidopsis centromeric histone CENH3 occurs mainly during G2 and requires the presence of the histone fold domain. Plant Cell 18 2443 2451
15. SchuhM
LehnerCF
HeidmannS
2007 Incorporation of Drosophila CID/CENP-A and CENP-C into centromeres during early embryonic anaphase. Curr Biol 17 237 243
16. HemmerichP
Weidtkamp-PetersS
HoischenC
SchmiedebergL
ErliandriI
2008 Dynamics of inner kinetochore assembly and maintenance in living cells. J Cell Biol
17. PidouxAL
ChoiES
AbbottJK
LiuX
KaganskyA
2009 Fission yeast Scm3: A CENP-A receptor required for integrity of subkinetochore chromatin. Mol Cell 33 299 311
18. KlineSL
CheesemanIM
HoriT
FukagawaT
DesaiA
2006 The human Mis12 complex is required for kinetochore assembly and proper chromosome segregation. J Cell Biol 173 9 17
19. OkadaM
CheesemanIM
HoriT
OkawaK
McLeodIX
2006 The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol 8 446 457
20. ErhardtS
MelloneBG
BettsCM
ZhangW
KarpenGH
2008 Genome-wide analysis reveals a cell cycle-dependent mechanism controlling centromere propagation. J Cell Biol 183 805 818
21. KwonMS
HoriT
OkadaM
FukagawaT
2007 CENP-C Is Involved in Chromosome Segregation, Mitotic Checkpoint Function, and Kinetochore Assembly. Mol Biol Cell
22. HoriT
AmanoM
SuzukiA
BackerCB
WelburnJP
2008 CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell 135 1039 1052
23. ZhangW
LeeHR
KooDH
JiangJ
2008 Epigenetic modification of centromeric chromatin: hypomethylation of DNA sequences in the CENH3-associated chromatin in Arabidopsis thaliana and maize. Plant Cell 20 25 34
24. ToppCN
ZhongCX
DaweRK
2004 Centromere-encoded RNAs are integral components of the maize kinetochore. Proc Natl Acad Sci U S A 101 15986 15991
25. JiangJ
BirchlerJA
ParrottWA
DaweRK
2003 A molecular view of plant centromeres. Trends Plant Sci 8 570 575
26. AllshireRC
KarpenGH
2008 Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat Rev Genet 9 923 937
27. WongLH
Brettingham-MooreKH
ChanL
QuachJM
AndersonMA
2007 Centromere RNA is a key component for the assembly of nucleoproteins at the nucleolus and centromere. Genome Res 17 1146 1160
28. ChuehAC
NorthropEL
Brettingham-MooreKH
ChooKH
WongLH
2009 LINE retrotransposon RNA is an essential structural and functional epigenetic component of a core neocentromeric chromatin. PLoS Genet 5 e1000354 doi:10.1371/journal.pgen.1000354
29. SugimotoK
YataH
MuroY
HimenoM
1994 Human centromere protein C (CENP-C) is a DNA-binding protein which possesses a novel DNA-binding motif. J Biochem (Tokyo) 116 877 881
30. YangCH
TomkielJ
SaitohH
JohnsonDH
EarnshawWC
1996 Identification of overlapping DNA-binding and centromere-targeting domains in the human kinetochore protein CENP-C. Mol Cell Biol 16 3576 3586
31. SugimotoK
KuriyamaK
ShibataA
HimenoM
1997 Characterization of internal DNA-binding and C-terminal dimerization domains of human centromere/kinetochore autoantigen CENP-C in vitro: role of DNA-binding and self-associating activities in kinetochore organization. Chromosome Res 5 132 141
32. TalbertPB
BrysonTD
HenikoffS
2004 Adaptive evolution of centromere proteins in plants and animals. J Biol 3 18
33. AnanievEV
PhillipsRL
RinesHW
1998 Chromosome-specific molecular organization of maize (Zea mays L.) centromeric regions. Proc Natl Acad Sci U S A 95 13073 13078
34. DuY
DaweRK
2007 Maize NDC80 is a constitutive feature of the central kinetochore. Chromosome Res 15 767 775
35. MarianCO
BordoliSJ
GoltzM
SantarellaRA
JacksonLP
2003 The maize Single myb histone 1 gene, Smh1, belongs to a novel gene family and encodes a protein that binds telomere DNA repeats in vitro. Plant Physiol 133 1336 1350
36. PeacockWJ
DennisES
RhoadesMM
PryorAJ
1981 Highly repeated DNA sequence limited to knob heterochromatin in maize. Proc Natl Acad Sci U S A 78 4490 4494
37. ZwickMS
Islam-FaridiMN
ZhangHB
HodnettGL
GomezMI
2000 Distribution and sequence analysis of the centromere-associated repetitive element CEN38 of Sorghum bicolor (Poaceae). Am J Bot 87 1757 1764
38. EspositoD
CraigieR
1999 HIV integrase structure and function. Adv Virus Res 52 319 333
39. FrameBR
ShouH
ChikwambaRK
ZhangZ
XiangC
2002 Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol 129 13 22
40. VerdelA
JiaS
GerberS
SugiyamaT
GygiS
2004 RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303 672 676
41. KanellopoulouC
MuljoSA
KungAL
GanesanS
DrapkinR
2005 Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev 19 489 501
42. LeeHR
NeumannP
MacasJ
JiangJ
2006 Transcription and evolutionary dynamics of the centromeric satellite repeat CentO in rice. Mol Biol Evol 23 2505 2520
43. CaroneDM
LongoMS
FerreriGC
HallL
HarrisM
2009 A new class of retroviral and satellite encoded small RNAs emanates from mammalian centromeres. Chromosoma 118 113 125
44. ShiJ
DaweRK
2006 Partitioning of the maize epigenome by the number of methyl groups on histone H3 lysines 9 and 27. Genetics 173 1571 1583
45. DaweRK
HenikoffS
2006 Centromeres put epigenetics in the driver's seat. Trends Biochem Sci 31 662 669
46. LambertSF
ThomasJO
1986 Lysine-containing DNA-binding regions on the surface of the histone octamer in the nucleosome core particle. Eur J Biochem 160 191 201
47. TwiningSS
GoryshinIY
BhasinA
ReznikoffWS
2001 Functional characterization of arginine 30, lysine 40, and arginine 62 in Tn5 transposase. J Biol Chem 276 23135 23143
48. TompaP
2002 Intrinsically unstructured proteins. Trends Biochem Sci 27 527 533
49. SugiyamaT
CamH
VerdelA
MoazedD
GrewalSI
2005 RNA-dependent RNA polymerase is an essential component of a self-enforcing loop coupling heterochromatin assembly to siRNA production. Proc Natl Acad Sci U S A 102 152 157
50. LiF
SchiemannAH
ScottMJ
2008 Incorporation of the noncoding roX RNAs alters the chromatin-binding specificity of the Drosophila MSL1/MSL2 complex. Mol Cell Biol 28 1252 1264
51. DaweRK
ReedLM
YuHG
MuszynskiMG
HiattEN
1999 A maize homolog of mammalian CENPC is a constitutive component of the inner kinetochore. Plant Cell 11 1227 1238
52. EarleyKW
HaagJR
PontesO
OpperK
JuehneT
2006 Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45 616 629
53. ZhangX
LiX
MarshallJB
ZhongCX
DaweRK
2005 Phosphoserines on maize CENTROMERIC HISTONE H3 and histone H3 demarcate the centromere and pericentromere during chromosome segregation. Plant Cell 17 572 583
54. LivakKJ
SchmittgenTD
2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 402 408
55. JuarezMT
KuiJS
ThomasJ
HellerBA
TimmermansMC
2004 microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428 84 88
Štítky
Genetika Reprodukční medicína
Článek Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the GenomeČlánek Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in MiceČlánek Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' DiseaseČlánek Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD TagsČlánek Wing Patterns in the Mist
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 2- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Scale of Population Structure in
- Allelic Exchange of Pheromones and Their Receptors Reprograms Sexual Identity in
- Genetic and Functional Dissection of and in Age-Related Macular Degeneration
- A Single Nucleotide Polymorphism within the Acetyl-Coenzyme A Carboxylase Beta Gene Is Associated with Proteinuria in Patients with Type 2 Diabetes
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Cdk2 Is Required for p53-Independent G/M Checkpoint Control
- Uncoupling of Satellite DNA and Centromeric Function in the Genus
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in the Clade
- Use of DNA–Damaging Agents and RNA Pooling to Assess Expression Profiles Associated with and Mutation Status in Familial Breast Cancer Patients
- Cheating by Exploitation of Developmental Prestalk Patterning in
- Replication and Active Demethylation Represent Partially Overlapping Mechanisms for Erasure of H3K4me3 in Budding Yeast
- Cdk1 Targets Srs2 to Complete Synthesis-Dependent Strand Annealing and to Promote Recombinational Repair
- A Genome-Wide Association Study Identifies Susceptibility Variants for Type 2 Diabetes in Han Chinese
- Genome-Wide Identification of Susceptibility Alleles for Viral Infections through a Population Genetics Approach
- Transcriptional Rewiring of the Sex Determining Gene Duplicate by Transposable Elements
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in
- Proteasome Nuclear Activity Affects Chromosome Stability by Controlling the Turnover of Mms22, a Protein Important for DNA Repair
- Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in Mice
- Structure, Function, and Evolution of the spp. Genome
- Human and Non-Human Primate Genomes Share Hotspots of Positive Selection
- A Kinase-Independent Role for the Rad3-Rad26 Complex in Recruitment of Tel1 to Telomeres in Fission Yeast
- Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' Disease
- Molecular Evolution and Functional Characterization of Insulin-Like Peptides
- Molecular Poltergeists: Mitochondrial DNA Copies () in Sequenced Nuclear Genomes
- Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD Tags
- Wing Patterns in the Mist
- DNA Binding of Centromere Protein C (CENPC) Is Stabilized by Single-Stranded RNA
- Genome-Wide Association Study Reveals Multiple Loci Associated with Primary Tooth Development during Infancy
- Mutations in , Encoding an Equilibrative Nucleoside Transporter ENT3, Cause a Familial Histiocytosis Syndrome (Faisalabad Histiocytosis) and Familial Rosai-Dorfman Disease
- Genome-Wide Identification of Binding Sites Defines Distinct Functions for PHA-4/FOXA in Development and Environmental Response
- Ku Regulates the Non-Homologous End Joining Pathway Choice of DNA Double-Strand Break Repair in Human Somatic Cells
- Nucleoporins and Transcription: New Connections, New Questions
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Nucleoporins and Transcription: New Connections, New Questions
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



