-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genome-Wide Identification of Susceptibility Alleles for Viral Infections through a Population Genetics Approach
Viruses have exerted a constant and potent selective pressure on human genes throughout evolution. We utilized the marks left by selection on allele frequency to identify viral infection-associated allelic variants. Virus diversity (the number of different viruses in a geographic region) was used to measure virus-driven selective pressure. Results showed an excess of variants correlated with virus diversity in genes involved in immune response and in the biosynthesis of glycan structures functioning as viral receptors; a significantly higher than expected number of variants was also seen in genes encoding proteins that directly interact with viral components. Genome-wide analyses identified 441 variants significantly associated with virus-diversity; these are more frequently located within gene regions than expected, and they map to 139 human genes. Analysis of functional relationships among genes subjected to virus-driven selective pressure identified a complex network enriched in viral products-interacting proteins. The novel approach to the study of infectious disease epidemiology presented herein may represent an alternative to classic genome-wide association studies and provides a large set of candidate susceptibility variants for viral infections.
Published in the journal: . PLoS Genet 6(2): e32767. doi:10.1371/journal.pgen.1000849
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000849Summary
Viruses have exerted a constant and potent selective pressure on human genes throughout evolution. We utilized the marks left by selection on allele frequency to identify viral infection-associated allelic variants. Virus diversity (the number of different viruses in a geographic region) was used to measure virus-driven selective pressure. Results showed an excess of variants correlated with virus diversity in genes involved in immune response and in the biosynthesis of glycan structures functioning as viral receptors; a significantly higher than expected number of variants was also seen in genes encoding proteins that directly interact with viral components. Genome-wide analyses identified 441 variants significantly associated with virus-diversity; these are more frequently located within gene regions than expected, and they map to 139 human genes. Analysis of functional relationships among genes subjected to virus-driven selective pressure identified a complex network enriched in viral products-interacting proteins. The novel approach to the study of infectious disease epidemiology presented herein may represent an alternative to classic genome-wide association studies and provides a large set of candidate susceptibility variants for viral infections.
Introduction
Infectious diseases represent one of the major threats to human populations, are still the first cause of death in developing countries [1], and are therefore a powerful selective force. In particular, viruses have affected humans before they emerged as a species, as testified by the fact that roughly 8% of the human genome is represented by recognizable endogenous retroviruses [2] which represent the fossil remnants of past infections. Also, viruses have probably acted as a formidable challenge to our immune system due to their fast evolutionary rates [3]. Indeed, higher eukaryotes have evolved mechanisms to sense and oppose viral infections; the recent identification of the antiviral activity of particular proteins such as APOBEC, tetherin, and TRIM5 has shed light on some of these mechanisms. Genes involved in anti-viral response have therefore been presumably subjected to an enormous, continuous selective pressure.
Despite the relevance of viral infection for human health, only few genome-wide association studies (GWAS) have been performed in the attempt to identify variants associated with increased susceptibility to infection or faster disease progression [4]–[5]. These studies have shown the presence of a small number of variants, mostly located in the HLA region. This possibly reflects the low power of GWAS to identify variants with a small effect. An alternative approach to discover variants that modulate susceptibility to viral infection is based on the identification of SNPs subjected to virus-driven selective pressure. Indeed, even a small fitness advantage can, on an evolutionary timescale, leave a signature on the allele frequency spectrum and allow identification of candidate polymorphisms. To this aim we exploited the availability of more than 660,000 SNPs genotyped in 52 human populations distributed world-wide (HGDP-CEPH panel) [6] and of epidemiological data stored in the Gideon database.
Results
Virus diversity is a reliable estimator of virus-driven selective pressure
Previous studies [7]–[9] have suggested that the number of the different pathogen species transmitted in a given geographic location is a good estimate of pathogen-driven selection for populations living in that area. Indeed, pathogen diversity is largely dependent on climatic factors [10] and might more closely reflect historical pressures than other estimates such as the prevalence of specific infections. We therefore reasoned that virus diversity can be used as a measure of the selective pressure exerted by virus-borne diseases on human populations and, as a consequence, that SNPs showing an unusually strong correlation with virus diversity can be considered genetic modulators of infection susceptibility or progression. To explore this possibility we used a large set of SNPs that have been genotyped in the HGDP-CEPH panel, a collection of DNAs from almost 950 individuals sampled throughout the world (Table 1). Virus diversity estimates were derived from the Global Infectious Disease and Epidemiology Network database: for each country where HGDP-CEPH populations are located we counted the number of different virus species (or genera/family as described in materials and methods) that are naturally transmitted (Table 1).
Tab. 1. Populations in the HGDP-CEPH panel and virus diversity estimates. 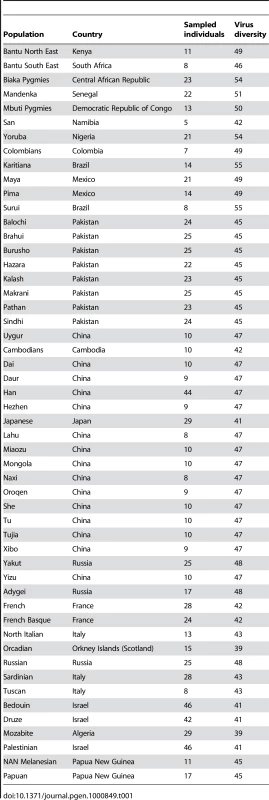
One simple prediction of our hypothesis whereby virus diversity is a reliable estimator of virus-driven selective pressure is that genes known to be involved in immune response are enriched in SNPs significantly associated with virus richness. In order to verify whether this is the case we analysed the InnateDB gene list which contains 2,915 genes involved in immune response and showing the presence of at least one SNP in the HGDP-CEPH panel. Correlations with virus richness were calculated using Kendall's partial rank correlation; since allele frequency spectra in human populations are known to be affected by demographic factors in addition to selective forces [11]–[12], each SNP was assigned a percentile rank in the distribution of τ values calculated for all SNPs having a minor allele frequency (MAF) similar (in the 1% range) to that of the SNP being analysed. A SNP was considered to be significantly associated with virus diversity if it displayed a significant correlation (after Bonferroni correction with α = 0.01) and a rank higher than 0.99. As shown in Table 2, 104 SNPs in InnateDB genes showed a significant association with virus diversity. All SNPs in InnateDB genes that correlated with virus diversity are listed in Table S1. By performing 10,000 re-samplings of 2,915 randomly selected human genes (see materials and methods for details) we verified that the empirical probability of obtaining 104 significantly associated SNPs amounts to 0.010, indicating that genes in the InnateDB list display more virus-associated SNPs than expected.
Tab. 2. Enrichment of SNPs significantly associated with virus diversity in different gene lists. 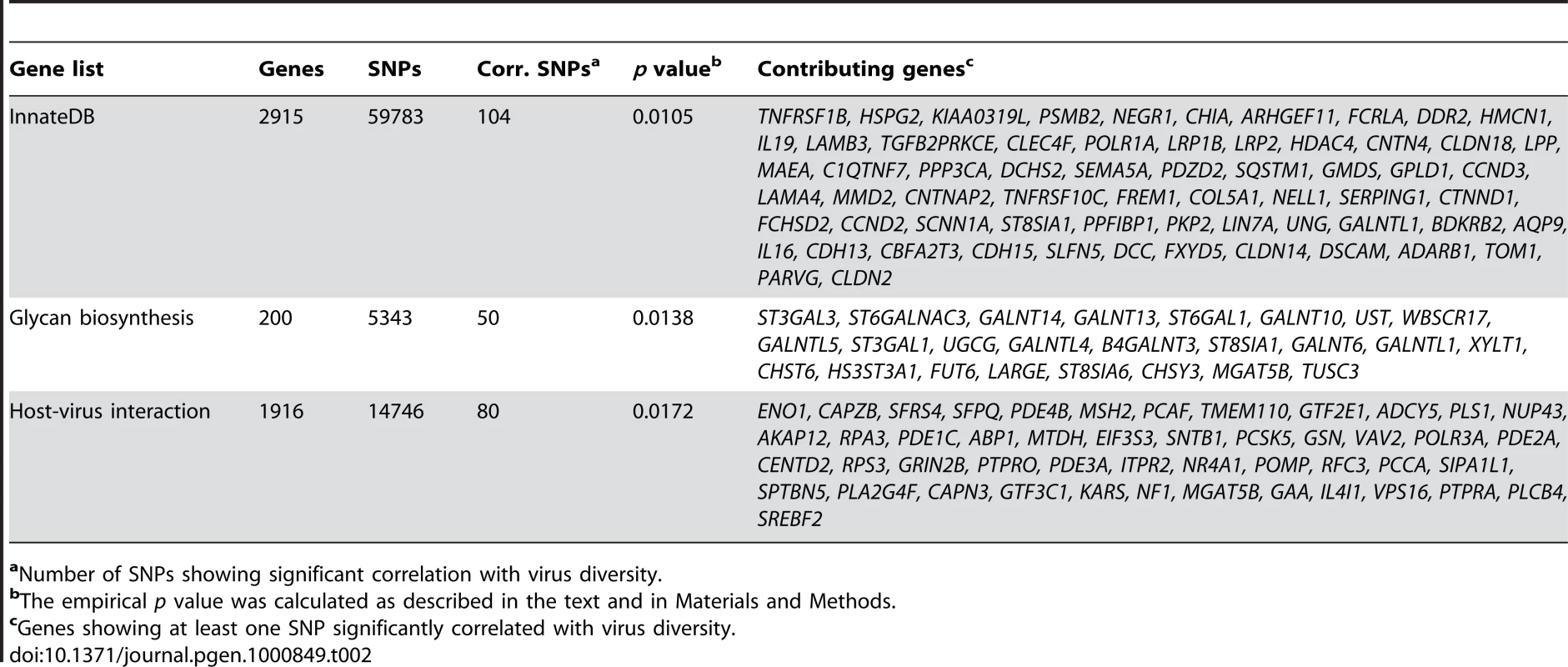
aNumber of SNPs showing significant correlation with virus diversity. It is worth mentioning that amongst these genes, UNG (MIM 191525), encoding uracil DNA glycosylase, functions downstream of APOBEC3G (MIM 607113) to mediate the degradation of nascent HIV-1 DNA [13]. SERPING1 (MIM 606860), a regulator of the complement cascade, is also involved in HIV-1 infection (MIM 609423) as its expression is dysregulated in immature dendritic cells by Tat [14]; moreover, the protein product of SERPING1 is cleaved by HCV and HIV-1 proteases [15]–[16].
Genes involved in the biosynthesis of glycan structures have also been considered as possible modulators of infection susceptibility. Indeed, since Haldane's prediction in 1949 [17] that antigens constituted of protein-carbohydrates molecules modulate the resistance/susceptibility to pathogen infection, protein glycolsylation has been shown to play a pivotal role in viral recognition of host targets [18], as well as in antigen uptake and processing and in immune modulation [19]–[20]. We therefore computed a list of genes involved in glycan biosynthesis from KEGG pathways and Gene Ontology annotations. Again these genes displayed significantly more virus-associated SNPs than expected if randomness alone were responsible (empirical p = 0.0138) (Table 2 and Table S2). Several virus-associated SNPs were located in genes coding for sialyltransferases (ST6GAL1 (MIM 109675), ST3GAL3 (MIM 606494), ST6GALNAC3 (MIM 610133), ST8SIA1 (MIM 601123), ST3GAL1 (MIM 607187) and ST8SIA6 (MIM 610139)). Notably, sialic acids represent the most prevalent terminal monosaccharides on the surface of human cells and determine the host range of different viruses including influenza A [21]–[22], polyomaviruses (i.e JCV and BKV in humans) [23], and rotaviruses (the leading cause of childhood diarrhea) [24].
Sialyltransferases also play central roles in B and T cell communication and function. In particular, the generation of influenza-specific humoral responses is impaired in mice lacking ST6GAL1 [25], while ST3GAL1 regulates apoptosis of CD8+ T cells [20]. Interestingly, ST8SIA6 is expressed in NK cells, possibly playing a role in the regulation of Siglec-7 lectin inhibitory function in these cells [26]. Four other genes (XYLT1 (MIM 608124), HS3ST3A1 (MIM 604057), UST (MIM 610752) and CHSY3 (MIM 609963)) carrying SNPs associated with virus diversity are involved in the biosynthesis of either heparan sulphate or chondroitin sulphate. The former is an ubiquitously expressed glycosaminoglycan serving as the cell entry route for herpesviruses [27], HTLV-1 [28] and papillomaviruses [29]. Chondroitin sulphate is similarly expressed on a wide array of cell types and functions as an auxiliary receptor for binding of herpes simplex virus [30] as well as a facilitator of HIV-1 entry into brain microvascular endothelial cells [31]. Finally, we identified LARGE (MIM 603590) among the genes subjected to virus-driven selective pressure (Table 2). Recent studies have demonstrated that the post-translational modification of α-dystroglycan by LARGE is critical for the binding of arenaviruses of different phylogenetic origin including Lassa fever virus and lymphocytic-choriomeningitis virus [32]–[33]. Therefore our data support the previously proposed hypothesis whereby viruses represent the selective pressure underlying the strong signal of positive selection at the LARGE locus [34].
Since genes involved in immune response and in the biosynthesis of glycan structures are likely to be subjected to selective pressures exerted by pathogens other than viruses, we verified whether a set of genes directly involved in interaction with viral proteins also displays more SNPs significantly correlated with virus diversity. To this aim we retrieved a list of 1,916 genes known to interact with at least one viral product and displaying at least one genotyped SNP in the HGDP-CEPH panel (see materials and methods). In order to perform a non-redundant analysis, genes included in the InnateDB list and involved in glycan biosynthesis were removed; the remaining 987 genes displayed 80 SNPs correlated with virus diversity, corresponding to an empirical p value of 0.017 (Table 2 and Table S3). Notably, when this same analysis was performed using the diversity of pathogens other than viruses (bacteria, protozoa and helminths), no significant excess of correlated SNPs was found (all empirical p values>0.05).
Genome-wide identification of variants subjected to virus-driven selective pressure
Given these results, we wished to identify SNPs significantly associated with virus richness on a genome-wide base. We therefore calculated Kendall's rank correlations between allele frequency and virus diversity for all the SNPs (n = 660,832) typed in the HGDP-CEPH panel. We next searched for instances which withstood Bonferroni correction (with α = 0.05) and displayed a τ percentile rank higher than the 99th among MAF-matched SNPs. A total of 441 SNPs mapping to 139 distinct genes satisfied both requirements. Table 3 shows the 30 top SNPs (or SNP clusters) located within genic regions and associated with virus diversity, while the full list of SNPs subjected to virus-driven selective pressure is available on Table S4. It is worth noting that the SNP dataset we used contains less than 200 variants mapping to HLA genes (both class I and II), therefore covering a minor fraction of genetic variability at these loci; as a consequence HLA genes cannot be expected to be identified as targets of virus-driven selective pressure using the approach we describe herein.
We next verified whether the correlations detected between the SNPs we identified and virus diversity could be secondary to climatic variables. Hence, for all countries where HGDP-CEPH populations are located we obtained (see materials and methods) the following parameters: average annual minimum and maximum temperature, and short wave (UV) radiation flux. Results showed that none of the SNPs associated with virus diversity significantly correlated with any of these variables (Table S5).
Tab. 3. Top 30 SNPs (or SNP clusters) correlated with virus diversity. 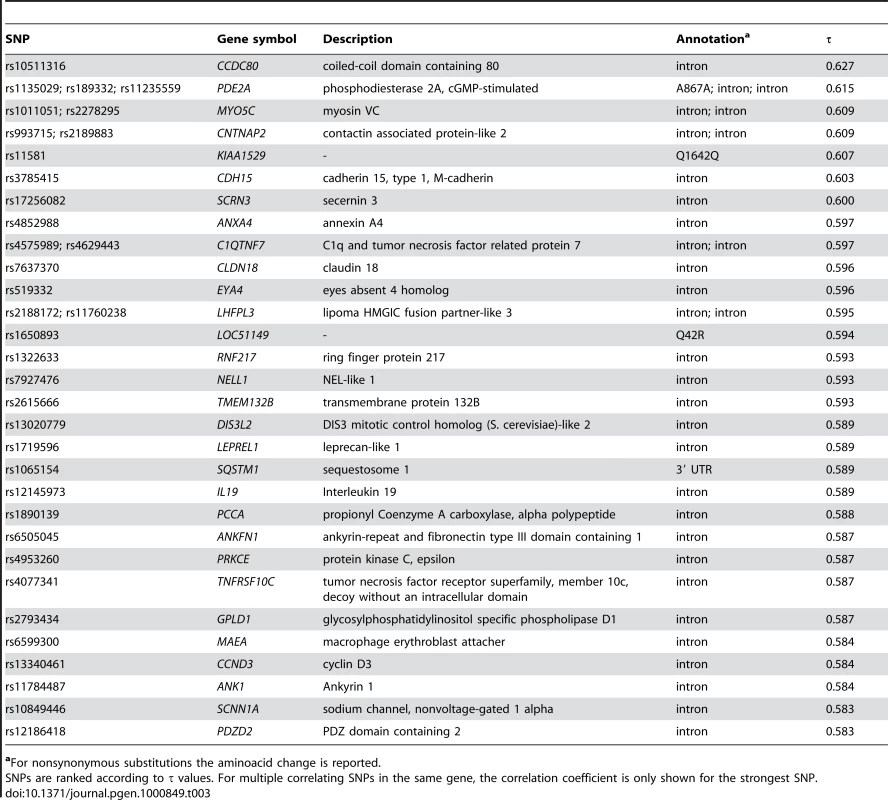
aFor nonsynonymous substitutions the aminoacid change is reported. Previous works have reported an enrichment of selection signatures within or in close proximity to human genes [12],[35]. In line with these data we verified that virus-associated SNPs are more frequently located within gene regions compared to a control set of MAF-matched variants (χ2 test, p = 0.026).
Functional characterization of genes subjected to virus-driven selective pressure
We investigated the role and functional relationship among genes subjected to virus-driven selective pressure using the Ingenuity Pathway Analysis (IPA, Ingenuity Systems) and the PANTHER classification system [36]–[37]. Unsupervised IPA analysis retrieved two networks with significant scores (p = 10−17 and p = 10−12) which were merged into a single interaction network (Figure 1). The network contains 23 genes showing a significant correlation with virus diversity and, among these, 10 encode proteins interacting with viral products (Figure 1). Based on the number of observed human-virus interactions, this finding is unlikely to occur by chance (χ2 test, p = 0.0013) as 2.88 human-virus interactions would be expected for 23 genes. Analysis of the whole network indicated that a 31 of 66 genes encode proteins interacting with viral products (Figure 1): again this number is higher than expected (expected interactions = 8.27; χ2 test, p = 2.8×10−10). Thus, the interaction network we have identified is enriched in genes subjected to virus-driven selective pressure and in genes coding for proteins interacting with viral products. It is worth mentioning that, in agreement with previous findings [38], many viral-interacting proteins represent hubs in the network. Conversely, most of the genes we found to be subjected to virus-driven selective pressure, irrespective of their ability to interact with viral proteins, tend to display very low connectivity (low-degree nodes). This observation might be consistent with previous indications [39]–[41] that in eukaryotes hub genes are more selectively constrained compared to low-degree nodes, these latter being more likely to evolve in response to environmental pressures.
Fig. 1. Network analysis of genes associated with virus diversity. 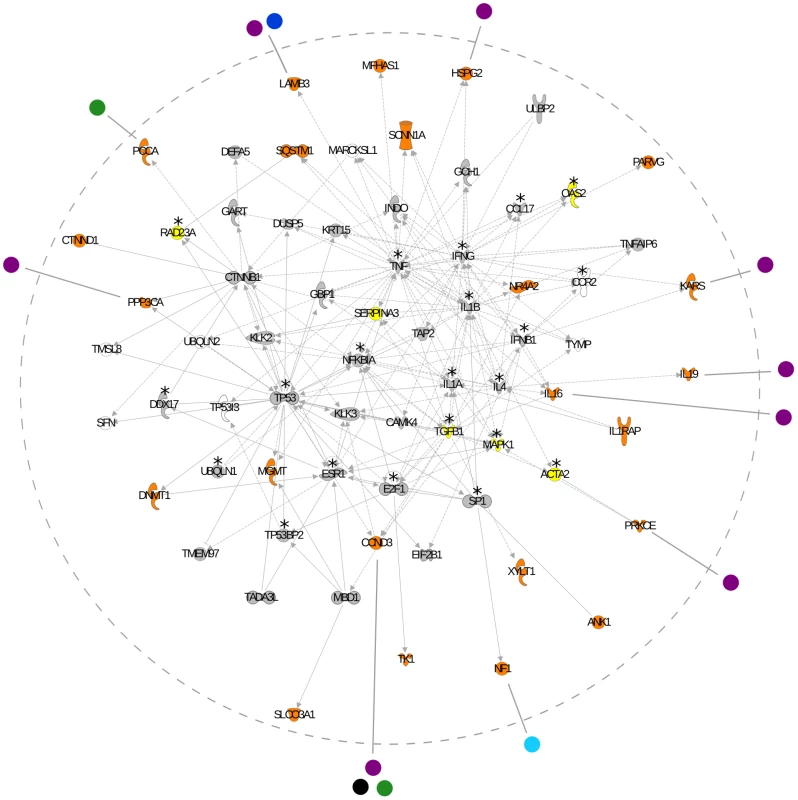
Interactions between human proteins are delimited by the hatched grey circle. Genes are represented as nodes; edges indicate known interactions (sold lines depicts direct and hatched lines depict indirect interaction). Human genes are colour-coded as follows: orange, genes with at least one SNP significantly associated with virus diversity; yellow, genes with at least one SNP that did not withstand genome-wide Bonferroni correction but displayed a rank higher than the 99th and a p value lower than 10−5 (these genes were not included in the input IPA list used to generate networks); grey, genes covered by at least one SNP in the HGDP-CEPH panel; white, genes with no SNPs in the panel. Virus-host interactions are shown for genes subjected to virus-driven selection only; genes interacting with viral products that display no SNP significantly associated with virus diversity are denoted with an asterisk. Viral products are reported outside the hatched circle and colour coded as follows: purple, HIV-1; green, Human herpesvirus; blue, Human rotavirus G3; cyan, Human adenovirus 2; black, Human T-lymphotropic virus 1. In addition to proteins directly interacting with viral products, several network genes showing correlation with virus diversity might play central roles during viral infection. DNMT1 (MIM 126375) and MGMT (MIM 156569) are involved in DNA methylation and repair, respectively, two processes that are often dysregulated during viral infection. In particular, altered expression of DNMT1 is induced by diverse viruses including HIV-1 [42], EBV [43], BKV and adenovirsuses [44]; also, DNMT1 plays a pivotal role in the expansion of effector CD8+ T cell following viral infection [45]. A relevant role in HIV-1 infection is also played by HSPG2 (MIM 142461), the gene coding for perlecan, a cell surface heparan sulfate proteoglycan which mediates the internalization of Tat protein [46].
We next investigated the over-representation of PANTHER classification categories among genes subjected to virus-driven selective pressure. Table 4 shows the significantly over-represented PANTHER molecular functions and biological processes with the contributing genes. In line with the results we reported above, genes involved in immune response, as well as genes coding for proteins involved in cell adhesion and extracellular matrix components, resulted to be over-represented; these latter genes might mediate viral-cellular interaction and facilitate viral entry.
Tab. 4. Significantly over-represented PANTHER categories. 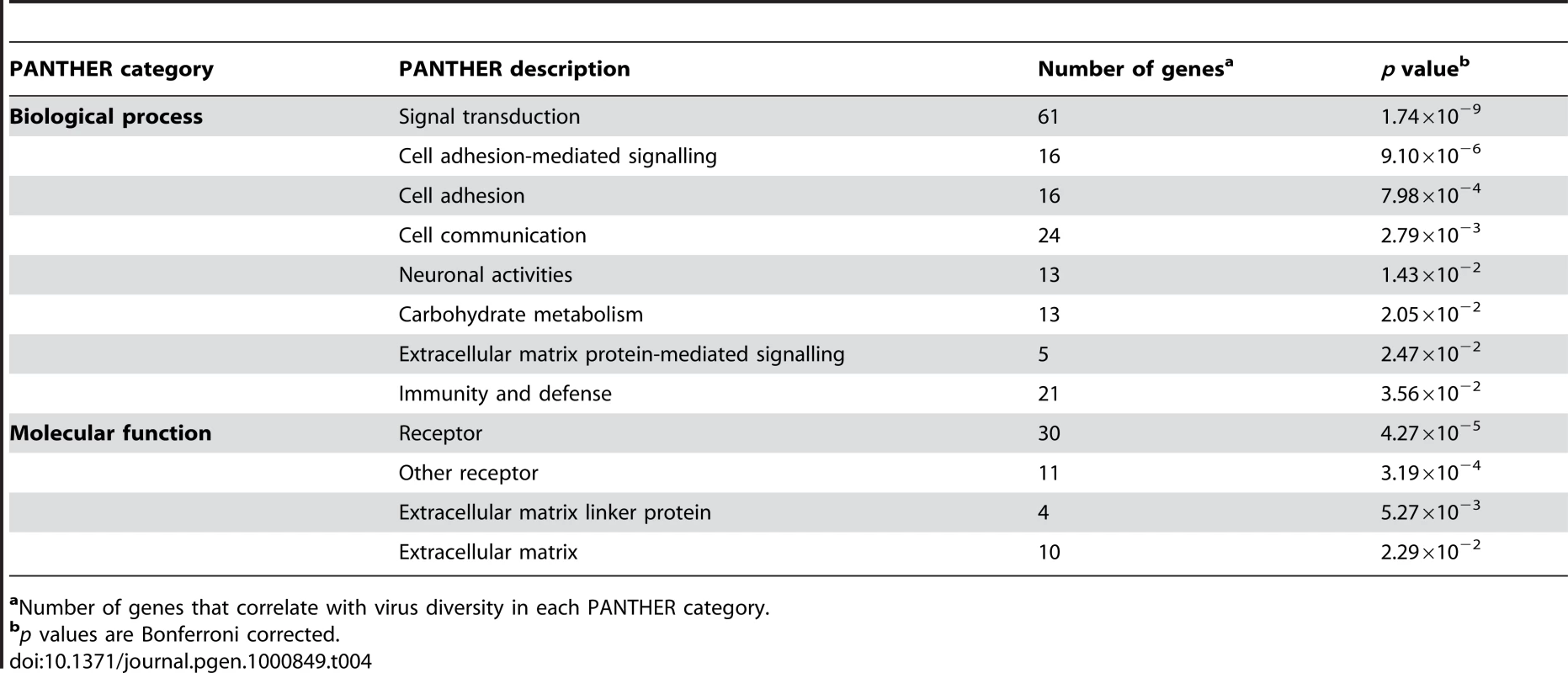
aNumber of genes that correlate with virus diversity in each PANTHER category. Discussion
The identification of non-neutrally evolving loci with a role in immunity can be regarded as a strategy complementary to classic clinical and epidemiological studies in providing insight into the mechanisms of host defense [47]. Here we propose that susceptibility genes for viral infections can be identified by searching for SNPs that display a strong correlation with the diversity of virus species/genera transmitted in different geographic areas. Similar approaches have previously been applied to study the adaptation to climate for genes involved in metabolism and sodium handling [48]–[50]. These analyses, including the one we describe herein, rely on similar assumptions and imply some caveats. First, we implicitly considered virus diversity, as we measure it nowadays, a good proxy for long-term selective pressure. This clearly represents an oversimplification, as new viral pathogens have recently emerged and the virulence of different viral species or genera might have changed over time. Still, previous studies have indicated that the geographic distribution of virus diversity is strongly influenced by climatic variables such as temperature and precipitation rates [10], suggesting that, despite significant changes in prevalence and virulence, virus diversity might have remained relatively constant across different geographic areas, possibly representing the best possible estimate of long-standing pressure. In line with these considerations, we calculated virus diversity as the number of all viral species (or genera/families) that can cause a disease in humans, irrespective of virulence or pathogenicity (Table S6).
The second issue relevant to the data we present herein is that environmental variables tend to co-vary across geographic regions: the distribution of different pathogens (e.g. parasitic worms and viruses/bacteria/protozoa) is correlated across HGDP-CEPH populations [9] and, as reported above, virus diversity is influenced by climatic factors. Therefore, our genome-wide search was preceded by analyses aimed at verifying whether virus diversity is a reliable and specific estimator of virus-driven selective pressure. In particular, we verified that genes involved in immune response and in the biosynthesis of glycans display significantly more variants associated with virus diversity than randomly selected human genes; this finding supports the idea that pathogens rather than climate or demography has driven the genetic variability at these loci. Notably, we also analysed genes that encode proteins interacting with viral components: since loci involved in immune response and in glycan biosynthesis were removed from this list, the remaining genes are expected to be specific targets of viral-driven selective pressure; consistently, we verified that a significant excess of SNPs correlating with virus diversity map to these loci. Conversely, a SNP excess was not noticed when the diversity of other human pathogens was used for the analysis, suggesting that, despite the correlation among different pathogen species across geographic locations [9], the selective pressure imposed by viruses can be distinguished from that exerted by other organisms.
As a further control for the possible confounding effects of other environmental factors, we verified that the variants we identified at the genome-wide level do not correlate with climate (temperature) and UV radiation. This analysis was motivated by the known association of virus diversity and biodiversity in general, with temperature [10],[51] and by the fact that both climate and UV exposure have long been considered among the strongest selective pressures in humans [52]. Since none of the SNPs we identified correlated with either short wave radiation flux or temperature, we consider that their geographic distribution is likely to have been shaped by virus-driven selective pressure. In this respect it is worth mentioning that UV irradiation has been shown to be immunosuppressive in mice (reviewed in [53]–[54]), but the effect of sun exposure on immune functions in humans is still poorly understood. Yet, herpes viruses (both simplex and zoster) and some papillomavirus types have been shown to be reactivated by UV exposure, suggesting that the link between short wave radiation flux and virus-driven selective pressure might be more complex than simply predicted on the basis of geographic variation.
Our genome wide search for genes subjected to virus-driven selection allowed the identification of a gene interaction network that is enriched in both genes associated with virus diversity and in genes encoding proteins that interact with viral products. Many of the genes included in the identified network are of great interest as they are known to be involved in the activation of mechanisms that have direct or indirect protective effects against viruses. Thus, beside the well known activities of IL1A (MIM 147760) and B (MIM 147720), IL4 (MIM 147780), TGFB1 (MIM 190180), IL16 (MIM 603035), IFNG (MIM 147570) and TNF (MIM 191160), OAS2 (MIM 603350) encodes a protein that activates latent RNases, resulting in the degradation of viral RNA and in the inhibition of viral replication [55]. CCL17 (MIM 601520) induces T lymphocytes chemotaxis, thus potentiating the immune responses, and PPP3CA (MIM 114105), also known as calcineurin, activates NFATc [56], a key factor in the up-regulation of IL2 (MIM 147680) [57], the main cytokine responsible for T lymphocytes growth and differentiation. Finally, ULBP2 (MIM 605698) encodes an MHC1-related protein that binds to NKG2D (MIM 602893) [58], an activating receptor expressed on CD8 T cells as well as on NK cells, NKT cells and γδ T cells. In the light of the viral pathogenesis of a growing number of neoplasia, it is very interesting that other members of the network play a well described role in the inhibition of tumoral growth. In particular, E2F1 (MIM 189971) is known to have a pivotal role in the control of cell cycle and in the activation of tumour suppressor proteins and, together with TP53I3, TADA3L, and TP53BP2 mediates p53-dependent and independent apoptosis [59]–[60]. CCND3 (MIM 123834) is involved in cell cycle progression through the G2 phase, whereas RAD23A (MIM 600061) up-regulates the nucleotide excision activity of 3-methyladenine-DNA glycosylase [61], therefore playing a role in DNA damage recognition in base excision repair. Finally, NR4A2 (MIM 601828) encodes a nuclear orphan receptor expressed in T cells and involved in apoptosis [62]. NR4A2 is also known to play a central role in eliciting the production of inflammatory cytokines in multiple sclerosis (MS (MIM 126200)) [63]. Notably, variants in PPP3CA (Figure 1) have recently been reported to correlate with MS severity as well [64]. We therefore investigated whether other genes carrying SNPs which correlate with virus diversity have been identified in GWAS for MS susceptibility or severity. Three additional genes, JMJD2C (MIM 605469), C20orf133 (also known as MACROD2, (MIM 611567)) and CSMD1 (MIM 608397) have been associated with MS [64] and display SNPs significantly correlated with virus diversity (Table S1). While the function of C20orf133 is unknown, JMJD2C encodes a histone demethylase expressed at very high levels in B cells and cytotoxic lymphocytes (see materials and methods), a pattern consistent with its being subjected to virus-driven selective pressure. Finally, CSMD1, in analogy to the aforementioned SERPING1, acts as a regulator of the complement system [65]; notably, complement activation plays a central role in both response to viruses and inflammatory reactions, particularly in the central nervous system [66].
Analysis of the 30 stronger associations (Table 3) indicated that several genes are part of the network described above or have been involved in immune response (see InnateDB gene list, Table 2). Conversely, others encode relatively unknown products (e.g. KIAA1529 (MIM 611258), LHFPL3 (MIM 609719), LOC51149, RNF217, TMEM132B, LEPREL1 (MIM 610341), ANKFN1, MYO5C (MIM 610022), ANXA4 (MIM 106491) and SCRN3). Among these genes, MYO5C, ANXA4 and SCRN3 are involved in membrane trafficking events along exocytotic and endocytotic pathways, suggesting that they might play a role in either viral cell entry [67] or lytic granule exocytosis; this might be the case for ANXA4 which is expressed at high levels in NK cells (see materials and methods). Most interestingly, EYA4 (MIM 603550) (Table 3) has recently been described as a phosphatase involved in triggering innate immune responses against viruses [68]. Finally, both PDE2A (MIM 602658) and SCNN1A (MIM 600228) might play a role in maintaining lung epithelial barrier homoeostasis during viral infection. Indeed, both genes can be induced by TNF-alpha in lung epithelial cells [69]–[70] and can influence lung fluid reabsorption and, therefore, edema formation. In line with these observations, expression of the amiloride-sensitive epithelial Na+ channel (SCNN1A codes for the α subunit) is affected by infection with influenza virus, severe acute respiratory syndrome coronavirus and respiratory syncitial virus.
In humans, resistance to infectious diseases is thought to be under complex, multigenic control with single loci playing a small protective role [47]. This concept also holds for viral infection as demonstrated by the role of genetic variants in modulating the susceptibility to HIV infection or disease progression (reviewed in [71]). Classic GWAS offer a powerful resource to identify susceptibility loci for infectious diseases; yet GWAS typically have limited power to detect variants with a low frequency or a small effect. Indeed, recent GWAS for SNPs determining the host control of HIV-1 [4]–[5] failed to identify most known loci with a role in AIDS progression. The alternative approach we have proposed here is based on the identification of variants subjected to virus-driven selective pressure. Similarly to the GWAS results mentioned above we did not identify well known antiviral-response genes. Still, we noticed that variants in TRIM5 (MIM 608487) (rs2291845, τ = 0.44, p = 1.86×10−5, rank = 0.97) and IFIH1 (MIM 606951) (also known as MDA5, rs10439256, τ = 0.51, p = 5.4×10−7, rank = 0.99) showed significant associations with virus-diversity, although they did not withstood genome-wide analysis. Also, it is worth mentioning that variants with a well established role in resistance to viral infections may be neutrally evolving; this is the case for the Δ32 allele of CCR5 (MIM 601373) for example, which confers protection against HIV-1 infection and possibly against other pathogens, but displays no selection signature [72]. This is possibly due to how long and how strong the selective pressure has been exerted. Conversely, variants subjected to selective pressure must have (or have had along human history) some selective advantage, indicating that the SNPs we have identified can be regarded as candidate modulators of infection susceptibility or disease progression.
Materials and Methods
Environmental variables
Virus absence/presence matrices for the 21 countries where HGDP-CEPH populations are located were derived from the Global Infectious Disease and Epidemiology Network database (Gideon, http://www.gideononline.com), a global infectious disease knowledge tool. Information in Gideon is weekly updated and derives from World Health Organization reports, National Health Ministries, PubMed searches and epidemiology meetings. The Gideon Epidemiology module follows the status of known infectious diseases globally, as well as in individual countries, with specific notes indicating the disease's history, incidence and distribution per country. We manually curated virus absence/presence matrices by extracting information from single Gideon entries. These may refer to either species, genera or families (in case data are not available for different species of a same genus/family). Following previous suggestions [7]–[9], we recorded only viruses that are transmitted in the 21 countries, meaning that cases of transmission due to tourism and immigration were not taken into account; also, species that have recently been eradicated as a result, for example, of vaccination campaigns, were recorded as present in the matrix. A total of 81 virus species/genera/families were retrieved (Table S6). The same approach was applied to calculate the diversity of other pathogens, namely bacteria, protozoa and helminths [9]. The annual minimum and maximum temperature were retrieved from the NCEP/NCAR database (http://www.ngdc.noaa.gov/ecosys/cdroms/ged_iia/datasets/a04/, Legates and Willmott Average, re-gridded dataset) using the geographic coordinates reported by HGDP-CEPH website for each population (http://www.cephb.fr/en/hgdp/table.php). Similarly, net short wave radiation flux data were obtained from NCEP/NCAR (http://www.esrl.noaa.gov/psd/data/gridded/data.ncep.reanalysis.surfaceflux.html, Reanalysis 1: Surface Flux); these data were read using Grid Analysis and Display System (GrADS, http://www.iges.org/grads/). Daily values for four years (1948–1951) were averaged to obtain an annual mean.
Since virus diversity, due to data organization in Gideon, can only be calculated per country (rather than per population), the same procedure was applied to climatic variables. Therefore the values of annual temperature and radiation flux were averaged for populations located in the same country. This assures that a similar number of ties is maintained in all correlation analyses.
Data retrieval and statistical analysis
Data concerning the HGDP-CEPH panel derive from a previous work [6]. Atypical or duplicated samples and pairs of close relatives were removed [73].
A SNP was ascribed to a specific gene if it was located within the transcribed region or no farther than 500 bp upstream the transcription start site. MAF for any single SNP was calculated as the average over all populations. The list of immune response genes was derived from the InnateDB website (http://www.innatedb.com/) and it contains a non-redundant list of 5,070 immune genes derived from ImmPort, IRIS, Septic Shock Group, MAPK/NFKB Network and Immunome Database; it only includes genes derived from curated immune gene lists.
Genes involved in glycan biosynthesis were obtained by merging genes from two KEGG pathways (“Glycan structures - biosynthesis 1” and “Glycan structures - biosynthesis 2”). Additional genes were identified by searching Gene Ontology categories for genes that act as glycosyltransferases (GO:0016757) and are located in either the Golgi or the endoplasmic reticulum (GO:0005783, GO:0005793 and GO:0005794). The list of human genes coding for proteins interacting with viral products was derived from three sources: a previously published study [38], the VirHostNet website [74] (http://pbildb1.univ-lyon1.fr/virhostnet/) and the HIV-1 Human Protein Interaction Database [75] (http://www.ncbi.nlm.nih.gov/RefSeq/HIVInteractions/).
Expression data were obtained from SymAtlas (http://symatlas.gnf.org/). The location of genomic elements that are highly conserved among vertebrates was derived from UCSC annotation tables (http://genome.ucsc.edu/; “PhastCons Conserved Elements, 44-way Vertebrate Multiz Alignment” track).
All correlations were calculated by Kendall's rank correlation coefficient (τ), a non-parametric statistic used to measure the degree of correspondence between two rankings. The reason for using this test is that even in the presence of ties, the sampling distribution of τ satisfactorily converges to a normal distribution for values of n larger than 10 [76].
In order to estimate the probability of obtaining n SNPs located within m genes and significantly associated with virus diversity, we applied a re-sampling approach: samples of m genes were randomly extracted from a list of all genes covered by at least one SNP in the HGDP-CEPH panel (number of genes = 15,280) and for each sample the number of SNPs significantly associated with virus diversity was counted. The empirical probability of obtaining n SNPs was then calculated from the distribution of counts deriving from 10,000 random samples. A SNP was ascribed to a gene if it was located within the transcribed region or in the 500 upstream nucleotides.
Analysis of PANTHER over-represented functional categories and pathways was performed using the “Compare Classifications of Lists” tool available at the PANTHER classification system website [77] (http://www.pantherdb.org/). Briefly, gene lists are compared to the reference list using the binomial test for each molecular function, biological process, or pathway term in PANTHER.
All calculation were performed in the R environment [78] (http://www.r-project.org/).
Network construction
Biological network analysis was performed with Ingenuity Pathways Analysis (IPA) software using an unsupervised analysis (www.ingenuity.com). IPA builds networks by querying the Ingenuity Pathways Knowledge Base for interactions between the identified genes and all other gene objects stored in the knowledge base; it then generates networks with a maximum network size of 35 genes/proteins. We used all genes showing at least one significantly associated SNP as the input set; in this case a SNP was ascribed to a gene if it was located within the transcribed region or in the 25 kb upstream. All network edges are supported by at least one published reference or from canonical information stored in the Ingenuity Pathways Knowledge Base. To determine the probability of the analysed genes to be found together in a network from Ingenuity Pathways Knowledge Base due to random chance alone, IPA applies a Fisher's exact test. The network score represents the -log (p value).
Supporting Information
Zdroje
1. MorensDM
FolkersGK
FauciAS
2004 The challenge of emerging and re-emerging infectious diseases. Nature 430(6996) 242 249
2. LanderES
LintonLM
BirrenB
NusbaumC
ZodyMC
2001 Initial sequencing and analysis of the human genome. Nature 409(6822) 860 921
3. BeutlerB
EidenschenkC
CrozatK
ImlerJL
TakeuchiO
2007 Genetic analysis of resistance to viral infection. Nat Rev Immunol 7(10) 753 766
4. LimouS
Le ClercS
CoulongesC
CarpentierW
DinaC
2009 Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS genomewide association study 02). J Infect Dis 199(3) 419 426
5. FellayJ
ShiannaKV
GeD
ColomboS
LedergerberB
2007 A whole-genome association study of major determinants for host control of HIV-1. Science 317(5840) 944 947
6. LiJZ
AbsherDM
TangH
SouthwickAM
CastoAM
2008 Worldwide human relationships inferred from genome-wide patterns of variation. Science 319(5866) 1100 1104
7. PrugnolleF
ManicaA
CharpentierM
GueganJF
GuernierV
2005 Pathogen-driven selection and worldwide HLA class I diversity. Curr Biol 15(11) 1022 1027
8. FumagalliM
CaglianiR
PozzoliU
RivaS
ComiGP
2009 Widespread balancing selection and pathogen-driven selection at blood group antigen genes. Genome Res 19(2) 199 212
9. FumagalliM
PozzoliU
CaglianiR
ComiGP
RivaS
2009 Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med 206(6) 1395 1408
10. GuernierV
HochbergME
GueganJF
2004 Ecology drives the worldwide distribution of human diseases. PLoS Biol 2 e141 doi:10.1371/journal.pbio.0020141
11. HandleyLJ
ManicaA
GoudetJ
BallouxF
2007 Going the distance: Human population genetics in a clinal world. Trends Genet 23(9) 432 439
12. CoopG
PickrellJK
NovembreJ
KudaravalliS
LiJ
2009 The role of geography in human adaptation. PLoS Genet 5 e1000500 doi:10.1371/journal.pgen.1000500
13. YangB
ChenK
ZhangC
HuangS
ZhangH
2007 Virion-associated uracil DNA glycosylase-2 and apurinic/apyrimidinic endonuclease are involved in the degradation of APOBEC3G-edited nascent HIV-1 DNA. J Biol Chem 282(16) 11667 11675
14. IzmailovaE
BertleyFM
HuangQ
MakoriN
MillerCJ
2003 HIV-1 tat reprograms immature dendritic cells to express chemoattractants for activated T cells and macrophages. Nat Med 9(2) 191 197
15. GerencerM
BurekV
2004 Identification of HIV-1 protease cleavage site in human C1-inhibitor. Virus Res 105(1) 97 100
16. DrouetC
BouilletL
CsopakiF
ColombMG
1999 Hepatitis C virus NS3 serine protease interacts with the serpin C1 inhibitor. FEBS Lett 458(3) 415 418
17. DronamrajuK
1990 Selected genetic papers of J.B.S. haldane. New York/London Garland Publishing
18. ImbertyA
VarrotA
2008 Microbial recognition of human cell surface glycoconjugates. Curr Opin Struct Biol 18(5) 567 576
19. ErbacherA
GiesekeF
HandgretingerR
MullerI
2009 Dendritic cells: Functional aspects of glycosylation and lectins. Hum Immunol 70(5) 308 312
20. Van DykenSJ
GreenRS
MarthJD
2007 Structural and mechanistic features of protein O glycosylation linked to CD8+ T-cell apoptosis. Mol Cell Biol 27(3) 1096 1111
21. SrinivasanA
ViswanathanK
RamanR
ChandrasekaranA
RaguramS
2008 Quantitative biochemical rationale for differences in transmissibility of 1918 pandemic influenza A viruses. Proc Natl Acad Sci U S A 105(8) 2800 2805
22. ChandrasekaranA
SrinivasanA
RamanR
ViswanathanK
RaguramS
2008 Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat Biotechnol 26(1) 107 113
23. NeuU
StehleT
AtwoodWJ
2009 The polyomaviridae: Contributions of virus structure to our understanding of virus receptors and infectious entry. Virology 384(2) 389 399
24. IsaP
AriasCF
LopezS
2006 Role of sialic acids in rotavirus infection. Glycoconj J 23(1-2) 27 37
25. ZengJ
JooHM
RajiniB
WrammertJP
SangsterMY
2009 The generation of influenza-specific humoral responses is impaired in ST6Gal I-deficient mice. J Immunol 182(8) 4721 4727
26. AvrilT
NorthSJ
HaslamSM
WillisonHJ
CrockerPR
2006 Probing the cis interactions of the inhibitory receptor siglec-7 with alpha2,8-disialylated ligands on natural killer cells and other leukocytes using glycan-specific antibodies and by analysis of alpha2,8-sialyltransferase gene expression. J Leukoc Biol 80(4) 787 796
27. ShuklaD
SpearPG
2001 Herpesviruses and heparan sulfate: An intimate relationship in aid of viral entry. J Clin Invest 108(4) 503 510
28. LambertS
BouttierM
VassyR
SeigneuretM
Petrow-SadowskiC
2009 HTLV-1 uses HSPG and neuropilin-1 for entry by molecular mimicry of VEGF165. Blood 113(21) 5176 5185
29. JohnsonKM
KinesRC
RobertsJN
LowyDR
SchillerJT
2009 Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J Virol 83(5) 2067 2074
30. MardbergK
TrybalaE
TufaroF
BergstromT
2002 Herpes simplex virus type 1 glycoprotein C is necessary for efficient infection of chondroitin sulfate-expressing gro2C cells. J Gen Virol 83(Pt 2) 291 300
31. ArgyrisEG
AcheampongE
NunnariG
MukhtarM
WilliamsKJ
2003 Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J Virol 77(22) 12140 12151
32. RojekJM
SpiropoulouCF
CampbellKP
KunzS
2007 Old world and clade C new world arenaviruses mimic the molecular mechanism of receptor recognition used by alpha-dystroglycan's host-derived ligands. J Virol 81(11) 5685 5695
33. KunzS
RojekJM
KanagawaM
SpiropoulouCF
BarresiR
2005 Posttranslational modification of alpha-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J Virol 79(22) 14282 14296
34. SabetiPC
VarillyP
FryB
LohmuellerJ
HostetterE
2007 Genome-wide detection and characterization of positive selection in human populations. Nature 449(7164) 913 918
35. BarreiroLB
LavalG
QuachH
PatinE
Quintana-MurciL
2008 Natural selection has driven population differentiation in modern humans. Nat Genet 40(3) 340 345
36. ThomasPD
CampbellMJ
KejariwalA
MiH
KarlakB
2003 PANTHER: A library of protein families and subfamilies indexed by function. Genome Res 13(9) 2129 2141
37. ThomasPD
KejariwalA
GuoN
MiH
CampbellMJ
2006 Applications for protein sequence-function evolution data: MRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res 34(Web Server issue) W645 50
38. DyerMD
MuraliTM
SobralBW
2008 The landscape of human proteins interacting with viruses and other pathogens. PLoS Pathog 4 e32 doi:10.1371/journal.ppat.0040032
39. AlbertR
2005 Scale-free networks in cell biology. J Cell Sci 118(Pt 21) 4947 4957
40. FraserHB
HirshAE
SteinmetzLM
ScharfeC
FeldmanMW
2002 Evolutionary rate in the protein interaction network. Science 296(5568) 750 752
41. PagelM
MeadeA
ScottD
2007 Assembly rules for protein networks derived from phylogenetic-statistical analysis of whole genomes. BMC Evol Biol 7 Suppl 1 S16
42. YoungbloodB
ReichNO
2008 The early expressed HIV-1 genes regulate DNMT1 expression. Epigenetics 3(3) 149 156
43. HinoR
UozakiH
MurakamiN
UshikuT
ShinozakiA
2009 Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res 69(7) 2766 2774
44. McCabeMT
LowJA
ImperialeMJ
DayML
2006 Human polyomavirus BKV transcriptionally activates DNA methyltransferase 1 through the pRb/E2F pathway. Oncogene 25(19) 2727 2735
45. ChappellC
BeardC
AltmanJ
JaenischR
JacobJ
2006 DNA methylation by DNA methyltransferase 1 is critical for effector CD8 T cell expansion. J Immunol 176(8) 4562 4572
46. ArgyrisEG
KulkoskyJ
MeyerME
XuY
MukhtarM
2004 The perlecan heparan sulfate proteoglycan mediates cellular uptake of HIV-1 tat through a pathway responsible for biological activity. Virology 330(2) 481 486
47. Quintana-MurciL
AlcaisA
AbelL
CasanovaJL
2007 Immunology in natura: Clinical, epidemiological and evolutionary genetics of infectious diseases. Nat Immunol 8(11) 1165 1171
48. HancockAM
WitonskyDB
GordonAS
EshelG
PritchardJK
2008 Adaptations to climate in candidate genes for common metabolic disorders. PLoS Genet 4 e32 doi:10.1371/journal.pgen.0040032
49. ThompsonEE
Kuttab-BoulosH
WitonskyD
YangL
RoeBA
2004 CYP3A variation and the evolution of salt-sensitivity variants. Am J Hum Genet 75(6) 1059 1069
50. YoungJH
ChangYP
KimJD
ChretienJP
KlagMJ
2005 Differential susceptibility to hypertension is due to selection during the out-of-africa expansion. PLoS Genet 1 e82 doi:10.1371/journal.pgen.0010082
51. AllenAP
BrownJH
GilloolyJF
2002 Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297(5586) 1545 1548
52. NovembreJ
Di RienzoA
2009 Spatial patterns of variation due to natural selection in humans. Nat Rev Genet 10(11) 745 755
53. SleijffersA
GarssenJ
Van LoverenH
2002 Ultraviolet radiation, resistance to infectious diseases, and vaccination responses. Methods 28(1) 111 121
54. NorvalM
2006 The effect of ultraviolet radiation on human viral infections. Photochem Photobiol 82(6) 1495 1504
55. JustesenJ
HartmannR
KjeldgaardNO
2000 Gene structure and function of the 2′-5′-oligoadenylate synthetase family. Cell Mol Life Sci 57(11) 1593 1612
56. CrabtreeGR
OlsonEN
2002 NFAT signaling: Choreographing the social lives of cells. Cell 109 Suppl S67 79
57. ShawJP
UtzPJ
DurandDB
TooleJJ
EmmelEA
1988 Identification of a putative regulator of early T cell activation genes. Science 241(4862) 202 205
58. SutherlandCL
ChalupnyNJ
SchooleyK
VandenBosT
KubinM
2002 UL16-binding proteins, novel MHC class I-related proteins, bind to NKG2D and activate multiple signaling pathways in primary NK cells. J Immunol 168(2) 671 679
59. SherrCJ
1998 Tumor surveillance via the ARF-p53 pathway. Genes Dev 12(19) 2984 2991
60. IrwinM
MarinMC
PhillipsAC
SeelanRS
SmithDI
2000 Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 407(6804) 645 648
61. MiaoF
BouzianeM
DammannR
MasutaniC
HanaokaF
2000 3-methyladenine-DNA glycosylase (MPG protein) interacts with human RAD23 proteins. J Biol Chem 275(37) 28433 28438
62. ChengLE
ChanFK
CadoD
WinotoA
1997 Functional redundancy of the Nur77 and nor-1 orphan steroid receptors in T-cell apoptosis. EMBO J 16(8) 1865 1875
63. DoiY
OkiS
OzawaT
HohjohH
MiyakeS
2008 Orphan nuclear receptor NR4A2 expressed in T cells from multiple sclerosis mediates production of inflammatory cytokines. Proc Natl Acad Sci U S A 105(24) 8381 8386
64. BaranziniSE
WangJ
GibsonRA
GalweyN
NaegelinY
2009 Genome-wide association analysis of susceptibility and clinical phenotype in multiple sclerosis. Hum Mol Genet 18(4) 767 778
65. KrausDM
ElliottGS
ChuteH
HoranT
PfenningerKH
2006 CSMD1 is a novel multiple domain complement-regulatory protein highly expressed in the central nervous system and epithelial tissues. J Immunol 176(7) 4419 4430
66. SpethC
DierichMP
GasqueP
2002 Neuroinvasion by pathogens: A key role of the complement system. Mol Immunol 38(9) 669 679
67. MercerJ
HeleniusA
2009 Virus entry by macropinocytosis. Nat Cell Biol 11(5) 510 520
68. OkabeY
SanoT
NagataS
2009 Regulation of the innate immune response by threonine-phosphatase of eyes absent. Nature
69. DagenaisA
FrechetteR
ClermontME
MasseC
PriveA
2006 Dexamethasone inhibits the action of TNF on ENaC expression and activity. Am J Physiol Lung Cell Mol Physiol 291(6) L1220 31
70. SeyboldJ
ThomasD
WitzenrathM
BoralS
HockeAC
2005 Tumor necrosis factor-alpha-dependent expression of phosphodiesterase 2: Role in endothelial hyperpermeability. Blood 105(9) 3569 3576
71. PiacentiniL
BiasinM
FeniziaC
ClericiM
2009 Genetic correlates of protection against HIV infection: The ally within. J Intern Med 265(1) 110 124
72. SabetiPC
WalshE
SchaffnerSF
VarillyP
FryB
2005 The case for selection at CCR5-Delta32. PLoS Biol 3 e378 doi:10.1371/journal.pbio.0030378
73. RosenbergNA
2006 Standardized subsets of the HGDP-CEPH human genome diversity cell line panel, accounting for atypical and duplicated samples and pairs of close relatives. Ann Hum Genet 70(Pt 6) 841 847
74. NavratilV
de ChasseyB
MeynielL
DelmotteS
GautierC
2009 VirHostNet: A knowledge base for the management and the analysis of proteome-wide virus-host interaction networks. Nucleic Acids Res 37(Database issue) D661 8
75. FuW
Sanders-BeerBE
KatzKS
MaglottDR
PruittKD
2009 Human immunodeficiency virus type 1, human protein interaction database at NCBI. Nucleic Acids Res 37(Database issue) D417 22
76. SalkindNJ
2007 Encyclopedia of measurement and statistics. Thousand Oaks, CA Sage Publications
77. ChoRJ
CampbellMJ
2000 Transcription, genomes, function. Trends Genet 16(9) 409 415
78. R Development Core Team 2008 R: A language and environment for statistical computing. Vienna, Austria.
Štítky
Genetika Reprodukční medicína
Článek Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the GenomeČlánek Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in MiceČlánek Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' DiseaseČlánek Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD TagsČlánek Wing Patterns in the Mist
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Scale of Population Structure in
- Allelic Exchange of Pheromones and Their Receptors Reprograms Sexual Identity in
- Genetic and Functional Dissection of and in Age-Related Macular Degeneration
- A Single Nucleotide Polymorphism within the Acetyl-Coenzyme A Carboxylase Beta Gene Is Associated with Proteinuria in Patients with Type 2 Diabetes
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Cdk2 Is Required for p53-Independent G/M Checkpoint Control
- Uncoupling of Satellite DNA and Centromeric Function in the Genus
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in the Clade
- Use of DNA–Damaging Agents and RNA Pooling to Assess Expression Profiles Associated with and Mutation Status in Familial Breast Cancer Patients
- Cheating by Exploitation of Developmental Prestalk Patterning in
- Replication and Active Demethylation Represent Partially Overlapping Mechanisms for Erasure of H3K4me3 in Budding Yeast
- Cdk1 Targets Srs2 to Complete Synthesis-Dependent Strand Annealing and to Promote Recombinational Repair
- A Genome-Wide Association Study Identifies Susceptibility Variants for Type 2 Diabetes in Han Chinese
- Genome-Wide Identification of Susceptibility Alleles for Viral Infections through a Population Genetics Approach
- Transcriptional Rewiring of the Sex Determining Gene Duplicate by Transposable Elements
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in
- Proteasome Nuclear Activity Affects Chromosome Stability by Controlling the Turnover of Mms22, a Protein Important for DNA Repair
- Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in Mice
- Structure, Function, and Evolution of the spp. Genome
- Human and Non-Human Primate Genomes Share Hotspots of Positive Selection
- A Kinase-Independent Role for the Rad3-Rad26 Complex in Recruitment of Tel1 to Telomeres in Fission Yeast
- Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' Disease
- Molecular Evolution and Functional Characterization of Insulin-Like Peptides
- Molecular Poltergeists: Mitochondrial DNA Copies () in Sequenced Nuclear Genomes
- Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD Tags
- Wing Patterns in the Mist
- DNA Binding of Centromere Protein C (CENPC) Is Stabilized by Single-Stranded RNA
- Genome-Wide Association Study Reveals Multiple Loci Associated with Primary Tooth Development during Infancy
- Mutations in , Encoding an Equilibrative Nucleoside Transporter ENT3, Cause a Familial Histiocytosis Syndrome (Faisalabad Histiocytosis) and Familial Rosai-Dorfman Disease
- Genome-Wide Identification of Binding Sites Defines Distinct Functions for PHA-4/FOXA in Development and Environmental Response
- Ku Regulates the Non-Homologous End Joining Pathway Choice of DNA Double-Strand Break Repair in Human Somatic Cells
- Nucleoporins and Transcription: New Connections, New Questions
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Nucleoporins and Transcription: New Connections, New Questions
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



