-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative
The global polio eradication initiative is the most ambitious and complex public health programme directed at a single disease in history with a projected cost of $16.5 billion. Of the three serotypes types 2 and 3 appear to have been eradicated in the wild and type 1 is mostly confined to a region of Pakistan and Afghanistan. There is a real probability of total eradication in the near future. The main vaccine used is a live attenuated virus, and our paper concerns one of the most intractable significant implications that this has for the polio endgame. We describe virological studies of a patient deficient in humoral immunity who has been excreting type 2 vaccine-derived poliovirus for 28 years. Our results show that the viruses are excreted at high titres, extremely virulent and antigenically drifted and raise questions about how the population may best be protected from them, particularly in the light of possible changes in vaccine production which are being encouraged to increase capability and reduce costs. The study has implications for the ecology of poliovirus in the human gut and highlights the risks that such vaccine-derived isolates pose for polio re-emergence in the post-eradication era.
Published in the journal: . PLoS Pathog 11(8): e32767. doi:10.1371/journal.ppat.1005114
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005114Summary
The global polio eradication initiative is the most ambitious and complex public health programme directed at a single disease in history with a projected cost of $16.5 billion. Of the three serotypes types 2 and 3 appear to have been eradicated in the wild and type 1 is mostly confined to a region of Pakistan and Afghanistan. There is a real probability of total eradication in the near future. The main vaccine used is a live attenuated virus, and our paper concerns one of the most intractable significant implications that this has for the polio endgame. We describe virological studies of a patient deficient in humoral immunity who has been excreting type 2 vaccine-derived poliovirus for 28 years. Our results show that the viruses are excreted at high titres, extremely virulent and antigenically drifted and raise questions about how the population may best be protected from them, particularly in the light of possible changes in vaccine production which are being encouraged to increase capability and reduce costs. The study has implications for the ecology of poliovirus in the human gut and highlights the risks that such vaccine-derived isolates pose for polio re-emergence in the post-eradication era.
Introduction
Despite difficulties in interrupting wild poliovirus transmission in the last few remaining endemic countries and recent drawbacks due to international spread of poliovirus in central Asia, central Africa and the Middle East [1], the global polio eradication appears to be within reach. Four of the six WHO regions have been certified polio-free and a country such as India, where massive poliomyelitis outbreaks were very common, interrupted circulation of endemic wild poliovirus in 2010. There has been no case of poliomyelitis caused by circulating wild type 2 poliovirus since 1999, no case of type 3 since November 2012 and the last case of type 1 in Africa was in August 2014, leaving some areas of Pakistan and Afghanistan as the main remaining reservoirs [2]. All type 2 poliomyelitis cases since 1999, except an isolated incident of 10 cases linked to a wild laboratory reference strain in India [3], are due to vaccine-related poliovirus strains in either recipients, their immediate contacts or after the vaccine virus has regained the ability to transmit and circulate freely. Vaccine-associated paralytic poliomyelitis occurs in a very small proportion of vaccinees [4] and can be prevented by using inactivated rather than live vaccine. Vaccine-derived poliovirus (VDPV) strains, defined as those with more than 1% (0.6% for serotype 2 poliovirus) sequence drift in the capsid VP1 gene with respect to the corresponding Sabin strain, can be generated and transmitted from person to person in populations with low immunity and have been associated with a number of poliomyelitis outbreaks around the world [5–9]. These circulating VDPVs (cVDPVs) behave very similarly to wild polioviruses and should therefore be eliminated by the same immunisation methods. In addition, some hypogammaglobulinaemic patients are known to excrete poliovirus for prolonged periods of time [10–12] but there is currently no effective strategy to deal with this problem. Although there has been some evidence of local virus transmission from these patients to unvaccinated children [13], VDPV strains from immunodeficient individuals (iVDPVs) have not yet been implicated in outbreaks in the same way that cVDPVs have [14]. The World Health Organization (WHO) and partners have prepared endgame plans for the global polio eradication initiative (GPEI) which include the elimination of the serotype 2 component from the Sabin live-attenuated oral poliovaccine (OPV) and the implementation of global use of inactivated poliovaccine (IPV) [15]. This represents a major change after more than 50 years of trivalent OPV use for routine immunisation although monovalent and bivalent vaccines are commonly used for campaigns on national immunisation days.
The risks posed by iVDPV strains and the prevalence of such cases globally are unknown so their relevance in the context of the GPEI endgame is not easy to assess. In order to better understand the growth and properties of iVDPV strains and their potential for transmission, we have characterised iVDPV isolates from an immunodeficient individual obtained during a period of more than 20 years. Although examples of long-term poliovirus excretion have been described before by us and others (reviewed in [14]), they have mostly included a small number of samples from paralytic cases as otherwise asymptomatic long-term excreters remain undetected. In previous cases patients died, stopped shedding virus or were lost to follow up relatively soon after the first virus isolation. Important gaps in the scientific knowledge of long-term poliovirus excretion by these individuals remained such as determining changes in excretion titres, antigenic structure and neurovirulence of poliovirus following many years of evolution in a single individual as well as estimating the efficacy of current vaccines at preventing paralysis and transmission induced by these viruses. Our paper provides relevant findings in these areas that indicate that VDPV isolates form these patients represent a real risk of polio re-emergence in the post-eradication era, particularly considering there is currently no effective strategy to treat these patients.
Results and Discussion
The first stool samples from this individual were tested between March and November 1995. At that time, type 2 VDPV isolates differing from the parental Sabin 2 OPV strain at between 9.9% and 11.3% of VP1 nucleotides were identified. A total of 185 subsequent samples have been obtained so far in the following years, all positive for iVDPV2 strains with virus titres shed in the stools typically around or above 4 log10 infectious particles per gram, comparable to virus titres shed by healthy vaccinees and paralytic cases infected with vaccine or wild poliovirus [16]. The latest isolate available was from 4th March 2015 showing a 17.7% VP1 sequence drift from Sabin 2 poliovirus. Phylogenetic analyses in the capsid region confirmed that the iVDPV strains were genetically related, sequentially evolved from Sabin 2 and distinct from other type 2 VDPVs and wild polioviruses (Fig 1).
Fig. 1. Sequence analysis of iVDPV strains. 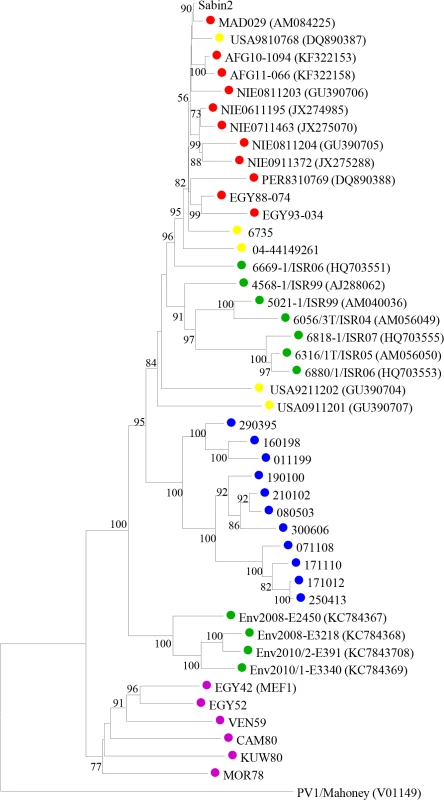
Neighbour-joining tree representing phylogenetic relationships through the entire capsid coding sequence (2637 nt) between iVDPV isolates from the case study (shown as a number that corresponds to the date of isolation in the format ddmmyy), Sabin 2 vaccine strain and other type 2 VDPV and wild polioviruses. EMBL Data Library accession numbers for published capsid sequences are shown in the tree. Numbers at nodes indicate the percentage of 1000 bootstrap pseudoreplicates supporting the cluster. The sequence of PV1-Mahoney reference strain was introduced as an outgroup for the correct rooting of the tree. Isolates from the patient are labelled with blue circles on the tree, other iVDPV isolates are indicated in yellow, cVDPVs in red, VDPVs found in sewage samples in green and wild polioviruses in purple. A Bayesian Monte Carlo Markov Chain (MCMC) phylogenetic analysis determined a mean evolutionary rate of 1.51×10−2 total substitutions/site/year [95% High Probability Distribution (HPD95) range = 1.26–1.77×10−2] in the VP1 gene, similar to previous estimates for poliovirus VP1 [17]. The date of the initiating OPV dose was estimated to be 11th March 1986 [HPD95 = 6th July 1983-11th January 1989], relatively close to 4th August 1986, the date of the patient’s last known OPV vaccination. It is therefore most likely that this individual has been excreting poliovirus for around 28 years. There was no apparent effect on the virus evolution rate suggesting bottleneck effects due to the anti-viral treatments that failed to interrupt virus excretion from this patient [18]. However, a much more detailed analysis of virus population dynamics should be conducted to determine any possible effect due to the different anti-viral interventions.
All iVDPV isolates showed reversion at the two known attenuation mutations of Sabin 2 vaccine strain: nucleotide 481 (from A to G) in the 5’ non-coding region (5’NCR) and capsid amino acid VP1-143 (from Isoleucine to Threonine) and were highly neurovirulent in transgenic mice expressing the human poliovirus receptor. The 50% paralytic dose (PD50) values were comparable to those determined for cVDPV and wild polioviruses while the Sabin 2 vaccine strain did not paralyse any animals at the highest dose that could be given (Fig 2).
Fig. 2. Neurovirulence of iVDPV strains. 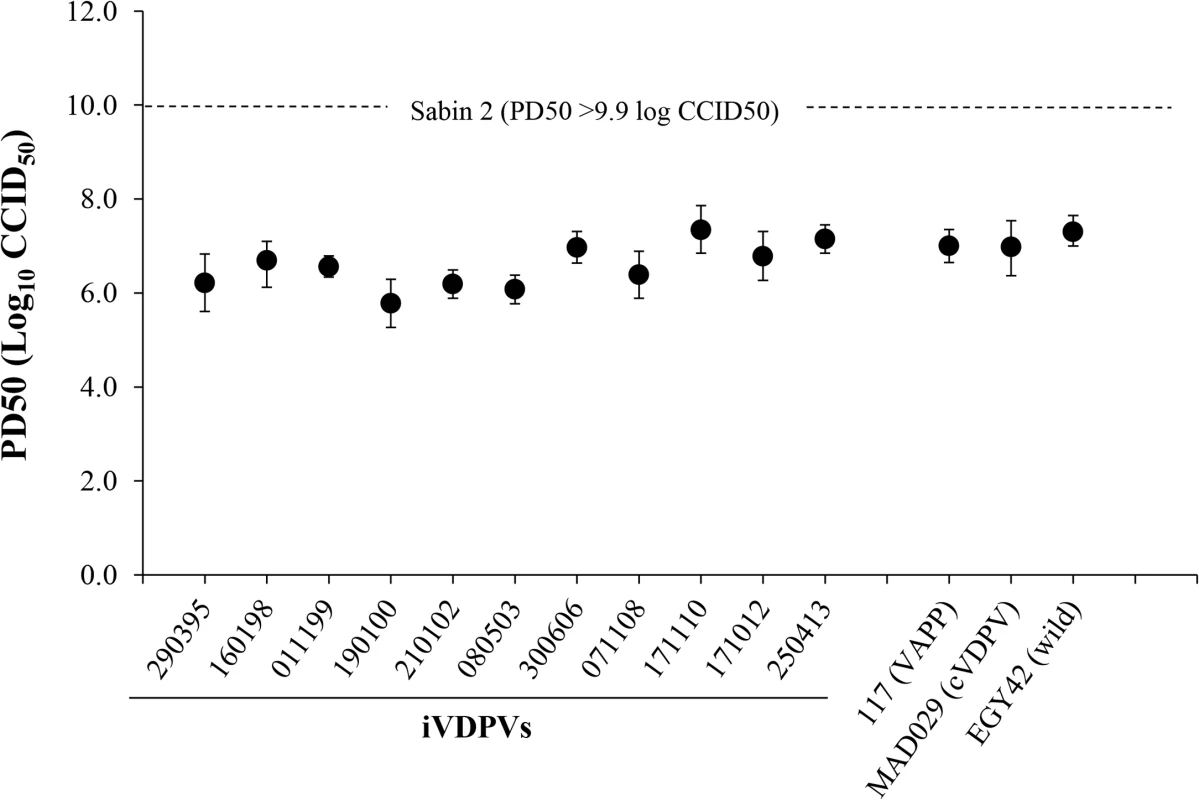
The graphic represents 50% Paralytic Doses (PD50) values (with 95% Confidence Intervals) for selected poliovirus strains determined in Tg21-bx mice using the Probit method. Isolates from the patient are shown underlined. Amino acid differences with respect to the Sabin 2 parental strain in the complete coding sequence of selected iVDPV isolates were determined (S1 Table). All iVDPV strains contained identical changes from Sabin 2 at 52 amino acid positions. Forty mutations were present in at least two iVDPV isolates and 24 amino acid changes were unique. The proportion of nucleotide mutations leading to amino acid changes was high for all iVDPV strains. This contrasts with the low proportion of non-synonymous changes from Sabin 2 identified in cVDPV strains and wild isolates as found here and elsewhere, particularly in capsid sequences. It is not clear whether any of the numerous additional mutations incorporated in the iVDPV isolates have any effect on neurovirulence but they do not seem to have an overall mitigation impact for any of the isolates tested as it has been reported for one highly drifted type 2 VDPV isolate found in a sewage sample in Israel [19]. Many of the sequence changes between the iVDPV strains and the Sabin 2 virus resulted in amino acid differences in known antigenic sites [20] (Table 1).
Tab. 1. Amino acid differences in antigenic sites between type 2 polioviruses. 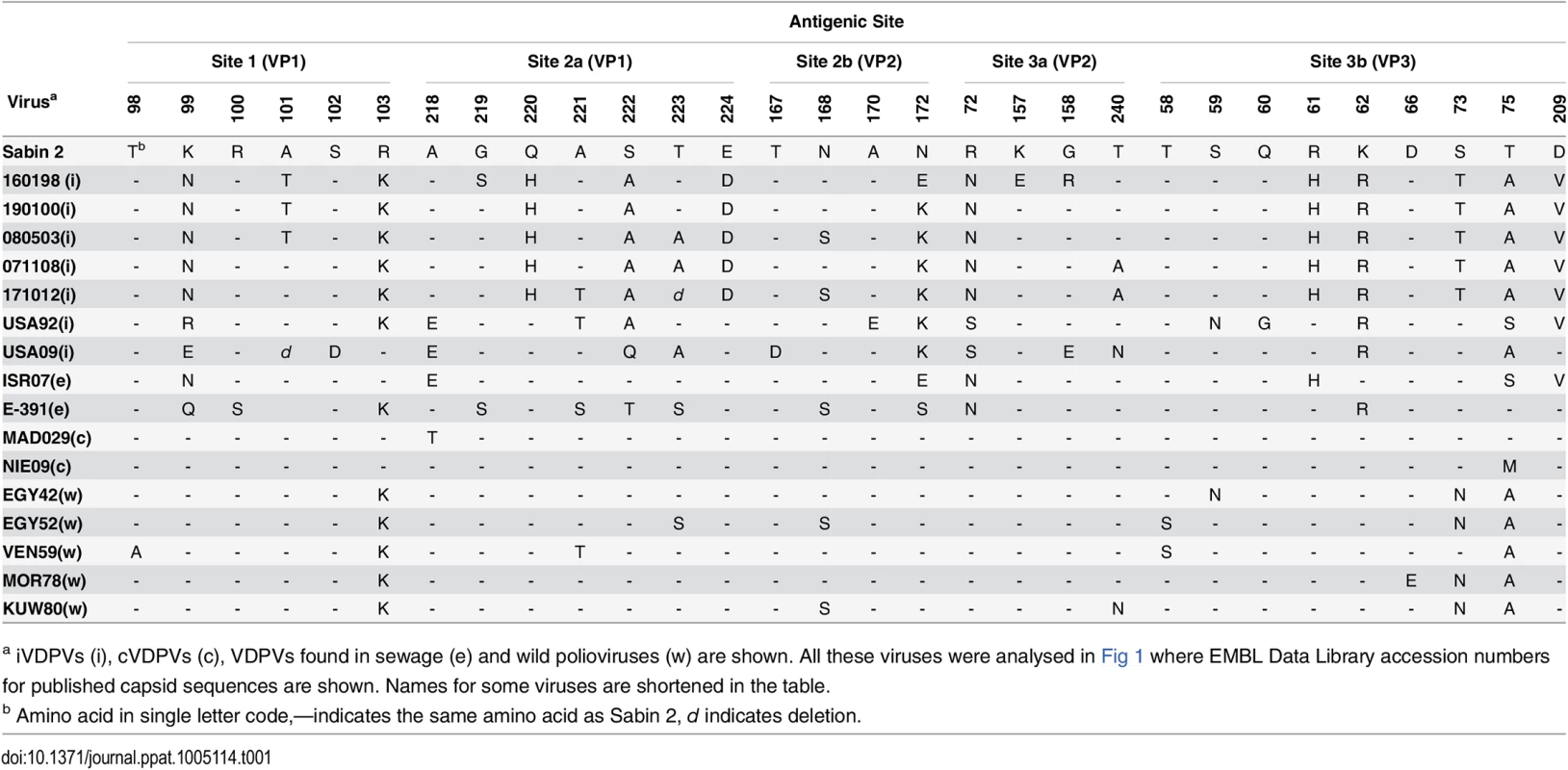
a iVDPVs (i), cVDPVs (c), VDPVs found in sewage (e) and wild polioviruses (w) are shown. All these viruses were analysed in Fig 1 where EMBL Data Library accession numbers for published capsid sequences are shown. Names for some viruses are shortened in the table. As a consequence, the iVDPV strains did not react at all with monoclonal antibodies against most of the known neutralising antibody sites (Fig 3). It was of interest that all isolates tested did react with antibodies specific for antigenic site 3b (1102 and 1103). In contrast, the wild polioviruses strains analysed, which span almost four decades in time and which were isolated in geographically distant locations, exhibited an antigenic structure much closer to that of Sabin 2 virus, reacting with at least one monoclonal antibody specific for each antigenic site (Fig 3). A cVDPV strain from Madagascar [21] also reacted with most monoclonal antibodies.
Fig. 3. Antigenic structure of iVDPV strains. 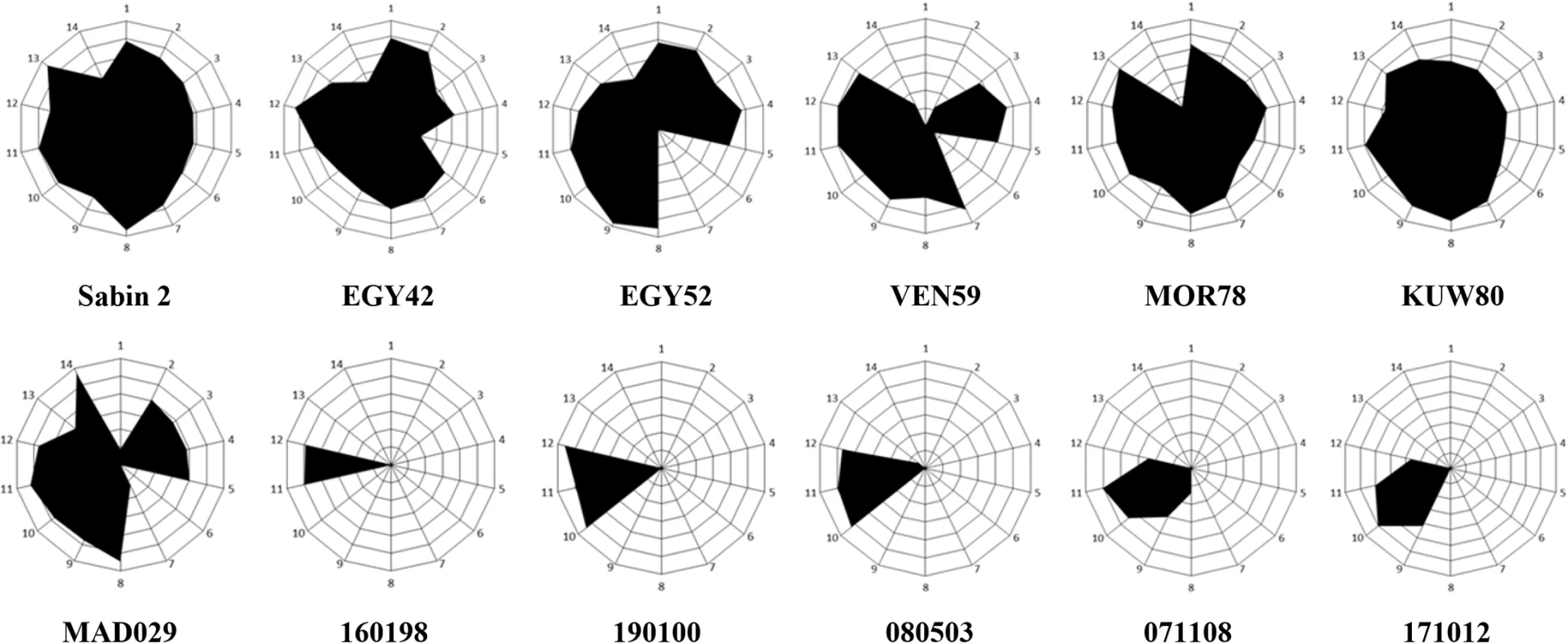
Radial diagrams representing the reactivity of poliovirus strains with Sabin 2-specific monoclonal antibodies. The results are shown as OD values at 492nm obtained in ELISA assays and expressed as normalised values relative to those obtained with antibody 1102 which reacted with all poliovirus strains. These values denote the average of two duplicate assays. Monoclonal antibodies used in the assay in the order 1 to 14 shown in the graph (from the top and clockwise), with antigenic site specificity shown in brackets, were: 969 (site 1), 435 (1), 433 (1), 434 (1), 436 (1), 1231 (2a), 1247 (2a), 1269 (2a), 1037 (2b), 1050 (3a), 1102 (3b), 1103 (3b), 1121 (3b) and 1051 (3b). Sabin 2 vaccine virus, iVDPV isolates from the patient (160198, 190100, 080503, 071108 and 171012), cVDPV strain MAD029 and wild strains (EGY42, EGY52, VEN59, MOR78 and KUW80) were used in the assays. There was no evidence of sequences derived from Sabin 1 or Sabin 3 poliovirus vaccine strains nor sequences derived from other polio or non-polio human enterovirus isolates in any iVDPV genome examined. There was therefore no indication of recombination with other enteroviruses, although recombination within the iVDPV population is quite possible [22,23]. In contrast, virtually all cVDPV and wild type polio strains are recombinants with other group C enteroviruses and include sequences from the 5’NCR and/or the non-structural coding region [21,24].
Despite the extensive antigenic changes found in iVDPV strains (Fig 4), human sera readily neutralised iVDPV isolate 160198, the most antigenically divergent strain (Fig 5). This isolate was the only one obtained by plaque purification so it might represent a minor variant with slightly different antigenic makeup.
Fig. 4. Location of mutations in iVDPV isolate 160198. 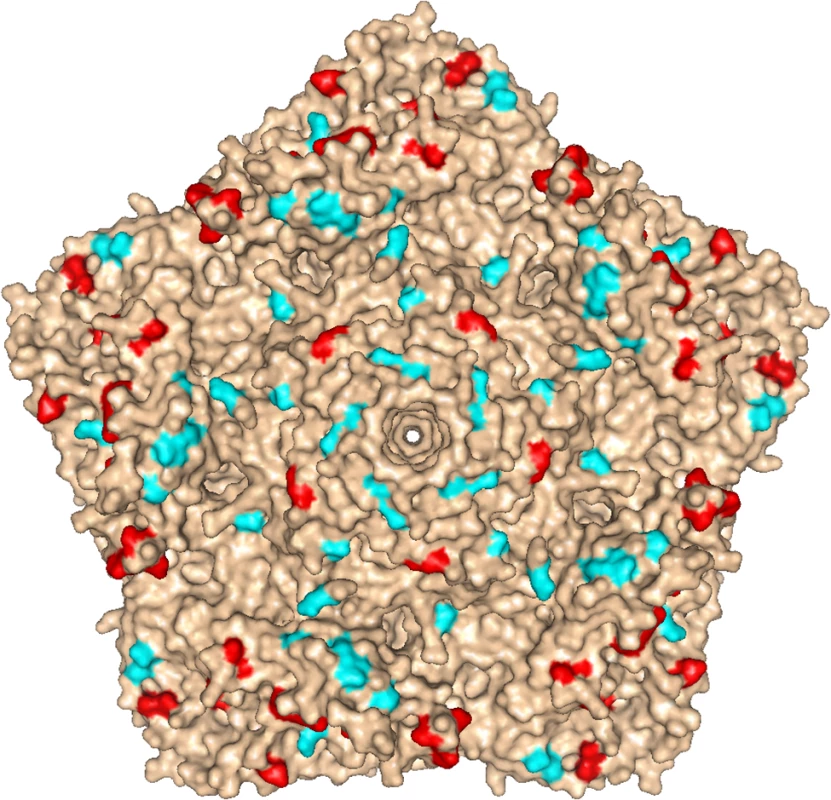
Molecular surface diagram of the three-dimensional structure of type 2 wild poliovirus strain Lansing viewed from the outside of the virion [25]. A pentameric unit is represented. The virus particle consists of 60 protomers. Each protomer contains a single copy of VP1, VP2, VP3, and VP4 arranged in icosahedral symmetry. The location of mutations found in known antigenic sites of iVDPV isolate 160198 with respect to Sabin 2 vaccine strain are shown in red, other amino acid changes from Sabin 2 are displayed in cyan. The image was generated using PyMOL Molecular Graphics System, Version 1.7.0.3 software (Schrödinger, LLC). Fig. 5. Neutralization of poliovirus strains by sera from fully immunised humans. 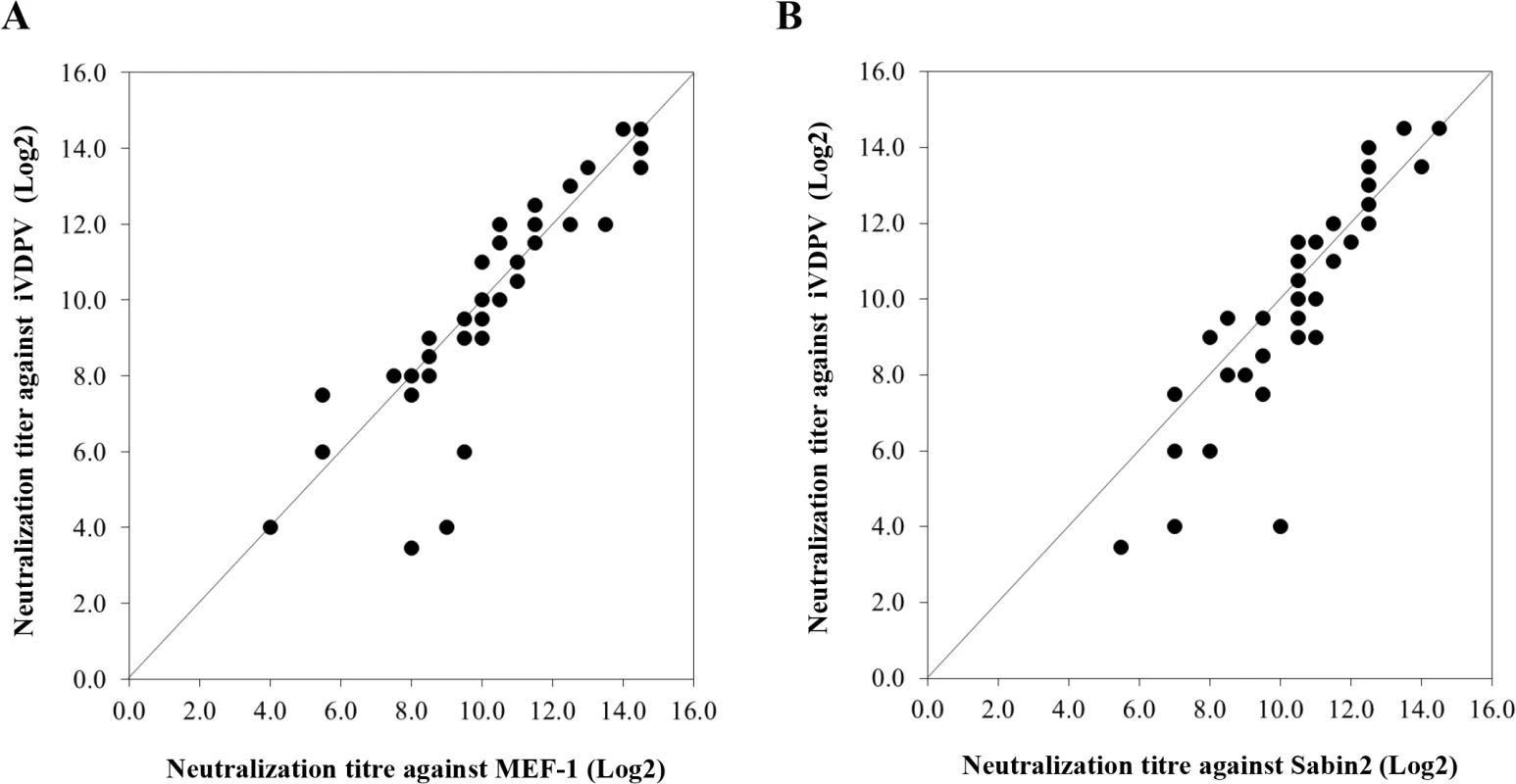
The graphs represent comparison of neutralization titres in 40 sera from UK adults against iVDPV isolate 160198 versus MEF-1 (A) or versus Sabin 2 (B) vaccine strains in cell culture assays. The values are expressed as reciprocals (Log2) of the highest dilution of serum that protected 50% of the cell cultures determined by the Karber formula. The log2 geometric mean titre (GMT) of antibodies neutralizing iVDPV virus 160198 in 40 serum samples from UK adults was 9.96±2.78 comparable to that against MEF-1 (log2 GMT = 10.20±2.40), the wild poliovirus strain used for IPV production, and Sabin 2 (log2 GMT = 10.49±2.02), used for OPV production. These differences were not statistically significant (P = 0.95 for iVDPV vs MEF-1 and P = 0.36 for iVDPV vs Sabin2, for paired results using the Wilcoxon signed-rank test). The results suggest that antibodies to antigenic site 3b in human sera, partially conserved in iVDPV strains, may be sufficient to neutralise the virus. Alternatively, other conserved antigenic epitopes not detected by our murine antibody panel but present in the iVDPV strains, could have contributed to the high neutralization levels shown in human sera. These results are reassuring in that they indicate that vaccinated humans are well protected against infection with these highly drifted iVDPV strains. However, the sera tested here correspond to a selected group of UK healthy adults between 28–65 years of age who had been vaccinated with a full course of four OPV doses plus at least one dose of IPV. The UK switched from OPV to IPV for polio immunisation in 2004 so it would be helpful to test sera from cohorts that have only received IPV immunisation. Israel, which also switched from OPV to IPV at a similar date (2005), has recently detected the widespread circulation of type 1 wild poliovirus through environmental surveillance. There were no paralytic cases but, like the iVDPVs reported here, isolates from Israel showed antigenic differences from the corresponding vaccine strain which may have contributed to their ability to circulate in the context of IPV immunity [26].
We used a transgenic mouse model [27] to test the ability of different IPV products to protect against paralysis caused by iVDPV strains. Both conventional IPV (cIPV) based on wild poliovirus strains and Sabin IPV (sIPV) based on OPV strains were used (Fig 6).
Fig. 6. Survival curve analysis of Tg21-bx mice immunised with IPV and challenged with paralytic doses of poliovirus. 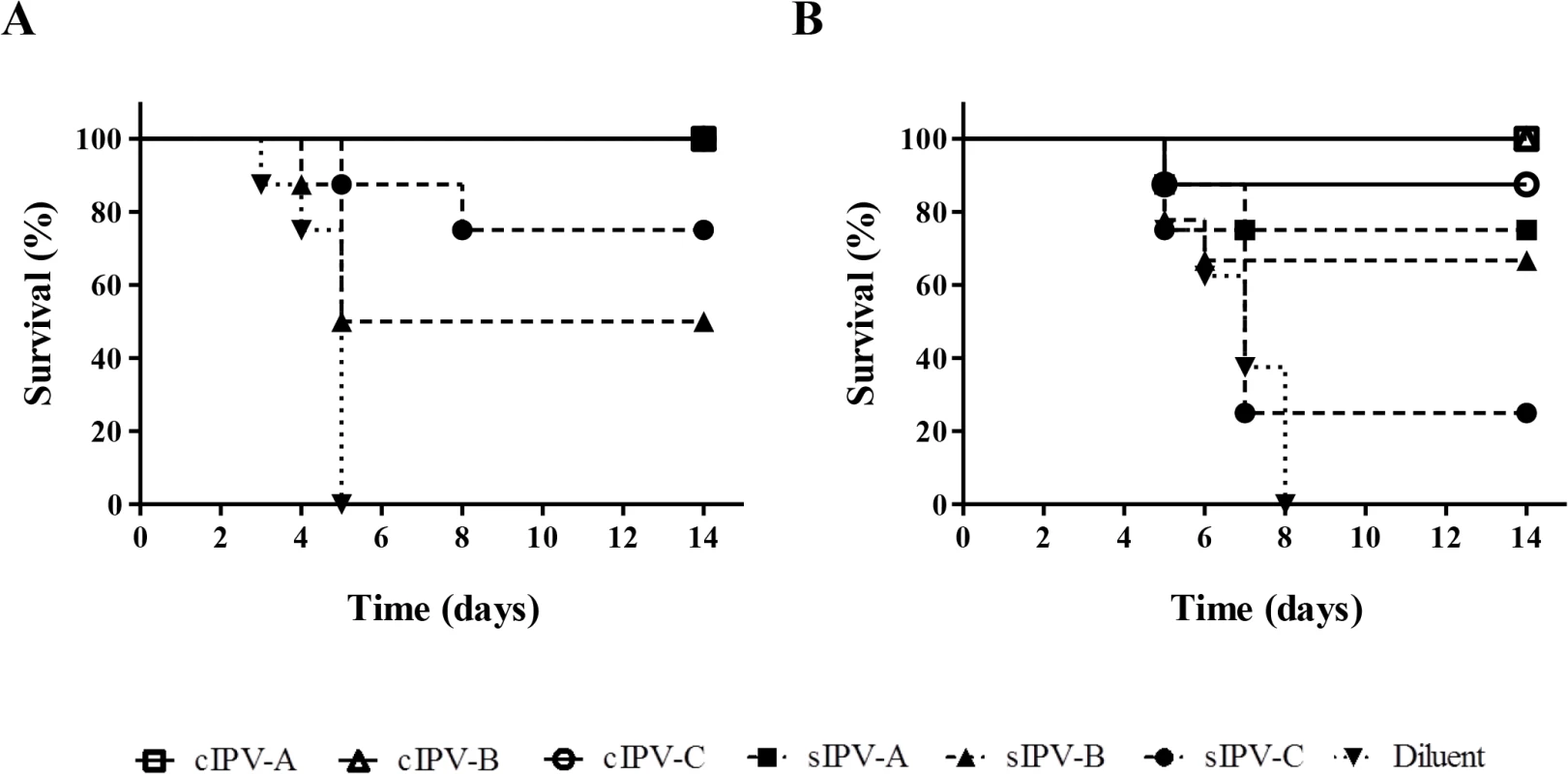
Survival curves showing animals protected against paralysis caused by MEF-1 (A) or iVDPV strain 171012 (B) following immunisation with conventional (cIPV) products are shown with opened symbols and continuous lines; results for Sabin IPV (sIPV) products are shown as dashed lines and closed symbols; and survival data for mice injected with diluent (control) are shown as dotted lines and inverted triangles. All three cIPV products showed 100% protection against challenge with both viruses with the exception of cIPV-C that protected 7 out of the 8 animals used in the test. sIPV-A vaccine also protected all immunised animals against challenge with the MEF-1 strain. All unimmunised control mice were severely paralysed by 5 days (MEF-1 virus) or 8 days (iVDPV strain) post-challenge. All three cIPV products protected against both challenge strains with only one animal developing paralysis. In contrast, sIPV products were less effective at protecting mice and showed variable responses between them. For all vaccines, the in vitro neutralizing antibody titers in sera from immunized mice were at least 7-fold lower against the iVDPV strain than they were against MEF-1 virus (Table 2).
Tab. 2. Neutralization titre against poliovirus in sera from transgenic mice immunised with IPV.a 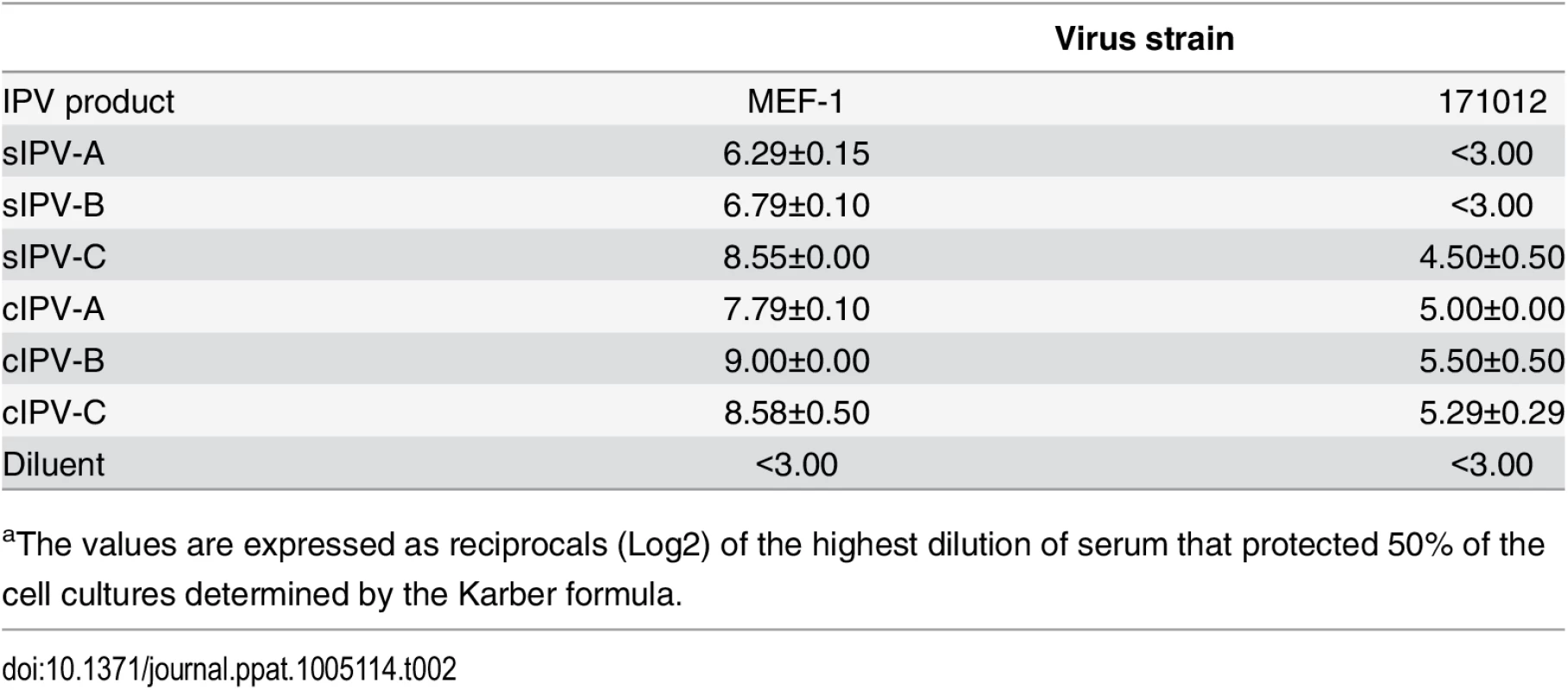
aThe values are expressed as reciprocals (Log2) of the highest dilution of serum that protected 50% of the cell cultures determined by the Karber formula. Our results highlight the need for improving the standardisation of sIPV products in terms of measuring vaccine potency and defining the protective human dose. The lower immunogenicity shown by type 2 sIPVs has been reported before [28,29].
In conclusion, we describe a patient who has been excreting highly virulent and antigenically modified type 2 poliovirus at high titres for a period estimated to be twenty eight years so far. This is by far the longest reported poliovirus excretion and represents the most comprehensive collection of iVDPV sequential isolates available. Provided antibody titres and immunisation coverage are maintained it is likely that the population will be protected against paralytic disease, but it is also possible that this virus could circulate in populations only using IPV as described in Israel for wild poliovirus [26], thus representing a possible source of polio re-emergence, particularly as these iVDPV strains are antigenically atypical and drifted from both Sabin 2 and MEF-1 vaccine strains. This is particularly relevant at present as there are imminent plans to remove type 2 poliovirus from OPV [15]. Moreover the use of IPV based on the Sabin strains is being encouraged by WHO for reasons of environmental safety and the data presented here suggest that it is less effective.
Of the total of 73 iVDPV cases that have been described between 1962 and 2014 [14], only seven of them involved infections lasting more than five years. The case described here represents the only individual of those seven known to be excreting at present. However, several highly drifted VDPV strains have recently been isolated from sewage samples in Slovakia, Finland, Estonia and Israel [30]. They included examples of all three poliovirus serotypes, although type 2 VDPVs were the most prevalent among them. These VDPV isolates showed molecular properties typical of iVDPVs described above indicating that an unknown number of these chronic excreters exist elsewhere. Interestingly, highly evolved type 1 and 2 VDPVs, which have been repeatedly isolated in Israel’s sewage in the last few years, do not appear to have spread widely as type 1 wild poliovirus did. Enhanced surveillance including sewage sampling and stool surveys should continue for as long as possible to search for the presence of iVDPV strains. The availability of efficient anti-viral treatments to interrupt virus replication in these individuals, actively being pursued at present [31], would also be vital as previous attempts have failed [18]. These measures are needed to be able to identify and manage the possible risks of iVDPV strains spreading and causing disease in patients and the general population, particularly in the light of changes in vaccination strategies as part of the polio eradication endgame and the absence of an established outbreak response strategy. Just as the use of new monovalent and bivalent vaccines proved essential to the elimination of wild poliovirus [32,33], novel vaccines unable to cause poliomyelitis would be useful at this stage of polio eradication. New polio vaccines such as those based on non-infectious virus-like particles or even new genetically designed stable live-attenuated versions [34–37] with no associated risk of producing VDPVs, might be required to resolve the “OPV paradox” that derives from using OPV to respond to outbreaks and generating new VDPVs as a consequence.
Materials and Methods
Case report
The patient is a white male from the UK. He received a full course of childhood immunisations, including OVP at 5, 7, and 12 months, with a booster at about 7 years of age. He was later diagnosed with common variable immunodeficiency (CVID) and started on intramuscular immunoglobulin therapy, which was changed to intravenous immunoglobulin after that [18].
Poliovirus isolation from stool samples
Poliovirus was isolated from 10% stool suspensions using HEp-2c cells. Type 2 iVDPV strains 6735 and 04–44149261, isolated from two other immunodeficient patients in the UK, and type 2 wild poliovirus strains EGY42 (MEF-1 strain used for IPV production), EGY52, VEN59, MOR78 and KUW80 isolated from paralytic cases in 1942, 1952, 1959, 1978 and 1980, respectively, were also characterised in this study.
Nucleotide sequencing of poliovirus genomes
VP1 gene
Purified viral RT-PCR DNA products were sequenced by the Sanger method on an ABI Prism DNA 377 Sequencer as specified by the manufacturer. Primers VDPV-F2 (5’-AGG GTT GTT GTC CCG YTG TCC AC-3’) and VDPV-R1 (5’-TAC ACA GCT GGY TAC AAA ATT TGC A-3’) were used to amplify and sequence VP1 gene sequences.
Full genome
Nearly full genomes of selected poliovirus isolates were sequenced using a deep sequencing method described before [38]. Sequence-independent amplification was performed to generate dsDNA templates using primers RA10-N8 (5´ - GAC CAT CTA GCG ACC TCC CAN NNN NNN N -3 and RA10 (5´ - GAC CAT CTA GCG ACC TCC CA -3´). Sequencing libraries were prepared using Nextera XT reagents and sequenced on a MiSeq using a 2 x 251 paired-end v2 Flow Cell and manufacturer’s protocols (Illumina). Raw sequence data were imported into Geneious R7 (Biomatters) and paired end reads combined. Data were filtered and aligned using a custom workflow with the following parameters: shotgun primer RA10 and Nextera adaptor/index sequences were trimmed from 5 and 3´ ends with a minimum 5 bp overlap; reads were trimmed to have an average error rate < 1%, no bases with a quality of < Q30 and no ambiguities. Reads were then mapped to the sequence of the Sabin 2 poliovirus reference (GenBank AY184220) and a consensus sequence obtained for each poliovirus strain. Nucleotide sequences of iVDPV and wild poliovirus isolates described in this paper have been deposited in the DDBJ/EMBL/GenBank and have been assigned accession numbers KR817050-KR817066.
Phylogenetic analysis
Poliovirus sequences were compared to those of other polioviruses in the DDBJ/EMBL/GenBank database. Representative related sequences were included in the phylogenetic analyses. Neighbour-joining phylogenetic analysis was performed with MEGA6 [39] using the maximum composite likelihood substitution model with gamma distributed substitution rates. In addition, a Bayesian Monte Carlo Markov Chain (MCMC) analysis of VP1 sequences (42 iVDPV isolates from the patient obtained throughout the period of virus excretion), as implemented in BEAST v1.8.1 [40], was used for the estimation of the rate of evolution and the date of the initiating OPV dose. The general time reversible (GTR) model of substitution with invariant sites was the best-fitting model of evolution as estimated by jMODELTEST [41]. Two independent chains of 10 million steps each were run under the strict clock model, assuming a constant substitution rate as estimated from the data set. The samples’ collection dates were included as temporal data. Effective sample size values were monitored for consistency using Tracer v1.6.
Neurovirulence in transgenic mice
Tg21-Bx transgenic mice expressing the human poliovirus receptor were inoculated intramuscularly (left hind limb) with 50 μl of 10-fold viral dilutions and daily clinical scores were recorded for 14 days. Eight mice were used for each viral dilution. The Probit method was used to calculate the 50% paralytic dose (PD50) and associated 95% confidence intervals for each poliovirus challenge [27].
Antigenic characterization
The antigenic properties of poliovirus isolates were studied by analysing their ability to bind Sabin 2-specific monoclonal antibodies in ELISA assays using testing formats described before [42]. Antibodies corresponding to antigenic sites 1, 2a, 2b and 3b were used in these assays. Solutions containing equivalent concentrations of poliovirus measured as 50% cell culture infectious doses (CCID50) per ml were selected. The results represent the OD values at 492nm and were expressed as normalised values relative to those obtained with antibody 1102 which reacted with all poliovirus strains.
Immunization/Challenge experiments in transgenic mice
Tg21-Bx mice (8 per test group) were immunised twice by intraperitoneal injection with IPV (using the equivalent of 1 human dose/mouse) or minimum essential medium (diluent control) at an interval of 2 weeks. Twenty-one days after the last dose, mice were challenged with the equivalent of 25 times the PD50 of live poliovirus and daily clinical scores were recorded for 14 days [27]. Vaccines used in these experiments were kindly donated by various manufacturers and were coded to maintain anonymity. This work was part of the characterisation of these vaccines as reference standards. It is important to note that given the high PD50 values observed for type 2 poliovirus strains in our transgenic mouse neurovirulence model, mice were challenged with very large amounts of virus (around 108 CCID50/mouse).
Titration of human and mouse sera for poliovirus neutralising antibodies
Neutralizing antibody titres in serum samples were determined by a standard microneutralization assay in 96-well plates. Two-fold serial dilutions of serum were preincubated with one hundred CCID50 of virus for 2 hours at 36°C. HEp-2C cells were added to each well, and survival at day 5 post-infection determined by staining with a 0.1% Naphthalene black solution. Antibody titres were expressed as reciprocals (Log2) of the highest dilution of serum that protected 50% of the cell cultures determined by the Karber formula. Virus challenge doses were confirmed by back-titration. The significance of pairwise differences in neutralization titres of human sera against MEF-1, Sabin 2 and iVDPV 160198 strain was determined using the Wilcoxon signed-rank test.
Ethics statement
The adult subject who provided stool samples gave written informed consent. Adults who provided blood samples also provided written informed consent. The work complies with the Caldicott Principles and Recommendations for patient confidentiality set up by the UK National Health Services (NHS). All links to personal details that could be used to identify individuals were removed and data were analysed anonymously when possible. No samples from children were involved in the study. The study was approved by NIBSC’s Ethics and Human Materials Advisory Committees. NIBSC’s Animal Welfare and Ethical Review Body approved the application for Procedure Project Licence Number 80/2478 which was approved by the UK Government Home Office and under which animal care and protocols shown in this paper were conducted. All animal care and protocols used at NIBSC adhere to UK regulations (Animals, scientific procedures, Act 1986 that regulates the use of animals for research in the UK) and to European Regulations (Directive 2010/63/Eu of the European Parliament on the protection of animals used for scientific purposes). The experiments in mice shown here were carried out following protocols 4 and 5 within Home Office Procedure Project Licence Number 80/2478 referred above.
Supporting Information
Zdroje
1. World Health Organization (2014) WHO statement on the meeting of the International Health Regulations Emergency Committee concerning the international spread of wild poliovirus. May 5, 2014.
2. World Health Organisation (2015) Polio Case Count.
3. Deshpande JM, Nadkarni SS, Siddiqui ZA (2003) Detection of MEF-1 laboratory reference strain of poliovirus type 2 in children with poliomyelitis in India in 2002 & 2003. Indian J Med Res 118 : 217–223. 14870793
4. Platt LR, Estivariz CF, Sutter RW (2014) Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis 210 Suppl 1: S380–389. doi: 10.1093/infdis/jiu184 25316859
5. Kew O, Morris-Glasgow V, Landaverde M, Burns C, Shaw J, et al. (2002) Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 296 : 356–359. 11896235
6. Burns CC, Shaw J, Jorba J, Bukbuk D, Adu F, et al. (2013) Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. J Virol 87 : 4907–4922. doi: 10.1128/JVI.02954-12 23408630
7. Shimizu H, Thorley B, Paladin FJ, Brussen KA, Stambos V, et al. (2004) Circulation of type 1 vaccine-derived poliovirus in the Philippines in 2001. J Virol 78 : 13512–13521. 15564462
8. Gumede N, Lentsoane O, Burns CC, Pallansch M, de Gourville E, et al. (2013) Emergence of vaccine-derived polioviruses, Democratic Republic of Congo, 2004–2011. Emerg Infect Dis 19 : 1583–1589. doi: 10.3201/eid1910.130028 24047933
9. Sharif S, Abbasi BH, Khurshid A, Alam MM, Shaukat S, et al. (2014) Evolution and circulation of type-2 vaccine-derived polioviruses in Nad Ali district of Southern Afghanistan during June 2009-February 2011. PLoS One 9: e88442. doi: 10.1371/journal.pone.0088442 24558390
10. Bellmunt A, May G, Zell R, Pring-Akerblom P, Verhagen W, et al. (1999) Evolution of poliovirus type I during 5.5 years of prolonged enteral replication in an immunodeficient patient. Virology 265 : 178–184. 10600590
11. Martin J, Dunn G, Hull R, Patel V, Minor PD (2000) Evolution of the Sabin strain of type 3 poliovirus in an immunodeficient patient during the entire 637-day period of virus excretion. J Virol 74 : 3001–3010. 10708414
12. Kew OM, Sutter RW, Nottay BK, McDonough MJ, Prevots DR, et al. (1998) Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J Clin Microbiol 36 : 2893–2899. 9738040
13. Alexander JP, Ehresmann K, Seward J, Wax G, Harriman K, et al. (2009) Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J Infect Dis 199 : 391–397. doi: 10.1086/596052 19090774
14. Burns CC, Diop OM, Sutter RW, Kew OM (2014) Vaccine-derived polioviruses. J Infect Dis 210 Suppl 1: S283–293. doi: 10.1093/infdis/jiu295 25316847
15. Sutter RW, Platt L, Mach O, Jafari H, Aylward RB (2014) The new polio eradication end game: rationale and supporting evidence. J Infect Dis 210 Suppl 1: S434–438. doi: 10.1093/infdis/jiu222 25316865
16. Fine PE, Carneiro IA (1999) Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am J Epidemiol 150 : 1001–1021. 10568615
17. Jorba J, Campagnoli R, De L, Kew O (2008) Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol 82 : 4429–4440. doi: 10.1128/JVI.02354-07 18287242
18. MacLennan C, Dunn G, Huissoon AP, Kumararatne DS, Martin J, et al. (2004) Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. Lancet 363 : 1509–1513. 15135598
19. Shulman LM, Sofer D, Manor Y, Mendelson E, Balanant J, et al. (2011) Antiviral activity of 3(2H) - and 6-chloro-3(2H)-isoflavenes against highly diverged, neurovirulent vaccine-derived, type2 poliovirus sewage isolates. PLoS One 6: e18360. doi: 10.1371/journal.pone.0018360 21904594
20. Patel V, Ferguson M, Minor PD (1993) Antigenic sites on type 2 poliovirus. Virology 192 : 361–364. 7685967
21. Rakoto-Andrianarivelo M, Guillot S, Iber J, Balanant J, Blondel B, et al. (2007) Co-circulation and evolution of polioviruses and species C enteroviruses in a district of Madagascar. PLoS Pathog 3: e191. 18085822
22. Odoom JK, Yunus Z, Dunn G, Minor PD, Martin J (2008) Changes in population dynamics during long-term evolution of sabin type 1 poliovirus in an immunodeficient patient. J Virol 82 : 9179–9190. doi: 10.1128/JVI.00468-08 18596089
23. Yang CF, Chen HY, Jorba J, Sun HC, Yang SJ, et al. (2005) Intratypic recombination among lineages of type 1 vaccine-derived poliovirus emerging during chronic infection of an immunodeficient patient. J Virol 79 : 12623–12634. 16188964
24. Dahourou G, Guillot S, Le Gall O, Crainic R (2002) Genetic recombination in wild-type poliovirus. J Gen Virol 83 : 3103–3110. 12466487
25. Lentz KN, Smith AD, Geisler SC, Cox S, Buontempo P, et al. (1997) Structure of poliovirus type 2 Lansing complexed with antiviral agent SCH48973: comparison of the structural and biological properties of three poliovirus serotypes. Structure 5 : 961–978. 9261087
26. Shulman LM, Martin J, Sofer D, Burns CC, Manor Y, et al. (2015) Genetic analysis and characterization of wild poliovirus type 1 during sustained transmission in a population with >95% vaccine coverage, Israel 2013. Clin Infect Dis 60 : 1057–1064. doi: 10.1093/cid/ciu1136 25550350
27. Martin J, Crossland G, Wood DJ, Minor PD (2003) Characterization of formaldehyde-inactivated poliovirus preparations made from live-attenuated strains. J Gen Virol 84 : 1781–1788. 12810872
28. Kersten G, Hazendonk T, Beuvery C (1999) Antigenic and immunogenic properties of inactivated polio vaccine made from Sabin strains. Vaccine 17 : 2059–2066. 10217607
29. Dragunsky EM, Ivanov AP, Wells VR, Ivshina AV, Rezapkin GV, et al. (2004) Evaluation of immunogenicity and protective properties of inactivated poliovirus vaccines: a new surrogate method for predicting vaccine efficacy. J Infect Dis 190 : 1404–1412. 15378432
30. Hovi T, Shulman L, van der Avoort H, Deshpande J, M R, et al. (2012) Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol Infection 1 : 1–13.
31. McKinlay MA, Collett MS, Hincks JR, Oberste MS, Pallansch MA, et al. (2014) Progress in the development of poliovirus antiviral agents and their essential role in reducing risks that threaten eradication. J Infect Dis 210 Suppl 1: S447–453. doi: 10.1093/infdis/jiu043 25316866
32. Grassly NC, Wenger J, Durrani S, Bahl S, Deshpande JM, et al. (2007) Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet 369 : 1356–1362. 17448821
33. Sutter RW, John TJ, Jain H, Agarkhedkar S, Ramanan PV, et al. (2010) Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet 376 : 1682–1688. doi: 10.1016/S0140-6736(10)61230-5 20980048
34. Burns CC, Campagnoli R, Shaw J, Vincent A, Jorba J, et al. (2009) Genetic inactivation of poliovirus infectivity by increasing the frequencies of CpG and UpA dinucleotides within and across synonymous capsid region codons. J Virol 83 : 9957–9969. doi: 10.1128/JVI.00508-09 19605476
35. Coleman JR, Papamichail D, Skiena S, Futcher B, Wimmer E, et al. (2008) Virus attenuation by genome-scale changes in codon pair bias. Science 320 : 1784–1787. doi: 10.1126/science.1155761 18583614
36. Macadam AJ, Ferguson G, Stone DM, Meredith J, Knowlson S, et al. (2006) Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: relevance to poliomyelitis eradication. J Virol 80 : 8653–8663. 16912313
37. Vignuzzi M, Wendt E, Andino R (2008) Engineering attenuated virus vaccines by controlling replication fidelity. Nat Med 14 : 154–161. doi: 10.1038/nm1726 18246077
38. Victoria JG, Kapoor A, Dupuis K, Schnurr DP, Delwart EL (2008) Rapid identification of known and new RNA viruses from animal tissues. PLoS Pathog 4: e1000163. doi: 10.1371/journal.ppat.1000163 18818738
39. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30 : 2725–2729. doi: 10.1093/molbev/mst197 24132122
40. Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29 : 1969–1973. doi: 10.1093/molbev/mss075 22367748
41. Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25 : 1253–1256. doi: 10.1093/molbev/msn083 18397919
42. Wilton T, Dunn G, Eastwood D, Minor PD, Martin J (2014) Effect of formaldehyde inactivation on poliovirus. J Virol 88 : 11955–11964. doi: 10.1128/JVI.01809-14 25100844
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type GenesČlánek Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion IsolatesČlánek -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated GlycolipidsČlánek Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- The Long and Winding Road (Apologies to the Beatles)
- The Ebola Virus: From Basic Research to a Global Health Crisis
- Riding the R Train into the Cell
- The Two-Phase Emergence of Non Pandemic HIV-1 Group O in Cameroon
- Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies
- E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3
- The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type Genes
- Hyperinvasive Meningococci Induce Intra-nuclear Cleavage of the NF-κB Protein p65/RelA by Meningococcal IgA Protease
- Active Transport of Phosphorylated Carbohydrates Promotes Intestinal Colonization and Transmission of a Bacterial Pathogen
- HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
- Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion Isolates
- Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus following European Invasion
- Inhibiting the Recruitment of PLCγ1 to Kaposi’s Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells
- Goblet Cell Derived RELM-β Recruits CD4 T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation
- HLA Class-II Associated HIV Polymorphisms Predict Escape from CD4+ T Cell Responses
- An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses
- Extracellular Adenosine Protects against Lung Infection by Regulating Pulmonary Neutrophil Recruitment
- : Adaptations to the Dixenous Life Cycle Analyzed by Genome Sequencing, Transcriptome Profiling and Co-infection with
- Which Way In? The RalF Arf-GEF Orchestrates Host Cell Invasion
- Intracellular Uropathogenic . Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells
- A Non-enveloped Virus Hijacks Host Disaggregation Machinery to Translocate across the Endoplasmic Reticulum Membrane
- Supporting Role for GTPase Rab27a in Hepatitis C Virus RNA Replication through a Novel miR-122-Mediated Effect
- -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids
- The Activation of Effector Avr3b by Plant Cyclophilin is Required for the Nudix Hydrolase Activity of Avr3b
- A Pyranose-2-Phosphate Motif Is Responsible for Both Antibiotic Import and Quorum-Sensing Regulation in
- Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation
- The Rsb Phosphoregulatory Network Controls Availability of the Primary Sigma Factor in and Influences the Kinetics of Growth and Development
- Inhibits Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis
- Illuminating Targets of Bacterial Secretion
- Chemical Signals and Mechanosensing in Bacterial Responses to Their Environment
- Interdisciplinarity and Infectious Diseases: An Ebola Case Study
- Fungi That Infect Insects: Altering Host Behavior and Beyond
- Plasticity and Redundancy in Proteins Important for Invasion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
- A Novel Virus Causes Scale Drop Disease in
- STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control
- Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
- Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative
- AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity
- Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 bZIP Factor Transgenic Mice
- Transgenic Rabbits Expressing Ovine PrP Are Susceptible to Scrapie
- Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of . Virulence Pathways
- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Serine Phosphorylation of HIV-1 Vpu and Its Binding to Tetherin Regulates Interaction with Clathrin Adaptors
- Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins the Activation of the MnK/eIF4E Pathway
- Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission
- Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection
- sRNA-Mediated Regulation of P-Fimbriae Phase Variation in Uropathogenic
- Evolutionary and Functional Analysis of Old World Primate TRIM5 Reveals the Ancient Emergence of Primate Lentiviruses and Convergent Evolution Targeting a Conserved Capsid Interface
- Hepcidin and Host Defense against Infectious Diseases
- Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis
- Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIV Rectal Challenge
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Illuminating Targets of Bacterial Secretion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



