-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity
AAV-mediated antibody delivery represents a promising alternative to classical vaccine approaches for the prevention of HIV/AIDS in humans. We used recombinant adeno-associated viral vectors (rAAV) as gene carrier to deliver anti-SIV (simian immunodeficiency virus) antibodies to monkeys. This non-classical immunization approach is independent of the host immune system and the AAV-transferred gene into muscle cells produces antibodies with known specificities. Our goal was to determine what serum levels of delivered antibodies can be achieved by using two different vector strategies, whether the delivered antibodies provoke an immune response, and to what degree the delivered non-neutralizing antibodies have the ability to protect against SIV virus challenge. This approach allowed for sustained levels of delivered antibodies in serum and exhibited significant protective effects against highly pathogenic SIVmac239 challenge. However, immune responses limited the concentration of circulating antibodies that could be achieved. This problem will need to be understood and overcome for the promise of this approach to be effectively realized.
Published in the journal: . PLoS Pathog 11(8): e32767. doi:10.1371/journal.ppat.1005090
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005090Summary
AAV-mediated antibody delivery represents a promising alternative to classical vaccine approaches for the prevention of HIV/AIDS in humans. We used recombinant adeno-associated viral vectors (rAAV) as gene carrier to deliver anti-SIV (simian immunodeficiency virus) antibodies to monkeys. This non-classical immunization approach is independent of the host immune system and the AAV-transferred gene into muscle cells produces antibodies with known specificities. Our goal was to determine what serum levels of delivered antibodies can be achieved by using two different vector strategies, whether the delivered antibodies provoke an immune response, and to what degree the delivered non-neutralizing antibodies have the ability to protect against SIV virus challenge. This approach allowed for sustained levels of delivered antibodies in serum and exhibited significant protective effects against highly pathogenic SIVmac239 challenge. However, immune responses limited the concentration of circulating antibodies that could be achieved. This problem will need to be understood and overcome for the promise of this approach to be effectively realized.
Introduction
There are good reasons for believing that development of an effective preventive vaccine against HIV-1 is going to be a very difficult task. HIV-1 has evolved a variety of immune evasion strategies that allow continuous virus replication in the face of apparently strong host immune responses, both cellular and humoral [1,2]. For a vaccine to be effective it will likely need to either completely block the initial infection or to provide immune responses that are more effective than those resulting from infection.
One promising, creative approach is to use adeno-associated virus (AAV) vector to deliver antibodies with potent and broadly neutralizing activity [3–7]. This method is an alternative to classical immunization and independent of the host immune system. Proteins delivered by AAV vector can persist at stable levels for years [8,9], and AAV vectors have proven to be safe and effective in human gene therapy trials against a number of diseases [10–13]. More than a dozen potent broadly neutralizing anti-HIV-1 monoclonal antibodies have already been characterized; they are human-derived, they can effectively neutralize more than 90% of circulating HIV-1 strains, and they represent a range of different specificities [14–21].
In a previous pioneering study performed in monkeys, anti-SIV immunoadhesins (antibody-like molecules) were delivered by AAV vector and a reasonable percentage of the monkeys exhibited apparent sterilizing immunity against intravenous (i.v.) challenge by SIVmac316 [4]. However, SIVmac316 is an easy-to-neutralize SIV strain, one third of the immunized monkeys developed antibody responses (anti-anti) to the delivered immunoadhesin, and monkeys with anti-anti responses were not protected against the challenge. It was not clear from that initial study to what extent anti-anti responses may have resulted from delivery of immunoadhesins, which do not represent an authentic IgG structure. We decided to build upon these studies by using AAV to deliver authentic IgG and to examine protection against the difficult-to-neutralize strain SIVmac239. At the time these studies were initiated, there were no monoclonal antibodies available that could neutralize SIVmac239. We thus converted the previously reported immunoadhesins 4L6 and 5L7 [4] into authentic IgG versions. These anti-SIV mAbs in IgG1 form contain only authentic rhesus IgG sequences. In this study we describe the AAV-mediated delivery of these full-length immunoglobulins to rhesus macaques and the protective effects of the non-neutralizing antibody 5L7 IgG1 against SIVmac239 challenge.
Results
AAV-mediated delivery of mAbs
Two fundamentally different types of AAV systems are available for vector-mediated delivery: single-stranded (ssAAV) vs. self-complementary (scAAV) [22]. scAAV has been reported to achieve considerably higher levels of transgene expression [23,24] but suffers from the drawback of not being able to accommodate sufficient genetic information for the expression of both heavy (H) and light (L) chains needed for authentic IgG [25]. We thus developed two strategies for AAV-mediated synthesis of authentic IgG for comparison purposes: ssAAV for the synthesis of matched H and L chains from a single vector vs. scAAV for the synthesis of H and L chains from separate vectors, an approach that we will call the two vector approach. The logic behind the two vector approach is that such a high quantity of AAV vector particles is inoculated into a highly localized area of muscle that it seems likely that many, perhaps even a majority, of cells will take up multiple AAV particles. We sought to deliver authentic IgG versions of the previously described monoclonal Fabs 346-16h and 347-23h [26] in order to allow comparison to the results of Johnson et al. that utilized immunoadhesins with these binding specificities (called 4L6 and 5L7, respectively) [4]. Since SIVmac239 was planned for challenge, this also allowed investigation of protective effects in the absence of virus-neutralizing activity. We employed IgG1 sequences in order to maximize antibody-dependent cellular cytotoxicity (ADCC) activity and other effector functions. We used AAV serotype 1 for intramuscular expression. The constructs shown in (S1 Fig) were demonstrated to express authentic rhesus IgG with expected properties prior to the initiation of monkey experiments.
In our first monkey study, three AAV1-negative monkeys were inoculated intramuscularly with 1.6 x 1013 ssAAV vector particles expressing both heavy and light chains of the authentic rhesus monkey IgG1 monoclonal antibody 5L7 (ssAAV-5L7 IgG1). On the same date, three AAV1-negative monkeys were inoculated with 0.8 x 1013 scAAV vector particles expressing 5L7 IgG1 heavy chain and 0.8 x 1013 scAAV vector particles expressing 5L7 kappa light chain (scAAV-5L7 IgG1 H + scAAV-5L7 kappa L). The levels of delivered 5L7 mAb in plasma were measured by a gp140 ELISA and purified mAb as standard. Considerable monkey-to-monkey variation was observed in the levels of 5L7 IgG1 that were achieved (Fig 1A). However, the levels in each individual monkey remained stable over the course of months leading up to the time of challenge. There were no clear differences in the persisting levels of 5L7 IgG1 mAb associated with the one vector vs. two vector delivery systems. While monkey 153–10 in the one vector group achieved persisting levels of 5L7 IgG1 mAb of only 1–5 μg/ml, monkey 84–05, also in the one vector group, achieved remarkable levels of approximately 270 μg/ml that have persisted now for more than two years. Three of the six monkeys in this first study had clear antibody responses to the delivered 5L7 IgG1 mAb (Fig 1C and S2 Fig). Monkey 153–10 with the lowest level of persisting 5L7 mAb had the strongest anti-anti response, while monkey 84–05 with the highest level of persisting 5L7 mAb had no detectable anti-anti response. The other two monkeys without detectable anti-anti responses (111–09 and 154–10) were in the two vector scAAV group.
Fig. 1. Serum concentration of the IgG1 mAbs 4L6 and 5L7, and emerging anti-mAb responses following recombinant AAV administration. 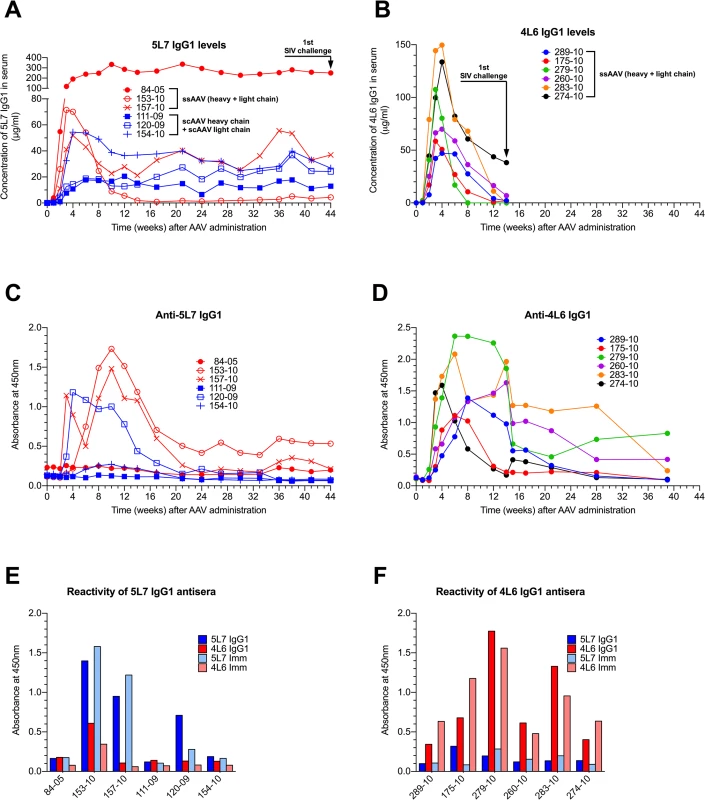
Levels of produced antibodies were measured in sera from immunized animals over time by a gp140 capture ELISA. SIV challenge is indicated by arrows. (A) Animals that received rAAV-5L7 IgG1. 84–05, 153–10 and 157–10 were given one ssAAV vector expressing both heavy and light chain of the antibody 5L7 IgG1. 111–09, 120–09 and 154–10 were given an equal mixture of two scAAV vectors expressing either heavy chain or light chain of the antibody 5L7 IgG1. (B) Animals that received rAAV-4L6 IgG1. All six animals were given one ssAAV vector expressing both heavy and light chain of the antibody 4L6 IgG1. (C and D) Antibody responses to 5L7 IgG1 and 4L6 IgG1, respectively, following recombinant AAV administration. Humoral immune responses were measured in sera from immunized animals over time by ELISA. The reactivity of serum was tested against homologous purified protein using a conjugated anti-lambda secondary antibody for detection since both 5L7 IgG1 and 4L6 IgG1 bear a kappa light chain. (E) Reactivity of sera from 5L7 IgG1 recipients to purified mAbs and immunoadhesins at week 10 after AAV administration. (F) Reactivity of sera from 4L6 IgG1 animals to purified mAbs and immunoadhesins at week 6 after AAV administration. In our second monkey study, we used the one vector ssAAV approach to deliver the 4L6 mAb in IgG1 form to six AAV1-negative monkeys. In this experiment, the levels of delivered 4L6 mAb peaked 3–4 weeks after administration and then fell precipitously (Fig 1B). All six of the recombinant AAV-4L6 recipients had antibody responses to the delivered 4L6 mAb whose appearance coincided with the precipitous decline in 4L6 mAb levels (Fig 1D and S3 Fig). The anti-anti responses in the 5L7 IgG1 recipients (Fig 1C) were largely specific for 5L7 while the anti-anti responses in the 4L6 IgG1 recipients (Fig 1D) were largely specific for 4L6; since the 5L7 and 4L6 IgG1s have the exact same constant regions, these results indicate that the responses were mostly directed to the variable domains (Fig 1E and 1F). By week 14, the time of SIV challenge, the levels of 4L6 mAb in two of the recipients (279–10 and 283–10) had dropped to undetectable levels, while the levels in the other four monkeys had dropped to 1–38 μg/ml (Fig 1B).
SIV challenge of monkeys
Six appropriately-matched control rhesus monkeys were enrolled in the challenge phase (S1 Table). Repeated, marginal dose, intravenous challenges were simultaneously initiated to control monkeys, to the 5L7 mAb group 44 weeks after their administration of recombinant AAV-5L7 IgG1, and to the 4L6 mAb group 14 weeks after their administration of recombinant AAV-4L6 IgG1. Administrations were performed every three weeks such that administrations were stopped when a monkey became infected from the previous exposure. A PBMC-grown stock of cloned SIVmac239 was used, one that has been carefully titered previously by the i.v. route in monkeys and has been used extensively by numerous investigators for controlled dose challenges [27–35]. One i.v. animal infectious dose was used through the first four administrations, 2 i.v. infectious doses for the fifth administration, and 10 i.v. infectious doses for the sixth administration.
Only one monkey of the 18 resisted all six challenges: monkey 84–05 with the highest levels of delivered mAb (averaging approximately 270 μg/ml of the 5L7 mAb through all of the challenges) (Fig 2A). Dozens of control monkeys have been challenged with the 10x dose of this exact same stock in numerous previous studies and not a single monkey has been refractory to infection [27–35]. The absence of anti-gp41 antibody responses in 84–05 even following the 10x challenge is consistent with the apparent sterilizing immunity in this animal (S4 Fig). Otherwise, no significant difference in the acquisition of SIV infection was observed among the test vs. control monkeys (Fig 2A).
Fig. 2. Repeated low-dose SIVmac239 challenge of AAV-immunized and control animals. 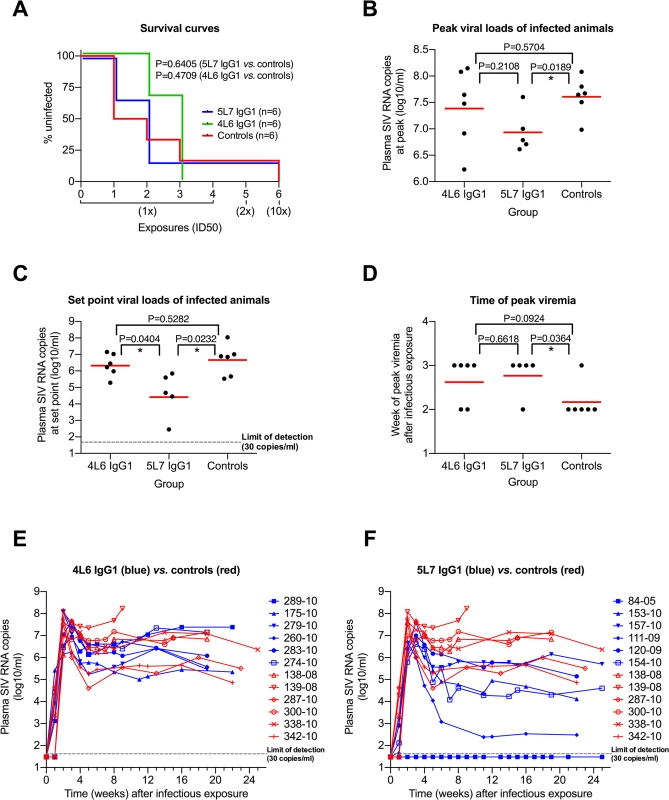
(A) Kaplan-Meier analysis of the three test groups. The percentage of animals remaining uninfected is plotted against the number of SIV exposures. SIV virus challenges were conducted in 3-week intervals. Four low-dose challenges with one half-maximal infectious dose (1x ID50) were followed by a two ID50 (2x) and a subsequent ten ID50 (10x) challenge. The survival curves are not significantly different (Mantel-Cox test). (B) Peak viral load comparison. The geometric mean (red line) is significantly different in the 5L7 IgG1 group compared to the control group (two-tailed, unpaired t test). (C) Set-point viral load analysis. All values within one group between weeks 8 and 25 were averaged, the resulting values were logarithmized (log10) and compared to each other. The geometric mean of the 5L7 IgG1 group differs significantly from the other two groups (two-tailed, unpaired t test). (D) Time of peak viremia. Most of the immunized animals show peak viremia at week 3, while 5 of 6 controls peak at week 2. (E and F) Viral loads in vivo after infectious exposure with SIVmac239. Viral loads in plasma of AAV animals (blue) and controls (red) measured as SIV RNA genome equivalent per ml are shown as a function of time since the infectious exposure. Control animal 139–08 was a rapid progressor and had to be sacrificed after 9 weeks due to AIDS-related symptoms. Test animal 84–05 remained uninfected after 6 SIV challenges. Given that levels of delivered 4L6 IgG1 mAb were driven to low or undetectable levels by the time of first challenge in most monkeys in this group, it is not surprising that plasma virion SIV RNA levels in the 4L6 mAb group as a function of weeks following the infectious exposure were not significantly different from control monkeys, neither at peak viremia nor during chronic phase infection (Fig 2B and 2C; Fig 2E). However, SIV plasma RNA loads in the 5L7 mAb group were significantly lower than those in the control group both at peak viremia (Δ of 0.67 logs) and during chronic phase infection (Δ of 2.06 logs) (Fig 2B and 2C; Fig 2F). The differences were significant whether or not the undetectable viral loads of monkey 84–05 were included in the analyses. The viral loads in the 5L7 mAb group at set point were also significantly lower than those in the 4L6 mAb group (Fig 2C). The duration in weeks from the time of the infectious exposure to peak plasma viral loads was also significantly different between the 5L7 mAb group and the control group (Fig 2D). While there is a suggestion that viral loads at peak height were inversely correlated with the levels of delivered 5L7 mAb, this correlation did not reach statistical significance (S5 Fig). There was no obvious association of measured 4L6 IgG1 levels in serum at the time of infectious exposure with the time to peak viremia (S6 Fig).
Effector functions
Both the 5L7 and the 4L6 mAb IgG1s lack detectable neutralizing activity against the challenge strain SIVmac239 (Fig 3A). In fact, even at 1 mg/ml of the 5L7 mAb IgG1, 50% neutralization of SIVmac239 was not achieved (Fig 3B). However, both the 5L7 and 4L6 mAbs had neutralizing activity against the neutralization-sensitive derivative of SIVmac239 called SIVmac316 (Fig 3C). The half-maximal inhibitory concentrations (IC50s) against SIVmac316 were 0.0015 μg/ml (4L6 IgG1) and 0.003 μg/ml (5L7 IgG1), which are consistent with previously described IC50s for Fab molecule and immunoadhesin versions of these mAbs [4,26]. The concentrations of mAb in sera shown in (Fig 1A) were measured biochemically with a gp140 ELISA using varying amounts of purified mAb produced by transfection of 293T cells in culture as standards. In order to validate the biochemically measured mAb concentrations and to confirm the expected neutralizing activity in serum against SIVmac316, serum samples were diluted to contain a starting concentration of 4 μg/ml 5L7 mAb based on the biochemically-measured concentration and neutralizing activity against SIVmac316 was measured (Fig 3D). The neutralizing activity in serum agreed spot-on with the biochemically-measured concentration of the 5L7 mAb for all six samples. As expected, sera from 84–05 lacked detectable neutralizing activity against SIVmac239 (Fig 3E). HEK 293T-expressed purified immunoadhesins and mAbs showed equivalent binding to SIVmac239 gp140 as determined by ELISA (Fig 3F).
Fig. 3. Neutralization of SIV and binding of purified proteins in vitro. 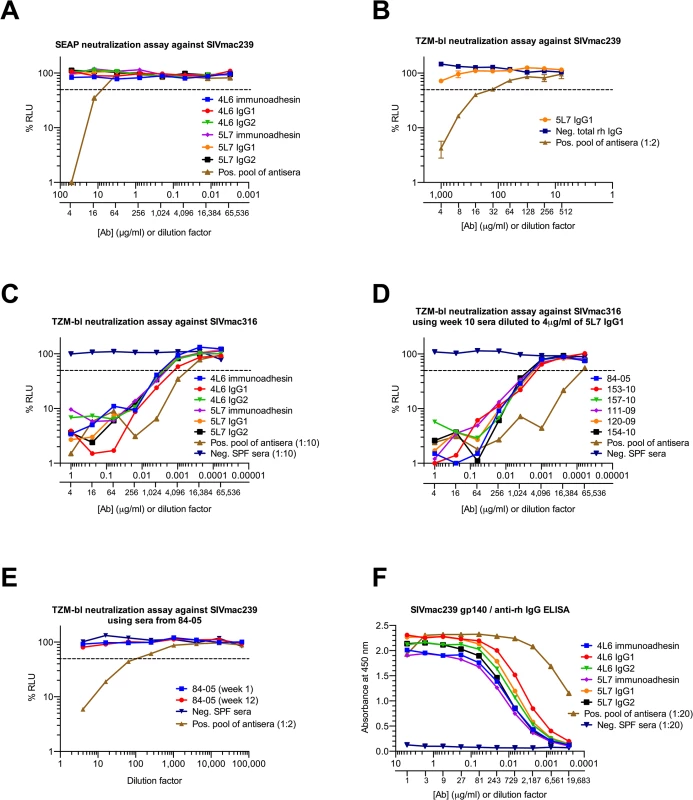
The dashed line indicates 50% RLU (relative light units) representing 50% neutralization activity against the tested SIV strain. Lowest RLU indicates highest neutralization. Binding of purified proteins was tested by a SIVmac239 gp140 ELISA, high absorbance indicates high binding. SPF serum (specific pathogen free) from naïve animals was used as negative control, a pool of antisera from SIV-infected animals served as positive control. (A) None of the antibodies or immunoadhesins showed neutralization against the resistant virus strain SIVmac239 tested at 50 μg/ml. Similar results were obtained in both the SEAP and TZMbl assays. (B) Neutralization curve of SIVmac239 with 5L7 IgG1 starting at 1mg/ml. (C) All of the tested antibodies and immunoadhesins showed equivalent neutralization activity against SIVmac316 with an average IC50 (half-maximal inhibitory concentration) of 0.002 μg/ml. (D) Animal sera from week 10 after rAAV administration (see Fig 1A) were diluted to 4 μg/ml based on previous ELISA quantitations, and tested for neutralizing activity against SIVmac316. The average IC50 measured corresponds to the IC50 of the purified proteins. (E) Animal sera of 84–05 from weeks 1 and 12 after AAV administration lacked detectable neutralizing activity against SIVmac239. (F) Antibodies and immunoadhesins were tested for their ability to bind SIVmac239 gp140. All of the antibody constructs showed equivalent binding activity as determined by absorbance at 450 nm. With this information in hand, we proceeded to measure ADCC activity in pre-challenge serum samples relative to the biochemically measured 5L7 mAb concentrations in them. We used an assay that measures natural killer (NK) cell-mediated ADCC activity of mAb or serum independent of neutralization or complement activity [36]. The assay uses a NK cell line that expresses macaque CD16 as effector cell and a CD4+ T cell line that expresses luciferase upon SIV infection as target. 5L7 IgG1 purified from 293T transfection was diluted to match the starting concentration of 5L7 IgG1 in the serum sample to which it was being compared. Serum from 84–05 was unusual in that it had markedly higher ADCC activity than the equivalent concentration of purified 5L7 IgG1 (Fig 4A and 4B). Pre-AAV serum from 84–05 contained no measurable activity capable of enhancing the ADCC activity of purified 5L7 IgG1 (S7 Fig). Four of the five remaining animals in the group had ADCC activity in their serum samples comparable to that of purified 5L7 IgG1 at equivalent concentrations (Fig 4A and 4B; S8 Fig). Serum from monkey 111–09, the monkey with the lowest post-challenge viremia, also had somewhat higher ADCC activity compared to purified 5L7 IgG1 at equivalent concentrations (S8 Fig).
Fig. 4. ADCC activity of purified proteins and sera against SIV-infected target cells in vitro. 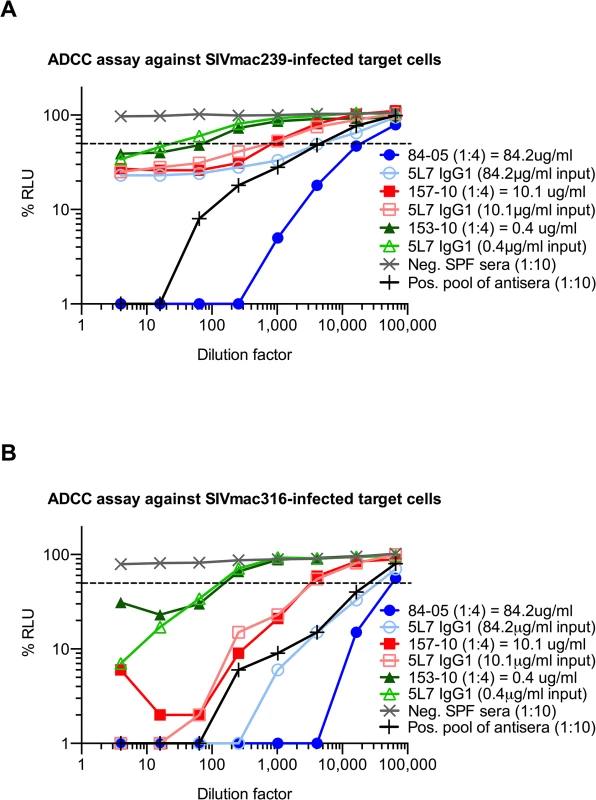
The dashed line indicates 50% RLU (relative light units) or 50% ADCC activity against SIV-infected target cells. ADCC was measured by the luciferase activity in SIV-infected cells after an 8 to 10 h incubation in the presence of a macaque CD16+ NK cell line and a serial dilution of antibodies or animal sera. The loss of RLU indicates the loss of virus-infected cells during the 8 to 10 h incubation period and represents a high ADCC activity. Purified 5L7 IgG1 was diluted to match the equivalent 5L7 IgG1 concentration of 5L7 IgG1-containing animal sera from week 21 post AAV administration, and compared for mediating ADCC towards (A) SIVmac239-infected cells and (B) SIVmac316-infected cells. The ADCC activities of sera from 153–10 and 157–10 overlapped with the purified protein at their corresponding concentration. 5L7 IgG1-containing animal serum from 84–05 mediated superior ADCC activity compared to purified 5L7 IgG1 at equivalent concentration of this antibody. Discussion
Given the current availability of a spectacular array of human mAbs with potent broadly-neutralizing activity against HIV-1, it has become possible to envision prevention scenarios in which cocktails of such antibodies could be used to provide a sterilizing barrier to infection against the vast majority of HIV-1 strains circulating in the population. However, it is not practically feasible to consider passive delivery of purified mAbs over a period of years for prevention purposes. AAV vectors are ideally suited for this purpose. The only protein expressed from AAV vectors is derived from the inserted transgene put into it. As long as the protein is viewed as self, protein expression can continue for prolonged periods. In fact, there are already examples where proteins delivered by AAV vector have persisted at stable levels for years [4,8,9,37], including animal 84–05 in our study. Since muscle cells exhibit little or no turnover, they are a preferred source for long-term delivery purposes. Little or no integration of AAV vector DNA into host genome sequences has been observed [38–40] and AAV vectors have proven safe when used in clinical settings to reverse hereditary absence of gene function [10–13].
The molecularly cloned SIVmac239 is a difficult-to-neutralize strain with many of the characteristics of a primary isolate. It has been extensively used as a challenge strain to gauge efficacy of vaccine approaches in monkeys, and it has generally proven quite difficult to achieve protection against [29,30,41–43]. Being a molecular clone, it avoids complications of interpretation that may arise when a heterogeneous mixture of sequences in an uncloned virus stock are used and when differences in neutralization sensitivity/resistance may exist within the mixture [44,45]. The presence of AAV-delivered 5L7 mAb in our experiments significantly delayed the time to peak viral loads and significantly lowered SIVmac239 viral loads both at peak viremia and at viral load set point. The lowering of viral load set points in the 5L7 mAb group was also significantly different from the 4L6 mAb group, lending additional support to the significance of the protective effects. It is also likely that the lack of SIVmac239 acquisition in monkey 84–05 is also a real effect of the high concentration of 5L7 mAb in this animal. The 10x (6th) challenge of this monkey utilized the same dose of the same stock that has been used previously in dozens of control monkeys without a failure of infection acquisition [27–35]. The protective effects in the 5L7 mAb group occurred in the absence of any detectable neutralizing activity of the 5L7 mAb against the SIVmac239 molecular clone even out to 1 mg/ml. To our knowledge, this is the first demonstration of significant protective effects of a mAb in the absence of an ability to neutralize the challenge virus [46,47].
Recent studies have emphasized the importance of Fc-mediated effector functions in contributing to the protection afforded by mAbs capable of neutralizing the SHIV used for challenge [48–52]. These studies modulated the Fc-mediated effector functions of a neutralizing mAb up or down in order to examine the effects on protective capacity. Some vaccine studies have also found associations of ADCC activity with protective efficacy [53–57]. Effector functions potentially contributing to the protective effects of the 5L7 IgG1 observed in our current study include but are not limited to ADCC activity and complement-mediated virolysis. Along these lines, it is fascinating that the 5L7 mAb circulating in monkey 84–05 was not only present at extraordinarily high levels, it had extraordinarily high ADCC activity on a per μg basis. Because of the nature of the ADCC assay that was used, it seems likely that the extraordinarily high ADCC activity in the serum of this one animal results from differences in post-translational modifications of the 5L7 mAb. Decreased core fucosylation or terminal sialylation have been shown to dramatically increase Fc-mediated ADCC activity [58–62].
One question addressed in our current studies was whether the anti-anti responses to the immunoadhesin constructs observed in Johnson et al. [4] could be avoided by delivery of authentic IgG. The answer is a clear no. Immune responses to the delivered IgG1 antibody described here were readily detected in 9 of the 12 test monkeys and these appeared to limit the levels of delivered antibody that could be achieved. This is especially true of the 4L6 group in which the levels of delivered 4L6 IgG1 mAb dropped precipitously coincident with the appearance of the anti-anti responses. In separate studies, we have observed strong antibody responses in rhesus monkeys to AAV-delivered, rhesusized anti-HIV-1 mAbs in 24 of 24 opportunities using a ssAAV1 vector design identical to the one used for the studies described here. This contrasts strikingly with studies in humans in which 13 sequential administrations of 1g of anti-HIV mAb failed to elicit anti-anti responses to the three different antibodies that were used in each of the 12 test subjects [63,64]. With our 5L7 and 4L6 experiments, it is possible that a lack of natural pairing of IgG heavy and light chains contributed to their immunogenicity. With our anti-HIV experiments referred to above, it is possible that the failure to rhesusize the variable regions contributed to their immunogenicity. Nonetheless, our results serve as a warning signal for the potential of immunogenicity problems when AAV is used to deliver anti-HIV mAbs in human trials.
Materials and Methods
Recombinant AAV vector constructs
Coding sequences (heavy chain, light chain or bicistronic) were designed in silico, codon-optimized and gene-synthesized (Genscript). 4L6 and 5L7 immunoadhesin sequences[4] served as a template and full-length antibodies were constructed by adding CH1 domain and CL domain of rhesus IgG to the already known immunoadhesin sequences. 4L6 and 5L7 sequences originate from recombinant anti-SIV Fab sequences derived from the bone marrow of SIV-infected rhesus monkeys[26]. Amino acid sequence alignments of 4L6 and 5L7 variable regions to the most closely corresponding rhesus genomic regions are depicted in (S9 Fig). Rhesus IgG1 sequence is based on accession no. AAF14058 and AAQ57555, and rhesus IgG2 sequence is based on AAF14060 and AAQ57567. Rhesus kappa light chain was designed using CL domain sequence from AAD02577. Synthesized fragments were then cloned into NotI site of scAAV or ssAAV vector plasmids[4]. Both 4L6 and 5L7 recognize gp120 and gp140 forms of the envelope glycoprotein.
Rhesus IgG proteins
HEK293T cells (ATCC) were transfected with rAAV vector plasmids and proteins were purified from cell culture supernatant using Protein A Plus (Pierce). Concentration of purified proteins was determined by NanoDrop (Thermo Scientific) A280 measurement and purity and integrity was verified by Coomassie staining (Life Technologies).
Binding assay
Immunoadhesins and mAbs were tested for their ability to bind SIVmac239 envelope glycoprotein by ELISA. Test plates were coated with recombinant gp140 of SIVmac239 (Immune Technology) for 1 h at 37°C. Plates were washed using PBS-Tween20 (Sigma-Aldrich) and subsequently blocked with 5% nonfat dry milk in PBS (Bio-Rad). Immunoadhesins, mAbs, positive and negative sera from macaques were serially diluted 1 : 3 in blocking buffer and added to the test plate. After 1 h of incubation at 37°C the plates were washed again and a HRP-conjugated goat anti-rhesus IgG H+L (SouthernBiotech) was then added for detection. The reaction was stopped after 1 h at 37°C and plates were washed 10 times. Subsequently, TMB substrate and stop solutions (SouthernBiotech) were added and Absorbance at 450 nm was measured in a microplate reader (PerkinElmer).
Virus neutralization assay
Neutralization activity of mAbs against tested SIV strains was measured by either a luciferase-based assay as described previously (TZMbl)[36] or an assay that is based on secreted alkaline phosphatase (SEAP)[36]. Virus and a serial dilution of Abs or serum were incubated for 1 h at 37°C before adding it to the TZMbl or SEAP cells (ATCC). Neutralization was assessed two days later by measuring luminescence in a 96-well plate reader (Perkin Elmer).
ADCC assay
Measurement of antibody effector function was performed by NK cell activity towards virus-infected target cells expressing luciferase[36]. A CD4+, CCR5+ T-cell line was infected with SIV four days prior assay readout. These target cells were washed three times on the day of the assay and co-cultured with a NK cell line expressing rhesus CD16 and a serial dilution of mAb or heat-inactivated serum for 8 to 10 hours. Cells were lysed afterwards and ADCC was measured as the loss of luciferase activity in the cell culture supernatant.
Recombinant AAV
Production of rAAV was conducted as described previously[65]. In short, HEK 293 cells were transfected with rAAV vector plasmid and two helper plasmids to allow generation of infectious AAV particles. After harvesting transfected cells and cell culture supernatant, rAAV was purified by three sequential CsCl centrifugation steps. Vector genome number was assessed by Real-Time PCR, and the purity of the preparation was verified by electron microscopy and silver-stained SDS-PAGE.
Animals and AAV immunization
The animals in our study were rhesus macaques of Indian-origin (Macaca mulatta). We purchased the 18 monkeys and housed them at the New England Primate Research Center of Harvard Medical School in a biocontainment facility in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care and the Harvard Medical School Animal Care and Use Committee. The animal samples used here were collected under experimental protocols approved by the Harvard Medical Area Standing Committee on Animals, and conducted in accordance to the Guide for the Care and Use of Laboratory Animals. All macaques were tested negative for the presence of antibodies to SIV and AAV1 capsid prior AAV administration. The weights of the animals ranged from 2.5 kg to 17.2 kg at the time of immunization. The six 5L7 IgG1 animals were split in two groups and each animal received a total of 1.6 x 1013 AAV vector genomes. One group received 1.6 x 1013 particles of ssAAV expressing the heavy and light chains of 5L7 IgG1, the other group received 0.8 x 1013 particles of scAAV expressing the heavy chain of 5L7 IgG1 plus 0.8 x 1013 particles of the scAAV expressing the kappa light chain of 5L7 IgG1. AAV administration was conducted by four equal and deep intramuscular injections, where each animal received two separate 0.5 ml injections into both quadriceps. All 6 animals that received 4L6 IgG1 were given a total of 2.5 x 1013 AAV vector genomes per monkey.
Ethics statement
The animal management program of Harvard Medical School is accredited by the American Association for the Accreditation of Laboratory Animal Care, and meets National Institutes of Health standards as set forth in the Guide for the Care and Use of Laboratory Animals (DHHS Publication No. (NIH)85-23 Revised 1985). The Institute also accepts as mandatory the PHS Policy on Humane Care and Use of Laboratory Animals by Awardee Institutions and NIH Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training. There is on file with the Office for Protection from Research Risks an approved Assurance of Compliance.
Animal facilities were administrated by the state and met Harvard standards for humane care and use of animals through a program of veterinary care, inspection and oversight. Animal care and welfare is the charge of the Committee on Animals, appointed by the Dean, consisting of 22 members comprising 4 veterinarians, 2 public representatives, and 16 doctoral level representatives of principal sites of animal use by Harvard Faculty. Additionally, local facilities are guided and monitored in daily activities by 8 departmental animal use committees. The procedure to avoid unnecessary discomfort, pain, or injury to animals are those prescribed in the aforementioned NIH “Guide” and additional detailed protocols for anesthesia, analgesia, tranquilization, euthanasia, or restraint have been developed and circulated by the Committee on Animals.
Rhesus macaques on study were housed in the biocontainment facility of the New England Primate Research Center of Harvard Medical School under approved protocol 04655 of the Institutional Animal Care and Use Committee (IACUC) of Harvard Medical School. The Harvard Office of Microbiological Safety oversaw the design and construction of this facility. It includes individual Hepa-filtered caging for monkeys and additional features such as a pass-through autoclave, footpedal-operated sink, and a special safety cabinet for surgical and necropsy procedures. The facility has restricted access. Individuals entering the room must suit-up with disposable cap, mask, coverall, gloves and shoe covers. Procedures used in the care and feeding of these animals are posted on the entry door. The animals were provided ad lib access to municipal source water, offered commercial monkey chow twice daily, and offered fresh produce a minimum of three times weekly. Light cycle was controlled at 12/12 hours daily. Containment facilities and procedures for macaques experimentally infected with RRV and SIV have been reviewed and approved by the Harvard Committee on Microbiological Safety.
Animals housed in the biocontainment facilities received a daily health check by both animal care technicians and veterinary professional staff. All animals received a complete physical examination on the average of once every four to six weeks. A comprehensive environmental enrichment and psychological well-being plan was in place for primates in the described studies and is available for inspection by the United States Animal and Plant Health Inspection Service (APHIS) and to officials of any pertinent organization. Euthanasia took place at defined experimental endpoints using protocols consistent with the American Veterinary Medical Association (AVMA) guidelines. Animals were first sedated with intramuscular ketamine hydrochloride at 20 mg/kg body followed by sodium pentobarbital (≥100 mg/kg) intravenously to achieve euthanasia.
Serum concentration of AAV-delivered mAb
To measure the concentration of 5L7 IgG1 and 4L6 IgG1 in vivo we performed a SIVmac239 gp140 (Immune Tech)/anti-rhesus IgG ELISA (Southern Biotech). Absorbance at 450 nm was compared to a serial dilution of purified mAb produced in HEK 293T cells, and the amount of antibody in serum was extrapolated based on the mAb standard curve. Reference protein was quantified by NanoDrop (Thermo Scientific) A280 measurement and purity was verified by Coomassie staining (Life Technologies). Levels of AAV-delivered antibody were measured up to the time of SIV challenge: 44 weeks (5L7 IgG1) and 14 weeks (4L6 IgG1) after AAV administration. The appearance of anti-env antibody responses following SIV infection obviated our ability to measure levels of delivered mAb at post-infection time points.
Anti-anti response
Humoral responses to the AAV-delivered mAb were measured by an antibody capture ELISA. Plates were coated with purified 5L7 IgG1 and 4L6 IgG1. After coating and blocking, we incubated the plates with antisera from the AAV-immunized monkeys. For detection, we probed with a HRP-conjugated anti-human lambda light chain antibody (Southern Biotech). This secondary antibody did not cross-react with the coated mAb on the plates since 5L7 IgG1 and 4L6 IgG1 harbor a kappa light chain. For detecting kappa anti-anti responses we produced recombinant versions of mAb that harbor a lambda light chain and detected kappa chain anti-mAb antibodies by probing with a HRP-conjugated anti-human kappa light chain antibody (Southern Biotech). To assess the target of the anti-anti response we tested the reactivity of antisera against AAV-delivered mAb and its immunoadhesin version as well as against antibody or immunoadhesin that was not given to one group of animals. For example, reactivity of antisera from 5L7 IgG1 recipient was tested against purified 5L7 IgG1, 5L7 immunoadhesin, 4L6 IgG1 and 4L6 immunoadhesin.
SIVmac239 challenge and viral load measurement
Infectious SIVmac239 was produced in PBMCs as described previously [27]. SIV challenges were conducted by repeated low dose intravenous injection with SIVmac239. The challenge dose was 1x AID50 for the first four injection and was elevated to 2x AID50 and 10x AID50 for the fifth and sixth challenge, respectively. The animals were exposed to SIV every three weeks or until positive viremia was measured. Challenge virus in plasma was detected by quantitative real-time RT-PCR using primers specific for SIV gag as described previously[66].
Reactivity of serum to gp41
Presence or absence of anti-SIV envelope antibodies following SIV challenge was assessed by measuring the reactivity of sera (before and after challenge) against purified SIVmac251 truncated gp41 protein (ImmunoDX) by ELISA.
Supporting Information
Zdroje
1. Desrosiers RC (1999) Strategies used by human immunodeficiency virus that allow persistent viral replication. Nat Med 5 : 723–725. 10395309
2. Evans DT, Desrosiers RC (2001) Immune evasion strategies of the primate lentiviruses. Immunol Rev 183 : 141–158. 11782254
3. Lewis AD, Chen R, Montefiori DC, Johnson PR, Clark KR (2002) Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J Virol 76 : 8769–8775. 12163597
4. Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, et al. (2009) Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med 15 : 901–906. doi: 10.1038/nm.1967 19448633
5. Balazs AB, Chen J, Hong CM, Rao DS, Yang L, et al. (2012) Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481 : 81–84.
6. Schnepp BC, Johnson PR (2014) Adeno-associated virus delivery of broadly neutralizing antibodies. Curr Opin HIV AIDS 9 : 250–256. doi: 10.1097/COH.0000000000000056 24638019
7. Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, et al. (2015) AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519 : 87–91. doi: 10.1038/nature14264 25707797
8. Fang J, Qian JJ, Yi S, Harding TC, Tu GH, et al. (2005) Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol 23 : 584–590. 15834403
9. Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, et al. (2005) Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 105 : 1424–1430. 15507527
10. Wang D, Zhong L, Nahid MA, Gao G (2014) The potential of adeno-associated viral vectors for gene delivery to muscle tissue. Expert Opin Drug Deliv 11 : 345–364. doi: 10.1517/17425247.2014.871258 24386892
11. Gaudet D, Methot J, Dery S, Brisson D, Essiembre C, et al. (2013) Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther 20 : 361–369. doi: 10.1038/gt.2012.43 22717743
12. Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, et al. (2012) Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther 20 : 443–455. doi: 10.1038/mt.2011.237 22068425
13. Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, et al. (2011) Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365 : 2357–2365. doi: 10.1056/NEJMoa1108046 22149959
14. Kwong PD, Mascola JR, Nabel GJ (2013) Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat Rev Immunol 13 : 693–701. doi: 10.1038/nri3516 23969737
15. Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, et al. (2013) HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A 110 : 16538–16543. doi: 10.1073/pnas.1315295110 24043801
16. Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, et al. (2013) Antibodies in HIV-1 vaccine development and therapy. Science 341 : 1199–1204. doi: 10.1126/science.1241144 24031012
17. Corti D, Lanzavecchia A (2013) Broadly neutralizing antiviral antibodies. Annu Rev Immunol 31 : 705–742. doi: 10.1146/annurev-immunol-032712-095916 23330954
18. Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, et al. (2012) HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492 : 118–122. doi: 10.1038/nature11604 23103874
19. Burton DR, Poignard P, Stanfield RL, Wilson IA (2012) Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science 337 : 183–186. doi: 10.1126/science.1225416 22798606
20. Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, et al. (2011) A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334 : 1097–1103. doi: 10.1126/science.1213256 21998254
21. Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, et al. (2011) Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477 : 466–470. doi: 10.1038/nature10373 21849977
22. Daya S, Berns KI (2008) Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev 21 : 583–593. doi: 10.1128/CMR.00008-08 18854481
23. Wang Z, Ma HI, Li J, Sun L, Zhang J, et al. (2003) Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther 10 : 2105–2111. 14625564
24. Gao GP, Lu Y, Sun X, Johnston J, Calcedo R, et al. (2006) High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J Virol 80 : 6192–6194. 16731960
25. McCarty DM (2008) Self-complementary AAV vectors; advances and applications. Mol Ther 16 : 1648–1656. doi: 10.1038/mt.2008.171 18682697
26. Johnson WE, Sanford H, Schwall L, Burton DR, Parren PW, et al. (2003) Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J Virol 77 : 9993–10003. 12941910
27. Lewis MG, Bellah S, McKinnon K, Yalley-Ogunro J, Zack PM, et al. (1994) Titration and characterization of two rhesus-derived SIVmac challenge stocks. AIDS Res Hum Retroviruses 10 : 213–220. 8198874
28. Lifson JD, Rossio JL, Piatak M Jr., Parks T, Li L, et al. (2001) Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol 75 : 10187–10199. 11581387
29. Jia B, Ng SK, DeGottardi MQ, Piatak M, Yuste E, et al. (2009) Immunization with single-cycle SIV significantly reduces viral loads after an intravenous challenge with SIV(mac)239. PLoS Pathog 5: e1000272. doi: 10.1371/journal.ppat.1000272 19165322
30. Bilello JP, Manrique JM, Shin YC, Lauer W, Li W, et al. (2011) Vaccine protection against simian immunodeficiency virus in monkeys using recombinant gamma-2 herpesvirus. J Virol 85 : 12708–12720. doi: 10.1128/JVI.00865-11 21900170
31. Evans DT, Bricker JE, Sanford HB, Lang S, Carville A, et al. (2005) Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239. J Virol 79 : 7707–7720. 15919923
32. Murphy CG, Lucas WT, Means RE, Czajak S, Hale CL, et al. (2000) Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J Virol 74 : 7745–7754. 10933680
33. Kaur A, Sanford HB, Garry D, Lang S, Klumpp SA, et al. (2007) Ability of herpes simplex virus vectors to boost immune responses to DNA vectors and to protect against challenge by simian immunodeficiency virus. Virology 357 : 199–214. 16962628
34. Horton H, Vogel TU, Carter DK, Vielhuber K, Fuller DH, et al. (2002) Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J Virol 76 : 7187–7202. 12072518
35. Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, et al. (2005) Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J Virol 79 : 15547–15555. 16306625
36. Alpert MD, Heyer LN, Williams DE, Harvey JD, Greenough T, et al. (2012) A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1 - or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J Virol 86 : 12039–12052. doi: 10.1128/JVI.01650-12 22933282
37. Buchlis G, Podsakoff GM, Radu A, Hawk SM, Flake AW, et al. (2012) Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood 119 : 3038–3041. doi: 10.1182/blood-2011-09-382317 22271447
38. Kaeppel C, Beattie SG, Fronza R, van Logtenstein R, Salmon F, et al. (2013) A largely random AAV integration profile after LPLD gene therapy. Nat Med 19 : 889–891. doi: 10.1038/nm.3230 23770691
39. Schnepp BC, Clark KR, Klemanski DL, Pacak CA, Johnson PR (2003) Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J Virol 77 : 3495–3504. 12610125
40. Li H, Malani N, Hamilton SR, Schlachterman A, Bussadori G, et al. (2011) Assessing the potential for AAV vector genotoxicity in a murine model. Blood 117 : 3311–3319. doi: 10.1182/blood-2010-08-302729 21106988
41. Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, et al. (2006) Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol 80 : 5875–5885. 16731926
42. Martins MA, Wilson NA, Piaskowski SM, Weisgrau KL, Furlott JR, et al. (2014) Vaccination with Gag, Vif, and Nef gene fragments affords partial control of viral replication after mucosal challenge with SIVmac239. J Virol 88 : 7493–7516. doi: 10.1128/JVI.00601-14 24741098
43. Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, et al. (2011) Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473 : 523–527. doi: 10.1038/nature10003 21562493
44. Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, et al. (2011) Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Sci Transl Med 3 : 81ra36. doi: 10.1126/scitranslmed.3002351 21543722
45. Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, et al. (2014) Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 505 : 502–508. doi: 10.1038/nature12893 24352234
46. Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, et al. (2011) Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A 108 : 11181–11186. doi: 10.1073/pnas.1103012108 21690411
47. Moog C, Dereuddre-Bosquet N, Teillaud JL, Biedma ME, Holl V, et al. (2014) Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol 7 : 46–56. doi: 10.1038/mi.2013.23 23591718
48. Moldt B, Schultz N, Dunlop DC, Alpert MD, Harvey JD, et al. (2011) A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcgamma receptors to define the role of effector functions in protection against HIV. J Virol 85 : 10572–10581. doi: 10.1128/JVI.05541-11 21849450
49. Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, et al. (2012) A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol 86 : 6189–6196. doi: 10.1128/JVI.00491-12 22457527
50. Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, et al. (2007) Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449 : 101–104. 17805298
51. Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, et al. (2009) Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15 : 951–954. doi: 10.1038/nm.1974 19525965
52. Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, et al. (2014) Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158 : 1243–1253. doi: 10.1016/j.cell.2014.08.023 25215485
53. Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M Jr., et al. (2012) ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog 8: e1002890. doi: 10.1371/journal.ppat.1002890 22927823
54. Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, et al. (2012) Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86 : 11521–11532. doi: 10.1128/JVI.01023-12 22896626
55. Lewis GK (2014) Role of Fc-mediated antibody function in protective immunity against HIV-1. Immunology 142 : 46–57. 24843871
56. Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, et al. (2009) Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol 182 : 3718–3727. doi: 10.4049/jimmunol.0803115 19265150
57. Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, et al. (2005) Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol 174 : 2185–2189. 15699150
58. Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, et al. (2013) Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest 123 : 2183–2192. doi: 10.1172/JCI65708 23563315
59. Ackerman ME, Dugast AS, Alter G (2012) Emerging concepts on the role of innate immunity in the prevention and control of HIV infection. Annu Rev Med 63 : 113–130. doi: 10.1146/annurev-med-050310-085221 22077718
60. Forthal D, Hope TJ, Alter G (2013) New paradigms for functional HIV-specific nonneutralizing antibodies. Curr Opin HIV AIDS 8 : 393–401. doi: 10.1097/COH.0b013e328363d486 23924999
61. Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, et al. (2011) Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A 108 : 12669–12674. doi: 10.1073/pnas.1108455108 21768335
62. Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS (2007) Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol 44 : 1524–1534. 17045339
63. Trkola A, Kuster H, Rusert P, Joos B, Fischer M, et al. (2005) Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med 11 : 615–622. 15880120
64. Joos B, Trkola A, Kuster H, Aceto L, Fischer M, et al. (2006) Long-term multiple-dose pharmacokinetics of human monoclonal antibodies (MAbs) against human immunodeficiency virus type 1 envelope gp120 (MAb 2G12) and gp41 (MAbs 4E10 and 2F5). Antimicrob Agents Chemother 50 : 1773–1779. 16641449
65. Mueller C, Ratner D, Zhong L, Esteves-Sena M, Gao G (2012) Production and discovery of novel recombinant adeno-associated viral vectors. Curr Protoc Microbiol Chapter 14: Unit14D 11.
66. Cline AN, Bess JW, Piatak M Jr., Lifson JD (2005) Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol 34 : 303–312. 16128925
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type GenesČlánek Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion IsolatesČlánek -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated GlycolipidsČlánek Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- The Long and Winding Road (Apologies to the Beatles)
- The Ebola Virus: From Basic Research to a Global Health Crisis
- Riding the R Train into the Cell
- The Two-Phase Emergence of Non Pandemic HIV-1 Group O in Cameroon
- Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies
- E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3
- The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type Genes
- Hyperinvasive Meningococci Induce Intra-nuclear Cleavage of the NF-κB Protein p65/RelA by Meningococcal IgA Protease
- Active Transport of Phosphorylated Carbohydrates Promotes Intestinal Colonization and Transmission of a Bacterial Pathogen
- HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
- Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion Isolates
- Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus following European Invasion
- Inhibiting the Recruitment of PLCγ1 to Kaposi’s Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells
- Goblet Cell Derived RELM-β Recruits CD4 T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation
- HLA Class-II Associated HIV Polymorphisms Predict Escape from CD4+ T Cell Responses
- An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses
- Extracellular Adenosine Protects against Lung Infection by Regulating Pulmonary Neutrophil Recruitment
- : Adaptations to the Dixenous Life Cycle Analyzed by Genome Sequencing, Transcriptome Profiling and Co-infection with
- Which Way In? The RalF Arf-GEF Orchestrates Host Cell Invasion
- Intracellular Uropathogenic . Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells
- A Non-enveloped Virus Hijacks Host Disaggregation Machinery to Translocate across the Endoplasmic Reticulum Membrane
- Supporting Role for GTPase Rab27a in Hepatitis C Virus RNA Replication through a Novel miR-122-Mediated Effect
- -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids
- The Activation of Effector Avr3b by Plant Cyclophilin is Required for the Nudix Hydrolase Activity of Avr3b
- A Pyranose-2-Phosphate Motif Is Responsible for Both Antibiotic Import and Quorum-Sensing Regulation in
- Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation
- The Rsb Phosphoregulatory Network Controls Availability of the Primary Sigma Factor in and Influences the Kinetics of Growth and Development
- Inhibits Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis
- Illuminating Targets of Bacterial Secretion
- Chemical Signals and Mechanosensing in Bacterial Responses to Their Environment
- Interdisciplinarity and Infectious Diseases: An Ebola Case Study
- Fungi That Infect Insects: Altering Host Behavior and Beyond
- Plasticity and Redundancy in Proteins Important for Invasion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
- A Novel Virus Causes Scale Drop Disease in
- STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control
- Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
- Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative
- AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity
- Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 bZIP Factor Transgenic Mice
- Transgenic Rabbits Expressing Ovine PrP Are Susceptible to Scrapie
- Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of . Virulence Pathways
- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Serine Phosphorylation of HIV-1 Vpu and Its Binding to Tetherin Regulates Interaction with Clathrin Adaptors
- Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins the Activation of the MnK/eIF4E Pathway
- Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission
- Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection
- sRNA-Mediated Regulation of P-Fimbriae Phase Variation in Uropathogenic
- Evolutionary and Functional Analysis of Old World Primate TRIM5 Reveals the Ancient Emergence of Primate Lentiviruses and Convergent Evolution Targeting a Conserved Capsid Interface
- Hepcidin and Host Defense against Infectious Diseases
- Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis
- Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIV Rectal Challenge
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Illuminating Targets of Bacterial Secretion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



