Intracellular Uropathogenic . Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells
Urinary tract infections (UTIs) are common and costly infectious diseases, affecting half of all women. Many women suffer from recurrent UTIs, for which no effective therapy currently exists. Intracellular persistence within bladder epithelial cells (BEC) by uropathogenic E. coli (UPEC) contributes to recurrent UTI in mouse models of infection. In the current study, we specifically asked whether and how UPEC co-opt any of the host proteins regulating vesicular trafficking for intracellular infection. Our study demonstrates a novel mechanism by which UPEC exploit a host endocytic recycling pathway protein (Rab35) to acquire the critical nutrient iron and to prevent lysosomal degradation, thereby promoting intracellular survival within BEC. The results of this study may highlight new avenues for therapeutic intervention in recurrent UTI. In addition, knowledge gained from this study can also be extended to understand the general principles by which other intracellular bacterial pathogens acquire essential nutrients, leading to additional strategies to combat these infectious diseases.
Published in the journal:
. PLoS Pathog 11(8): e32767. doi:10.1371/journal.ppat.1005083
Category:
Research Article
doi:
https://doi.org/10.1371/journal.ppat.1005083
Summary
Urinary tract infections (UTIs) are common and costly infectious diseases, affecting half of all women. Many women suffer from recurrent UTIs, for which no effective therapy currently exists. Intracellular persistence within bladder epithelial cells (BEC) by uropathogenic E. coli (UPEC) contributes to recurrent UTI in mouse models of infection. In the current study, we specifically asked whether and how UPEC co-opt any of the host proteins regulating vesicular trafficking for intracellular infection. Our study demonstrates a novel mechanism by which UPEC exploit a host endocytic recycling pathway protein (Rab35) to acquire the critical nutrient iron and to prevent lysosomal degradation, thereby promoting intracellular survival within BEC. The results of this study may highlight new avenues for therapeutic intervention in recurrent UTI. In addition, knowledge gained from this study can also be extended to understand the general principles by which other intracellular bacterial pathogens acquire essential nutrients, leading to additional strategies to combat these infectious diseases.
Introduction
Urinary tract infections (UTIs) are one of the most common bacterial infections in humans, affecting at least 50% of women at some point in their lifetime. UTIs constitute significant morbidity and economic burden, accounting for more than 1 million hospitalizations and $2.4 billion in medical expenses in the USA alone annually [1,2]. Most (>80%) UTIs are caused by Escherichia coli, thus the term uropathogenic E. coli (UPEC) [3]. After an initial infection, 25% of patients suffer a recurrence within 6 months, with 68% of these UTIs apparently caused by the original strain, despite appropriate antibiotic therapy [4,5]. Mouse models of UTI have been used by many groups to elucidate mechanisms underlying UPEC pathogenesis [6–8]. Experimentally infected mice also suffer episodes of recurrent UTI subsequent to clearance of bacteriuria following antibiotic therapy [9]. These recurrent infections are due to UPEC that persist within urinary bladder epithelial cells. UPEC have been described to form several types of intracellular populations in vivo, such as intracellular bacterial communities (IBCs) and quiescent intracellular reservoirs (QIRs), that contribute to phenotypic antibiotic resistance and evasion of the host immune response [10]. QIRs, in particular, cause persistent infections lasting for months in mice [11], while IBCs and other intracellular bacteria have been detected in the urine of both adult and pediatric human UTI patients [12–15]. Therefore, understanding the molecular mechanisms underlying the establishment of the intracellular bacterial reservoirs is critical for the development of efficient therapeutic strategies to control recurrent UTIs.
UPEC utilize type 1 pili, which carry the FimH adhesin at their distal tips, to specifically bind to mannosylated surface proteins (including uroplakins [16] and α3 and β1 integrins [17] on the surface of bladder epithelial cells [18]). Upon binding, UPEC are internalized into cyclic AMP- modulated Rab27b/CD63+ vesicles [19]. Internalization requires components of the host cell cytoskeleton and activation of Rho GTPases, various kinases, and other signaling intermediates [17,20–24]. Most of the internalized bacteria are expelled out of urothelial cells by Rab27b-mediated exocytosis [25]. In vivo, however, some bacteria escape their enclosing vesicle, enter the cytosol, and subsequently multiply to form biofilm-like IBCs that contain 104−105 bacteria each [26,27]. As a host countermeasure, infected epithelial cells undergo an apoptotic-like cell death, detaching from the urothelium to be expelled in the urine with the internalized bacteria [28]. However, this exposes deeper layers of the urothelium to infection, which is thought to lead to QIR formation. In contrast to IBCs, QIRs are smaller structures (6–8 bacteria), enclosed in host membranes (LAMP1, ATG16L1, and LC3- positive and CathepsinD negative), and found in immature epithelial cells [11,29]. However, the molecular profiles of these bacterial reservoirs and the interplay between UPEC and the host resulting in their formation are still in need of further characterization.
For successful intracellular survival, UPEC must obtain sufficient nutrients from the host cell. In mammals, iron is tightly bound to plasma and cellular proteins such as hemoglobin, transferrin, lactoferrin, or ferritin, which limit its availability to microbial pathogens, a phenomenon termed nutritional immunity [30]. In order to acquire iron, UPEC, like other bacteria, synthesize several siderophores (catecholates enterobactin and salmochelin, the hydroxamate aerobactin, and yersiniabactin, a mixed-type siderophore) and their cognate receptors [31]. These iron acquisition systems are important for virulence in mouse models of UTI and are among the most highly upregulated genes in both mouse [32,33] and human infections [34,35]. They are therefore being actively pursued as vaccine candidates [36–39]. With only one exception, all of these studies have focused on the expression of iron acquisition systems by extracellular bacteria. Notably, in the sole intracellular study [32], host cell transferrin receptor (TfR) levels increased within IBC-containing epithelial cells and epithelial cells near IBC-containing cells. Transferrin is the major iron carrier protein in the blood. Transferrin bound iron (Fe3+) is internalized from the circulation by the ubiquitously expressed TfR in almost all cells [40]. TfR trafficking is regulated in host cells by Rab GTPases [41]. Rab proteins are a subfamily of the Ras superfamily of GTPases that regulate various aspects of intracellular membrane trafficking [42]. Many intracellular pathogens that reside within host cell-derived vacuoles selectively recruit Rab proteins to facilitate trafficking of nutrient-rich vesicles to their vacuolar niches. Several pathogens also use Rab recruitment to prevent fusion of their vacuoles with degradative lysosomal compartments [43]. The connection between access to iron, transferrin, and Rab proteins was first noted in Mycobacterium tuberculosis, which associates with Rab11, a key regulator of the transferrin recycling pathway, to acquire iron [44]. Recent studies have identified another Rab protein, Rab35, a component of the rapid endocytic recycling pathway, that plays an important role in the recycling of endocytosed transferrin receptors (TfR) to the cell surface [45,46]. Upon internalization, the iron loaded transferrin-TfR complex is delivered to the early endosomes, where the Fe3+ dissociates from the transferrin. The receptor and transferrin are then recycled to the cell surface by Rab35 positive recycling endosomes to initiate a new round of iron uptake [47,48]. Inside the endosome, Fe3+ is reduced to Fe2+, which is subsequently released into the labile iron pool (LIP) in the cytosol, where it may be utilized or stored in complex with ferritin [49].
Similar to Rab11 recruitment by Mycobacteria, Rab35 has been shown to be recruited to the vacuoles of Anaplasma phagocytophilum [50], although its functional relevance in the intracellular persistence of pathogens has not yet been investigated. We hypothesized that Rab35 might play a role in iron acquisition during intracellular infection by UPEC. We found that UPEC infecting cultured bladder epithelial cells do indeed recruit Rab35 to their enclosing vesicles, structures we term the UPEC containing vacuoles (UCV). In a mouse model of persistent UPEC infection, UPEC within the uroepithelium also associates with Rab35. We found that Rab35 recruitment leads to increased TfR association with the UCV, which supports UPEC survival through the provision of iron. Finally, Rab35 recruitment serves a second function for UPEC survival by avoidance of UCV fusion with degradative lysosomes. Therefore, Rab35 recruitment is a key feature of the UPEC strategy for exploiting host vesicular trafficking during intracellular infection.
Results
Rab35 is recruited to the UPEC containing vacuole during intracellular infection of urinary bladder cells
To identify host cell molecules and pathways utilized by UPEC for intracellular survival within BEC, we first focused on membrane trafficking pathway proteins. Rab GTPases are critical regulators of mammalian membrane trafficking pathways and many pathogenic bacteria are known to exploit these proteins for their intracellular survival within the host [43] by recruiting (or excluding) specific Rab proteins to bacteria containing compartments. We hypothesized that UPEC might subvert Rab GTPases for intracellular survival, possibly by recruiting specific Rab proteins to intracellular UPEC-containing compartments. To examine this, we initially focused on a subset of 15 human Rab GTPase proteins [50] and assessed their localization patterns during the UPEC intracellular infection, using the well-established bladder epithelial cell line 5637 (BEC5637) based infection model system [19]. BEC cells over-expressing GFP/EGFP-tagged Rab GTPases were infected with UPEC (CI5 strain) for various time intervals (4, 24 and 48 h). Based on previously reported data [11], we reasoned that intracellular bacterial levels at 4 h post-infection would represent the number of bacteria that had invaded the BEC or intracellular bacteria levels during the early stages of infection, while the number of intracellular bacteria at 24 and 48 h (or ≥ 24 h post-infection) post-infection would represent the surviving bacterial population (or bacterial levels during late stages of infection). In comparison to other tested Rab proteins (S1A Fig), Rab35 showed a rather striking degree of localization to UPEC-enriched vesicular structures, which we termed UPEC-containing vacuoles (UCV). BEC5637 cells over expressing GFP-tagged Rab35 protein were infected with UPEC for 4, 24 and 48 h. As shown in the graph (Fig 1A), a major fraction of Rab35 was found to associate with UCV at all the tested time points, as observed by confocal microscopy. There was a notable increase in the percentage of Rab35 localizing to the UCV membrane from 4 h to 24 h; but thereafter this value remained fairly constant. Representative confocal images of infected (24 h post-infection) and uninfected samples are also shown. Depending on the stage of UCV development, we observed three categories of associations of Rab35 with UCV/UPEC at the 24 h time point. (1) UCV with ~2–10 bacteria, showing a distinct rim-like demarcation of Rab35 enriched at the periphery of UCV membrane (Fig 1A, marked as 1 in the merged image); (2) UCV with ~1–2 bacteria, where Rab35 may not always outline a complete rim-like structure, but is nonetheless enriched at the periphery of UCV membrane (Fig 1A, marked as 2 in the merged image); (3) Single UPEC where an UCV is not very well developed, but the bacteria appear to be associated with cytoplasmic patches of Rab35 (S1B Fig, % seen = 30 ± 8). The first two categories were counted as the positive population (% seen = 64 ± 8) for Rab35 association with the UCV. On the other hand, UPEC-infected BEC5637 cells transfected with GFP showed diffuse green fluorescence throughout the cell with no specific GFP signal association with UCV (S1C Fig). Interestingly, comparison of infected and uninfected GFP-Rab35 expressing cells revealed that UPEC infection resulted in a distinct pattern of Rab35’s localization and membrane association within the infected cells (Fig 1A). While the uninfected cells showed a rather uniform distribution of Rab35 in the cytoplasm, UPEC infection induced a notable recruitment leading to increased presence of Rab35 around the UCVs. Rab35 recruitment was specific for UPEC as a commensal K12 E. coli strain that expresses type 1 pili (MG1655) was unable to recruit Rab35 (S1D Fig). In addition, we also found that heat-killed UPEC was unable to recruit Rab35 (S1E Fig). Rab35 is involved in the rapid endocytic recycling pathway and regulates membrane traffic via cyclical conversion between its active GTP-bound state and its inactive GDP-bound state [51]. We reasoned that if UPEC co-opts Rab35 to modulate a cellular process, it might preferentially associate with the GTP-bound, functionally active form of Rab35. Indeed, we observed that UPEC preferentially associated with the constitutively active Rab35 Q67L mutant (GTP locked) as compared to dominant negative Rab35 S22N (GDP bound) (2 fold difference, p = 0.03) (Fig 1B). This suggests that not only do UPEC recruit Rab35 to its vacuole, but it also possibly utilizes the transport pathways regulated by Rab35 for its survival. We also found an increase in the expression of Rab35 mRNA (2.2 fold at 24h, p = 0.008; Fig 1C) as well as protein (3 fold at 24h, p = 0.005; Fig 1D) with the progression of infection.
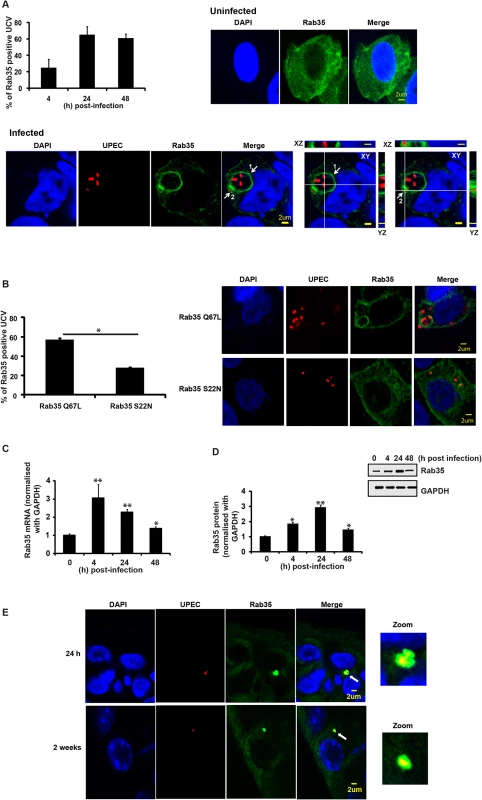
We also examined whether Rab35 is associated with intracellular UPEC during in vivo infection using a well-established murine UTI infection model [52]. Mice were infected with a cystitis isolate of UPEC, and bladders were removed at 24 h and 2 week time points, fixed, sectioned, and stained for UPEC and Rab35. We found that approximately 25% of the UPEC in bladder sections, found in small intracellular collections resembling QIRs, were associated with Rab35 (n = 4 sections/mouse bladder, n = 3 mice per experiment). An example of this association is shown in Fig 1E (top panels shows results from 24 h and bottom panel shows results from 2 weeks post-infection). As not all UPEC were colocalized with Rab35, we could conclude that the Rab35 staining was not due to nonspecific binding of the Rab35 antibody to UPEC (S1F Fig). Previous in vivo studies have shown that UPEC resides in a Lysosomal-associated membrane protein 1 (LAMP1, a late endosomal/early lysosomal marker) positive and Cathepsin D (late lysosomal marker) negative compartment in bladder cells during persistent infection (QIRs) [11]. We also observed that the Rab35-positive QIRs were also positive for LAMP1 (S2A Fig). Interestingly, similarly to ATG16L1 and LC3, which associate with both QIRs at 2 weeks post-infection and with IBCs at 6 h post-infection [29], we found that Rab35 also colocalized with immature IBCs at 6 h post-infection (S2B Fig).
Rab35 plays a role in intracellular UPEC infection of urinary bladder epithelial cells
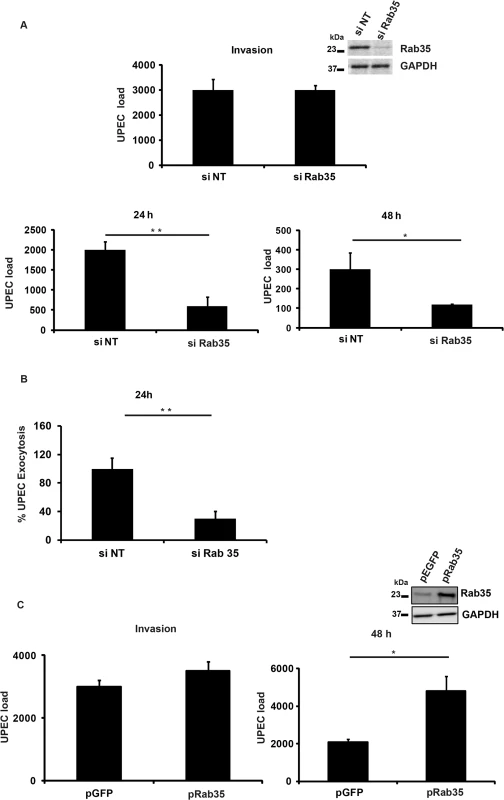
Since we observed localization of Rab35 to UCV in vitro and to IBCs and QIRs in vivo, we next asked whether Rab35 played a functional role during UPEC infection of bladder epithelial cells. Using a short interfering RNA (siRNA) mediated gene-silencing approach, we knocked down the expression of Rab35 in BEC5637 cells and then infected them with UPEC. The ability of these cells to support UPEC infection was assessed at various time points by cfu enumeration. Gene knock down was confirmed by Western blot analysis (Fig 2A, inset). Rab35 silencing did not significantly affect the invasion/entry (4 h) of UPEC into bladder cells (Fig 2A). Remarkably, we found that silencing of Rab35 led to a significant reduction in the intracellular bacterial load of BEC5637 cells at 24 and 48 h post infection (3.3 fold at 24 h, p = 0.01 and 2.5 fold at 48 h, p = 0.04) (Fig 2A). Infected BECs have the capacity to expel UPEC [19]. We also measured the levels of UPEC that are expelled from Rab35 silenced cells at 4 h and at 24 h post-infection. There was no change in the % of UPEC expelled from Rab35 silenced cells compared to control at 4 h post-infection (S2C Fig). At 24 h post-infection, there were reduced levels of UPEC expulsion from Rab35 silenced cells (Fig 2B). This experiment ruled out the possibility that the observed reduction in the intracellular UPEC load in Rab35 silenced cells is due to enhanced expulsion of bacteria; instead, the reduction appears to be due to reduced survival of intracellular UPEC. Consistent with the knockdown data, ectopic overexpression of Rab35 protein (GFP-Rab35) led to enhanced levels of UPEC (2.3-fold, p = 0.03) in bladder epithelial cells, as assessed at 48 h, although invasion of UPEC into BEC cells was not affected (Fig 2C). Expression levels of Rab35 were assessed by Western blot analysis (Fig 2C inset). These results demonstrate that Rab35 plays a role in the intracellular survival of UPEC in bladder epithelial cells.
UPEC are targeted to a Cathepsin D-positive compartment in the absence of Rab35 expression
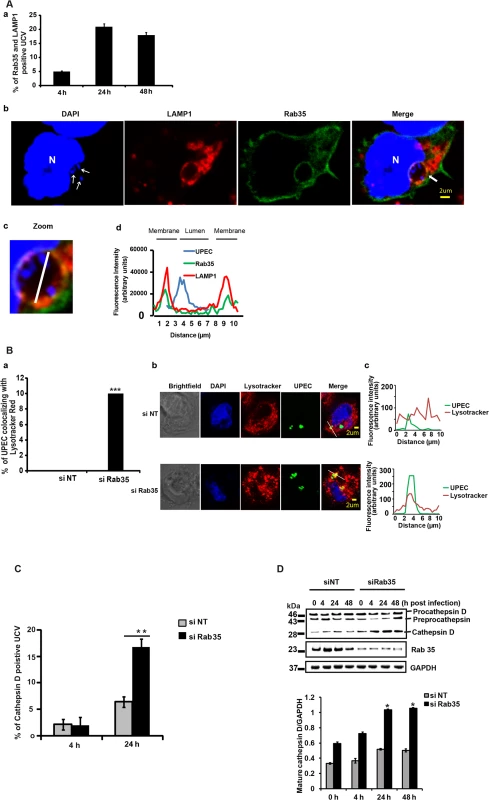
Because UPEC intracellular survival was compromised within Rab35 knocked down cells, we examined the fate of internalized bacteria in Rab35-deficient cells. Previous reports [11] and our current data show that UPEC in QIRs reside in a LAMP1 positive compartment in bladder cells in vivo. We also observed that UCVs in vitro were positive for both LAMP1 and Rab35. The percentage of Rab35 and LAMP1 positive UCV increased from 4–24 h, but thereafter this value remained fairly constant (Fig 3A). The Mander’s overlap coefficient of Rab35 and LAMP1 on UCV was 0.65 ± 0.09 (n ≥ 50) at 24 h. Since UPEC was apparently unable to sustain its intracellular growth in the absence of Rab35, we hypothesized that the bacteria might be directed to the lysosomal compartment for degradation. To test this, we first studied the colocalization of UPEC with lysosomes in normal and Rab35-depleted cells, using lyso-Tracker Red, which stains late endosomes and lysosomes [53]. Very few UPEC colocalized with lyso-Tracker Red stained compartments in Rab35 sufficient cells. However, we observed that in Rab35-deficient cells, a significantly higher number of intracellular UPEC colocalized with lyso-Tracker Red at 24 h post infection (Fig 3B). Since LysoTracker Red cannot reliably distinguish endosomes and lysosomes, we performed further experiments to probe the exact nature of the compartment occupied by UPEC in the absence of Rab35. The lysosomal protease cathepsin D is first synthesized as an inactive precursor (procathepsin D) that is then cleaved to form active cathepsin D in lysosomes [54,55]. Hence, colocalization of UPEC with cathepsin D, and the processing status of cathepsin D, could be used as further indicators of UCV’s fusion with lysosomes. Subsequent colocalization experiments revealed a significant increase in the colocalization of UPEC with cathepsin D in Rab35-silenced cells, compared to controls (2.6 fold, p = 0.001; Fig 3C). In addition, we also found that the levels of mature/activated form of cathepsin D increased in Rab35 knockdown cells with the progression of infection (Fig 3D). These results indicate that UPEC are transported to the degradative lysosomal compartment in the absence of Rab35.
Iron acquisition via the Rab35-Transferrin receptor 1 pathway is critical for the intracellular survival of UPEC within BEC
We next sought to determine the mechanism by which Rab35 is utilized by UPEC to avoid fusion of UCV with lysosomes, thus promoting its intracellular survival within BEC. Rab35 is involved in the fast recycling of TfR, the protein critical for the cellular uptake of iron, and UPEC is known to up-regulate TfR1 levels during intracellular infection of BEC [32]. Because of its involvement in TfR recycling, we hypothesized that Rab35 might be recruited by UPEC in order to acquire iron (a highly limiting nutrient) in addition to its role in avoidance of fusion with lysosomes. We performed several experiments to address this possibility. First, we re-visited the requirement of iron for the multiplication of UPEC in cell-free cultures [56]. As seen in S3A Fig, addition of iron significantly increased UPEC levels in cell-free cultures, while the addition of the iron chelating compound deferoxamine reversed this effect. Although previous studies have reported the induction of iron acquisition-associated genes in UPEC during IBC formation [32,57], the role of iron in the intracellular survival of UPEC has not yet been studied in detail. We therefore determined whether iron is critical for the intracellular survival of UPEC. We found that supplementation of iron in the form of holotransferrin indeed significantly increased the intracellular levels of UPEC in BEC cells at 24 h (2.4 fold, p = 0.006, Fig 4A). Conversely, treatment with deferoxamine reduced the intracellular survival of UPEC (2.4 fold, p = 0.004, Fig 4A). Accordingly, we also observed increased fusion of UCV with lysosomes in deferoxamine treated cells (S3B Fig).
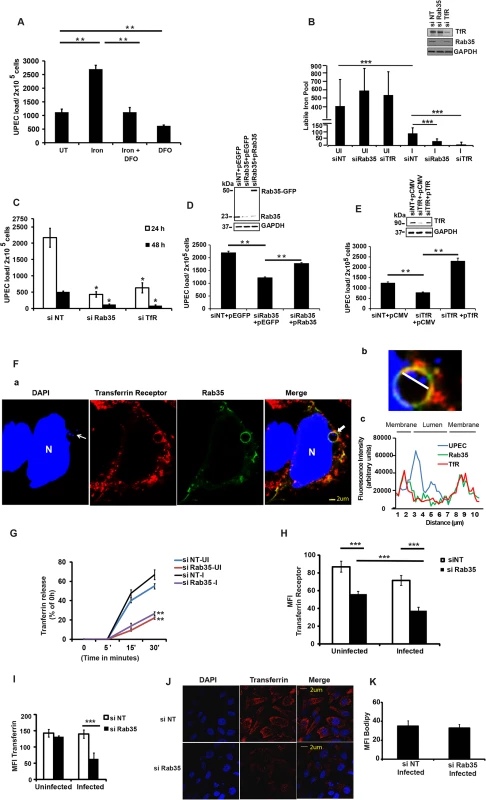
To determine whether a Rab35-mediated pathway is exploited by UPEC to meet their iron requirements through TfR, we first analyzed the role of Rab35 and TfR1 in modulating the levels of metabolically available iron or the labile iron pool (LIP) in BEC, within the context of UPEC infection. Knock down of either Rab35 or TfR1 did not significantly alter LIP in uninfected cells at both 4 h (S4A Fig) and 24 h (Fig 4B). Notably, UPEC infection led to a severe reduction of the LIP in negative control siRNA treated BEC cells (3.26 fold, p = 0.001) at 24 h (Fig 4B), but not 4 h (S4A Fig), post infection. This data argues that UPEC utilize the bulk of the pre-existing LIP of BEC during infection. Remarkably, this reduction of LIP was further aggravated in both Rab35 (2.8 fold reduction in comparison to infected negative control siRNA treated samples, p = 0.0001) and TfR1 knocked down cells (11.4 fold reduction in comparison to infected negative control siRNA treated samples, p = 0.0001) at 24 h post UPEC infection (Fig 4B). Interestingly, silencing of TfR1, but not Rab35, also reduced the LIP at 4 h post-infection (S4A Fig), although UPEC load was unaffected at this time point (S4B Fig). Since the reduction of LIP upon UPEC infection was much greater in Rab35 and TfR1 silenced cells at 24 h than in infected control cells, we reasoned that UPEC is dependent on Rab35 and TfR1 to actively acquire iron from the extracellular milieu to replenish intracellular iron levels for its survival at later stages (24 h) of infection.
Further highlighting the association between Rab35 and TfR1 during UPEC infection, we found that similar to Rab35 knock down cells, the reduced iron pool in TfR1 knockdown cells (Fig 4B) was also associated with reduced survival of UPEC at 24 and 48 h post infection (3.4 fold at 24 h, p = 0.02, Fig 4C). These experiments demonstrate that UPEC exploits Rab35 and TfR1 to acquire iron during later stages of intracellular infection (≥ 24 h) in bladder cells.
To ensure the specificity of the siRNAs used, we also performed siRNA phenotype rescue experiments. Rab35 or TfR was knocked down using siRNAs targeting their 3’UTR regions, followed by ectopic overexpression of Rab35/TfR respective coding regions, and followed by UPEC infection. It was found that ectopic overexpression of non-degradable Rab35 (Fig 4D) or TfR (Fig 4E) rescued the growth defect observed in Rab35/TfR-deficient cells.
In order to determine the specificity of the requirement of Rab35 in meeting the iron requirement of persistent UPEC infection, we examined whether Rab14, which is also known to be involved in TfR recycling [58] is utilized by UPEC for intracellular survival. Rab14 was knocked down in BEC cells and the intracellular UPEC levels were determined at various time points (4, 24 and 48 h). As seen in S5 Fig, we found that Rab14 was not essential for the UPEC survival within BEC.
Rab35 and Transferrin Receptor 1 co-localize at the UPEC-containing vacuole
Since both Rab35 and TfR1 are critical for UPEC intracellular survival, we hypothesized that UPEC recruits Rab35 to meet its iron nutrient requirements through the regulation of TfR transport towards bacteria containing vacuoles. To test this, we first examined whether TfR1 and Rab35 protein colocalized with UCV during infection. Confocal microscopy analysis showed that TfR1 colocalized with Rab35 at the cell surface during UPEC infection (S4C Fig). More interestingly, TfR1 shows very obvious colocalization with UPEC-containing Rab35 positive UCVs (Fig 4F) with Mander’s overlap coefficient of 0.85 ± 0.12 (n ≥ 50). To further characterize the nature of UCV, we performed colocalization studies with another marker of early endosomes (early endosomal antigen, EEA1) and found no obvious colocalization of UCV with EEA1 (S4D Fig). A recent study has reported that UPEC in QIRs in vivo survive within an autophagosome-like compartment [29]. Consistent with this, we found that Rab35-positive UCV in vitro were also associated with the autophagy marker LC3 (S6 Fig). The Mander’s overlap coefficient of Rab35 and LC3 in the infected cells was determined to be 0.60 ± 0.10 (n ≥ 50).
Rab35 is required for the recycling and maintenance of cellular surface levels of TfR during UPEC infection
We next examined whether UPEC infection affects TfR recycling in the presence and absence of Rab35 expression. It was found that UPEC infection per se did not affect TfR recycling (as determined by measuring transferrin release) in Rab35 sufficient (siNT) cells compared to uninfected cells (Fig 4G). We then examined whether absence of Rab35 expression affects TfR recycling in the context of UPEC infection. Consistent with previous reports, we found that Rab35 silencing significantly reduced TfR recycling in uninfected BEC cells (2.5 fold at 30’, p = 0.002), compared to uninfected control siRNA treated cells (Fig 4G). Similarly, the recycling of TfR to the cell surface was significantly reduced (2.53 fold at 30', p = 0.002) in UPEC infected Rab35 knockdown BEC cells (Fig 4G). We also quantified the surface expression of TfR in similar conditions, using immuno-staining based fluorescence microscopy. Rab35 knockdown resulted in a 1.6-fold (p = 0.0001) and 1.9-fold (p = 0.0001) reduction in the levels of cell surface TfR in uninfected and infected cells, respectively, compared to control siRNA treated cells (Fig 4H). Although there was no significant difference in the surface expression of TfR during infection in the siNT group, there was a moderate but significant reduction (1.5-fold, p = 0.0007, Fig 4H) in the TfR surface expression in infected cells compared to uninfected cells in the Rab35 knockdown group, indicating a requirement for Rab35 in maintaining the TfR surface levels during UPEC infection.
Additionally, consistent with the reduction of the surface TfR receptor levels and their reduced ability to accumulate a labile iron pool within persistently infected cells, Rab35 knock-down cells also showed a significantly reduced uptake of Alexa Fluor labeled Transferrin at 24 h post infection (2.2-fold, p = 0.0001, Fig 4I and 4J). There was no reduction in the uptake of Alexa labeled Transferrin upon infection of wild type cells, in comparison with uninfected cells. Similarly, Rab35 knockdown alone did not reduce Transferrin uptake in uninfected cells. In order to confirm that the reduced uptake of transferrin was not due to a general defect in endocytosis due to infection, we analyzed the uptake of a lipid (MFI Bodipy) in Rab35 silenced cells. As shown in Fig 4K, UPEC infected Rab35 knockdown cells were not defective in bulk endocytosis. The above-described observations demonstrate that Rab35 is critical for the maintenance of the cellular surface levels of TfR and the subsequent iron-transferrin uptake by BEC cells during UPEC infection.
Iron supplementation is unable to support intracellular UPEC survival in the absence of host Rab35 or TfR
The data provided earlier showed that iron supplementation by the addition holotransferrin enhanced the intracellular UPEC levels within normal cells. We next investigated whether iron supplementation can rescue the growth defect observed in Rab35/TfR-silenced cells. Holotransferrin was added to the culture media of control cells, TfR- or Rab35-knockdown cells and UPEC infected cells. We found that iron supplementation was unable to rescue the UPEC growth defect in both TfR and Rab35-knock down cells. As shown in Fig 5A, UPEC infected control (si-NT) cells showed significantly higher bacterial load in the presence of extracellular iron (holotransferrin) treatment (2.4 fold, p = 0.006). However the TfR/Rab35-silenced cells did not show any increase in the bacterial load even with iron supplementation, most likely due to the failure of these cells to accumulate iron from the extracellular milieu. These results collectively suggest that iron is important for UPEC survival inside bladder cells, and the Rab35-TfR pathway plays a major role in meeting the iron requirements of intracellular UPEC.
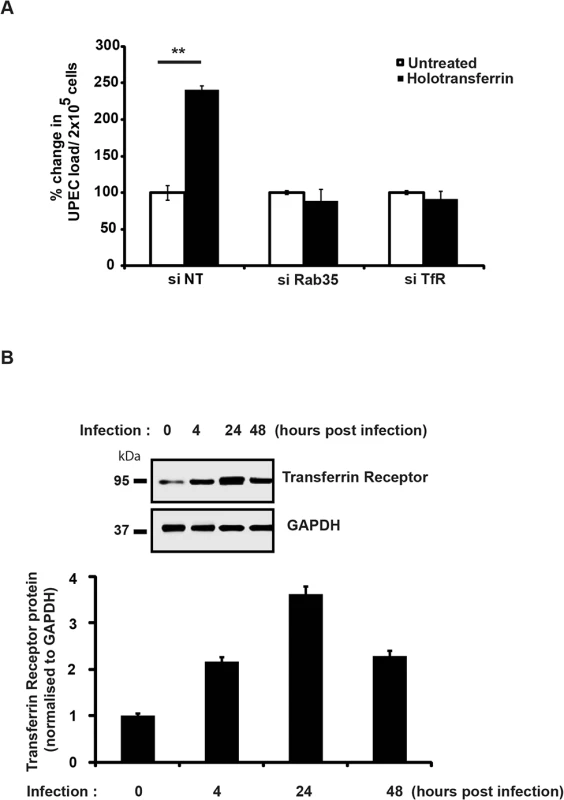
Earlier studies have shown that the expression levels of TfR are negatively regulated by intracellular iron content [59]. Since UPEC-infected cells had reduced intracellular iron levels (LIP) at 24 h post-infection (Fig 4B), we hypothesized that TfR levels might be elevated in these cells. Consistent with our hypothesis, we observed increased expression of TfR1 upon UPEC infection, with a maximal induction observed at 24 h (Fig 5B). This suggests that during infection, the observed consumption and depletion of cellular labile iron pools by UPEC will in turn trigger increased expression levels of TfR. This upregulation of TfR expression might facilitate increased internalization of Fe-Transferrin complexes into the cell.
Genes involved in iron acquisition are differentially expressed by UPEC in the absence of Rab35
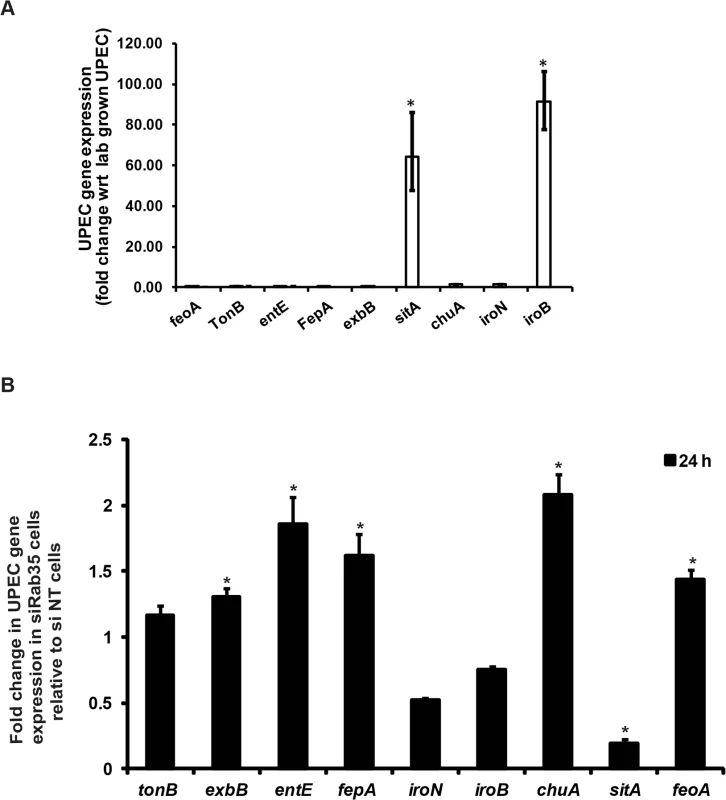
Because there is a constant struggle between the host and bacteria to secure iron for their respective uses, we wondered how the genetic regulatory system in UPEC would respond to the significant reduction in availability of cellular iron caused by Rab35 depletion. In response to iron limitation in the urinary tract, UPEC produce various siderophores (eg, EntE) and siderophore receptors (eg. FepA, IroN) as well as components of energy transduction systems required for siderophore uptake (eg. TonB, ExbB). In addition, UPEC can also utilize ferrous iron via the iron transporter FeoA and host heme stores (via ChuA, the receptor for hemin) [32,33]. We first determined the levels of several genes known to be involved in iron acquisition in UPEC (feoA, tonB, entE, fepA, exbB, sitA, chuA, iroN, and iroB) at 24 h post-infection in Rab35-sufficient BEC cells by qRT-PCR analysis. We found that expression of two of the nine genes tested were significantly up-regulated in UPEC at 24 h post-infection in comparison to lab grown bacteria: sitA, encoding an ABC type iron transporter subunit; and iroB, encoding a putative glycosyltransferase involved in salmochelin glucosylation (Fig 6A). In Rab35-silenced cells, five of the UPEC genes tested were up-regulated at 24 h post-infection: exbB, entE, fepA, chuA, and feoA (Fig 6B). Intriguingly, the expression of the two genes sitA and iroB that were up-regulated in infected control cells were found to be either down-regulated (sitA) or did not change significantly (iroB) in Rab35 silenced cells at 24 h post-infection. Thus, intracellular UPEC display a specific response to iron limitation resulting from the abrogation of Rab35 expression, further supporting a role for Rab35 in facilitating iron acquisition during intracellular UPEC infection.
Discussion
Numerous studies have described the complexities and consequences of intracellular UPEC infections in mice, humans, and cell culture. In general, studies on intracellular UPEC infections resembling those found in vivo have been limited partly because in vitro models do not seem to recreate the same structures. Several features of in vitro infections of cultured 5637 cells, however, have been validated using mouse models of infection, particularly for early stages of invasion [60,61]. We have used this in vitro system to identify a role for host Rab35 in intracellular UPEC survival.
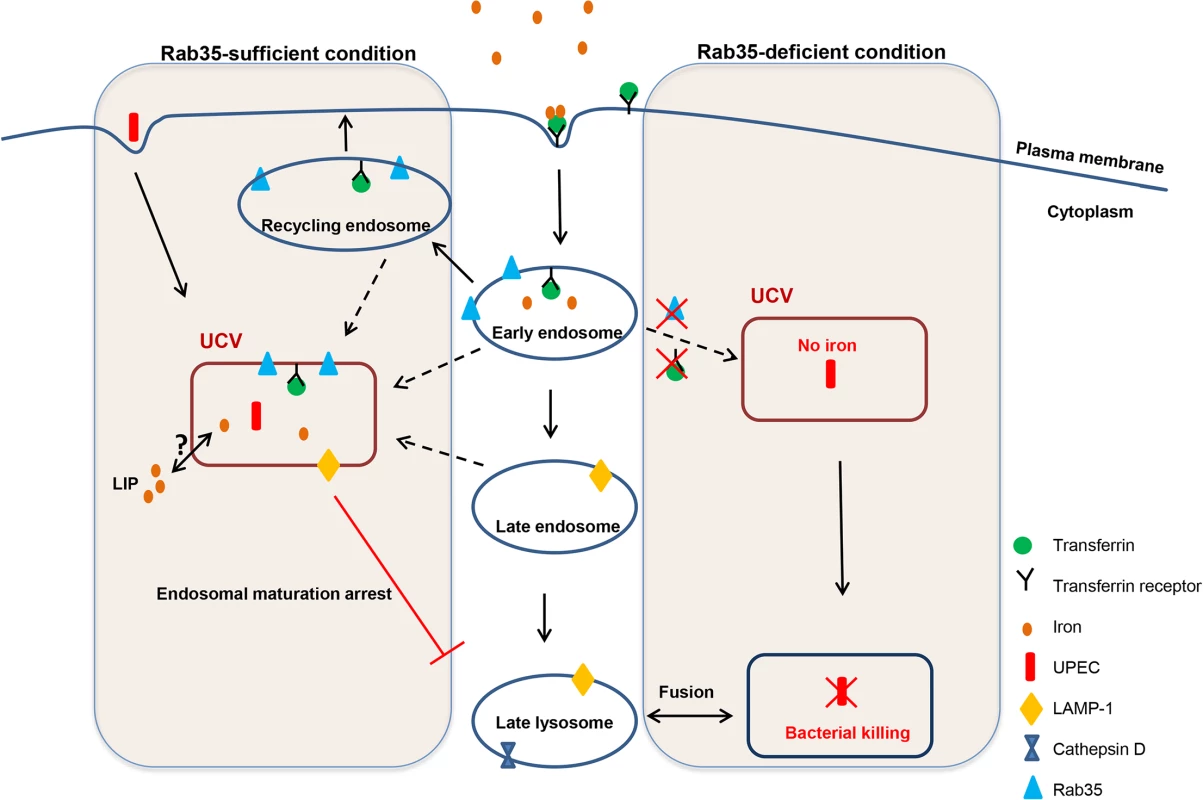
At late time points (> 2 weeks) during experimental mouse infections, UPEC is reported to reside within LAMP1-positive and Cathepsin D-negative compartments (resembling late endosomes) in vivo [11,62]. How UPEC survive within these acidic environments and how the phagosome is modified or phagosome maturation is arrested has yet to be fully determined. In our in vitro cell culture infection system, we also found that UCVs are LAMP1 positive. The UCVs are also positive for Rab35 and TfR; therefore, the UCV compartment shows some characteristics of late endosome and early/recycling endosomes. Accordingly, we found that in experimentally infected mice, UPEC within the uroepithelium also colocalize with Rab35 and the QIR marker LAMP1. The observation that UCV’s were also associated with the autophagy marker LC3 also indicate that UCV may also possess some characteristics of autophagosomes, although more studies are required to characterize this further. We therefore add Rab35 to the list of QIR markers in vivo (LAMP1-positive, LC3-positive, ATG16L1-positive, and Cathepsin D-negative) and for the first time demonstrate that UCVs found during in vitro infection of 5637 cells carry similar vesicular markers, suggesting that intracellular infection of 5637 cells may be useful as a model for QIR development and therefore recurrent UTI.
Survival of UPEC within UCVs carrying these unique vesicular markers could be one of the mechanisms by which the bacteria avoid endosomal maturation/lysosomal fusion. It is also worth noting that Rab35 deficiency resulted in reduced survival of UPEC within bladder cells in culture and this is supported by the observation of an increased percentage of UPEC-containing Cathepsin D-positive compartments (late lysosomal/phagolysosomal) upon Rab35 silencing. The percentage of UCV positive for Rab35 continuously increased throughout the course of infection further highlighting the requirement of this pathway for UPEC persistence within bladder cells. A model for how Rab35 promotes UPEC survival within BEC is given in Fig 7. The compartment occupied by UPEC resembles that of A. phagocytophilum where the bacterium has been shown to preferentially recruit Rab GTPases (including Rab35) associated with the endocytic recycling pathway to its vacuole in order to facilitate intracellular survival [50]. For the intracellular bacteria M. avium [44] and M. tuberculosis [63], bacteria-containing phagosomes are also known to fuse with recycling/early endosomes to acquire iron and to halt the phagosome maturation process. The recruitment of host Rab35 to the UCV observed during UPEC infection hints at the possibility that it is a bacteria-induced phenomenon. Future studies will focus on the exact mechanism by which UCV hijacks Rab35 to its vacuole and the bacterial proteins (if any) that are involved in mediating this process.
As a part of host defense mechanisms against bacterial infection, mucosal surfaces like the urinary bladder mucosa often limit the availability of essential nutrients, such as iron. To counteract this, bacteria including UPEC have developed or acquired apparently redundant systems to acquire free or host protein-bound iron, including siderophores, outer membrane iron compound receptors and TonB-dependent receptors. At least one of the two major siderophores (enterobactin or aerobactin) were required for the efficient host colonization of UPEC [38]. Our study provides a preliminary understanding of the expression status of various UPEC genes associated with iron acquisition during intracellular survival of UPEC within urinary bladder cells. It is interesting to note that only two bacterial genes, sitA and iroB (out of 9 tested), were significantly up-regulated in intracellular UPEC infection in comparison to the extracellular form. Intriguingly, UPEC in Rab35 silenced cells showed a significant down-regulation in the expression of sitA, while the expression of iroB remained unchanged. The significance of this observation is currently unclear; although functional redundancy of the iron acquisition systems in UPEC [64] could be one of the contributing factors. In contrast, the Rab35 silenced cells showed an increase in the mRNA levels of genes such as tonB, fepA (a TonB-dependent active transporter that recognizes extracellular ferric enterobactin and translocates it into the periplasm), entE (enterobactin synthetase), chuA (heme-derived ferric iron receptor), feoA (ferrous iron transport protein), and exbB (component of TonB complex). Increases in the mRNA levels of these genes were also previously reported in a comparison of UPEC in IBCs with UPEC in the distal intestine, further supporting their role in iron acquisition by intracellular UPEC [32].
Until now, only a few reports have examined the requirement of bacterial iron acquisition systems in the extracellular and IBC forms of UPEC [32–35], and there has been only one study to report the up-regulation of host genes associated with iron regulation (TfR and Lcn2) in the urothelial cells associated with IBCs [32]. Our study demonstrates for the first time that UPEC adopts a unique strategy by exploiting the fast endocytic recycling pathway component Rab35 to sequester TfR, in turn will increasing the availability of iron for the intracellular UPEC. In the absence of Rab35 expression, the TfR will not be efficiently recycled to the cell surface, leading to insufficient replenishment of intracellular iron, ultimately hampering the growth of UPEC (by the fusion of UCV with lysosomal compartments). The iron supplementation experiments confirmed the notion that iron is critical for the survival of both extracellular and intracellular UPEC. The intracellular labile iron pool within bladder epithelial cells decreased to ~38% within the first 24 h following UPEC infection, suggesting that UPEC derives majority of its iron from the labile iron pool of the host cell. Two major sources for the labile iron pool are (i) transferrin-bound iron, internalized via transferrin receptors on the cell surface and (ii) ferritin, the intracellular iron storage protein. Silencing of TfR almost completely eliminated the labile iron pool at 24 h, suggesting that this is the major pathway by which the iron pool is maintained within the UPEC-infected cell. In further support of this, silencing of Rab35 also reduced intracellular iron. The fact that Rab35/TfR-silenced cells were unable to take up extracellular iron and support UPEC growth also illustrates the requirement of Rab35 in transferrin receptor-mediated iron uptake during UPEC infection. TfR level is controlled by levels of intracellular iron. When the cellular iron pool drops, iron-regulatory proteins IRP1 and IRP2 are activated, and they bind iron-responsive elements (IRE) to upregulate TfR by a post-transcriptional mRNA stability mechanism [65]. We have indeed observed an increased TfR protein expression during intracellular UPEC infection.
Since our studies have identified that intracellular UPEC utilize iron for survival within BEC, a major question that remains to be resolved is the source of iron utilized by the Rab35/TfR positive intravacuolar bacteria. Iron taken up by cells through the TfR-Tf complex first reaches endosomes from where it is exported into cytoplasm, leading to the formation of the cellular labile iron pool. Thus, intravacuolar pathogens can acquire iron from two possible sources: (a) extracellular (transferrin-bound iron) that is used “directly” within the vacuole; or (b) intracellular (the cytoplasmic labile iron pool). Future studies will determine whether Rab35/TfR+ve UCV utilizes vacuolar iron or the cytoplasmic labile iron pool.
Given the molecular similarities with QIRs, UCVs may also contain UPEC that are not actively replicating. If the acquired iron is not utilized for growth, an interesting question that remains to be resolved is the purpose of acquisition of iron by UPEC at this stage. UPEC at this stage could be comparable to intravacuolar persistent stage of M. tuberculosis where the bacterium attains a seemingly non-replicating (quiescent) or slowly replicating state of growth [66]. Bacteria in a quiescent state display nominal metabolic capacity, maintain membrane potential, and do not undergo obvious morphological differentiation [67,68]. This state allows the bacteria to escape from host-stress responses such as low iron, low oxygen, and low pH, and to maintain a viable bacterial population during the stress period. When conditions are favorable, these bacteria will then resume growth and start multiplying actively. Our studies show that iron is one of the major limiting nutrients that UPEC exploits to survive within the vacuole of BEC. The iron sequestered by the UPEC may be used for maintaining the basal metabolism of the persistent bacteria. In support of this notion, our data shows that iron depletion reduces the survival of UPEC, while supplementation of iron triggers multiplication/ bacterial growth.
On the other hand, it should be noted that too much iron is also detrimental to the bacteria, since iron levels and oxidative stress are directly linked [69]. Iron itself is a mediator of oxidative stress, as it chemically generates hydroxyl radicals that are capable of damaging DNA and proteins [70,71]. To prevent iron-dependent cytotoxicity, bacteria also store excess iron (similar to the host) with ferritin, bacterioferritin, and Dps proteins [72] to be utilized when conditions are favorable. Thus the bacterial iron storage proteins store iron as well as protect the bacteria from iron-mediated oxidative stress conditions. Supporting this, it was reported that ferritins, Dps, or bacterioferritins enhance the growth of iron-deprived pathogens such as E. coli [73], Campylobacter jejuni [74], and Mycobacterium tuberculosis [75], protect them against oxidative stress and support their survival within the host [76]. UPEC in the UCVs could therefore be actively sequestering iron from the host to meet one or more of the following purposes: (1) maintaining its basal metabolism and survival during stressed conditions (low iron, low oxygen, or low pH); (2) sequestering excessive Fe as a stress resistance mechanism as well as for storage; and (3) for active multiplication when conditions are favorable. Further studies are necessary to distinguish among these possibilities. There is therefore a delicate balance between the host response (by sequestering iron to prevent bacterial multiplication) and the bacterial response (by exploiting iron to a level at which it is not toxic to the bacteria).
The identification and characterization of Rab35 as a critical regulator of UPEC intracellular survival may open up new avenues for the therapeutic intervention for the elimination of chronic or persistent UPEC infections.
Materials and Methods
Ethics statement
All animal experiments were performed in accordance with protocols that were reviewed and approved by the A*STAR Biological Resource Center Institutional Animal Care and Use Committee (protocol #130853). These protocols were approved to be in accordance with the prevailing Singapore National Advisory Committee for Laboratory Animal Research (NACLAR) guidelines.
Bacterial strain and growth conditions
The clinical UTI isolate E. coli CI5 strain [77,78] was kindly provided by Prof. Soman N Abraham (Duke and Duke-NUS) and was used throughout the in vitro study. The prototypic cystitis strain UTI89 [52] transformed with plasmid pANT4 expressing GFP [79] (referred to as strain SLC-295) was used for in vivo infection experiments in mice. The K12/mCherry strain (SLC-720) is E. coli K12 substr. MG1655 transformed with an mCherry expression vector (pSLC-229). pSLC-229 was generated by replacing the GFP gene in plasmid pANT4 [79] with mCherry amplified from pXJ40_2 (a kind gift from Sohail Ahmed). Briefly, mCherry was amplified using PCR (using the primers 5’-GACTCTGAATTCATGGTGAGCAAGGGCGAGGA and 5’-GATCCTGCATGCTTACTTGTACAGCTCGTCCATGC). The expected 700bp product was digested with EcoRI and SphI and cloned into the same sites of pANT4 to yield pSLC-229; the correct predicted sequence was verified by sequencing the ligation junctions and the entire mCherry gene. pSLC-229 was then transformed into MG1655 to yield SLC-720.
Cell line and growth conditions
Human Bladder epithelial cell line 5637 (ATCC HTB-9) was maintained at 37°C and 5% CO2 in RPMI 1640 media (Gibco) supplemented with 10% Fetal Calf Serum (Gibco).
Plasmid constructs
All the Rab GTPase plasmids used in this study including the EGFP tagged wild type Rab35, and mutants (Rab35 Q67l and Rab35 S22N) were as described before [50]. The other GFP/RFP tagged Rab GTPases used for the microscopy-based study were: Rab1, Rab2A, Rab3A, Rab4A, Rab5, Rab6A, Rab8A, Rab10, Rab11A, Rab14, Rab18, Rab22A, Rab27A, and Rab33A. RFP-tagged LAMP1 and Cathepsin D plasmids were obtained from (Addgene). Transferrin receptor plasmid was a kind gift from Dr. Kuni Matsumoto.
Antibodies
Human Rab35 (9690) and Cathepsin D antibody (2284) were obtained from Cell Signaling Technology. Transferrin Receptor (sc-32271)) and LAMP1 (sc-20011) antibodies was obtained from Santa Cruz. Anti GFP antibody (ab540) was obtained from Abcam. Mouse Rab35 (NB20042) antibody was purchased from Novus Biologicals. Rabbit polyclonal antibody to LC3 (ab58610) was purchased from Abcam. Mouse LAMP1 antibody was obtained from Abcam (ab25245).
Intracellular bacterial survival assay
BEC 5637 cell monolayers grown in 24-well tissue culture plates were infected with CI5 UPEC at a multiplicity of infection of 500 bacteria per host cell. For generation of heat-killed UPEC, the bacteria were suspended in PBS and heated at 100°C for 30 min. The non-viability of bacteria was confirmed by plating the bacteria on LB agar plates. To facilitate and synchronize bacterial contact with the host cells, plates were centrifuged at 600 × g for 5 min. After 2 h incubation at 37°C, cells were washed three times with RPMI to remove nonadherent bacteria. Monolayers were then incubated for 1 h with complete RPMI medium plus 100 μg/ml of gentamicin (Gibco) to kill extracellular bacteria. Subsequently cells were washed and further incubated in fresh medium containing gentamicin (10 μg/ml) for the entire duration of the experiment. At designated times monolayers were washed with PBS and subsequently lysed in PBS plus 0.1% Triton X-100, and bacteria present within the lysates were enumerated by plating serial dilutions on LB agar plates. To check for invasion, cells were washed with PBS directly after 1h of 100 μg/ml of gentamicin treatment and lysed in PBS-Triton–X 100.
UPEC exocytosis assay
Bacteria exocytosis assay was performed as described before [19]. Briefly, cells were infected as described above. After Gentamycin (100μg/ml) treatment, cells were washed twice and left in fresh culture medium containing 100mM methyl-α-D-mannopyranoside (to prevent reattachment and entry of bacteria into BEC). At indicated time points, the culture medium was collected and plated for CFU counts.
siRNA, overexpression and transfection studies
The small interfering RNA targeting human Rab35 (5’-GCUCACGAAGAACAGUAAA-3’) [80] was purchased from Sigma. Two siRNAs targeting human Transferrin Receptor (5’-GCACAGCUCUCCUAUUGAA-3’ and 5’-GCUGAAAGCUUAAAUGCAA-3’ [81] were purchased from SABio. The TfR siRNAs were pooled and used for experiments. The siRNAs targeting the 3’UTR of Rab35 were (5’-GCGAGGGUGUGCUUGCAAAUU-3’ and 5’-GCAAAUUCAAGCAAUAAGAUU-3’) and siRNAs targeting the 3’UTR of TfR were (5’-CAGAAACCAGTTATGTGAATGATCT-3’, 5’- GGTTCAACTGTTGATTGCAGGAATA-3’, 5’-CAGACTCAGTTTGTCAGACTTTAAA-3’, and 5’- TCGGAGACAGTGATCTCCATATGTT-3’). The siRNAs were pooled and used for the experiments. The non-targeting siRNA (si-NT) from SABio was used as a negative control. Cells were transfected with siRNA using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions and were used for further experiments 48 h post transfection.
For transfection experiments, BEC-5637 cells were plated in a 24 well plate at a density of 1 x 105 cells/ well. Next day cells were transfected with 1 μg DNA using Polyethylenimine (PEI) (1:3 ratio DNA: PEI). Cells were washed 6 h after transfection and incubated in fresh culture medium for 20 h, before proceeding for further experiments.
For siRNA rescue experiments, BEC cells were transfected with 3’ UTR siRNA against Rab35 or TfR using Lipofectamine 3000 (Invitrogen) according to manufacturer’s instructions. 24 h later cells were washed and transfected with 1μg control plasmid (pEGFPC1 for Rab35 plasmid and pCMV-Flag for TfR plasmid) or Rab35/TfR expression plasmids. 24 h later cells were washed and left in serum free media for 4 h. Cells were subsequently infected with UPEC (MOI 500) under serum free conditions. The cells were finally left in 10 μg/ml Gentamycin—RPMI with 3% serum. 24 h later the intracellular bacterial load was determined as described earlier.
Confocal microscopy
Bacterial colocalization with various markers was performed using Confocal Microscopy. Briefly, cells were grown on coverslips and transfected with plasmids for various fluorescently tagged proteins as described above. After 20 h cells were washed and infected with UPEC. At different time points, cells were fixed with 4% paraformaldehyde and stained for nuclei with DAPI. The coverslips were mounted using Prolong Gold Antifade (Molecular Probes) and examined using Zeiss confocal Microscope with appropriate filter sets. For cell surface colocalization of Rab35 and Transferrin receptor, cells were transfected with GFP Rab35 expressing plasmid. After infection the cells were fixed and cell surface Transferrin Receptor was stained with transferrin receptor antibody. The samples were analyzed by confocal microscopy as mentioned above. For the colocalization of bacteria with lysosomal compartment, infected cells were treated with LysoTracker Red (Life Technologies) for 2 h at 37°C. Cells were then fixed and analyzed by confocal microscopy. All the images were acquired on LSM 710 Carl Zeiss microscope using Plan-Apochromat 63x/1.40 oil DIC objective. Images were acquired at 16 bit depth at a resolution of 1024x1024 pixels. Z sections of images were acquired at 63X magnification and were analyzed using Zen 2010 software and were processed using Adobe photoshop7 software. For images with XZ/ YZ projections, serial Z sections of the Z stack were taken at 0.5μm thickness.
Quantitative colocalization analysis
The selected UCVs, containing detectable Rab35 and membrane marker were used for pixel quantification and statistical analysis. Quantification of the colocalization of Rab35 with LAMP1 or TfR was done using Zen 2010 software (Zeiss), which calculates the Mander’s overlap coefficient (R) [82]. The values for the overlap coefficient range from 0 to 1. An Overlap Coefficient with a value of 1 indicates high colocalization and 0 represents low colocalization. The overlap coefficients were calculated using data from at least 3 independent experiments (n ≥ 50).
Transferrin uptake and transferrin receptor recycling
Cells grown on coverslips were analyzed for transferrin uptake at 24 h post infection. Cells were washed with RPMI and were incubated in serum free media for 1h to remove any traces of Transferrin and then were exposed to 50 μg/ml tansferrin conjugated to Alexa Flour 548 (invitrogen) at 37°C for 20’. Internalization was stopped by chilling the cells on ice. External transferrin was removed by washing with PBS, which was followed with washes with PBS (pH 5) to remove bound transferrin. Cells were finally washed with PBS (pH 7) and analyzed for transferrin uptake by confocal microscopy as described above. Fluorescence intensity was analyzed by Zen software.
For Transferrin recycling cells were first allowed to uptake Transferrin as mentioned above. Following final wash with PBS (pH 7), cells were incubated in RPMI in presence of 50μg/ml holotransferrin. Cells were fixed with 4% paraformaldehyde after 5, 15 and 30 minutes. Intracellular Transferrin was analyzed by confocal microscopy as described above. Transferrin receptor recycling/ Transferrin release was measured as (Intracellular Transferrin at 0h - Intracellular Transferrin at that time point).
RNA isolation from host cells and bacteria
At designated time points, BEC-5637 cells were lysed in RLT buffer (Qiagen) and RNA was isolated using RNeasy kit (Qiagen). For isolating RNA from bacteria from infected BEC-5637, cells were seeded and infected in 6 well plates in triplicates. At designated time points, cells were lysed by passage through 20G needle 6–7 times. The lysate was spun at 1000xg for 5 minutes to remove the cell debris. The supernatant was then spun at 10,000xg for 20 minutes to pellet down the bacteria. The bacterial pellet was subsequently lysed in RLT buffer and processed for RNA isolation as described above. cDNA synthesis was carried out by iScript cDNA synthesis kit (Bio-Rad) as per manufacturer’s instructions.
PCR amplification of Rab35 and bacterial genes
Rab35 and the bacterial genes were amplified from cDNA by q PCR. Primer sequences for iron-related genes of UPEC were as described before [32]. Sequences for all other primers used are listed in the S1 Table.
Reactions were performed in Roche light cycler 480 Real-time PCR system under following conditions—94°C/2 minutes for 1 cycle; 94°C/30 seconds, 55°C/30seconds, 72°C/60 seconds for 40 cycles; 72°C/7 minutes for 1 cycle. Results were analyzed using delta-delta-Ct algorithm.
Protein expression analysis
For protein expression analysis cells were lysed at 4°C for 15 minutes in RIPA buffer—(20 mM Tris-HCl (pH 7.5),150 mM NaCl, 1 mM Na2EDTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate) containing protease and phosphatase inhibitor cocktail (Roche). The supernatant of the lysate spun at 12000 g for 10’ was estimated for protein concentration using Lowry assay. Equal protein concentrations were run on 12% SDS gel and analyzed for protein expression by Western blotting.
Iron uptake assay
Labile iron pool was calculated by using standard Calcein-AM protocol [83]. Briefly cells were washed with PBS-BSA (1mg/ml)-Hepes (20mM) and then treated with 0.15uM Calcein (life technologies) in PBS-BSA-Hepes for 10’ at 37°C. Cells were washed thrice with PBS-BSA (1mg/ml)-Hepes (20mM) and resuspended in same buffer. Fluorescence was measured in iTecan Fluorimeter at Excitation- 488nm, Emission- 520nm for 10 minutes to establish stable baseline signal (F1). After this, 1000uM Dipyridyl (Sigma) in PBS-BSA (1mg/ml)-Hepes (20mM) was added to cells. Fluorescence was measured again until a stable base line signal was established (F2). Labile Iron Pool was calculated as F2-F1.
Holotransferrin/deferroxamine treatment of BEC-5637
siNT, siRab35 and siTfR cells were infected with UPEC under serum free conditions. After gentamycin (100μg/ml) treatment, cells were left in 10μg/ml gentamycin containing RPMI alone or along with Holotransferrin (50μg/ml) or deferroxamine (100μM). The bacterial load was enumerated at 24 h post infection.
Iron treatment of UPEC
UPEC cultures were diluted to OD600 of 0.01 in 3 ml RPMI medium with vehicle control, 10μM Ferric chloride (Sigma) or 200μM deferoxamine. Bacteria were grown at 37°C with shaking. OD600 was recorded at serial intervals.
In vivo mouse experiments
A GFP-expressing derivative of UTI89 (SLC-295) was grown at 37°C in 10mL of LB media without shaking for 24h, followed by a 1:1000 dilution and a second 24h of growth at 37°C in LB without shaking. Bacteria were harvested by centrifugation and resuspended in PBS to an OD600 of 0.5 (1–2×107 CFU/50μL) and used directly as the inoculum. 7–8 week old female C57BL/6 mice (In Vivos, Singapore) were transurethrally inoculated with 50μL of this inoculum or sterile PBS (negative control). At 6h (5 mice), 24 h (5 mice) or 2 weeks (5 mice) post-infection, mice were sacrificed and their bladders aseptically harvested. Bladders were then hemisected and incubated in 10% formalin at 4°C overnight.
Histologic analysis and immunofluorescence assay
The processing of the bladder samples was carried out at the A*STAR/IMCB histology facility (A*STAR, Singapore). Briefly, the bladder tissues were embedded in 2% agar for paraffin processing. For IFA, 4- to 5μm serial sections were cut longitudinally, deparaffinized in xylene (twice for 5 min at room temperature), rehydrated in 100% ethanol (twice for 2 min at room temperature), 95% ethanol (for 1minute), and 80% ethanol (for 1 minute). Antigen retrieval was performed by boiling slides for 30 min in 1mM EDTA buffer, pH 8. Slides were blocked in 1% FBS 0.4% TritonX-100 for 1 h at room temperature, and subsequently incubated overnight at 4°C with the following primary antibodies- goat anti GFP antibody (Abcam 1:500 dilution in blocking buffer) and rabbit anti Rab35 antibody (Novus biological, 1in 50 dilution in blocking buffer). For LAMP1 triple staining, the sections were additionally stained with rat anti LAMP1 antibody (abcam 1:200 blocking buffer). Sections were washed thrice with blocking buffer and were subsequently incubated with Alexa Fluor 488 (anti-rabbit), Alexa 594 (anti-goat) and Alexa 350 (anti-rat) conjugated secondary antibodies (1:500; Molecular Probes). The sections were then stained with DAPI and were analyzed by confocal microscopy.
Statistics
P values were determined by unpaired two-tailed Student’s t test. P values less than 0.05 were considered statistically significant.
Accession numbers
RAB35 (Species: Homo sapiens, Gene ID: 11021)
Transferrin receptor (Species: Homo sapiens, Gene ID: 7037)
Supporting Information
Zdroje
1. Foxman B (2010) The epidemiology of urinary tract infection. Nat Rev Urol 7: 653–660. doi: 10.1038/nrurol.2010.190 21139641
2. Litwin MS, Saigal CS, Yano EM, Avila C, Geschwind SA, et al. (2005) Urologic diseases in America Project: analytical methods and principal findings. J Urol 173: 933–937. 15711342
3. Ronald A (2003) The etiology of urinary tract infection: traditional and emerging pathogens. Dis Mon 49: 71–82. 12601338
4. Brauner A, Jacobson SH, Kuhn I (1992) Urinary Escherichia coli causing recurrent infections—a prospective follow-up of biochemical phenotypes. Clin Nephrol 38: 318–323. 1468162
5. Russo TA, Stapleton A, Wenderoth S, Hooton TM, Stamm WE (1995) Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis 172: 440–445. 7622887
6. Hung CS, Dodson KW, Hultgren SJ (2009) A murine model of urinary tract infection. Nat Protoc 4: 1230–1243. doi: 10.1038/nprot.2009.116 19644462
7. Sivick KE, Mobley HL (2010) Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect Immun 78: 568–585. doi: 10.1128/IAI.01000-09 19917708
8. Ulett GC, Totsika M, Schaale K, Carey AJ, Sweet MJ, et al. (2013) Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Curr Opin Microbiol 16: 100–107. doi: 10.1016/j.mib.2013.01.005 23403118
9. Schilling JD, Lorenz RG, Hultgren SJ (2002) Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun 70: 7042–7049. 12438384
10. Hunstad DA, Justice SS (2010) Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol 64: 203–221. doi: 10.1146/annurev.micro.112408.134258 20825346
11. Mysorekar IU, Hultgren SJ (2006) Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A 103: 14170–14175. 16968784
12. Robino L, Scavone P, Araujo L, Algorta G, Zunino P, et al. (2014) Intracellular Bacteria in the Pathogenesis of Escherichia coli Urinary Tract Infection in Children. Clin Infect Dis.
13. Robino L, Scavone P, Araujo L, Algorta G, Zunino P, et al. (2013) Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathog Dis 68: 78–81. doi: 10.1111/2049-632X.12047 23733378
14. Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ (2007) Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 4: e329. 18092884
15. Khasriya R, Sathiananthamoorthy S, Ismail S, Kelsey M, Wilson M, et al. (2013) Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. J Clin Microbiol 51: 2054–2062. doi: 10.1128/JCM.03314-12 23596238
16. Zhou G, Mo WJ, Sebbel P, Min G, Neubert TA, et al. (2001) Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci 114: 4095–4103. 11739641
17. Eto DS, Jones TA, Sundsbak JL, Mulvey MA (2007) Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog 3: e100. 17630833
18. Hung CS, Bouckaert J, Hung D, Pinkner J, Widberg C, et al. (2002) Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol Microbiol 44: 903–915. 12010488
19. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, et al. (2007) Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med 13: 625–630. 17417648
20. Dhakal BK, Kulesus RR, Mulvey MA (2008) Mechanisms and consequences of bladder cell invasion by uropathogenic Escherichia coli. Eur J Clin Invest 38 Suppl 2: 2–11. doi: 10.1111/j.1365-2362.2008.01986.x 18616559
21. Duncan MJ, Li G, Shin JS, Carson JL, Abraham SN (2004) Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem 279: 18944–18951. 14976212
22. Eto DS, Gordon HB, Dhakal BK, Jones TA, Mulvey MA (2008) Clathrin, AP-2, and the NPXY-binding subset of alternate endocytic adaptors facilitate FimH-mediated bacterial invasion of host cells. Cell Microbiol 10: 2553–2567. doi: 10.1111/j.1462-5822.2008.01229.x 18754852
23. Martinez JJ, Hultgren SJ (2002) Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli. Cell Microbiol 4: 19–28. 11856170
24. Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ (2000) Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J 19: 2803–2812. 10856226
25. Song J, Bishop BL, Li G, Grady R, Stapleton A, et al. (2009) TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci U S A 106: 14966–14971. doi: 10.1073/pnas.0900527106 19706440
26. Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, et al. (2003) Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301: 105–107. 12843396
27. Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, et al. (2004) Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A 101: 1333–1338. 14739341
28. Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, et al. (1998) Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282: 1494–1497. 9822381
29. Wang C, Mendonsa GR, Symington JW, Zhang Q, Cadwell K, et al. (2012) Atg16L1 deficiency confers protection from uropathogenic Escherichia coli infection in vivo. Proc Natl Acad Sci U S A 109: 11008–11013. doi: 10.1073/pnas.1203952109 22715292
30. Skaar EP (2010) The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6: e1000949. doi: 10.1371/journal.ppat.1000949 20711357
31. Henderson JP, Crowley JR, Pinkner JS, Walker JN, Tsukayama P, et al. (2009) Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog 5: e1000305. doi: 10.1371/journal.ppat.1000305 19229321
32. Reigstad CS, Hultgren SJ, Gordon JI (2007) Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem 282: 21259–21267. 17504765
33. Snyder JA, Haugen BJ, Buckles EL, Lockatell CV, Johnson DE, et al. (2004) Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect Immun 72: 6373–6381. 15501767
34. Bielecki P, Muthukumarasamy U, Eckweiler D, Bielecka A, Pohl S, et al. (2014) In vivo mRNA profiling of uropathogenic Escherichia coli from diverse phylogroups reveals common and group-specific gene expression profiles. MBio 5: e01075–01014. doi: 10.1128/mBio.01075-14 25096872
35. Hagan EC, Lloyd AL, Rasko DA, Faerber GJ, Mobley HL (2010) Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog 6: e1001187. doi: 10.1371/journal.ppat.1001187 21085611
36. Alteri CJ, Hagan EC, Sivick KE, Smith SN, Mobley HL (2009) Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog 5: e1000586. doi: 10.1371/journal.ppat.1000586 19806177
37. Hagan EC, Mobley HL (2007) Uropathogenic Escherichia coli outer membrane antigens expressed during urinary tract infection. Infect Immun 75: 3941–3949. 17517861
38. Torres AG, Redford P, Welch RA, Payne SM (2001) TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect Immun 69: 6179–6185. 11553558
39. Sivick KE, Mobley HL (2009) An "omics" approach to uropathogenic Escherichia coli vaccinology. Trends Microbiol 17: 431–432. doi: 10.1016/j.tim.2009.07.003 19758805
40. Zhao N, Enns CA (2012) Iron transport machinery of human cells: players and their interactions. Curr Top Membr 69: 67–93. doi: 10.1016/B978-0-12-394390-3.00003-3 23046647
41. Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525. doi: 10.1038/nrm2728 19603039
42. Mizuno-Yamasaki E, Rivera-Molina F, Novick P (2012) GTPase networks in membrane traffic. Annu Rev Biochem 81: 637–659. doi: 10.1146/annurev-biochem-052810-093700 22463690
43. Brumell JH, Scidmore MA (2007) Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol Mol Biol Rev 71: 636–652. 18063721
44. Halaas O, Steigedal M, Haug M, Awuh JA, Ryan L, et al. (2010) Intracellular Mycobacterium avium intersect transferrin in the Rab11(+) recycling endocytic pathway and avoid lipocalin 2 trafficking to the lysosomal pathway. J Infect Dis 201: 783–792. doi: 10.1086/650493 20121435
45. Chua CE, Lim YS, Tang BL (2010) Rab35—a vesicular traffic-regulating small GTPase with actin modulating roles. FEBS Lett 584: 1–6. doi: 10.1016/j.febslet.2009.11.051 19931531
46. Patino-Lopez G, Dong X, Ben-Aissa K, Bernot KM, Itoh T, et al. (2008) Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem 283: 18323–18330. doi: 10.1074/jbc.M800056200 18450757
47. Grant BD, Donaldson JG (2009) Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10: 597–608. doi: 10.1038/nrm2755 19696797
48. Mayle KM, Le AM, Kamei DT (2012) The intracellular trafficking pathway of transferrin. Biochim Biophys Acta 1820: 264–281. doi: 10.1016/j.bbagen.2011.09.009 21968002
49. Ponka P, Lok CN (1999) The transferrin receptor: role in health and disease. Int J Biochem Cell Biol 31: 1111–1137. 10582342
50. Huang B, Hubber A, McDonough JA, Roy CR, Scidmore MA, et al. (2010) The Anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes. Cell Microbiol 12: 1292–1307. doi: 10.1111/j.1462-5822.2010.01468.x 20345488
51. Chesneau L, Dambournet D, Machicoane M, Kouranti I, Fukuda M, et al. (2012) An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr Biol 22: 147–153. doi: 10.1016/j.cub.2011.11.058 22226746
52. Mulvey MA, Schilling JD, Hultgren SJ (2001) Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 69: 4572–4579. 11402001
53. Al-Younes HM, Rudel T, Meyer TF (1999) Characterization and intracellular trafficking pattern of vacuoles containing Chlamydia pneumoniae in human epithelial cells. Cell Microbiol 1: 237–247. 11207556
54. Delbruck R, Desel C, von Figura K, Hille-Rehfeld A (1994) Proteolytic processing of cathepsin D in prelysosomal organelles. Eur J Cell Biol 64: 7–14. 7957314
55. Rijnboutt S, Stoorvogel W, Geuze HJ, Strous GJ (1992) Identification of subcellular compartments involved in biosynthetic processing of cathepsin D. J Biol Chem 267: 15665–15672. 1322403
56. Hagan EC, Mobley HL (2009) Haem acquisition is facilitated by a novel receptor Hma and required by uropathogenic Escherichia coli for kidney infection. Mol Microbiol 71: 79–91. doi: 10.1111/j.1365-2958.2008.06509.x 19019144
57. Berry RE, Klumpp DJ, Schaeffer AJ (2009) Urothelial cultures support intracellular bacterial community formation by uropathogenic Escherichia coli. Infect Immun 77: 2762–2772. doi: 10.1128/IAI.00323-09 19451249
58. Linford A, Yoshimura S, Nunes Bastos R, Langemeyer L, Gerondopoulos A, et al. (2012) Rab14 and its exchange factor FAM116 link endocytic recycling and adherens junction stability in migrating cells. Dev Cell 22: 952–966. doi: 10.1016/j.devcel.2012.04.010 22595670
59. Wang J, Pantopoulos K (2011) Regulation of cellular iron metabolism. Biochem J 434: 365–381. doi: 10.1042/BJ20101825 21348856
60. Song J, Bishop BL, Li G, Duncan MJ, Abraham SN (2007) TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe 1: 287–298. 17710226
61. Thumbikat P, Berry RE, Zhou G, Billips BK, Yaggie RE, et al. (2009) Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog 5: e1000415. doi: 10.1371/journal.ppat.1000415 19412341
62. Eto DS, Sundsbak JL, Mulvey MA (2006) Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell Microbiol 8: 704–717. 16548895
63. Kyei GB, Vergne I, Chua J, Roberts E, Harris J, et al. (2006) Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. EMBO J 25: 5250–5259. 17082769
64. Garcia EC, Brumbaugh AR, Mobley HL (2011) Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect Immun 79: 1225–1235. doi: 10.1128/IAI.01222-10 21220482
65. Pantopoulos K (2004) Iron metabolism and the IRE/IRP regulatory system: an update. Ann N Y Acad Sci 1012: 1–13. 15105251
66. Rittershaus ES, Baek SH, Sassetti CM (2013) The normalcy of dormancy: common themes in microbial quiescence. Cell Host Microbe 13: 643–651. doi: 10.1016/j.chom.2013.05.012 23768489
67. Gengenbacher M, Rao SP, Pethe K, Dick T (2010) Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology 156: 81–87. doi: 10.1099/mic.0.033084-0 19797356
68. Rao SP, Alonso S, Rand L, Dick T, Pethe K (2008) The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 105: 11945–11950. doi: 10.1073/pnas.0711697105 18697942
69. Touati D (2000) Iron and oxidative stress in bacteria. Arch Biochem Biophys 373: 1–6. 10620317
70. Davies MJ (2005) The oxidative environment and protein damage. Biochim Biophys Acta 1703: 93–109. 15680218
71. Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57: 395–418. 14527285
72. Andrews SC, Robinson AK, Rodriguez-Quinones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27: 215–237. 12829269
73. Zhao G, Ceci P, Ilari A, Giangiacomo L, Laue TM, et al. (2002) Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli. J Biol Chem 277: 27689–27696. 12016214
74. Wai SN, Nakayama K, Umene K, Moriya T, Amako K (1996) Construction of a ferritin-deficient mutant of Campylobacter jejuni: contribution of ferritin to iron storage and protection against oxidative stress. Mol Microbiol 20: 1127–1134. 8809765
75. Pandey R, Rodriguez GM (2012) A ferritin mutant of Mycobacterium tuberculosis is highly susceptible to killing by antibiotics and is unable to establish a chronic infection in mice. Infect Immun 80: 3650–3659. doi: 10.1128/IAI.00229-12 22802345
76. Cassat JE, Skaar EP (2013) Iron in infection and immunity. Cell Host Microbe 13: 509–519. doi: 10.1016/j.chom.2013.04.010 23684303
77. Abraham SN, Babu JP, Giampapa CS, Hasty DL, Simpson WA, et al. (1985) Protection against Escherichia coli-induced urinary tract infections with hybridoma antibodies directed against type 1 fimbriae or complementary D-mannose receptors. Infect Immun 48: 625–628. 2860067
78. Mehershahi KS, Abraham SN, Chen SL (2015) Complete Genome Sequence of Uropathogenic Escherichia coli Strain CI5. Genome Announc 3.
79. Lee AK, Falkow S (1998) Constitutive and inducible green fluorescent protein expression in Bartonella henselae. Infect Immun 66: 3964–3967. 9673287
80. Kouranti I, Sachse M, Arouche N, Goud B, Echard A (2006) Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol 16: 1719–1725. 16950109
81. Messaritou G, East L, Roghi C, Isacke CM, Yarwood H (2009) Membrane type-1 matrix metalloproteinase activity is regulated by the endocytic collagen receptor Endo180. J Cell Sci 122: 4042–4048. doi: 10.1242/jcs.044305 19861500
82. Manders EMM, Verbeek FJ, Aten JA (1993) Measurement of Colocalization of Objects in Dual-Color Confocal Images. Journal of Microscopy-Oxford 169: 375–382.
83. Jones DT, Trowbridge IS, Harris AL (2006) Effects of transferrin receptor blockade on cancer cell proliferation and hypoxia-inducible factor function and their differential regulation by ascorbate. Cancer Res 66: 2749–2756. 16510596
Štítky
Hygiena a epidemiologie Infekční lékařství LaboratořČlánek vyšel v časopise
PLOS Pathogens
2015 Číslo 8
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Jak souvisí postcovidový syndrom s poškozením mozku?
Nejčtenější v tomto čísle
- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
- Hepcidin and Host Defense against Infectious Diseases
