-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Riding the R Train into the Cell
article has not abstract
Published in the journal: . PLoS Pathog 11(8): e32767. doi:10.1371/journal.ppat.1005036
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005036Summary
article has not abstract
A wise traveler arriving in an unfamiliar city would be ill-advised to blaze a new route from the outskirts, but rather should rely on existing, well-lit transit systems to journey to the city center while avoiding the brigands lurking in the shadows. So too, a wise virus entering a cell will exploit the well-established trafficking routes that maintain cellular life.
Incoming virus particles face enormous challenges to initiate infection. After the virion binds and enters the cell, the capsid proteins must transport the viral genome to the site where it is released, expressed, and replicated. This is often a difficult and hazardous journey with membranes to traverse and cellular defenses to avoid. A non-enveloped DNA virus must navigate from the cell surface into the nucleus, where viral gene expression and genome replication occur, while crossing cell membranes, undergoing disassembly, and avoiding cellular innate immune defenses en route [1].
Among the cellular pathways that transport material from the periphery deep into the cell, the vesicular retrograde transport system is a likely candidate for conveying incoming DNA viruses. The Golgi apparatus is a major sorting hub of the cell where newly synthesized proteins are modified and distributed to the cell surface or other locations in the cell, such as the endosome. The transport of proteins from the Golgi apparatus often depends on carrier proteins that ferry cellular cargos to their destinations, a process that depletes the carriers from the Golgi. These carriers are retrieved back to the Golgi in a retrograde fashion for reuse after they have delivered their cargo [2]. Retromer, a conserved cytoplasmic protein complex, plays a central role in retrograde endosome-to-Golgi transport (as well as in endosome-to-plasma membrane transport) [2–4]. Retromer is a complex of three proteins (VPS29, VPS35, and VPS26) that recognizes sorting signals in the cytoplasmic domains of transmembrane proteins at the endosomal membrane. Retromer also associates with sorting nexins—peripheral membrane proteins that bind the endosomal membrane and help shape it into transport vesicles containing the cargo—which bud off of the endosomal membrane. These transport vesicles then travel in a microtubule-dependent fashion to their target organelle, where they fuse with the organelle membrane to deliver the cargo. Thus, cells have an elaborate vesicular system to transfer proteins from the endosome to the Golgi. Certain plant and bacterial toxins utilize this retrograde pathway to enter cells [1,5], so it seems logical that some viruses would also exploit this pathway to make the same journey.
The first evidence implicating retromer in virus entry came from studies of human papillomavirus type 16 (HPV16), a non-enveloped DNA virus. HPV are responsible for approximately 5% of human cancer, but little is known about their trafficking during cell entry. Because the identification of cellular proteins involved in HPV entry would elucidate the trafficking pathway involved in this process and identify possible targets for novel anti-viral approaches, we conducted a genome-wide siRNA screen for cellular factors required for the entry of HPV16 pseudovirus into cultured human epithelial cells [6]. This screen and the follow-up validation experiments identified numerous cellular proteins as being required for HPV16 entry, including several components of the retrograde transport pathway and all three retromer subunits. One explanation for these findings is that HPV16 entry requires one or more cellular proteins that must undergo retromer-mediated retrograde transport. Indeed, retrograde transport systems have been implicated in infection by other viruses. The coatomer complex COPI, which plays a role in trafficking between the Golgi and the endoplasmic reticulum (ER), has been identified in RNA interference screens for cellular factors required for infection by several RNA viruses. However, COPI does not necessarily directly mediate intracellular trafficking of the incoming virus particle but rather may play an indirect role in virus infection. For example, it has been proposed that COPI components are required to establish membrane-associated replication compartments for poliovirus and hepatitis C virus [7,8].
Alternatively, incoming HPV16 itself, or infectious components thereof, may undergo retrograde transport. At first glance, this possibility seemed unlikely because HPV lacks transmembrane proteins, which were the only known retromer cargos [3,4]. In addition, the prevailing view in the HPV field was that incoming virus was transported out of the endosome directly into the cytoplasm [9]. However, several recent pieces of evidence suggest that HPV16 is transported in a vesicular fashion by the retrograde pathway during entry. Localization studies revealed viral capsid proteins and viral DNA in membrane-bound organelles prior to entry of viral DNA and the minor capsid protein L2 into the nucleus [6,10]. At early times of infection, viral components are present in the early endosome, and, at later times, in the trans-Golgi network, the Golgi apparatus, and the ER (Fig 1) [11,12]. Virus at these sites appears to be undergoing infectious entry because genetic knockdown of retrograde transport factors (such as Rab proteins) and pharmacologic inhibition of retrograde transport block the delivery of viral components to the distal sites in the pathway and prevent viral gene expression [6,10,13]. Similarly, knockdown or inhibition of the cellular protease γ-secretase blocks infection after the L2 protein and the viral DNA exit the endosome (but prior to their arrival in the Golgi), and some mutations in the L2 protein trap the viral genome in the Golgi and abort infection [12,14]. Finally, TRAPPC8 and SNX17, cellular proteins involved in vesicular trafficking, form stable complexes with HPV L2 and are required for proper intracellular trafficking of HPV16 during entry [15,16].
Fig. 1. Transit pathways used by small DNA viruses. 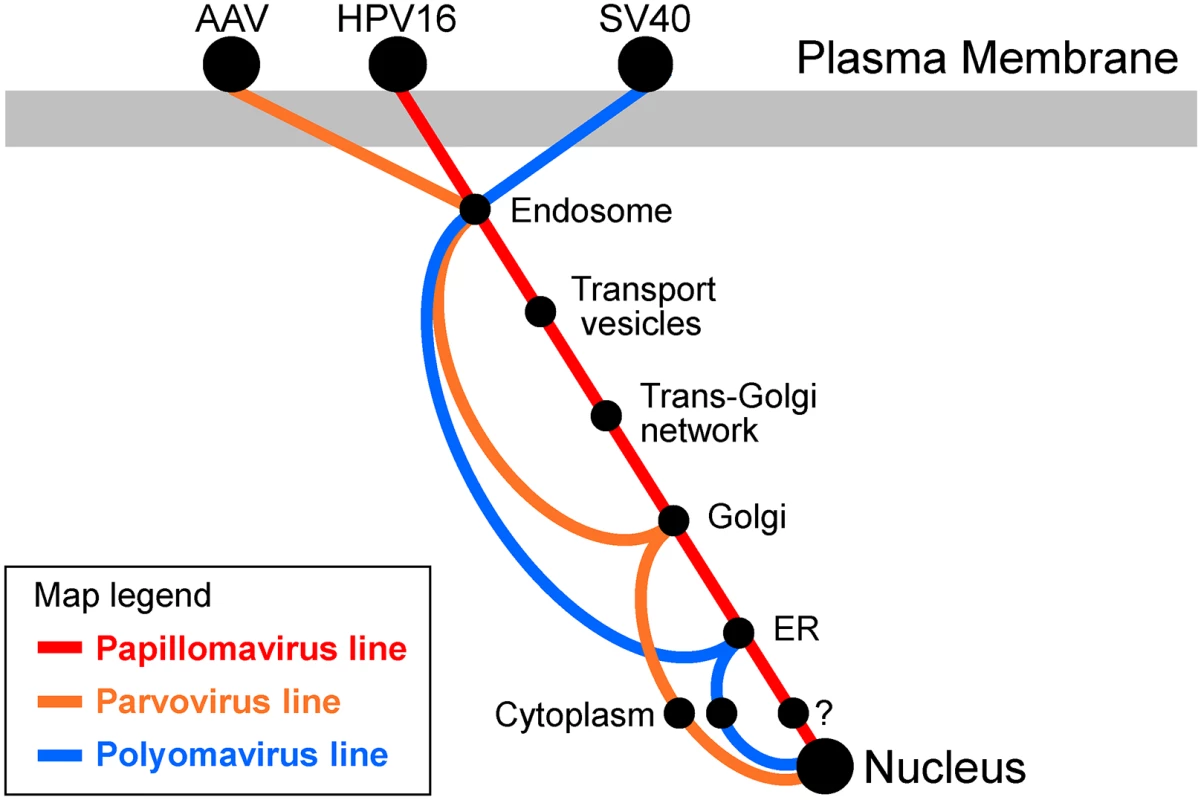
The map shows our current understanding of the intracellular trafficking pathways used during entry by three families of small DNA viruses: human papillomaviruses (red), parvoviruses (orange), and polyomaviruses (blue). A prototype member of each virus family is shown at the top, where it binds to a distinct receptor at the cell surface. Receptors and vesicular sites implicated in virus entry are represented by the black spots. Parvoviruses and polyomaviruses are thought to exit the vesicular pathway into the cytoplasm prior to nuclear entry. HPV engages the retromer to exit the endosome, but it is unclear if there is a distinct cytoplasmic phase. Most strikingly, knockdown of retromer expression or some mutations in the C-terminal segment of the HPV16 L2 protein causes the accumulation of the L2 protein in the endosome and prevents its arrival in the Golgi [6,17]. The L2 sequences required for endosome exit are highly conserved in different HPV types and resemble sorting motifs in cellular proteins that bind retromer. Indeed, the entry defect of these L2 mutants could be rescued by replacing the viral element with a known retromer sorting signal from a cellular protein [17,18]. Furthermore, the L2 element was able to support the transport of a marker protein from the endosome to the Golgi in a retromer-dependent fashion. Finally, in vitro binding experiments demonstrated that retromer binds directly to the motifs in L2 that are required for endosome exit [17]. Taken together, these findings establish that HPV16 L2 (and presumably associated viral DNA) is a novel type of retromer cargo, which is transported by the retrograde pathway into the Golgi. Although retrograde trafficking of other HPV types has not been studied in detail, all sequenced HPV types contain a retromer binding motif, and recent evidence suggests that multiple high-risk HPV types utilize similar entry pathways [17,19]. It is not known how incoming HPV16 in the endosome lumen engages retromer in the cytoplasm because L2 is not a classic transmembrane protein, but it is possible that the C-terminus of L2, containing retromer binding sites, protrudes through the endosomal membrane into the cytoplasm, where it is bound by retromer. Consistent with this model, this segment of the L2 protein has pH-dependent membrane-destabilizing activity and can target a reporter protein to cell membranes [20].
Although the studies cited above were the first to forge a link between retromer and virus entry, retromer has been implicated in other aspects of virus infection. For the lentivirus HIV-1, intracellular trafficking of newly synthesized envelope glycoprotein (Env) regulates virus assembly during the late stage of infection. Groppelli showed that the cytoplasmic tail of the gp41 Env subunit binds retromer, an interaction that is required for normal Env trafficking [21]. Inhibition of retromer function resulted in increased cell surface expression of Env, increased incorporation of Env into nascent virions, and enhanced infectivity. In addition, the Herpesvirus Saimiri (HVS) tyrosine kinase-interacting oncoprotein Tip binds to retromer and inhibits its retrograde transport activity [22]. This ultimately results in down-regulation of cell surface CD4 expression and enhanced in vitro immortalization activity of HVS in primary human T cells.
HPV are not the only DNA viruses that engage the retrograde transport system during entry. Polyomaviruses such as SV40 and JC virus have long been known to transit to the ER, where important capsid disassembly steps take place [23–25]. Polyomavirus trafficking to the ER is inhibited by pharmacologic inhibitors of retrograde transport [26] and by inhibition of COPI function [27,28]. However, polyomaviruses have not been detected in the Golgi apparatus and polyomavirus entry does not require retromer, so the route these viruses take to the ER is not known (Fig 1) [12,29]. The parvovirus Adeno-associated virus (AAV) also utilizes the retrograde pathway during intracellular trafficking. After internalization, AAV travels in maturing endosomal compartments to the Golgi apparatus [30], prior to exit into the cytoplasm (Fig 1). Golgi trafficking and infectivity of AAV in epithelial cells are inhibited by chemical inhibitors of retrograde transport or by knockdown of the tSNARE syntaxin 5 (STX5), but do not require retromer or retrograde transport factors Rab7, Rab9, Rab11, STX6, or STX16 [31]. These results suggest that AAV utilizes a novel retrograde transport pathway to enter epithelial cells. In neurons, retrograde transport of AAV9 appears to require Rab7, so the mechanism of AAV trafficking may show important cell type differences [32,33].
A vesicular retrograde entry pathway provides several benefits to incoming virus particles. First, it might be the most direct or efficient route to a crucial intracellular location while targeting the virion away from dead-end sites, such as the lysosome, where degradation would occur. Second, it might allow the incoming capsid to reach a vesicular location where some other essential entry process or factor resides, such as enzymes that catalyze crucial capsid rearrangement or proteolytic cleavage events, or factors that mediate membrane penetration or nuclear import. Finally, a vesicular route will shield the virus and viral DNA from cytoplasmic innate immune sensors that might otherwise inhibit infection. Eventually, the viral genome must leave the retrograde pathway and enter the nucleus. This step presumably involves one or more membrane penetration or membrane fusion events and may include the passage of viral components through the cytoplasm, but consideration of this important topic is outside the scope of this brief review.
The results summarized above demonstrate that viruses can traffic during entry via non-canonical retrograde transport pathways or via known retrograde pathways in unusual ways. Thus, further analysis of virus entry will elucidate novel features of cellular trafficking pathways and processes. Because these processes may well be utilized by the cell to sort cellular proteins, as well as to mediate virus trafficking, studies of virus entry are likely to provide unexpected new insights into fundamental cell biology.
Zdroje
1. Inoue T, Moore P, Tsai B. How viruses and toxins disassemble to enter host cells. Annu Rev Microbiol. 2011;65 : 287–305. doi: 10.1146/annurev-micro-090110-102855 21682643
2. Lu L, Hong W. Review: From endosomes to the trans-Golgi network. Sem Cell Dev Biol. 2014;31 : 30–39.
3. Burd C, Cullen PJ. Retromer: a master conductor of endosome sorting. Cold Spring Harb Perspect Biol. 2014;6: pii: a016774. doi: 10.1101/cshperspect.a016774 24492709
4. Seaman MN. The retromer complex—endosomal protein recycling and beyond. J Cell Sci. 2012;125 : 4693–4702. doi: 10.1242/jcs.103440 23148298
5. Sandvig K, van Deurs B. Delivery into cells: lessons learned from plant and bacterial toxins. Gene Ther. 2005;12 : 865–872. 15815697
6. Lipovsky A, Popa A, Pimienta G, Wyler M, Bhan A, Kuruvilla L, et al. Genome-wide siRNA screen identifies the retromer as a cellular entry factor for human papillomavirus. Proc Natl Acad Sci U S A. 2013;110 : 7452–7457. doi: 10.1073/pnas.1302164110 23569269
7. Li H, Yang X, Yang G, Hong Z, Zhou L, Yin P, et al. Hepatitis C virus NS5A hijacks ARFGAP1 to maintain a phosphatidylinositol 4-phosphate-enriched microenvironment. J Virol. 2014;88 : 5956–5966. doi: 10.1128/JVI.03738-13 24623438
8. Richards AL, Soares-Martins JA, Riddell GT, Jackson WT. Generation of unique poliovirus RNA replication organelles. mBio. 2014;5: e00833–00813. doi: 10.1128/mBio.00833-13 24570367
9. Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118: S12–17. doi: 10.1016/j.ygyno.2010.04.004 20494219
10. Day PM, Thompson CD, Schowalter RM, Lowy DR, Schiller JT. Identification of a role for the trans-Golgi network in human papillomavirus 16 pseudovirus infection. J Virol. 2013;87 : 3862–3870. doi: 10.1128/JVI.03222-12 23345514
11. Laniosz V, Dabydeen SA, Havens MA, Meneses PI. Human papillomavirus type 16 infection of human keratinocytes requires clathrin and caveolin-1 and is brefeldin a sensitive. J Virol. 2009;83 : 8221–8232. doi: 10.1128/JVI.00576-09 19494002
12. Zhang W, Kazakov T, Popa A, DiMaio D. Vesicular trafficking of incoming human papillomavirus type 16 to the Golgi apparatus and endoplasmic reticulum requires gamma-secretase activity. mBio. 2014;5: e01777–01714. doi: 10.1128/mBio.01777-14 25227470
13. Aydin I, Weber S, Snijder B, Samperio Ventayol P, Kuhbacher A, et al. Large scale RNAi reveals the requirement of nuclear envelope breakdown for nuclear import of human papillomaviruses. PLoS Pathog. 2014;10: e1004162. doi: 10.1371/journal.ppat.1004162 24874089
14. DiGiuseppe S, Bienkowska-Haba M, Hilbig L, Sapp M. The nuclear retention signal of HPV16 L2 protein is essential for incoming viral genome to transverse the trans-Golgi network. Virology. 2014;458–459 : 93–105.
15. Ishii Y, Nakahara T, Kataoka M, Kusumoto-Matsuo R, Mori S, Takeuchi T, et al. Identification of TRAPPC8 as a host factor required for human papillomavirus cell entry. PLoS One. 2013;8: e80297. doi: 10.1371/journal.pone.0080297 24244674
16. Bergant Marusic M, Ozbun MA, Campos SK, Myers MP, Banks L. Human papillomavirus L2 facilitates viral escape from late endosomes via sorting nexin 17. Traffic. 2012;13 : 455–467. doi: 10.1111/j.1600-0854.2011.01320.x 22151726
17. Popa A, Zhang W, Harrison MS, Goodner K, Kazakov T, Goodwin EC, et al. Direct binding of retromer to human papillomavirus type 16 minor capsid protein L2 mediates endosome exit during infection. PLos Pathog. 2015;11: e1004699. doi: 10.1371/journal.ppat.1004699 25693203
18. Seaman MNJ. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci. 2007;120 : 2378–2389. 17606993
19. Spoden G, Kuhling L, Cordes N, Frenzel B, Sapp M, Boller K, et al. Human papillomavirus types 16, 18, and 31 share similar endocytic requirements for entry. J Virol. 2013;87 : 7765–7773. doi: 10.1128/JVI.00370-13 23616662
20. Kamper N, Day PM, Nowak T, Selinka HC, Florin L, Bolscher J, et al. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. J Virol. 2006;80 : 759–768. 16378978
21. Groppelli E, Len AC, Granger LA, Jolly C. Retromer regulates HIV-1 envelope glycoprotein trafficking and incorporation into virions. PLoS Pathog. 2014;10: e1004518. doi: 10.1371/journal.ppat.1004518 25393110
22. Kingston D, Chang H, Ensser A, Lee HR, Lee J, Lee SH, et al. Inhibition of retromer activity by herpesvirus saimiri tip leads to CD4 downregulation and efficient T cell transformation. J Virol. 2011;85 : 10627–10638. doi: 10.1128/JVI.00757-11 21849449
23. Kartenbeck J, Stukenbrok H, Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989;109 : 2721–2729. 2556405
24. Goodwin EC, Lipovsky A, Inoue T, Magaldi TG, Edwards APB, Van Goor KE, et al. BiP and multiple DNAJ molecular chaperones in the endoplasmic reticulum are required for efficient simian virus 40 infection. mBio. 2011;2 : 101–111.
25. Schelhaas M, Malmstrom J, Pelkmans L, Haugstetter J, Ellgaard L, Grunewald K, et al. Simian Virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell. 2007;131 : 516–529. 17981119
26. Nelson CD, Carney DW, Derdowski A, Lipovsky A, Gee GV, O'Hara B, et al. A retrograde trafficking inhibitor of ricin and shiga-like toxins inhibits infection of cells by human and monkey polyomaviruses. mBio. 2013;4 (6): e00729–00713. doi: 10.1128/mBio.00729-13 24222489
27. Norkin LC, Anderson HA, Wolfrom SA, Oppenheim A. Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J Virol. 2002;76 : 5156–5166. 11967331
28. Richards AA, Stang E, Pepperkok R, Parton RG. Inhibitors of COP-mediated transport and cholera toxin action inhibit simian virus 40 infection. Mol Biol Cell. 2002;13 : 1750–1764. 12006667
29. Norkin LC, Kuksin D. The caveolae-mediated sv40 entry pathway bypasses the golgi complex en route to the endoplasmic reticulum. Virol J. 2005;2 : 38. 15840166
30. Bantel-Schaal U, Hub B, Kartenbeck J. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J Virol. 2002;76 : 2340–2349. 11836412
31. Nonnenmacher ME, Cintrat JC, Gillet D, Weber T. Syntaxin 5-dependent retrograde transport to the trans-Golgi network is required for adeno-associated virus transduction. J Virol. 2015;89 : 1673–1687. doi: 10.1128/JVI.02520-14 25410859
32. Castle MJ, Perlson E, Holzbaur EL, Wolfe JH. Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment. Mol Ther. 2014;22 : 554–566. doi: 10.1038/mt.2013.237 24100640
33. Keiser NW, Yan Z, Zhang Y, Lei-Butters DC, Engelhardt JF. Unique characteristics of AAV1, 2, and 5 viral entry, intracellular trafficking, and nuclear import define transduction efficiency in HeLa cells. Hum Gene Ther. 2011;22 : 1433–1444. doi: 10.1089/hum.2011.044 21574868
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type GenesČlánek Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion IsolatesČlánek -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated GlycolipidsČlánek Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- The Long and Winding Road (Apologies to the Beatles)
- The Ebola Virus: From Basic Research to a Global Health Crisis
- Riding the R Train into the Cell
- The Two-Phase Emergence of Non Pandemic HIV-1 Group O in Cameroon
- Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies
- E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3
- The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type Genes
- Hyperinvasive Meningococci Induce Intra-nuclear Cleavage of the NF-κB Protein p65/RelA by Meningococcal IgA Protease
- Active Transport of Phosphorylated Carbohydrates Promotes Intestinal Colonization and Transmission of a Bacterial Pathogen
- HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
- Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion Isolates
- Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus following European Invasion
- Inhibiting the Recruitment of PLCγ1 to Kaposi’s Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells
- Goblet Cell Derived RELM-β Recruits CD4 T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation
- HLA Class-II Associated HIV Polymorphisms Predict Escape from CD4+ T Cell Responses
- An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses
- Extracellular Adenosine Protects against Lung Infection by Regulating Pulmonary Neutrophil Recruitment
- : Adaptations to the Dixenous Life Cycle Analyzed by Genome Sequencing, Transcriptome Profiling and Co-infection with
- Which Way In? The RalF Arf-GEF Orchestrates Host Cell Invasion
- Intracellular Uropathogenic . Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells
- A Non-enveloped Virus Hijacks Host Disaggregation Machinery to Translocate across the Endoplasmic Reticulum Membrane
- Supporting Role for GTPase Rab27a in Hepatitis C Virus RNA Replication through a Novel miR-122-Mediated Effect
- -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids
- The Activation of Effector Avr3b by Plant Cyclophilin is Required for the Nudix Hydrolase Activity of Avr3b
- A Pyranose-2-Phosphate Motif Is Responsible for Both Antibiotic Import and Quorum-Sensing Regulation in
- Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation
- The Rsb Phosphoregulatory Network Controls Availability of the Primary Sigma Factor in and Influences the Kinetics of Growth and Development
- Inhibits Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis
- Illuminating Targets of Bacterial Secretion
- Chemical Signals and Mechanosensing in Bacterial Responses to Their Environment
- Interdisciplinarity and Infectious Diseases: An Ebola Case Study
- Fungi That Infect Insects: Altering Host Behavior and Beyond
- Plasticity and Redundancy in Proteins Important for Invasion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
- A Novel Virus Causes Scale Drop Disease in
- STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control
- Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
- Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative
- AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity
- Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 bZIP Factor Transgenic Mice
- Transgenic Rabbits Expressing Ovine PrP Are Susceptible to Scrapie
- Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of . Virulence Pathways
- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Serine Phosphorylation of HIV-1 Vpu and Its Binding to Tetherin Regulates Interaction with Clathrin Adaptors
- Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins the Activation of the MnK/eIF4E Pathway
- Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission
- Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection
- sRNA-Mediated Regulation of P-Fimbriae Phase Variation in Uropathogenic
- Evolutionary and Functional Analysis of Old World Primate TRIM5 Reveals the Ancient Emergence of Primate Lentiviruses and Convergent Evolution Targeting a Conserved Capsid Interface
- Hepcidin and Host Defense against Infectious Diseases
- Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis
- Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIV Rectal Challenge
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Illuminating Targets of Bacterial Secretion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



