-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPlasticity and Redundancy in Proteins Important for Invasion
article has not abstract
Published in the journal: . PLoS Pathog 11(8): e32767. doi:10.1371/journal.ppat.1005069
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005069Summary
article has not abstract
Phenotypic Plasticity to Preserve Essential Functions
Phenotypic plasticity encompasses the versatile abilities of an organism to modify its phenotype according to changes in the environment. Such adaptations, in response to a decrease of fitness, are commonly exploited by microorganisms and plants that cannot easily escape their environment and, hence, are at risk of extinction. Functional redundancy and plasticity is therefore anticipated for obligate intracellular parasites, including members of the phylum Apicomplexa, to ensure successful host cell invasion—a critical step for their survival.
The phylum Apicomplexa includes many medically and veterinary-relevant parasites, most notably the human pathogens Plasmodium and Toxoplasma gondii, responsible for malaria and toxoplasmosis, respectively. These parasites are the most studied of the phylum, not only because of their prevalence but also because of their accessibility to genetic manipulation [1,2], which has allowed the investigation of several aspects of their lytic cycle, particularly their mechanisms of entry into the target cell.
Invasion by most Apicomplexa is a multistep process consisting of recognition, attachment, and active penetration into host cells, which has been dissected in depth in Toxoplasma tachyzoites [3]. Parasite propulsion into the host cell is powered by a myosin motor complex termed the glideosome. During motility and invasion, T. gondii myosin A (TgMyoA) presumably translocates adhesins at the moving junction (MJ) from the apical to the posterior pole of the parasite [4]. The MJ forms a constriction around the parasite at the point of entry into the host cell that appears as a ring structure. Apical membrane antigen 1 (AMA1) in association with a complex of rhoptry neck proteins (RONs) that are anchored into the host cortical cytoskeleton [5] are the currently known components of this MJ.
Insults imposed upon the parasite by experimental genome editing strategies, such as ablation of TgMyoA and TgAMA1 genes, significantly compromised the fitness of the parasites and have now been demonstrated to elicit phenotypic plasticity. Such plasticity has uncovered informative redundancy and adaptation mechanisms. Hereafter, we compare and contrast the methodologies available to investigate the function of crucial genes in T. gondii and discuss how some experimental approaches contribute to or even favor the emergence of alternative mechanisms to ensure completion of the vital step of host cell invasion.
A Plethora of Tools to Tackle the Function of Critical Genes in Toxoplasma gondii
The amenability of T. gondii to genetic manipulation has led to the development of various experimentally regulated expression systems to investigate the function of genes critical for parasite survival and, notably, for invasion [2].
Recently, the generation of gene knockouts by homologous recombination was greatly facilitated by the elimination of random integration events in parasites lacking Ku80, a protein involved in the non-homologous end-joining pathway of DNA repair [6,7]. Furthermore, Cre recombinase, either expressed upon transient transfection [8] or stably as a dimerizable Cre (DiCre) activated by rapamycin [9], efficiently excises genes flanked by LoxP sites. These refined technologies allow the generation of null mutants; however, the clonal isolation procedure and subsequent propagation of severely impaired mutants is prone to selection of spurious adaptation events resulting in an attenuation of the phenotype severity. In contrast, strategies aimed at an acute conditional depletion of a protein in a clonal population over a relatively short period of time prior to phenotypic assessment minimizes the risks of emergence of compensatory mechanisms. This holds true for the tetracycline (tet)-inducible transactivator system [10], the FKBP-destabilization domain [11], and the conditional DiCre-mediated excision system [9]. The drawback or limit of these approaches is the quasi-impossibility of fully eliminating a protein even if it falls below detectable levels, and in consequence, should never be considered or interpreted as a null mutant.
TgMyoA and TgAMA1 were initially investigated using the regulatable tet-inducible system [10,12] and shown to be critical for invasion (Table 1). More recently, null mutants for TgMyoA and TgAMA1 [9,13] were isolated despite their severe phenotypes. While these genes can now be qualified as dispensable for parasite survival in vitro, subsequent studies revealed a considerable level of plasticity towards compensatory mechanisms that ensured invasion by these mutants [14–16]. These two examples are detailed below and underscore the complexity in interpreting data and the need to carefully take into account the capacity of parasite adaptation. In turn, these data reveal critical information about the function of other related genes, shedding light on their potential for functional redundancy.
Tab. 1. Summary of the phenotype and adaptation observed in the cell lines discussed in this review according to the technology used to investigate the function of the corresponding gene. 
TgMyoA-KO and Recruitment of Another Myosin
The MyoA-glideosome refers to the machinery anchored to the parasite pellicle and acting as a component of gliding motility that critically contributes to invasion and egress (Fig 1A) [10]. Ablation of the TgMyoA gene (TgMyoA-KO) [9] raised legitimate questions regarding the validity of the gliding motility model for invasion and, alternatively, suggested the possibility of functional redundancy given the existence of 11 myosins in the T. gondii genome, with six of them belonging to the same class XIV as TgMyoA [17]. Pertinently, attempts to simultaneously disrupt TgMyoA and its close homologue TgMyoC failed [14]. Indeed, it has not been possible to isolate TgMyoA-KO clones in which TgMyoC was already deleted (TgMyoA-cKO/TgMyoC-KO). Moreover, conditional depletion of TgMyoC in TgMyoA-KO/TgMyoC-cKD caused a further decrease in invasion, as well, clearly indicating that TgMyoC accounts for the residual invasion seen in the TgMyoA-KO (Table 1) [14,15].
Fig. 1. A. Schematic representation of a T. gondii tachyzoite highlighting the localization and composition of the three glideosomes. 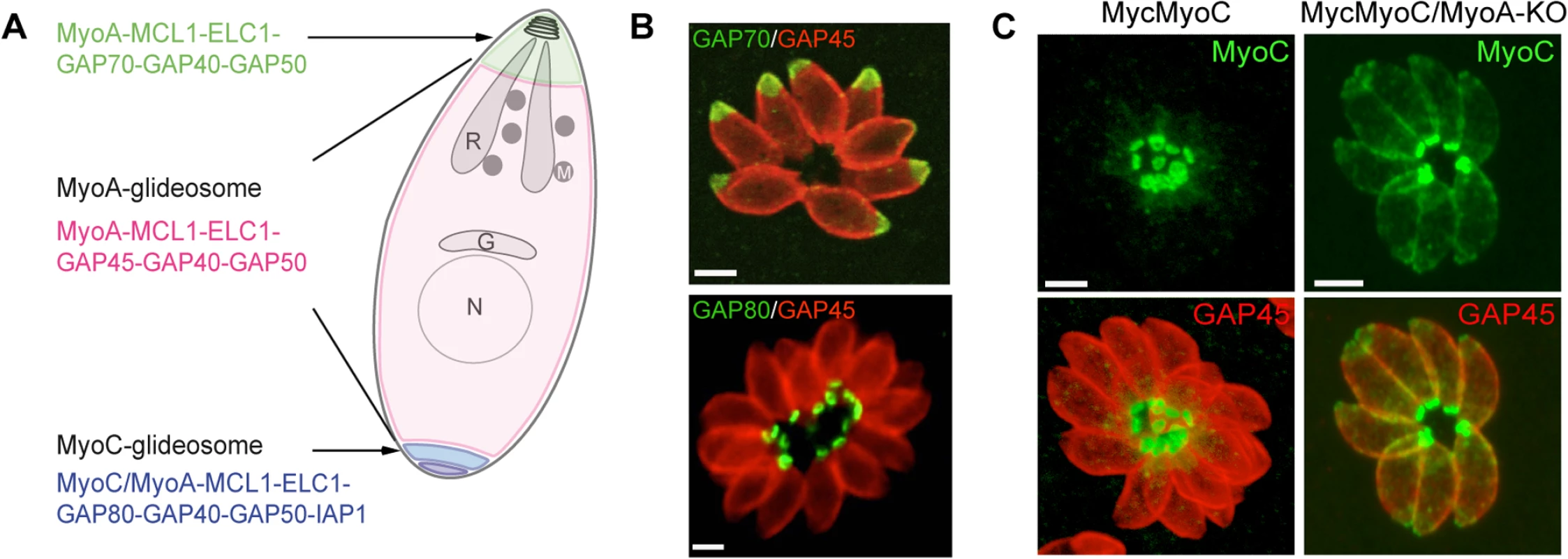
B. Localization of three glideosomes by immunofluorescence using the endogenously tagged TgGAP70 and TgGAP80 and the anti-TgGAP45 antibodies. Scale bars: 2 μm. C. Relocalization of MycMyoC to the periphery of the tachyzoite in addition to its basal localization in absence of TgMyoA in the MycMyoC-cKD/MyoA-KO strain. Scale bars: 2 μm. Further characterization of TgMyoC revealed that this motor belongs to the MyoC-glideosome, a complex localized to the basal polar ring, that shares an overall similar architecture with the MyoA-glideosome as well as the common myosin light chains MLC1 and ECL1 (Fig 1A and 1B) [15,18]. In parasites lacking TgMyoA, TgMyoC strikingly relocalizes along the parasite pellicle where TgMyoA normally resides (Fig 1C) [15]. This is achieved via interaction with GAP45, which typically anchors TgMyoA to the pellicle but which functionally replaces GAP80 in the recruitment of TgMyoC to the pellicle. Further evidence for plasticity was also observed in parasites lacking TgGAP80, in which TgGAP45 brings TgMyoC to the posterior pole [15,19].
Relocalization of TgMyoC immediately ensures survival of TgMyoA-KO parasites, and continuous passaging of these parasites in culture reveals an improved fitness with time and partial restoration of the invasion process.
TgAMA1-KO and the Up-Regulation of Other AMA Family Members
Conditional knockdown of AMA1 (TgAMA1-cKD) caused a strong impairment in invasion resulting from the inability to form an intimate interaction between the parasite and the host cell at the MJ (Table 1) [12,20]. In contrast, and rather unexpectedly, TgAMA1-KO parasites were still able to enter host cells at the same speed as wild-type parasites through the establishment of a visible MJ [13]. Intriguingly, the invasion efficiency of the TgAMA1-KO was superior to that of the TgAMA1-cKD, suggesting that a compensatory mechanism was selected during the continuous passaging of the mutant [13,16]. With the view that the MJ serves as a platform for the transmission of the force generated by the glideosome [21], TgAMA1 had to be replaced by a functionally redundant protein capable of bridging the RONs and the parasite actomyosin system. In this context, two homologues of TgAMA1 were readily identified: TgAMA2 and the sporozoite AMA1 (TgAMA3), which specifically interacts with the sporozoite RON2 (TgRON2L2) [22]. Both proteins represent plausible, functionally redundant candidates. Concordantly, in the TgAMA1-KO, TgAMA2 expression was shown to be up-regulated at the transcript level as monitored by qPCR [13,16] and was detectable at the MJ interacting with TgRON2 in place of TgAMA1 [16]. In addition, continuous in vitro culture over 12 months showed an increasing invasion rate and, therefore, an improved fitness. Interestingly, in the TgAMA1-KO/TgAMA2-KO double mutant, TgAMA4, which also shares sequence similarities with TgAMA1 and TgAMA3, was found to be overexpressed. This overexpression was accompanied by the concomitant up-regulation of the specific TgAMA4 ligand TgRON2L1 [16].
This series of AMA-RON2 pairs that can be mobilized upon successive gene deletions highlights the considerable degree of parasite plasticity and the critical role of the MJ for host cell entry.
Plasticity Operates in Other Apicomplexa and in Processes Outside of Invasion
A striking and comparable example of genome plasticity related to the invasion process has been reported for the etiologic agent of human malaria, Plasmodium falciparum. Indeed, upon gene deletion, this parasite is able to redeploy a machinery composed of adhesin ligands organized hierarchically to ensure invasion via alternative pathways [23].
Carbon metabolism generates metabolites critically needed for biomass production and cell survival. Upon genetic intervention, the parasites are also expected to adjust and compensate for the deletion of genes implicated in any of these vital processes. For example, the pool of cytoplasmic acetyl-CoA is an essential metabolite needed for acetylation of proteins and lipogenesis and can be produced from citrate through the action of ATP citrate lyase (ACL) or from acetate by acetyl-CoA synthetase (ACS). In Toxoplasma tachyzoites, TgACS and TgACL can be individually deleted without noticeable phenotypes but cannot be deleted in combination. While TgACL is barely detectable in wild-type parasites, it is readily up-regulated in TgACS-KO parasites to adjust the level of acetyl-CoA required for proper growth [24]. This response to loss of fitness illustrates a rapid adaptation to unfavorable conditions by up-regulation of a complementary metabolic pathway.
Lastly, organelle biogenesis and cell division are fundamental events that can also challenge the faculty of tachyzoite adaptation. Such an adaptation was reported upon the disruption of the membrane occupation and recognition nexus protein 1 (TgMORN1), a protein that associates with several structures implicated in cell division [25]. While conditional depletion of TgMORN1 (TgMORN1-cKD) showed impairment in basal complex formation, cytokinesis, and apicoplast (plastid-like organelle) inheritance [26], a TgMORN1-KO mutant, isolated following Cre recombinase excision, presented an attenuation of the phenotype severity, in particular for apicoplast segregation [8]. The apicoplast hosts essential metabolic pathways [27] and is rapidly lost upon multiple rounds of division in the TgMORN1-cKD, indicating that MORN1 is critical for the survival of the parasite. It is therefore surprising that TgMORN1-KO parasites can be propagated in culture with only a quarter of them without apicoplast. These results suggest an adaptation phenomenon possibly because of a functional redundancy since other MORN-containing proteins are present in the T. gondii genome.
Concluding Remarks
T. gondii is an experimentally versatile member of the Apicomplexan phylum that needs to be taken seriously when it comes to the emergence and study of compensatory mechanisms. The few examples discussed here only show the tip of the iceberg. The frequency at which the compensatory changes occur has not yet been determined, and thus the mechanisms responsible have not yet been unraveled; however, they might include epigenetic processes such as chromatin remodeling or changes in protein conformation and stability.
The gene knockout technologies available for Toxoplasma [2] and Plasmodium [1] are further potentiated by the CRISPR/Cas9-based genome editing strategy [28–32]. This genome editing engenders off-target effects, and thus it becomes even more crucial to be aware of the strengths and weaknesses of these approaches. The acute, conditional depletion of a protein remains the panacea to study its function. As illustrated above, the complete deletion of a gene opens a new dimension of investigations that deserve to be globally scrutinized prior to drawing conclusions. We are entering an area where global phenotypic analyses become feasible and should be used to embrace the phenotypic complexity of a single gene deletion and, incidentally, to avoid pitfalls.
Zdroje
1. de Koning-Ward TF, Gilson PR, Crabb BS. Advances in molecular genetic systems in malaria. Nat Rev Microbiol. 2015;13(6):373–87. doi: 10.1038/nrmicro3450 25978707
2. Jimenez-Ruiz E, Wong EH, Pall GS, Meissner M. Advantages and disadvantages of conditional systems for characterization of essential genes in Toxoplasma gondii. Parasitology. 2014;141(11):1390–8. doi: 10.1017/S0031182014000559 24926834
3. Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Current opinion in microbiology. 2007;10(1):83–9. 16837236
4. Tyler JS, Treeck M, Boothroyd JC. Focus on the ringleader: the role of AMA1 in apicomplexan invasion and replication. Trends in parasitology. 2011;27(9):410–20. doi: 10.1016/j.pt.2011.04.002 21659001
5. Besteiro S, Michelin A, Poncet J, Dubremetz JF, Lebrun M. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS pathogens. 2009;5(2):e1000309. doi: 10.1371/journal.ppat.1000309 19247437
6. Fox BA, Ristuccia JG, Gigley JP, Bzik DJ. Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryot Cell. 2009;8(4):520–9. doi: 10.1128/EC.00357-08 19218423
7. Huynh MH, Carruthers VB. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell. 2009;8(4):530–9. doi: 10.1128/EC.00358-08 19218426.
8. Heaslip AT, Dzierszinski F, Stein B, Hu K. TgMORN1 is a key organizer for the basal complex of Toxoplasma gondii. PLoS pathogens. 2010;6(2):e1000754. doi: 10.1371/journal.ppat.1000754 20140195
9. Andenmatten N, Egarter S, Jackson AJ, Jullien N, Herman JP, Meissner M. Conditional genome engineering in Toxoplasma gondii uncovers alternative invasion mechanisms. Nature methods. 2012. E-pub ahead of print.
10. Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298(5594):837–40. 12399593
11. Herm-Gotz A, Agop-Nersesian C, Munter S, Grimley JS, Wandless TJ, Frischknecht F, et al. Rapid control of protein level in the apicomplexan Toxoplasma gondii. Nature methods. 2007;4(12):1003–5. 17994029
12. Mital J, Meissner M, Soldati D, Ward GE. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Molecular biology of the cell. 2005;16(9):4341–9. 16000372
13. Bargieri DY, Andenmatten N, Lagal V, Thiberge S, Whitelaw JA, Tardieux I, et al. Apical membrane antigen 1 mediates apicomplexan parasite attachment but is dispensable for host cell invasion. Nature communications. 2013;4 : 2552. doi: 10.1038/ncomms3552 24108241
14. Egarter S, Andenmatten N, Jackson AJ, Whitelaw JA, Pall G, Black JA, et al. The toxoplasma Acto-MyoA motor complex is important but not essential for gliding motility and host cell invasion. PloS one. 2014;9(3):e91819. doi: 10.1371/journal.pone.0091819 24632839
15. Frenal K, Marq JB, Jacot D, Polonais V, Soldati-Favre D. Plasticity between MyoC - and MyoA-glideosomes: an example of functional compensation in Toxoplasma gondii invasion. PLoS pathogens. 2014;10(10):e1004504. doi: 10.1371/journal.ppat.1004504 25393004
16. Lamarque MH, Roques M, Kong-Hap M, Tonkin ML, Rugarabamu G, Marq JB, et al. Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nature communications. 2014;5 : 4098. doi: 10.1038/ncomms5098 24934579
17. Foth BJ, Goedecke MC, Soldati D. New insights into myosin evolution and classification. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(10):3681–6. Epub 2006/03/01. 16505385
18. Jacot D, Frenal K, Marq JB, Sharma P, Soldati-Favre D. Assessment of phosphorylation in Toxoplasma glideosome assembly and function. Cellular microbiology. 2014;16(10):1518–32. doi: 10.1111/cmi.12307 24779470
19. Frenal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D. Functional dissection of the apicomplexan glideosome molecular architecture. Cell host & microbe. 2010;8(4):343–57.
20. Lamarque M, Besteiro S, Papoin J, Roques M, Vulliez-Le Normand B, Morlon-Guyot J, et al. The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS pathogens. 2011;7(2):e1001276. doi: 10.1371/journal.ppat.1001276 21347343
21. Bichet M, Joly C, Henni A, Guilbert T, Xemard M, Tafani V, et al. The toxoplasma-host cell junction is anchored to the cell cortex to sustain parasite invasive force. BMC biology. 2014;12(1):773. doi: 10.1186/s12915-014-0108-y 25551479
22. Poukchanski A, Fritz HM, Tonkin ML, Treeck M, Boulanger MJ, Boothroyd JC. Toxoplasma gondii sporozoites invade host cells using two novel paralogues of RON2 and AMA1. PloS one. 2013;8(8):e70637. doi: 10.1371/journal.pone.0070637 23940612
23. Baum J, Maier AG, Good RT, Simpson KM, Cowman AF. Invasion by P. falciparum merozoites suggests a hierarchy of molecular interactions. PLoS pathogens. 2005;1(4):e37. 16362075
24. Tymoshenko S, Oppenheim RD, Agren R, Nielsen J, Soldati-Favre D, Hatzimanikatis V. Metabolic Needs and Capabilities of Toxoplasma gondii through Combined Computational and Experimental Analysis. PLoS Comput Biol. 2015;11(5):e1004261. doi: 10.1371/journal.pcbi.1004261 26001086
25. Gubbels MJ, Vaishnava S, Boot N, Dubremetz JF, Striepen B. A MORN-repeat protein is a dynamic component of the Toxoplasma gondii cell division apparatus. J Cell Sci. 2006;119(Pt 11):2236–45.
26. Lorestani A, Sheiner L, Yang K, Robertson SD, Sahoo N, Brooks CF, et al. A Toxoplasma MORN1 null mutant undergoes repeated divisions but is defective in basal assembly, apicoplast division and cytokinesis. PloS one. 2010;5(8):e12302. doi: 10.1371/journal.pone.0012302 20808817
27. Fichera ME, Roos DS. A plastid organelle as a drug target in apicomplexan parasites. Nature. 1997;390(6658):407–9. 9389481
28. Shen B, Brown KM, Lee TD, Sibley LD. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. mBio. 2014;5(3):e01114–14. doi: 10.1128/mBio.01114-14 24825012
29. Sidik SM, Hackett CG, Tran F, Westwood NJ, Lourido S. Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PloS one. 2014;9(6):e100450. doi: 10.1371/journal.pone.0100450 24971596
30. Ghorbal M, Gorman M, Macpherson CR, Martins RM, Scherf A, Lopez-Rubio JJ. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat Biotechnol. 2014;32(8):819–21. doi: 10.1038/nbt.2925 24880488
31. Lee MC, Fidock DA. CRISPR-mediated genome editing of Plasmodium falciparum malaria parasites. Genome Med. 2014;6(8):63. doi: 10.1186/s13073-014-0063-9 25473431
32. Wagner JC, Platt RJ, Goldfless SJ, Zhang F, Niles JC. Efficient CRISPR-Cas9-mediated genome editing in Plasmodium falciparum. Nature methods. 2014;11(9):915–8. doi: 10.1038/nmeth.3063 25108687
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type GenesČlánek Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion IsolatesČlánek -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated GlycolipidsČlánek Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
-
Všechny články tohoto čísla
- The Long and Winding Road (Apologies to the Beatles)
- The Ebola Virus: From Basic Research to a Global Health Crisis
- Riding the R Train into the Cell
- The Two-Phase Emergence of Non Pandemic HIV-1 Group O in Cameroon
- Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies
- E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3
- The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type Genes
- Hyperinvasive Meningococci Induce Intra-nuclear Cleavage of the NF-κB Protein p65/RelA by Meningococcal IgA Protease
- Active Transport of Phosphorylated Carbohydrates Promotes Intestinal Colonization and Transmission of a Bacterial Pathogen
- HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
- Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion Isolates
- Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus following European Invasion
- Inhibiting the Recruitment of PLCγ1 to Kaposi’s Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells
- Goblet Cell Derived RELM-β Recruits CD4 T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation
- HLA Class-II Associated HIV Polymorphisms Predict Escape from CD4+ T Cell Responses
- An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses
- Extracellular Adenosine Protects against Lung Infection by Regulating Pulmonary Neutrophil Recruitment
- : Adaptations to the Dixenous Life Cycle Analyzed by Genome Sequencing, Transcriptome Profiling and Co-infection with
- Which Way In? The RalF Arf-GEF Orchestrates Host Cell Invasion
- Intracellular Uropathogenic . Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells
- A Non-enveloped Virus Hijacks Host Disaggregation Machinery to Translocate across the Endoplasmic Reticulum Membrane
- Supporting Role for GTPase Rab27a in Hepatitis C Virus RNA Replication through a Novel miR-122-Mediated Effect
- -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids
- The Activation of Effector Avr3b by Plant Cyclophilin is Required for the Nudix Hydrolase Activity of Avr3b
- A Pyranose-2-Phosphate Motif Is Responsible for Both Antibiotic Import and Quorum-Sensing Regulation in
- Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation
- The Rsb Phosphoregulatory Network Controls Availability of the Primary Sigma Factor in and Influences the Kinetics of Growth and Development
- Inhibits Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis
- Illuminating Targets of Bacterial Secretion
- Chemical Signals and Mechanosensing in Bacterial Responses to Their Environment
- Interdisciplinarity and Infectious Diseases: An Ebola Case Study
- Fungi That Infect Insects: Altering Host Behavior and Beyond
- Plasticity and Redundancy in Proteins Important for Invasion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
- A Novel Virus Causes Scale Drop Disease in
- STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control
- Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
- Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative
- AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity
- Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 bZIP Factor Transgenic Mice
- Transgenic Rabbits Expressing Ovine PrP Are Susceptible to Scrapie
- Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of . Virulence Pathways
- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Serine Phosphorylation of HIV-1 Vpu and Its Binding to Tetherin Regulates Interaction with Clathrin Adaptors
- Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins the Activation of the MnK/eIF4E Pathway
- Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission
- Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection
- sRNA-Mediated Regulation of P-Fimbriae Phase Variation in Uropathogenic
- Evolutionary and Functional Analysis of Old World Primate TRIM5 Reveals the Ancient Emergence of Primate Lentiviruses and Convergent Evolution Targeting a Conserved Capsid Interface
- Hepcidin and Host Defense against Infectious Diseases
- Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis
- Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIV Rectal Challenge
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Illuminating Targets of Bacterial Secretion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



