-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
Arthropods are commonly infected with heritable bacteria, and some of these symbionts can protect their hosts against infection and/or be reproductive parasites. Which of these traits evolves will depend on whether the trait is costly to the symbiont and the host. Using a panel of strains of the symbiont Wolbachia in the fruit fly Drosophila simulans, we found that the beneficial effect of antiviral protection and the parasitic phenotype of cytoplasmic incompatibility occur independently across the strains. We found that high antiviral protection is associated with high symbiont densities and strong reductions in other life-history traits affecting the fitness of both the symbiont and the host. In contrast cytoplasmic incompatibility did not induce costs on these traits. This trade-off between antiviral protection and other fitness components may select for reduced antiviral protection, which would endanger the long-term success of programs using Wolbachia to block the transmission of mosquito-borne viruses.
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1005021
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005021Summary
Arthropods are commonly infected with heritable bacteria, and some of these symbionts can protect their hosts against infection and/or be reproductive parasites. Which of these traits evolves will depend on whether the trait is costly to the symbiont and the host. Using a panel of strains of the symbiont Wolbachia in the fruit fly Drosophila simulans, we found that the beneficial effect of antiviral protection and the parasitic phenotype of cytoplasmic incompatibility occur independently across the strains. We found that high antiviral protection is associated with high symbiont densities and strong reductions in other life-history traits affecting the fitness of both the symbiont and the host. In contrast cytoplasmic incompatibility did not induce costs on these traits. This trade-off between antiviral protection and other fitness components may select for reduced antiviral protection, which would endanger the long-term success of programs using Wolbachia to block the transmission of mosquito-borne viruses.
Introduction
Heritable symbionts are frequent in insects and their evolutionary success relies on various strategies. By sharing a common route of transmission with their host’s genes, they benefit from increasing host fitness. Consequently, numerous endosymbiotic bacteria evolved towards mutualism, for example by complementing their host diet [1,2], increasing tolerance to environmental stresses [3] or protecting against natural enemies [4–9]. However, because most of these heritable bacteria are maternally-transmitted, the evolutionary interests of host and symbiont are not perfectly aligned since only females transmit the symbiont. This has led to many symbionts evolving selfish strategies that consist of parasitic manipulation of their host’s reproduction by inducing female-biased sex-ratios or cytoplasmic incompatibility (CI) [10]. CI is a sperm modification that results in embryonic mortality in crosses between uninfected females and males harboring the symbiont, thus giving a competitive advantage to infected females that can rescue the sperm modification. Mutualism and reproductive manipulation are not mutually exclusive, and some symbionts display both [11]. However, the balance between the benefits and costs of these extended phenotypes to the symbiont’s fitness, as well as the genetic correlations between them, will determine which of these strategies is favoured by natural selection.
Wolbachia, which are common maternally-transmitted bacterial symbionts of arthropods, can be both parasites and mutualists. Wolbachia has been shown to protect Drosophila and mosquitoes against several RNA viruses—including Dengue and Chikungunya viruses [7,9,12–15]. Some strains also protect insects against filarial nematodes [16], Plasmodium parasites [12,17,18] and pathogenic bacteria [19]. Although it is unclear how important antiviral protection is in nature and whether it is under strong selection, some protective Wolbachia strains are able to invade host populations while inducing no other known phenotypes [20,21]. In addition, Wolbachia has the ability to spread rapidly through insect populations by parasitically manipulating reproduction, in particular by CI [22]. This combination of traits makes Wolbachia an attractive tool for blocking disease transmission by mosquitoes, as CI allows it to spread through vector populations while its antiviral effects can prevent them from transmitting arboviruses [23,24].
Levels of both antiviral protection and CI may evolve rapidly. During the 20th century in natural populations of D. melanogaster the Wolbachia strain wMelCS, which provides strong antiviral protection, was partially replaced by wMel [25,26], which provides weaker protection [27]. In North American populations of D. simulans, field and experimental data suggest that the strain wRi has evolved to produce weaker levels of CI within a few decades [28].
Efforts to use Wolbachia to block the transmission of viruses have focused largely on the mosquito Aedes aegypti, which is the primary vector of dengue virus. Wolbachia has been successfully introduced into two Australian populations of Aedes aegypti [29], and three years post-release it had reached a stable and high prevalence in the field despite having a negative effect on the fecundity of mosquitoes [30]. Both antiviral protection and levels of CI were maintained over time [30,31].
In the long-term, the presence of fitness costs is expected to select for both host genes and bacterial genes that reduce these costs [32]. In accordance with this prediction, the Wolbachia strain wRi evolved from reducing the fecundity of the flies to increasing it within two decades in North American populations of D. simulans [33]. It is possible that the evolution of lower costs could be achieved by a decrease in bacterial densities, as costly Wolbachia tend to have high bacterial densities [27,34,35]. Since a high Wolbachia density may be required for the expression of both antiviral protection [14,27,34,36–38] and CI [35,39–42], the evolution of reduced Wolbachia density might translate into a correlated decrease in the ability to block arbovirus transmission and invade insect populations.
To investigate these questions, we used sixteen Wolbachia strains in a common host genetic background to measure the level of CI induced and effects on other fitness-related traits, and have tested for correlations between these traits and antiviral protection. Our results demonstrate that antiviral protection is independent of CI but that it is associated with reduction on other fitness components. Furthermore, this trade-off can be explained by the density of the bacteria in the somatic tissues of the insect. Overall, our study suggests that newly introduced Wolbachia infections may evolve towards weaker protection in the field.
Results
To compare multiple symbiont strains independent of host genetic effects, we used a panel of Wolbachia strains that had been transferred from different Drosophila species into a single inbred line of D. simulans (Fig 1F). To avoid effects of using an inbred fly line, we crossed these flies to a different inbred fly line and used the F1 progeny in our experiments. Vertical transmission rates were previously estimated and were 100% for all Wolbachia strains used in this study [14].
Fig. 1. Phylogenetic distribution of CI levels and Wolbachia effects on egg hatch rates, fecundity and lifespan. 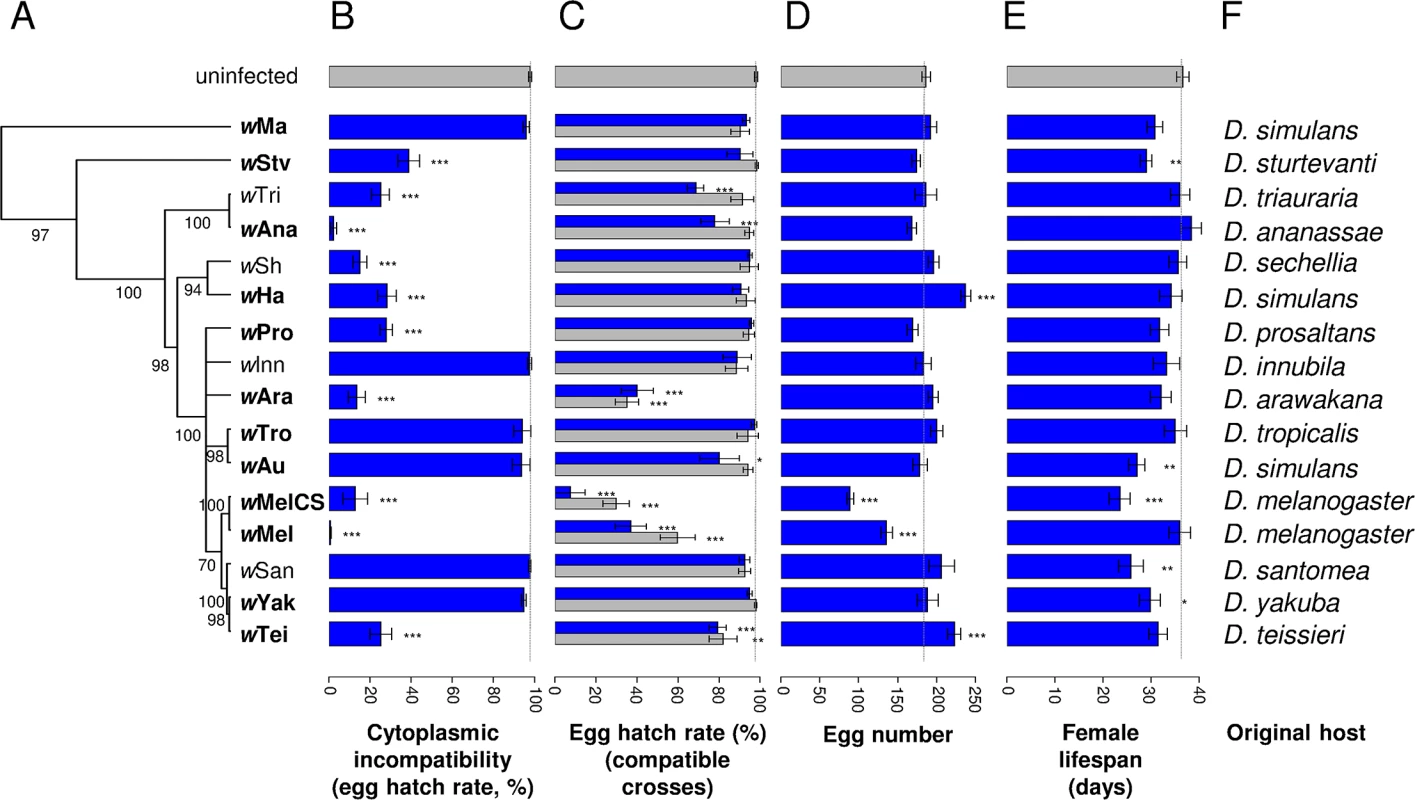
(A) The phylogeny based on the MLST genes 16S rRNA, aspC, atpD, ftsZ, sucB, groEL, coxA and fbpA was inferred using ClonalFrame v1.2 [43] as in [14]. Strains in bold conferred significant antiviral protection [14]. Branch labels represent posterior support values. Nodes with less than 50% support were collapsed. Branch lengths indicate relative time. (B) CI measured as egg hatch rates in crosses between uninfected females and Wolbachia-infected males. (C) Egg hatch rates in crosses between Wolbachia-infected females and Wolbachia-infected males (blue bars) or uninfected males (grey bars). (D) Fecundity of Wolbachia-infected females. (E) Lifespan of Wolbachia-infected females. Error bars are standard errors. *: significance relative to the Wolbachia-free line (Dunnett’s test; *: P < 0.05; **: P < 0.01; ***: P < 0.001). The dotted line indicates for each trait the mean value in the Wolbachia-free controls. (F) Original host species of the Wolbachia strains. Cytoplasmic incompatibility and antiviral protection are independent traits
Cytoplasmic incompatibility causes an excess of embryonic mortality in crosses between symbiont-infected males and uninfected females. Therefore, in order to measure levels of CI induced by different Wolbachia strains, we crossed infected males of each strain with uninfected females and counted the number of eggs that hatched (9,432 eggs from 380 females). There was a significant effect of Wolbachia (Deviance = 681.81; df = 16; P < 0.0001) with a clear division between 10 strains that induce CI and six that do not (Fig 1B). The strength of CI also varied among the 10 CI strains, ranging from just 0.5% of the eggs hatching in incompatible crosses involving the wMel strain, to 38.7% of the eggs hatching with wStv.
We have previously shown that these strains provide varying levels of protection against the viruses DCV and FHV [14], and using this data we found that there was no correlation between CI and the antiviral effects of Wolbachia. This was the case regardless of which virus the flies are infected with or whether antiviral protection is measured in terms of increased survival (black line in Fig 2A and 2B) or reduced viral titre (black line in S1A and S1B Fig). This conclusion also holds if we only analyse the 10 strains that induce significant CI (red line in Fig 2A and 2B; S1A and S1B Fig). Since a decrease in hatch rate in incompatible crosses can be due not only to CI but also to an induced cost on male fertility, we also analysed the correlation between protection and levels of CI corrected for differences in male fertility (the hatch rates of infected females mated with infected males relative to hatch rates when mated with uninfected males). Similar to the uncorrected estimate, these corrected levels of CI did not show any significant correlation with antiviral protection, whether measured as survival after infection (Pearson’s correlation test: All strains: DCV: P = 0.28 and FHV: P = 0.86; CI-inducing strains: DCV: P = 0.67 and FHV: P = 0.71) or as viral titre (Pearson’s correlation test: All strains: DCV: P = 0.58 and FHV: P = 0.95; CI-inducing strains: DCV: P = 0.87 and FHV: P = 0.75).
Fig. 2. Correlation between CI and antiviral protection. 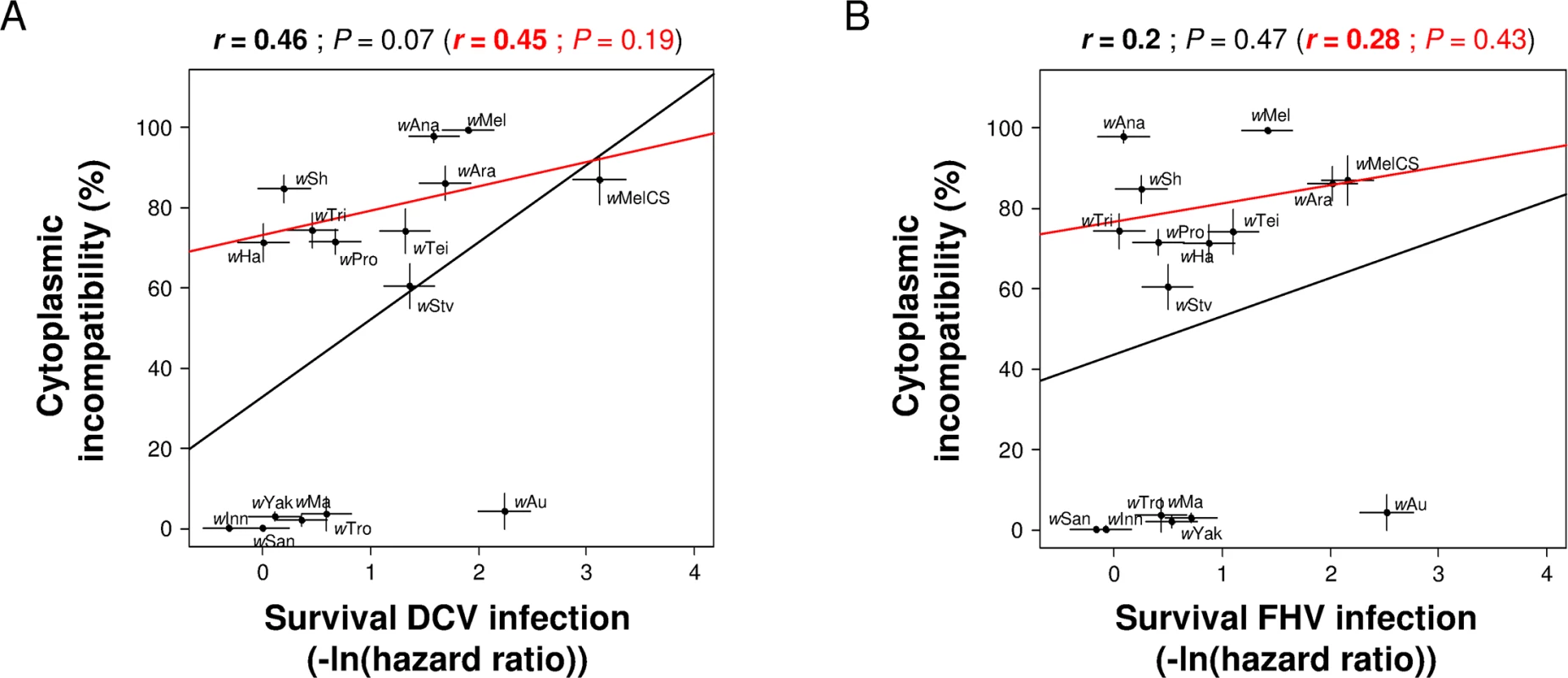
Levels of CI estimated as the percentage of unhatched eggs relative to the mean hatch rate in crosses between uninfected females and uninfected males. Level of protection measured as survival in [14] upon infection with (A) DCV and (B) FHV (0 and positive values mean no difference and increase in survival compared to Wolbachia-free control respectively). Means and standard errors are shown. Solid lines show predicted values from linear regressions using all strains (black) or only CI-inducing strains (red). r is the Pearson’s correlation coefficient between traits. Antiviral protection is costly
As Wolbachia is vertically transmitted, reductions in the survival or fecundity of Wolbachia-infected females will reduce the fitness of both the host and the symbiont. To estimate these costs, we measured egg hatch rates (in parallel to the CI crosses, 16,469 eggs from 555 females), early-life fecundity (280,260 eggs from 1,548 females) and female lifespan (913 females) of flies infected with the 16 different Wolbachia strains.
We found significant variation in egg hatch rates between fly lines infected with different Wolbachia strains (Fig 1C; Deviance = 340,97; df = 16; P < 0.0001). When the father was uninfected, four strains caused a significant reduction in hatch rates, with three of them resulting in less than 40% of the eggs hatching (Fig 1C, grey bars). Additionally, when both the mother and father were infected, there was a trend towards even lower hatch rates, with two more strains becoming significant (Fig 1C, blue bars). This suggests that male fertility is also being reduced by Wolbachia or that rescue of CI is not perfect for some of the strains (ie the modification of sperm in males that is required for CI still causes embryonic mortality when the egg is infected).
Fecundity and lifespan are also affected by Wolbachia. For fecundity, two strains increased and two strains reduced the number of eggs laid (Deviance = 250.55; df = 16; P < 0.0001; Fig 1D). Wolbachia also affected female survival (Deviance = 52.37; df = 16; P < 0.0001), with five of the sixteen strains significantly shortening lifespan (Fig 1E).
The strains that provide the greatest protection against viruses (measured as survival) tended to cause the greatest reductions in the other life-history traits of the flies. Hatch rates of Wolbachia-infected females were significantly reduced in flies carrying the symbionts providing the highest levels of protection against both DCV and FHV, whatever the Wolbachia-infection status of males (Fig 3A and 3B; S2A and S2B Fig). Because the tested traits are not phylogenetically independent, we reanalyzed these correlations using phylogenetic independent contrasts (see methods). The correlations between hatch rates and level of protection were robust to the phylogenetic non-independence of the data (S1 Table). Higher levels of antiviral protection were also associated with reduced male fertility (Fig 3C and 3D) and lower fecundity (Fig 3E and 3F), but these correlations were only significant in case of DCV. Phylogenetic independent contrasts analyses also showed that correlations with male fertility and fecundity were significant but it strongly depended on the branch length used in the linear models (S1 Table). No correlation with the level of protection and female lifespan was detected (S2C and S2D Fig; note the smaller sample sizes for this trait). Interestingly, wAu, which is a native strain of D. simulans, provides high antiviral protection yet induced little reduction in hatch rates or fecundity.
Fig. 3. Correlations between antiviral protection and other host life-history traits. 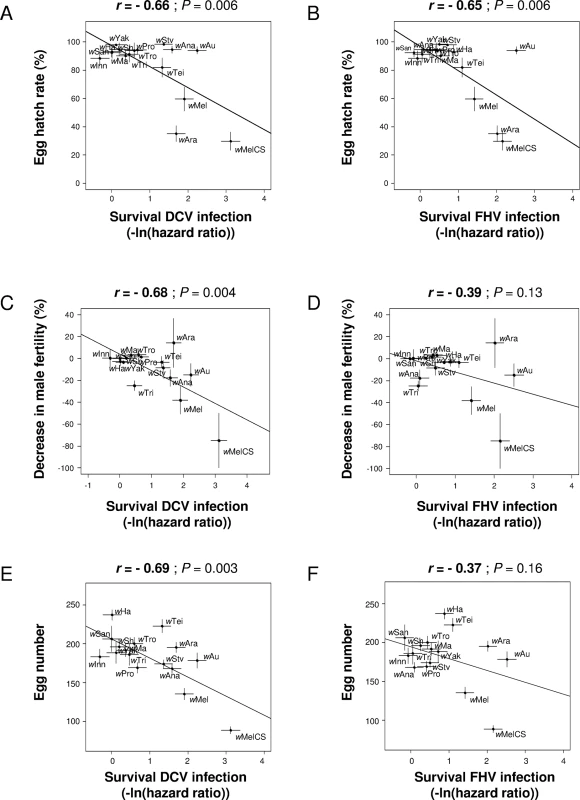
A and B: correlation between survival after viral infection and egg hatch rates in crosses between Wolbachia-free males and Wolbachia-infected females. Virus infections used (A) DCV and (B) FHV [14] (0 and positive values mean no difference and increase in survival compared to Wolbachia-free control respectively). C and D: correlation between decrease in male fertility in crosses between Wolbachia-infected parents and survival after infection with (C) DCV and (D) FHV. E and F: correlation between egg number and survival after infection with (E) DCV and (F) FHV. Means and standard errors are shown. Solid lines show predicted values from linear regressions. r is the Pearson’s correlation coefficient between traits. If the antiviral effects of Wolbachia were measured as changes in viral titres rather than survival, most of the correlations became non-significant or marginally-significant, but the direction of the relationships remained the same, with low viral titres associated with stronger costs (S3A–S3J Fig). Again, costs induced by wAu on hatch rates were generally lower than expected by the correlations with viral titres.
Cytoplasmic incompatibility is not costly
Similar to antiviral protection, we tested for correlations between levels of CI and other components of host fitness. There was no significant correlation between the level of CI and male fertility, female fecundity, lifespan or the hatch rate of eggs from crosses between Wolbachia-infected females and uninfected males (Fig 4A–4C; S4B Fig). In crosses where both parents were Wolbachia-infected, the level of CI was negatively correlated with hatch rates (S4A Fig). This was only the case when both CI inducing and non-CI inducing strains were analyzed, and it may reflect incomplete rescue of cytoplasmic incompatibility.
Fig. 4. Correlations between CI and other host life-history traits. 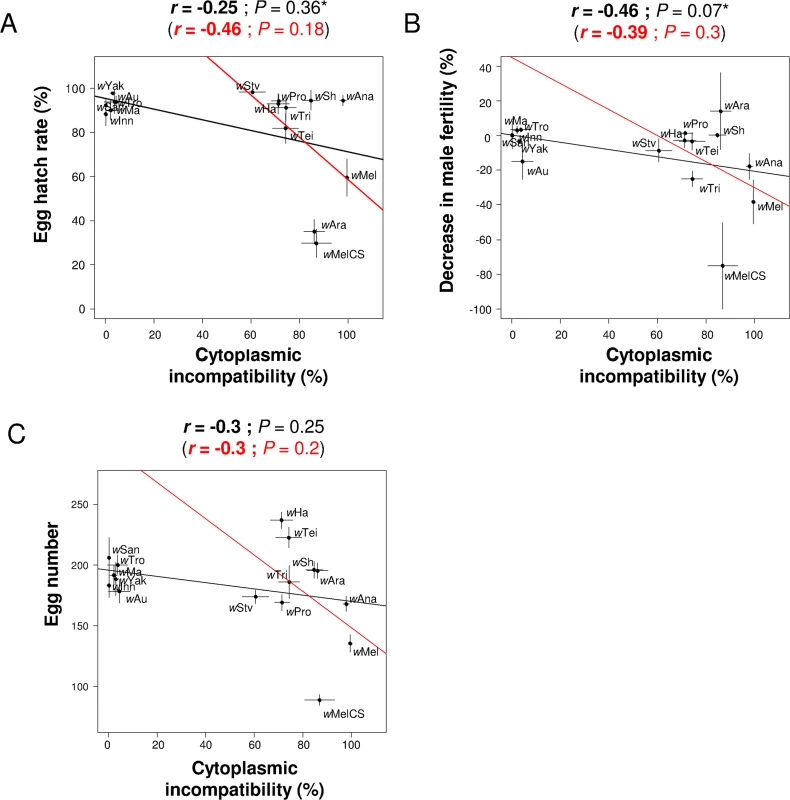
The level of CI is correlated with (A) the egg hatch rates in crosses with Wolbachia-free males, (B) the decrease in male fertility and (C) the egg number. Means and standard errors are shown. Solid lines show predicted values from linear regressions using all strains (black) or only CI-inducing strains (red). r is the Pearson’s or Spearman’s (*) correlation coefficient between traits. Wolbachia density mediates the trade-off between protection and cost
We hypothesized that Wolbachia must infect the germline to induce CI and somatic tissues to provide antiviral protection, so differences in tissue tropism between symbiont strains may partly explain why they have different phenotypic effects on their hosts. To examine this, we measured Wolbachia density in somatic tissues (head and thorax of females), testes and freshly laid eggs (as a proxy for the female germline).
There were large between-strain differences in density (Fig 5A–5C). For example, in somatic tissues the Wolbachia copy number varies over a 19-fold range. Furthermore, the strains have different tissue tropisms, with a significant strain-by-tissue interaction (Fig 5A–5C). The density in the testes and head + thorax tended to be tightly correlated (Pearson’s correlation test: r = 0.89; P < 0.0001), and frequently differed from the density in eggs (Pearson’s correlation test: head + thorax–eggs: r = 0.63; P = 0.01; testes–eggs: r = 0.61; P = 0.013).
Fig. 5. Wolbachia tissue tropism. 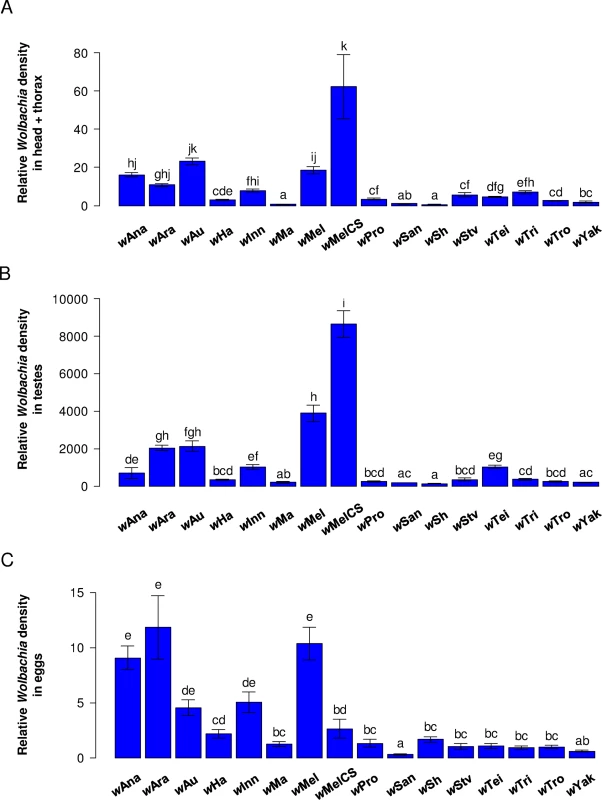
Mean Wolbachia density in (A) head and thorax of females, (B) testes and (C) freshly laid eggs. Error bars are standard errors. Letters indicate significant differences based on a Tukey’s honest significance test on ln-transformed data. All tissues were analyzed in a single linear model to test for difference in tissue tropism: strain effect: F15,427 = 131. 1; P < 0.0001; tissue effect: F2,427 = 4448. 8; P < 0.0001; strain × tissue effect: F30,427 = 11.5; P < 0.0001. Variation in Wolbachia density can explain between-strain differences in antiviral protection but not differences in CI. Protection against DCV and FHV was positively correlated with Wolbachia density in head and thorax, whether measured as survival (Fig 6A and 6B) or viral titres (S5A and S5B Fig), even after removing potential phylogenetic effects (S1 Table). This holds when both the density in the soma and eggs are included as predictive variables: protection shows a significant partial correlation with density in the soma but not with density in the eggs (only marginally significant for FHV titre; S2 Table). On the contrary, there is no correlation between levels of CI and density in the somatic tissues (Fig 6C), in the testes or in the eggs (S6A–S6B Fig). The only exception to this was when only analyzing CI-inducing strains, levels of CI were positively correlated to the Wolbachia density in eggs (red line in S6B Fig; note eggs are uninfected in the CI cross).
Fig. 6. Correlations between Wolbachia density in somatic tissues and antiviral protection, CI or other host life-history traits. 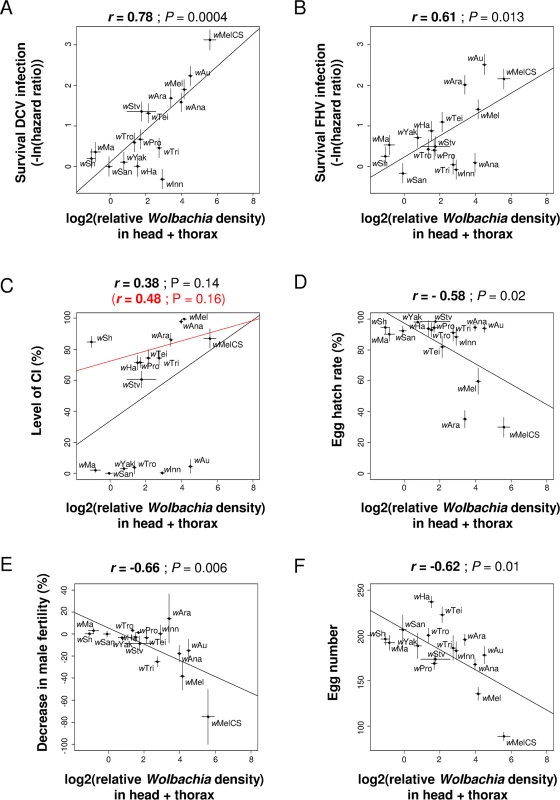
The relative Wolbachia density in head and thorax of females is correlated with survival [14] upon infection with (A) DCV or (B) FHV (0 and positive values mean no difference and increase in survival compared to Wolbachia-free control respectively), (C) the level of CI, (D) the egg hatch rate in crosses with Wolbachia-free males, (E) the decrease in male fertility and (F) the egg number. Means and standard errors are shown. Solid lines show predicted values from linear regressions. r is the Pearson’s correlation coefficient between traits. The negative effects of Wolbachia on host life-history traits are related to the symbiont density, with hatch rates, male fertility and fecundity all negatively correlated to the Wolbachia density in the somatic tissues (Fig 6D–6F) but not with the density in the eggs (Pearson’s correlation test: Hatch rate with uninfected father: P = 0.08; hatch rate with infected father: P = 0.06; male fertility: P = 0.58; fecundity: P = 0.27). The same conclusion holds when controlling for the Wolbachia phylogeny (S1 Table), although for male fertility and fecundity significance depends on the branch length used for the linear model. When these traits are analyzed with a multiple regression, they show significant partial correlations with density in the soma but not with density in the eggs (S2 Table). There was no correlation between female lifespan and Wolbachia density in any of the tissues (Pearson’s correlation test: head + thorax: P = 0.73; testes: P = 0.32; eggs: P = 0.13).
Discussion
Heritable bacterial symbionts have successfully colonized a wide range of arthropods by using a diversity of strategies ranging from mutualism to parasitism. Typically the evolution of these symbiont strategies has been considered in isolation, but this can be misleading if there are trade-offs between these traits and other components of host or symbiont fitness. Identifying these trade-offs is not only a prerequisite to understand the evolution of symbiosis, but will also inform the use of symbionts in applied programs. Using a set of Wolbachia strains that provide varying levels of protection against viral pathogens, we found that this mutualistic effect was independent of the ability to parasitically manipulate host reproduction. Antiviral protection relies on the bacteria reaching high densities in somatic tissues and is associated with strong reductions in several host life-history traits, while reproductive parasitism is not linked to symbiont density in somatic tissues and not costly to infected females.
While some symbionts are mutualists that spread through populations by increasing host fitness and others are parasites that manipulate host reproduction, others simultaneously have both effects [11]. It is already well known that in Wolbachia antiviral protection and CI are highly genetically variable traits [14,27,37,44]. However, to our knowledge, our study is the first to assess both traits in a wide array of strains in a common host genetic background. We found no correlation between the expressions of these phenotypes, with four strains only providing protection, two strains only inducing CI, eight strains inducing both protection and CI, and two strains showing neither phenotype. Therefore, these traits have independent evolutionary trajectories. Some strains may also rely on alternative strategies to be maintained in populations, such as enhancing the host fecundity or other fitness components [45]. For instance, two of the tested strains in our study were associated with increased fecundity.
Besides antiviral protection and reproductive manipulation, Wolbachia infections can induce fitness costs, with important life-history traits being affected such as lifespan, fecundity, egg viability or larval development and competitiveness [30,46–53]. In accordance with previous studies, we found Wolbachia-induced costs on several traits that should reduce both the fitness of the host and of Wolbachia. In some cases these costs could be very large–for example some strains result in the majority of infected eggs never hatching, suggesting that those strains might not be able to invade natural host populations.
We found that antiviral protection trade-offs with egg hatch rates, female fecundity and male fertility. In many cases highly protective strains induced substantial reductions in these fitness components. Because Wolbachia relies on host reproduction for its transmission, these trade-offs will affect both the host and symbiont, as both partners benefit from antiviral protection and both will suffer from reduced female reproduction. Further evidence that antiviral protection is costly comes from a comparison of the two main Wolbachia genotypes in D. melanogaster populations, which showed that the genotype that provided the greatest antiviral protection also shortened the lifespan of infected flies (Chrostek et al. 2013). Similarly, when wAu is transferred into D. melanogaster it reaches high densities, provides strong protection against viruses and shortens the lifespan of flies (Chrostek et al. 2014). Interestingly, using a similar experimental design to ours, another study showed that high levels of protection conferred by the symbiont Hamiltonella defensa against parasitoids in aphids are associated with less costly symbiont strains contrary to what we found [54]. While the mechanisms of protection in Wolbachia remain to be elucidated, in H. defensa it is known that protection relies on the presence of a bacteriophage encoding a toxin [55,56]. It is likely that different mechanisms of protection lead to different trade-offs with host life-history traits.
The reason that Wolbachia-mediated antiviral protection is so costly appears to be that it requires high symbiont densities. The density of Wolbachia in host tissues have been repeatedly shown to be involved in the ability of the bacteria to protect against viruses [12,14,27,36–38,57,58], and this was also the case in the present study with high protection being associated with higher densities in the somatic tissues of the flies. Using our sixteen Wolbachia strains we were able to test for a correlation between density and costs, and found that high densities of the bacteria in somatic tissues correlate with lower egg hatch rates, male fertility and fecundity. Harboring high loads of Wolbachia might be harming flies due to a re-allocation of resources from host to symbiont or pathological effects of the symbiont infection. Accordingly, wMelPop, a mutant strain that over-replicates causes a severe life-shortening effect [38,48,59] and other high density Wolbachia genotypes in D.melanogaster are associated with reduced lifespan [27,34]. The correlations between antiviral protection, costs on life-history traits and Wolbachia density remained when controlling for phylogenetic effects, which supports the hypothesis that there is a causal link between antiviral protection and costs that is mediated by symbiont density.
Contrary to antiviral protection, we did not observe any trade-off between the expression of CI and the other host fitness components. The explanation for this is likely that CI levels were not correlated to the density of Wolbachia in somatic tissues (note that our sample size is limited if considering just CI inducing strains). CI is thought to be the result of a sperm modification causing improper segregation of the paternal chromosomes after fertilization of the egg [60]. Rather than the overall density of Wolbachia in the somatic tissues, it is the ability of the bacteria to specifically colonize sperm cysts that is thought to allow the expression of CI [39,42]. For this reason we investigated whether differences in tissue tropism between strains might affect whether they cause CI. While tissue tropism did vary, there was no correlation between density in testes and CI, but it may be that this is a poor proxy for the number of sperm cysts that are infected. However, we found that, among CI-inducing strains, levels of CI were positively correlated with the bacterial density in eggs (our measure of female germline density), similar to what was found in another study [40]. It is possible that higher density in the eggs might correlate with bacteria targeting the germ line in developing male embryos. Alternatively, the egg is the site of the rescue activity that prevents the expression of CI in Wolbachia-infected embryos [60], so strains inducing high levels of CI may have evolved towards higher density in the egg to overcome the effect of the sperm modification.
Our findings have important implications regarding the evolution of Wolbachia symbioses, as trade-offs will act as a constraint on the evolution of mutualism (protection) but not reproductive parasitism (CI). Selection will act on both host and parasite genes to reduce the cost of Wolbachia infection, and alone this is likely to lead to the evolution of lower bacterial densities and therefore reduced antiviral protection. Thus, unless antiviral protection is sufficiently strongly selected for, it may reach lower levels or even disappear as the two partners coevolved towards less harmful Wolbachia infection. This prediction is supported by the partial replacement of the highly protective strain wMelCS by wMel, a lower density strain inducing lower protection, in populations of D. melanogaster [25–27]. Strikingly, in the pathogenic strain wMelPop, the symbiont density and the associated level of protection and costs on other life-history traits have been shown to evolve quickly, over a few host generations, suggesting that such changes may rapidly occur in nature [38]. As the most protective strains are very costly, they may only be favoured when there is very strong selection by viruses.
Over the long term, selection may sometimes be able to break a trade-off [61] and lead to the evolution of Wolbachia strains that provide the benefits of antiviral protection but without the associated costs. Because we transferred most of the symbiont strains from other species into D. simulans, the control of the bacterial density and associated costs is expected to be inefficient due to a lack of coevolution between the two partners. This situation therefore reflects new associations that have arisen by horizontal transmission (as frequently occurs during the evolution of Wolbachia). We had one highly protective strain that naturally occurs in D. simulans, and this strain induced little cost on egg hatch rates despite showing rather high bacterial density and strong protection. This strain does not induce CI and yet shows rapid spread in natural populations [20,21] suggesting that protection might be the selective force driving the evolution of this strain. While this suggests that natural selection may be able to break the association between antiviral protection and cost, this may not be inevitable as naturally occurring protective Wolbachia strains in D. melanogaster still reduce the lifespan of flies [27].
Whether CI or antiviral protection is favored by selection will depend not only on the costs of these traits but also the strength of selection favouring the trait. Selection on the symbiont to evolve CI may often be very weak–there is no selection for the phenotype in males in panmictic populations [32,62], and its evolution relies on population structure generating local relatedness [63] [64] (see [65] for an alternative explanation). Our observation that CI is not associated with costly changes in the phenotype of infected females (the transmitting sex) means there may often be little selection on the symbiont to reduce the strength of CI, making it stable over evolutionary time even when population structure is weak.
Finally, our results have implications for the control of vector-borne viral diseases by the introduction of Wolbachia into mosquito populations, as such efforts may fail if selection to reduce the cost of infection leads to reduced symbiont density and therefore the loss of antiviral protection [66]. This is even more likely if viruses cause little harm to its vector or are rare in the vector population, thus inducing little selective pressure on protection [23]. This is the case for the main target of these control efforts, dengue virus, which is thought to only decrease the fitness of mosquitoes by a few percent [67] and its prevalence in mosquito populations is low [68]. Therefore, the long-term maintenance of protection may rely on selection by the wider community of viruses favouring protection. The first releases of Wolbachia infected Aedes aegypti mosquitoes took place in 2011 [29], and one year later the Wolbachia strain still protected against dengue virus infection [31]. Only further monitoring over future years will determine whether this is truly an ‘evolution proof’ method of disease control.
Methods
Drosophila lines and Wolbachia strains
All Wolbachia strains were in the D. simulans STCP line that was generated by six generations of sib matings [69]. Wolbachia was previously backcrossed or microinjected into the STCP line [14,44,69,70].
Flies were maintained on a cornmeal diet at 25°C, 12 hours light/dark and 70% relative humidity. To minimize inbreeding effects, before each experiment STCP females were crossed to males of a different Wolbachia-free isofemale line (14021–0251.175, Dsim\wild-type, San Diego Drosophila Species Stock Center). Groups of 30 first instar F1 larvae were then transferred to new vials to ensure a constant larval density. Measurements of fitness traits were carried out on emerging F1 adults. Except for the fecundity measurements, F1 larvae were raised on a standard cornmeal diet (agar: 1%, dextrose: 8.75%, maize: 8.75%, yeast: 2%, nipagin: 3%) with 100 μl of 15% liquid yeast on the top of the food. For the fecundity experiment (see below), F1 larvae developed on a diet depleted in maize (4.4%) and dextrose (4.4%) with no added yeast to create less favorable conditions. Two generations before the experiments, Wolbachia infection statuses were checked by PCR using primers wsp81F and wsp691R [71].
Hatch rates and cytoplasmic incompatibility
Virgin F1 male and female flies were collected and aged for 3 and 5 days respectively. Because multiple male matings can decrease the strength of CI [72,73], a male and female were placed in a vial for 4–8 hours. In D. simulans, remating does not occur within 8 hours after the first copulation (Nina Wedell, personal communication). Females were then placed individually on a 50 mm diameter Petri dish with standard cornmeal diet containing food coloring with 15 μl of 15% liquid yeast on the top of the food. Around 20 hours later, females were removed and eggs were counted. Females that laid five or less eggs were discarded. Hatch rates were estimated by counting unhatched eggs about 35 hours later. The compatible crosses between uninfected males and uninfected females showed a mean egg hatch rate of 98%, thus suggesting that most females in this experiment were mated. Moreover, for Wolbachia-infected lines, discarding potentially non-mated females for which none of the eggs hatched did not change the significance of correlations with the other traits as mean hatch rates with or without those females were strongly correlated (Pearson’s correlation test: r ≈ 0.99, df = 14, P < 0.0001).
Fecundity
F1 larvae were raised on our poor diet, and 0 to 2-day-old flies were placed on standard cornmeal food with live yeast on the surface to stimulate egg maturation. After 2 days (2 - to 4-day-old), 3 males and 3 females were placed in petri dishes of colored poor diet. Over 6 days, flies were anaesthetized with CO2 and transferred onto a new dish every 24 hours. The number of eggs was recorded by photographing the Petri dish and counting eggs using a multi-point counter tool in ImageJ [74].
Female lifespan
As for the hatch rate experiment, F1 larvae were raised on our standard cornmeal diet. Five male and 5 female freshly emerged flies were placed per vial on poor diet. Flies were tipped onto fresh food every 3 days and the number of dead female flies recorded daily for 72 days until all flies died.
Dissections
To investigate Wolbachia tissue tropism, F1 larvae were reared on standard diet, and virgin males and females aged to 3 and 5-day-old respectively. Males were then anaesthetized on ice and dissected in Ringer’s solution [75]. For each Wolbachia strain, 10 pools of 5 pairs of testes were collected.
Five-day-old females were allowed to mate with 2 - to 4-day-old virgin Wolbachia-free STCP males for 24 hours. Females were then isolated and 10 replicates of 3 females per strain were placed in Petri dishes onto grape agar food with 15 μl of 15% liquid yeast on the top. After 6 to 8 hours, 20 eggs were harvested from each Petri dish and transferred into a microcentrifuge tube. In parallel, the head and thorax was separated from the abdomen of 6-day-old females. For each Wolbachia strain, 10 replicates, each consisting of a pool of head and thorax collected from 10 females were transferred into microcentrifuge tubes. All tissues were frozen at -80°C for DNA extraction.
DNA extraction and quantitative PCR
DNA was extracted from the tissue samples using EconoSpin All-In-One Silica Membrane Mini Spin Columns (Epoch Biolabs) and the QIAamp DNA Micro kit (Qiagen). Using the extracted DNA, quantitative PCR (qPCR) was used to determine the Wolbachia density in the carcasses (head and thorax), testes and eggs. For carcasses and testes, the amount of the Wolbachia gene atpD (atpDQALL_F: 5’-CCTTATCTTAAAGGAGGAAA-3’; atpDQALL_R: 5’-AATCCTTTATGAGCTTTTGC-3’) relative to the endogenous control gene actin 5C (Forward primer: 5’-GACGAAGAAGTTGCTGCTCTGGTTG-3’; Reverse primer: 5’-TGAGGATACCACGCTTGCTCTGC-3’) was quantified using the SensiFAST SYBR & Fluorescein kit (Bioline). The Wolbachia density was estimated as: 2ΔCt, where Ct is the cycle threshold and ΔC t = Ctactin5C-CtatpD. The PCR cycle was 95°C for 2 min, followed by 40 cycles of 95°C for 5 s, 55°C for 10 s, 72°C for 5 s. Since embryo mortality due to Wolbachia was observed in our experiment on hatch rate, the Wolbachia density in eggs was estimated as the amount of the gene atpD in a sample relative to the amount of the same gene in a positive control placed on every qPCR plate as follow: 2ΔCt, where Ct is the cycle threshold and ΔC t = Ctpositive control-CtatpD. For each sample, two qPCR reactions (technical replicates) were carried out and a linear model was used to correct for plate effects.
Statistical analysis
Statistical analyses were performed using R [76]. Hatch rates were analyzed using mixed effect generalized linear models with a logit link function and the effect of individual mothers treated as random (package lme4). Fecundity was analyzed using a linear model with the total number of eggs laid over 6 days as a response and a random temporal block effect. Female lifespan was analyzed with a generalized linear model with the Wolbachia infection status as a fixed effect and vial as a random effect. To test the effects of Wolbachia strain individually on these traits we performed multiple comparisons with the control cross (uninfected flies) using Dunnett’s test (package multcomp).
Wolbachia densities within tissues were log2-transformed and analyzed with a linear model including the effect of the Wolbachia strain, tissue, and their interaction. Between-strain differences in density were tested using multiple comparisons (Tukey’s HSD test, package multcomp).
Between-trait correlations were tested with Pearson’s correlation tests unless the assumptions of normality and homoscedasticity were not reached, in which case Spearman’s tests were used. In order to take into account the phylogenetic non-independence of the data, significant correlations were further analyzed using independent contrasts [77] with the function crunch (R package caper) [78] (See S1 Table).
Supporting Information
Zdroje
1. Douglas AE. The microbial dimension in insect nutritional ecology. Funct Ecol. 2009;23 : 38–47.
2. Moran N, Tran P, Gerardo N. Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl Environ Microbiol. 2005;71 : 8802–8810. 16332876
3. Russell J, Moran N. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc R Soc B Biol Sci. 2006;273 : 603–10.
4. Oliver KM, Russell JA, Moran NA, Hunter MS. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. 2003;100 : 1803–1807. 12563031
5. Xie JL, Vilchez I, Mateos M. Spiroplasma Bacteria Enhance Survival of Drosophila hydei Attacked by the Parasitic Wasp Leptopilina heterotoma. PLoS One. 2010;5: e12149. doi: 10.1371/journal.pone.0012149 20730104
6. Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science. 2010;329 : 212–5. doi: 10.1126/science.1188235 20616278
7. Teixeira L, Ferreira A, Ashburner M. The Bacterial Symbiont Wolbachia Induces Resistance to RNA Viral Infections in Drosophila melanogaster. Plos Biol. 2008;6 : 2753–2763.
8. Scarborough CL, Ferrari J, Godfray HCJ. Aphid protected from pathogen by endosymbiont. Science. United States; 2005;310 : 1781. 16357252
9. Hedges L, Brownlie J, O’Neill S, Johnson K. Wolbachia and virus protection in insects. Science. 2008;322 : 702. doi: 10.1126/science.1162418 18974344
10. Engelstädter J, Hurst GDD. The Ecology and Evolution of Microbes that Manipulate Host Reproduction. Annu Rev Ecol Evol Syst. 2009;40 : 127–149.
11. Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science. 2011;332 : 254–6. doi: 10.1126/science.1199410 21474763
12. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139 : 1268–78. doi: 10.1016/j.cell.2009.11.042 20064373
13. Glaser RL, Meola MA. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One. 2010;5: e11977. doi: 10.1371/journal.pone.0011977 20700535
14. Martinez J, Longdon B, Bauer S, Chan Y-S, Miller WJ, Bourtzis K, et al. Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Wolbachia Strains. Schneider DS, editor. PLoS Pathog. 2014;10: e1004369. doi: 10.1371/journal.ppat.1004369 25233341
15. Van den Hurk AF, Hall-Mendelin S, Pyke AT, Frentiu FD, McElroy K, Day A, et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis. 2012;6: e1892. doi: 10.1371/journal.pntd.0001892 23133693
16. Kambris Z, Cook P, Phuc H, Sinkins S. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326 : 134–136. doi: 10.1126/science.1177531 19797660
17. Kambris Z, Blagborough AM, Pinto SB, Blagrove MSC, Godfray HCJ, Sinden RE, et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 2010;6: e1001143. doi: 10.1371/journal.ppat.1001143 20949079
18. Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7: e1002043. doi: 10.1371/journal.ppat.1002043 21625582
19. Ye YH, Woolfit M, Rancès E, O’Neill SL, McGraw E a. Wolbachia-Associated Bacterial Protection in the Mosquito Aedes aegypti. PLoS Negl Trop Dis. 2013;7.
20. Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR. Rapid Sequential Spread of Two Wolbachia Variants in Drosophila simulans. PLoS Pathog. 2013;9: e1003607. doi: 10.1371/journal.ppat.1003607 24068927
21. Hoffmann A, Clancy D, Duncan J. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity. 1996;76 : 1–8. 8575931
22. Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nature. 2008;6 : 741–751.
23. Bull JJ, Turelli M. Wolbachia versus dengue: Evolutionary forecasts. Evol Med Public Heal. 2013;2013 : 197–201.
24. McGraw E a, O’Neill SL. Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol. 2013;11 : 181–93. doi: 10.1038/nrmicro2968 23411863
25. Riegler M, Sidhu M, Miller WJ, O’Neill SL. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biol. 2005;15 : 1428–33. 16085497
26. Richardson MF, Weinert LA, Welch JJ, Linheiro RS, Magwire MM, Jiggins FM, et al. Population genomics of the Wolbachia endosymbiont in Drosophila melanogaster. PLoS Genet. 2012;8: e1003129. doi: 10.1371/journal.pgen.1003129 23284297
27. Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, et al. Wolbachia Variants Induce Differential Protection to Viruses in Drosophila melanogaster: A Phenotypic and Phylogenomic Analysis. Malik HS, editor. PLoS Genet. 2013;9: e1003896. doi: 10.1371/journal.pgen.1003896 24348259
28. Carrington LB, Lipkowitz JR, Hoffmann AA, Turelli M. A re-examination of Wolbachia-induced cytoplasmic incompatibility in California Drosophila simulans. PLoS One. 2011;6: e22565. doi: 10.1371/journal.pone.0022565 21799900
29. Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature; 2011;476 : 454–7. doi: 10.1038/nature10356 21866160
30. Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips BL, Billington K, Axford JK, et al. Stability of the wMel Wolbachia Infection following Invasion into Aedes aegypti Populations. PLoS Negl Trop Dis. 2014;8: e3115. doi: 10.1371/journal.pntd.0003115 25211492
31. Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014;8: e2688. doi: 10.1371/journal.pntd.0002688 24587459
32. Turelli M. Evolution of incompatibility-inducing microbes and their hosts. Evolution. 1994;48 : 1500–1513.
33. Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. From Parasite to Mutualist: Rapid Evolution of Wolbachia in Natural Populations of Drosophila. Plos Biol. 2007;5 : 997–1005.
34. Chrostek E, Marialva MSP, Yamada R, O’Neill SL, Teixeira L. High Anti-Viral Protection without Immune Upregulation after Interspecies Wolbachia Transfer. PLoS One. 2014;9: e99025. doi: 10.1371/journal.pone.0099025 24911519
35. McMeniman CJ, Lane AM, Fong AWC, Voronin D a, Iturbe-Ormaetxe I, Yamada R, et al. Host adaptation of a Wolbachia strain after long-term serial passage in mosquito cell lines. Appl Environ Microbiol. 2008;74 : 6963–9. doi: 10.1128/AEM.01038-08 18836024
36. Osborne SE, Iturbe-Ormaetxe I, Brownlie JC, O’Neill SL, Johnson KN. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl Environ Microbiol. 2012;78 : 6922–9. doi: 10.1128/AEM.01727-12 22843518
37. Osborne SE, Leong YS, O’Neill SL, Johnson KN. Variation in Antiviral Protection Mediated by Different Wolbachia Strains in Drosophila simulans. Plos Pathog. 2009;5 : 9.
38. Chrostek E, Teixeira L. Mutualism Breakdown by Amplification of Wolbachia Genes. PLOS Biol. 2015;13: e1002065. doi: 10.1371/journal.pbio.1002065 25668031
39. Veneti Z, Clark ME, Zabalou S, Karr TL, Savakis C, Bourtzis K. Cytoplasmic incompatibility and sperm cyst infection in different Drosophila-Wolbachia associations. Genetics. 2003;164 : 545–52. 12807775
40. Veneti Z, Clark M, Karr T. Heads or tails: host-parasite interactions in the Drosophila-Wolbachia system. Appl Environ Microbiol. 2004;70 : 5366–5372. 15345422
41. Clancy DJ, Hoffmann AA. Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia-infected Drosophila simulans. Entomol Exp Appl. 1998;86 : 13–24.
42. Clark M, Veneti Z, Bourtzis K, Karr T. Wolbachia distribution and cytoplasmic incompatibility during sperm development: the cyst as the basic cellular unit of CI expression. Mech Dev. 2003;120 : 185–198. 12559491
43. Didelot X, Falush D. Inference of Bacterial Microevolution Using Multilocus Sequence Data. Genet. 2007;175 : 1251–1266.
44. Zabalou S, Apostolaki A, Pattas S, Veneti Z, Paraskevopoulos C, Livadaras I, et al. Multiple rescue factors within a Wolbachia strain. Genetics. 2008;178 : 2145–60. doi: 10.1534/genetics.107.086488 18430940
45. Harcombe W, Hoffmann AA. Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. J Invertebr Pathol. 2004;87 : 45–50. 15491598
46. De Almeida F, Moura AS, Cardoso AF, Winter CE, Bijovsky AT, Suesdek L. Effects of Wolbachia on fitness of Culex quinquefasciatus (Diptera; Culicidae). Infect Genet Evol. 2011;11 : 2138–43. doi: 10.1016/j.meegid.2011.08.022 21907309
47. McMeniman CJ, O’Neill SL. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl Trop Dis. 2010;4: e748. doi: 10.1371/journal.pntd.0000748 20644622
48. Min K, Benzer S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci U S A. 1997;94 : 10792–10796. 9380712
49. Gavotte L, Mercer DR, Stoeckle JJ, Dobson SL. Costs and benefits of Wolbachia infection in immature Aedes albopictus depend upon sex and competition level. J Invertebr Pathol. Elsevier Inc.; 2010;105 : 341–6. doi: 10.1016/j.jip.2010.08.005 20807539
50. Yeap HL, Mee P, Walker T, Weeks AR, O’Neill SL, Johnson P, et al. Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics. 2011;187 : 583–95. doi: 10.1534/genetics.110.122390 21135075
51. Suh E, Dobson SL. Reduced competitiveness of Wolbachia infected Aedes aegypti larvae in intra - and inter-specific immature interactions. J Invertebr Pathol. Elsevier Inc.; 2013;114 : 173–177. doi: 10.1016/j.jip.2013.08.001 23933013
52. Turley AP, Zalucki MP, O’Neill SL, McGraw EA. Transinfected Wolbachia have minimal effects on male reproductive success in Aedes aegypti. Parasit Vectors. Parasites & Vectors; 2013;6 : 36.
53. Joshi D, McFadden MJ, Bevins D, Zhang F, Xi Z. Wolbachia strain wAlbB confers both fitness costs and benefit on Anopheles stephensi. Parasit Vectors. 2014;7 : 336. doi: 10.1186/1756-3305-7-336 25041943
54. Cayetano L, Rothacher L, Simon J, Vorburger C. Cheaper is not always worse: strongly protective isolates of a defensive symbiont are less costly to the aphid host. Proc R Soc B Biol Sci. 2015;282.
55. Oliver KM, Degnan PH, Hunter MS, Moran NA. Bacteriophages Encode Factors Required for Protection in a Symbiotic Mutualism. Science. 2009;325 : 992–994. doi: 10.1126/science.1174463 19696350
56. Weldon SR, Strand MR, Oliver KM. Phage loss and the breakdown of a defensive symbiosis in aphids. Proc Biol Sci. 2013;280 : 20122103. doi: 10.1098/rspb.2012.2103 23193123
57. Frentiu FD, Robinson J, Young PR, McGraw EA, O’Neill SL. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS One. 2010;5: e13398. doi: 10.1371/journal.pone.0013398 20976219
58. Lu P, Bian G, Pan X, Xi Z. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl Trop Dis. 2012;6: e1754. doi: 10.1371/journal.pntd.0001754 22848774
59. McGraw EA, Merritt DJ, Droller JN, O’Neill SL. Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci U S A. 2002;99 : 2918–23. 11880639
60. Tram U, Ferree PM, Sullivan W. Identification of Wolbachia-host interacting factors through cytological analysis. Microbes Infect. 2003;5 : 999–1011. 12941392
61. Blows MW, Hoffmann AA. A reassessment of genetic limits to evolutionary change. Ecology. 2005;86 : 1371–1384.
62. Prout T. Some evolutionary possibilities for a microbe that causes incompatibility in its host. Evolution. 1994;48 : 909–911.
63. Frank SA. Cytoplasmic Incompatibility and Population Structure. J Theor Biol. 1997;184 : 327–330.
64. Haygood R, Turelli M. Evolution of incompatibility-inducing microbes in subdivided host populations. Evolution. United States; 2009;63 : 432–447.
65. Hurst L, McVean GT. Clade Selection, Reversible Evolution and the Persistence of Selfish Elements: The Evolutionary Dynamics of Cytoplasmic Incompatibility. Proc R Soc London Ser B-Biological Sci. 1996;263 : 97–104.
66. Vavre F, Charlat S. Making (good) use of Wolbachia: what the models say. Curr Opin Microbiol. 2012;15 : 263–8. doi: 10.1016/j.mib.2012.03.005 22494817
67. Maciel-de-Freitas R, Koella JC, Lourenço-de-Oliveira R. Lower survival rate, longevity and fecundity of Aedes aegypti (Diptera: Culicidae) females orally challenged with dengue virus serotype 2. Trans R Soc Trop Med Hyg. 2011;105 : 452–458. doi: 10.1016/j.trstmh.2011.05.006 21700303
68. Yoon IK, Getis A, Aldstadt J, Rothman AL, Tannitisupawong D, Koenraadt CJM, et al. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012;6: e1730. doi: 10.1371/journal.pntd.0001730 22816001
69. Poinsot D, Bourtzis K, Markakis G, Savakis C. Wolbachia Transfer from Drosophila melanogaster into D. simulans: Host Effect and Cytoplasmic Incompatibility Relationships. Genetics. 1998;150 : 227–237. 9725842
70. Veneti Z, Zabalou S, Papafotiou G, Paraskevopoulos C, Pattas S, Livadaras I, et al. Loss of reproductive parasitism following transfer of male-killing Wolbachia to Drosophila melanogaster and Drosophila simulans. Heredity. 2012;109 : 306–12. doi: 10.1038/hdy.2012.43 22892635
71. O’Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci U S A. 1992;89 : 2699–702. 1557375
72. Awrahman ZA, Champion de Crespigny F, Wedell N. The impact of Wolbachia, male age and mating history on cytoplasmic incompatibility and sperm transfer in Drosophila simulans. J Evol Biol. 2013; 1–10.
73. Reynolds KT, Hoffmann AA. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet Res. 2002;80 : 79–87. 12534211
74. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved.; 2012;9 : 671–675.
75. Sullivan W, Ashburner M, Hawley R. Drosophila Protocols. New York: Cold Spring Harbor Laboratory Press; 2000.
76. R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Pimenta PF, editor. Vienna, Austria; 2013. Available: http://www.r-project.org
77. Felsenstein J. Phylogenies and the Comparative Method. The American Naturalist. 1985. pp. 1–15.
78. Orme D. The caper package: comparative analysis of phylogenetics and evolution in R. 2013.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



