-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
Erythrocytes infected by parasites causing severe P. falciparum malaria often form rosettes by binding a number of uninfected erythrocytes. Several of the parasite proteins involved are known, whereas the identity of the corresponding host receptor(s) on the surrounding erythrocytes is not. Although formation of rosettes often depends on non-immune IgM also binding to the infected erythrocytes, that does not by itself lead to formation of rosettes. Here, we report that the serum protein α2-macroglobulin (α2M) is able to induce rosetting in several in vitro and ex vivo parasite isolates. In contrast to IgM, α2M supports rosetting on its own, while presence of IgM markedly lowers the concentration of α2M required. These findings are explainable by the ability of α2M to crosslink at least four individual PfEMP1 molecules, indicating that the role of α2M in rosetting is to align multiple parasite adhesion proteins, thereby increasing their combined avidity for carbohydrate receptors on surrounding erythrocytes. Our study suggests a new mechanism whereby P. falciparum exploits soluble host proteins to avoid immune destruction, by using them to facilitate adhesion of infected erythrocytes to low-affinity carbohydrate receptors, and points to new strategies to interfere with a major pathogenic mechanism of this devastating parasite.
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1005022
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005022Summary
Erythrocytes infected by parasites causing severe P. falciparum malaria often form rosettes by binding a number of uninfected erythrocytes. Several of the parasite proteins involved are known, whereas the identity of the corresponding host receptor(s) on the surrounding erythrocytes is not. Although formation of rosettes often depends on non-immune IgM also binding to the infected erythrocytes, that does not by itself lead to formation of rosettes. Here, we report that the serum protein α2-macroglobulin (α2M) is able to induce rosetting in several in vitro and ex vivo parasite isolates. In contrast to IgM, α2M supports rosetting on its own, while presence of IgM markedly lowers the concentration of α2M required. These findings are explainable by the ability of α2M to crosslink at least four individual PfEMP1 molecules, indicating that the role of α2M in rosetting is to align multiple parasite adhesion proteins, thereby increasing their combined avidity for carbohydrate receptors on surrounding erythrocytes. Our study suggests a new mechanism whereby P. falciparum exploits soluble host proteins to avoid immune destruction, by using them to facilitate adhesion of infected erythrocytes to low-affinity carbohydrate receptors, and points to new strategies to interfere with a major pathogenic mechanism of this devastating parasite.
Introduction
About 630,000 (0.3%) of the approximately 200 million malaria cases each year are fatal [1]. The majority occur among African children below the age of five years, who die of severe Plasmodium falciparum malaria [2]. The particular virulence of P. falciparum parasites is related to the expression of adhesive proteins on the surface of the erythrocytes they infect, and the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family appears to be of particular importance in this respect. Each parasite genome encodes about 60 antigenically diverse PfEMP1 proteins composed of a series of Duffy binding-like (DBL) and Cysteine-rich inter-domain region (CIDR) domains. The PfEMP1 proteins are expressed on knob-like protrusions on the infected erythrocyte (IE) surface, where they mediate adhesion of IEs to a range of host endothelial receptors [3–6]. IE sequestration can cause inflammation and organ dysfunction, and can lead to severe and life-threatening complications [7]. In addition to adhesion to receptors in various organs, some P. falciparum-IEs also have the capacity to bind receptors on uninfected erythrocytes, leading to IE/erythrocyte aggregates called rosettes [8,9]. Although the mechanisms and functional significance of rosetting remain unclear, it has repeatedly been associated with parasites causing severe P. falciparum malaria [10–12]. Rosetting involves soluble factors in human serum, in apparent contrast to other PfEMP1-mediated IE adhesion to clinically important endothelial protein receptors such as intercellular cell adhesion molecule 1 (ICAM-1) and endothelial protein C receptor [4,6,13]. Several candidates have been proposed (reviewed in ref. [14]), but only pentameric IgM has repeatedly been found to be necessary, although not sufficient, for rosetting to occur [15–17]. The molecular details of this interaction between PfEMP1 and IgM have been described [17,18]. PfEMP1 interaction with the rosetting receptors on surrounding erythrocytes is mediated by N-terminal DBL1 α1.5/6/7/8 domains [19–21], whereas rosette-facilitating IgM binds to the membrane-proximal, C-terminal end of PfEMP1 [17,18]. How IgM can facilitate rosetting despite binding the opposite end of the PfEMP1 molecule facing the erythrocyte receptor is not known. Based on studies of the IgM-binding and rosette-mediating PfEMP1 protein HB3VAR06, we recently proposed that the function of IgM in rosetting could be cross-linking of PfEMP1 molecules [17], thereby overcoming their low individual affinity for their erythrocyte receptors, which are suspected to be mainly sulfated carbohydrate moieties [12,22–24]. However, we found that IgM alone was not sufficient to facilitate HB3VAR06-mediated rosetting, in line with earlier findings [15,16], and we therefore set out to identify the missing serum component(s) involved in rosetting and describe their involvement in the interaction.
Results
Identification of the human serum protein α2M binding to the PfEMP1 protein HB3VAR06
Pentameric IgM facilitates HB3VAR06-mediated rosetting, although additional unidentified serum components are required [17]. To identify these additional components, we used His-tagged recombinant full-length HB3VAR06 (FV6) coupled to magnetic epoxy resin beads to identify serum proteins with affinity for FV6. The proteins isolated by this pull-down technique were separated by 2-dimensional (2D) gel electrophoresis (Fig 1A). Two candidate spots with an apparent molecular weight of 180 kDa and a third candidate spot at 118kDa were detected, in addition to the expected 75-kDa band corresponding to the IgM heavy chain (Fig 1A, left). These spots were not observed in parallel experiments with a recombinant, His-tagged DBL domain (DBLβ3_D5) of a PfEMP1 protein (PFD1235w) not mediating rosetting [25,26] (Fig 1A, right). Matrix-assisted laser desorption/ionization (MALDI) time-of-flight (TOF)/TOF mass spectrometry analysis identified all three candidate spots as monomer subunits of the human protease inhibitor α2-macroglobulin (α2M). α2M is a 720-kDa homotetrameric glycoprotein composed of four disulfide-linked 180-kDa subunits that form a cage-like structure [27], which circulates at physiological concentrations of 3–4 μM (2–3 mg)/mL [28]. Among its many roles in regulation and transport, it is best known as a broad-spectrum protease inhibitor (reviewed in ref. [29]). The observed affinity of α2M for HB3VAR06 was confirmed by enzyme-linked immunosorbent assay (ELISA) and flow cytometry, demonstrating binding of α2M to FV6 and to native HB3VAR06 on IEs (Fig 1B and 1C). In contrast, α2M did not bind to the VAR2CSA-type PfEMP1 IT4VAR04, neither recombinant full-length protein (FV2) nor native IT4VAR04 on IEs (Fig 1B and 1C). Fluorescence microscopy of α2M-labeled HB3VAR06+ IEs revealed a punctate pattern of fluorescence (Fig 1D, top panels) resembling that characteristic of antibody-labeled PfEMP1 [30,31], whereas parallel samples including the detection antibodies but omitting α2M did not produce this pattern (Fig 1D, lower panels).
Fig. 1. Identification of α2M as the soluble serum factor binding HB3VAR06. 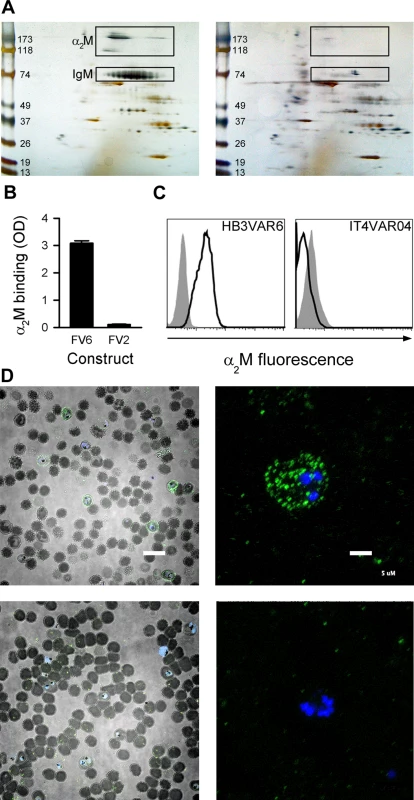
(A) 2D gel electrophoresis of serum components pulled down with recombinant full-length HB3VAR06 (FV6; left) or PFD1235w-DBLβ3_D5 (right). Spots subsequently identified as α2M and IgM in the left panel are boxed. Molecular weight (kDa) markers are shown along the left margins. (B) Binding of α2M to recombinant full-length HB3VAR06 (FV6; left) and IT4VAR04 (FV2; right), measured by ELISA. Means and SD are indicated. (C) Binding of α2M to HB3VAR06+ IEs (left) and IT4VAR04+ IEs (right), measured by flow cytometry. Control sample labeling (no α2M added) is indicated by gray shading. (D) Fluorescence micrographs of DAPI-labeled HB3VAR06+ IEs in the presence (top) and absence (bottom) of fluorescein isothiocyanate-labeled α2M at low (scale bar: 20 μm; left) and high (scale bar: 5 μm; right) magnification are shown. HB3VAR06 can bind native α2M
Circulating native α2M has a serum half-life of several hours [32]. It contains four “bait” regions that are highly susceptible to proteases, and an internal thioester that is sensitive to cleavage by small nucleophiles such as methylamine (MA). Cleavage of either the “bait” regions or the thioester results in a Venus flytrap-like conformational change in α2M. This exposes the thioester, which then covalently “captures” the protease in the cage-like structure and exposes the receptor-binding sites in α2M. In vivo, the serum half-life of this “activated” α2M is therefore short (2–4 min), as it is rapidly cleared from circulation when the receptor-binding sites bind to the α2M receptor low density lipoprotein receptor-related protein 1 (CD91) [32,33]. The conformation of α2M activated either by protease cleavage of the bait region or by nucleophile cleavage of the thioester are structurally and functionally similar and both bind CD91 (reviewed in ref. [34]). We therefore used native α2M and MA-activated α2M (α2M-MA) to examine which of the two conformational states could bind HB3VAR06. ELISA analysis showed that FV6 bound approximately four times more native α2M than α2M-MA (Fig 2A). Corresponding flow cytometry data showed concentration-dependent binding of native α2M binding to HB3VAR06+ IEs, whereas α2M-MA did not bind well to IEs, even at high concentrations (Fig 2B).
Fig. 2. Binding of native and MA-activated α2M to HB3VAR06. 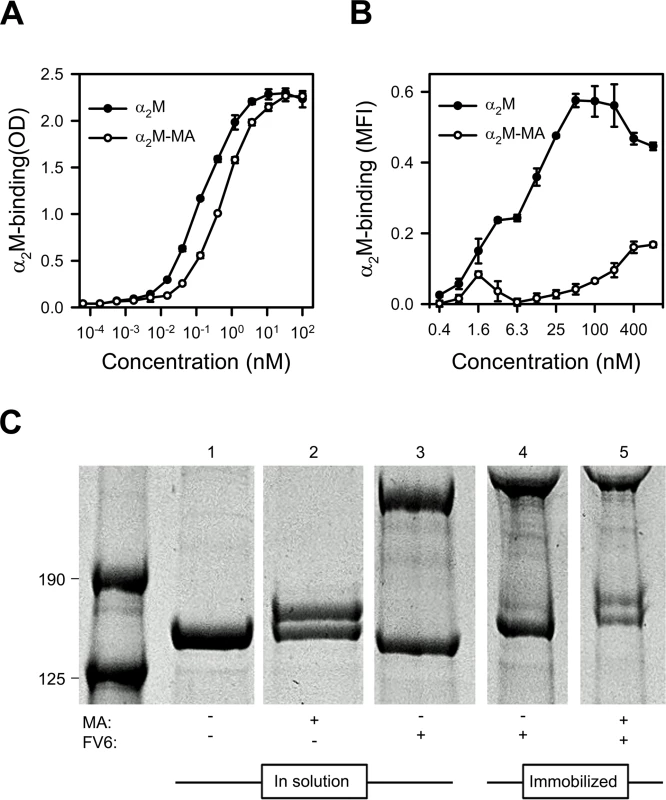
(A) Titration of binding of native α2M (black circles) and α2M-MA (white circles) to recombinant full-length HB3VAR06 measured by ELISA. Means and SD are indicated. (B) Titration of binding of native α2M (black circles) and α2M-MA (white circles) to HB3VAR06+ IEs measured by flow cytometry. Means and SD are indicated. (C) Activation of α2M measured by SDS gel electrophoresis of soluble and immobilized α2M in the presence of mPEG: soluble α2M alone (lane 1), soluble α2M and MA (lane 2), soluble α2M and FV6 (lane 3), bead-immobilized α2M-FV6 complexes alone (lane 4), and bead-immobilized α2M-FV6 complexes and MA (lane 5). While native α2M was detectable in all lanes, activated α2M having a higher molecular weight than native α2M due to incorporation of mPEG was only detected in the presence of MA (lanes 2 and 5). α2M is a promiscuous protein with about one hundred binding partners listed in the BioGRID database [35], and in most cases these interactions trigger activation of α2M. Methyl-poly(ethylene glycol) maleimide (mPEG) forms a covalent bond with the thiol group liberated following α2M activation. This can be detected by denaturing SDS gel electrophoresis as an increase in the molecular weight of activated α2M. We used this system to test whether interaction with HB3VAR06 activated α2M. In solution and in the presence of mPEG, MA activated native α2M as expected (Fig 2C, lane 2), whereas FV6 did not (lane 3). Immobilization of FV6-bound α2M to epoxy beads did not cause activation of α2M (lane 4), but the α2M in the immobilized complex could be activated by MA (lane 5).
α2M binds the penultimate C-terminal DBLξ2 domain of HB3VAR06
HB3VAR06 is composed of an N-terminal segment followed by eight DBL and CIDR domains (Fig 3A). In an earlier study, we used recombinant DBL domain constructs from HB3VAR06 to show that IgM binds to the penultimate DBL domain (D8; DBLξ2) near the C-terminus [17]. Taking a similar approach here, we could show that α2M also bound exclusively to recombinant single-, double-, and triple-domain constructs containing DBLξ2 (Fig 3B). False signals due to contaminating IgM in the primary or secondary antibodies used to detect α2M were ruled out in control experiments without α2M (S1 Fig).
Fig. 3. Identification and characterization of the α2M-binding domain in HB3VAR06. 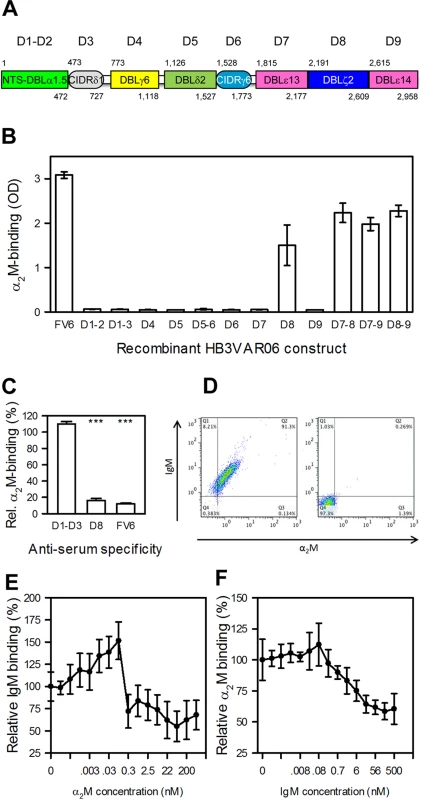
(A) Schematic representation of the domain structure of HB3VAR06. Domain nomenclature as described by Rask et al. [37], as well as the first and last amino acid in each domain are indicated. (B) Binding of α2M to recombinant HB3VAR06 single-, double-, and triple-domain constructs (labeled as in panel A) as well as to full-length HB3VAR06 (FV6) measured by ELISA. Means and SD are indicated. (C) Inhibition of α2M binding to HB3VAR06+ IEs by anti-sera raised against the N-terminal head structure (D1–D3), DBLξ2 (D8), and full-length HB3VAR06 (FV6), respectively, measured by flow cytometry. Means and SD relative to binding without anti-serum are indicated. (D) Simultaneous labeling of HB3VAR06+ IEs by α2M and IgM (left), measured by flow cytometry. A control experiment with all detecting antibodies present but without α2M and IgM is shown to the right. (E) Inhibition of IgM binding to HB3VAR06+ IEs by increasing concentrations of α2M, measured by flow cytometry. Means and SD relative to binding in the absence of α2M are indicated. (F) Inhibition of α2M binding to HB3VAR06+ IEs by increasing concentrations of IgM, measured by flow cytometry. Means and SD relative to binding in the absence of IgM are indicated. Flow cytometry analysis of HB3VAR06+ IEs showed that FV6 - and DBLξ2-specific antisera significantly reduced α2M binding, whereas an NTS-DBLα-CIDRδ-specific anti-serum did not (Fig 3C). Thus, IgM and α2M bound to the same domain in HB3VAR06. IgM and α2M are high-molecular weight proteins (about 900 kD and 720 kD, respectively), but either could bind HB3VAR06+ IEs in the presence of the other (Fig 3D). In competitive binding experiments, each protein reduced the binding of the other by approximately two-fold (Fig 3E and 3F). The results indicate that IgM and α2M bind to separate HB3VAR06 molecules with similar affinities, although we cannot rule out that individual HB3VAR06 molecules might be able to accommodate both IgM and α2M simultaneously.
α2M is required for HB3VAR06-mediated rosetting and acts synergistically with IgM
Size-exclusion chromatography analysis showed that saturation of native α2M by FV6 occurred at a α2M:FV6 ratio of at least 1 : 4, whereas saturation of α2M-MA binding to FV6 was reached already at a 1 : 1 ratio (Fig 4A and 4B). In the absence of serum, HB3VAR06+ IEs do not form rosettes [17], but native α2M alone could support rosette formation in serum-free medium in a concentration-dependent manner (Fig 4C). In contrast, α2M-MA had no effect on rosetting (Fig 4C). This supports our hypothesis that HB3VAR06 rosetting requires cross-linking of multiple PfEMP1 molecules to overcome the low affinity for receptors on erythrocytes [17], although it may simply reflect the inability of α2M-MA to bind to IEs (Fig 2B). Furthermore, the higher multimerization potential of α2M than IgM was consistent with our observation that α2M could induce rosetting in the absence of other serum factors (Fig 4C), in contrast to IgM [17]. IgM lowered the concentration of α2M required for rosetting, indicating that the two serum factors can act in synergy (Fig 4C). This was supported by the observation that rosetting rates were higher when α2M and IgM were added together at equimolar concentrations than the theoretical rates calculated as the sum of rosetting rates in medium with either component alone (S2 Fig). In our earlier study [17], we found that IgM-depleted serum did not support rosetting although α2M was not intentionally removed. However, addition of exogenous native α2M at concentrations lower than those required in serum-free medium restored the ability of IgM-depleted serum to support rosetting (Fig 4D). Thus, endogenous α2M levels in IgM-depleted serum appeared to be too low to sustain rosetting, but sufficient to augment IgM-dependent rosetting, when subsequently added.
Fig. 4. Capacity of α2M to induce resetting. 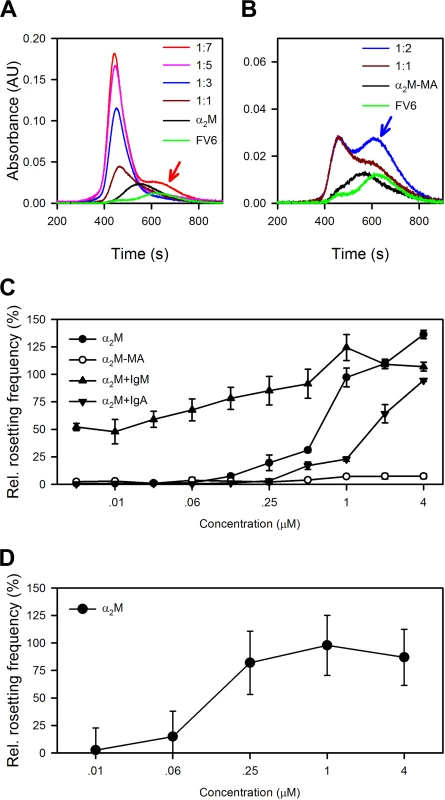
(A) Size-exclusion chromatography of α2M alone (black), recombinant full-length HB3VAR06 alone (FV6; green), or the two together at α2M:FV6 molar ratios of 1:1 (brown), 1:3 (blue), 1:5 (pink), and 1:7 (red), measured by size-exclusion chromatography. The prominent shoulder of unbound FV6 at 1:7 is indicated by a red arrow. (B) Size-exclusion chromatography of α2M-MA alone (black), recombinant full-length HB3VAR06 alone (FV6; green), or the two together at α2M-MA:FV6 molar ratios of 1:1 (brown) and 1:2 (blue), measured by size-exclusion chromatography. The prominent shoulder of unbound FV6 at 1:2 is indicated by a blue arrow. (C) Rosetting of HB3VAR06+ IEs in Albumax medium at different concentrations of α2M (black circles), α2M-MA (○), and α2M in the presence of fixed concentration (3 mg/mL) IgM (black point-up triangles) or IgA (black point-down triangles). Means and SDs relative to rosetting in serum-containing medium are indicated. (D) Ability of α2M to restore the capacity of IgM-depleted serum to support rosetting of HB3VAR06+ IEs. Means and SD are indicated. α2M-binding is not restricted to HB3VAR06
Late-stage erythrocytes infected with five of eight laboratory lines selected to express different rosette-mediating PfEMP1 variants contained sub-populations binding α2M, evaluated by flow cytometry (Fig 5A). α2M induced serum-free rosetting in one (TM284VAR1(R+)) of three α2M-binding lines tested, but not in the two others (PF13 and VarO) (Fig 5B). PF13 IEs express the PfEMP1 protein PF13_0003, which does not contain a DBLξ domain with potential to bind IgM, and this parasite did not form any rosettes when grown in serum-free Albumax medium. VarO+ IEs have previously been reported to require at least 5% serum to form rosettes [36], but in our hands this parasite line rosetted at low levels (~10%) under serum-free conditions. Although the penultimate C-terminal DBL domain in VarO is a DBLξ domain according to the classification algorithms employed by Rask et al. [37] used here, not all such domains support Fc-mediated IgM binding (Jeppesen et al., submitted for publication). For both PF13 and VarO, it is tempting to speculate that the inefficiency of α2M to induce rosetting in these two parasites is related to absence of synergy between α2M and IgM. As expected, α2M had no effect on rosetting rates in two lines (HB3VAR03 and IT4VAR09) not binding α2M. Ex vivo analysis of IEs from 12 Ghanaian children with P. falciparum malaria showed α2M binding in four and IgM binding in five (Fig 5C and 5D). The two phenotypes were highly correlated (P(r = 0.75) <0.005), and IEs from all the α2M-binding isolates also bound IgM (Fig 5D). Four children were clinically categorized as suffering from severe P. falciparum malaria (two had multiple convulsions, one was prostrate, one had respiratory distress). There were no statistically significant associations between clinical presentation (including parasitemia at admission) and either α2M - or IgM-binding phenotype (P(r) >0.4 for all). However, our study was neither designed nor powered to thoroughly investigate whether these phenotypes are preferentially found among erythrocytes infected by parasites obtained from patients with severe disease. Together, these data show that the ability to bind α2M is a common PfEMP1 phenotype, rather than restricted to HB3VAR06.
Fig. 5. α2M binding in parasites not expressing HB3VAR06. 
(A) Binding of α2M to erythrocytes infected by eight genotypically and/or phenotypically different parasite lines, measured by flow cytometry. Control sample labeling (secondary antibody only) is indicated by gray shading. (B) Rosetting frequencies of erythrocytes infected by six genotypically or phenotypically different parasite lines at increasing concentrations of α2M but without serum, measured by flow cytometry. Means and SD relative to rosetting in the presence of serum are indicated. (C) Ex vivo binding of α2M (left) and IgM (right) to erythrocytes infected by a P. falciparum patient isolate (P25). (D) Correlation of ex vivo binding of α2M and IgM to erythrocytes infected by P. falciparum parasites from 12 patients with uncomplicated malaria. Isolate P25 shown in panel C is indicated by an arrow. Discussion
Serum factors are required for PfEMP1-mediated rosetting to occur in the majority of genetically and phenotypically distinct P. falciparum isolates (reviewed in ref. [14]). Pentameric IgM has been a recurring candidate [38,39], and we recently proposed that its role in rosetting is linked to its ability to bind multiple PfEMP1 proteins [17]. However, pentameric IgM only accommodates two HB3VAR06 proteins per IgM molecule, limiting its multimerization potential, and our results showed that additional unidentified serum component(s) are indeed needed for HB3VAR06-mediated rosetting to occur. We therefore set out to close this gap in understanding, and we report here that the unknown serum factor is the abundant protease inhibitor α2M. This is the first report that α2M plays a role in the pathogenesis of P. falciparum malaria, and the first demonstration of a single, soluble serum component that is both necessary and sufficient for rosetting of P. falciparum-IEs.
Antigen-specific pull-down and mass-spectrometry showed that α2M bound HB3VAR06, and this finding was confirmed by ELISA and flow cytometry employing recombinant and native HB3VAR06, respectively (Fig 1). We next showed that native α2M bound HB3VAR06 much better than activated α2M, that this binding did not lead to activation of α2M, and that native α2M retained its capacity for activation after binding to PfEMP1 (Fig 2). This is similar to what has previously been observed in human-pathogenic Group G Streptococci [40,41], where it has been proposed that surface-bound native α2M can protect the bacteria from host protease attack [42]. It is possible that α2M-binding to PfEMP1 might provide IEs with a similar type of protection, but this is presently an unconfirmed hypothesis.
α2M bound to DBLξ2 near the C-terminus of HB3VAR06, which anchors it in the IE membrane (Fig 3). This is the same domain previously shown to interact with the Fc part of IgM [17]. We previously proposed that IgM facilitates rosetting by aligning or “cross-linking” multiple PfEMP1 molecules to increase their combined avidity for receptors on the surrounding erythrocytes, because we found that IgM could bind up to two HB3VAR06 molecules [17]. This “cross-linking” hypothesis is markedly strengthened by the findings reported here. While native α2M (which binds at least four PfEMP1 molecules; Fig 4A) supports rosetting on its own, our data also show that α2M and IgM act synergistically in facilitating rosetting (Fig 4C and 4D). Thus, IgM allows α2M-dependent rosetting to occur at much lower concentrations of α2M than would otherwise be required, although IgM on its own does not support rosetting [17]. Activated α2M (which cannot bind multiple PfEMP1 molecules; Fig 4B) appears to play no role in rosetting (Fig 4C). Finally, our results showed that α2M binding is not restricted to HB3VAR06, as several other genotypically distinct P. falciparum laboratory lines expressing a variety of PfEMP1 proteins on the IE surface could be labeled by α2M (Fig 5A), and that this in some cases led to rosetting (Fig 5B). Furthermore, α2M bound to the surface of IEs from Ghanaian P. falciparum malaria patients (Fig 5C), and the ability to bind α2M and IgM were linked phenotypes (Fig 5D). Rosetting has been linked to parasites causing severe malaria in several studies [10,12,43], but in our small data set we did not find significant correlation between α2M-binding and severe malaria as defined by the WHO criteria [44]. Future studies are therefore required to determine whether the α2M-binding rate is higher among parasites isolated from severe malaria patients, and whether it is associated with particular clinical syndromes and/or expression of particular PfEMP1 proteins.
In this paper we have provided comprehensive evidence that α2M is an important component in PfEMP1-mediated rosetting. While the requirement for soluble serum factors in rosetting has long been recognized, their role has not been fully understood. An early study [38], predating the discovery of PfEMP1, suggested that soluble serum factors can form bridges between the IE and the erythrocytes bound to it in the rosette. While our evidence does not formally rule out that possibility, it suggests an alternative mechanism where the soluble serum proteins bind multiple PfEMP1 proteins at their C-terminus, further organizing the knob-specific display of PfEMP1 on the IE surface. The putative α2M—(and IgM-) mediated cross-linking of PfEMP1 might improve IE binding avidity by refining the overall topological organization of PfEMP1 or by facilitating intermolecular dimerization of constituent DBL domains. With respect to the former possibility, it is known that the topological and spatial organization of both mannose binding lectin (MBL3 oligomers) and its ligand (mannose) contribute to the strength of their adhesive interaction [45]. With respect to the latter, closer packing of multiple PfEMP1 head structures might allow intermolecular dimerization of N-terminal DBL domains. DBL dimerization has been proposed to be “conserved in DBL-domain receptor engagement” and important for the adhesive interaction of DBL domains and their receptors [46,47]. Evidence that the binding sites for Blood Group A antigen and heparin are situated on opposing sides of the head structure of the PfEMP1 protein VarO is compatible with this model, as soluble heparin disrupts VarO-dependent rosetting [36]. However, experimental validation of either hypothesis will require further investigation.
Whether α2M binding to PfEMP1 arose to subvert a host immune response mechanism, as might be suggested by the protective function of the complement factor C3 homolog TEP1 in mosquito immunity to these parasites [48], or conversely evolved as a parasite immune-evasive strategy similar to that of Group G Streptococci [40,41] is another question that will require further investigation. In either case, it is plausible that the net result is an increased repertoire of host receptors available for IE adhesion. This is likely to enhance IE retention in tissues, either directly (by enabling IE adhesion to low-affinity vascular receptors, e.g., endothelial Blood Group A antigen [49]) or indirectly (through retention of rosetting IEs in the microvasculature). Either way, this would help the P. falciparum parasites to avoid destruction in the spleen. We propose that this at least partially explains the correlation between rosetting and severe malaria. Since the proposed α2M-mediated cross-linking of PfEMP1 does not depend on the host receptor specificity of the PfEMP1 ligands involved, our finding may open new avenues for PfEMP1-based immune intervention against IE adhesion that target α2M/IgM-binding rather than host receptor-binding epitopes. Such anti-adhesive intervention would have a broader scope than has been possible to date.
Materials and Methods
Ethics statement
The collection of human plasma samples was approved by the Institutional Review Board of Noguchi Memorial Institute for Medical Research, University of Ghana (Study Number 038/10-11), and by the Regional Research Ethics Committees, Capital Region of Denmark (Protocol H-4-2013-083). All donors were adults and provided written informed consent. All the animal experiments were conducted according to Danish Law and approved (permit 2012-15-2934-00567) by the Danish Animal Procedures Committee (“Dyreforsøgstilsynet”).
Recombinant parasite proteins, animal anti-sera, human serum, and α2M
All recombinant parasite proteins were cloned, expressed, and purified as previously described [50]. In brief, the ectodomains of HB3VAR06, IT4VAR60 (Met1 to Ser2,136) and IT4VAR09 (Met1 to Cys2,345) were codon-optimized for insect cell expression by GeneArt (Regensburg, Germany). Full-length, single-, double-, and triple-domain constructs were cloned into the pAcGP67-A vector (BD biosciences, San Jose, CA), transfected and amplified in Sf9 insect cells before being purified from the supernatant of High-Five insect cells via affinity chromatography on HisTrap HP columns (GE Healthcare, Fairland, CT). Recombinant proteins representing PFD1235w-DBLβ3_D5 and the full-length VAR2CSA-type PfEMP1 IT4VAR04 (FV2) were expressed as previously described [26,51]. The ICAM-1-binding full-length PfEMP1 protein IT4VAR13 (FV13) [52] was a kind gift from Thomas Lavstsen. PfEMP1 domain boundaries and sequences not explicitly given above can be obtained from the VarDOM server (http://genome.cbs.dtu.dk/services/VarDom/) [37].
Animal anti-sera specific for recombinant HB3VAR06, IT4VAR09, and IT4VAR60 constructs were raised in rats and rabbits as previously described [17]. All the animal experiments were conducted according to Danish Law and approved by the Danish Animal Procedures Committee (“Dyreforsøgstilsynet”) (permit 2012-15-2934-00567). The mouse monoclonal antibodies specific for the PfEMP1proteins VarO (D1568) and PF13 (J321) were a kind gift from Inès Vigan-Womas and Odile Mercereau-Puijalon. Anti-serum specific for the N-terminal head structure (NTS-DBLα-CIDRα1.4) of HB3VAR03 has been described previously [53] and was a kind gift from Alex Rowe. Human serum was collected and pooled from ten anonymous and healthy blood bank donors without previous exposure to P. falciparum antigens.
α2-macroglobulin (α2M) (Sigma) and MA-activated α2M (α2M-MA; BioMac, Leipzig, Germany) were purchased or extracted from human serum by Zn2+-chelate affinity as described [54]. This was followed by gel filtration on a Superdex 200 (GE Healthcare) according to the manufacturer’s instructions. MA-induced conversion of serum-purified native α2M to activated α2M (α2M-MA) in the presence of iodoacetamide was carried out as described before [55].
Pull-down and identification of α2M
FV6 or PFD1235w DBLβ3_D5 (100 μg) were coupled to M-270 epoxy Dynabeads (3 mg; Life technologies, Carlsbad, CA) overnight at 4°C according to manufacturer’s instructions. Following washing (3× in 0.1 M NaPO4 buffer containing 0.1% Ig-free bovine serum albumin (BSA;Rockland, USA; pH7.4), the beads were incubated in non-heat-inactivated human serum (500 μL, 1 h, 4°C) on a rotating mixer. Finally, the beads were washed (3× in NaPO4 buffer with 0.05% Tween 20) before bound proteins were eluted in citrate (50 μL, 0.01 M, pH 3.1). Eluted products were extensively dialyzed against NaPO4 buffer (0.1 M) before the protein concentration was assessed by measurement of absorbance at 280 nm, and 5 μg added to rehydration buffer (8 μL, immobilized pH gradient (IPG) buffer pH 3–10; Life Technologies) with dithioerythritol (0.05 g). The 7 cm IPG strips (Life Technologies) for 2D gel electrophoresis were rehydrated (14 h) before a three-step electrophoresis program was run on an IPGPhor machine (Amersham, Buckinghamshire, UK) as described [56]. Strips were equilibrated and second dimension electrophoresis run using pre-cast 4–12% Bis-Tris Zoom gels according to manufacturer’s instructions (Life Technologies).
Gels were silver stained by sequential incubations in fixation solution (10% C2H4O2, 40% C2H6O, 30 min), sensitizing solution (30% C2H6O, 0.2% Na2S2O3, 0.8 M NaOAc, 30 min), dH2O (3×5 min), silver stain (0.25% w/v AgNO3 solution, 20 min), dH2O (2×1 min), developing solution (0.0074% w/v CH2O, 0.25 M Na2CO3, 5 min), and stopping solution (1.46% ethylene-diamine-tetra-acetic acid-Na2, 10 min).
Mass spectrometry
Gel spots of interest were excised and cut into 1 mm3 cubes, de-stained by washing in acetonitrile, and subjected to reduction and alkylation before in-gel digestion with trypsin at 37°C using a ProGest Investigator in-gel digestion robot (Digilab, Marlborough, MA) and standard protocols [57]. Digested peptides were extracted with 10% formic acid, and applied (0.5 μL) to the MALDI target along with α-cyano-4-hydroxycinnamic acid matrix (0.5 μL, 10 mg/mL, 50 : 50 acetonitrile: 0.1% trifluoroacetic acid) and allowed to dry. MALDI-mass spectrometry data were acquired, using a 4800 MALDI TOF/TOF analyzer (ABSciex, Cheshire, UK) equipped with a Nd:YAG 355 nm laser and calibrated using a mixture of peptides. The most intense peptides were selected for mass spectrometry (MS)/MS analysis using GPS Explorer (ABSciex) to interface with the Mascot 2.4 search engine (Matrix Science) and the MS/MS data using Mascot 2.4 directly. Swiss-Prot (Dec 2012) or NCBInr (Aug 2013) databases were interrogated using Homo sapiens as species restriction. The data were searched with tolerances of 100 parts-per-million for the precursor ions and 0.5 Da for the fragment ions, trypsin as the cleavage enzyme, assuming up to one missed cleavage, carbamidomethyl modification of cysteines as a fixed modification and methionine oxidation selected as a variable modification.
P. falciparum parasites, and in vitro culture and selection
P. falciparum HB3 parasites [58] were maintained in vitro and selected for rosetting and expression of HB3VAR06 expression as described [17]. For rosetting laboratory isolates, we used a combination of rosette enrichment (sedimentation in gelatin as described by [59]) and PfEMP1-specific antibody selection [60]. We used rabbit anti-sera raised against the relevant recombinant full-length PfEMP1 proteins to select P. falciparum IT4 to express IT4VAR60 (such parasites have variously been described in the literature as FCR3S1.2 and PAR+; see [61]) and IT4VAR09 (also known as R29; [62]). P. falciparum HB3 was selected to express HB3VAR03 using rabbit-antisera against HB3VAR03-NTS-DBLα-CIDRα. P. falciparum 3D7 expressing PF13_0003 (PF13) and VarO (Genbank: EU9082205) were selected by mouse monoclonal antibodies J321 and D1568 respectively, as described elsewhere [21]. For the isolate TM284var1 (Genbank: JQ684046 and [63]), no PfEMP1-specific anti-sera were available to us. Instead, we used a combination of rosette enrichment and IgM selection. Rosetting in the isolate MUZ12 (Genbank: JQ684048) was maintained by gelatin selection, but no further PfEMP1-specific selection was used. For IgM selection, IEs were incubated in culture medium supplemented with 10% human serum (15 min, rotating plate) before being washed and enriched using rabbit anti-human IgM (Dako, A0426) coupled to Protein A beads (Life technologies). PfEMP1 expression was regularly monitored by flow cytometry using PfEMP1-specific antisera, and only IEs where 60–95% of IEs were specifically labeled were used in the experiments described in this study. All cultures were kept synchronous by twice-weekly sorbitol treatment as described [64]. The genotypic identity and the absence of Mycoplasma infection were verified regularly as described [26]. P. falciparum IT4 was selected for IE surface expression of the PfEMP1 protein IT4VAR32b by human monoclonal antibody AB01 [65]. IEs from 12 children with P. falciparum malaria were obtained from venous blood samples collected at Hohoe Municipal Hospital, Hohoe, Ghana. The samples were incubated overnight in candle jars at 37°C, and transported to Accra for analysis by ex vivo flow cytometry (see below). These samples were collected with the permission of the Institutional Review Board at Noguchi Memorial Institute for Medical research, University of Ghana (file 026/13-14) and the Ethical Review Committee of the Ghana Health Services (file GHS-ERC 08/05/14).
Measurements of α2M and Fc-mediated IgM binding to PfEMP1
To assess α2M binding by ELISA, 96-well, flat-bottomed MaxiSorp plates (Thermo scientific) were coated with recombinant PfEMP1 constructs (18nM, overnight, 4°C). Following blocking (2 h) in TSM buffer with 1% Ig free BSA, plates were washed and incubated with α2M or α2M-MA (10 nM). Binding was detected with polyclonal goat-anti-α2M (Abcam, Cambridge, UK 7337 1 : 5,000) or monoclonal mouse-anti-α2M (Abcam, 1 : 1,000), followed by rabbit-anti-goat horseradish peroxidase (1 : 6,000, Dako, Glostrup, Denmark) or rabbit-anti-mouse horseradish peroxidase (1 : 2,000, Dako).
Binding of IgM, α2M and α2M-MA to IEs was detected by flow cytometry, essentially as described [65]. In brief, late-stage IEs (1x105) purified by magnet-activated cell sorting were incubated (30 min, room temperature) with either α2M or α2M-MA (10 nM). The IEs were washed and incubated first with primary (polyclonal goat-anti-α2M, 1 : 3,000), then fluorescein isothiocyanate-conjugated secondary antibody (polyclonal rabbit-anti-goat, 1 : 150) (Vector, Peterborough, UK, FI-5000) and ethidium bromide (2 μg/mL). In competition assays, α2M, IgM, or HB3VAR06-specific anti-sera (1 : 20) were added in separate steps. Ethidium bromide labeling was omitted in experiments detecting IgM binding using phycoerythrin-conjugated donkey-anti-human IgM (Jackson ImmunoResearch, Newmarket, UK, 1 : 400). In assays employing simultaneous surface labeling of IEs by IgM, α2M, and HB3VAR06-specific IgG, we used PerCP-conjugated donkey-anti-rabbit antibody (Jackson ImmunoResearch,1 : 50) to detect HB3VAR06-specific rabbit IgG and Alexa 488-conjugated donkey-anti-goat antibody (Life Technologies, 1 : 10,000) to detect α2M. IE surface labeling was assessed by flow cytometry using a Beckman coulter FC500 instrument (Beckman Coulter, Fullerton, CA) in Copenhagen or a Becton Dickinson FACSCalibur (BD Biosciences, San Jose, CA) in Ghana. List-mode data files were analyzed using FlowJo (v 7.6; Treestar, Ashton, OR) and WinList (v. 6; Verity Software House, Topsham, ME) software.
Immunofluorescence microscopy
HB3VAR06+ IEs (1.2 mL, 5% hematocrit) were pelleted, washed (3×, PBS with 1% Ig-free BSA), and incubated with α2M (500 μL, 100 nM, room temperature, 30 min). Following additional washing, the IEs were incubated with polyclonal goat-anti - α2M (500 μL, 1 : 3,000) then Alexa 488-conjugated donkey-anti-goat (Life Technologies, 1 : 10,000) and DAPI (1 μg/mL). Micrographs of live, unfixed IEs were obtained using a Nikon TE 2000-E confocal microscope equipped with a 60× oil immersion lens (N.A. 1.4) and Nikon EZ-C1 3.5 software (Nikon Instruments, Amsterdam, Netherlands). Images were analyzed using Image J64 software (http://imagej.nih.gov/ij/) [66].
Detection of α2M activation
To test whether binding of α2M to FV6 induced thiol-ester conversion of α2M to activated α2M, FV6 and α2M (10 μg) were incubated together in NaPO4 buffer (pH 8), in the presence of 0.75 mM mPEG, MW 5000 (mPEG; Laysan Bio, Arab, AL). Incorporation of mPEG was assessed by denaturing SDS-PAGE (5 μL load), comparing activation to that obtained by MA (150 mM; Sigma) in the absence of iodoacetamide. To assess whether α2M could be activated after binding to FV6, the FV6: α2M complex was immobilized on epoxy beads as described above for the α2M pull-down, but using purified α2M (200 μg) instead of serum. After removal of unbound α2M by thorough washing, FV6-bound α2M was incubated with mPEG with or without methylamine as above in NaPO4 buffer (pH8, 2 h, 4°C). After additional washing, the beads were incubated with SDS loading buffer and DTT (50 μL), heated (70°C, 5 min) before loading (20 μL) on denaturing SDS gels and processed as above.
Analytical size-exclusion chromatography
Size-exclusion chromatography (SEC) was performed using a 24-mL Superdex 200 10/30 HR column (GE Healthcare) equilibrated with 50 mM Tris-HCL and 150 mM NaCl (pH 7.4). α2M (150 μg) alone and in combination with molar ratios of FV6 were incubated on ice (15 min) and subjected to SEC analysis using a flow rate of 0.5 mL/min and absorbance detection at 280 nm.
Rosetting assessment
Late-stage IEs (5% parasitemia) were diluted in Albumax medium to 2% hematocrit to achieve a total volume of 20 μL in a 384-well plate. Rosetting agents (α2M, α2M-MA, IgM, IgA, serum) were concentrated to ≥10 mg/mL and dialyzed against Albumax medium before dilution to the required concentration. The effect of each reagent on IEs (20 μL culture) was tested in triplicate in 384-well plates (20 μL/well, 1 h, 37°C). IEs were labeled with ethidium bromide (2 μg/mL), and rosetting (≥2 erythrocytes bound per IE) assessed by fluorescence microscopy of 200 IEs. In each assay, the rosetting frequency was compared to the positive control (10% serum).
Supporting Information
Zdroje
1. World Health Organization (2013) World Malaria Report 2013.
2. Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379 : 413–431. doi: 10.1016/S0140-6736(12)60034-8 22305225
3. Barnwell JW, Asch AS, Nachman RL, Yamaya M, Aikawa M, Ingravallo P (1989) A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest 84 : 765–772. 2474574
4. Berendt AR, Simmons DL, Tansey J, Newbold CI, Marsh K (1989) Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature 341 : 57–59. 2475784
5. Treutiger CJ, Heddini A, Fernandez V, Muller WA, Wahlgren M (1997) PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med 3 : 1405–1408. 9396614
6. Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, Avril M, Brazier AJ, Freeth J, Jespersen JS, Nielsen MA, Magistrado P, Lusingu J, Smith JD, Higgins MK, Theander TG (2013) Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498 : 502–505. doi: 10.1038/nature12216 23739325
7. Turner GDH, Morrison H, Jones M, Davis TME, Looareesuwan S, Buley ID, Gatter KC, Newbold CI, Pukrittayakamee S, Nagachinta B, White NJ, Berendt AR (1994) An immunohistochemical study of the pathology of fatal malaria: evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol 145 : 1057–1069. 7526692
8. Udomsangpetch R, Wåhlin B, Carlson J, Berzins K, Torii M, Aikawa M, Perlmann P, Wahlgren M (1989) Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med 169 : 1835–1840. 2654325
9. Adams Y, Kuhnrae P, Higgins MK, Ghumra A, Rowe JA (2014) Rosetting Plasmodium falciparum-infected erythrocytes bind to human brain microvascular endothelial cells in vitro, demonstrating a dual adhesion phenotype mediated by distinct P. falciparum erythrocyte membrane protein 1 domains. Infect Immun 82 : 949–959. doi: 10.1128/IAI.01233-13 24343658
10. Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, Wahlgren M (1990) Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336 : 1457–1460. 1979090
11. Treutiger CJ, Hedlund I, Helmby H, Carlson J, Jepson A, Twumasi P, Kwiatkowski D, Greenwood BM, Wahlgren M (1992) Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg 46 : 503–510. 1599043
12. Rowe A, Obeiro J, Newbold CI, Marsh K (1995) Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun 63 : 2323–2326. 7768616
13. Moxon CA, Wassmer SC, Milner DA Jr., Chisala NV, Taylor TE, Seydel KB, Molyneux ME, Faragher B, Esmon CT, Downey C, Toh CH, Craig AG, Heyderman RS (2013) Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 122 : 842–851. doi: 10.1182/blood-2013-03-490219 23741007
14. Mercereau-Puijalon O, Guillotte M, Vigan-Womas I (2008) Rosetting in Plasmodium falciparum: A cytoadherence phenotype with multiple actors. Transfus Clin Biol 15 : 62–71. doi: 10.1016/j.tracli.2008.04.003 18514562
15. Scholander C, Carlson J, Kremsner PG, Wahlgren M (1998) Extensive immunoglobulin binding of Plasmodium falciparum-infected erythrocytes in a group of children with moderate anemia. Infect Immun 66 : 361–363. 9423881
16. Somner EA, Black J, Pasvol G (2000) Multiple human serum components act as bridging molecules in rosette formation by Plasmodium falciparum-infected erythrocytes. Blood 95 : 674–682. 10627479
17. Stevenson L, Huda P, Jeppesen A, Laursen E, Rowe JA, Craig A, Streicher W, Barfod L, Hviid L (2015) Investigating the function of Fc-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting. Cell Microbiol 17 : 819–831. doi: 10.1111/cmi.12403 25482886
18. Ghumra A, Semblat J-P, McIntosh RS, Raza A, Rasmussen IB, Braathen R, Johansen F-E, Sandlie I, Mongini PK, Rowe JA, Pleass RJ (2008) Identification of residues in the Cμ4 domain of polymeric IgM essential for interaction with Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1). J Immunol 181 : 1988–2000. 18641336
19. Rowe JA, Moulds JM, Newbold CI, Miller LH (1997) P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388 : 292–295. 9230440
20. Chen Q, Barragan A, Fernandez V, Sundstrom A, Schlichtherle M, Sahlen A, Carlson J, Datta S, Wahlgren M (1998) Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J Exp Med 187 : 15–23. 9419207
21. Vigan-Womas I, Guillotte M, Le Scanf C, Igonet S, Petres S, Juillerat A, Badaut C, Nato F, Schneider A, Lavergne A, Contamin H, Tall A, Baril L, Bentley GA, Mercereau-Puijalon O (2008) An in vivo/in vitro model of rosetting and autoagglutination mediated by Plasmodium falciparum VarO, a group A var gene encoding a frequent serotype. Infect Immun 76 : 5565–5580. doi: 10.1128/IAI.00901-08 18809668
22. Barragan A, Fernandez V, Chen Q, von Euler A, Wahlgren M, Spillmann D (2000) The duffy-binding-like domain 1 of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a heparan sulfate ligand that requires 12 mers for binding. Blood 95 : 3594–3599. 10828049
23. Barragan A, Kremsner PG, Wahlgren M, Carlson J (2000) Blood group A antigen is a co-receptor in Plasmodium falciparum rosetting. Infect Immun 68 : 2971–2975. 10768996
24. Vogt AM, Winter G, Wahlgren M, Spillmann D (2004) Heparan sulphate identified on human erythrocytes: a Plasmodium falciparum receptor. Biochem J 381 : 593–597. 15209561
25. Jensen ATR, Magistrado PA, Sharp S, Joergensen L, Lavstsen T, Chiucchiuini A, Salanti A, Vestergaard LS, Lusingu JP, Hermsen R, Sauerwein R, Christensen J, Nielsen MA, Hviid L, Sutherland C, Staalsoe T, Theander TG (2004) Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by Group A var genes. J Exp Med 199 : 1179–1190. 15123742
26. Bengtsson A, Joergensen L, Rask TS, Olsen RW, Andersen MA, Turner L, Theander TG, Hviid L, Higgins MK, Craig A, Brown A, Jensen AT (2013) A novel domain cassette identifies Plasmodium falciparum PfEMP1 proteins binding ICAM-1 and is a target of cross-reactive, adhesion-inhibitory antibodies. J Immunol 190 : 240–249. doi: 10.4049/jimmunol.1202578 23209327
27. Marrero A, Duquerroy S, Trapani S, Goulas T, Guevara T, Andersen GR, Navaza J, Sottrup-Jensen L, Gomis-Ruth FX (2012) The crystal structure of human α2-macroglobulin reveals a unique molecular cage. Angew Chem Int Ed Engl 51 : 3340–3344. doi: 10.1002/anie.201108015 22290936
28. Tunstall AM, Merriman JM, Milne I, James K (1975) Normal and pathological serum levels of α2-macroglobulins in men and mice. J Clin Pathol 28 : 133–139. 47865
29. Rehman AA, Ahsan H, Khan FH (2013) Alpha-2-macroglobulin: a physiological guardian. J Cell Physiol 228 : 1665–1675. doi: 10.1002/jcp.24266 23086799
30. Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ (1995) Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82 : 77–87. 7541722
31. Joergensen L, Bengtsson DC, Bengtsson A, Ronander E, Berger SS, Turner L, Dalgaard MB, Cham GK, Victor ME, Lavstsen T, Theander TG, Arnot DE, Jensen AT (2010) Surface co-expression of two different PfEMP1 antigens on single Plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathog 6: e1001083. doi: 10.1371/journal.ppat.1001083 20824088
32. Imber MJ, Pizzo SV (1981) Clearance and binding of two electrophoretic "fast" forms of human α2-macroglobulin. J Biol Chem 256 : 8134–8139. 6167573
33. Kristensen T, Moestrup SK, Gliemann J, Bendtsen L, Sand O, Sottrup-Jensen L (1990) Evidence that the newly cloned low-density-lipoprotein receptor related protein (LRP) is the α2-macroglobulin receptor. FEBS Lett 276 : 151–155. 1702392
34. Sottrup-Jensen L (1989) Alpha-macroglobulins: structure, shape, and mechanism of proteinase complex formation. J Biol Chem 264 : 11539–11542. 2473064
35. Stark C, Breitkreutz BJ, Reguly T, Boucher L, Breitkreutz A, Tyers M (2006) BioGRID: a general repository for interaction datasets. Nucleic Acids Res 34: D535–D539. 16381927
36. Vigan-Womas I, Guillotte M, Juillerat A, Hessel A, Raynal B, England P, Cohen JH, Bertrand O, Peyrard T, Bentley GA, Lewit-Bentley A, Mercereau-Puijalon O (2012) Structural basis for the ABO blood-group dependence of Plasmodium falciparum rosetting. PLoS Pathog 8: e1002781. doi: 10.1371/journal.ppat.1002781 22807674
37. Rask TS, Hansen DA, Theander TG, Pedersen AG, Lavstsen T (2010) Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes—divide and conquer. PLoS Comput Biol 6: e1000933. doi: 10.1371/journal.pcbi.1000933 20862303
38. Scholander C, Treutiger CJ, Hultenby K, Wahlgren M (1996) Novel fibrillar structure confers adhesive property to malaria - infected erythrocytes. Nat Med 2 : 204–208. 8574966
39. Clough B, Atilola FA, Black J, Pasvol G (1998) Plasmodium falciparum: the importance of IgM in the rosetting of parasite-infected erythrocytes. Exp Parasitol 89 : 129–132. 9603499
40. Chhatwal GS, Muller HP, Blobel H (1983) Characterization of binding of human alpha 2-macroglobulin to group G streptococci. Infect Immun 41 : 959–964. 6193068
41. Muller HP, Rantamaki LK (1995) Binding of native alpha 2-macroglobulin to human group G streptococci. Infect Immun 63 : 2833–2839. 7542633
42. Rasmussen M, Muller HP, Bjorck L (1999) Protein GRAB of streptococcus pyogenes regulates proteolysis at the bacterial surface by binding alpha2-macroglobulin. J Biol Chem 274 : 15336–15344. 10336419
43. Kun JF, Schmidt-Ott RJ, Lehman LG, Lell B, Luckner D, Greve B, Matousek P, Kremsner PG (1998) Merozoite surface antigen 1 and 2 genotypes and rosetting of Plasmodium falciparum in severe and mild malaria in Lambaréné, Gabon. Trans R Soc Trop Med Hyg 92 : 110–114. 9692171
44. Warrell DA, Molyneux ME, Beales PF (1990) Severe and complicated malaria. Second edition. Trans R Soc Trop Med Hyg 84 (suppl. 2): 1–65. 2219249
45. Gjelstrup LC, Kaspersen JD, Behrens MA, Pedersen JS, Thiel S, Kingshott P, Oliveira CL, Thielens NM, Vorup-Jensen T (2012) The role of nanometer-scaled ligand patterns in polyvalent binding by large mannan-binding lectin oligomers. J Immunol 188 : 1292–1306. doi: 10.4049/jimmunol.1103012 22219330
46. Tolia NH, Enemark EJ, Sim BK, Joshua-Tor L (2005) Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell 122 : 183–193. 16051144
47. Batchelor JD, Zahm JA, Tolia NH (2011) Dimerization of Plasmodium vivax DBP is induced upon receptor binding and drives recognition of DARC. Nat Struct Mol Biol 18 : 908–914. doi: 10.1038/nsmb.2088 21743458
48. Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, Kafatos FC (2001) Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 104 : 709–718. 11257225
49. Szulman AE (1960) The histological distribution of blood group substances A and B in man. J Exp Med 111 : 785–800. 13774694
50. Ampomah P, Stevenson L, Ofori MF, Barfod L, Hviid L (2014) B-cell responses to pregnancy-restricted and -unrestricted Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) antigens in Ghanaian women naturally exposed to malaria parasites. Infect Immun 82 : 1860–1871. doi: 10.1128/IAI.01514-13 24566620
51. Khunrae P, Dahlbäck M, Nielsen MA, Andersen G, Ditlev SB, Resende M, Pinto VV, Theander TG, Higgins MK, Salanti A (2010) Full-length recombinant Plasmodium falciparum VAR2CSA binds specifically to CSPG and induces potent parasite adhesion-blocking antibodies. J Mol Biol 397 : 826–834. doi: 10.1016/j.jmb.2010.01.040 20109466
52. Brown A, Turner L, Christoffersen S, Andrews KA, Szestak T, Zhao Y, Larsen S, Craig AG, Higgins MK (2013) Molecular architecture of a complex between an adhesion protein from the malaria parasite and intracellular adhesion molecule 1. J Biol Chem 288 : 5992–6003. doi: 10.1074/jbc.M112.416347 23297413
53. Claessens A, Adams Y, Ghumra A, Lindergard G, Buchan CC, Andisi C, Bull PC, Mok S, Gupta AP, Wang CW, Turner L, Arman M, Raza A, Bozdech Z, Rowe JA (2012) A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci U S A 109: E1772–E1781. doi: 10.1073/pnas.1120461109 22619330
54. Sottrup-Jensen L, Petersen TE, Magnusson S (1980) A thiol-ester in α2-macroglobulin cleaved during proteinase complex formation. FEBS Lett 121 : 275–279. 6161841
55. Andersen GR, Jacobsen L, Thirup S, Nyborg J, Sottrup-Jensen L (1991) Crystallization and preliminary X-ray analysis of methylamine-treated α2-macroglobulin and 3 α2-macroglobulin-proteinase complexes. FEBS Lett 292 : 267–270. 1720400
56. Higon M, Cowan G, Nausch N, Cavanagh D, Oleaga A, Toledo R, Stothard JR, Antunez O, Marcilla A, Burchmore R, Mutapi F (2011) Screening trematodes for novel intervention targets: a proteomic and immunological comparison of Schistosoma haematobium, Schistosoma bovis and Echinostoma caproni. Parasitology 138 : 1607–1619. doi: 10.1017/S0031182011000412 21729355
57. Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, Mann M (1996) Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature 379 : 466–469. 8559255
58. Bhasin VK, Trager W (1984) Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am J Trop Med Hyg 33 : 534–537. 6383092
59. Handunnetti SM, Gilladoga AD, van Schravendijk M - R, Nakamura K-I, Aikawa M, Howard RJ (1992) Purification and in vitro selection of rosette-positive (R+) and rosette-negative (R-) phenotypes of knob-positive Plasmodium falciparum parasites. Am J Trop Med Hyg 46 : 371–381. 1575284
60. Staalsoe T, Nielsen MA, Vestergaard LS, Jensen ATR, Theander TG, Hviid L (2003) In vitro selection of Plasmodium falciparum 3D7 for expression of variant surface antigens associated with severe malaria in African children. Parasite Immunol 25 : 421–427. 14651589
61. Robson KJ, Walliker D, Creasey A, McBride J, Beale G, Wilson RJM (1992) Cross-contamination of Plasmodium cultures. Parasitol Today 8 : 38–39.
62. Roberts DJ, Craig AG, Berendt AR, Pinches R, Nash G, Marsh K, Newbold CI (1992) Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357 : 689–692. 1614515
63. Carlson J, Ekre HP, Helmby H, Gysin J, Greenwood BM, Wahlgren M (1992) Disruption of Plasmodium falciparum erythrocyte rosettes by standard heparin and heparin devoid of anticoagulant activity. Am J Trop Med Hyg 46 : 595–602. 1599054
64. Moll K., Ljungström I., Perlmann H., Scherf A., and Wahlgren M. (2008) Methods in malaria research. Manassas, VA: MR4/ATCC.
65. Barfod L, Dalgaard MB, Pleman ST, Ofori MF, Pleass RJ, Hviid L (2011) Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1. Proc Natl Acad Sci U S A 108 : 12485–12490. doi: 10.1073/pnas.1103708108 21746929
66. Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9 : 671–675. 22930834
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



