-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
Staphylococcus aureus persistently colonizes ~20% of the human population, and 40–60% of humans are intermittently colonized by this bacterium. The most common reservoir for S. aureus is the anterior nares, and the incidence of staphylococcal disease in higher in individuals who are colonized. Rectal colonization by S. aureus isolates, reflecting gastrointestinal (GI) carriage, has recently been recognized as an important reservoir from which person to person transmission occurs. We developed a murine model of S. aureus GI colonization to investigate bacterial factors that promote staphylococcal colonization of the gut. We identified several surface-associated S. aureus antigens that modulate colonization of the GI tract and identified a surface glycopolymer (cell wall teichoic acid) as critical for the early steps in colonization. The failure of the teichoic acid mutant to colonize the GI tract can be attributed to its defects in bacterial adherence and to its enhanced susceptibility to mammalian host defenses unique to the gastrointestinal tract. Efforts to develop antimicrobials that target WTA may lead to an overall reduction in asymptomatic colonization by antibiotic-resistant S. aureus and may impact the incidence of invasive disease.
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1005061
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005061Summary
Staphylococcus aureus persistently colonizes ~20% of the human population, and 40–60% of humans are intermittently colonized by this bacterium. The most common reservoir for S. aureus is the anterior nares, and the incidence of staphylococcal disease in higher in individuals who are colonized. Rectal colonization by S. aureus isolates, reflecting gastrointestinal (GI) carriage, has recently been recognized as an important reservoir from which person to person transmission occurs. We developed a murine model of S. aureus GI colonization to investigate bacterial factors that promote staphylococcal colonization of the gut. We identified several surface-associated S. aureus antigens that modulate colonization of the GI tract and identified a surface glycopolymer (cell wall teichoic acid) as critical for the early steps in colonization. The failure of the teichoic acid mutant to colonize the GI tract can be attributed to its defects in bacterial adherence and to its enhanced susceptibility to mammalian host defenses unique to the gastrointestinal tract. Efforts to develop antimicrobials that target WTA may lead to an overall reduction in asymptomatic colonization by antibiotic-resistant S. aureus and may impact the incidence of invasive disease.
Introduction
Staphylococcus aureus is a bacterial pathogen that commonly colonizes the nose, skin, and mucosal surfaces of healthy individuals. However, S. aureus may also cause a variety of superficial and invasive infections in hospitalized patients, as well as in individuals within the community who lack the risk factors commonly associated with nosocomial infections [1,2]. Although the anterior nares are the most common anatomic site of S. aureus carriage, ~20% of adults are positive for intestinal carriage of S. aureus [3]. The gastrointestinal (GI) tract has been shown to be a potentially important reservoir for S. aureus in several clinical studies [4–6]. Although nasal carriage apparently predisposes the host to intestinal carriage, ~37% of intestinal carriers are not positive for S. aureus nasal colonization [3]. Compared to nasal colonization only, simultaneous nasal and intestinal colonization was associated with a significant increase in the frequency of positive skin cultures [7]. Squier et al. [8] observed that critically ill patients who had both rectal and nasal carriage were significantly more likely to develop staphylococcal infection (40% infection rate) than those with nasal carriage only (18% infection rate).
Patients positive for staphylococcal GI colonization often contaminate their environment with S. aureus [3,9]. Thus, intestinal carriage may serve as an important reservoir for S. aureus transmission, contributing to bacterial dissemination and subsequent risk of infection [3]. Faden et al. compared methicillin-resistant S. aureus (MRSA) nasal and rectal colonization rates in children with staphylococcal skin abscesses and a control group of children without staphylococcal disease [10]. Whereas rates of nasal colonization were equivalent for both groups of children, MRSA was detected significantly more often in the rectum of children with skin abscesses (47%) compared with controls (1%). Moreover, S. aureus recovered from the abscesses and rectum were identical in 88% of cases, compared with 75% of nasal isolates. Almost all abscess isolates (57/60) were USA300 strains, whereas only 2 of 22 isolates from the control groups were USA300. In a study of HIV-infected men who have sex with men, Szumowski et al. reported that perianal (but not nasal) colonization by MRSA was significantly associated with skin abscess formation [11]. These studies suggest that rectal colonization by S. aureus, probably reflecting gastrointestinal carriage, is an important reservoir from which person to person transmission occurs.
Host factors that facilitate staphylococcal colonization of the GI tract are poorly understood. Intestinal carriage occurs at a high frequency within the first six months of life, after which the incidence drops [3]. Additional factors, such as decreased stomach acidity, antibiotics that disrupt the indigenous microbiota, or the administration of cyclophosphamide or prednisone, may also influence acquisition of S. aureus in the human GI tract [4–6].
Whereas staphylococcal factors that promote GI colonization have not been reported, several independent investigations have identified surface antigens that impact S. aureus nasal colonization in rodent models. Mutants deficient in either clumping factor B or cell wall teichoic acid (WTA) showed reduced nasal carriage in experimentally inoculated rats or mice [12,13], and clumping factor B promoted nasal colonization of humans [14].
In this investigation, we sought to develop and characterize a reliable murine model of S. aureus GI carriage to better understand its relevance as a risk factor for subsequent infection and its potential for transmission and spread of this pathogen. S. aureus antigens important for nasal colonization were assessed to determine whether they might also play a role in colonization of the GI tract. We identified several surface-associated S. aureus antigens that modulated colonization of the GI tract and identified WTA as critical for the early steps in colonization. The failure of the WTA mutant (ΔtagO) to colonize the GI tract correlated with its defects in bacterial adherence and greater susceptibility to antimicrobial factors within the mouse gut.
Results
Establishment of an S. aureus gastrointestinal colonization model in mice
The mouse intestinal tract is comprised of diverse commensal bacteria, including Bacteroides, Clostridia, segmented filamentous bacteria, members of the Enterobacteriaceae, Lactobacilli, and Enterococci [15,16]. These normal flora provide some protection against invading pathogens, as evidenced by the fact that we were unable to establish stable (≥ 1 week) GI colonization by S. aureus in conventional mice in the absence of selective antibiotic pressure (S1 Fig). In previous studies, we maintained stable nasal colonization of mice with S. aureus by supplementing their drinking water with streptomycin (Sm) and inoculating with Sm-resistant (Smr) staphylococcal isolates [12,17], and a similar approach was taken here. Fecal cultures performed on mice prior to inoculation were negative for Smr S. aureus (lower limit of detection ~3 log CFU S. aureus/g stool). Awake mice maintained on Sm water and administered intranasal inocula of Smr S. aureus Newman ranging from 8 x 108 to 2 x 105 CFU showed stable colonization of the GI tract for at least 3 wks (Table 1). Recovery of Smr S. aureus Newman from the stool cultures consistently averaged ~105 CFU/g stool. Differences in GI colonization of the WT strain were not observed when we compared inoculation by the intranasal route with inoculation by oral gavage. A subset of animals were given Sm water during week 1 and then given regular drinking water thereafter. These mice maintained GI colonization for at least 3 weeks at levels (~105 CFU/g stool) similar to those of mice maintained for 3 wks on Sm water (S1 Fig).
Tab. 1. Quantitative stool cultures from mice (n = 4–15 per group) that were inoculated with Smr S. aureus strain Newman on day zero. 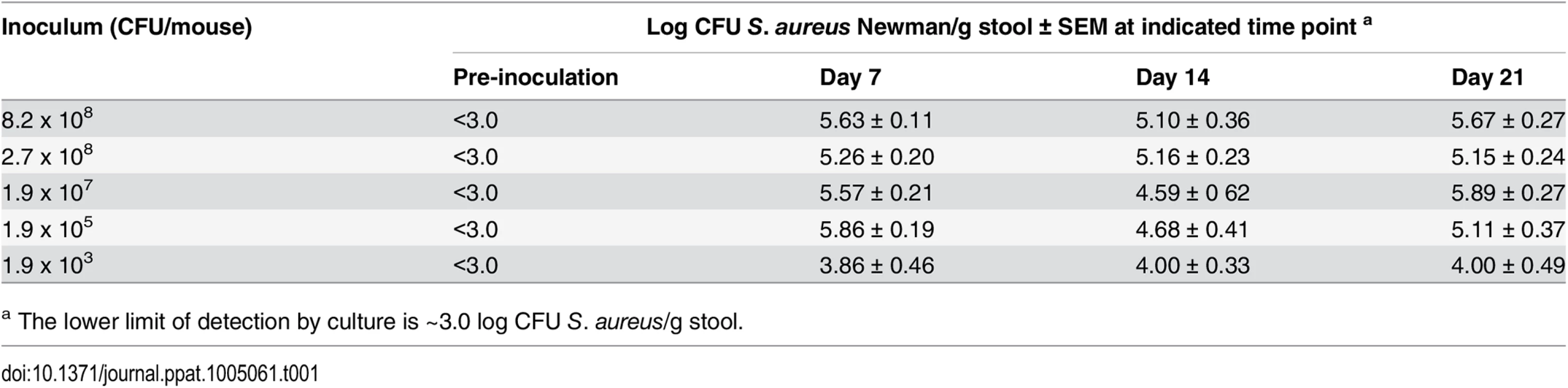
a The lower limit of detection by culture is ~3.0 log CFU S. aureus/g stool. To determine where S. aureus resided in the GI tract of conventional mice, we inoculated animals with ~109 CFU of S. aureus, and euthanized the animals on days 1, 2, 4 and 7. Segments of the GI tract were removed and cultured quantitatively. The colonized staphylococcal density in the conventional mice was quite variable in the small intestine with the highest being observed in the distal segment of the small intestine (1.3 x 105 CFU/g tissue), cecum (3.9 x 105 CFU/g tissue) and colon (3.2 x 105 CFU/g).
To measure the impact of the commensal GI flora on S. aureus colonization, germfree Swiss Webster mice (n = 8) were inoculated with ~109 CFU of S. aureus, and quantitative stool cultures were performed on days 1, 5, 7, 12, and 14. In contrast to conventional mice, germfree mice were readily colonized by S. aureus as early as day 1 without the need for Sm drinking water. Quantitative cultures yielded concentrations ranging from 1 x 107 to 9.7 x 108 CFU/g stool over the course of the two-week time period. Four to five mice were euthanized on day 7 or 14, and segments of the GI tract were removed, homogenized, and cultured quantitatively. Whereas S. aureus was detected throughout the GI tract at concentrations >104 cfu/g, significantly higher staphylococcal densities were achieved in the distal portions of the GI tract (Table 2). These findings corroborated those that we found in conventional mice, i.e., the highest bacterial burdens were localized to the mouse cecum and colon.
Tab. 2. Quantitative cultures of GI segments from germ-free mice inoculated with S. aureus and evaluated on days 7 or 14. 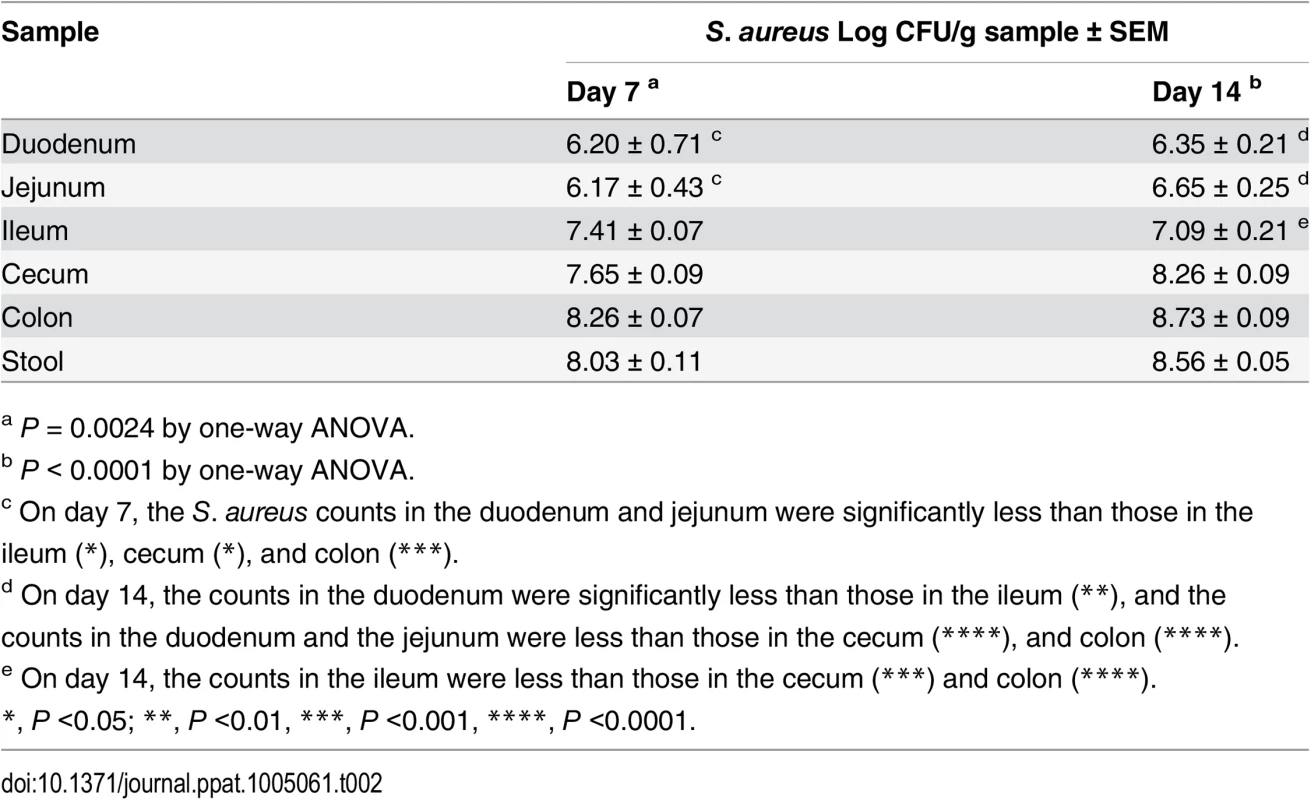
a P = 0.0024 by one-way ANOVA. Bacterial factors that promote colonization of the GI tract
Previous studies revealed that S. aureus mutants deficient in sortase (ΔsrtA; affects all cell wall anchored proteins), clumping factor B, the serotype 5 capsule, and WTA were defective in their ability to colonize the nasal cavity of rodents [12,13,17,18]. Evaluation of microbial fitness for colonization of the GI tract is often performed in the context of competition experiments [19–21]. When we evaluated the relative fitness of S. aureus mutants vs. the parental strain, we observed that the acapsular Newman cap5G mutant showed enhanced fitness, colonizing the mouse GI tract in numbers greater than that of the parental type 5 capsule positive strain (Fig 1). The sortase A mutant and a clumping factor A (clfA) mutant showed impaired fitness in vivo, since both were out-competed by the wild type (WT) strain Newman (Fig 1). To validate the latter finding, we repeated the competition experiment between strain Newman and its clfA mutant with inoculation by oral gavage. Similar to our initial findings, the clfA mutant again showed a significant colonization defect between 7 and 21 days after inoculation (S2 Fig). Newman Δica, Newman ΔclfB, Newman Δspa, and Newman ΔsdrCDE showed no colonization defect (S3 Fig).
Fig. 1. S. aureus factors that impact colonization of the murine GI tract. 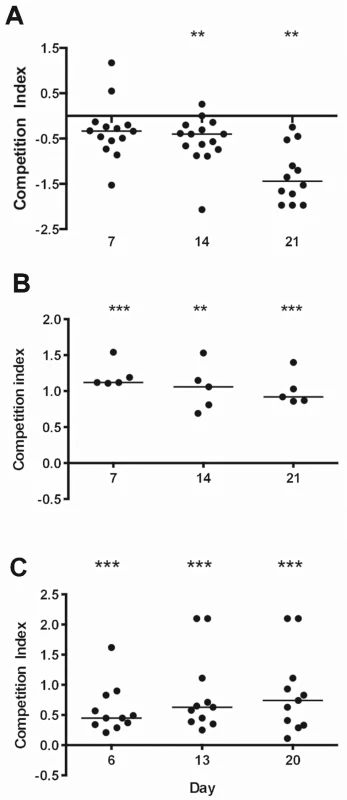
A) The Newman serotype 5 capsule-negative mutant showed a competitive fitness advantage relative to the wild type Newman capsule-positive strain. B) A sortase A mutant and C) a clumping factor A (ClfA) mutant showed a competitive defect compared to the parental strain Newman. The competitive index (CI) was defined as the log10 output ratio/ input ratio. A CI <0 indicates a mutant with a colonization advantage over the WT strain, and a CI >0 indicates a mutant with a colonization disadvantage compared to the WT. Horizontal bars represent the median competitive index for groups of 5–15 mice. P-values were determined by Wilcoxon signed-rank test. *P < 0.05; ** P < 0.01; *** P < 0.001. When we inoculated separate groups of 5 to 10 mice with the parental strain Newman or isogenic mutants lacking the serotype 5 capsule, sortase, or ClfA, we observed no differences between the colonization levels achieved by the parental or mutant strains (S4 Fig). Thus, the colonization defects of these three mutants were only evident as measured by in vivo competition experiments. Likewise, a mutant defective in beta hemolysin (Hlb) colonized the mouse gut at levels consistent with the respective parental strains (S4 Fig).
Weidenmaier et al. reported that a tagO mutant failed to colonize the nasal cavity of cotton rats [13]. To elucidate the role of WTA in GI colonization, separate groups of mice were inoculated with S. aureus SA113 or Newman or their isogenic tagO or dltA mutants. Both SA113 ΔtagO and Newman ΔtagO showed a significant (P <0.01) reduction in GI colonization as early as one week after intranasal inoculation compared to the parental strain (Fig 2A), despite the fact that the growth of the mutant in vitro was comparable to that of the parental strain [22]. To confirm these findings, additional groups of mice were inoculated by oral gavage with strain Newman or its tagO mutant, and GI colonization was monitored up to 72 h. By 24 h after inoculation, there were significantly fewer Newman ΔtagO recovered from the stools of mice inoculated with Newman ΔtagO compared to the WT strain (S5 Fig). These data suggest that the WTA polymer is a critical determinant for colonization of the GI tract by S. aureus. To determine whether modifications of the WTA backbone affect GI colonization, we inoculated mice with dltA mutants of strain SA113 or Newman, which lack the ester-linked alanine substituent on staphylococcal teichoic acid. The dltA mutants showed no colonization defect (Fig 2A). Similarly, a ΔtarM ΔtarS double mutant of S. aureus RN4220, which lacks alpha - and beta-O-linked GlcNAc modifications of WTA, colonized mice at levels similar to the WT strain (S6 Fig).
Fig. 2. Effects of WTA on S. aureus GI colonization and the influence of surface antigens on in vitro adherence of S. aureus to T84 cells. 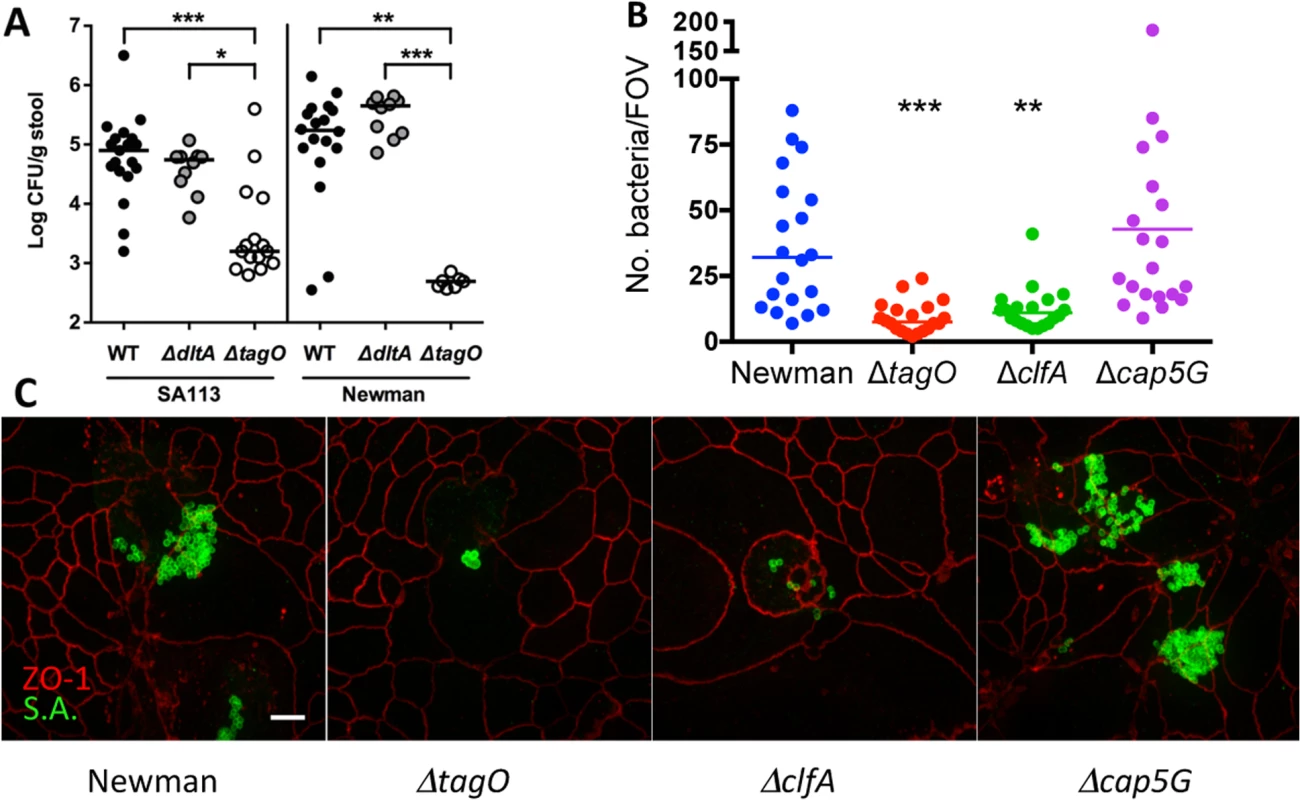
A) Fecal cultures were performed 7 d after bacterial inoculation with 109 CFU S. aureus. Each dot indicates the CFU S. aureus/g stool for a single mouse, and the median of each group of animals is indicated by a horizontal line. The lower limit of detection by culture was ~2.5 log CFU/g stool. P-values were determined by Kruskal-Wallis test with Dunn’s multiple comparison test. B) At an MOI of 100, Newman ΔtagO and ΔclfA were less adherent in vitro to T84 intestinal epithelial cells than WT strain Newman, as measured by the number of adherent S. aureus per field of view (FOV). * P < 0.05; ** P < 0.01; *** P < 0.001. C) The relative adherence of strain Newman and its isogenic mutants lacking WTA (ΔtagO), ClfA, or capsular polysaccharide (Δcap5G) to T84 cells in vitro was viewed by confocal microscopy. Immunostaining was performed with mouse anti-ZO-1 (red) to visualize the T-84 apical tight junctions and S. aureus rabbit antiserum to identify the bacteria (green). Scale bar, 10 microns. WTA has been reported to promote staphylococcal adherence to human nasal epithelial cells and endothelial cells [13,23]. As a result, we compared the relative in vitro adherence of strains Newman and Newman ΔtagO to T84 human intestinal epithelial cells. As shown in Fig 2B and 2C, strain Newman showed significantly (P = 0.0009) greater adherence to the T84 cells than did its ΔtagO mutant. Likewise, the clfA mutant, which showed an in vivo fitness defect (Fig 1C), also showed impaired adherence to T84 cells (Fig 2B and 2C; P = 0031). The acapsular cap5G mutant, on the other hand, showed a modest increase in overall adherence to the epithelial cells, consistent with previous studies showing that the capsule can mask adhesins that mediate attachment to mammalian cells [24,25].
Competition between Newman and Newman ΔtagO within the GI tract
To determine whether a WTA-deficient mutant was competent for transit through the stomach and duodenum to establish a colonization niche in the intestines, we performed several short-term in vivo experiments. Mice were inoculated with either strain Newman or the Newman tagO mutant and euthanized after 1 h. Recovery of S. aureus by quantitative cultures of the stomach and four different segments of the GI tract were quite variable among different mice. For this reason, we performed subsequent experiments in a competition format wherein mice were inoculated with a 1 : 1 mixture of Newman and Newman ΔtagO. A control in vitro competition experiment whereby Newman and its tagO mutant were cultured together for 1 h showed a CI of 0. Strain Newman and its tagO isogenic mutant were co-inoculated into mice, and after 1 h segments of the gut were homogenized and cultured quantitatively. The Newman ΔtagO mutant exhibited a substantial fitness defect in vivo with median CIs of 0.33 and 0.92 in the murine stomach and duodenum, respectively (P < 0.05) (Fig 3A). Few S. aureus colonies were recovered from the fourth segment of the small intestine, the cecum, or the colon, and consequently these data were not analyzed further. Our findings indicate that the ΔtagO mutant is significantly impaired in surviving transit through the mouse stomach and duodenum, and that its failure to colonize the GI tract may reflect its inherent susceptibility to antimicrobial factors in the gut.
Fig. 3. The reduced GI fitness of the ΔtagO mutant correlates with its susceptibility to bile, defensins, and proteases. 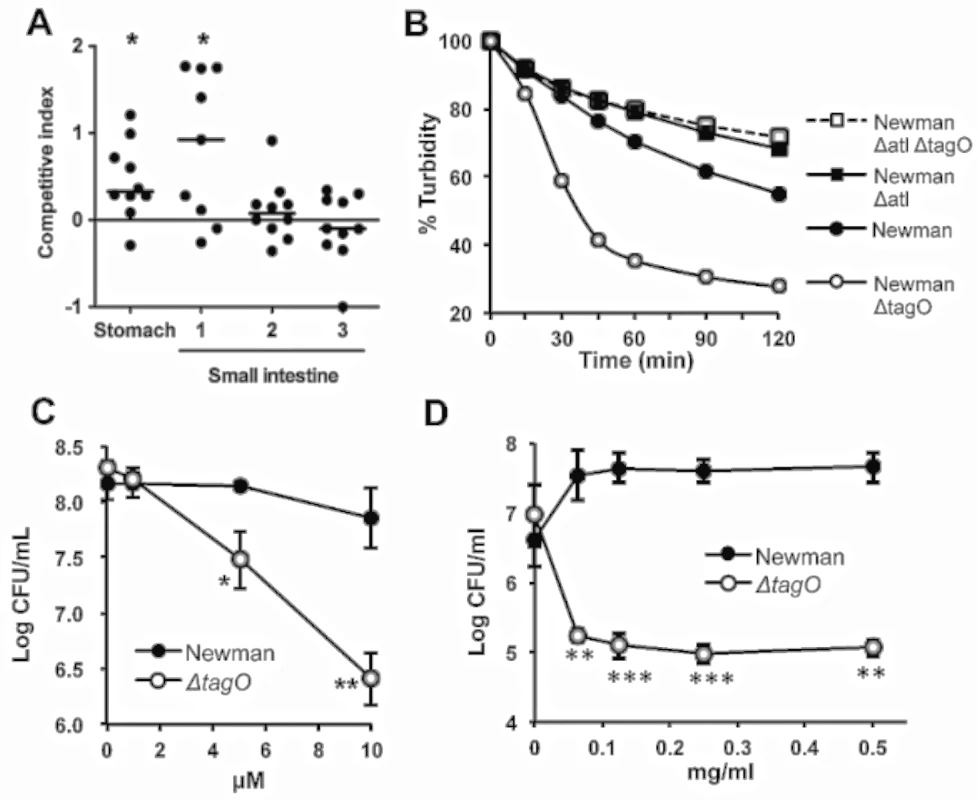
A) Mice were inoculated with a 1:1 mixture of the WT and ΔtagO mutant. After 1 h segments of the gut were homogenized and cultured quantitatively. The average S. aureus bacterial burden in the stomach was 3.2 x 106 CFU/g, and the first three segments of the small intestine yielded S. aureus concentrations of 2.6 x 105, 5.3 x 105 and 3.4 x 107 CFU/g, respectively. Horizontal bars represent the median competitive index for groups of 9–10 mice. P values were determined by Wilcoxon signed-rank test. B) S. aureus suspensions were exposed in vitro to bile salts (0.075% sodium deoxycholate), and quantitative cultures were performed to evaluate bacterial survival compared with samples lacking deoxycholate. Data are representative of 3 separate determinations. C) S. aureus were exposed for 2 h to increasing concentrations of α-defensin 5. The data are means ± SEM of 3 experiments. D) S. aureus at 1 x 106 CFU/ml were incubated at 37°C in the presence of increasing concentrations of proteinase K, and bacterial viability was measured after 24 h. The data are means ± SEM of 3 independent experiments. P-values were determined by the Student t-test. *P < 0.05, **P < 0.01, ***P < 0.001. Relative susceptibility of the ΔtagO mutant to antibacterial factors in the mouse GI tract
Because the tagO mutant survived poorly in vivo after inoculation, we hypothesized that Newman ΔtagO might be more susceptible than the WT strain to bile salts or low pH. As shown in Fig 3B, the tagO mutant was more susceptible than strain Newman to killing by bile salts (0.075% sodium deoxycholate), and the killing was rapid (~1 h). Because the concentration of bile salts in hepatic bile can reach concentrations as high as 1.66% [26], the enhanced susceptibility of the tagO mutant may contribute to its poor recovery from the gut after only 1 h in vivo. In contrast, both the parental and mutant strains were killed under low pH conditions (glycine buffer, pH 3.0–3.6), and no differences in their relative susceptibilities to acidic conditions were observed (S7 Fig).
The antimicrobial peptide α-defensin 5, secreted by mammalian Paneth cells, plays a role in the intestinal host defense against bacterial pathogens. Furthermore, α-defensins demonstrate homeostatic control of the host commensal microbiota [27]. Our in vitro studies indicated that the Newman ΔtagO mutant was killed to a significantly greater extent than the WT strain after a 2 h exposure to α-defensin 5 concentrations ranging from 5–10 μM (Fig 3C), which are far below the 14–70 μM concentrations found in the human intestinal lumen [28].
Bacteria transiting the GI tract are also exposed to proteolytic enzymes. Although we could not demonstrate consistent differences in susceptibility during short-term exposures to proteases (S8 Fig), the tagO mutant was more sensitive than the parental strain Newman to overnight treatments with proteinase K (Fig 3D), pepsin (at low pH) and trypsin (S8 Fig).
Bactericidal activity of staphylococcal supernatants for Newman ΔtagO
As noted above, when 109 CFU of Newman and its tagO mutant were incubated together in vitro for 1 h at 37°C in tryptic soy broth (TSB; Difo, Sparks, MD), the competitive index was 0, i.e., both strains were recovered in equivalent numbers. However, if the two strains were incubated together overnight in TSB, the tagO mutant could not be recovered from the culture (>107 reduction in CFU compared to strain Newman). Similar experiments performed with WT strain SA113 and its tagO mutant yielded comparable results (~105 reduction in the SA113 ΔtagO CFU compared with SA113). Filter-sterilized culture supernatants of S. aureus Newman (but not culture supernatants from the tagO mutant) also showed bactericidal activity toward Newman ΔtagO within 1 to 3 h of incubation (Fig 4A and 4B). The bactericidal activity of the Newman culture supernatant was lost if it was boiled for 5 min prior to inoculation with Newman ΔtagO (Fig 4A). In contrast, the addition of a protease inhibitor cocktail to the supernatant had no effect on its killing activity (Fig 4A). Consistent with this finding, we observed no differences in the protease activity of culture supernatants derived from the WT or the ΔtagO mutant strain when measured on casein agar plates, azocasein assays, or the PDQ protease assay [Athena Environmental Sciences, Inc., Baltimore, MD] (S9 Fig). Peptidoglycan fragments added to filter-sterilized culture supernatants of strain Newman inhibited killing of the ΔtagO mutant in a dose dependent manner (Fig 4B). This finding suggested that autolysins present in the Newman culture supernatant might be lysing the tagO mutant. Indeed, zymogram analysis revealed that the Newman culture supernatant showed greater autolytic activity than that of the tagO mutant (Fig 4C). That the bactericidal effect on the WTA mutant was due to exogenous autolytic activity was supported by the observation that both the tagO and an autolysin (atl) tagO double mutant showed a >2-log reduction in CFU/ml following a 3-h exposure to the Newman culture supernatant (Fig 4D). In contrast, neither strain that produced WTA (the atl mutant nor Newman ΔtagO complemented with a plasmid carrying the cloned tagO gene [ΔtagO C’]) were killed after incubation with the Newman supernatant. The susceptibility of the tagO mutant to the bactericidal activity of culture supernatants was not limited to strain Newman, since Newman ΔtagO was also killed by supernatants harvested from S. aureus strains USA300 LAC and ST80, but not from Sanger 252 (Fig 4E). None of the culture supernatants were bactericidal against the parental strain Newman.
Fig. 4. Culture supernatants of WT S. aureus were bactericidal for Newman ΔtagO. 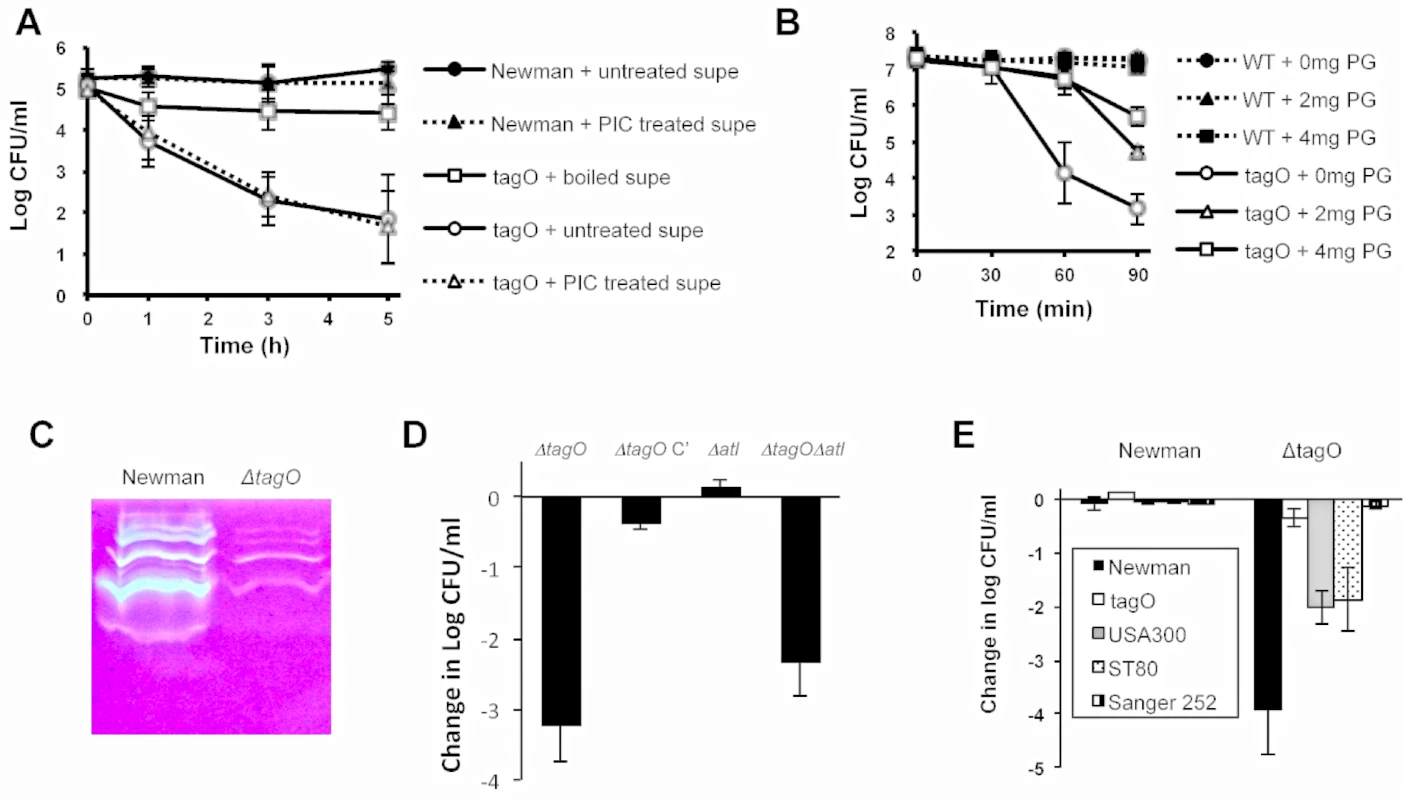
A) Newman supernatants (untreated), boiled, or supplemented with a protease inhibitor cocktail (PIC) were inoculated with Newman or Newman ΔtagO and incubated at 37°C. Bactericidal activity was measured by quantitative cultures at the indicated time points. B) Newman culture supernatants were supplemented with 0, 2, or 4 mg peptidoglycan and inoculated with either Newman or Newman ΔtagO. CFU/ml determinations were performed to evaluate bacterial viability. The data represent means ± SEM of 3 independent experiments. C) Zymogram of Newman and ΔtagO culture supernatants. The samples were concentrated and adjusted to equivalent protein concentrations before electrophoresis. D) Newman mutants were inoculated into filter-sterilized Newman culture supernatants, and bactericidal activity was measured after 3 h. ΔtagO C’ carried pRBtagO, which restored WTA production to the tagO mutant. E) Newman or Newman ΔtagO was added to culture supernatants of S. aureus Newman, ΔtagO, ST80, or Sanger 252, and bactericidal activity was measured at 3 h. The data represent means ± SEM of 3–4 experiments. Koprivnjak et al. reported that S. aureus SA113 ΔtagO was more sensitive than the WT strain to Triton X-100 at concentrations >0.1% [29]. We observed that Newman ΔtagO also was hypersusceptible to Triton X-100 mediated bacterial lysis at concentrations as low as 0.05% (Fig 5A). As expected, a Newman atl mutant and an atl tagO double mutant were resistant to Triton X-100 induced autolysis (Fig 5A). Because factors that stimulate autolytic activity include detergents, proteolytic enzymes, and cationic peptides [30], we hypothesized that the Newman ΔtagO might be more sensitive than the WT strain to factors that promote staphylococcal autolysis. This hypothesis is consistent with the observation made by Atilano et al [31] that peptidoglycan prepared from a ΔtagO mutant shows reduced cross-linking compared to the WT strain. Autolysin extracts from strain Newman and Newman ΔtagO showed similar lytic activity toward peptidoglycan prepared from the strain Lafferty (S10 Fig). However, peptidoglycan prepared from the Newman ΔtagO mutant was more susceptible to Newman autolysin extracts than a peptidoglycan preparation from the parental strain Newman (Fig 5B).
Fig. 5. The relative susceptibilities of S. aureus strains and their peptidoglycan preparations to autolysis. 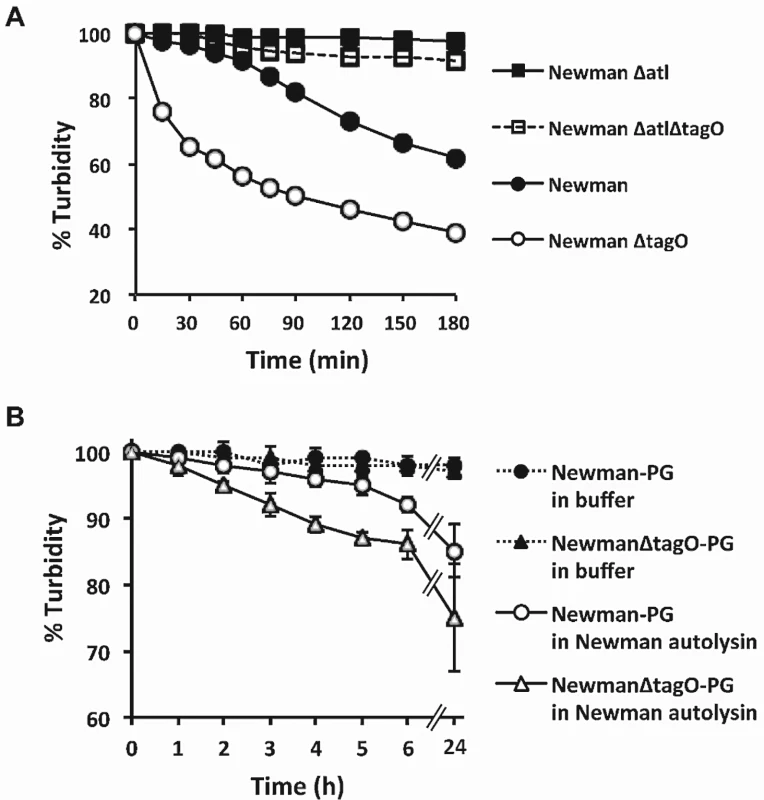
A) Triton X-100 induced autolysis of S. aureus Newman, ΔtagO, Δatl, and ΔtagOΔatl mutants. The results shown are representative of 3 independent determinations. B) An S. aureus Newman autolysin extract (25 μg protein/ml) was prepared and incubated with peptidoglycan isolated from Newman and Newman ΔtagO. The data represent means ± SEM of 3 independent experiments. Lysis was expressed as a percentage of the OD580 nm at time zero. GI colonization by a WTA mutant resistant to autolysis (Δatl ΔtagO)
To clarify the relationship between the defect in GI colonization of the tagO mutant and its susceptibility to autolysis, we inoculated separate groups of mice with Newman, Newman Δatl, Newman ΔtagO, or an atl tagO double mutant; GI colonization was assessed up to 14 days after inoculation. The Newman Δatl mutant colonized the mouse GI tract as well as the WT strain, but the ΔtagO and the Δatl ΔtagO double mutant failed to colonize (S11 Fig). When the experiment was repeated, we observed that both the tagO and the atl tagO double mutant were recovered in reduced numbers from the stool as early as two days after inoculation, and by day five neither mutant could be detected in the murine stool cultures (Fig 6). Similar results were obtained in mice inoculated by oral gavage (S5 Fig). In a competition format, four mice were inoculated with a 1 : 1 mixture of Newman and the Newman double mutant and euthanized for quantitative cultures after 1 h. Like the tagO mutant, Newman Δatl ΔtagO exhibited a substantial fitness defect in vivo with a median CI of 1.9 in the stomach and CIs ranging from 0.98 to 1.6 in the proximal small intestine. These results indicate that the failure of S. aureus lacking WTA to colonize the GI tract is not explained by its enhanced autolytic response (lacking in the double mutant), but instead correlates with its poor adherence and susceptibility to bactericidal factors within the mouse gut.
Fig. 6. Both Newman ΔtagO and Newman Δatl ΔtagO fail to persistently colonize the mouse GI tract. 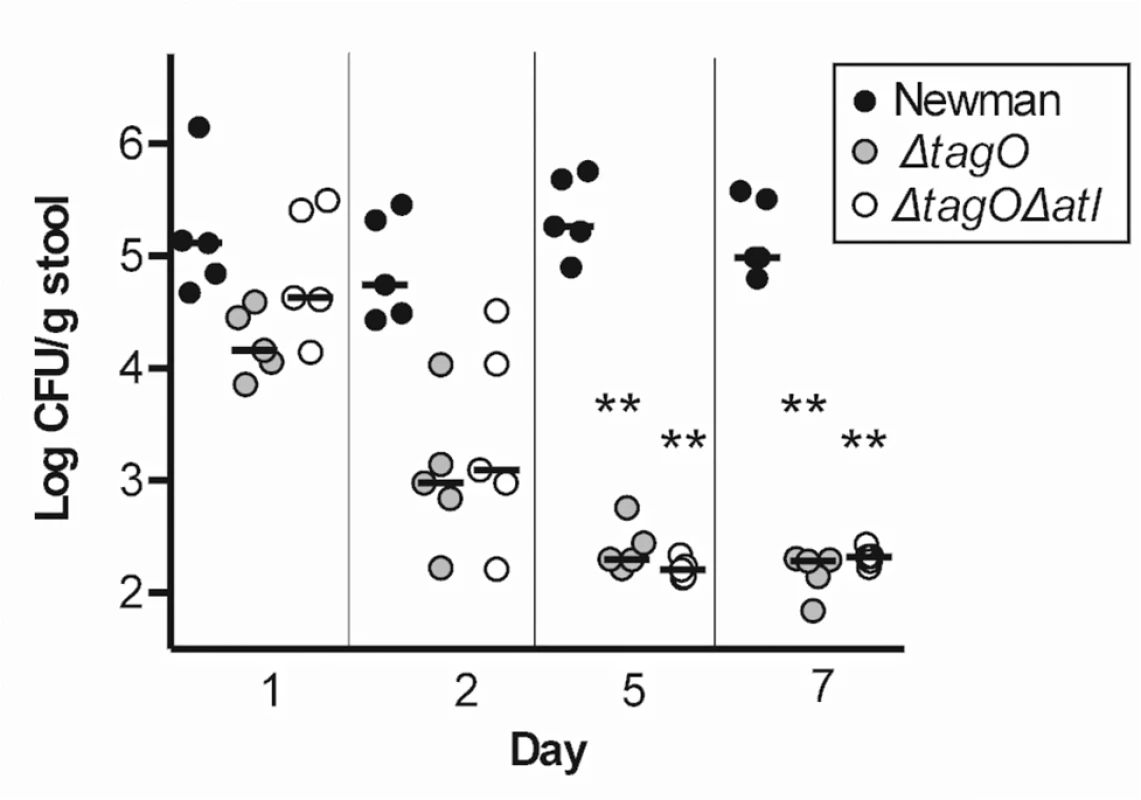
Mice were inoculated on day 0 with Newman, Newman ΔtagO, or Newman Δatl ΔtagO, and stool samples were cultured quantitatively 1, 2, 5, and 7 days after inoculation. Each symbol represents a stool culture from an individual mouse. P values were determined by Wilcoxon signed-rank test. **P < 0.01. Discussion
In addition to its niche within the nares, in the throat, and on the skin of humans, S. aureus may also colonize the GI tract of ~20% of otherwise healthy individuals [3]. While no disease is clearly associated with GI colonization by staphylococci, it serves as a source of recolonization after eradication of nasal colonization. In a hospital setting or in the community, fecal contamination associated with diarrhea or incontinence could contaminate the environment and facilitate transmission among individuals. As such, the GI tract is an under-appreciated reservoir for methicillin-resistant and-sensitive strains of S. aureus. Among patients in an intensive care unit or transplant unit, those positive for both rectal and nasal carriage of S. aureus were more likely to develop a staphylococcal infection than those with nasal carriage alone [8]. Other reports have similarly suggested that GI carriage is an important risk factor for S. aureus infections [3,4,9,10]. Importantly, staphylococcal skin and soft tissue infections are linked to rectal, but not nasal, colonization by MRSA strains in children [10]. Molecular typing experiments indicated that rectal, nares, and infecting isolates (including blood isolates) were clonally identical in 82% of the patients with S. aureus infections [8]. A comprehensive understanding of the factors involved in asymptomatic carriage by S. aureus in humans is critical to our ability to control the incidence of infection.
Kernbauer et al. [32] recently reported that mice challenged intravenously with S. aureus develop staphylococcal colonization of the GI tract, and that fecal shedding resulted in S. aureus transmission to cohoused naïve mice. They noted that GI colonization resulted in no obvious signs of infectious abscesses or inflammation. In our studies, healthy mice inoculated intranasally developed persistent S. aureus GI colonization only when they were administered Sm in their drinking water for at least one week. This suggests that transient suppression of the indigenous intestinal flora may reduce bacterial interference and created an environment amenable to stable S. aureus colonization. The concentration of S. aureus in the cecum, colon, and stool was not high in conventional mice, achieving levels ~105 CFU/g, very similar to those reported by Kernbauer et al. [32]. When germ-free mice that lacked a competing indigenous flora were inoculated with S. aureus, the concentrations in the intestinal contents and stool rose to ~108 CFU/g.
To investigate bacterial factors that impact GI colonization, we assessed colonization of mice inoculated with a single bacterial strain, and we also performed competition experiments between WT and mutant S. aureus strains. A Newman type 5 capsule-negative mutant was able to persist in the gut in higher numbers than the WT encapsulated parental strain in competition experiments, consistent with previous studies showing that the capsule can impede factors critical for colonization [24,25]. The Newman sortase and clfA mutants each showed impaired fitness in the mouse gut. In contrast, strains with mutations in clfB, spa, sdrCDE, or the ica locus showed no fitness defect in the GI colonization model. This suggests that ClfA and perhaps other wall-anchored protein adhesins (possibly masked by the capsule) play a role in promoting S. aureus colonization of the GI tract. Competition experiments are commonly used to assess microbial fitness for colonization of the GI tract [19–21], probably because they are more sensitive and may reveal more subtle competitive advantages demonstrated by a particular wild type or mutant strain.
More striking, however, was our observation that tagO mutants of strains SA113 and Newman that fail to produce WTA were unable to colonize the nose or the GI tract of mice. This colonization defect was evident in groups of conventional mice inoculated with pure cultures of the mutant strain; competition assays were not necessary to demonstrate this impaired colonization phenotype in vivo. S. aureus mutants that lacked the D-ala or GlcNAc substituents of WTA, on the other hand, showed no defects in GI colonization. These findings suggest that the glycopolymer itself, and not its modifying groups, are critical for interactions with the host.
In vitro assays demonstrated that both the tagO and clfA mutants of strain Newman were less adherent to T84 cells in vitro than the parental strain Newman. These data suggest a critical role for these surface antigens in GI colonization. Baur et al. [33] recently reported that S. aureus WTA interacts with a scavenger receptor (SREC-I; scavenger receptor expressed by endothelial cell-I) detected on nasal epithelial cells to promote bacterial adherence. Whether such a receptor is found on intestinal epithelial cells remains to be determined.
In addition to its adherence defects, the tagO mutant showed fitness defects in transit through the mouse stomach and intestines. In an in vivo competition study, the tagO mutant was outcompeted by the WT strain within 1 h after inoculation. This finding is consistent with our in vitro observations that the tagO mutant was more susceptible than the parental strain to bile acids, proteases, and alpha defensin 5, which would be encountered in the GI tract. Like humans, mice have Paneth cells in the small intestinal crypts, and these cells secrete alpha defensins that show bactericidal activity against a number of microbes, including S. aureus [34].
S. aureus autolysins are activated by detergents (Triton X-100 and bile acids such as deoxycholate), defensins, and proteases [30]. Because these factors are present in the GI tract, we postulated that the Newman tagO mutant did not survive well because it is more susceptible to stress-induced autolysis than the wild-type strain. To address this, we created an atl deficient mutant of strain Newman. Atl is the major S. aureus autolytic enzyme, and it binds preferentially at the septum site (where WTA is less abundant) to facilitate cell division. However, Atl loses its selective localization to division sites in a tagO mutant. In the absence of WTA, Atl binding was reported to be evenly distributed on the cell surface, which explains the increased fragility and susceptibility of the ΔtagO mutant to autolysis [31,35]. As expected, the atl and the atl tagO double mutant were both resistant to Triton X-100-induced lysis (activation of endogenous autolysins), whereas both the tagO and atl tagO double mutant were highly susceptible to exogenous autolysins (in Newman culture supernatants).
Our final in vivo colonization studies showed that the WT strain Newman and its isogenic atl mutant were both proficient for GI colonization. However, neither the tagO nor the atl tagO double mutant colonized the mouse gut. These results indicate that the enhanced sensitivity of the tagO mutant to antimicrobial factors in the GI tract, as well as its poor adherence characteristics, likely contribute to its impaired fitness in vivo. However, the enhanced vulnerability of the tagO mutant to clearance from the GI tract is apparently not due to activation of its endogenous autolysins by host-induced bacterial stress. Previous studies have characterized the in vitro susceptibility of S. aureus ΔtagO to a variety of host immune defenses. Compared to the parental strains, a ΔtagO mutant showed increased susceptibility to antimicrobial fatty acids on human skin [36], but it was not more susceptible to killing by neutrophils, lysozyme [37], lactoferrin, or cationic antimicrobial peptides such as hNP1-3, LL37, or magainin II amide. Mutants lacking WTA were more resistant to mammalian group IIA phospholipase A2 and human β-defensin 3 (HBD-3) than the WT strain [29]. Additional studies will be necessary to further delineate the essential role of WTA in S. aureus colonization of the mammalian GI tract.
The murine colonization model may be useful in further characterization of factors that impact S. aureus carriage in the GI tract, with the acknowledged limitation that the human intestinal tract and its flora have characteristics distinct from those of the mouse [38]. Nonetheless, our results show that S. aureus WTA contributes to staphylococcal fitness within the GI tract, providing resistance to host bactericidal factors and promoting bacterial adherence to epithelial cells. A better understanding of mechanisms that lead to asymptomatic colonization by S. aureus may lead to preventive therapies that impact transmission among humans. Recent efforts to develop antimicrobials that target WTA [39–41] may lead to effective agents that diminish both nasal and GI colonization by MRSA and may impact invasive disease [23].
Materials and Methods
Bacterial strains
The S. aureus strains tested in the murine gastrointestinal colonization model are listed in Table 3; each was resistant to Sm. Mutations in the S. aureus ica locus, spa, and atl were moved into Sm-resistant Newman by transduction with phage 80α or phage 85 from the original antibiotic marked mutant strains [42–44]. The tagO::tet mutation was moved from S. aureus RN4220 into Newman Δatl as described previously [22]. Mutants were confirmed by PCR or Southern blot analysis and were phenotypically identical to the parental strains in terms of the growth rate, hemolysis on sheep blood agar plates, and the metabolic profile on API Staph test strips (Biomerieux, Inc., Durham, NC). S. aureus strains were cultivated in TSB to the logarithmic growth phase, unless otherwise noted. Sm (0.5 mg/ml; Sigma Chemical Co., St. Louis, Mo.), erythromycin (Em; 5 μg/ml; Sigma), tetracycline (Tc; 2.5 μg/ml; Sigma), or spectinomycin (Spc; 100 μg/ml; MP Biomedicals, Solon, OH) was added to culture medium for selection where appropriate.
Tab. 3. Sm-resistant S. aureus strains used in this study. 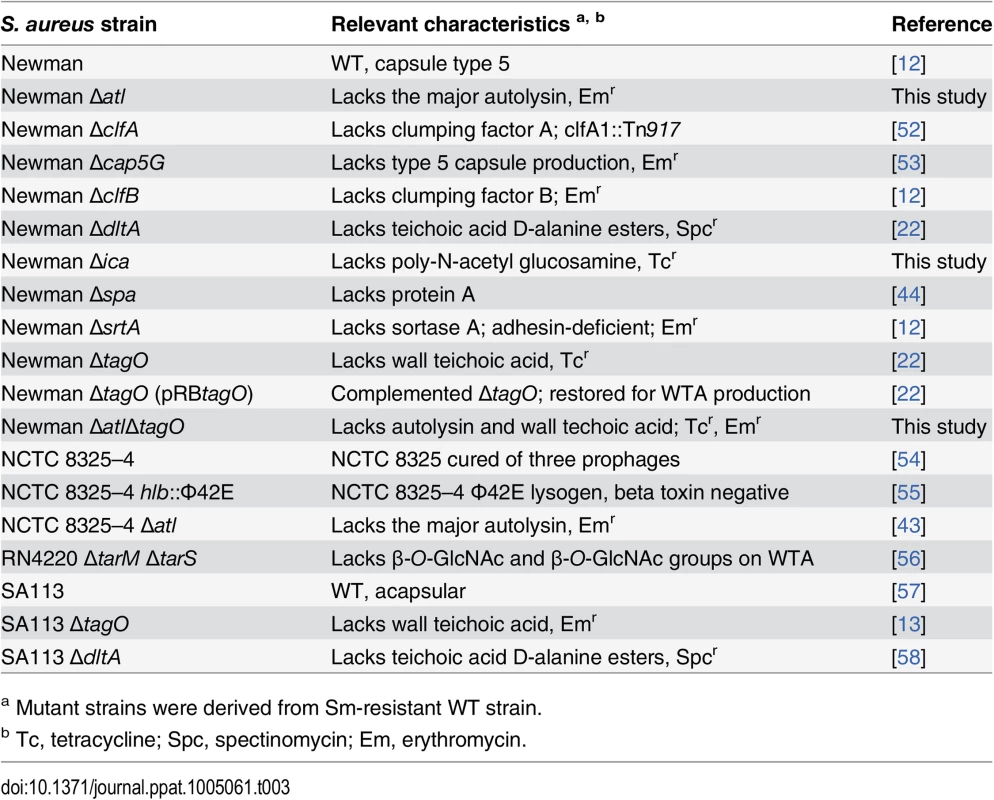
a Mutant strains were derived from Sm-resistant WT strain. Ethics statement
Animal experiments were approved by the Longwood Medical Area's Institutional Animal Care and Use Committee under protocol 86–02131. All studies were performed in strict accordance with the National Institutes of Health standards as set forth in "Guide for the Care and Use of Laboratory Animals" (DHSS Publication No. (NIH) 85–23).
Murine model of gastrointestinal colonization
Conventional female ICR mice aged 4–6 wks old were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN) or Charles River Laboratories (Wilmington, MA). The mice were given drinking water containing Sm (0.5 g/L) one day prior to inoculation and for the course of experiment (unless otherwise noted), and the drinking water and cages were changed twice a week. Conventional mice were inoculated without prior anesthesia by the intranasal route with 10 μl of an S. aureus suspension as described [12]. Gastrointestinal colonization by S. aureus was evaluated by quantitative cultures of representative mouse stool pellets that were collected prior to inoculation and weekly thereafter. The samples were weighed, suspended in 2–5 ml of TSB, diluted, and plated quantitatively on mannitol salt agar (MSA) containing 0.5 mg/ml Sm. The plates were incubated aerobically for 48 h at 37°C, the colonies were enumerated, and the data were expressed as CFU/g stool. Significant (P < 0.05) differences between quantitative culture results were determined by the two-tailed Student t test, and the Welch correction was applied to pairs with unequal variances. P-values for experiments having three or more groups were determined by the Kruskal-Wallis test with Dunn’s multiple comparison analysis (Prism; GraphPad Software, La Jolla, CA).
Germfree Swiss-Webster female mice (3–4 weeks old) were purchased from Taconic Farms (Hudson, NY) and maintained in a negative-pressure BL2 isolator by personnel at the gnotobiotic core facility of the Harvard Digestive Diseases Center. S. aureus suspensions were drawn into a sterile syringe with a neonatal feeding tube, and ~ 200 μl (~109 CFU) was orally fed to the mice. Gnotobiotic mice were given sterile (no Sm) drinking water, and their stools were cultured on nonselective medium (tryptic soy agar [TSA] plates).
For competition experiments, the mutant and parental strains were mixed in equal numbers (total 109 CFU/mouse) prior to inoculation of conventional mice. The input CFU ratio was calculated by dividing the wild type inoculum CFU by the mutant inoculum CFU (WT/mutant). At various time points, stool samples were collected and plated quantitatively on MSA + Sm plates. Colonies of the mutant strains were distinguished from those of the parental strain by transferring (via sterile toothpicks) ~100 colonies to TSA and TSA + antibiotic (Tc, Em, or Spc) plates. The output CFU ratio was determined by dividing the WT CFU by the mutant CFU for each fecal sample. A competitive index (CI) was calculated as described [45] by dividing the output ratio by the input ratio, and the CI was expressed as log10. A CI = 0 indicates similar numbers of CFU of the two competing strains recovered in vivo, suggesting comparable fitness of the strains in vivo. A CI >1 indicates the fitness advantage of the parental strain over the mutant, whereas a CI <1 indicates that the mutant is more fit than the parental strain. A Wilcoxon signed-rank test was used to determine whether the CI values were significantly different from the hypothetical value of zero. For in vitro competition experiments, the parental and mutant strains were inoculated together into 5 ml of TSB, grown with aeration for either 2 or 24 h at 37°C, and subsequently plated quantitatively. Colonies of the ΔtagO mutant were distinguished from those of the parental strain by their antibiotic resistance as described above, and the CI was calculated.
Competition between Newman and Newman ΔtagO in transit through the GI tract
To determine whether the tagO mutant survived as well as the parental strain in its transit through the mouse stomach, rodent chow was withheld for 3 h prior to inoculation of mice with either S. aureus Newman, Newman ΔtagO, or Newman ΔtagO Δatl. The mice were euthanized by CO2 asphyxiation after 1 h, and quantitative cultures were performed on homogenates made from different segments of the GI tract. However, variations in the recovery of S. aureus among individual mice were substantial, and we were unable to draw meaningful conclusions from these data. To circumvent this problem, competition experiments were performed as described above in which equal numbers (~5 x 108 CFU) of the two strains were mixed before inoculation. One hour after bacterial challenge, the mice were euthanized, the GI tract was excised, and the stomach, small intestine (4 segments), cecum, and colon were separated. Each segment was weighed, homogenized in 1 ml TSB, and cultured quantitatively on MSA + Sm plates. Colonies of the ΔtagO mutant were distinguished from those of the parental strain by replica plating on TSA + Tc or TSA + Em, and the CI was calculated.
Adherence of Newman vs. ΔtagO to T84 cells
Polarized monolayers of human intestinal T84 cells were cultured on 0.33 cm2 polyester Transwell inserts (Corning, Acton, MA) as previously described [46]. Log-phase TSB cultures of S. aureus Newman or its isogenic mutants were washed in Hanks Balanced Salt Solution (HBSS) and incubated at a multiplicity of infection of 100 on the apical surfaces of polarized T84 monolayers for 1 h at 37°C. Inserts were washed five times with HBSS and processed for confocal microscopy as previously described [47]. Briefly, the T84 cell monolayers were fixed in 4% paraformaldehyde for 20 min. The cells were washed 3x in HBSS, permeabilized with 0.2% Saponin/HBSS solution, washed again, and blocked with 10% normal goat serum (NGS)/HBSS solution. Immunostaining was performed with mouse anti-ZO-1 (1 : 200; Invitrogen) and S. aureus rabbit antiserum (raised to whole killed bacteria and diluted 1 : 400) in 10% NGS/HBSS. The cells were incubated with AlexaFluor 568 labeled goat anti-mouse conjugate and AlexaFluor 488 labeled goat anti-rabbit conjugate (both 1 : 400 in 10% NGS/HBSS solution; Invitrogen) for 1 h at room temperature. Samples were imaged by confocal microscopy on a Nikon TE2000 inverted microscope (Nikon Instruments, Melville, NY) coupled to a Perkin-Elmer spinning disk confocal unit (Boston, MA), using a Nikon PlanFluor 40× (1.3 NA) and Nikon PlanApo 60x (1.4 NA) oil immersion objective lens and an Orca AG scientific-grade cooled CCD camera (Hamamatsu Photonics K.K., Japan). Confocal images, collected en face to the apical membrane, were 3D stacks collapsed into a single projection. Adherence was quantified by counting the total bacteria in 20 random fields of view (FOV) among triplicate samples with a 63X objective and a 10X ocular lens.
Bactericidal assays
Newman WT and ΔtagO were cultivated in TSB to log phase at 37°C, washed in 10 mM phosphate buffer (pH 7.4), and suspended to 1–3 x 108 CFU/ml in either buffer, bile salts (sodium deoxycholate [DOC]; Sigma), or human α-defensin 5 (HD5) (Peptide Institute, Inc., Osaka, Japan). Susceptibility to low pH was assessed by suspending S. aureus strains in glycine buffer (pH 3.0, 3.3, or 3.6) or in phosphate buffer at neutral pH. Bacterial suspensions were incubated at 37°C for 1 to 2 h, and viable counts were determined. Susceptibility to proteases was assessed by preparing serial dilutions in optimal buffers (proteinase K in 10 mM phosphate buffer, pH 7.4; pepsin in glycine buffer, pH 3.6; trypsin in 10 mM phosphate, pH 8.0 buffer). The protease solutions were then inoculated with logarithmic-phase S. aureus to a final concentration of 1 x 106 CFU/ml, and viability counts were performed after incubation for 24 h at 37°C.
The bactericidal activity of S. aureus culture supernatants was assessed by culturing the S. aureus strains in TSB at 37°C for ~21 h. Filter-sterilized culture supernatants were inoculated to a final 105 CFU/ml with strain Newman or ΔtagO, ΔtagO (pRBtagO) [22], Δatl, or Δatl ΔtagO mutants. CFU/ml determinations were performed after incubation for 1 to 5 h at 37°C. For some assays, the Newman supernatant was boiled for 5 min, treated with a protease inhibitor cocktail (Sigma P-8465), or supplemented with 0 to 4 mg peptidoglycan purified from S. aureus Lafferty [48] as described [22] before inoculation with strain Newman or its tagO mutant.
S. aureus autolysin activity
Triton X-100-induced autolysin assays were performed as described by Shaw et al. [49]. Briefly, Newman WT and ΔtagO strains were grown to logarithmic phase, and the bacterial cells were washed twice and suspended to OD650 nm of 1.0 in 50 mM Tris-HCl (pH 7.6), 2 mM CaCl2, 0.05% Triton X-100. The suspensions were incubated in a 37°C shaking water bath, and the OD580 nm was monitored for 3 h.
Zymography was used to detect the lytic activities in culture supernatants from Newman and Newman ΔtagO, as described by Groicher et al. [50]. Overnight culture supernatants, concentrated with Centricon-5 centrifugal filter units (Millipore) to a protein concentration of 2.65 mg/ml, were electrophoresed in a 10% polyacrylamide SDS gel supplemented with 1 mg/ml S. aureus cell walls. After washing the gel in 1% Triton X-100 and 25 mM Tris-HCl buffer overnight to allow cell wall hydrolysis, the gel was stained with 1% methylene blue and destained in water. Lytic zones in the gel indicated proteins with peptidoglycan hydrolase activity.
S. aureus autolysin extracts were prepared by 3 M LiCl treatment as described [51]. Peptidoglycan samples, prepared from strains Newman or Newman ΔtagO as described previously [22], were suspended in 0.01 M KHPO4 buffer (pH 7.1) to OD580 nm of 0.5 to 0.6. The suspensions were mixed with an S. aureus Newman autolysin extract (25 μg/ml protein), incubated in a 30°C water bath, and OD580 nm readings were taken at 1 h intervals. Lytic activity was expressed as a percentage of the OD580 nm at time zero.
Supporting Information
Zdroje
1. Archer GL (1998) Staphylococcus aureus: A well-armed pathogen. Clin Infect Dis 26 : 1179–1181. 9597249
2. Creech CB 2nd, Talbot TR, Schaffner W (2006) Community-associated methicillin-resistant Staphylococcus aureus: the way to the wound is through the nose. J Infect Dis 193 : 169–171. 16362879
3. Acton DS, Plat-Sinnige MJ, van Wamel W, de Groot N, van Belkum A (2009) Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J Clin Microbiol Infect Dis 28 : 115–127. doi: 10.1007/s10096-008-0602-7 18688664
4. Boyce JM, Havill NL, Maria B (2005) Frequency and possible infection control implications of gastrointestinal colonization with methicillin-resistant Staphylococcus aureus. J Clin Microbiol 43 : 5992–5995. 16333087
5. Ray AJ, Pultz NJ, Bhalla A, Aron DC, Donskey CJ (2003) Coexistence of vancomycin-resistant enterococci and Staphylococcus aureus in the intestinal tracts of hospitalized patients. Clin Infect Dis 37 : 875–881. 13130397
6. Rimland D, Roberson B (1986) Gastrointestinal carriage of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 24 : 137–138. 3722359
7. Bhalla A, Aron DC, Donskey CJ (2007) Staphylococcus aureus intestinal colonization is associated with increased frequency of S. aureus on skin of hospitalized patients. BMC Infect Dis 7 : 105. 17848192
8. Squier C, Rihs JD, Risa KJ, Sagnimeni A, Wagener MM, et al. (2002) Staphylococcus aureus rectal carriage and its association with infections in patients in a surgical intensive care unit and a liver transplant unit. Infect Control Hosp Epidemiol 23 : 495–501. 12269445
9. Boyce JM, Havill NL, Otter JA, Adams NM (2007) Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 28 : 1142–1147. 17828690
10. Faden H, Lesse AJ, Trask J, Hill JA, Hess DJ, et al. (2010) Importance of colonization site in the current epidemic of staphylococcal skin abscesses. Pediatrics 125: e618–624. doi: 10.1542/peds.2009-1523 20156893
11. Szumowski JD, Wener KM, Gold HS, Wong M, Venkataraman L, et al. (2009) Methicillin-resistant Staphylococcus aureus colonization, behavioral risk factors, and skin and soft-tissue infection at an ambulatory clinic serving a large population of HIV-infected men who have sex with men. Clin Infect Dis 49 : 118–121. doi: 10.1086/599608 19480576
12. Schaffer AC, Solinga RM, Cocchiaro J, Portoles M, Kiser KB, et al. (2006) Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect Immun 74 : 2145–2153. 16552044
13. Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, et al. (2004) Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med 10 : 243–245. 14758355
14. Wertheim HF, Walsh E, Choudhurry R, Melles DC, Boelens HA, et al. (2008) Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Medicine 5: e17. doi: 10.1371/journal.pmed.0050017 18198942
15. Savage DC, Dubos R, Schaedler RW (1968) The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med 127 : 67–76. 4169441
16. Saha S, Jing X, Park SY, Wang S, Li X, et al. (2010) Peptidoglycan recognition proteins protect mice from experimental colitis by promoting normal gut flora and preventing induction of interferon-gamma. Cell Host Microbe 8 : 147–162. doi: 10.1016/j.chom.2010.07.005 20709292
17. Kiser KB, Cantey-Kiser JM, Lee JC (1999) Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect Immun 67 : 5001–5006. 10496870
18. Weidenmaier C, Kokai-Kun JF, Kulauzovic E, Kohler T, Thumm G, et al. (2008) Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int J Med Microbiol 298 : 505–513. doi: 10.1016/j.ijmm.2007.11.006 18221914
19. Pierce JV, Kumamoto CA (2012) Variation in Candida albicans EFG1 expression enables host-dependent changes in colonizing fungal populations. MBio 3: e00117–00112. doi: 10.1128/mBio.00117-12 22829676
20. Skurnik D, Roux D, Cattoir V, Danilchanka O, Lu X, et al. (2013) Enhanced in vivo fitness of carbapenem-resistant oprD mutants of Pseudomonas aeruginosa revealed through high-throughput sequencing. Proc Natl Acad Sci U S A 110 : 20747–20752. doi: 10.1073/pnas.1221552110 24248354
21. Coyne MJ, Chatzidaki-Livanis M, Paoletti LC, Comstock LE (2008) Role of glycan synthesis in colonization of the mammalian gut by the bacterial symbiont Bacteroides fragilis. Proc Natl Acad Sci U S A 105 : 13099–13104. doi: 10.1073/pnas.0804220105 18723678
22. Weidenmaier C, McLoughlin RM, Lee JC (2010) The zwitterionic cell wall teichoic acid of Staphylococcus aureus provokes skin abscesses in mice by a novel CD4+ T-cell-dependent mechanism. PLoS One 5: e13227. doi: 10.1371/journal.pone.0013227 20949105
23. Weidenmaier C, Peschel A, Xiong YQ, Kristian SA, Dietz K, et al. (2005) Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J Infect Dis 191 : 1771–1777. 15838806
24. Risley AL, Loughman A, Cywes-Bentley C, Foster TJ, Lee JC (2007) Capsular polysaccharide masks clumping factor A-mediated adherence of Staphylococcus aureus to fibrinogen and platelets. J Infect Dis 196 : 919–927. 17703424
25. Pohlmann-Dietze P, Ulrich M, Kiser KB, Doring G, Lee JC, et al. (2000) Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect Immun 68 : 4865–4871. 10948098
26. Begley M, Gahan CG, Hill C (2005) The interaction between bacteria and bile. FEMS Microbiol Rev 29 : 625–651. 16102595
27. Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, et al. (2010) Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 11 : 76–83. doi: 10.1038/ni.1825 19855381
28. Ghosh D, Porter E, Shen B, Lee SK, Wilk D, et al. (2002) Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol 3 : 583–590. 12021776
29. Koprivnjak T, Weidenmaier C, Peschel A, Weiss JP (2008) Wall teichoic acid deficiency in Staphylococcus aureus confers selective resistance to mammalian group IIA phospholipase A(2) and human beta-defensin 3. Infect Immun 76 : 2169–2176. doi: 10.1128/IAI.01705-07 18347049
30. Mani N, Tobin P, Jayaswal RK (1993) Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J Bacteriol 175 : 1493–1499. 8095258
31. Atilano ML, Pereira PM, Yates J, Reed P, Veiga H, et al. (2010) Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc Natl Acad Sci U S A 107 : 18991–18996. doi: 10.1073/pnas.1004304107 20944066
32. Kernbauer E, Maurer K, Torres VJ, Shopsin B, Cadwell K (2015) Gastrointestinal dissemination and transmission of Staphylococcus aureus following bacteremia. Infect Immun 83 : 372–378. doi: 10.1128/IAI.02272-14 25385792
33. Baur S, Rautenberg M, Faulstich M, Grau T, Severin Y, et al. (2014) A nasal epithelial receptor for Staphylococcus aureus WTA governs adhesion to epithelial cells and modulates nasal colonization. PLoS Pathog 10: e1004089. doi: 10.1371/journal.ppat.1004089 24788600
34. Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, et al. (2000) Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1 : 113–118. 11248802
35. Schlag M, Biswas R, Krismer B, Kohler T, Zoll S, et al. (2010) Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol Microbiol 75 : 864–873. doi: 10.1111/j.1365-2958.2009.07007.x 20105277
36. Kohler T, Weidenmaier C, Peschel A (2009) Wall teichoic acid protects Staphylococcus aureus against antimicrobial fatty acids from human skin. J Bacteriol 191 : 4482–4484. doi: 10.1128/JB.00221-09 19429623
37. Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, et al. (2007) Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J Bacteriol 189 : 280–283. 17085565
38. Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, et al. (2012) Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149 : 1578–1593. doi: 10.1016/j.cell.2012.04.037 22726443
39. Suzuki T, Swoboda JG, Campbell J, Walker S, Gilmore MS (2011) In vitro antimicrobial activity of wall teichoic acid biosynthesis inhibitors against Staphylococcus aureus isolates. Antimicrob Agents Chemother 55 : 767–774. doi: 10.1128/AAC.00879-10 21098254
40. Suzuki T, Campbell J, Swoboda JG, Walker S, Gilmore MS (2011) Role of wall teichoic acids in Staphylococcus aureus endophthalmitis. Invest Ophthalmol Vis Sci 52 : 3187–3192. doi: 10.1167/iovs.10-6558 21345983
41. Sewell EW, Brown ED (2014) Taking aim at wall teichoic acid synthesis: new biology and new leads for antibiotics. J Antibiot (Tokyo) 67 : 43–51.
42. Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F (1999) The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67 : 5427–5433. 10496925
43. Foster SJ (1995) Molecular characterization and functional analysis of the major autolysin of Staphylococcus aureus 8325/4. J Bacteriol 177 : 5723–5725. 7559367
44. Mempel M, Schmidt T, Weidinger S, Schnopp C, Foster T, et al. (1998) Role of Staphylococcus aureus surface-associated proteins in the attachment to cultured HaCaT keratinocytes in a new adhesion assay. J Invest Dermatol 111 : 452–456. 9740240
45. Diep BA, Stone GG, Basuino L, Graber CJ, Miller A, et al. (2008) The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J Infect Dis 197 : 1523–1530. doi: 10.1086/587907 18700257
46. Lencer WI, Moe S, Rufo PA, Madara JL (1995) Transcytosis of cholera toxin subunits across model human intestinal epithelia. Proc Natl Acad Sci U S A 92 : 10094–10098. 7479732
47. Saslowsky DE, Lencer WI (2008) Conversion of apical plasma membrane sphingomyelin to ceramide attenuates the intoxication of host cells by cholera toxin. Cell Microbiol 10 : 67–80. 18052945
48. Bayer AS, Sullam PM, Ramos M, Li C, Cheung AL, et al. (1995) Staphylococcus aureus induces platelet aggregation via a fibrinogen - dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infect Immun 63 : 3634–3641. 7642301
49. Shaw LN, Golonka E, Szmyd G, Foster SJ, Travis J, et al. (2005) Cytoplasmic control of premature activation of a secreted protease zymogen: deletion of staphostatin B (SspC) in Staphylococcus aureus 8325–4 yields a profound pleiotropic phenotype. J Bacteriol 187 : 1751–1762. 15716447
50. Groicher KH, Firek BA, Fujimoto DF, Bayles KW (2000) The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J Bacteriol 182 : 1794–1801. 10714982
51. Qoronfleh MW, Wilkinson BJ (1986) Effects of growth of methicillin-resistant and-susceptible Staphylococcus aureus in the presence of beta-lactams on peptidoglycan structure and susceptibility to lytic enzymes. Antimicrob Agents Chemother 29 : 250–257. 2872855
52. McDevitt D, Francois P, Vaudaux P, Foster TJ (1994) Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol 11 : 237–248. 8170386
53. Kneidinger B, O'Riordan K, Li J, Brisson JR, Lee JC, et al. (2003) Three highly conserved proteins catalyze the conversion of UDP-N-acetyl-D-glucosamine to precursors for the biosynthesis of O antigen in Pseudomonas aeruginosa O11 and capsule in Staphylococcus aureus type 5. Implications for the UDP-N-acetyl-L-fucosamine biosynthetic pathway. J Biol Chem 278 : 3615–3627. 12464616
54. Novick R (1967) Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33 : 155–166. 4227577
55. Bramley AJ, Patel AH, O'Reilly M, Foster R, Foster TJ (1989) Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun 57 : 2489–2494. 2744856
56. Brown S, Xia G, Luhachack LG, Campbell J, Meredith TC, et al. (2012) Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc Natl Acad Sci U S A 109 : 18909–18914. doi: 10.1073/pnas.1209126109 23027967
57. Iordanescu S, Surdeanu M (1976) Two restriction and modification systems in Staphylococcus aureus NCTC8325. J Gen Microbiol 96 : 277–281.
58. Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, et al. (1999) Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274 : 8405–8410. 10085071
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



