-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCross Kingdom Activators of Five Classes of Bacterial Effectors
article has not abstract
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1004944
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004944Summary
article has not abstract
Introduction
Toxin production by pathogenic microorganisms likely serves to (i) protect against phagocytosis by predatory cells, (ii) aid in penetrating tissue barriers, (iii) promote nutrient release, or (iv) alter cellular architecture and metabolism in ways that facilitate the establishment of a niche for colonization and replication. Useful enzymatic targets for some bacterial toxins may be similar between prokaryotic and eukaryotic environments, necessitating a need for eukaryotic-specific cofactors to regulate toxin activity. Host cell—derived factors may impart localization properties to an effector, induce folding events, provide a platform for the inhibition of cellular processes or support greater substrate promiscuity (Fig 1). The aim of this review is to describe the diversity of bacterial effectors known to require, or to be stimulated by eukaryotic cofactors and to integrate new ideas regarding the structural and functional implications of this relationship (Table 1).
Fig. 1. Examples of cofactor regulation of secreted bacterial enzymes. 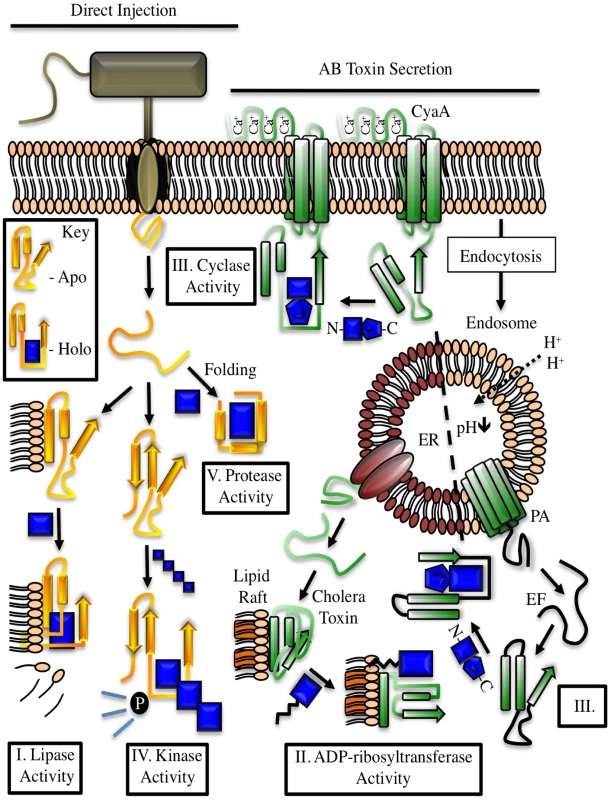
Toxins delivered to their target cell by either direct injection (yellow) or cell surface binding and translocation (green) can be host cofactor activated. These enzymes generally contain dynamic structures that assume a catalytically active fold upon complex formation with a host factor. The key depicts this process through cofactor-mediated organization (blue) of an unstructured sequence. Apo, the apoenzyme catalytically-inactive state. Holo, the holoenzyme active state in which the toxin is in complex with its cofactor. CyaA, the plasma membrane-localized nucleotide cyclase toxin from Bordetella pertussis complexed to calcium ions and calmodulin. EF, edema factor from Bacillus anthracis, binds to calmodulin in a different orientation than CyaA. PA, protective antigen. The cofactor for cholera toxin (ARF) is shown in the myristolated, GTP-bound form. ER, endoplasmic reticulum, with cholera toxin peptide being secreted through ER protein channels. Tab. 1. Cross kingdom enzyme stimulators for secreted bacterial toxins. 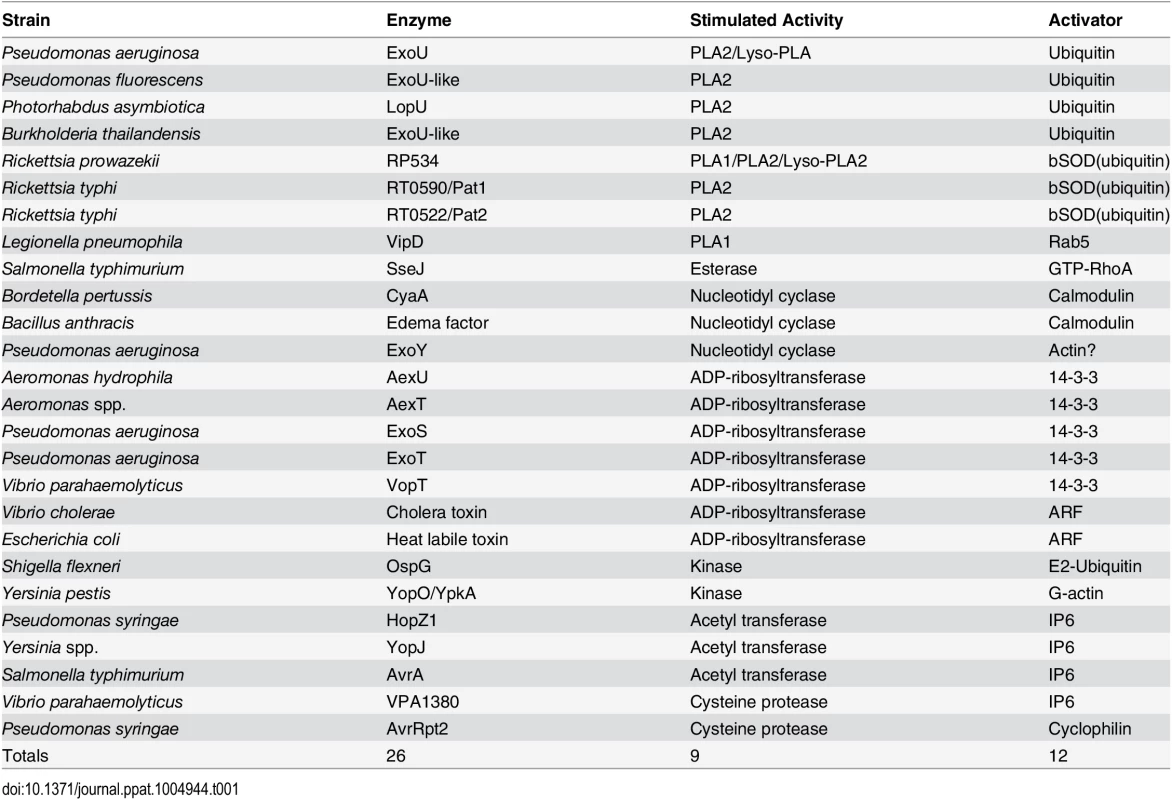
I. Phospholipases
Many bacterial toxins contain catalytic domains with homology to plant patatins, which are lipid acyl hydrolases found in potato tubers. Cofactor activation of phospholipase activity is best characterized for ExoU, a lipid membrane—hydrolyzing protein encoded by the opportunistic pathogen P. aeruginosa. Monoubiquitin, ubiquitin polymers, and ubiquitylated proteins are capable of activating ExoU [1]. Bioinformatic analyses identified at least 17 additional bacterial patatin-like phospholipases that fit the criteria for ubiquitin-mediated activation [2]. Functional studies of a selected subset of enzymes demonstrated that ubiquitin activates phospholipases from P. asymbiotica, B. thailandensis, and P. fluorescens [2].
ExoU orthologs are also found in frank pathogens within the Rickettsiae, Legionellae, and Salmonellae. R. typhus encodes ExoU homologs, Pat1 and Pat2 [3,4]. Both proteins are activated by preparations of bovine SOD1 (bSOD1 [3,4]), which may provide a source of ubiquitin [1]. Additionally, R. prowazekii, the etiologic agent of typhus, displays enhanced enzymatic activity in the presence of bSOD1 (ubiquitin, [5]). Rickettsial-derived PLA enzymes function in cellular entry [6], phagosomal escape, and noncytolytic free fatty acid release [7].
VipD lipase from L. pneumophila displays a Rab5-dependent PLA1 activity [8] that targets the enzyme to endosomes resulting in inhibition of phagosome maturation. SseJ, a glycerophospholipid-cholesterol acyltransfersase (GCAT)-like enzyme from Salmonella, is activated by GTP-RhoA [9] likely for the temporal regulation of cholesterol metabolism in infected cells [10]. The Y. enterocolitica homolog to SseJ, YspM, may have a similar function [11].
II. Transferases
Most cofactor-stimulated transferases catalyze the transfer of an ADP-ribose moiety from nicotinamide adenine dinucleotide to a target protein. Exoenzyme S from P. aeruginosa was one of the first identified bacterial ADP-ribosyltransferases requiring a cofactor for activation [12]. Members of the 14-3-3 family of eukaryotic scaffolding proteins stimulate ADP-ribosylation of a variety of targets causing disruptions in cytoskeletal integrity and vesicular trafficking [13]. Homologous enzymes are present in A. hydrophila [14] and V. parahaemolyticus [15].
Another enzyme from P. aeruginosa, ExoT, is similar (76% identity) to ExoS and requires 14-3-3 proteins as cofactors, but has limited target recognition and is not overtly cytotoxic [16]. CrkI and CrkII are the only proteins modified by ExoT [17]. ADP-ribosylation uncouples integrin signaling and alters the cytoskeleton. A. hydrophila and A. salmonicida encode enzymes similar to ExoT/S termed AexT [18].
Bacterial effectors with acetyltransferase activity include YopJ, a type III secreted enzyme from Yersinia that inhibits immune responses through acetylation of several residues in the kinases involved in the MAPK and NFκB pathways [19]. Acetyltransferase activity is stimulated by a highly abundant eukaryotic signaling carbohydrate, inositol hexakisphosphate (IP6, [20]). P. syringae secretes an IP6-activated acetyltransferase, HopZ1, which interferes with plant cell cytoskeletal networks and downstream cell wall—mediated immune responses by acetylating microtubules [21]. The binding of IP6 to S. typhimurium AvrA suggests that stimulation of acetyltransferase activity in this family of enzymes likely involves allosteric conformational changes [20]. Structural investigations of V. cholerae RTX toxin [22] and Clostridium difficile toxin A [23] suggest that IP6 interactions with bacterial enzymes, and their subsequent allosteric conformational changes, may be a conserved strategy.
ADP-ribosylation factors (ARFs) have been identified as stimulators of type I and type II heat labile toxin (LT) activity from enteric bacteria. Cholera toxin from V. cholerae, LT-I and LT-II from E. coli, are the most studied enzymes of this family to date; each are highly homologous in sequence and structure. Cholera toxin A1 subunit was shown to fold on host cell lipid rafts [24] before interacting with ARF proteins in their GTP-bound state [25]. Activated enzymes ADP-ribosylate the alpha subunit of the heterotrimeric G stimulatory protein (Gs), locking it into the GTP-bound form. This stimulates adenylyl cyclase activity and generates supraphysiological amounts of cAMP, deregulating the chloride balance in the intestinal lumen to cause diarrhea.
III. Nucleotide Cyclases
Cofactor-stimulated nucleotide cyclases include the adenylyl cyclases edema factor (EF) of anthrax toxin and CyaA of B. pertussis. These enzymes contain structurally similar components but differ in affinity and binding mechanism for their eukaryotic activator, calmodulin (CaM). Edema factor has three domains: a protective antigen-binding domain, the adenylyl cyclase core domain, and an alpha-helical domain. The helical domain interacts with the core domain to maintain an unstructured activation loop. The N-terminal domain of CaM interacts with the EF helical domain, causing a conformational switch between the helical and core domains [26]. The C-terminal domain of CaM then docks into a crevice between the helical and core domains, stabilizing the catalytic loop [27].
In contrast to soluble EF, CyaA localizes to the cytosolic interface at the plasma membrane. The CaM binding domain involves completely different sequences with an affinity that is roughly 100-fold stronger as compared to EF. N - and C-terminal domains of CaM participate in binding, stabilization, and activation of CyaA. The C-terminal domain stabilizes a catalytic loop, and the N-terminal domain is postulated to bind a β-hairpin motif of CyaA to promote substrate (ATP) binding within the catalytic chamber [28].
Exoenzyme Y is a type III-secreted, cofactor-stimulated adenylyl cyclase from P. aeruginosa [29]. Functionally, ExoY cyclase activity disrupts the actin cytoskeleton and is correlated with Tau hyperphosphorylation, which leads to microtubule breakdown, endothelial cell gap formation, and increased tissue permeability [30]. The cofactor for ExoY remains to be established, but preliminary work suggests that host cell actin stimulates cyclase activity [31]. Sensitive detection methods demonstrate that ExoY, EF, and CyaA have broad nucleotidyl cyclase activity and catalyze the formation of cCMP and cUMP (or cGMP and cUMP with ExoY) in addition to cAMP [32,33]. The production of cCMP and cUMP during in vivo infection may provide an experimental system to explore the roles of these cyclic nucleotides as new second messengers [34].
IV. Kinases
Yersinia species inject actin-stimulated effector kinases into host cells [35]. YopO or YpkA binds to actin in a manner that does not interfere with the association of other actin-targeting proteins, while simultaneously blocking actin incorporation into a filament. Thus, actin facilitates catalysis by stabilizing the enzyme’s catalytic loop while serving as a platform for phosphorylation of other actin-binding proteins [36].
Another kinase characterized from Shigella species, but also present in strains of Yersinia and enterohemorrhagic E. coli, is OspG [37–39]. OspG is involved in attenuating NFκB signaling by interfering with IκBα degradation [40]. Ubiquitin and ubiquitin chains were first identified as stimulators of OspG kinase activity in vitro [39]. Two groups subsequently solved structures of OspG in complex with E2 ubiquitin-conjugating enzymes covalently linked to monoubiquitin, UbcH5a~Ub [38] and UbcH7~Ub [37]. At least 10 different E2~Ub conjugates appear to be able to activate OspG, suggesting that there may not be a strict preference towards a particular E2.
V. Proteases
V. parahaemolyticus has recently been shown to secrete an IP6-activated enzyme, VPA1380, with homology to the cysteine protease domains of multifunctional-autoprocessing RTX (MARTX) domains from Vibrio and large clostridial toxins A and B [41]. The mechanism of activation, toxicity, and target substrates are yet to be elucidated. P. syringae secretes a protease, AvrRpt2, which is homologous to the staphopain cysteine proteases that are activated through interactions with cyclophilins [42]. AvrRpt2 is predominantly in an unstructured coil conformation until binding to a host cyclophilin, which induces folding into a stable conformer [43]. Analysis of protease activity supports a model in which AvrRpt2 must be in complex with cyclophilin cofactor for maximal enzymatic activity [44].
Conclusions
Numerous secreted bacterial enzymes interact with host cell factors to ensure enzymatic activation in the correct environment, and in some cases, trafficking to a specific location within the host. Common properties of the activators are that they are ubiquitously distributed throughout the eukaryotic kingdom, are present in high concentration, and play roles in cell signaling. The prevailing theme of a high cellular concentration may facilitate the required conditions for toxins to establish a cross kingdom binding partner as they evolved [45]. In this way, highly regulated and flexible proteins that are easily translocated across lipid barriers could be allosterically reorganized to a stable and active conformation. Importantly, bacterial utilization of host cell machinery to ensure maximal toxin activity in the correct environment minimizes the maintenance of bacterial genetic information.
Zdroje
1. Anderson DM, Schmalzer KM, Sato H, Casey M, Terhune SS, Haas AL, et al. Ubiquitin and ubiquitin-modified proteins activate the Pseudomonas aeruginosa T3SS cytotoxin, ExoU. Molecular microbiology. 2011;82(6):1454–67. doi: 10.1111/j.1365-2958.2011.07904.x 22040088
2. Anderson DM, Sato H, Dirck AT, Feix JB, Frank DW. Ubiquitin activates patatin-like phospholipases from multiple bacterial species. Journal of bacteriology. 2015;197(3):529–41. doi: 10.1128/JB.02402-14 25404699
3. Rahman MS, Ammerman NC, Sears KT, Ceraul SM, Azad AF. Functional characterization of a phospholipase A(2) homolog from Rickettsia typhi. Journal of bacteriology. 2010;192(13):3294–303. doi: 10.1128/JB.00155-10 20435729
4. Rahman MS, Gillespie JJ, Kaur SJ, Sears KT, Ceraul SM, Beier-Sexton M, et al. Rickettsia typhi possesses phospholipase A2 enzymes that are involved in infection of host cells. PLoS pathogens. 2013;9(6):e1003399. doi: 10.1371/journal.ppat.1003399 23818842
5. Housley NA, Winkler HH, Audia JP. The Rickettsia prowazekii ExoU homologue possesses phospholipase A1 (PLA1), PLA2, and lyso-PLA2 activities and can function in the absence of any eukaryotic cofactors in vitro. Journal of bacteriology. 2011;193(18):4634–42. doi: 10.1128/JB.00141-11 21764940
6. Silverman DJ, Santucci LA, Meyers N, Sekeyova Z. Penetration of host cells by Rickettsia rickettsii appears to be mediated by a phospholipase of rickettsial origin. Infection and immunity. 1992;60(7):2733–40. 1612741
7. Winkler HH, Daugherty RM. Phospholipase A activity associated with the growth of Rickettsia prowazekii in L929 cells. Infection and immunity. 1989;57(1):36–40. 2491840
8. Gaspar AH, Machner MP. VipD is a Rab5-activated phospholipase A1 that protects Legionella pneumophila from endosomal fusion. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(12):4560–5. doi: 10.1073/pnas.1316376111 24616501
9. Christen M, Coye LH, Hontz JS, LaRock DL, Pfuetzner RA, Megha, et al. Activation of a bacterial virulence protein by the GTPase RhoA. Science signaling. 2009;2(95):ra71. doi: 10.1126/scisignal.2000430 19887681
10. Kolodziejek AM, Miller SI. Salmonella modulation of the phagosome membrane, role of SseJ. Cellular microbiology. 2015;17(3):333–41. doi: 10.1111/cmi.12420 25620407
11. Witowski SE, Walker KA, Miller VL. YspM, a newly identified Ysa type III secreted protein of Yersinia enterocolitica. Journal of bacteriology. 2008;190(22):7315–25. doi: 10.1128/JB.00861-08 18805975
12. Coburn J, Kane AV, Feig L, Gill DM. Pseudomonas aeruginosa exoenzyme S requires a eukaryotic protein for ADP-ribosyltransferase activity. The Journal of biological chemistry. 1991;266(10):6438–46. 1901061
13. Fu H, Coburn J, Collier RJ. The eukaryotic host factor that activates exoenzyme S of Pseudomonas aeruginosa is a member of the 14-3-3 protein family. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(6):2320–4. 8460141
14. Sha J, Wang SF, Suarez G, Sierra JC, Fadl AA, Erova TE, et al. Further characterization of a type III secretion system (T3SS) and of a new effector protein from a clinical isolate of Aeromonas hydrophila—part I. Microbial pathogenesis. 2007;43(4):127–46. 17644303
15. Kodama T, Rokuda M, Park KS, Cantarelli VV, Matsuda S, Iida T, et al. Identification and characterization of VopT, a novel ADP-ribosyltransferase effector protein secreted via the Vibrio parahaemolyticus type III secretion system 2. Cellular microbiology. 2007;9(11):2598–609. 17645751
16. Yahr TL, Barbieri JT, Frank DW. Genetic relationship between the 53 - and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. Journal of bacteriology. 1996;178(5):1412–9. 8631719
17. Sun J, Barbieri JT. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. The Journal of biological chemistry. 2003;278(35):32794–800. 12807879
18. Vilches S, Wilhelms M, Yu HB, Leung KY, Tomas JM, Merino S. Aeromonas hydrophila AH-3 AexT is an ADP-ribosylating toxin secreted through the type III secretion system. Microbial pathogenesis. 2008;44(1):1–12. 17689917
19. Orth K, Palmer LE, Bao ZQ, Stewart S, Rudolph AE, Bliska JB, et al. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science. 1999;285(5435):1920–3. 10489373
20. Mittal R, Peak-Chew SY, Sade RS, Vallis Y, McMahon HT. The acetyltransferase activity of the bacterial toxin YopJ of Yersinia is activated by eukaryotic host cell inositol hexakisphosphate. The Journal of biological chemistry. 2010;285(26):19927–34. doi: 10.1074/jbc.M110.126581 20430892
21. Lee AH, Hurley B, Felsensteiner C, Yea C, Ckurshumova W, Bartetzko V, et al. A bacterial acetyltransferase destroys plant microtubule networks and blocks secretion. PLoS pathogens. 2012;8(2):e1002523. doi: 10.1371/journal.ppat.1002523 22319451
22. Lupardus PJ, Shen A, Bogyo M, Garcia KC. Small molecule-induced allosteric activation of the Vibrio cholerae RTX cysteine protease domain. Science. 2008;322(5899):265–8. doi: 10.1126/science.1162403 18845756
23. Pruitt RN, Chagot B, Cover M, Chazin WJ, Spiller B, Lacy DB. Structure-function analysis of inositol hexakisphosphate-induced autoprocessing in Clostridium difficile toxin A. The Journal of biological chemistry. 2009;284(33):21934–40. doi: 10.1074/jbc.M109.018929 19553670
24. Ray S, Taylor M, Banerjee T, Tatulian SA, Teter K. Lipid rafts alter the stability and activity of the cholera toxin A1 subunit. The Journal of biological chemistry. 2012;287(36):30395–405. doi: 10.1074/jbc.M112.385575 22787142
25. Kahn RA, Gilman AG. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. The Journal of biological chemistry. 1986;261(17):7906–11. 3086320
26. Drum CL, Yan SZ, Bard J, Shen YQ, Lu D, Soelaiman S, et al. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature. 2002;415(6870):396–402. 11807546
27. Tang WJ, Guo Q. The adenylyl cyclase activity of anthrax edema factor. Molecular aspects of medicine. 2009;30(6):423–30. doi: 10.1016/j.mam.2009.06.001 19560485
28. Guo Q, Jureller JE, Warren JT, Solomaha E, Florian J, Tang WJ. Protein-protein docking and analysis reveal that two homologous bacterial adenylyl cyclase toxins interact with calmodulin differently. The Journal of biological chemistry. 2008;283(35):23836–45. doi: 10.1074/jbc.M802168200 18583346
29. Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(23):13899–904. 9811898
30. Ochoa CD, Alexeyev M, Pastukh V, Balczon R, Stevens T. Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. The Journal of biological chemistry. 2012;287(30):25407–18. doi: 10.1074/jbc.M111.301440 22637478
31. Schneider EH, Seifert R. Report on the Third Symposium "cCMP and cUMP as New Second Messengers". Naunyn-Schmiedeberg's archives of pharmacology. 2015;388(1):1–3. doi: 10.1007/s00210-014-1072-3 25471064
32. Beckert U, Wolter S, Hartwig C, Bahre H, Kaever V, Ladant D, et al. ExoY from Pseudomonas aeruginosa is a nucleotidyl cyclase with preference for cGMP and cUMP formation. Biochemical and biophysical research communications. 2014;450(1):870–4. doi: 10.1016/j.bbrc.2014.06.088 24971548
33. Gottle M, Dove S, Kees F, Schlossmann J, Geduhn J, Konig B, et al. Cytidylyl and uridylyl cyclase activity of Bacillus anthracis edema factor and Bordetella pertussis CyaA. Biochemistry. 2010;49(26):5494–503. doi: 10.1021/bi100684g 20521845
34. Bahre H, Hartwig C, Munder A, Wolter S, Stelzer T, Schirmer B, et al. cCMP and cUMP occur in vivo. Biochemical and biophysical research communications. 2015.
35. Trasak C, Zenner G, Vogel A, Yuksekdag G, Rost R, Haase I, et al. Yersinia protein kinase YopO is activated by a novel G-actin binding process. The Journal of biological chemistry. 2007;282(4):2268–77. 17121817
36. Lee WL, Grimes JM, Robinson RC. Yersinia effector YopO uses actin as bait to phosphorylate proteins that regulate actin polymerization. Nature structural & molecular biology. 2015;22(3):248–55.
37. Grishin AM, Condos TE, Barber KR, Campbell-Valois FX, Parsot C, Shaw GS, et al. Structural basis for the inhibition of host protein ubiquitination by Shigella effector kinase OspG. Structure. 2014;22(6):878–88. doi: 10.1016/j.str.2014.04.010 24856362
38. Pruneda JN, Smith FD, Daurie A, Swaney DL, Villen J, Scott JD, et al. E2~Ub conjugates regulate the kinase activity of Shigella effector OspG during pathogenesis. The EMBO journal. 2014;33(5):437–49. doi: 10.1002/embj.201386386 24446487
39. Zhou Y, Dong N, Hu L, Shao F. The Shigella type three secretion system effector OspG directly and specifically binds to host ubiquitin for activation. PloS one. 2013;8(2):e57558. doi: 10.1371/journal.pone.0057558 23469023
40. Kim DW, Lenzen G, Page AL, Legrain P, Sansonetti PJ, Parsot C. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):14046–51. 16162672
41. Calder T, Kinch LN, Fernandez J, Salomon D, Grishin NV, Orth K. Vibrio type III effector VPA1380 is related to the cysteine protease domain of large bacterial toxins. PloS one. 2014;9(8):e104387. doi: 10.1371/journal.pone.0104387 25099122
42. Coaker G, Falick A, Staskawicz B. Activation of a phytopathogenic bacterial effector protein by a eukaryotic cyclophilin. Science. 2005;308(5721):548–50. 15746386
43. Coaker G, Zhu G, Ding Z, Van Doren SR, Staskawicz B. Eukaryotic cyclophilin as a molecular switch for effector activation. Molecular microbiology. 2006;61(6):1485–96. 16968222
44. Aumuller T, Jahreis G, Fischer G, Schiene-Fischer C. Role of prolyl cis/trans isomers in cyclophilin-assisted Pseudomonas syringae AvrRpt2 protease activation. Biochemistry. 2010;49(5):1042–52. doi: 10.1021/bi901813e 20050698
45. Spitzer J, Poolman B. The role of biomacromolecular crowding, ionic strength, and physicochemical gradients in the complexities of life's emergence. Microbiology and molecular biology reviews: MMBR. 2009;73(2):371–88. doi: 10.1128/MMBR.00010-09 19487732
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



