-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIntraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
The transmissible spongiform encephalopathies (TSEs) are a group of infectious neurodegenerative diseases affecting both humans and animals. The prion hypothesis postulates that prions are protein conformation based infectious agents responsible for TSE infectivity. Prions have been synthetically generated in vitro, but it remains unclear whether the properties of synthetically generated prion are the same as those of TSE agents and whether the disease caused by synthetically generated prion is identical to naturally occurring TSEs. In this study, we demonstrated that similar to the classical TSE agents, the synthetically generated prion has a titratable infectivity and is able to cause prion disease in wild-type mice via routes other than direct intra-cerebral inoculation. More importantly, we showed that the synthetically generated prion induced pathological changes, including the dissemination of disease-specific prion protein accumulation and the route and mechanism of neuroinvasion, were all typical of classical TSEs. These results demonstrate the similarity of synthetically generated prion to the infectious agent in TSEs, providing strong evidence supporting the prion hypothesis.
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1004958
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004958Summary
The transmissible spongiform encephalopathies (TSEs) are a group of infectious neurodegenerative diseases affecting both humans and animals. The prion hypothesis postulates that prions are protein conformation based infectious agents responsible for TSE infectivity. Prions have been synthetically generated in vitro, but it remains unclear whether the properties of synthetically generated prion are the same as those of TSE agents and whether the disease caused by synthetically generated prion is identical to naturally occurring TSEs. In this study, we demonstrated that similar to the classical TSE agents, the synthetically generated prion has a titratable infectivity and is able to cause prion disease in wild-type mice via routes other than direct intra-cerebral inoculation. More importantly, we showed that the synthetically generated prion induced pathological changes, including the dissemination of disease-specific prion protein accumulation and the route and mechanism of neuroinvasion, were all typical of classical TSEs. These results demonstrate the similarity of synthetically generated prion to the infectious agent in TSEs, providing strong evidence supporting the prion hypothesis.
Introduction
Transmissible Spongiform Encephalopathies (TSEs, also known as prion diseases) are fatal transmissible neurodegenerative disorders, which include bovine spongiform encephalopathy (BSE) in cattle, scrapie in sheep and goats, chronic wasting disease (CWD) in deer and elk, and Creutzfeldt-Jakob disease (CJD) in humans [1,2]. The prion hypothesis postulates that the infectious agent in TSEs is a prion, a misfolded conformer of host encoded normal prion protein (PrPC) [3]. Because of its seeding capability, the pathogenic PrP conformer seeds the conversion of host PrPC to more pathogenic PrP form, resulting in prion amplification, accumulation, and neurodegeneration.
Previous studies have established that PrPC and disease specific abnormal PrP are different conformers of the prion protein. PrPC is mainly composed of α-helical structures, soluble in mild detergents and sensitive to protease K (PK) digestion [4–8]. The diseased brains contain various PrP conformers that are different from PrPC [9]. The classical disease specific PrP conformer is rich in β-sheet structures, aggregated and partially resistant to PK digestion [4–8]. The development of more sensitive assays, such as the conformation-dependent immunoassay (CDI) [10], or antibodies specifically recognizing diseased PrP conformers, such as 15B3 antibody [11,12], led to the discovery of various PrP conformers in the diseased brains that are distinct from PrPC [13,14]. Notably, some of these PrP conformers are completely sensitive to PK digestion [13–16] and it was estimated that up to 90% of the disease specific PrP conformers in sporadic CJD cases are PK sensitive PrP conformers [13]. These findings are consistent with the notion that PrP is able to exist in multiple thermodynamically stable conformations [17–19] either by itself or by forming complex with other factors, but whether only one or multiple of these PrP conformers are associated with the prion infectivity remains unclear.
Of all the disease specific conformers, the classical C-terminal PK-resistant PrP conformer, PrP-res, is the best-established disease marker. The association between the presence of PrP-res in tissue homogenates or partially purified PrP-res and prion infectivity, measured by eliciting a prion disease in an animal or chronically infecting susceptible cell lines, is well supported by experimental evidence [1,20]. Despite the fact that the amount of PrP-res did not always correlate with the titer or severity of infectivity [21] and in some cases, prion infectivity was detected without biochemically or pathologically detectable PrP-res [22], PrP-res remains the best biochemically tractable PrP conformer and has been used widely to test the prion hypothesis.
The prion hypothesis explains the infectivity by the seeding capability of disease specific PrP conformers [1,3]. The cell-free conversion assay, in which partially purified PrP-res was used to seed the conformational change of purified PrPC, established the seeding capability of PrP-res [23,24]. This conclusion was confirmed by the more efficient serial protein misfolding cyclic amplification (sPMCA) assay [25,26], in which a small amount of diseased tissue homogenate was mixed with large amount of normal brain homogenates and the whole mixture was subjected to successive cycles of sonication and incubation [27]. The sPMCA allows an efficient and almost indefinite replication of the PrP-res conformation from a minute amount of PrP-res seed [28], which led to the demonstration that the PrP-res conformation and prion infectivity are simultaneously propagated via sPMCA [26], establishing a correlation between PrP-res and prion infectivity. Notably, in the absence of PrP-res seed, the PrP-res conformer and prion infectivity were generated by subjecting normal mouse brain homogenates or purified PrPC plus polyanions to sPMCA [29,30], which further strengthens the association between PrP conformational change and the generation of prion infectivity.
Recently, several groups have successfully demonstrated that infectious prions can be synthetically generated from purified bacterially expressed recombinant PrP (recPrP) in biochemically defined in vitro systems [31–37]. Although the infectivity varied, these studies have established that purified recPrP can be converted into infectious conformers and cause prion disease in wild-type rodents via intra-cerebral (i.c.) inoculation [31–37].
Despite the advance, it remains unclear whether the rec-Prion share the same pathogenic properties of naturally occurring prions, such as the ability to transmit disease via routes other than the most efficient i.c. inoculation [38–40], the neuroinvasion pathway, and the ability to induce TSE specific lesions. Answers to these questions are important for establishing the prion as a disease-causing agent in TSEs. In this study, we determined the titer of the rec-Prion using end-point titration assays, infected wild-type mice with the rec-Prion via an intraperitoneal (i.p.) route, and performed detailed pathological phenotype analysis. We found that the rec-Prion inoculated mice developed a prion disease with properties, including survival times, pathological lesions, visceral dissemination, and neuroinvasion pathways closely resembling those found for naturally occurring scrapie and CWD.
Results
Determining the titer for rec-Prion
We previously showed that highly infectious rec-Prions can be generated in vitro by subjecting a substrate mixture consisting of recPrP plus two cofactors, synthetic 1-palmitoyl-2-oleoylphosphatidylglycerol (POPG) and total RNA isolated from normal mouse liver, to sPMCA [31]. The rec-Prion possesses the biochemical hallmarks of the disease specific PrP-res conformer and efficiently causes prion disease in wild-type mice after i.c. inoculation [31]. To determine whether the rec-Prion generated via our protocol contains a titratable infectivity, we performed end-point titration bioassay (Table 1). The rec-Prion inoculum was prepared by sPMCA as previously described [31]. At the end of preparation, the sPMCA product was subject to a 10-fold serial dilution and each diluted sPMCA products was inoculated into wild-type CD-1 mice via an i.c. route. All mice were monitored for the development of prion disease for 500 days post inoculation (dpi). The prion infectivity titer was determined according to the Spearman-Karber formula, which showed that the rec-Prion contained a titer of 103.84 LD50 (i.c.) (50% of lethal dose by i.c. route) / μg total rPrP. Thus, we conclude that our sPMCA protocol is able to produce rec-Prion containing an infectivity titer around 104 LD50 (i.c.) / μg, confirming that the rec-Prion contains a titratable infectivity.
Tab. 1. End point titration bioassay. 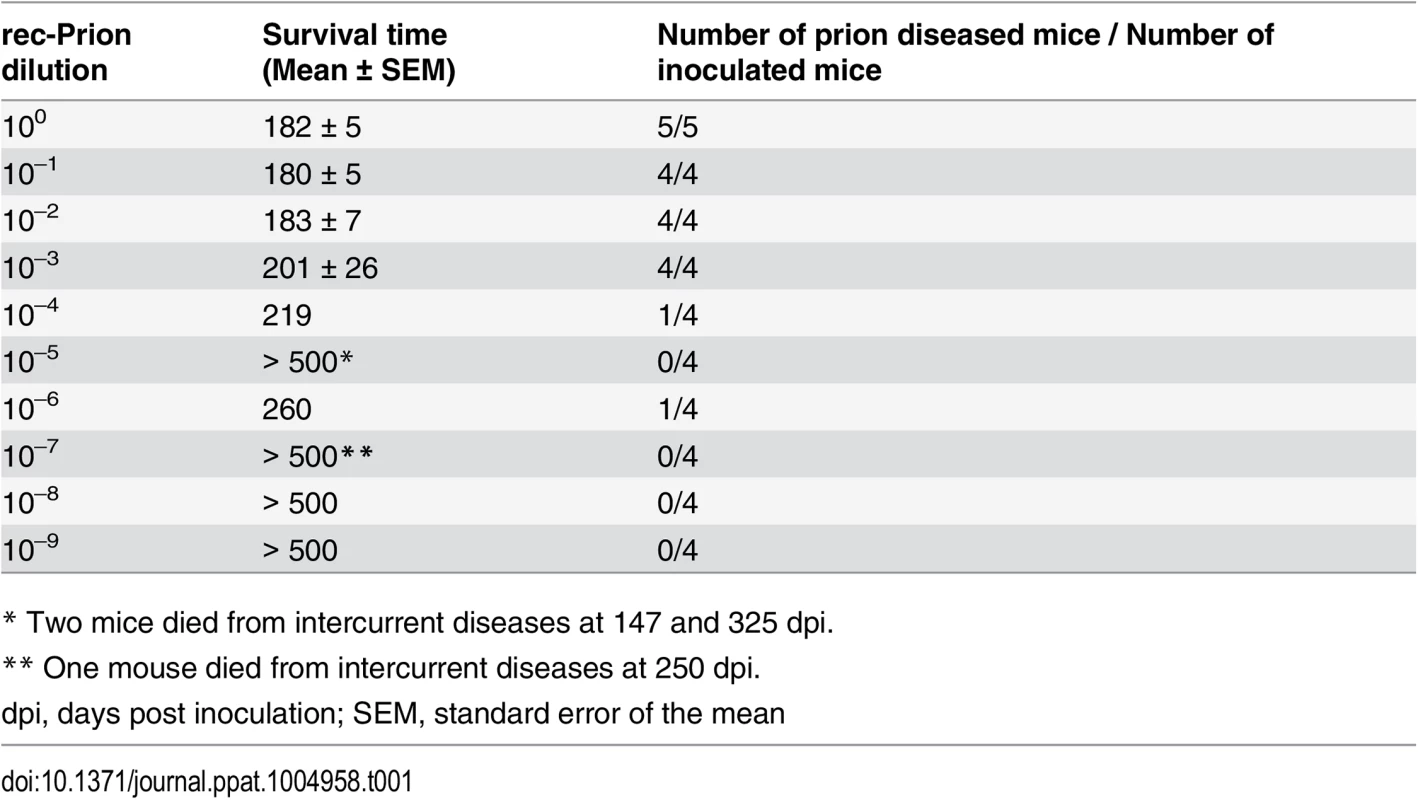
* Two mice died from intercurrent diseases at 147 and 325 dpi. Intraperitoneal (i.p.) inoculation of rec-Prion in wild-type CD-1 mice
To determine whether rec-Prions can cause disease via routes other than i.c. inoculation, we challenged wild-type CD-1 mice with rec-Prion via an i.p. route. Three negative control inocula were used in this experiment, which were the same controls used in our previous report of i.c. inoculation of rec-Prion [31] (listed in the S1 Table). Control 1, the sPMCA substrate mixture without recPrP, was subjected to the same sPMCA procedure that prepared rec-Prion inoculum. Control 2, the sPMCA substrate mixture, was incubated at 37°C for 24 days without sonication. Control 3 was prepared by directly mixing recPrP, POPG and RNA in inoculum diluent. The amount of each component in control 3 is equal to that in the rec-Prion inoculum.
Except for one mouse (IP#152) that died unexpectedly at 96 days post inoculation (dpi), all other rec-Prion inoculated mice developed clinical signs around 170–200 dpi, including restlessness, hindlimb paralysis, rough coat and weight loss (supporting S1 Fig). The disease progressed rapidly and mice generally reached terminal stage within 2–3 weeks. The average survival time for rec-Prion inoculated mice was 206.8 ± 3.82 dpi (IP#152 mouse was not included). Mice that received control inocula were monitored for 500 dpi and no sign of disease was observed (Fig 1A and Table 2 experiment 1).
Fig. 1. Intraperitoneal inoculation of wild-type CD-1 mice with rec-Prion. 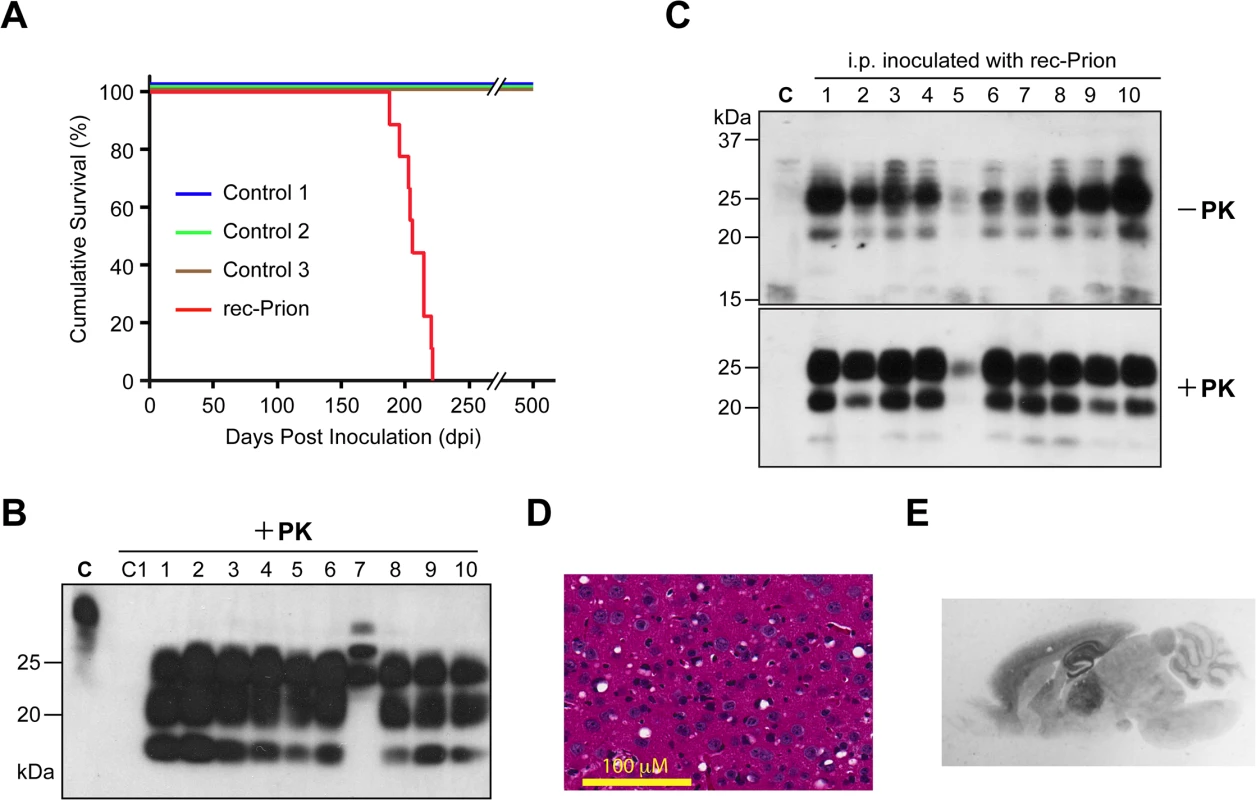
(A) Survival curves of wild-type CD-1 mice that received i.p. inoculations of rec-Prion or control inocula. (B) Immunoblot analysis of PK-resistant PrP in the brain of all 10 mice that received i.p. inoculation of rec-Prion. C, undigested brain homogenate prepared from a rec-Prion inoculated mouse as a control. C1, PK digested brain homogenate prepared from an age-matched, un-inoculated CD-1 mice. PrP was detected by the POM1 monoclonal anti-PrP antibody and a peroxidase conjugated goat anti-mouse IgG (Bio Rad). (C) The spleen homogenates prepared from all 10 mice that received i.p. inoculation of rec-Prion were digested with or without PK as indicated. The PrP in the spleen homogenates was detected by the polyclonal M20 anti-PrP antibody (Santa Cruz Biotech. Inc.) and a peroxidase conjugated donkey anti-goat IgG (Santa Cruz Biotech. Inc.). C, homogenate prepared from a spleen of an un-inoculated CD-1 mouse as a control. (D) H&E stain of the frontal cortical region of a mouse i.p. challenged with rec-Prion. (E) PET blot analysis to show PK-resistant PrP-res deposition in the brain of a mouse received i.p. rec-Prion inoculation. Tab. 2. Intraperitoneal transmission studies in wild-type mice. 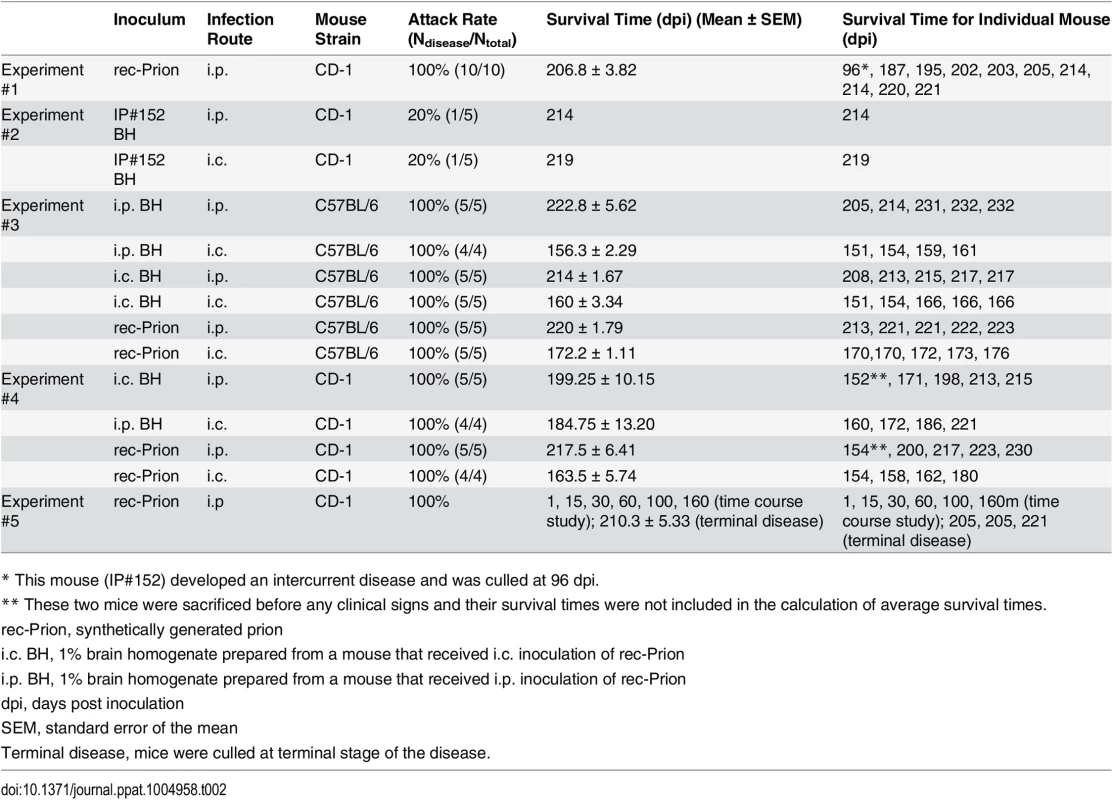
* This mouse (IP#152) developed an intercurrent disease and was culled at 96 dpi. Mouse brain homogenates were subjected to PK digestion and except for the IP#152 mouse, the classic PrP-res signal was detected in all 9 mice that received i.p. rec-Prion inoculation (Fig 1B). The brain of IP#152 mouse contained PK-resistant bands of higher molecular weights (Fig 1B, lane 7), which were confirmed as cross-reacting bands recognized by the secondary antibody (S2 Fig). No PrP-res was detected in the brains of mice that received control inocula. For all 10 mice that were challenged i.p. with rec-Prions, PrP-res was also detected in the spleen (Fig 1C).
Spongiosis (Fig 1D), astrogliosis and microgliosis (S3 Fig) were detected in the brains of 9 i.p. inoculated mice (The IP#152 brain was not available for histological examination). The PK-resistant PrP-res deposition was confirmed by paraffin-embedded tissue (PET) blot (Fig 1E), revealing that PrP-res was deposited widely in the brain and the most prominent deposition was in hippocampus and thalamus, a pattern similar to that of mice that received i.c. rec-Prion inoculation [31]. No spongiosis or PrP-res were detected in the mouse brains inoculated with any of the control inocula (S4 Fig).
Second round transmission
The detection of PrP-res in the spleen, but not in the brain of IP#152 mouse suggests that it was at an early stage of prion disease and died from an intercurrent disease. Consistent with this notion, second round transmission with IP#152 brain homogenate, either via i.p. or i.c. route, only had an attack rate of 20% (Table 2 Experiment 2; S5 Fig). Nonetheless, the presence of prion infectivity in IP#152 mouse confirmed a 100% attack rate for i.p. rec-Prion challenge in wild-type mice.
Second round transmission of other rec-Prion inoculated mice were performed via i.p. or i.c. route in either C57BL/6 (Table 2, experiment 3) or CD-1 mice (Table 2, experiment 4). As controls, freshly prepared rec-Prions were used to inoculate C57BL/6 or CD-1 mice via either i.c. or i.p. route in all these experiments (Table 2, experiments 3 and 4). All inoculations led to a 100% attack rate. The survival times were 156–172 dpi for i.c. and 214–222 dpi for i.p. inoculation in C57BL/6 mice. In CD-1 mice, the survival times were 163–184 for i.c. and 199–217 for i.p. inoculation.
All inoculated CD-1 and C57BL/6 mice developed signs of prion disease and accumulated PrP-res (Fig 2A and 2B). Notably, at early stage of the disease, more PrP-res accumulated in the spleen than in the brain (Fig 2A lanes 6 (brain) and 5 (spleen); Fig 2B lanes 5 (brain and spleen). These two mice were sacrificed before clinical signs became apparent, suggesting that PrP-res may accumulate first in the spleen. The fact that spleen PrP-res accumulation was detected in both i.p. and i.c. challenged mice (Fig 2) suggests that the rec-Prion is a lymphotropic prion, which is able to replicate and accumulate in peripheral lymphoid organs. The accumulation of PrP-res in lymphoid organs following i.c. rec-Prion inoculation provides a disease phenotype that is similar to that of naturally occurring contagious prions [41–44].
Fig. 2. Brain and spleen PrP-res accumulation in rec-Prion inoculated wild-type CD-1 mice. 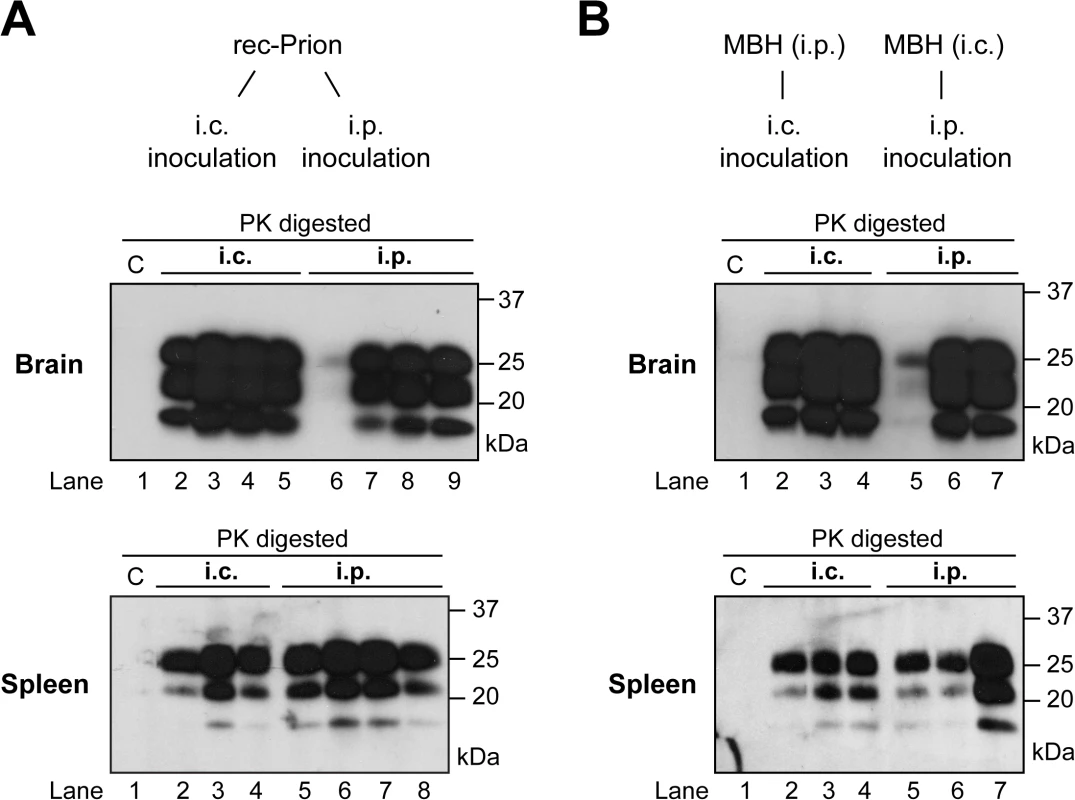
(A) PrP-res in brain (top panel) and spleen (bottom panel) of mice that received i.p. or i.c rec-Prion inoculation as indicated. (B) PrP-res in brain (top panel) and spleen (bottom panel) of mice that received i.p. or i.c inoculation of mouse brain homogenate (MBH) prepared from mice that received rec-Prion transmission. Two inoculated mice were sacrificed before any clinical signs (154 and 152 dpi), and their PK digested samples were separated in lanes 6 (brain) and 5 (spleen) of (A), and lanes 5 (brain and spleen) of (B). C, PK-digested brain or spleen homogenates prepared from age-matched wild-type CD-1 mice as controls. Pathology of rec-Prion caused disease
The ability to infect mice via the i.p route allows us to determine the pathogenic changes in rec-Prion caused disease, including disease pathology in central nervous system (CNS) and lymphoreticular system (LRS), and the route and mechanism of neuroinvasion. Detailed pathological analyses and a time course study were performed (Table 2 experiment 5), which allows us to compare the i.p. rec-Prion challenge induced disease to that of classical rodent scrapie. Figs 3 and 4 shows the patterns of vacuolation and immunohistochemically detected aberrant PrP deposition (PrP-d, the disease specific PrP form detected by histological methods) in terminally diseased mice. PrP-d in CNS and spinal cord was mainly found as diffuse punctuate forms with frequent granular intra-cellular accumulations (Fig 4) and occasional small plaque-like deposits. Thus, both the nature and patterns of vacuolation and PrP-d accumulation are consistent with those found in different strains and isolates of laboratory murine scrapie and supports the contention that rec-Prions causes bona fide prion disease in rodents [31].
Fig. 3. Vacuolar lesion profile of CD-1 mice challenged by i.p. inoculation of rec-Prions. 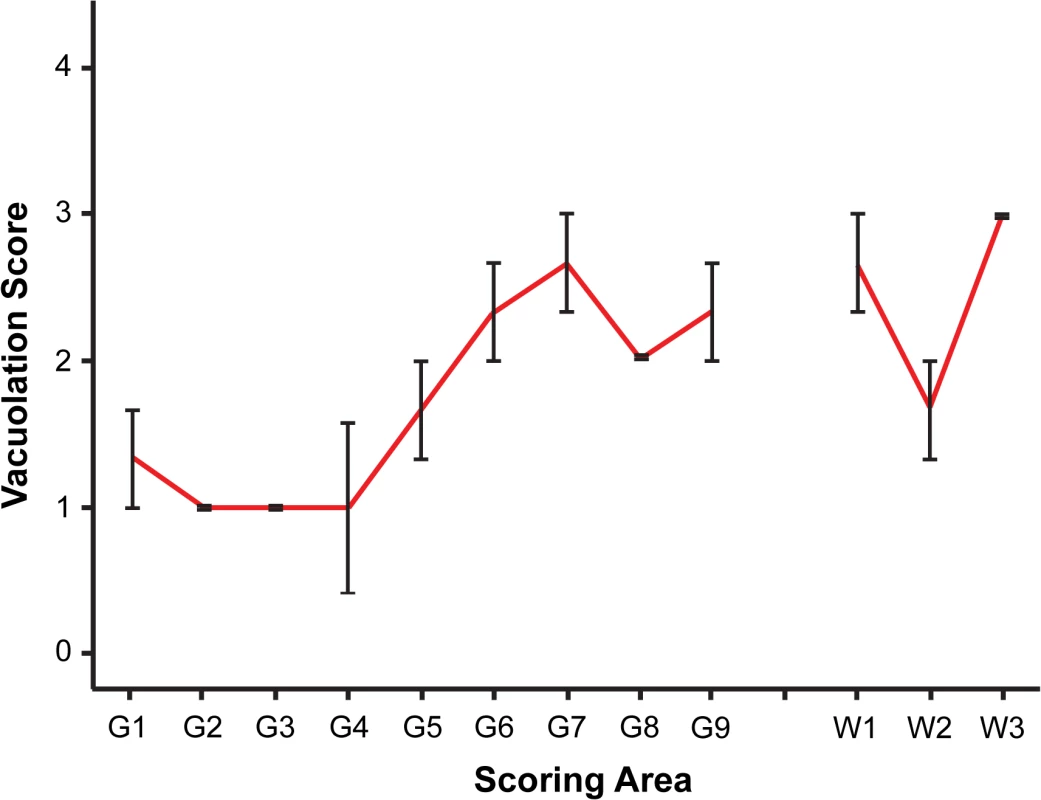
Spongiosis in the following brain regions were scored. Grey matter regions: G1, dorsal medulla; G2, cerebellar cortex; G3, superior colliculus; G4, hypothalamus; G5, medial thalamus; G6, hippocampus; G7, septum; G8, parietal cerebral cortex; G9, frontal cerebral cortex. White matter regions: W1, cerebellar white matter; W2, mesencephalic tegmentum; W3, pyramidal tract. Error bars represent SEM (standard error of the mean). Fig. 4. PrP-d accumulation in CNS. 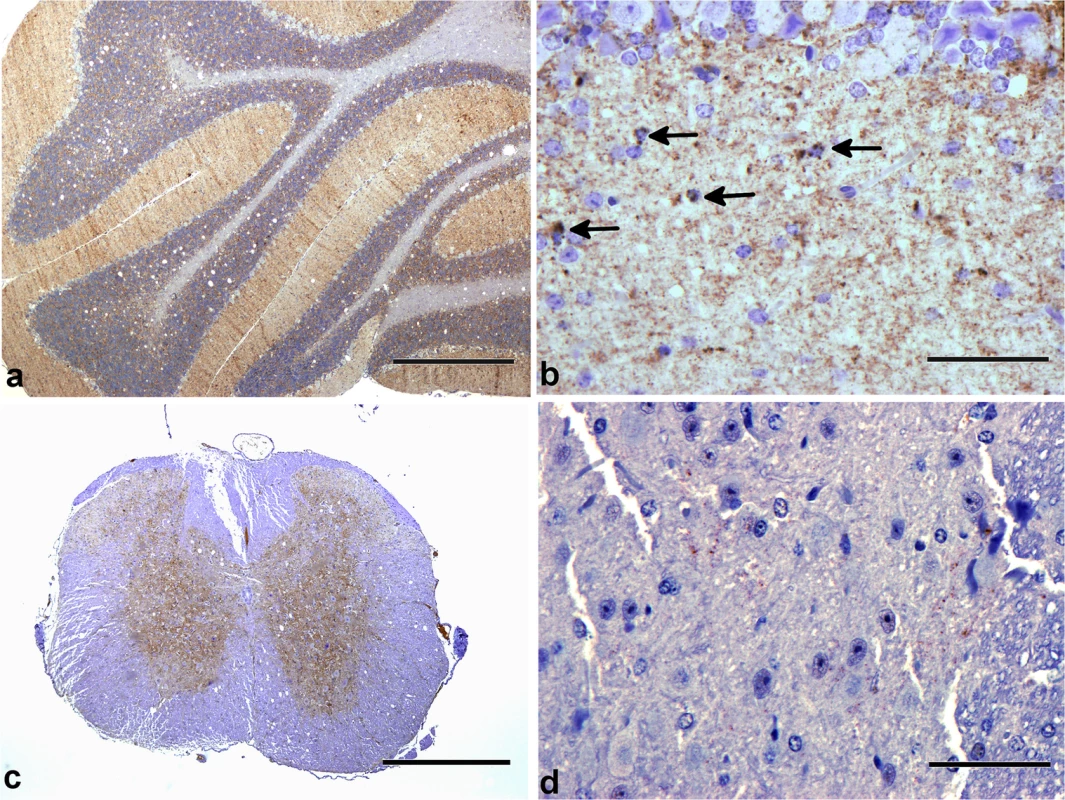
Patterns of PrP-d accumulation in brains of wild-type CD-1 mice i.p. challenged with rec-Prions. (a) Cerebellum at terminal disease stage showing diffuse PrP-d accumulations in the molecular and granule cell layers, and moderate vacuolation of white matter and granule cell layers. Bar = 500 μm. (b) Cerebellum at higher magnification showing intracellular accumulation of PrP-d in glial cells (arrows) and diffuse punctuate nature of accumulation in the molecular layer. Bar = 50 μm. (c) Cervical spinal cord showing diffuse PrP-d accumulation throughout grey matter (and moderate white matter vacuolation). Bar = 500 μm. (d) Thoracic spinal cord showing sparse punctuate PrP-d accumulation in the intermediolateral column of a mouse sacrificed at 100 dpi. Bar = 50 μm. Classical forms of prion disease in rodents, farm animals and man are associated with highly specific PrP-d co-localized ultrastructural membrane changes and intra-lysosomal PrP-d accumulation [13,45]. These features are characteristic and unique to prion disease. Using immunogold electron microscopy, we confirmed these distinctive features in rec-Prion challenged mice examined at terminal stage disease (Fig 5), including PrP-d associated membrane ruffling, branched membrane invaginations with sub-membrane coating on the ends of branches (Fig 5A and 5B) and spiral membraneinvaginations with continuous coating of spiral membranes and buds (Fig 5C). The membrane invaginations were encountered on both dendrites and axon terminals. Lysosomal PrP-d accumulation was also identified in glia (Fig 5D) and neurons. These ultrastructural pathological features confirm the phenotypic similarity of rec-Prion caused disease with classical prion diseases.
Fig. 5. Ultrastructural changes in rec-Prion challenged CD-1 mice detected by immunogold electron microscopy. 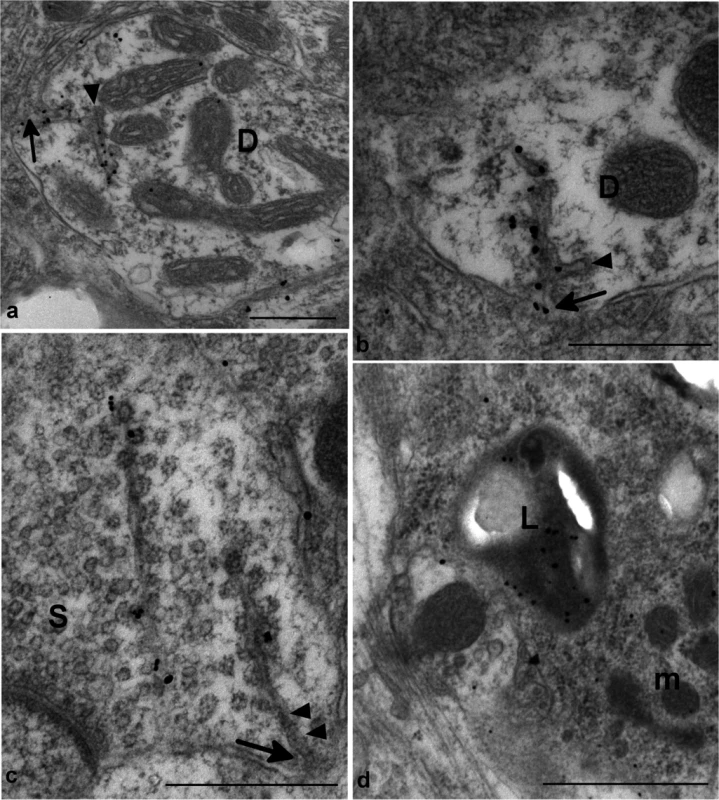
(a) and (b) show dendritic membrane invaginations (arrows) in two dendrites (D). The reflected membrane invagination is labelled for PrP-d. The membrane invaginations are branched and the ends of the branches show a membrane coating on the cytoplasmic face of the membrane (arrowheads). Bars = 500 nm. (c) shows two weakly PrP-d labelled spiral membrane invaginations with continuous membrane undercoated buds in an axon terminal containing numerous synaptic vesicles (S). The spiral nature of the invaginations can be seen between arrowheads. One invagination is tangentially sectioned and lies apparently free within the cytoplasm. The other is visibly connected to the membrane (arrow). Bar = 500nm. (d) shows immunogold labelling for PrP-d within a lysosome (L) of a microglial process (m). Bar = 1 μm. All images were taken from medulla of i.p. challenged terminal disease affected mouse and 1A8 antibody was used to perform the immunogold detection of PrP-d. In lymphoid tissues PrP-d was confined to germinal centers of lymph nodes, Peyer’s patches of the intestine and spleen where it was associated with both tingible body macrophages (TBMs) and follicular dendritic cells (Fig 6). Immunogold electron microscopy localized PrP-d to the lysosomes of TBMs (Fig 6) and the cell membranes of the dendrites of follicular dendritic cells. In the enteric nervous system PrP-d was in the cytoplasm of enteric neurons and satellite cells (Fig 6).
Fig. 6. PrP-d accumulation in viscera of CD-1 mice i.p. challenged with rec-Prions. 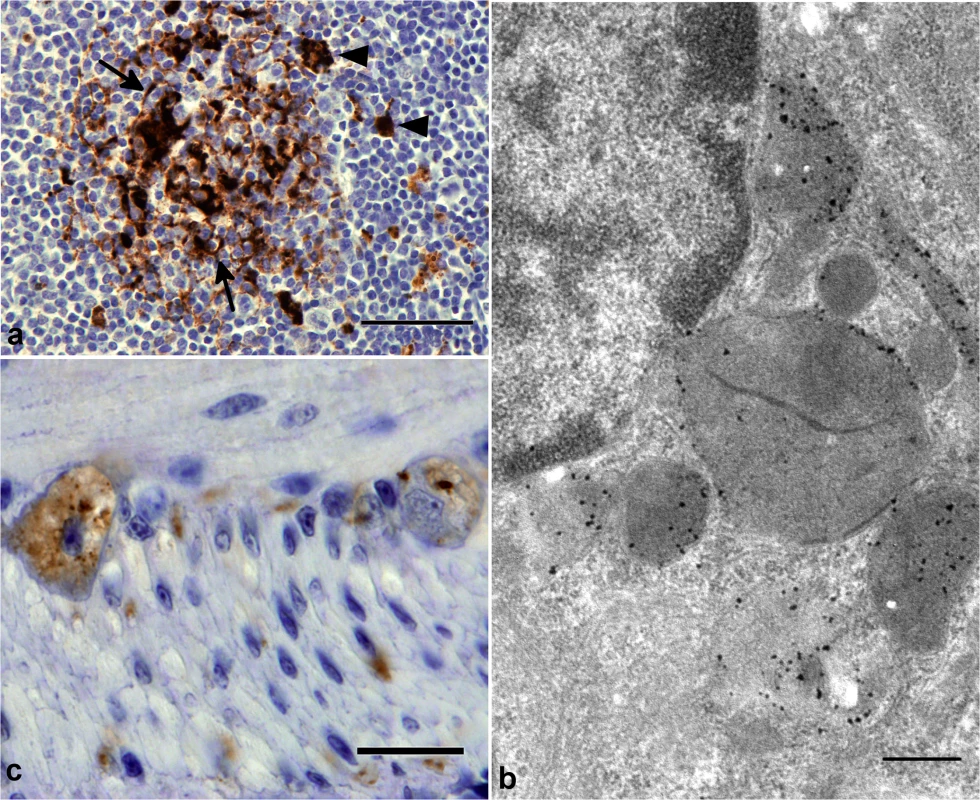
(a) Terminal disease lymph node tissue showing granular PrP-d accumulation in Tingible body macrophages (TBMS, arrowheads) and diffuse punctuate PrP-d accumulation in association with follicular dendritic cells (arrow). Bar = 50μm. (b) Immunogold electron microscopy of terminal disease lymph node shows PrP-d (black) in lysosomes and endo-lysosomes of TBMs. The 1A8 antibody was used for immunogold staining. Bar = 500 nm. (c) Terminal disease intestine showing granular PrP-d accumulation in neurons of enteric nerve plexuses. Bar = 20 μm. Collectively, the patterns of cellular and sub-cellular localization of PrP-d in i.p. rec-Prion challenged mice in the CNS, LRS and in the enteric nervous system (ENS) are indistinguishable from classical forms of experimental rodent prion disease and of naturally occurring contagious TSEs [46], confirming the diagnosis of rec-Prion caused disease as a TSE or prion disease.
Pathogenesis of i.p. rec-Prion challenge caused prion disease
A time course study was performed to determine the sequential pathogenic changes caused by rec-Prion. The temporal sequence of the spread was determined by immunohistochemistry on selected viscera and CNS tissues at 1, 15, 30, 60, 100, 160 dpi, and terminal disease stages (Table 3). PrP-d accumulation was first detected in lymphoid tissues at 30 dpi, while the first PrP-d accumulation in the CNS were found at 100 dpi (Table 3). In spinal cord at 100 dpi, sparse puncta of PrP-d was confined to the intermediolateral column of the thoracic spinal cord (Fig 4) in 3 of 5 mice, but not the cervical or lumbar spinal cord. (In the other 2 mice, the PrP-d distribution in spinal cord was more widespread at 100 dpi.) The first PrP-d accumulation in the brain was found in white matter tracts of the medulla and midbrain. Thus the initial sites and dissemination of PrP-d in spinal cord are consistent with previous oral and natural prion disease pathogenesis studies in laboratory rodents [47] and in sheep [48,49], suggesting neuroinvasion ascended to this site via autonomic nerves of the abdomen.
Tab. 3. Temporal sequence of PrP-d accumulation in various tissues after i.p. rec-Prion inoculation (PrP-d positive samples / Total samples examed). 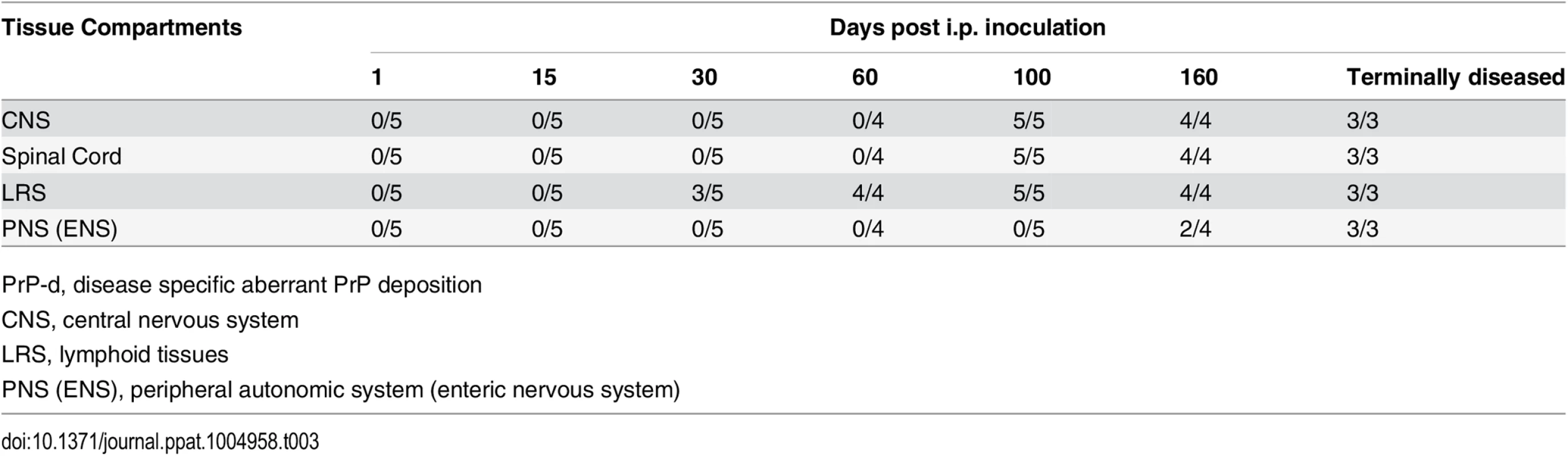
PrP-d, disease specific aberrant PrP deposition PrP-d was initially detected in the lymphoid tissues of 3 out of 5 mice at 30 dpi (Tables 3 and 4). By 60 dpi PrP-d accumulation was detected in almost all LRS tissues (Table 4), but not in the ENS. The most conspicuous early amplification occurred in the spleen at 30 dpi and affected most lymphoid tissues by 60 dpi. The rapid involvement of all LRS tissues combined with the absence of any physical connection between them argues for a hematogenous dissemination of infectivity at a relatively early stage of infection, which is consistent with serial pathogenesis studies of sheep scrapie and BSE [48–50]. The first detection of PrP-d in the ENS occurred at 160 dpi and affected all mice only at terminal stage disease (Table 3), suggesting a centrifugal spread of PrP-d from CNS.
Tab. 4. Temporal sequence of PrP-d accumulation in lymphoid tissues after i.p. rec-Prion inoculation (PrP-d positive samples / Total samples examed). 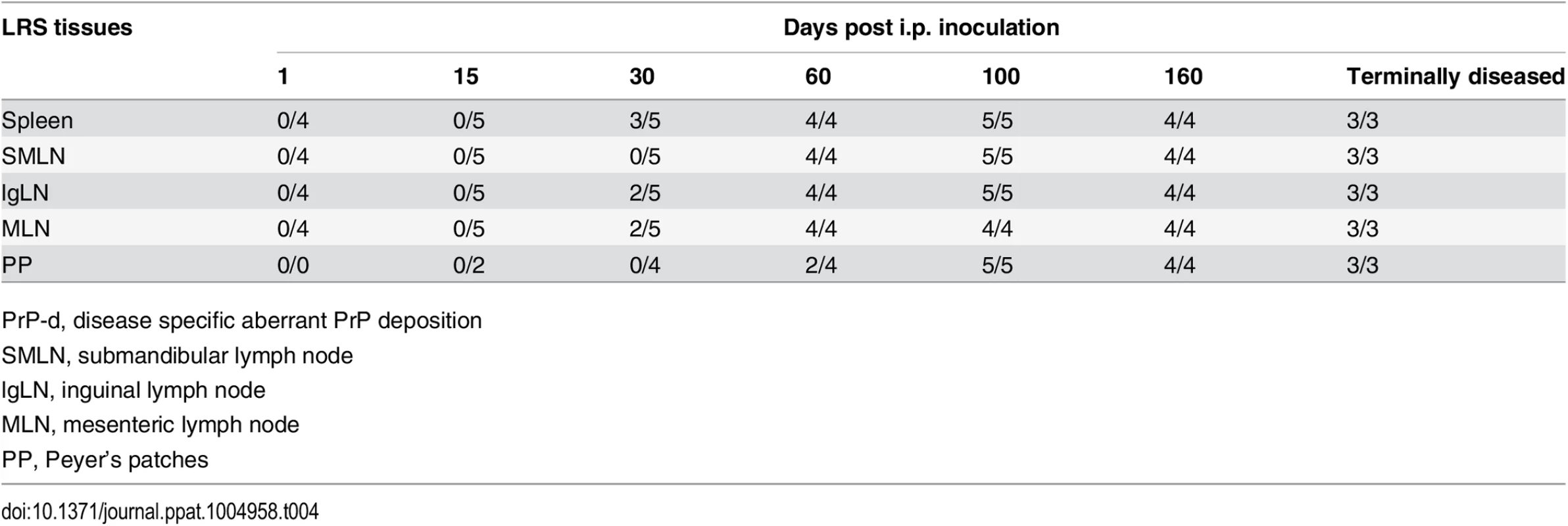
PrP-d, disease specific aberrant PrP deposition Discussion
In this study, we showed for the first time that i.p. inoculation of rec-Prion is sufficient to cause prion disease in wild-type mice. Moreover, detailed pathological analyses confirmed that the characteristics of rec-Prion caused disease are consistent with naturally occurring contagious TSEs. Together with other properties of rec-Prion demonstrated in previous studies [31,34], our data support the concept that infectivity in TSEs can be the result of a protein conformation based infectious agent.
In natural sheep scrapie, CWD of deer and following experimental oral challenge with these agents in laboratory rodents, prion infectivity is found in CNS, LRS tissues and the autonomic nervous systems [46,51]. In naturally occurring contagious TSEs and following experimental oral challenge, infectivity of the gut associated lymphoid tissues precedes generalized hematogenous distribution to other lymphoid tissues [48,51]. Mice i.p challenged with rec-Prions in the present experiments also showed generalized involvement of LRS tissues at a relatively early stage of disease, but in contrast to naturally contagious TSEs, rec-Prion challenged mice showed an early accumulation in spleen and a relatively delayed accumulation in Peyer’s patches (Table 4). This pattern of distribution likely reflects an initial dispersal of the inoculum by peritoneal macrophages rather than intestinal trans-epithelial absorption of infectivity such as that occurs following oral exposure [52].
Following oral or natural exposure [47,49], PrP-d in CNS tissues is first detected in the intermediolateral column of the thoracic spinal cord and the dorsal parasympathetic nucleus of the vagal nerve (DMNV) in the brain medulla, sites corresponding to neuroinvasion by ascending infection of autonomic nerves of the abdomen and intestine respectively [47–49]. Neuroinvasion in the spinal cord of rec-Prion challenged mice also corresponds to an ascending infection via abdominal autonomic nerves. However there was no specific involvement of the DMNV at any stage suggesting a single site of neuroinvasion by rec-Prions and corresponding to late involvement of Peyer’s patches. The initial PrP-d location in the medulla and midbrain probably reflects an ascending infection from the spinal cord. Thus, i.p. inoculated rec-Prions have more closely replicated naturally occurring scrapie and CWD, insofar as the nature of rapid hematogenous dispersal of infectivity through LRS tissues and ascending neural infectivity found in the present experiments are in agreement with naturally contagious TSEs.
Prions are similar to viruses insofar as their infectivity can be determined by end-point titration. Here we showed that the titer of rec-Prion generated by our methodology [31,34,53] can be determined by end-point titration. It is important to note that sPMCA is influenced by several uncontrollable factors, particularly the stochastic events during the sonication step, which may cause the titer of each rec-Prion batch to vary. Nonetheless, our result does show that end-point titration can be used to titrate the infectivity associated with rec-Prion and our protocol is able to generate an infectivity around 104 LD50 (i.c.) / μg. These values are lower than those obtained for some highly adapted experimental rodent strains [54], but are only one log lower than those found for some highly pathogenic sources such as experimental transmission of BSE in sheep and mice [55].
Numerous naturally occurring and laboratory strains of prion disease can be recognized in sheep [56,57] and laboratory rodents [58]. More than 20 strains of scrapie have been identified in mice using selected properties of disease [58,59]. The mechanisms by which a misfolded protein can code for these strain specific properties is unclear, but it has been postulated that tertiary and/or quaternary conformation of the prion may carry such information [1]. The disease phenotype of rec-Prion has minor difference from other commonly used laboratory strains of scrapie, in particular the white matter vacuolation was unusually prominent [60]. The nature of PrP-d deposition and low abundance of plaques readily distinguishes the rec-Prion disease generated here from other diseases initiated by inoculation of misfolded synthetic PrP generated by other means [61,62]. These data, and the absence of disease in mice challenged with control inocula, provide confidence that the present disease transmissions were not caused by laboratory contamination.
The rec-Prion was generated several times in the present study and the disease phenotype, insofar as it was characterized in each experiment, was relatively constant, indicating that our method of producing rec-Prion may constrain the nature of rPrP misfolding process. The rec-Prion seed used in our sPMCA may guide the misfolding of recPrP. In addition, the cofactors used in our protocol, both of which alter recPrP conformation [63–67], may limit the options available to recPrP for misfolding. Thus, instead of a stochastic misfolding process, the recPrP aggregate generated with our method may follow rather limited misfolding possibilities, resulting in a rather constant disease phenotype in mice.
Collectively, our study showed for the first time that rec-Prions are able to cause bona fide prion disease in wild-type mice not only through i.c. inoculation, but also via the i.p. route. Moreover, our results established that rec-Prion caused disease shares the pathogenic and pathologic properties with rodent adapted prions and naturally occurring contagious scrapie and CWD, confirming that rec-Prion-caused disease is a prion disease. The relatively simple, yet powerful in vitro system to generate rec-Prion will help us to elucidate the molecular mechanism of prion infectivity in future studies.
Materials and Methods
Mouse bioassay
For end-point titration, the sPMCA product was 10-fold serially diluted with the inoculation diluent (PBS containing 1 mg BSA/mL). Thirty microliter of inoculum, undiluted or diluted sPMCA product, was inoculated into a mouse via an i.c. route. The i.c. injection, animal monitoring, biochemical and histopathological analyses were performed as previously described [31,53].
For i.p. inoculation, the inoculum was prepared as previously described [31,53]. Briefly, the sPMCA product was washed twice with PBS through pelleting-and-resuspension, and the final pellet was resuspended in the inoculating diluent. The volume of final resuspension was 1/10 (experiment 1) or 1/1 (other experiments 2, 3, 4 and 5) of the original volume of the sPMCA product. Each mouse was intraperitoneally injected with 50 μL of the inoculum. The inoculated mice were monitored three times a week. Once neurological signs were clearly identified, mice were monitored daily and euthanized at terminal disease stage. Second round transmission was performed as previously described [31,53].
Ethics statement
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committees of the Van Andel Research Institute (Assurance Number A4383-01) and the Ohio State University (Assurance Number A3261-01).
In vitro rec-Prion preparation
For experiment 1, the expression and purification of recPrP and the conditions for sPMCA were exactly the same as originally described [31,53]. For other experiments (Table 2), expression and purification of recPrP and the sPMCA protocol were slightly modified [34]. Briefly, the sPMCA was carried out with a substrate mixture containing 25 μg/mL recPrP (recPrP concentration was determined by OD280 reading and the Є280 molar extinction coefficient of recombinant murine PrP 23–230 with one disulfide bond), 22.2 μg/mL POPG (Avanti Polar Lipids), and 150 μg/mL total RNA isolated from normal mouse liver (RNA concentration was determined by OD260 reading). The sPMCA substrate was prepared as previously described [31,53]. One round of sPMCA consists of 48 cycles of 30-second sonication followed by 29.5-minute incubation at 37°C. At end of each round, 1/10 sPMCA product was transferred to a fresh tube containing 90 μL of sPMCA substrate and then proceeded to a new round of sPMCA. All rec-Prion inocula used in this study were prepared with seeded sPMCA, which are 1–3 rounds of sPMCA seeded with previously generated rec-Prions.
The recPrP purification in experiment 1 was exactly the same as originally described [31,53]. For recPrP purification in other experiments, E. Coli BL21 (DE3) cells were transformed with the pET-22b-moPrP23-230, which expresses the un-tagged version of murine PrP 23–230. Detailed protocol for purifying untagged recombinant murine PrP23-230 is described in the supporting S1 Method.
Pathological analyses
In Experiments 1–4 brains were removed from the calvarium, sagittally sectioned and half brains frozen for subsequent immunoblotting. The remaining half brains were embedded in paraffin wax and stained by HE, immunohistochemistry, and PET blot as previously described [31,53,68]. From experiment 5, brains, five lymphoid tissues (spleen, mesenteric lymph node, inguinal lymph node, sub-mandibular lymph node) and four samples of intestine, including ileum with Peyer’s patches, were fixed in formalin. Brains were coronally sectioned at 2–3 mm intervals and processed through paraffin wax for haematoxylin and eosin staining. Vacuolar lesion profiles were constructed on nine grey, and three white matter sites according to standard methods [69]. Sections were also immunolabelled for PrP-d using the routine methods [70]. The antibodies SAF84, 1C5 and 2G11 were used on each section. All illustrated results are from the antibody 2G11 that gave the least background results.
Ultrastructural immunohistochemical labelling
Additional terminally diseased mice were perfusion fixed using 4% paraformaldehyde / 0.1% gluteraldehyde for ultrastructural analysis. Light microscopical resin immunolabelling — Selected brain areas (cerebellar cortex, medulla and midbrain), mesenteric and inguinal lymph nodes of 2 rec-Prion inoculated mice and 2 control mice (i.p. inoculated with sPMCA substrate) were processed into epoxy resin as described previously [71] and sections cut at 1 μm. The avidin-biotin complex immunohistochemical staining method was applied to the etched and pre-treated 1 μm sections using 1C5 PrP antibody at a 1 : 100 or 1A8 antibody at 1 : 2000 dilution. Sections were examined by light microscopy and selected blocks with appropriate immunolabelled follicles and control blocks were then taken for sub-cellular studies. The method employed in the study of TSE pathology in resin embedded tissues does not show any PrPC labelling in control tissues, therefore PrP detected in these tissues is by definition, disease associated. Immunolabelling was consistently found to be superior in mesenteric lymph nodes therefore only this lymph node was taken for further EM analysis. Multiple sections from at least 2 blocks containing follicles were studied from each animal. Ultrastructural immunohistochemical labelling — 65 nm sections were taken from resin blocks previously found to show PrP-d labelling (or controls containing follicles) and immunolabelled as described previously [71]. PrP-d was detected using primary antibody 1C5 at a 1 : 10 or 1A8 at 1 : 500 dilution in incubation buffer. A pre-immune serum was used as a control. At least two 1mm2 sections were studied from each animal.
PK digestion and immunoblot analysis
PK digestion and immunoblot analysis were performed similarly as previously described [31,68]. For analyzing PrP-res in mouse tissues, 20 μg brain homogenate or 40 μg spleen homogenate were subjected to 50 μg / mL PK digestion at 37°C for 1 hour. PrP-res was detected by immunoblot analysis with the POM1 anti-PrP antibody [72] (for brain) or M20 polyclonal anti-PrP antibody (Santa Cruz Biotechnology, Inc.) (for spleen).
Anti-PrP antibodies
The following antibodies were used for detecting PrP in this study. The SAF84 monoclonal antibody recognizing an epitope of residue 160–170 of hamster PrP, and the 2G11 monoclonal antibody raised against a synthetic peptide (146–182) of the ovine PrP were purchased from Cayman Chemical. The M20 antibody (Santa Cruz Biotechnology, Inc.) is a purified goat polyclonal antibody raised against C-terminus of mouse PrP. The 1C5 antibody recognizes an epitope of residue 119–130 of hamster PrP [73]. The 1A8 is a polyclonal antibody against PrP [74,75]. POM1 recognizes a discontinuous epitope at C-terminus of PrP [72,76].
Supporting Information
Zdroje
1. Prusiner SB (1998) Prions. Proc Natl Acad Sci U S A 95 : 13363–13383. 9811807
2. Aguzzi A, Baumann F, Bremer J (2008) The prion's elusive reason for being. Annu Rev Neurosci 31 : 439–477. doi: 10.1146/annurev.neuro.31.060407.125620 18558863
3. Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216 : 136–144. 6801762
4. Meyer RK, McKinley MP, Bowman KA, Braunfeld MB, Barry RA, et al. (1986) Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A 83 : 2310–2314. 3085093
5. Caughey B, Neary K, Buller R, Ernst D, Perry LL, et al. (1990) Normal and scrapie-associated forms of prion protein differ in their sensitivities to phospholipase and proteases in intact neuroblastoma cells. J Virol 64 : 1093–1101. 1968104
6. Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, et al. (1991) Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry 30 : 7672–7680. 1678278
7. Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, et al. (1993) Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A 90 : 10962–10966. 7902575
8. Smirnovas V, Baron GS, Offerdahl DK, Raymond GJ, Caughey B, et al. (2011) Structural organization of brain-derived mammalian prions examined by hydrogen-deuterium exchange. Nat Struct Mol Biol 18 : 504–506. doi: 10.1038/nsmb.2035 21441913
9. Hope J, Morton LJ, Farquhar CF, Multhaup G, Beyreuther K, et al. (1986) The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J 5 : 2591–2597. 3096712
10. Safar J, Wille H, Itri V, Groth D, Serban H, et al. (1998) Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4 : 1157–1165. 9771749
11. Korth C, Stierli B, Streit P, Moser M, Schaller O, et al. (1997) Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390 : 74–77. 9363892
12. Nazor KE, Kuhn F, Seward T, Green M, Zwald D, et al. (2005) Immunodetection of disease-associated mutant PrP, which accelerates disease in GSS transgenic mice. Embo J 24 : 2472–2480. 15962001
13. Kim C, Haldiman T, Cohen Y, Chen W, Blevins J, et al. (2011) Protease-sensitive conformers in broad spectrum of distinct PrPSc structures in sporadic Creutzfeldt-Jakob disease are indicator of progression rate. PLoS Pathog 7: e1002242. doi: 10.1371/journal.ppat.1002242 21931554
14. Kim C, Haldiman T, Surewicz K, Cohen Y, Chen W, et al. (2012) Small protease sensitive oligomers of PrPSc in distinct human prions determine conversion rate of PrP(C). PLoS Pathog 8: e1002835. doi: 10.1371/journal.ppat.1002835 22876179
15. Sajnani G, Silva CJ, Ramos A, Pastrana MA, Onisko BC, et al. (2012) PK-sensitive PrP is infectious and shares basic structural features with PK-resistant PrP. PLoS Pathog 8: e1002547. doi: 10.1371/journal.ppat.1002547 22396643
16. Pastrana MA, Sajnani G, Onisko B, Castilla J, Morales R, et al. (2006) Isolation and characterization of a proteinase K-sensitive PrPSc fraction. Biochemistry 45 : 15710–15717. 17176093
17. Collinge J, Clarke AR (2007) A general model of prion strains and their pathogenicity. Science 318 : 930–936. 17991853
18. Weissmann C (2012) Mutation and selection of prions. PLoS Pathog 8: e1002582. doi: 10.1371/journal.ppat.1002582 22479179
19. Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C (2010) Darwinian evolution of prions in cell culture. Science 327 : 869–872. doi: 10.1126/science.1183218 20044542
20. Klohn PC, Stoltze L, Flechsig E, Enari M, Weissmann C (2003) A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc Natl Acad Sci U S A 100 : 11666–11671. 14504404
21. Barron RM, Campbell SL, King D, Bellon A, Chapman KE, et al. (2007) High titers of transmissible spongiform encephalopathy infectivity associated with extremely low levels of PrPSc in vivo. J Biol Chem 282 : 35878–35886. 17923484
22. Lasmezas CI, Deslys JP, Robain O, Jaegly A, Beringue V, et al. (1997) Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science 275 : 402–405. 8994041
23. Kocisko DA, Come JH, Priola SA, Chesebro B, Raymond GJ, et al. (1994) Cell-free formation of protease-resistant prion protein. Nature 370 : 471–474. 7913989
24. Bessen RA, Kocisko DA, Raymond GJ, Nandan S, Lansbury PT, et al. (1995) Non-genetic propagation of strain-specific properties of scrapie prion protein. Nature 375 : 698–700. 7791905
25. Saborio GP, Permanne B, Soto C (2001) Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411 : 810–813. 11459061
26. Castilla J, Saa P, Hetz C, Soto C (2005) In vitro generation of infectious scrapie prions. Cell 121 : 195–206. 15851027
27. Castilla J, Saa P, Morales R, Abid K, Maundrell K, et al. (2006) Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol 412 : 3–21. 17046648
28. Morales R, Duran-Aniotz C, Diaz-Espinoza R, Camacho MV, Soto C (2012) Protein misfolding cyclic amplification of infectious prions. Nat Protoc 7 : 1397–1409. doi: 10.1038/nprot.2012.067 22743831
29. Deleault NR, Harris BT, Rees JR, Supattapone S (2007) Formation of native prions from minimal components in vitro. Proc Natl Acad Sci U S A 104 : 9741–9746. 17535913
30. Barria MA, Mukherjee A, Gonzalez-Romero D, Morales R, Soto C (2009) De novo generation of infectious prions in vitro produces a new disease phenotype. PLoS Pathog 5: e1000421. doi: 10.1371/journal.ppat.1000421 19436715
31. Wang F, Wang X, Yuan CG, Ma J (2010) Generating a prion with bacterially expressed recombinant prion protein. Science 327 : 1132–1135. doi: 10.1126/science.1183748 20110469
32. Makarava N, Kovacs GG, Bocharova O, Savtchenko R, Alexeeva I, et al. (2010) Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol 119 : 177–187. doi: 10.1007/s00401-009-0633-x 20052481
33. Kim JI, Cali I, Surewicz K, Kong Q, Raymond GJ, et al. (2010) Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J Biol Chem 285 : 14083–14087. doi: 10.1074/jbc.C110.113464 20304915
34. Wang F, Zhang Z, Wang X, Li J, Zha L, et al. (2012) Genetic informational RNA is not required for recombinant prion infectivity. J Virol 86 : 1874–1876. doi: 10.1128/JVI.06216-11 22090130
35. Deleault NR, Piro JR, Walsh DJ, Wang F, Ma J, et al. (2012) Isolation of phosphatidylethanolamine as a solitary cofactor for prion formation in the absence of nucleic acids. Proc Natl Acad Sci U S A 109 : 8546–8551. doi: 10.1073/pnas.1204498109 22586108
36. Deleault NR, Walsh DJ, Piro JR, Wang F, Wang X, et al. (2012) Cofactor molecules maintain infectious conformation and restrict strain properties in purified prions. Proc Natl Acad Sci U S A 109: E1938–1946. doi: 10.1073/pnas.1206999109 22711839
37. Zhang Z, Zhang Y, Wang F, Wang X, Xu Y, et al. (2013) De novo generation of infectious prions with bacterially expressed recombinant prion protein. FASEB J 27 : 4768–4775. doi: 10.1096/fj.13-233965 23970796
38. Hoinville LJ (1996) A review of the epidemiology of scrapie in sheep. Rev Sci Tech 15 : 827–852. 9025137
39. Wilesmith JW, Ryan JB, Atkinson MJ (1991) Bovine spongiform encephalopathy: epidemiological studies on the origin. Vet Rec 128 : 199–203. 1823120
40. Will RG (2003) Acquired prion disease: iatrogenic CJD, variant CJD, kuru. Br Med Bull 66 : 255–265. 14522863
41. Tatzelt J, Groth DF, Torchia M, Prusiner SB, DeArmond SJ (1999) Kinetics of prion protein accumulation in the CNS of mice with experimental scrapie. J Neuropathol Exp Neurol 58 : 1244–1249. 10604749
42. Crozet C, Lezmi S, Flamant F, Samarut J, Baron T, et al. (2007) Peripheral circulation of the prion infectious agent in transgenic mice expressing the ovine prion protein gene in neurons only. J Infect Dis 195 : 997–1006. 17330790
43. Inoue Y, Yamakawa Y, Sakudo A, Kinumi T, Nakamura Y, et al. (2005) Infection route-independent accumulation of splenic abnormal prion protein. Jpn J Infect Dis 58 : 78–82. 15858284
44. Shearin H, Bessen RA (2014) Axonal and transynaptic spread of prions. J Virol.
45. Jeffrey M, McGovern G, Siso S, Gonzalez L (2011) Cellular and sub-cellular pathology of animal prion diseases: relationship between morphological changes, accumulation of abnormal prion protein and clinical disease. Acta Neuropathol 121 : 113–134. doi: 10.1007/s00401-010-0700-3 20532540
46. Jeffrey M, Gonzalez L (2007) Classical sheep transmissible spongiform encephalopathies: pathogenesis, pathological phenotypes and clinical disease. Neuropathol Appl Neurobiol 33 : 373–394. 17617870
47. McBride PA, Schulz-Schaeffer WJ, Donaldson M, Bruce M, Diringer H, et al. (2001) Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J Virol 75 : 9320–9327. 11533195
48. van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA (2000) Pathogenesis of natural scrapie in sheep. Arch Virol Suppl: 57–71. 11214935
49. Jeffrey M, Ryder S, Martin S, Hawkins SA, Terry L, et al. (2001) Oral inoculation of sheep with the agent of bovine spongiform encephalopathy (BSE). 1. Onset and distribution of disease-specific PrP accumulation in brain and viscera. J Comp Pathol 124 : 280–289. 11437504
50. Gonzalez L, Pitarch JL, Martin S, Thurston L, Moore J, et al. (2014) Identical pathogenesis and neuropathological phenotype of scrapie in valine, arginine, glutamine/valine, arginine, glutamine sheep infected experimentally by the oral and conjunctival routes. J Comp Pathol 150 : 47–56. doi: 10.1016/j.jcpa.2013.06.006 24035191
51. Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O'Rourke KI, et al. (1999) Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol 80 (Pt 10): 2757–2764. 10573172
52. Jeffrey M, Gonzalez L, Espenes A, Press CM, Martin S, et al. (2006) Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J Pathol 209 : 4–14. 16575799
53. Wang F, Wang X, Ma J (2011) Conversion of bacterially expressed recombinant prion protein. Methods 53 : 208–213. doi: 10.1016/j.ymeth.2010.12.013 21176786
54. Casaccia P, Ladogana A, Xi YG, Pocchiari M (1989) Levels of infectivity in the blood throughout the incubation period of hamsters peripherally injected with scrapie. Arch Virol 108 : 145–149. 2512893
55. Gonzalez L, Chianini F, Martin S, Siso S, Gibbard L, et al. (2007) Comparative titration of experimental ovine BSE infectivity in sheep and mice. J Gen Virol 88 : 714–717. 17251591
56. Gonzalez L, Siso S, Monleon E, Casalone C, van Keulen LJ, et al. (2010) Variability in disease phenotypes within a single PRNP genotype suggests the existence of multiple natural sheep scrapie strains within Europe. J Gen Virol 91 : 2630–2641. doi: 10.1099/vir.0.022574-0 20538906
57. Gonzalez L, Martin S, Begara-McGorum I, Hunter N, Houston F, et al. (2002) Effects of agent strain and host genotype on PrP accumulation in the brain of sheep naturally and experimentally affected with scrapie. J Comp Pathol 126 : 17–29. 11814318
58. Bruce ME (1993) Scrapie strain variation and mutation. Br Med Bull 49 : 822–838. 8137131
59. Bruce ME, McBride PA, Farquhar CF (1989) Precise targeting of the pathology of the sialoglycoprotein, PrP, and vacuolar degeneration in mouse scrapie. Neurosci Lett 102 : 1–6. 2550852
60. Bruce ME, Fraser H (1991) Scrapie strain variations and its implications. Current Topics in Microbiology and Immunology 172 : 125–138. 1810707
61. Makarava N, Kovacs GG, Savtchenko R, Alexeeva I, Ostapchenko VG, et al. (2012) A new mechanism for transmissible prion diseases. J Neurosci 32 : 7345–7355. doi: 10.1523/JNEUROSCI.6351-11.2012 22623680
62. Jeffrey M, McGovern G, Makarava N, Gonzalez L, Kim YS, et al. (2014) Pathology of SSLOW, a transmissible and fatal synthetic prion protein disorder, and comparison with naturally occurring classical transmissible spongiform encephalopathies. Neuropathol Appl Neurobiol 40 : 296–310. doi: 10.1111/nan.12053 23578208
63. Ma J (2012) The role of cofactors in prion propagation and infectivity. PLoS Pathog 8: e1002589. doi: 10.1371/journal.ppat.1002589 22511864
64. Wang F, Yang F, Hu Y, Wang X, Jin C, et al. (2007) Lipid interaction converts prion protein to a PrPSc-like proteinase K-resistant conformation under physiological conditions. Biochemistry 46 : 7045–7053. 17503780
65. Wang F, Yin S, Wang X, Zha L, Sy MS, et al. (2010) Role of the highly conserved middle region of prion protein (PrP) in PrP-lipid interaction. Biochemistry 49 : 8169–8176. doi: 10.1021/bi101146v 20718504
66. Gomes MP, Millen TA, Ferreira PS, e Silva NL, Vieira TC, et al. (2008) Prion protein complexed to N2a cellular RNAs through its N-terminal domain forms aggregates and is toxic to murine neuroblastoma cells. J Biol Chem 283 : 19616–19625. doi: 10.1074/jbc.M802102200 18456654
67. Gomes MP, Vieira TC, Cordeiro Y, Silva JL (2012) The role of RNA in mammalian prion protein conversion. Wiley Interdiscip Rev RNA 3 : 415–428. doi: 10.1002/wrna.118 22095764
68. Wang X, Bowers SL, Wang F, Pu XA, Nelson RJ, et al. (2009) Cytoplasmic prion protein induces forebrain neurotoxicity. Biochim Biophys Acta 1792 : 555–563. doi: 10.1016/j.bbadis.2009.02.014 19281844
69. Fraser H, Dickinson AG (1968) The sequential development of the brain lesion of scrapie in three strains of mice. J Comp Pathol 78 : 301–311. 4970192
70. Gonzalez L, Martin S, Houston FE, Hunter N, Reid HW, et al. (2005) Phenotype of disease-associated PrP accumulation in the brain of bovine spongiform encephalopathy experimentally infected sheep. J Gen Virol 86 : 827–838. 15722546
71. McGovern G, Brown KL, Bruce ME, Jeffrey M (2004) Murine scrapie infection causes an abnormal germinal centre reaction in the spleen. J Comp Pathol 130 : 181–194. 15003476
72. Polymenidou M, Moos R, Scott M, Sigurdson C, Shi YZ, et al. (2008) The POM monoclonals: a comprehensive set of antibodies to non-overlapping prion protein epitopes. PLoS One 3: e3872. doi: 10.1371/journal.pone.0003872 19060956
73. Choi JK, Park SJ, Jun YC, Oh JM, Jeong BH, et al. (2006) Generation of monoclonal antibody recognized by the GXXXG motif (glycine zipper) of prion protein. Hybridoma (Larchmt) 25 : 271–277. 17044782
74. Farquhar CF, Somerville RA, Ritchie LA (1989) Post-mortem immunodiagnosis of scrapie and bovine spongiform encephalopathy. J Virol Methods 24 : 215–221. 2569471
75. Liu WG, Brown DA, Fraser JR (2003) Immunohistochemical comparison of anti-prion protein (PrP) antibodies in the CNS of mice infected with scrapie. J Histochem Cytochem 51 : 1065–1071. 12871988
76. Baral PK, Wieland B, Swayampakula M, Polymenidou M, Rahman MH, et al. (2012) Structural studies on the folded domain of the human prion protein bound to the Fab fragment of the antibody POM1. Acta Crystallogr D Biol Crystallogr 68 : 1501–1512. doi: 10.1107/S0907444912037328 23090399
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



