-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
Trophozoites of E.
histolytica represent an excellent model to study endosomal-sorting complex required for transport components due to their high endocytic activity and vesicle trafficking. The key role of EhVps32 on phagocytosis is supported by: i) its presence on phagosomes, ii) its interaction with EhADH (an erythrocytes receptor), Gal/GalNac lectin and actin, iii) the higher rate of erythrophagocytosis showed by EhVps32 overexpressing trophozoites, iv) the diminish rate of phagocytosis in EhVps32-silenced G3 trophozoites, and v) its location in erythrocytes-containing acidic phagosomes. Here, we discovered the presence of membranous concentric helicoidally and tunnel-like structures constituted by EhVps32 and EhADH that may have a dynamic role in membrane remodeling and in the generation of intraluminal vesicles in the phagosomes. Elucidating molecular mechanisms of endocytosis-exocytosis pathways will help us to better understand the pathogenic process of E. histolytica and develop new drugs for diagnosis and vaccine methods.
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1005079
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005079Summary
Trophozoites of E.
histolytica represent an excellent model to study endosomal-sorting complex required for transport components due to their high endocytic activity and vesicle trafficking. The key role of EhVps32 on phagocytosis is supported by: i) its presence on phagosomes, ii) its interaction with EhADH (an erythrocytes receptor), Gal/GalNac lectin and actin, iii) the higher rate of erythrophagocytosis showed by EhVps32 overexpressing trophozoites, iv) the diminish rate of phagocytosis in EhVps32-silenced G3 trophozoites, and v) its location in erythrocytes-containing acidic phagosomes. Here, we discovered the presence of membranous concentric helicoidally and tunnel-like structures constituted by EhVps32 and EhADH that may have a dynamic role in membrane remodeling and in the generation of intraluminal vesicles in the phagosomes. Elucidating molecular mechanisms of endocytosis-exocytosis pathways will help us to better understand the pathogenic process of E. histolytica and develop new drugs for diagnosis and vaccine methods.Introduction
Entamoeba histolytica is the protozoan responsible for human amoebiasis, considered the third cause of death in the world due to parasitic diseases [1]. Phagocytosis is a key factor in the parasite virulence and several proteins involved in this event have been already unveiled [2–9], among them the Gal/GalNac lectin [10], EhC2PK, EhCaBP1, EhAK1 [4,11,12] and the EhCPADH complex, formed by a protease (EhCP112) and an adhesin (EhADH) [2], which is a member of the ALIX (apoptosis-linked gene 2-interacting protein X) family [13]. In addition to the Bro1 domain located at its N-terminus, EhADH possesses an adherence epitope at the C-terminus which functions as a receptor during adherence to and phagocytosis of erythrocytes [2,13,14]. BRO1 was described as endosome associated protein that functions in the multivesicular bodies (MVBs) pathway in Saccharomyces cerevisiae [15]. EhADH interacts with EhVps32 [16], a protein described in mammals as a member of the endosomal sorting complex required for transport (ESCRT). ESCRT is a system composed by class E vacuolar protein sorting (Vps) factors and it is highly involved in endocytosis [17]. Additionally, ESCRT participates in a number of cellular events such as cell division and autophagy, among others [18–20].
In eukaryotes, nascent endosomes undergo a maturation process that is controlled by fusion and fission events [21]. Early endosomes mature to intermediate endosomes, which fuse to MVBs where cargo molecules and receptors are segregated to be digested or recycled. Then, late endosomes and endolysosomes are generated. During this process, endosomes acquire different pH, size, appearance and lipid and protein composition [22,23]. Hybrids with characteristics of both intermediate and late endosomes and lysosomes are also formed [24].
In general, assembly of the ESCRT machinery begins with recognition of monoubiquitinated cargo by ESCRT-0 (Vps27 and Hse1). Then, ESCRT-0 interacts with ESCRT-I (Vps20, Vps23, Vps37 and Mvb12) that binds to endosomal membranes [25]. ESCRT-I activates ESCRT-II (Vps22, Vps25 and Vps36), producing membrane invagination to form intraluminal vesicles (ILVs). At this point, ESCRT-III subunits (Vps2, Vps20, Vps24 and Vps32) are recruited, leading to the generation of oligomers that regulate formation and release of ILVs [26] and acting as scission machinery in preformed vesicle necks. Subsequently, Vps4 AAA ATPase catalyzes the dissociation of ESCRT-III components from the membrane to re-start the cycle [27,28]. In other cases, the Alix protein mediates the ubiquitin-independent, but ESCRT-III-dependent endocytosis [29]. ESCRT-III members have coiled-coil protein-protein interaction domains common to the Snf7 family [30]. Its main component, Vps32 (Snf7 in S. cerevisiae [31] and CHMP4 in humans [32]), has a positively charged N-terminus that binds to negatively charged lipids. N-terminus also binds to the negatively charged C-terminus domain to generate the EhVps32 auto-inhibited form. Vps32 and Vps20 form the ESCRT-III sub-complex I, which is in direct contact with endosomes. Afterward, they recruit Vps2 and Vps24 that form sub-complex II [33].
E. histolytica possesses the genes encoding ESCRT proteins [34] and those encoding EhVps4 AAA ATPase and EhADH, both ESCRT associated proteins [13,35]. Here, we show the participation of EhVps32 in both receptor-mediated and non-specific phagocytosis as well as in pinocytosis; we also revealed its co-localization with EhADH, Gal/GalNac lectin and actin during erythrophagocytosis. Besides, we identified the presence of membranous helicoidally and tunnel-like structures in trophozoites constituted by EhVps32 and EhADH that seem to be involved in the dynamic membrane remodeling during phagocytosis. These events are crucial for target cells destruction during parasite invasion to host tissues.
Results
In resting conditions, EhVps32 is localized adjacent to plasma membrane and in cytoplasmic vesicles
As a tool to study the location and function of EhVps32 in E. histolytica trophozoites, we produced antibodies against the EhVps32 recombinant protein (rEhVps32) [16]. By western blot assays, αrEhVps32 antibodies recognized a 32 kDa band in trophozoite lysates, in cytoplasm and in membrane fractions (Fig 1A and 1B). However, after membrane fractionation by ultracentrifugation, EhVps32 was only detected in internal membranes (Fig 1B). In the same nitrocellulose filter, the αGal/GalNac lectin antibodies identified a 170 kDa band [36], in both plasma and internal membrane fractions. The Gal/GalNac lectin has been described as a membrane protein marker [10]. Through confocal microscopy and TEM, EhVps32 appeared in cytoplasmic vesicles of distinct size, some of them, close to the plasma membrane (Fig 1C and 1D). In addition, Gal/GalNac lectin and EhVps32 co-localized close to the plasma membrane. Little signal was observed in cells treated with preimmune serum and in non-permeabilized cells (Fig 1C and 1D), confirming that EhVps32 is not a plasma membrane protein, but it is located in cytoplasmic vesicles, some of them adjacent to the plasma membrane.
Fig. 1. Detection and localization of EhVps32 protein in trophozoites lysates and fixed trophozoites. 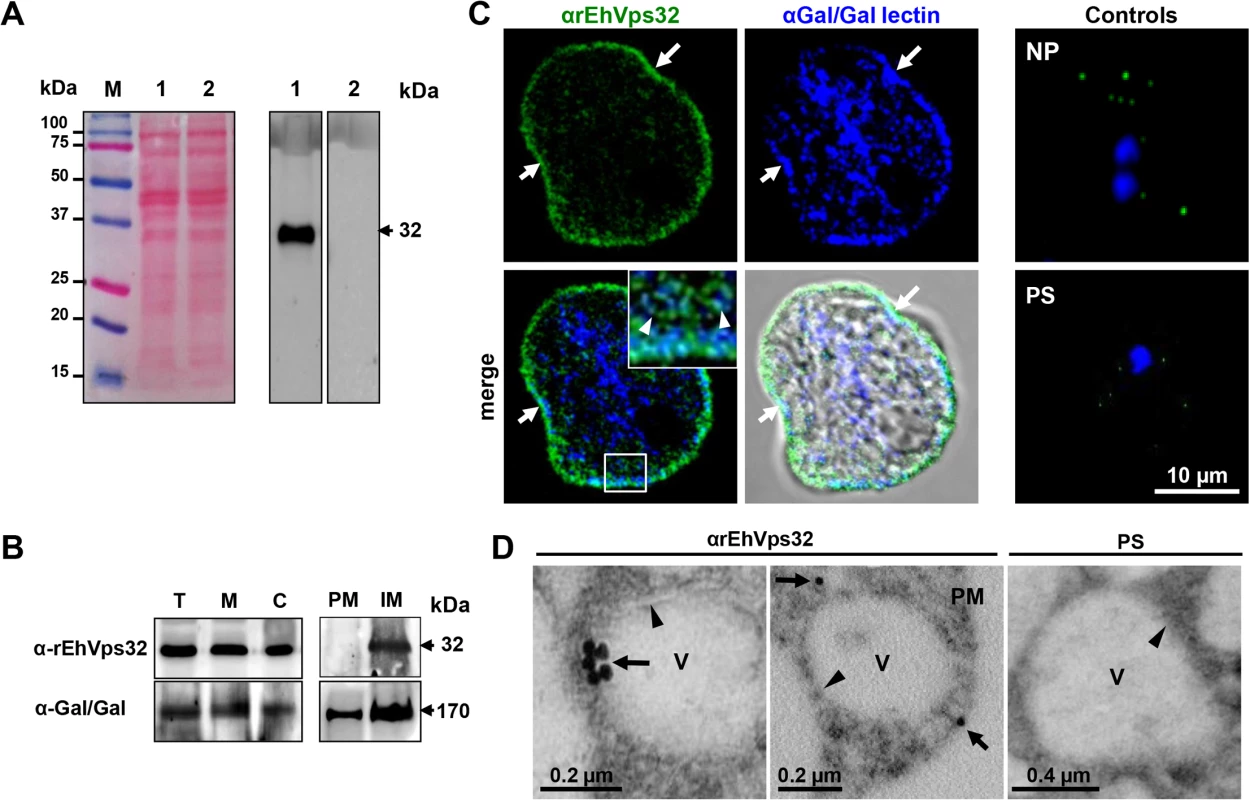
(A) At left, Ponceau stained nitrocellulose membrane of transferred 12% SDS-PAGE separated trophozoites lysates (lanes 1 and 2) and molecular markers (lane M). At right, western blot assay using αrEhVps32 antibodies (lane 1) or preimmune serum (lane 2), followed by HRP-labeled secondary antibodies. (B) Western blot assays of trophozoites extracts (T), membrane fraction (M) and cytoplasmic fraction (C). Membrane fraction was ultracentrifuged to separate plasma membrane (PM) and internal membranes (IM). (C) Confocal microscopy using αrEhVps32 and αGal/GalNac lectin antibodies, followed by FITC-labeled and Pacific blue-labeled secondary antibodies, respectively. Controls: non-permeabilized trophozoites (NP) and trophozoites treated with preimmune serum (PS) and secondary antibodies. Nuclei were counterstained with DAPI (blue fluorescence). Arrows: plasma membrane. Arrowheads: vesicles close to plasma membrane magnified in white square. (D) TEM of trophozoites incubated with αrEhVps32 antibodies or PS and gold-labeled secondary antibodies. V: vesicles. Arrows: gold particles. Arrowheads: vesicle membrane. PM: plasma membrane. During erythrophagocytosis, EhVps32 co-localizes with EhADH, Gal/GalNac lectin and actin
The involvement of EhVps32 in erythrophagocytosis was studied by confocal microscopy through kinetics from 0 (resting conditions) to 60 min. In parallel, we investigated its co-localization with EhADH, Gal/GalNac lectin and actin, three proteins involved in endocytosis [2,10,37]. EhADH is a receptor for erythrocytes during target cell adherence and it has been found on phagosomes [2,14,38]. Additionally, in vitro, it binds to EhVps32 through its Bro1 domain [16]. At resting conditions EhVps32 co-localized with EhADH close to the plasma membrane (Fig 2A), with a similar pattern to that observed with Gal/GalNac lectin (Fig 1C). According to the results obtained with non permeabilized cells and membrane fractionation, EhVps32 might be adjacent to the plasma membrane (Fig 1C), and, as it has been reported, EhADH could be facing the extracellular space [16].
After 2 min, EhVps32 also decorated phagocytic cups, where it co-localized with EhADH, but EhVps32 did not appear surrounding the erythrocytes, whereas EhADH decorated adhered erythrocytes (Fig 2A and 2B). Between 5 to 30 min of phagocytosis, EhVps32 presented different patterns on erythrocyte-containing phagosomes: some erythrocytes remained without fluorescence, while others appeared completely covered by the αrEhVps32 antibodies (Fig 2A). At this time, EhVps32 and EhADH co-localized on some phagosomes; although other phagosomes recognized by αEhADH antibodies were not stained by αrEhVps32 antibodies (Fig 2A). At 60 min, when digestion had advanced, the majority of EhVps32 returned to its resting position and its presence on phagosomes diminished, whereas EhADH remained in them (Fig 2A). Nevertheless, at this time, many trophozoites had ingested more than 20 erythrocytes per trophozoite distributed in phagosomes with a distinct number of erythrocytes inside.
Pearson’s coefficient showed that the EhVps32 and EhADH co-localization was 0.47 at 0 min, at 30 min it reached 0.65, while at 60 min it diminished to 0.3 (Fig 2C). The proportion of erythrocytes inside phagosomes decorated by αEhADH or αrEhVps32 antibodies with relation to total ingested erythrocytes confirmed that, whereas the majority of phagosomes were stained by EhADH, EhVps32 was detected only in 20% of them at 2 min and in 58% at 30 min, dropping close to zero after this time (Fig 2D).
Fig. 2. Co-localization and interaction of EhVps32 and EhADH during erythrophagocytosis. 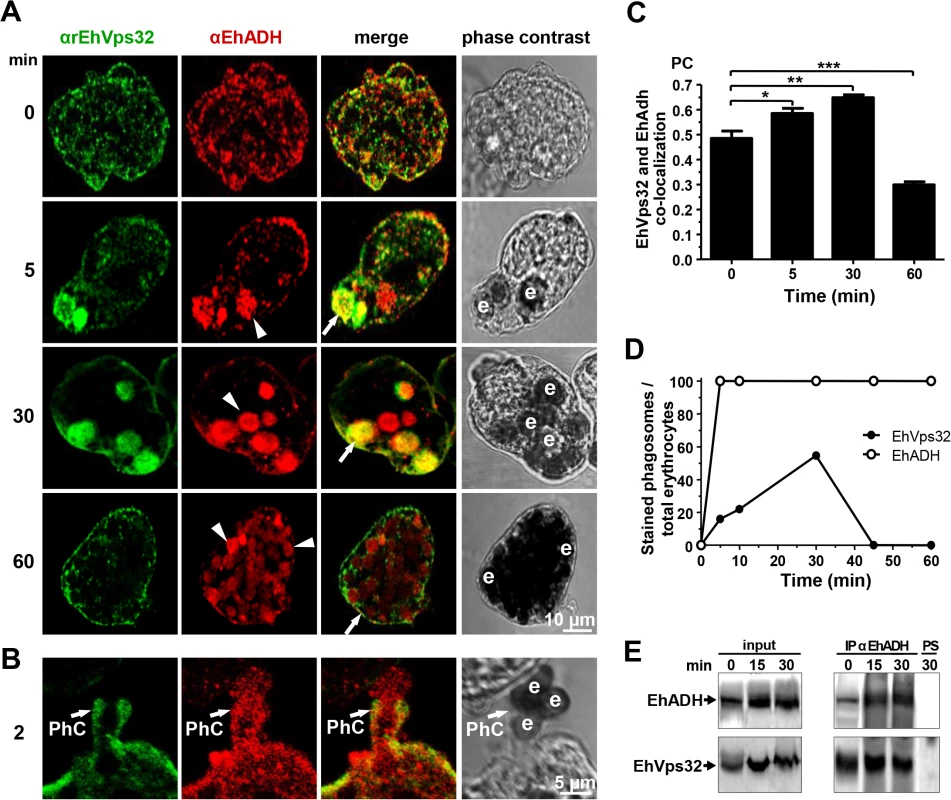
(A,B) Trophozoites were incubated with erythrocytes for different times and analyzed through confocal microscopy. (A) After erythrophagocytosis, trophozoites were incubated with αrEhVps32 and αEhADH antibodies, then with FITC and TRITC-labeled secondary antibodies, respectively. (B) Illustration of a phagocytic cup (PhC). Arrows: co-localization of EhVps32 and EhADH. Arrowheads: EhADH in phagosomes without EhVps32 signal. e: erythrocytes. (C) Pearson’s coefficient (PC) to quantify EhVps32 and EhADH co-localization in the entire cell. (*) p<0.05, (**) p<0.01 and (***) p<0.001. (D) Proportion of erythrocytes inside phagosomes decorated by αrEhVps32 and αEhADH antibodies with relation to total ingested erythrocytes per trophozoite. (E) Immunoprecipitation of trophozoites lysates at different times of erythrophagocytosis using αEhADH antibodies or preimmune serum (PS). Immunoprecipitation assays using αEhADH antibodies confirmed the association of EhVps32 with EhADH in resting conditions and during phagocytosis (Fig 2E). By these experiments we could not accurately distinguish differences in the amount of both interacting proteins at different phagocytosis times. Nevertheless, altogether our results suggest that during erythrophagocytosis EhADH recruits EhVps32, probably after adherence to target cells and before their digestion. As the phagosome maturation process is fast, continuous and non-synchronous, it is difficult to observe EhVps32 in all phagosomes at a given time. Additionally, we cannot discard the participation of both proteins in other functions distinct to phagocytosis.
Gal/GalNac lectin is another E. histolytica protein involved in adherence and phagocytosis. It is located in the plasma membrane and in the endosomes generated during the endocytic process [10]. Furthermore, in resting conditions it co-localized with EhVps32 (Fig 1C), adjacent to the plasma membrane. Then, we investigated the location of EhVps32 and Gal/GalNac lectin during erythrophagocytosis. At 2 min, we found both proteins in the plasma membrane and in the phagocytic cups, close to adhered erythrocytes which would be ingested (Fig 3A). In addition, both proteins co-localized with actin, detected by phalloidin (Fig 3A). Interestingly, 20 min after incubation with erythrocytes, the three proteins appeared together at some points of the plasma membrane and around the ingested erythrocytes, confirming the participation of them in erythrophagocytosis (Fig 3A). Pearson’s coefficient of EhVps32 and Gal/GalNac lectin co-localization at plasma membrane, at resting conditions it was 0.38, after 2 min of phagocytosis it was 0.42, and after 20 min it was 0.32; whereas in the entire cell, Pearson’s coefficients were 0.27, 0.23 and 0.29, respectively (Fig 3B). These results suggest that in addition to EhADH, EhVps32 interacts with Gal/GalNac lectin and actin during erythrophagocytosis.
Fig. 3. Co-localization of EhVps32, Gal/GalNac lectin, actin and Lysotracker during erythrophagocytosis. 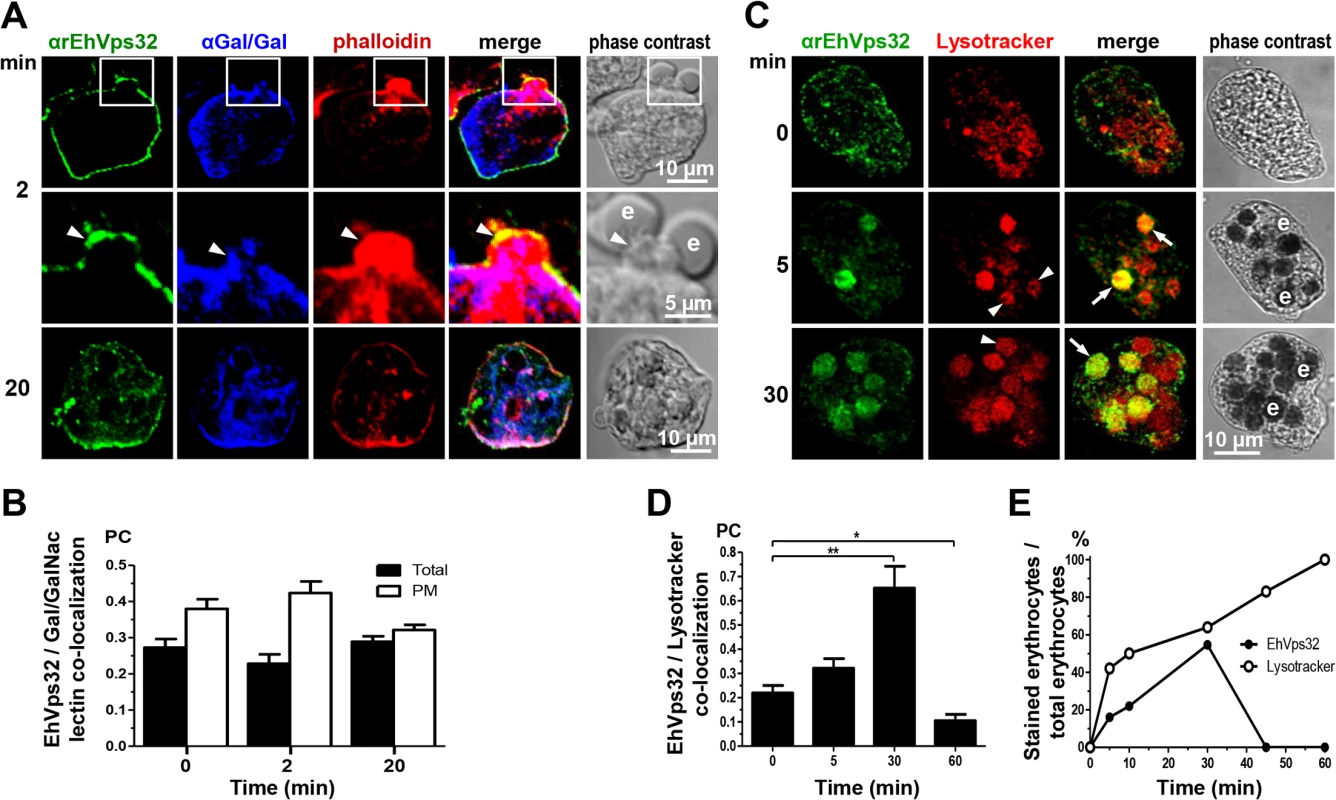
Trophozoites were incubated with erythrocytes for different times and analyzed through confocal microscopy. (A) After erythrophagocytosis, trophozoites were incubated with rabbit αrEhVps32 and mouse αGal/GalNac lectin antibodies and rhodamine-phalloidin, and then, with α-rabbit FITC and α-mouse Pacific blue-labeled secondary antibodies, respectively. Arrowheads: co-localization at phagocytic cup. Arrows: co-localization at phagosome membrane. e: erythrocytes. (B) Pearson’s coefficient (PC) to quantify EhVps32 and Gal/GalNac lectin co-localization in the entire cell (total) and in the plasma membrane (PM). (C) Confocal microscopy of trophozoites incubated with Lysotracker and treated with αrEhVps32 and FITC-labeled secondary antibodies. Arrows: co-localization of EhVps32 and Lysotracker. Arrowheads: Lysotracker-stained phagosomes without EhVps32 signal. e: erythrocytes. (D) Pearson’s coefficient (PC) to quantify EhVps32 and Lysotracker co-localization in the entire cell. (*) p<0.05 and (**) p<0.01. (E) Proportion of erythrocytes inside phagosomes decorated by αrEhVps32 antibodies and Lysotracker with relation to total ingested erythrocytes per trophozoite. Interestingly, in non-specific phagocytosis of latex microspheres, EhVps32 was detected in all phagosomes containing fluorescent microspheres with a Pearson’s coefficient at 5 min of 0.43; at 30 min, 0.63; and at 60 min, 0.68 (S1 Fig). However, fluorescent microspheres and EhADH exhibited poor co-localization, with a Pearson’s coefficient lower than 0.3 at all times tested (S1 Fig). In contrast to erythrophagocytosis findings, EhVps32 participates in the whole process of non-specific phagocytosis, whereas EhADH participation appeared to be minimal.
EhVps32 is localized in acidic vesicles
In mammalian cells, intermediate endocytosis is characterized by formation of typical MVBs, that once formed, rapidly acidify reaching a pH of 5.5 [39]. In resting conditions, many small vesicles were positive for Lysotracker in the cytoplasm (Fig 3C), indicating that trophozoites possess a significant amount of acidic vesicles, probably due to their basal endocytosis. Between 5 to 30 min, Lysotracker stained all erythrocytes-containing phagosomes that were positive for αEhVps32 antibodies. On the other hand, other phagosomes were stained only by Lysotracker (Fig 3C and 3E). At 45 and 60 min, EhVps32 appeared in the cytoplasm and adjacent to the plasma membrane, whereas Lysotracker decorated almost all erythrocytes-containing phagosomes (Fig 3E). Pearson’s coefficient showed that association between EhVps32 and Lysotracker augmented from 0.22 at 0 time to 0.65 at 30 min, and it diminished to 0.1 after this time (Fig 3D).
During phagocytosis, EhVps32 appears in phagosomes and membranous concentric structures
Between 10 and 30 min, gold-labeled antibodies detected through TEM the EhVps32 protein in phagosome membranes (Fig 4B), in concentric membranous structures close to ingested erythrocytes (Fig 4C, 4D and 4E) and in erythrocyte fragments (Fig 4D and 4E). Trophozoites treated only with gold labeled secondary antibodies gave no signal (Fig 4A). At 60 min, TEM images exhibited erythrocytes inside phagosomes with putative ILVs that could correspond to MVBs or to other unidentified structures. At this time, EhVps32 scarcely appeared in late phagosomes, which exhibited erythrocyte fragments in advanced phases of digestion (Fig 4F). These results corroborate that EhVps32 is in phagosomes of acidic nature, but it is poorly located in phagolysosomes.
Fig. 4. Detection by TEM immunogold of EhVps32 on phagosomes and membranous concentric structures. 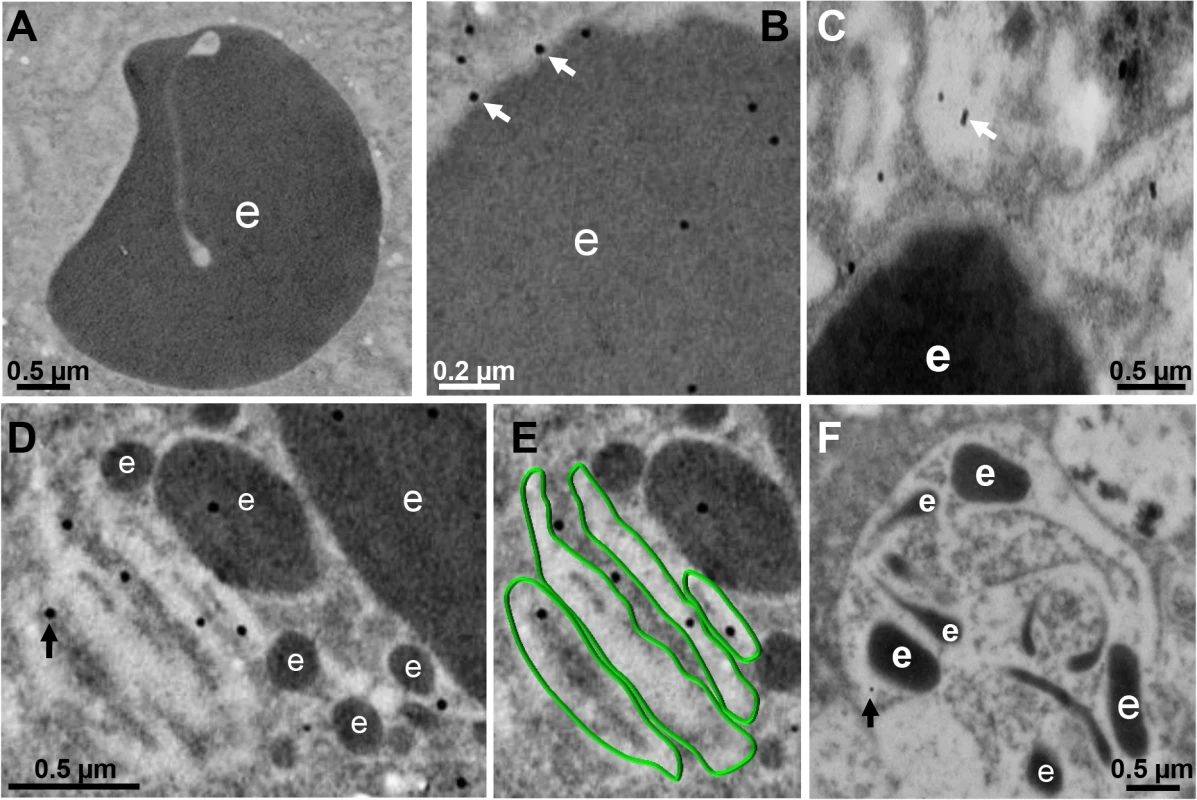
TEM of trophozoites incubated with erythrocytes after 10 (A-C), 30 min (D,E) and 60 min (F), treated with αrEhVps32 antibodies and gold-labeled secondary antibodies. (A) Control treated only with gold-labeled secondary antibodies. (E) Membranous concentric structures observed in (D) were colored in green. e: erythrocytes and erythrocytes fragments. Arrows: gold particles. During pinocytosis, EhVps32 co-localizes with EhADH and Gal/GalNac lectin
In other organisms, the role of Vps32 has been elucidated in pinocytosis, but not in phagocytosis [40]. To confirm the participation of EhVps32 in pinocytosis we studied the relationship of EhVps32 with EhADH and Gal/GalNac lectin during FITC-labeled dextran uptake. Confocal images showed that at 30 min incubation, EhVps32, EhADH and Gal/GalNac lectin clearly co-localized in dextran-containing endosomes (Fig 5A and 5B). This pinosomes appeared larger at 60 min (Fig 5A). However, detection of actin co-localizing with dextran and EhVps32 was less evident (Fig 5C).
Fig. 5. Co-localization of EhVps32 with EhADH and Gal/GalNac lectin during pinocytosis. 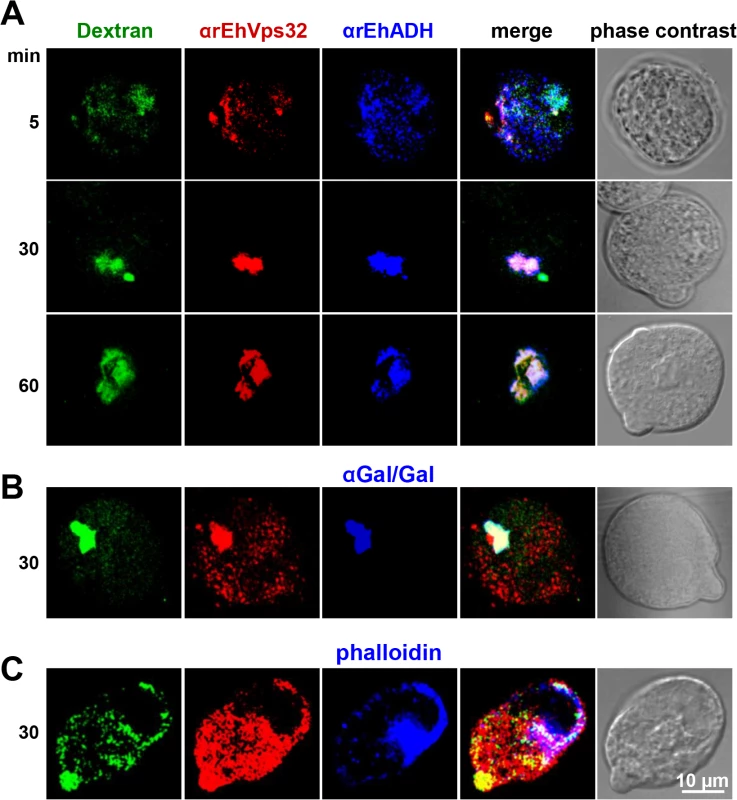
(A) Confocal microscopy of trophozoites incubated with FITC-dextran for different times and then, treated with mouse αrEhVps32 and rabbit αEhADH antibodies, followed by α-mouse TRITC-labeled and α-rabbit Cy5-labeled secondary antibodies, respectively. (B) Trophozoites incubated with rabbit αrEhVps32 and mouse αGal/GalNac lectin antibodies, and then with α-rabbit TRITC-labeled and α-mouse Pacific blue-labeled antibodies, respectively. (C) Trophozoites incubated with mouse αrEhVps32 antibodies and rhodamine-phalloidin, followed by incubation with α-mouse Pacific blue-labeled secondary antibodies. Only for this last experiment, blue and red channels were digitally inverted. rEhVps32 forms oligomers in vitro and EhVps32 monomers are located in the cytoplasm
According to results presented above, EhVps32 seems to be involved in phagocytosis and pinocytosis. However, to carry out its function as scission factor and generate ILVs during erythrophagocytosis, EhVps32 needs to form oligomers on curved membranes of phagosomes, as it has been described for this protein in other systems [41]. First, we explored in vitro whether rEhVps32 was able to form oligomers, employing TEM negative staining assays of purified rEhVps32. Images showed the presence of long filaments (10–75 nm width) with ramifications and many small rings (0.1–0.15 μm) that presumably could augment in size and complexity by continuous oligomerization of the protein (Fig 6A and 6F). Fig 6A exhibits a long ring (0.7 x 0.65 μm), containing other smaller rings (0.1–0.15 μm) and Fig 6B and 6C showed concentric structures. Antibodies against rEhVps32 recognized these structures (Fig 6D and 6F). Size exclusion chromatography of the rEhVps32 purified protein followed by western blot analysis confirmed the presence of oligomers, with a migration rate (Rf) corresponding to EhVps32 multiples (Fig 6G and 6I). However, little differences were found in the western blot patterns obtained from the fractions containing the larger oligomerized molecules and the one containing the monomer. We attributed this to the fast polymerization of rEhVps32 in the tube.
Fig. 6. In vitro oligomerization of rEhVps32 and cellular localization of EhVps32 monomers. 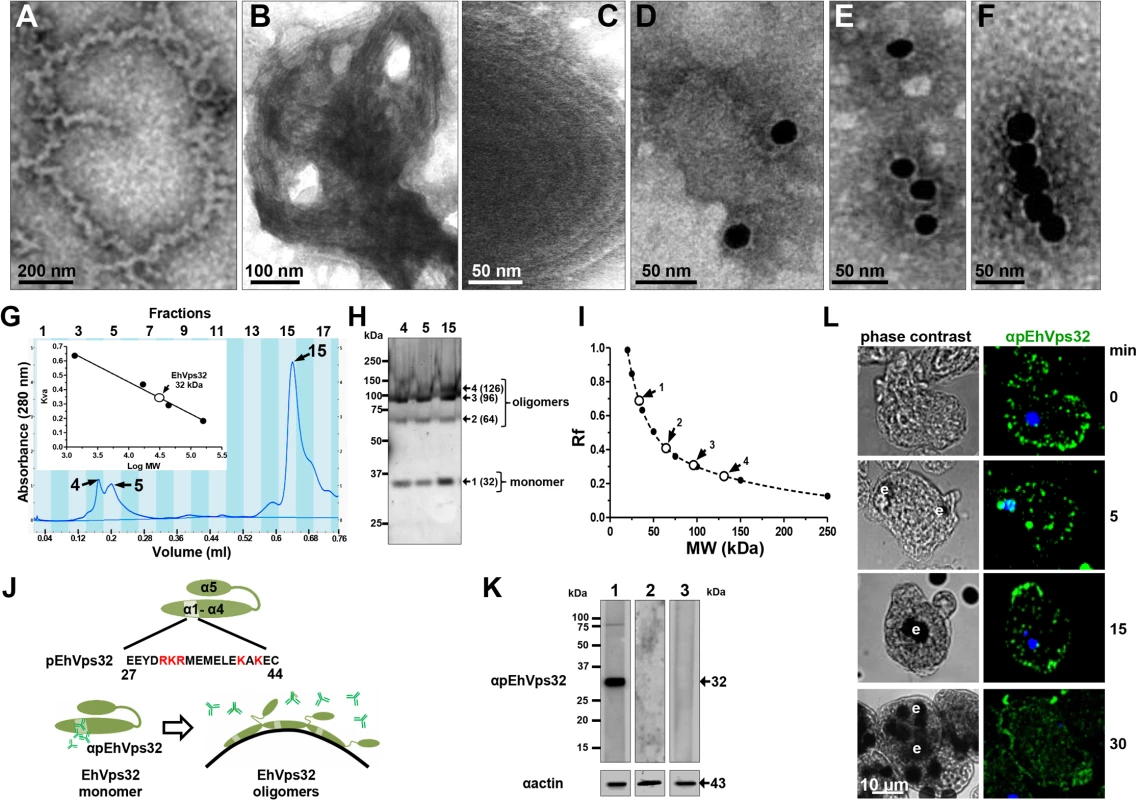
(A-C) TEM of purified rEhVps32 (without GST-tag) negatively stained. (D-F) Samples were treated with αrEhVps32 antibodies, followed by gold-labeled secondary antibodies. (G) Gel filtration chromatographic profiles of purified rEhvps32 (without GST-tag). Inset: calibration curve using molecular weight standards to estimate the profile of EhVps32 monomer. (H) Eluted fractions contained the EhVps32 profiles indicated in the graph (G) were submitted to western blot analysis, using αrEhVps32 antibodies. (I). Rf of molecular weight markers (●) and bands (○) revealed by αrEhVps32 antibodies in (H). (J) Schematic representation of close and open conformations of EhVps32 showing the position of pEhVps32 polypeptide used to generate αpEhVps32 antibodies. Red letters: positively charged residues. Scheme shows the EhVps32 monomers in close conformation and the EhVps32 oligomers in open conformation on the phagosome membrane. (K) Western blot assays of trophozoites lysates, using αpEhVps32 antibodies alone (lane 1), competed with rEhVps32 purified protein (lane 2) or competed with αrEhVps32 antibodies (lane 3). As a loading control, the same membranes were re-blotted with αactin antibodies. (L) Confocal microscopy of trophozoites incubated with erythrocytes during different times, treated with αpEhVps32 and FITC-labeled secondary antibodies. Nuclei were counterstained with DAPI. e, erythrocytes. To investigate the location of EhVps32 monomers and oligomers in trophozoites, we generated polyclonal antibodies (pEhVps32) directed against an antigenic region formed by 18 amino acids located in the first alpha helix at the EhVps32 amino terminus (Fig 6J). According to reports in other systems [41,42], this peptide may be in contact with the phagosome membrane because it contains positively charged amino acids that bind to negatively charged membrane lipids [43]. Thus, it is predictable that αpEhVps32 antibodies would react with the exposed epitope in EhVps32 monomers, but not with oligomers, in which this region is hidden (Fig 6J). In western blot assays, αpEhVps32 antibodies recognized the 32 kDa protein in trophozoites lysates and competed with the rEhVps32 purified protein and with the αrEhVps32 antibodies (Fig 6K), evidencing their specificity. In confocal microscopy experiments, αpEhVps32 antibodies decorated cytoplasmic small vesicles but not phagosomes containing erythrocytes (Fig 6L). These results suggest that, as in other systems [44], in vivo EhVps32 forms oligomers on the phagosome membranes, which would be necessary to function as scission machinery during ILVs formation in endocytosis.
EhVps32 overexpression increases rate of erythrophagocytosis and induces formation of helicoidally concentric structures
We searched for further insights on EhVps32 function using pNeoEhvps32-HA transiently-transfected trophozoites. Transfected trophozoites were viable for 72 h, probably due to the excessive oligomerization of EhVps32. However, until this time, they appeared healthy, with active movement, pleomorphic and able to divide. Twelve hours after transfection we confirmed by western blot experiments that pNeoEhvps32-HA transfected cells expressed 1.6 to 2 fold more EhVps32 protein than pNeo-transfected and non-transfected cells, respectively (Fig 7A and 7B). The rate of erythrophagocytosis was evaluated 12 h after transfection in cultures with 95% of viability. pNeoEhvps32-HA-transfected trophozoites ingested between 56 and 105% more erythrocytes than pNeo transfected trophozoites (Fig 7C and 7D). Number of erythrocytes inside trophozoites was counted by light microscopy in 3,3’ diaminobenzidine-stained preparations (Fig 7C) and by the amount of hemoglobin inside the trophozoites at different times of phagocytosis, with similar results (Fig 7D).
Fig. 7. Expression and localization of EhVps32 and rate of erythrophagocytosis in pNeoEhvps32-HA transfected trophozoites. 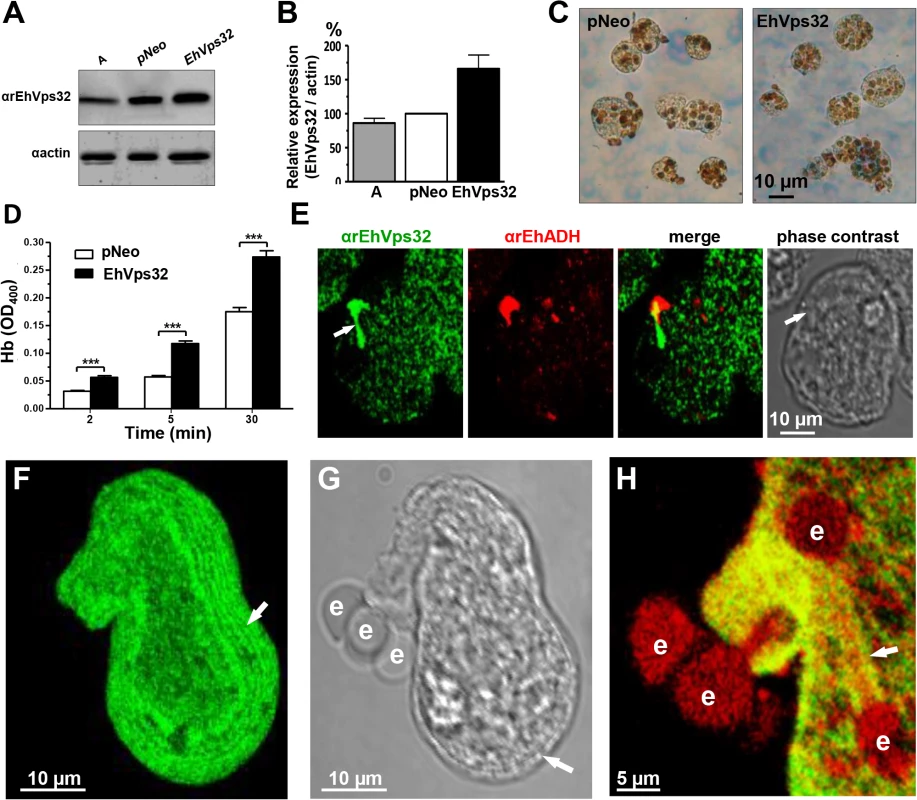
(A) Western blot assays of trophozoites lysates from wild type clone A (lane A) and pNeo (lane pNeo) and pNeoEhvps32-HA (lane EhVps32) transfected cells, using the αrEhVps32 antibodies. As a loading control, the same membrane was re-blotted with αactin antibodies. (B) Densitometry analysis of bands showed in (A), normalized against actin band. (C) Diaminobenzidine-stained trophozoites that ingested erythrocytes for 30 min. (D) Rate of erythrophagocytosis of transfected trophozoites. (***) p<0.001. (E) Confocal microscopy of pNeoEhvps32-HA transfected trophozoites in resting conditions, using αrEhVps32 and αEhADH antibodies, followed by FITC-labeled and TRITC-labeled secondary antibodies. Arrows: membrane projections. (F-H) Trophozoites overexpressing EhVps32 incubated with erythrocytes for 2 min treated with αrEhVps32 and αEhADH antibodies and FITC- and TRITC-secondary antibodies, respectively. (F) Maximum projection of a transfected trophozoite evidencing the concentric arrays (arrow) formed by EhVps32. (G) Phase contrast. Arrow: concentric arrays. (H) Merging image (αrEhVps32 and αEhADH antibodies). Arrow: tunnel-like structure. e: erythrocytes. Confocal microscopy images of resting pNeoEhvps32-HA-transfected trophozoites revealed the presence of structures of distinct size protruding from the plasma membrane, which were recognized by αrEhVps32 and αEhADH antibodies (Fig 7E). Trophozoites exhibited structures decorated in one end by αrEhVps32 antibodies and in the opposite end by αEhADH antibodies, whereas in the middle, both proteins merged. We do not know the significance of this protein distribution. However, these results reinforce the hypothesis that both proteins interact even in resting trophozoites, and that this interaction was more evident when EhVps32 is overexpressed. During phagocytosis in some trophozoites, αrEhVps32 antibodies decorated membranous concentric structures that were located inside cells and forming part of the phagocytic cup (Fig 7F, 7G and 7H). They were visible in the cytoplasm forming filaments or tunnel-like structures extended to the ingested erythrocytes and contacting phagosomes (Fig 7F, 7G and 7H). EhADH appeared around adhered erythrocytes, in erythrocytes in process of ingestion and in erythrocytes inside phagosomes (Fig 7H).
We investigated the appearance of these structures on the trophozoite surface by SEM. Images of pNeoEhvps32-HA-transfected trophozoites revealed the presence of a high amount of membrane rings of 0.9–1.6 μm diameters, with 0.3 to 0.7 μm holes (Fig 8B), probably corresponding to the extreme of the tunnel-like and other structures observed in immunofluorescence experiments. Trophozoites exhibited membrane projections of 2.2 to 2.4 μm length, and 1.5 to 1.9 μm widths (Fig 8C) similar to those recognized by αrEhVps32 antibodies in confocal microscopy experiments (Figs 7E and S2A–S2F). They also appeared, although in fewer 2amount and with less diversity, in the wild type strain (S2K and S2L Fig) and in pNeo transfected trophozoites (S2G–S2J Fig). Thus, EhVps32 seems to form oligomers by the assembly of rings and filaments that protruded from the plasma membrane, which, in excess, might kill the cells.
Fig. 8. SEM and TEM of membranous helicoidally structures in pNeoEhvps32-HA transfected trophozoites. 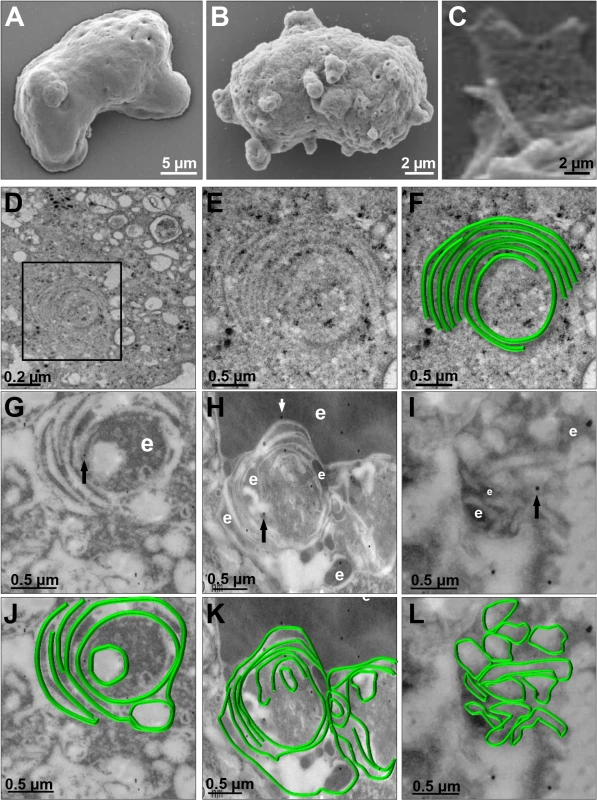
(A-C) SEM of pNeo (A) and pNeoEhvps32-HA (B,C) transfected trophozoites. (C) Magnification of conical helicoidally structures protruding from plasma membrane. (D-F) TEM of glutaraldehyde fixed trophozoites transfected with pNeoEhvps32-HA plasmid embedded in Polybed epoxy resin. Square in (D), magnified in (E,F). (F) A drawing in green superposed to the concentric structure in (E). (G-L) TEM of pNeoEhvps32-HA transfected trophozoites, fixed by PFA and glutaraldehyde, embedded in LR White resin and incubated with αrEhVps32 antibodies followed by gold-labeled secondary antibodies. Arrows: gold particles. e: erythrocytes fragments. (J-L) Helicoidally structures in (G-I) were drawn in green. We explored by TEM the ultrastructure of the arrays detected by confocal microscopy and SEM. Thin sections of pNeoEhvps32-HA-transfected trophozoites, exhibited helicoidally structures up to 2.12 x 1.75 μm in diameters, formed by 5 to 7 concentric filaments of 75–100 nm width (Fig 8D, 8E and 8F). Then, we prepared thin sections of trophozoites embedded in LR White resin, to better allow the access of αrEhVps32 antibodies. Antibodies recognized these structures, confirming the presence of EhVps32 in them (Fig 8G–8L). Additionally, these structures were similar to those formed in vitro and detected by TEM in negative stain preparations of the rEhVps32 purified protein (S3 Fig).
EhVps32 knock down drastically diminishes rate of erythrophagocytosis
To get more evidence on the role of EhVps32 in phagocytosis, we employed trophozoites of the G3 strain [45] to transcriptional silence the Ehvps32 gene. EhVps32-silenced G3 trophozoites, grown in 7 μg/ml of G418, presented a growth rate similar to G3 strain and both were used to determine the level of expression of EhVps32. Western blot assays showed 80% reduction in the amount of EhVps32 protein in EhVps32-silenced trophozoites compared with G3 strain (Fig 9A and 9B). EhVps32-silenced trophozoites showed a poor capacity to ingest erythrocytes, presenting 80% less amount of ingested erythrocytes than the G3 strain (Fig 9C and 9D) (a mean of 1.27 OD400 hemoglobin per G3 trophozoites vs 0.26 OD400 hemoglobin per EhVps32-silenced trophozoites, after 20 min phagocytosis). The 3,3’ diaminobenzidine-stained images clearly showed these differences (Fig 9C).
Confocal microscopy images also evidenced differences between G3 strain and EhVps32-silenced trophozoites. Differences of αrEhVps32 fluorescence intensity between G3 and EhVps32-silenced trophozoites were evident at 0 and 20 min phagocytosis. Whereas in G3 trophozoites, EhVps32, EhADH and actin co-localized in the phagocytic cups and around phagosomes, in EhVps32-defficent trophozoites, EhADH presented a diffused pattern around phagosomes and actin was re-distributed in actin points beside the phagosomes, but not around them (Fig 9E).
Fig. 9. Detection and localization of EhVps32 and erythrophagocytosis rate of EhVps32-silenced G3 trophozoites. 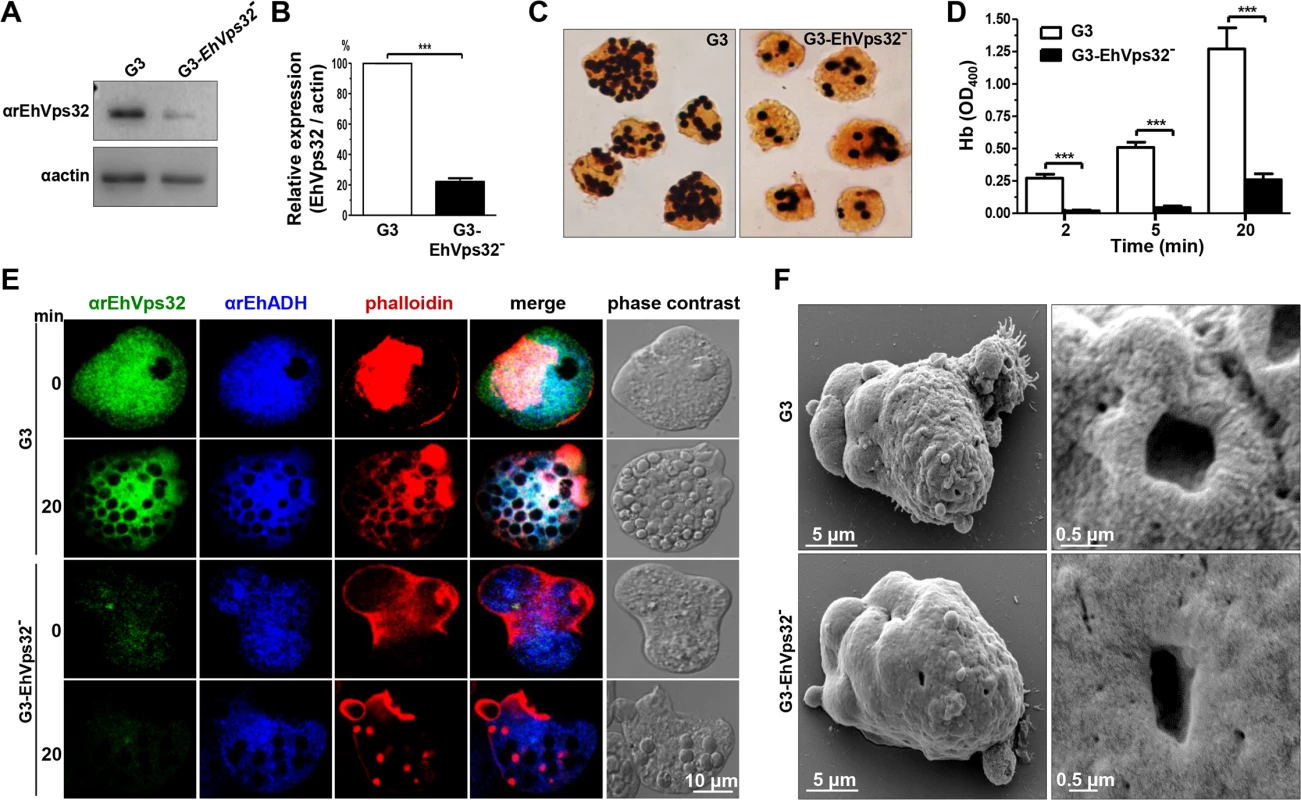
(A) Western blot assays of trophozoites lysates from G3 strain and pNeoEhvps32-HA (EhVps32-) silenced G3 cells, using the αrEhVps32 antibodies. As a loading control, the same membrane was re-blotted with αactin antibodies. (B) Densitometry analysis of bands showed in (A) normalized against actin protein. (C) Diaminobenzidine-stained trophozoites that ingested erythrocytes for 20 min. (D) Rate of erythrophagocytosis of G3 trophozoites. (***) p<0.001. (E) Confocal microscopy of G3 trophozoites in resting conditions and at 20 min of erythrophagocytosis, using mouse αrEhVps32 and rabbit αEhADH antibodies and rhodamine-phalloidin, followed by α-mouse FITC-labeled and α-rabbit Cy5-labeled secondary antibodies. (F) SEM of G3 trophozoites. Right panels: magnification of holes. By SEM we also observed changes in the EhVps32-silenced trophozoites surface. They contained less projections and roughness than the G3 trophozoites (Fig 9F). Additionally, the doughnut structures showed a flat appearance with fewer edges.
In summary, our results show the involvement of EhVps32 protein in phagocytosis, suggesting that the ESCRT machinery participates in this virulence event. Association of EhVps32 with EhADH, Gal/GalNac lectin and actin, all of them proteins involved in phagocytosis, gives further support to this assumption.
Discussion
In this paper, we identified EhVps32 protein in E. histolytica trophozoites, which in eukaryotes acts as a scission machinery during the ILVs formation in endocytosis. We showed here the association of EhVps32 with EhADH, Gal/GalNac lectin and actin in trophozoites under resting condition and during erythrophagocytosis. Additionally, we discovered in this parasite membranous tunnel-like and helicoidally structures formed by EhVps32. Filaments, rings, spirals, circular arrays and helicoidally structures resemble those described in COS7 cells and yeast, transfected with CHMP4 and Snf7, respectively [46,47]. Vps32 has been widely studied during endocytosis in other eukaryotes, however, as far as we know, this is the first study showing its participation in phagocytosis. Furthermore, studies on ESCRT machinery have been performed in yeast and complex organisms that are not professional phagocytes. The unique membrane exchange and constitutive endocytosis of the unicellular protozoan E. histolytica provide an excellent model to investigate novel roles of ESCRT machinery and other molecules involved in phagocytosis.
The molecular weight of EhVps32 appeared larger (32 kDa) than the expected one from its amino acid sequence (24 kDa). Accordingly, Snf7 migrates at 35 kDa when its predicted molecular weight is 27 kDa [33]. This has been attributed to the electric charge of the protein [33], and EhVps32 is also rich in charged residues.
The presence of EhVps32 adjacent to the plasma membrane (Fig 1), as well as its polarization in phagocytic cups during phagocytosis, support the hypothesis that EhVps32 is activated since trophozoites initiate the cargo recognition (Fig 2). The direct participation of EhVps32 in erythrophagocytosis started to be visible at 5 min, when EhVps32 interacted with EhADH on erythrocytes-containing phagosomes (Fig 2). Its co-localization with Gal/GalNac lectin and actin in phagocytic cups, in phagosome membrane and in phagosomes (Fig 3), strengthened this hypothesis. EhVps32 is present in phagosomes until 30 min of phagocytosis, but during advanced lysis of erythrocytes, it was poorly detected in phagosomes stained with Lysotracker and αEhADH antibodies (Figs 2 and 3). These findings corroborated the role of EhVps32 in phagocytosis, when ILVs are formed and released.
In yeast, MVBs formation is restricted to intermediate and late endocytosis; while in mammals, typical and uniform MVBs appear during intermediate endocytosis, although multivesicular vacuoles are found in all stages of the endocytic pathway [39]. E. histolytica trophozoites also present structures similar to MVBs (Fig 4) [13,48], but neither the time of their formation, nor molecules participating have been fully identified [16,35]. EhADH has been found in putative MVBs [16] and here we demonstrated its co-localization with EhVps32 in phagosomes between 5 to 30 min of phagocytosis, these structures may correspond to MVBs-like bodies. However, EhVps32 appeared poorly in late phagosomes and phagolysosomes that are formed after 30 min phagocytosis (Fig 4). MVBs possess an acidic pH (~5.5), and in humans, they mediate microtubule-dependent transport toward late endosomes [28]. However, in E. histolytica, suitable endosomal markers are not available and the existence of microtubules has been demonstrated only in dividing nuclei of trophozoites [49]. According to previous reports [50], MVBs-like structures could be also formed in trophozoites before erythrocytes digestion (Fig 4). Further studies are needed to deeply analyze putative MVBs in trophozoites and to precisely define the EhVps32 participation during formation of these structures.
In mammalian cells, an alternative ubiquitin-independent MVBs formation pathway has been reported, in which Alix and ESCRT-III proteins are involved [29]. In human, PAR1-activated receptor directly binds to Alix; then, Alix recruits CHMP4B and the rest of the ESCRT-III subunits, in an ubiquitin-independent manner [29]. This also could be happening in E. histolytica, where EhADH acts as an erythrocyte receptor by its adherence domain located at the carboxyl terminus, whereas by its Bro1 domain in the amino terminus, it recruits EhVps32 [2,13], as it has been experimentally proved by pull down experiments [16]. This interaction is also happening in resting conditions, probably it is due to constitutive endocytosis, as it has been seen in immunofluorescence and immunoprecipitation assays (Fig 2).
EhVps32 also participates during pinocytosis, co-localizing with EhADH and Gal/GalNac lectin, but not with actin (Fig 5). This is in agreement with many reports describing Vps32 participation in endocytosis [40] and with other reports indicating that actin is involved only in phagocytosis of E. histolytica [51].
Interestingly, EhVps32 participates in phagosomes formation since the beginning of the non-specific phagocytosis pathway, whereas EhADH participation appeared diminished there (S1 Fig). This gives further evidence of the presence of different mechanisms for cargo ingestion by trophozoites and distinct functions for both proteins in these pathways. Negatively stained preparations observed by TEM showed that rEhVps32 forms oligomers in vitro (Fig 6) that are similar to those formed by Vps32 protein in other cell types [47]. Recognition of these structures by αrEhVps32 antibodies, confirmed the capacity of EhVps32 to form oligomers. Size exclusion chromatography corroborated that EhVps32 purified protein forms oligomers. Thus, it is logical to assume that fluorescence due to αrEhVps32 antibodies, detected on phagosomes during phagocytosis, may correspond to EhVps32 oligomers in vivo. This assumption was strengthened by the differential arrays observed in trophozoites by confocal microscopy using αpEhVps32 and αrEhVps32 antibodies (Fig 6), that might specifically detected monomers and oligomers, respectively.
Confocal microscopy, TEM and SEM assays using pNeoEhvps32-HA transfected trophozoites evidenced the presence of tunnel-like and helicoidally arrays of different size (Figs 7 and 8). Some of these arrays are similar to those described for Vps32 and its homologues in other systems [46,47]. The filaments forming these structures (75–100 nm) appeared wider than the filaments formed in vitro by oligomerization of the purified rEhVps32 (10–75 nm). This may be explained because, in vivo, in addition to EhVps32, other proteins may be in these structures that were more abundant during phagocytosis, forming part of phagocytic cups and connecting phagosomes (Fig 7). Nakada-Tsukui, et al (2009) also visualized tunnel-like structures in E. histolytica trophozoites during phagocytosis using the PtdIns(3)P biomarker [3]. However, further studies are necessary to define the relationship of structures reported here with those reported by them.
An excess of EhVps32 in the cell and its permanent expression due to the presence of pNeoEhvps32-HA plasmid leads to an increase of membrane rings and filaments, whose fine structure was revealed by ultrastructural studies, in which EhVps32 was present (Fig 8). Differences in size of these arrays could be due to distinct stages of oligomerization. Growth in size and an increase in number of these structures may eventually lead to cell lysis, explaining why the pNeoEhvps32-HA transfection produced healthy trophozoites only until 72 h transfection. In non-transfected trophozoites, EhVps32 oligomerization may be controlled by the EhVps4 AAA ATPase activity [35], that in other eukaryotes catalyzes the dissociation of ESCRT-III components [27,28]. In pNeoEhvps32-HA transfected trophozoites, the excess of EhVps32 could alter the equilibrium between EhVps32 and EhVps3 AAA ATPase.
Although we cannot discard the presence of class E vacuoles formed by alterations in the phagocytosis process due to EVps32 overexpression, the membranous concentric structures found here do not seem to correspond to class E vacuoles. This assumption is based on: i) overexpression of EhVps32 promoted a higher rate of phagocytosis and did not abolish it, as it was the case for Bro1-truncated transfected trophozoites that resulted to be dominant negative mutants [16]. In these trophozoites, Bro-1 recruited important proteins for phagocytosis and leaded to the formation of class E vacuoles. ii) We did not detect empty phagosomes (without erythrocytes) stained by αrEhVps32 or αEhADH antibodies. iii) The novel structures described here appeared on erythrocytes-containing phagosomes of wild type and transfected trophozoites, although they are in smaller number in wild type trophozoites. v) These structures are very similar to those reported in other systems as ESCRT III structures involved in endocytosis [46]. vi) EhVps32-silenced G3 trophozoites exhibited a low rate of erythrophagocytosis. In addition to the low expression of EhVps32, the EhADH and actin re-localization and the morphological alterations (Fig 9), may explain this erythrophagocytosis activity.
A number of studies have identified at least 50 Vps genes and proteins in yeast and mammals. All they are involved in vesicle trafficking, forming complexes known as ESCRT, retromer, CORVET, HOPS, GARP and PI3K-III [52]. Except for a study where Nakada-Tsukui et al [53] have characterized a retromerlike complex formed by Vps26, Vps29 and Vps35, all them EhRab7A-binding proteins, little is known about the orthologues of Vps proteins in E. histolytica. Among proteins of ESCRT complex, only EhVps32 (in this paper, [16]), EhADH and Vps4 AAAtpase [16,35] have been studied. Therefore, it is relevant to characterize the Vps proteins in a unicellular organism with a very active membrane fusion and fission. Knowledge of Vps’s will provide a basis for understanding these events in E. histolytica. Learning more on the vesicular trafficking across species, starting with an antique protozoan parasite will supply a basis for further addressing specific roles of Vps, not only in E. histolytica, but also in other organisms.
In conclusion, EhVps32 is a vacuolar protein of the ESCRT-III complex that formed oligomers as it does its homologues in humans and yeast. Besides, this protein was involved in phagocytosis, interacted with EhADH in acidic vesicles, co-localized with Gal/GalNac lectin and actin and formed structures unveiled here. There are many reports on the role of Vps32 in endocytosis; however, this is the first study of the Vps32 role during phagocytosis in a unicellular eukaryotic organism, and its active participation in this event that is crucial for virulence expression of the parasite.
Materials and Methods
E. histolytica cultures
Trophozoites of E. histolytica (strain HM1:IMSS) clones A and G3 (kindly provided by Dr. David Mirelman, from Weizmann Institute of Science, Israel) were axenically cultured in TYI-S-33 medium at 37°C [45,54] and harvested in logarithmic growth phase. All experiments presented here were performed at least three times by duplicate. EhVps32-silenced G3 trophozoites were initially selected by adding 1 μg/ml of Neomycin (G418, Gibco) to the medium and then cultured in 7 μg/ml G418, before performing the experiments.
Generation of polyclonal antibodies against EhVps32
Escherichia coli BL21 (DE3) pLysS bacteria (Invitrogen) were transformed with the pGEX-5X-1-EhVps32 plasmid containing the full open reading frame of Ehvps32 gene to produce a GST-tagged EhVps32 recombinant protein (rEhVps32), which was purified as described [16]. rEhVps32 (50 or 150 μg for each animal, respectively) emulsified in Titer-Max Classic adjuvant (Sigma) was subcutaneously and intramuscularly inoculated into Balb/cJ male mice and into New Zealand male rabbits. Two more doses of rEhVps32 (25 or 100 μg for each animal, respectively) were injected at 20 days intervals and then, animals were bled to obtain αrEhVps32 antibodies; preimmune serum was obtained before immunization. Additionally, EEYDRKRMEMELEKAKEC polypeptide (27 to 44 residues in EhVps32) was synthesized together with KLH (Keyhole Limpet Hemocyanin) tag to increase the immunogenicity (GenScript). Rabbits were immunized with 100 μg of this polypeptide and then, they received two more immunizations (50 μg each) to generate αpEhVps32 antibodies.
Cell fractionation
Trophozoites (108) were harvested, washed twice with 19 mM potassium phosphate buffer, pH 7.2, and 0.27 M NaCl (PD solution). Cellular pellet was resuspended to 2 × 107 cells/mL in PD solution containing 10 mM MgCl2 and mixed with an equal volume of 1 mg/mL concanavalin A. After 5 min, cells were spun at 50 g for 1 min. The supernatant was discarded and cellular pellet was resuspended in 12 mL of 10 mM Tris-HCl buffer, pH 7.5, containing 2 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mM MgCl2. After 10 min swelling in hypotonic buffer, cells were homogenized by 20 strokes in a glass Dounce homogenizer with a tight-fitting pestle (Wheaton Scientific Div.). Cellular lysis and membrane sheets formation were verified by phase-contrast microscopy. The homogenate was layered over a two-step gradient consisting of 8 mL of 0.5 M mannitol over 4 mL of 0.58 M sucrose, both in Tris buffer, and spun at 250 ×g for 30 min. Material remaining at the top of 0.5 M mannitol was centrifuged at 40 000 ×g for 1 h to separate soluble molecules (cytoplasmic fraction) from small membrane fragments and vesicles (internal membranes). Large plasma membrane fragments and other heavy debris formed a tight pellet at the bottom of the gradient (crude membrane fraction). This pellet was resuspended in 1 mL Tris buffer containing 1 M α-methyl mannoside and left on ice for 40 min with occasional mixing. Plasma membranes free of concanavalin A were diluted into three volumes of Tris buffer, homogenized by 80 strokes with a glass Dounce homogenizer, layered on a 20% sucrose Tris gradient and spun for 30 min at 250 g. Vesiculated plasma membranes floating above the initial sucrose layer were collected and then concentrated by centrifugation at 40,000 g for 1 h. The pellet, enriched in plasma membranes, was resuspended in Tris buffer. All steps were performed at 4°C [55].
Western blot experiments
Trophozoites lysates (30 μg) or cytoplasmic or membrane fractions or internal membranes or plasmatic membranes obtained as described [2,55] were separated in 12% SDS-PAGE, transferred to nitrocellulose membranes and probed with mouse αrEhVps32 (1 : 500), rabbit αpEhVps32 (1 : 3 000), mouse αGal/GalNac lectin (kindly given by Dr. William A. Petri Jr, University of Virginia, USA) (1 : 100) or mouse αactin (1 : 2 000) antibodies. Then, membranes were incubated with the corresponding α-mouse or α-rabbit HRP-labeled secondary antibodies (Zymed; 1 : 10 000), respectively, and revealed with ECL Prime western blotting detection reagent (GE-Healthcare). For some experiments, αpEhVps32 antibodies were pre-incubated overnight (ON) with 100 μg of rEhVps32 purified protein or membranes were pre-incubated with αrEhVps32 antibodies before incubation with αpEhVps32 antibodies.
Laser confocal microscopy assays
Trophozoites were grown on coverslips, fixed with 4% paraformaldehyde (PFA) at 37°C for 1 h, permeabilized with 0.2% Triton X-100 or non-permeabilized, and blocked with 10% fetal bovine serum (FBS) in PBS. Then, cells were incubated with mouse αrEhVps32 (1 : 100) or rabbit αpEhVps32 (1 : 100) antibodies, at 37°C for 1 h, followed by incubation for 1 h with α-mouse or α-rabbit FITC-labeled secondary antibodies (Zymed; 1 : 100), respectively. For co-localization experiments, samples were incubated first with mouse αrEhVps32 and rabbit αEhADH (1 : 100) or rabbit αrEhVps32 (1 : 100) and mouse αGal/GalNac lectin (1 : 25), followed by the corresponding α-mouse FITC-labeled, α-rabbit FITC-labeled, α-mouse Pacific blue-labeled, α-rabbit TRITC-labeled, α-mouse TRITC-labeled and α-rabbit Cy5 (Zymed, 1 : 100) secondary antibodies. For some experiments, Rhodamine-phalloidin (Sigma, 1 : 100) was employed to detect actin. For co-localization with Lysotracker, live trophozoites were incubated ON with 2 μM Lysotracker red (Molecular Probes) and then, with mouse αrEhVps32 antibodies as described above. In some experiments, nuclei were counterstained with 2.5 μg/ml 4’, 6-diamidino-2-phenylindole (DAPI; Zymed) for 5 min. All preparations were preserved using Vectashield antifade reagent (Vector), examined through a Carl Zeiss LMS 700 confocal microscope and processed with ZEN 2009 Light Edition software (Zeiss). To quantify co-localization, 1 μm z-stacks of entire cells or an area around plasma membrane were analyzed using the Just Another Co-localization Plugin (JACoP) [56] in the Image J 1.48i software [57]. Each point represented an average of 12–25 cells and values are given as means ± standard error.
Phagocytosis and pinocytosis assays
Trophozoites were incubated with human erythrocytes (1 : 25 ratio) or with 2 μg/ml FITC-dextran (70 kDa, Sigma) for 5, 10, 30, 45 and 60 min at 37°C and then, processed for immunofluorescence assays as described above. For erythrophagocytosis assay, hemoglobin concentration was quantified by spectrophotometry at OD400 [50]. In parallel, samples of all interaction times were stained by 2 mg/ml 3,3’ diaminobenzidine (Sigma) [58]. For other experiments, the proportion of erythrocytes inside phagosomes (decorated by αEhVps32 or αEhADH antibodies or Lysotracker or FITC-microspheres) with relation to total ingested erythrocytes per trophozoite was evaluated in at least 20 confocal images. For non-specific phagocytosis assays, trophozoites were incubated with FITC-label latex microspheres (1 μm diameter; 1 : 100; Molecular Probes) at 37°C for different times and then, exhaustively washed and processed for immunofluorescence.
Immunoprecipitation assays
Trophozoites were lysed with 10 mM Tris-HCl, 50 mM NaCl and 100 mM protease inhibitors (PHMB, IA, NEM and TLCK), followed by cycles of freeze-thawing in liquid nitrogen and vortexing. In parallel, 200 μl of recombinant protein G-agarose (rProtein-G; Invitrogen) were incubated with 100 μg of rabbit αEhADH antibodies or preimmune serum for 2 h at 4° C, with gentle stirring. Then, rProtein-G beads were washed with 0.5% BSA in PBS, followed by additional washes with PBS for 5 min, under gentle stirring and centrifuged at 11,600 g for 2 min. Trophozoites lysates (1 mg) were pre-cleared with 200 μl of rProtein-G (previously blocked with 2% BSA) and incubated 2 h at 4°C under gentle stirring. Samples were centrifuged at 11, 600 g to obtain the supernatant that was added to rProtein-G previously incubated with antibodies. Preparations were incubated ON at 4° C and then, beads were recovered by centrifugation. After washes with PBS, 60 μl of 4 x sample buffer (40% glycerol, 240 mM Tris-HCl pH 6.8, 8% SDS, 0.04% bromophenol blue and 5% β-mercaptoethanol) were added. Samples were boiled for 3 min and centrifuged again at 11,600 g for 2 min at 4° C. Supernatant (30 μl) was loaded into 12% SDS–PAGE and subjected to western blot assays.
Transmission electron microscopy (TEM)
Samples were fixed with 2.5% (v/v) glutaraldehyde in 0.1M sodium cacodylate buffer, pH 7.2, for 60 min. Then, they were postfixed for 60 min with 1% (w/v) osmium tetroxide in the same buffer. After dehydration with increasing concentrations of ethanol and propylene oxide, samples were embedded in Polybed epoxy resins and polymerized at 60°C for 24 h. Thin sections (60 nm) were contrasted with uranyl acetate and lead citrate before being examined in a Joel JEM-1011 transmission electron microscope. For gold immunolabeling experiments, trophozoites were fixed with 4% PFA and 0.5% glutaraldehyde in PBS for 1 h at room temperature (RT). Samples were embedded in LR White resin (London Resin Co) and polymerized under UV at 4°C ON. Thin sections were incubated ON with mouse αrEhVps32 antibodies (1 : 20) and then, ON at RT with α-mouse IgGs antibodies conjugated to 20 nm gold particles (Ted Pella Inc; 1 : 60).
Negative staining
rEhVps32 was purified as described [16] and the GST-tag was removed using Factor Xa protease (GE-Healthcare), according to manufacturer’s instructions. rEhVps32 (2.5 μg in 5 μl) was pipetted onto the surface of the formvar - coated copper grids. After 5 min, samples were blotted off with filter paper and stained with 2.5% uranyl acetate. Grids were then left to air dry and carbon coated. In some experiments, samples were treated with αrEhVps32 antibodies, followed by gold-labeled secondary antibodies (30 nm). Preparations were examined through a JEM-1011 transmission electron microscope.
Scanning electron microscopy (SEM)
Glutaraldehyde fixed samples were dehydrated with increasing concentrations of ethanol and CO2 critically point dried in a Samdri apparatus. Then, they were gold coated in an ion sputtering device (Jeol-JFC-1100) and examined with a Jeol JSM-7100F field emission scanning electron microscope.
Gel filtration chromatography
Purified rEhVps32 without GST-tag was subjected to size exclusion chromatography using a gel filtration column (2.5 cm x 30 cm), packed with 45 ml of Sephacryl-HR 100 (GE Healthcare), previously equilibrated with buffer A (20 mM Tris-HCl pH 8.0, 100 mM NaCl and 1 mM EDTA) and resolved at a flow rate of 1 ml/min using a NGC Q10 chromatographic system (Bio-Rad). The column was equilibrated with gel filtration standards containing thyroglobulin (670 kDa), γ-globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa) and vitamin B12 (1.35 kDa) (Bio-Rad). The elution volume (Ve) of the ovalbumin, myoglobin and vitamin B12 were used to obtain the calibration curve by plotting the Log MW vs Kva. The elution volume of the first peak (thyroglobulin and γ-globulin) was taken as the void volume (Vo) to estimate Kva. Eluted fractions were separated by 12% SDS-PAGE and submitted to western blot assays, using αrEhVps32 antibodies.
Generation of pNeoEhvps32-HA transfected trophozoites
PCR-amplified Ehvps32 full gene with the hemagglutinin (HA) tag in the 3’ end were cloned into pJET1.2/blunt plasmid (Fermentas), accordingly to manufacturer’s instructions. Then, Ehvps32-HA gene was subcloned into BamHI and KpnI sites of pExEhNeo (pNeo) plasmid [59], producing the pNeoEhvps32-HA construct. E. coli DH5α bacteria were transformed with pNeoEhvps32-HA or pNeo plasmids. Plasmids were purified using Qiagen Midi kit (Qiagen) and automatically sequenced. To perform transfection, 3 x 105 trophozoites were cultivated ON with 5% CO2, then, washed with M199 medium (Sigma) and incubated with M199 medium supplemented with 15% FBS. Subsequently, transfection mix (20 μg of plasmid, 20 μl of Superfect [Qiagen] in 100 μl of M199 medium) was added and incubated 10 min at RT. Trophozoites were cultivated for 3 h at 37°C and 5% CO2. Finally, cells were cooled and transferred to a tube with 10 ml of TYI pre-warmed medium and cultivated for 12 h at 37°C.
Generation of EhVps32-silenced trophozoites
The first 431 bp from the 5' end of the Ehvps32 gene were PCR-amplified and cloned into pJET1.2/blunt plasmid and then, subcloned into pSAP2/Gunma plasmid, downstream of the 5' upstream segment of the EhAp-A gene [45], using a 5' StuI site and a 3' SacI site with the following primers: forward, 5'-AGCTAGGCCTATGTCTTGGTTCAGAAGAAATACT-3'; reverse, 5'-GCATGAGCTCATGTCTTGTAAATCTTCACCTAAA-3' (restriction sites are underlined). Trophozoites of G3 clone were transfected with pSAP2/GunmaEhVps32-431 plasmid, using the Superfect-based method as stated above.
Statistical analysis
Statistically analyses were performed by t-Student test, using GraphPad Prism 5.0 software. The scores showing statistically significant differences are indicated with asterisks in the graphs. The corresponding p values are indicated in the figure legends.
Accession numbers
EhVps32 (C4M1A5/EHI_169820), EhVps2 (C4LZV3/EHI_194400), EhVps20 (C4MAC7/EHI_066730), EhVps22 (C4LXI0/EHI_131120), EhVps23 (C4LUR9/EHI_135460), EhVps24 (C4M2Y2/EHI_048690), EhVps26 (Q53UB0/EHI_137860), EhVps27 (C4LYX5/EHI_117910), EhVps29 (Q9BI08/EHI_025270) EhVps32 (C4M1A5/EHI_169820), EhVps35 (Q6Y0Y5/EHI_002990), EhVps36 (CALTE5/EHI_045320), EhVps37A (C4MAH4/EHI_077870), EhVps37D (C4M6J5/EHI_060400), EhHse1 (C4M9E1/EHI_091530), EhVta1 (C4M0R8/EHI_010040), EhADH112 (Q9U7F6/EHI_181220), EhCP112 (Q9U7F7/EHI_181230), Gal/Gal lectin (C4LTM0/EHI_012270), EhC2PK (C4M3C4/EHI_053060), EhCaBP1 (C4M7Q6/EHI_120900), EhAK1 (C4M9G9/EHI_105830).
Supporting Information
Zdroje
1. Mortimer L, Chadee K (2010) The immunopathogenesis of Entamoeba histolytica. Exp Parasitol 126 : 366–380. doi: 10.1016/j.exppara.2010.03.005 20303955
2. García-Rivera G, Rodríguez MA, Ocádiz R, Martínez-López MC, Arroyo R, et al. (1999) Entamoeba histolytica: a novel cysteine protease and an adhesin form the 112 kDa surface protein. Molecular Microbiology 33 : 556–568. 10417646
3. Nakada-Tsukui K, Okada H, Mitra BN, Nozaki T (2009) Phosphatidylinositol-phosphates mediate cytoskeletal reorganization during phagocytosis via a unique modular protein consisting of RhoGEF/DH and FYVE domains in the parasitic protozoon Entamoeba histolytica. Cell Microbiol 11 : 1471–1491. doi: 10.1111/j.1462-5822.2009.01341.x 19496789
4. Mansuri MS, Bhattacharya S, Bhattacharya A (2014) A novel alpha kinase EhAK1 phosphorylates actin and regulates phagocytosis in Entamoeba histolytica. PLoS Pathog 10: e1004411. doi: 10.1371/journal.ppat.1004411 25299184
5. Seigneur M, Mounier J, Prevost MC, Guillen N (2005) A lysine - and glutamic acid-rich protein, KERP1, from Entamoeba histolytica binds to human enterocytes. Cell Microbiol 7 : 569–579. 15760457
6. Santi-Rocca J, Weber C, Guigon G, Sismeiro O, Coppee JY, et al. (2008) The lysine - and glutamic acid-rich protein KERP1 plays a role in Entamoeba histolytica liver abscess pathogenesis. Cell Microbiol 10 : 202–217. 17711481
7. Vishwakarma RA, Anand MT, Arya R, Vats D, Bhattacharya A (2006) Glycosylated inositol phospholipid from Entamoeba histolytica: identification and structural characterization. Mol Biochem Parasitol 145 : 121–124. 16242191
8. Teixeira JE, Huston CD (2008) Participation of the serine-rich Entamoeba histolytica protein in amebic phagocytosis of apoptotic host cells. Infect Immun 76 : 959–966. 18086807
9. Laughlin RC, McGugan GC, Powell RR, Welter BH, Temesvari LA (2004) Involvement of raft-like plasma membrane domains of Entamoeba histolytica in pinocytosis and adhesion. Infect Immun 72 : 5349–5357. 15322032
10. Petri WA Jr., Haque R, Mann BJ (2002) The bittersweet interface of parasite and host: lectin-carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu Rev Microbiol 56 : 39–64. 12142490
11. Somlata, Bhattacharya S, Bhattacharya A (2011) A C2 domain protein kinase initiates phagocytosis in the protozoan parasite Entamoeba histolytica. Nat Commun 2 : 230. doi: 10.1038/ncomms1199 21407196
12. Sahoo N, Labruyere E, Bhattacharya S, Sen P, Guillen N, et al. (2004) Calcium binding protein 1 of the protozoan parasite Entamoeba histolytica interacts with actin and is involved in cytoskeleton dynamics. J Cell Sci 117 : 3625–3634. 15252130
13. Bañuelos C, García-Rivera G, Mendoza L, González-Robles A, López-Reyes I, et al. (2011) EhADH is a Bro1 domain-containing protein involved in the Entamoeba histolytica endocytic pathway. Journal of Biochemistry & Cell.
14. Bañuelos C, García-Rivera G, López-Reyes I, Orozco E (2005) Functional characterization of EhADH112: An Entamoeba histolytica Bro1 domain-containing protein. Exp Parasitol 110 : 292–297. 15955327
15. Odorizzi G, Katzmann DJ, Babst M, Audhya A, Emr SD (2003) Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J Cell Sci 116 : 1893–1903. 12668726
16. Banuelos C, Garcia-Rivera G, Lopez-Reyes I, Mendoza L, Gonzalez-Robles A, et al. (2012) EhADH112 is a Bro1 domain-containing protein involved in the Entamoeba histolytica multivesicular bodies pathway. J Biomed Biotechnol 2012 : 657942. doi: 10.1155/2012/657942 22500103
17. Rothman JH, Howald I, Stevens TH (1989) Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. EMBO J 8 : 2057–2065. 2676511
18. Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, et al. (2007) Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J 26 : 4215–4227. 17853893
19. Guizetti J, Schermelleh L, Mantler J, Maar S, Poser I, et al. (2011) Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 331 : 1616–1620. doi: 10.1126/science.1201847 21310966
20. Rusten TE, Vaccari T, Stenmark H (2011) Shaping development with ESCRTs. Nat Cell Biol 14 : 38–45. doi: 10.1038/ncb2381 22193162
21. Fairn GD, Grinstein S (2012) How nascent phagosomes mature to become phagolysosomes. Trends Immunol 33 : 397–405. doi: 10.1016/j.it.2012.03.003 22560866
22. Dunn KW, McGraw TE, Maxfield FR (1989) Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol 109 : 3303–3314. 2600137
23. Piper RC, Luzio JP (2001) Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2 : 612–621. 11555415
24. Gruenberg J, Griffiths G, Howell KE (1989) Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol 108 : 1301–1316. 2538480
25. Curtiss M, Jones C, Babst M (2007) Efficient cargo sorting by ESCRT-I and the subsequent release of ESCRT-I from multivesicular bodies requires the subunit Mvb12. Mol Biol Cell 18 : 636–645. 17135292
26. Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD (2002) Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell 3 : 283–289. 12194858
27. Obita T, Saksena S, Ghazi-Tabatabai S, Gill DJ, Perisic O, et al. (2007) Structural basis for selective recognition of ESCRT-III by the AAA ATPase Vps4. Nature 449 : 735–739. 17928861
28. Lata S, Schoehn G, Solomons J, Pires R, Gottlinger HG, et al. (2009) Structure and function of ESCRT-III. Biochem Soc Trans 37 : 156–160. doi: 10.1042/BST0370156 19143622
29. Dores MR, Chen B, Lin H, Soh UJ, Paing MM, et al. (2012) ALIX binds a YPX(3)L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. J Cell Biol 197 : 407–419. doi: 10.1083/jcb.201110031 22547407
30. Winter V, Hauser MT (2006) Exploring the ESCRTing machinery in eukaryotes. Trends Plant Sci 11 : 115–123. 16488176
31. Teis D, Saksena S, Emr SD (2008) Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev Cell 15 : 578–589. doi: 10.1016/j.devcel.2008.08.013 18854142
32. Katoh K, Shibata H, Hatta K, Maki M (2004) CHMP4b is a major binding partner of the ALG-2-interacting protein Alix among the three CHMP4 isoforms. Arch Biochem Biophys 421 : 159–165. 14678797
33. Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD (2002) Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell 3 : 271–282. 12194857
34. López-Reyes I, Bañuelos C, Betanzos A, Orozco E (2011) A bioinformatical approach to study the endosomal sorting complex required for transport (ESCRT) machinery in protozoan parasites: the Entamoeba histolytica case. In: Mahdavi MA, editor. Bioinformatics. Second ed. Rijeka, Croatia: Intech Open Access Publisher. pp. 289–312.
35. López-Reyes I, García-Rivera G, Bañuelos C, Herranz S, Vincent O, et al. (2010) Detection of the Endosomal Sorting Complex Required for Transport in Entamoeba histolytica and Characterization of the EhVps4 Protein. J Biomed Biotechnol 2010 : 890674. doi: 10.1155/2010/890674 20508821
36. Petri WA Jr., Jackson TF, Gathiram V, Kress K, Saffer LD, et al. (1990) Pathogenic and nonpathogenic strains of Entamoeba histolytica can be differentiated by monoclonal antibodies to the galactose-specific adherence lectin. Infect Immun 58 : 1802–1806. 1692809
37. Marion S, Laurent C, Guillen N (2005) Signalization and cytoskeleton activity through myosin IB during the early steps of phagocytosis in Entamoeba histolytica: a proteomic approach. Cell Microbiol 7 : 1504–1518. 16153248
38. Arroyo R, Orozco E (1987) Localization and identification of an Entamoeba histolytica adhesin. Mol Biochem Parasitol 23 : 151–158. 2883572
39. Scott CC, Vacca F, Gruenberg J (2014) Endosome maturation, transport and functions. Semin Cell Dev Biol 31C: 2–10.
40. Weiss P, Huppert S, Kolling R (2009) Analysis of the dual function of the ESCRT-III protein Snf7 in endocytic trafficking and in gene expression. Biochem J 424 : 89–97. doi: 10.1042/BJ20090957 19725809
41. Henne WM, Buchkovich NJ, Zhao Y, Emr SD (2012) The endosomal sorting complex ESCRT-II mediates the assembly and architecture of ESCRT-III helices. Cell 151 : 356–371. doi: 10.1016/j.cell.2012.08.039 23063125
42. Shim S, Kimpler LA, Hanson PI (2007) Structure/function analysis of four core ESCRT-III proteins reveals common regulatory role for extreme C-terminal domain. Traffic 8 : 1068–1079. 17547705
43. Henne WM, Stenmark H, Emr SD (2013) Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol 5.
44. Bajorek M, Schubert HL, McCullough J, Langelier C, Eckert DM, et al. (2009) Structural basis for ESCRT-III protein autoinhibition. Nat Struct Mol Biol 16 : 754–762. doi: 10.1038/nsmb.1621 19525971
45. Bracha R, Nuchamowitz Y, Mirelman D (2003) Transcriptional silencing of an amoebapore gene in Entamoeba histolytica: molecular analysis and effect on pathogenicity. Eukaryot Cell 2 : 295–305. 12684379
46. Shen QT, Schuh AL, Zheng Y, Quinney K, Wang L, et al. (2014) Structural analysis and modeling reveals new mechanisms governing ESCRT-III spiral filament assembly. J Cell Biol 206 : 763–777. doi: 10.1083/jcb.201403108 25202029
47. Hanson PI, Roth R, Lin Y, Heuser JE (2008) Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J Cell Biol 180 : 389–402. doi: 10.1083/jcb.200707031 18209100
48. Okada M, Huston CD, Oue M, Mann BJ, Petri WA Jr., et al. (2006) Kinetics and strain variation of phagosome proteins of Entamoeba histolytica by proteomic analysis. Mol Biochem Parasitol 145 : 171–183. 16290089
49. Orozco E, Solis FJ, Dominguez J, Chavez B, Hernandez F (1988) Entamoeba histolytica: cell cycle and nuclear division. Exp Parasitol 67 : 85–95. 2901981
50. Mora-Galindo J, Gutierrez-Lozano M, Anaya-Velazquez F (1997) Entamoeba histolytica: kinetics of hemolytic activity, erythrophagocytosis and digestion of erythrocytes. Arch Med Res 28 Spec No: 200–201. 9033071
51. Labruyere E, Guillen N (2006) Host tissue invasion by Entamoeba histolytica is powered by motility and phagocytosis. Arch Med Res 37 : 253–258. 16380326
52. Li Z, Blissard G (2014) The vacuolar protein sorting genes in insects: A comparative genome view. Insect Biochem Mol Biol.
53. Nakada-Tsukui K, Saito-Nakano Y, Ali V, Nozaki T (2005) A retromerlike complex is a novel Rab7 effector that is involved in the transport of the virulence factor cysteine protease in the enteric protozoan parasite Entamoeba histolytica. Mol Biol Cell 16 : 5294–5303. 16120649
54. Diamond LS, Harlow DR, Cunnick CC (1978) A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg 72 : 431–432. 212851
55. Aley SB, Scott WA, Cohn ZA (1980) Plasma membrane of Entamoeba histolytica. J Exp Med 152 : 391–404. 6249883
56. Bolte S, Cordelieres FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224 : 213–232. 17210054
57. Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9 : 671–675. 22930834
58. Vacca LL, Hewett D, Woodson G (1978) A comparison of methods using diaminobenzidine (DAB) to localize peroxidases in erythrocytes, neutrophils, and peroxidase-antiperoxidase complex. Stain Technol 53 : 331–336. 89720
59. Hamann L, Nickel R, Tannich E (1995) Transfection and continuous expression of heterologous genes in the protozoan parasite Entamoeba histolytica. Proc Natl Acad Sci U S A 92 : 8975–8979. 7568055
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



