-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
Within mosquito populations, genetic differences between individuals affect their ability to transmit human diseases such as malaria, dengue fever, and lymphatic filariasis. In the mosquito Aedes aegypti, some individuals are genetically resistant to Brugia malayi, a mosquito-vectored parasite that causes a debilitating tropical disease called lymphatic filariasis. To characterize the genetic basis of resistance, we identified resistant and susceptible mosquitoes from a wild Kenyan population, and sequenced the protein-coding region of their genomes (the exome). This allowed us to locate a single region of the mosquito genome that is causing resistance and to identify genes that may be controlling the trait. To understand the mechanisms of resistance, we measured gene expression. The susceptible mosquitoes have reduced expression of immunity genes after they are infected with B. malayi, including genes known to kill this group of parasites. This is possibly because their immune response is being suppressed by the parasites. We conclude that resistance is controlled by a single locus and show that resistance results in an increased immune response.
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004765
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004765Summary
Within mosquito populations, genetic differences between individuals affect their ability to transmit human diseases such as malaria, dengue fever, and lymphatic filariasis. In the mosquito Aedes aegypti, some individuals are genetically resistant to Brugia malayi, a mosquito-vectored parasite that causes a debilitating tropical disease called lymphatic filariasis. To characterize the genetic basis of resistance, we identified resistant and susceptible mosquitoes from a wild Kenyan population, and sequenced the protein-coding region of their genomes (the exome). This allowed us to locate a single region of the mosquito genome that is causing resistance and to identify genes that may be controlling the trait. To understand the mechanisms of resistance, we measured gene expression. The susceptible mosquitoes have reduced expression of immunity genes after they are infected with B. malayi, including genes known to kill this group of parasites. This is possibly because their immune response is being suppressed by the parasites. We conclude that resistance is controlled by a single locus and show that resistance results in an increased immune response.
Introduction
The rate at which parasites are transmitted by mosquitoes is an important determinant of the prevalence of vector-borne diseases in human populations. Alongside factors like the number of mosquitoes and their biting preferences, the rate of transmission depends on the ability of mosquitoes to acquire the parasite when feeding on an infected person and subsequently transmit it. This is referred to as their vector competence, and is affected by both environmental and genetic factors [1,2]. Even within a population of a single mosquito species there can be tremendous genetic variation in vector competence, often as a result of differences in the immune response of the mosquito to the parasites they are vectoring [2]. For example, variation exists in susceptibility of Anopheles gambiae to the malaria parasite Plasmodium falciparum [3,4] and in Aedes aegypti to dengue and filarial nematodes [5,6]. This has attracted much attention as it could one day lead to be better disease control by manipulating mosquito populations to reduce vector competence. For example, field trials are underway that are releasing Ae. aegypti mosquitoes carrying the bacterial symbiont Wolbachia, which reduces the mosquitoes’ ability to transmit dengue virus [7].
The tropical disease lymphatic filariasis, or elephantiasis, is a leading cause of morbidity and disability worldwide, with especially high parasite burdens in Africa and south and south-east Asia [8]. It is estimated to affect 120 million people worldwide, and symptoms include lymphedema and swelling of the extremities [9]. In humans, the disease is caused by the filarial nematodes Wuchereria bancrofti, Brugia malayi and Brugia timori, and is vectored by a range of mosquitoes, including species of Culex, Mansonia, Anopheles and Aedes [9]. W. bancrofti is the major cause of filariasis worldwide, leading to 90% of the cases of lymphatic filariasis, and Brugia species, which are only found in Asia, cause the remaining 10% [8]. B. malayi is the main laboratory model for studying lymphatic filariasis, and it grows readily in some strains of the mosquito Ae. aegypti. Despite having overlapping ranges, Ae. aegypti does not naturally vector any of the nematodes that cause lymphatic filariasis in humans. It is however a natural vector of Dirofilaria, which causes filariasis in dogs [10].
B. malayi, along with other filarial nematodes, are heteroxenous, requiring both a vertebrate host and a mosquito vector for their life cycle [8,9]. Humans, cats, and monkeys can all serve as vertebrate hosts for B. malayi [10]. Male and female worms reproduce sexually in the vertebrate, producing microfilariae which circulate in the bloodstream and are ingested by mosquitoes during blood feeding. After penetrating the mosquito midgut, the filarial nematodes develop inside various tissues within the mosquito. In the case of B. malayi, the microfilariae migrate to the thoracic muscles of the mosquito, where they undergo successive molts until they become L3 larvae [11]. They then migrate to the mosquito proboscis, where they are transferred to the vertebrate host during blood feeding.
Beginning in the 1960’s, mosquito strains and species have been identified that are naturally refractory (resistant) to infection by filarial nematodes [12]. Proposed mechanisms of resistance include reduced ingestion of parasites, physical killing of parasites in the foregut, barriers to penetration of the midgut, and hemolymph factors that kill the parasite in the thoracic cavity and lead to melanotic encapsulation [13]. Some species such as Armigeres subalbatus, a natural vector of Brugia pahangi, are completely refractory to infection by B. malayi while being highly susceptible to B. pahangi [14]. Others, such as Ae. aegypti, are polymorphic within species for resistance [15]. In laboratory lines of Ae. aegypti, genetic variation in resistance to B. malayi has a simple genetic basis, and is primarily determined by a single dominant locus on the first chromosome [16]. This genetic resistance extends to some other species of nematodes, such as B. pahangi and W. bancrofti, but not to Dirofilaria, for which Ae. aegypti is a natural vector [16]. In this mosquito, sex is also determined by a region on the first chromosome, and the resistance locus is tightly linked to the sex-determining region [17–19].
The immune responses of Ae. aegypti have been extensively studied, but it remains unknown which factors are important in killing filarial nematodes and whether genetic differences in susceptibility are caused by differences in immune responses. Despite the mechanisms being unclear, the mosquito immune response does appear to control filarial nematodes. Fewer parasites reached the L3 stage when the immune system was upregulated by inoculating mosquitoes with bacteria before they fed on blood carrying microfilariae [20]. Similarly, parasite numbers were reduced when the mosquito was infected with the bacterium Wolbachia, which also upregulated the immune response [21]. Anti-microbial peptide (AMP) production may be responsible for these effects as cecropin negatively affects worm motility [22]. However, activation of the two main immune signaling pathways, Toll and IMD, by RNAi depletion of their negative regulators, Cactus and Caspar, produced no measurable effect on resistance to B. malayi [11].
Genetic mapping of parasite resistance in mosquitoes has so far been done by individually testing markers [3,6,18] or with high-density genotyping using SNP arrays or RAD-sequencing [19,23]. These approaches often utilize randomly selected markers sparsely interspersed in the genome and rely on markers being in linkage disequilibrium with the causative polymorphism, which itself is unlikely to be sampled. In species like An. gambiae, linkage disequilibrium extends very short distances in wild populations [24], and it is preferable to concentrate efforts on regions that are likely to be involved in the trait of interest. In humans, the solution has been to use exome capture to sequence only protein-coding regions of the genome, which has been met with much success in identifying the mutations that cause Mendelian diseases [25]. This is especially desirable in species like humans and Ae. aegypti, where the large and repetitive genomes mean that whole genome sequencing is prohibitively costly and that much of the non-coding sequence cannot be investigated because relatively short sequence reads cannot be uniquely mapped to the genome.
We have investigated the genetic and mechanistic basis of resistance to B. malayi in Ae. aegypti using a combination of genomic and transcriptomic approaches. First, we resequenced the exome using probes we designed for Ae. aegypti and performed an association study to map the locus causing resistance with unprecedented precision. Using RNA-seq, we then measured gene expression in resistant and susceptible genotypes of the mosquito to understand how this locus alters the transcriptional response to filarial nematode infection. To minimize the contribution of random genetic differences between the resistant and susceptible lines, we performed genetic crosses to isolate the resistance locus in a common genetic background. This allowed us to identify differences in immune and non-immune response gene expression that will facilitate our understanding of mechanisms of resistance.
Materials and Methods
Mosquito Strains and Rearing
A wild outcrossed population was established for association mapping. Mosquito eggs were collected in July 2010 from a 120 km stretch between Kilifi, Malindi, and Mombasa in coastal Kenya using oviposition traps [26]. Each trap consisted of a black plastic cup, hay infused water (4 g dried grass in 1 L of water for 4 days) and a strip of creped cardboard paper. Eggs from each collection site (median of 42 eggs/trap with 1–16 traps used per collection site) were hatched in the laboratory and reared separately. Strains were established from two collection sites near Kilifi (St. Thomas and Mabarikani) and one site each near Malindi (Muthangani) and Mombasa (Mtwapa). At the F2 generation all strains were reciprocally crossed to each other and to themselves, with similar numbers of males and females in each group. Fifteen males and fifteen females from each cross (480 individuals total) were used to start an outcrossed population, where they were allowed to mate randomly for six generations. Each generation was maintained at a minimum population size of 900 adults and was not allowed to overlap with the previous generation.
We measured the effect of the resistance locus on gene expression by taking advantage of sex linkage to generate susceptible and resistant mosquitoes that are genetically equivalent across most of their genome. Resistance has previously been mapped to approximately 4-21cM from the sex-determination locus [17,19] and is dominant in action. The Liverpool IB12 (LVP-IB12R) strain of Ae. aegypti is a highly inbred line that was used for the genome sequencing project [27] and was previously found to be resistant to infection[19]. It is derived from the Liverpool strain which has been maintained in culture since 1936 and was originally collected from West Africa [12]. A strain of Liverpool susceptible to infection by B. malayi (LVP-FR3S) [19] was obtained from the NIAID/NIH Filariasis Research Reagent Resource Center (FR3, Atlanta, Georgia, USA). We refer to the strains as LVPR or LVPS from this point on. To obtain resistant progeny, we crossed LVPR virgin females to LVPS males and backcrossed F1 males to LVPS virgin females. To obtain susceptible progeny, we crossed LVPS virgin females to LVPR males and backcrossed F1 males to LVPS virgin females.
All mosquitoes were reared at a larval density of 200 individuals in 1.8 L of water. They were fed liver powder as larvae and 10% w/v fructose with 0.1% para-aminobenzoic acid (PABA) as adults and kept at 28°C (± 1°C) with 75% (±5%) humidity and a 12 hour light:dark cycle. Females were blood fed using an artificial membrane feeder (Hemotek Limited, UK) with donated human blood obtained from Blood Transfusion Services at Addenbrooke’s Hospital, Cambridge, UK. The temperature of the blood was maintained at 37°C in the feeders.
Infections and Phenotyping
To infect mosquitoes for association mapping, B. malayi was obtained from Darren Cook and Mark Taylor at the Liverpool School of Tropical Medicine (LSTM), where they were reared in gerbils. Microfilariae were harvested into RPMI medium, which was then centrifuged at 700 rpm for 5 minutes and 0.5 mL of the pellet was transferred to 40 mL of blood. Microfilariae were incubated in the blood at 37°C for at least one hour prior to feeding. Outcrossed and control LVPS mosquitoes were fed on blood containing parasites at a concentration of 457 microfilariae per 20 μl of blood. Female mosquitoes were 6 to 9 days old on the day of infection. Unfed mosquitoes were discarded, and infected mosquitoes were maintained on a 10% fructose solution with 0.1% PABA for 10–11 days post-infection. To check for infection, individual mosquitoes were separated at the head and thorax at 10 or 11 days after infection and incubated in 100 μl of 1X phosphate buffered saline (PBS) for one hour at 37°C. We found this caused L3 larvae to migrate into the PBS and gave similar estimates of infection as individually dissecting mosquitoes. The supernatant was transferred to a microscope slide, the number of L3 parasites was counted, and the mosquito carcasses were stored at -80°C until DNA extraction could be performed. Mosquitoes were classified as susceptible to infection if they had one or more L3 parasites and were classified as resistant if they had none.
For measuring gene expression, resistant and susceptible progeny from the crosses described in the previous section were collected from the following treatments: immediately prior to blood feeding and 12 and 48 hours post-feeding with either a control blood meal or a blood meal containing microfilariae. Microfilariae were harvested into 50 mL RPMI medium and incubated overnight with 0.5 mL gentamicin (10 mg/ml in water) at 28°C, and 0.5 mL of the pellet formed overnight was transferred to 16 mL of blood. The infective blood meal contained 160 microfilariae per 20 ul of blood. A non-infective control of 50 mL RPMI with 0.5 mL gentamicin was also incubated in the same manner, and 0.5 mL of solution was transferred to 16 mL of blood. Both blood vials were then incubated at 37°C for at least one hour prior to feeding. Female mosquitoes were 4 to 8 days old on the day of blood feeding. Three to four replicate cages were maintained for each treatment and all time points were collected from the same cages. After blood feeding, mosquitoes were maintained in paper cups in groups of 8 individuals and were given 10% fructose with 0.1% PABA after collection of the 12 hour time point. We dissected five individual mosquitoes of each genotype at 24, 48, and 72 hours after infection to follow the progression of B. malayi development in resistant versus susceptible mosquitoes. Pools of 8 individuals for each treatment were snap frozen at each time point and stored at -80°C prior to RNA extraction.
Library Preparation and Sequencing
DNA was extracted from single mosquitoes using QiaAmp MicroDNA kit (Qiagen) with the following modifications. Tissues were incubated with RNAse post-homogenization and no carrier RNA was used. DNA was eluted in 50 μl AE buffer and 1 μl of eluate was quantified with a Qubit 2.0 fluorimeter (Invitrogen). Total RNA was extracted using Trizol (Invitrogen) and was treated with Turbo DNAse (Ambion) prior to library preparation. RNA integrity was assessed using a Bioanalyzer (Agilent).
We sequenced the exomes of individual mosquitoes. DNA sequencing libraries were made using TruSeq DNA Sample Preparation kits (Illumina). Genomic DNA (600ng to 1ug of starting material) was sheared to 500bp fragment sizes via sonication, and libraries were prepared following the instructions from the manufacturer. Exome capture was then performed to enrich for coding sequences using custom SeqCap EZ Developer probes (Nimblegen). Overlapping probes covering the protein coding sequence (not including UTRs) in the AaegL1.3 gene annotations [27] were produced by Nimblegen based on exonic coordinates specified by us. In total, 26.7Mb of the genome (2%) was targeted for enrichment. Exome capture coordinates are available at https://www.jiggins.gen.cam.ac.uk/data/Aaegypti_exome.bed. Captures were performed on pools of 24 uniquely barcoded individuals, and the target enriched libraries were sequenced with either 100bp paired-end HiSeq2000 or 150bp paired-end MiSeq (see S1 Table). Library preparation, exome capture, and sequencing were performed by the High-Throughput Genomics Group at the Wellcome Trust Centre for Human Genetics (Oxford, UK).
In addition to the exome sequencing, we also produced low coverage whole genome sequences from some mosquitoes (these were largely different individuals but were from the same experiment, see S1 Table). For production of these libraries, DNA was sheared and PCR adapters were added in a single transposase mediated ligation step using the Nextera Library kit (Illumina). Fifty ng of genomic DNA was used per individual and libraries were prepared following the instructions from the manufacturer. Libraries were pooled in groups of 21–25 uniquely barcoded individuals and sequenced with 100bp paired-end HiSeq2000 by the Biosciences Core Laboratory at King Abdullah University of Science of Technology (KAUST) (Thuwal, Saudi Arabia).
RNA sequencing libraries were made using the TruSeq RNA Sample Preparation kit version 1 (Illumina) starting with 3 ug of total RNA per library. Libraries from different treatments were balanced between lanes (see S2 Table), pooled in groups of 8–10 libraries per lane, and sequenced with four lanes of 100 bp paired-end HiSeq2000 by the Eastern Sequence and Informatics Hub (EASIH) (Addenbrooke’s, Cambridge, UK).
Association Study
Sequences from DNA sequencing libraries were quality trimmed from the 3’ end using Trimmomatic version 0.30 [28] when average quality scores in sliding windows of 4 base pairs dropped below 20 or when the quality score at the end of the read dropped below 20. Sequences less than 50 base pairs in length and unpaired reads were discarded. Sequences were aligned to the reference genome (AaegL1, Oct 2005) [27] with BWA version 0.6.1-r104 [29] using the default parameters. Alignments for individuals sequenced across different lanes were merged into single BAM files using Picard version 1.93. Alignments were sorted, indexed, and assigned read groups using SAMtools version 0.1.18 [30] and Picard. Indels were realigned using GATK version 2.3 [31], and PCR and optical duplicates were removed using Picard. We have deposited the raw sequencing reads to the Short Read Archive with Accession Number SRP044393.
We performed association mapping using a combination of high and low coverage sequences. Average exome coverage from whole genome sequenced libraries was 0.73X per sample while average exome coverage from exome captured libraries was 32X for HiSeq sequencing and 2.3X for MiSeq sequencing. For this reason, we tested for associations with infection status using genotype posterior probabilities, which incorporate uncertainty in genotype calls, rather than calling individual genotypes prior to mapping [32] using the doAsso function in ANGSD version 0.539 [33]. BAM files were used as input for ANGSD. All SNPs called with a LRT statistic greater than 24 (P<10–6) were tested for association with susceptibility to Brugia. Only bases with a minimum base quality greater than 20 and only reads that were uniquely mapped and with a mapping quality greater than 20 were included. Major and minor alleles were inferred from genotype likelihoods using the genotype likelihood model implemented in SAMtools [34], and allele frequencies were estimated assuming a known minor allele using an EM algorithm [35].
Associations were tested under an additive model with logistic regression, a dominance model or a recessive model. The dominance and recessive models test for associations with infection status assuming the minor allele is dominant or recessive respectively. In addition, the additive model was reimplemented setting the most significant marker from the original test as a covariate (supercont1.398, position 175496) to test for the presence of a second locus. Only individuals with full genotypic information at this SNP with a posterior probability of 0.7 were included (73 of 140 individuals), and the covariate was coded under the dominant model. At least 15 individuals were required to have each genotypic class for the additive, dominance, and covariate models, and at least 10 individuals were required to have each genotypic class for the recessive model. To obtain a genome-wide significance threshold for each model that is corrected for multiple tests we permuted the phenotypes and repeated the analysis 200 times, each time retaining the lowest P-value across all variants to generate a null distribution. This was used to set a genome-wide significance cutoff of P<0.01 and P<0.05. We also tested whether any indels were associated with resistance. ANGSD can only test SNPs for associations directly from BAM files, so we provided indel genotype probabilities, which are used in an intermediate step in ANGSD, to test for associations. Genotype probabilities for indels were produced using GATK’s UnifiedGenotyper and ProduceBeagleInput. Only the additive model was tested using this method, and significance was assessed by permutation as described for SNPs. The variant effect predictor [36] was used to assign variants to genes and classify their effects (non-synonymous, synonymous, etc) using gene annotation set AaegL1.3.
We found that multiple SNPs in linkage disequilibrium were associated with resistance, so we excluded variants that explained the infection data significantly less well than our top hit (supercont1.398, position 175496). Using only the HiSeq exome sequenced individuals, we fitted a generalized linear model with a logit link function, where the response was the probability of a mosquito being infected with an L3 worm, and the predictor variables were the ‘top hit’ SNP and the SNP in question. A SNP was rejected if it was not significant but the ‘top hit’ SNP was.
RNA-seq Analysis
Sequences from RNA sequencing libraries were quality trimmed using the same method as used for DNA sequencing libraries, except that sequences less than 25 base pairs in length were discarded. An average of 42 million paired-end reads were obtained from each of the 36 libraries (S2 Table). Reads were aligned to predicted transcripts in the Ae. aegypti transcriptome (gene annotation set AaegL2.0) with Bowtie2 version 2.1.0 [37] using TopHat2 version 2.0.9 [38] with 10 mismatches allowed, read gap length and read edit distance set to 5, and no novel junctions allowed. Reads were mapped to the B. malayi genome (Ensembl version 3.0.19) [39] using TopHat2 as described above, but gene expression was not analyzed further due to low coverage. We have deposited the raw sequencing reads to the Short Read Archive with Accession Number SRP044393.
Differential expression analysis was performed using edgeR [40] after enumerating the number of reads per transcript with HTSeq [41]. We made the following comparisons: 1) Differential expression in response to infection, performed separately for each genotype and time point; 2) Differential expression between genotypes prior to infection to measure constitutive expression differences; 3) Difference in response to infection between genotypes (interaction model), performed separately for each time point. In all cases, we filtered out lowly expressed genes by requiring that each gene included in our comparison have at least 0.1 count per million (0.1 cpm) in enough samples to equal our smallest replicate size for that comparison (n = 2–4). All pairwise comparisons were made using exact tests, and the interaction models were fit using general linear models that accounted for genotype and infection status. Significance was assessed either as having an experiment-wide FDR<0.20 (pairwise comparisons) or an individual gene significance of P<0.01 (interaction model). The biological coefficient of variation (BCV), a measure of biological variability between replicates, ranged from 0.179 to 0.455 (S3 Table). After excluding the four libraries with the lowest library amplifications (S2 Table), the BCV ranged from 0.179 to 0.353. The number of genes meeting our filtering criteria ranged from 12,549 to 13,594 (of 17,165) after excluding poor libraries (S3 Table).
We used the RNA-seq data and Popoolation2 [42] to measure differentiation (FST) between resistant and susceptible progeny on a per SNP and per gene basis. BAM alignments from all treatments from the same cross (yielding either resistant or susceptible progeny) were merged prior to analysis.
We compared our data on gene expression patterns with previously published microarray data for the Toll and IMD pathways [43] and JAK-STAT pathway [44]. To determine which pathways were activated by infection, we examined gene expression patterns in response to B. malayi in those genes that were previously shown to be differentially expressed as a result of perturbation of each pathway. We also compared expression patterns with the response to infection by Wolbachia strain wMelPop-CLA [45]. Data for this comparison was downloaded from VectorBase [46], and only genes that were significant at P<0.01 were used for comparison. We classified immunity genes using ImmunoDB (http://cegg.unige.ch/Insecta/immunodb/) and manual curation by ourselves of more recently identified immune genes.
Results
Exome Sequencing of a Kenyan Ae. aegypti Population Identifies a Single Dominant Locus That Controls Susceptibility to B. malayi
To create a population that varied in susceptibility to B. malayi, we collected Ae. aegypti eggs from the coastal region of Kenya where there is known to be a mixture of genetically resistant and susceptible individuals [15]. These eggs were used to create a large outcrossed population that was maintained in the laboratory for 6 generations. The mosquitoes were then fed on human blood containing B. malayi microfilariae, which resulted in 23% (88 of 388) becoming infected with L3 larvae, with an average of 2.4 L3’s in each infected mosquito. This is a considerably lower infection rate than in the susceptible control line (86% of mosquitoes infected, 19 of 22, with an average of 2.5 L3’s per infected mosquito), suggesting that there is genetic variation in susceptibility within our population.
To identify genes associated with resistance, we used a combination of exome sequencing or low coverage whole-genome sequencing. The Ae. aegypti genome is large and repetitive, so exome sequencing provided us with far higher coverage of the exonic regions than was possible with the whole genome sequencing. The exome capture was highly efficient, resulting in 100 times greater coverage of the exome regions (26.7 MB, 2% of the genome) compared with the non-exome regions. So that we could combine the high and low coverage data, we performed our association mapping using an approach based on genotype probabilities at each site (as opposed to calling genotypes and then testing each site for an association with infection). In total we sequenced 67 L3-infected and 73 uninfected mosquitoes, which we classified as susceptible and resistant respectively.
We found that susceptibility to Brugia has a simple genetic basis in our population, with a small number of sites highly significantly associated with infection (Fig. 1A). Of the sites that have a known position in the genome, all of those with a genome-wide significance of P<0.01 were clustered together at 0 cM on chromosome 1 (Fig. 1A). To test whether there were multiple genes affecting susceptibility to Brugia, we repeated the association study including the most significant variant from the first analysis as a covariate. This resulted in no significant associations (Fig. 1B). Furthermore, quantile-quantile (qq) plots comparing expected and observed P-values in the analysis with the top SNP as a covariate confirm that there are no additional associations (S1 Fig). Therefore we can conclude that there is a single variant causing the differences in susceptibility, and all the significant associations are caused by sites in linkage disequilibrium with this variant.
Fig. 1. Association mapping demonstrates that a single dominant locus controls resistance to B. malayi in a Kenyan population of Ae. aegypti. 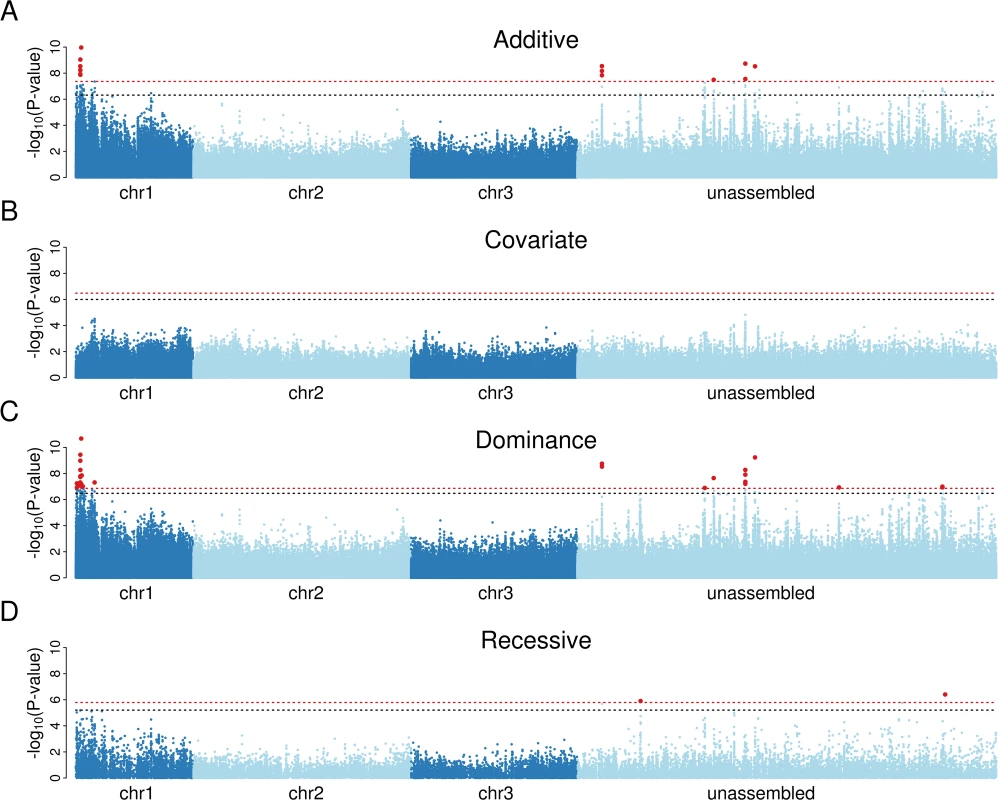
A) Mapping with an additive model of inheritance produces a small number of significant associations on chromosome 1 and in regions unassigned to chromosomes. B) Including the most significant marker in panel A as a covariate in the analysis results in no significant associations, suggesting that a single locus controls infection. C) A dominance model of inheritance produces highly significant associations, whereas D) a recessive model does not. Red-dotted line shows genome-wide significance at P<0.01; black-dotted line shows significance at P<0.05. The x-axis represents a physical map (bp) made by arranging scaffolds along the genetic map [19], with scaffolds mapping to the same genetic map position being ordered randomly. To correct genome misassemblies, scaffolds that contain any gene with at least 10 segregating sites and an FST greater than 0.1 in the RNA-seq data were moved to the unassembled region. In the dominance model, 13 sites on 4 scaffolds map to 0 cM and a single site maps to 12 cM. To test whether resistance is dominant, we repeated the association study using a model that assumed the minor allele to be either dominant or recessive. The dominant model resulted in a cluster of significant associations on chromosome 1, and the top associations were more significant than the previous analysis that assumed additive effects (Fig. 1C). In contrast, the recessive model generated only two marginally significant associations (Fig. 1D), and inspection of these showed that they were caused by linkage disequilibrium with the highly significant dominant variants. The minor allele of the most significant site was associated with increased resistance, so we can conclude that resistance is caused by a single dominant locus at 0 cM on chromosome 1 (positions 1p23 and 1p25 on the physical map [47]). This is within the same region that we previously genetically mapped in crosses between laboratory lines from West Africa (0 to 12 cM) [19], and in the same physical region where a sex-linked, dominant resistance locus was approximately mapped (resistance: 1p31, sex determining region: 1q21) [18,48], so the associations we detected are likely to be caused by the same resistance region. Therefore, the previously identified laboratory QTL is present at an appreciable frequency in East Africa in the wild.
Identification of Candidate Genes Associated with Resistance
We next examined whether the variant causing resistance could be identified in our dataset. We found 26 SNPs associated with infection below a genome-wide significance threshold of 1% and an additional 27 below 5% (Dominance Model; Fig. 1C and S1 Dataset). No indels were significantly associated with infection. Because of our much higher coverage of the exome, all of the significant associations were in or near to genes.
We used two criteria to exclude SNPs from the list of candidate loci. First, our data only provides evidence for a single causative variant, so we are able to reduce this list from 53 SNPs to 19 SNPs by excluding variants that explain the phenotypic data significantly less well than our top hit (see methods for details; based on HiSeq exome sequences alone, S1 Dataset). Second, in the experiments described below we find that worms never develop in mosquitoes carrying the resistance allele, and only 7 of the 19 SNPs follow this pattern (allowing a 10% phenotyping error and using only HiSeq exome data, S1 Dataset). None of these SNPs alter the protein sequence (6 synonymous and 1 intronic, S1 Dataset). Furthermore, these SNPs occur in five different genes, none of which have known immune functions (gamma-tubulin complex component 3 [AAEL008465], cysteine synthase [AAEL008467], putative latent nuclear antigen [AAEL002082], conserved hypothetical protein [AAEL008350], and chaoptin [AAEL008940]). Therefore, we cannot identify a single variant that causes resistance.
A combination of two factors has likely prevented us from identifying the gene that is causing mosquitoes to be resistant. First, linkage disequilibrium in our mapping population means that significant associations are found across multiple scaffolds, sometimes with identical segregation patterns. This is visible in quantile-quantile plots where there is a large excess of sites with differing frequencies in resistant and susceptible chromosomes (S1 Fig). Second, low sequencing coverage means we have limited power to detect associations in non-coding sequence. Therefore, if the variant causing resistance is in a low coverage region we may not detect it, but it will generate significant associations in nearby genes in linkage disequilibrium.
While we did not identify a causal variant, the list of sites and genes associated with resistance are strong candidates. Even if none of these genes prove to be causing resistance, we have narrowed the region down to the 14 scaffolds that either map to the 0 cM genetic position on chromosome 1 (scaffolds 1.48b, 1.97b, 1.166, 1.222a, 1.267, 1.272, 1.296, 1.327, 1.398, 1.461, 1.487, 1.512, 1.696, and 1.970, containing 175 genes and 15.0 Mb) or the 6 scaffolds with SNPs in the top 1% of hits whose location in the genome is uncertain (scaffolds 1.226, 1.360, 1.676, 1.1219, 1.257, 1.389, containing at most 94 genes and 6.6 Mb). FST data obtained from our RNA-seq experiment (described in the following section) supports four of these six scaffolds as being located on chromosome 1 (1.360, 1.676, 1.257, and 1.389). This conflicts with a previous analysis showing that two of these scaffolds (1.360 and 1.257) are on chromosome 2 [19], but this is not unexpected as the genome has a high misassembly rate, and many scaffolds are chimeras of sequences found in different places in the genome [19].
Brugia malayi Development in Resistant and Susceptible Genotypes of Ae. aegypti
To examine the effects of the B. malayi resistance locus on mosquito gene expression and worm development we used two laboratory mosquito lines, one resistant (LVPR) and one susceptible (LVPS). When comparing these lines it is desirable to homogenize the genetic background in which the two alleles of the locus are found. To do this we took advantage of the locus being dominant and sex-linked. By crossing resistant (LVPR) males with susceptible (LVPS) females or vice-versa, and then backcrossing the F1 males to susceptible female (LVPS) mosquitoes, we generated resistant and susceptible mosquitoes that are genetically similar except for the effects of other dominant sex-linked genes. We checked that this was the case by calling SNPs from the RNA-seq data (described below) and measuring differentiation between the backcross progeny using FST, which ranges from 0 (complete allele sharing) to 1 (complete differentiation). As expected, we found that the first chromosome was differentiated between the backcross progeny from the resistant grandmother compared to progeny from the susceptible grandmother (mean FST = 0.081). The second and third chromosomes were not differentiated (mean FST = 0.011 and 0.009 respectively) (S2 Fig). The region of differentiation extended across the length of the first chromosome (S2 Fig), which is likely to be due to the low rate of recombination in Ae. aegypti.
We found that resistance closely segregated with the sex-determining locus (Fig. 2 A-B), with no L3 worms (0 of 48) developing in the backcross progeny from the resistant (LVPR) grandmother. In contrast, in the backcross progeny from the susceptible (LVPS) grandmother, 89% (24 of 27) of the mosquitoes were infected, and these had an average load of 5.1 L3’s per mosquito (Fig. 2 A-B). The infection rates of these backcrosses were similar to those of the parental lines (LVPS and LVPR; Fig. 2 A-B). The sex determining locus is at approximately 21 cM [19], and we observe no recombinants between the sex and resistance loci (0 of 48, Fig. 2 A-B), although some recombinants may not be detected because not all susceptible individuals develop an infection (83%, 10 of 12). These results suggest that the difference in resistance between these lines is being controlled by the same dominant sex-linked locus described in the Kenyan population in the previous section. We will subsequently refer to these as the resistant and susceptible genotypes.
Fig. 2. Resistance to B. malayi is dominant and sex-linked, and it impairs early stages of B. malayi development. 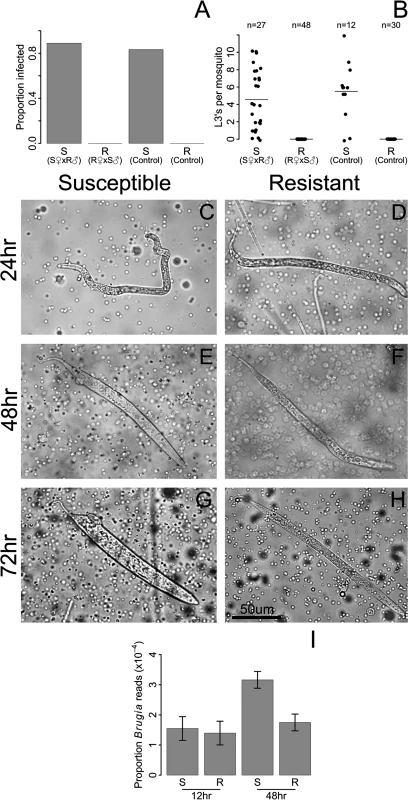
Female progeny from a backcross inherited the resistance phenotype of their maternal grandparent, whether resistance was measured as A) the proportion of mosquitoes infected with L3 stage parasites or B) the number of L3 stage parasites at 10 days after infection. S♀ x R♂ (referred to as susceptible from this point forward) were created by crossing an LVPS female with an LVPR male, followed by backcrossing the F1 progeny to LVPS. R♀ x S♂ (referred to as resistant) were created in the same way except an LVPR female was crossed with an LVPS male in the parental generation. By 24 hours after infection, many B. malayi microfilariae have migrated from the midgut to the thoracic tissues in both C) susceptible and D) resistant hosts. At 48 hours after infection, microfilariae are molting into the non-feeding L1 developmental stage in E) susceptible hosts, whereas growth is arrested in F) resistant hosts. By 72 hours after infection, nearly all surviving B. malayi are in the L1 stage in G) susceptible hosts, whereas they are dead or dying in H) resistant hosts. I) Genome-wide gene expression levels of B. malayi are comparable in resistant and susceptible hosts at 12 hours after infection, whereas by 48 hours, gene expression, and presumably growth, of B. malayi is higher in susceptible mosquitoes. B. malayi gene expression is estimated by dividing the total number of RNA-seq reads mapping to the B. malayi genome by the total number of reads mapping to the Ae. aegypti genome. Using these genetically similar mosquitoes we examined the stage in B. malayi development being targeted by resistant mosquitoes. In susceptible Ae. aegypti, microfilariae penetrate the midgut and migrate to the thorax from 1–24 hours following infection, enter a non-feeding L1 stage of development from 6–72 hours, and eventually exit the mosquito as L3 larvae after approximately ten to twelve days [11,49]. Microfilariae are approximately 200–300 um in size at the time of ingestion and L3’s can reach 1–3 mm in size [11], and the numbers of worms decrease from an average of 17 microfilariae per mosquito at the time of ingestion to 6 at the L3 stage [11]. We find microfilariae and L1’s in the thorax of both resistant and susceptible mosquitoes for several days after infection (Fig. 2 C-H). At 24 hours we found that microfilariae have migrated from the midgut to the thoracic tissues in both genotypes (Fig. 2 C-D). We continue to find live microfilariae and L1’s in both genotypes at 48 and 96 hours (Fig. 2 E-H), which is consistent ongoing migration until blood digestion ends at around 72 hours after feeding [50]. By 48 hours and especially by 96 hours after infection there are clear differences between the genotypes, with microfilariae molting into the non-feeding L1 larvae in susceptible hosts, whereas growth is arrested in resistant hosts (Fig. 2 E-H). The microfilariae and L1’s in the resistant genotype are phenotypically distinguishable from those in susceptible genotype, and appear to be smaller and thinner (Fig. 2 G-H). We could not accurately compare microfilariae numbers using our dissections, so we instead compared gene expression (see next paragraph). We sometimes see worms being melanized in both genotypes, but usually they are not.
We can also follow worm development from the number of RNA-seq reads mapping to the B. malayi genome. At 12 hours, B. malayi gene expression levels are comparable in resistant and susceptible genotypes, suggesting similar growth and numbers at this time point. By 48 hours, gene expression is much higher in the susceptible genotypes, suggesting greater parasite growth in the susceptible host [51] (Fig. 2I). This suggests that parasite development is aborted in resistant mosquitoes between 12 and 48 hours after infection, although languishing microfilariae and L1’s can be found in resistant mosquitoes for several more days. Our results corroborate previous studies which show that parasites in refractory Ae. aegypti decline in numbers by 48 hours after infection [49,51,52]. Therefore, parasites are able to reach thorax of resistant mosquitoes, but are killed in the microfilariae and L1 stages. This suggests that the resistance mechanism plays an important role after invasion of the thorax.
Resistant and Susceptible Mosquitoes Have a Similar Transcriptional Response to Infection by B. malayi
We used RNA-seq to investigate the transcriptional response of Ae. aegypti to B. malayi (using mosquitoes from the same experiment as shown in Fig. 2). We chose to measure gene expression early in the infection when differences in worm development in susceptible and resistant mosquitoes appear. For each genotype separately, we measured differential expression between infected and uninfected mosquitoes. We found a strong correlation between genotypes in the induced responses at both time points (Pearson’s R2 at 12hr: 0.51, 48hr: 0.58; Fig. 3), suggesting a similar response to B. malayi infection in resistant and susceptible mosquitoes.
Fig. 3. The induced response to B. malayi is highly correlated between resistant and susceptible mosquitoes. 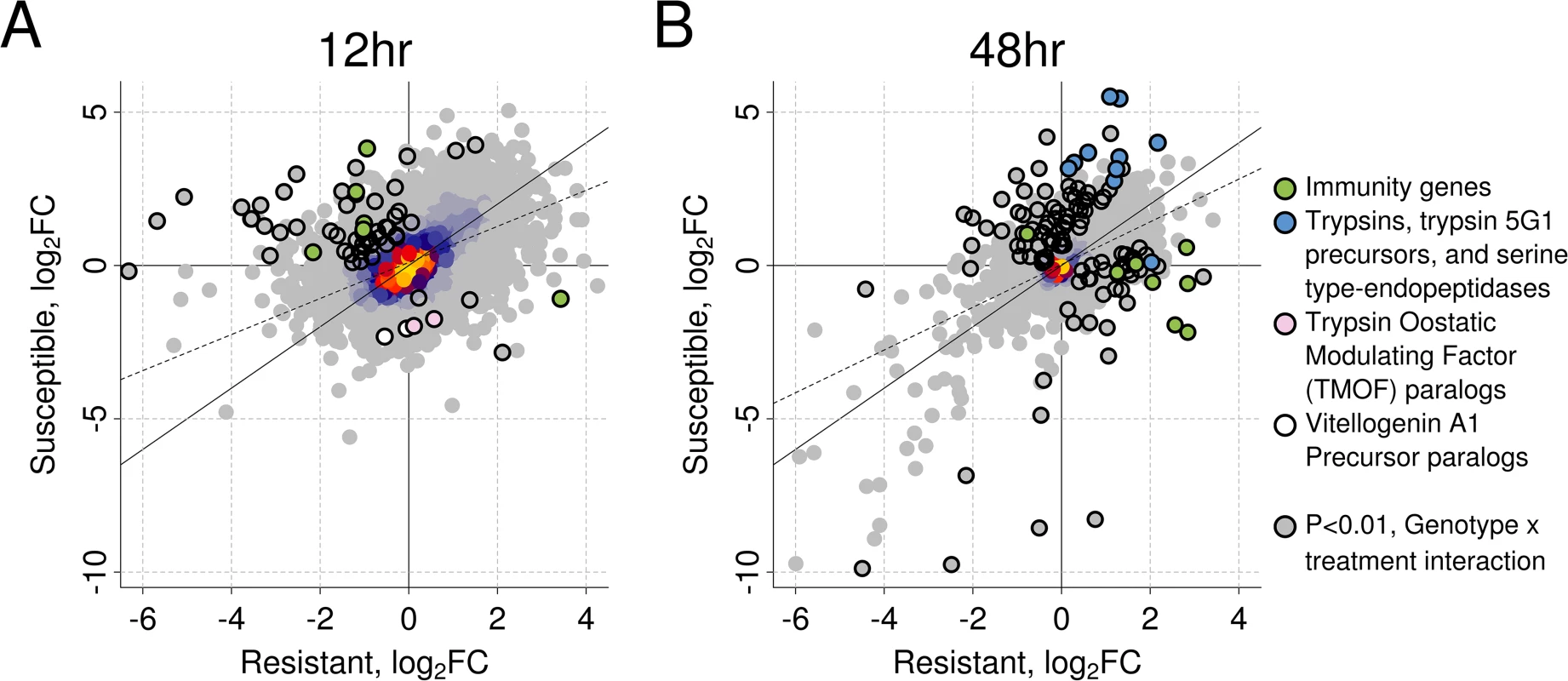
The correlation in log2 fold change between infected versus uninfected mosquitoes is shown at (A) 12 hours (Pearson's R2 = 0.51) and (B) 48 hours (Pearson's R2 = 0.58) post infection for resistant and susceptible mosquitoes. The genes highlighted with black circles differ in the direction or magnitude of expression (interaction term significant at a P<0.01). The black diagonal line shows the relationship expected with a perfect correlation, and the dotted line shows the best fit line from the observed data. Warmer colors (uncircled) indicate a higher density of superimposed points. Our first time-point was 12 hours post-infection, when microfilariae are migrating from the midgut to the thorax and beginning to enter the L1 stage. B. malayi infection significantly regulated 459 genes in the resistant line (238 induced and 221 repressed) and 636 genes in the susceptible line (535 induced and 101 repressed) (S2 Dataset). A total of 97 genes were significantly upregulated in both resistant and susceptible mosquitoes in response to infection, and 26 were significantly downregulated in both genotypes.
Our second time point was 48 hours post-infection, when over half of the parasites will be L1’s in susceptible mosquitoes [11] and parasites are beginning to die in resistant mosquitoes. At this time, Brugia infection significantly regulated 1,029 genes (725 genes induced and 304 genes repressed) in the resistant line and 609 genes (467 genes induced and 142 genes repressed) in the susceptible line (S2 Dataset). A total of 274 genes were significantly upregulated in both genotypes and 91 genes were downregulated in both genotypes.
Similarities in the Immune Response of Resistant and Susceptible Mosquitoes following Infection by B. malayi
There is a clear transcriptional response of immune-related genes to B. malayi at both 12 and 48 hours after infection (Figs. 3, 4). The large majority of the immune-related genes are upregulated in the infected relative to the uninfected mosquitoes, indicating that the immune system is being activated (Fig. 4). Furthermore, this upregulation of immune genes is apparent at 12 hours post-infection, which conflicts with an earlier report that very little transcriptional response is seen until 5 days after infection in susceptible mosquitoes [11]. The previous study used microarrays and pooled across several time points so may be less sensitive than our approach.
Fig. 4. Immune-related genes that are differentially expressed in response to B. malayi infection. 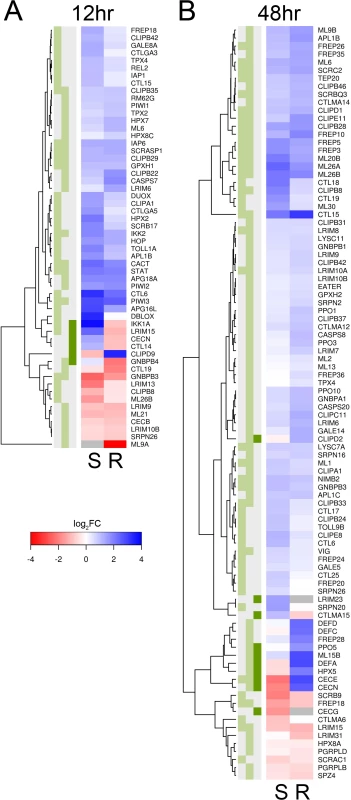
Immune-related genes are from the ImmunoDB database or manual curation, and only those that are significantly differentially expressed for either or both genotypes (or have a significant interaction term) are shown. Expression is log2 fold-change in response to B. malayi, with genes in blue being upregulated and genes in red being downregulated. Grey means insufficient coverage to estimate expression. (A) At 12 hours post infection, almost all genes are being expressed in the same direction and with similar magnitudes. (B) At 48 hours, a subset of genes are being expressed differently in response to infection. The green boxes indicate statistical significance in susceptible mosquitoes (left, FDR<0.2), resistant mosquitoes (middle, FDR<0.2) or a significant interaction (dark green right, P<0.01). There is a striking concordance between the transcriptional response of immune genes in the resistant and susceptible genotypes (Fig. 4). Among the immune genes significantly differentially regulated 12 hours post-infection in at least one of the genotypes, almost invariably the gene is differentially regulated in the same direction in the other genotype (even if this is not statistically significant) (Fig. 4). At 48 hours there is still a similar response in the two genotypes, although some differences are emerging (see below).
The genes that are differentially regulated in response to Brugia infection in both genotypes have a range of immune functions (Fig. 4). These include melanization (CLIPB8, CLIPB29, and CLIPB35 [53]), hemocyte-mediated immunity (CLIPD1, FREP3, FREP5, FREP10, and a Nimrod homolog [54]), antioxidant protection (GPXH1, HPX3), the Toll pathway (CACT, GNBPB3), IMD pathway (IKK2), and JAK-STAT pathway (STAT). Melanization of B. malayi was occasionally observed at early stages of infection in both genotypes and is controlled by clip serine protease cascades and phenoloxidases which are released by hemocytes [53]. It is associated with the production of reactive oxygen species, which is expected to trigger the production of antioxidants. The activation of the Toll, IMD and JAK-STAT pathway by Brugia infection has never been previously described.
A Small Number of Genes Respond Differently to Early Stages of Infection in Resistant and Susceptible Mosquitoes
In the resistant and susceptible mosquitoes a subset of genes responded to B. malayi infection in different directions or with a significantly different magnitude, and these may give clues as to the mechanism of resistance (Figs. 3, S3). At 12 hours post-infection we detected 86 such genes (N = 13,156) by testing for a significant interaction between genotype and infection (S2 Dataset). Of these, 62 had sufficient data to be tested for differential expression separately within each genotype. Contrary to our expectation, we found that most (53 of 62) of these genes were more highly expressed in the susceptible line, with 48 of these being upregulated in the susceptible line and downregulated in the resistant (Fig. 3).
There is little evidence to suggest that resistance is due to immune gene expression at the early 12 hour time point, as the susceptible mosquitoes upregulated more immune-related genes than the resistant ones (Figs. 3, 4). Immune genes upregulated in susceptible and downregulated in resistant mosquitoes include a cecropin (CECN), a lectin (CTL14), a gram-negative binding protein (GNBP4), the IMD signaling pathway molecule IKK1A, and a leucine-rich repeat protein (LRIM15), all of which may be related to the immune response (Fig. 4). In contrast, the resistant line only upregulated expression of a single clip-domain serine protease (CLIPD9) (Fig. 4). Furthermore, 10 genes that are induced by infection by the Wolbachia strain wMelPop-CLA, which is known to make mosquitoes more resistant to filarial nematodes [21,45], are upregulated in the susceptible line but downregulated in the resistant line (S5 Fig).
At the early time point, the non-immunity genes that had a different transcriptional response to B. malayi in the two genotypes had a diversity of functions, but many were related to metabolism, with the susceptible line upregulating transport and digestion-related genes and the resistant line downregulating digestion and upregulating vitellogenin (egg yolk protein) (Figs. 3, S4). Of the 48 genes upregulated in the susceptible genotype and downregulated in the resistant genotype, a number were transporter, transmembrane, or gut structural genes (amino acid transporter PAT1 (AAEL007191), innexin (AAEL014846), integrin (AAEL014846), an insect major allergen/G12 family member (AAEL009166), and a monocarboxylate transporter (AAEL013915)). A translational repressor that is important in starvation responses was also induced only in the susceptible line (eukaryotic initiation factor 4E binding protein, AAEL001864) [55]. Only nine genes had higher induction in the resistant genotype. Two were paralogs of a vitellogenin A1 precursor (AAEL006126 and AAEL006138) and two were paralogs of trypsin modulating oostatic factor (TMOF) (AAEL006670 and AAEL014561). TMOF is expressed in the ovaries and binds to receptors in the midgut where it turns off trypsin production [56] and downregulates the production of vitellogenin. The other genes were an immune modulator (CLIPD9), an AMP dependent ligase (AAEL006823), a putative pupal cuticle protein (AAEL010127) and two genes with unknown functions (AAEL008449 and AAEL000681).
Susceptible Mosquitoes Downregulate Some Immune-Related Genes 48 Hours after B. malayi Infection
At 48 hours post-infection, the time point where parasites are being killed in resistant but not susceptible mosquitoes, 152 genes (out of 13,594) responded to B. malayi infection differently in the susceptible and resistant mosquitoes (Figs. 3, S3 and S2 Dataset). Of the 122 genes with sufficient data to be tested for differential expression in response to infection within genotype, 75 had higher expression in the susceptible line and 47 had higher expression in the resistant line.
In contrast to the earlier time-point, by 48 hours there were differences in the induced immune response between resistant and susceptible mosquitoes. The 152 genes that behaved differently between the two genotypes were significantly enriched for immune response categories (S4 Fig) and these immune response genes tended to have higher expression in the infected resistant mosquitoes (Figs. 3, 4, S3). These included antimicrobial peptides (CECE: AAEL000611, CECN: AAEL000621, DEFA: AAEL003841, DEFC: AAEL003832, and DEFD: AAEL003857), a prophenoloxidase (PPO5: AAEL013492), JNK (AAEL008622), CLIPD2 (AAEL004979), FREP28 (AAEL003156), a Niemann Pick-type C gene ML15B (AAEL009556), and the heme peroxidase HPX5 (AAEL002354). The only immune gene that had higher expression in susceptible mosquitoes was DSCAM (AAEL011284). Unexpectedly, many immune genes were downregulated in susceptible mosquitoes in response to B. malayi infection while being upregulated in the resistant ones (Fig. 4). Two Cecropin genes were found to be suppressed in susceptible mosquitoes at 48–72 hours post-infection in a previous study [11], supporting our results. This pattern therefore suggests parasite suppression of these immune response genes or dysregulation within susceptible mosquitoes.
At 48 hours after infection, the susceptible genotype appears to have higher upregulation of genes that are highly induced by blood feeding [50] and are potentially related to metabolism and digestion. This include trypsins (AAEL013703, AAEL013707, AAEL013712, and AAEL013715) [57], several chymotrypsin-related serine proteases expressed in the midgut (AAEL001674, AAEL001690, AAEL001693, AAEL001701, AAEL008769, and AAEL008780) [57], a putative serine collagenase (AAEL007432) [57], a sphingomyelin phosphodiesterase (AAEL013717) [27], and methylenetetrahydrofolate dehydrogenases (AAEL006085 and AAEL014871) [27]. This is consistent with trypsin modulating oostatic factor (TMOF) paralogs, which inhibit late trypsin production, being downregulated in the susceptible line but not the resistant line at 12 hours, leading to a longer period of expression of trypsins [56].
The Transcriptional Response to B. malayi Implicates the Toll, IMD and JAK-STAT Pathways
The Toll, IMD, and JAK-STAT pathways play a central role in controlling the immune response of insects, so we investigated whether they were activated by B. malayi, and whether patterns of activation differ between resistant and susceptible mosquitoes. To do this we compared our results to microarray datasets from mosquitoes where REL1 or REL2 had been overexpressed (the transcription factors activated by the Toll and IMD pathways respectively) [43] or PIAS, a negative regulator of the JAK-STAT pathway, had been knocked down [44].
At 12 hours post-infection, the transcriptional response of both resistant and susceptible genotypes suggests they have activated their Toll and/or IMD pathways (Figs. 5A, S5). Consistent with our earlier findings of an induced immune response, the susceptible genotype appears to be activating these pathways to a higher degree (Figs. 5A, S5). As has been previously reported, overexpression of REL1 and REL2 elicits similar responses in an overlapping set of genes (S6 Fig) [43], so these patterns could be generated by B. malayi activating the Toll, IMD or both pathways.
The susceptible genotype appears to activate the JAK-STAT pathway at 12 hours post infection, while the resistant genotype does not show evidence for this (Figs. 5A, S5). In particular, the insect major allergen/G12 gene AAEL009166 appears to be induced in the susceptible line but strongly downregulated by the resistant line. This gene is induced by the JAK-STAT pathway, and is highly expressed in the midgut following a bloodmeal and is thought to be involved in digestion [58,59]. This JAK-STAT-like response is distinct from the other pathways we investigated, as of the 10 genes that overlap between the Toll and JAK-STAT datasets, 9 go in opposite directions (S6 Fig). These 9 genes include genes that respond differently to B. malayi in the two genotypes: the antimicrobial peptides CECE and DEFC (upregulated by the Toll pathway and downregulated by the JAK-STAT pathway [44]) and four insect major allergen genes (AAEL009166, AAEL010429, AAEL010436, and AAEL013118; downregulated by the Toll pathway and upregulated by the JAK-STAT pathway).
The pattern of immune pathway activation 48 hours post-infection is less clear. In both the resistant and susceptible mosquitoes, B. malayi infection increases the expression of genes normally upregulated by active Toll, IMD and JAK-STAT pathways (Figs. 5A, S5). This is reflected in our earlier result that both genotypes show signs of an induced immune response. However, many genes that are normally downregulated by these pathways are upregulated in both genotypes. This upregulation appears to be higher in the susceptible genotype, especially in the case of the IMD pathway (Figs. 5A, S5), including trypsins, a trypsin 5G1 precursor, and serine-type endopeptidases (Fig. 3).
There Are Strong Correlations between the Transcriptional Responses to B. malayi and the Bacterial Endosymbiont Wolbachia
Ae. aegypti has been artificially infected with a bacterial symbiont called Wolbachia [45], and the wMelPop-CLA strain increases resistance to B. malayi [21]. Additionally, B. malayi harbors its own obligate strain of Wolbachia wBm [60], which may also interact with the Ae. aegypti immune response. We therefore compared the transcriptional responses to Wolbachia and B. malayi using previously published data for Wolbachia (see Methods).
At the early time point there are striking differences between the resistant and susceptible genotypes. In the resistant genotype there is a strong negative correlation in the responses to B. malayi and Wolbachia wMelPop-CLA, with genes tending to respond in opposite directions (Figs. 5A, S5). In contrast, in the susceptible genotype there is a positive correlation (Figs. 5A, S5).
At 48 hours post-infection, the transcriptional response to B. malayi in both the susceptible and resistant mosquitoes is strongly correlated to the response to the wMelPop-CLA (Figs. 5A, S5). A subset of these genes behaved differently in the two genotypes, including the downregulation of antimicrobial peptides in susceptible mosquitoes that is described above (S5 Fig).
Constitutive Differences in Gene Expression between Resistant and Susceptible Genotypes of Ae. aegypti
Until now we have only investigated induced responses to B. malayi, but it is possible that constitutive gene expression differences might contribute to resistance. As in the previous section, we identified genes that were upregulated or downregulated by stimulation of the Toll, IMD, or JAK-STAT pathways, or by Wolbachia infection (Fig. 5B). We found that the resistant line had significantly higher constitutive expression of genes under the control of the Toll pathway and genes induced by the Wolbachia strain wMelPop-CLA (Fig. 5B). Overall, it appears that some immune pathways are upregulated in the resistant genotype prior to infection, suggesting that constitutive differences in gene expression could potentially play a role in resistance.
Fig. 5. Response to infection of genes in the Toll, IMD, or JAK-STAT pathways, or those responsive to infection with Wolbachia strain wMelPop-CLA. 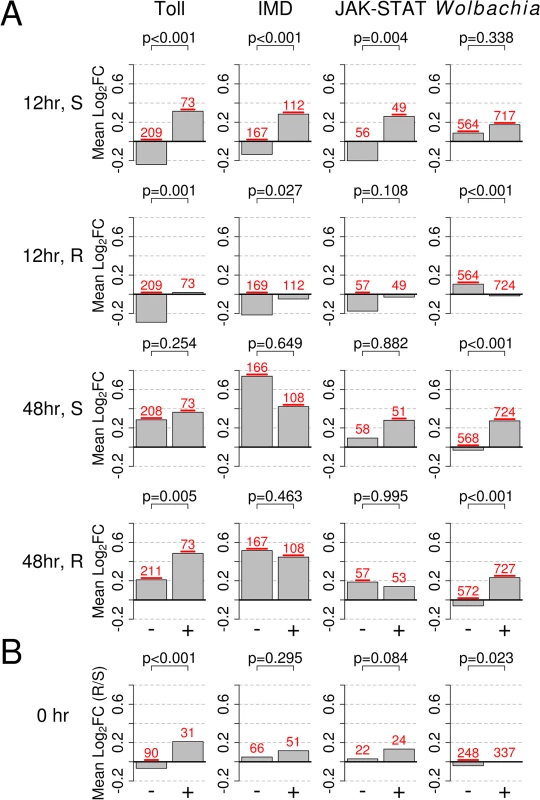
The bars show mean log2 fold change in expression (Mean Log2FC). + or - indicates genes that are upregulated or downregulated by the category shown at the top of each column and were obtained from [43–45]. A) Expression changes in response to B. malayi in susceptible and resistant genotypes at 12 and 48 hours after infection. B) Constitutive expression of pathways prior to infection (0 hr; resistant versus susceptible), with positive values indicating higher expression in the resistant genotype and vice versa. In B), only genes mapping to the second and third chromosomes, which are not biased with regards to sequence mapping, are shown. The numbers of genes in each category are shown is red above each bar and are underlined if their mean is significantly different from 0 (t-test, P<0.05). P-values from a Mann-Whitney test comparing up and down regulated genes are shown above each bar plot. We confirmed that these results are robust to the confounding effects of biases in mapping sequence reads. Reads will not map to the reference genome if SNPs cause a significant number of mismatches to the reference genome. Despite homogenizing the genetic background of our mosquitoes, the resistant and susceptible genotypes are still genetically different in sex-linked regions. We found that these differences are biasing our estimates of gene expression, as genetic differentiation (FST) between the genotypes correlated with differences in expression levels (details in S7 Fig). We therefore repeated our comparison of constitutive expression using only genes found on the second or third chromosomes which are not affected by this bias, and found that the results of our pathway comparisons were qualitatively unchanged. It is important to note that this bias does not affect the analysis of induced responses to B. malayi as these rely on comparisons within genotype rather than between genotypes.
Expression Profiles of Candidate Resistance Genes from the Association Study
The polymorphism that confers resistance could be in a gene whose expression changes in response to B. malayi infection. Therefore, we examined our gene expression data for differentially expressed genes in the genomic region controlling resistance (Fig. 6). Specifically, we chose genes that were either differentially expressed in response to B. malayi infection or constitutively differentially expressed between the genotypes (note the caveat above that this comparison can be confounded by mapping biases). This generated a list of 41 genes (Fig. 6).
Fig. 6. Differentially expressed genes in the genomic region controlling resistance. 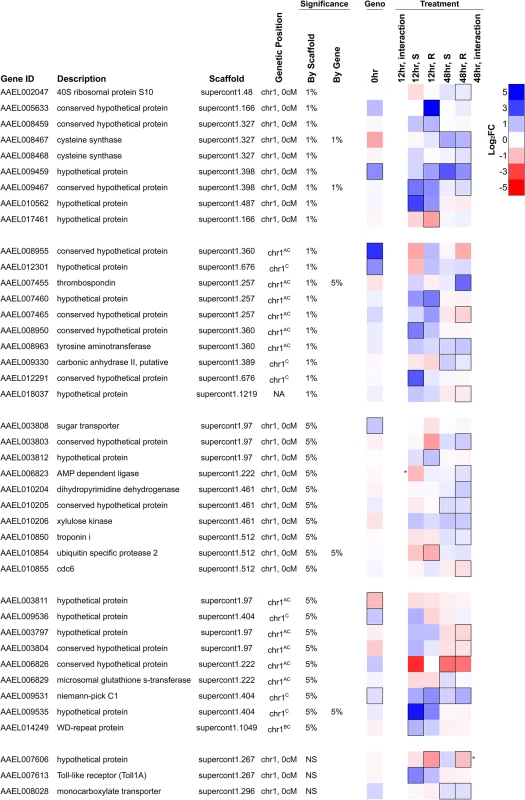
Genes shown either map to the genetic map position associated with resistance (chromosome 1, 0 cM) or are on an unassembled scaffold with a significant association at a genome-wide significance of P<0.01. They are significantly differentially expressed either 1) between resistant and susceptible genotypes prior to infection (FDR<0.20; black border) or 2) in response to infection in either genotype at 12 or 48 hours after infection (FDR<0.20; black border), or 3) respond to infection differently between the genotypes (* indicates a significant interaction between genotype and infection at P<0.01). Several genes have been assigned to chromosome 1 but have an unknown genetic map position. AOn a misassembled scaffold, BMapped to chromosome 1 previously without positional information [19], CNewly mapped to chromosome 1 using FST estimates from RNA-seq data. NA indicates no genetic mapping information. The most significant SNP in the association study is within the intron of the gene AAEL009467, and this gene is induced in response to B. malayi infection by both genotypes at the early time point (non-significant in the resistant genotype) and by the resistant genotype only at the later time point (Fig. 6). This gene encodes a protein that contains an actin-depolymerizing factor homology (ADF-H) domain, which mediates actin binding. It has 1 : 1 orthologs in other mosquito vectors including Anopheles gambiae and Culex quinquefasciatus. This SNP is unlikely to be causing resistance as it does not have the expected segregation pattern (see above). We had high sequence coverage and thus high power for this gene across the entire coding sequence so it is unlikely that the casual mutation is protein coding. It is possible that a non-coding variant affecting gene expression could be causal.
An unnamed gene (AAEL009459) encoding a hypothetical protein is located 80kb away from the most significant hit and is strongly induced by infection in resistant mosquitoes at the early time point and in both genotypes at the later time point (Fig. 6). It also has higher constitutive expression in the resistant genotype, although high FST suggests that this may be an artifact of mapping biases. This gene is also strongly upregulated by Wolbachia wMelPop-CLA and has no known orthologs in other species. This gene appears to have a neighboring unannotated paralog that is expressed in our RNA-seq data, and the duplicate has two distinct alleles in the resistant and susceptible genotypes from the laboratory crosses. Unfortunately, because it is unannotated, sequence coverage is extremely low in the association study samples, so it is unclear if these alleles are associated with resistance.
Two immune-related genes, a Niemann Pick-type C1 (NPC1) gene (AAEL009531) and Toll1A (AAEL007613), are also found in the region associated with resistance and were upregulated in response to infection in one or both genotypes. The Niemann Pick-type C1 gene is expressed in the mosquito midgut [61] and here is induced in response to B. malayi infection. Knockdown of this gene inhibits dengue infection, and it has been proposed to be a negative regulator of the immune response [61]. Toll1A is a homolog to the Drosophila Toll [62], and the scaffold 1.267, which contains Toll1A, also contains several other Toll genes.
Discussion
We have found that a single, dominant locus underlies resistance to B. malayi in a wild outcrossed population of Ae. aegypti from Kenya. The locus maps to the same region of the genome as the fm locus [12,18], which was identified in laboratory lines established from West Africa in 1936, suggesting that this laboratory-observed variant is present at appreciable frequencies in the wild and has been maintained for at least 80 years. By exploiting the power of exome sequencing to allow association studies in large and repetitive genomes, we have narrowed the causal region to a single genetic position and identified candidate resistance genes. By studying gene expression in resistant and susceptible genotypes, we found a reduced immune response in the susceptible compared to the resistant genotype at the time of parasite killing, which could potentially result from the parasite suppressing host immunity. We have also found differences in the expression of reproductive and digestion related genes, suggesting that resource allocation may be affected differently between the genotypes.
Resistance to B. malayi Has a Simple Genetic Basis
Resistance of Ae. aegypti to B. malayi has a simple genetic basis. By combining exome and whole-genome sequencing, we found that resistance to B. malayi in a Kenyan population of Ae. aegypti is controlled by a single dominant locus. This locus is located in the same genomic region as a dominant QTL controlling B. malayi resistance that was mapped in crosses between laboratory lines from West Africa [12,18]. In quantitative genetics it is commonly seen that major-effect QTL from lab crosses are an artifact caused by multiple closely linked loci of smaller effect [63], and these are broken up using high-resolution approaches like ours. We found that a model accounting for the most significant SNP gave no evidence for any additional associations, which is most parsimoniously explained by resistance to B. malayi being controlled by a single causative locus. An alternative explanation is that multiple resistance genes are being held in tight linkage disequilibrium by an unknown chromosomal inversion. It is also normal in QTL studies to identify different loci when using different crosses [64], and for QTL that are important in the laboratory to be unimportant in the wild [65,66]. Again that appears not to be the case here, as we found that the locus identified from West Africa is common in an East African population.
The simple genetics of B. malayi resistance is striking as most quantitative traits tend to be affected by many genes, each of which only has a small phenotypic effect [63]. However, there are a number of studies suggesting that susceptibility to infection in insects often has a very simple genetic basis. For example, in Drosophila a small number of major effect loci control resistance to viruses [67], and the same is probably true for resistance to parasitoid wasps [68,69]. Similarly, it has been suggested that in humans susceptibility to infection has a simpler underlying genetic basis than other quantitative traits due to strong selection by pathogens [70]. It has been suggested that this simple genetics is a result of directional selection driving major effect resistance alleles through populations [67].
The polymorphism we have investigated in African mosquitoes is not the result of B. malayi selecting for resistance, because the parasite is only found in south and south-east Asia and is not thought to be naturally vectored by Ae. aegypti. It is known that this locus also confers resistance to the filarial nematodes B. pahangi and Wuchereria bancrofti, but not to Dirofilaria immitis, the cause of dog heartworm [16]. It is possible that selection for resistance to another closely related but unidentified nematode is responsible for the maintenance of this polymorphism. Any selection for resistance is likely to vary among populations, as genotypes that are susceptible to B. pahangi are only known to be common in East Africa [15,71]. Thus it remains to be seen what has maintained this polymorphism in the wild since at least 1936, but understanding this experimentally tractable system may yield insights into natural mosquito-parasite associations. In mosquito-filarial systems, it is commonly seen that resistance is sex-linked and dominant, including a separate locus that confers resistance of Ae. aegypti to D. immitis and to the bullfrog filarial nematode Waltonella flexicauda [72,73]. It is tempting to speculate that this could be the result of sexually-antagonistic selection favoring resistance only in females.
Towards Identifying the Resistance Gene
Our association study mapped the locus causing resistance with a high precision, but we did not identify the causative gene. Due to large effective population sizes, linkage disequilibrium in insect populations can sometimes extend only a few tens of base pairs [74]. This means that association studies like ours would often be expected to identify the exact genes involved. However, we found high levels of linkage disequilibrium in our mapping population and multiple loci with identical segregation patterns. This could be due to difficulties in establishing African Ae. aegypti in the laboratory resulting in a relatively small number of individuals founding our mapping population. This could also occur if the resistance locus falls within a chromosomal inversion, which may result in long-distance linkage disequilibrium in natural populations and might prevent mapping to the level of a single gene. Despite this, we localized the locus with a very high resolution to a single genetic position (0 cM) and identified a small number of strong candidate genes, one of which may cause resistance. The availability of detailed genetic maps combined with the density of genetic markers allowed by whole-exome sequencing has enabled us to compile the first complete list of candidate resistance genes. Several steps are needed to identify the exact variant(s) causing resistance. First, additional association studies on populations with less linkage disequilibrium may narrow identify far fewer genes. Second, gene knockouts, RNAi and other functional studies can confirm the role of these genes in defense against filarial worms.
In our association study, we identified polymorphisms in five different genes which best explained resistance and had the segregation pattern expected for single dominant resistance locus. None of these genes have any known involvement in the immune response, but it is still possible that one of them is responsible for resistance. We also identified candidates in the correct region of the genome with expression differences between the resistant and susceptible genotypes or in response to infection. These candidates did not always have significant associations, but it is possible that the true causative site was untested if it is non-coding or lacked sufficient coverage. Two genes identified this way, a niemann-pick C1 gene and Toll1A, are thought to be involved in the immune response. The niemann-pick C1 gene, which is upregulated in response to Brugia infection, has recently been shown to be required for successful dengue infection [61]. Toll1A is one of four Ae. aegypti genes homologous to Drosophila Toll (Toll-1) [62,75], and it is induced following infection. In Drosophila Toll is crucial for signaling through the Toll pathway, and while Toll1A does not play the same role in Ae. aegypti, there is some evidence to suggest it may play a role in modulating the immune response [62]. Lastly, the top hit in our association study is an unnamed gene that is induced by infection (AAEL009467). This gene encodes a protein with an actin-binding ADF-H domain and Brugia develops in the thoracic muscle fibers of Ae. aegypti, where actin dynamics are expected to be important.
The Ae. aegypti Immune Response to B. malayi Differs in Resistant and Susceptible Mosquitoes
We found that Ae. aegypti upregulates immune-related genes in response to B. malayi as early as 12 hours after infection. Many of these immune genes are known to be under the control of the Toll and/or IMD signaling pathways, so it is likely that B. malayi is activating one or both of these pathways (although the pattern is less clear-cut if genes downregulated by these pathways are considered; see Results). The role of the immune response in resistance to filarial infection is unclear, as it has only been studied in susceptible mosquitoes and the outcome of those studies is not entirely clear-cut. Activation of the immune system prior to infection with B. malayi confers a protective effect, with susceptible Ae. aegypti mosquitoes that are given sham infections or non-lethal bacterial infections sustaining a significantly lower intensity of infection [20]. Similarly, infection with the bacterium Wolbachia wMelPop-CLA also induces an immune response and provides some protection to susceptible mosquitoes [21]. However, RNAi knockdown of the Toll pathway and over-activation of the Toll or IMD pathways in susceptible mosquitoes did not influence the response to infection [11]. This could simply be that using RNAi to induce an immune response is less efficient than using bacteria, or it may mean Toll - and IMD-independent aspects of immunity are controlling filarial infections. Only by repeating these assays in resistant mosquitoes will it be possible to determine which pathways underlie protection in genetically resistant individuals. At 48 hours after infection, the genes most responsive to B. malayi infection are those that are induced by Wolbachia wMelPop-CLA infection. It is possible that the mosquito immune response may be launched not solely against the worm itself, but also in response to the Wolbachia harbored by the nematode, as is the case in the vertebrate host [76], or to midgut bacteria that enter the hemocoel after B. malayi penetration.
By comparing gene expression in both resistant and susceptible mosquitoes, we find that resistant mosquitoes are upregulating a number of immune effector genes at the time point where the parasites are dying, whereas the susceptible mosquitoes are not. At 12 hours post infection the immune response of the two genotypes is similar, but by 48 hours post infection, when parasites are dying in resistant mosquitoes, differences in the immune response are clear. At 48 hours, effector molecules of the immune response, including anti-microbial peptides (AMPs) and prophenoloxidases (PPOs), are upregulated in resistant mosquitoes and either downregulated in susceptible mosquitoes or induced to a much lower degree. Expression of the AMP cecropin was previously seen to be downregulated 48–72 hours after infection in susceptible mosquitoes, supporting what we’ve seen here [11]. Although AMPs are often thought to be primarily antibacterial and antifungal, cecropins have been shown to kill B. panhangi in vitro and in vivo [22], and cecropin and defensin overexpression kills Plasmodium in Ae. aegypti [77].
The downregulation of immune effectors like antimicrobial peptides in susceptible mosquitoes suggests that the differences in the immune response may be driven by immune suppression by the parasite. Parasitic nematodes have mechanisms that circumvent the humoral immune responses of their hosts, including proteinases that directly inhibit cecropins and cuticle factors that sequester host hemolymph proteins and prevent the activation of the immune response (reviewed in [78]). This is likely to be the case for B. malayi, as the closely related parasite B. pahangi reduces the melanization response of Ae. aegypti [79]. If parasite suppression of the immune response is causing the weaker immune response seen in susceptible mosquitoes, then this pattern could either be a cause or consequence of resistance. The resistance locus that we mapped might cause the mosquitoes to mount a stronger immune response to B. malayi, perhaps by circumventing the parasite suppression, and this in turn is the cause of resistance. Alternatively nematodes in the resistant mosquitoes may be unable to suppress the immune response because they are dying for some other reason, such as if resistant mosquitoes are metabolically inhospitable or lack a receptor necessary for nematode migration. In this case, the stronger reduction in immune response seen in susceptible mosquitoes may be a consequence of having more and healthier nematodes.
Resistant mosquitoes also constitutively express immune genes to a higher level, which supports a direct role for the immune system in resistance. We found that resistant mosquitoes have higher constitutive expression of genes under the control of the Toll pathway as well as genes expressed in response to infection with Wolbachia wMelPop-CLA. As described earlier, both experimental stimulation of the immune response and infection with Wolbachia strain wMelPop-CLA provide some protection to Brugia infection, although not complete resistance [20,21].
B. malayi Alters the Expression of Genes Involved in Digestion, Nutrient Transport and Egg Production
Genes involved in digestion, nutrient transport and egg production are expressed differently between the resistant and susceptible mosquitoes after infection by B. malayi. Susceptible mosquitoes appear to be upregulating genes involved in digestion and nutrient transport, and downregulating the egg yolk precursor protein vitellogenin. Many of the genes that are differently induced are genes that respond to blood feeding [58]. The target of rapamycin (TOR) pathway is central to coordinating this blood-feeding response, and is thought to detect the influx of amino acids and upregulate the expression of genes related to digestion, nutrient transport, and vitellogenesis [80]. Several of the genes that respond differently to infection in the two genotypes are in or under the control of the TOR pathway, including a known translational repressor of the pathway 4E-BP [55], the amino acid transporter PAT1 [81], and Vitellogenin A1 precursors [80]. Amino acids and other nutrients that are ingested in a blood meal are normally used for egg production. In infected mosquitoes, competition between the host and parasite for nutrients can result in a reduction of egg production [82], such as seen with Wolbachia [83] and Plasmodium [84]. There can also be a conflict between the immune system and egg production, where the same resources are needed for both processes [85]. Altered expression of these genes could reflect active or passive manipulation of the host resources by the parasite, or competition within the mosquito for resources.
Vitellogenin expression is strongly downregulated by B. malayi in susceptible mosquitoes at 12 hours post-infection and strongly upregulated in resistant mosquitoes at 48 hours-post infection. Despite this, no difference in the number of eggs laid is seen in either genotype in response to infection (Cristina Ariani, personal communication), although other mosquito-filarial worm combinations do lead to a reduction [85–87]. In An. gambiae mosquitoes, vitellogenin production is negatively regulated downstream of Cactus/REL1/REL2, leading to the arrest of oogenesis when the immune system in activated [88]. Vitellogenin interferes with TEP1 binding to the surface of developing Plasmodium ookinetes, so downregulation of vitellogenin production boosted the efficiency of parasite killing [88].
A key molecule underlying the different responses to B. malayi in resistant and susceptible mosquitoes might be trypsin modulation oostatic factor (TMOF). TMOF is produced in the ovaries and turns off egg production and late trypsin production by binding to midgut receptors [56]. Susceptible mosquitoes have decreased TMOF production at 12 hours, and are expressing trypsins and serine proteases at higher levels at 48 hours. Trypsin production in the midgut is highly upregulated by blood feeding to allow quick digestion of proteins in the blood meal [57], and tryptic activity has long been speculated to be a source of conflict with the parasite. Trypsin activity has been suggested to change nutrient availability, or to either digest or activate parasite proteins as they pass through the midgut [89,90]. Indeed, inhibition of trypsin in Ae. aegypti increases dengue virus titres [89] and decreases Plasmodium load [91].
In conclusion, by developing exome sequencing we were able to conduct the first large-scale association study in Ae. aegypti, a species with a large and repetitive genome. This allowed us to identify a major-effect locus conferring resistance to B. malayi. This locus alters the transcriptional response of mosquitoes to B. malayi, with resistant individuals expressing immune effectors to a higher degree. Our results suggest that this pattern may be driven by B. malayi suppressing the immune response in susceptible mosquitoes.
Supporting Information
Zdroje
1. Lefèvre T, Vantaux A, Dabiré KR, Mouline K, Cohuet A. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 2013;9: e1003365. doi: 10.1371/journal.ppat.1003365 23818841
2. Beerntsen BT, James AA, Christensen BM. Genetics of mosquito vector competence. Microbiol Mol Biol Rev. 2000;64 : 115–137. 10704476
3. Riehle MM, Markianos K, Niaré O, Xu J, Li J, Touré AM, et al. Natural malaria infection in Anopheles gambiae is regulated by a single genomic control region. Science. 2006;312 : 577–9. 16645095
4. Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, Miller LH, et al. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234 : 607–610. 3532325
5. Macdonald WW. The selection of a strain of Aedes aegypti susceptible to infection with semi-periodic Brugia malayi. Ann Trop Med Parasitol. 1962;56 : 368–372.
6. Bosio CF, Fulton RE, Salasek ML, Beaty BJ, Black WC IV. Quantitative trait loci that control vector competence for dengue-2 virus in the mosquito Aedes aegypti. Genetics. 2000;156 : 687–698. 11014816
7. Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476 : 454–7. doi: 10.1038/nature10356 21866160
8. Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376 : 1175–85. doi: 10.1016/S0140-6736(10)60586-7 20739055
9. Bockarie MJ, Pedersen EM, White GB, Michael E. Role of vector control in the global program to eliminate lymphatic filariasis. Annu Rev Entomol. 2009;54 : 469–87. doi: 10.1146/annurev.ento.54.110807.090626 18798707
10. Dissanaike AS. Zoonotic aspects of filarial infections in man. Bull World Health Organ. 1979;57 : 349–57. 314349
11. Erickson SM, Xi Z, Mayhew GF, Ramirez JL, Aliota MT, Christensen BM, et al. Mosquito infection responses to developing filarial worms. PLoS Negl Trop Dis. 2009;3: e529. doi: 10.1371/journal.pntd.0000529 19823571
12. Macdonald WW. The genetic basis of susceptibility to infection with semi-periodic Brugia malayi to Aedes aegypti. Ann Trop Med Parasitol. 1962;56 : 373–382.
13. Kobayashi M, Ogura N, Yamamoto H. Studies on filariasis VIII: Histological observation on the abortive development of Brugia malayi larvae in the thoracic muscles of the mosquitoes, Armigeres subalbatus. Jpn J Santi Zool. 1986;37 : 127–132.
14. Aliota MT, Fuchs JF, Mayhew GF, Chen C-C, Christensen BM. Mosquito transcriptome changes and filarial worm resistance in Armigeres subalbatus. BMC Genomics. 2007;8 : 463. 18088420
15. Paige C, Craig G Jr. Variation in filarial susceptibility among east African populations of Aedes aegypti. J Med Entomol. 1975;12 : 485–493. 1223294
16. Macdonald WW, Ramachandran CP. The influence of the gene fm (filarial susceptibility, Brugia malayi) on the susceptibility of Aedes aegypti to seven strains of Brugia, Wuchereria and Dirofilaria. Ann Trop Med Parasitol. 1965;59 : 64–73. 14297358
17. Macdonald WW, Sheppard PM. Cross-over values in the sex chromosomes of the mosquito Aedes aegypti and evidence for the presence of inversions. Ann Trop Med Parasitol. 1965; 74–87.
18. Severson DW, Mori A, Zhang Y, Christensen BM. Chromosomal mapping of two loci affecting filarial worm susceptibility in Aedes aegypti. Insect Mol Biol. 1994;3 : 67–72. 7987523
19. Juneja P, Osei-Poku J, Ho YS, Ariani C V, Palmer WJ, Pain A, et al. Assembly of the genome of the disease vector Aedes aegypti onto a genetic linkage map allows mapping of genes affecting disease transmission. PLoS Negl Trop Dis. 2014;8: e2652. doi: 10.1371/journal.pntd.0002652 24498447
20. Lowenberger CA, Ferdig MT, Bulet P, Khalili S, Hoffmann J a, Christensen BM. Aedes aegypti: induced antibacterial proteins reduce the establishment and development of Brugia malayi. Exp Parasitol. 1996;83 : 191–201. doi: 10.1006/expr.1996.0066 8682188
21. Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326 : 134–6. doi: 10.1126/science.1177531 19797660
22. Chalk R, Townson H, Ham P. Brugia panhangi: The effects of cecropins on microfilariae in vitro and in Aedes aegypti. Exp Parasitol. 1995;80 : 401–406. 7729475
23. Stathopoulos S, Neafsey DE, Lawniczak MKN, Muskavitch MAT, Christophides GK. Genetic dissection of Anopheles gambiae gut epithelial responses to Serratia marcescens. PLoS Pathog. 2014;10: e1003897. doi: 10.1371/journal.ppat.1003897 24603764
24. Weetman D, Wilding CS, Steen K, Morgan JC, Simard F, Donnelly MJ. Association mapping of insecticide resistance in wild Anopheles gambiae populations: major variants identified in a low-linkage disequilbrium genome. PLoS One. 2010;5: e13140. doi: 10.1371/journal.pone.0013140 20976111
25. Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson D a, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12 : 745–55. doi: 10.1038/nrg3031 21946919
26. Osei-Poku J. The evolution and genetics of vector competence in mosquito disease vectors. Ph. D Thesis, The University of Cambridge. Available: https://www.repository.cam.ac.uk/bitstream/handle/1810/245011/Osei-PokuPhD%20thesis.pdf. 2012. p. 226.
27. Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316 : 1718–23. 17510324
28. Lohse M, Bolger AM, Nagel A, Fernie AR, Lunn JE, Stitt M, et al. RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res. 2012;40: W622–7. doi: 10.1093/nar/gks540 22684630
29. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25 : 1754–60. doi: 10.1093/bioinformatics/btp324 19451168
30. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25 : 2078–9. doi: 10.1093/bioinformatics/btp352 19505943
31. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20 : 1297–1303. doi: 10.1101/gr.107524.110 20644199
32. Skotte L, Korneliussen TS, Albrechtsen A. Association testing for next-generation sequencing data using score statistics. Genet Epidemiol. 2012;36 : 430–7. doi: 10.1002/gepi.21636 22570057
33. Korneliussen TS, Albrechtsen A, Nielsen R. ANGSD: Analysis of Next Generation Sequencing Data. BMC Bioinformatics. 2014;15 : 356. 25420514
34. Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27 : 2987–93. doi: 10.1093/bioinformatics/btr509 21903627
35. Kim SY, Lohmueller KE, Albrechtsen A, Li Y, Korneliussen T, Tian G, et al. Estimation of allele frequency and association mapping using next-generation sequencing data. BMC Bioinformatics. BioMed Central Ltd; 2011;12 : 231. doi: 10.1186/1471-2105-12-231 21663684
36. McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010;26 : 2069–70. doi: 10.1093/bioinformatics/btq330 20562413
37. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9 : 357–9. doi: 10.1038/nmeth.1923 22388286
38. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. BioMed Central Ltd; 2013;14: R36. doi: 10.1186/gb-2013-14-4-r36 23618408
39. Ghedin E, Wang S, Spiro D, Caler E, Zhao Q, Crabtree J, et al. Draft genome of the filarial nematode parasite Brugia malayi. Science. 2007;317 : 1756–60. 17885136
40. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26 : 139–40. doi: 10.1093/bioinformatics/btp616 19910308
41. Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, et al. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat Protoc. 2013;8 : 1765–86. doi: 10.1038/nprot.2013.099 23975260
42. Kofler R, Pandey RV, Schlötterer C. PoPoolation2: identifying differentiation between populations using sequencing of pooled DNA samples (Pool-Seq). Bioinformatics. 2011;27 : 3435–6. doi: 10.1093/bioinformatics/btr589 22025480
43. Zou Z, Souza-Neto J, Xi Z, Kokoza V, Shin SW, Dimopoulos G, et al. Transcriptome analysis of Aedes aegypti transgenic mosquitoes with altered immunity. PLoS Pathog. 2011;7: e1002394. doi: 10.1371/journal.ppat.1002394 22114564
44. Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A. 2009;106 : 17841–6. doi: 10.1073/pnas.0905006106 19805194
45. Rancès E, Ye YH, Woolfit M, McGraw E a, O’Neill SL. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012;8: e1002548. doi: 10.1371/journal.ppat.1002548 22383881
46. Lawson D, Arensburger P, Atkinson P, Besansky NJ, Bruggner R V, Butler R, et al. VectorBase: a data resource for invertebrate vector genomics. Nucleic Acids Res. 2009;37: D583–7. doi: 10.1093/nar/gkn857 19028744
47. Timoshevskiy VA, Kinney NA, DeBruyn BS, Mao C, Tu Z, Severson DW, et al. Genomic composition and evolution of Aedes aegypti chromosomes revealed by the analysis of physically mapped supercontigs. BMC Biol. 2014;12 : 27. doi: 10.1186/1741-7007-12-27 24731704
48. Timoshevskiy VA, Severson DW, Debruyn BS, Black WC, Sharakhov I V, Sharakhova M V. An integrated linkage, chromosome, and genome map for the yellow fever mosquito Aedes aegypti. PLoS Negl Trop Dis. 2013;7: e2052. doi: 10.1371/journal.pntd.0002052 23459230
49. Ramachandran CP. Biological aspects in the transmission of Brugia malayi by Aedes aegypti in the laboratory. J Med Entomol. 1966;3 : 239–252. 4964879
50. Dissanayake SN, Ribeiro JM, Wang M-H, Dunn WA, Yan G, James AA, et al. aeGEPUCI: a database of gene expression in the dengue vector mosquito, Aedes aegypti. BMC Res Notes. 2010;3 : 248. doi: 10.1186/1756-0500-3-248 20920356
51. Choi Y-J, Aliota MT, Mayhew GF, Erickson SM, Christensen BM. Dual RNA-seq of parasite and host reveals gene expression dynamics during filarial worm-mosquito interactions. PLoS Negl Trop Dis. 2014;8: e2905. doi: 10.1371/journal.pntd.0002905 24853112
52. Rodriguez PH, Torres C, Marotta JA. Comparative development of Brugia malayi in susceptible and refractory genotypes of Aedes aegypti. J Parasitol. 1984;70 : 1001–2. 6527177
53. Zou Z, Shin SW, Alvarez KS, Kokoza V, Raikhel AS. Distinct melanization pathways in the mosquito Aedes aegypti. Immunity. Elsevier Ltd; 2010;32 : 41–53. doi: 10.1016/j.immuni.2009.11.011 20152169
54. Choi Y-J, Fuchs JF, Mayhew GF, Yu HE, Christensen BM. Tissue-enriched expression profiles in Aedes aegypti identify hemocyte-specific transcriptome responses to infection. Insect Biochem Mol Biol. Elsevier Ltd; 2012;42 : 729–38. doi: 10.1016/j.ibmb.2012.06.005 22796331
55. Roy SG, Raikhel AS. Nutritional and hormonal regulation of the TOR effector 4E-binding protein (4E-BP) in the mosquito Aedes aegypti. FASEB J. 2012;26 : 1334–42. doi: 10.1096/fj.11-189969 22159149
56. Borovsky D. Trypsin-modulating oostatic factor: a potential new larvicide for mosquito control. J Exp Biol. 2003;206 : 3869–3875. 14506222
57. Brackney DE, Isoe J, Black WC IV, Zamora J, Foy BD, Miesfeld RL, et al. Expression profiling and comparative analyses of seven midgut serine proteases from the yellow fever mosquito, Aedes aegypti. J Insect Physiol. Elsevier Ltd; 2010;56 : 736–44. doi: 10.1016/j.jinsphys.2010.01.003 20100490
58. Bonizzoni M, Dunn WA, Campbell CL, Olson KE, Dimon MT, Marinotti O, et al. RNA-seq analyses of blood-induced changes in gene expression in the mosquito vector species, Aedes aegypti. BMC Genomics. 2011;12 : 82. doi: 10.1186/1471-2164-12-82 21276245
59. Randall TA, Perera L, London RE, Mueller GA. Genomic, RNAseq, and molecular modeling evidence suggests that the major allergen domain in insects evolved from a homodimeric origin. Genome Biol Evol. 2013;5 : 2344–58. doi: 10.1093/gbe/evt182 24253356
60. Bandi C, Anderson TJC, Genchi C, Blaxter ML, Celoria V. Phylogeny of Wolbachia in filarial nematodes. Proc R Soc B Biol Sci. 1998;265 : 2407–2413. 9921679
61. Jupatanakul N, Sim S, Dimopoulos G. Aedes aegypti ML and Niemann-Pick type C family members are agonists of dengue virus infection. Dev Comp Immunol. Elsevier Ltd; 2014;43 : 1–9. doi: 10.1016/j.dci.2013.10.002 24135719
62. Shin SW, Bian G, Raikhel AS. A Toll receptor and a cytokine, Toll5A and Spz1C, are involved in toll antifungal immune signaling in the mosquito Aedes aegypti. J Biol Chem. 2006;281 : 39388–95. 17068331
63. Mackay TFC, Stone EA, Ayroles JF. The genetics of quantitative traits: challenges and prospects. Nat Rev Genet. 2009;10 : 565–77. doi: 10.1038/nrg2612 19584810
64. Wilfert L, Schmid-Hempel P. The genetic architecture of susceptibility to parasites. BMC Evol Biol. 2008;8 : 187. doi: 10.1186/1471-2148-8-187 18590517
65. Schwartz A, Koella JC. Melanization of Plasmodium falciparum and C-25 Sephadex beads by field-caught Anopheles gambiae (Diptera: Culicidae) from southern Tanzania. J Med Entomol. 2002;39 : 84–88. 11931276
66. Macdonald SJ, Long AD. A potential regulatory polymorphism upstream of hairy is not associated with bristle number variation in wild-caught Drosophila. Genetics. 2004;167 : 2127–31. 15342546
67. Magwire MM, Fabian DK, Schweyen H, Cao C, Longdon B, Bayer F, et al. Genome-wide association studies reveal a simple genetic basis of resistance to naturally coevolving viruses in Drosophila melanogaster. PLoS Genet. 2012;8: e1003057. doi: 10.1371/journal.pgen.1003057 23166512
68. Poirie M, Frey F, Hita M, Huguet E, Lemeunier F, Periquet G, et al. Drosophila resistance genes to parasitoids: chromosomal location and linkage analysis. Proc R Soc B Biol Sci. 2000;267 : 1417–1421. 10983825
69. Orr HA, Shannon I. The genetics of adaptation: the genetic basis of resistance to wasp parasitism in Drosophila melanogaster. Am Nat. Society for the Study of Evolution; 1997;51 : 1877–1885.
70. Hill AVS. Evolution, revolution and heresy in the genetics of infectious disease susceptibility. Philos Trans R Soc B Biol Sci. 2012;367 : 840–849. doi: 10.1098/rstb.2011.0275 22312051
71. Rodriguez P, Craig G Jr. Susceptibility to Brugia pahangi in geographic strains of Aedes aegypti. Am J Trop Med Hygeine. 1973;22 : 53–61. 4684889
72. Terwedow HA, Craig GB. Infection of female and male Aedes aegypti (Diptera: Culicidae) with the filarial parasite Waltonella flexicauda. J Med Entomol. 1977;14 : 421–424. 305482
73. Sulaiman I, Townson H. The genetic basis of susceptibility to infection with Dirofilaria immitis in Aedes aegypti. Ann Trop Med Parasitol. 1980;74 : 635–646. 7458468
74. Mackay TFC, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, et al. The Drosophila melanogaster Genetic Reference Panel. Nature. 2012;482 : 173–8. doi: 10.1038/nature10811 22318601
75. Waterhouse RM, Kriventseva E V, Meister S, Xi Z, Alvarez KS, Bartholomay LC, et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316 : 1738–43. doi: 10.1126/science.1139862 17588928
76. Turner JD, Langley RS, Johnston KL, Gentil K, Ford L, Wu B, et al. Wolbachia lipoprotein stimulates innate and adaptive immunity through Toll-like receptors 2 and 6 to induce disease manifestations of filariasis. J Biol Chem. 2009;284 : 22364–78. doi: 10.1074/jbc.M901528200 19458089
77. Kokoza V, Ahmed A, Woon Shin S, Okafor N, Zou Z, Raikhel AS. Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2010;107 : 8111–6. doi: 10.1073/pnas.1003056107 20385844
78. Castillo JC, Reynolds SE, Eleftherianos I. Insect immune responses to nematode parasites. Trends Parasitol. Elsevier Ltd; 2011;27 : 537–47. doi: 10.1016/j.pt.2011.09.001 21982477
79. Christensen BM, Lafond MM. Parasite-induced suppression of the immune response in Aedes aegypti to Brugia pahangi. J Parasitol. 1986;72 : 216–219. 3734990
80. Hansen IA, Attardo GM, Park J-H, Peng Q, Raikhel AS. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc Natl Acad Sci U S A. 2004;101 : 10626–31. 15229322
81. Evans AM, Aimanova KG, Gill SS. Characterization of a blood-meal-responsive proton-dependent amino acid transporter in the disease vector, Aedes aegypti. J Exp Biol. 2009;212 : 3263–71. doi: 10.1242/jeb.029553 19801431
82. Hurd H, Hogg JC, Renshaw M. Interactions between bloodfeeding, fecundity and infection in mosquitoes. Parasitol Today. 1995;11 : 411–416.
83. Caragata EP, Rancès E, O’Neill SL, McGraw EA. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb Ecol. 2014;67 : 205–18. doi: 10.1007/s00248-013-0339-4 24337107
84. Sylvestre G, Gandini M, Maciel-de-Freitas R. Age-dependent effects of oral infection with dengue virus on Aedes aegypti (Diptera: Culicidae) feeding behavior, survival, oviposition success and fecundity. PLoS One. 2013;8: e59933. doi: 10.1371/journal.pone.0059933 23555838
85. Ferdig MT, Beerntsen BT, Spray FJ, Li J, Christensen BM. Reproductive costs associated with resistance in a mosquito-filarial worm system. Am J Trop Med Hygeine. 1993;49 : 756–762. 7904130
86. Gaaboub A. Observations on the basal follicle numbers developed per female of two strains of Aedes aegypti after being fed on hosts with different levels of microfilariae of Brugia pahangi. J Invertebr Pathol. 1976;28 : 203–207. 965782
87. Javadian E, Macdonald WW. The effect of infection with Brugia pahangi and Dirofilaria repens on the egg-production of Aedes aegypti. Ann Trop Med Parasitol. 1974;68 : 477–81. 4480018
88. Rono MK, Whitten MMA, Oulad-Abdelghani M, Levashina EA, Marois E. The major yolk protein vitellogenin interferes with the anti-plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol. 2010;8: e1000434. doi: 10.1371/journal.pbio.1000434 20652016
89. Brackney DE, Foy BD, Olson KE. The effects of midgut serine proteases on dengue virus type 2 infectivity of Aedes aegypti. Am J Trop Med Hyg. 2008;79 : 267–74. 18689635
90. Molina-Cruz A, Gupta L, Richardson J, Bennett K, Black W IV, Barillas-Mury C. Effect of mosquito midgut trypsin activity on dengue-2 virus infection and dissemination in Aedes aegypti. Am J Trop Med Hyg. 2005;72 : 631–7. 15891140
91. Shahabudin M, Criscio M, Kaslow D. Unique specificity of in vitro inhibition of mosquito midgut trypsin-like activity correlates with in vivo inhibition of malaria parasite infectivity. Exp Parasitol. 1995;80 : 212–219. 7534722
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



