-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
Hepatitis C virus (HCV) replicates its genome in close association to cellular membranes which serve as assembly site of multi-subunit replication complexes. The process of replication complex maturation must be properly controlled to prevent the non-functional maturation/assembly of these complexes. In this process, the temporal regulation of viral polyprotein processing often plays a pivotal role as exemplified by the strict requirement for NS2-NS3 cleavage for HCV genome replication. We demonstrate here that a conserved hydrophobic NS3 surface patch activates the NS2 protease to stimulate NS2-NS3 cleavage. By dissecting the role of these NS3 surface residues in viral RNA replication, we show that one of these NS3 residues is also a critical determinant for HCV genome replication by negatively regulating NS5A hyperphosphorylation. Surprisingly, further experiments revealed that the NS2-NS3 cleavage is a prerequisite for NS5A hyperphosphorylation. To fulfill the requirements for gradual assembly into functional replication complexes, an ordered cascade of molecular events takes place: in uncleaved NS2-NS3, the hydrophobic NS3 surface patch promotes NS2 protease stimulation; upon NS2-NS3 cleavage, this surface region becomes available for functional replicase assembly. As a consequence, the hydrophobic surface patch on free NS3 can promote NS5A hyperphosphorylation as an indication of functional replicase assembly.
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004736
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004736Summary
Hepatitis C virus (HCV) replicates its genome in close association to cellular membranes which serve as assembly site of multi-subunit replication complexes. The process of replication complex maturation must be properly controlled to prevent the non-functional maturation/assembly of these complexes. In this process, the temporal regulation of viral polyprotein processing often plays a pivotal role as exemplified by the strict requirement for NS2-NS3 cleavage for HCV genome replication. We demonstrate here that a conserved hydrophobic NS3 surface patch activates the NS2 protease to stimulate NS2-NS3 cleavage. By dissecting the role of these NS3 surface residues in viral RNA replication, we show that one of these NS3 residues is also a critical determinant for HCV genome replication by negatively regulating NS5A hyperphosphorylation. Surprisingly, further experiments revealed that the NS2-NS3 cleavage is a prerequisite for NS5A hyperphosphorylation. To fulfill the requirements for gradual assembly into functional replication complexes, an ordered cascade of molecular events takes place: in uncleaved NS2-NS3, the hydrophobic NS3 surface patch promotes NS2 protease stimulation; upon NS2-NS3 cleavage, this surface region becomes available for functional replicase assembly. As a consequence, the hydrophobic surface patch on free NS3 can promote NS5A hyperphosphorylation as an indication of functional replicase assembly.
Introduction
Hepatitis C virus (HCV) is a single-stranded positive-sense RNA virus belonging to the family Flaviviridae and is with 170 million infected individuals worldwide an important cause of chronic liver disease [1]. The positive sense RNA genome contains a 5’-untranslated region (UTR), a single open reading frame (ORF) that encodes both structural as well as non-structural (NS) viral proteins and a 3’ UTR. Cap-independent translation of the viral genome yields a single polyprotein that is co - and posttranslationally processed into the individual proteins by host signal peptidases and two viral proteases NS2-NS3 and NS3-4A. The host signal peptidases cleave at the junctions of Core/E1, E1/E2, E2/p7 and p7/NS2 [2–4]. The NS3/NS4A serine protease complex mediates the cleavages of the non-structural proteins NS3-NS5B [5,6]. The chymotrypsin-like serine protease domain residing in the N - terminal 180 amino acids of NS3 requires NS4A as a cofactor for full activity [5,7,8]. NS3 harbors downstream of the protease domain ATPase and helicase activities [9].
The NS2 protein (217 amino acids, aa) is membrane-associated via its N-terminal domain that consists of three putative transmembrane segments with a perinuclear ER localization [10,11]. The C-terminal protease domain (aa 94–217) resides on the cytoplasmic face of the ER membrane [10,12] and, with the N-terminal domain of NS3, forms the NS2-NS3 autoprotease that catalyzes the cleavage at the NS2/NS3 site [7,13,14]. The putative catalytic triad of the NS2-NS3 protease resides entirely in NS2 and autocleavage at the NS2/NS3 junction is independent of the NS3 serine protease activity [7,15,16]. The NS2 protease domain is highly conserved among HCV genotypes and its crystal structure indicates that a dimer forms a composite active site with a catalytic triad analogous to those of cysteine proteases [16]. Recently, NS2, followed by only two residues of NS3, has been shown to be a bona fide protease exhibiting low-level intrinsic protease activity. This NS2 protease activity is stimulated by the NS3 serine protease domain (residues 1–180) defining this domain as stimulatory cofactor for NS2 [17]. Cleavage between NS2 and NS3 is essential for RNA genome replication of the full-length virus and subgenomic NS2-NS5B replicons but not for NS3/4A serine protease activity [18–20]. Furthermore, the NS2 protein, but not its proteolytic activity, is required for the production of infectious virus [21–29]. The crystal structure of the NS2 protease domain represents the post-cleavage conformation, which likely differs from the one of the NS2-NS3 precursor [16]. Due to the lack of the NS2-NS3 structure, little is known about the NS2-NS3 cleavage mechanism and its regulation by NS3. Mechanistically, it was proposed that conserved surface areas of NS2 and NS3 may interact to contribute to a functional catalytic NS2-NS3 environment and correct positioning of the scissile bond [16].
To identify molecular features that are critical for NS2 protease stimulation by NS3, we conducted a comprehensive mutagenesis screen of the entire NS3 protease domain. We identified a conserved hydrophobic NS3 surface patch that is essential for efficient NS2 protease activation in the context of uncleaved NS2-NS3 and demonstrate that NS2 protease stimulation mainly depends on hydrophobic protein-protein interactions. Furthermore, mutational analysis of this NS3 surface patch revealed that this area is also pivotal for viral RNA replication and NS5A hyperphosphorylation. Based on these findings we propose a sequential cascade of molecular events where the hydrophobic NS3 surface patch orchestrates NS2 protease stimulation, NS5A hyperphosphorylation and viral RNA replication. Moreover, this study defines an unexpected effect of the NS2-NS3 cleavage and offers a molecular understanding why efficient NS2-NS3 cleavage and the generation of free NS3 are essential for viral RNA replication.
Results
Identification of amino acids in the NS3 protease domain required for the activation of the NS2 protease by alanine scanning mutagenesis
The NS2 protease has a low-level intrinsic ability to cleave the NS2-3 precursor protein and this function is strongly enhanced by the NS3 protease domain (aa 1–180) [17,30]. The NS2-NS3 cleavage can be analyzed in a cell-based assay by expressing HCV NS2-NS3 polyprotein fragments from T7 promoter of pcite-FLAG-NS2-NS3(1–172)GST plasmid in T7 polymerase producing Huh-7/T7 cells additionally boosted by the MVA-T7pol vaccinia virus system followed by Western blot analysis (Fig. 1A and 1B).
Fig. 1. Di-alanine scanning mutagenesis of the NS3 protease domain identified amino acids important for the NS2-protease activation by NS3. 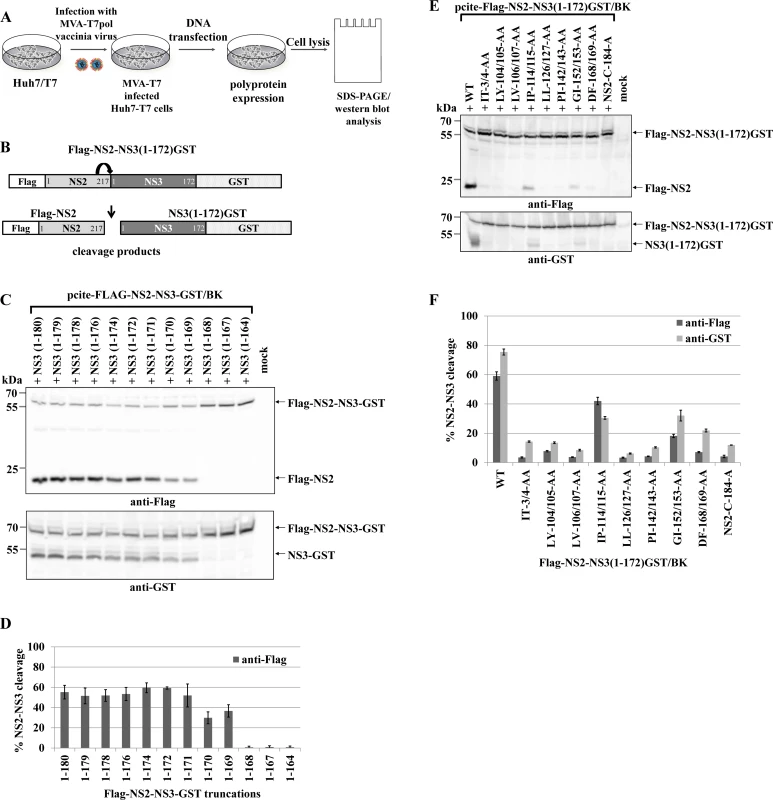
(A) The workflow to identify NS3 mutations that inhibit NS2-NS3 cleavage. (B) Scheme of the HCV NS2-NS3 cleavage assay. NS2-mediated cleavage of expressed FLAG-NS2-NS3(1–172)GST/BK polyprotein fragments results in the generation of FLAG-NS2 and NS3(1–172)GST cleavage products. (C) Effect of NS2-NS3 truncations on NS2-NS3 autoprocessing. Plasmids with the indicated NS3 C-terminal truncations were expressed and NS2-NS3 cleavage was detected by Western blotting. NS3(1–180) refers to FLAG-NS2-NS3(1–180)GST/BK. Mock indicates cells transfected with a pcite2a vector control. The positions of FLAG-NS2-NS3-GST, FLAG-NS2 and NS3-GST proteins are indicated by arrows. (D) Levels of NS2-NS3 cleavage were quantified from Western blots. (E) Inhibition of NS2-NS3 cleavage by selected alanine mutations in NS3. Plasmids encoding FLAG-NS2-NS3(1–172)GST with the indicated NS3 mutations were expressed and NS2-NS3 cleavage was detected by Western blotting. WT refers to wild type FLAG-NS2-NS3(1–172), and NS2-C184-A refers to a plasmid expressing NS2-NS3 polyprotein with a NS2-protease active site mutation. Mock indicates cells transfected with a vector control. The positions of the FLAG-NS2-NS3(1–172)GST, NS3(1–172)GST, and FLAG-NS2 proteins are indicated by arrows. (F) Signals of Flag-NS2-NS3GST and NS3GST derivatives were quantified by ImageJ software from three Western blots to calculate the percentage of NS2-NS3 cleavage. To investigate the mechanism of NS2-NS3 cleavage, we aimed at the identification of residues in the NS3 protease domain that are critical for NS2 stimulation. Accordingly, we established an experimental set-up that retains robust NS2-NS3 cleavage but yet is sensitive to perturbations of NS2 activation when mutating residues in NS3. To this end, analysis of C-terminal truncations of the NS3 protease domain revealed that NS3 residues 1–169 were able to detectably activate NS2-NS3 cleavage. Furthermore, a NS3 protease domain consisting of aa 1–172 was found to be sufficient to stimulate NS2-NS3 cleavage to a level comparable with NS2-NS3(1–180) (Fig. 1C and 1D). Therefore, we used pcite-FLAG-NS2-NS3(1–172)GST as the basis for our di-alanine scanning mutagenesis of the entire (genotype 1b)-derived NS3 protease domain and determined the impact of these di-alanine NS3 mutations on the NS2 activation by measuring the NS2-NS3 cleavage by Western blotting (Fig. 1A and 1B).
Among all tested NS3 di-alanine mutants we identified 8 candidates that either strongly interfere with (IP114/115AA and GI152/153AA) or inhibit (IT3/4AA, LY104/105AA, LV106/107AA, LL126/127AA, PL142/143AA and DF168/169AA) the NS2-NS3 cleavage (Fig. 1E and 1F). To rule out that these NS3 mutations affect NS3 protein function(s) not related to the NS2-activation, we introduced the inhibitory mutations into pcite-NS3(1–172)GST/BK and determined their NS3 serine protease activity in a trans-cleavage assay in the presence of the NS4A cofactor with a NS4B/NS5A serine protease substrate (S1A Fig.). While the NS3 mutations IT3/4AA and IP114/115AA exhibit serine protease activity comparable to wild type NS3 in this trans-cleavage assay, mutations LY104/105AA and LL126/127AA reduced NS3 serine protease activity but still allowed for detectable cleavage of the NS4B/NS5A substrate (S1B and S1C Fig.). In contrast, the NS3 double mutations LV106/107AA, PL142/143AA, GI152/153AA and DF168/169AA did not display detectable NS3 serine protease activity in this assay (S1B and S1C Fig.). Mapping of these NS3 residues on a NS3 structure revealed that residues LV106/107, PL142/143, GI152/153 and DF168/169 are directed towards the center of the NS3 protein suggesting that changing these amino acids pairwise to alanine might affect proper NS3 protein folding (S2 Fig.). Based on these observations only the NS3 mutations IT3/4AA, LY104/105AA, IP114/115AA and LL126/127AA block the NS2 protease activating function of NS3 but still allow for NS3/NS4A serine protease activity. Accordingly, NS3 mutations LV106/107AA, PI142/143AA, GI152/153AA and DF168/169AA were not further analyzed.
A hydrophobic surface patch in the NS3 protease domain is the main determinant for the NS2 protease stimulation by NS3
Since activation of NS2 by NS3 should require interaction of NS2 with NS3, we hypothesized that the amino acids involved, should be localized on the NS3 surface. Mapping of NS3 mutations that selectively blocked NS2 protease activation onto the NS3 crystal structure [31] revealed that I3, Y105, P115 and L127 constitute a continuous hydrophobic surface patch, while the residues T4, L104, I114 and L126 are directed more towards the protein core (Fig. 2A).
Fig. 2. A conserved hydrophobic NS3 surface patch consisting of residues Y105, P115 and L127 is important for NS2-activation by NS3. 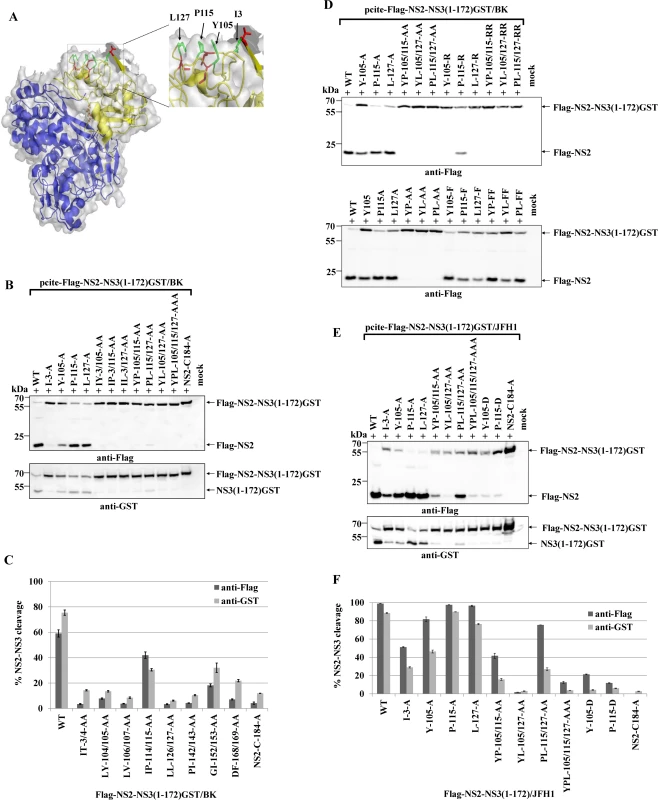
(A) Location of the residues I3, Y105, P115 and L127 in the NS3 structure of the genotype 1b. The surface residues are shown in green stick representation. The residues T4, L104, I114 and L126 are depicted in red stick representation. The overall NS3 structure is shown in grey surface representation. Carbon and backbone ribbon are colored in yellow for the protease domain and blue for the helicase domain, respectively. An enlargement of the NS3 surface patch consisting of I3, Y105, P115 and L127 is shown on the right. The figure was generated using Pymol version 1.10 and the coordinates of PDB code 1CU1 [30]. (B) Effect of alanine substitutions in NS3 of HCV strain BK on NS2-NS3 cleavage efficiency. Plasmids expressing either wild type (WT) or mutant FLAG-NS2-NS3(1–172)GST/BK were transfected into Huh7/T7 cells and the cleavage activity was analyzed by Western blot analysis. (C) Quantification of NS2-NS3 cleavage from Western blots related to Fig. 2B using ImageJ software. (D) The hydrophobic character of the NS3 surface area is important for the NS2 protease stimulation by NS3. The cleavage efficiencies of the indicated BK NS2-NS3 polyprotein fragments carrying either charged (arginine) or hydrophobic (phenylalanine) NS3 amino acid substitutions were determined by Western blot analysis. Quantification of these Western blots is presented in S3 Fig. (E) The importance of hydrophobic NS3 surface residues Y105, P115 and L127 for NS2-activation by NS3 is conserved in HCV genotype 2a (JFH1). Plasmids expressing either wild type (WT) or mutant FLAG-NS2-NS3(1–172)GST/JFH1 were transfected into Huh-7/T7 cells and NS2-NS3 cleavage was analyzed by Western blot analysis. Blots shown are representative from three different experiments. (F) Quantification of NS2-NS3 cleavage from Western blots related to Fig. 2E. To investigate if this surface area is critical for the NS2 protease stimulation by NS3, we mutated these residues individually as well as simultaneously and analyzed their impact on NS2-NS3 cleavage. While the individual NS3 mutations Y105A, P115A and L127A allowed for NS2-NS3 cleavage to different degrees, their combinations inhibited NS2-NS3 processing, indicating that the identified hydrophobic patch is indeed pivotal for the NS3-mediated NS2 protease stimulation (Fig. 2B). Since the I3A mutation is located in the proximity of the NS2/NS3 site and thus might act as a cleavage site mutant we did not focus on this residue. In order to evaluate if the hydrophobic character or the amino acid identity are critical for NS2 activation, we mutated the surface amino acids Y105, P115 and L127 individually and simultaneously to either phenylalanine or arginine. While all single or double phenylalanine exchanges were functionally tolerated to various degrees (Fig. 2D), the introduction of a single charged amino acid at position 105 (Y105R) or 127 (L127R) strongly inhibited NS2 protease activation (Fig. 2D and S3 Fig.). In contrast, a single proline-to-arginine exchange at position 115 (P115R) had only a moderate effect on NS2-NS3 cleavage indicating more flexibility at this position provided that the surrounding area remains hydrophobic (Fig. 2D and S3 Fig.). These results revealed that the hydrophobicity of the amino acid side chains rather than their identities determine the function of this NS3 surface area for NS2 protease stimulation. The fact that the NS3 residues Y105, P115 and L127 are conserved among all HCV genotypes suggested that this hydrophobic patch and its role in the NS3-mediated NS2 activation represents a conserved feature in the HCV life cycle. To confirm this assumption, we introduced the mutations into pcite-FLAG-NS2-NS3(1–172)GST/JFH1 that is based on genotype 2a (JFH1) NS2-NS3 sequence and determined the extent of the NS2-NS3 cleavage. Overall, the efficiency of the NS2-NS3 cleavage in the presence of the minimal NS3-cofactor domain (aa 1–172) for JFH1 appears to be increased when compared to the genotype 1b (compare Fig. 2B and 2D). As observed for genotype 1b, individual exchanges of P115A and L127A had a minor influence on the NS2-NS3 cleavage efficiency in this genotype 2a context with Y105A displaying a moderate effect (Fig. 2F). In contrast, mutating two (YP105/115AA, YL105/127AA and to a lesser extent PL115/127AA) or all three (YPL105/115/127AAA) of the conserved surface residues simultaneously to alanine, strongly (YP105/115AA, YL105/127AA and YPL105/115/127AAA) or moderately (PL115/127AA) reduced the NS2 activation by NS3 (Fig. 2D). To further confirm this observation, the Flag-NS2-NS3(1–172)GST derivatives with either single (Y105A and P115A) or double alanine substitutions (YP105/115AA) in the hydrophobic patch of two different HCV genotypes, genotype 1b (BK) and genotype 2a (JFH1), were analyzed by a RIP assay. The WT and the NS2/C184A derivatives of both genotypes served as positive and negative controls, respectively. As shown in Fig. 3, the impact of the NS3 mutations on NS2-NS3 cleavage is in agreement with our Western blot results (compare Fig. 3B with Fig. 2C and 2F). Furthermore, the corresponding mutations in both genotypes have a similar impact on the NS2-NS3 cleavage when compared to their respective WT derivate (Fig. 3B). This demonstrates that the mechanism of the NS3-mediated NS2 protease stimulation is conserved among different genotypes.
Fig. 3. Mutational analysis of the NS3-mediated NS2 protease stimulation demonstrates that this process is conserved among different HCV genotypes. 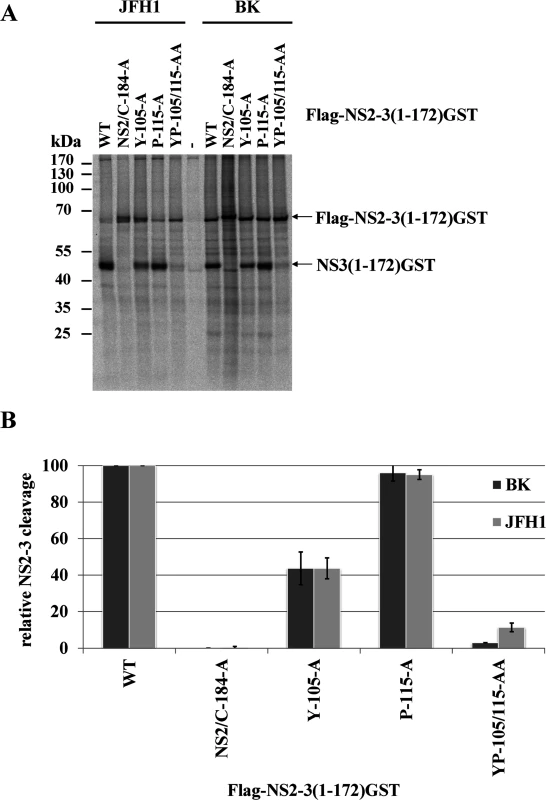
(A) Transient expression of Flag-NS2-3(1–172)GST derivatives from genotype 1b (BK) and genotype 2a (JFH1). After transient expression in the presence of [35S] methionine/cysteine by use of the T7 vaccinia virus system, the cell lysates were subjected to a radioimmunoprecipitation analysis using an anti-GST antibody. The transfected pcite Flag-NS2-NS3(1–172)GST plasmids encoding NS2-3 polyprotein fragments of genotype 1b (BK) or genotype 2a (JFH1) are indicated. WT, wild type; NS2/C184A, NS2-protease inactive mutant; mock, lysates of MVA-T7pol-infected cells were used for RIP assay. The positions of Flag-NS2-NS3(1–172)GST and NS3(1–172)GST are indicated by arrowheads. The positions of the mass standards are shown on the left. (B) Quantification of two independent RIP assays by phosphoimaging. The NS3 L127 residue of the hydrophobic surface patch plays an important role in regulating NS5A hyperphosphorylation and viral RNA replication
Assuming that the hydrophobic patch on the NS3 surface functions only in NS3-cofactor mediated NS2-stimulation, surface mutations in the context of a NS3-5B/JFH1 replicon should not affect polyprotein processing or RNA replication. To test this hypothesis, we introduced the NS3 mutations into the luciferase-expressing genotype 2a reporter replicon pFKI389-Luc/NS3-3’ and determined the RNA replication kinetics of the mutant RNAs relative to the wild type (WT) and non-replicative (GND) RNAs. Besides the four single mutations (I3A, Y105A, P115A and L127A), two simultaneous mutations (YP105/115AA and YPL105/115/127AAA) were analyzed. The majority of the single alanine mutations (I3A, Y105A or P115A) as well as the combination of Y105 and P115 mutations (YP105/115AA) had no detectable effect on RNA replication, indicating that the I3, Y105 and P115 surface exchanges do not inhibit any of the NS3 functions required for RNA replication in the NS3-NS5B/JFH1 replicon context (Fig. 4A). In contrast, the L127A exchange resulted in a strongly reduced RNA replication level when present either alone or in combination with Y105A or P115A mutations in the NS3-5B/JFH1 replicon, suggesting that this residue plays a critical role in HCV genome replication (Fig. 4A). To characterize the replication phenotype of L127A in more detail, we first determined if this mutation is affecting the NS3-4A serine protease activity. Accordingly, we introduced the entire set of NS3 mutations into a pcite-NS3-3’/JFH1 plasmid which allows the replication-independent expression of the NS3-5B polyprotein by using the MVA-T7pol vaccinia virus system. Western blot analysis confirmed that all NS3 surface mutations (including L127A) had no detectable effects on NS3 stability or NS3-NS5B polyprotein processing in this system (Fig. 4B). In contrast, the analysis of the NS5A phospho-form distribution led to an intriguing observation regarding levels of hyperphosphorylated NS5A. While the NS3 mutations I3A, Y105A, P115A and YP105/115AA exhibited NS5A hyperphosphorylation levels comparable to wild type, the L127A mutation individually or in combination with Y105A, P115A or YP105/115AA showed a strong reduction in NS5A hyperphosphorylation (Fig. 4B). Strikingly, the L127A-replication phenotype correlated with the significant reduction in the ratio of hyper - to basally phosphorylated NS5A (Fig. 4A and 4C). However, we cannot rule out that L127A also inhibits other NS3 function(s), not linked to its serine protease activity, which may also contribute to the negative replication phenotype.
Fig. 4. Mutational analysis of the hydrophobic NS3 surface area in the context of a HCV genotype 2a NS3-5B replicon. 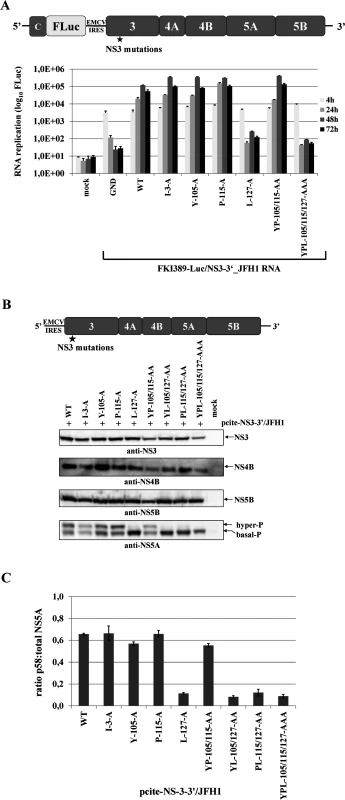
(A) Top, schematic representation of the HCV genotype 2a NS3-5B replicon. Bottom, NS3 mutations were engineered into pFKI389-Luc/NS3-3’_JFH1 (WT), in vitro transcribed RNA was electroporated into Huh7 Lunet cells, and luciferase activity was measured at 4, 24, 48 and 72 h post electroporation (pe). Mean values of three independent experiments are shown. Error bars indicate standard deviations. (B) Top, schematic of the expression construct pcite-NS3-3’/JFH1. This plasmid encodes the NS3 to NS5B sequence of the HCV genotype 2a (JFH1) as indicated. NS3 mutations were introduced into pcite-NS3-3’/JFH1 and plasmids were transfected into Huh-7/T7cells infected with MVA-T7pol vaccinia virus. Bottom, effect of the NS3 mutations on NS3-NS5B polyprotein processing and NS5A hyperphosphorylation. Cell lysates were prepared 20 h post transfection (pt) and analyzed by Western blotting with antibodies directed against HCV NS3, NS4B, NS5A and NS5B. The position of hyper and basally phosphorylated NS5A is indicated on the right. (C) Western blot signals of NS5A p56 and p58 forms of three Western blots were quantified by ImageJ software and the ratio of hyperphosphorylation (p58) to total NS5A was calculated. WT, wild type; GND, polymerase inactive mutant; mock, cells transfected with vector control. NS3 mutations defective in NS2 protease stimulation block RNA replication in a NS2-5B replicon system
NS2-NS3 cleavage is essential to liberate NS3, a step that has been shown to be critical for viral RNA replication [32]. Accordingly, NS3 mutations that interfere with the NS2 protease activation in the context of the NS2-5B/JFH1 polyprotein should not only reduce the NS2-NS3 cleavage but also inhibit RNA replication similar to what has been demonstrated with NS2 mutations inactivating the NS2 protease [32]. To test this assumption, we introduced the NS3 mutations into the genotype 2a replicon pFKI389-Luc/NS2-3’ and determined the RNA replication kinetics of the mutant RNAs relative to the wild type (WT) and the non-replicative (GND) replicon RNAs. A replicon carrying a NS2-active site mutation (NS2/C-184-A) was used as a further non-replicative control which is defective in NS2-NS3 cleavage but retains its serine protease activity. The single mutations I3A, Y105A and P115A in the NS2-5B replicon RNA allowed for RNA replication to different degrees: the P115A mutant replicon RNA replicated to wild type levels whereas the mutant replicon RNAs I3A and Y105A showed a greater than 100-fold reduction in RNA replication (Fig. 5A). The L127A mutation in the NS2-5B/JFH1 replicon inhibited RNA replication to levels similar to the ones observed in the NS3-5B/JFH1 replicon. Most interestingly, the NS2-5B/JFH1 replicon with the YP105/115AA mutation that interferes with the NS2 activation by NS3 did not detectably replicate indicating that inefficient NS2-NS3 cleavage triggered by NS3 surface mutations blocks viral RNA replication (Fig. 5A).
Fig. 5. Mutational analysis of the NS3 mutations in the context of a HCV genotype 2a subgenomic NS2-5B replicon revealed NS2-NS3 cleavage is a prerequisite for NS5A hyperphosphorylation. 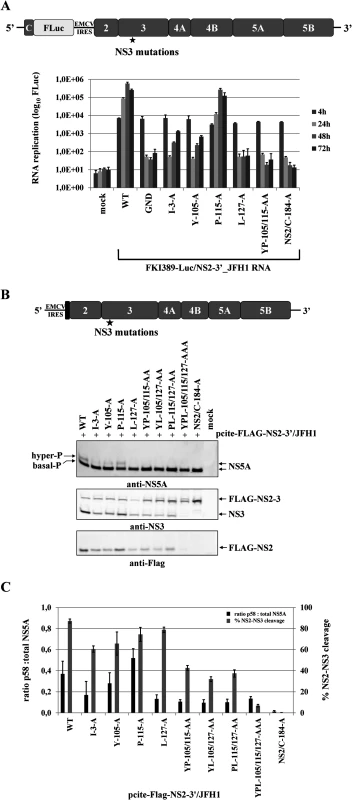
(A) Top, schematic representation of the FKI389-Luc/NS2-3’_JFH1 replicon. Bottom, the NS3 mutations were introduced into pFKI389-Luc/NS2-3’_JFH1, in vitro transcribed RNA was electroporated into Huh7 Lunet cells, and luciferase activity was measured at 4, 24, 48 and 72 h pe. Mean values of three independent experiments are shown. Error bars indicate standard deviations. (B) Top, schematic of the expression construct pcite-FLAG-NS2-3’/JFH1. This plasmid encodes the T7 promotor sequence fused to the EMCV IRES followed by a FLAG epitope and NS2 to NS5B sequence of the HCV isolate 2a. Indicated NS3-cofactor mutations were engineered into pcite-NS2-3’/JFH1 and plasmids were transfected into Huh-7/T7cells infected with MVA-T7pol vaccinia virus. Bottom, effects of the NS3-cofactor mutations on NS2/3 cleavage, polyprotein processing and NS5A hyperphosphorylation in the context of NS2-5B. Cell lysates were prepared 20 h pt and analyzed by Western blotting with antibodies against FLAG-epitope, NS3 and NS5A. The positions of FLAG-NS2-3, FLAG-NS2, NS3 as well as the hyper and basally phosphorylated NS5A are indicated on the right. (C) Signals of Flag-NS2-3 and NS3 were quantified by ImageJ software from two Western blots to calculate the percentage of NS2-3 cleavage. Signals of NS5A p56 and p58 forms of two Western blots were quantified by ImageJ software and the ratio of hyperphosphorylation (p58) to total NS5A was calculated. WT, wild type; GND, polymerase-inactive mutant; NS2/C-184-A, NS2-protease inactive mutant; mock, cells transfected with vector control. Efficient NS2-3 cleavage is a prerequisite for NS5A hyperphosphorylation and RNA replication in a NS2-5B replicon system
Several determinants for NS5A hyperphosphorylation have been mapped to NS3, NS4A and NS4B and expression of the NS3-5A polyprotein is required for this process. As demonstrated above, the NS3 mutation L127A strongly reduced NS5A hyperphosphorylation as well as RNA replication (Fig. 4). At the same time, L127, together with Y105 and P115, constitutes the NS3 surface patch that is required for activation of the NS2 protease in the context of uncleaved NS2-NS3 (Fig. 2). Therefore, we asked whether NS2-NS3 cleavage in a NS2-5B polyprotein is mechanistically linked to NS5A hyperphosphorylation. This hypothesis was based on the assumption that the accessibility of L127 on the NS3 surface should be compromised in the context of uncleaved NS2-NS3 with respect to its function in NS5A hyperphosphorylation. To assess the role of NS2-NS3 cleavage efficiency in regulating NS5A hyperphosphorylation experimentally, a panel of pcite-FLAG-NS2-3’/JFH1 plasmids was used for NS2-NS5B polyprotein expression in Huh-7/T7cells. For a rigorous test, a pcite-FLAG-NS2-3’/JFH1 variant carrying an NS2 protease active-site mutation (NS2/C-184-A) was used. As expected, the analysis of the NS2/C-184-A mutant showed that NS2-NS3 cleavage was abrogated without affecting polyprotein processing downstream of NS3 by the serine protease activity (Fig. 5B). In addition, the abrogation of NS2-NS3 cleavage correlated with a complete loss of NS5A hyperphosphorylation (Fig. 5B). Therefore, the NS3 release from uncleaved NS2-NS3 appears to be a prerequisite for NS5A hyperphosphorylation.
Further analyses revealed that single NS3 mutations had small but detectable effects on NS2-NS3 cleavage efficiency compared to wild type, while combinations of NS3 mutations (YP105/115AA, PL115/127AA and YL105/127AA) further decreased NS2-NS3 cleavage (Fig. 5B and 5C) and the triple mutation (YPL105/115/127AAA) almost abolished cleavage. Importantly, for double and triple mutants with decreased NS2-NS3 cleavage efficiency the analysis of NS5A revealed strongly reduced levels of hyperphosphorylated NS5A (Fig. 5B and 5C). In addition, analysis of NS5A hyperphosphorylation revealed that the small but detectable effects on NS2-NS3 cleavage efficiency for the single NS3 mutants I3A and Y105A also correlated with a reduced NS5A hyperphosphorylation compared to either wild type or P115A. In contrast, L127A decreased the production of NS5A hyperphosphorylation to levels comparable with the double or triple mutations (Fig. 5B). Thus, the effect of L127A is similar to what has been observed in the NS3-5B polyprotein. The quantification of NS2-NS3 cleavage and NS5A hyperphosphorylation indicates that an efficient NS2-NS3 cleavage increases the ratio of hyper - to basal-phosphorylation of NS5A (Fig. 5B and 5C). Together, these results strengthen our observation that NS5A hyperphosphorylation is functionally linked to NS2-NS3 cleavage in a NS2-5B polyprotein context.
The dual function of the NS3 surface patch in stimulating NS2 activation and regulating NS5A hyperphosphorylation is conserved among HCV genotypes
Recent work characterizing NS5A hyperphosphorylation in replicons of HCV genotype 1b (Con1) and genotype 2a (JFH1) isolates revealed significant differences concerning the degree of correlation between NS5A hyperphosphorylation and viral RNA replication [33–36]. Therefore, we determined if L127 is also critical for NS5A hyperphosphorylation in the context of the NS3-5B/Con1 replicase. Accordingly, the NS3 mutations were introduced into a NS3-5B/Con1 luciferase reporter replicon and analyzed for their effect on RNA replication (Fig. 6A). NS3-5B/Con1 polyprotein processing as well as the NS5A phospho-form distribution were determined by Western blotting using Huh-7/T7 cells and the MVA-T7pol vaccinia virus system (Fig. 6B). In agreement with the results obtained for the NS3-5B/JFH1 replicon, the NS3-5B/Con1 replicons encoding for the NS3 mutants Y105A, P115A and YP105/115AA showed robust RNA replication, comparable to wild type, while a replicon encoding the NS3 L127A exchange was replication-deficient (Fig. 6A). Transient protein expression in cells transfected with the corresponding set of mutant pcite-NS3-5B constructs followed by immunoblotting assays did not reveal a substantial inhibition of the NS3-5B/Con1 polyprotein processing indicating that no apparent defect in the NS3-NS4A serine protease was induced by the NS3 mutations (Fig. 6B). In agreement with our results for JFH1, only the substitution L127A significantly reduced the ratio of hyper - to basal phosphorylated NS5A relative to wild type in the context of the NS3-5B/Con1 polyprotein (Fig. 6B).
Fig. 6. NS3-L127A inhibits RNA replication and suppresses NS5A hyperphosphorylation in the context of a HCV genotype 1b NS3-5B replicon. 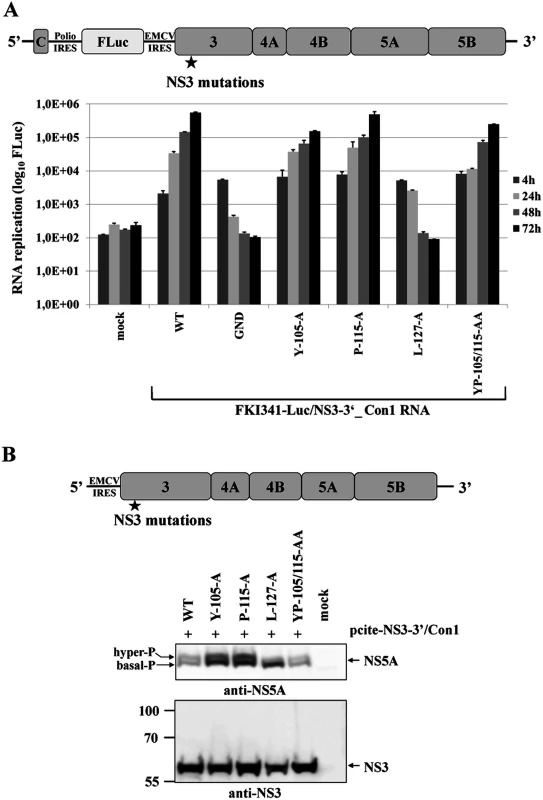
(A) Top, schematic of the HCV genotype 1b NS3-5B replicon. Bottom, the NS3 mutations were engineered into pFKI341-Luc/NS3-3’_Con1 (WT), transcribed RNA was electroporated into Huh7 Lunet cells. Luciferase activity was measured at 4, 24, 48 and 72 h pe. Mean values of three independent experiments are shown, error bars indicate standard deviations. (B) Top, schematic of pcite-NS3-3’/Con1. This plasmid encodes the NS3 to NS5B sequence of the HCV isolate 1b (Con1). NS3 mutations were introduced into pcite-NS3-3’/Con1 and plasmids were transfected into Huh-7/T7cells infected with MVA-T7pol vaccinia virus. Bottom, effects of the NS3 mutations on NS3-5B polyprotein processing and NS5A hyperphosphorylation. Cell lysates were prepared 20 h pt and analyzed by Western blotting with anti NS3 and NS5A antibodies. The position of hyper and basally phosphorylated NS5A is indicated on the left. WT, wild type; GND, polymerase-inactive mutant; mock, cells transfected with vector control. Next, we also tested in the Con1 replicon system if a correlation between the NS2-NS3 cleavage efficiency and NS5A hyperphosphorylation exists. To this end we first determined the replication capacities of mutant NS2-5B/Con1 replicons in a transient replication assay. Among the mutant NS2-5B/Con1 replicons that were examined only the one encoding the NS3 P115A exchange replicated to wild type level (Fig. 7A). All other NS2-5B/Con1 replicons examined did not replicate to detectable levels. The observation that the NS2-5B/Con1 replicons with the NS2/C184A, the YP105/115AA and the L127A mutations showed no replication corroborated our earlier observations in the NS2-5B/JFH1 replicon system. The fact that the NS2-5B/Con1 Y105A replicon derivative did also fail to replicate was somewhat unexpected and differs from the Y105A NS2-5B/JFH1 mutant replicon that still replicates at low level compared to WT (Fig. 5A). Processing of the respective Y105A NS2-5B/Con1 polyprotein upon expression by the MVA-T7 pol vaccinia virus system revealed that the NS2-NS3 cleavage was already strongly reduced compared to either wild type or the NS3 P115A mutant NS2-5B (Fig. 7B and 7C). Moreover, in cells expressing the NS2-5B/Con1 Y105A polyprotein, NS5A hyperphosphorylation was almost undetectable in our Western blot system when compared to the wild type or NS2-5B/Con1 (P115A) polyprotein (Fig. 7B and 7C). Together, these findings again emphasize the functional link between NS2-NS3 cleavage efficiency and NS5A hyperphosphorylation.
Fig. 7. Mutational analysis of NS3 surface residues in a HCV genotype 1b NS2-5B replicon confirm the critical role of efficient NS2-NS3 cleavage for NS5A hyperphosphorylation. 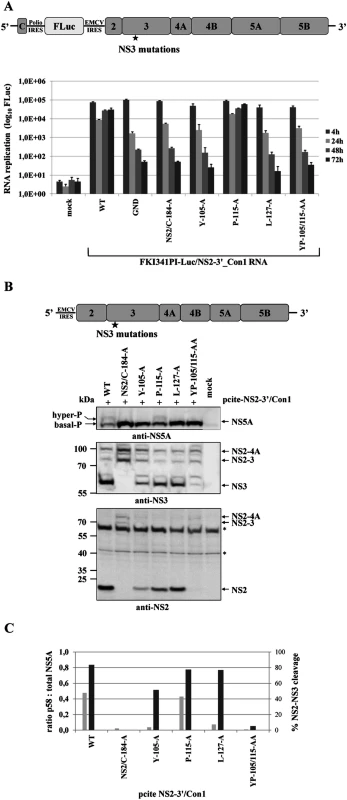
(A) Top, schematic of the HCV genotype 1b NS2-5B replicon. Bottom, the NS3 mutations were built into pFKI341-Luc/NS2-3’_Con1 and transcribed RNA was electroporated into Huh7 Lunet cells. Luciferase activity was measured at 4, 24, 48 and 72 h pe. Mean values of three independent experiments are shown. Error bars indicate standard deviations. (B) Top, schematic of pcite-NS2-3’/Con1. This plasmid encodes the NS2 to NS5B sequence of the HCV isolate 1b as indicated. NS3 mutations were introduced into pcite-NS2-3’/Con1 and plasmids were transfected into Huh-7/T7cells infected with MVA-T7pol vaccinia virus. Bottom, effects of the NS3 mutations on NS2-5B polyprotein processing and NS5A hyperphosphorylation. Cell lysates were prepared 20 h pt and analyzed by Western blotting with antibodies directed against NS2, NS3 and NS5A. The position of hyper and basally phosphorylated NS5A is indicated. (C) Signals of NS2-3 and NS3 were quantified by ImageJ software from two Western blots to calculate the percentage of NS2-3 cleavage. Signals of NS5A p56 and p58 forms of two Western blots were quantified by ImageJ software and the ratio of hyperphosphorylation (p58) to total NS5A was calculated. WT, wild type; GND, polymerase-inactive mutant; NS2/C-184-A, NS2-protease inactive mutant; mock, lysates from cells transfected with vector control. Collectively, these results point to a conserved and, so far, unappreciated role of efficient NS2-NS3 cleavage as a prerequisite for the NS5A hyperphosphorylation during the biogenesis of the HCV replication complex in different HCV genotypes in a process that is critical for viral genome replication.
The hydrophobic NS3 surface residues required for NS2 protease stimulation are not critical for NS2/NS3 interactions and virus assembly in the context of a bicistronic genome
NS2 is required for virion assembly and this function has been assigned to both the N-terminal membrane association - and the C-terminal protease domain [19,21,22,37,38]. Accordingly, the NS2 protease domain not only catalyzes the NS2-NS3 cleavage but also provides determinants for virion morphogenesis. This observation together with the finding that mature NS2 is interacting with NS3 in a way that has been implicated to coordinate virion assembly [24], prompted us to test whether mutations in the hydrophobic NS3 surface patch have effects on infectious virus production. To this end we introduced the NS3 mutations Y105A, P115A, L127A and YP105/115AA into the JFH1ad-R2a_NS2EI3 genome (S4 and S5 Figs.). In this bicistronic context, virus assembly can be analyzed independently from NS2-NS3 cleavage and thus uncouples NS2-NS3 processing from replication and virus assembly. Both single mutations replicated to wild type level, whereas the double mutation (YP105/115AA) exhibited slightly reduced replication (S4B Fig.). The L127A mutation did not replicate in this bicistronic context (S5 Fig.) confirming our earlier observation that this mutation inhibited RNA replication (Figs. 4A and 5A). The replication-competent NS3 mutants (Y105A, P115A and YP105/115AA) have no detectable effect on NS5A hyperphosphorylation in the context of the bicistronic JFH1ad-R2a_NS2EI3 genome (S6 Fig.) as expected from our transient expression experiments with the NS3-5B polyprotein (Fig. 4B). Infectious virus production was similar to wild type for both single mutants, while the double mutant showed a reduction of infectious titers of about 10-fold (S4C and S4D Fig.). When we calculated the infectivity release efficiency as a ratio of infectivity release and replication the double mutation exhibited a 5-fold reduction compared to wild type, whereas the single mutations showed efficiencies of about 60% of wild type level (S4E Fig.).
The double mutant YP105/115AA strongly reduces NS2 protease stimulation by NS3 (Fig. 5B) most likely by disturbing surface interactions between NS2 and NS3 in the NS2-NS3 precursor protein. The same mutations mildly reduce the infectivity release efficiency to 20% of wild type in a bicistronic background (S4E Fig.). We also examined whether the NS3 mutations influence binding between NS2 and NS3, which could, in case of the double mutant YP105/115AA, explain the observed moderate reduction in virus production. To this end, Huh7.5 cells were transfected with wild type JFH1ad_HAF-NS2EI3 (carrying a HA-Flag epitope sequence, HAF, in NS2; S7A Fig.) or mutant RNAs encoding individual NS3 substitutions. After 72 hours cells were harvested, lysed and lysates were used for immunoprecipitation. Western blot analysis of immunoprecipitated HAF-NS2 protein as well as the co-immunoprecipitated NS3 indicated that the mutations in NS3 did not detectably influence NS2:NS3 interaction (S7B Fig.). Together, these results showed that virus mutants with substitutions in the hydrophobic NS3 surface patch are still capable of virus production albeit at slightly (Y105A and P115A) or moderately (YP105/115AA) reduced levels compared to wild type. This indicates that the hydrophobic NS3 surface residues required for NS2 protease stimulation are not critical for virus assembly in the context of a bicistronic genome.
Discussion
Polyprotein processing is a key step in the HCV life cycle and the NS2-NS3 autocleavage has been demonstrated to be essential for RNA replication of full-length virus and subgenomic NS2-5B replicons [18,32]. NS2 in its cleaved form is also required for virion morphogenesis [19,22,37–39]. Previous studies have shown that the NS2 protease activity critically depends on the presence of the NS3 cofactor [17] and that the formation of an NS2 dimer is required for NS2-NS3 cleavage due to the unique composite nature of the NS2 protease active site [12,16,20]. Since the structure of the NS2-NS3 protein in its pre-cleavage conformation has not been determined, the molecular mechanism of the NS2-activation and its regulation remained enigmatic.
Our finding that a conserved hydrophobic NS3 surface patch composed of Y105, P115 and L127 is essentially involved in NS2 protease stimulation reveals that NS2 protease stimulation is mainly directed by specific hydrophobic NS3 surface residues (Fig. 2). This observation is supported by our mutational characterization of this surface area. While the introduction of single charged amino acids in this hydrophobic core strongly reduces (P115R) or abolishes (Y105R, L127R) NS2 protease activation, more conservative phenylalanine exchanges of Y105, P115 and L127 were tolerated with regard to NS2-NS3 cleavage (Fig. 2B). Accordingly, the hydrophobic character of this surface patch is pivotal for the NS3 cofactor function in NS2 protease activation. The finding that only combinations of these amino acids inhibit NS2-NS3 cleavage suggests that multiple hydrophobic NS3 residues are required to create a continuous hydrophobic NS3 surface area that interacts with NS2 to stimulate NS2 protease activity (Fig. 2A). This NS2/NS3 interaction in uncleaved NS2-NS3 likely promotes the efficient formation of a functional conformation required for productive NS2-NS3 autoprocessing. Interestingly, the NS3 surface area implicated in the formation of a proteolytically active conformation of the NS2-NS3 complex is situated in the previously defined NS3 Zn2+-binding domain [17]. This domain was shown to represent the functional NS2-protease activating domain and can also activate NS2-NS3 cleavage in trans [17]. Our results indicate that intrinsic structural preferences in the NS2-NS3 precursor have important regulatory roles in the NS2-NS3 autocleavage reaction. Mechanistically, it has been proposed that the intramolecular NS2-NS3 cleavage is performed by active NS2-NS3 dimers in the course of co - and posttranslational polyprotein processing [40]. Although critical residues for NS2 dimerization are not known [16], the detection of NS2 interactions in mammalian cells by co-immunoprecipitation in the absence of NS3 suggests that NS3 is not essential for NS2 dimer formation [40]. In theory, the formation of a proteolytically active NS2-NS3 precleavage complex could be promoted by either intermolecular or intramolecular interactions between NS2 and NS3 (i.e., between the NS2 moiety of one molecule of NS2-NS3 and the NS3 moiety of a second NS2-NS3 molecule or intramolecular of NS2-NS3 molecules which form dimers). Future experiments will aim at the identification of corresponding interaction sites on NS2 and to investigate if the stimulation of NS2 by NS3 in uncleaved NS2-NS3 is based on intra - or intermolecular protein interactions. Such regulation of polyprotein processing is also seen in other polyprotein-encoding viruses. For instance, the regulation of P23 polyprotein processing in alphaviruses is achieved by extensive intramolecular contacts between nsP2 and nsP3 which may function in positioning and recognition of the P2/3 cleavage site [41,42]. In contrast, poliovirus 3CD has no intramolecular contacts and cleavage of the solvent exposed cleavage site is regulated by intermolecular contacts between 3CD molecules [43].
A major finding of this study is that the hydrophobic NS3 surface involved in activating the NS2 protease has a second important function in the regulation of viral RNA replication. The L127A mutation in NS3 massively reduced NS5A hyperphosphorylation and this defect strongly correlated with an inhibition of viral RNA replication of NS3-5B replicons of different HCV genotypes without affecting the NS3-4A serine protease activity (Figs. 4 and 6). Determinants for NS5A hyperphosphorylation have been mapped to NS3, NS4A and NS4B and expression of the NS3-5A polyprotein is required for this process [44–47]. These findings suggest that the molecular basis for the multifactorial nature of NS5A hyperphosphorylation is its dependency on the assembly of the viral replicase.
These results point to a surprising functional overlap of NS3 surface determinants that are involved in NS2 protease activation and NS5A hyperphosphorylation, a process depending on viral replicase assembly, with L127 being a structural constituent of both NS3 functions. The dual role of L127 in promoting NS2-NS3 cleavage and regulating NS5A hyperphosphorylation prompted us to test whether NS2-NS3 cleavage is a prerequisite for NS5A hyperphosphorylation. The detailed mechanism underlying the NS5A hyperphosphorylation is still poorly understood [34,35,48,49]. It was hypothesized that the generation of authentic NS2 by NS2-NS3 cleavage is important for NS5A hyperphosphorylation [50]. However, hyperphosphorylated NS5A was also detected in the absence of NS2 suggesting that the authentic N-terminus of NS3, generated by NS2-NS3 cleavage, represents the critical determinant for NS5A hyperphosphorylation [44,51]. We could demonstrate that mutations either inactivating the NS2 protease (NS2 C184A) or blocking the NS2 protease cofactor function of NS3 (YP105/115AA) were blocking or strongly reducing NS5A hyperphosphorylation in NS2-5B polyproteins of two different HCV genotypes. Reductions in the NS2-NS3 cleavage efficiency correlated not only with a decrease in NS5A hyperphosphorylation but also with a block in viral RNA replication of the respective NS2-5B replicons (Figs. 5 and 7). This correlation became apparent by using the NS2-5B replicons of genotype 1b and 2a. When we compared the impact of the NS3 mutations on RNA replication in these systems, we observed that the Y105A mutation inhibited NS2-5B/Con1 replication but was still allowing for RNA replication in the NS2-5B JFH1 background (compare Figs. 5A and 7A). This difference is most likely due to the significantly lower replication capacity of the genotype 1b replicon RNA and is also reflected by the detectable NS5A hyperphosphorylation only in the case of the NS2-5B/JFH1 (Figs. 5B and 7B). These results are in line with the recent observation that, although NS2-NS3 cleavage is not limiting Con1 replication, the formation of the membranous HCV replication complexes (RC) might be less efficient in Con1 compared to the biogenesis of JFH1 RCs [52].
Based on our data, we propose the following order of events: NS2 in uncleaved NS2-NS3 interacts with the hydrophobic NS3 surface area that includes L127 resulting in NS2 protease stimulation. As a consequence, in uncleaved NS2-NS3 the NS3 surface surrounding L127 is most likely not accessible for protein-protein interactions since this region is engaged in interactions with the NS2 protease domain. Upon NS2 release the NS3 surface area around L127 becomes available for novel protein-protein interactions that finally allow NS5A hyperphosphorylation to occur. We propose that this process is functionally linked to the assembly of the viral replicase complex and likely involves interactions of NS3 with NS4A [32,47]. Moreover, after NS2 release NS3 can undergo a structural change to adopt a conformation required for its function within the viral replicase. These changes are suggested to promote inter-domain co-operations between NS3 helicase and serine protease domain [53] and lead to efficient NS4A binding [32]. Interestingly, such a scenario is supported by the observation that uncleaved NS2-NS3 exhibits a lower affinity to NS4A when compared to NS3 [32]. In this context it is remarkable that mutations in the NS4A acidic domain could be rescued by second site mutations in the NS3 protease domain [47]. One of these residues is in close proximity to L127 on the NS3 surface supporting our finding that this region is critical for NS5A hyperphosphorylation and likely for the assembly of the viral replication complex. Together, our data indicate that NS2-NS3 cleavage is mechanistically linked to NS5A hyperphosphorylation as well as the assembly of the viral replicase. These intriguing observations reveal an unexpected function for the NS2-NS3 cleavage and might explain why an efficient liberation of functional NS3 is a prerequisite for viral genome replication.
The identification of a hydrophobic surface segment on NS3 as an essential module of the NS2 protease cofactor in NS3 which also plays a critical role in replicase assembly is an important step towards a better understanding of the sequential processes involved in the functional assembly of the HCV RNA replication complex at the molecular level which are most certainly accompanied by conformational transitions within the different macromolecular complexes.
Methods
Cell culture
Huh7 Lunet [44] and Huh7/T7 [54] cells were maintained in Dulbecco's modified minimal essential medium supplemented with 10% FCS, 100 U penicillin/100 μg/ml streptomycin, and 2 mM L-glutamine. Huh-7/T7cells were cultured in the presence of 400 μg/ml G418.
Plasmids and mutagenesis
Genomes Con1 [44,55], BK [56], JFH1 [57] have been described. All mutations were introduced via QuikChange (QC) mutagenesis. Details concerning the generation of constructs and their properties can be found in the supplementary material.
In vitro transcription and electroporation of HCV RNAs
The experimental procedures used to generate in vitro transcripts from cloned HCV sequences and transfection of Huh-7 cells by electroporation have been described [37]. After electroporation, cells were immediately transferred to complete DMEM and seeded as required for the assay.
Luciferase assay
At each time point (4, 24, 48, and 72 h), cells were washed with PBS, scraped into 1 ml of PBS and collected by centrifugation. The cells were lysed in 40 μl of lysis buffer (PJK-GmbH). 20 μl of the lysate was analyzed using the Beetle Juice luciferase assay system (PJK-GmbH) and measured in a luminometer (Junior LB9509, Berthold).
Vaccinia virus infection, DNA transfection and transient protein expression
The applied procedures have been described [58]. HCV nonstructural proteins were expressed from pcite plasmids. Briefly, 2 x 106 Huh-7/T7 cells were infected with MVA-T7pol vaccinia virus [59] and subsequently transfected with 4 or 8 μg of plasmid DNA by using Superfect reagent (QIAGEN).
SDS-PAGE and western blotting
Proteins were separated in polyacrylamide-Tricine gels. After SDS-PAGE, proteins were transferred onto a nitrocellulose membrane (Pall, USA). The membrane was blocked with 5% (w/v) dried skim milk in phosphate-buffered saline with 0.05% (v/v) Tween 20 (Invitrogen). For antigen detection, anti-NS5A 9E10 [60], mouse monoclonal antibody against NS3 of the JFH-1 isolate (4D11) (generated in a cooperation between Harish Ramanathan, Michael Engle, Michael S. Diamond and Brett D. Lindenbach) or anti-NS3 (2E3) [61], anti-NS2 (YAL-4-70-8, Cell Essentials), anti-NS4B [62], anti-FLAG (Sigma), anti-V5 (Invitrogen), anti-HA (HA.11 clone 16B12, Covance) and anti-GST (GE Healthcare), antibodies were used in 2% (w/v) dried skim milk in phosphate-buffered saline with 0.05% (v/v) Tween 20. For primary antibody detection, horseradish peroxidase-conjugated species-specific secondary antibodies (Dianova) were used at a 1 : 3000 dilution and Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer) was applied prior to imaging using a LAS 4000 imaging system (Biorad, Munich). Quantifications of Western blots were carried out using ImageJ 1.47t software (NIH, Bethesda).
Metabolic labeling of proteins
HCV nonstructural proteins were expressed from pcite plasmids. For transient protein expression, 2 x 106 Huh-7/T7 cells were infected with MVA-T7pol vaccinia virus [59] and subsequently transfected with 4 μg of plasmid DNA by using Superfect reagent (QIAGEN). Cells were kept in DMEM culturing medium for 2 h. Medium was changed to DMEM (lacking L-Methionine, L-Cysteine and L-Glutamine) supplemented with 1% Glutamax (Gibco). After 30 min the medium was changed again for DMEM (lacking L-Methionine, L-Cysteine and L-Glutamine) supplemented with 1% Glutamax (Gibco), containing 70 μCi (1Ci = 37GBq) [35S]-labeled methionine/ cysteine (Hartmann Analytics).
RIP
After 6 h cells were lysed in 250 μl RIPA(G) [150 mM NaCl, 1% (vol/vol) Nonidet NP40, 0.5% (wt/vol) deoxycholate, 0.1% (wt/vol)SDS, 50 mM Tris (pH 8)], containing 1 mM PefablocSC (Roth). The following steps were performed at 4°C, mixing was ensured by placing samples on a spinning wheel. Lysates were incubated for 30 min and then centrifuged for 30 min at 16.000 g. The supernatants were incubated with anti-GST antibody (GE Healthcare) 1 : 400 in RIPA(G) for 1 h, then 50 μl of a 20% (vol/vol) Protein-A-Sepharose suspension were added and incubated for another hour. The Protein-A-Sepharose was pelleted at 16.000 g and washed 3 x with RIPA(G). Proteins were denatured in sample buffer containing 5% β-Mercaptoethanol at 95°C for 10 min and then separated in polyacrylamide-tricine gels with 8% polyacrylamide. SDS-Gels were fixed in a solution containing 40% methanol and 10% acetic acid, dried and exposed to Imaging screens (Fuji) for 1–3 days. Readouts were performed using Phosphorimager (Fuji BAS). Quantifications were carried out with AIDA Image Analyzer (Version 3.52) software using a background substraction method.
Supporting Information
Zdroje
1. Thomas DL (2013) Global control of hepatitis C: where challenge meets opportunity. Nat Med 19 : 850–858. doi: 10.1038/nm.3184 23836235
2. McLauchlan J, Lemberg MK, Hope G, Martoglio B (2002) Intramembrane proteolysis promotes trafficking of hepatitis C virus core protein to lipid droplets. EMBO J 21 : 3980–3988. 12145199
3. Hijikata M, Kato N, Ootusyama Y, Nakagawa M, Shimotohno K (1991) Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci USA 88 : 5547–5551. 1648221
4. Lin C, Lindenbach BD, Prágal BM, McCourt DW, Rice CM (1994) Processing in the Hepatitis C Virus E2-NS2 region: identification of p7 and two distinct E2 - specific products with different C termini. J Virol 68 : 5063–5073. 7518529
5. Failla C, Tomei L, de Francesco R (1994) Both NS3 and NS4A are required for proteolytic processing of hepatitis C virus nonstructural proteins. J Virol 68 : 3753–3760. 8189513
6. Kolykhalov AA, Agapov E V, Rice C (1994) Specificity of the Hepatitis C Virus NS3 serine protease: Effects of substitutions at the 3/4A, 4A/4B, and 5A/5B cleavage sites on polyprotein processing. J Virol 68 : 7525–7533. 7933136
7. Hijikata M, Mizushima H, Tanji Y, Komoda Y, Hirowatari Y, et al. (1993) Proteolytic processing and membrane association of putative nonstructural proteins of hepatitis C virus. Proc Natl Acad Sci U S A 90 : 10773–10777. 7504283
8. Bartenschlager R, Ahlborn-Laake L, Mous J, Jacobsen H (1994) Kinetic and structural analyses of hepatitis C virus polyprotein processing. J Virol 68 : 5045–5055. 8035505
9. Kim DW, Kim J, Gwack Y, Han JH, Choe J (1997) Mutational analysis of the hepatitis C virus RNA helicase. J Virol 71 : 9400–9409. 9371600
10. Santolini E, Pacini L, Fipaldini C, Migliaccio G, Monica N (1995) The NS2 protein of hepatitis C virus is a transmembrane polypeptide. J Virol 69 : 7461–7471. 7494252
11. Kim JE, Song WK, Chung KM, Back SH, Jang SK (1999) Subcellular localization of hepatitis C viral proteins in mammalian cells. Arch Virol 144 : 329–343. 10470257
12. Pallaoro M, Lahm A, Biasiol G, Brunetti M, Nardella C, et al. (2001) Characterization of the hepatitis C virus NS2/3 processing reaction by using a purified precursor protein. J Virol 75 : 9939–9946. 11559826
13. Grakoui A, Wychowsky C, Lin C, Feinstone S, Rice C (1993) Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol 67 : 1385–1395. 7679746
14. Grakoui A, McCourt DW, Wychowski C, Feinstone SM, Rice C (1993) A second hepatitis C virus-encoded proteinase. Proc Natl Acad Sci USA 90 : 10583–10587. 8248148
15. Hijikata M, Mizushima H, Akagi T, Mori S, Kakiuchi N, et al. (1993) Two distinct proteinase activities required for the processing of a putative nonstructural precursor protein of hepatitis C virus. J Virol 67 : 4665–4675. 8392606
16. Lorenz IC, Marcotrigiano J, Dentzer TG, Rice CM (2006) Structure of the catalytic domain of the hepatitis C virus NS2-3 protease. Nature 442 : 831–835. 16862121
17. Schregel V, Jacobi S, Penin F, Tautz N (2009) Hepatitis C virus NS2 is a protease stimulated by cofactor domains in NS3. Proc Natl Acad Sci U S A 106 : 5342–5347. doi: 10.1073/pnas.0810950106 19282477
18. Kolykhalov AA, Mihalik K, Feinstone SM, Rice CM (2000) Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3’ nontranslated region are essential for virus replication in vivo. J Virol 74 : 2046–2051. 10644379
19. Jones CT, Murray CL, Eastman DK, Tassello J, Rice CM (2007) Hepatitis C virus p7 and NS2 proteins are essential for production of infectious virus. J Virol 81 : 8374–8383. 17537845
20. Welbourn S, Pause A (2007) The hepatitis C virus NS2/3 protease. Curr Issues Mol Biol 9 : 63–69. 17263146
21. Dentzer TG, Lorenz IC, Evans MJ, Rice CM (2009) Determinants of the hepatitis C virus nonstructural protein 2 protease domain required for production of infectious virus. J Virol 83 : 12702–12713. doi: 10.1128/JVI.01184-09 19812162
22. Phan T, Beran RKF, Peters C, Lorenz I, Lindenbach BD (2009) Hepatitis C virus NS2 protein contributes to virus particle assembly via opposing epistatic interactions with the E1-E2 glycoprotein and NS3-4A enzyme complexes. J Virol 83 : 8379–8395. doi: 10.1128/JVI.00891-09 19515772
23. De la Fuente C, Goodman Z, Rice CM (n.d.) Genetic and functional characterization of the N-terminal region of the hepatitis C virus NS2 protein. J Virol 87 : 4130–4145. doi: 10.1128/JVI.03174-12 23408609
24. Stapleford KA, Lindenbach BD (2011) Hepatitis C virus NS2 coordinates virus particle assembly through physical interactions with the E1-E2 glycoprotein and NS3-NS4A enzyme complexes. J Virol 85 : 1706–1717. doi: 10.1128/JVI.02268-10 21147927
25. Jirasko V, Montserret R, Lee JY, Gouttenoire J, Moradpour D, et al. (2010) Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog 6: e1001233. doi: 10.1371/journal.ppat.1001233 21187906
26. Jirasko V, Montserret R, Appel N, Janvier A, Eustachi L, et al. (2008) Structural and functional characterization of nonstructural protein 2 for its role in hepatitis C virus assembly. J Biol Chem 283 : 28546–28562. doi: 10.1074/jbc.M803981200 18644781
27. Ma Y, Anantpadma M, Timpe JM, Shanmugam S, Singh SM, et al. (2010) Hepatitis C Virus NS2 Protein Serves as a Scaffold for Virus Assembly by Interacting with both Structural and Nonstructural Proteins. J Virol 85 : 86–97. doi: 10.1128/JVI.01070-10 20962101
28. Popescu CI, Callens N, Trinel D, Roingeard P, Moradpour D, et al. (2011) NS2 Protein of Hepatitis C Virus Interacts with Structural and Non-Structural Proteins towards Virus Assembly. PLoS Pathog 7: e1001278. doi: 10.1371/journal.ppat.1001278 21347350
29. Yi M, Ma Y, Yates J, Lemon SM (2009) Trans-complementation of an NS2 defect in a late step in hepatitis C virus (HCV) particle assembly and maturation. PLoS Pathog 5: e1000403. doi: 10.1371/journal.ppat.1000403 19412343
30. Tedbury PR, Harris M (2007) Characterisation of the role of zinc in the hepatitis C virus NS2/3 auto-cleavage and NS3 protease activities. J Mol Biol 366 : 1652–1660. 17239391
31. Yao N, Reichert P, Taremi SS, Prosise WW, Weber PC (1999) Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease-helicase. Structure 7 : 1353–1363. 10574797
32. Welbourn S, Green R, Gamache I, Dandache S, Lohmann V, et al. (2005) Hepatitis C virus NS2/3 processing is required for NS3 stability and viral RNA replication. J Biol Chem 280 : 29604–29611. 15980068
33. Fridell RA, Valera L, Qiu D, Kirk MJ, Wang C, et al. (2013) Intragenic complementation of hepatitis C virus NS5A RNA replication-defective alleles. J Virol 87 : 2320–2329. doi: 10.1128/JVI.02861-12 23236071
34. Ross-Thriepland D, Harris M (2014) Insights into the complexity and functionality of hepatitis C virus NS5A phosphorylation. J Virol 88 : 1421–1432. doi: 10.1128/JVI.03017-13 24257600
35. Appel N, Zayas M, Miller S, Krijnse-Locker J, Schaller T, et al. (2008) Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog 4: e1000035. doi: 10.1371/journal.ppat.1000035 18369481
36. Evans MJ, Rice CM, Goff SP (2004) Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication. Proc Natl Acad Sci U S A 101 : 13038–13043. 15326295
37. Jirasko V, Montserret R, Appel N, Janvier A, Eustachi L, et al. (2008) Structural and functional characterization of nonstructural protein 2 for its role in hepatitis C virus assembly. J Biol Chem 283 : 28546–28562. doi: 10.1074/jbc.M803981200 18644781
38. Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, et al. (2006) Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci U S A 103 : 7408–7413. 16651538
39. Pacini L, Graziani R, Bartholomew L, De Francesco R, Paonessa G (2009) Naturally occurring hepatitis C virus subgenomic deletion mutants replicate efficiently in Huh-7 cells and are trans-packaged in vitro to generate infectious defective particles. J Virol 83 : 9079–9093. doi: 10.1128/JVI.00308-09 19587042
40. Dimitrova M, Imbert I, Kieny MP, Schuster C (2003) Protein-protein interactions between hepatitis C virus nonstructural proteins. J Virol 77 : 5401–5414. 12692242
41. Shin G, Yost SA, Miller MT, Elrod EJ, Grakoui A, et al. (2012) Structural and functional insights into alphavirus polyprotein processing and pathogenesis. Proc Natl Acad Sci U S A 109 : 16534–16539. doi: 10.1073/pnas.1210418109 23010928
42. Lulla A, Lulla V, Merits A (2012) Macromolecular assembly-driven processing of the 2/3 cleavage site in the alphavirus replicase polyprotein. J Virol 86 : 553–565. doi: 10.1128/JVI.05195-11 22031949
43. Marcotte LL, Wass AB, Gohara DW, Pathak HB, Arnold JJ, et al. (2007) Crystal structure of poliovirus 3CD protein: virally encoded protease and precursor to the RNA-dependent RNA polymerase. J Virol 81 : 3583–3596. 17251299
44. Koch JO, Bartenschlager R (1999) Modulation of hepatitis C virus NS5A hyperphosphorylation by nonstructural proteins NS3, NS4A, and NS4B. J Virol 73 : 7138–7146. 10438800
45. Asabe S-I, Tanji Y, Satho S, Kaneko T, Kimura K, et al. (1997) The N-terminal region of hepatitis C virus-encoded NS5A is important for NS4A-dependent phosphorylation. J Virol 71 : 790–796. 8985418
46. Kaneko T, Tanji Y, Satoh S, Hijikata M, Asabe S, et al. (1994) Production of two phosphoproteins from the NS5A region of the hepatitis C viral genome. Biochem Biophys Res Comm 205 : 320–326. 7999043
47. Lindenbach BD, Pragai BM, Montserret R, Beran RK, Pyle AM, et al. (2007) The C terminus of hepatitis C virus NS4A encodes an electrostatic switch that regulates NS5A hyperphosphorylation and viral replication. J Virol 81 : 8905–8918. 17581983
48. Huang Y, Staschke K, De Francesco R, Tan S-L (2007) Phosphorylation of hepatitis C virus NS5A nonstructural protein: a new paradigm for phosphorylation-dependent viral RNA replication? Virology 364 : 1–9. 17400273
49. Appel N, Pietschmann T, Bartenschlager R (2005) Mutational analysis of hepatitis C virus nonstructural protein 5A: potential role of differential phosphorylation in RNA replication and identification of a genetically flexible domain. J Virol 79 : 3187–3194. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15709040. 15709040
50. Liu Q, Bhat RA, Prince AM, Zhang P (1999) The hepatitis C virus NS2 protein generated by NS2-3 autocleavage is required for NS5A phosphorylation. Biochem Biophys Res Comm 254 : 572–577. 9920780
51. Neddermann P, Clementi A, De Francesco R (1999) Hyperphosphorylation of the hepatitis C virus NS5A protein requires an active NS3 protease, NS4A, NS4B, and NS5A encoded on the same polyprotein. J Virol 73 : 9984–9991. 10559312
52. Madan V, Paul D, Lohmann V, Bartenschlager R (2014) Inhibition of HCV Replication by Cyclophilin Antagonists is Linked to Replication Fitness and Occurs by Inhibition of Membranous Web Formation. Gastroenterology 146 : 1361–1372. doi: 10.1053/j.gastro.2014.01.055 24486951
53. Beran RK, Pyle AM (2008) Hepatitis C viral NS3-4A protease activity is enhanced by the NS3 helicase. J Biol Chem 283 : 29929–29937. doi: 10.1074/jbc.M804065200 18723512
54. Schultz DE, Honda M, Whetter LE, McKnight KL, Lemon SM (1996) Mutations within the 5’ nontranslated RNA of cell culture-adapted hepatitis A virus which enhance cap-independent translation in cultured African green monkey kidney cells. J Virol 70 : 1041–1049. 8551562
55. Lohmann V, Korner F, Koch J, Herian U, Theilmann L, et al. (1999) Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science (80-) 285 : 110–113. 10390360
56. Takamizawa A, Mori C, Fuke I, Manabe S, Murakami S, et al. (1991) Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol 65 : 1105–1113. 1847440
57. Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, et al. (2005) Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11 : 791–796. 15951748
58. Isken O, Langerwisch U, Schonherr R, Lamp B, Schroder K, et al. (2014) Functional characterization of bovine viral diarrhea virus nonstructural protein 5A by reverse genetic analysis and live cell imaging. J Virol 88 : 82–98. doi: 10.1128/JVI.01957-13 24131714
59. Sutter G, Ohlmann M, Erfle V (1995) Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett 371 : 9–12. 7664891
60. Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, et al. (2005) Complete replication of hepatitis C virus in cell culture. Science 309 : 623–626. 15947137
61. Backes P, Quinkert D, Reiss S, Binder M, Zayas M, et al. (2010) Role of annexin A2 in the production of infectious hepatitis C virus particles. J Virol 84 : 5775–5789. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20335258. doi: 10.1128/JVI.02343-09 20335258
62. Paul D, Romero-Brey I, Gouttenoire J, Stoitsova S, Krijnse-Locker J, et al. (2011) NS4B self-interaction through conserved C-terminal elements is required for the establishment of functional hepatitis C virus replication complexes. J Virol 85 : 6963–6976. doi: 10.1128/JVI.00502-11 21543474
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



