-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
γδ T cells are unconventional T lymphocytes that play a unique role in host protection against pathogens. Human Cytomegalovirus (HCMV) is a widespread virus that can cause severe organ disease such as hepatitis and pneumonitis in immune-compromised patients. Our decade-long study conveys compelling evidence for the implication of human γδ T cells in the immune response against HCMV, but their protective role could not be formally demonstrated in humans. In the present study we use the murine model of CMV infection which allows the spatial and temporal analysis of viral spread and anti-viral immune responses. We show that, in the absence of αβ T cells, γδ T cells control MCMV-induced hepatitis, pneumonitis and death by restricting viral load in the liver, lungs and spleen. γδ T cells expand in these organs and display memory features that could be further incorporated into vaccination strategies. In conclusion, γδ T cells represent an important arm in the immune response against CMV infection that could be particularly important in the context of αβ T cell immune-suppression.
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004702
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004702Summary
γδ T cells are unconventional T lymphocytes that play a unique role in host protection against pathogens. Human Cytomegalovirus (HCMV) is a widespread virus that can cause severe organ disease such as hepatitis and pneumonitis in immune-compromised patients. Our decade-long study conveys compelling evidence for the implication of human γδ T cells in the immune response against HCMV, but their protective role could not be formally demonstrated in humans. In the present study we use the murine model of CMV infection which allows the spatial and temporal analysis of viral spread and anti-viral immune responses. We show that, in the absence of αβ T cells, γδ T cells control MCMV-induced hepatitis, pneumonitis and death by restricting viral load in the liver, lungs and spleen. γδ T cells expand in these organs and display memory features that could be further incorporated into vaccination strategies. In conclusion, γδ T cells represent an important arm in the immune response against CMV infection that could be particularly important in the context of αβ T cell immune-suppression.
Introduction
Human CMV (HCMV) is a universally distributed pathogen that infects 50–90% of the world's population. Asymptomatic in healthy people, HCMV infection may lead to increased morbidity and mortality in immunocompromised individuals. Overall survival following transplantation is decreased when either the donor or the recipient is HCMV-seropositive [1,2,3]. Because of drug-related adverse effects and drug resistance there is growing interest for immunotherapy as an adjunct to antiviral therapy. Understanding the mechanisms developed by the immune system to control HCMV is therefore critical to enable the design of new curative or preemptive protocols aimed at enhancing patient immune defense against this virus.
Effective immune control of HCMV has been compellingly shown to rely on both conventional lymphocytes and NK cells [4]. However, as we initially reported, HCMV also induces a robust γδ T cell response in organ transplant recipients [5]; and later, γδ T cell response to HCMV was extended to several other situations not always associated to immunosuppression; such as immunodeficiencies, bone marrow transplantation, pregnancy, elderly and also in healthy individuals [6,7,8,9,10,11,12]. HCMV-mediated persistent expansion of γδ T cells in transplant recipients is associated with infection resolution [13], and implies tissue-associated Vδ2-negative γδ T cells which acquire a terminally differentiated phenotype upon HCMV pressure [10,14]. When isolated in vitro, these lymphocytes were shown to kill HCMV-infected cells, limit virus propagation and produce IFNγ through recognition of opsonized viruses [15,16].
Several features of γδ T cells might explain their specific relationship to HCMV: (i) they are not MHC restricted, and thus not affected by HCMV strategies to inhibit HLA molecules, (ii) they recognize self-antigens on the surface of stressed cells such as virus infected cells [17,18] and (iii) they are located at external body surfaces (eg gut and lung) and organs (eg liver) involved in HCMV transmission and replication [19]. Moreover, HCMV-reactive γδ T cells exhibit dual reactivity against tumor cells, due to the recognition of stress-induced self-antigens shared by HCMV-infected and tumor cells [15,18,20]. In agreement with this, HCMV-infection and/or γδ T cell expansion have been associated with reduced cancer risk in kidney transplant recipients [21] and with graft-versus leukemia effect in bone marrow transplant recipients [22,23,24].
All these specificities are consistent with an antiviral protective role of γδ T cells against HCMV and they thus represent valuable candidates for anti-HCMV immunotherapy especially in immunocompromised patients vulnerable to neoplasia. However, their role in protection and specific contribution within the global anti-CMV immune response has not been firmly established, nor their anatomical sites of activation and intervention. The aim of the present study was therefore to take advantage of the murine model of CMV infection to address these questions and to assess the respective ability of αβ and γδ T cells alone to protect mice from CMV infection. Murine CMV (MCMV) has been widely used to model the immune response to HCMV in mice since it reproduces with reasonable accuracy the antiviral response of CD8 T cells and NK cells [25]. Murine γδ T cells have been implicated in MCMV infection only once [26], and their sufficiency for protection has not yet been addressed. We show herein that γδ T cells are as competent as αβ T cells to control MCMV infection and protect mice from death encouraging the development of novel anti-viral immunotherapeutic protocols based on γδ T cell manipulation.
Results
γδ T cells are as efficient as αβ T cells to protect mice from MCMV-induced death
In mice, MCMV-specific αβ T cells control viral spread and protect infected mice from death [27] but little is known regarding the implication of γδ T cells. To evaluate the respective contribution of αβ and γδ T cells to the immune response against MCMV, mice deficient for γδ T cells (TCRδ−/−), for αβ T cells (TCRα−/−) or for both T cell subsets (CD3ε−/−) were challenged with 105 plaque forming units (PFU) of salivary gland MCMV. This dose was reported to be sublethal for C57BL/6 mice (as described at http://mutagenetix.utsouthwestern.edu/protocol/protocol_rec.cfm?protocolid=5). Accordingly, 100% of CD3ε+/− control mice survived MCMV infection, whereas CD3ε−/− died about 4 weeks after viral challenge (Fig. 1A), confirming the critical role of T cells in controlling MCMV infection. CD3ε−/− mice were extremely sensitive to MCMV despite the presence of NK cells [28] since they died at doses of MCMV as low as 2.103 PFU (Fig. 1B). Unexpectedly, both TCRδ−/− and TCRα−/− mice survived as long as CD3ε+/− control mice. These results reveal that the presence of either αβ or γδ T cell subset was sufficient to protect mice from MCMV infection, disclosing the potentially critical function of γδ T cells in the immune response against MCMV.
Fig. 1. γδ T cells prevent mice from MCMV-induced mortality. 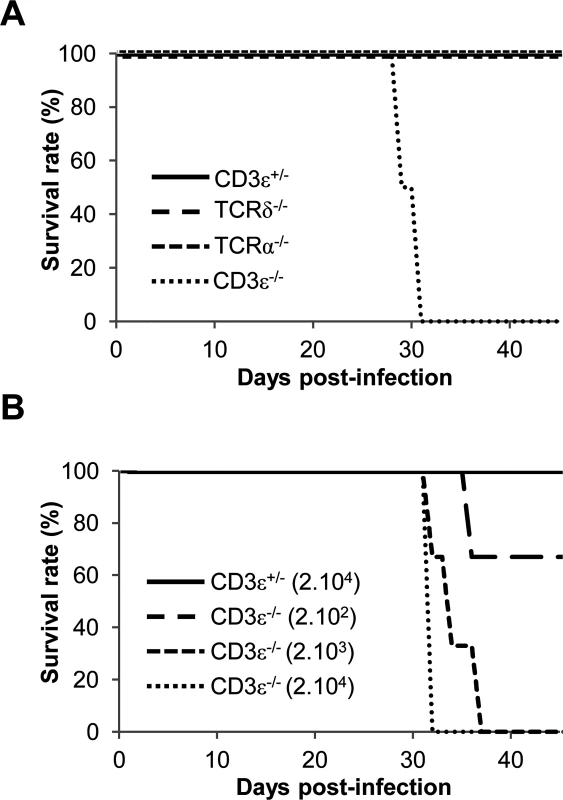
A. TCRδ−/−, TCRα−/− CD3ε+/− and CD3ε−/− mice (10 of each) were infected i.p. with 1.105 PFU of MCMV at day 0 and monitored every other day for mortality. Data are from one representative of 3 independent experiments. B. CD3ε+/− and CD3ε−/− mice (4 of each) were infected i.p. with indicated doses of MCMV at day 0 and monitored every day for mortality. Data are from one experiment. γδ T cells control viral loads in organs
To examine whether this protection against CMV by γδ T cells relies on the control of viral loads, the kinetics of MCMV spread in T cell deficient versus T cell competent mice was determined in various organs. Comparison between each mouse line is shown in Fig. 2 and comparison between different time points is shown in S1 Fig. In the absence of T cells, MCMV DNA copy numbers increased substantially from day 3 to 24, with up to 107 copies (/100ng DNA) in the spleen and lungs of CD3ε−/− mice before death. Interestingly, γδ T cells alone (in TCRα−/− mice) were sufficient to prevent an increase of viral load in all organs, except the salivary glands which are known to support prolonged virus replication even in wild-type mice (S1 Fig.). At the end of these experiments, MCMV copies were much lower in T cell bearing mice than in mice without T cells (Fig. 2), underlining the inability of C57BL/6 mice to control MCMV infection in the absence of T cells. It was of particular interest to see that in the lungs γδ T cells were as potent as αβ T cells to control the viral load except at day 14. As a whole, these results suggest independent control of MCMV spread by the αβ and γδ T cell subsets, revealing that γδ T cells are sufficient to control viral load and can substitute for the absence of αβ T cells.
Fig. 2. MCMV dissemination in lungs, spleen, liver, intestine and salivary glands from T cell competent and T cell deficient mice. 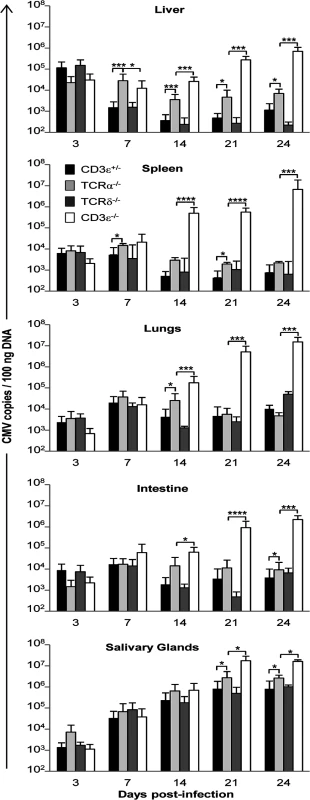
TCRδ−/−, TCRα−/−, CD3ε+/−, and CD3ε−/− mice were infected i.p. with 2.103 PFU of MCMV. At indicated days post-infection, 4 mice of each mouse line were dissected and MCMV gB was quantified in organs by real time PCR. The experiment was repeated 3 times under similar conditions. Histograms represent means of MCMV DNA copy number (per 100 ng genomic DNA) ± SD of all mice from the three experiments (n = 4x3 mice). Statistical differences between viral loads in TCRα−/− versus CD3ε+/− mice, and in TCRα−/− versus CD3ε−/− mice are shown. γδ T cell-dependent control of viral load associates with reduced organ damage
Hepatitis and pneumonitis are common features of CMV pathogenesis in both humans and mice. Hepatitis can be assessed in living infected mice through the quantification of transaminase levels in the serum. As shown in Fig. 3A, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) only increased in the absence of all T cells (CD3ε−/− mice), reaching up to 8 fold the basal level before death of CD3ε−/− mice. Accordingly, histological analysis of livers from CD3ε−/− infected mice before death (day 22) showed typical features of active hepatitis, with many large granulomas mainly composed of histiocytic cells associated with multiple apoptotic hepatocytes (Fig. 3B). In contrast, only a few small granulomas were observed in TCRα−/− mice livers at that time point. Furthermore, CD3ε−/− mice presented an active pneumopathy with large granulomas and hemorrhagic foci at day 22, while TCRα−/− lung histology was close to normal with only a slight increase of inflammatory cells in the inter-alveolar septa (Fig. 3B). In conclusion, CD3ε−/− mice showed clear evidences of both liver and lung diseases 3 weeks post MCMV infection, in agreement with the high viral loads found at that time in these organs. In contrast, liver and lung disorders were not observed in TCRα−/− mice, emphasizing the ability of γδ T cells to control MCMV infection and associated organ disease. Whether γδ T cells limit organ disease only as a consequence of viral replication control or also by producing mediators of tissue repair deserves further attention.
Fig. 3. γδ T cell control of MCMV infection is associated with reduced organ damage. 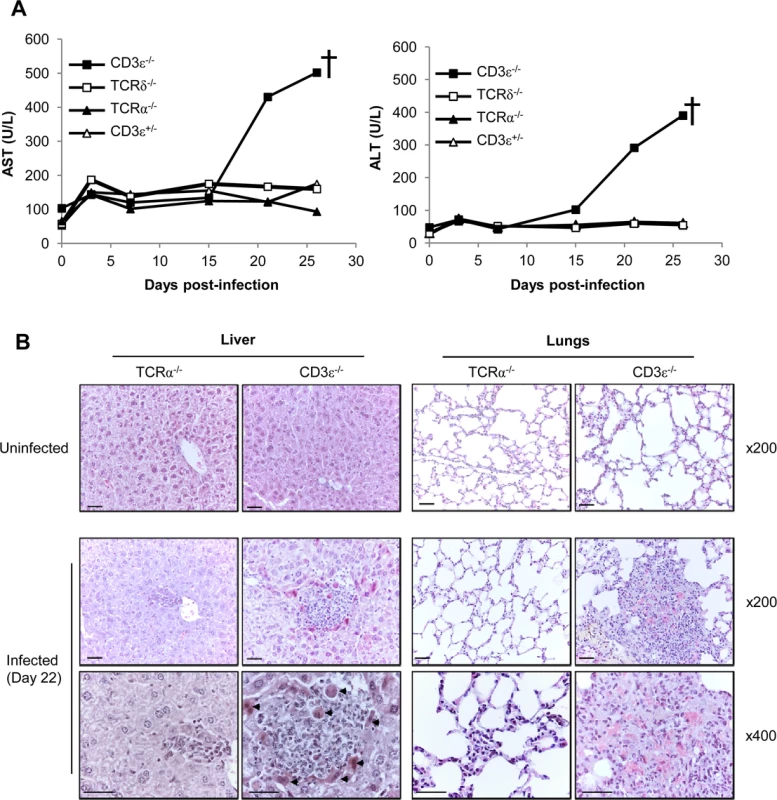
A. TCRδ−/−, TCRα−/−, CD3ε+/− and CD3ε−/− mice were infected i.p. as indicated in Fig. 1A. 3 mice/group were bled at days 0, 3, 7, 15, 21 and just before death for biochemical analyses of AST and ALT in serums. The experiment was repeated twice and data obtained for one representative mouse/group are shown. † death of CD3ε−/−. B. TCRα−/− and CD3ε−/− mice were uninfected, or i.p. infected with 2.103 pfu of MCMV. Uninfected and Day 22-infected mice were sacrificed and the liver and lungs were embedded in paraffin for HES staining. Apoptotic hepatocytes are shown (arrowheads). Scale bar = 200 mm. Magnifications are indicated in the right-hand side of the figure. The data are from one representative of 3 mice for each condition. Expansion of γδ T cells in the liver and lungs of MCMV-infected TCRα−/− mice
We next sought to analyze whether the control of MCMV spread was associated with an amplification of γδ T cells in infected organs. S2 Fig. shows the gating strategy used for γδ T cell flow cytometry analysis. After a slight decrease at day 3, γδ T cell numbers increased importantly in the lungs until day 21 (approximately 8 fold), and this rise persisted until the end of the experiment. A significant but more modest and transient increase was also observed in the liver (approximately 2 fold from day 3 to 7). By contrast and to our surprise given their preponderance in gut intraepithelial lymphocytes, no significant variation of γδ T cells was observed in the intestine. In the spleen, γδ T cells levels remained stable until day 21 when they decreased (Fig. 4A). In conclusion, control of MCMV infection by γδ T cells in TCRα−/− mice is associated with a transient γδ T cell increase in the liver, and a delayed but strong and persistent expansion of γδ T cells in the lungs.
Fig. 4. Mobilization of γδ T cells in MCMV-infected organs from TCRα−/− mice. 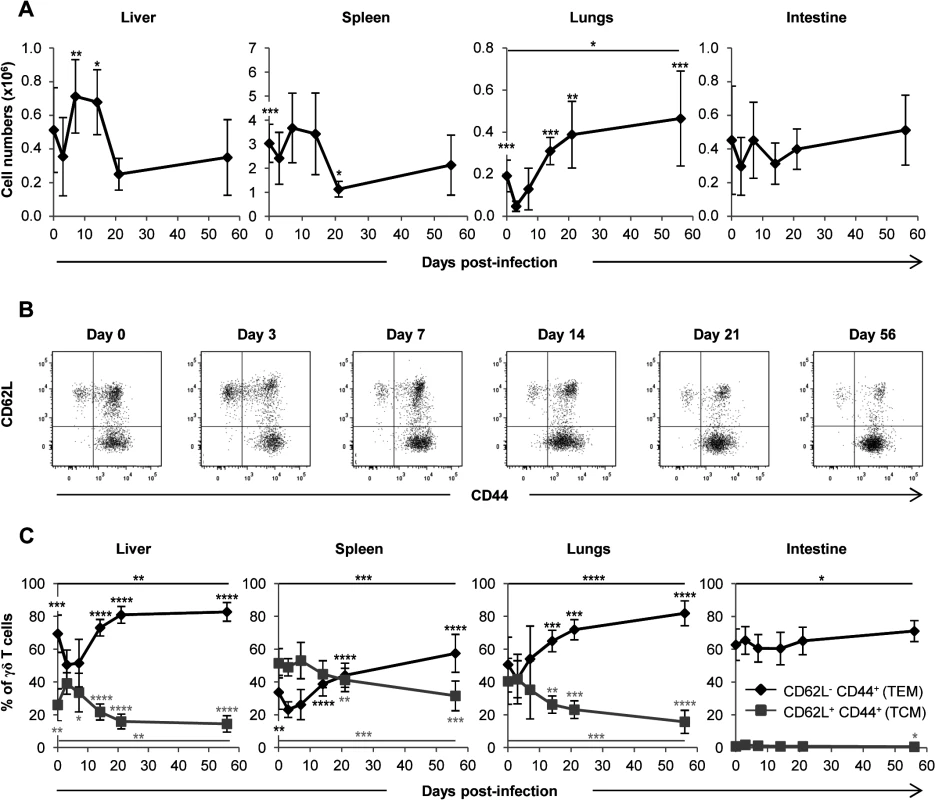
TCRα−/− mice were infected i.p. with 2.103 PFU of MCMV. At indicated post-infection days, 5–9 mice were sacrificed, immune cells prepared from each organ and γδ T cells stained as shown in S2 Fig. A. Kinetics of absolute γδ T cell numbers determined as described in methods. Presented data are mean ± SEM of 8–9 mice from one representative of 2 experiments. B. CD62L and CD44 expression by lymphocytes was evaluated by flow cytometry, with the presented gating strategy (lungs shown as example). C. Longitudinal analysis of γδ T cell phenotype in all organs. Results are pooled from 2 independent experiments representing a total of 13–14 mice (means ± SEM). Statistical differences of cell numbers and percentages between day 3 and other time points are shown, as well as statistical differences between days 0 and 56 (solid line). γδ T cells responding to MCMV display an effector-memory phenotype
We next asked whether γδ T cells responding to MCMV differentiate into effector-memory cells as we observed previously in humans [10,14]. After a transient decrease early post MCMV challenge, the proportion of effector memory (EM, CD44+CD62L−) γδ T cells increased in the spleen, liver and lungs concomitantly with a decrease of central memory (CM, CD44+CD62L+) γδ T cells. Effector memory γδ T cells reached more than 80% in the liver and lungs at day 56 (Fig. 4B and 4C). Consistent with the absence of variation in γδ T cell numbers in the intestine, no modification of γδ T cells phenotype could be observed in this organ. These results confirm that MCMV induces a marked response of γδ T cells in the lungs and liver, which is more modestly seen in the spleen and absent from the intestine.
Vγ1+ and Vγ4+ γδ T cell subsets are both involved in the response to MCMV
The subsets of murine γδ T lymphocytes expressing the Vγ1 or Vγ4 chains of the TCR predominate in the spleen, liver and lungs, whereas intestinal γδ T cells are almost exclusively Vγ7+ (nomenclature of Heilig and Tonegawa [29]). We assessed the quantity, repertoire and memory phenotype of these γδ T lymphocyte subsets in the liver, spleen and lungs. Not surprisingly, low proportions of Vγ1+ γδ T cells were found in the intestine (S2 Fig.). As observed in Fig. 5A, the expansion of γδ T cells in the lungs and liver after day 3 concerned mainly Vγ1+ but also Vγ4+ γδ T cells. Both subsets followed the kinetics of total γδ T cells (Fig. 4A). Analysis of subsets also showed a response of Vγ1+, but not Vγ4+ T cells, in the spleen (Fig. 4A and Fig. 5A). The proportion of EM cells among both Vγ1+ and Vγ4+ γδ T cells increased after day 3 in the lungs, liver and spleen (Fig. 5B). In contrast, Vγ7+ γδ T cell numbers/memory phenotype did not vary significantly upon MCMV infection (Fig. 5A and Fig. 5B), as could be expected from the analysis of the whole γδ T cell population in the intestine (Fig. 4A and Fig. 4C). The complementary-determining-region (CDR3)γ1 and CDR3γ4 length profile of liver, spleen and lung-derived γδ T cells were not different between uninfected and infected mice for 14 days (S3 Fig. and S4 Fig.), indicating that there were no major changes in these CDR3 repertoires after expansion.
Fig. 5. Both Vγ1 and Vγ4 subset are involved in γδ T cell response to MCMV. 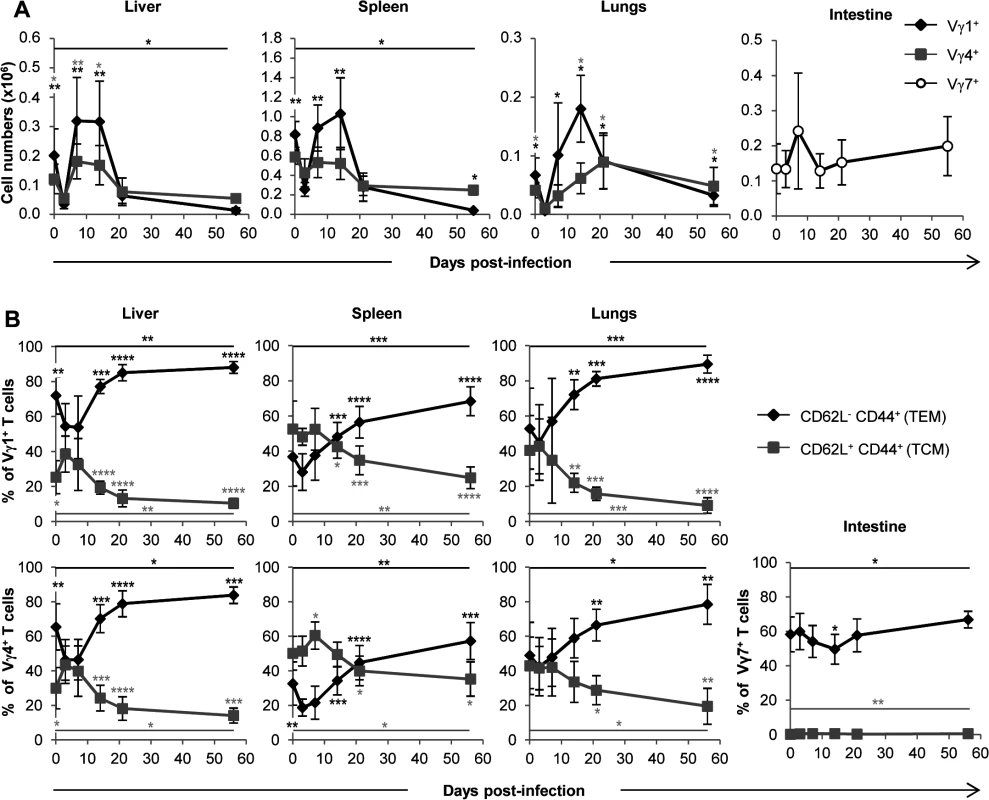
TCRα−/− mice were infected i.p. with 2.103 PFU of MCMV. At indicated days post-infection, 5–9 mice were sacrificed and immune cells were prepared from each organ. Expression of Vγ1, Vγ4 and Vγ7 chains by lymphocytes was evaluated by flow cytometry (S2 Fig.). A. Kinetics of absolute cell numbers for each subset. Presented data are mean ± SEM of 8–9 mice from one representative of 2 experiments. B. CD62L and CD44 expression by γδ T cell subsets was evaluated by flow cytometry in all organs. Results are pooled from 2 independent experiments representing a total of 13–14 mice (means ± SEM). Statistical differences of cell numbers and percentages between day 3 and other time points are shown, as well as statistical differences between days 0 and 56 (solid line). γδ T cells recovery rescues CD3ε−/− mice from MCMV-induced death
γδ T cells development in CD3ε−/− mice was reconstituted by bone marrow (BM) transfer experiments using TCRα−/− mice as donors (referred to as TCRα−/− > CD3ε−/− mice). This method allowed the generation of the BM-derived Vγ1+ and Vγ4+ γδ T cell subsets that were increased upon MCMV infection. Control BM transplants were also performed with TCRδ−/− donors (TCRδ−/− > CD3ε−/− mice) and with CD3ε+/− donors (CD3ε+/− > CD3ε−/− mice). γδ and/or αβ T cell reconstitution was allowed to establish for 3 months before MCMV infection of the mice. γδ T cell subset percentages were analyzed in blood from live mice throughout reconstitution (Fig. 6A). Two months after grafting, the percentages of blood γδ and/or αβ T cells (among total lymphocytes) had reached a plateau (Fig. 6A). The proportion of peripheral blood γδ T cells in CD3ε+/− > CD3ε−/− mice was lower than that found in TCRα−/− > CD3ε−/− mice (Fig. 6A, lower panel), in accordance with previous findings which showed that γδ T cells in TCRα−/− outnumbered γδ T cells in C57BL/6 mice [30]. When infected with MCMV at 3 months post-graft, TCRα−/− > CD3ε−/− mice survived MCMV infection as efficiently as CD3ε+/− > CD3ε−/− and TCRδ−/− > CD3ε−/− mice, in marked contrast with CD3ε−/− > CD3ε−/− mice (Fig. 6B).
Fig. 6. γδ T cell recovery rescues CD3ε−/− mice from MCMV-induced death. 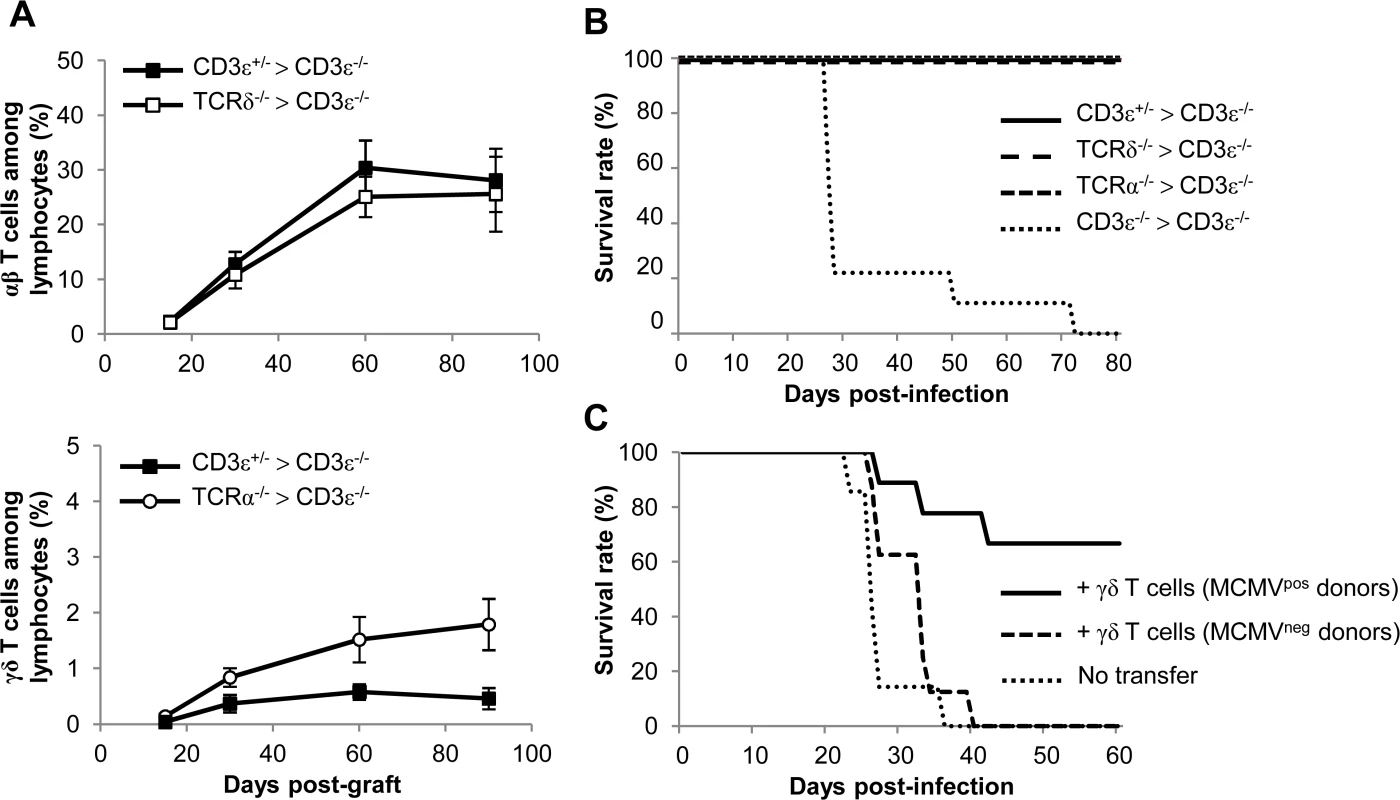
A. Bone marrows (BM) from TCRδ−/−, TCRα−/−, CD3ε+/− and CD3ε−/− mice (10 of each) were transferred into CD3ε−/− recipient mice at day 0 (1 donor BM/recipient). At days 15, 30, 60 and 90, blood samples were collected (5 for each grafted mouse line) in order to follow αβ/γδ T cell reconstitution in peripheral blood. The evolution of the proportions of αβ T cells in CD3ε+/− > CD3ε−/− and TCRδ−/− > CD3ε−/− mice are shown (top) as well as the evolution of γδ T cells in CD3ε+/− > CD3ε−/− and TCRα−/− > CD3ε−/− mice (bottom). Results are expressed as percentages among peripheral blood lymphocytes ± SD. B. Three months post-graft, CD3ε+/− > CD3ε−/−, TCRα−/− > CD3ε−/−, TCRδ−/− > CD3ε−/− and CD3ε−/− > CD3ε−/− mice (10 of each) were infected i.p. with 2.103 PFU of MCMV and monitored daily for mortality. This experiment was repeated twice with concordant results. C. γδ T cells from uninfected or 14-days infected TCRα−/− mice were purified and i.v. transferred (8–9.105 cells, 92–93% purity) into CD3ε−/− mice (8–9 recipients). 24h after transfer, reconstituted CD3ε−/− mice were challenged with 2.103 PFU of MCMV and monitored daily for mortality. 7 untransferred CD3ε−/− were used as controls. This experiment was repeated twice. In a second experimental scenario γδ T cells were purified from TCRα−/− splenocytes and injected intravenously (i.v.) into CD3ε−/− hosts one day before MCMV infection. Surprisingly, very low protection was obtained when γδ T cells isolated from control mice were transferred, whereas γδ T cells from MCMV-infected mice conferred good protection (Fig. 6C).
All together our results confirm the protective anti-CMV role of BM-derived γδ T cells, and show that priming of splenic γδ T cells with MCMV in donor mice is necessary for protection against MCMV after their adoptive transfer.
γδ T cells are not the main producers of IFNγ and cytolytic granules during early acute MCMV infection
We next sought to gain insight into the mechanism by which γδ T cells exert their antiviral function. CD27 expression was shown to segregate γδ T cells into two functional subsets in mice: CD27+ γδ T cells being the main producers of the antiviral cytokine IFNγ and CD27− γδ T cells being prone to secrete IL-17A which is not classically considered as important in antiviral responses [31] [32]. To determine which of these subsets respond to CMV, we analyzed their evolution in organs from MCMV-infected mice. As evidenced in S5A Fig., CD27− cells dominated the γδ T cell response in the lungs, while CD27+ and CD27− subtypes were roughly equally implicated in the liver. However, IL-17A transcripts were barely detected in these organs (S5B Fig.). By contrast, IFNγ was expressed in both these organs but noticeably peaked as early as day 3, before the rise of γδ T cell numbers and Cδ transcripts (S5B Fig.).
Since the presence of IFNγ transcripts in organs from TCRα−/− infected mice could be attributed to NK cells, we determined IFNγ production at the cellular level by intracellular staining of lymphocytes and using the gating strategy shown in S6 Fig. As shown in Fig. 7A, the proportion of IFNγ-producing NK cells peaked at day 3 in all organs. IFNγ-producing γδ T cells also peaked 3 to 7 days post-infection (Fig. 7A), but represented a minor population when compared to IFNγ-producing NK cells at similar time points (Fig. 7B). Consequently, NK cells were the largely preponderant producers of IFNγ during early acute MCMV infection (Fig. 7B), accounting for 2% of lymphocytes at day 3 in the liver and lungs (i.e. when the relative expression of IFNγ was the highest, S5B Fig.). Similarly, during the course of infection, the proportions of CD107a+ NK cells were higher than that of CD107a+ γδ T lymphocytes (S7 Fig.). These experiments are in accord with the substantial role of NK cells in the control of early MCMV infection through IFNγ production and cytotoxicity [33], and suggest that the antiviral role of γδ T cells might not principally rely on these two functions.
Fig. 7. γδ T cells are not the main producers of IFNγ during early acute MCMV infection. 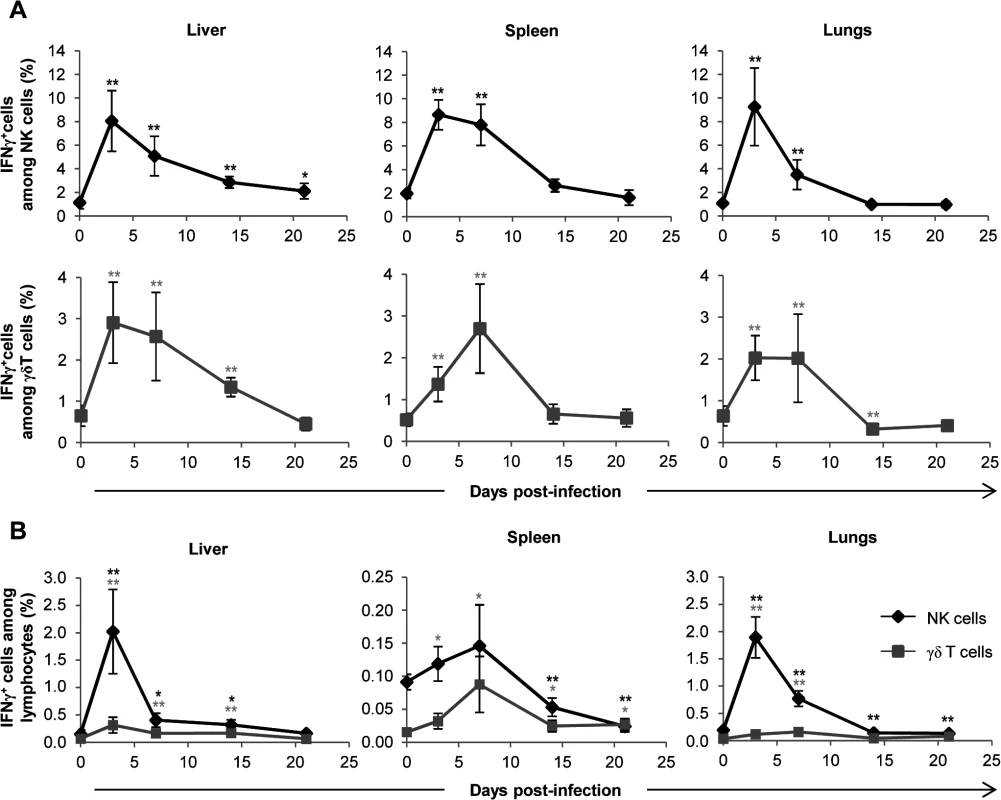
TCRα−/− mice were infected i.p. with 2.103 PFU of MCMV. At indicated days post-infection, 6–8 mice were sacrificed and immune cells were isolated from each organ for ex-vivo analysis of IFNγ production by live (7AAD−) CD3ε−NKp46+ and CD3ε+γδ+ cells. A. Proportions of IFNγ producing cells for each NK or γδ T cell subtype are shown. B. Percentages of IFNγ producing NK and γδ T cells among lymphocytes. Data are from 1 representative of 2 independent experiments and are expressed as mean percentages ± SEM of 6–8 mice. Statistical differences between day 0 and other time points are shown. NK-independent antiviral protective effect of γδ T cells
Considering the above results we hypothesized that γδ T cells could exert an indirect antiviral effect by promoting NK cells accumulation as has been previously reported [34]. We therefore compared the evolution of NK cell numbers early post-MCMV infection in TCRα−/− and CD3ε−/− mice. For both mouse lines and as depicted in C57BL/6 wt mice, the overall kinetic was organ-specific with an early decrease of NK cells in the spleen in contrast to liver (Fig. 8A) [35][36]. In contrast to our hypothesis and despite the MCMV-induced death of CD3ε−/− mice, NK cell numbers were globally higher in CD3ε−/− mice than in TCRα−/− mice at all early time points tested (Fig. 8A), showing that γδ T cells antiviral activity was not due to an early increase of NK cells. In addition, when transferred into B/NK/T cells immunodeficient Rag−/−γc−/− mice, MCMV-primed γδ T cells were also strikingly sufficient to long term protect these mice from death (Fig. 8B). At day 56, γδ T cells could easily be detected in the liver, spleen and lungs of Rag−/−γc−/− recipient mice in contrast to NK cells, demonstrating that the protective function of γδ T cells could act in the total absence of NK cells (Fig. 8C).
Fig. 8. NK-independent antiviral protective effect of γδ T cells. 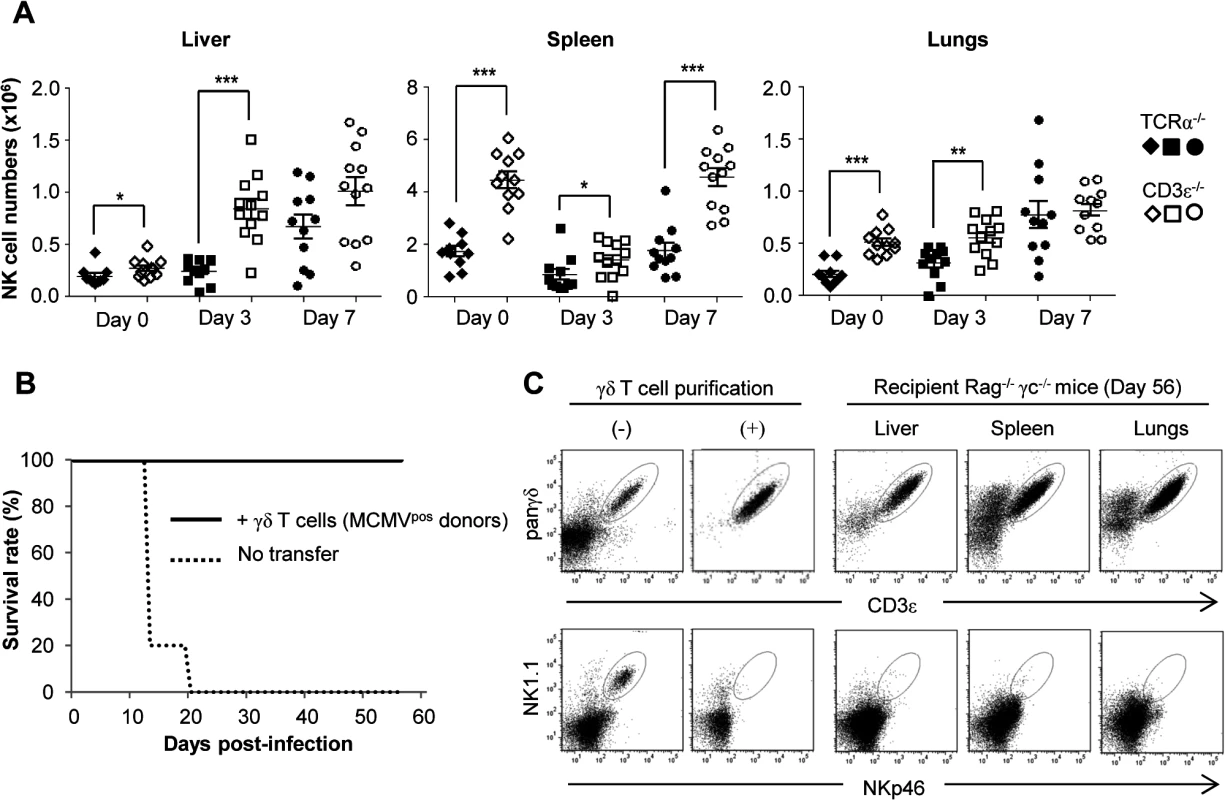
A. TCRα−/− and CD3ε−/− mice were uninfected (Day 0) or infected i.p. with 2.103 PFU of MCMV. At indicated days post-infection, 5–6 mice were sacrificed and immune cells were isolated from each organ for flow cytometry analysis. Absolute numbers of NK cells were calculated as described in methods. Black and white symbols represent individual TCRα−/− and CD3ε−/− mice respectively, and horizontal lines represent the mean of 10–12 mice pooled from 2 independent experiments. Differences were evaluated using the Mann-Whitney test: * = p<0,05, ** = p<0,01, *** = p<0,001. B. γδ T cells from 14-days infected TCRα−/− mice were purified and i.v. transferred (1.106 cells, 97% purity) into Rag−/−γc−/− mice (10 recipients). 24h after transfer, reconstituted Rag−/−γc−/− mice were challenged with 2.103 PFU of MCMV and monitored daily for mortality. 5 untransferred Rag−/−γc−/− mice were used as controls. Results are from one representative of 2 independent experiments. C. Left: flow cytometry analysis of live (7AAD−) CD3ε+panγδ+ T cells (upper panels) and NKp46+NK1.1+ cells (lower panels) in splenocytes from TCRα−/−donors, before (-) and after (+) purification of γδ T cells as described in methods. Right: At day 56 post-infection of Rag−/−γc−/− recipients, 3 mice were sacrificed; organs removed and immune cells isolated for flow cytometry analysis of γδ and NK cells. Results are from one representative mouse. Discussion
Previous work conveys compelling evidence for the implication of human Vδ2neg γδ T cells in the immune response against HCMV infection [5,6,7,9]. However, key questions that cannot easily be addressed in humans remain unanswered, such as the spatial and temporal regulation of the anti-HCMV γδ T cell response and its protective role. Because of its similarity with the human CMV pathogenesis and immune response, the mouse model of MCMV infection has been extensively used and is well characterized. The goal of this study was to take advantage of this model to address these questions concerning the protective role and localization of the γδ T cell response. Herein, we show that γδ T cells are as competent as αβ T cells to protect against CMV challenge, a finding that can be of particular relevance in clinical settings, situations or diseases where αβ T lymphocytes are compromised (hypomorphic Rag1 mutations, individuals treated with immunosuppressive drugs, foetuses or neonates, …) and where γδ T cells have already been shown to expand [6,7,8,9,10,11,12]. This protective function of γδ T cells, under conditions of suboptimal αβ T cell response, has previously been observed earlier in mice in the context of infection by Herpes Simplex Virus type 1 (HSV-1) [37] or by the gut coccidian parasite Eimeria vermiformis [38]. These results also corroborate the conserved level of protection against infection observed in patients lacking TCR αβ T cells due to a mutation in the gene coding the TCR α chain [39]. Since γδ T cells have been shown to play an important role in young mice in other infectious models, it would be interesting to evaluate this role in the context of MCMV infection [40]. In addition to extending our results to more a “natural setting” of suboptimal αβ T cells responses, it would allow analysis of the role of non BM-derived γδ T cell subtypes [41]. Finally this MCMV model could be used to evaluate the importance of γδ versus αβ T cells in the context of immunosuppression as used in transplant recipients.
After administration of MCMV via the intraperitoneal route, MCMV targets the liver and spleen as cell free viruses within the first hours before dissemination to the other organs [42]. Accordingly, viral loads were the highest at day 3 in the liver and spleen but peaked at day 7 in the lungs and intestine in all mouse lines tested in the present study. In TCRα−/− mice, viral loads were the lowest at day 14 in the liver and spleen and at day 21 in the lungs (Fig. 2), i.e. after the significant increase of both Vγ1+ and Vγ4+ γδ T cell subsets in the liver and lungs (Fig. 4A), and of Vγ1+ γδ T cells in the spleen (Fig. 5A). Three weeks post-MCMV infection, high viral loads and liver/lung injury were evidenced in CD3ε−/− mice despite normal development and function of NK cells in these mice [28]. In contrast, liver and lung disorders were not observed in TCRα−/− mice at that time. These results are consistent with a role for γδ T cell response/expansion in these organs to control virus multiplication and associated organ damage in the absence of αβ T cells. The protective role of γδ T cells was ascertained by reconstituting γδ T cells in CD3ε−/− mice by bone marrow transplantation, or by adoptive transfer of splenic γδ T cells from TCRα−/− MCMV infected mice. However, when isolated from the spleen of TCRα−/− uninfected mice, γδ T cells were inefficient to induce protection in CD3ε−/− recipients. We can exclude the possibility that lack of protection in CD3ε−/− mice which received naïve γδ T cells was due to an absence of engraftment, because both naïve and MCMV-primed γδ T cells were found in the liver, spleen and lungs of recipient mice (S8 Fig.). The absence of protection by non-primed γδ T cells purified from splenocytes may be due to a delay of reconstitution/differentiation in recipient mice that allow the virus to overwhelm the γδ T cell response. Infection of donor mice by CMV most likely prime γδ splenocytes to readily respond to CMV once transferred in CD3ε−/− mice, compensating this reconstitution limitation.
The development of the anti-CMV immune response involves a complex network of cells from the innate and adaptive immunity that act sequentially to favor health over disease. Research in mice has paid a lot of attention to the early control of MCMV by NK cells, which are responsible for the enhanced resistance of the C57BL/6 mouse strain when compared to BALBc mice. In C57BL/6 mice, NK cell antiviral activity relies on both perforin and IFNγ-release that control viral loads in the liver, spleen and lungs [33,43]. Our ex vivo analysis of lymphocytes from C57BL/6 TCRα−/− infected organs show that the early boost (days 3–7) of IFNγ expression and cytotoxic granule exocytosis is mostly due to NK cells, while γδ T cells participate only modestly to these functions (Fig. 7 and S7 Fig.). Thus, although we cannot exclude that this modest contribution might help in controlling MCMV loads, these results rather raise the possibility that γδ T cells operate either by regulating other immune cells or through the production of unknown antiviral mediators. Strikingly, however, our adoptive transfer experiment into Rag−/−γc−/− immunodeficient hosts showed that γδ T cell antiviral protective function can be independent of NK/B/αβ T cells. This emphasizes their efficiency and opens interesting perspectives for their possible manipulation in clinical situations where other immune cells are defective.
The kinetics of γδ T cell response was organ specific, with a progressive increase and accumulation of γδ T cells in the lungs, whereas γδ T cells quickly increased and dropped at day 21 in the liver and spleen (Fig. 4A). The persistence within the lungs of memory γδ T cells contrasts with the transient increase of pulmonary γδ T cells that was observed in other murine infectious contexts [44,45,46]. However it reproduces the persistence of γδ T cell expansion in human blood during HCMV-infection which could result from persistent activation of γδ T cells in chronically infected tissues [5,10]. This suggests that the lungs could be an anatomical site for replication of HCMV and chronic activation of γδ T cells, consistent with the fact that HCMV is frequently found in lungs of solid organ transplant patients where it can induce tissue invasive disease [4].
The γδ T cell response to MCMV implicates bone marrow derived Vγ1+ and Vγ4+ T cells. It will be interesting in the future to determine whether these subsets play similar functions in the response to MCMV, since evidence for distinct roles of Vγ1+ and Vγ4+ T cells in the protection and/or pathogenesis during infection of mice has been reported [46,47,48]. The involvement of several subsets in the response to MCMV is in agreement with the implication of diverse Vδ2neg T cell subsets (Vδ1, Vδ3, Vδ5) in the response to HCMV [5]. In contrast to long term HCMV-induced γδ T cells that display a restricted CDR3δ length repertoire [5], the CDR3γ1 and γ4 length repertoire of liver, spleen and lung-derived γδ T cells was equivalent in 14-days MCMV-infected and uninfected TCRα−/− mice (S3 Fig. and S4 Fig.). This could reflect a TCR-independent innate-like response of γδ T cells and/or high frequencies of MCMV-specific γδ T cells already existing in naïve mice. However, we cannot exclude the presence of a shared antigen-recognition motif in CDR3γ of different lengths (as observed for the CDR3δ of T22-specific γδ T cells [49]). The number of CDR3γ1 peaks (4 or 5) confirms previous analysis of CDR3 repertoire in mice [50].
Another interesting question concerns the memory function of γδ T cells during MCMV infection, as recently described for CD44+CD27− γδ T cells in mouse models of bacterial infections [51,52]. Adaptive and innate like γδ T cells could both participate to memory, in light of the emerging role for innate cells in this context [53]. Previous contact with HCMV induced a rapid recall expansion of effector memory Vδ2neg γδ T cells, which coincided with better infection resolution of HCMV reactivation in transplant recipients [10]. CMV infection in mice also induces CD44+CD62L− effector memory γδ T cells that are maintained and outnumber CD44+CD62L+ central memory γδ T cells at day 56 in all organs (Fig. 4B and Fig. 4C). By definition, effector memory cells are prone to exert rapid functions at the aggression site and the results shown here support the hypothesis that peripheral blood effector-memory human Vδ2neg γδ T cells are re-circulating cells that originate from CMV-targeted organs. It remains to be investigated whether murine γδ T cells recognize self-encoded stress-regulated antigens on CMV-infected cells, as demonstrated for human γδ T cells [18].
Acute infections with HCMV can result in serious disease in infected neonates and in the context of immunosuppression linked to transplantation. Inducing or enhancing the antiviral response of γδ T cells in this context is an attractive objective. Our findings open new perspectives for the use of the murine model of MCMV infection to define the precise mechanism of antiviral activity of γδ T cells and to develop new strategies to induce their activation in vivo. Their absence of MHC restriction, their combination of conventional adaptive and innate-like responses, their particular anatomical localization and their dual reactivity against infected and tumor cells, are specific features that place γδ T cells as unique effectors for clinical manipulation. In conjunction with the identification of stress antigens recognized by γδ T cells on infected cells, these results open new avenues for clinical manipulation of γδ T cells against CMV-mediated disease.
Materials and Methods
Ethics statement
All experimental procedures involving animals were conducted according to European Union guidelines (European Directive 2010/63/UE) (http://ec.europa.eu/environment/chemicals/lab_animals/home_en.htm) and approved by the local ethics committee: Comité d'éthique pour l'expérimentation animale de Bordeaux (CE50), [project n° 50120197-A].
Mice
We used C57BL/6 mice. CD3ε−/− [54], TCRα−/− [30] and Rag−/−γc−/− mice [55] were from the CDTA (Centre de Distribution, Typage et Archivage Animal, Orléans, France). TCRδ−/− [56] were a gift from Dr Malissen (Centre d’Immunologie de Marseille Luminy, France). Mice were used between 8–12 weeks of age and kept under pathogen-free conditions (Animalerie spécialisée, Université Bordeaux Segalen, France). CD3ε−/− were bred to C57BL/6 mice (C57BL/6J, Charles Rivers laboratory, Larbresle, France) to obtain CD3ε+/− control mice. MCMV-infection was performed in an appropriate animal facility (Animalerie A2, Université Bordeaux Segalen, France).
Virus stock and infection of mice
MCMV was acquired from the American Type Culture Collection (Smith strain, ATCC VR-194) and propagated into BALBc mice (BALBcBy/J, Charles Rivers laboratory, Larbresle, France) to generate MCMV salivary gland extracts. Virus titers were defined by standard plaque assay on monolayers of mouse embryonic fibroblasts (MEF). Unless indicated, infections were performed by i.p. administration of 2.103 PFU of the salivary gland viral stock.
AST and ALT quantifications
Mice were bled via the retroorbital sinus after anesthesia (one eye every other week) and the serums collected and frozen. AST and ALT were quantified using standard enzymological methods (laboratoire de Biochimie, CHU Bordeaux, France).
Tissue processing and histology
Mice were euthanized by cervical dislocation. Liver and lungs were removed, fixed for 24 h in 3.7% neutral-buffered formalin (Sigma-Aldrich), followed by standard histological processing and paraffin embedding. Sections of 4 μm thickness were processed for Hematoxylin/Eosin/Safran (HES) staining (following standard protocols).
Quantification of MCMV loads
Genomic DNA was isolated from organs using Nucleospin tissue purification kit (Macherey Nagel). Real time PCR to quantify MCMV was performed in Step one plus thermocycler (Applied biosystem) using GoTaq qPCR Master Mix (Promega) with primers specific for MCMV glycoprotein B (gB) (gi330510, forward primer: AGGCCGGTCGAGTACTTCTT and reverse primer: GCGCGGAGTATCAATAGAGC). Known quantities of plasmid comprising MCMV gB were used for the titration curve.
Relative quantification of transcripts by real time PCR and spectratyping
Total RNA from immune cells was prepared with Nucleospin RNAII kit (Macherey Nagel). Goscript reverse transcriptase (Promega) was used to generate cDNA. Real time PCR was performed in CFX 384 (BioRad). The relative expression of transcripts was determined using the GAPDH reference gene. For spectratyping analysis, PCR (40 cycles) was performed with Vγ1 and Cγ4 or with Vγ4 and Cγ1 primers, resulting in amplification of the sequences containing the CDR3γ1 or CDR3γ4, respectively. Then a run-off reaction (one cycle) was performed using a fluorescently labeled Jγ4-FAM primer for CDR3γ1 and with a Jγ1-FAM primer for CDR3γ4 (primers sequences from [50]). The labeled reaction products were run on a capillary sequencer (ABI3730xl analyzer) at ImmuneHealth (Gosselies, Belgium). The fluorescence intensity was analyzed using Peak Scanner 1.0 (Applied Biosystems).
List of primer Fw (forward) and Rv (Reverse):
GAPDH (Genbank NM_008084):
Fw 5’-AATGGGGTGAGGCCGGTGCT-3’
Rv 5’-CACCCTTCAAGTGGGCCCCG-3’
IFNγ (NM_008337.3)
Fw: 5’-ACTGGCAAAAGGATGGTGAC-3’
Rv 5’-TGAGCTCATTGAATGCTTGG-3’
IL17-A (NM_010552.3)
Fw 5’-TCATCTGTGTCTCTGATGCTGTT-3’
Rv 5’-TTGGACACGCTGAGCTTTGA-3’
Cδ (X12729.1)
Fw 5’-CTGTGCACTCGACTGACTTTGAACC-3’
Rv 5’-CCCAGCACCGTGAGGGACATC-3’
CDR3γ1
Fw Vγγ1 5'-CCGGCAAAAAGCAAAAAAGT-3
Rv Cγ4 5’-AAGGAGACAAAGGTAGGTCCCAGC-3’
Jγ4-FAM 5'-TACGAGCTTTGTCCCTTTG-3'
CDR3γ4
Fw Vγ4 5’-CTTGCAACCCCTACCCATAT-3’
Rv Cγ1 5’-CCACCACTCGTTTCTTTAGG-3’
Jγ1-FAM 5'-CTTAGTTCCTTCTGCAAATACC-3’
Preparation of immune cells from organs and numeration
We used cell strainers to mash the spleens and livers in RPMI-1640 with 8% FBS; red blood cells were lysed with NH4Cl. For the liver, immune cells were isolated by centrifugation (2000 rpm, 20 min) over a 40/80% discontinuous Percoll gradient (GE Healthcare). Pulmonary mononuclear cells were isolated as described [57]. Intestinal intraepithelial mononuclear cells were isolated as described elsewhere [58]. Total organ live cells (unstained with Trypan blue) were then counted using a hemocytometer (Malassez chamber). The proportion of γδ T cells (CD3ε+panγδ+) and NK cells (NK1.1+NKp46+) among total organ live cells (7AAD−) was evaluated by FACS using a large FSC/SSC gate that included all cells but debris. This proportion was then multiplied by total organ cell number to obtain the absolute number of γδ T cells and NK cells.
Antibodies and flow cytometry
The following monoclonal antibodies were from BD Pharmingen: anti-CD3ε (145–2C11), anti-TCRδ (GL3), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-CD27 (LG.3A10), anti-NK1.1 (PK136) and anti-NKp46 (29A1.4). Anti-IFNγ (XMG1.2), anti-CD107a (1D4B) and respective isotype control mAbs: Rat IgG1κ (eBRG1) and Rat IgG2aκ (eBR2a) were purchased from eBioscience. Anti-Vγ1 (2.11), anti-Vγ4 (49.2) and anti-Vγ7 (F2.64) mAbs were a kind gift from P. Pereira (Institut Pasteur, Paris). For flow cytometry analysis, immune cells were first incubated with anti-mouse CD16/32 (eBioscience) and stained with relevant antibodies and 7-AAD (BD Pharmingen). Fixed cells were acquired using a LSRFortessa (BD Biosciences), and analyzed using FlowJo software (Tree Star). For intracellular IFNγ staining, cells were incubated in complete medium for 2h at 37°C; 10μg/ml of Brefeldin A (Sigma-Aldrich) was added during the last hour. Intracellular staining was performed after cell surface staining, using BD Cytofix/Cytoperm Fixation/Permeabilization Kit and according to the manufacturer’s instruction (BD Biosciences). For CD107a staining, cells were incubated in complete medium for 2h at 37°C; 10μg/ml Brefeldin A (Sigma-Aldrich) and anti-CD107a or isotype control mAb were added during the last hour. Cells were then stained with relevant monoclonal antibodies.
Bone marrow transplant experiments
Mice femora and tibia from CD3ε+/−, TCRα−/−, TCRδ−/− and CD3ε−/− were isolated and the BM was flushed with 1 ml of IMDM with FBS (1%). BM cells from one donor were injected to one CD3ε−/− mice (8–10 per group), intravenously (i.v.) through the retrobulbar sinus in a volume of 0.2 mL IMDM. Mice were conditioned by i.p. injections of Busulfan 22.5 mg/kg (Pierre Fabre laboratory) two days and one day prior to transplantation [59].
Adoptive transfer experiments
10 TCRα−/− mice were uninfected, or 14 days infected with 2.103 PFU of MCMV. Immune cells were prepared from spleens and pooled before γδ T cell sorting using the TCRγ/δ+ T Cell Isolation kit (Miltenyi Biotec). Purity was verified by flow cytometry and 8.105 to 1.106 γδ T cells i.v. transferred into CD3ε−/− or Rag−/−γc−/− recipients. 24h after γδ T cell transfer, recipient mice were infected i.p. with 2.103 PFU of MCMV and followed daily. 2–3 months after infection, recipient mice were sacrificed to verify the presence of γδ/NK cells in organs.
Statistical analysis
Differences were evaluated by the Mann-Whitney test and represented as follows: * = p<0.05, ** = p<0.01, *** = p<0.001, **** = p<0.0001.
Supporting Information
Zdroje
1. Broers AE, van Der Holt R, van Esser JW, Gratama JW, Henzen-Logmans S, et al. (2000) Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 95 : 2240–2245. 10733491
2. Craddock C, Szydlo RM, Dazzi F, Olavarria E, Cwynarski K, et al. (2001) Cytomegalovirus seropositivity adversely influences outcome after T-depleted unrelated donor transplant in patients with chronic myeloid leukaemia: the case for tailored graft-versus-host disease prophylaxis. Br J Haematol 112 : 228–236. 11167809
3. Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, et al. (2013) CMV serostatus has still an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Blood 122 : 3359–3364. doi: 10.1182/blood-2013-05-499830 24037724
4. Crough T, Khanna R (2009) Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev 22 : 76–98, Table of Contents. doi: 10.1128/CMR.00034-08 19136435
5. Dechanet J, Merville P, Lim A, Retiere C, Pitard V, et al. (1999) Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest 103 : 1437–1449. 10330426
6. Ehl S, Schwarz K, Enders A, Duffner U, Pannicke U, et al. (2005) A variant of SCID with specific immune responses and predominance of gamma delta T cells. J Clin Invest 115 : 3140–3148. 16211094
7. Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, et al. (2010) Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J Exp Med 207 : 807–821. doi: 10.1084/jem.20090348 20368575
8. Fornara C, Lilleri D, Revello MG, Furione M, Zavattoni M, et al. (2011) Kinetics of effector functions and phenotype of virus-specific and gammadelta T lymphocytes in primary human cytomegalovirus infection during pregnancy. J Clin Immunol 31 : 1054–1064. doi: 10.1007/s10875-011-9577-8 21847524
9. Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, et al. (2010) The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 116 : 2164–2172. doi: 10.1182/blood-2010-01-255166 20576814
10. Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, et al. (2008) Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 112 : 1317–1324. doi: 10.1182/blood-2008-01-136713 18539896
11. Roux A, Mourin G, Larsen M, Fastenackels S, Urrutia A, et al. (2013) Differential Impact of Age and Cytomegalovirus Infection on the gammadelta T Cell Compartment. J Immunol 191 : 1300–1306. doi: 10.4049/jimmunol.1202940 23817410
12. Wistuba-Hamprecht K, Frasca D, Blomberg B, Pawelec G, Derhovanessian E (2013) Age-associated alterations in gammadelta T-cells are present predominantly in individuals infected with Cytomegalovirus. Immun Ageing 10 : 26. doi: 10.1186/1742-4933-10-26 23822093
13. Lafarge X, Merville P, Cazin MC, Berge F, Potaux L, et al. (2001) Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. J Infect Dis 184 : 533–541. 11494158
14. Couzi L, Pitard V, Netzer S, Garrigue I, Lafon ME, et al. (2009) Common features of gammadelta T cells and CD8(+) alphabeta T cells responding to human cytomegalovirus infection in kidney transplant recipients. J Infect Dis 200 : 1415–1424. doi: 10.1086/644509 19780672
15. Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, et al. (2005) Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 201 : 1567–1578. 15897274
16. Couzi L, Pitard V, Sicard X, Garrigue I, Hawchar O, et al. (2012) Antibody-dependent anti-cytomegalovirus activity of human gammadelta T cells expressing CD16 (FcgammaRIIIa). Blood 119 : 1418–1427. doi: 10.1182/blood-2011-06-363655 22180442
17. Vantourout P, Hayday A (2013) Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol 13 : 88–100. doi: 10.1038/nri3384 23348415
18. Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, et al. (2012) Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat Immunol 13 : 872–879. doi: 10.1038/ni.2394 22885985
19. Bonneville M, O'Brien RL, Born WK (2010) Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 10 : 467–478. doi: 10.1038/nri2781 20539306
20. Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, et al. (2013) gammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 27 : 1328–1338. doi: 10.1038/leu.2012.374 23277330
21. Couzi L, Levaillant Y, Jamai A, Pitard V, Lassalle R, et al. (2010) Cytomegalovirus-induced gammadelta T cells associate with reduced cancer risk after kidney transplantation. J Am Soc Nephrol 21 : 181–188. doi: 10.1681/ASN.2008101072 19713314
22. Godder KT, Henslee-Downey PJ, Mehta J, Park BS, Chiang KY, et al. (2007) Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant 39 : 751–757. 17450185
23. Behrendt CE, Rosenthal J, Bolotin E, Nakamura R, Zaia J, et al. (2009) Donor and recipient CMV serostatus and outcome of pediatric allogeneic HSCT for acute leukemia in the era of CMV-preemptive therapy. Biol Blood Marrow Transplant 15 : 54–60. doi: 10.1016/j.bbmt.2008.10.023 19135943
24. Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, et al. (2011) Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 118 : 1402–1412. doi: 10.1182/blood-2010-08-304121 21540462
25. Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S (2003) Pathogenesis of murine cytomegalovirus infection. Microbes Infect 5 : 1263–1277. 14623023
26. Ninomiya T, Takimoto H, Matsuzaki G, Hamano S, Yoshida H, et al. (2000) Vgamma1+ gammadelta T cells play protective roles at an early phase of murine cytomegalovirus infection through production of interferon-gamma. Immunology 99 : 187–194. 10692035
27. Trgovcich J, Stimac D, Polic B, Krmpotic A, Pernjak-Pugel E, et al. (2000) Immune responses and cytokine induction in the development of severe hepatitis during acute infections with murine cytomegalovirus. Arch Virol 145 : 2601–2618. 11205107
28. Renard V, Ardouin L, Malissen M, Milon G, Lebastard M, et al. (1995) Normal development and function of natural killer cells in CD3 epsilon delta 5/delta 5 mutant mice. Proc Natl Acad Sci U S A 92 : 7545–7549. 7638228
29. Heilig JS, Tonegawa S (1986) Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 322 : 836–840. 2943999
30. Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, et al. (1992) Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science 256 : 1448–1452. 1604321
31. Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, et al. (2009) CD27 is a thymic determinant of the balance between interferon-gamma - and interleukin 17-producing gammadelta T cell subsets. Nat Immunol 10 : 427–436. doi: 10.1038/ni.1717 19270712
32. Schmolka N, Serre K, Grosso AR, Rei M, Pennington DJ, et al. (2013) Epigenetic and transcriptional signatures of stable versus plastic differentiation of proinflammatory gammadelta T cell subsets. Nat Immunol 14 : 1093–1100. doi: 10.1038/ni.2702 23995235
33. Loh J, Chu DT, O'Guin AK, Yokoyama WM, Virgin HWt (2005) Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol 79 : 661–667. 15596864
34. Gardner T, Chen Q, Jin Y, Ajuebor MN (2009) Characterization of the role of TCR gammadelta in NK cell accumulation during viral liver inflammation. Exp Mol Pathol 86 : 32–35. doi: 10.1016/j.yexmp.2008.10.005 19028491
35. Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, et al. (2001) Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2 : 951–956. 11550009
36. Robbins SH, Tessmer MS, Mikayama T, Brossay L (2004) Expansion and contraction of the NK cell compartment in response to murine cytomegalovirus infection. J Immunol 173 : 259–266. 15210783
37. Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA (1997) T cell receptor-gamma/delta cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med 185 : 1969–1975. 9166426
38. Smith AL, Hayday AC (2000) An alphabeta T-cell-independent immunoprotective response towards gut coccidia is supported by gammadelta cells. Immunology 101 : 325–332. 11106935
39. Morgan NV, Goddard S, Cardno TS, McDonald D, Rahman F, et al. (2011) Mutation in the TCRalpha subunit constant gene (TRAC) leads to a human immunodeficiency disorder characterized by a lack of TCRalphabeta+ T cells. J Clin Invest 121 : 695–702. doi: 10.1172/JCI41931 21206088
40. Ramsburg E, Tigelaar R, Craft J, Hayday A (2003) Age-dependent requirement for gammadelta T cells in the primary but not secondary protective immune response against an intestinal parasite. J Exp Med 198 : 1403–1414. 14597739
41. Vermijlen D, Prinz I (2014) Ontogeny of Innate T Lymphocytes—Some Innate Lymphocytes are More Innate than Others. Front Immunol 5 : 486. doi: 10.3389/fimmu.2014.00486 25346734
42. Hsu KM, Pratt JR, Akers WJ, Achilefu SI, Yokoyama WM (2009) Murine cytomegalovirus displays selective infection of cells within hours after systemic administration. J Gen Virol 90 : 33–43. doi: 10.1099/vir.0.006668-0 19088270
43. Sumaria N, van Dommelen SL, Andoniou CE, Smyth MJ, Scalzo AA, et al. (2009) The roles of interferon-gamma and perforin in antiviral immunity in mice that differ in genetically determined NK-cell-mediated antiviral activity. Immunol Cell Biol 87 : 559–566. doi: 10.1038/icb.2009.41 19564888
44. Dieli F, Ivanyi J, Marsh P, Williams A, Naylor I, et al. (2003) Characterization of lung gamma delta T cells following intranasal infection with Mycobacterium bovis bacillus Calmette-Guerin. J Immunol 170 : 463–469. 12496432
45. Kirby AC, Newton DJ, Carding SR, Kaye PM (2007) Evidence for the involvement of lung-specific gammadelta T cell subsets in local responses to Streptococcus pneumoniae infection. Eur J Immunol 37 : 3404–3413. 18022862
46. Dodd J, Riffault S, Kodituwakku JS, Hayday AC, Openshaw PJ (2009) Pulmonary V gamma 4+ gamma delta T cells have proinflammatory and antiviral effects in viral lung disease. J Immunol 182 : 1174–1181. 19124761
47. Huber SA, Graveline D, Newell MK, Born WK, O'Brien RL (2000) V gamma 1+ T cells suppress and V gamma 4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol 165 : 4174–4181. 11035049
48. Welte T, Lamb J, Anderson JF, Born WK, O'Brien RL, et al. (2008) Role of two distinct gammadelta T cell subsets during West Nile virus infection. FEMS Immunol Med Microbiol 53 : 275–283. doi: 10.1111/j.1574-695X.2008.00430.x 18513355
49. Sandstrom A, Scharf L, McRae G, Hawk AJ, Meredith SC, et al. (2012) gammadelta T cell receptors recognize the non-classical major histocompatibility complex (MHC) molecule T22 via conserved anchor residues in a MHC peptide-like fashion. J Biol Chem 287 : 6035–6043. doi: 10.1074/jbc.M111.333153 22215668
50. Andrew EM, Newton DJ, Dalton JE, Egan CE, Goodwin SJ, et al. (2005) Delineation of the function of a major gamma delta T cell subset during infection. J Immunol 175 : 1741–1750. 16034115
51. Sheridan BS, Romagnoli PA, Pham QM, Fu HH, Alonzo F 3rd, et al. (2013) gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity 39 : 184–195. doi: 10.1016/j.immuni.2013.06.015 23890071
52. Murphy AG, O'Keeffe KM, Lalor SJ, Maher BM, Mills KH, et al. (2014) Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection. J Immunol 192 : 3697–3708. doi: 10.4049/jimmunol.1303420 24623128
53. Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL (2013) Natural killer cells: walking three paths down memory lane. Trends Immunol 34 : 251–258. doi: 10.1016/j.it.2013.02.005 23499559
54. Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, et al. (1995) Altered T cell development in mice with a targeted mutation of the CD3-epsilon gene. EMBO J 14 : 4641–4653. 7588594
55. Goldman JP, Blundell MP, Lopes L, Kinnon C, Di Santo JP, et al. (1998) Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol 103 : 335–342. 9827902
56. Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, et al. (1993) T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell 72 : 337–348. 8381716
57. Sauer KA, Scholtes P, Karwot R, Finotto S (2006) Isolation of CD4+ T cells from murine lungs: a method to analyze ongoing immune responses in the lung. Nat Protoc 1 : 2870–2875. 17406546
58. Capone M, Lees RK, Finke D, Ernst B, Meerwijk JP, et al. (2003) Selective absence of CD8+ TCRalpha beta+ intestinal epithelial cells in transgenic mice expressing beta2-microglobulin-associated ligands exclusively on thymic cortical epithelium. Eur J Immunol 33 : 1471–1477. 12778464
59. Robert-Richard E, Ged C, Ortet J, Santarelli X, Lamrissi-Garcia I, et al. (2006) Human cell engraftment after busulfan or irradiation conditioning of NOD/SCID mice. Haematologica 91 : 1384. 17018389
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



