-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
article has not abstract
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004644
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004644Summary
article has not abstract
An innate immune response is essential for survival of the host upon infection, yet excessive inflammation can result in harmful complications [1]. Inhibitory signaling evolved to limit host responses and prevent inflammatory pathology [2,3]. Given the significance of inhibitory pathways for immunity and homeostasis, they provide ideal targets for manipulation by bacterial pathogens. Recent evidence highlights that bacteria have developed diverse strategies to exploit these inhibitory pathways to avoid host defense for their own benefit. In this review, we cover these different immune evasion strategies for the first time. The recent literature discussed emphasizes that bacteria subvert host immune responses not only by direct engagement of inhibitory receptors (i.e., often through “molecular mimicry” of host ligands [4,5]) but also through virulence factors that resemble intermediates of host inhibitory signaling and interfere with defense functions [6–8]. Understanding how bacteria manipulate inhibitory signaling affords promising opportunities to counteract these escape strategies and tip the balance in favor of the host. In addition, these understandings may provide useful insights on the functional roles of inhibitory pathways in limiting host responses and preventing pathology.
Inhibitory Signaling Controls Inflammatory Responses in Host Immune Cells
In response to infection, the host immune system initiates swift and robust inflammatory responses to protect the host from the spread of invading microbes. Inflammation is launched when front-line defense cells, such as epithelial cells, macrophages, and neutrophils, detect alarm signals. The sensing of microbes through pattern recognition receptors (PRRs) activates the inflammatory functions of sentinel cells. However, if the initial host response is overamplified, inflammation results in host tissue damage and can lead to severe complications. Inhibitory pathways control host immune responses upon infection and prevent collateral tissue damage. Inhibitory immune receptors attenuate cellular signaling delivered by activating receptors, including Toll-like receptors (TLRs) [3] and Fc receptors (FcRs), directly or through their downstream signaling intermediates (see review [9] and Fig. 1). Inhibitory receptors contain specific sequence motifs in their intracellular tails to recruit signaling molecules. The most common inhibitory motif is the immunoreceptor tyrosine-based inhibitory motif (ITIM). Engagement of ITIM-bearing receptors results in ITIM tyrosine phosphorylation and recruitment of downstream mediators containing Src homology 2 (SH2) domains, such as SHP-1, SHP-2, SHIP, and Csk [9]. Next, dephosphorylating signaling intermediates causes them to act on their respective targets to dampen inflammatory signals relayed by activating receptors. ITIM-containing receptors mostly, but not exclusively [3], attenuate immunoreceptor tyrosine-based activation motif (ITAM)–associated receptors, such as Fcγ receptors (FcγRs).
Fig. 1. Negative modulation of inflammatory responses against pathogens by ITIM-bearing inhibitory receptors. 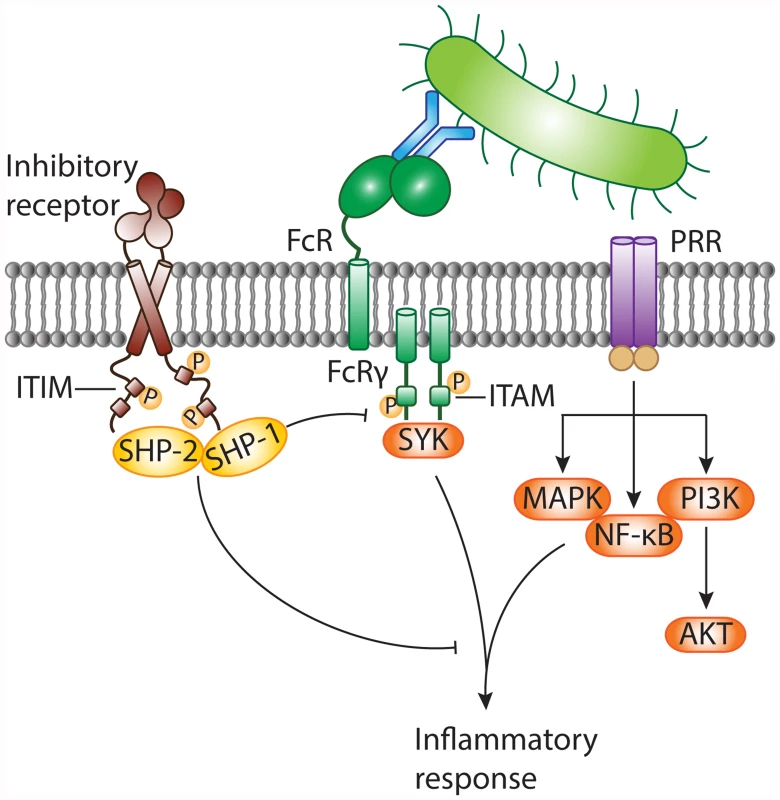
Invasion of the host by bacteria results in the appearance of pathogen-associated molecular patterns (PAMPs). These danger signals are sensed by pattern recognition receptors (PRRs), including TLRs, on the surface of sentinel cells. Bacteria can be opsonized with antibodies and are recognized by cell surface Fc receptors (FcRs) associated with the immunoreceptor tyrosine-based activation motif (ITAM)-containing FcR common γ chain. FcRs generally transmit activating signals through activation of the protein tyrosine kinase SYK, while diverse signaling cascades (such as activation of MAPK, NF-κB, and PI3K) are relayed by PRRs. The inflammatory response against non-self is essential to combat invading bacteria. On the other hand, the antibacterial response needs to be controlled to prevent collateral tissue damage. Inhibitory receptors often possess immunoreceptor tyrosine-based inhibitory motifs (ITIMs) within their intracellular tails. Following receptor engagement, tyrosine residues within the ITIMs are phosphorylated and become docking sites for cytosolic protein tyrosine phosphatases, such as SHP-1 and SHP-2. These negative regulatory proteins terminate activating signals delivered by PRRs and/or ITAM-coupled FcRs and contribute to dampening of the inflammatory response. MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor κB; PI3K, phosphoinositide 3-kinase. Undesirable outcomes may arise, however, when bacteria take advantage of host inhibitory signaling. Some bacterial pathogens use surface ligands to directly engage ITIM-bearing receptors, which they can co-ligate with activated PRRs (such as TLRs) or ITAM-paired receptors to suppress cellular activation and increase bacterial survival (Fig. 2a). For instance, following inhalation of Moraxella catarrhalis or Neisseria meningitidis, pulmonary epithelial cells release IL-8 and GM-CSF in a TLR-2-dependent manner, to recruit neutrophils. To evade immune clearance, the virulence proteins UspA1 of M. catarrhalis and Opa of N. meningitidis ligate the ITIM-containing receptor carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) with TLR-2 on epithelial cells, thus inhibiting co-engaged TLR-2 signaling and cytokine release [10]. Similarly, Nakayama et al. reported that Staphylococcus aureus targets the murine ITIM-bearing inhibitory receptor paired Ig-like receptor B (PIR-B) through the essential cell wall component lipoteichoic acid (LTA) to blunt TLR-induced inflammatory cytokine release by macrophages in response to the bacteria [5,11]. S. aureus, a major source of mortality in hospitals, can spread to the bloodstream and cause life-threatening sepsis. Following challenge with S. aureus, Pirb-knockout mice show enhanced inflammatory responses, and are better at clearing the bacteria and resistant to S. aureus-induced sepsis.
Fig. 2. Bacterial pathogens evade host defense responses by manipulating inhibitory signaling. 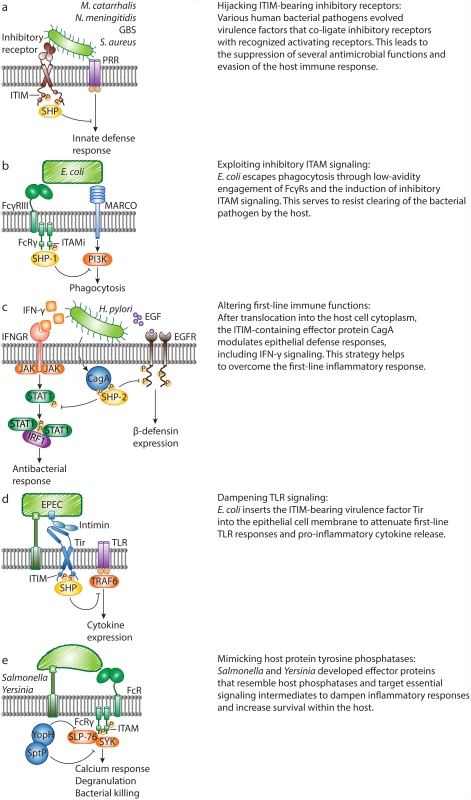
A. M. catarrhalis, N. meningitidis, Group B Streptococcus and Staphylococcus aureus evolved specific virulence factors to engage inhibitory receptors, which co-ligate with and attenuate pattern recognition receptor (PRR) signaling. B. Escherichia coli escapes macrophage receptor with collagenous structure (MARCO)–dependent killing through hijacking of inhibitory ITAM signaling. Non-opsonized E. coli binds to FcγRIII with low affinity and induces weak phosphorylation of the FcR common γ chain (FcRγ), leading to recruitment of SHP-1. In turn, SHP-1 dephosphorylates PI3K and abrogates MARCO-dependent phagocytosis. C. Upon infection, Helicobacter pylori translocates the ITIM-containing virulence protein, CagA, into host cells, and CagA-SHP-2 interactions lead to dephosphorylation of activated STAT1 and epidermal growth factor receptor (EGFR). This abrogates IFN-γ signaling and human β-defensin 3 (hBD3) synthesis, and enhances bacterial survival. D. During infection with the bacterium enteropathogenic E. coli (EPEC), the intimin receptor (Tir) translocates into the epithelial cell. The intracellular tail of EPEC Tir recruits host cell phosphatases SHP-1 and SHP-2. As a result, the activation of TRAF6 is inhibited, and EPEC-induced expression of pro-inflammatory cytokines is suppressed. E. Salmonella and Yersinia secrete protein tyrosine phosphatases SptP and YopH, respectively. SptP targets the protein tyrosine kinase SYK in mast cells and suppresses degranulation. During in vivo infection, YopH targets the signaling adaptor SLP-76 in neutrophils. This leads to reduced calcium responses and IL-10 production. In addition, Group B Streptococcus (GBS) uses an evasion strategy to oppose ITAM-mediated inflammatory responses. The surface β-protein and sialic acid of GBS both suppress host defense by engaging inhibitory sialic acid–binding Ig-like lectins (Siglecs). GBS, an important cause of neonatal infections, targets ITIM-bearing Siglec-5 and Siglec-9 to recruit SHP-2 and escape killing by monocytes and/or neutrophils in vitro [12,13]. In line with these findings, mice lacking Siglec-E (the orthologue of human Siglec-9) clear GBS more quickly than wild-type mice [4]. Recently, the ITAM-coupled Siglec-14 was shown to counteract Siglec-5–dependent host immune suppression by GBS, thus forming a paired receptor system that balances inflammatory responses to bacterial pathogens [14]. Together, an increasing number of studies demonstrate that bacterial pathogens target ITIM-containing inhibitory receptors to suppress immune cell function and increase their survival within the host.
ITAMs That Deliver Inhibitory Signals—And Their Manipulation by E. coli
Although ITAM motifs generally deliver activating signals, growing evidence now supports a role for ITAM-associated receptors in mediating inhibitory signals. ITAMs are found in the cytoplasmic domains of host receptors but also in certain transmembrane adaptors, such as the FcR common γ chain (FcRγ) in myeloid cells, that pair with specific receptors. ITAM-mediated cell activation requires high-avidity ligation of the ITAM-coupled receptors. In contrast, low-avidity ligation of these receptors generates inhibitory signals [15,16]. An ITAM that is functioning in an inhibitory mode is referred to as an “ITAMi”. Bacterial pathogens can hijack ITAMi signaling as a means to subvert defense responses. Specifically, E. coli binds FcγRIII directly in an antibody-independent manner, and this low-avidity interaction induces FcRγ phosphorylation, followed by SHP-1 recruitment (Fig. 2b). In turn, recruitment of SHP-1 is associated with a reduction in phosphorylation of PI3K, which is thereby unable to support MARCO-mediated phagocytosis of E. coli [17]. Consequently, mice deficient in FcγRIII or FcRγ have increased survival rates in models of sepsis, and this is attributed, in part, to their enhanced ability to clear E. coli. Thus, E. coli can overcome the inflammatory response through manipulation of the FcγRIII-FcRγ signaling complex, resulting in severe consequences during sepsis. To date, the identity of the FcγRIII-interacting ligand of E. coli remains unknown. Also, since these findings are not exclusive to pathogenic E. coli, it is conceivable that this crosstalk instead evolved to limit and control unwarranted inflammatory responses to commensal organisms, such as gram-negative bacteria in the gut.
H. pylori and Enteropathogenic E. coli Hack into Host Inflammatory Signaling Through ITIM-Containing Effector Secretion
The above-mentioned examples include strategies where bacterial cell surface ligands directly interact with ITIM-bearing or ITAMi-paired host receptors to overcome host defenses. Successful bacterial pathogens may also use virulence factors that are delivered into host cells via specialized secretion systems and interfere with cellular signaling. These bacterial proteins are commonly referred to as “effectors”. Remarkably, recent studies have revealed that bacterial pathogens secrete effectors to relay inhibitory signals. Enteropathogenic E. coli (EPEC) and H. pylori release effectors containing ITIM-like motifs within target cells to suppress immune responses [8,18–20], while Yersinia and Salmonella attenuate inflammatory signaling through secretion of effectors that bear resemblance to host cellular protein tyrosine phosphatases (PTPases) [6,7].
The first identified bacterially encoded effector containing tyrosine-based motifs resembling ITIMs, is the major virulence factor cytotoxin-associated gene A (CagA) from H. pylori, a cause of gastric inflammation. During H. pylori infection, a type IV secretion system is formed that exports CagA into host cells. Translocated CagA undergoes tyrosine phosphorylation in the host cells and directly mediates SHP-2 activation by binding to SH2 domains in a phosphorylation-dependent manner [21]. Work by Wang et al. described that the effector CagA modulates epithelial cell inflammatory responses by preventing the induction of IFN-γ–dependent STAT1 phosphorylation and IRF1 transactivation in targeted epithelial cells (Fig. 2c) [8]. Similarly, H. pylori counteracts the expression of the antimicrobial defensin peptide human β-defensin 3 (hBD3), to which H. pylori is highly susceptible (Fig. 2c) [20]. Following activation by CagA, SHP-2 dephosphorylates the intracellular domains of EGFR, thereby abrogating hBD3 synthesis and increasing bacterial survival.
Bioinformatics approaches revealed that the bacterial effector translocated intimin receptor (Tir) of enterohaemorrhagic E. coli (EHEC) and EPEC encodes similar ITIM-like motifs [22]. EPEC uses a strategy where it injects bacterial Tir into the epithelial cell membrane (Fig. 2d). The extracellular part of Tir is engaged by the bacterial surface ligand intimin, while the intracellular part of Tir contains a region with similarity to host ITIMs. The bacterial Tir ITIMs recruit the host tyrosine phosphatases SHP-1 and SHP-2 [18,19], which enhance its binding to TRAF6. The resulting interaction inhibits the ubiquitination and activation of TRAF6 and thereby suppresses EPEC-induced expression of inflammatory cytokines.
Salmonella and Yersinia Break Down Host Defense Responses through Bacterial PTPases
Salmonella and Yersinia contain effector proteins that resemble host PTPases and target central inflammatory signaling to shut down host immune cells (Fig. 2e) [6,7]. Like CagA and Tir, PTPase-like bacterial effectors were discovered by sequence homology studies [23]. The structurally related effectors SptP and YopH share homology with eukaryotic PTPases [23] and are essential for virulence of Salmonella and Yersinia, respectively. Bacterial PTPases have a myriad of known host targets and other well-established functions. In this review, we focus on the bacterial strategies that disrupt inflammatory responses through dephosphorylation of signaling intermediates by bacterial PTPases. We do not cover general tactics used by bacteria to interfere with inflammatory signaling through inactivation of signaling molecules by other effector proteins, such as the Yersinia leucine-rich repeat effector YopM [24,25]. Choi et al. demonstrated that Salmonella typhimurium secretes SptP to impede inflammatory responses [6]. SYK is an essential protein tyrosine kinase for IgE-mediated mast cell degranulation. WT S. typhimurium suppresses IgE-induced phosphorylation of SYK, whereas an isogenic ΔsptP mutant is not able to do so. In vivo, mast cells fail to degranulate and recruit neutrophils upon infection with S. typhimurium. In the absence of SptP, however, mast cells do degranulate and neutrophils are rapidly recruited to sites of infection, demonstrating a direct role for SptP in suppressing mast cell activation, neutrophil influx and bacterial clearance.
Neutrophils are also recruited to inflammatory lesions after infection with Yersinia pseudotuberculosis (Yptb), another gram-negative human pathogen. In a recent study, it was shown that the PTPase-like effector YopH affects phosphorylation of the crucial signaling adaptor SLP-76 and activation of its downstream effectors in recruited neutrophils, dampening calcium responses and IL-10 production [7]. Depletion of neutrophils allows the outgrowth of a mutant lacking YopH, indicating that YopH is critical for attenuating neutrophil bactericidal functions to enhance survival of Yptb.
Concluding Remarks
Since the first descriptions of an ITIM in FcγRIIB and CD22 over 15 years ago [26,27], many inhibitory receptors are still being discovered by the presence of intracellular inhibitory motifs. To date, genomic and proteomic informatics revealed more than 300 ITIM-bearing proteins [28,29], and many of them still await demonstration of function. Studying the mechanisms of bacterial manipulation of inhibitory signaling may provide useful insights on the functional roles of novel ITIM-bearing receptors. Clearly, bacteria have evolved sophisticated strategies to successfully instigate host inhibitory signaling, allowing evasion of immune defense mechanisms. Insight regarding these strategies is crucial to design approaches to control infection. In an era of growing resistance to antibiotics, blocking of subverted host receptors or counteracting the virulence factors involved affords promising approaches to overcome immune evasion by pathogens.
Zdroje
1. Cohen J (2002) The immunopathogenesis of sepsis. Nature 420 : 885–891. 12490963
2. Zhu Y, Yao S, Chen L (2011) Cell surface signaling molecules in the control of immune responses: a tide model. Immunity 34 : 466–478. doi: 10.1016/j.immuni.2011.04.008 21511182
3. Lu R, Pan H, Shively JE (2012) CEACAM1 negatively regulates IL-1β production in LPS activated neutrophils by recruiting SHP-1 to a SYK-TLR4-CEACAM1 complex. PLoS Pathog 8: e1002597. doi: 10.1371/journal.ppat.1002597 22496641
4. Chang Y-C, Olson J, Beasley FC, Tung C, Zhang J, et al. (2014) Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo. PLoS Pathog 10: e1003846. doi: 10.1371/journal.ppat.1003846 24391502
5. Nakayama M, Kurokawa K, Nakamura K, Lee BL, Sekimizu K, et al. (2012) Inhibitory receptor paired Ig-like receptor B is exploited by Staphylococcus aureus for virulence. J Immunol Baltim Md 1950 189 : 5903–5911. doi: 10.4049/jimmunol.1201940 23152562
6. Choi HW, Brooking-Dixon R, Neupane S, Lee C - J, Miao EA, et al. (2013) Salmonella typhimurium impedes innate immunity with a mast-cell-suppressing protein tyrosine phosphatase, SptP. Immunity 39 : 1108–1120. doi: 10.1016/j.immuni.2013.11.009 24332031
7. Rolán HG, Durand EA, Mecsas J (2013) Identifying Yersinia YopH-targeted signal transduction pathways that impair neutrophil responses during in vivo murine infection. Cell Host Microbe 14 : 306–317. doi: 10.1016/j.chom.2013.08.013 24034616
8. Wang Y-C, Chen C-L, Sheu B-S, Yang Y-J, Tseng P-C, et al. (2014) Helicobacter pylori Infection Activates Src Homology-2 Domain-Containing Phosphatase 2 To Suppress IFN-γ Signaling. J Immunol Baltim Md 1950 193 : 4149–4158. doi: 10.4049/jimmunol.1400594 25225672
9. Vivier E, Daëron M (1997) Immunoreceptor tyrosine-based inhibition motifs. Immunol Today 18 : 286–291. 9190115
10. Slevogt H, Zabel S, Opitz B, Hocke A, Eitel J, et al. (2008) CEACAM1 inhibits Toll-like receptor 2-triggered antibacterial responses of human pulmonary epithelial cells. Nat Immunol 9 : 1270–1278. doi: 10.1038/ni.1661 18836450
11. Nakayama M, Underhill DM, Petersen TW, Li B, Kitamura T, et al. (2007) Paired Ig-like receptors bind to bacteria and shape TLR-mediated cytokine production. J Immunol Baltim Md 1950 178 : 4250–4259. 17371981
12. Carlin AF, Uchiyama S, Chang Y-C, Lewis AL, Nizet V, et al. (2009) Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113 : 3333–3336. doi: 10.1182/blood-2008-11-187302 19196661
13. Carlin AF, Chang Y-C, Areschoug T, Lindahl G, Hurtado-Ziola N, et al. (2009) Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J Exp Med 206 : 1691–1699. doi: 10.1084/jem.20090691 19596804
14. Ali SR, Fong JJ, Carlin AF, Busch TD, Linden R, et al. (2014) Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med 211 : 1231–1242. doi: 10.1084/jem.20131853 24799499
15. Wang L, Gordon RA, Huynh L, Su X, Park Min K H, et al. (2010) Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity 32 : 518–530. doi: 10.1016/j.immuni.2010.03.014 20362473
16. Pinheiro da Silva F, Aloulou M, Benhamou M, Monteiro RC (2008) Inhibitory ITAMs: a matter of life and death. Trends Immunol 29 : 366–373. doi: 10.1016/j.it.2008.05.001 18602341
17. Pinheiro da Silva F, Aloulou M, Skurnik D, Benhamou M, Andremont A, et al. (2007) CD16 promotes Escherichia coli sepsis through an FcR gamma inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat Med 13 : 1368–1374. 17934470
18. Yan D, Wang X, Luo L, Cao X, Ge B (2012) Inhibition of TLR signaling by a bacterial protein containing immunoreceptor tyrosine-based inhibitory motifs. Nat Immunol 13 : 1063–1071. doi: 10.1038/ni.2417 23001144
19. Yan D, Quan H, Wang L, Liu F, Liu H, et al. (2013) Enteropathogenic Escherichia coli Tir recruits cellular SHP-2 through ITIM motifs to suppress host immune response. Cell Signal 25 : 1887–1894. doi: 10.1016/j.cellsig.2013.05.020 23707390
20. Bauer B, Pang E, Holland C, Kessler M, Bartfeld S, et al. (2012) The Helicobacter pylori virulence effector CagA abrogates human β-defensin 3 expression via inactivation of EGFR signaling. Cell Host Microbe 11 : 576–586. doi: 10.1016/j.chom.2012.04.013 22704618
21. Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, et al. (2002) SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295 : 683–686. 11743164
22. Barrow AD, Trowsdale J (2006) You say ITAM and I say ITIM, let’s call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol 36 : 1646–1653. 16783855
23. Kaniga K, Uralil J, Bliska JB, Galán JE (1996) A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol Microbiol 21 : 633–641. 8866485
24. LaRock CN, Cookson BT (2012) The Yersinia Virulence Effector YopM Binds Caspase-1 to Arrest Inflammasome Assembly and Processing. Cell Host Microbe 12 : 799–805. doi: 10.1016/j.chom.2012.10.020 23245324
25. Ye Z, Gorman AA, Uittenbogaard AM, Myers-Morales T, Kaplan AM, et al. (2014) Caspase-3 Mediates the Pathogenic Effect of Yersinia pestis YopM in Liver of C57BL/6 Mice and Contributes to YopM’s Function in Spleen. PLoS ONE 9: e110956. doi: 10.1371/journal.pone.0110956 25372388
26. Daëron M, Latour S, Malbec O, Espinosa E, Pina P, et al. (1995) The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity 3 : 635–646. 7584153
27. Doody GM, Justement LB, Delibrias CC, Matthews RJ, Lin J, et al. (1995) A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science 269 : 242–244. 7618087
28. Staub E, Rosenthal A, Hinzmann B (2004) Systematic identification of immunoreceptor tyrosine-based inhibitory motifs in the human proteome. Cell Signal 16 : 435–456. 14709333
29. Daëron M, Jaeger S, Du Pasquier L, Vivier E (2008) Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev 224 : 11–43. doi: 10.1111/j.1600-065X.2008.00666.x 18759918
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



