-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Inducible Deletion of CD28 Prior to Secondary Infection Impairs Worm Expulsion and Recall of Protective Memory CD4 T Cell Responses
IL-13 driven Th2 immunity is indispensable for host protection against infection with the gastrointestinal nematode Nippostronglus brasiliensis. Disruption of CD28 mediated costimulation impairs development of adequate Th2 immunity, showing an importance for CD28 during the initiation of an immune response against this pathogen. In this study, we used global CD28−/− mice and a recently established mouse model that allows for inducible deletion of the cd28 gene by oral administration of tamoxifen (CD28−/loxCre+/−+TM) to resolve the controversy surrounding the requirement of CD28 costimulation for recall of protective memory responses against pathogenic infections. Following primary infection with N. brasiliensis, CD28−/− mice had delayed expulsion of adult worms in the small intestine compared to wild-type C57BL/6 mice that cleared the infection by day 9 post-infection. Delayed expulsion was associated with reduced production of IL-13 and reduced serum levels of antigen specific IgG1 and total IgE. Interestingly, abrogation of CD28 costimulation in CD28−/loxCre+/− mice by oral administration of tamoxifen prior to secondary infection with N. brasiliensis resulted in impaired worm expulsion, similarly to infected CD28−/− mice. This was associated with reduced production of the Th2 cytokines IL-13 and IL-4, diminished serum titres of antigen specific IgG1 and total IgE and a reduced CXCR5+ TFH cell population. Furthermore, total number of CD4+ T cells and B220+ B cells secreting Th1 and Th2 cytokines were significantly reduced in CD28−/− mice and tamoxifen treated CD28−/loxCre+/− mice compared to C57BL/6 mice. Importantly, interfering with CD28 costimulatory signalling before re-infection impaired the recruitment and/or expansion of central and effector memory CD4+ T cells and follicular B cells to the draining lymph node of tamoxifen treated CD28−/loxCre+/− mice. Therefore, it can be concluded that CD28 costimulation is essential for conferring host protection during secondary N. brasiliensis infection.
Published in the journal: . PLoS Pathog 10(2): e32767. doi:10.1371/journal.ppat.1003906
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003906Summary
IL-13 driven Th2 immunity is indispensable for host protection against infection with the gastrointestinal nematode Nippostronglus brasiliensis. Disruption of CD28 mediated costimulation impairs development of adequate Th2 immunity, showing an importance for CD28 during the initiation of an immune response against this pathogen. In this study, we used global CD28−/− mice and a recently established mouse model that allows for inducible deletion of the cd28 gene by oral administration of tamoxifen (CD28−/loxCre+/−+TM) to resolve the controversy surrounding the requirement of CD28 costimulation for recall of protective memory responses against pathogenic infections. Following primary infection with N. brasiliensis, CD28−/− mice had delayed expulsion of adult worms in the small intestine compared to wild-type C57BL/6 mice that cleared the infection by day 9 post-infection. Delayed expulsion was associated with reduced production of IL-13 and reduced serum levels of antigen specific IgG1 and total IgE. Interestingly, abrogation of CD28 costimulation in CD28−/loxCre+/− mice by oral administration of tamoxifen prior to secondary infection with N. brasiliensis resulted in impaired worm expulsion, similarly to infected CD28−/− mice. This was associated with reduced production of the Th2 cytokines IL-13 and IL-4, diminished serum titres of antigen specific IgG1 and total IgE and a reduced CXCR5+ TFH cell population. Furthermore, total number of CD4+ T cells and B220+ B cells secreting Th1 and Th2 cytokines were significantly reduced in CD28−/− mice and tamoxifen treated CD28−/loxCre+/− mice compared to C57BL/6 mice. Importantly, interfering with CD28 costimulatory signalling before re-infection impaired the recruitment and/or expansion of central and effector memory CD4+ T cells and follicular B cells to the draining lymph node of tamoxifen treated CD28−/loxCre+/− mice. Therefore, it can be concluded that CD28 costimulation is essential for conferring host protection during secondary N. brasiliensis infection.
Introduction
CD28 is considered to be the main co-stimulator of T cells, providing a critical signal for activation of naive T cells [1], [2], [3]. Interactions between CD28 and its ligands CD80/CD86 enhances cytokine production, prevents T cell anergy and protects against apoptosis [4], [5]. These CD28 dependent interactions are important during the initiation of T cell mediated immunity against a number of infections. Mice deficient in CD28 failed to develop adequate Th2 immune response during infection with S. mansoni [6], L. major [7] and N. brasiliensis [8], [9]. In contrast, infection of CD28−/− mice with H. polygyrus did not hamper normal development of Th2 immune response [10].
The absence of CD28 alters the organisation of secondary lymphoid tissue by affecting recruitment of T cells to B cell follicles, impairing germinal centre development [11], [12], [13], isotype switching, B cell maturation and development of memory B cells. This is linked to diminished recruitment of CXCR5+ TFH cells which localise within the B cell follicles [14], [15], [16], [17]. TFH cells produce IL-21, a key cytokine involved in isotype switching and differentiation of plasma cells [15]. CD28−/− mice infected with S. mansoni [6] or Leishmania major [7] failed to produce antigen specific type 2 antibodies IgG1 and IgE. Taken together this demonstrates an important role for CD28 in co-ordinating B cell responses.
Studies suggest that CD28 is not required during recall of memory T cell responses to infection with N. brasiliensis [8] and H. polygyrus [18]. Furthermore, infection of CD28−/− mice with fungi B. dermatitidis revealed maintenance of memory T cells is CD28 independent [19]. In fact, some studies suggested that recall of memory responses may be dependent on other co-stimulatory molecules such as inducible costimulator (ICOS) or 4-1BB [20], [21], [22]. In contrast, development of effector and memory CD4+ T cells was reduced in the absence of CD28 during T. gondii infection [23]. Recall of memory responses to persistent viral infections is dependent on CD28 [24], [25]. Therefore, the importance of CD28 during development and recall of memory responses remains controversial. There have been attempts to address this issue by blocking CD80 and CD86 or by transfer of memory T cells into CD80/CD86 deficient mice [26]. However, both approaches deprive CTLA-4 (CD152) of its ligands thus caution must be exercised when interpreting these data. Hence, new approaches that don't suffer from these additional effects are required to solve the conundrum surrounding the contribution of CD28 during recall of memory responses to infections.
Infection of mice with Nippostrongylus brasiliensis triggers a host protective immune response characterised by increased production of Th2 cytokines IL-13 and IL-4 [27], [28], [29], goblet cell hyperplasia [30] eosinophilia [31] and elevated levels of serum IgG1 and IgE [28], [32]. Infection with N. brasiliensis begins when third stage larvae (L3) penetrate the skin and migrate, via the circulatory system, into the lungs. Larvae enter the airways, from which they are coughed up and swallowed. The larvae mature into adult worms that produce eggs upon reaching the small intestinal lumen. Immune-competent BALB/c mice clear N. brasiliensis infection after approximately 9 days post-infection [33]. Secondary infection with N. brasiliensis induces a potent memory immune response that results in poor worm maturation and inhibits egg production [34].
Our understanding of mechanisms mediating recall of protective immunity to N. brasiliensis infection is continuously expanding. Studies conducted in mice defective of eosinophilopoeisis showed the importance of eosinophils in driving recall of protective immunity to N. brasiliensis re-infection [35], [36], [37]. Furthermore, recent studies have demonstrated an essential role of lung-resident CD4+ T cells in mediating host resistance to N. brasiliensis re-infection [38], [39], and this was shown to be dependent on IL-4Rα signalling [39]. Earlier studies conducted in B-cell deficient mice suggested that B cells do not play a role in the resolution N. brasiliensis secondary infection [40]. However, a recent study by us showed that interfering with IL-4Rα signalling specifically on B cells impaired worm expulsion during secondary challenge with N. brasiliensis, suggesting that IL-4/IL-13 responsive B cells are required for recall of protective immunity [41]. This was shown to be related to impaired IL-13 production by B cells and reduced expression of markers associated with antigen presentation [41].
The aim of this study was to evaluate the importance of CD28 in initiating protective Th2 immunity against both primary and secondary infections with N. brasiliensis. Our findings demonstrate that CD28 is required for initiation of protective Th2 immunity against primary infection with N. brasiliensis. Furthermore, the absence of CD28 impairs development of memory CD4+ T cell responses resulting in failure to clear adult N. brasiliensis worms during secondary infection. Failure to resolve infection was associated with reduced production of Th2 cytokines, particularly IL-13 and IL-4, abrogated humoral immunity and failure to expand the memory CD4+ T cell compartment.
Results
CD28 costimulatory signalling is required for optimal immunity against N. brasiliensis infection
In order to investigate whether CD28 is required for the development of protective immunity against N. brasiliensis infection, CD28−/− mice were infected with 500 infective N. brasiliensis larvae and killed 9 and 12 days post-infection (Fig. 1A). Infected CD28−/− mice had significantly higher intestinal adult worm burdens at day 9 post-infection compared to infected wild-type C57BL/6 mice (Fig. 1B). At day 12 post-infection, infected C57BL/6 mice cleared the worms as well as most of the infected CD28−/− mice (Fig. 1B). Associated with transiently increased worm burdens in infected CD28−/− mice was the number of mucus producing goblet cells that were reduced at day 9 post-infection in this mouse strain compared to control mice (Fig. 1C). Histological examination of periodic-acid Schiff (PAS) stained intestinal sections further confirmed that infected CD28−/− mice had impaired goblet cell hyperplasia at day 9 post-infection compared to infected C57BL/6 mice (Fig. 1C). Mucus production by goblet cells is crucial for mediating expulsion of adult N. brasiliensis worms [28], [42], [43], [44].
Fig. 1. Delayed worm expulsion in CD28 deficient mice during primary N. brasiliensis infection. 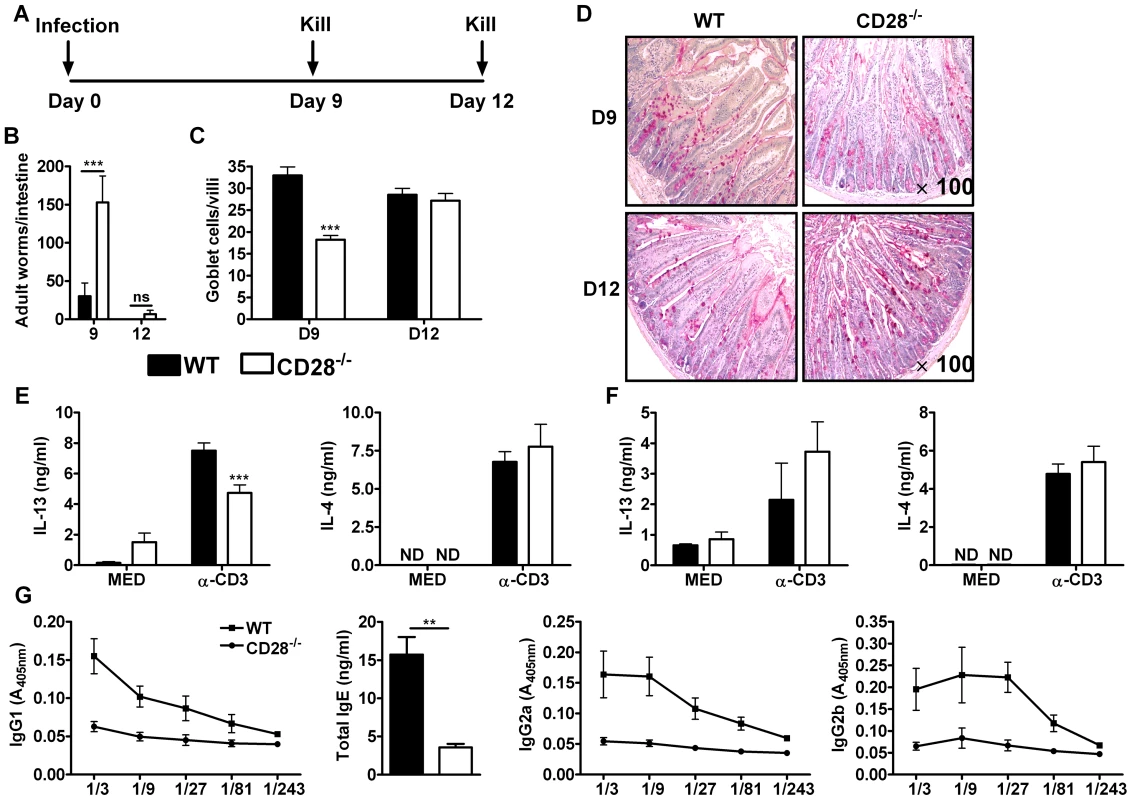
Wild-type (C57BL/6) and CD28−/− mice were infected with 500 L3 N. brasiliensis and killed 9 and 12 days post-infection. (A), Schematic showing experimental set-up. (B), Intestinal worm burdens were quantified. (C), Goblet cells per villi were enumerated. (D), PAS staining of mucus producing goblet cells in the intestinal tissue. Cytokine production by total mesenteric lymph node cells collected 9 days (E) or 12 days (F) post-infection was determine by ELISA after re-stimulation of total cells in media or with 20 µg/ml α-CD3 for 72 h at 37°C. (G), Serum antibody titres of N. brasiliensis specific IgG1, IgG2a, IgG2b and total IgE were determined by ELISA. Data are representative of two independent experiments. n = 4–6 mice per group. *P<0.05, **P<0.01, and ***P<0.001 vs C75BL/6 mice using two-tailed Mann-Whitney non-parametric Student's t-test or Two-Way ANOVA with Bonferroni's post test. Production of Th2 cytokines, particularly IL-13, drives the development of host protective immunity to N. brasiliensis [28]. To investigate the impact of CD28 deficiency in the production of host protective Th2 cytokines, cytokine production by total mesenteric lymph node (MLN) cells cultured in media or in the presence of 20 µg/ml α-CD3 was determined by sandwich ELISA. Total MLN cells re-stimulated with α-CD3 resulted in significantly reduced production of IL-13 by CD28−/− mice at day 9 post-infection (Fig. 1E) and was similar at day 12 (Fig. 1F) compared to wild-type mice, a cytokine crucial for worm clearance [27], [28], [39], [42], [45]. Production of IL-4 was unaltered between the two groups (Fig. 1E and F). Little or no cytokine production was detected in media control supernatants from all groups of mice (Fig. 1E and F). Finally, serum titers of both type 1 (IgG2a and IgG2b) and type 2 (IgG1 and total IgE) antibodies were markedly reduced in CD28−/− mice compared to C57BL/6 mice (Fig. 1G), suggesting that CD28 deficiency results in a general attenuation of the humoral immune response during N. brasiliensis infection. Together, these data demonstrated that CD28 is required for optimal immunity against N. brasiliensis primary infection.
CD28 is required for protective memory responses to N. brasiliensis infection
In order to investigate the contribution of CD28 in host protective memory responses, we took advantage of inducible CD28 deleting mouse strain CD28−/lox, rosa (CreERT2), here referred to (CD28−/loxCre+/−), a suitable experimental model system, recently generated by us [46], [47]. CD28−/lox mice (exon 2 and 3 are flanked by loxP) were intercrossed with rosaCreERT2 mice to generate CD28−/lox, rosa (CreERT2) mice. Oral administration of estrogen analogue tamoxifen allowed for translocation of estrogen-receptor-Cre fusion protein into the nucleus, where Cre recombinase carried out efficient deletion of the cd28 gene [46], [47], [48].
C57BL/6, CD28−/loxCre+/− and CD28−/− mice were infected with 500 infective N. brasiliensis larvae, treated with Ivermectin and rested for 21 days (Fig. 2A). At day 29, CD28−/loxCre+/− mice were treated with tamoxifen in vegetable oil (CD28−/loxCre+/−+TM) for four consecutive days (Fig. 2A). At day 35, mice were re-infected with 500 L3 N. brasiliensis and killed 5 days post-infection (Fig. 2A). Efficient deletion of CD28 was confirmed by flow cytometric analysis of CD4+ T cells. Here, oral administration of tamoxifen in CD28−/loxCre+/− mice resulted in similar levels of CD28 expression as was found in infected CD28−/− mice (Fig. 2B, Fig. S1A). Since eosinophils are important mediators of anti-helminth immunity and activated human eosinophils were shown to express CD28 [35], [36], [49], we tested for CD28 surface expression by this cell type in infected mice. Mouse eosinophils were negative in CD28 surface expression, as shown by flow cytometric analysis of CD28 expression by eosinophils from CD28−/− mice and C57BL/6 mice (data not shown). CD28−/loxCre+/− mice treated with tamoxifen prior to secondary challenge with N. brasiliensis adult worms exhibited high intestinal worm burdens similar to CD28−/− mice (Fig. 2C). Both infected tamoxifen treated CD28−/loxCre+/− mice and CD28−/− mice had significantly higher worm burdens than infected wild-type C57BL/6 mice (Fig. 2C) or infected untreated CD28−/loxCre+/− littermate control mice (Fig. S1B). Goblet cell hyperplasia was similar between wild-type C57BL/6 mice and tamoxifen treated CD28−/loxCre+/− mice (Fig. 2D). In contrast, CD28−/− mice showed reduced goblet cell hyperplasia indicated by reduced mucus producing cells compared to wild-type control mice (Fig. 2D). Therefore, these data suggest that CD28 costimulation is essential for host protection during N. brasiliensis secondary infection.
Fig. 2. CD28 is required for development of protective recall responses to re-infection with N. brasiliensis. 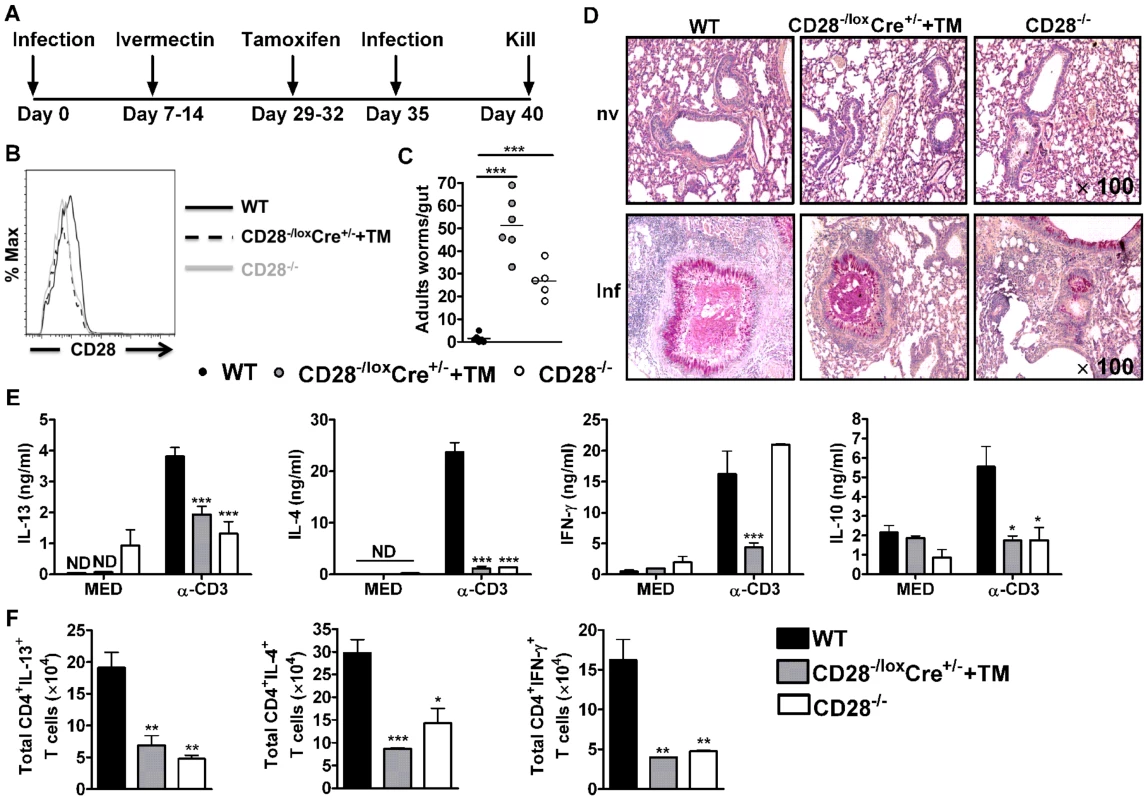
Wild-type (C57BL/6), tamoxifen treated CD28−/loxCre+/− and CD28−/− mice were infected with 500 L3 N. brasiliensis, treated with Ivermectin, then re-infected with 500 L3 N. brasiliensis at day 35 and killed 5 days post-infection. (A), Schematic showing experimental set-up. (B), Histogram showing the efficiency of CD28 deletion on CD4+ T cells. (C), Intestinal worm burdens were quantified. (D), PAS staining of pulmonary mucus producing goblet cells from naive and infected mice (E), Cytokine production by total mediastinal lymph node cell re-stimulated in media or in the presence of 20 µg/ml α-CD3 was determined by ELISA. (F), Total number of CD4+ T cells producing IL-13, IL-4 and IFN-γ after restimulation of total MST cells with 50 ng/ml PMA, 250 ng/ml ionomycin and 200 µM monensin in vitro. Data is representative of two independent experiments. n = 5–6 mice per group. *P<0.05, **P<0.01, and ***P<0.001 vs C57BL/6 using One-Way or 2-Way ANOVA with Bonferroni's post test. To investigate the mechanism resulting in impaired protection in mice deficient of CD28, we determined cytokine production by ELISA after in vitro restimulation of total MST or lung cells cultured in media or in the presence of 20 µg/ml α-CD3. Little or no cytokine production was detected from total MST (Fig. 2E) or lung (Fig. S2A) cells stimulated in media across all groups. However, production of IL-4, IL-13 and IL-10 by total MST (Fig. 2E) or lung (Fig. S2A) cells stimulated with α-CD3 was significantly reduced in infected CD28−/− mice and infected tamoxifen treated CD28−/loxCre+/− mice compared to infected wild-type mice. Impaired Th2 cytokine production was further confirmed by intracellular flow cytometry, with infected CD28 deficient mice exhibiting reduced total number of CD4+ T cells producing Th1 and Th2 cytokines after restimulation of total MST (Fig. 2F) or lung (Fig. S2B) cells with PMA/Ionomycin in vitro. Furthermore, secretion of IFN-γ was abrogated in infected tamoxifen-treated CD28−/loxCre+/− mice compared to infected C57BL/6 mice, while it was similar between CD28−/− mice and wild-type mice (Fig. 2E). In contrast, α-CD3 stimulated lung cells from infected CD28−/− mice and infected tamoxifen treated CD28−/loxCre+/− mice had significantly increased IFN-γ production compared to infected C57BL/6 mice (Fig. S2A). Total MST (Fig. S3) or lung (Fig. S2C) cells from naive mice cultured in media or in the presence α-CD3 produced no detectable cytokines except for IFN-γ that was detected in all mice strains after stimulating cells with α-CD3. Taken together, these data suggest that abrogating CD28 costimulation impairs production of host protective Th2 cytokines during N. brasiliensis secondary infection.
Development of TFH cells and memory CD4+ T cells requires CD28 during secondary infection with N. brasiliensis
To further investigate possible cellular mechanisms, CD4+ T cell subsets were compared at 5 days post secondary infection. The absolute number of CD4+ T cells was significantly reduced in infected tamoxifen-treated CD28−/loxCre+/− mice compared to infected wild-type mice (Fig. 3A) and untreated CD28−/loxCre+/− littermate control (Fig. S1D). Similarly, infected CD28−/− mice had reduced numbers of CD4+ T cells compared to infected C57BL/6 mice (Fig. 3A), while there was no difference in the number of CD4+ T cells between infected CD28−/− mice and infected untreated CD28−/loxCre+/− littermate control mice (Fig. S1D). Furthermore, the absolute number of T follicular helper cells (TFH) expressing CXCR5 and ICOS was significantly reduced in infected CD28−/loxCre+/− mice that had CD28 deleted by oral administration of tamoxifen prior to secondary N. brasiliensis challenge and infected CD28−/− compared to infected wild-type mice (Fig. 3B). Although naive untreated CD28−/loxCre+/− mice had significantly increased number of CD4+ T cells, the number of TFH cells expressing CXCR5 and ICOS was similar to naive C57BL/6 mice (Fig. S4A and B). The absolute number of CD4+ T cells and TFH cells was significantly reduced in naive CD28−/− mice compared to both naive untreated CD28−/loxCre+/− mice and naive wild-type C57BL/6 mice, suggesting that the absence of CD28 during thymic differentiation of CD4+ T cells hampers expansion of TFH cells (Fig. S4A and B). These data demonstrate that the development of TFH during recall responses to N. brasiliensis infection is critically reliant on CD28 costimulation during secondary infection.
Fig. 3. CD28 is necessary for development of TFH cells and optimal activation of CD4+ T cells. 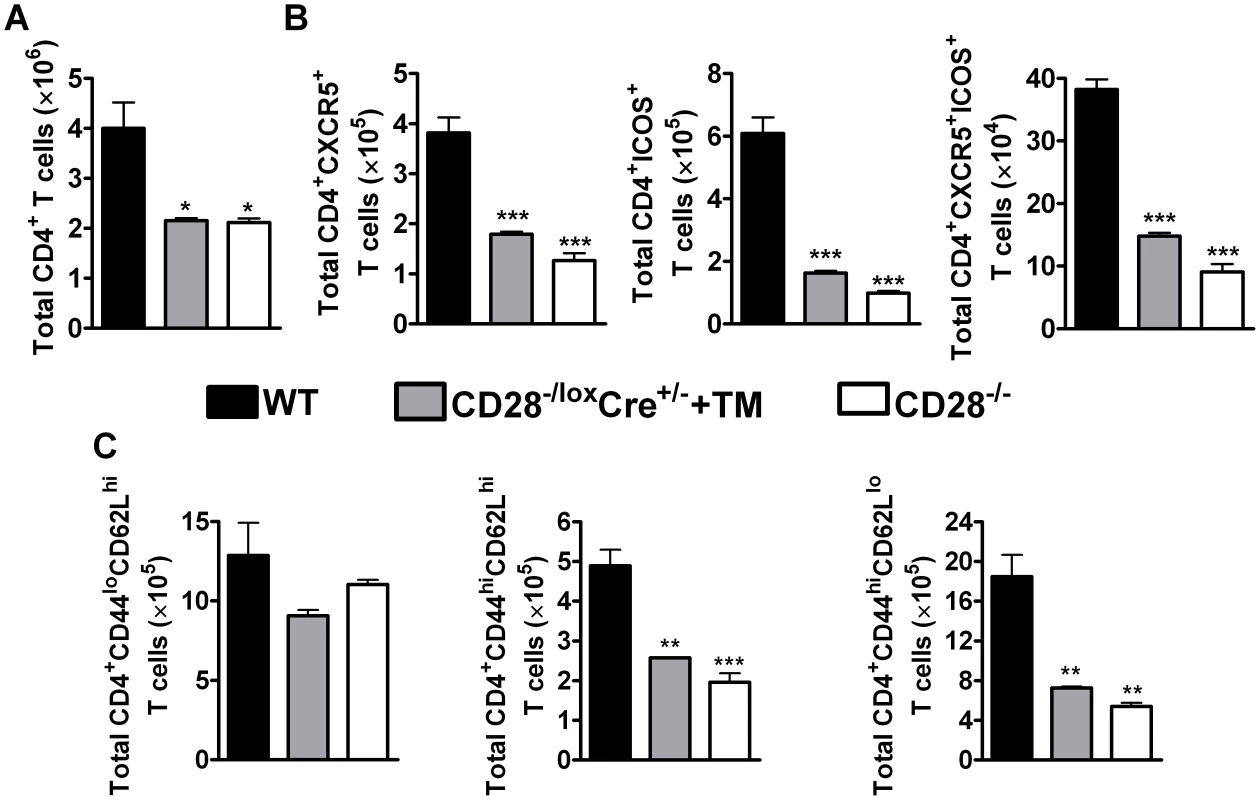
Single cell suspension was prepared from mediastinal lymph node and cells were stained for flow cytometric analysis. (A), Absolute numbers of CD4+ T cells in the lymph node. (B), Absolute numbers of CD4+CXCR5+, CD4+CXCR5+ICOS+ and CD4+ICOS+ T cells recruited to the mediastinal lymph node. (C) Total number of T cell subsets infiltrating the draining lymph node. T cells subsets were differentiated based on the following markers: naive (CD4+CD44loCD62Lhi), central memory (CD4+CD44hiCD62Lhi) and effector memory (CD4+CD44hiCD62Llo) T cells. Data represents two independent experiments. n = 5–6 mice per experiment. *P<0.05, **P<0.01, and ***P<0.001 vs C57BL/6 mice using One-Way ANOVA with Bonferroni's post test. There is evidence suggesting that memory T cells develop directly from effector CD4+ T cells [50]. Memory T cells are defined by the expression of lymph node homing receptor L-selectin (CD62L) [51], [52], CD44 and CD45RB in mice [53]. Flow cytometric analysis of memory T cells was conducted to investigate the development of memory T cells during recall responses to N. brasiliensis secondary infection. The total number of naive CD4+ T cells (CD4+CD62LhiCD44lo) was similar amongst all infected groups of mice (Fig. 3C). Interestingly, interfering with CD28 costimulation prior to secondary infection with N. brasiliensis in infected tamoxifen-treated CD28−/loxCre+/− mice had a profound impact on the recruitment and/or expansion of central memory (CD4+CD62LhiCD44hi) and effector memory (CD4+CD62LloCD44hi) T cells, as indicated by significantly reduced numbers compared to infected C57BL/6 mice (Fig. 3C). Infected CD28−/− mice had reduced numbers of central and effector memory CD4+ T cells compared to infected C57BL/6 mice (Fig. 3C). Importantly, there was no difference in the number of naive and effector memory CD4+ T cells between naive C57BL/6 mice and naive untreated CD28−/loxCre+/− littermate control mice, except that naive littermate control mice had significantly more central memory CD4+ T cells than naive C57BL/6 mice (Fig. S4C). Naive CD28−/− mice had reduced numbers of naive, central and effector memory CD4+ T cells compared to naive C57BL/6 mice and naive untreated CD28−/loxCre+/− mice (Fig. S4C). Together, these data suggest that CD28 is essential for the expansion of central and effector memory CD4+ T cells during re-infection with N. brasiliensis.
CD28 influences B cell development in the draining lymph node during secondary infection with N. brasiliensis
To investigate whether impairing CD28 signalling on CD4+ T cells alters B cell function, we determined serum antibody titers by ELISA. Interestingly, infected CD28−/− mice and infected tamoxifen-treated CD28−/loxCre+/− mice had significantly reduced antigen-specific type 1 and type 2 antibody isotypes including total IgE compared to infected wild-type mice (Fig. 4A), or infected untreated CD28−/loxCre+/− littermate control (Fig. S1C). This data suggests that mice deficient of CD28 costimulation have impaired humoral immunity. Moreover, we determined the ability of B220+ B cells to produce cytokines by stimulating total MST cells with PMA/Ionomycin and detecting cytokine secretion by intracellular flow cytometric analysis. The absolute number of B220+ B cells secreting IL-4, IL-13 and IFN-γ were significantly reduced in both infected tamoxifen treated CD28−/loxCre+/− mice and infected CD28−/− mice compared to infected wild-type mice (Fig. 4B). These data suggest that interfering with CD28 costimulation on CD4+ T cells impacts on the ability of B cells to produce antibodies and cytokines during N. brasiliensis infection.
Fig. 4. B cell development in the mediastinal lymph node is affected by CD28 deletion on CD4+ T cells. 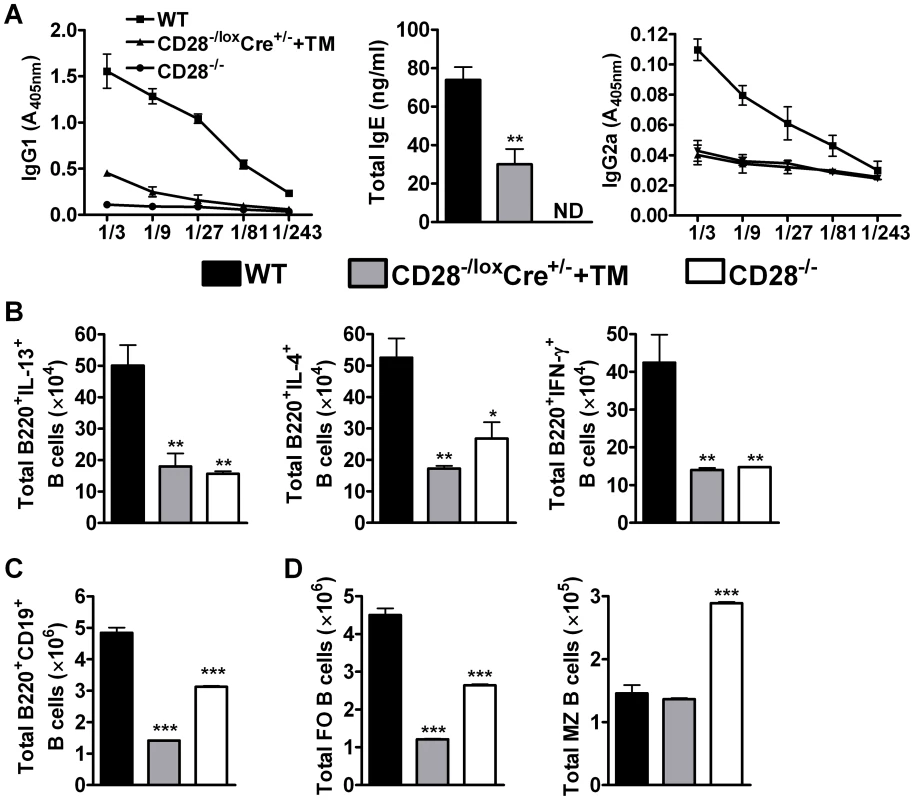
Blood was collected from reinfected mice by cardiac puncture and centrifuged at 8×g for 10 min in serum separator tubes. (A), Serum antibody titres of N. brasiliensis specific IgG1, IgG2a and total IgE were determined by ELISA. Single cells suspension was prepared from mediastinal lymph nodes and cells stained for flow cytometric analysis. (B), Total number of cytokine producing B220+ B cells was determined by intracellular flow cytometric analysis after re-stimulation of total MST cells with 50 ng/ml PMA, 250 ng/ml ionomycin and 200 µM monensin for 4 h at 37°C. (C) Total numbers of CD19+B220+ B cells population recruited into the mediastinal lymph node. (D)Absolute numbers of different B cell subsets recruited into the draining mediastinal lymph node. B cell subsets were differentiated based on the following markers: follicular B cells (FO, CD19+B220+CD21hiCD23hi) and marginal zone B cells (MZ, CD19+B220+CD21hiCD23lo). Data represents two independent experiments. n = 5–6 mice per group. *P<0.05 and **P<0.01 vs C75BL/6 using One-Way ANOVA with Bonferroni post test. We further analysed the development of B cell subsets in the MST of infected mice by flow cytometry. Infected CD28−/− mice had a reduced number of CD19+B220+ B cells (Fig. 4C) and follicular B cells (Fig. 4D) while the number of marginal zone B cells (Fig. 4D) was doubled compared to infected wild-type mice. There was no difference in the number of CD19+B220+ B cells between infected CD28−/− and infected CD28−/loxCre+/− littermate mice (Fig. S1G), although the number of follicular and non-follicular B cells was significantly reduced in infected CD28−/− mice compared to infected littermate control mice (Fig. S1G). Infected CD28−/loxCre+/− mice given tamoxifen prior to secondary infection had a reduced number of CD19+B220+ B cells (Fig. 4C) due to strikingly reduced numbers of follicular B cells compared to infected wild-type mice (Fig. 4D) or infected untreated CD28−/loxCre+/− littermate control mice (Fig. S1G). In contrast, the absolute number of marginal zone B cells was similar between infected tamoxifen treated CD28−/loxCre+/− mice and infected wild-type mice (Fig. 4D). The similarly reduced numbers of follicular B cells in infected CD28−/− mice and infected tamoxifen-treated CD28−/loxCre+/− mice, suggests that the expansion and/or recruitment of follicular B cells requires interaction between B cells and CD4+ T cells responsive to CD28 costimulation during secondary infection. Interestingly, naive C57BL/6 mice and naive untreated CD28−/loxCre+/− mice had similar numbers of CD19+B220+ B cells (S5A), follicular B cells (Fig. S5B) and marginal zone B cells (Fig. S5B). In contrast, naive CD28−/− had significantly reduced number of CD19+B220+ B cells (Fig. S5A), follicular B cells (Fig. S5B) and an increased number of marginal zone B cells (Fig. S5B) compared to both naive C57BL/6 mice and naive untreated CD28−/loxCre+/− mice. Together, these data suggest that cellular interactions between CD4+ T cells expressing CD28 and CD19+B220+ B cells are required for the expansion and/or recruitment of follicular B cells into the lymphoid tissue during N. brasiliensis secondary infection.
Discussion
The contribution of CD28 costimulation during recall of memory responses to infections has remained controversial despite numerous attempts to study it. Some studies have suggested that CD28 costimulation is not required for recall of protective memory responses to nematode infections [8], [18]. Furthermore, infection of CD28−/− mice with the fungi B. dermatitidis showed that development of memory responses is CD28 independent [19]. In contrast, the absence of CD28 costimulation during recall of memory responses to T. gondii infection [23] and viral infections [24], [25] inhibited the development of protective memory responses. The controversy surrounding the requirement of CD28 for recall of protective memory responses may depend on the pathogen itself. To address these questions for nematode infections, we utilised CD28−/− mice, and more importantly a recently established conditional CD28 deleting mouse strain (CD28−/loxCre+/−), where CD28 deletion is induced by oral administration of the estrogen analogue tamoxifen [47]. The latter mouse model allowed for abrogation of CD28 costimulation after primary infection with N. brasiliensis, ensuring that priming of the immune response occurred in the presence of CD28 costimulation.
Primary infection in CD28−/− mice with N. brasiliensis showed that CD28 costimulation is essential for development of optimal T cell immunity, as CD28−/− mice had delayed adult worm expulsion in the small intestines. This was associated with impaired IL-13 production, known to play a crucial role in driving worm expulsion during N. brasiliensis infection [28], [33]. Blocking CD28 costimulation in infected wild-type mice using a novel mouse anti-mouse CD28 (E18) monoclonal antibody impaired expulsion of the worms during primary N. brasiliensis infection and hampered optimal development of Th2 cytokine responses (our unpublished data). This has been further confirmed by other studies that have demonstrated that interfering with CD28 costimulation during primary infection with nematodes impairs optimal development of Th2 cytokine responses [8], [18]. Interestingly, analysis of the antibody responses showed reduced titres of both type 1 and type 2 antibody isotypes, indicating a general abrogation of humoral immunity in the absence of CD28 costimulation. In contrast, CD28−/− mice inoculated with H. polygyrus primary infection mounted a sufficient humoral immune response [10]. Hence, the requirement of CD28 costimulation in driving development of humoral immunity seems to depend on parasites causing the infection. In fact, infection with H. polygyrus seems to be sufficient to induce polyclonal antibody responses even in the absence of CD28 costimulation, supporting the suggestion that parasites can trigger polyclonal B cells responses [54].
Abrogating CD28 costimulation only during secondary infection in inducible CD28 deficient mice led to failure in mounting a protective memory response, strongly suggesting that CD28 costimulation is needed for efficient recall of protective immunity to N. brasiliensis. This conclusion is in contrast with previous findings using CTLA4-Ig to block the CD28 ligands CD80 and CD86 [8]. A possible explanation is that CTLA4-Ig exerts additional effects which may confound the situation. Furthermore, this fusion protein also prevents ligation of endogenous CTLA-4 expressed by regulatory and activated T-cells, which may partially counterbalance the desired effect. Failure to recall protective memory responses in inducible CD28 deficient mice was accompanied by strikingly reduced production of Th2 cytokines, particularly IL-4 and IL-13 by total MST or lung cells as well as impaired cytokine production by CD4+ T cells and B220+ B cells. These results are in agreement with our current knowledge that CD28 costimulation enhances IL-4 receptor sensitivity and subsequently Th2 CD4+ T cell differentiation [55], [56], the latter being involved in mediating protective immunity against re-infection with N. brasiliensis [38] by lung-resident CD4+ T cells. This was further confirmed by a recent study from our laboratory that demonstrated the importance of lung-resident CD4+ T cells in driving recall of protective immunity to Nippostrongylus brasiliensis re-infection after blocking T cells migration into the lungs with Fingolimod (FTY720) [39].
Previous studies have suggested a number of pathways governing the development of memory CD4+ T cells. In a study by Hu and colleagues, memory CD4+ T cells were shown to develop directly from effector CD4+ T cells that reverted to a resting state, suggesting a linear pathway for memory T cells generation [50]. However, other studies have suggested a more complex pathway for central memory T cell generation comprised of heterogeneous memory T cell populations [57]. In our study, the absolute number of CD4+ T cells was reduced in infected CD28−/loxCre+/− mice that had CD28 deleted prior to secondary N. brasiliensis challenge and infected CD28−/− mice compared to infected C57BL/6 mice. These data imply that CD28 co-stimulation is important for expanding the CD4+ T cell compartment in the lymphoid tissue. Furthermore, the total number of central and effector memory CD4+ T cells was markedly reduced in the absence of CD28 costimulation during secondary N. brasiliensis infection, regardless of whether CD28 was constitutively missing or deleted after priming. Moreover, naive CD28−/− mice had a reduced number of CD4+ T cells compared to naive C57BL/6 mice and naive untreated CD28−/loxCre+/− littermate control mice. Interestingly, naive untreated CD28−/loxCre+/− mice and naive CD57BL/6 mice showed similar numbers of CD4+ T cell subsets except for an increased number of CD4+ T cells and central memory cells in littermate control mice, demonstrating that untreated CD28−/loxCre+/− littermate control mice are phenotypically similar to wild-type mouse until cd28 gene deletion is induced with tamoxifen prior to re-infection. These findings suggest that CD28 costimulation is required for the expansion of the CD4+ T cell compartment during thymic differentiation. Furthermore, it demonstrates the importance of CD28 in the expansion of memory CD4+ T cells during N. brasiliensis re-infection.
Interruption of CD28 costimulation also affected humoral immunity, as shown by reduced serum titres of type 1 and type 2 antibody isotypes in infected CD28−/− mice and infected tamoxifen-treated CD28−/loxCre+/− mice compared to infected C57BL/6 mice. This data concurred with a previous study, that found reduced total IgE titres in sera from mice treated with CTLA4-Ig during re-infection with N. brasiliensis [8]. In contrast, serum levels of antigen-specific IgG1 and IgE were increased in CD28−/− mice during H. polygyrus secondary challenge [18]. Therefore, the importance of CD28 costimulation in sustaining CD4+ T cells dependent memory antibody responses seems to be differentially affected by parasite causing the infection.
In a study by Zaretsky and colleagues, IL-4 producing Th2 cells were shown to possess the capacity to differentiate into TFH cells during immunisation with S. mansoni antigens [58]. This was further confirmed in a study by King and Mohrs that demonstrated that in a Th2 setting induced by infection with H. polygyrus, the majority of IL-4 producing CD4+ T cells in the reactive lymph nodes co-express canonical TFH cells markers and localised within the B cell follicles [59]. Our data showed that in the absence of CD28 costimulation, the expansion of TFH cells expressing the canonical markers CXCR5 and ICOS were reduced during re-infection with N. brasiliensis. ICOS has been shown to play an essential role in maintaining the expression of CXCR5 on CD4+ T cells during SRBC immunisation and enhances GC formation and antibody production [60]. Hence, we concluded that CD28 costimulation is important for development of TFH cells in the reactive lymph nodes during re-infection with the nematode N. brasiliensis. Together, these findings strongly demonstrate an important role played by CD28 costimulation during recall of protective memory responses to N. brasiliensis infection. CD28 costimulation seems to be required throughout the infection period to sustain the development of protective memory responses. These findings are in stark contrast to the normal development of protective memory responses exhibited by CD28−/− mice infected with H. polygyrus [18]. A recent study by Harvie and colleagues showed that the lungs are a crucial site harbouring protective immunity against N. brasiliensis re-infection [38]. The differences observed in the requirement of CD28 costimulation during recall of memory responses to N. brasiliensis and H. polygyrus may be due to different migration patterns of the parasites within the host. H. polygyrus is a completely enteric parasite while N. brasiliensis migrates via the lungs to the intestines.
The mechanisms involved in recall of protective immunity to N. brasiliensis re-infection seem to involve both the innate and adaptive arm of the immune response. Voehringer and colleagues showed that mice deficient in eosinophils failed to expel N. brasiliensis adult worms at day 7 after secondary exposure despite the presence of Th2 cells in the lungs [36]. This was further corroborated by a subsequent study by Knott and colleagues, showing that eosinophils contribute to host resistance to N. brasiliensis secondary infection by limiting the number of worms reaching the lungs early during re-infection [35]. In contrast, it was shown that eosinophils in the gut are not required for worm expulsion in either primary or secondary N. brasiliensis infection [35]. Alternatively activated macrophages do not play a role in the expulsion of N. brasiliensis adult worms during primary infection in LysMcreIL-4Rα−/lox mice [61]. However, a recent study has shown that alternatively activated macrophages are involved in the killing of Strongloides stercoralis in vivo and this killing effect involves a collaboration with neutrophils and complement in vitro [62]. Nuocytes have been shown to mediate type 2 immunity during N. brasiliensis infection by producing IL-13 early during infection [63]; thus, it would be interesting to determine their contributions to protective immunity during secondary infection.
The interactions between CXCR5+ TFH cells and follicular B cells are crucial for germinal center formation in the lymphoid tissues, an important site for the development of memory B cells and antibody producing plasma cells [64], [65]. In our study, infected CD28−/− mice and infected tamoxifen treated CD28−/loxCre+/− mice had significantly reduced absolute numbers of follicular B cells compared to infected C57BL/6 control mice. In contrast, infected CD28−/− mice had increased number of marginal zone B cells while there was no difference in the number of marginal zone B cells between infected C57BL/6 mice and infected tamoxifen treated CD28−/loxCre+/− mice. Impaired expansion and/or recruitment of follicular B cells in infected CD28−/− mice and infected tamoxifen treated CD28−/loxCre+/− mice might provide a plausible explanation for abrogated humoral immunity after both primary and secondary N. brasiliensis infection. Therefore, interfering with CD28 costimulation appears to alter the ability of CD4+ T cells to provide cognate help to B cells necessary for optimal antibody production and isotype class-switching.
In conclusion, our study demonstrates the essential role played by CD28 costimulation during recall of protective memory responses to N. brasiliensis infection. CD28 costimulation was shown to confer protection against primary infection with N. brasiliensis using CD28−/− mice. Failure to expel adult N. brasiliensis worms during secondary infection was associated with diminished Th2 cytokine responses and abrogated humoral immunity particularly the production of IgG1 and total IgE. Importantly, the deficiency of CD28 costimulation impaired recruitment of memory CD4+ T cell sub-populations and expansion of T follicular helper cells crucial for providing help to follicular B cells.
Materials and Methods
Mice
C57/BL6 background CD28−/− and CD28−/lox, rosa (CreERT2) (CD28lox/−Cre+/−) mice were obtained from Prof. T. Hünig at the University of Würzburg, Germany. The mice were bred and maintained in specific pathogen-free barrier conditions in individually ventilated cages at the University of Cape Town Animal Facility. All experimental mice were age and sex matched and used between 8–12 weeks of age.
Ethics statement
This study was conducted under strict recommendation of the South African national guidelines and of the University of Cape Town practice for laboratory animal procedures. All mouse experiments were carried out in accordance to protocols approved by the Animals Research Ethics Committee of the Health Sciences Faculty, University of Cape Town (Project Number: 011/008). Care was taken to minimize suffering of the animals.
N. brasiliensis infection
Primary infection
Mice were injected subcutaneously with 500 N. brasiliensis L3 suspended in 0.65–0.9% NaCl using 21-G needle (Braun, Melsungen, Germany). Mice were killed 9 and 12 days post-infection and adults worms were enumerated using a previously described method [27].
Secondary infection
Mice were initially injected with 500 N. brasiliensis L3, orally treated with 10 mg/ml Ivermectin in drinking water at seven days post-infection and shelved for 21 days prior to a secondary subcutaneous infection with 500 N. brasiliensis L3. Mice were killed 5 days post secondary infection by halothane inhalation and exsanguination.
N. brasiliensis intestinal worm counts
Small intestines were removed from infected mice; the lumen was exposed by dissection and suspended in 0.65% NaCl. The intestines were incubated at 37°C for 4 h to allow for migration of the worms out of the lumen after which they were enumerated under a dissecting microscope (Nikon Eclipse).
Histology
Tissue sample were fixed in buffered 4% (v/v) formaldehyde, embedded in paraffin wax and cut into 5 µm sections. The sections were stained with periodic acid-Schiff reagent (PAS) in order to visualize mucus producing goblet cells. The sections were analysed under a light microscope.
Determination of antibody titres
N. brasiliensis antigen-specific serum antibody isotypes and total IgE titres from infected mice were determined as previously described [66]. Briefly, blood was collected in serum separator tubes (BD Bioscience, San Diego, CA) and centrifuged at 8 000×g for 10 min at 4°C to separate serum. The flat-bottom 96-well plates were coated with 10 µg/ml somatic N. brasiliensis antigen (NAg), blocked with 2% (w/v) milk powder for 2 h at 37°C and samples were loaded and incubated overnight at 4°C. Alkaline phosphatase labelled secondary antibody was added and incubated for 2 h at 37°C. The plates were developed by addition of 4-nitrophenyl substrate (Sigma). The absorbance was read at 405 nm using VersaMax microplate spectrophotometer (Molecular Devices, Germany).
Ex vivo restimulation of cells and cytokines detection
Single cell suspensions were prepared by pressing the draining lymph nodes through 70 µM cell-strainers. Cells were resuspended in complete IMDM (Gibco) supplemented with 10% FCS (Gibco) and penicillin and streptomycin (100 U/ml and 100 µg/ml, Gibco). The cells were cultured at 1×106 cells/ml in 96-well plates coated with α-CD3 (20 µg/ml) and incubated at 37°C in a humidified atmosphere containing 5% CO2. Supernatants were collected after 72 h and cytokines were measured by ELISA. Quantities of IL-4, IL-10, IFN-γ and IL-13 were measured by sandwich ELISA as previously described [66].
Inducible deletion in conditional knock-out mice
CD28−/loxCre+/− mice were given 2.5 mg Tamoxifen (Sigma, Deisenhofen, Germany) in vegetable oil for four consecutive days by forced feeding.
Flow cytometry
The following antibodies comprising the B cells antibody panel were used: B220-V500 (RA36B2), CD19-PerCP Cy5.5 (ID3), CD23-PE (B3B4), CD21-APC (7G6), CD24-PECy7 (M1/69), CD80-V450 (19-10A1), MHCII-FITC (2G9) and IgM-Biotin (RMM-1) (BD Bioscience, Erembodegem, Belgium). T cells panel consisted of the following antibodies: CD4-PerCP (RM4-5), CD62L-V500 (MEL-14), CD44-FITC (IM7), CD28-PE (37.51), CXCR5-V450 (2G8) and CD278-Biotin (7E.17G9) (BD Bioscience, Erembodegem, Belgium). Cells were acquired on a FACS Fortessa machine (BD Immunocytometry system, San Jose, CA, USA) and data was analyzed using Flowjo software (Treestar, Ashland, OR, USA).
Intracellular flow cytometry
For detection of intracellular cytokines, MST or lung cells from N. brasiliensis infected mice were plated at 2×106 cells/well and stimulated at 37°C for 4 h with 50 ng/ml phorbol (myristate acetate), 250 ng/ml ionomycin and 200 µM monensin in complete IMDM (all purchased from Sigma-Aldrich). Cells were stained with extracellular markers [CD4 PercP Cy5.5 (RM4-5) and B220 FITC (RA3-6B2)], fixed for 30 min on ice in 2% (w/v) paraformaldehyde and permeabilised with 0.5% saponin buffer and stained with PE-labelled anti-mouse IL-4, IL-13, and IFN-γ for 30 min. Acquisition was performed using a FACSCalibur (BD Immunocytometry Systems, San Jose, CA, USA) and data were analysed using FlowJo software (Treestar, Ashland, OR, USA).
Statistics
Statistical analysis was conducted using GraphPad Prism 4 software (http://www.prism-software.com). Data were calculated as mean ± SD. Statistical significant was determined using the unpaired Student's t test, One-Way or Two-Way ANOVA with Bonferroni's post test, defining differences to C57BL/6 or untreated CD28lox−Cre+/− as significant (*, p≤0.05; **, p≤0.01; ***, p≤0.001).
Supporting Information
Zdroje
1. HathcockKS, HodesRJ (1996) Role of the CD28-B7 costimulatory pathways in T cell-dependent B cell responses. Adv Immunol 62 : 131–166.
2. NoelPJ, BoiseLH, GreenJM, ThompsonCB (1996) CD28 costimulation prevents cell death during primary T cell activation. J Immunol 157 : 636–642.
3. SalomonB, BluestoneJA (2001) Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 19 : 225–252.
4. BluestoneJA (1995) New perspectives of CD28-B7-mediated T cell costimulation. Immunity 2 : 555–559.
5. SharpeAH (1995) Analysis of lymphocyte costimulation in vivo using transgenic and ‘knockout’ mice. Curr Opin Immunol 7 : 389–395.
6. KingCL, XianliJ, JuneCH, AbeR, LeeKP (1996) CD28-deficient mice generate an impaired Th2 response to Schistosoma mansoni infection. Eur J Immunol 26 : 2448–2455.
7. CorryDB, ReinerSL, LinsleyPS, LocksleyRM (1994) Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol 153 : 4142–4148.
8. HarrisNL, PeachRJ, RoncheseF (1999) CTLA4-Ig inhibits optimal T helper 2 cell development but not protective immunity or memory response to Nippostrongylus brasiliensis. Eur J Immunol 29 : 311–316.
9. LiuZ, LiuQ, PesceJ, WhitmireJ, EkkensMJ, et al. (2002) Nippostrongylus brasiliensis can induce B7-independent antigen-specific development of IL-4-producing T cells from naive CD4 T cells in vivo. J Immunol 169 : 6959–6968.
10. GauseWC, ChenSJ, GreenwaldRJ, HalvorsonMJ, LuP, et al. (1997) CD28 dependence of T cell differentiation to IL-4 production varies with the particular type 2 immune response. J Immunol 158 : 4082–4087.
11. LaneP, BurdetC, HubeleS, ScheideggerD, MullerU, et al. (1994) B cell function in mice transgenic for mCTLA4-H gamma 1: lack of germinal centers correlated with poor affinity maturation and class switching despite normal priming of CD4+ T cells. J Exp Med 179 : 819–830.
12. FergusonSE, HanS, KelsoeG, ThompsonCB (1996) CD28 is required for germinal center formation. J Immunol 156 : 4576–4581.
13. LenschowDJ, WalunasTL, BluestoneJA (1996) CD28/B7 system of T cell costimulation. Annu Rev Immunol 14 : 233–258.
14. WalkerLS, Gulbranson-JudgeA, FlynnS, BrockerT, RaykundaliaC, et al. (1999) Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med 190 : 1115–1122.
15. KingC, TangyeSG, MackayCR (2008) T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol 26 : 741–766.
16. CampbellDJ, KimCH, ButcherEC (2001) Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat Immunol 2 : 876–881.
17. SchaerliP, WillimannK, LangAB, LippM, LoetscherP, et al. (2000) CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 192 : 1553–1562.
18. EkkensMJ, LiuZ, LiuQ, FosterA, WhitmireJ, et al. (2002) Memory Th2 effector cells can develop in the absence of B7-1/B7-2, CD28 interactions, and effector Th cells after priming with an intestinal nematode parasite. J Immunol 168 : 6344–6351.
19. WuthrichM, WarnerT, KleinBS (2005) CD28 is required for optimal induction, but not maintenance, of vaccine-induced immunity to Blastomyces dermatitidis. Infect Immun 73 : 7436–7441.
20. BertramEM, DawickiW, SedgmenB, BramsonJL, LynchDH, et al. (2004) A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. J Immunol 172 : 981–988.
21. BertramEM, DawickiW, WattsTH (2004) Role of T cell costimulation in anti-viral immunity. Semin Immunol 16 : 185–196.
22. DawickiW, BertramEM, SharpeAH, WattsTH (2004) 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol 173 : 5944–5951.
23. VillegasEN, EllosoMM, ReichmannG, PeachR, HunterCA (1999) Role of CD28 in the generation of effector and memory responses required for resistance to Toxoplasma gondii. J Immunol 163 : 3344–3353.
24. FuseS, ZhangW, UsherwoodEJ (2008) Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J Immunol 180 : 1148–1157.
25. NdejembiMP, TeijaroJR, PatkeDS, BingamanAW, ChandokMR, et al. (2006) Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol 177 : 7698–7706.
26. BorowskiAB, BoesteanuAC, MuellerYM, CarafidesC, TophamDJ, et al. (2007) Memory CD8+ T cells require CD28 costimulation. J Immunol 179 : 6494–6503.
27. BarnerM, MohrsM, BrombacherF, KopfM (1998) Differences between IL-4R alpha-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol 8 : 669–672.
28. UrbanJFJr, Noben-TrauthN, DonaldsonDD, MaddenKB, MorrisSC, et al. (1998) IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8 : 255–264.
29. FowellDJ, MagramJ, TurckCW, KilleenN, LocksleyRM (1997) Impaired Th2 subset development in the absence of CD4. Immunity 6 : 559–569.
30. KhanWI, CollinsSM (2004) Immune-mediated alteration in gut physiology and its role in host defence in nematode infection. Parasite Immunol 26 : 319–326.
31. KopfM, Le GrosG, BachmannM, LamersMC, BluethmannH, et al. (1993) Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 362 : 245–248.
32. FinkelmanFD, Shea-DonohueT, GoldhillJ, SullivanCA, MorrisSC, et al. (1997) Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol 15 : 505–533.
33. UrbanJFJr, MaddenKB, SveticA, CheeverA, TrottaPP, et al. (1992) The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev 127 : 205–220.
34. KatonaIM, UrbanJ, FinkelmanFD (1988) The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol 140 : 3206–3211.
35. KnottML, MatthaeiKI, GiacominPR, WangH, FosterPS, et al. (2007) Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol 37 : 1367–1378.
36. VoehringerD, ReeseTA, HuangX, ShinkaiK, LocksleyRM (2006) Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med 203 : 1435–1446.
37. GiacominPR, GordonDL, BottoM, DahaMR, SandersonSD, et al. (2008) The role of complement in innate, adaptive and eosinophil-dependent immunity to the nematode Nippostrongylus brasiliensis. Mol Immunol 45 : 446–455.
38. HarvieM, CamberisM, TangSC, DelahuntB, PaulW, et al. (2010) The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun 78 : 3753–3762.
39. ThawerSG, HorsnellWG, DarbyM, HovingJC, DewalsB, et al. (2013) Lung-resident CD4 T cells are sufficient for IL-4Ralpha-dependent recall immunity to Nippostrongylus brasiliensis infection. Mucosal Immunol doi:10.1038/mi.2013.40
40. LiuQ, KreiderT, BowdridgeS, LiuZ, SongY, et al. (2010) B cells have distinct roles in host protection against different nematode parasites. J Immunol 184 : 5213–5223.
41. HorsnellWGC, DarbyM, HovingJC, NieuwenhuizenN, BobatS, et al. (2013) IL-4Rα associated antigen processing by B cells promotes immunity in Nippostrongylus brasiliensis infection. PLoS Pathog 9 DOI:10.1371/journal.ppat.1003662
42. HorsnellWG, CutlerAJ, HovingJC, MearnsH, MyburghE, et al. (2007) Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice. PLoS Pathog 3: e1.
43. KhanWI, AbeT, IshikawaN, NawaY, YoshimuraK (1995) Reduced amount of intestinal mucus by treatment with anti-CD4 antibody interferes with the spontaneous cure of Nippostrongylus brasiliensis-infection in mice. Parasite Immunol 17 : 485–491.
44. McKenzieGJ, BancroftA, GrencisRK, McKenzieAN (1998) A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol 8 : 339–342.
45. HorsnellWG, ViraA, KirsteinF, MearnsH, HovingJC, et al. (2011) IL-4Ralpha-responsive smooth muscle cells contribute to initiation of TH2 immunity and pulmonary pathology in Nippostrongylus brasiliensis infections. Mucosal Immunol 4 : 83–92.
46. HunigT, LuhderF, ElfleinK, GogishviliT, FrohlichM, et al. (2010) CD28 and IL-4: two heavyweights controlling the balance between immunity and inflammation. Med Microbiol Immunol 199 : 239–246.
47. GogishviliT, LuhderF, KirsteinF, NieuwenhuizenNE, GoebbelsS, et al. (2012) Interruption of CD28-mediated costimulation during allergen challenge protects mice from allergic airway disease. J Allergy Clin Immunol 130 : 1394–403.e4.
48. SeiblerJ, ZevnikB, Kuter-LuksB, AndreasS, KernH, et al. (2003) Rapid generation of inducible mouse mutants. Nucleic Acids Res 31: e12.
49. WoerlyG, LacyP, YounesAB, RogerN, LoiseauS, et al. (2002) Human eosinophils express and release IL-13 following CD28-dependent activation. J Leukoc Biol 72 : 769–779.
50. HuH, HustonG, DusoD, LepakN, RomanE, et al. (2001) CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nat Immunol 2 : 705–710.
51. SallustoF, LangenkampA, GeginatJ, LanzavecchiaA (2000) Functional subsets of memory T cells identified by CCR7 expression. Curr Top Microbiol Immunol 251 : 167–171.
52. SallustoF, LenigD, ForsterR, LippM, LanzavecchiaA (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401 : 708–712.
53. BingamanAW, PatkeDS, ManeVR, AhmadzadehM, NdejembiM, et al. (2005) Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur J Immunol 35 : 3173–3186.
54. FinkelmanFD, HolmesJ, KatonaIM, UrbanJFJr, BeckmannMP, et al. (1990) Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol 8 : 303–333.
55. KuboM, YamashitaM, AbeR, TadaT, OkumuraK, et al. (1999) CD28 costimulation accelerates IL-4 receptor sensitivity and IL-4-mediated Th2 differentiation. J Immunol 163 : 2432–2442.
56. TaoX, ConstantS, JorritsmaP, BottomlyK (1997) Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol 159 : 5956–5963.
57. SallustoF, LanzavecchiaA (2001) Exploring pathways for memory T cell generation. J Clin Invest 108 : 805–806.
58. ZaretskyAG, TaylorJJ, KingIL, MarshallFA, MohrsM, et al. (2009) T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med 206 : 991–999.
59. KingIL, MohrsM (2009) IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med 206 : 1001–1007.
60. AkibaH, TakedaK, KojimaY, UsuiY, HaradaN, et al. (2005) The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol 175 : 2340–2348.
61. HerbertDR, HolscherC, MohrsM, ArendseB, SchwegmannA, et al. (2004) Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20 : 623–635.
62. Bonne-AnneeS, KerepesiLA, HessJA, O'ConnellAE, LokJB, et al. (2013) Human and mouse macrophages collaborate with neutrophils to kill larval Strongyloides stercoralis. Infect Immun 81 : 3346–3355.
63. NeillDR, WongSH, BellosiA, FlynnRJ, DalyM, et al. (2010) Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464 : 1367–1370.
64. BreitfeldD, OhlL, KremmerE, EllwartJ, SallustoF, et al. (2000) Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med 192 : 1545–1552.
65. KimCH, RottLS, Clark-LewisI, CampbellDJ, WuL, et al. (2001) Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med 193 : 1373–1381.
66. MohrsM, LedermannB, KohlerG, DorfmullerA, GessnerA, et al. (1999) Differences between IL-4 - and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol 162 : 7302–7308.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic HelixČlánek Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron MobilizationČlánek AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for ImmunityČlánek Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden HamstersČlánek Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Viral Enhancer Mimicry of Host Innate-Immune Promoters
- The Epstein-Barr Virus-Encoded MicroRNA MiR-BART9 Promotes Tumor Metastasis by Targeting E-Cadherin in Nasopharyngeal Carcinoma
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- The Consequences of Reconfiguring the Ambisense S Genome Segment of Rift Valley Fever Virus on Viral Replication in Mammalian and Mosquito Cells and for Genome Packaging
- Substrate-Induced Unfolding of Protein Disulfide Isomerase Displaces the Cholera Toxin A1 Subunit from Its Holotoxin
- Male-Killing Induces Sex-Specific Cell Death via Host Apoptotic Pathway
- Highly Active Antiretroviral Therapies Are Effective against HIV-1 Cell-to-Cell Transmission
- The microRNAs in an Ancient Protist Repress the Variant-Specific Surface Protein Expression by Targeting the Entire Coding Sequence
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Type III Secretion Protein MxiI Is Recognized by Naip2 to Induce Nlrc4 Inflammasome Activation Independently of Pkcδ
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
- Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Strains
- Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic Helix
- Foxp3 Regulatory T Cells Delay Expulsion of Intestinal Nematodes by Suppression of IL-9-Driven Mast Cell Activation in BALB/c but Not in C57BL/6 Mice
- Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron Mobilization
- MicroRNA Editing Facilitates Immune Elimination of HCMV Infected Cells
- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Identification of Host-Targeted Small Molecules That Restrict Intracellular Growth
- A Cyclophilin Homology Domain-Independent Role for Nup358 in HIV-1 Infection
- Engagement of NKG2D on Bystander Memory CD8 T Cells Promotes Increased Immunopathology following Infection
- Suppression of RNA Silencing by a Plant DNA Virus Satellite Requires a Host Calmodulin-Like Protein to Repress Expression
- CIB1 Synergizes with EphrinA2 to Regulate Kaposi's Sarcoma-Associated Herpesvirus Macropinocytic Entry in Human Microvascular Dermal Endothelial Cells
- A Gammaherpesvirus Bcl-2 Ortholog Blocks B Cell Receptor-Mediated Apoptosis and Promotes the Survival of Developing B Cells
- Metabolic Reprogramming during Purine Stress in the Protozoan Pathogen
- The Post-transcriptional Regulator / Activates T3SS by Stabilizing the 5′ UTR of , the Master Regulator of Genes, in
- Tailored Immune Responses: Novel Effector Helper T Cell Subsets in Protective Immunity
- AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for Immunity
- Epstein-Barr Virus Large Tegument Protein BPLF1 Contributes to Innate Immune Evasion through Interference with Toll-Like Receptor Signaling
- The Major Cellular Sterol Regulatory Pathway Is Required for Andes Virus Infection
- Insights into the Initiation of JC Virus DNA Replication Derived from the Crystal Structure of the T-Antigen Origin Binding Domain
- Domain Shuffling in a Sensor Protein Contributed to the Evolution of Insect Pathogenicity in Plant-Beneficial
- Lectin-Like Bacteriocins from spp. Utilise D-Rhamnose Containing Lipopolysaccharide as a Cellular Receptor
- A Compositional Look at the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects
- Exploits Asparagine to Assimilate Nitrogen and Resist Acid Stress during Infection
- Interleukin-33 Increases Antibacterial Defense by Activation of Inducible Nitric Oxide Synthase in Skin
- Protective Vaccination against Papillomavirus-Induced Skin Tumors under Immunocompetent and Immunosuppressive Conditions: A Preclinical Study Using a Natural Outbred Animal Model
- Gem-Induced Cytoskeleton Remodeling Increases Cellular Migration of HTLV-1-Infected Cells, Formation of Infected-to-Target T-Cell Conjugates and Viral Transmission
- Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden Hamsters
- Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
- Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung
- Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response
- Human Genome-Wide RNAi Screen Identifies an Essential Role for Inositol Pyrophosphates in Type-I Interferon Response
- The Master Regulator of the Cellular Stress Response (HSF1) Is Critical for Orthopoxvirus Infection
- Code-Assisted Discovery of TAL Effector Targets in Bacterial Leaf Streak of Rice Reveals Contrast with Bacterial Blight and a Novel Susceptibility Gene
- Competitive and Cooperative Interactions Mediate RNA Transfer from Herpesvirus Saimiri ORF57 to the Mammalian Export Adaptor ALYREF
- The Type III Secretion Chaperone Slc1 Engages Multiple Early Effectors, Including TepP, a Tyrosine-phosphorylated Protein Required for the Recruitment of CrkI-II to Nascent Inclusions and Innate Immune Signaling
- Yeasts: How Many Species Infect Humans and Animals?
- Clustering of Pattern Recognition Receptors for Fungal Detection
- Distinct Antiviral Responses in Pluripotent versus Differentiated Cells
- Igniting the Fire: Virulence Factors in the Pathogenesis of Sepsis
- Inactivation of the Host Lipin Gene Accelerates RNA Virus Replication through Viral Exploitation of the Expanded Endoplasmic Reticulum Membrane
- Inducible Deletion of CD28 Prior to Secondary Infection Impairs Worm Expulsion and Recall of Protective Memory CD4 T Cell Responses
- Clonal Expansion during Infection Dynamics Reveals the Effect of Antibiotic Intervention
- The Secreted Triose Phosphate Isomerase of Is Required to Sustain Microfilaria Production
- Unifying Viral Genetics and Human Transportation Data to Predict the Global Transmission Dynamics of Human Influenza H3N2
- ‘Death and Axes’: Unexpected Ca Entry Phenologs Predict New Anti-schistosomal Agents
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



