-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Male-Killing Induces Sex-Specific Cell Death via Host Apoptotic Pathway
Some symbiotic bacteria cause remarkable reproductive phenotypes like cytoplasmic incompatibility and male-killing in their host insects. Molecular and cellular mechanisms underlying these symbiont-induced reproductive pathologies are of great interest but poorly understood. In this study, Drosophila melanogaster and its native Spiroplasma symbiont strain MSRO were investigated as to how the host's molecular, cellular and morphogenetic pathways are involved in the symbiont-induced male-killing during embryogenesis. TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining, anti-cleaved-Caspase-3 antibody staining, and apoptosis-deficient mutant analysis unequivocally demonstrated that the host's apoptotic pathway is involved in Spiroplasma-induced male-specific embryonic cell death. Double-staining with TUNEL and an antibody recognizing epidermal marker showed that embryonic epithelium is the main target of Spiroplasma-induced male-specific apoptosis. Immunostaining with antibodies against markers of differentiated and precursor neural cells visualized severe neural defects specifically in Spiroplasma-infected male embryos as reported in previous studies. However, few TUNEL signals were detected in the degenerate nervous tissues of male embryos, and the Spiroplasma-induced neural defects in male embryos were not suppressed in an apoptosis-deficient host mutant. These results suggest the possibility that the apoptosis-dependent epidermal cell death and the apoptosis-independent neural malformation may represent different mechanisms underlying the Spiroplasma-induced male-killing. Despite the male-specific progressive embryonic abnormality, Spiroplasma titers remained almost constant throughout the observed stages of embryonic development and across male and female embryos. Strikingly, a few Spiroplasma-infected embryos exhibited gynandromorphism, wherein apoptotic cell death was restricted to male cells. These observations suggest that neither quantity nor proliferation of Spiroplasma cells but some Spiroplasma-derived factor(s) may be responsible for the expression of the male-killing phenotype.
Published in the journal: . PLoS Pathog 10(2): e32767. doi:10.1371/journal.ppat.1003956
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003956Summary
Some symbiotic bacteria cause remarkable reproductive phenotypes like cytoplasmic incompatibility and male-killing in their host insects. Molecular and cellular mechanisms underlying these symbiont-induced reproductive pathologies are of great interest but poorly understood. In this study, Drosophila melanogaster and its native Spiroplasma symbiont strain MSRO were investigated as to how the host's molecular, cellular and morphogenetic pathways are involved in the symbiont-induced male-killing during embryogenesis. TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining, anti-cleaved-Caspase-3 antibody staining, and apoptosis-deficient mutant analysis unequivocally demonstrated that the host's apoptotic pathway is involved in Spiroplasma-induced male-specific embryonic cell death. Double-staining with TUNEL and an antibody recognizing epidermal marker showed that embryonic epithelium is the main target of Spiroplasma-induced male-specific apoptosis. Immunostaining with antibodies against markers of differentiated and precursor neural cells visualized severe neural defects specifically in Spiroplasma-infected male embryos as reported in previous studies. However, few TUNEL signals were detected in the degenerate nervous tissues of male embryos, and the Spiroplasma-induced neural defects in male embryos were not suppressed in an apoptosis-deficient host mutant. These results suggest the possibility that the apoptosis-dependent epidermal cell death and the apoptosis-independent neural malformation may represent different mechanisms underlying the Spiroplasma-induced male-killing. Despite the male-specific progressive embryonic abnormality, Spiroplasma titers remained almost constant throughout the observed stages of embryonic development and across male and female embryos. Strikingly, a few Spiroplasma-infected embryos exhibited gynandromorphism, wherein apoptotic cell death was restricted to male cells. These observations suggest that neither quantity nor proliferation of Spiroplasma cells but some Spiroplasma-derived factor(s) may be responsible for the expression of the male-killing phenotype.
Introduction
Symbiotic microorganisms are ubiquitously associated with diverse insects, and affect their host biology in a variety of ways [1], [2]. Some symbionts play important biological roles such as provisioning of essential nutrients to their hosts [3], helping food digestion for their hosts [4], or improving the fitness of their hosts under specific ecological conditions [5]. Other symbionts like Wolbachia, Cardinium and Spiroplasma are generally parasitic rather than beneficial to their hosts, often causing negative fitness effects and also inducing reproductive phenotypes like cytoplasmic incompatibility, male-killing, parthenogenesis or feminization, by which these symbionts are able to spread their own infections into the host populations in a selfish manner [6]–[8].
Members of the genus Spiroplasma, belonging to the class Mollicutes, are wall-less bacteria associated with diverse arthropods and plants [9]. Some Spiroplasma species and strains are known to cause male-killing phenotypes in fruit flies, ladybird beetles and butterflies, wherein infected females produce all-female or female-biased offspring due to male-specific mortality during embryogenesis and/or larval development [10], [11]. Male-killing symbiotic bacteria belonging to Spiroplasma poulsonii [12] have been identified from fruit flies of the genus Drosophila, which are represented by the strains WSRO from D. willistoni, NSRO from D. nebulosa, MSRO from D. melanogaster and others [13], [14].
While the Drosophila-Wolbachia symbiosis represents one of the best-studied model symbiotic systems [8], [15], the Drosophila-Spiroplasma symbiosis has also been well-studied as another model system of infection dynamics [16]–[18], immune regulation [19]–[21], vertical transmission [22], [23] and male-killing expression [24]–[27]. However, molecular and cellular mechanisms underlying the Spiroplasma-induced male-specific embryonic pathology are still not well understood. Histological observations, mosaic analysis and in vitro culturing have suggested that nervous system is among the major target sites of Spiroplasma-induced male-killing in Drosophila embryos [28]–[32]. In D. melanogaster, Spiroplasma-infected mutants deficient in dosage compensation complex genes fail to show male-killing phenotype, indicating that a functional dosage compensation complex is required for expression of the Spiroplasma-induced make-killing [24]. In D. nebulosa infected with its native Spiroplasma strain NSRO, dying male embryos exhibit widespread TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) signals, suggesting possible involvement of host's pathway of programmed cell death or apoptosis [25].
In this study, we performed detailed investigation of the male-killing process during embryogenesis of D. melanogaster infected with its native Spiroplasma strain MSRO. In particular, we focused on host's molecular, cellular and morphogenetic pathways that may potentially be involved in the male-killing phenotype by utilizing the wealth of genetic resources available in D. melanogaster. Our observations unveiled several previously unknown aspects of Spiroplasma-induced male-killing, which include unequivocal demonstration of male-specific up-regulation of apoptotic pathway, identification of embryonic epithelium as the main target of male-specific apoptosis, male-specific malformation of embryonic nervous system independent of apoptosis, and specific killing of male cells in gynandromorphic embryos.
Results and Discussion
TUNEL-based detection of ectopic programmed cell death in Spiroplasma-infected male embryos
In Spiroplasma-infected female embryos (sexed according to Sex-lethal [Sxl] expression, see Materials and Methods and Fig. S1A and B), TUNEL-positive cells were scarcely found by stage 9 (Fig. 1A), and first appeared at stage 10 in the cephalic region (Fig. 1B, arrowhead). Subsequently, TUNEL-labeled cells spread to the other regions (Fig. 1C and D) and reached a peak level at stages 12–13 (Fig. 1K). These patterns are typical of normal programmed cell death in Drosophila development [33]. Actually, the Spiroplasma-infected female embryos exhibited no substantial differences in the spatiotemporal appearance of TUNEL-positive cells in comparison with uninfected male and female embryos (Fig. 1C; Fig. S1C–E). The Spiroplasma-infected female embryos developed normally (Fig. 1E), and finally emerged as first instar larvae. By contrast, in Spiroplasma-infected male embryos, ectopic TUNEL signals were observed at stage 10: in addition to the dense signals at the cephalic region (Fig. 1G, arrowhead), TUNEL-positive cells were detected throughout the embryonic body (Fig. 1G). Subsequently, the excessive TUNEL signals became more prominent and progressively increased during germ band retraction (Fig. 1H, I and K). From stage 13 and on, the Spiroplasma-infected male embryos started to disintegrate with massive cell death, wherein segmentation and other morphological traits of the embryos became difficult to recognize (Fig. 1J), and finally died. These results indicate that Spiroplasma-infected Drosophila males exhibit ectopic programmed cell death from the early stage of embryonic development. A previous study reported that, in D. nebulosa and its natural Spiroplasma strain NSRO, Spiroplasma-infected male embryos exhibit developmental arrest between stages 12 and 13 with segmentation failure, disintegrated embryonic morphology, and widespread apoptosis as testified by TUNEL staining [25]. Our observations with D. melanogaster and its natural Spiroplasma strain MSRO are highly concordant with the previous observations, suggesting that the same molecular and cellular processes are operating under the symbiont-induced male-specific cell death in the different host species.
Fig. 1. Ectopic cell death in Spiroplasma-infected male embryos of D. melanogaster. 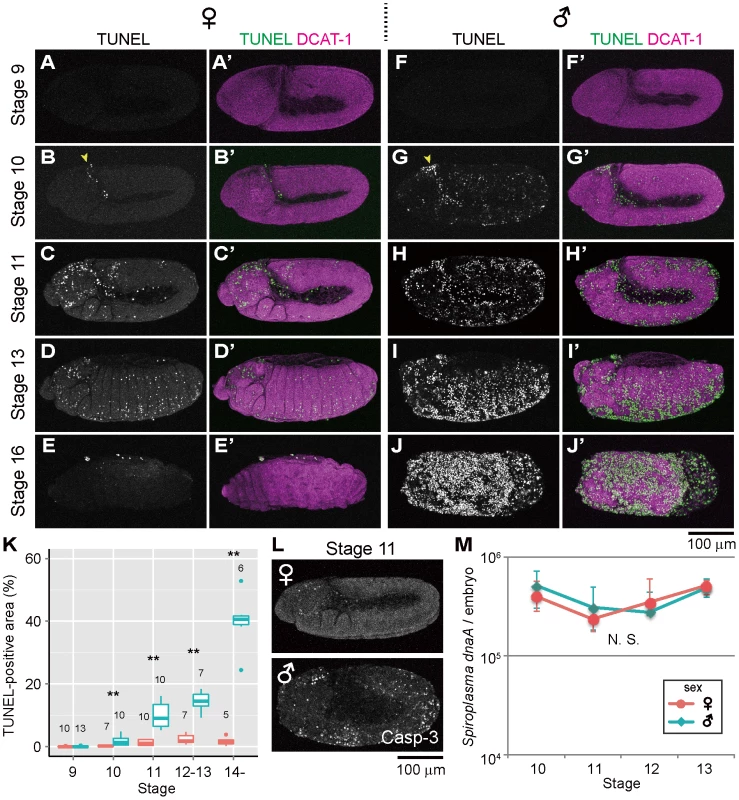
(A–E) Spiroplasma-infected female embryos and (F–J) Spiroplasma-infected male embryos stained with TUNEL and an antibody against Drosophila α-Catenin (DCAT-1) for visualization of embryonic morphology. Developmental stages are indicated on the left side. Yellow arrowheads indicate the cephalic region at stage 10 where TUNEL positive cells appear first. (K) Quantification of TUNEL-positive area in Spiroplasma-infected female embryos (red) and male embryos (blue). Medians and interquartile ranges are shown with sample sizes (Wilcoxon rank sum test; **, P<0.01). (L) Anti-cleaved Caspase-3 antibody (Casp-3) staining of Spiroplasma-infected female and male embryos at stage 11. (M) Infection dynamics of the male-killing Spiroplasma in developing female embryos (red) and male embryos (blue) from stage 10 to stage 13. Infection densities are indicated in terms of symbiont dnaA gene copies per embryo. Medians and interquartile ranges of 12 measurements are shown. No significant difference was observed between sexes or stages (Kruskal-Wallis test followed by Scheffe test; P>0.05). TUNEL-independent detection of ectopic programmed cell death in Spiroplasma-infected male embryos
Previous studies have demonstrated that normal programmed cell death in Drosophila development requires the activity of Caspase-9-like initiator caspase Dronc (Drosophila Nedd2-like caspase) [34]–[38], and an antibody against cleaved-Caspase-3 can detect the Dronc activity [39]. When probed with the anti-cleaved-Caspase-3 antibody, Spiroplasma-infected male embryos exhibited more immunopositive signals than Spiroplasma-infected female embryos as well as uninfected male and female embryos, and the spatiotemporal patterns of the signals looked similar to those of the TUNEL signals (Fig. 1L). These results suggest that Spiroplasma-infected Drosophila males exhibit ectopic programmed cell death during embryonic development, at least in part by activating the caspase-dependent apoptotic pathway.
Population dynamics of Spiroplasma in Drosophila embryos
Spiroplasma-infected male embryos and female embryos were individually subjected to quantitative PCR targeting Spiroplasma dnaA gene copies. Throughout the embryonic stages examined (from 10 to 13), Spiroplasma titers per embryo remained almost constant, exhibiting no significant differences between male embryos and female embryos (Fig. 1M). By contrast, Spiroplasma titers per host elongation factor 1α 100E (EF1a) gene copy exhibited higher values in male embryos than in female embryos (Fig. S1F), which was attributable to lower EF1α gene titers in male embryos presumably because of male-killing phenotype (Fig. S1G). These results strongly suggest that the ectopic programmed cell death specific to male embryos entails no Spiroplasma proliferation during embryogenesis. Meanwhile, it should be noted that, since quantitative PCR detects not live bacterial cells but DNA molecules, the possibility cannot be ruled out that titers of live Spiroplasma cells may change during embryogenesis and/or between male embryos and female embryos.
Host's apoptotic pathway is involved in ectopic cell death in Spiroplasma-infected male embryos
Previous studies have demonstrated that programmed cell death in normal Drosophila development requires proapoptotic genes reaper (rpr), head involution defective (hid) and grim, which are collectively termed RHG genes [40]–[42]. In Drosophila mutant H99 deficient in all these genes, apoptotic cell death is almost completely blocked during embryogenesis [40]. RHG proteins bind to Drosophila inhibitor of apoptosis protein 1 (DIAP1) and disrupt its ability to inhibit caspase activity, by which apoptosis is triggered [43]–[46]. When Spiroplasma-infected H99 mutant embryos were subjected to the TUNEL assay, TUNEL-positive cells were observed neither in female embryos nor in male embryos at stages 11 and 12 (Fig. 2A and C–F). These results strongly suggest that the majority of the ectopic programmed cell death observed in Spiroplasma-infected male embryos is induced via host's apoptotic pathway.
Fig. 2. Involvement of host's apoptotic pathway in ectopic cell death in Spiroplasma-infected male embryos of D. melanogaster. 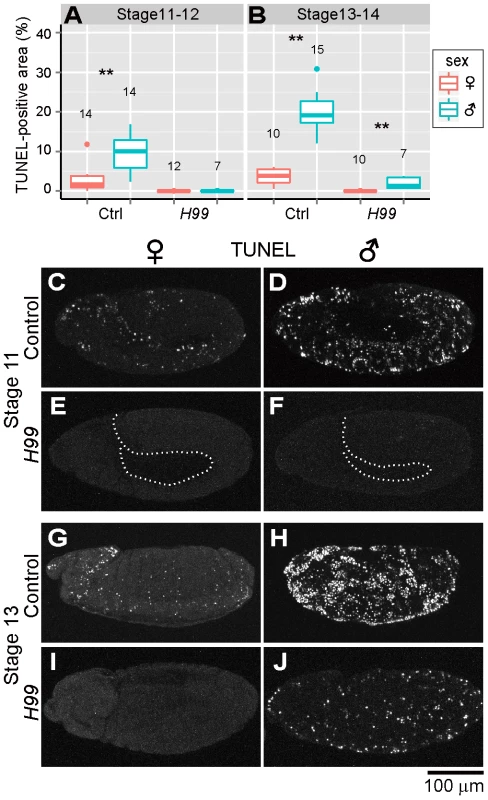
(A and B) Quantification of TUNEL-positive area per embryo in control embryos (Ctrl: H99/+ genotype) and apoptosis-deficient embryos (H99: H99/H99 genotype) at stage 11–12 (A) and stage 13–14 (B) (Wilcoxon rank sum test; **, P<0.01). (C–J) TUNEL signals in the Spiroplasma-infected control embryos and apoptosis-deficient embryos at stage 11 (C–F) and stage 13 (G–J). The internal edge of the epidermis is highlighted by a dashed line in (E) and (F). Residual ectopic cell death in Spiroplasma-infected and apoptosis-suppressed male embryos
In later stages of embryogenesis (stages 13 and 14), some TUNEL-positive cells were detected specifically in Spiroplasma-infected H99 male embryos, although the level of the signals was significantly lower than that in Spiroplasma-infected control male embryos (Fig. 2B and G–J). The residual TUNEL-positive cells may implicate the presence of a minor pathway of Spiroplasma-induced male-specific cell death independent of RHG proteins. Alternatively, the residual TUNEL-positive cells may be due to attenuated clearance of dead cells in the infected embryos.
Host's Hox genes and segment polarity genes are not involved in ectopic cell death in Spiroplasma-infected male embryos
One of the major mechanisms of apoptosis regulation in Drosophila development is the expression control of RHG genes [44], [47]. Notably, it was reported that several Hox proteins, such as Deformed (Dfd) and Abdominal B (Abd-B), may directly activate the expression of rpr by binding to its enhancer elements [48]. It was also reported that some segment polarity genes may regulate cell survival and death to establish morphological patterns during embryogenesis [49]. However, when we performed immunohistochemical visualization of Hox proteins Antennapedia (Antp), Ultrabithorax (Ubx) and Abd-B, and several segment polarity proteins Wingless (Wg) and Engrailed (En) in Spiroplasma-infected male embryos and female embryos, no sex-related differences were observed in their localization patterns (Fig. S2). These results refute the possibility that Spiroplasma infection may induce ectopic cell death by affecting such developmental signals as Hox genes and segment polarity genes in male embryos.
Ectopic cell death occurs in epithelial cells of Spiroplasma-infected male embryos
In Drosophila, atypical protein kinase C (aPKC) localizes to the subapical region (SAR) in the epithelial junctions, thereby establishing apical-basal cell polarity (Fig. 3A) [50], [51]. We visualized embryonic epithelial cells by immunostaining with anti-aPKC antibody. In Spiroplasma-infected female embryos, aPKC showed normal junctional localization and subapical localization in epithelial cells (Fig. 3B–D). In Spiroplasma-infected male embryos, by contrast, junctional localization of aPKC was significantly impaired (Fig. 3E and F), and concentrated TUNEL signals were observed at the level of the subapical region of epithelial cells (Fig. 3G). Notably, intersegmental furrows, which were normally formed in Spiroplasma-infected female embryos (Fig. 3B–D, brackets), became obscure in Spiroplasma-infected male embryos (Fig. 3E–G). These results indicate that embryonic epithelium is among the target tissues wherein Spiroplasma-associated male-specific programmed cell death is induced. Spiroplasma-infected male embryos show ambiguous segments after germ band retraction and die (Fig. 1I) [25]. The loss of segmentation may be relevant to the epithelial damage due to the male-specific apoptosis.
Fig. 3. Ectopic cell death in epithelial cells of Spiroplasma-infected male embryos of D. melanogaster. 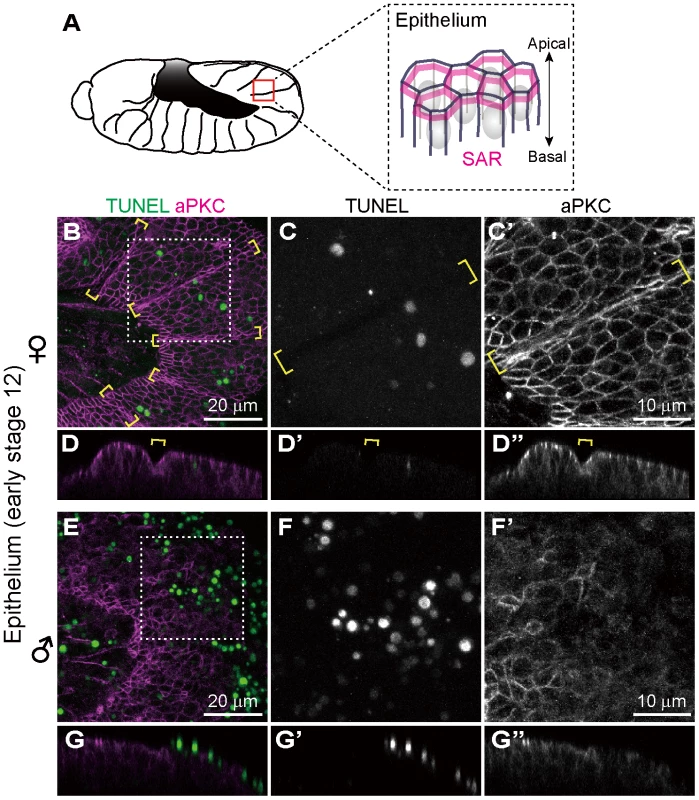
(A) Schematic diagram of epithelial constitution at early stage 12 when segmentation of embryonic body becomes evident. Each epithelial cell develops a distinct apical-basal polarity. A portion of the apical membrane constitutes the subapical region (SAR; magenta), which shares molecular similarities with the vertebrate tight junction [50]. (B–G) Spiroplasma-infected female embryos (B–D) and male embryos (E–G) at early stage 12 stained with TUNEL and anti-aPKC antibody that visualizes the SAR of epithelial cells. The boxed regions in (B) and (E) are magnified in (C) and (F), respectively. Z-sections of (B) and (E) are shown in (D) and (G), respectively. Intersegmental furrows are indicated by yellow brackets. Ectopic cell death does not occur in differentiated neural cells of Spiroplasma-infected male embryos
Previous histological observations, mosaic analysis and in vitro culturing have suggested that nervous system is among the major target sites of Spiroplasma-induced male-killing in Drosophila embryos [28]–[32]. In Drosophila embryos, Elav (embryonic lethal abnormal vision) protein is specifically expressed in differentiated neural cells [52], [53]. We performed immunostaining of developing embryos with anti-Elav antibody, which clearly visualized neurons in central nervous system (CNS) and also neurons in peripheral nervous system (PNS) (Fig. 4A). In Spiroplasma-infected male embryos, both CNS and PNS were disorganized (Fig. 4E and F), whereas these neural structures were intact in Spiroplasma-infected female embryos (Fig. 4C and D). It is noteworthy that, despite the remarkable structural disorder, the regions of concentrated TUNEL-positive cells in Spiroplasma-infected male embryos did not agree with the locations of CNS and PNS (Fig. 4E and F). These results suggest that Spiroplasma infection certainly disrupts the formation of normal nervous system specifically in male embryos, but the neural defects are not due to apoptosis of already differentiated neural cells.
Fig. 4. Ectopic cell death and neural defects in Spiroplasma-infected male embryos of D. melanogaster. 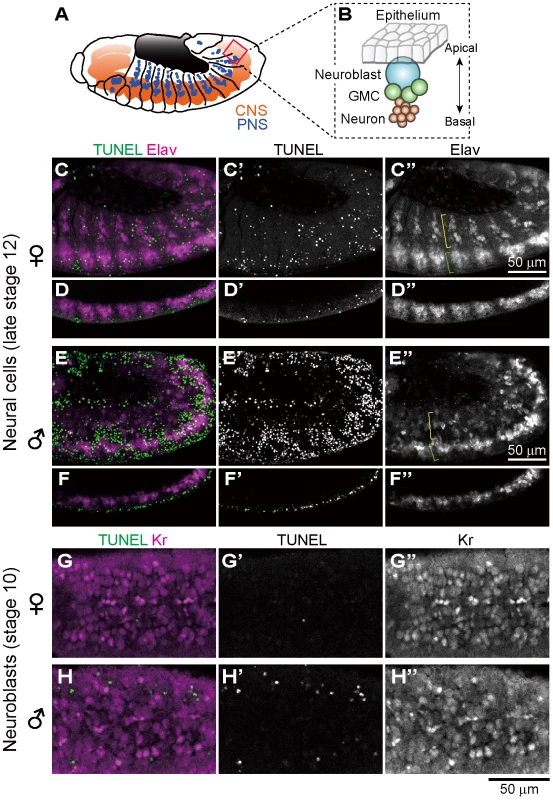
(A) Schematic diagram of the embryonic nervous system at late stage 12, wherein the central nervous system (CNS) and the peripheral nervous system (PNS) are depicted in red and blue, respectively. (B) Schematic diagram of neurogenesis at the cellular level: blue, neuroblast (or precursor neural cell); green, ganglion mother cells (or GMC); and red, neurons (or differentiated neural cells). (C–F) Lateral views of Spiroplasma-infected female embryos (C–D) and male embryos (E–F) at late stage 12 stained with TUNEL and anti-Elav antibody that visualizes differentiated neural cells. Single optical-sections through CNS of (C) and (E) are shown in (D) and (F), respectively. Green brackets and yellow brackets indicate CNS and PNS, respectively. (G–H) Dorsal views of Spiroplasma-infected female embryos (G) and male embryos (H) at stage 10 stained with TUNEL and anti-Krüppel (Kr) antibody that visualizes precursor neural cells or neuroblasts. Single optical sections through the neuroblast layer are selected. Ectopic cell death does not occur in precursor neural cells of Spiroplasma-infected male embryos
In Drosophila embryos, precursor neural cells, or neuroblasts, delaminate basally from neuroectoderm and undergo asymmetric cell division to generate neuroblast itself and ganglion mother cells (GMC). GMCs divide once to give rise to two neurons (Fig. 4B). Neuroblasts express several transcription factors sequentially to generate diverse populations of neurons, one of which is the transcription factor Krüppel (Kr) [54]. Spiroplasma-infected embryos at stage 10 were immunostained with anti-Kr antibody to observe neuroblasts at the beginning of male-specific ectopic cell death. Numerous Kr signals were identified in the neuroblast layer of both Spiroplasma-infected female embryos and male embryos, but they did not co-localize with the TUNEL signals (Fig. 4G and H). These results suggest that Spiroplasma infection disrupts the formation of normal nervous system specifically in male embryos, but the neural defects are unlikely due to direct killing of precursor neural cells via apoptosis.
Spiroplasma-infected male embryos exhibit neural malformation even when apoptosis is suppressed
In the light of these results, it is of focal interest how the epithelial apoptosis and the neural malformation in the Spiroplasma-infected male embryos are interconnected to each other. Are there any causal relationships between them, or do they represent independent processes leading to the embryonic male lethality? To address this question, we observed the development of nervous system in Spiroplasma-infected H99 mutant embryos, in which apoptotic cell death is almost completely blocked [40], by immunostaining with anti-Elav antibody. In Spiroplasma-infected H99 female embryos, CNS and PNS developed normally (Fig. 5A and C), while in Spiroplasma-infected H99 male embryos, remarkable neural malformation was observed (Fig. 5B and D). These results strongly suggest that host's apoptotic pathway is not required for expression of the neural malformation in Spiroplasma-infected male embryos. Plausibly, the male-specific neural malformation may occur independently of the male-specific epithelial apoptosis in the Spiroplasma-infected embryos. If so, the Spiroplasma-induced male-killing entails at least two independent mechanisms: one targets male epithelial cells via host's apoptotic pathway and another targets male nervous system via unknown pathway(s). Alternatively, the defects in neural tissues may somehow influence the organization of adjacent epithelial cells, thereby causing the male-specific epithelial apoptosis secondarily, or vise versa.
Fig. 5. Neural defects in Spiroplasma-infected male embryos of apoptosis-deficient mutant of D. melanogaster. 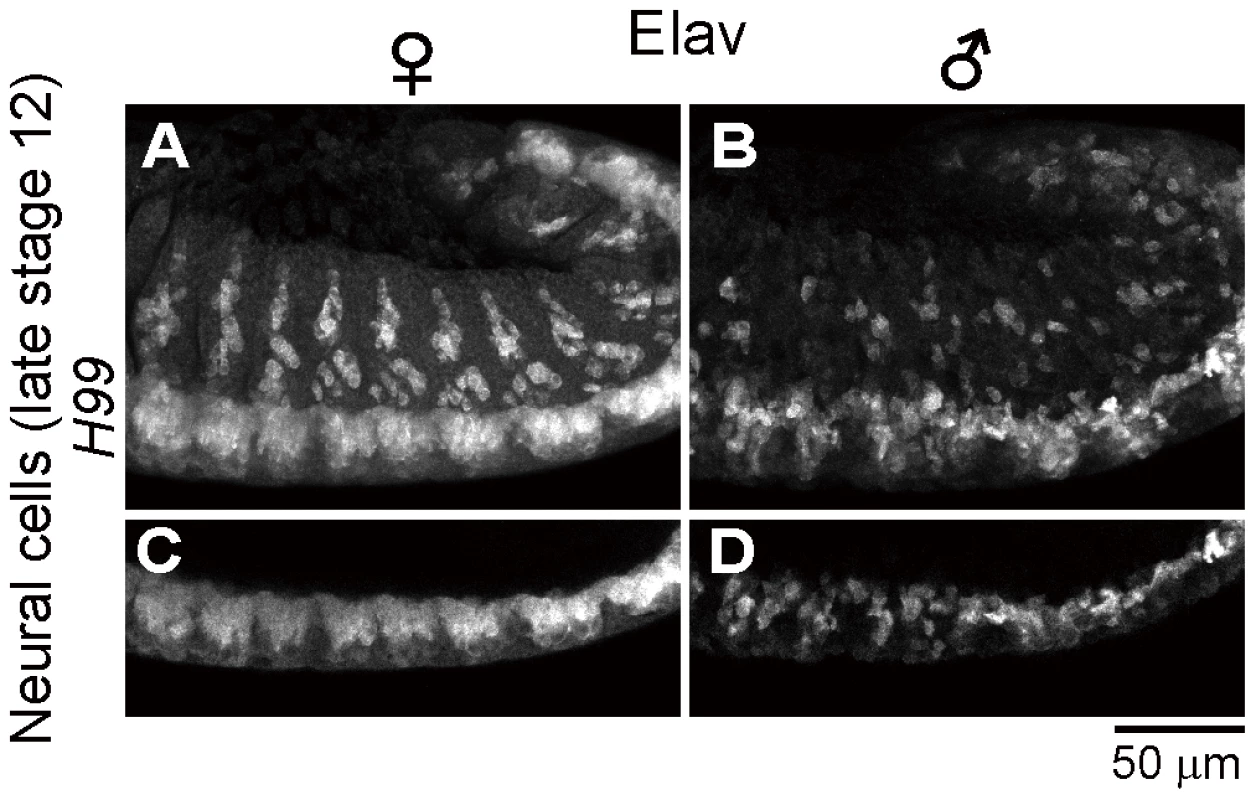
Spiroplasma-infected female embryos (A and C) and male embryos (B and D) of the apoptosis-deficient H99/H99 genotype at late stage 12 stained with anti-Elav antibody to visualize CNS and PNS. Single optical-sections through CNS of (A) and (B) are shown in (C) and (D), respectively. Identification of gynandromorphic Spiroplasma-infected embryos, and male-specific cell death within the embryos
In the survey of Spiroplasma-infected embryos, we occasionally identified gynandromorphic embryos with mosaic expression of Sxl (Fig. 6). Sxl-mosaic embryos were observed at stage 12 and later (3/162; 1.9%), but not found at earlier stages (stage 9 to 11; 0/142). In D. melanogaster, spontaneous gynandromorphism has been reported to occur at frequencies between 0.02 to 0.1% in XX zygotes [55]. While symbiont-induced gynandromorphism has been reported from Wolbachia-infected moth, butterfly, planthopper, wasp and wood louse [56]–[60], it requires further verification whether or not the infrequent occurrence of gynandromorphism in D. melanogaster is induced by Spiroplasma infection. Interestingly, apoptotic cells labeled with anti-cleaved-Caspase-3 antibody were restricted to Sxl-negative, presumable male areas in the gynandromorphic embryos (Fig. 6B and C). These results provide strong evidence that Spiroplasma infection selectively acts on male cells but not on female cells, thereby causing male-killing phenotype.
Fig. 6. Spiroplasma-infected gynandromorphic embryo of D. melanogaster wherein apoptotic cell death is associated with male cells. 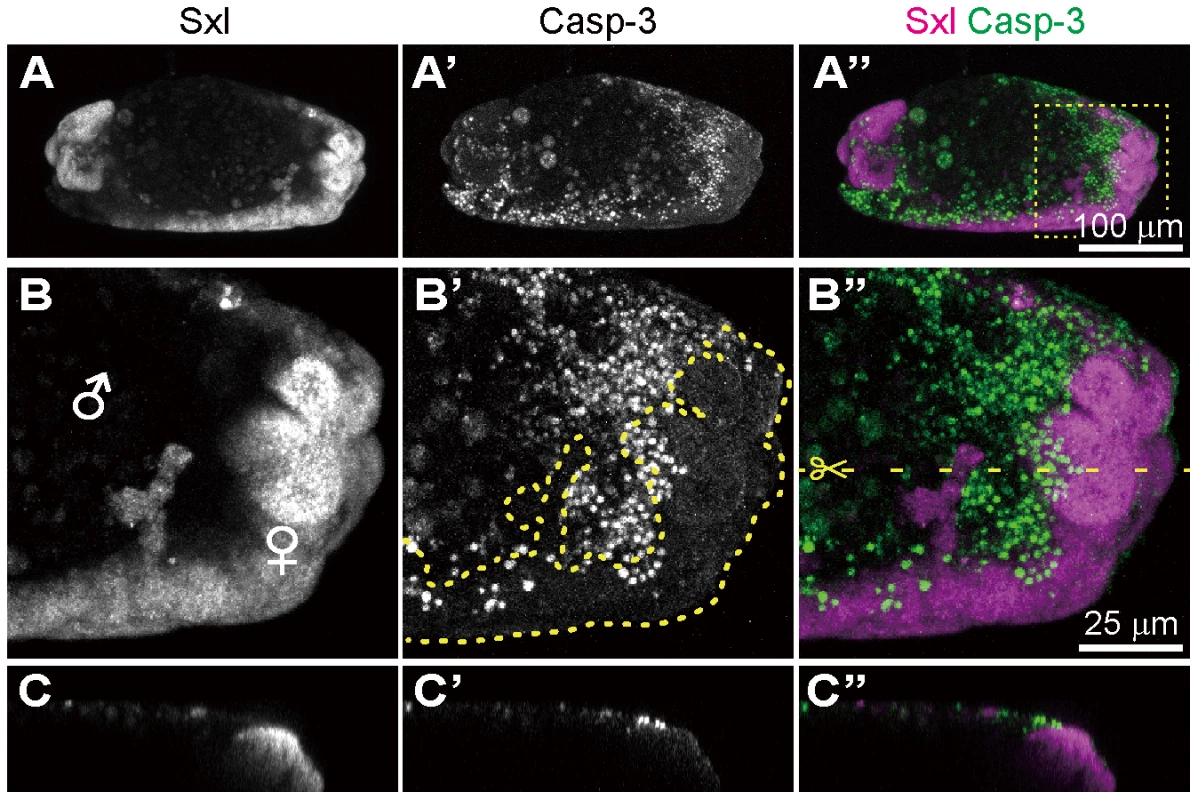
This is most likely a stage 13 embryo, although its precise developmental stage cannot be determined because of severe morphological defects. (A–C) Images of immunostaining with anti-Sxl antibody by which female cells are specifically stained. (A′–C′) Images of immunostaining with anti-cleaved-Casp-3 antibody by which apoptotic cells are selectively visualized. (A″–C″) Merged images. The boxed region in (A″) is magnified in (B)–(B″). Z-sections of dotted yellow line in (B″) is shown in (C)–(C″). In (B′), the area of Sxl-expressing female cells is highlighted by dotted yellow line. Conclusion and perspective
In conclusion, our study unveiled previously unknown molecular and cellular aspects underlying the Spiroplasma-induced male-killing in D. melanogaster. We demonstrated that in Spiroplasma-infected Drosophila embryos (i) host's apoptotic pathway is up-regulated in a male-specific manner, (ii) the male-specific apoptosis mainly targets embryonic epithelial cells, (iii) as previously reported, remarkable neural malformation is observed in male embryos, (iv) however, neither differentiated neural cells nor precursor neural cells exhibit apoptosis in male embryos, (v) the male-specific neural malformation occurs even when host's apoptotic pathway is disrupted, and (vi) therefore, the apoptosis-dependent epidermal cell death and the apoptosis-independent neural malformation may represent different mechanisms underlying the Spiroplasma-induced lethality in male embryos. We also found that (vii) Spiroplasma titers remain almost constant throughout the embryonic development and across male and female embryos, (viii) although at a low frequency (∼2%), gynandromorphic embryos are found in the Spiroplasma-infected embryos, (ix) in these embryos, apoptotic cell death is preferentially observed in male cells, and (x) therefore, neither quantity nor proliferation of Spiroplasma but some Spiroplasma-derived factor(s) selectively acting on host's male cells may be responsible for the expression of male-killing phenotype. These findings highlight complex molecular and cellular interactions in the Spiroplasma-Drosophila symbiosis, and provide invaluable clues to our deeper understanding of the symbiont-induced manipulation of host's development and reproduction.
Materials and Methods
Fly stocks and genetics
The following laboratory strains of D. melanogaster were raised at 25°C on a standard cornmeal diet in plastic tubes unless otherwise indicated. Oregon-R (wild-type strain) was provided by Takehide Murata (the Institute of Physical and Chemical Research, RIKEN). Sxl-Pe-EGFP G78b [61] and Df(3L)H99, kniri-1, pp/TM3, Sb1 [40] were obtained from the Bloomington Stock Center, USA, and the Drosophila Genetic Resource Center (DGRC) at Kyoto Institute of Technology, Japan, respectively. After tetracycline treatment for curing bacterial infections as described [62], the fly strains were infected with the Spiroplasma strain MSRO by hemolymph injection as described [16]. The MSRO-containing hemolymph was collected from naturally infected D. melanogaster strain Ug-SR derived from Uganda [63], which was gifted by John Jaenike (University of Rochester, USA). Since the MSRO-infected fly strains produce all-female offspring, these strains were maintained by supplying males from corresponding uninfected fly stocks. H99 mutant strain was re-balanced with GFP-tagged balancer (TM3, ActGFP, Ser1) and homozygous mutant individuals were identified by immunostaining with anti-GFP antibody.
Immunohistochemistry
Spiroplasma-infected female flies within three days after eclosion were allowed to mate with male flies for three days in plastic tubes. These insects were kept with grape juice agar plates for embryo collection. Embryos at different developmental stages were dechorionated, fixed in 4% formaldehyde and heptane for 20 min, and devitellinized by vigorously shaking in heptane and methanol. In this study, female-specific expression of Sxl, the master regulator of the sex determination system in Drosophila [64], [65], was used for sexing of embryos (Fig. S1A and B). The following primary antibodies were used for immunohistochemical staining: mouse anti-Sex-lethal (M18; 1∶20 dilution; Developmental Studies Hybridoma Bank [DSHB]), rabbit anti-cleaved-Caspase-3 (1∶100; Cell signaling Technology, Inc.), rat anti-Dα-Catenin (DCAT-1; 1∶10; DSHB), rabbit anti-PKCζ (C-20; 1∶100; Santa Cruz Biotechnology, Inc.), mouse anti-Elav (9F8A9; 1∶20; DSHB), rat anti-Elav (7E8A10; 1∶20; DSHB), chicken anti-GFP (GFP-1020; 1∶400; Aves Labs, Inc.), mouse anti-Wingless (4D4; 1∶20; DSHB), mouse anti-Engrailed/Invected (4D9; 1∶20; DSHB), mouse anti-Antp (8C11; 1∶20; DSHB), mouse anti-Ubx (FP3.38; 1∶20; DSHB), mouse anti-Abd-B (1A2E9; 1∶20; DSHB), and guinea pig anti-Krüppel (#573; 1∶300; Asian Distribution Center for Segmentation Antibodies) [66]. Fluorochrome-labeled secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. and Molecular Probes. Nuclear DNA was stained with SYTOX Orange Nucleic Acid Stain (S-11368; 1∶20,000; Molecular Probes). TUNEL staining was performed to detect DNA fragmentation associated with programmed cell death or apoptosis [67], [68] by In Situ Cell Death Detection Kit, TMR red (Roche Applied Science) as described [69]. Images were taken on a confocal microscope (Zeiss LSM 5 Pascal and LSM 510 META). Serial Z-sections of confocal images were compiled to create projection images (maximum intensity projection) unless otherwise described, using a custom macro in ImageJ software (National Institutes of Health, USA).
Quantitative PCR
After dechorionization, developmental stages and sexes of Sxl-Pe-EGFP embryos were determined visually under a stereoscopic fluorescent microscope (Leica M165 FC). Each of 12 embryos of both sexes, which were collected at stage 10, 11, 12 or 13, was individually subjected to DNA extraction using QIAamp DNA mini kit (Qiagen). The DNA samples were subjected to real-time quantitative PCR using SYBR Green (Takara) and Mx3000P qPCR system (Stratagene) essentially as described [16]. Spiroplasma titers in terms of dnaA gene copies were quantified using the primers 5′-TGA AAA AAA CAA ACA AAT TGT TAT TAC TTC-3′ and 5′-TTA AGA GCA GTT TCA AAA TCG GG-3′. The copy numbers of the host EF1α gene were also quantified using the primers 5′-TTA ACA TTG TGG TCA TTG GCC A-3′ and 5′-CTT CTC AAT CGT ACG CTT GTC G-3′. The reaction mixture consisted of 1× AmpliTaq Gold buffer, 1.5 mM MgCl2, 0.2 mM each of dATP, dGTP, dCTP and dUTP, 0.3 µM each of the forward and reverse primers, 1/100,000 SYBR green, and 0.02 U/µl AmpliTaq Gold DNA polymerase (Applied Biosystems). PCR was performed under a temperature profile of 95°C for 10 min followed by 38 cycles of 95°C for 1 min, 60°C for 1 min and 72°C for 1 min. The data were statistically analyzed using the software R version 2.15.0 (R Foundation for Statistical Computing). Multiple comparison was performed using non-parametric Kruskal-Wallis test followed by Scheffe test.
Imaging analysis
Quantitative analyses of TUNEL signals were performed by custom R scripts with EBImage package for image processing [70]. Briefly, maximum projections of confocal slices stained with TUNEL and Sxl antibody were obtained. Images showing lateral view of the embryos were selected for further processing. Embryonic regions were determined by binarization of projected images of Sxl. Processed images were visually checked and signals derived from other objects (e.g. flanking embryos, backgrounds etc.) were manually blacked out to obtain the area of each embryo precisely (mask image). TUNEL signals were also binarized and signals inside the mask image were calculated by image integration. For normalization, TUNEL signals in each embryo were divided by the embryonic area calculated by mask image. Statistical test was performed using Wilcoxon rank sum test.
Supporting Information
Zdroje
1. Buchner P (1965) Endosymbiosis of Animals with Plant Microorganisms. New York, NY, USA: Interscience Publishers. 909 p.
2. Bourtzis K, Miller TA (2003) Insect Symbiosis. Boca Raton, FL: CRC Press. 156 p.
3. MoranNA, McCutcheonJP, NakabachiA (2008) Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42 : 165–190 doi:10.1146/annurev.genet.41.110306.130119
4. OhkumaM (2003) Termite symbiotic systems: efficient bio-recycling of lignocellulose. Appl Microbiol Biotechnol 61 : 1–9 doi:10.1007/s00253-002-1189-z
5. OliverKM, DegnanPH, BurkeGR, MoranNA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55 : 247–266 doi:10.1146/annurev-ento-112408-085305
6. O'Neill SL, Hoffmann AA, Werren JH (1997) Influential Passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford, UK: Oxford University Press. 214 p.
7. Zchori-FeinE, PerlmanSJ, KellySE, KatzirN, HunterMS (2004) Characterization of a “Bacteroidetes” symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): proposal of “Candidatus Cardinium hertigii.”. Int J Syst Evol Microbiol 54 : 961–968 doi:10.1099/ijs.0.02957-0
8. WerrenJH, BaldoL, ClarkME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6 : 741–751 doi:10.1038/nrmicro1969
9. WhitcombRF (1981) The biology of spiroplasmas. Ann Rev Entomol 26 : 397–425 doi:10.1146/annurev.en.26.010181.002145
10. HurstGDD, JigginsFM (2000) Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg Infect Dis 6 : 329–336 doi:10.3201/eid0604.000402
11. Hurst GDD, Jiggins FM, Majerus MEN (2003) Inherited Microorganisms That Selectively Kill Male Hosts: The Hidden Players of Insect Evolution? Insect Symbiosis. Boca Raton, FL: CRC Press. pp. 177–197.
12. WilliamsonDL, SakaguchiB, HackettKJ, WhitcombRF, TullyJG, et al. (1999) Spiroplasma poulsonii sp. nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int J Syst Bacteriol 49 : 611–618 doi:10.1099/00207713-49-2-611
13. HaselkornTS (2010) The Spiroplasma heritable bacterial endosymbiont of Drosophila. Fly (Austin) 4 : 80–87 doi:10.4161/fly.4.1.10883
14. AnbutsuH, FukatsuT (2011) Spiroplasma as a model insect endosymbiont. Environ Microbiol Rep 3 : 144–153 doi:10.1111/j.1758-2229.2010.00240.x
15. SerbusLR, Casper-LindleyC, LandmannF, SullivanW (2008) The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet 42 : 683–707 doi:10.1146/annurev.genet.41.110306.130354
16. AnbutsuH, FukatsuT (2003) Population dynamics of male-killing and non-male-killing spiroplasmas in Drosophila melanogaster. Appl Environ Microbiol 69 : 1428–1434 doi:10.1128/AEM.69.3.1428-1434.2003
17. AnbutsuH, FukatsuT (2006) Tissue-specific infection dynamics of male-killing and nonmale-killing spiroplasmas in Drosophila melanogaster. FEMS Microbiol Ecol 57 : 40–46 doi:10.1111/j.1574-6941.2006.00087.x
18. AnbutsuH, GotoS, FukatsuT (2008) High and low temperatures differently affect infection density and vertical transmission of male-killing Spiroplasma symbionts in Drosophila hosts. Appl Environ Microbiol 74 : 6053–6059 doi:10.1128/AEM.01503-08
19. HurstGDD, AnbutsuH, KutsukakeM, FukatsuT (2003) Hidden from the host: Spiroplasma bacteria infecting Drosophila do not cause an immune response, but are suppressed by ectopic immune activation. Insect Mol Biol 12 : 93–97 doi:10.1046/j.1365-2583.2003.00380.x
20. AnbutsuH, FukatsuT (2010) Evasion, suppression and tolerance of Drosophila innate immunity by a male-killing Spiroplasma endosymbiont. Insect Mol Biol 19 : 481–488 doi:10.1111/j.1365-2583.2010.01008.x
21. HerrenJK, LemaitreB (2011) Spiroplasma and host immunity: activation of humoral immune responses increases endosymbiont load and susceptibility to certain Gram-negative bacterial pathogens in Drosophila melanogaster. Cell Microbiol 13 : 1385–1396 doi:10.1111/j.1462-5822.2011.01627.x
22. NikiY (1988) Ultrastructural study of the sex ratio organism (SRO) transmission into oocytes during oogenesis in Drosophila melanogaster. Jpn J Genet 63 : 11–21 doi:10.1266/jjg.63.11
23. HerrenJK, ParedesJC, SchüpferF, LemaitreB (2013) Vertical transmission of a Drosophila endosymbiont via cooption of the yolk transport and internalization machinery. MBio 4: e00532–12 doi:10.1128/mBio.00532-12
24. VenetiZ, BentleyJK, KoanaT, BraigHR, HurstGDD (2005) A functional dosage compensation complex required for male killing in Drosophila. Science 307 : 1461–1463 doi:10.1126/science.1107182
25. BentleyJK, VenetiZ, HeratyJ, HurstGDD (2007) The pathology of embryo death caused by the male-killing Spiroplasma bacterium in Drosophila nebulosa. BMC Biol 5 : 9 doi:10.1186/1741-7007-5-9
26. KageyamaD, AnbutsuH, ShimadaM, FukatsuT (2007) Spiroplasma infection causes either early or late male killing in Drosophila, depending on maternal host age. Naturwissenschaften 94 : 333–337 doi:10.1007/s00114-006-0195-x
27. KageyamaD, AnbutsuH, ShimadaM, FukatsuT (2009) Effects of host genotype against the expression of Spiroplasma-induced male killing in Drosophila melanogaster. Heredity (Edinb) 102 : 475–482 doi:10.1038/hdy.2009.14
28. CounceSJ, PoulsonDF (1962) Developmental effects of the sex-ratio agent in embryos of Drosophila willistoni. J Exp Zool 151 : 17–31 doi:10.1002/jez.1401510103
29. Tsuchiyama-OmuraS, SakaguchiB, KogaK, PoulsonDF (1988) Morphological features of embryogenesis in Drosophila melanogaster infected with a male-killing Spiroplasma. Zoolog Sci 5 : 375–383.
30. KurodaY, ShimadaY, SakaguchiB, OishiK (1992) Effects of sex-ratio (SR)-Spiroplasma infection on Drosophila primary embryonic cultured cells and on embryogenesis. Zoolog Sci 9 : 283–291.
31. TsuchiyamaS, SakaguchiB, OishiK (1978) Analysis of gynandromorph survivals in Drosophila melanogaster infected with the male-killing SR organisms. Genetics 89 : 711–721.
32. KoanaT, MiyakeT (1983) Effects of the sex ratio organism on in vitro differentiation of Drosophila embryonic cells. Genetics 104 : 113–122.
33. AbramsJM, WhiteK, FesslerLI, StellerH (1993) Programmed cell death during Drosophila embryogenesis. Development 117 : 29–43.
34. QuinnLM, DorstynL, MillsK, ColussiPA, ChenP, et al. (2000) An essential role for the caspase dronc in developmentally programmed cell death in Drosophila. J Biol Chem 275 : 40416–40424 doi:10.1074/jbc.M002935200
35. ChewSK, AkdemirF, ChenP, LuW-J, MillsK, et al. (2004) The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila. Dev Cell 7 : 897–907 doi:10.1016/j.devcel.2004.09.016
36. DaishTJ, MillsK, KumarS (2004) Drosophila caspase DRONC is required for specific developmental cell death pathways and stress-induced apoptosis. Dev Cell 7 : 909–915 doi:10.1016/j.devcel.2004.09.018
37. XuD, LiY, ArcaroM, LackeyM, BergmannA (2005) The CARD-carrying caspase Dronc is essential for most, but not all, developmental cell death in Drosophila. Development 132 : 2125–2134 doi:10.1242/dev.01790
38. WaldhuberM, EmotoK, PetritschC (2005) The Drosophila caspase DRONC is required for metamorphosis and cell death in response to irradiation and developmental signals. Mech Dev 122 : 914–927 doi:10.1016/j.mod.2005.04.003
39. FanY, BergmannA (2010) The cleaved-Caspase-3 antibody is a marker of Caspase-9-like DRONC activity in Drosophila. Cell Death Differ 17 : 534–539 doi:10.1038/cdd.2009.185
40. WhiteK, GretherME, AbramsJM, YoungL, FarrellK, et al. (1994) Genetic control of programmed cell death in Drosophila. Science 264 : 677–683 doi:10.1126/science.8171319
41. GretherME, AbramsJM, AgapiteJ, WhiteK, StellerH (1995) The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev 9 : 1694–1708 doi:10.1101/gad.9.14.1694
42. ChenP, NordstromW, GishB, AbramsJM (1996) grim, a novel cell death gene in Drosophila. Genes Dev 10 : 1773–1782 doi:10.1101/gad.10.14.1773
43. SalvesenGS, AbramsJM (2004) Caspase activation - stepping on the gas or releasing the brakes? Lessons from humans and flies. Oncogene 23 : 2774–2784 doi:10.1038/sj.onc.1207522
44. StellerH (2008) Regulation of apoptosis in Drosophila. Cell Death Differ 15 : 1132–1138 doi:10.1038/cdd.2008.50
45. XuD, WoodfieldSE, LeeTV, FanY, AntonioC, et al. (2009) Genetic control of programmed cell death (apoptosis) in Drosophila. Fly (Austin) 3 : 78–90 doi:10.4161/fly.3.1.7800
46. MiuraM (2012) Apoptotic and nonapoptotic caspase functions in animal development. Cold Spring Harb Perspect Biol 4: pii: a008664 doi:10.1101/cshperspect.a008664
47. LinN, ZhangC, PangJ, ZhouL (2009) By design or by chance: cell death during Drosophila embryogenesis. Apoptosis 14 : 935–942 doi:10.1007/s10495-009-0360-8
48. LohmannI, McGinnisN, BodmerM, McGinnisW (2002) The Drosophila Hox gene Deformed sculpts head morphology via direct regulation of the apoptosis activator reaper. Cell 110 : 457–466 doi:10.1016/S0092-8674(02)00871-1
49. PazderaTM, JanardhanP, MindenJS (1998) Patterned epidermal cell death in wild-type and segment polarity mutant Drosophila embryos. Development 125 : 3427–3436.
50. KnustE, BossingerO (2002) Composition and formation of intercellular junctions in epithelial cells. Science 298 : 1955–1959 doi:10.1126/science.1072161
51. SuzukiA, OhnoS (2006) The PAR-aPKC system: lessons in polarity. J Cell Sci 119 : 979–987 doi:10.1242/jcs.02898
52. RobinowS, WhiteK (1988) The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol 126 : 294–303 doi:10.1016/0012-1606(88)90139-X
53. RobinowS, WhiteK (1991) Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol 22 : 443–461 doi:10.1002/neu.480220503
54. IsshikiT, PearsonB, HolbrookS, DoeCQ (2001) Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell 106 : 511–521 doi:10.1016/S0092-8674(01)00465-2
55. Ashburner M, Golic KG, Hawley RS (2005) 27 Mosaics. Drosophila: A Laboratory Handbook. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. pp. 961–1006.
56. BouchonD, RigaudT, JuchaultP (1998) Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proc Biol Sci 265 : 1081–1090 doi:10.1098/rspb.1998.0402
57. KageyamaD, OhnoS, HoshizakiS, IshikawaY (2003) Sexual mosaics induced by tetracycline treatment in the Wolbachia-infected adzuki bean borer, Ostrinia scapulalis. Genome 46 : 983–989 doi:10.1139/g03-082
58. NegriI, PellecchiaM, MazzoglioPJ, PatettaA, AlmaA (2006) Feminizing Wolbachia in Zyginidia pullula (Insecta, Hemiptera), a leafhopper with an XX/X0 sex-determination system. Proc Biol Sci 273 : 2409–2416 doi:10.1098/rspb.2006.3592
59. NaritaS, KageyamaD, NomuraM, FukatsuT (2007) Unexpected mechanism of symbiont-induced reversal of insect sex: feminizing Wolbachia continuously acts on the butterfly Eurema hecabe during larval development. Appl Environ Microbiol 73 : 4332–4341 doi:10.1128/AEM.00145-07
60. TulgetskeGM, StouthamerR (2012) Characterization of intersex production in Trichogramma kaykai infected with parthenogenesis-inducing Wolbachia. Naturwissenschaften 99 : 143–152 doi:10.1007/s00114-011-0880-2
61. ThompsonJ, GrahamP, SchedlP, PulakR (2004) Sex-specific GFP-expression in Drosophila embryos and sorting by COPAS flow cytometry technique. 45th ADRC Available: http://www.unionbio.com/publications/detail.aspx?id=143.
62. GotoS, AnbutsuH, FukatsuT (2006) Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl Environ Microbiol 72 : 4805–4810 doi:10.1128/AEM.00416-06
63. PoolJE, WongA, AquadroCF (2006) Finding of male-killing Spiroplasma infecting Drosophila melanogaster in Africa implies transatlantic migration of this endosymbiont. Heredity (Edinb) 97 : 27–32 doi:10.1038/sj.hdy.6800830
64. BoppD, BellLR, ClineTW, SchedlP (1991) Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes Dev 5 : 403–415 doi:10.1101/gad.5.3.403
65. SchüttC, NöthigerR (2000) Structure, function and evolution of sex-determining systems in Dipteran insects. Development 127 : 667–677.
66. KosmanD, SmallS, ReinitzJ (1998) Rapid preparation of a panel of polyclonal antibodies to Drosophila segmentation proteins. Dev Gene Evol 208 : 290–294 doi:10.1007/s004270050184
67. WyllieAH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284 : 555–556 doi:10.1038/284555a0
68. AramaE, StellerH (2006) Detection of apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and acridine orange in Drosophila embryos and adult male gonads. Nat Protoc 1 : 1725–1731 doi:10.1038/nprot.2006.235
69. KrieserRJ, MooreFE, DresnekD, PellockBJ, PatelR, et al. (2007) The Drosophila homolog of the putative phosphatidylserine receptor functions to inhibit apoptosis. Development 134 : 2407–2414 doi:10.1242/dev.02860
70. PauG, FuchsF, SklyarO, BoutrosM, HuberW (2010) EBImage–an R package for image processing with applications to cellular phenotypes. Bioinformatics 26 : 979–981 doi:10.1093/bioinformatics/btq046
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic HelixČlánek Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron MobilizationČlánek AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for ImmunityČlánek Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden HamstersČlánek Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Viral Enhancer Mimicry of Host Innate-Immune Promoters
- The Epstein-Barr Virus-Encoded MicroRNA MiR-BART9 Promotes Tumor Metastasis by Targeting E-Cadherin in Nasopharyngeal Carcinoma
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- The Consequences of Reconfiguring the Ambisense S Genome Segment of Rift Valley Fever Virus on Viral Replication in Mammalian and Mosquito Cells and for Genome Packaging
- Substrate-Induced Unfolding of Protein Disulfide Isomerase Displaces the Cholera Toxin A1 Subunit from Its Holotoxin
- Male-Killing Induces Sex-Specific Cell Death via Host Apoptotic Pathway
- Highly Active Antiretroviral Therapies Are Effective against HIV-1 Cell-to-Cell Transmission
- The microRNAs in an Ancient Protist Repress the Variant-Specific Surface Protein Expression by Targeting the Entire Coding Sequence
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Type III Secretion Protein MxiI Is Recognized by Naip2 to Induce Nlrc4 Inflammasome Activation Independently of Pkcδ
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
- Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Strains
- Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic Helix
- Foxp3 Regulatory T Cells Delay Expulsion of Intestinal Nematodes by Suppression of IL-9-Driven Mast Cell Activation in BALB/c but Not in C57BL/6 Mice
- Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron Mobilization
- MicroRNA Editing Facilitates Immune Elimination of HCMV Infected Cells
- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Identification of Host-Targeted Small Molecules That Restrict Intracellular Growth
- A Cyclophilin Homology Domain-Independent Role for Nup358 in HIV-1 Infection
- Engagement of NKG2D on Bystander Memory CD8 T Cells Promotes Increased Immunopathology following Infection
- Suppression of RNA Silencing by a Plant DNA Virus Satellite Requires a Host Calmodulin-Like Protein to Repress Expression
- CIB1 Synergizes with EphrinA2 to Regulate Kaposi's Sarcoma-Associated Herpesvirus Macropinocytic Entry in Human Microvascular Dermal Endothelial Cells
- A Gammaherpesvirus Bcl-2 Ortholog Blocks B Cell Receptor-Mediated Apoptosis and Promotes the Survival of Developing B Cells
- Metabolic Reprogramming during Purine Stress in the Protozoan Pathogen
- The Post-transcriptional Regulator / Activates T3SS by Stabilizing the 5′ UTR of , the Master Regulator of Genes, in
- Tailored Immune Responses: Novel Effector Helper T Cell Subsets in Protective Immunity
- AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for Immunity
- Epstein-Barr Virus Large Tegument Protein BPLF1 Contributes to Innate Immune Evasion through Interference with Toll-Like Receptor Signaling
- The Major Cellular Sterol Regulatory Pathway Is Required for Andes Virus Infection
- Insights into the Initiation of JC Virus DNA Replication Derived from the Crystal Structure of the T-Antigen Origin Binding Domain
- Domain Shuffling in a Sensor Protein Contributed to the Evolution of Insect Pathogenicity in Plant-Beneficial
- Lectin-Like Bacteriocins from spp. Utilise D-Rhamnose Containing Lipopolysaccharide as a Cellular Receptor
- A Compositional Look at the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects
- Exploits Asparagine to Assimilate Nitrogen and Resist Acid Stress during Infection
- Interleukin-33 Increases Antibacterial Defense by Activation of Inducible Nitric Oxide Synthase in Skin
- Protective Vaccination against Papillomavirus-Induced Skin Tumors under Immunocompetent and Immunosuppressive Conditions: A Preclinical Study Using a Natural Outbred Animal Model
- Gem-Induced Cytoskeleton Remodeling Increases Cellular Migration of HTLV-1-Infected Cells, Formation of Infected-to-Target T-Cell Conjugates and Viral Transmission
- Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden Hamsters
- Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
- Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung
- Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response
- Human Genome-Wide RNAi Screen Identifies an Essential Role for Inositol Pyrophosphates in Type-I Interferon Response
- The Master Regulator of the Cellular Stress Response (HSF1) Is Critical for Orthopoxvirus Infection
- Code-Assisted Discovery of TAL Effector Targets in Bacterial Leaf Streak of Rice Reveals Contrast with Bacterial Blight and a Novel Susceptibility Gene
- Competitive and Cooperative Interactions Mediate RNA Transfer from Herpesvirus Saimiri ORF57 to the Mammalian Export Adaptor ALYREF
- The Type III Secretion Chaperone Slc1 Engages Multiple Early Effectors, Including TepP, a Tyrosine-phosphorylated Protein Required for the Recruitment of CrkI-II to Nascent Inclusions and Innate Immune Signaling
- Yeasts: How Many Species Infect Humans and Animals?
- Clustering of Pattern Recognition Receptors for Fungal Detection
- Distinct Antiviral Responses in Pluripotent versus Differentiated Cells
- Igniting the Fire: Virulence Factors in the Pathogenesis of Sepsis
- Inactivation of the Host Lipin Gene Accelerates RNA Virus Replication through Viral Exploitation of the Expanded Endoplasmic Reticulum Membrane
- Inducible Deletion of CD28 Prior to Secondary Infection Impairs Worm Expulsion and Recall of Protective Memory CD4 T Cell Responses
- Clonal Expansion during Infection Dynamics Reveals the Effect of Antibiotic Intervention
- The Secreted Triose Phosphate Isomerase of Is Required to Sustain Microfilaria Production
- Unifying Viral Genetics and Human Transportation Data to Predict the Global Transmission Dynamics of Human Influenza H3N2
- ‘Death and Axes’: Unexpected Ca Entry Phenologs Predict New Anti-schistosomal Agents
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



