-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Interleukin-33 Increases Antibacterial Defense by Activation of Inducible Nitric Oxide Synthase in Skin
Interleukin-33 (IL-33) is associated with multiple diseases, including asthma, rheumatoid arthritis, tissue injuries and infections. Although IL-33 has been indicated to be involved in Staphylococcus aureus (S. aureus) wound infection, little is known about how IL-33 is regulated as a mechanism to increase host defense against skin bacterial infections. To explore the underlying intricate mechanism we first evaluated the expression of IL-33 in skin from S. aureus-infected human patients. Compared to normal controls, IL-33 was abundantly increased in skin of S. aureus-infected patients. We next developed a S. aureus cutaneous infection mouse model and found that IL-33 was significantly increased in dermal macrophages of infected mouse skin. The expression of IL-33 by macrophages was induced by staphylococcal peptidoglycan (PGN) and lipoteichoic acid (LTA) via activation of toll-like receptor 2(TLR2) –mitogen-activated protein kinase (MAPK)-AKT-signal transducer and activator of transcription 3(STAT3) signaling pathway as PGN and LTA failed to induce IL-33 in Tlr2-deficient peritoneal macrophages, and MAPK,AKT, STAT3 inhibitors significantly decreased PGN - or LTA-induced IL-33. IL-33, in turn, acted on macrophages to induce microbicidal nitric oxygen (NO) release. This induction was dependent on inducible nitric oxide synthase (iNOS) activation, as treatment of macrophages with an inhibitor of iNOS, aminoguanidine, significantly decreased IL-33-induced NO release. Moreover, aminoguanidine significantly blocked the capacity of IL-33 to inhibit the growth of S. aureus, and IL-33 silencing in macrophages significantly increased the survival of S. aureus in macrophages. Furthermore, the administration of IL-33-neutralizing antibody into mouse skin decreased iNOS production but increased the survival of S. aureus in skin. These findings reveal that IL-33 can promote antimicrobial capacity of dermal macrophages, thus enhancing antimicrobial defense against skin bacterial infections.
Published in the journal: . PLoS Pathog 10(2): e32767. doi:10.1371/journal.ppat.1003918
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003918Summary
Interleukin-33 (IL-33) is associated with multiple diseases, including asthma, rheumatoid arthritis, tissue injuries and infections. Although IL-33 has been indicated to be involved in Staphylococcus aureus (S. aureus) wound infection, little is known about how IL-33 is regulated as a mechanism to increase host defense against skin bacterial infections. To explore the underlying intricate mechanism we first evaluated the expression of IL-33 in skin from S. aureus-infected human patients. Compared to normal controls, IL-33 was abundantly increased in skin of S. aureus-infected patients. We next developed a S. aureus cutaneous infection mouse model and found that IL-33 was significantly increased in dermal macrophages of infected mouse skin. The expression of IL-33 by macrophages was induced by staphylococcal peptidoglycan (PGN) and lipoteichoic acid (LTA) via activation of toll-like receptor 2(TLR2) –mitogen-activated protein kinase (MAPK)-AKT-signal transducer and activator of transcription 3(STAT3) signaling pathway as PGN and LTA failed to induce IL-33 in Tlr2-deficient peritoneal macrophages, and MAPK,AKT, STAT3 inhibitors significantly decreased PGN - or LTA-induced IL-33. IL-33, in turn, acted on macrophages to induce microbicidal nitric oxygen (NO) release. This induction was dependent on inducible nitric oxide synthase (iNOS) activation, as treatment of macrophages with an inhibitor of iNOS, aminoguanidine, significantly decreased IL-33-induced NO release. Moreover, aminoguanidine significantly blocked the capacity of IL-33 to inhibit the growth of S. aureus, and IL-33 silencing in macrophages significantly increased the survival of S. aureus in macrophages. Furthermore, the administration of IL-33-neutralizing antibody into mouse skin decreased iNOS production but increased the survival of S. aureus in skin. These findings reveal that IL-33 can promote antimicrobial capacity of dermal macrophages, thus enhancing antimicrobial defense against skin bacterial infections.
Introduction
Interleukin-33 (IL-33), previously known as a nuclear factor from high endothelial venules [1], is a chromatin-associated nuclear cytokine from the IL-1 family that functions as an “alarmin” [2], [3]. It has been shown to be constitutively expressed in the nuclei of endothelial and epithelial cells in vivo [4] or to be released into the extracellular space after tissue damage [5], [6] to stimulate innate immune responses. Recently emerging evidence has shown that IL-33 is also a potent inducer of pro-inflammatory cytokines and chemokines by mast cells [7], [8], resulting in the development or exacerbation of asthma or atopic allergy and anaphylaxis [9]. In addition to functioning as an alarmin or inducer of proinflammatory cytokines, IL-33 plays an important protective role during parasitic infection via the induction of type 2 immune responses [10], [11] or promotes neutrophil proliferation and recruitment against Staphylococcus aureus (S.aureus) wound infection [12]. Although IL-33 has been shown to play a significant role during infection, little is known about how IL-33 is regulated as a mechanism to increase host defense against skin bacterial infections.
S.aureus skin infection is a major bacterial infection of the skin and has become an enormous public health problem due to the emergence of methicillin-resistant S.aureus (MRSA). Without treatment, S.aureus skin infection can disseminate and promote life-threatening infections. Remarkably, in the United States the estimated number of deaths caused by MRSA infection is around 18,500 per year, exceeding the number of deaths associated with human immunodeficiency virus infection/acquired immunodeficiency syndrome (HIV/AIDS) [13], [14]. Due to this rapidly emerging epidemic and the growing problem of antibiotic resistance, it is necessary to understand protective immune responses against S.aureus skin infection, thereby developing immune-based antibacterial therapies to combat S.aureus infection.
Recently several cytokines have been shown to enhance an effective immune response against S.aureus infection [14]. For example, IL-17 from skin γδ T cells stimulates keratinocytes to produce pro-inflammatory cytokines, chemokines and adhesion molecules that mediate neutrophil recruitment to the site of S.aureus infection to promote bacterial clearance [15]. IL-1 family cytokines IL-1α and IL-1β initiate an IL-1 receptor signaling loop to produce neutrophil-attracting chemokines, such as chemokine (C-X-C-motif) ligand 1 (CXCL1), CXCL2 and CXCL8 in human keratinocytes, leading to neutrophil recruitment to the site of S.aureus infection [16]. Furthermore, IL-18, another IL-1 family cytokine, protects burn-injured mice from MRSA by enhancing neutrophil function [17]. However, whether IL-33, a new member of IL-1 family, will regulate innate immune responses other than neutrophil functions against S.aureus skin infection remains largely unknown.
Given that the emergence of antibiotic-resistant S.aureus requires the development of new antibacterial therapies, and IL-1 family cytokines play important protective roles in S.aureus infections, we set out to investigate whether IL-33 is expressed after S.aureus cutaneous infection and determine if it exhibits a significant functional relevance in this system. Our findings uncover a vital protective role of IL-33 against S.aureus infection and delineate a previously unknown mechanism in host defense.
Results
Staphylococcus aureus infection induces IL-33 expression in skin
Although IL-33 has been shown to be involved in host defense against intestinal nematode infection [11], [18] and S.aureus wound infection [12], the underlying intricate mechanism by which IL-33 is regulated to protect the host from bacterial skin infection remains largely unknown. To explore the role of IL-33 in bacterial infection we first evaluated the expression of IL-33 in skin from three S.aureus-infected human patients. Compared to normal controls, IL-33 was abundantly increased in skin of S.aureus-infected patients (Figure 1A). The immunofluorescence analysis showed that IL-33 protein was expressed both in epidermis and dermis of S.aureus-infected human skin (Figure 1B). To further confirm that the increase in cutaneous IL-33 production demonstrates a causal relationship with S.aureus infection, we evaluated IL-33 expression in a S.aureus cutaneous infection mouse model and found that both protein and mRNA of IL-33 were markedly increased in infected mouse skin (Figure 1C–1E). The formation of skin lesions caused by S.aureus was time-dependent and reached its peak at day-3 post-infection (Figure 1D). Consistent with skin lesions, the induction of IL-33 mRNA was time-dependent with the maximum induction observed in day-3-infected skin (Figure 1D). IL-33 protein expression was detectable by immunofluorescent staining primarily localized to dermal macrophages in infected skin (Figure 1E). Furthermore, to determine which cell type is a major producer of IL-33 during skin infection we used heat-inactivated S.aureus to stimulate primary keratinocytes, mast cells, neutrophils and macrophages in vitro. Heat-inactivated S.aureus significantly increased IL-33 mRNA in macrophages (Figure 1F) but not in mast cells and neutrophils (Figure S1A and S1B). To our surprise, in primary murine and human keratinocytes heat-inactivated S.aureus slightly induced IL-33 mRNA expression, but it markedly increased IL-33 protein production (Figure S1C–S1F). Taken together, these data demonstrate that S.aureus infection increases IL-33 in epidermal keratinocytes and dermal macrophages.
Fig. 1. IL-33 expression in skin after Staphylococcus aureus infection. 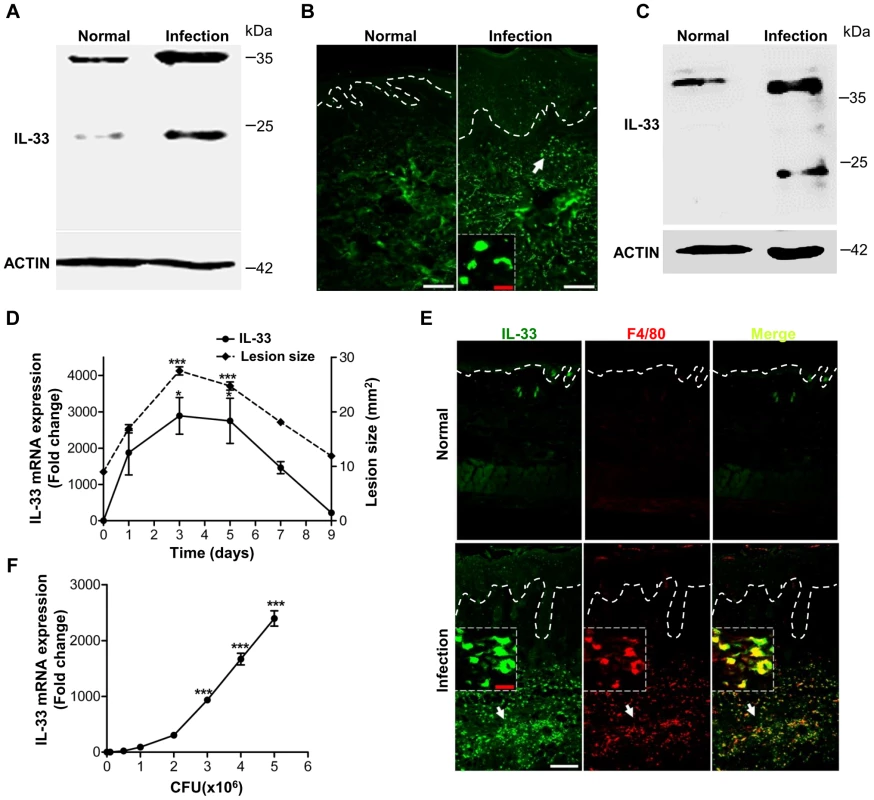
(A) Western blot of IL-33 in skin extracts from S.aureus-infected patients. Samples from 3 S.aureus-infected patients with abscess were pooled together. (B) Immunofluorescence analysis of IL-33 in skin of S.aureus-infected patients. This image is representative of three patients with abscess. Dotted white lines indicate the location of dermal-epidermal junction. Red scale bar represents 10 µm and white scale bars represent 50 µm. The arrow designates region of 200× magnification shown in inset. (C) Western blot of IL-33 in skin extracts from S.aureus-infected mice. Samples from 3 S.aureus-infected mice were pooled together. (D) Quantification of IL-33 expression in skin and the lesional size of skin lesions after mouse back skin infected with 1–2×107 CFU S.aureus. Normal skin without infection was collected as control. (E) Immunofluorescence analysis of IL-33 and F4/80 (macrophage marker) in mouse skin after S.aureus infection. This image is representative of six mice with abscess. Dotted white lines indicate the location of dermal-epidermal junction. Red scale bar represents 10 µm and white scale bar represents 50 µm. Arrows designate region of 200× magnification shown in insets. (F) IL-33 expression in RAW264.7 cells stimulated with different doses of heat-inactivated S.aureus for 24 hours. *P<0.05 and ***P<0.001. P values were analyzed by one-way analysis of variance (ANOVA). Data are the means ± SEM and representative of two to three independent experiments with n = 3–6 per group. PGN and LTA are two major molecules of Staphylococcus aureus to induce IL-33 in macrophages
We have observed that S.aureus infection enhanced IL-33 expression in keratinocytes and macrophages, next we sought to identify which molecules from S. aureus would be inducers of IL-33. Since S.aureus induced both mRNA and protein of IL-33 consistently in macrophages (Figure 1C–1F), and peptidoglycan (PGN) and lipoteichoic acid (LTA) are two major constituents of S.aureus cell wall, we thereby used S.aureus-derived PGN and LTA to stimulate macrophages in vitro. PGN, as well as LTA, significantly induced IL-33 mRNA in time - and dose - dependent manners (Figure 2A–2D). In addition to mRNA, both commercial PGN and PGN purified from S.aureus CMCC(B)26003, the strain used to infect mice, increased full-length IL-33 protein in pure primary peritoneal macrophages by western blot analysis (Figure 2E and 2F, Figure S2A). This production of IL-33 was detected in both the nucleus and cytoplasm following the nucleocytoplasmic separation analysis (Figure 2G). The immunofluorescence analysis also confirmed this observation that PGN induced the expression of IL-33 in both cytoplasm and nucleus (Figure 2H). Furthermore, IL-33 was detectable in cell culture media (Figure 2I). Taken together, these results show that PGN and LTA are two major molecules from S.aureus to directly induce IL-33 in macrophages.
Fig. 2. IL-33 expression induced by S.aureus-derived PGN and LTA in macrophages. 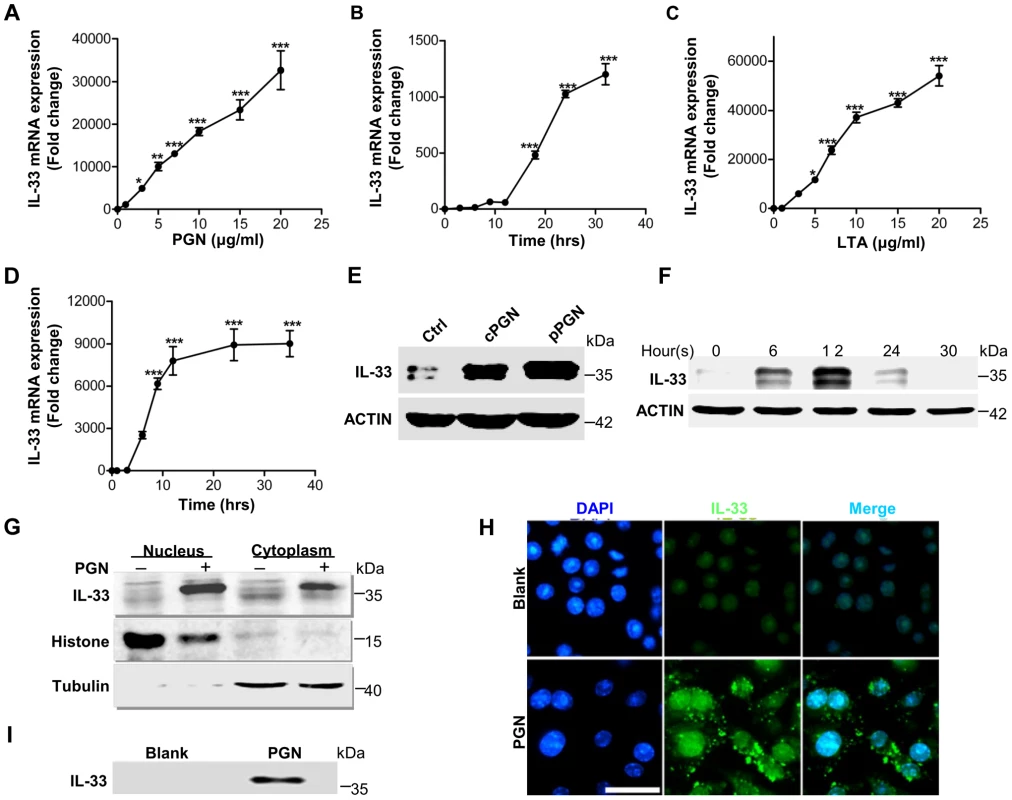
(A&B) Quantification of IL-33 expression in macrophages RAW264.7 treated with different doses of PGN (A) or treated with 10 µg/ml PGN for different times (B). (C&D) Quantification of IL-33 expression in macrophages RAW264.7 treated with different doses of LTA (C) or treated with 10 µg/ml LTA for different times (D). (E) Western blot of IL-33 induced by commercial PGN (cPGN) and PGN purified from S.aureus CMCC(B)26003 (pPGN) in total cell lysate of primary peritoneal macrophages. (F) Western blot of IL-33 induced by PGN in total cell lysate of primary peritoneal macrophages at different time points. (G) Western blot of IL-33 from the cytoplasm or nucleus of macrophages RAW264.7 treated with 10 µg/ml PGN. Histone was used as an endogenous control for nuclear proteins and tubulin was used as an endogenous control for cytoplasmic proteins. (H) Immunofluorescent staining of IL-33 in macrophages RAW264.7 treated with PGN for 24 hours. Scale bar represents 50 µm. (I) IL-33 in cell culture media. Cell culture media of macrophages RAW264.7 treated with 10 µg/ml PGN was incubated with protein G beads coupled with IL-33 antibody for overnight. Next day, IL-33 captured by IL-33 antibody was eluted from beads for western blot. *P<0.05 and ***P<0.001. P values were determined by one-way ANOVA. Data are the means ± SEM of n = 3 and representative of three independent experiments. Staphylococcus aureus activates TLR2-MAPK-AKT-STAT3 signaling pathway to induce IL-33
Having identified PGN and LTA from S.aureus as two major inducers of IL-33 in macrophages, we next sought to explore the molecular mechanisms involved in the induction of IL-33 by PGN and LTA in macrophages. Since Toll-like receptor 2 (TLR2) is a well-known receptor of Gram-positive bacteria, we hypothesized that the activation of TLR2 was required for S.aureus to induce IL-33. To test this, we infected wild-type (WT) and Tlr2-deficient (Tlr2−/−) mice with S.aureus. Compared to wild-type mice, the bacterial burden and survival of S.aureus was much higher in Tlr2−/− mice (Figure 3A and 3B), while both mRNA and protein of IL-33 were decreased in infected skin of Tlr2−/− mice (Figure 3C–3E). Furthermore, PGN induced IL-33 mRNA and protein in a time-dependent manner in isolated primary peritoneal macrophages from wild-type mice, but not in Tlr2−/− primary peritoneal macrophages (Figure 3F and 3G). In addition to PGN, LTA significantly induced IL-33 mRNA expression in wild-type but not Tlr2−/− primary peritoneal macrophages (Figure S2B). Altogether, these data demonstrate that S.aureus signals through TLR2 to induce IL-33 expression in macrophages.
Fig. 3. TLR2 is required for Staphylococcus aureus to induce IL-33. 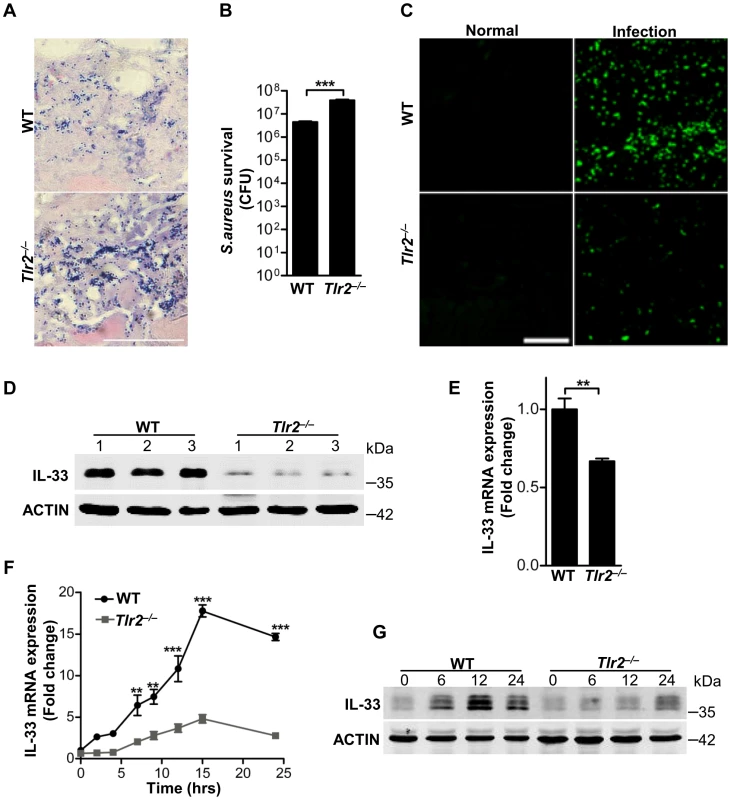
(A) Gram-positive staining of mouse skin infected with S.aureus. Scale bar represents 50 µm. (B) Local S.aureus survival in skin lesions at day-3 post-infection. (C) Immunofluorescent staining of IL-33 in wild-type and Tlr2−/− mouse skin infected with S.aureus. This image is representative of six mice with abscess. Scale bar represents 50 µm. (D) Western blot of IL-33 in skin lesions caused by S.aureus infection at 3 days. 1, 2, 3 represent three mice of each group were used. (E) Quantification of IL-33 mRNA expression in skin of wild-type and Tlr2−/− mice infected with S.aureus. (F) IL-33 mRNA expression in primary peritoneal macrophages from wild-type and Tlr2−/− mice stimulated with 10 µg/ml PGN. (G) Western blot of IL-33 in primary peritoneal macrophages from wild-type and Tlr2−/− mice stimulated with 10 µg/ml PGN for different times. **P<0.01 and ***P<0.001. P values were analyzed by two-tailed t tests in (B & E) or two-way ANOVA in (F). All data are representative of two independent experiments with n = 6 per group and are means ± SEM. Next we further explored TLR2-mediated downstream signaling pathways in macrophages. We used inhibitors of several key pathways downstream of TLR2 to treat macrophages in the presence or absence of PGN or LTA. Among these inhibitors, mitogen-activated protein kinase (MAPK) inhibitors, AKT inhibitor and signal transducers and activators of transcription 3 (STAT3) inhibitor significantly decreased the expression of PGN - or LTA-induced both mRNA and protein of IL-33 (Figure 4A–4C). PGN and LTA induced phosphorylation of p38 MAPK, AKT and STAT3 in a time-dependent manner in macrophages (Figure 4D). Besides p38 MAPK, PGN increased the phosphorylation of c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) (Figure 4E). Furthermore, PGN induced phosphorylation of p38 MAPK in wild-type peritoneal macrophages, but this effect was lost in Tlr2−/− peritoneal macrophages (Figure 4F); p38-MAPK inhibitor dampened PGN-induced AKT phosphorylation while AKT inhibitor did not have the capacity to block PGN-induced p38-MAPK phosphorylation (Figure 4G). Finally, AKT inhibitor prevented PGN-induced STAT3 phosphorylation, but not vice versa (Figure 4H). Taken together, these results suggest that S.aureus activates the TLR2-MAPK-AKT-STAT3 signaling pathway to regulate the production of IL-33 in macrophages.
Fig. 4. Staphylococcus aureus activates MAPKs-AKT-STAT3 signaling to induce IL-33. 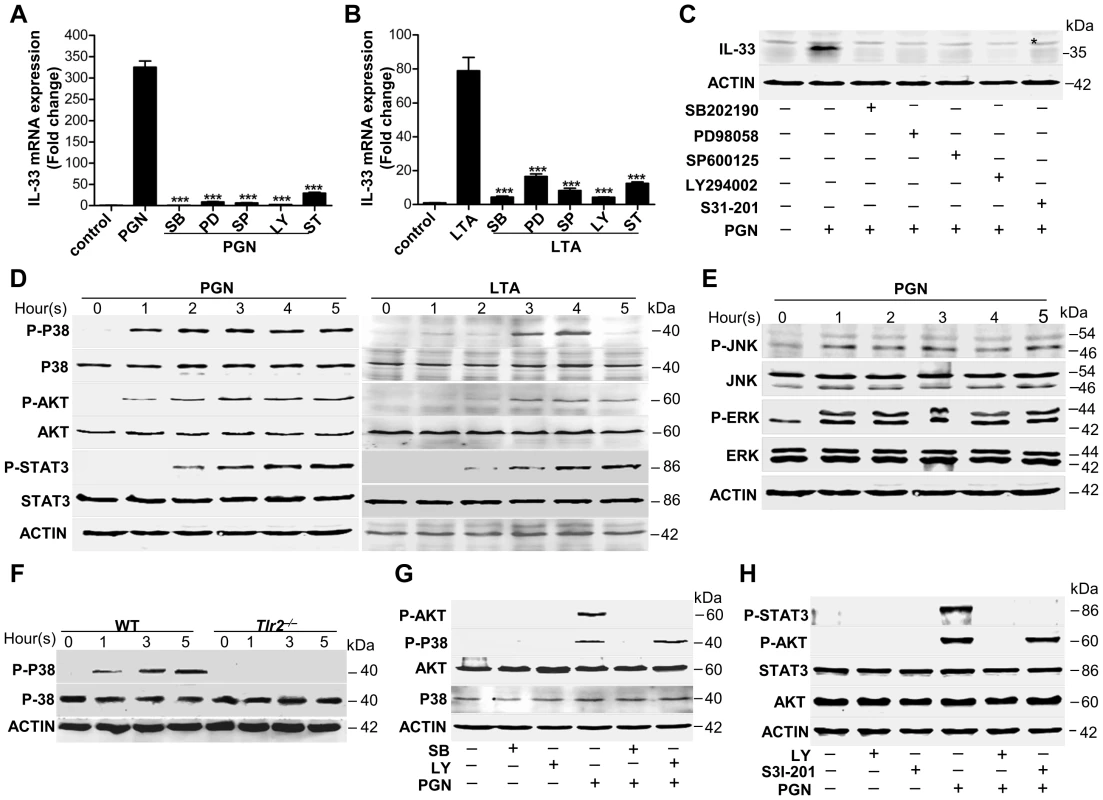
(A&B) Quantification of IL-33 mRNA of macrophages RAW264.7 treated with 10 µg/ml PGN (A) or LTA (B) in the presence or absence of different inhibitors. SB: p38 MAPK inhibitor SB202190 (5 µM); PD: MEK1 inhibitor PD98059 (20 µM); SP: JNK inhibitor SP600125 (15 µM); Ly: AKT inhibitor Ly294002 (50 µM); ST: STAT3 inhibitor S31-201 (50 µM). (C) Western blot of IL-33 in macrophages RAW264.7 stimulated by PGN in the presence or absence of inhibitors. * Unspecific bands. (D) Phosphorylation of p38 MAPK (Thr180/Tyr182), AKT (Ser473) and STAT3 (Tyr705) after RAW264.7 cells treated with 10 µg/ml PGN or LTA for 1–5 h. (E) Phosphorylation of JNK (Thr183/Tyr185) and ERK (Thr202/Tyr204) after RAW264.7 cells treated with 10 µg/ml PGN for 1–5 h. (F) Phosphorylation of p38 MAPK (Thr180/Tyr182) after wild-type and Tlr2−/− primary peritoneal macrophages treated with 10 µg/ml PGN for 1–5 h. (G) Phosphorylation of p38 MAPK (Thr180/Tyr182) and AKT (Ser473) after macrophages RAW264.7 treated with 10 µg/ml PGN in the presence of p38MAPK inhibitor SB202190 or AKT inhibitor Ly294002. (H) Phosphorylation of AKT (Ser473) and STAT3 (Tyr705) after macrophages RAW264.7 treated with 10 µg/ml PGN in the presence of AKT inhibitor Ly294002 or STAT3 inhibitor S31-201. ***P<0.001. P values were analyzed by one-way ANOVA in (A&B). All data are representative of two experiments with n = 3 per group and are means ± SEM. IL-33 activates ST2-AKT-β-catenin to induce iNOS
Since we have delineated a key mechanism by which S.aureus induces IL-33 in macrophages, we next wanted to establish an in vivo role of IL-33 in S.aureus skin infection. Previous observations have suggested that IL-1 induces nitric oxygen synthase (NOS) to release nitric oxygen (NO), and IL-33 has been shown to induce NO production in endothelial cells [19], [20], [21]. Since we observed that heat-inactivated S.aureus, PGN and LTA stimulated the production of inducible form of NOS (iNOS) and NO in both time - and dose - dependent manners in macrophages (Figure S3), we hypothesized that IL-33 might be an intermediate for S.aureus to induce iNOS and NO in macrophages. To test this, we first determined if heat-inactivated S.aureus induced the expression of iNOS after IL-33 gene silencing. Heat-inactivated S.aureus significantly increased the expression of iNOS, and this increase was significantly inhibited after IL-33 was silenced using IL-33 siRNAs (Figure 5A). Consistent with this observation, iNOS induced by PGN and LTA was also significantly decreased after IL-33 was silenced (Figure 5B and 5C). Moreover, S.aureus completely failed to induce iNOS expression in Tlr2−/− primary peritoneal macrophages compared to that in wild-type primary peritoneal macrophages (Figure 5D), and the inactivation of MAPKs, AKT and STAT3 significantly decreased the expression of PGN - and LTA-induced iNOS (Figure S4A and S4B). To further confirm that IL-33 can directly active iNOS, we stimulated macrophages with recombinant full-length IL-33 and processed IL-33 (Ser109-Ile266) in vitro and found that both mRNA and protein of iNOS was induced by recombinant full-length and processed IL-33 in a time-dependent manner in primary peritoneal macrophages(Figure 5E–5H). Consistently, recombinant processed IL-33 increased both mRNA and protein of iNOS in time - and dose-dependent manners in macrophage cell line RAW264.7 (Figure S4C–S4E). Collectively, these data confirm that IL-33 is an important cytokine induced by S.aureus to increase iNOS expression.
Fig. 5. IL-33 induces iNOS in macrophages. 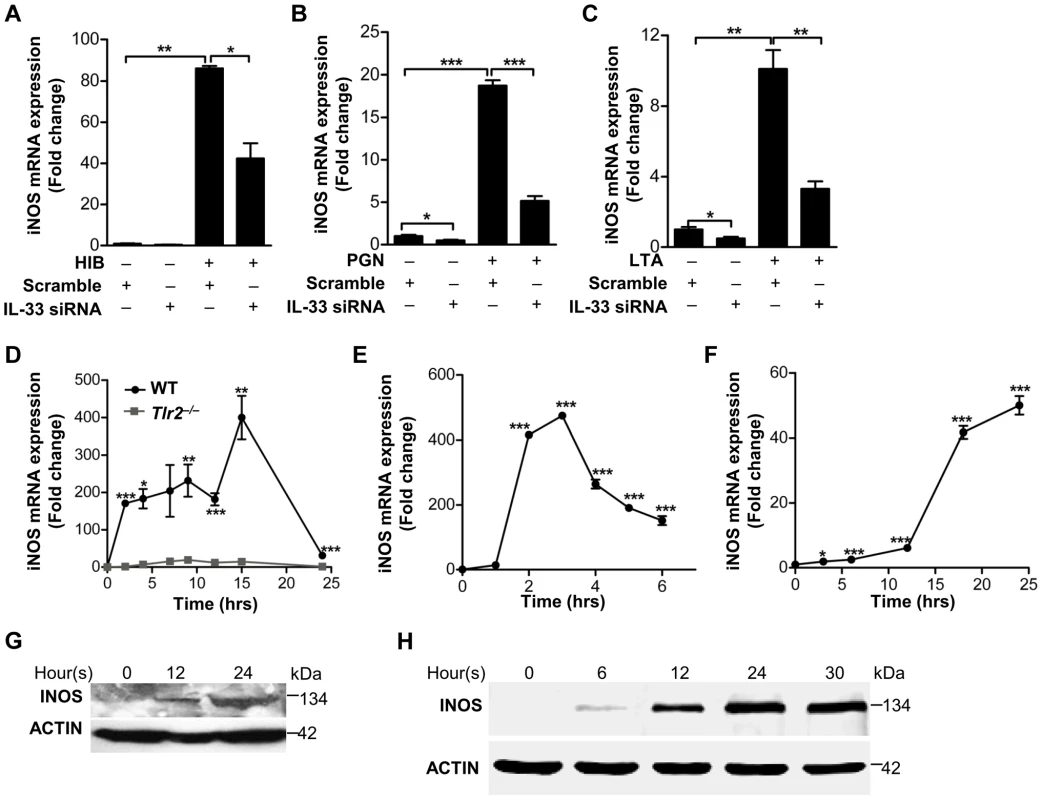
(A–C) iNOS expression induced by heat-inactivated S.aureus (A) or PGN (B) or LTA(C) before or after IL-33 was silenced in primary peritoneal macrophages. (D) Quantification of iNOS mRNA expression of WT and Tlr2−/− primary peritoneal macrophages treated with 10 µg/ml PGN. (E&F) iNOS mRNA expression in BMDMs treated with 30 ng/ml full-length IL-33(E) or in primary peritoneal macrophages treated with 30 ng/ml processed IL-33(Ser109-Ile266) (F) for various times. (G&H) Western blot of iNOS in BMDMs treated with 30 ng/ml full-length IL-33(G) or in primary peritoneal macrophages treated with 30 ng/ml processed IL-33(Ser109-Ile266) (H) for various times. *P<0.05, **P<0.01 and ***P<0.001. P values were determined by one-way ANOVA (A–C, E&F) or two-way ANOVA (D). All data are means ± SEM of n = 3 and representative of two to three independent experiments. It has been reported that IL-33 mediates its biological effects via its receptor ST2 [1]. We observed that recombinant processed IL-33 significantly increased ST2 in time - and dose-dependent manners in macrophages (Figure S5A and S5B). Silencing of ST2 significantly decreased the expression of iNOS induced by recombinant processed IL-33 (Figure 6A). Moreover, recombinant processed IL-33 induced iNOS protein production in WT peritoneal macrophages and bone marrow-derived macrophages (BMDMs), while this induction was completely or partially abrogated in ST2−/− or IL1R−/− peritoneal macrophages and BMDMs (Figure 6B and Figure S5C). To identify the key downstream pathways of ST2 activated by IL-33, we treated macrophages with multiple inhibitors in the presence or absence of recombinant processed IL-33. Among this inhibitors, AKT inhibitors (Ly294002 and the AKT1/2 specific inhibitor) and β-catenin inhibitor (cardamonin) significantly inhibited iNOS expression by recombinant processed IL-33 (Figure 6C and 6D). To confirm that AKT is a critical downstream signaling molecule for ST2, we next evaluated if recombinant processed IL-33 activated AKT after ST2 gene silencing. Recombinant processed IL-33 markedly increased the phosphorylation of AKT in a time-dependent manner (Figure S5D and S5E) and this increase was markedly inhibited after ST2 was silenced using ST2 shRNAs (Figure 6E and Figure S5F). Furthermore, AKT inhibitors completely inhibited IL-33-induced AKT phosphorylation or β-catenin phosphorylation and accumulation while β-catenin inhibitor failed to block IL-33-induced AKT phosphorylation (Figure 6F and 6G, Figure S5G). All these data demonstrate that IL-33 activates the ST2-AKT-β-catenin signaling pathway to regulate iNOS in macrophages.
Fig. 6. IL-33 activates ST2-AKT-β-catenin to induce iNOS. 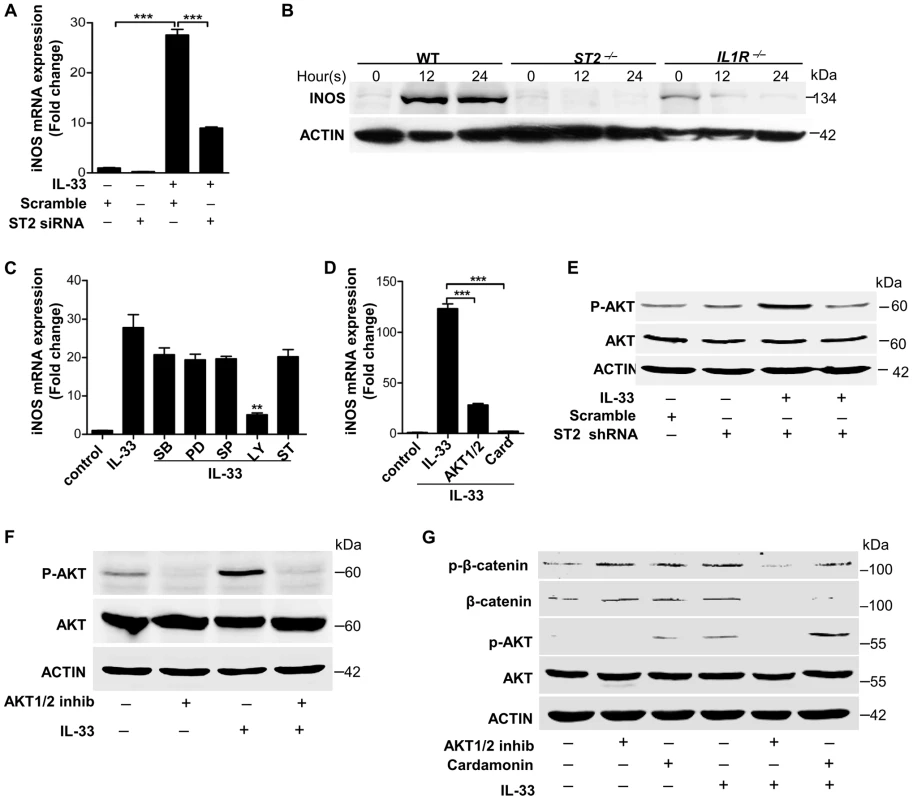
(A) iNOS mRNA expression induced by 30 ng/ml processed IL-33(Ser109-Ile266) before or after ST2 was silenced in primary peritoneal macrophages. (B) Western blot of iNOS in WT, ST2−/− and IL1R−/− primary peritoneal macrophages treated with 30 ng/ml processed IL-33(Ser109-Ile266) for different times. (C) iNOS mRNA expression induced by 30 ng/ml processed IL-33(Ser109-Ile266) in the presence or absence of different inhibitors. SB: p38 MAPK inhibitor SB202190 (5 µM); PD: MEK1 inhibitor PD98059 (20 µM); SP: JNK inhibitor SP600125 (15 µM); Ly: AKT inhibitor Ly294002 (50 µM); ST: STAT3 inhibitor S3I-201 (50 µM). (D) iNOS mRNA expression induced by 30 ng/ml processed IL-33(Ser109-Ile266) in the presence or absence of AKT1/2 specific inhibitor (8 µM) or β-catenin inhibitor cardamonin (9 µM). (E) Phosphorylation of AKT (Ser473) induced by 30 ng/ml processed IL-33(Ser109-Ile266) before and after ST2 was silenced in primary peritoneal macrophages. (F) Phosphorylation of AKT (Ser473) induced by 30 ng/ml processed IL-33(Ser109-Ile266) in the presence or absence of AKT1/2 specific inhibitor (8 µM) in primary peritoneal macrophages. (G) Phosphorylation of AKT (Ser473) and β-catenin (Ser675) induced by 30 ng/ml processed IL-33(Ser109-Ile266) in the presence or absence of AKT1/2 specific inhibitor (8 µM) or β-catenin inhibitor cardamonin (9 µM) in BMDMs. The phosphorylation of β-catenin at Ser675 resulted in the accumulation of total β-catenin in cells. **P<0.01 and *** P<0.001. P values were determined by one-way ANOVA. All data are means ± SEM of n = 3 and representative of two independent experiments. IL-33 activates iNOS to release NO against Staphylococcus aureus infection
To understand the immunological relevance of iNOS induced by IL-33 and infection, we next set out to elucidate the biological role of IL-33. It is known that iNOS regulates NO production and NO plays an important role in host defense against intracellular pathogens [22], [23]. Furthermore, it has been reported that topically application of NO is a potentially useful preventive and therapeutic strategy against superficial skin infections, including MRSA skin infection [24]. Consistent with these observations, we observed that acidified nitrite significantly inhibited S.aureus growth in vitro (Figure 7A). To test whether the activation of iNOS by IL-33 would release NO against S.aureus infection, we first investigated the capacity of IL-33 to induce NO. Recombinant processed IL-33 induced NO release in a dose-dependent manner (Figure 7B). This induction was dependent on iNOS activation, as treatment of macrophages with a relatively selective inhibitor of iNOS, aminoguanidine, significantly decreased NO release (Figure 7C). Moreover, aminoguanidine significantly blocked the capacity of IL-33 to inhibit the growth of intracellular S.aureus in macrophages (Figure 7D), and IL-33 silencing in macrophages not only significantly decreased the production of NO induced by S.aureus (Figure 7E) but also increased the survival of S.aureus in macrophages (Figure 7F). Since we have shown that the induction of IL-33 by S.aureus (including PGN and LTA) or iNOS by IL-33 was dependent on AKT activation, we next used the AKT inhibitor to block the production of IL-33 or NO. We found that the AKT inhibitor significantly increased the survival of S.aureus and blocked the capacity of IL-33 to inhibit the growth of S.aureus (Figure 7G).
Fig. 7. IL-33 activates iNOS to release NO against Staphylococcus aureus infection. 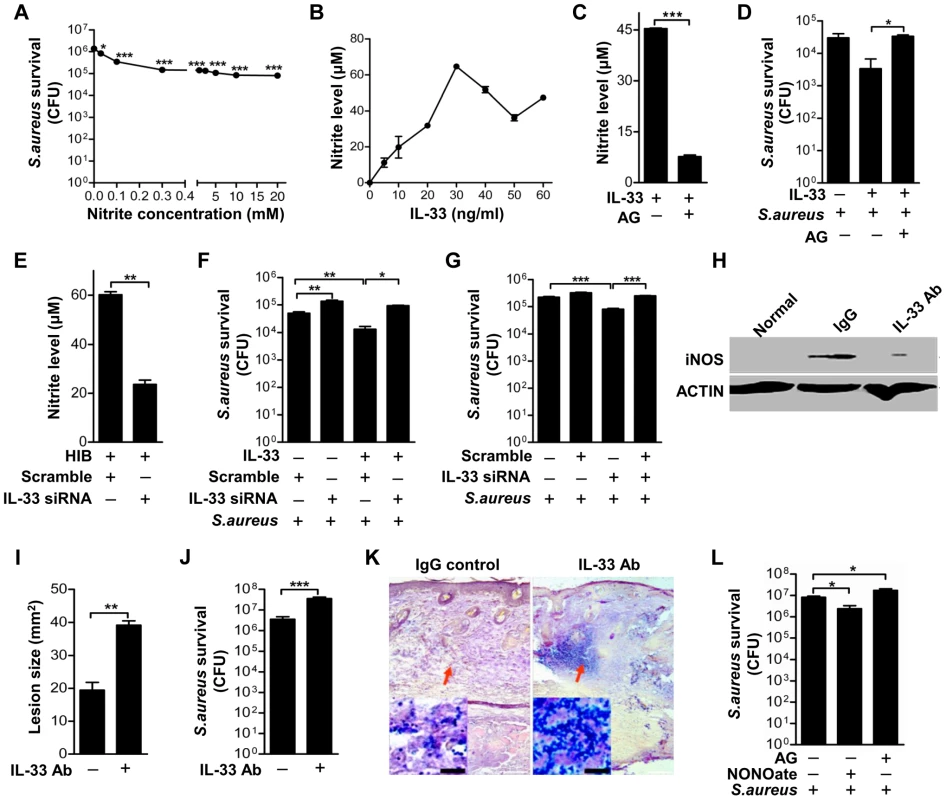
(A) Acidified nitrite inhibited S.aureus growth in vitro. (B) Different doses of processed IL-33(Ser109-Ile266) induced NO release in RAW264.7 cells. (C) The release of NO by 30 ng/ml processed IL-33(Ser109-Ile266) in the presence or absence of iNOS inhibitor aminoguanidine. (D) The survival of S.aureus in the intracellular of RAW264.7 cells after stimulated with 30 ng/ml processed IL-33(Ser109-Ile266) in the presence or absence of aminoguanidine. (E) The release of NO by heat-inactivated S.aureus after IL-33 was silenced. (F) The survival of S.aureus in RAW264.7 cells stimulated by 30 ng/ml processed IL-33(Ser109-Ile266) before and after IL-33 was silenced. (G) The survival of S.aureus in RAW264.7 cells after treated with 30 ng/ml processed IL-33(Ser109-Ile266) in the presence or absence of AKT inhibitor Ly294002 (50 µM). (H) Western blot of iNOS in skin lesions caused by S.aureus infection at days 3 with or without IL-33 blockade. (I) Image J analysis of the lesion size of skin lesions treated as in (H). (J) Local S.aureus survival in skin lesions treated as in (H). (K) Gram-positive staining of S.aureus in infected skin of (H). (L) Local S.aureus survival in skin of mice treated with NO-donor NONOate or iNOS inhibitor aminoguanidine. *P<0.05, **P<0.01 and ***P<0.001. P values were determined by one-way ANOVA in (A, D, F, G and L) or two-tailed t tests in (C, E, I and J). All data are representative of two to three independent experiments with n = 3–6 per group and are means ± SEM. To further address the role of IL-33 in S.aureus infection, we treated mice with IL-33 neutralizing antibody before mice were intradermally injected with S.aureus. S.aureus infection increased iNOS production in macrophages (Figure 7H, Figure S6A) and neutrophil recruitment (Figure S6B) while the blockage of IL-33 by its neutralizing antibody markedly inhibited macrophage-derived iNOS production (Figure 7H and Figure S6A) and neutrophil recruitment (Figure S6B). Consistent with this, mice treated with IL-33 neutralizing antibody exhibited larger infectious skin lesions when compared with control mice injected with IgG control (Figure 7I). Mouse skin treated with IL-33 neutralizing antibody exhibited greater S.aureus survival at the local site of infection (Figure 7J) and a greater S.aureus bacterial burden in skin lesion (Figure 7K). Moreover, the presence of an iNOS inhibitor increased S.aureus survival in mouse skin, while treatment with NO-donor DETA-NONOate reduced the bacterial load in vivo (Figure 7L). Thus, these data confirm that IL-33 activates iNOS to release NO against S.aureus infection.
Discussion
Innate immunity is the first line of defense to protect the host from bacterial skin infection. IL-33, as a cytokine from innate immune cells, has been shown to be protective against parasitic and bacterial infection [10], [11], [12], but the underlying intricate mechanism by which IL-33 is regulated to increase host defenses against bacterial skin infection remains largely unknown. Here we observed that IL-33 increased host innate defense via the induction and activation of iNOS to release microbicidal NO. Our results demonstrate that PGN and LTA, two major cell wall components of S.aureus, induce IL-33 via activation of TLR2-MAPK-AKT-STAT3 signaling pathway. Furthermore, the intradermal administration of IL-33 neutralizing antibody or iNOS inhibitor in skin decreases NO release, which leads to increased survival of S.aureus, while the direct administration of NO-donor DETA-NONOate decreases the bacterial survival in skin lesions. The mechanism for IL-33-mediated antibacterial function involves NO release through the binding to its receptor ST2, followed by the activation of AKT-β-catenin and iNOS, events we show have a major role in inhibiting S.aureus infection in skin. Therefore, the identification of IL-33 as a stimulus for NO, and the elucidation of its mechanism of induction and action, provides a new mechanism for understanding innate immune responses during bacterial skin infections. These findings also offer potential immune-based antibacterial therapies to combat bacterial skin infections by targeting IL-33 and downstream NO induction.
When a pathogen breaches the skin barrier, neutrophils are quickly recruited to the site of infection followed by the infiltration of macrophages. At the initial stage of the host immune response to infection, macrophages and neutrophils are generally thought to function primarily as phagocytic cells [25]. Although these two professional phagocytes possess overlapping and complementary characteristics during infection, their distinct antimicrobial properties that stem from their innate immune lineage make them irreplaceable as individual components of the innate immune system [26]. Neutrophils are equipped with an array of microbicidal mechanisms and employ several granular antimicrobial molecules, including defensins and cathelicidin to combat pathogenic infections [27], [28]. Macrophages exhibit remarkable phagocytic abilities compared to neutrophils, which enhance their contribution to direct antimicrobial activities [29]. Here we provide a previously unidentified mechanism by which macrophages exert their primary antimicrobial defense against S. aureus infection by induction of IL-33 to activate iNOS for NO release into the intracellular and extracellular milieu. In response to S.aureus infection, TLR2 present on the cell surface of macrophages allows for the recognition of PGN and LTA, followed by activation of p38 MAPK-AKT-STAT3 signaling to induce IL-33 production. In turn, IL-33 induces and activates iNOS to release NO in macrophages. This previously unknown function of IL-33 in macrophages sheds new light on the function of macrophages in innate host defense. Interestingly, the increase of IL-33 mRNA by PGN or LTA is more than 1000 fold higher in macrophage RAW264.7 while only 4–15 fold higher in primary peritoneal macrophages compared to vehicle treated, which might be due to the differences between cell line and primary cells. Besides induction of microbicidal NO, IL-33 recruits neutrophils to the site of S.aureus infection (Figure S6B) but does not induce iNOS in neutrophils (data not shown), which is consistent with the previous observation [12]. In addition to murine macrophages, we analyzed IL-33 expression induced by S.aureus in other cell types, including mast cells, neutrophils and keratinocytes. S.aureus markedly induces IL-33 protein production but slightly induces mRNA expression in keratinocytes (Figure S1C–S1F) or completely fails to induce IL-33 expression in neutrophils and mast cells. Therefore, our whole study is primarily focused on murine macrophages.
The expression of IL-33 is primarily localized to nonhematopoietic cells, particularly in endothelial and epithelial cells [2]. Full-length, unprocessed IL-33 has been shown to localize within the nucleus of epithelial and endothelial cells [4]. Here we show that IL-33 is also expressed in hematopoietic-derived macrophages as well as epidermal keratinocytes after S.aureus infection, although there is certain difference between mRNA and protein of IL-33 in epidermal keratinocytes (Figure S1C–S1F). It is known that in principle, unprocessed proteins of IL-1 family such as IL-1α should remain in the cellular compartment in which they are synthesized [30]. However, we observe that, upon PGN stimulation, full-length IL-33 is detectable not only inside macrophages (including cytoplasm and nucleus) but also in cell culture media. How full-length, unprocessed IL-33 is excreted from macrophages remains unclear. We speculate that full-length, unprocessed IL-33 is passively released by necrotic cells into the extracellular milieu as an alarmin molecule to indicate cellular stressors (i.e. infection or injury). Alternatively, S.aureus infection may induce acute extracellular accumulation of ATP, and this autocrine ATP activates purinergic receptors to induce full-length, unprocessed IL-33 release [31]. Moreover, both full-length IL-33 and processed IL-33 are detected in skin during S.aureus infection, while heat-inactivated S.aureus only induces full-length IL-33 in macrophages. One may assume that upon stimulation, neutrophils release cathepsin G and elastase to process full-length IL-33 to mature bioactive forms in vivo [5], [32] while in vitro macrophages fail to perform this process due to lack of the above proteases. However, it is also possible that full-length IL-33 is processed by an unknown mechanism in vivo that does not exist in macrophages. Furthermore, although the previous report shows that the activity of processed IL-33 in the induction of inflammatory cytokines is around 10-fold higher than full-length IL-33, in our system full-length IL-33 exhibits the similar activity as processed IL-33 in the induction of iNOS (Figure 5E–5H). In spite of this discrepancy, both data suggest that the functional domain of IL-33 is primarily at the C-terminus of IL-33.
Like other members of IL-1 family, IL-33 acts as a double-edged sword. Accumulating evidence shows that IL-33 has an important role during the development or exacerbation of airway inflammation. The direct administration of IL-33 to mouse lung induces IL-5-producing T cells, thus exacerbating allergen-induced airway inflammation [33]. In addition to the initiation of inflammation, IL-33 can effectively attenuate sepsis by mobilizing the innate cells, neutrophils, to the site of infection, helping to clear the pathogens [34]. The above evidence clearly shows that IL-33 has a dual role in different inflammatory disease processes. It is logical that IL-33 would exert these distinct functions depending on the immune mechanism underlying the pathogenesis of each disease condition. However, it is unknown whether this dual role of IL-33 exists in other skin diseases, such as atopic dermatitis. It has been shown that IL-33 mounts potent Th2 inflammatory responses and induces the degranulation of IgE-sensitized mast cells in skin, resulting in exacerbation of atopic dermatitis [35]. Usually, patients with atopic dermatitis exhibit greater colonization by S.aureus. Here, we show that S.aureus infection increases IL-33 expression in macrophages, and IL-33 in turn induces and activates iNOS to release NO, thus limiting bacterial growth and/or survival during skin infection. The different functions of IL-33 in mast cells and macrophages suggest that IL-33 may play a dual role during S.aureus infection in atopic dermatitis patients. Therefore, targeting IL-33 as an alternative treatment strategy for atopic dermatitis patients should be approached with caution. The key will be to evoke a reduction in the detrimental aspects of IL-33 without increasing the risk of infection in atopic dermatitis patients.
Nitric oxide, as a free radical gas molecule, exhibits potent antimicrobial activities against multiple bacteria including S.aureus [36], [37]. The administration of NO-donor compounds on S.aureus-infected soft tissue significantly inhibited the growth of S.aureus and effectively cleared infection in chronic wounds [36]. It is known that all major cell types in the skin, such as keratinocytes, fibroblasts, endothelial cells and macrophages, can produce NO under different stimuli [38], [39], [40]. Multiple cytokines, including IL-1β and IFNγ, have been shown to regulate iNOS activation to induce NO production; however, the administration of iNOS inhibitors exacerbates the overwhelming majority of infections [41], [42]. Here we observe that IL-33, a new member of the IL-1 family, stimulates NO production in macrophages via ST2-AKT-β-catenin-dependent iNOS activation, which is consistent with previous observations of endothelia NOS (eNOS) activation in endothelial cells [21], [43]. To our surprise, we have also observed that the induction of iNOS by IL-33 is dependent on IL-1R as well as ST2 in macrophages (Figure 6B and Figure S5C). One may assume that the deficiency of IL-1R impairs the function of its accessory protein (IL-1RAP), that forms a heterodimeric receptor complex with ST2 for IL-33 binding [9]. The other speculation is that IL-33 induces IL-1α and IL-1β (data not shown), and then IL-1α and IL-1β in turn activate IL-1R to induce iNOS in macrophages [20]. Furthermore, the release of NO by IL-33 is 10–60 µM (Figure 7B) while 30 µM NO in vitro inhibited around 50% of S.aureus growth (Figure 7A), suggesting that skin-derived NO induced by IL-33 might be sufficient to protect host against S.aureus infection. Moreover, treatment of macrophages with IL-33 leads to an increase in iNOS expression as well as NO production (Figure 5E–5H, and Figure 7B), while blockade of IL-33 by IL-33 neutralizing antibody or inhibition of iNOS with a specific inhibitor in vivo significantly prevents IL-33-induced bacterial clearance(Figure 7J–7L). Thus, the evidence presented here clearly shows that macrophage-derived NO induced by IL-33 plays a critical role in host innate defense against S.aureus infection. However, whether NO induced by IL-33 is biologically relevant or IL-33 activates other innate immune effectors to control S.aureus infection needs further investigation.
In conclusion, our study provides evidence that IL-33 is important to protect the host from bacterial skin infections, specifically S.aureus infection. IL-33 is an intermediate molecule embedded within the cross-talk between microbes and the host, and may function as a previously unknown key element in the treatment of S.aureus skin infection. Our findings implicate the potential of IL-33 as a therapeutic target in skin bacterial infections. The identification of IL-33 function in the skin provides new insights into pathways contributing to antimicrobial defense and may ultimately lead to the development of novel treatment regimens for skin infection and inflammation.
Materials and Methods
Human samples, mouse and bacterial strains
All human skin samples were obtained from 3 methicillin resistant S.aureus-infected adult patients (including one female and two male patients) in Changhai Hospital, Second Military Medical University, China. MRSA caused abscess in all these patients. 2 mm skin around lesional sites was taken for analyzing IL-33 expression and skin far from lesional sites was used as control. Due to the limited skin samples, protein from 3 patients was pooled together for western blot analysis. Tlr2−/− mouse breeding pair was purchased from the Jackson Laboratory (USA). C57BL/6 mice and age-matched Tlr2−/− mice were housed in the animal facility at East China Normal University. ST2−/− and IL1R−/− mice were housed in the animal facility at University of Orleans, France. Staphylococcus aureus CMCC(B)26003 with methicillin resistance was purchased from the National Center for Medical Culture Collections in China.
Ethics statement
All human sample acquisitions were approved by the ethical committee of Changhai Hospital, Second Military Medical University, China, and performed in accordance with the declaration of Helsinki Principles. All participants provided written informed consent which was obtained before enrolment in the study.
All animal experiments were performed according to the protocol approved by the East China Normal University (ECNU) Animal Care and Use Committee and in direct accordance with Ministry of Science and Technology of the People's Republic of China on Animal Care guidelines. The protocol was approved by ECNU Animal Care and Use Committee (Protocol ID: AR2012/12017). All surgeries were performed under anesthesia and all efforts were made to minimize suffering.
Cell culture and stimulation
RAW264.7 cells and mast cells (Chinese Academy of Sciences) were cultured in DMEM (Invitrogen) medium containing 10% FBS (GIBCO), 50 U.ml−1 penicillin and 50 ug.ml−1 streptomycin (GIBCO) under standard culture conditions. Neonatal human epidermal keratinocytes (Cascade biologics) were cultured as previously reported [44] and primary murine epidermal keratinocytes were isolated and cultured as our previous report [45]. Primary peritoneal neutrophils were isolated from six WT mice by mice by i.p. injection of 3 ml thioglycolate medium (Sigma). Cells were harvested 4 hours later by peritoneal lavage with cold PBS, followed by washing with RPMI1640 medium (GIBCO). Primary peritoneal macrophages were isolated from six to nine WT and Tlr2−/− mice by i.p. injection of 1 ml thioglycolate medium (Sigma). Cells were harvested 3 days later by peritoneal lavage with cold PBS, followed by washing with DMEM medium (GIBCO). After cultured in medium DMEM for 7 days, we did FACS analysis by using F4/80 antibody to determine the purity of primary peritoneal macrophages. Bone marrow-derived macrophages from 3 WT, ST2−/− and IL1R−/− mice were cultured as the standard protocol from Cold Spring Harbor Laboratory [46].
For all cell stimulation experiments, 2×105 cells were seeded in each well of 24-well or 8×105 cells were seeded in each well of 6-well plates. When cells were grown to 80% confluence, the indicated doses of heat-inactivated S.aureus, PGN (Sigma), LTA (InvivoGen), recombinant murine IL-33 (R&D) or human IL-33 (Sino Biological Inc) or different inhibitors under concentrations without cytotoxicity or low cytotoxicity (Figure S7A–S7D) were used to stimulate cells. After 24 hours treatment, cells were collected for RNA isolation or western blot.
shRNA preparation and targeting gene knockdown
Oligonucleotides encoding mouse IL-33 siRNA and ST2 shRNA were designed. Blast search was performed by using the National Center for Biotechnology Information (NCBI) database to ensure that siRNAs or shRNA constructs were targeting only mouse IL-33 and ST2, respectively. For ST2 shRNA constructs, the oligonucleotides were annealed and cloned into the pLL3.7 vector according to the manufacturer. 4 ug of pLL3.7 constructs containing shRNAs, 4 ug of packaging plasmid psPAX2 (Addgene), and 2 ug of envelope plasmid pMD2.G (Addgene) were used to transfected HEK293T cells by calcium phosphate precipitation method. 48 h later, lentiviruses containing targeted gene shRNA were collected and used to transfect macrophages. Or IL-33 siRNAs were directly transfected with macrophages by using GenEscort transfection reagent (Wisegen Biotechnology Corporation). The cytotoxicity of siRNA and shRNA in macrophages was tested by MTT assay (Figure S7E–S7H)
Real-time quantitative RT-PCR
Real-time RT-PCR specific primers were used to evaluate gene expression. RNA analysis was done as previously reported [45].
Fractionation of cytoplasmic and nuclear proteins
Cells were treated with IL-33 for indicated times and then lysed by a buffer (pH = 7.4) containing 10 mM HEPES, 10 mM NaCl, 1 mM KH2PO4, 5 mM NaCO3, 5 mM EDTA-2Na, 1 mM CaCl2, 0.5 mM MgCl2, 1 mM PMSF, 1 mM NaF and NaVO3. The lysate was collected and added with 2 M sucrose, then centrifuged at 6500×g for 15 minutes (4°C). The supernatant containing the cytoplasmic fraction was harvested and the pellet containing the nuclear fraction was resuspended in a buffer (pH = 7.4) containing 10 mM Tris, 300 mM sucrose, 1 mM EDTA, 0.1% NP-40. The protein concentration was determined by BCA Protein Assay Kit (Thermo) for immunoblotting.
Immunoblotting and immunofluorescent staining
2 mm mouse skin taken from the margin of murine wounds or cells stimulated as described was lysed by using RIPA buffer (pH 7.4) containing protease inhibitor cocktail (Roche). To concentrate IL-33 from cell culture medium, mouse IL-33 antibody (R&D) immunocomplexed to Protein A/G agarose (Abmart) was used to capture IL-33. 10 µg of total protein was used for Western blot. IL-33, iNOS, p38 MAPK, AKT and β-catenin were detected by immunoblot with IL-33 antibody (R&D), iNOS antibody (Abcam), p38 MAPK antibody (Cell Signaling), AKT antibody (Cell Signaling) and β-catenin antibody (Cell signaling), respectively.
5 µm of formalin-fixed, paraffin-embedded tissue sections was mounted on glass slides and used for immunohistochemistry and staining. 4% PFA was used to fixate the samples. After 10-min fixation and subsequent pretreated with antigen retrieval solution, the sections were stained with IL-33 (R&D) antibody or iNOS (Abcam) antibody and F4/80 antibody (Santa Cruz) or Ly6G/Gr antibody (Abcam). The sections were reprobed with anti-Goat IgG FITC (Jackson immunoresearch) or anti-Rat IgG TRITC conjugate antibody (KPL), and then mounted in ProLong Gold antifade reagent with DAPI (Invitrogen) and visualized them by the microscope (Leica).
Determination of the microbiocidal activity of acidified nitrite
The microbiocidal activity of acidified nitrite was determined as previous reported [47]. Briefly, potassium nitrite solutions were prepared with final concentrations of 0, 0.03, 0.1, 0.3, 1, 2.5, 5, 10, 20 µmol ml−1. S.aureus was adjusted to 1×106 CFU per microwell. The pH was adjusted to 4.5 by hydrochloric acidification. The microwells were filled in sequence with: 60 µl nitrite solution, 60 µl bacterial suspension and 120 µl acidified broth. Plates were covered with self-adhesive sterile Sealplates and incubated at 37°C for 5 hours. Bacteria from the microwells were plated on tryptic soy broth agar plates. The microbiocidal activity of acidified nitrite was determined by counting the bacterial colonies.
iNOS inhibition
Macrophages were pretreated with 0.36 mM aminoguanidine (AG) (Sigma) for 3 hours before PGN, LTA or IL-33 stimulation. The nitrite concentration in the culture supernatant was assayed by NO assay kit (Nanjing Jiancheng biotechnology Ltd. Co) according to the manufacturer's instructions.
In vitro killing assay
RAW264.7 cells were pretreated with 0.36 mM aminoguanidine (Sigma) for 3 hours before 30 ng ml−1 mIL-33 was added to stimulate cells for 20 hours. 106 CFU S.aureus was added and co-incubated with cells for another 5 hours at 37°C. Bacteria from cell culture medium and lysate were plated on tryptic soy broth agar. The next day, bacterial colonies were counted.
IL-33 neutralization in vivo
50 µg of monoclonal mouse IL-33 antibody (MBL) was intraperitoneally injected into each mouse 24 hours before infection. Next day, 50 µg of monoclonal mouse IL-33 antibody (MBL) was intradermally injected into mouse back skin. 4 hours later, 1–2×107 CFU S.aureus was interadermally injected into second IL-33-injected sites. 3 days later, skin around the lesions was taken for CFU assay.
Cutaneous infection in vivo
The backs of age-matched adults were shaved and hair was removed by using chemical depilation as previous described [45]. In the same day S.aureus was grown in Trypocase Soy Broth (TSB) for overnight. Next day, 1 mL of overnight culture of S.aureus was re-inoculated into 30 mL fresh TSB. When the bacterium grew to logarithmic phase (OD600 = 0.7–0.8), 50 µl of live S.aureus (1–2×107 CFU) was complexed to the same volume of cytodex beads (Sigma) as carrier and then intradermally injected into mouse back skin where the iNOS inhibitor aminoguanidine (100 mg per kg body weight) or the NO donor DETA - NONOate (10 mg per kg body weight) was intradermally injected one hour before. Mice were euthanized at the indicated timepoints. In some experiments, skin around the lesions was collected and homogenized in 1× PBS to determine the number of surviving S.aureus. In other experiments, RNA or protein from normal or infected skin was collected either for real-time RT-PCR or for western blot, or stored in 4% paraformaldehyde for immunostaining (Shanghai Sanjing Gongmao Ltd.Co).
Histopathological diagnose and detection of Staphylococcus aureus
Paraffin embedded skin sections were used by haematoxylin-eosin staining for the histopathological diagnosis, and modified Gram-staining [48] was used to detect S.aureus in infected lesions. This analysis was performed using conventional optical microscopy (Leica). The bacteria were characterized by coccoid characteristics.
Statistical analysis
All data are present as mean ± SEM. We used two-tailed t tests to determine significances between two groups. For multiple groups, we employed a one-way or two-way ANOVA with Bonferroni post test using GraphPad prism version 5. For all statistical tests, we considered P value<0.05 to be statistically significant.
Supporting Information
Zdroje
1. SchmitzJ, OwyangA, OldhamE, SongY, MurphyE, et al. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23 : 479–490.
2. CarriereV, RousselL, OrtegaN, LacorreDA, AmerichL, et al. (2007) IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A 104 : 282–287.
3. RousselL, ErardM, CayrolC, GirardJP (2008) Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep 9 : 1006–1012.
4. MoussionC, OrtegaN, GirardJP (2008) The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One 3: e3331.
5. CayrolC, GirardJP (2009) The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A 106 : 9021–9026.
6. LuthiAU, CullenSP, McNeelaEA, DuriezPJ, AfoninaIS, et al. (2009) Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31 : 84–98.
7. AliS, HuberM, KolleweC, BischoffSC, FalkW, et al. (2007) IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc Natl Acad Sci U S A 104 : 18660–18665.
8. AllakhverdiZ, SmithDE, ComeauMR, DelespesseG (2007) Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol 179 : 2051–2054.
9. LiewFY, PitmanNI, McInnesIB (2010) Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 10 : 103–110.
10. MirchandaniAS, SalmondRJ, LiewFY (2012) Interleukin-33 and the function of innate lymphoid cells. Trends Immunol 33 : 389–396.
11. YasudaK, MutoT, KawagoeT, MatsumotoM, SasakiY, et al. (2012) Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci U S A 109 : 3451–3456.
12. YinH, LiX, HuS, LiuT, YuanB, et al. (2013) IL-33 promotes Staphylococcus aureus-infected wound healing in mice. Int Immunopharmacol 17 : 432–438.
13. KlevensRM, MorrisonMA, NadleJ, PetitS, GershmanK, et al. (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298 : 1763–1771.
14. MillerLS, ChoJS (2011) Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 11 : 505–518.
15. ChoJS, PietrasEM, GarciaNC, RamosRI, FarzamDM, et al. (2010) IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 120 : 1762–1773.
16. OlaruF, JensenLE (2010) Staphylococcus aureus stimulates neutrophil targeting chemokine expression in keratinocytes through an autocrine IL-1alpha signaling loop. J Invest Dermatol 130 : 1866–1876.
17. KinoshitaM, MiyazakiH, OnoS, InatsuA, NakashimaH, et al. (2011) Enhancement of neutrophil function by interleukin-18 therapy protects burn-injured mice from methicillin-resistant Staphylococcus aureus. Infect Immun 79 : 2670–2680.
18. HumphreysNE, XuD, HepworthMR, LiewFY, GrencisRK (2008) IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol 180 : 2443–2449.
19. CorbettJA, McDanielML (1995) Intraislet release of interleukin 1 inhibits beta cell function by inducing beta cell expression of inducible nitric oxide synthase. J Exp Med 181 : 559–568.
20. KannoK, HirataY, ImaiT, IwashinaM, MarumoF (1994) Regulation of inducible nitric oxide synthase gene by interleukin-1 beta in rat vascular endothelial cells. Am J Physiol 267: H2318–2324.
21. ChoiYS, ChoiHJ, MinJK, PyunBJ, MaengYS, et al. (2009) Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood 114 : 3117–3126.
22. BeckermanKP, RogersHW, CorbettJA, SchreiberRD, McDanielML, et al. (1993) Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol 150 : 888–895.
23. GreenSJ, MeltzerMS, HibbsJBJr, NacyCA (1990) Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol 144 : 278–283.
24. OrmerodAD, ShahAA, LiH, BenjaminNB, FergusonGP, et al. (2011) An observational prospective study of topical acidified nitrite for killing methicillin-resistant Staphylococcus aureus (MRSA) in contaminated wounds. BMC Res Notes 4 : 458.
25. FosterTJ (2005) Immune evasion by staphylococci. Nat Rev Microbiol 3 : 948–958.
26. SilvaMT (2010) When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol 87 : 93–106.
27. BorregaardN, CowlandJB (1997) Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89 : 3503–3521.
28. SegalAW (2005) How neutrophils kill microbes. Annu Rev Immunol 23 : 197–223.
29. SilvaMT, Correia-NevesM (2012) Neutrophils and macrophages: the main partners of phagocyte cell systems. Front Immunol 3 : 174.
30. CartaS, LavieriR, RubartelliA (2013) Different Members of the IL-1 Family Come Out in Different Ways: DAMPs vs. Cytokines? Front Immunol 4 : 123.
31. KouzakiH, IijimaK, KobayashiT, O'GradySM, KitaH (2011) The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol 186 : 4375–4387.
32. LefrancaisE, RogaS, GautierV, Gonzalez-de-PeredoA, MonsarratB, et al. (2012) IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A 109 : 1673–1678.
33. Kurowska-StolarskaM, KewinP, MurphyG, RussoRC, StolarskiB, et al. (2008) IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol 181 : 4780–4790.
34. Alves-FilhoJC, SonegoF, SoutoFO, FreitasA, VerriWAJr, et al. (2010) Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med 16 : 708–712.
35. PushparajPN, TayHK, H'NgSC, PitmanN, XuD, et al. (2009) The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A 106 : 9773–9778.
36. GhaffariA, JaliliR, GhaffariM, MillerC, GhaharyA (2007) Efficacy of gaseous nitric oxide in the treatment of skin and soft tissue infections. Wound Repair Regen 15 : 368–377.
37. GhaffariA, MillerCC, McMullinB, GhaharyA (2006) Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide 14 : 21–29.
38. WangR, GhaharyA, ShenYJ, ScottPG, TredgetEE (1996) Human dermal fibroblasts produce nitric oxide and express both constitutive and inducible nitric oxide synthase isoforms. J Invest Dermatol 106 : 419–427.
39. MannickJB, AsanoK, IzumiK, KieffE, StamlerJS (1994) Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein-Barr virus reactivation. Cell 79 : 1137–1146.
40. XieQW, ChoHJ, CalaycayJ, MumfordRA, SwiderekKM, et al. (1992) Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256 : 225–228.
41. VouldoukisI, Riveros-MorenoV, DugasB, OuaazF, BecherelP, et al. (1995) The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the Fc epsilon RII/CD23 surface antigen. Proc Natl Acad Sci U S A 92 : 7804–7808.
42. MacMickingJ, XieQW, NathanC (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15 : 323–350.
43. GongK, ZhouF, HuangH, GongY, ZhangL (2012) Suppression of GSK3beta by ERK mediates lipopolysaccharide induced cell migration in macrophage through beta-catenin signaling. Protein Cell 3 : 762–768.
44. LaiY, Di NardoA, NakatsujiT, LeichtleA, YangY, et al. (2009) Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 15 : 1377–1382.
45. LaiY, LiD, LiC, MuehleisenB, RadekKA, et al. (2012) The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity 37 : 74–84.
46. WeischenfeldtJ, PorseB (2008) Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. CSH Protoc 2008 pdb prot5080.
47. DykhuizenRS, FrazerR, DuncanC, SmithCC, GoldenM, et al. (1996) Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense. Antimicrob Agents Chemother 40 : 1422–1425.
48. HuckerGJ (1921) A New Modification and Application of the Gram Stain. J Bacteriol 6 : 395–397.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic HelixČlánek Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron MobilizationČlánek AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for ImmunityČlánek Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden HamstersČlánek Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Viral Enhancer Mimicry of Host Innate-Immune Promoters
- The Epstein-Barr Virus-Encoded MicroRNA MiR-BART9 Promotes Tumor Metastasis by Targeting E-Cadherin in Nasopharyngeal Carcinoma
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- The Consequences of Reconfiguring the Ambisense S Genome Segment of Rift Valley Fever Virus on Viral Replication in Mammalian and Mosquito Cells and for Genome Packaging
- Substrate-Induced Unfolding of Protein Disulfide Isomerase Displaces the Cholera Toxin A1 Subunit from Its Holotoxin
- Male-Killing Induces Sex-Specific Cell Death via Host Apoptotic Pathway
- Highly Active Antiretroviral Therapies Are Effective against HIV-1 Cell-to-Cell Transmission
- The microRNAs in an Ancient Protist Repress the Variant-Specific Surface Protein Expression by Targeting the Entire Coding Sequence
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Type III Secretion Protein MxiI Is Recognized by Naip2 to Induce Nlrc4 Inflammasome Activation Independently of Pkcδ
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
- Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Strains
- Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic Helix
- Foxp3 Regulatory T Cells Delay Expulsion of Intestinal Nematodes by Suppression of IL-9-Driven Mast Cell Activation in BALB/c but Not in C57BL/6 Mice
- Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron Mobilization
- MicroRNA Editing Facilitates Immune Elimination of HCMV Infected Cells
- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Identification of Host-Targeted Small Molecules That Restrict Intracellular Growth
- A Cyclophilin Homology Domain-Independent Role for Nup358 in HIV-1 Infection
- Engagement of NKG2D on Bystander Memory CD8 T Cells Promotes Increased Immunopathology following Infection
- Suppression of RNA Silencing by a Plant DNA Virus Satellite Requires a Host Calmodulin-Like Protein to Repress Expression
- CIB1 Synergizes with EphrinA2 to Regulate Kaposi's Sarcoma-Associated Herpesvirus Macropinocytic Entry in Human Microvascular Dermal Endothelial Cells
- A Gammaherpesvirus Bcl-2 Ortholog Blocks B Cell Receptor-Mediated Apoptosis and Promotes the Survival of Developing B Cells
- Metabolic Reprogramming during Purine Stress in the Protozoan Pathogen
- The Post-transcriptional Regulator / Activates T3SS by Stabilizing the 5′ UTR of , the Master Regulator of Genes, in
- Tailored Immune Responses: Novel Effector Helper T Cell Subsets in Protective Immunity
- AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for Immunity
- Epstein-Barr Virus Large Tegument Protein BPLF1 Contributes to Innate Immune Evasion through Interference with Toll-Like Receptor Signaling
- The Major Cellular Sterol Regulatory Pathway Is Required for Andes Virus Infection
- Insights into the Initiation of JC Virus DNA Replication Derived from the Crystal Structure of the T-Antigen Origin Binding Domain
- Domain Shuffling in a Sensor Protein Contributed to the Evolution of Insect Pathogenicity in Plant-Beneficial
- Lectin-Like Bacteriocins from spp. Utilise D-Rhamnose Containing Lipopolysaccharide as a Cellular Receptor
- A Compositional Look at the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects
- Exploits Asparagine to Assimilate Nitrogen and Resist Acid Stress during Infection
- Interleukin-33 Increases Antibacterial Defense by Activation of Inducible Nitric Oxide Synthase in Skin
- Protective Vaccination against Papillomavirus-Induced Skin Tumors under Immunocompetent and Immunosuppressive Conditions: A Preclinical Study Using a Natural Outbred Animal Model
- Gem-Induced Cytoskeleton Remodeling Increases Cellular Migration of HTLV-1-Infected Cells, Formation of Infected-to-Target T-Cell Conjugates and Viral Transmission
- Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden Hamsters
- Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
- Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung
- Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response
- Human Genome-Wide RNAi Screen Identifies an Essential Role for Inositol Pyrophosphates in Type-I Interferon Response
- The Master Regulator of the Cellular Stress Response (HSF1) Is Critical for Orthopoxvirus Infection
- Code-Assisted Discovery of TAL Effector Targets in Bacterial Leaf Streak of Rice Reveals Contrast with Bacterial Blight and a Novel Susceptibility Gene
- Competitive and Cooperative Interactions Mediate RNA Transfer from Herpesvirus Saimiri ORF57 to the Mammalian Export Adaptor ALYREF
- The Type III Secretion Chaperone Slc1 Engages Multiple Early Effectors, Including TepP, a Tyrosine-phosphorylated Protein Required for the Recruitment of CrkI-II to Nascent Inclusions and Innate Immune Signaling
- Yeasts: How Many Species Infect Humans and Animals?
- Clustering of Pattern Recognition Receptors for Fungal Detection
- Distinct Antiviral Responses in Pluripotent versus Differentiated Cells
- Igniting the Fire: Virulence Factors in the Pathogenesis of Sepsis
- Inactivation of the Host Lipin Gene Accelerates RNA Virus Replication through Viral Exploitation of the Expanded Endoplasmic Reticulum Membrane
- Inducible Deletion of CD28 Prior to Secondary Infection Impairs Worm Expulsion and Recall of Protective Memory CD4 T Cell Responses
- Clonal Expansion during Infection Dynamics Reveals the Effect of Antibiotic Intervention
- The Secreted Triose Phosphate Isomerase of Is Required to Sustain Microfilaria Production
- Unifying Viral Genetics and Human Transportation Data to Predict the Global Transmission Dynamics of Human Influenza H3N2
- ‘Death and Axes’: Unexpected Ca Entry Phenologs Predict New Anti-schistosomal Agents
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



