-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIgniting the Fire: Virulence Factors in the Pathogenesis of Sepsis
article has not abstract
Published in the journal: . PLoS Pathog 10(2): e32767. doi:10.1371/journal.ppat.1003871
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003871Summary
article has not abstract
Sepsis is a devastating disease process characterized by a systemic inflammatory response in the host, evoked by a known or suspected pathogen. Staphylococcus aureus has emerged as a leading etiologic agent of sepsis, owing to its propensity to cause deep-seated tissue infection and bacteremia [1]. S. aureus harbors an arsenal of virulence factors to facilitate tissue adhesion, immune evasion, and host cell injury. In the bloodstream, these factors cause inflammation, impair immune cell function, alter coagulation, and compromise vascular integrity. This review will discuss key secreted and surface-anchored proteins required for S. aureus infection in the hostile host environment of the bloodstream, emphasizing mechanistic insights on virulence factor function that illustrate the complex nature of the host–pathogen interaction. While we currently lack a clear understanding of the temporal and spatial integration of these virulence factors in the bloodstream, it is apparent that S. aureus triggers pathophysiologic disturbances that are further amplified by the host inflammatory response, culminating in the severe clinical manifestations of sepsis and septic shock.
Inflammation: An Early S. aureus Insult in Sepsis
The clinical manifestations of sepsis span a continuum of severity, in the most extreme form termed “septic shock,” in which vascular insults and systemic inflammation lead to compromised cardiac function and blood pressure, culminating in impaired oxygen delivery to the tissues and organ failure. In the United States, ∼750,000 individuals suffer from sepsis per year, with mortality rates that approach or even exceed 50% in severe disease [2]. Multiple clinical trials aimed at curbing the host inflammatory response to severe infection have yielded limited clinical success [3]. The mainstay of therapy for sepsis and septic shock remains 2-fold: (1) rapid treatment of the underlying infection and (2) early resuscitation to blunt physiologic abnormalities that potentiate disease progression [4]. The nature of these beneficial interventions focuses attention on rigorously defining the inciting insult caused by the pathogen.
Multiple S. aureus proteins and cell wall components are pro-inflammatory, eliciting host responses similar to gram-negative lipopolysaccharide (LPS) [5]. The production of cytokines, including TNF-α and IL-6, results from the action of S. aureus lipoproteins on mononuclear phagocytes through TLR-2 pathway activation [6], [7]. Furthermore, bloodstream exposure of rat hosts to peptidoglycan and lipoteichoic acid leads to induction of IL-1 and IFN-γ [8]. Toxin-induced cellular injury also elicits prominent host inflammatory responses. In the bloodstream, circulating immune cells and the vascular endothelium are primary targets of staphylococcal virulence factors. Among the longest studied of these toxins are the staphylococcal superantigens that potently stimulate non-specific T-cell proliferation and activation and potentiate the host inflammatory response associated with sepsis [9]. A family of bi-component leukotoxins including Panton-Valentine Leukocidin (PVL), Leukocidin AB/GH (LukAB/GH), Leukocidin ED (LukED), and γ-hemolysin (Hlg) injure an array of leukocytes including neutrophils, mononuclear phagocytes, and T cells [10]. Also contributing to leukocyte injury is the family of cytolytic peptides termed phenol soluble modulins (PSMs) [11] and the small pore-forming toxin α-hemolysin (α-toxin, Hla) (Figure 1a) [12]. Genetic regulatory control that leads to increased production of PVL, PSMs, and α-toxin in highly virulent methicillin-resistant S. aureus (MRSA) strains has been described as a molecular mechanism that underlies increased disease severity observed upon infection with these isolates [13]–[15]. These virulence factors are potent stimulants of leukocyte inflammatory responses [10], [12], [16]. S. aureus mutants engineered to lack expression of even one of these toxins exhibit virulence defects in experimental infection [11], [17]–[19], suggesting that the collective impact of this group of toxins on inflammation and destabilization of the host during bloodstream infection is substantial.
Fig. 1. Overview of S. aureus virulence factors that contribute to the pathogenesis of sepsis. 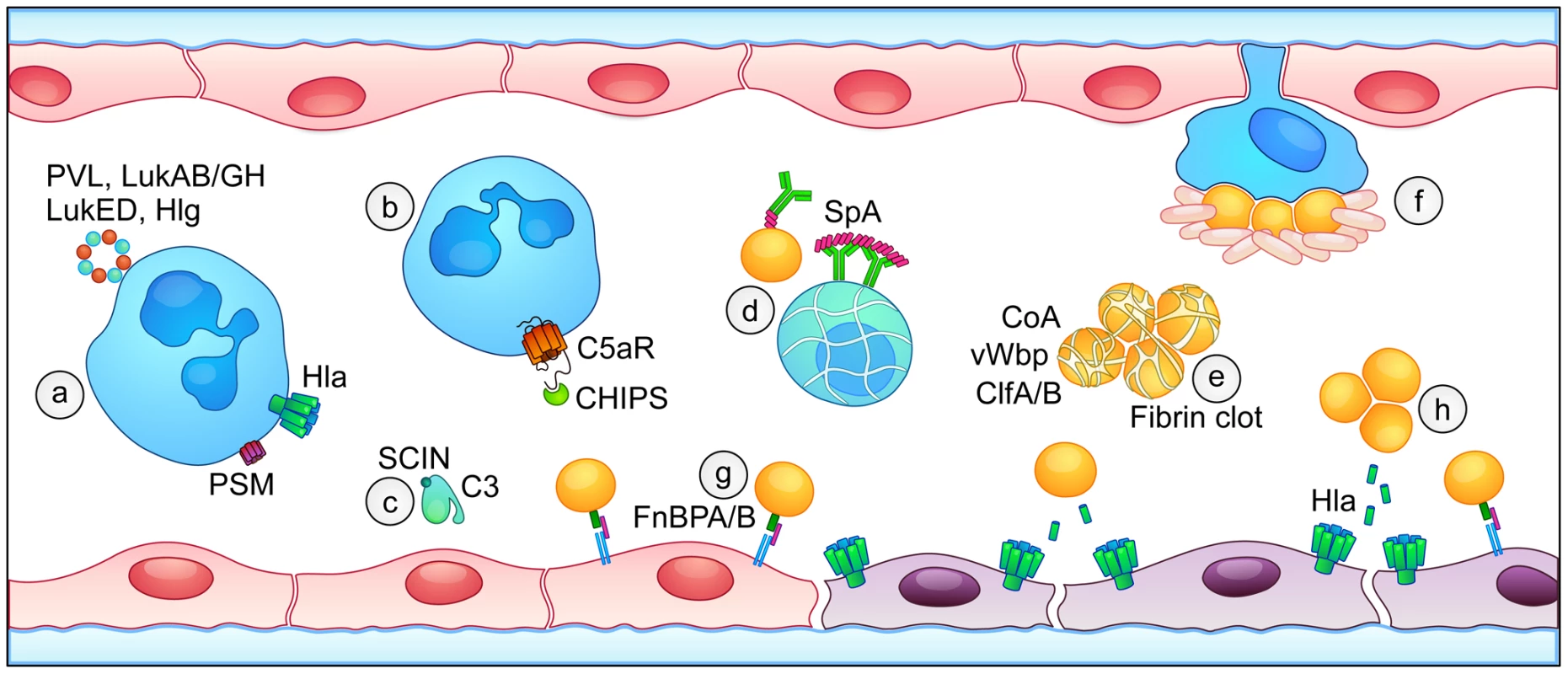
(a) Leukocytes are targeted and injured by bi-component leukocidins (PVL, LukAB/GH, LukED, and Hlg, blue and orange), phenol-soluble modulins (PSM, purple), and α-toxin (Hla, green). (b) Inhibition of host complement pathways occurs through Chemotaxis Inhibitory Protein of Staphylococci (CHIPS) binding to the C5a receptor and (c) Staphylococcal Complement Inhibitor (SCIN)-mediated blockade of C3 convertase activity. (d) Staphylococcal protein A (SpA) binds to host antibodies, preventing opsonophagocytosis and contributing to apoptotic death of B cells. (e) Coagulase (Coa) and von Willebrand factor binding protein (vWbp) initiate fibrin clot formation, facilitating the formation of staphylococcal aggregates in the blood through the action of clumping factors A and B (ClfA/B). (f) Platelet traps surround staphylococci that adhere to macrophage-like Kupffer cells in the liver sinusoid. (g) Fibronectin-binding proteins A and B (FnBPA/B) bind to integrin α5β1, enabling the tethering of S. aureus to endothelial cells in the context of blood flow. (h) Expression of S. aureus α-toxin (Hla) causes direct injury to the endothelium, disrupting the integrity of the endothelial barrier. Escape from Innate Immune Cells: A Key to Bloodstream Survival
In concert with direct leukocyte injury, S. aureus utilizes a number of strategies to modulate the innate host immune response and prevent bacterial clearance in the bloodstream [20]. Most clinical S. aureus isolates express a polysaccharide capsule that affords protection against phagocyte-mediated clearance; furthermore, the pathogen is able to resist killing in the phagocyte. S. aureus also secretes factors that function to dampen leukocyte recruitment or prevent opsonophagocytic uptake as a predecessor to intracellular killing. Two of the best-studied of these proteins are CHemotaxis Inhibitory Protein of Staphylococci (CHIPS) and Staphylococcal Complement INhibitor (SCIN). CHIPS binds to the cellular receptors for C5a and N-formyl peptides, diminishing the ability of both bacterial peptides and complement activation to function as leukocyte chemoattractants (Figure 1b) [21], [22]. SCIN potently inhibits human complement pathway defenses by blocking C3 convertase activity, reducing the deposition of the C3b opsonin on S. aureus and decreasing neutrophil uptake and killing [23] (Figure 1c). Both of these staphylococcal virulence factors display human specificity and are secreted during early bacterial growth to provide rapid evasion of innate immunity [24].
To limit the function of innate immune cells that successfully circumvent anti-chemotactic signals, S. aureus utilizes the cell-wall–anchored Staphylococcal protein A (SpA) to preclude antibody-driven opsonophagocytic clearance. SpA binds to the Fc and Fab regions of host antibodies [25], preventing staphylococcal antigen recognition and Fc-mediated effector functions. SpA also engages the B cell receptor and initiates activation-induced apoptotic death of VH3+ B cells (Figure 1d) [26]. S. aureus SpA mutants display virulence defects in an intraperitoneal model of lethal infection as well as intravenous infection that leads to arthritis and renal abscess formation [27]–[29]. Immunization with an inactive, “non-toxinogenic” SpA variant is protective in lethal S. aureus sepsis, promoting opsonophagocytic clearance in the blood [28]. In addition to these immunoevasion strategies and the broadly toxic effects of the array of staphylococcal toxins on host immune cells [10]–[12], recent studies indicate a prominent role for LukED in bloodstream infection by virtue of its ability to injure monocytes and lymphocytes, diminishing phagocytic uptake and promoting pathogen dissemination [18], [30]. Collectively, these bacterial defense mechanisms increase the burden of staphylococci in the blood, further compromising the septic host.
Modulation of Intravascular Coagulation: A Host–Pathogen Tug-of-War
Coagulopathy is another hallmark of septic shock, manifest as pathologic clotting within the microvasculature and a predisposition toward systemic bleeding [3]. S. aureus encodes virulence factors that modulate both soluble and cell-mediated pathways of coagulation. Physiologic coagulation in response to injury requires the rapid, localized activation of platelets, together with activation of the soluble clotting cascade. This serine protease-based cascade culminates in the enzymatic conversion of prothrombin to thrombin, in turn promoting the cleavage of fibrinogen to soluble fibrin monomers that polymerize into insoluble fibrin. Platelet-fibrin matrices form a physical substrate for plugging of the injured vasculature. While these processes are tightly regulated to ensure hemostasis yet avoid untoward intravascular thrombosis, bacterial virulence factors and the underlying host inflammatory state in sepsis induce pathologic alterations of coagulation. The staphylococcal virulence factors coagulase (Coa) and von Willebrand factor binding protein (vWbp) promote the non-catalytic activation of prothrombin, yielding cleavage of soluble fibrinogen to engender fibrin clot formation in the absence of an inciting injury [31], [32]. Fibrin clots promote clumping factor protein-mediated (ClfA and ClfB) aggregation of staphylococci (Figure 1e) [27]. Agglutination of S. aureus in the blood promotes bacterial survival, noted by the significant virulence defect in S. aureus strains that lack expression of Coa, vWbp, and ClfA [33]. In this context, survival is favored by protection of the aggregated organisms against phagocytic clearance. As activation of host coagulation pathways is proinflammatory [34], manipulation of this pathway by S. aureus likely contributes to the systemic inflammatory response.
Recent observations indicate the importance of thrombosis in immunodefense [35], demonstrating that platelets confer anti-staphylococcal immunity to bloodstream infection [36]. Intravital imaging revealed that platelets form aggregates around staphylococci adhered to macrophage-like Kupffer cells associated with the liver sinusoidal endothelium, entrapping S. aureus and facilitating pathogen clearance (Figure 1f) [36]. Suggesting the host-protective role of these traps, experimental platelet depletion leads to increased mortality from bloodstream infection [36]. While the role of platelets as innate immune cells has recently gained considerable attention, the modulation of normal platelet function by S. aureus has long been suggested by the ability of α-toxin to initiate platelet activation and aggregation [37]. α-toxin–induced platelet activation would thereby seem to promote the formation of platelet traps and support bacterial clearance—a paradoxical “anti-virulence” effect, highlighting the need to further investigate the precise molecular mechanisms by which staphylococcal virulence factors modulate platelet function in innate immunity.
The Microvascular Endothelium: A Site of Coordination?
Blood flow presents a major hurdle to both the pathogen and the host during intravascular infection. The ability of immune cells to identify and then contain S. aureus in the context of a branching vascular tree and the dilutional effects of blood flow is challenged. Conversely, the pathogen is challenged to (1) constrain the delivery of soluble virulence factors that initiate pathologic coagulation in flowing blood and (2) overcome fluid shear stress to adhere to the vascular wall and promote dissemination. Fibronectin-binding protein A (FnBPA) is a surface-displayed protein that facilitates endothelial adherence (Figure 1g) [38]. Variations in FnBPA underlie differences in fibronectin-binding affinity; high-affinity variants enhance endothelial cell binding and correlate with increased endothelial invasion in bloodstream infection [38]. The initial tethering of S. aureus to the endothelium may favor the establishment of a microenvironment in the small vessels or slow-flow vessels, such as liver sinusoids, in which S. aureus–induced coagulation and aggregate formation enhances the localized elaboration of virulence factors (Figure 1h) [39].
A primary molecular mechanism for bacterial-induced vascular permeability is endothelial disruption due to extreme inflammation [34]. In addition to its role in leukocyte and platelet injury, recent studies suggest that α-toxin co-opts the function of its cellular receptor A Disintegrin and Metalloprotease 10 (ADAM10) to disrupt the endothelial barrier by promoting the untimely cleavage of vascular endothelial (VE)-cadherin, destroying the inter-cellular junction that is required for vascular integrity [17]. As an intact endothelium forms the principal physical barrier to intravascular dissemination of bacterial pathogens, disruption of this barrier is expected to promote dissemination, a common and severe consequence of S. aureus sepsis. Damaged endothelium is also a potent stimulus for the rapid recruitment of platelets and activation of soluble clotting cascades. The microvascular endothelial surface may, thus, form a site wherein the pathogen, its armamentarium of virulence factors, platelets, leukocytes, and host coagulation proteins are co-localized. This “coordination” site may simultaneously trigger microvascular occlusion and increased vascular permeability—events that decrease effective blood flow to the tissues and precipitate sepsis-associated vital organ failure.
Towards the Future: Insights That May Change S. aureus Sepsis
The devastating mortality of sepsis and the inability of current clinical approaches to mitigate disease testify to our limited understanding of the complex host–pathogen interaction in the bloodstream. While the virulence factors discussed herein each contributes to sepsis pathogenesis, loss of any one factor is insufficient for complete protection against experimental S. aureus challenge. Similarly, vaccine approaches that target isolated virulence factors do not provide complete protection against lethal sepsis [40]. Together, these observations highlight our need to understand the temporospatial regulation of virulence factor expression and action in vivo. While exaggerated host inflammatory responses are associated with the progression of severe septic shock, insults directly delivered by S. aureus—coagulopathy, immune cell injury, microvascular occlusion, and barrier damage—collectively mirror disease endpoints in sepsis. These insults likely initiate the very pathophysiologic state that is then exacerbated by an untoward host response. While multiple blood-borne bacteria incite endothelial injury and lead to a coagulopathic state manifested as sepsis [41], whether a deliberate pathogen-driven coordination of these events occurs to promote virulence requires further study. The host–pathogen interaction in the bloodstream has proven extremely challenging to redirect in favor of the host, in spite of the commonality of these observed physiologic disturbances. The essential role of bacterial virulence factors as catalysts of sepsis, however, suggests that a keen focus on understanding how these factors are integrated in time and space within the vasculature should yield new insights for sepsis prevention and therapy in the coming years.
Zdroje
1. LowyFD (1998) Staphylococcus aureus infections. N Engl J Med 339 : 520–532.
2. AngusDC, Linde-ZwirbleWT, LidickerJ, ClermontG, CarcilloJ, et al. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29 : 1303–1310.
3. RussellJA (2006) Management of sepsis. N Engl J Med 355 : 1699–1713.
4. CannonCM, HolthausCV, ZubrowMT, PosaP, GunagaS, et al. (2012) The GENESIS Project (GENeralized Early Sepsis Intervention Strategies): A Multicenter Quality Improvement Collaborative. J Intensive Care Med 28 : 355–368.
5. SalomaoR, BrunialtiMK, RapozoMM, Baggio-ZappiaGL, GalanosC, et al. (2012) Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock 38 : 227–242.
6. HashimotoM, TawaratsumidaK, KariyaH, KiyoharaA, SudaY, et al. (2006) Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J Immunol 177 : 3162–3169.
7. Bubeck WardenburgJ, WilliamsWA, MissiakasD (2006) Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc Natl Acad Sci U S A 103 : 13831–13836.
8. De KimpeSJ, KengatharanM, ThiemermannC, VaneJR (1995) The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci U S A 92 : 10359–10363.
9. Salgado-PabonW, BreshearsL, SpauldingAR, MerrimanJA, StachCS, et al. (2013) Superantigens are critical for Staphylococcus aureus Infective endocarditis, sepsis, and acute kidney injury. MBio 4: e00494–13.
10. YoongP, TorresVJ (2013) The effects of Staphylococcus aureus leukotoxins on the host: cell lysis and beyond. Curr Opin Microbiol 16 : 63–69.
11. WangR, BraughtonKR, KretschmerD, BachT-HL, QueckSY, et al. (2007) Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nature Medicine 13 : 1510–1514.
12. BerubeBJ, Bubeck WardenburgJ (2013) Staphylococcus aureus alpha-Toxin: Nearly a Century of Intrigue. Toxins (Basel) 5 : 1140–1166.
13. LiM, DiepBA, VillaruzAE, BraughtonKR, JiangX, et al. (2009) Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 106 : 5883–5888.
14. ChuaKY, SeemannT, HarrisonPF, MonagleS, KormanTM, et al. (2011) The dominant Australian community-acquired methicillin-resistant Staphylococcus aureus clone ST93-IV [2B] is highly virulent and genetically distinct. PLoS One 6: e25887 doi:10.1371/journal.pone.0025887
15. DeLeoFR, KennedyAD, ChenL, Bubeck WardenburgJ, KobayashiSD, et al. (2011) Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci U S A 108 : 18091–18096.
16. MalachowaN, KobayashiSD, BraughtonKR, WhitneyAR, ParnellMJ, et al. (2012) Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis 206 : 1185–1193.
17. PowersME, KimHK, WangY, Bubeck WardenburgJ (2012) ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis 206 : 352–356.
18. AlonzoF3rd, BensonMA, ChenJ, NovickRP, ShopsinB, et al. (2012) Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol Microbiol 83 : 423–435.
19. DumontAL, NygaardTK, WatkinsRL, SmithA, KozhayaL, et al. (2011) Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol 79 : 814–825.
20. NizetV (2007) Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy Clin Immunol 120 : 13–22.
21. de HaasCJ, VeldkampKE, PeschelA, WeerkampF, Van WamelWJ, et al. (2004) Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199 : 687–695.
22. PostmaB, PoppelierMJ, van GalenJC, ProssnitzER, van StrijpJA, et al. (2004) Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J Immunol 172 : 6994–7001.
23. RooijakkersSH, RuykenM, RoosA, DahaMR, PresanisJS, et al. (2005) Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol 6 : 920–927.
24. RooijakkersSH, RuykenM, van RoonJ, van KesselKP, van StrijpJA, et al. (2006) Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell Microbiol 8 : 1282–1293.
25. LindmarkR, Thoren-TollingK, SjoquistJ (1983) Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J Immunol Methods 62 : 1–13.
26. GoodyearCS, SilvermanGJ (2004) Staphylococcal toxin induced preferential and prolonged in vivo deletion of innate-like B lymphocytes. Proc Natl Acad Sci U S A 101 : 11392–11397.
27. ChengAG, KimHK, BurtsML, KrauszT, SchneewindO, et al. (2009) Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. Faseb J 23 : 3393–3404.
28. KimHK, ChengAG, KimHY, MissiakasDM, SchneewindO (2010) Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med 207 : 1863–1870.
29. PalmqvistN, FosterT, TarkowskiA, JosefssonE (2002) Protein A is a virulence factor in Staphylococcus aureus arthritis and septic death. Microb Pathog 33 : 239–249.
30. Reyes-RoblesT, AlonzoF3rd, KozhayaL, LacyDB, UnutmazD, et al. (2013) Staphylococcus aureus Leukotoxin ED Targets the Chemokine Receptors CXCR1 and CXCR2 to Kill Leukocytes and Promote Infection. Cell Host Microbe 14 : 453–459.
31. PanizziP, FriedrichR, Fuentes-PriorP, RichterK, BockPE, et al. (2006) Fibrinogen substrate recognition by staphylocoagulase (pro)thrombin complexes. J Biol Chem 281 : 1179–1187.
32. BjerketorpJ, JacobssonK, FrykbergL (2004) The von Willebrand factor-binding protein (vWbp) of Staphylococcus aureus is a coagulase. FEMS Microbiol Lett 234 : 309–314.
33. McAdowM, KimHK, DedentAC, HendrickxAP, SchneewindO, et al. (2011) Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog 7: e1002307 doi:10.1371/journal.ppat.1002307
34. SchoutenM, WiersingaWJ, LeviM, van der PollT (2008) Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol 83 : 536–545.
35. EngelmannB, MassbergS (2013) Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 13 : 34–45.
36. WongCH, JenneCN, PetriB, ChrobokNL, KubesP (2013) Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol 14 : 785–792.
37. BhakdiS, MuhlyM, MannhardtU, HugoF, KlapettekK, et al. (1988) Staphylococcal alpha toxin promotes blood coagulation via attack on human platelets. J Exp Med 168 : 527–542.
38. EdwardsAM, PottsJR, JosefssonE, MasseyRC (2010) Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within FnBPA. PLoS Pathog 6: e1000964 doi:10.1371/journal.ppat.1000964
39. CarnesEC, LopezDM, DoneganNP, CheungA, GreshamH, et al. (2010) Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat Chem Biol 6 : 41–45.
40. BagnoliF, BertholetS, GrandiG (2012) Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol 2 : 16.
41. LemichezE, LecuitM, NassifX, BourdoulousS (2010) Breaking the wall: targeting of the endothelium by pathogenic bacteria. Nat Rev Microbiol 8 : 93–104.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic HelixČlánek Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron MobilizationČlánek AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for ImmunityČlánek Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden HamstersČlánek Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
-
Všechny články tohoto čísla
- Viral Enhancer Mimicry of Host Innate-Immune Promoters
- The Epstein-Barr Virus-Encoded MicroRNA MiR-BART9 Promotes Tumor Metastasis by Targeting E-Cadherin in Nasopharyngeal Carcinoma
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- The Consequences of Reconfiguring the Ambisense S Genome Segment of Rift Valley Fever Virus on Viral Replication in Mammalian and Mosquito Cells and for Genome Packaging
- Substrate-Induced Unfolding of Protein Disulfide Isomerase Displaces the Cholera Toxin A1 Subunit from Its Holotoxin
- Male-Killing Induces Sex-Specific Cell Death via Host Apoptotic Pathway
- Highly Active Antiretroviral Therapies Are Effective against HIV-1 Cell-to-Cell Transmission
- The microRNAs in an Ancient Protist Repress the Variant-Specific Surface Protein Expression by Targeting the Entire Coding Sequence
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Type III Secretion Protein MxiI Is Recognized by Naip2 to Induce Nlrc4 Inflammasome Activation Independently of Pkcδ
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
- Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Strains
- Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic Helix
- Foxp3 Regulatory T Cells Delay Expulsion of Intestinal Nematodes by Suppression of IL-9-Driven Mast Cell Activation in BALB/c but Not in C57BL/6 Mice
- Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron Mobilization
- MicroRNA Editing Facilitates Immune Elimination of HCMV Infected Cells
- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Identification of Host-Targeted Small Molecules That Restrict Intracellular Growth
- A Cyclophilin Homology Domain-Independent Role for Nup358 in HIV-1 Infection
- Engagement of NKG2D on Bystander Memory CD8 T Cells Promotes Increased Immunopathology following Infection
- Suppression of RNA Silencing by a Plant DNA Virus Satellite Requires a Host Calmodulin-Like Protein to Repress Expression
- CIB1 Synergizes with EphrinA2 to Regulate Kaposi's Sarcoma-Associated Herpesvirus Macropinocytic Entry in Human Microvascular Dermal Endothelial Cells
- A Gammaherpesvirus Bcl-2 Ortholog Blocks B Cell Receptor-Mediated Apoptosis and Promotes the Survival of Developing B Cells
- Metabolic Reprogramming during Purine Stress in the Protozoan Pathogen
- The Post-transcriptional Regulator / Activates T3SS by Stabilizing the 5′ UTR of , the Master Regulator of Genes, in
- Tailored Immune Responses: Novel Effector Helper T Cell Subsets in Protective Immunity
- AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for Immunity
- Epstein-Barr Virus Large Tegument Protein BPLF1 Contributes to Innate Immune Evasion through Interference with Toll-Like Receptor Signaling
- The Major Cellular Sterol Regulatory Pathway Is Required for Andes Virus Infection
- Insights into the Initiation of JC Virus DNA Replication Derived from the Crystal Structure of the T-Antigen Origin Binding Domain
- Domain Shuffling in a Sensor Protein Contributed to the Evolution of Insect Pathogenicity in Plant-Beneficial
- Lectin-Like Bacteriocins from spp. Utilise D-Rhamnose Containing Lipopolysaccharide as a Cellular Receptor
- A Compositional Look at the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects
- Exploits Asparagine to Assimilate Nitrogen and Resist Acid Stress during Infection
- Interleukin-33 Increases Antibacterial Defense by Activation of Inducible Nitric Oxide Synthase in Skin
- Protective Vaccination against Papillomavirus-Induced Skin Tumors under Immunocompetent and Immunosuppressive Conditions: A Preclinical Study Using a Natural Outbred Animal Model
- Gem-Induced Cytoskeleton Remodeling Increases Cellular Migration of HTLV-1-Infected Cells, Formation of Infected-to-Target T-Cell Conjugates and Viral Transmission
- Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden Hamsters
- Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
- Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung
- Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response
- Human Genome-Wide RNAi Screen Identifies an Essential Role for Inositol Pyrophosphates in Type-I Interferon Response
- The Master Regulator of the Cellular Stress Response (HSF1) Is Critical for Orthopoxvirus Infection
- Code-Assisted Discovery of TAL Effector Targets in Bacterial Leaf Streak of Rice Reveals Contrast with Bacterial Blight and a Novel Susceptibility Gene
- Competitive and Cooperative Interactions Mediate RNA Transfer from Herpesvirus Saimiri ORF57 to the Mammalian Export Adaptor ALYREF
- The Type III Secretion Chaperone Slc1 Engages Multiple Early Effectors, Including TepP, a Tyrosine-phosphorylated Protein Required for the Recruitment of CrkI-II to Nascent Inclusions and Innate Immune Signaling
- Yeasts: How Many Species Infect Humans and Animals?
- Clustering of Pattern Recognition Receptors for Fungal Detection
- Distinct Antiviral Responses in Pluripotent versus Differentiated Cells
- Igniting the Fire: Virulence Factors in the Pathogenesis of Sepsis
- Inactivation of the Host Lipin Gene Accelerates RNA Virus Replication through Viral Exploitation of the Expanded Endoplasmic Reticulum Membrane
- Inducible Deletion of CD28 Prior to Secondary Infection Impairs Worm Expulsion and Recall of Protective Memory CD4 T Cell Responses
- Clonal Expansion during Infection Dynamics Reveals the Effect of Antibiotic Intervention
- The Secreted Triose Phosphate Isomerase of Is Required to Sustain Microfilaria Production
- Unifying Viral Genetics and Human Transportation Data to Predict the Global Transmission Dynamics of Human Influenza H3N2
- ‘Death and Axes’: Unexpected Ca Entry Phenologs Predict New Anti-schistosomal Agents
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



