-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Secreted Triose Phosphate Isomerase of Is Required to Sustain Microfilaria Production
Human lymphatic filariasis is a major tropical disease transmitted through mosquito vectors which take up microfilarial larvae from the blood of infected subjects. Microfilariae are produced by long-lived adult parasites, which also release a suite of excretory-secretory products that have recently been subject to in-depth proteomic analysis. Surprisingly, the most abundant secreted protein of adult Brugia malayi is triose phosphate isomerase (TPI), a glycolytic enzyme usually associated with the cytosol. We now show that while TPI is a prominent target of the antibody response to infection, there is little antibody-mediated inhibition of catalytic activity by polyclonal sera. We generated a panel of twenty-three anti-TPI monoclonal antibodies and found only two were able to block TPI enzymatic activity. Immunisation of jirds with B. malayi TPI, or mice with the homologous protein from the rodent filaria Litomosoides sigmodontis, failed to induce neutralising antibodies or protective immunity. In contrast, passive transfer of neutralising monoclonal antibody to mice prior to implantation with adult B. malayi resulted in 60–70% reductions in microfilarial levels in vivo and both oocyte and microfilarial production by individual adult females. The loss of fecundity was accompanied by reduced IFNγ expression by CD4+ T cells and a higher proportion of macrophages at the site of infection. Thus, enzymatically active TPI plays an important role in the transmission cycle of B. malayi filarial parasites and is identified as a potential target for immunological and pharmacological intervention against filarial infections.
Published in the journal: . PLoS Pathog 10(2): e32767. doi:10.1371/journal.ppat.1003930
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003930Summary
Human lymphatic filariasis is a major tropical disease transmitted through mosquito vectors which take up microfilarial larvae from the blood of infected subjects. Microfilariae are produced by long-lived adult parasites, which also release a suite of excretory-secretory products that have recently been subject to in-depth proteomic analysis. Surprisingly, the most abundant secreted protein of adult Brugia malayi is triose phosphate isomerase (TPI), a glycolytic enzyme usually associated with the cytosol. We now show that while TPI is a prominent target of the antibody response to infection, there is little antibody-mediated inhibition of catalytic activity by polyclonal sera. We generated a panel of twenty-three anti-TPI monoclonal antibodies and found only two were able to block TPI enzymatic activity. Immunisation of jirds with B. malayi TPI, or mice with the homologous protein from the rodent filaria Litomosoides sigmodontis, failed to induce neutralising antibodies or protective immunity. In contrast, passive transfer of neutralising monoclonal antibody to mice prior to implantation with adult B. malayi resulted in 60–70% reductions in microfilarial levels in vivo and both oocyte and microfilarial production by individual adult females. The loss of fecundity was accompanied by reduced IFNγ expression by CD4+ T cells and a higher proportion of macrophages at the site of infection. Thus, enzymatically active TPI plays an important role in the transmission cycle of B. malayi filarial parasites and is identified as a potential target for immunological and pharmacological intervention against filarial infections.
Introduction
Continued survival of parasitic helminths within their mammalian host requires that they neutralise potentially protective immune responses, generate energy and reproduce. Filarial nematodes are particularly long-lived, tissue-dwelling parasites which evade immunity and maintain transmission over many years [1]. Over 100 million people are infected with lymphatic filariae, such as Brugia malayi, and no vaccine is available for human use [2], [3]. Transmission occurs when blood-borne microfilarial larvae are taken up by a mosquito vector, generating infective third-stage larvae which enter humans on a subsequent blood-meal. Hence, any immunological means of blocking microfilarial release would interrupt transmission.
As extracellular pathogens, the interaction of live parasites with both the host and each other is likely to occur through a combination of excretory/secretory (ES) products and surface molecules [4], [5]. Given the presumed involvement of ES molecules in a range of processes essential for successful parasitism, they represent attractive vaccine and drug targets. Because of this, we and others have taken a proteomic approach to characterise the complex mixture of proteins secreted by the human filarial nematode Brugia malayi (B. malayi ES, BES) [6]–[9]. This revealed that the most abundant ES protein of adult B. malayi is the glycolytic enzyme triose phosphate isomerase (Bm-TPI, EC 5.3.1.1), predominantly from female worms. Detailed analysis of the secretions of all life cycle stages has revealed that TPI is also released by moulting L3 larvae early in infection [8].
TPI catalyses the interconversion of the triose phosphates glyceraldehyde 3-phosphate and dihydroxyacetone phosphate, an essential step in glycolysis and gluconeogenesis [10]. Whilst TPI has been detected in the ES products of other worms, such as the cercariae and eggs of Schistosoma mansoni [11], [12] and adult Haemonchus contortus [13], the levels appear low compared to the large amounts released by adult B. malayi [6]–[8]. Furthermore, there is little or no secretion of other glycolytic enzymes, implying that TPI is selectively secreted through an active process, rather than simply leaching from compromised worms. This was supported by the demonstration that TPI is approximately 20-fold enriched in BES compared to a soluble extract of adult B. malayi [6].
However, it is unclear why Brugia and other nematodes secrete TPI given that glycolysis occurs in the cytosol. In this regard, several reports indicate that TPI is a multifunctional protein. For instance, TPI binds to the intracellular tail of the integrin aIIb in platelets and may regulate integrin signalling [14]. TPI can also function as an extracellular adherence molecule, and in this way mediates the interaction of the fungal pathogen Paracoccidiodes brasiliensis with both host epithelial cells and the extracellular matrix proteins laminin and fibronectin [15]. Similarly, surface associated TPI of Staphylococcus aureus contains a lectin activity that can bind fungal sugars and promote bacterial adherence to, and subsequent killing of, Cryptococcus neoformans [16], [17]. Additionally, studies of human TPI deficiency have shown that exogenous TPI can complement TPI-deficient cells, suggesting that the secreted enzyme may be taken up in a functional form by surrounding cells [18], [19].
Glycolytic enzymes, including TPI, have also been tested as targets of protective immunity. Thus, a neutralizing monoclonal antibody to S. mansoni TPI can confer up 40–50% reduction in worm burden in mice [20] and DNA vaccination with S. japonicum TPI reduces worm and egg burdens in experimentally infected pigs and water buffalo [21], [22]. Successful vaccination with schistosome TPI is consistent with its induction of IFNg, a cytokine associated with protective immunity against the larval schistosomula [23]. Even in the Th2-dominated environment that develops following schistosome egg production in mice, TPI preferentially stimulates Th1 cytokines [24]. S. mansoni TPI can also induce Th1 differentiation by T cells from unexposed humans [25]. In other helminth species, certain glycolytic enzymes have been similarly tested as vaccine candidates: for example, Onchocerca volvulus fructose 1,6-biphosphate aldolase is strongly recognised by antibodies from exposed but uninfected subjects, and can elicit a 50% reduction in larval survival in vaccinated mice [26]. However, studies on B. malayi have to date focussed solely on the biochemical properties of glycolytic enzymes with a view to development of new pharmacological inhibitors [27], [28].
Since glycolysis plays a key role in filarial worm energy metabolism [29]–[31], coupled with the unusually high level of secretion of TPI, we investigated the role of Bm-TPI in B. malayi infection. We confirmed that Bm-TPI is highly preferentially secreted, enzymatically active, and an antibody target in both infected mice and humans. Whilst vaccination with filarial TPI failed to confer protection against challenge infection with B. malayi or Litomosoides sigmodontis, antibody-mediated neutralisation of Bm-TPI shows it is required for the optimal survival of microfilariae within the mammalian host. As such, this parasite enzyme represents a novel and rational target for intervention by immunological or pharmacological means.
Results
Cloning and characterisation of recombinant Bm-TPI and Ls-TPI
To study the role of filarial triose phosphate isomerases, we first cloned the cDNA encoding this enzyme for expression as recombinant proteins. Full-length Bm-TPI cDNA was amplified by PCR from mixed adult B. malayi cDNA, cloned and confirmed as identical to the annotated B. malayi gene (Bm1_29130 [32]). The encoded 247-aa protein lacks a signal sequence, and has a predicted molecular weight of 27,097 Da. Sequence analysis shows a high degree of amino acid conservation with human (61% identity), S. mansoni (58%) and C. elegans (76%) proteins, including the AYEPVWAIGTG active loop and catalytic E165 (corresponding to E166 in human TPI; [33]), as well as the other active site residues, N10 (human N12), K12 (K14) and H94 (H96) (Fig. 1 A).
Fig. 1. Bm-TPI is conserved in sequence and enzymatic function. 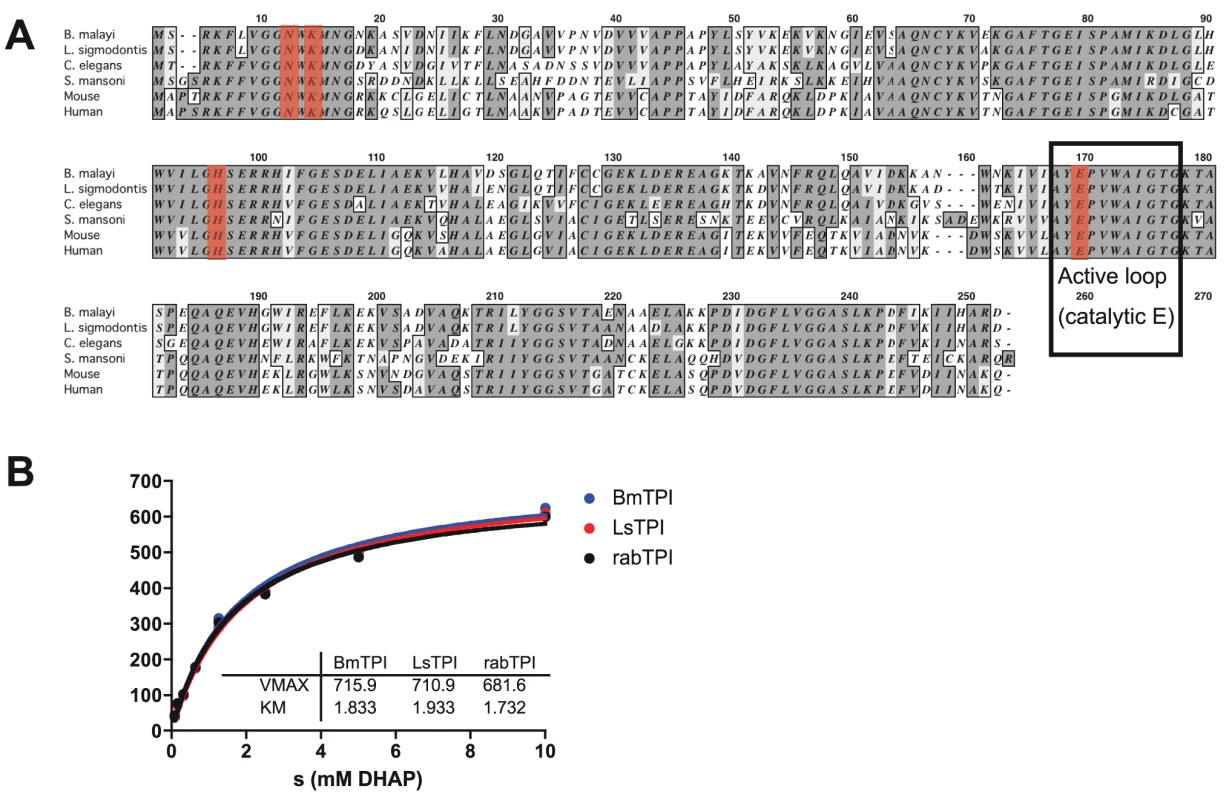
A. Amino acid alignment of Bm-TPI (XP_001897269) with Ls-TPI (Hx2000032586), Ce-TPI (NP_496563), Sm-TPI (P48501), mouse TPI (NP_033441) and human TPI (NP_000356). B. Michaelis-Menten kinetics comparing activity of recombinant Bm- and Ls-TPI with native rabbit TPI. Results are representative of multiple batches of enzymes. Recombinant Bm-TPI was expressed in bacteria and purified by nickel resin affinity chromatography, appearing as a single band of approx. 28 kDa by SDS-PAGE and a dominant molecular species by mass spectrometry of 28,030 (data not shown). Functional activity of recombinant Bm-TPI was confirmed by enzymatic assay, in which it displayed typical Michaelis-Menten kinetics indistinguishable from rabbit TPI with a Vmax of 715 U/mg and a Km of 1.8 mM (Fig. 1 B). The activity of Bm-TPI was compared to the homologous enzyme from the mouse filarial parasite Litomosoides sigmodontis [34]. Ls-TPI has 94% (233/247) amino acid identity to Bm-TPI (Fig. 1A), and following cloning of the corresponding cDNA and bacterial expression, recombinant protein showed similar enzyme kinetics to both Bm-TPI and rabbit TPI (Fig. 1 B).
Abundant expression of Bm-TPI
Previous proteomic studies have indicated Bm-TPI is amongst the most abundant proteins secreted by adult female B. malayi [6]–[8]. Preferential secretion was confirmed by Western blot using a polyclonal antiserum raised against rBm-TPI, which showed significant enrichment of Bm-TPI in BES compared to somatic extracts of adult worms, L3 larvae and microfilariae (Fig. 2 A). Native secreted Bm-TPI was shown to be enzymatically active by comparing BES with varying amounts of recombinant Bm-TPI. This revealed that each mg of BES had equivalent enzymatic activity to 370±86 ng recombinant protein (Fig. 2 B). Enzymatic activity in BES was abolished by heat denaturation (Fig. 2 B).
Fig. 2. Adult worms preferentially secrete enzymatically active Bm-TPI. 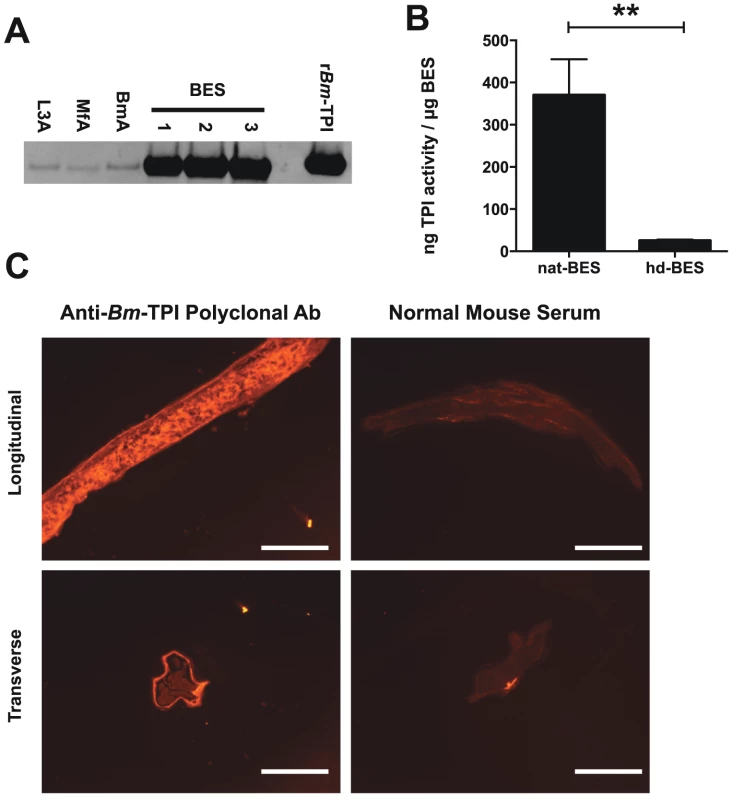
A. Western blot for Bm-TPI of 1 mg parasite extract (somatic extacts of L3 (L3A), Mf (MfA) and adult (BmA) or three independent batches of adult BES. Recombinant Bm-TPI included as a positive control. B. TPI activity in multiple independent batches of native BES or heat-denatured (hd)–BES. ** p<0.01 by t-test. C. Immunofluorescence of adult B. malayi female with polyclonal mouse anti-Bm-TPI (left panels) and control normal mouse serum (right panels) applied to longitudinal (Upper panels) and transverse (lower panels) sections. Scale bar represents 100 mm. Immunohistochemistry of sections of adult male and female B. malayi showed ubiquitous somatic expression pattern expected of a glycolytic enzyme, but provided no clues as to the source of secreted Bm-TPI by adult females (Fig. 2 C). Additionally, surface staining of intact whole worms was not seen, indicating that Bm-TPI is not shed from the cuticle of the parasite (data not shown).
Antibody recognition of Bm-TPI by infected subjects
An important question is whether the prominent expression of Bm-TPI results in a strong antibody response in infected patients. We analysed serum samples from a cohort of B. malayi-exposed residents of Rengat, Sumatra, Indonesia that were classified into presumed uninfected subjects (“endemic normals”), asymptomatic microfilarial carriers, and patients with chronic filarial pathology who are generally amicrofilaraemic [35], [36]. Individuals within each exposed group were found with positive IgG responses against Bm-TPI compared to sera from unexposed UK residents (Fig. 3 A). However, a greater proportion of infected individuals suffering from lymphatic pathology were seropositive (76%) compared to asymptomatic microfilaremics (48%) and endemic normals (42%), and the majority of strong responders were within the filarial pathology group. In contrast, no antibody reactivity was detected against mammalian TPI (using rabbit TPI which has 245/249 amino acid identity with the human protein) (Fig. 3 B). An isotype analysis of anti-Bm-TPI antibodies showed that reactivity was confined to the IgG1 and IgG4 isotypes (Fig. 3 C–F); notably IgG4 levels were higher to Bm-TPI in the pathology group, although the Mf+ individuals display far higher IgG4 levels to total B. malayi somatic antigens [35]. As we had previously detected little anti-Bm-TPI antibody reactivity using 2-D Western blots [6], the high level of reactivity found by ELISA indicated that the epitopes are predominantly conformational and denatured by SDS-PAGE electrophoresis, a conclusion supported by the lack of immunoreactivity in the vast majority of individuals to heat-treated Bm-TPI (Fig. S1 A–D).
Fig. 3. Anti-Bm-TPI antibody levels in B. malayi-infected human filariasis patients. 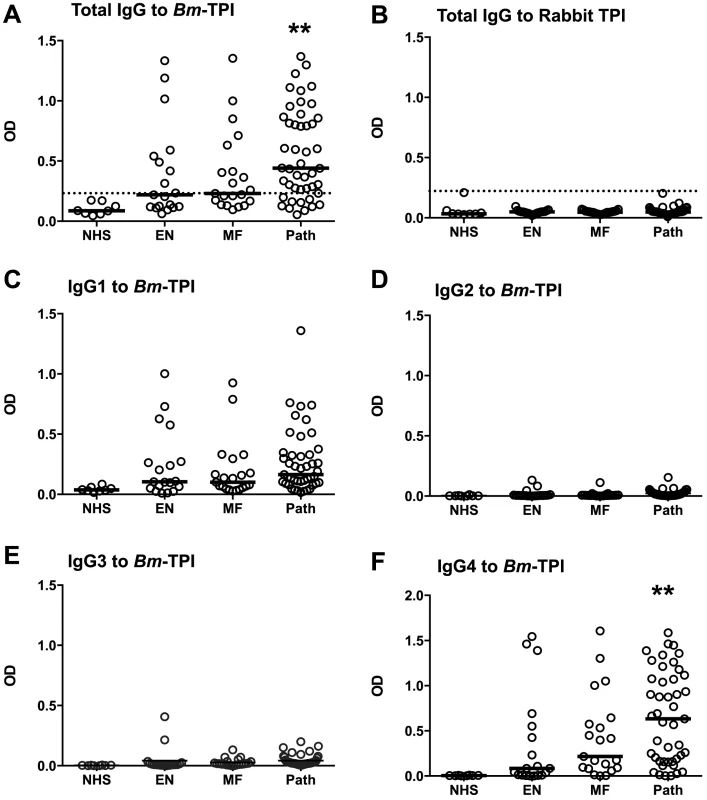
A. Human anti-Bm-TPI IgG levels measured by ELISA (OD values). Each data point represents an individual normal human serum (NHS) or serum from filariasis-exposed subjects classified as endemic normal (EN), microfilaraemic (MF) or chronic pathology (Path). B. IgG ELISA responses of the same patient groups to mammalian (rabbit) TPI. C–F. IgG1-IgG4 isotype-specific anti-Bm-TPI levels in the same patient groups. ** p<0.01, significance compared to NHS by ANOVA. Lines in (A–F) represent median values. Vaccination of rodents with filarial TPI does not confer protection against challenge infection
We next assessed whether vaccination of Mongolian jirds (Meriones unguiculatus), which are fully permissive to B. malayi infection [37], with Bm-TPI would generate protective immunity against challenge infection. In an initial experiment, animals were vaccinated three times with either Bm-TPI or control protein (BSA) in alum. Animals were infected intraperitoneally, with serum antibody titres and worm burdens being determined at 8 weeks post-challenge. All infected animals made strong IgG1 responses against a somatic extract of B. malayi adults (data not shown), but prior immunization with Bm-TPI induced >10 times higher IgG1 titres against this protein (Fig. 4 A). Despite this potent antibody response, and as shown in Fig. 4 B, there was no significant reduction in worm burdens at 8 weeks of infection in the Bm-TPI-immunized jirds (44±6.5, vs BSA, 55±7.0, p = 0.290). In a further experiment, we reasoned that a longer duration of infection might be required to see any protective effects induced by vaccination with a largely adult-specific secretory product. As such, jirds were immunised with Bm-TPI or BSA as before, challenged and assessed 21 weeks later. Vaccination again induced high titers of anti-TPI IgG1 (Fig. 4 C), but failed to provide any protection, and indeed both adult worm (Fig. 4 D) and peritoneal microfilariae numbers (Fig. 4 E) were slightly elevated compared to the BSA control.
Fig. 4. Vaccination with Bm-TPI does not curtail infection. 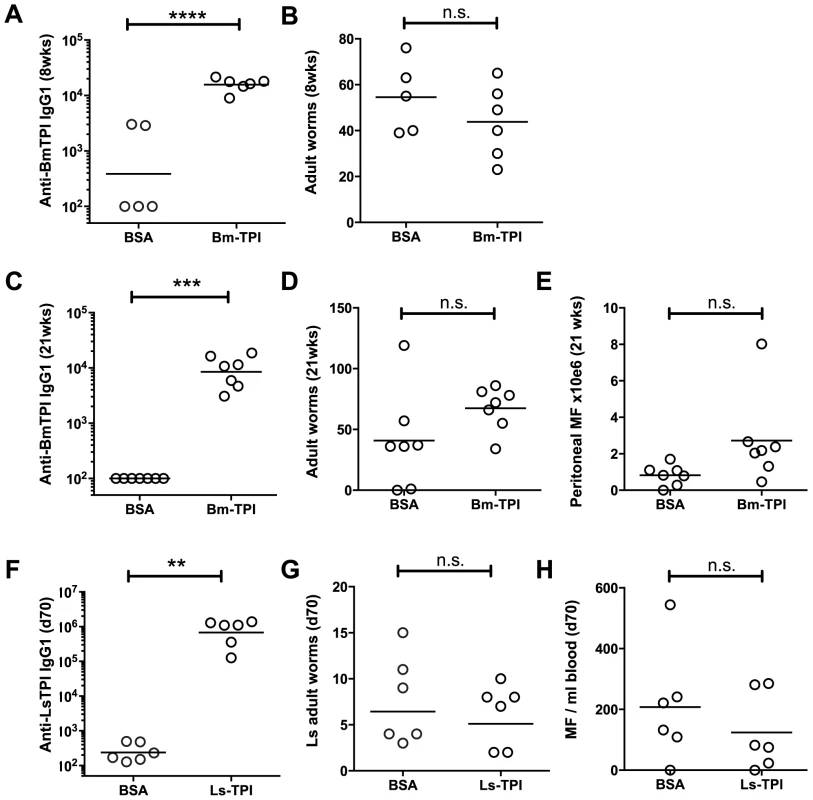
A. Immunisation induces high titers of week 8 post-challenge anti-Bm-TPI IgG1 antibodies in vaccinated jirds, compared to animals immunised with BSA control. B. Week 8 post-challenge adult B. malayi worm burdens in Bm-TPI and BSA vaccinated jirds. C. Anti-Bm-TPI IgG1 titers remain high by week 21 post-challenge in vaccinated jirds, compared to BSA control animals. D. Week 21 post-challenge adult B. malayi worm burdens in Bm-TPI and BSA vaccinated jirds. E. Peritoneal B. malayi microfilarial counts in jirds at week 21 post-infection previously vaccinated with BSA or Bm-TPI. F. Immunisation induces high titers of day 70 post-challenge anti-Ls-TPI IgG1 antibodies in vaccinated BALB/c mice, compared to animals immunised with BSA control. G. Day 70 post-challenge adult L. sigmodontis worm burdens in Ls-TPI and BSA vaccinated BALB/c mice. H. Day 70 post-challenge blood L. sigmodontis microfilarial counts in Ls-TPI and BSA vaccinated BALB/c mice. Dotted lines in A, C and F represent background antibody titers naïve jird or mouse sera. n.s. non-significant, ** p>0.01, *** p>0.001, **** p>0.0001, by t-test. The jird model is one that tests immunity of a rodent host to a human parasite, with parasites resident in the peritoneal cavity rather than their physiological niche of lymphatic vessels (for adult worms) and blood (for microfilariae). We therefore conducted a parallel test of protective capacity of TPI in a natural murine model of filarial infection, L. sigmodontis, which resides in the pleural cavity [34]. Mice were vaccinated three times with Ls-TPI in alum, and then challenged with L. sigmodontis L3. The results were similar to those observed with B. malayi in the jird: while specific anti-TPI antibodies were strongly boosted (Fig. 4 F), adult worm numbers were unchanged at day 70 post-challenge (Fig. 4 G) and when worm lengths were measured no differences were seen (data not shown). Moreover, circulating microfilarial numbers were not significantly diminished in immunized animals (Fig. 4 H).
Blockade of Bm-TPI does not impair parasite survival in vitro
One explanation for the poor level of protection induced by vaccination with Bm-TPI is the failure to generate high titres of neutralizing antibodies. Indeed, the sera from vaccinated jirds with high anti-Bm-TPI titres (Fig. 4 B) had limited ability to block Bm-TPI catalytic activity, leaving 75% of isomerase activity intact (Fig. 5 A). Similarly, polyclonal mouse serum raised to Bm-TPI effected only a slight reduction in enzyme activity (Fig. 5 B), whilst human sera from individuals strongly reactive to Bm-TPI (Fig. 3) failed to inhibit the enzyme at all (Fig. 5 C). This suggested that both immunisation and natural infection generated only limited amounts of anti-Bm-TPI antibodies directed at the active site. To confirm this, a panel of mouse monoclonal antibodies specific for Bm-TPI were generated. Only 2 of 23 (9%) anti-Bm-TPI clones were capable of blocking enzyme activity (Fig. 5 D), of which one anti-Bm-TPI mAb (clone 1.11.1, IgG1 isotype) was used in subsequent experiments.
Fig. 5. Generation of neutralizing antibodies to Bm-TPI. 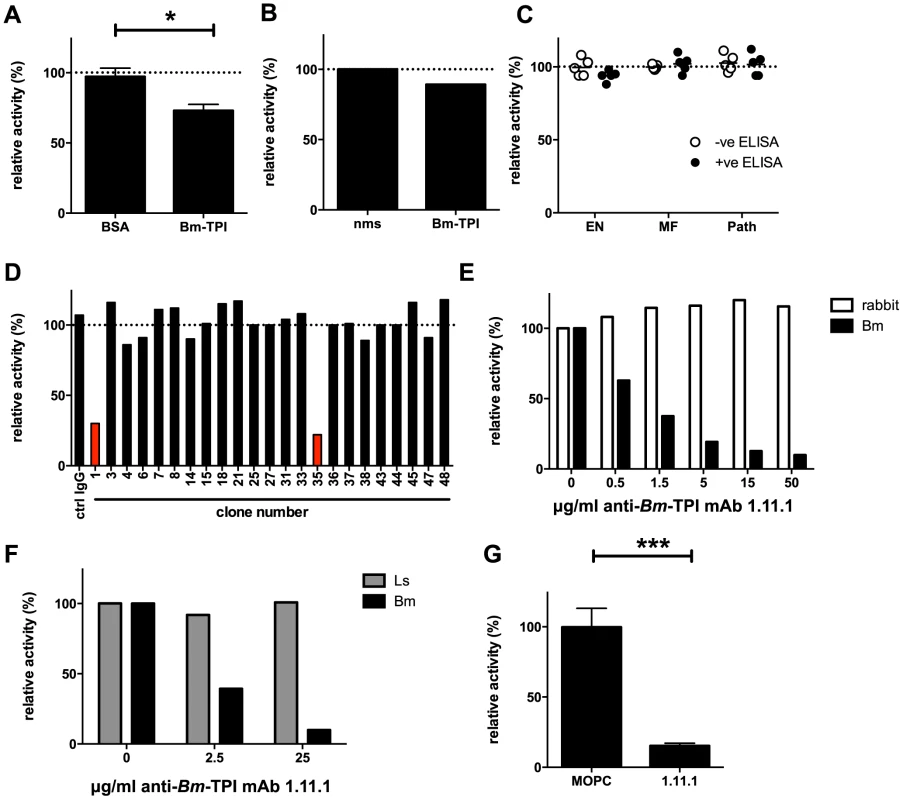
A. TPI enzyme activity in presence of 10% serum from jirds infected for 21 weeks with B. malayi following immunisation with BSA or Bm-TPI. * p<0.05 by t-test. B. TPI enzyme activity in presence of 10% polyclonal anti-Bm-TPI serum from BALB/c mice or naive mouse serum (nms). C. TPI enzyme activity in presence of 10% serum from human filariasis patients. Serum was used from the 5 strongest anti-Bm-TPI reactors (black circles) or 5 non-reactors (white circles) from each group (Fig. 3). D. Generation of antibody specificities that neutralise Bm-TPI activity is a relatively rare event. Ability of a panel of murine mAb specific for Bm-TPI (data not shown) to inhibit enzyme activity was determined. Clones with neutralising capacity are shown in red. E. Specificity of neutralizing mAb 1.11.1 for Bm-TPI (black bars) and not rabbit TPI (white bars). F. Specificity of neutralizing mAb 1.11.1 for Bm-TPI (black bars) and not Ls-TPI (grey bars). A, B, E and F are representative of multiple batches of recombinant enzyme. G. Neutralisation of native Bm-TPI activity in BES (4 independent batches) by mAb 1.11.1. MOPC31C IgG1 myeloma protein was used as a control. Dotted lines in (A–D) represent enzyme activity in the absence of serum or antibody (normalised to 100%).*** p<0.001 by t-test. MAb 1.11.1 was able to effectively block recombinant Bm-TPI enzymatic activity in a dose-dependent manner (Fig. 5 E), with a calculated Ki of <1 mg/ml for 100 ng Bm-TPI. In contrast, no blockade of either mammalian (Fig. 5 E), or perhaps surprisingly, L. sigmodontis TPI (Fig. 5 F) was seen. Most importantly, anti-Bm-TPI blocked native Bm-TPI as assessed by its ability to inhibit TPI activity present in BES (Fig. 5 G).
We then tested whether antibody inhibition of TPI enzymatic activity could cause parasite death in vitro. However, adult male and female B. malayi were able to survive in culture for sustained periods (≥3 days) in the presence of up to 500 mg/ml mAb clone 1.11.1 (data not shown). This suggested that whilst the antibody can inhibit secreted TPI, it cannot act directly on the parasite, and that in vitro worm survival over this period does not depend on TPI activity in the culture medium.
In vivo neutralization of Bm-TPI reduces microfilaraemia
Next, we tested whether MAb 1.11.1, with specific neutralizing ability, would alter the course of filarial infection in vivo. Mice were implanted intraperitoneally with 10 B. malayi adults (8 female, 2 male) and treated every 1–2 days with 200 mg anti-Bm-TPI mAb or IgG1 isotype control. Transfer of mAb 1.11.1 mAb established serum anti-Bm-TPI titres 35-fold greater than develop normally in response to infection, as seen in mice given the isotype control antibody (Fig. 6 A). Furthermore, transfer of 1.11.1 mAb conferred on recipient serum the ability to effectively block TPI enzymatic activity in vitro (Fig. 6 B). Despite this, live adult worms were recovered from the peritoneal cavities of both groups of infected mice after 28 days (Fig. 6 C) with no significant differences in male or female numbers (data not shown). In contrast, numbers of peritoneal microfilariae (Mf) were significantly reduced in anti-Bm-TPI-treated mice, being 69.5% lower than in animals given isotype control (Fig. 6 D) indicating that Bm-TPI activity is required for either the release or survival of live Mf.
Fig. 6. In vivo Bm-TPI blockade reduced microfilarial production by adult females. 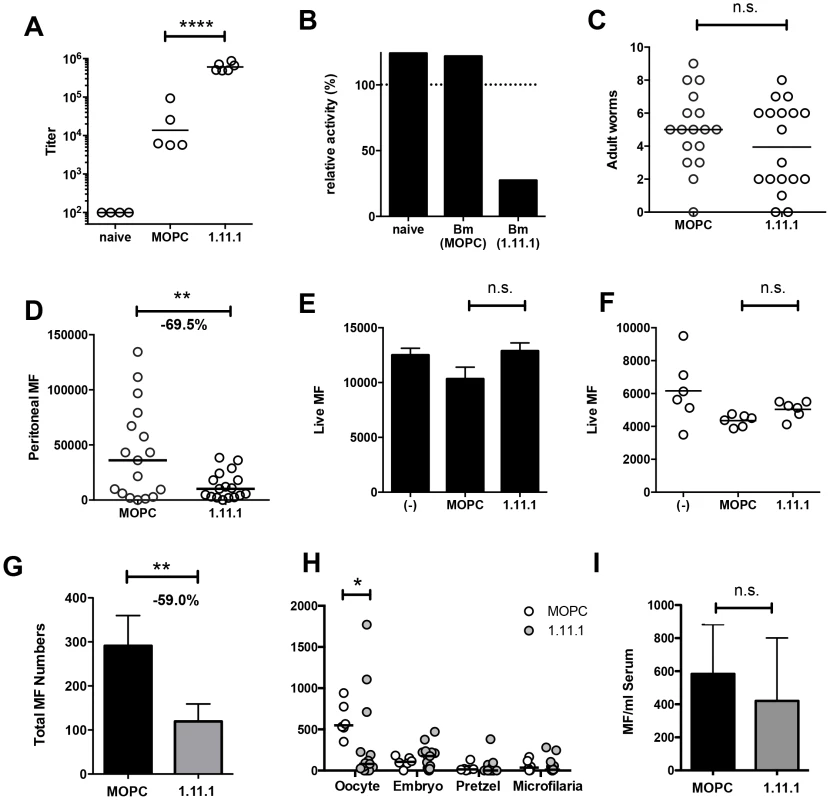
A. Serum titers of anti-Bm-TPI antibodies following multiple injections (16×) with 1.11.1 anti-Bm-TPI mAb or control IgG1 myeloma protein MOPC31C in mice 28 days following transplant of adult B. malayi worms. Representative of two experiments. **** p<0.0001 by ANOVA. B. Ability of serum from recipient mice to neutralize Bm-TPI activity. Sera were compared from naïve mice or day 28 post-Bm adult implant mice treated with MOPC or 1.11.1 anti-Bm-TPI mAb as (A). Dotted line indicates enzyme activity in absence of serum. C. Day 28 peritoneal worm burdens in recipients of MOPC31C or 1.11.1 anti-Bm-TPI Mab as (A). D. Day 28 peritoneal microfilarial counts in recipients of MOPC31C or 1.11.1 anti-Bm-TPI Mab as (A). Data in C and D are combined results from 3 independent experiments, with 5–6 mice per group. ** p<0.01 by t-test. E. Live MF numbers following 3 day in vitro culture alone, with MOPC31C or with 1.11.1. Initial MF input was 15,000 and data is from 3–4 wells per treatment, and is representative of two experiments. F. Live MF numbers recovered from individual female worms obtained from the peritoneal cavity of untreated jirds. Worms were cultured for 2 days alone, with MOPC31C or with 1.11.1, and is representative of two experiments. G. Live Mf numbers by individual female worms (2 day cultured) obtained from the peritoneal cavity of mice injected multiple times with MOPC31C or with 1.11.1. for 14 days. * p<0.05 by Mann-Whitney. Data are pooled from two independent experiments. H. Embryogram of uterine contents of individual adult female B. malayi parasites recovered from peritoneal cavity of mice (n = 4) injected multiple times for 14 days with MOPC31C or 1.11.1 anti-TPI antibody. I. Microfilarial numbers in blood 24 hours following i.v. transfer into mice receiving control MOPC31C and 1.11.1 anti-TPI antibodies. Using Mf obtained from non-immunised jirds, we next demonstrated that in vitro Bm-TPI blockade was unable to kill Mf (Fig. 6 E), indicating that the antibody is not directly toxic to Mf, and that neutralisation of TPI is not detrimental to this stage of the parasite. Likewise, in vitro Bm-TPI blockade did not reduce Mf production by adult females obtained from non-immunised jirds (Fig. 6 F). Instead, when we cultured adult females from the peritoneal cavity of mice following in vivo anti-Bm-TPI or isotype treatment, parasites from anti-Bm-TPI treated mice produced ∼60% fewer MF in vitro, consistent with Bm-TPI blockade compromising parasite fitness in terms of female reproductive output in vivo (Fig. 6 G). In particular, a much greater proportion of adult female worms from anti-TPI-treated jirds completely failed to release live Mf during in vitro culture (45.2% compared to 12.5% in isotype-treated controls).
Analysis of uterine contents from individual female B. malayi revealed that TPI blockade led to a significant decrease in the number of unfertilised oocytes (Fig. 6 H). Whilst oocytes were the predominant developmental stage in females obtained from isotype treated mice (Fig. S2 A), anti-Bm-TPI treatment led to the accumulation of smaller developmental stages that appeared damaged or partially degraded (Fig. S2 B). In contrast, no significant difference was observed in the number of Mf transferred directly into antibody-treated mice (Fig. 6 I) again implying that TPI blockade targets female egg production, thereby reducing subsequent release of live Mf.
Macrophage expansion in TPI-neutralised mice
To establish if the stronger immunity in the TPI-neutralised setting is associated with changes in immune cell types, we studied the frequency and phenotype of key cell populations in B. malayi-implanted mice. Administration of 1.11.1 did not affect overall cell recruitment to the peritoneal cavity infection site (Fig. 7 A), but TPI blockade resulted in a greater proportion of CD11b+F4/80+ macrophages within this compartment (Fig. 7 B). Analysis of markers of alternative activation of macrophages showed that Chi3L3 (Ym1) and RELMα expression was uniformly high in CD11b+ cells from all infected mice, irrespective of treatment (Fig. 7 C), indicating alterations in quantitative expansion rather than the qualitative nature of the macrophage population. In contrast to increased frequencies of macrophages, we also found reduced eosinophilia in the anti-TPI-treated mice (Fig. 7 D).
Fig. 7. Altered T cell, eosinophil and macrophage responses in mice receiving neutralising anti-Bm-TPI monoclonal antibody. 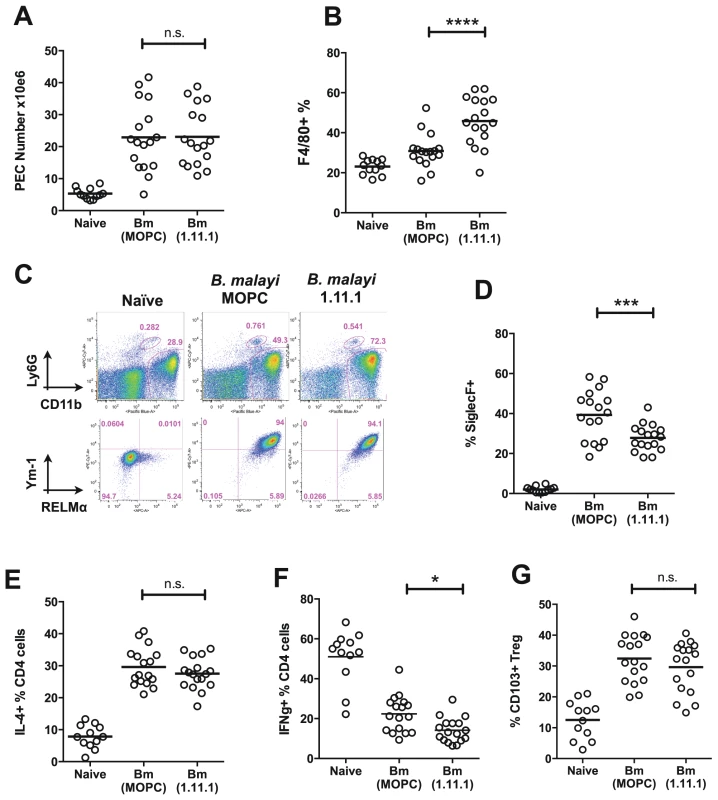
A. Peritoneal cell recruitment in recipients of 1.11.1 anti-Bm-TPI monoclonal antibody or control IgG1 myeloma protein MOPC31C, in mice 28 days following transplant of adult B. malayi worms. B. Peritoneal CD11b+ F4/80+ macrophages as in (A). C. Intracellular expression of Ym-1 and RELMa by peritoneal macrophages (CD11b+ F4/80+ Ly6G− siglecF−) in mice 14 days following transplant of adult B. malayi parasites with 1.11.1 anti-Bm-TPI monoclonal antibody or control IgG1 myeloma protein MOPC31C. Representative of 4 mice per group. D. Peritoneal CD11b+ siglecF+ cells as in (A). E. Intracellular IL-4 production by CD4+ peritoneal T cells as in (A) F. Intracellular IFN-γ production by CD4+ peritoneal T cells as in (A). G. Frequency of CD103+ expression among Foxp3+ Tregs as in (A). Data in (A–B, D–G) are pooled from 4 independent experiments. Within the CD4+ T cell subset, Th2 responses measured by total IL-4-expressing CD4+ T cells, also showed little difference in antibody recipients (Fig. 7 E), but there was a significant reduction in IFN-γ expression, indicating diminished Th1 responsiveness in the absence of TPI enzymatic activity (Fig. 7 F). To test whether Bm-TPI directly stimulates the expansion of Th1 cells, transgenic DO11.10 ovalbumin-specific T cells were co-cultured with ovalbumin peptide and TPI, or co-transferred to naïve mice together with ovalbumin antigen and TPI i.p. However, cytokine responses in TPI recipients were unaltered both in vitro and in vivo (Fig. S3) arguing that the effects of TPI on the effector T cell compartment occur in the setting of an active parasite infection. Finally, we examined the activation of Foxp3-expressing regulatory T cells in mice receiving B. malayi implants which has been previously reported, including the induction of CD103 expression [38]. Frequencies of Foxp3+ Tregs expressing the marker CD103 were elevated in all infected mice irrespective of 1.11.1 administration (Fig. 7 G).
Discussion
Parasite mediated-immunomodulation relies on products secreted into the environment of the pathogen in vivo, and the analysis of in vitro released “excretory-secretory” (ES) proteins has provided an approximation of the spectrum of released macromolecular components. We and others have identified Bm-TPI as a dominant product secreted by adult B. malayi worms [6]–[9], and here confirm not only the preferential secretion of this enzyme by live adult worms in vitro, but show, by its antigenicity in infected mice and humans, that it is exposed to the immune system in vivo. How TPI is secreted, in the absence of a signal peptide, remains unclear and its ubiquitous expression throughout the somatic tissues of B. malayi does not provide any pointers to a particular route of secretion. Possibly, as secretion is far higher in female worms than males [7], TPI could be released along with microfilariae from the female genital tract.
Filarial TPIs, from both B. malayi and L. sigmodontis, are active enzymes with catalytic properties very similar to those of mammalian homologues. Immunologically, however, TPIs from mammals and nematodes are non-cross-reactive, and indeed we generated monoclonal antibodies capable of distinguishing between the two filarial enzymes. Hence, in human infections anti-Bm-TPI antibodies were not found to be auto-reactive with self TPI, suggesting that the pathologies of lymphatic inflammation, edema and fever are not linked to an autoimmune reaction against human TPII. In this respect, filarial infection differs from that of Trypanosoma cruzi, which induces auto-antibody production against host TPI [39].
When comparing the levels of anti-Bm-TPI between infected individuals we noted that titres are greater in cases of filarial pathology in whom circulating microfilariae are generally absent. Because of this, we tested the potential of Bm-TPI to provoke protective immunity against B. malayi in animal models. In the jird, M. unguiculatus, which is fully susceptible to infection with the mosquito-borne L3 stage, both total worm and Mf numbers were unchanged in vaccinated animals following peritoneal infection. However, vaccination did not generate high levels of neutralizing antibody in terms of the catalytic activity of the enzyme, which may be more efficient than removal of TPI by complexing and opsonization. By screening a large panel of murine monoclonals, we selected a neutralizing antibody that conferred, in a mouse peritoneal implantation model, immunity against the Mf stage.
We also noted that TPI blockade inhibits the development of eggs within the adult female worm, reflecting a loss of fitness in the parasite that may have either or both a metabolic or immunological cause. Several other regimes have been shown to limit the release of viable microfilariae by filarial parasites. In this regard, antibiotic-mediated depletion of endosymbiotic Wolbachia causes degeneration of B. malayi oocytes, embryos and microfilariae [40], [41], although in this setting parasite metabolism is likely to be more heavily compromised. An immunological cause of reduced parasite fecundity can also be illustrated in mice immunised with L. sigmodontis Mf, >70% of which fail to develop microfilaraemia, due to an inhibition of embryogenesis to the pretzel stage [42].
The selective effect on microfilarial levels recapitulates a consistent, but unexplained, feature of filarial nematode infections. In both humans and animal models, cryptic amicrofilaraemic infections can occur in which immunity appears to operate only against the microfilarial stage. For example, cats infected with B. pahangi often became Mf-negative and yet remained seropositive for circulating filarial antigen and were found to harbour live adult worms at autopsy [43]. Similarly, circulating antigen tests in humans identify a significant proportion of Mf-negative infected subjects. However, in the majority of cases, it appears that natural infection does not generate blocking antibody to TPI suggesting the possibility that the active site has in some manner evolved to minimise stimulation of neutralising antibody. The surprising finding that such antibodies do not cross-react between the highly conserved TPIs from two related filarial species reflects a further unusual property of these proteins.
To establish whether TPI enzyme activity is essential for its extracellular function, and whether only blocking antibodies can be protective, it would be desirable to test multiple panels of monoclonals and to conduct experiments with pharmacological inhibitors of filarial TPI. Future studies may also develop blocking antibodies against L. sigmodontis TPI which would permit the analysis of the biological role of TPI during the full course of infection in mice, as the murine antibodies we describe here cannot be administered to other rodent species for extended periods of time.
In addition, species-specific chemical TPI inhibitors have been described which target non-conserved amino acids, particularly at the dimer interface e.g. Trypanosoma cruzi [44], Trypanosoma brucei [45], Plasmodium falciparum [46] and Giardia lamblia [47]. Small molecule inhibitors may be superior to antibody-mediated blocking as they offer the advantage of penetration into the parasite itself, rather than just inhibition of secreted form, and are likely to be more effective against the adult worms which, as we show, survive even in the face of very high neutralising Ab titres.
The in vivo consequences of TPI neutralisation may link poor microfilarial survival with shifts in the anti-parasite immune response. Thus, the reduction in IFN-g production by peritoneal cavity CD4+ T cells may be an indirect effect of diminished numbers of microfilariae, as this stage (unusually) induces Th1 responsiveness [48]–[50]. Similarly, the reduced eosinophilia could reflect weaker stimulation from the microfilarial stage. Interestingly, this argument would suggest that microfilariae normally dampen macrophage expansion in vivo, a point which has yet to be experimentally investigated.
The finding that TPI is also secreted by plant-parasitic nematodes [51], and the importance of glycolysis in adult B. malayi [31], are consistent with TPI release facilitating adult worm metabolism and being required for optimal fecundity and Mf production in vivo. It is also possible that the heightened frequency of alternatively-activated macrophages imposes immunological damage on adult worms, thereby reducing their ability to reproduce, and such damage may of course be more easily achieved if the target is also metabolically compromised. In any event, our data present a remarkable immunological strategy for transmission in the filarial nematode: adult females release TPI which promotes oogenesis, and so increases the number of their offspring. Hence, in the mouse model at least, TPI neutralisation inhibits this process and reduces the microfilarial burden.
Materials and Methods
Animals, human samples, parasites and production of BES
BALB/c mice and Meriones unguiculatus jirds were bred in-house. B. malayi (obtained originally from TRS Laboratories, Athens, Georgia, USA) was maintained in Aedes aegypti mosquitos and jirds. Infective larvae (L3) were recovered from mosquitos 12–14 days following feeding on microfiariae - (Mf-) containing blood, as detailed previously [52]. Jirds were infected with up to 600 L3; adult worms and microfilariae were recovered from the peritoneum approximately 4 months later. The Litomosoides sigmodontis life cycle was maintained, and L3 larvae or adult worms recovered, as described previously [53]. Somatic extracts of worms (from adults, BmA; from L3, L3A and from Mf, MfA) and BES were produced as previously described [6].
Ethics statement
All animal protocols adhered to the guidelines of the UK Home Office, complied with the Animals (Scientific Procedures) Act 1986, were approved by the University of Edinburgh Ethical Review Committee, and were performed under the authority of the UK Home Office Project Licence number 60/4105. Human serum samples were taken from archived stocks derived from a study in Rengat, Indonesia that has been previously described [54] and in which informed consent was obtained from all patients before clinical and parasitologic investigation and blood withdrawal in accordance with the guidelines of the Indonesian Department of Health and Human Services.
Cloning and recombinant expression of Bm-TPI and Ls-TPI
Total RNA was extracted from adult mixed sex B. malayi and L. sigmodontis using TRIzol (Invitrogen), and reverse transcribed with MMLV reverse transcriptase (Stratagene) using standard protocols. A partial sequence (nt 1–664) for Ls-TPI (LS00587) was obtained from NEMBASE v4 (http://www.nematodes.org/nembase4/index.shtml). The missing 3′ end was obtained by 3′ RACE using Invitrogen Gene Racer Core kit with RACE-ready cDNA and the forward primer ATG TCT CGA AAG TTT CTA GTT as previously described [55]. The resultant full-length nucleotide sequence has been submitted to the European Nucleotide Archive with the Accession Number HG329626. The following PCR primers were used for amplification; Bm-TPI forward primer CAT ATG TCG CGA AAA TTT CTT, Ls-TPI forward primer CAT ATG TCT CGA AAG TTT CTA GTT, Bm-TPI and Ls-TPI reverse primer CTC GAG ATC ACG TGC ATG AAT AAT TT (restriction sites are underlined). PCR conditions were as follows: 35 cycles of 95°C 30 sec, 60°C 30 sec, and 72°C 2 min. Reaction products were separated on 1% agarose gels, visualised using ethidium bromide, and the 750-bp amplicons excised and purified (QIAquick gel extraction, Qiagen). PCR products were cloned into pGEM-T vector (Promega) and transformed into E. coli JM109 (Promega) for overnight colony formation. Minipreps from positive colonies were sequenced. An internal Nde1 site was removed in both Bm-TPI and Ls-TPI by PCR-based site-directed mutagenesis (CATATG replaced with CATACG, a synonymous mutation, in-frame codon underlined), using 50 ng of parental plasmid, Deep Vent DNA polymerase (New England Biolabs) and the following PCR primers : Bm-TPI forward primer CCT TAT TTA TCA TAC GTT AAG GAG AAA, Bm-TPI reverse primer TTT CTC CTT AAC GTA TGA TAA ATA AGG, Ls-TPI forward primer CCA TAT TTG TCA TAC GTC AAG GAA AAA GTT, Ls-TPI reverse primer AAC TTT TTC CTT GAC GTA TGA CAA ATA TGG. PCR conditions were as follows: 18 cycles of 95°C 30 sec, 55°C 1 min, and 75°C 8 min. Reaction products were digested with Dpn1 (New England Biolabs) for 2 hours at 37°C to remove parental plasmid, purified as for plasmid preps, and then used to transform E. coli JM109, and grown as before. Coding sequences for Bm-TPI and Ls-TPI were ligated into linearised pET29c (Novagen) following digestion with Nde1 and Xho1. Protein expression was induced in BL21(DE3) cells with 1 mM IPTG for 3 hours at 37°C. Bacteria were pelleted and lysed in Bug Buster supplemented with 25 U/ml benzonase (Novagen) for 20 min at room temperature. C-terminal His-tagged proteins were purified by metal affinity chromatography using Hi-Trap chelating Hp columns on an AKTAprime (GE Healthcare). Eluted fractions containing recombinant TPI protein were pooled and dialysed into PBS. Endotoxin was removed using Detoxi-Gel Endotoxin Removing Columns (Thermo Scientific). Recombinant TPI was stored at 2 mg/ml at −80°C.
Enzymatic assays
Enzymatic activity of TPI was determined in the reverse direction (conversion of DHAP to G3P) as described by Lambeir et al [56]. Standard reaction conditions were 100 mM TEA-HCl pH 7.6, 1 mM EDTA, 0.16–10 mM DHAP (1.5 mM if not indicated), 1 mM NAD+, 5 mM disodium hydrogen arsenate and 10 mg rabbit GAPDH. Reactions were initiated with 100 ng TPI in a total volume of 150 ml. Rabbit TPI and all above reagents were from Sigma. The initial reaction rate was calculated by the change in NADH absorbance at 340 nm with a Nanodrop 2000 (Thermo Scientific). Enzyme activity was calculated in units (1 U = 1 mmol substrate formation min−1 mg−1 enzyme at 25°C). Calculations were confirmed using a NADH standard curve (Sigma). To determine the level of TPI activity in BES, reactions were initiated with 500 ng of BES or heat denatured BES (95°C 30 min), and compared to 25–400 ng of Bm-TPI. To assess the inhibitory potential of serum from immunised animals, enzymatic assays were performed in the presence of 10% test or control serum. Enzyme blockade by monoclonal antibodies was assessed in the range 0.5–50 mg/ml, and an isotype control mouse IgG1 MOPC31C was used where indicated. In these instances, enzymatic activity was calculated as percentage activity compared to the relevant control.
Human, jird and mouse antibody and cytokine ELISA
Recombinant Bm-TPI, rabbit TPI (Sigma), BSA or BmA were coated (1 mg/ml in 0.06 M carbonate buffer pH 9.6) onto Maxisorb 96 well Immunoplates (Nunc) overnight at 4°C. Plates were blocked in 2% BSA (mouse and human sera) or 1% casein (jird sera) in tris-buffered saline/0.05% tween 20 (TBST) for 2 hours at 37°C. Human IgG responses to Bm-TPI were tested using sera (1/100 dilution) from a previously characterised Indonesian B. malayi endemic study population [57]. Individuals were classified into three clinical groups, elephantiasis (pathology) patients, asymptomatic microfilaraemics, and endemic normals. Normal human sera (NHS) were obtained from non-exposed UK residents. IgG binding was detected using peroxidase labelled anti-human IgG (1/1000, DakoCytomation), or anti-human IgG1, IgG2, IgG3 or IgG4 (1/5000, The Binding Site). Plates were developed using ABTS peroxidase substrate (KPL), and measured with an Emax microplate reader (Molecular Devices). Samples were considered positive if they exceeded the mean value plus 3 standard deviations of nonendemic human serum samples. Jird sera (1/100 dilution) antibody levels were measured using peroxidase labelled anti-mouse IgG1 (1/2000, Southern Biotech). BALB/c anti-Bm-TPI titres were determined with doubling dilutions of sera (1/50 onwards) and anti-mouse Ig peroxidase (1/2000, DakoCytomation).
Polyclonal and monoclonal antibody production
For polyclonal antibody production, BALB/c mice were immunised i.p. with 50 mg Bm-TPI in alum, and then boosted with 10 mg Bm-TPI i.p. in alum on days 28 and 35. Serum was recovered on day 42. For monoclonal antibody production, BALB/c mice were immunised with 50 mg Bm-TPI, and then boosted with 1 mg Bm-TPI in PBS i.v. on days 28, 29 and 30. Spleens were recovered on day 32 and fused with SP2 as before [58]. Cells were screened for Bm-TPI binding by ELISA, and positive wells were tested for Bm-TPI blocking ability in the above enzymatic assay. From this a blocking hybridoma was obtained, and cloned through two rounds of limiting dilution, resulting in anti-Bm-TPI clone 1.11.1. This was found to be an IgG1 using mouse antibody isotype kit (Isostrip, Roche). Monoclonal antibody was purified from culture supernatants using HiTrap protein G HP columns and an AKTAprime, and dialysed into PBS. Control mouse IgG1 MOPC31C was produced in the same way from cells sourced from ECACC.
Western blots
BES and somatic extracts of L3, MF and mixed adult B. malayi (1 mg) were run on SDS-PAGE gels and blotted onto nitrocellulose membranes as previously described [6]. Following blocking in 5% milk powder/TBST (2 hours room temperature), membranes were probed overnight at 4°C with 1/500 mouse polyclonal anti-Bm-TPI, washed extensively in TBST and the incubated with 1/2000 rabbit anti-mouse Ig HRP (1 h, room temperature; DakoCytomation). Following further washing in TBST, blots were developed using ChemiGlow West (Alpha Innotech) and imaged using a FluorChem SP (Alpha Innotech).
Immunohistochemistry
Adult B. malayi were mounted in Cryo-M-Bed (Bright Instruments), frozen on dry ice, and 5 mm sections cut using a Leica CM1510S cryotstat. Air dried sections were fixed in 100% acetone (10 min), washed twice with PBS (20 min), and stained in a humidified chamber with 1/100 dilution mouse anti-Bm-TPI sera (generated as above) in 1% FCS/PBS (1 hour at room temperature). Control sections were similarly treated with naïve mouse sera. Following extensive washing in PBS, sections were incubated with 1/100 goat anti-mouse IgG TRITC (Sigma) as above, washed in PBS, then mounted with Vectashield (Vector labs). Sections were analysed with an Olympus BX50 fluorescent microscope and Openlab software (PerkinElmer).
Vaccination regimes
Meriones unguiculatus jirds were immunized with 200 mg of Bm-TPI or BSA i.p. in alum adjuvant and then boosted with sub-cutaneous injections of 50 mg protein in alum at weeks 5 and 6. Jirds were challenged with 190 B. malayi L3 i.p. at week 8 post-immunisation. Infection was allowed to progress 8 weeks in the first experiment and 16 weeks in the second experiment. For Ls-TPI vaccination, BALB/c mice were immunised with 50 mg Ls-TPI or BSA in alum, and then boosted on weeks 4 and 5 with 25 mg protein in alum. Mice were infected sub-cutaneously on week 7 post-immunisation with 30 L. sigmodontis L3, and infections were terminated at week 10 post-infection.
In vivo Bm-TPI neutralisation and embryograms
Surgical implant of adult B. malayi (8 females and 2 males) into the peritoneal cavity of BALB/c mice was performed as previously described [50]. Mice were given 200 mg of anti-Bm-TPI clone 1.11.1 mAb or MOPC31C isotype control every 1–2 days over the course of a 14–28 day infection. Embryograms were performed on recovered female parasites exactly as [42]. For in vivo microfilariae transfer, Mf from the peritoneal cavity of infected jirds (1×105) were transferred i.v. in 200 ml RPMI1640 into BALB/c recipients, which were then injected with 200 mg antibody as above. Circulating Mf numbers were determined by tail bleed 24 hours later.
FACS analysis, cell culture and transfer
Cells were recovered from infected mice by peritoneal wash [38], washed into FACS buffer (PBS +0.5% BSA +0.05% sodium azide) Fc receptors blocked with 0.5 mg/ml rat IgG on ice for 10 minutes, then stained variously with anti-CD11b Pacific Blue (Biolegend; clone M1/70) anti-siglecF (PE or PE CF-594 conjugates, BD Pharmingen; clone E50-2440), anti-Ly6G APC-Cy7 (Biolegend; clone 1A8) and anti-F4/80 (FITC or PerCP conjugates, Biolegend; clone BM8). For macrophage alternate activation analysis, cells were fixed and permeablised (eBioscience, as per manufacturer's instructions) before intracellular staining with anti-RELMα (unlabeled rabbit polyclonal; PeproTech, followed by rabbit Ig labeling reagent; Invitrogen) and anti-Ym-1 (biotin-conjugated mouse chitinase 3-like 3; R&D, followed by streptavidin PE-Cy7; Biolegend). For intracellular cytokine staining, peritoneal cells were re-stimulated ex vivo in complete RPMI1640 media (supplemented with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin) with 1 mg/ml ionomycin, 500 ng/ml PMA and 10 mg/ml Brefeldin A (all Sigma) for 4 hours at 37°C. Following FcR block, cells were surface stained with anti-CD4 PerCP (clone RM4-5), fixed and permeabilised (BD Pharmingen Cytofix/Cytoperm) and then intracellular stained with anti-IFNg APC (clone XMG1.2) and anti-IL-4 PE (clone 11B11). Relevant isotype control stains were included. Alternatively, CD4+ cells were purified from the spleens of naïve BALB/c mice using MACS beads and columns (Miltenyi Biotec), according to the manufacturer's instruction, and stimulated in complete RPMI1640 for 3 days in the presence of 1 mg/ml anti-CD3 (clone 145-2C11) and 0.5 mg/ml anti-CD28 (clone 37.51) with varying amounts of recombinant Bm-TPI. Cells were then washed, resuspended in fresh media and stimulated with PMA and ionomycin in the presence of Brefeldin A as above. For in vivo transfers of ovalbumin-specific DO11.10 cells, BALB/c mice were injected i.p. with 2×10e6 DO11.10 splenocytes (equivalent to approx 4×105 CD4+ cells, data not shown). The next day, mice were given 0.5×106 dendritic cells i.p. pulsed overnight with LPS (100 ng/ml, Sigma) and ovalbumin peptide (pOVA) residues 323–339 (20 mg/ml, Invivogen), then subsequently injected on days 0, 1, 3 and 5 with 100 mg r Bm-TPI or PBS control. Control mice were given PBS rather than dendritic cells. At day 7, spleens and peritoneal cells were harvested, stimulated as above and stained with biotin anti-mouse TCR DO11.10 (clone KJ1-26) followed by streptavidin-APC conjugate. Dendritic cells were generated in vitro from mouse bone marrow in the presence of GM-CSF [59]. Antibodies were from Biolegend unless stated.
Statistical analysis
Statistical significance was determined using Prism 6 (Graphpad Software Inc.). For comparison between two groups, unpaired Student's t-test or Mann-Whitney U-test was used dependent on data normality. Multiple comparisons used one-way ANOVA followed by Tukey's test.
Supporting Information
Zdroje
1. BabuS, NutmanTB (2012) Immunopathogenesis of lymphatic filarial disease. Semin Immunopathol 34 : 847–861.
2. GregoryWF, AtmadjaAK, AllenJE, MaizelsRM (2000) The abundant larval transcript 1/2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infect Immun 68 : 4174–4179.
3. BabayanSA, AllenJE, TaylorDW (2012) Future prospects and challenges of vaccines against filariasis. Parasite Immunol 34 : 243–253.
4. HewitsonJP, GraingerJR, MaizelsRM (2009) Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol Biochem Parasitol 167 : 1–11.
5. JohnstonMJG, MacdonaldJA, McKayDM (2009) Parasitic helminths: a pharmacopeia of anti-inflammatory molecules. Parasitology 136 : 125–147.
6. HewitsonJP, HarcusYM, CurwenRS, DowleAA, AtmadjaAK, et al. (2008) The secretome of the filarial parasite, Brugia malayi : proteomic profile of adult excretory-secretory products. Mol Biochem Parasitol 160 : 8–21.
7. MorenoY, GearyTG (2008) Stage - and gender-specific proteomic analysis of Brugia malayi excretory-secretory products. PLoS Negl Trop Dis 2: e326.
8. BennuruS, SemnaniR, MengZ, RibeiroJM, VeenstraTD, et al. (2009) Brugia malayi excreted/secreted proteins at the host/parasite Interface: stage - and gender-specific proteomic profiling. PLoS Negl Trop Dis 3: e410.
9. BennuruS, MengZ, RibeiroJM, SemnaniRT, GhedinE, et al. (2011) Stage-specific proteomic expression patterns of the human filarial parasite Brugia malayi and its endosymbiont Wolbachia. Proc Natl Acad Sci U S A 108 : 9649–9654.
10. KnowlesJR (1991) To build an enzyme. Philos Trans R Soc Lond B Biol Sci 332 : 115–121.
11. CurwenRS, AshtonPD, SundaralingamS, WilsonRA (2006) Identification of novel proteases and immunomodulators in the secretions of schistosome cercariae that facilitate host entry. Mol Cell Proteomics 5 : 835–844.
12. CassCL, JohnsonJR, CaliffLL, XuT, HernandezHJ, et al. (2007) Proteomic analysis of Schistosoma mansoni egg secretions. Mol Biochem Parasitol 155 : 84–93.
13. YatsudaAP, KrijgsveldJ, CornelissenAWCA, HeckAJ, De VriesE (2003) Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J Biol Chem 278 : 16941–16951.
14. LiuQY, CorjayM, FeuersteinGZ, NambiP (2006) Identification and characterization of triosephosphate isomerase that specifically interacts with the integrin alphaIIb cytoplasmic domain. Biochem Pharmacol 72 : 551–557.
15. PereiraLA, BaoSN, BarbosaMS, da SilvaJL, FelipeMS, et al. (2007) Analysis of the Paracoccidioides brasiliensis triosephosphate isomerase suggests the potential for adhesin function. FEMS Yeast Res 7 : 1381–1388.
16. IkedaR, SaitoF, MatsuoM, KurokawaK, SekimizuK, et al. (2007) Contribution of the mannan backbone of cryptococcal glucuronoxylomannan and a glycolytic enzyme of Staphylococcus aureus to contact-mediated killing of Cryptococcus neoformans. J Bacteriol 189 : 4815–4826.
17. FuruyaH, IkedaR (2009) Interaction of triosephosphate isomerase from the cell surface of Staphylococcus aureus and alpha-(1→3)-mannooligosaccharides derived from glucuronoxylomannan of Cryptococcus neoformans. Microbiology 155 : 2707–2713.
18. AtionuA, HumphriesA, LallozMR, AryaR, WildB, et al. (1999) Reversal of metabolic block in glycolysis by enzyme replacement in triosephosphate isomerase-deficient cells. Blood 94 : 3193–3198.
19. SchneiderAS (2000) Triosephosphate isomerase deficiency: historical perspectives and molecular aspects. Baillieres Best Pract Res Clin Haematol 13 : 119–140.
20. HarnDA, GuW, OliginoLD, MitsuyamaM, GebremichaelA, et al. (1992) A protective monoclonal-antibody specifically recognizes and alters the catalytic activity of schistosome triose-phosphate isomerase. J Immunol 148 : 562–567.
21. ZhuY, SiJ, HarnDA, XuM, RenJ, et al. (2006) Schistosoma japonicum triose-phosphate isomerase plasmid DNA vaccine protects pigs against challenge infection. Parasitology 132 : 67–71.
22. Da'daraAA, LiYS, XiongT, ZhouJ, WilliamsGM, et al. (2008) DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine 26 : 3617–3625.
23. WilsonRA, CoulsonPS, BettsC, DowlingM-A, SmythiesLE (1996) Impaired immunity and altered pulmonary responses in mice with a disrupted interferon-g receptor gene exposed to the irradiated Schistosoma mansoni vaccine. Immunology 87 : 275–282.
24. ReynoldsSR, DahlCE, HarnDA (1994) T and B cell epitope determination and analysis of multiple antigenic peptides for the Schistosoma mansoni experimental vaccine triose-phosphate isomerase. J Immunol 152 : 193–200.
25. ReisEA, Mauadi CarmoTA, AthanazioR, ReisMG, HarnDAJr (2008) Schistosoma mansoni triose phosphate isomerase peptide MAP4 is able to trigger naive donor immune response towards a type-1 cytokine profile. Scand J Immunol 68 : 169–176.
26. McCarthyJS, WiesemanM, TropeaJ, KaslowD, AbrahamD, et al. (2002) Onchocerca volvulus glycolytic enzyme fructose-1,6-bisphosphate aldolase as a target for a protective immune response in humans. Infect Immun 70 : 851–858.
27. RaverdyS, ZhangY, FosterJ, CarlowCK (2007) Molecular and biochemical characterization of nematode cofactor independent phosphoglycerate mutases. Mol Biochem Parasitol 156 : 210–216.
28. SinghAR, JoshiS, AryaR, KayasthaAM, SrivastavaKK, et al. (2008) Molecular cloning and characterization of Brugia malayi hexokinase. Parasitol Int 57 : 354–361.
29. BarrettJ, MendisAH, ButterworthPE (1986) Carbohydrate metabolism in Brugia pahangi (Nematoda: Filarioidea). Int J Parasitol 16 : 465–469.
30. KöhlerP (1991) The pathways of energy generation in filarial parasites. Parasitol Today 7 : 21–25.
31. TielensAG (1994) Energy generation in parasitic helminths. Parasitol Today 10 : 346–352.
32. GhedinE, WangS, SpiroD, CalerE, ZhaoQ, et al. (2007) Draft genome of the filarial nematode parasite Brugia malayi. Science 317 : 1756–1760.
33. BannerDW, BloomerAC, PetskoGA, PhillipsDC, PogsonCI, et al. (1975) Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5 angstrom resolution using amino acid sequence data. Nature 255 : 609–614.
34. HoffmannWH, PetitG, Schulz-KeyH, TaylorDW, BainO, et al. (2000) Litomosoides sigmodontis in mice: reappraisal of an old model for filarial research. Parasitol Today 16 : 387–389.
35. KurniawanA, YazdanbakhshM, van ReeR, AalberseR, SelkirkME, et al. (1993) Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol 150 : 3941–3950.
36. YazdanbakhshM, PaxtonWA, KruizeYCM, SartonoE, KurniawanA, et al. (1993) T cell responsiveness correlates differentially with antibody isotype levels in clinical and asymptomatic filariasis. J Infect Dis 167 : 925–931.
37. AshLR (1973) Chronic Brugia pahangi and Brugia malayi infections in Meriones unguiculatus. J Parasitol 59 : 442–447.
38. McSorleyHJ, HarcusYM, MurrayJ, TaylorMD, MaizelsRM (2008) Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite, Brugia malayi. J Immunol 181 : 6456–6466.
39. Cortes-FigueroaAA, Perez-TorresA, SalaizaN, CabreraN, Escalona-MontanoA, et al. (2008) A monoclonal antibody that inhibits Trypanosoma cruzi growth in vitro and its reaction with intracellular triosephosphate isomerase. Parasitol Res 102 : 635–643.
40. BandiC, McCallJW, GenchiC, CoronaS, VencoL, et al. (1999) Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int J Parasitol 29 : 357–364.
41. GhedinE, HailemariamT, DePasseJV, ZhangX, OksovY, et al. (2009) Brugia malayi gene expression in response to the targeting of the Wolbachia endosymbiont by tetracycline treatment. PLoS Negl Trop Dis 3: e525.
42. ZiewerS, HubnerMP, DubbenB, HoffmannWH, BainO, et al. (2012) Immunization with L. sigmodontis microfilariae reduces peripheral microfilaraemia after challenge infection by inhibition of filarial embryogenesis. PLoS Negl Trop Dis 6: e1558.
43. DenhamDA, MedeirosF, BaldwinC, KumarH, MidwinterICT, et al. (1992) Repeated infection of cats with Brugia pahangi : parasitological observations. Parasitology 104 : 415–420.
44. Olivares-IllanaV, Rodriguez-RomeroA, BeckerI, BerzunzaM, GarciaJ, et al. (2007) Perturbation of the dimer interface of triosephosphate isomerase and its effect on Trypanosoma cruzi. PLoS Negl Trop Dis 1: e1.
45. KuntzDA, OsowskiR, SchudokM, WierengaRK, MullerK, et al. (1992) Inhibition of triosephosphate isomerase from Trypanosoma brucei with cyclic hexapeptides. Eur J Biochem 207 : 441–447.
46. SinghSK, MaithalK, BalaramH, BalaramP (2001) Synthetic peptides as inactivators of multimeric enzymes: inhibition of Plasmodium falciparum triosephosphate isomerase by interface peptides. FEBS Lett 501 : 19–23.
47. Enriquez-FloresS, Rodriguez-RomeroA, Hernandez-AlcantaraG, De la Mora-De la MoraI, Gutierrez-CastrellonP, et al. (2008) Species-specific inhibition of Giardia lamblia triosephosphate isomerase by localized perturbation of the homodimer. Mol Biochem Parasitol 157 : 179–186.
48. PearlmanE, HazlettFEJr, BoomWH, KazuraJW (1993) Induction of murine T-helper-cell responses to the filarial nematode Brugia malayi. Infect Immun 61 : 1105–1112.
49. LawrenceRA, AllenJE, OsborneJ, MaizelsRM (1994) Adult and microfilarial stages of the filarial parasite Brugia malayi stimulate contrasting cytokine and immunoglobulin isotype responses in BALB/c mice. J Immunol 153 : 1216–1224.
50. ZangXX, AtmadjaAK, GrayP, AllenJE, GrayCA, et al. (2000) The serpin secreted by Brugia malayi microfilariae, Bm-SPN-2, elicits strong, but short-lived, immune responses in mice and humans. J Immunol 165 : 5161–5169.
51. BellafioreS, ShenZ, RossoMN, AbadP, ShihP, et al. (2008) Direct identification of the Meloidogyne incognita secretome reveals proteins with host cell reprogramming potential. PLoS Pathog 4: e1000192.
52. GregoryWF, BlaxterML, MaizelsRM (1997) Differentially expressed, abundant trans-spliced cDNAs from larval Brugia malayi. Mol Biochem Parasitol 87 : 85–95.
53. TaylorM, Le GoffL, HarrisA, MaloneE, AllenJE, et al. (2005) Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol 174 : 4924–4933.
54. SartonoE, KruizeYCM, KurniawanA, van der MeidePH, PartonoF, et al. (1995) Elevated cellular responses and interferon-γ release after long-term diethylcarbamazine treatment of patients with human lymphatic filariasis. J Infect Dis 171 : 1683–1687.
55. McSorleyHJ, GraingerJR, HarcusYM, MurrayJ, NisbetA, et al. (2010) daf-7-related TGF-β homologues from trichostrongyloid nematodes show contrasting life cycle expression patterns. Parasitology 137 : 159–171.
56. LambeirAM, OpperdoesFR, WierengaRK (1987) Kinetic properties of triose-phosphate isomerase from Trypanosoma brucei brucei. A comparison with the rabbit muscle and yeast enzymes. Eur J Biochem 168 : 69–74.
57. Kurniawan-AtmadjaA, SartonoE, PartonoF, YazdanbakhshM, MaizelsRM (1998) Specificity of predominant IgG4 antibodies to adult and microfilarial stages of Brugia malayi. Parasite Immunol 20 : 155–162.
58. HewitsonJP, FilbeyKJ, GraingerJR, DowleAA, PearsonM, et al. (2011) Heligmosomoides polygyrus elicits a dominant nonprotective antibody response directed at restricted glycan and peptide epitopes. J Immunol 187 : 4764–4777.
59. LutzMB, KukutschN, OgilvieAL, RossnerS, KochF, et al. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223 : 77–92.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic HelixČlánek Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron MobilizationČlánek AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for ImmunityČlánek Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden HamstersČlánek Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Viral Enhancer Mimicry of Host Innate-Immune Promoters
- The Epstein-Barr Virus-Encoded MicroRNA MiR-BART9 Promotes Tumor Metastasis by Targeting E-Cadherin in Nasopharyngeal Carcinoma
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- The Consequences of Reconfiguring the Ambisense S Genome Segment of Rift Valley Fever Virus on Viral Replication in Mammalian and Mosquito Cells and for Genome Packaging
- Substrate-Induced Unfolding of Protein Disulfide Isomerase Displaces the Cholera Toxin A1 Subunit from Its Holotoxin
- Male-Killing Induces Sex-Specific Cell Death via Host Apoptotic Pathway
- Highly Active Antiretroviral Therapies Are Effective against HIV-1 Cell-to-Cell Transmission
- The microRNAs in an Ancient Protist Repress the Variant-Specific Surface Protein Expression by Targeting the Entire Coding Sequence
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Type III Secretion Protein MxiI Is Recognized by Naip2 to Induce Nlrc4 Inflammasome Activation Independently of Pkcδ
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
- Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Strains
- Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic Helix
- Foxp3 Regulatory T Cells Delay Expulsion of Intestinal Nematodes by Suppression of IL-9-Driven Mast Cell Activation in BALB/c but Not in C57BL/6 Mice
- Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron Mobilization
- MicroRNA Editing Facilitates Immune Elimination of HCMV Infected Cells
- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Identification of Host-Targeted Small Molecules That Restrict Intracellular Growth
- A Cyclophilin Homology Domain-Independent Role for Nup358 in HIV-1 Infection
- Engagement of NKG2D on Bystander Memory CD8 T Cells Promotes Increased Immunopathology following Infection
- Suppression of RNA Silencing by a Plant DNA Virus Satellite Requires a Host Calmodulin-Like Protein to Repress Expression
- CIB1 Synergizes with EphrinA2 to Regulate Kaposi's Sarcoma-Associated Herpesvirus Macropinocytic Entry in Human Microvascular Dermal Endothelial Cells
- A Gammaherpesvirus Bcl-2 Ortholog Blocks B Cell Receptor-Mediated Apoptosis and Promotes the Survival of Developing B Cells
- Metabolic Reprogramming during Purine Stress in the Protozoan Pathogen
- The Post-transcriptional Regulator / Activates T3SS by Stabilizing the 5′ UTR of , the Master Regulator of Genes, in
- Tailored Immune Responses: Novel Effector Helper T Cell Subsets in Protective Immunity
- AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for Immunity
- Epstein-Barr Virus Large Tegument Protein BPLF1 Contributes to Innate Immune Evasion through Interference with Toll-Like Receptor Signaling
- The Major Cellular Sterol Regulatory Pathway Is Required for Andes Virus Infection
- Insights into the Initiation of JC Virus DNA Replication Derived from the Crystal Structure of the T-Antigen Origin Binding Domain
- Domain Shuffling in a Sensor Protein Contributed to the Evolution of Insect Pathogenicity in Plant-Beneficial
- Lectin-Like Bacteriocins from spp. Utilise D-Rhamnose Containing Lipopolysaccharide as a Cellular Receptor
- A Compositional Look at the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects
- Exploits Asparagine to Assimilate Nitrogen and Resist Acid Stress during Infection
- Interleukin-33 Increases Antibacterial Defense by Activation of Inducible Nitric Oxide Synthase in Skin
- Protective Vaccination against Papillomavirus-Induced Skin Tumors under Immunocompetent and Immunosuppressive Conditions: A Preclinical Study Using a Natural Outbred Animal Model
- Gem-Induced Cytoskeleton Remodeling Increases Cellular Migration of HTLV-1-Infected Cells, Formation of Infected-to-Target T-Cell Conjugates and Viral Transmission
- Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden Hamsters
- Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
- Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung
- Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response
- Human Genome-Wide RNAi Screen Identifies an Essential Role for Inositol Pyrophosphates in Type-I Interferon Response
- The Master Regulator of the Cellular Stress Response (HSF1) Is Critical for Orthopoxvirus Infection
- Code-Assisted Discovery of TAL Effector Targets in Bacterial Leaf Streak of Rice Reveals Contrast with Bacterial Blight and a Novel Susceptibility Gene
- Competitive and Cooperative Interactions Mediate RNA Transfer from Herpesvirus Saimiri ORF57 to the Mammalian Export Adaptor ALYREF
- The Type III Secretion Chaperone Slc1 Engages Multiple Early Effectors, Including TepP, a Tyrosine-phosphorylated Protein Required for the Recruitment of CrkI-II to Nascent Inclusions and Innate Immune Signaling
- Yeasts: How Many Species Infect Humans and Animals?
- Clustering of Pattern Recognition Receptors for Fungal Detection
- Distinct Antiviral Responses in Pluripotent versus Differentiated Cells
- Igniting the Fire: Virulence Factors in the Pathogenesis of Sepsis
- Inactivation of the Host Lipin Gene Accelerates RNA Virus Replication through Viral Exploitation of the Expanded Endoplasmic Reticulum Membrane
- Inducible Deletion of CD28 Prior to Secondary Infection Impairs Worm Expulsion and Recall of Protective Memory CD4 T Cell Responses
- Clonal Expansion during Infection Dynamics Reveals the Effect of Antibiotic Intervention
- The Secreted Triose Phosphate Isomerase of Is Required to Sustain Microfilaria Production
- Unifying Viral Genetics and Human Transportation Data to Predict the Global Transmission Dynamics of Human Influenza H3N2
- ‘Death and Axes’: Unexpected Ca Entry Phenologs Predict New Anti-schistosomal Agents
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



