-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
Cancer is a complex disease that is characterized by the sequential accumulation of genetic mutations. Exposure to environmental agents, such as solar ultraviolet, induces such alterations and thus contributes to the development of genomic instability. The tumor suppressor p53 has a central role in orchestrating cellular responses to genotoxic stress. In response to DNA-damage, p53 is stabilized and activated to direct cell fate decisions. Cells in which p53 stabilization is compromised become more vulnerable to mutagenic agents and hence the mutation rate increases, which promotes tumor development. Stabilization of p53 is thus a critical step towards cancer prevention. Using a genetic approach, we demonstrate that the ubiquitous transcription factor Upstream Stimulatory factor 1 (USF1) is required for immediate p53 stabilization and appropriate cell fate decisions following genotoxic stress. Furthermore, we show that this involves a novel function of USF1 that underscores its critical role as a stress sensor. The loss of USF1 expression should thus be considered as a potential initiator of tumorigenesis in the context of environmental insults.
Published in the journal: . PLoS Genet 10(5): e32767. doi:10.1371/journal.pgen.1004309
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004309Summary
Cancer is a complex disease that is characterized by the sequential accumulation of genetic mutations. Exposure to environmental agents, such as solar ultraviolet, induces such alterations and thus contributes to the development of genomic instability. The tumor suppressor p53 has a central role in orchestrating cellular responses to genotoxic stress. In response to DNA-damage, p53 is stabilized and activated to direct cell fate decisions. Cells in which p53 stabilization is compromised become more vulnerable to mutagenic agents and hence the mutation rate increases, which promotes tumor development. Stabilization of p53 is thus a critical step towards cancer prevention. Using a genetic approach, we demonstrate that the ubiquitous transcription factor Upstream Stimulatory factor 1 (USF1) is required for immediate p53 stabilization and appropriate cell fate decisions following genotoxic stress. Furthermore, we show that this involves a novel function of USF1 that underscores its critical role as a stress sensor. The loss of USF1 expression should thus be considered as a potential initiator of tumorigenesis in the context of environmental insults.
Introduction
Genomic instability is a central hallmark of cancer, where DNA damaging agents play an important role [1]–[2]. The transformation of normal cells into cancer cells requires the succession of several genetics alterations within the genome that alter key physiological regulatory processes.
In DNA-damaged eukaryotic cells, genome integrity is maintained by an immediate and inducible protective program. This program requires dedicated sensors that drive and regulate the cellular response, by monitoring DNA-repair and if required by forcing damaged-cells into cell death pathways [3]. When these sensors are compromised, sensitivity to mutagenic agents is increased and the mutation rate speeds up, allowing tumor development. The extent of DNA lesions and the capacity of dedicated sensors to direct a proper response are thus determining parameters of cell fate.
To date, the tumor suppressor p53 is the most important sensor [4], being a central and early regulator of the DNA-damage response. Upon recognition of DNA damage, p53 induces a transient growth arrest by holding the cell cycle at the G1/S regulation point. p53 acts through activating the expression of the cell-cycle arrest gene CDKN1A (p21) [5], [6], [7], allowing DNA repair and thereby preventing the development of cancer. This p53-dependent transient cell cycle arrest is thus a decisive step in cell fate that requires the stabilization of p53 protein. Indeed, in the absence of cellular stress, p53 is maintained at low steady-state levels by the dynamic p53-MDM2 feedback loop [8]. In response to DNA-damage signaling, the p53 protein undergoes extensive post-translational modifications including phosphorylation by DNAPK, ATM and ATR, all members of the PI3K family [9]. These modifications nucleate subsequent changes in the repertoire of proteins interacting with p53 and in particular abolish the p53-MDM2 interaction [10]. This results in the immediate increase in p53 levels and transcriptional activity, thereby directing cell fate decisions [11], [12], [13].
The Upstream Stimulatory Factor 1 (USF1) is an ubiquitous transcription factor of the basic-helix-loop-helix leucine-zipper (bHLH-LZ) family that operates as a stress sensor. USF1 is a direct target of the p38 stress-activated kinase and genetic studies demonstrate that USF1 is a transcriptional rheostat for the stress response [14], [15], [16]. In response to UV-radiation, a physiological source of direct DNA-damage, known as the major risk factor for skin cancers [17], [18], [19], [20], USF1 regulates the expression of pigmentation genes [15], and genes of the nucleotide excision repair pathway (NER) [21]. This protective function of USF1 is important since the repair of DNA damage is central to the maintenance of genome stability.
The USF1 and p53 pathways both have pivotal roles in the response to stress, where they participate in the immediate molecular and cellular responses. They regulate common biological processes to mitigate deleterious effects. Both pathways have been studied in detail, but little is known about any crosstalk between them, although in vitro studies suggested that USF1 may regulate the basal transcription of the p53 gene [22], [23]. We thus examined whether USF1 could contribute to the canonical p53 stress response by directing proper cell fate decisions. We used a combination of in vivo and in vitro genetic approaches to test for the presence of a coordinated USF1/p53 program. We demonstrate that in the presence of DNA damage, USF1 is necessary for immediate p53 protein stabilization and that the p53-mediated cell cycle arrest requires USF1. We report evidence that USF1 is a central regulator of p53 to direct cell fate decisions, identifying thereby a new functional and unexpected role for USF1. Collectively, these findings have important and broad consequences for our understanding of mechanisms that maintain stress-induced DNA damage and cancer promotion.
Results
USF1-deficient mouse skin is unable to up-regulate p53 in presence of DNA damage
To identify a coordinated USF1/p53 program, we first examined p53 expression (by assaying mRNA and protein levels) and the p53 acute stress response in Usf1-/- mice. Mice were challenged with UVB irradiation, a physiological inducer of direct DNA-damage, known to activate the p53 pathway [24]. We quantified Trp53 mRNA in skin cells from Usf1 KO mice and WT littermates (n = 9 for each genotype) and found no significant differences between the two genotypes both before and 5 hours after UVB radiation (Figure 1A). Similarly, the basal level of the p53 protein was low, with no statistical difference (Wilcoxon Mann-Whitney test with W = 0,98) between the two genotypes (n = 16 and n = 11 for respectively Usf1 KO mice and WT littermates). However, while a significant and reproducible 2-fold increase of the p53 protein was observed in WT littermates 5 hours post-UVB irradiation, p53 protein-levels remained low and unchanged in Usf1-/- mice (Figure 1B). Phosphorylation of the H2AX histone (γH2AX), a substrate of the DNAPK/ATM/ATR axis [25], [26], increased following irradiation in both genotypes confirming comparable signal transmission of UVB-induced DNA damage (Figure 1B). Levels of p53 remained low in Usf1-/- mice compared to their WT littermates 12 h post-irradiation (Figure S1A). This ruled out the possibility that the p53 response in Usf1-/- mice was simply delayed. Following UVB-irradiation, the p21, 14-3-3 sigma and PCNA genes were less strongly induced in Usf1-/- than control mouse skin both in vivo (Usf1-/- mouse skin; Figure 1C) and ex vivo (Usf1-/- cultured skin biopsies; Figure S1B). Thus, the absence of induction of p53 in the Usf1-/- mice was accompanied by weaker up-regulation of some p53 target genes required for the DNA-damage response, 5 hours post-irradiation. In addition, and in accordance with the use of the mice minimal erythema dose (MED), Bax and Puma pro-apoptotic genes were not up-regulated 5 hours post-irradiation in both genotypes (data not shown).
Fig. 1. Usf1 KO mice present defective induction of p53 protein. 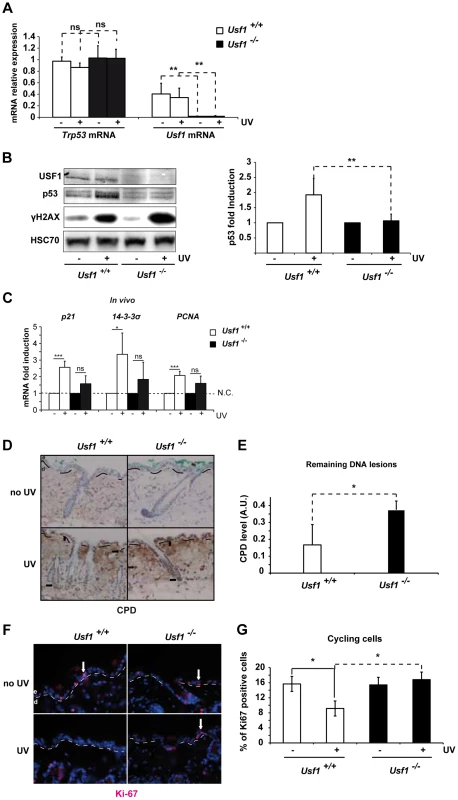
The back of Usf1 KO mice (Usf1-/-) and WT mice (Usf1+/+) were irradiated or not irradiated with an UVB dose corresponding to the mice MED (5 kJ/m2) and the skin was analyzed 5 h later. (A) RT-qPCR analysis of Trp53 and Usf1 mRNA relative level (expressed as a ratio to the value for the Hprt transcript) in skin extracts from protected (-) and UV-exposed (+) areas. Error bars: SD, n>9. (B) Western blot showing USF1, p53, γH2AX and HSC70 (loading control) immunoreactivity 5 h after skin irradiated or not irradiated with UVB. The graph reports the mean ratio between the p53 signal (normalized to that for HSC70) in skin-exposed areas versus non-irradiated areas (controls). Error bars: SD, n = 8 for each condition. (C) Usf1+/+ (Usf1 WT) and Usf1-/- (Usf1 KO) skins were or were not irradiated with UVB (5 kJ/m2) and analyzed for the induction of transcripts in vivo. RT-qPCR analysis of CDKN1a (p21), SFN (14-3-3σ) and PCNA transcripts in UVB-irradiated skin and non-exposed controls; values reported were normalized to those for the Hprt transcript. Transcripts were assayed in vivo 5 hours after irradiation. Error bars: SD, n = 4 in vivo (D) Immunohistochemical labeling of cyclobutane pyrimidine dimers (CPD) showing their localization and abundance in skin areas (x100) exposed or not exposed to UVB. Dashed lines indicate the boundary between the dermis (d) and the epidermis (e), and arrows indicate positive nuclei. (E) The level of CPDs in total DNA extracts from skin was quantified by ELISA. The graph shows the mean difference in the CPD absorbance values between for exposed and protected skin areas. Error bars: SD, n = 4. (F) Immunofluorescence staining with the Ki-67 antibody of inter-follicular cycling cells in skin areas (x100) exposed or not exposed to UVB. (G) The graph shows the mean percentage of cycling cells (calculated as Ki-67-positive cells/total Dapi-stained cells) in protected and UV-exposed skin areas. Error bars: SD, n = 3. Student's t test was used to test the significance of differences (*, p <0.05, **, p<0.01, ***, p<0.001). Trp53-deficient mice have reduced DNA repair ability and impaired cell cycle arrest in response to DNA-damaging agents [27], [28]. We therefore used immunohistochemistry (IHC) to examine the effect of USF1 deficiency on these processes. Levels of cyclobutane pyrimidine dimers (CPDs) in the epidermis and dermis and in the bulge region, 5 hours post-irradiation, were higher in Usf1-/- mice than WT littermates (Figure 1D). This was confirmed by ELISA, which showed that there was twice as much CPD in Usf1-/- mouse skin (5 h post-UV; n = 4, p<0.05) (Figure 1E). We next examined the proliferation index of epidermal cells by IHC using Ki-67, the cellular marker of cycling cells [29]. In non UV-exposed skin, the proliferation index in the inter-follicular areas was comparable in the two genotypes. In response to UVB irradiation, however, the proliferation index remained constant in Usf1-/- mice whereas it decreased by approximately 50% in WT littermates (Figure 1, F and G). The defect of DNA repair (Figure S1C and S1D) and the absence of cell cycle control (Figure S1E) in reponse to UVB was also observed in cultured skin biopsies of Usf1-/- mice, up to 24 h after irradiation. Thus, in addition to defective induction of p53 protein upon UVB exposure, Usf1 deficient cells fail to down-regulate their cell cycle despite the presence of DNA damage.
USF1 is required for p53-dependent G1/S arrest upon genotoxic stress
To decipher the specific contribution of USF1 and p53 proteins to the regulation of cell cycle progression upon genotoxic stress, we generated stable knock-down (KD) cell lines using the B16 mice melanoma cells that express active p53 and USF1 pathways. The effectiveness of the shRNAs used to knock down Usf1 and Trp53 was verified (Figure 2, A and B). Levels of Trp53 mRNA were comparable in Usf1 KD and control cells (sh-CT) and remained unchanged in response to UVB, whereas the levels of the p53 protein increased only in UVB-irradiated control cells (Figure 2, A and B). The mRNA and protein levels of p21, the p53-dependent effector of the G1/S arrest, remained low in both Usf1 KD and Trp53 KD cells in response to UVB, whereas they increased in control cells. Furthermore, consistent with findings for Usf1-/- mice, time course experiments showed that there was no delayed UV-induced p53 and p21 up-regulation in Usf1 and Trp53 KD cells (Figure S3A). These findings showed that the KD cell culture models reproduced features of Usf1-/- mice. To examine S phase progression upon genotoxic stress, cells were synchronized and we followed the synthesis of DNA by measuring the incorporation of a thymidine analogue (BrdU). The results show that the proliferation rates of synchronized Usf1 and Trp53 KD cells were similar to that of control cells (Figure 2C). However, in the UVB-irradiated condition, while the number of BrdU-incorporating cells remained unchanged and comparable for the Usf1 and Trp53 KD cells, irradiated control cells exhibited a significant reduction, of 50%, in the number of BrdU-incorporating cells. Similar results were obtained using primary fibroblasts isolated from Usf1-/- mice and Usf1+/+ littermates (Figure S2). These data are consistent with the in vivo results (Figure 1, F and G), highlighting a general mechanism. In addition, USF1 levels did not differ between Trp53 KD cells and controls, indicating that USF1 expression is not dependent on p53 (Figure 2, A and B). This also suggests that the deficiency in cell cycle arrest of Usf1 KD cells in response to genotoxic stress may be the result of the absence of increased levels and/or activity of p53 and p21 [5], [6], [7], [30], [31].
Fig. 2. USF1 mediates p53-dependent cell cycle arrest. 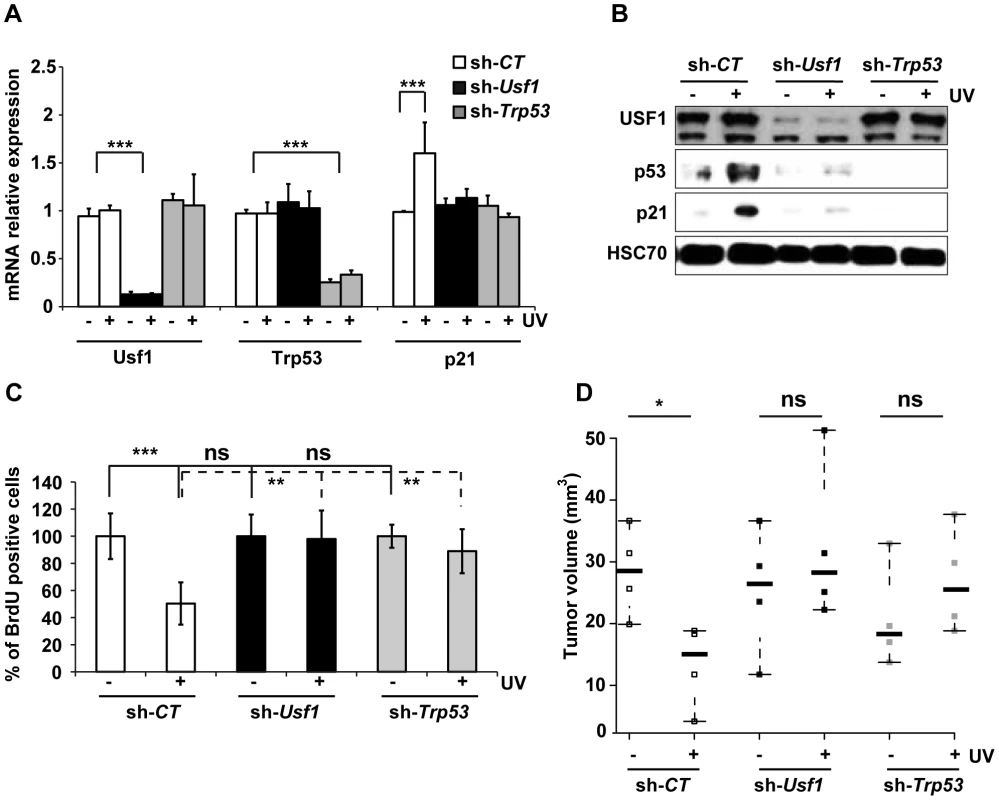
B16 melanoma cells knocked down for Usf1 (sh-Usf1) or p53 (sh-Trp53) and control cells (sh-CT) were synchronized in G1/early S phase. The cells were then irradiated or not irradiated with UVB (0.3 kJ/m2) and the cell cycle released. (A) Trp53, Usf-1 and p21 mRNAs in cells irradiated (+) or not irradiated (-) with UVB were quantified by RT-qPCR 3 hours after the release of the cell cycle; results are reported relative to the values for the Hprt transcript. Error bars: SD, n = 3. (B) Western blot analysis of USF1, p53, p21 and HSC70 (loading control) in protein extracts from cells treated as in A. (C) BrdU incorporation assay in cells irradiated or not irradiated with UVB. The values plotted are mean percentages of BrdU incorporating cells after UVB irradiation compared to those for non-irradiated cells. Error bars: SD, n = 3. (D) Stripchart plot showing the volume of tumors formed 12 days after subcutaneous injections of 2.104 B16 melanoma cells (sh-Usf1, sh-Trp53 or sh-CT). UVB (0.3 kJ/m2) irradiated or control cells, for which cell viability had been controled and was identical, were injected into the two sides of the back of NOD/SCID mice. Error bars: SD, n = 4 for mice injected with sh-CT and sh-Trp53, and n = 5 for mice injected with sh-Usf1 cells. Student's t test was used for statistical analysis (*, p <0.05, **, p<0.01, ***, p<0.001). The loss of p53 is a critical event that promotes tumor growth. We therefore investigated whether loss of USF1 favors tumor growth in vivo under stress conditions. To this end, we injected NOD/SCID mice subcutaneously with mock - or UVB-irradiated, viable Usf1 and Trp53 KD cells, and examined tumor growth 12 days later. The tumors produced by UVB-irradiated control cells were half the size of those produced by mock-irradiated control cells (Figure 2D). Usf1 and Trp53 KD cells both generated massive tumors and their sizes were not modified by UV-pretreatment (Figure 2D). This demonstrates that USF1, like p53, is required for the transient cell cycle arrest in order to delay cell proliferation in response to induced DNA damage.
USF1 is critical for p53 protein stabilization
We next investigated how USF1 controls p53 protein levels. USF1 was re-expressed in Usf1 KD cells and we showed that this restored the induction of p53 protein (Figure 3A) and p53 transcriptional activity in response to stress (Figure S3B). The effects of re-expressing USF1 were independent of Trp53 transcript levels (data not shown) and similar results were obtained with USF1 mutants lacking the DNA binding domain as well as the transcriptional activation domain (Figure S3C). These observations suggest that USF1 positively regulates p53 protein levels and activity independently of its transcription factor function. Therefore, USF1 may act through translational and/or post-translational mechanisms to modulate p53 availability. Treatment of Usf1 KD and control cells with MG132 (an inhibitor of proteasome activity) resulted in immediate and similar increases of p53 protein levels in the two types of cell lines (Figure 3B). This indicates that USF1 prevents the degradation of p53 rather than inducing p53 synthesis. Furthermore, the abundance of USF1 protein in control cells remained unchanged when proteasome activity was inhibited (Figure 3B), validating the use of the MG132 inhibitor as a powerfull in vitro tool to further investigate the mechanism of p53 stabilization in the Usf1 KD background.
Fig. 3. USF1 is required to stabilize p53 protein following genotoxic stress. 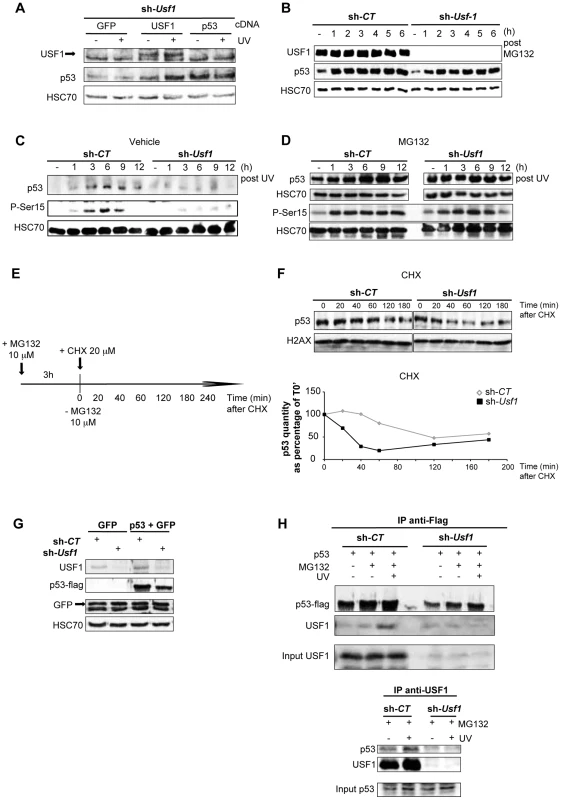
B16 melanoma cells knocked down for Usf1 (sh-Usf1) and their controls (sh-CT) were analyzed for post-translational regulation of p53. (A) Western blot analysis of the effect of USF1 re-expression on p53 protein levels in sh-Usf1 cells irradiated or not irradiated with UVB and tested 6 h after irradiation. Cells were transfected with the cDNA indicated (as described in the materials and methods) and analyzed for USF1, p53 and HSC70 (loading control). (B) Western blot showing USF1, p53 and HSC70 immunoreactivity in sh-CT and sh-Usf1 cells at the indicated time following treatment with MG132 (10 µM). (C–D) Time course of p53 accumulation and Ser15-phosphorylation in sh-CT and sh-Usf1 cells treated with vehicle (DMSO) in C or MG132 (10 µM) plus UVB (0.3 kJ/m2) irradiation in D. (E–F) p53 degradation in sh-CT and sh-Usf1 cells pretreated for 3 h with MG132 (10 µM) and then with cycloheximide (CHX 20 µM) (E). Cells were analyzed at the time points indicated after addition of CHX. The graphs show the results of densitometric analysis of p53 immunoreactive bands (normalized to the loading controls H2AX or HSC70). (G) Western blot showing flag-tagged p53 and GFP proteins in sh-CT or sh-Usf1 cells after co-transfection of the corresponding cDNA. (H, upper panel) Immunoprecipitation analysis to assay flag-tagged p53 after transfection of sh-CT or sh-Usf1 with the corresponding cDNA. Cells were treated with MG132, were or were not irradiated with UVB and analyzed 3 hours later. (H, lower panel) sh-CT and sh-Usf1 cells were treated with 10 µM MG132 for 3 hours then irradiated or not irradiated with UVB. Western blot analysis of proteins immunoprecipitated from cell lysates with USF1 antibody and blotted with p53 (1C12) and USF1 antibodies. Phosphorylation of p53 is important for its stabilization and is dependent on the activation of the DNA damage signal transducers, DNAPK, ATM and ATR. Since the phosphorylation of serine 15 (Ser15) in the p53 protein is required to mediate interactions with other proteins to block contact with its inhibitor, MDM2 [32], [33], we specifically examined this modification. Usf1 KD and control cells were pre-treated with vehicle or MG132 to stabilize the p53 protein and exposed to UVB. In the absence of MG132 pre-treatment, UVB-induced phosphorylation of Ser15 and stabilization of p53 occurred only in control and not in Usf1 KD cells (Figure 3C). Inhibition of the proteasome degradation pathway in the presence UVB resulted in comparable levels of phosphorylated Ser15 and stabilization of p53 in Usf1 KD cells and control cells (Figure 3D). These results, together with data showing that phosporylation of Chk1, a downstream target of the ATM/ATR pathway implicated in p53 activation [34], is maintained in Usf1-/- mice (Figure S3D) and in the Usf1 KD cells in response to UV (Figure S3E). This suggests that while upstream mechanisms of transduction of the DNA-damage signal, targeting p53-stabilization, are functional in Usf1 KD cells, the absence of USF1 prevents full stabilization of p53.
We next examined whether USF1 modulates the half-life of p53. Cells were pre-treated with MG132 (for 3 hours) to stabilize p53, and time course experiments were performed with the protein translation inhibitor, cycloheximide (CHX) (Figure 3E). The half-life of the p53 protein in Usf1 KD cells was 30 min, and in control cells was 110 min (sh-CT) (Figure 3F). To confirm these results Usf1 KD and control cells were co-transfected with a vector encoding a flag-tag p53 construct and a GFP control construct. GFP was expressed at the same level in the two cell lines, but p53 levels in Usf1 KD cells were half that in control cells (Figure 3G). These in vitro results together with work from the Levine group [35], [36] suggest that the steady state level of p53 depends on the experimental systems used (ie cell tranfection, chemical compound), which are known to challenge cells. We next examined the half-life of p53 by irradiating cells before CHX addition and our results show that the half-life of p53 was over 180 min in control cells but only 60 min in Usf1 KD cells (Figure S4A). Together this supports a role for USF1 in modulating the half-life of p53 under conditions of stress.
To examine whether impairment of p53 stabilization could be associated with the binding of USF1 with p53, overexpressed flag-tag p53 was immuno-precipitated from both Usf1 KD and control cells transfected as above (Figure 3G) and treated with or without MG132 and UVB. We observed an interaction of p53 with USF1 only in control cells and this interaction is notably increased after UV irradiation when the p53 protein is stabilized (Figure 3H, upper panel). In order to confirm this interaction between p53 and USF1, we performed immunoprecipitations assays with USF1 antibody in Usf1 KD and control cells, pre-treated with MG132 and following exposure to UVB. Again, only in the presence of USF1 was an interaction observed between USF1 and p53 which was particularly evident after UV irradiation (Figure 3H, lower panel). These results highlight the potential function of the USF1 transcription factor in stabilizing the p53 protein through a direct interaction.
USF1 associates with p53 and inhibits MDM2-mediated p53 degradation
Since stabilization of p53 in response to genotoxic-stress is dependent on the regulation of its proteasomal degradation, we measured the rate of p53-ubiquitination in the absence of USF1. The basal level of ubiquitinated flag-tag p53 was approximately three times higher in Usf1 KD than control cells (Figure 4A). Following MG132 treatment there was a substantial accumulation of ubiquitinated flag-tag p53 in Usf1 KD cells. Irradiation following MG132 treatment had almost no effect on the levels of ubiquitinated flag-tag p53 in Usf1 KD cells but this level was almost half in control cells (Figure 4A). These investigations demonstrate that USF1 interferes with the process of p53 ubiquitination and thereby maintains p53 stability following exposure to genotoxic agents.
Fig. 4. USF1 counteracts MDM2-mediated p53 degradation upon cellular stress. 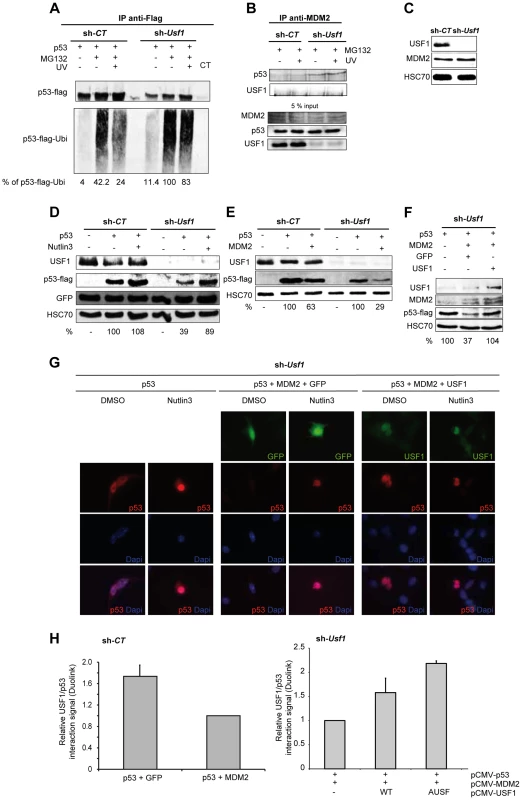
p53 protein-protein interactions and MDM2 mediated p53 degradation were studied in B16 melanoma cells knocked down for Usf1 (sh-Usf1) and their controls (sh-CT). (A–B) sh-CT and sh-Usf1 cells were treated with 10 µM MG132 for 3 hours then irradiated or not irradiated with UVB. Western blot analysis of proteins immunoprecipitated from cell lysates (A) Immunoprecipitation analysis to assay ubiquitinated flag-tagged p53 after transfection of sh-CT or sh-Usf1 with the corresponding cDNA. Cells were treated with MG132, were or were not irradiated with UVB and analyzed 3 hours later. The values reported indicate the level of p53 ubiquitination (normalized to the total amount of flag-tagged protein recovered). p53 expressing sh-Usf1 cells treated with MG132 has been arbitrary chosen as the reference (100%) since it is the condition where normalized-level of p53-ubiquitinated protein is the highest. (B) sh-CT and sh-Usf1 cells were treated with 10 µM MG132 for 3 hours then irradiated or not irradiated with UVB. Western blot analysis of proteins immunoprecipitated from cell lysates with a mix of two MDM2 antibodies (SMP14 and 3G9) and blotted with p53 antibody (1C12). (C) Western blot analysis showing basal levels of USF1, MDM2 and HSC70 (loading control) in sh-CT and sh-Usf1 cell lysates. (D) Western blot showing the effect of Nutlin-3 (10 µM, 6 h) treatment on the levels of flag-tagged p53 and GFP proteins in sh-CT and sh-Usf1 cells; antibodies to USF1, p53, GFP and HSC70 (loading control) were used. (E) Western blot analysis of p53, MDM2 and HSC70 (loading control) in sh-CT and sh-Usf1 cells over-expressing either p53 or p53 plus MDM2. (F) Same experiment as in D but in sh-Usf1 KD cells over-expressing either GFP or USF1. (G) Immunofluorescence analysis of p53 expression and localization in sh-Usf1 KD cells treated as in D and stimulated with vehicle (DMSO) or Nutlin-3 (10 µM) for 6 hours. Experiments have been done in triplicate and 15 to 20 microscopic fields analyzed per condition. (H) Quantification of the level of p53 and USF1 interaction in B16 melanoma cells using Thermo Scientific Cellomics HCS Solution (fluorescent microscopy) using Duolink PLA technology. Quantification of p53-USF1 interaction level using specific primary antibodies and Duolink PLA technology in B16 melanoma sh-CT cells over-expressing either p53 or p53 plus MDM2 (left panel). The graph represents the cumulative level of fluorescence observed in B16 cells under specific spotted form. p53 plus GFP is used as control condition. Quantification of p53-USF1 interaction level in B16 melanoma sh-Usf1 cells over-expressing p53 plus MDM2 and or not different forms of USF1 (wild type or negative dominant (AUSF)) (right panel). p53 plus MDM2 is used as control condition. MDM2 is the E3-ubiquitin ligase that interacts with p53 to promote p53 degradation by the proteasome and is therefore a central regulator of p53 stability [8]. We thus examined whether USF1 protects p53 from interacting with MDM2 and consequently preventing its degradation, by using immunoprecipitation assays performed with antibodies to MDM2 (Figure 4B). The anti-MDM2 antibody precipitated p53 with MDM2 from Usf1 KD cells but not from the control cells and UVB irradiation had no significant effect (Figure 4B). These results suggest that in the absence of USF1, the interaction between p53 and MDM2 is favored. These immunoprecipitation experiments showed that USF1 and MDM2 did not interact with one another. Also, MDM2 was similarly abundant in both control and knock down cell lines under all conditions tested, indicating that silencing Usf1 did not interfere with MDM2 levels (Figure 4, B and C). In addition, we demonstrate that the level of the MDM2 protein remains unchanged under the cell culture conditions previously tested (Figure S4 B–D). Together these results suggest that USF1 and MDM2 could bind p53 in a competitive manner.
To confirm that the interaction of MDM2 was responsible for the increased degradation of p53 in Usf1 KD cells, we examined the levels of flag-tag p53 in the presence of Nutlin-3, a specific inhibitor of the MDM2-p53 interaction (Figure 4D). Consistent with our previous results, the expression of flag-tag p53 in Usf1 KD cells was half that in control cells. A short treatment (6 h) with Nutlin-3 almost completely restored the level of flag-tag p53 in Usf1 KD cells. We next compared the ability of MDM2 to degrade p53 in the presence and absence of USF1. Control and Usf1 KD cells were transiently co-transfected with vectors expressing p53 and MDM2. Whereas co-expression with MDM2 led to a decrease of approximately 70% of p53 protein in Usf1 KD cells, there was only a 37% decrease in control cells (Figure 4E). When we restored USF1 expression, the degradation of p53 mediated by MDM2 was completly counteracted (Figure 4F). These results provide further evidence that USF1 contributes to protecting p53 from MDM2-mediated degradation.
MDM2 has been reported to promote nuclear export of p53 and thereby targetting it for degradation [35]. We therefore determined whether USF1 can interfere with MDM2 mediation of p53 cellular localization under conditions described in Figure 4F and using Nutlin-3. In the presence of Nutlin-3, the levels of p53 were exclusively nuclear and higher compared to control (vehicle). When p53 and MDM2 were co-expressed, p53 was completely degraded confirming the activity of MDM2 on p53. As expected, the presence of Nutlin-3 almost completely counteracted the MDM2-mediated degradation of p53 and led to accumulation of p53 in the nucleus. Finally, similar to the results obtained for Nutlin-3, co-expression of USF1 with p53 and MDM2 abolished p53 degradation and maintained the nuclear localization of the p53 protein (Figure 4G). Under comparable conditions of overexpression, we quantified the interaction between p53 and USF1 using the Duolink PLA techonology, and show that the number of p53/USF1 interactions decreased when MDM2 was co-expressed (Figure 4H). In addition, re-expression of USF1 (WT or AUSF forms), with p53 and MDM2 led to a significant increase of the number of p53/USF1 interactions (Figure 4H, right panel). Together, these data demonstrate that USF1 interferes with MDM2-mediated nuclear export of p53 and its subsequent degradation by directly interacting with p53.
Discussion
Activation of the p53 pathway in response to DNA damage is a critical mechanism that selectively directs cells to transient cell cycle arrest to favor DNA repair or to promote cell death when DNA damage is irreparable. Disruption of this protective pathway leads to an increase in cells' mutation load promoting genomic instability and frequently cancer development [36]. Our study demonstrates a new role for USF1 as a key upstream regulator of the p53 pathway. We provide compelling evidence that USF1 binds to p53 under UV stress conditions, preventing MDM2-mediated p53 degradation. Under stress conditions, USF1 thereby ensures the stability and tumor suppressor activity of the p53 protein. We thus propose that USF1 directs appropriate p53-dependent cell fate decisions in response to genotoxic stress (Figure 5).
Fig. 5. Model of regulation of p53 stabilization by USF1 in response to stress. 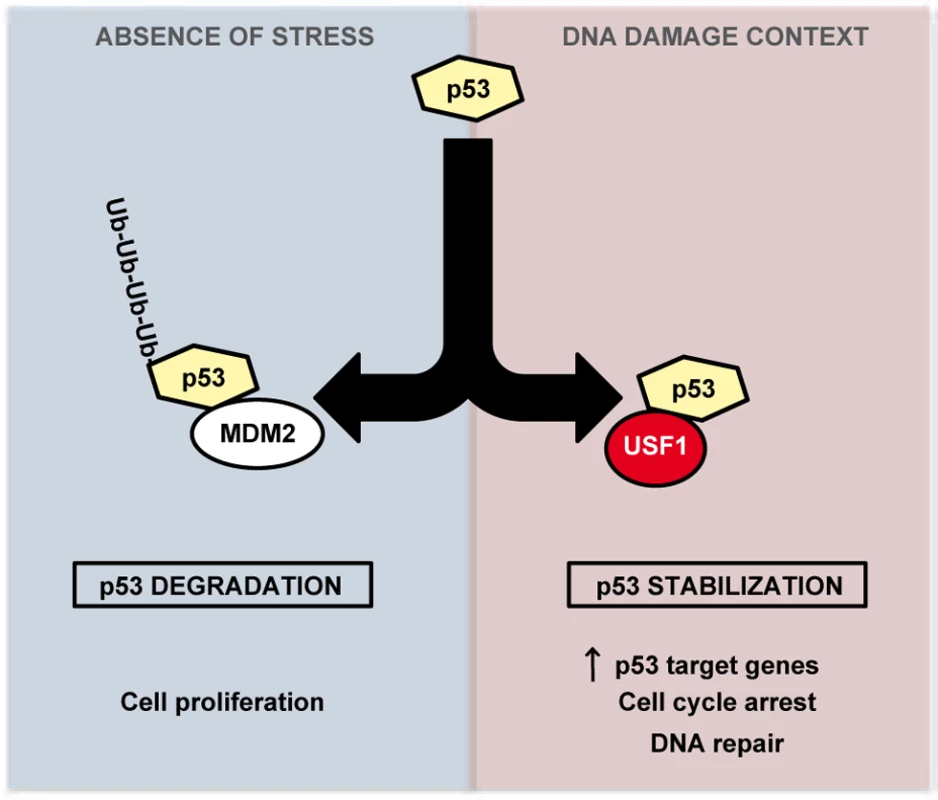
USF1 prevents MDM2-mediated p53 degradation under stress conditions, thereby ensuring the stability and tumor suppressor activity of the p53 protein. Left Panel, in the absence of stress, p53 is targeted to proteasomal degradation after binding to MDM2, maintaining cell proliferation. Right Panel, under DNA-damage context, USF1 counteracts MDM2 function by interacting with p53 thereby increasing its transcriptional activity to control transient cell cycle arrest and DNA repair processes. In the absence of USF1, p53 stabilization is abolished abrogating cell cycle control in response to DNA damage and thereby favoring genomic instability. The transcription factors USF1 and p53 are both associated with the stress response and with cell proliferation [37], [38], [39] and while there is evidence suggesting a link between them, this had not been demonstrated in vivo. For example, USF1 and p53 regulate the transcription of common target genes including APC, BRCA2 and hTERT [18], [40], [41], [42], [43], [44], and in vitro studies implicated USF1 in the basal regulation of the Trp53 promoter [22], [23]. Here, we report that, in mice skin and in B16 melanoma cells, USF1 drives DNA damage-induced cell cycle arrest by regulating p53 protein stability and function. Thus in vivo loss of USF1 parallels p53 deficency [27], [45], with altered proliferation control in the presence of DNA-damage. And like Trp53-/- [46], [47] or p21-/- cells [5], Usf1-/- primary fibroblasts bypass the transient cell cycle arrest triggered by DNA damaging radiation. We further found that under basal conditions the skin of WT and Usf1-/- mice contained similar levels of p53 mRNA and protein, but observed differences in p53 protein levels between the two genotypes after UVB challenge. Despite previous suggestions, we could not demonstrate a transcriptional link between USF1 and p53 [22], [23]. This could possibly be attributed to cell specificities or stress-dependent contexts in which levels and activities of transcriptional factors, required to drive Trp53 gene expression may vary [48]. Indeed, in studies involving human skin and keratinocytes UV-induced p53 was shown to be regulated only at the post-translational level but not at the mRNA level, while irritants that promote erythema induced p53 mRNA expression [49], [50]. These observations indicate that USF1 may act through more than one route to increase p53 levels according to cell type. In skin and in response to UV-induced DNA damage USF1 controls the p53 protein stability. This is expected to be of significant impact, since one attractive strategy for cancer therapy is based on p53 reactivation in cancers encoding normal but inactivated p53 protein, as observed frequently in melanomas [51], [52], [53].
Our evidence that USF1 stabilizes the p53 protein suggests that it may have functions independent of its well-defined role as a transcriptional regulator. Indeed, USF1 regulates gene expression through binding E-box regulatory elements in the promoters of target genes or by acting as a docking platform to recruit chromatin-modifying enzymes such as CBP/p300, PCAF, Set7/9, HDAC9 [54], [55], [56], [57], [58]. We now demonstrate that USF1 physically associates with the p53 complex. The ability of USF1 to promote p53 function appears to be independent from its ability to bind DNA. We cannot however exclude the possibility that the interaction between these two transcription factors may be important to bring p53 in the vicinity of p53 DNA-responsive element. Together this provides new prospects for how USF1 and p53 may regulate the expression of common target genes. In addition, it shows that USF1 can function through a new and unexpected mechanism to control cellular processes, broadening the role of USF1 and of members of the b-HLH-LZ transcription factors family.
Here we demonstrate that, in response to stress, USF1 associates with p53 to ensure p53 function. USF1 thereby prevents MDM2-mediated ubiquitination and subsequent degradation of p53. This mechanism relies on a stress-dependent association of USF1 with the p53 protein. Other stress-inducible transcription factors have been reported to contribute to the regulation of p53. For example, the transcription factor YY1 [59] enhances MDM2-mediated ubiquitination of p53 while ATF3 [60] prevents p53 ubiquitination and TAFII31 [61] prevents MDM2 association with p53. Although these transcription factors share a common role with USF1 in mediating p53 stability, the function of USF1 is not redundant since loss of USF, on its own, impedes p53 stabilization. The importance of USF1 in regulating p53 function may first be attributed to their hierarchical relation. Trp53 KD cells express normal levels of USF1 but they are not able to direct cell cycle arrest as observed for Usf1 KD cells. Similarly, overexpression of USF1 in p53-null Saos2-cells is not able to mimic the effect of p53 on cell proliferation, while USF1 promotes p53 function in p53 expressing cells [62]. USF1 is thus proposed to operate as an upstream regulator of p53 stability and function. Second, the abundance of USF1 may also support its critical role in directing p53 function. Indeed, while USF1 is constitutively expressed, ATF3 and YY1 mediated p53-interaction requires their induction in response to genotoxic stress [59], [60]. This suggests that USF1 is an immediate regulator of p53 stabilization in response to genotoxic stress. This however does not exclude the possibility that these transcription factors could act sequentially. Why and how the association of one factor with p53 is favored over another remains however to be elucidated. One possibility could be that the nature and intensity of the DNA damage regulate this to influence p53-directed cell fate [13], [63].
To date, the implication of USF1 in cancer development has been investigated through only one angle, its function as a transcription factor. SNPs affecting USF1 binding to the Pten promoter have been found to be associated with Cowden syndrome [64]; the loss of USF1 transcriptional activity has been described in several breast cancer cell lines [65] and the activation of the hTERT gene in oral tumors is associated with the decreased expression of USF1 and USF2 [66]. Together, this supports the transcriptional role of USF1 in cancer development, although no association has been reported between mutations in the USF1 coding sequence and UV-induced cancer or other cancers [67], [68], [69]. Cancers are associated with exposure to environmental and biological carcinogens, including virus infection, tobacco smoke and sunlight, both of which promote DNA hypermethylation [70]. Interestingly, oncogenic transformation by Helicobacter pylori infection is associated with the methylation of the USF1 promoter and subsequent inhibition of USF1 protein production [71]. In addition, Helicobacter pylori infection has been also shown to impair p53 protein stability [72]. It remains to be elucidated whether this mechanism of stress-induced epigenetic transformation contributes to silencing of USF1 and thus impairing p53 stability and whether it is a new mechanism of how p53 loss of function may occur in cancer cells.
In this work we demonstrate that USF1 is a critical stress sensor required to direct appropriate p53-dependent cell fate decisions. USF1 operates through a new and unexpected function revealing additional functions for bHLH-LZ factors. Finally, our findings suggest that the loss of USF1 expression should be consider as a potential initiator of tumorigenesis in the context of environmental insults.
Materials and Methods
Mouse skin irradiation
Usf1-/- (KO) and Usf1+/+ (WT) mice were kindly provided by Sophie Vaulont (Cochin Institute) [73]. Animals 8–12 weeks old were used for UV irradiation experiments. Mice were maintained under specific pathogen-free (SPF) conditions in our accredited animal facilities (A 35 – 238 – 40). For in vivo irradiation, the backs of the mice were shaved, and one area was protected (non-exposed control) and another irradiated (exposed area). For ex vivo analysis, skin biopsies (0.8 cm diameter) were recovered from the back of WT and Usf1-/- mice and maintained in culture as previously described (Baron Y. et al., 2012). Skins were irradiated with a single UVB dose (312 nm, 5 kJ/m2) using the Stratalinker apparatus (Stratagene). This dose corresponds to the minimal erythema dose (MED) of these mice, inducing erythema 24 h later.
Ethics statement
The present animal study follows the 3R legislation (Replace-Reduce-Refine). It has been declared and approved by the French Government Board (N°5347). Animal welfare is a constant priority: animals were thus sacrificed under anesthesia.
Cell culture, small hairpin (sh) RNA transductions, and inhibitor treatments
Mice primary fibroblasts were isolated by collagenase dissociation of skin dermis from Usf1-/- and Usf1+/+ mice [74]. Cells were cultured in DMEM (Invitrogen) medium containing 10% FBS and 1% penicillin-streptomycin at 37°C under a 5% CO2 atmosphere for one week before the irradiation protocol. Cells were then irradiated with 0.6 kJ/m2 UVB and harvested at the indicated time points.
B16 mice melanoma cells were transduced with lentiviral particles containing a vector carrying an shRNA (Sigma) targeting the murine Usf1 mRNA (sh-Usf1 SHCLNV-NM_009480 clone TRCN0000302005) or the Trp53 mRNA (sh-Trp53; SHCLNV-NM_011640 clone TRCN000030210844), or carrying scrambled shRNA (sh-CT (SHC002V). After infection, cells were maintained under selection in the presence of puromycin (Invitrogen). Cells were then routinely cultured in RPMI (Invitrogen) supplemented with 10% FBS and 1% penicillin-streptomycin at 37°C under a 10% CO2 atmosphere. Cell were irradiated with 0.3 kJ/m2 UVB and harvested at the indicated time points. For MG132 assays, cells were treated with 10 µM Z-Leu-Leu-al (Sigma) in RPMI (Invitrogen) medium. For cycloheximide (CHX) treatment, after 3 h of MG132 treatment the culture medium was removed and replaced by medium containing 20 µM CHX (Sigma). For Nutlin-3 treatments, cells were stimulated with 10 µM of Nutlin-3 (Santa cruz).
Cell cycle synchronization, cell viability and BrdU incorporation analysis
B16 melanoma cells were synchronized in G1/S phase following a double thymidine/aphidicolin block (16 h with 2 mM thymidine, released for 9 hours and then 16 h with 5 µg/ml aphidicolin).Cell viability following exposure to UV was measured using MTT test as previously described [21].
BrdU analysis was carried using an in situ BrdU detection kit (BD Biosciences): as recommended by the manufacturer. Positively stained cells (BrdU positives) and total cells (hematoxylin stained) in 10 randomly selected microscopic fields (x100) were counted for each condition.
Gene expression analysis
RNA extraction and RT-PCRq were as previously described [21]. Relative amounts of transcripts were determined using the delta Ct method. Data were normalized to the values for the HPRT transcript. Forward (F) and reverse (R) primers were designed using the Universal Probe Library Assay Design Center (Roche) and their efficiency has been confirmed.
Western blotting and immunoprecipitation
Mouse skin proteins were extracted by pottering 8 mm diameter skin biopsies in liquid nitrogen; the resulting powders were lysed in a lysis buffer containing 10 mM EDTA, 50 mM pH 8 Tris-HCl, 0.5% Empigen BB, 1% SDS, 25 mM NaF, 1 mM orthovanadate, 25 mM β-glycerophosphate, and 1x protease inhibitor cocktail (Roche Diagnostics). Cell culture protein lysates were obtained by scraping off cells in NP40 lysis buffer: 20 mM Tris pH 7.5, 100 mM NaCl, 20 mM β-glycerophosphate, 5 mM MgCl2, 0.2% NP-40, 10% glycerol, 1 mM NaF, 0.5 mM DTT, 1x protease inhibitor cocktail. Equal amounts of protein (30 µg), quantified using the BCA protein assay (Sigma) were denaturated in Laemmli buffer for 5 min at 95°C and resolved by 15% SDS-PAGE. Membranes were probed with appropriate antibodies and signals detected using the LAS-3000 Imaging System (Fujifilm) were quantified with ImageJ (http://rsbweb.nih.gov/ij/). The following primary antibody were used: anti-USF1 (C:20), anti-HSC70 (B-6), anti-MDM2 (SMP14), anti-GFP (Santa Cruz), anti-CPD (TDM2) (MBL), anti-p53 (1C12), anti-phospho H2AX Ser139 (γH2AX) (Cell Signaling), anti-total histone H2AX, anti-p21 (Abcam), and anti-MDM2 (3G9) (Millipore) and anti-Ubiquitin (Dako).
Co-immuno-precipitation experiments were performed using 1 mg of protein with 2 µg of Rabbit IgG (Jackson ImmunoResearch, West Grove, PA) as negative control or with 2 µg of USF1 antibodies (C:20) or MDM2 antibodies (SMP14 and 3G9) and incubated overnight at 4°C. Flag-tag proteins were immunoprecipitated using the flag immunoprecipitation kit (Sigma). Immunocomplexes were isolated using Protein A-G sepharose beads.
Luciferase activity and transitory transfections
To analyze the transcriptional activity of p53, B16 melanoma cells in 10 cm-diameter dishes were transiently transfected with 5 µg of pG13-Luc (carrying a p53-responsive element; [75]) alone or in combination with 6 µg of pCMV GFP (encoding the GFP) or pCMV-USF1 (WT, T153E, T153A, AUSF (negative dominant; [15]), or pCAG3.1 (encoding p53; [76]) and incubated for 24 h. Cells were then passaged in 12-well plates and irradiated 24 h post passage (a total of 48 h post transfection). Luciferase analysis was conducted using the DUAL-Luciferase reporter assay kit according to the manufacturer's recommendations (Promega).
To study p53 degradation in the presence of MDM2 B16 melanoma cells in 6-well plates were transfected with 1 µg of a plasmid encoding flag-tagged p53 protein (Flag-p53/pRK5; Addgen, Plasmid 39237) alone or in combination with 2 µg of a plasmid encoding the MDM2 protein (pCMV-myc3-HDM2; Addgen, Plasmid 20935). For p53 stabilization rescue analysis in sh-Usf1 KD cells, cells were co-transfected with 2 µg of plasmid encoding GFP protein or USF1 wild type protein [15], together with 1 µg of Flag-p53/pRK5 plus 2 µg of pCMV-myc3-HDM2. The amount of plasmid DNA used for transfection was adjusted with empty pCMV plasmid to be equal in every case.
CPD quantification by ELISA
CPD in skin tissues was assayed by ELISA, according to Cosmo bio recommendations (Cosmo Bio Co., LTD., Japan). DNA was purified with Nucleospin tissue extraction kits (Macherey-Nagel, Düren, Germany). Briefly, 200 ng aliquots of denatured DNA were distributed into 96-well plates precoated with protamine sulfate (Polyvinylchloride flat-bottom). DNA lesions were detected with specific mouse anti-CPD antibodies [21], and bound antibody was revealed with a biotin/peroxidase-streptovidin system. The absorbance at 492 nm was used for quantitative measurements.
Immuno-histochemistry and immunofluorescence staining
Skin biopsies were fixed in formalin (4%) and embedded in paraffin. Paraffin-embedded tissu was cut into 4 µm-thick slices, mounted on slides and dried at 58°C for 60 minutes. A Discovery Automated IHC stainer and the Ventana DABMap detection kit (Ventana Medical Systems) were used for immunohistochemical staining. For DAB, detection slides were incubated with rabbit monoclonal anti-CPD antibody (TDM, MBL) and bound antibody was revealed with biotinylated goat anti-rabbit secondary antibody (Vector laboratory, USA). Slides were then counterstained for 4 minutes with hematoxylin and rinsed. For fluorescence analysis, slides were incubated with rabbit monoclonal Ki-67 (SP6, bioscience leasanton CA) and bound antibody was detected with secondary anti-rabbit FITC-conjugated antibody. Slides were coverslipped in aqueous mounting medium containing DAPI. The numbers of Ki-67-positive cells were evaluated by counting the percentage of interfollicular-positive cells (Ki-67 among Dapi-stained cells) in 10 different microscope fields (x40) per skin section.
For cyto-immunofluorecence staining, cells grown on coverslip chambers were fixed with formalin (4%) for 20 min, washed with PBS, quenched with 50 mM NH4Cl for 20 min and washed once with PBS. The cells were permabilized with 0.1% Triton and saturated with 1% PBS/BSA; 15 min later, the primary antibody was added. Bound antibody was reveled with anti-rabbit Alexa 488 or anti-mouse FITC 588 secondary antibodies (Jackson). Experiments have been done in triplicate and 15 to 20 microscopic fields analyzed per condition. A minimum of one hundered cells were analyzed per condition.
Detection of protein interactions with Duolink using PLA technology (Sigma)
B16 melanoma were transiently or not transfected with pCAG3.1 (encoding p53; [76]), pCMV-MDM2 (encoding MDM2 protein), and pCMV-GFP (encoding the GFP protein) or various pCMV-USF1 expressing vector (WT and AUSF forms; [15]), and incubated for 24 h. Cells were then passaged in 96-well plates and fixed using PFA 24 h post passage (a total of 48 h post transfection). Protein-protein (USF1 and p53) or (p53 and MDM2) interactions in B16 melanoma cells were then analyzed following recommanded protocol by manufacturer (Sigma Aldrich) and visualized in collaboration with the ImPACcell plateform (SFR Biosit, University of Rennes, France) using Thermo Scientific Cellomics HCS Solution. For quantification, a minimum of 15 microscopic fields were analyzed and the signal were counted in a minimum of 60 cells. The following primary antibody were used: rabbit anti-USF1 (C:20), mouse anti-MDM2 and mouse anti-p53 (1C12) or rabbit anti-p53 (Fl-393).
Supporting Information
Zdroje
1. HanahanD, WeinbergRA (2011) Hallmarks of cancer: the next generation. Cell 144 : 646–674.
2. LawrenceMS, StojanovP, PolakP, KryukovGV, CibulskisK, et al. (2013) Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499 : 214–218.
3. SancarA, Lindsey-BoltzLA, Unsal-KacmazK, LinnS (2004) Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 73 : 39–85.
4. OrenM (2003) Decision making by p53: life, death and cancer. Cell Death Differ 10 : 431–442.
5. BrugarolasJ, ChandrasekaranC, GordonJI, BeachD, JacksT, et al. (1995) Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377 : 552–557.
6. DengC, ZhangP, HarperJW, ElledgeSJ, LederP (1995) Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82 : 675–684.
7. HarperJW, AdamiGR, WeiN, KeyomarsiK, ElledgeSJ (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75 : 805–816.
8. FuchsSY, AdlerV, BuschmannT, WuX, RonaiZ (1998) Mdm2 association with p53 targets its ubiquitination. Oncogene 17 : 2543–2547.
9. KruseJP, GuW (2009) Modes of p53 regulation. Cell 137 : 609–622.
10. MeekDW, AndersonCW (2009) Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harb Perspect Biol 1: a000950.
11. Gomez-LazaroM, Fernandez-GomezFJ, JordanJ (2004) p53: twenty five years understanding the mechanism of genome protection. J Physiol Biochem 60 : 287–307.
12. OlssonA, ManzlC, StrasserA, VillungerA (2007) How important are post-translational modifications in p53 for selectivity in target-gene transcription and tumour suppression? Cell Death Differ 14 : 1561–1575.
13. PietenpolJA, StewartZA (2002) Cell cycle checkpoint signaling: cell cycle arrest versus apoptosis. Toxicology 181–182 : 475–481.
14. CorreS, PrimotA, BaronY, Le SeyecJ, GodingC, et al. (2009) Target gene specificity of USF-1 is directed via p38-mediated phosphorylation-dependent acetylation. J Biol Chem 284 : 18851–18862.
15. CorreS, PrimotA, SviderskayaE, BennettDC, VaulontS, et al. (2004) UV-induced expression of key component of the tanning process, the POMC and MC1R genes, is dependent on the p-38-activated upstream stimulating factor-1 (USF-1). J Biol Chem 279 : 51226–51233.
16. GalibertMD, CarreiraS, GodingCR (2001) The Usf-1 transcription factor is a novel target for the stress-responsive p38 kinase and mediates UV-induced Tyrosinase expression. EMBO J 20 : 5022–5031.
17. IkehataH, OnoT (2011) The mechanisms of UV mutagenesis. J Radiat Res 52 : 115–125.
18. NarayananDL, SaladiRN, FoxJL (2010) Ultraviolet radiation and skin cancer. Int J Dermatol 49 : 978–986.
19. RavanatJL, DoukiT, CadetJ (2001) Direct and indirect effects of UV radiation on DNA and its components. J Photochem Photobiol B 63 : 88–102.
20. ZaidiMR, DayCP, MerlinoG (2008) From UVs to metastases: modeling melanoma initiation and progression in the mouse. J Invest Dermatol 128 : 2381–2391.
21. BaronY, CorreS, MouchetN, VaulontS, PrinceS, et al. (2012) USF-1 is critical for maintaining genome integrity in response to UV-induced DNA photolesions. PLoS Genet 8: e1002470.
22. ReismanD, RotterV (1993) The helix-loop-helix containing transcription factor USF binds to and transactivates the promoter of the p53 tumor suppressor gene. Nucleic Acids Res 21 : 345–350.
23. HaleTK, BraithwaiteAW (1995) Identification of an upstream region of the mouse p53 promoter critical for transcriptional expression. Nucleic Acids Res 23 : 663–669.
24. CampbellC, QuinnAG, AngusB, FarrPM, ReesJL (1993) Wavelength specific patterns of p53 induction in human skin following exposure to UV radiation. Cancer Res 53 : 2697–2699.
25. AuclairY, RougetR, Affar elB, DrobetskyEA (2008) ATR kinase is required for global genomic nucleotide excision repair exclusively during S phase in human cells. Proc Natl Acad Sci U S A 105 : 17896–17901.
26. BonnerWM, RedonCE, DickeyJS, NakamuraAJ, SedelnikovaOA, et al. (2008) GammaH2AX and cancer. Nat Rev Cancer 8 : 957–967.
27. JimenezGS, NisterM, StommelJM, BeecheM, BarcarseEA, et al. (2000) A transactivation-deficient mouse model provides insights into Trp53 regulation and function. Nat Genet 26 : 37–43.
28. LiG, MitchellDL, HoVC, ReedJC, TronVA (1996) Decreased DNA repair but normal apoptosis in ultraviolet-irradiated skin of p53-transgenic mice. Am J Pathol 148 : 1113–1123.
29. McCormickD, ChongH, HobbsC, DattaC, HallPA (1993) Detection of the Ki-67 antigen in fixed and wax-embedded sections with the monoclonal antibody MIB1. Histopathology 22 : 355–360.
30. AndorferP, SchwarzmayrL, RothenederH (2011) EAPP modulates the activity of p21 and Chk2. Cell Cycle 10 : 2077–2082.
31. Hyka-NouspikelN, DesmaraisJ, GokhalePJ, JonesM, MeuthM, et al. (2012) Deficient DNA damage response and cell cycle checkpoints lead to accumulation of point mutations in human embryonic stem cells. Stem Cells 30 : 1901–1910.
32. FerreonJC, LeeCW, AraiM, Martinez-YamoutMA, DysonHJ, et al. (2009) Cooperative regulation of p53 by modulation of ternary complex formation with CBP/p300 and HDM2. Proc Natl Acad Sci U S A 106 : 6591–6596.
33. ShiehSY, IkedaM, TayaY, PrivesC (1997) DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91 : 325–334.
34. ShiehSY, AhnJ, TamaiK, TayaY, PrivesC (2000) The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev 14 : 289–300.
35. TaoW, LevineAJ (1999) P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl Acad Sci U S A 96 : 6937–6941.
36. NakanishiM, NiidaH, MurakamiH, ShimadaM (2009) DNA damage responses in skin biology—implications in tumor prevention and aging acceleration. J Dermatol Sci 56 : 76–81.
37. JungHS, KimKS, ChungYJ, ChungHK, MinYK, et al. (2007) USF inhibits cell proliferation through delay in G2/M phase in FRTL-5 cells. Endocr J 54 : 275–285.
38. Loayza-PuchF, DrostJ, RooijersK, LopesR, ElkonR, et al. (2013) p53 induces transcriptional and translational programs to suppress cell proliferation and growth. Genome Biol 14: R32.
39. LuoX, SawadogoM (1996) Antiproliferative properties of the USF family of helix-loop-helix transcription factors. Proc Natl Acad Sci U S A 93 : 1308–1313.
40. DavisPL, MironA, AndersenLM, IglehartJD, MarksJR (1999) Isolation and initial characterization of the BRCA2 promoter. Oncogene 18 : 6000–6012.
41. GoueliBS, JanknechtR (2003) Regulation of telomerase reverse transcriptase gene activity by upstream stimulatory factor. Oncogene 22 : 8042–8047.
42. JaiswalAS, NarayanS (2001) Upstream stimulating factor-1 (USF1) and USF2 bind to and activate the promoter of the adenomatous polyposis coli (APC) tumor suppressor gene. J Cell Biochem 81 : 262–277.
43. KanayaT, KyoS, HamadaK, TakakuraM, KitagawaY, et al. (2000) Adenoviral expression of p53 represses telomerase activity through down-regulation of human telomerase reverse transcriptase transcription. Clin Cancer Res 6 : 1239–1247.
44. WuK, JiangSW, CouchFJ (2003) p53 mediates repression of the BRCA2 promoter and down-regulation of BRCA2 mRNA and protein levels in response to DNA damage. J Biol Chem 278 : 15652–15660.
45. IkehataH, OkuyamaR, OgawaE, NakamuraS, UsamiA, et al. (2010) Influences of p53 deficiency on the apoptotic response, DNA damage removal and mutagenesis in UVB-exposed mouse skin. Mutagenesis 25 : 397–405.
46. HuangLC, ClarkinKC, WahlGM (1996) Sensitivity and selectivity of the DNA damage sensor responsible for activating p53-dependent G1 arrest. Proc Natl Acad Sci U S A 93 : 4827–4832.
47. KuerbitzSJ, PlunkettBS, WalshWV, KastanMB (1992) Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A 89 : 7491–7495.
48. Saldana-MeyerR, Recillas-TargaF (2011) Transcriptional and epigenetic regulation of the p53 tumor suppressor gene. Epigenetics 6 : 1068–1077.
49. HealyE, ReynoldsNJ, SmithMD, CampbellC, FarrPM, et al. (1994) Dissociation of erythema and p53 protein expression in human skin following UVB irradiation, and induction of p53 protein and mRNA following application of skin irritants. J Invest Dermatol 103 : 493–499.
50. LiuM, DhanwadaKR, BirtDF, HechtS, PellingJC (1994) Increase in p53 protein half-life in mouse keratinocytes following UV-B irradiation. Carcinogenesis 15 : 1089–1092.
51. GembarskaA, LucianiF, FedeleC, RussellEA, DewaeleM, et al. (2012) MDM4 is a key therapeutic target in cutaneous melanoma. Nat Med 18 : 1239–47.
52. JiZ, KumarR, TaylorM, RajaduraiA, Marzuka-AlcalaA, et al. (2013) Vemurafenib Synergizes with Nutlin-3 to Deplete Survivin and Suppress Melanoma Viability and Tumor Growth. Clin Cancer Res 19 : 4383–91.
53. LuM, BreyssensH, SalterV, ZhongS, HuY, et al. (2013) Restoring p53 function in human melanoma cells by inhibiting MDM2 and cyclin B1/CDK1-phosphorylated nuclear iASPP. Cancer Cell 23 : 618–633.
54. ChatterjeeTK, IdelmanG, BlancoV, BlomkalnsAL, PiegoreMGJr, et al. (2011) Histone deacetylase 9 is a negative regulator of adipogenic differentiation. J Biol Chem 286 : 27836–27847.
55. Crusselle-DavisVJ, VieiraKF, ZhouZ, AnantharamanA, BungertJ (2006) Antagonistic regulation of beta-globin gene expression by helix-loop-helix proteins USF and TFII-I. Mol Cell Biol 26 : 6832–6843.
56. HuangS, LiX, YusufzaiTM, QiuY, FelsenfeldG (2007) USF1 recruits histone modification complexes and is critical for maintenance of a chromatin barrier. Mol Cell Biol 27 : 7991–8002.
57. WangY, WongRH, TangT, HudakCS, YangD, et al. (2013) Phosphorylation and recruitment of BAF60c in chromatin remodeling for lipogenesis in response to insulin. Mol Cell 49 : 283–297.
58. WongRH, ChangI, HudakCS, HyunS, KwanHY, et al. (2009) A role of DNA-PK for the metabolic gene regulation in response to insulin. Cell 136 : 1056–1072.
59. SuiG, Affar elB, ShiY, BrignoneC, WallNR, et al. (2004) Yin Yang 1 is a negative regulator of p53. Cell 117 : 859–872.
60. YanC, LuD, HaiT, BoydDD (2005) Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. EMBO J 24 : 2425–2435.
61. BuschmannT, LinY, AithmittiN, FuchsSY, LuH, et al. (2001) Stabilization and activation of p53 by the coactivator protein TAFII31. J Biol Chem 276 : 13852–13857.
62. QyangY, LuoX, LuT, IsmailPM, KrylovD, et al. (1999) Cell-type-dependent activity of the ubiquitous transcription factor USF in cellular proliferation and transcriptional activation. Mol Cell Biol 19 : 1508–1517.
63. ChangD, ChenF, ZhangF, McKayBC, LjungmanM (1999) Dose-dependent effects of DNA-damaging agents on p53-mediated cell cycle arrest. Cell Growth Differ 10 : 155–162.
64. PezzolesiMG, ZbukKM, WaiteKA, EngC (2007) Comparative genomic and functional analyses reveal a novel cis-acting PTEN regulatory element as a highly conserved functional E-box motif deleted in Cowden syndrome. Hum Mol Genet 16 : 1058–1071.
65. IsmailPM, LuT, SawadogoM (1999) Loss of USF transcriptional activity in breast cancer cell lines. Oncogene 18 : 5582–5591.
66. ChangJT, YangHT, WangTC, ChengAJ (2005) Upstream stimulatory factor (USF) as a transcriptional suppressor of human telomerase reverse transcriptase (hTERT) in oral cancer cells. Mol Carcinog 44 : 183–192.
67. Cleary SP, Jeck WR, Zhao X, Kuichen Selitsky SR, et al.. (2013) Identification of driver genes in hepatocellular carcinoma by exome sequencing. Hepatology.
68. KrauthammerM, KongY, HaBH, EvansP, BacchiocchiA, et al. (2012) Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 44 : 1006–1014.
69. MorinRD, MungallK, PleasanceE, MungallAJ, GoyaR, et al. (2013) Mutational and structural analysis of diffuse large B-cell lymphoma using whole genome sequencing. Blood 122 : 1256–65.
70. ZeilingerS, KuhnelB, KloppN, BaurechtH, KleinschmidtA, et al. (2013) Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One 8: e63812.
71. BussiereFI, MichelV, MemetS, AveP, VivasJR, et al. (2010) H. pylori-induced promoter hypermethylation downregulates USF1 and USF2 transcription factor gene expression. Cell Microbiol 12 : 1124–1133.
72. WeiJ, NagyTA, VilgelmA, ZaikaE, OgdenSR, et al. (2010) Regulation of p53 tumor suppressor by Helicobacter pylori in gastric epithelial cells. Gastroenterology 139 : 1333–1343.
73. ValletVS, CasadoM, HenrionAA, BucchiniD, RaymondjeanM, et al. (1998) Differential roles of upstream stimulatory factors 1 and 2 in the transcriptional response of liver genes to glucose. J Biol Chem 273 : 20175–20179.
74. LichtiU, AndersJ, YuspaSH (2008) Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc 3 : 799–810.
75. KernSE, PietenpolJA, ThiagalingamS, SeymourA, KinzlerKW, et al. (1992) Oncogenic forms of p53 inhibit p53-regulated gene expression. Science 256 : 827–830.
76. SaitoS, GoodarziAA, HigashimotoY, NodaY, Lees-MillerSP, et al. (2002) ATM mediates phosphorylation at multiple p53 sites, including Ser(46), in response to ionizing radiation. J Biol Chem 277 : 12491–12494.
Štítky
Genetika Reprodukční medicína
Článek Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin PathwayČlánek Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human DiseasesČlánek G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify LongevityČlánek PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial MatrixČlánek Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus ConflictsČlánek The Impact of Population Demography and Selection on the Genetic Architecture of Complex TraitsČlánek Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar AcidificationČlánek The Case for Junk DNA
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 5- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Genetic Interactions Involving Five or More Genes Contribute to a Complex Trait in Yeast
- A Mutation in the Gene in Dogs with Hereditary Footpad Hyperkeratosis (HFH)
- Loss of Function Mutation in the Palmitoyl-Transferase HHAT Leads to Syndromic 46,XY Disorder of Sex Development by Impeding Hedgehog Protein Palmitoylation and Signaling
- Heterogeneity in the Frequency and Characteristics of Homologous Recombination in Pneumococcal Evolution
- Genome-Wide Nucleosome Positioning Is Orchestrated by Genomic Regions Associated with DNase I Hypersensitivity in Rice
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Single Nucleotide Variants in Transcription Factors Associate More Tightly with Phenotype than with Gene Expression
- Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin Pathway
- Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human Diseases
- Epistatically Interacting Substitutions Are Enriched during Adaptive Protein Evolution
- Meiotic Drive Impacts Expression and Evolution of X-Linked Genes in Stalk-Eyed Flies
- G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify Longevity
- Population Genomic Analysis of Ancient and Modern Genomes Yields New Insights into the Genetic Ancestry of the Tyrolean Iceman and the Genetic Structure of Europe
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
- Whole Exome Re-Sequencing Implicates and Cilia Structure and Function in Resistance to Smoking Related Airflow Obstruction
- Allelic Expression of Deleterious Protein-Coding Variants across Human Tissues
- R-loops Associated with Triplet Repeat Expansions Promote Gene Silencing in Friedreich Ataxia and Fragile X Syndrome
- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- The Impairment of MAGMAS Function in Human Is Responsible for a Severe Skeletal Dysplasia
- Octopamine Neuromodulation Regulates Gr32a-Linked Aggression and Courtship Pathways in Males
- Mlh2 Is an Accessory Factor for DNA Mismatch Repair in
- Activating Transcription Factor 6 Is Necessary and Sufficient for Alcoholic Fatty Liver Disease in Zebrafish
- The Spatiotemporal Program of DNA Replication Is Associated with Specific Combinations of Chromatin Marks in Human Cells
- Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus Conflicts
- Genome-Wide Inference of Ancestral Recombination Graphs
- Mutations in Four Glycosyl Hydrolases Reveal a Highly Coordinated Pathway for Rhodopsin Biosynthesis and N-Glycan Trimming in
- SHP2 Regulates Chondrocyte Terminal Differentiation, Growth Plate Architecture and Skeletal Cell Fates
- The Impact of Population Demography and Selection on the Genetic Architecture of Complex Traits
- Retinoid-X-Receptors (α/β) in Melanocytes Modulate Innate Immune Responses and Differentially Regulate Cell Survival following UV Irradiation
- Genetic Dissection of the Female Head Transcriptome Reveals Widespread Allelic Heterogeneity
- Genome Sequencing and Comparative Genomics of the Broad Host-Range Pathogen AG8
- Copy Number Variation Is a Fundamental Aspect of the Placental Genome
- GOLPH3 Is Essential for Contractile Ring Formation and Rab11 Localization to the Cleavage Site during Cytokinesis in
- Hox Transcription Factors Access the RNA Polymerase II Machinery through Direct Homeodomain Binding to a Conserved Motif of Mediator Subunit Med19
- Drosha Promotes Splicing of a Pre-microRNA-like Alternative Exon
- Predicting the Minimal Translation Apparatus: Lessons from the Reductive Evolution of
- PAX6 Regulates Melanogenesis in the Retinal Pigmented Epithelium through Feed-Forward Regulatory Interactions with MITF
- Enhanced Interaction between Pseudokinase and Kinase Domains in Gcn2 stimulates eIF2α Phosphorylation in Starved Cells
- A HECT Ubiquitin-Protein Ligase as a Novel Candidate Gene for Altered Quinine and Quinidine Responses in
- dGTP Starvation in Provides New Insights into the Thymineless-Death Phenomenon
- Phosphorylation Modulates Clearance of Alpha-Synuclein Inclusions in a Yeast Model of Parkinson's Disease
- RPM-1 Uses Both Ubiquitin Ligase and Phosphatase-Based Mechanisms to Regulate DLK-1 during Neuronal Development
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- Heritable Transmission of Stress Resistance by High Dietary Glucose in
- Revertant Mutation Releases Confined Lethal Mutation, Opening Pandora's Box: A Novel Genetic Pathogenesis
- Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar Acidification
- A Genome-Wide Assessment of the Role of Untagged Copy Number Variants in Type 1 Diabetes
- Selectivity in Genetic Association with Sub-classified Migraine in Women
- A Lack of Parasitic Reduction in the Obligate Parasitic Green Alga
- The Proper Splicing of RNAi Factors Is Critical for Pericentric Heterochromatin Assembly in Fission Yeast
- Discovery and Functional Annotation of SIX6 Variants in Primary Open-Angle Glaucoma
- Six Homeoproteins and a linc-RNA at the Fast MYH Locus Lock Fast Myofiber Terminal Phenotype
- EDR1 Physically Interacts with MKK4/MKK5 and Negatively Regulates a MAP Kinase Cascade to Modulate Plant Innate Immunity
- Genes That Bias Mendelian Segregation
- The Case for Junk DNA
- An In Vivo EGF Receptor Localization Screen in Identifies the Ezrin Homolog ERM-1 as a Temporal Regulator of Signaling
- Mosaic Epigenetic Dysregulation of Ectodermal Cells in Autism Spectrum Disorder
- Hyperactivated Wnt Signaling Induces Synthetic Lethal Interaction with Rb Inactivation by Elevating TORC1 Activities
- Mutations in the Cholesterol Transporter Gene Are Associated with Excessive Hair Overgrowth
- Scribble Modulates the MAPK/Fra1 Pathway to Disrupt Luminal and Ductal Integrity and Suppress Tumour Formation in the Mammary Gland
- A Novel CH Transcription Factor that Regulates Expression Interdependently with GliZ in
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics
- Spermatid Cyst Polarization in Depends upon and the CPEB Family Translational Regulator
- Insights into the Genetic Structure and Diversity of 38 South Asian Indians from Deep Whole-Genome Sequencing
- Intron Retention in the 5′UTR of the Novel ZIF2 Transporter Enhances Translation to Promote Zinc Tolerance in
- A Dominant-Negative Mutation of Mouse Causes Glaucoma and Is Semi-lethal via LBD1-Mediated Dimerisation
- Biased, Non-equivalent Gene-Proximal and -Distal Binding Motifs of Orphan Nuclear Receptor TR4 in Primary Human Erythroid Cells
- Ras-Mediated Deregulation of the Circadian Clock in Cancer
- Retinoic Acid-Related Orphan Receptor γ (RORγ): A Novel Participant in the Diurnal Regulation of Hepatic Gluconeogenesis and Insulin Sensitivity
- Extensive Diversity of Prion Strains Is Defined by Differential Chaperone Interactions and Distinct Amyloidogenic Regions
- Fine Tuning of the UPR by the Ubiquitin Ligases Siah1/2
- Paternal Poly (ADP-ribose) Metabolism Modulates Retention of Inheritable Sperm Histones and Early Embryonic Gene Expression
- Allele-Specific Genome-wide Profiling in Human Primary Erythroblasts Reveal Replication Program Organization
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



