-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Allelic Expression of Deleterious Protein-Coding Variants across Human Tissues
Gene expression is a fundamental cellular process that contributes to phenotypic diversity. Gene expression can vary between alleles of an individual through differences in genomic imprinting or cis-acting regulatory variation. Distinguishing allelic activity is important for informing the abundance of altered mRNA and protein products. Advances in sequencing technologies allow us to quantify patterns of allele-specific expression (ASE) in different individuals and cell-types. Previous studies have identified patterns of ASE across human populations for single cell-types; however the degree of tissue-specificity of ASE has not been deeply characterized. In this study, we compare patterns of ASE across multiple tissues from a single individual using whole transcriptome sequencing (RNA-Seq) and a targeted, high-resolution assay (mmPCR-Seq). We detect patterns of ASE for rare deleterious and loss-of-function protein-coding variants, informing the frequency at which allelic expression could modify the functional impact of personal deleterious protein-coding across tissues. We demonstrate that these interactions occur for one third of such variants however large direction flips in allelic expression are infrequent.
Published in the journal: . PLoS Genet 10(5): e32767. doi:10.1371/journal.pgen.1004304
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004304Summary
Gene expression is a fundamental cellular process that contributes to phenotypic diversity. Gene expression can vary between alleles of an individual through differences in genomic imprinting or cis-acting regulatory variation. Distinguishing allelic activity is important for informing the abundance of altered mRNA and protein products. Advances in sequencing technologies allow us to quantify patterns of allele-specific expression (ASE) in different individuals and cell-types. Previous studies have identified patterns of ASE across human populations for single cell-types; however the degree of tissue-specificity of ASE has not been deeply characterized. In this study, we compare patterns of ASE across multiple tissues from a single individual using whole transcriptome sequencing (RNA-Seq) and a targeted, high-resolution assay (mmPCR-Seq). We detect patterns of ASE for rare deleterious and loss-of-function protein-coding variants, informing the frequency at which allelic expression could modify the functional impact of personal deleterious protein-coding across tissues. We demonstrate that these interactions occur for one third of such variants however large direction flips in allelic expression are infrequent.
Introduction
Recent genome sequencing studies have highlighted that healthy individuals carry multiple loss-of-function and rare deleterious variants whose interpretation is expected to inform individual disease risk and facilitate precision medicine [1]–[3]. However, accurate interpretation of these variants remains a considerable challenge as phenotypic effects remain difficult to predict. Furthermore, even when a specific function can be ascribed to a genetic variant, the variable penetrance and trait expressivity of genetic variants may yield important differences. In this respect, an important modifier of a coding allele's effect is its level of expression (Figure 1). This type of modification is likely to have considerable impact on interpretation of coding variant effects as genetic analyses of gene expression have reported that allele specific expression (ASE) influences at least 30% of genes for any given cell type [4], [5] and variability in allelic expression of pathogenic coding alleles has already been implicated in contributing to clinical variability for several diseases [6]–[10]. However, the degree to which deleterious and loss-of-function coding variants, routinely found through individual exome and genome sequencing, are allelically-expressed across multiple tissue types remains unexplored.
Fig. 1. Schematic of allele-specific expression. 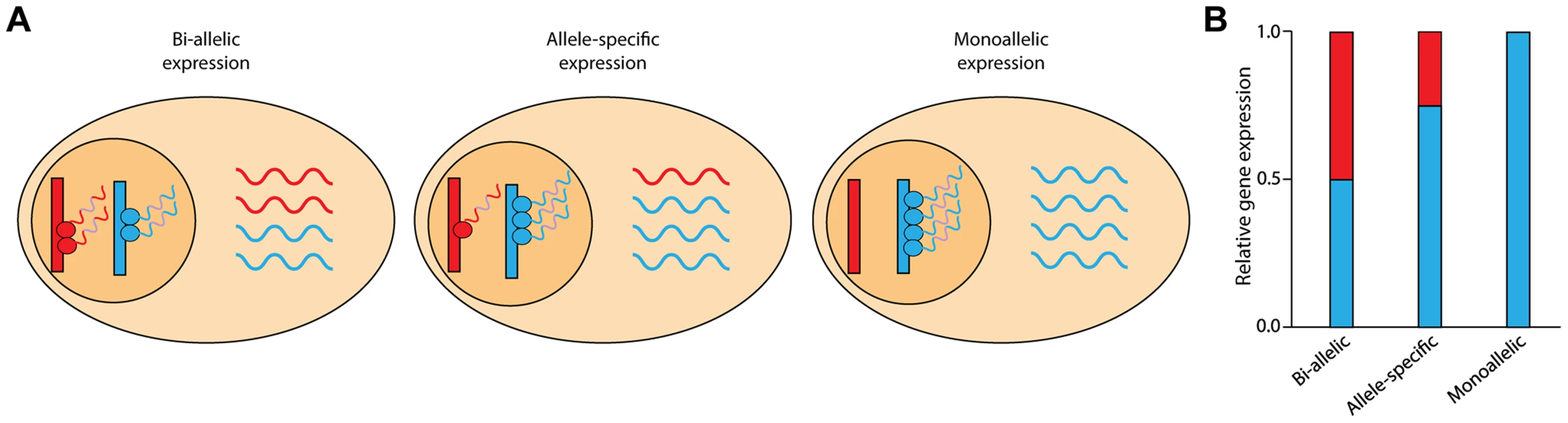
(A) The two chromosomal copies (alleles) of a gene are shown in red and blue. In most cases, both alleles are transcribed; this is known as bi-allelic expression (left panel). In the case of allele-specific expression (middle panel), one allele exhibits greater expression than the other allele. When only one allele of a gene is actively transcribed, gene expression is termed monoallelic expression (right panel). (B) RNA-Seq reads across heterozygous sites can discriminate between the two alleles and quantify the relative abundance of expression. Although the relative gene expression levels may be similar, the allelic ratios can vary. In this study we investigated patterns of gene expression and ASE for rare deleterious and loss-of-function variants across multiple tissues using both RNA-Seq and mmPCR-Seq, a targeted and high-resolution sequencing assay for measuring allelic ratios [11]. A major advantage of mmPCR-Seq is that it uncouples a gene's expression level, which can characteristically vary across tissues, from the power to measure allele-specific expression. Using this approach, we obtain 1000s of reads per heterozygous site per tissue to robustly quantify ASE. By comparing patterns of gene expression to allelic expression, we observed higher variability of allelic expression between tissues suggesting that expression level alone may be insufficient to predict the exposure of a damaging allele. Furthermore, we report patterns of ASE across tissues for both rare deleterious and loss-of-function protein-coding variants. These results demonstrate the extent to which regulatory variation can modify the functional impact of protein-coding variation across tissues, as well as the importance of using ASE for the interpretation of heterozygous variants in clinical sequencing analyses.
Results and Discussion
Collection of Deleterious and Loss-of-Function Variants
To map patterns of ASE for deleterious and loss-of-function coding variants, we sequenced the exome from two tissues (frontal lobe and small intestine) and RNA from ten tissues (cerebellum, frontal lobe, pancreas, stomach, small intestine, colon, heart, lungs, liver, and skeletal muscle) from a single individual. From the exome data, we identified 51,875 SNPs, of which 45,058 had consistent genotypes across tissues and were defined as “high-confidence” variants (Table S1). We identified 2,767 high-confidence variants that are private and not previously found in dbSNP [12], the 1000 Genomes Project [13], or the NHLBI Exome Sequencing Project (ESP) [14] (Table S2). Of these, 91 were heterozygous derived nonsynonymous variants classified by Sift [15] and Polyphen [16] as “damaging” and “deleterious”, respectively. Complementing these variants, we identified 106 SNPs that introduce premature stop-codons in exons, of which 75 SNPs were predicted to cause complete loss of function of all known transcripts using previously described prediction methods [1].
Quantification of Allele-Specific Expression by RNA-Seq and mmPCR-Seq
We performed RNA sequencing (RNA-Seq) for each tissue (Figures S1, S2, S3) and intersected this data with high-confidence heterozygous variants to identify ASE patterns (Figure S4). ASE was determined on a per-heterozygote per-tissue basis using a binomial test where p is the empirical probability that a reference allele maps to the genome compared to a non-reference allele across all sites (Figure S5). Quality control filtering (by depth, p-value, bi-allelic expression and intragenic location) was performed to identify high-confident ASE sites across all tissues (Figure S6 and Table S3). The detailed method is available at http://montgomerylab.stanford.edu/resources.html.
The measurements of ASE by RNA-Seq are influenced by the depth of coverage of a gene in the assayed tissue [17], introducing challenges for ASE comparisons across tissues where genes are characteristically differentially expressed. To more accurately quantify ASE, we also applied our recently developed method that couples microfluidics-based multiplex PCR and next generation sequencing (mmPCR-Seq) [11]. We applied this technique to 74 deleterious, 50 nonsense and 205 control variants (Figure S7). Seventeen deleterious and 25 nonsense sites were excluded because they showed no evidence of expression in any of the ten tissues. For each tissue, we performed two technical replicates and mapped the merged sequence reads since we target-sequenced specific loci (Figure S8, S9). We applied the same pipeline and filters to detect ASE as those used for RNA-Seq. We further evaluated the correlation of effect size between technical replicates and observed high technical reproducibility (Figure S10). The small intestine and skeletal muscle have the greatest reproducibility (Pearson Correlation, R>0.93). The tissue with the lowest reproducibility is the pancreas (R>0.70), which contains a high concentration of nucleases and other enzymes that can degrade RNA. The variability of effect size between the replicates was also quantified for each tissue at varying read depths (Figure S11). As expected, sites with higher read depths have less variability between replicates. With the exception of the pancreas and frontal lobe, which are two tissues known to have low RNA quality post-mortem. Regardless, the variability of allelic ratios between replicates was well below 0.2 across all samples read depths. For tested sites, mmPCR-Seq provided greater depth and power to detect ASE and in many cases facilitated estimates for sites immeasurable without extreme RNA-seq coverage (Figures S12, S13). For instance, for 598 measurements which had no reads with RNA-Seq, we obtained an average of 2639 reads for mmPCR-Seq. Furthermore, only 73 measurements had greater than 100 reads for RNA-Seq compared to 817 for mmPCR-Seq.
Differential Gene and Allele-Specific Expression
We next examined the sharing of gene expression and allelic effects across different tissues. Shared patterns of gene expression are detectable for tissues with shared functional roles or embryonic origins (Figure 2A, inset). For instance, the small intestine and colon, which are both digestive system organs derived from the endoderm layers, have a high degree of correlation (Spearman Correlation, R = 0.92). Likewise, the frontal lobe and cerebellum, which are both neural tissues derived from the ectoderm, have a high degree of shared gene expression (R = 0.91). To test the degree of correlation of allelic expression across tissues, we measured concordance of allelic ratios between pairwise tissues using the high-depth mmPCR-Seq data. Here, allelic ratios are defined as the ratio of the non-reference allele to the sum of the non-reference allele and the reference allele. We observed that the concordance of ASE between tissues does not as strongly reflect the relationships seen for shared gene expression or shared embryonic origin (Figure 2B). The range of pairwise tissue correlation for allelic effects ranges between 0.46 and 0.80, with the small intestine and colon having the most similarity (R = 0.80). We also compared in detail the pairwise correlation coefficients for expression and allelic ratios for tissue pairs of highly similar embryonic origin (Figure S14). We compared two neural tissues (frontal lobe and cerebellum) both derived the ectoderm and two intestinal tissues (small intestine and colon) both derived from the mesoderm. Irrespective of read depth and sequencing technology, the correlation of expression for tissues is consistently greater than the correlation of allelic effects across tissues. This observation suggests that allelic effects exhibit more variability than gene expression across tissues.
Fig. 2. Correlation of gene expression and allelic ratios across ten somatic tissues. 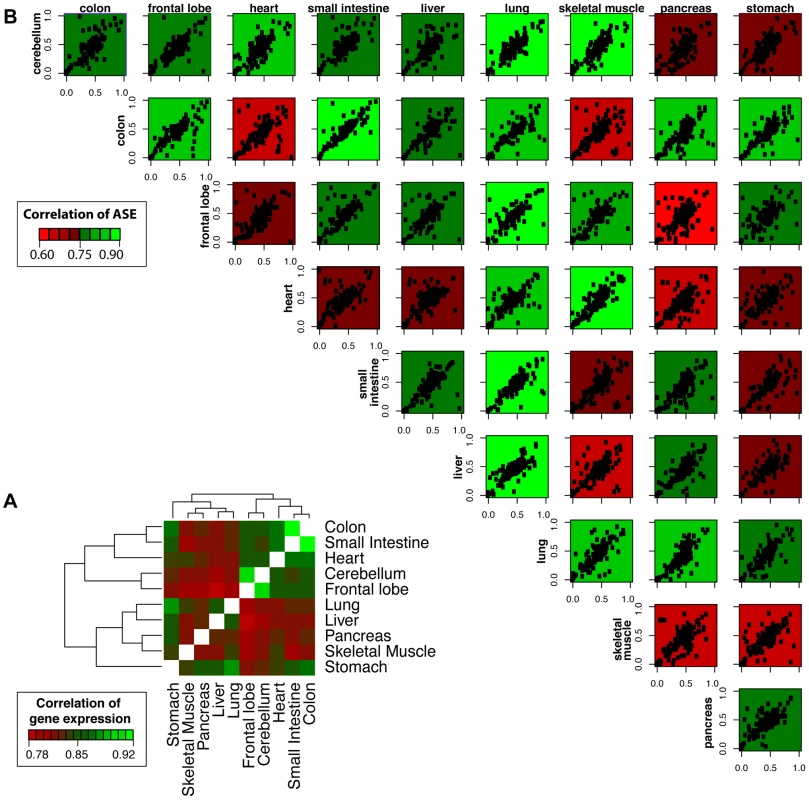
(A) Shared patterns of gene expression were detected for tissues with shared functional roles or embryonic origins. For example, the small intestine and colon are both digestive system organs derived from the endoderm and have a high degree of pairwise correlation (Spearman Correlation, R = 0.92). Likewise, the frontal lobe and cerebellum, which are both vital tissues nervous system derived from the ectoderm, have a high degree of shared expression (R = 0.91). The hierarchical clustering was generated using pairwise Spearman correlation coefficients of FPKM expression values for all genes. (B) Shared patterns of ASE were detected by mmPCR-Seq. The concordance of ASE between tissues does not as strongly reflect the relationships seen for shared gene expression or shared embryonic origin. The allelic ratio is calculated as the alternate allele reads divided by the total reads. Each data point represents a single heterozygous site tested for ASE with a total read depth greater than 200. The plots are colored by the degree of correlation of allelic bias between the pairwise tissues. These results indicate that relationships of allelic expression across tissues are much more complex than those of total expression level. We also investigated the sharing of monoallelic expression across tissues (Figure 1). We identified five genes (NDN, MAP2K3, FRG1B, IGSF3, and DUSP22) that showed monoallelic expression across all testable tissues (N≥5) in the RNA-Seq data. Two of these genes were mono-allelically expressed across all ten tissues: NDN, which is a known maternally imprinted gene [18], and MAP2K3, which has known allele-specific expression bias [19]. For all five genes, the same allele was mono-allelically expressed in all testable tissues suggesting that these genes are not imprinted in a tissue-specific manner.
Patterns of Allele-Specific Expression across Tissues
The majority of sites tested by mmPCR-Seq have equal expression of both alleles, as expected. However, many sites exhibit consistent or variable allelic patterns across different tissues (Figure S15). By comparing the mean and variance of allelic ratios as quantified through mmPCR-Seq across tissues, we stratified sites into those that exhibited no ASE, shared ASE and variable ASE across tissues. Due to the inherent nature of the binomial test, minor deviations from equal allelic expression will appear significant with high read coverage and therefore p-value significance alone is not sufficient for distinguishing between these classes. Therefore, we also took effect size (allelic ratios) into account when classifying sites as ASE. However, the definition of what constitutes a biologically important allelic effect is not easily discernable; therefore, to distinguish between each group, we accounted for previously reported definitions of ASE [20], [21] and applied cutoffs based on the reproducibility of both the allelic ratio and its variance across replicates (Figure S16). Variants were classified as non-ASE sites if the allelic expression was balanced (mean allelic ratio = 0.5+/−0.15) and if there was low variance (σ2<0.2) of the allelic ratios for all tissues tested. Variants were classified as shared ASE sites if they had a significant p-value (p<0.01), an imbalanced mean allelic ratio (0.35<mean allelic ratio <0.65), and non-variable allelic ratios (σ2<0.2) across all tissues. Lastly, variants were classified as variable (tissue-specific) ASE sites if they had a significant p-value (p<0.01) and variable allelic ratios (σ2>0.2) across tissues. The reproducibility of the groups between replicates was tested at varying allelic ratio and variance cut-offs (Figure S16) and was also assessed when the pancreas and frontal lobe, two tissues that had high variability between replicates, were removed (Figure S11). The concordance between replicates increases as the variance cut-off increases and reaches a plateau of ∼95% at a variance of 0.2. Since the greatest reproducibility is observed when the ASE cutoff is <0.35 or >0.65, the variance cutoff is 0.2, and the pancreas is removed, these cut-offs were chosen for Figure 3. Using these cut-offs, the reproducibility between replicates for the three groups (non-ASE, shared ASE and variable ASE) is 93.3%. The reproducibility between replicates for the classification of non-ASE and ASE (shared ASE plus variable ASE) is 95.7%. In total, for sites tested with mmPCR-Seq, 172 showed no ASE across tissues, 52 showed shared ASE, and 8 showed variable ASE (Figure 3A). These proportions are similar to those obtained with RNA-Seq (Figure S17). We then tested if sites exhibiting shared or variable ASE are more likely to be deleterious sites compared to sites exhibiting no ASE. Of the sites exhibiting no ASE, only 25.0% are deleterious. Comparatively, we find no significant enrichment in deleteriousness among sites which exhibit variable ASE compared to non-ASE sites(p = 0.423, Fisher's exact test; not significant); however, a significantly higher proportion of shared ASE sites (42.3%) are deleterious compared to non-ASE sites (p = 0.022; Fisher's exact test).
Fig. 3. Patterns of ASE across tissues and their influence on rare deleterious variant interpretation. 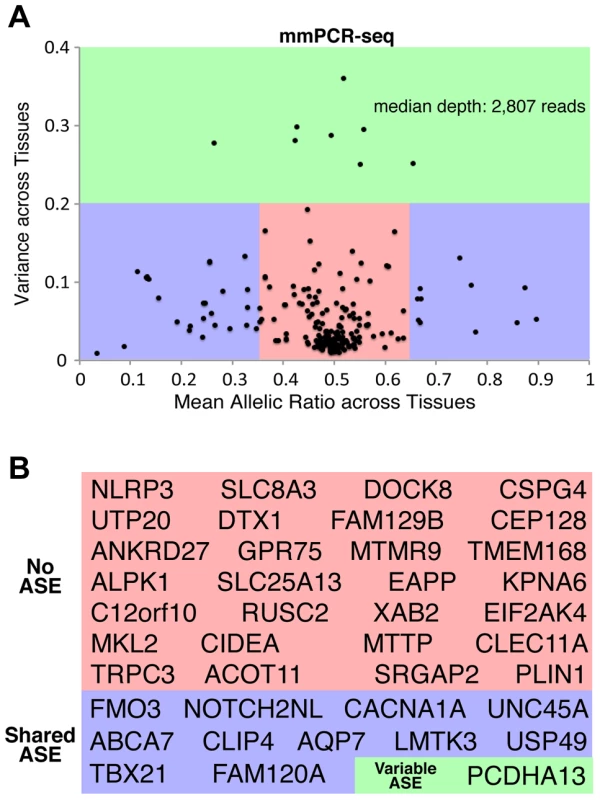
(A) The distribution of allelic ratios across tissues indicates that most heterozygous sites have bi-allelic expression across all tissues (no ASE, red). A subset of sites exhibits ASE that is consistent between all tissues (shared ASE, blue). However, a small fraction of sites exhibit ASE that is tissue-specific (variable ASE, green). The mean allelic ratio is on the x-axis and the variance (standard deviation) of the allelic ratio is on the y-axis. Allelic ratios were calculated for all sites tested by mmPCR-Seq. The reproducibility between replicates for the three groups (non-ASE, shared ASE and variable ASE), as well as the classification of non-ASE and ASE (shared ASE plus variable ASE) is is 93.3% and 95.7%, respectively. (B) Genes with rare and deleterious nsSNPs were stratified into those that exhibited no ASE (red), shared ASE (blue), and variable ASE (green) across different tissues. The reproducibility of genes classified as shared ASE and variable ASE between replicates is 100%. Next, we investigated the relationship between ASE effect sizes and direction of effect across tissues. Figure S15 highlights the range of effect sizes and directions of effect seen across tissues. By focusing on the range of allelic ratios for variants tested in three or more tissues, we further reviewed the distribution of minimum and maximum allelic ratios observed across all tested tissues (Figure S18). As expected, most sites have an allelic ratio around 0.5, and imbalanced loci show similar direction of effect. Interestingly, several sites exhibit opposing directions of effect in different tissues. For example, heterozygous sites in genes PCDHA13, SCRIB, and PDE4DIP have a major flip in direction of effect from an alternate allele ratio less than 0.2 to an alternate allele ratio greater than 0.8 across tissues. Four additional heterozygous sites have a large directional flip from an alternate allele ratio less than 0.4 to greater than 0.8, and five more heterozygous sites have a directional flip from an alternate allele ratio less than 0.2 to greater than 0.6.
To determine if gene expression level informed allelic expression level, we investigated the relationship between gene expression and allelic expression level as measured by mmPCR-Seq (Figure S19). As expected, due to the nature of mmPCR-Seq, no general pattern between absolute expression levels and ASE was observed. Four sites (circled in Figure S19) did have noticeably lower non-reference allele ratios and lower gene expression levels in the pancreas, stomach and lung; however these outliers were not enriched in any variant class and did not influence distinction of variable versus shared ASE.
Allele-Specific Expression of Rare Deleterious Variants across Tissues
By focusing on patterns of ASE for rare deleterious variants in this individual, we identified 40 sites corresponding to 40 unique genes which were quantified by mmPCR-Seq across three or more tissues. Of these genes 28 exhibited no ASE across tissues, 11 exhibited shared patterns of ASE across tissues and 1 exhibited variable ASE (Figure 3B; Figure S20). We next investigated if genes with different patterns of ASE have relevant disease associations using the Online Mendelian Inheritance in Man (OMIM) database of heritable diseases (Table S4) [22]. Although the OMIM database a limited catalog of genomic variants, OMIM variants serve as examples of the pathogenic consequences of deleterious alleles. Among those that exhibit shared ASE is the FMO3 gene, which encodes a monooxygenase enzyme responsible for hepatic metabolism and whose deficiency causes the rare Mendelian disorder trimethylaminuria that is manifested in a range of phenotypes (OMIM 602079; Figure S20) [23], [24]. Here, the shared allelic effect is detectable in all tissues, but the strongest effect against the deleterious allele is detected in the liver (non-ref to ref allelic ratio = 0.16; Figure S20). In contrast, no ASE patterns are observed for a deleterious SNP located in the gene encoding a cryopyrin (NLRP3), which is associated with the Mendelian disease Muckle-Wells Syndrome (OMIM 191900) and associated with inflammasome function and immune responses. The single deleterious site that demonstrates variable ASE is a gene encoding a protocadherin (PCDHA13). In the skeletal muscle and heart, the deleterious allele exhibits greater expression than the normal allele, but in the liver and colon the deleterious alleles exhibits less expression. Of interest, PCDHA13, which is known to play a critical role in establishing specific cell-cell connections in the brain, shows no strong patterns of ASE in the two disease-relevant neural tissues, frontal lobe and cerebellum. While the consequences of allelic expression of this individual's deleterious alleles are unknown, different patterns of allelic expression across tissues highlight the potential importance of testing multiple tissues to better elucidate the functional context of rare, deleterious alleles.
Allele-Specific Expression of Loss-of-Function Variants across Tissues
Loss-of-function alleles that introduce premature stop codons have been identified to exhibit patterns of allelic expression indicating nonsense-mediated decay (NMD) [1]. We sought to test the extent of this impact across different tissues. Indeed, comparison of ASE data using mmPCR-Seq for nonsense (stop-gained) and control sites indicates considerable reduction in the expression of the nonsense allele across all tissues (Figure 4A and Table S5). We also observed lowered expression of rare, deleterious alleles at heterozygous sites compared to control sites (p<0.05, student's t-test). This observation has been previously reported in a single cell-type, with a possible explanation for this phenomenon being that lowly-expressed alleles can better tolerate the fitness impact of deleterious protein-coding alleles [25], [26]. Furthermore, we identified that rare (MAF<5%) nonsense alleles exhibited even stronger evidence of nonsense-mediated decay than common alleles (Figure 4B). To ensure that genotype errors and mappability did not affect this observation, we compared RNA allelic bias to DNA allelic bias from exome-sequencing. Nonsense variants were removed from the analysis if the alternative allelic ratio was below 0.2 in both tissues. This filtration step ensures that genotyping and mappability of non-reference variants did not influence our observation that rare nonsense variants have decreased allelic expression compared to common nonsense variants. This observation suggests that haplotypes that harbor rare nonsense variants are either considerably unlikely to be expressed or altered transcripts are being efficiently degraded by the NMD machinery.
Fig. 4. ASE analysis of rare deleterious nsSNPs and nonsense variants by mmPCR-Seq. 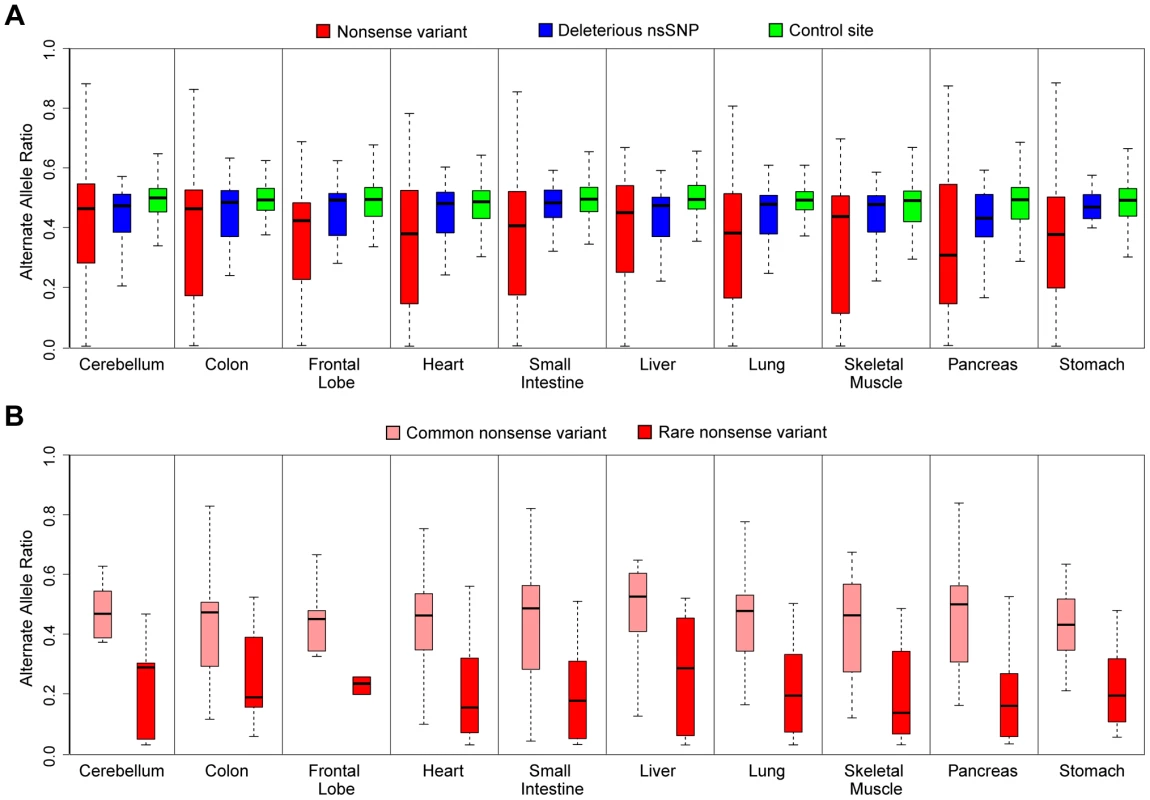
(A) ASE analysis of nonsense variants (red), rare deleterious nsSNPs (blue), and control sites (green) tested by mmPCR-Seq in different tissues. The control sites are random heterozygous sites in the individual's genome. Rare, deleterious nsSNPs and nonsense alleles have significantly reduced expression compared to controls. This observation is most significant for loss-of-function variants where the nonsense allele is likely removed through nonsense-mediated decay (student's t-test, p<0.05, see Table S5). (B) ASE analysis of rare (red) and common (pink) nonsense variants tested by mmPCR-Seq data across different tissues. Common nonsense variants are defined as those with a minor allele frequency greater than 5% across the 1000 Genomes population data. Rare nonsense alleles show significantly reduced expression compared to common nonsense alleles (student's t-test, p<0.05). In conclusion, despite the feasibility of sequencing individual genomes, the functional impact of potentially pathogenic protein-coding variants remains difficult to ascertain by DNA sequencing or computational prediction methods alone. The incorporation of transcriptome data can enhance the interpretation of such variants by providing insight into their patterns of ASE. We demonstrate the advantage of ASE for interpretation of pathogenic protein-coding allele by generated high resolution measurements of ASE for these variants across multiple tissues. Such data enables us to identify the extent to which these alleles are modified by regulatory effects and the extent to which this effect is detectable across tissues. We highlight as many as a 1/3 of all deleterious alleles are imbalanced and that nonsense alleles show characteristic and consistently lower expression across multiple tissues. Ultimately, by coupling interpretation of personal genomes with their corresponding transcriptomes, these results highlight that it may be possible to better understand the impact of pathogenic protein-coding variants within different tissues of an individual.
Materials and Methods
Collection of Tissue Samples
In order to investigate the differential allelic effects of divergent tissues in a single individual, we obtained the genomic DNA and RNA for ten somatic tissues (cerebellum, frontal lobe, pancreas, stomach, small intestine, colon, heart, lungs, liver, and skeletal muscle) from Biochain Institute, Inc (Newark, CA, USA). The samples were collected post-mortem from a healthy 25-year-old male with no significant medical history.
Whole Exome Sequencing
Genomic DNA from the frontal lobe and small intestine were prepared for exome sequencing. The enrichment of targeted regions (consensus coding sequence definition of exons and flanking introns, ∼50 Mb) was performed using the Agilent SureSelect Human All Exon 50 Mb Kit (Agilent Technologies, Santa Clara, CA, USA) following the manufacturer's recommended protocol. Paired-end libraries were constructed using the Illumina Paired End Sample Prep Kit following the manufacturer's instructions and sequencing was carried out using the Illumina HiSeq 2000 platform (Illumina, San Diego, CA, USA). Exome sequence data was processed through a pipeline based on Picard (http://picard.sourceforge.net/) with base quality score recalibration and local realignment at known indels and BWA [27], for mapping reads to the human reference genome (build hg19). GATK version v2.3-13 [28] was used for SNP calling, with the default filters, and the additional parameters: -T UnifiedGenotyper; –downsample_to_coverage 75; –genotype_likelihoods_model BOTH; -contamination 0.0; -nct 1. For ASE detection (described below), we filtered for heterozygous variants that were present in both the frontal lobe and small intestine.
Whole Transcriptome Sequencing
Paired-end RNA-Seq libraries were prepared using the Illumina TruSeq RNA Sample Preparation kit. PolyA+ RNA was isolated using Sera-Mag oligo(dT) beads (Thermo) and fragmented with the Ambion Fragmentation Reagents kit. Complementary DNA (cDNA) synthesis, end repair, A-base addition and ligation of the Illumina-indexed adaptors were performed according to Illumina's protocol. Each sample was barcoded and all samples were sequenced on one lane of the Illumina HiSeq 2000 platform (2×100-nt read length). In total, we obtained 13.3±3.7 (mean ± SD) million paired end reads per sample. We assessed the sequence quality using the publicly available software FastQC. For each sample, we examined per-base quality scores across the length of the reads to ensure that >95% of the reads had >Q60 for bases 1–100. Reads were mapped by TopHat (version 2.0.0) to the known transcriptome (-G option; Gencode version 7 annotations) the human reference genome (hg19) using default parameters [29]. Cufflinks (version 2.0.2) was used to quantify gene expression for known transcripts (-G option; Gencode version 7 annotations) using the default parameters [30].
Targeted Allelic Sequencing by mmPCR-Seq
To quantify allele-specific expression at lowly expressed site, we applied a high-throughput method that couples microfluidics-based multiplex PCR and deep sequencing (mmPCR-Seq) [11]. We designed primers and applied this technique to 74 deleterious nonsynonymous variants, 50 nonsense variants, and 205 control variants. The control sites are common (MAF>0.05), non-deleterious variants. First, multiplexed PCR reactions were carried out using the Fluidigm Access Array for each sample. Then, the PCR products were indexed using barcoded adaptor primers via a single PCR reaction for each tissue sample. All indexed samples were pooled and purified using a Qiagen RNeasy. Six picomoles were loaded into one lane of an Illumina MiSeq for deep sequencing. The sequence reads were mapped to the human reference genome (hg19) using the Spliced Transcripts Alignment to a Reference (STAR, version 3.2) aligner [31]. Since we targeted together specific heterozygous sites in the genome, the default parameters were modified (minimum score and match filters lowered from 0.66 to 0.3) to increase the number of mapped reads.
Allele-Specific Expression
Allele-specific expression was determined on a per-heterozygote-site per-tissue basis using the pipeline depicted in Figure S4 and available online (http://montgomerylab.stanford.edu/resources.html). First, mapped reads were sorted using the Samtools (version 0.1.7) [32]. Next, Samtools mpileup was used to call variants from the aligned reads using a list of known heterozygous sites from the individual. Heterozygous sites with a base quality score (MAQ) below 10, individual allele read depth below 5 and a total (both alleles) read depth below 20 were filtered out. Next, we calculated the reference to non-reference allele mapping ratio for each tissue. To test for ASE, we performed a binomial statistical test for each heterozygous site in each tissue modifying p to be the empirical probability of observing a reference versus non-reference allele across all sites. A significance cut-off of 0.05 and 0.01 were used for the RNA-Seq and mmPCR-Seq data, respectively.
Data Access
The raw mmPCR-seq data has been submitted to the NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE51769. The code for ASE detection pipeline can be found online (http://montgomerylab.stanford.edu/resources.html).
Supporting Information
Zdroje
1. MacArthurDG, BalasubramanianS, FrankishA, HuangN, MorrisJ, et al. (2012) A systematic survey of loss-of-function variants in human protein-coding genes. Science 335 : 823–828.
2. KeinanA, ClarkAG (2012) Recent explosive human population growth has resulted in an excess of rare genetic variants. Science 336 : 740–743.
3. MacArthurDG, Tyler-SmithC (2010) Loss-of-function variants in the genomes of healthy humans. Human Molecular Genetics 19: R125–130.
4. GeB, PokholokDK, KwanT, GrundbergE, MorcosL, et al. (2009) Global patterns of cis variation in human cells revealed by high-density allelic expression analysis. Nature Genetics 41 : 1216–1222.
5. VerlaanDJ, GeB, GrundbergE, HobermanR, LamKCL, et al. (2009) Targeted screening of cis-regulatory variation in human haplotypes. Genome Research 19 : 118–127.
6. BerlivetS, MoussetteS, OuimetM, VerlaanDJ, KokaV, et al. (2012) Interaction between genetic and epigenetic variation defines gene expression patterns at the asthma-associated locus 17q12-q21 in lymphoblastoid cell lines. Human Genetics 131 : 1161–1171.
7. JentarraGM, RiceSG, OlfersS, RajanC, SaffenDM, et al. (2012) Skewed allele-specific expression of the NF1 gene in normal subjects: a possible mechanism for phenotypic variability in neurofibromatosis type 1. Journal of Child Neurology 27 : 695–702.
8. FinchN, CarrasquilloMM, BakerM, RutherfordNJ, CoppolaG, et al. (2011) TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology 76 : 467–474.
9. EmisonES, Garcia-BarceloM, GriceEA, LantieriF, AmielJ, et al. (2010) Differential contributions of rare and common, coding and noncoding Ret mutations to multifactorial Hirschsprung disease liability. American Journal of Human Genetics 87 : 60–74.
10. MaiaA-T, AntoniouAC, O'ReillyM, SamarajiwaS, DunningM, et al. (2012) Effects of BRCA2 cis-regulation in normal breast and cancer risk amongst BRCA2 mutation carriers. Breast Cancer Research 14: R63–R63.
11. ZhangR, RamawamiG, SmithK, GustavoT, MontgomerySB, et al. (2013) Quantifying RNA allelic ratios by microfluidic multiplex PCR and sequencing. Nature Methods [Available online 24 November 2013].
12. SherryST, WardMH, KholodovM, BakerJ, PhanL, et al. (2001) dbSNP: the NCBI database of genetic variation. Nucleic Acids Research 29 : 308–311.
13. AbecasisGR, AltshulerD, AutonA, BrooksLD, DurbinRM, et al. (2010) A map of human genome variation from population-scale sequencing. Nature 467 : 1061–1073.
14. NHLBI GO Exome Sequencing Project (ESP). Available: https://esp.gs.washington.edu/drupal/. Accessed 8 April 2014.
15. NgPC, HenikoffS (2003) SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Research 31 : 3812–3814.
16. AdzhubeiIA, SchmidtS, PeshkinL, RamenskyVE, GerasimovaA, et al. (2010) A method and server for predicting damaging missense mutations. Nature Methods 7 : 248–249.
17. DegnerJF, MarioniJC, PaiAA, PickrellJK, NkadoriE, et al. (2009) Effect of read-mapping biases on detecting allele-specific expression from RNA-sequencing data. Bioinformatics 25 : 3207–3212.
18. JayP, RougeulleC, MassacrierA, MonclaA, MatteiMG, et al. (1997) The human necdin gene, NDN, is maternally imprinted and located in the Prader-Willi syndrome chromosomal region. Nat Genet 17 : 357–361.
19. TuskanRG, TsangS, SunZ, BaerJ, RozenblumE, et al. (2008) Real-time PCR analysis of candidate imprinted genes on mouse chromosome 11 shows balanced expression from the maternal and paternal chromosomes and strain-specific variation in expression levels. Epigenetics 3 : 43–50.
20. PantPV, TaoH, BeilharzEJ, BallingerDG, CoxDR, et al. (2006) Analysis of allelic differential expression in human white blood cells. Genome Res 16 : 331–339.
21. ZhangK, LiJB, GaoY, EgliD, XieB, et al. (2009) Digital RNA allelotyping reveals tissue-specific and allele-specific gene expression in human. Nature Methods 6 : 613–618.
22. BoyadjievSA, JabsEW (2000) Online Mendelian Inheritance in Man (OMIM) as a knowledgebase for human developmental disorders. Clin Genet 57 : 253–266.
23. MayatepekE, KohlmüllerD (1998) Transient trimethylaminuria in childhood. Acta Paediatrica 87 : 1205–1207.
24. DolphinCT, JanmohamedA, SmithRL, ShephardEA, PhillipsIR (1997) Missense mutation in flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nature Genetics 17 : 491–494.
25. LappalainenT, MontgomerySB, NicaAC, DermitzakisET (2011) Epistatic Selection between Coding and Regulatory Variation in Human Evolution and Disease. American Journal of Human Genetics 89 : 459–463.
26. GibsonG (2011) Rare and common variants: twenty arguments. Nature Reviews Genetics 13 : 135–145.
27. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
28. McKennaA, HannaM, BanksE, SivachenkoA, CibulskisK, et al. (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research 20 : 1297–1303.
29. TrapnellC, PachterL, SalzbergSL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics (Oxford, England) 25 : 1105–1111.
30. TrapnellC, WilliamsBa, PerteaG, MortazaviA, KwanG, et al. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature biotechnology 28 : 511–515.
31. DobinA, DavisCa, SchlesingerF, DrenkowJ, ZaleskiC, et al. (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics (Oxford, England) 29 : 15–21.
32. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
Štítky
Genetika Reprodukční medicína
Článek Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin PathwayČlánek Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human DiseasesČlánek G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify LongevityČlánek PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial MatrixČlánek Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus ConflictsČlánek The Impact of Population Demography and Selection on the Genetic Architecture of Complex TraitsČlánek Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar AcidificationČlánek The Case for Junk DNA
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 5- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
-
Všechny články tohoto čísla
- Genetic Interactions Involving Five or More Genes Contribute to a Complex Trait in Yeast
- A Mutation in the Gene in Dogs with Hereditary Footpad Hyperkeratosis (HFH)
- Loss of Function Mutation in the Palmitoyl-Transferase HHAT Leads to Syndromic 46,XY Disorder of Sex Development by Impeding Hedgehog Protein Palmitoylation and Signaling
- Heterogeneity in the Frequency and Characteristics of Homologous Recombination in Pneumococcal Evolution
- Genome-Wide Nucleosome Positioning Is Orchestrated by Genomic Regions Associated with DNase I Hypersensitivity in Rice
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Single Nucleotide Variants in Transcription Factors Associate More Tightly with Phenotype than with Gene Expression
- Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin Pathway
- Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human Diseases
- Epistatically Interacting Substitutions Are Enriched during Adaptive Protein Evolution
- Meiotic Drive Impacts Expression and Evolution of X-Linked Genes in Stalk-Eyed Flies
- G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify Longevity
- Population Genomic Analysis of Ancient and Modern Genomes Yields New Insights into the Genetic Ancestry of the Tyrolean Iceman and the Genetic Structure of Europe
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
- Whole Exome Re-Sequencing Implicates and Cilia Structure and Function in Resistance to Smoking Related Airflow Obstruction
- Allelic Expression of Deleterious Protein-Coding Variants across Human Tissues
- R-loops Associated with Triplet Repeat Expansions Promote Gene Silencing in Friedreich Ataxia and Fragile X Syndrome
- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- The Impairment of MAGMAS Function in Human Is Responsible for a Severe Skeletal Dysplasia
- Octopamine Neuromodulation Regulates Gr32a-Linked Aggression and Courtship Pathways in Males
- Mlh2 Is an Accessory Factor for DNA Mismatch Repair in
- Activating Transcription Factor 6 Is Necessary and Sufficient for Alcoholic Fatty Liver Disease in Zebrafish
- The Spatiotemporal Program of DNA Replication Is Associated with Specific Combinations of Chromatin Marks in Human Cells
- Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus Conflicts
- Genome-Wide Inference of Ancestral Recombination Graphs
- Mutations in Four Glycosyl Hydrolases Reveal a Highly Coordinated Pathway for Rhodopsin Biosynthesis and N-Glycan Trimming in
- SHP2 Regulates Chondrocyte Terminal Differentiation, Growth Plate Architecture and Skeletal Cell Fates
- The Impact of Population Demography and Selection on the Genetic Architecture of Complex Traits
- Retinoid-X-Receptors (α/β) in Melanocytes Modulate Innate Immune Responses and Differentially Regulate Cell Survival following UV Irradiation
- Genetic Dissection of the Female Head Transcriptome Reveals Widespread Allelic Heterogeneity
- Genome Sequencing and Comparative Genomics of the Broad Host-Range Pathogen AG8
- Copy Number Variation Is a Fundamental Aspect of the Placental Genome
- GOLPH3 Is Essential for Contractile Ring Formation and Rab11 Localization to the Cleavage Site during Cytokinesis in
- Hox Transcription Factors Access the RNA Polymerase II Machinery through Direct Homeodomain Binding to a Conserved Motif of Mediator Subunit Med19
- Drosha Promotes Splicing of a Pre-microRNA-like Alternative Exon
- Predicting the Minimal Translation Apparatus: Lessons from the Reductive Evolution of
- PAX6 Regulates Melanogenesis in the Retinal Pigmented Epithelium through Feed-Forward Regulatory Interactions with MITF
- Enhanced Interaction between Pseudokinase and Kinase Domains in Gcn2 stimulates eIF2α Phosphorylation in Starved Cells
- A HECT Ubiquitin-Protein Ligase as a Novel Candidate Gene for Altered Quinine and Quinidine Responses in
- dGTP Starvation in Provides New Insights into the Thymineless-Death Phenomenon
- Phosphorylation Modulates Clearance of Alpha-Synuclein Inclusions in a Yeast Model of Parkinson's Disease
- RPM-1 Uses Both Ubiquitin Ligase and Phosphatase-Based Mechanisms to Regulate DLK-1 during Neuronal Development
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- Heritable Transmission of Stress Resistance by High Dietary Glucose in
- Revertant Mutation Releases Confined Lethal Mutation, Opening Pandora's Box: A Novel Genetic Pathogenesis
- Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar Acidification
- A Genome-Wide Assessment of the Role of Untagged Copy Number Variants in Type 1 Diabetes
- Selectivity in Genetic Association with Sub-classified Migraine in Women
- A Lack of Parasitic Reduction in the Obligate Parasitic Green Alga
- The Proper Splicing of RNAi Factors Is Critical for Pericentric Heterochromatin Assembly in Fission Yeast
- Discovery and Functional Annotation of SIX6 Variants in Primary Open-Angle Glaucoma
- Six Homeoproteins and a linc-RNA at the Fast MYH Locus Lock Fast Myofiber Terminal Phenotype
- EDR1 Physically Interacts with MKK4/MKK5 and Negatively Regulates a MAP Kinase Cascade to Modulate Plant Innate Immunity
- Genes That Bias Mendelian Segregation
- The Case for Junk DNA
- An In Vivo EGF Receptor Localization Screen in Identifies the Ezrin Homolog ERM-1 as a Temporal Regulator of Signaling
- Mosaic Epigenetic Dysregulation of Ectodermal Cells in Autism Spectrum Disorder
- Hyperactivated Wnt Signaling Induces Synthetic Lethal Interaction with Rb Inactivation by Elevating TORC1 Activities
- Mutations in the Cholesterol Transporter Gene Are Associated with Excessive Hair Overgrowth
- Scribble Modulates the MAPK/Fra1 Pathway to Disrupt Luminal and Ductal Integrity and Suppress Tumour Formation in the Mammary Gland
- A Novel CH Transcription Factor that Regulates Expression Interdependently with GliZ in
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics
- Spermatid Cyst Polarization in Depends upon and the CPEB Family Translational Regulator
- Insights into the Genetic Structure and Diversity of 38 South Asian Indians from Deep Whole-Genome Sequencing
- Intron Retention in the 5′UTR of the Novel ZIF2 Transporter Enhances Translation to Promote Zinc Tolerance in
- A Dominant-Negative Mutation of Mouse Causes Glaucoma and Is Semi-lethal via LBD1-Mediated Dimerisation
- Biased, Non-equivalent Gene-Proximal and -Distal Binding Motifs of Orphan Nuclear Receptor TR4 in Primary Human Erythroid Cells
- Ras-Mediated Deregulation of the Circadian Clock in Cancer
- Retinoic Acid-Related Orphan Receptor γ (RORγ): A Novel Participant in the Diurnal Regulation of Hepatic Gluconeogenesis and Insulin Sensitivity
- Extensive Diversity of Prion Strains Is Defined by Differential Chaperone Interactions and Distinct Amyloidogenic Regions
- Fine Tuning of the UPR by the Ubiquitin Ligases Siah1/2
- Paternal Poly (ADP-ribose) Metabolism Modulates Retention of Inheritable Sperm Histones and Early Embryonic Gene Expression
- Allele-Specific Genome-wide Profiling in Human Primary Erythroblasts Reveal Replication Program Organization
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



