-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaOctopamine Neuromodulation Regulates Gr32a-Linked Aggression and Courtship Pathways in Males
To mate or fight? When meeting other members of their species, male fruit flies must determine whether a second fly is male or female and proceed with the appropriate behavioral patterns. The taste receptor, Gr32a, has been reported to respond to chemical messages (pheromones) that are important for gender recognition, as eliminating Gr32a function impairs both male courtship and aggressive behavior. Here we demonstrate that different subsets of Gr32a-expressing neuron populations mediate these mutually exclusive behaviors and the male Gr32a-mediated behavioral response is amplified through neurons that contain the neuromodulator octopamine (OA, an invertebrate equivalent of norepinephrine). Gr32a-expressing neurons connect functionally and synaptically with distinct OA neurons indicating these amine neurons may function as early as a second-order step in a chemosensory-driven circuit. Our results contribute to understanding how an organism selects an appropriate behavioral response upon receiving external sensory signals.
Published in the journal: . PLoS Genet 10(5): e32767. doi:10.1371/journal.pgen.1004356
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004356Summary
To mate or fight? When meeting other members of their species, male fruit flies must determine whether a second fly is male or female and proceed with the appropriate behavioral patterns. The taste receptor, Gr32a, has been reported to respond to chemical messages (pheromones) that are important for gender recognition, as eliminating Gr32a function impairs both male courtship and aggressive behavior. Here we demonstrate that different subsets of Gr32a-expressing neuron populations mediate these mutually exclusive behaviors and the male Gr32a-mediated behavioral response is amplified through neurons that contain the neuromodulator octopamine (OA, an invertebrate equivalent of norepinephrine). Gr32a-expressing neurons connect functionally and synaptically with distinct OA neurons indicating these amine neurons may function as early as a second-order step in a chemosensory-driven circuit. Our results contribute to understanding how an organism selects an appropriate behavioral response upon receiving external sensory signals.
Introduction
Organisms live in complicated environments requiring successful interaction with their surroundings for reproduction and survival. Information about the environment is transformed into neural activity by specialized sensory organs that detect signals via touch-, taste-, vibration-, odor - and image-sensitive neurons. Pheromones commonly used as olfactory or contact signals in social behavior like courtship and aggression provide information about gender, receptivity, or conspecificity [1]–[3]. In many systems, chemosensory signal-detecting systems are regulated by biogenic amines including dopamine, serotonin, and norepinephrine (or octopamine, its invertebrate analog) acting as neuromodulators [4]–[6]. Despite extensive investigation in a wide variety of organisms, it has proven difficult to assign specific roles to individual amines in the circuitry concerned with social behavior [7]–[10]. In this study, we directly connect amine regulation to pheromonal communication by identifying specific chemosensory to octopamine neuron contacts and then investigating their tissue-specific functional roles in male aggression and courtship selection.
In Drosophila, pheromonal signals are communicated primarily via cuticular hydrocarbons (CHC) and long carbon chain esters that trigger olfactory (volatile) or gustatory (contact) receiving pathways in conspecifics [11]–[13]. Contact pheromones are detected by gustatory receptor-expressing sensory neurons (GRNs) found in taste sensilla in mouth, leg, and wing segments. Despite the importance of this non-volatile sensory information, only a small number of gustatory receptors (GRs) have been reported to be involved in the perception of pheromones that regulate social behavior. In one well-studied example, the behavior of males lacking the gustatory receptor Gr32a is altered in at least three ways; levels of male courtship towards females are reduced, levels of male courtship towards second males are elevated, and aggression as measured by the numbers of lunges (a key higher level behavioral pattern) is reduced [14]–[16]. In addition, a recent study describes a role of tarsal/leg Gr32a-expressing neurons in the inhibition of interspecies courtship between Drosophila species [17]. To transduce pheromonal stimuli, axons of Gr32a-expressing neurons project to distinct zones in the suboesophageal ganglion (SOG) [15], [18], and other sites within the central nervous system [19]. The SOG is a central brain region that in addition to axons of gustatory neurons contains extensive neuronal processes of octopamine neurons [20]–[22].
Reduced levels of the amine octopamine (OA) yield phenotypes similar to those seen in flies lacking Gr32a function [23]–[25]. Males without OA exhibit increased male-male courtship [23] and a delay in the initiation of male aggressive behavior [25], as do Gr32a loss-of-function flies [16]. OA function is also necessary for males to make correct choices between courtship and aggression [21], [23] and OA has been suggested to be essential for the display of higher-level aggression [24], [25]. As studies in multiple systems reveal that the context of sensory information and internal states are often shaped molecularly by neuromodulators, we tested the hypothesis that the structural composition of the Gr32a pheromonal network includes synaptic connections to OA neuromodulatory neurons.
We used behavioral assays, Ca2+ imaging, and the GRASP (GFP Reconstitution Across Synaptic Partners) method [26], [27] to demonstrate the existence of functional and putative synaptic connections between Gr32a neurons and octopaminergic SOG neurons. Removing Gr32a-expressing neurons, eliminating OA, and altering both simultaneously confirmed essential roles for these chemosensory and OA neuronal groups on male aggression initiation and courtship selection. A role for the labellar Gr32a subpopulation in male aggression was revealed by functionally and anatomically separating Gr32a-expressing neurons into mouth and leg populations. Ca2+ imaging experiments demonstrate that OA-expressing neurons in the SOG respond to male cuticular hydrocarbon extracts and this response is eliminated in the absence of Gr32a neurons. Finally, GRASP connectivity between Gr32a neurons and three OA neurons that co-express the male forms of Fruitless (FruM), link anatomical characterization with previous functional data [21] and indicate that this small subset of aminergic neurons is important to provide male selective modulation of behavior. The results presented here begin to decipher social behavior at the level of small subsets of sensory and neuromodulatory neurons and provide insight into how amine-expressing neurons anatomically contribute to circuitry directing sex-specific behavior.
Results
Gr32a neurons contact OA neurons in the suboesophageal ganglion
To test the hypothesis that OA neurons might anatomically function in the Gr32a pheromonal input pathway, we generated a Tdc2-LexA:VP16 line and utilized this tool with the split-GFP system developed in C. elegans [26] and adapted for Drosophila [27]. In invertebrates, OA is synthesized from the amino acid tyrosine via the action of tyrosine decarboxylase (TDC) and tyramine β-hydroxylase (Tβh). The Tdc2 gene encodes the neuronal TDC [28] and the Tdc2-LexA line can be used to label and manipulate OA neurons ([29], Figure S1) and possibly a small population of tyramine (TA)-expressing neurons [20]. The Gr32a receptor is expressed in sensory neurons in the mouth (labellum - a gustatory organ of the proboscis and pharynx) and in tarsal segments of all three legs [14], [15], [30]. Axons of Gr32a-expressing neurons project through three peripheral nerves to the SOG (Figure 1A,B) [18], [31]–[33]. Peripheral chemosensory neuron expression of OA has not been detected in this study or previously [28]. However, within the central brain, individual OA neurons project extensive arborizations targeting multiple neuropil regions including the SOG, which functions at least in part, to receive key contact pheromone information (Figure 1C,D, Figure S1D) [20], [21], [28].
Fig. 1. Gr32a neurons contact OA neurons in the suboesophageal ganglion. 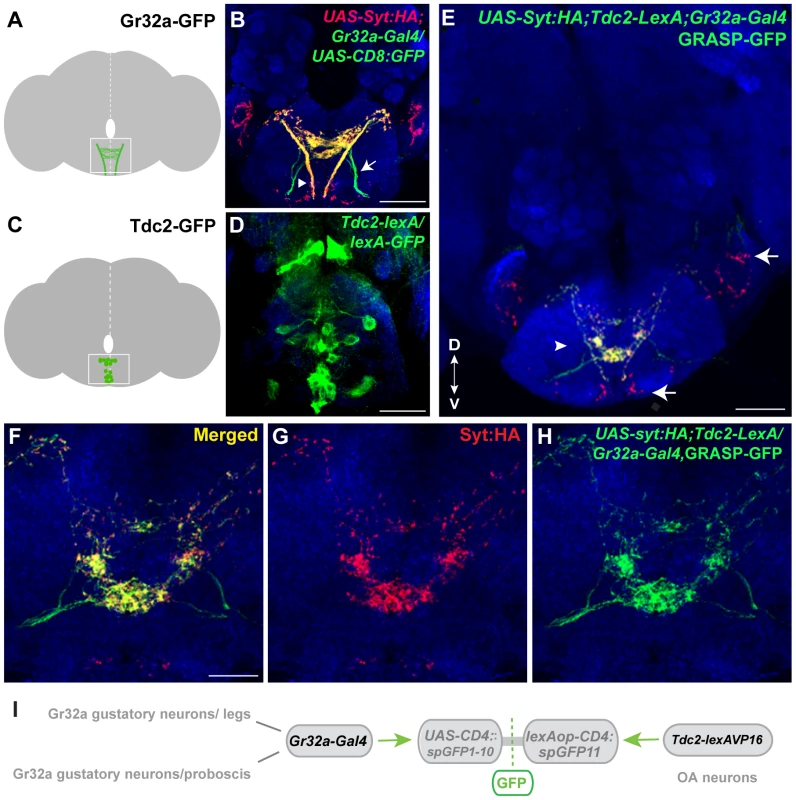
(A) Schematic depicting the SOG region targeted by Gr32a axons visualized in panel B. (B) Axons and presynaptic terminals of Gr32a-expressing neurons identified by immunofluorescence to CD8:GFP and the synaptotagmin:HA fusion protein in UAS-sytHA;;UAS-CD8:GFP/Gr32a-Gal4 progeny (green, anti-CD8, Invitrogen; red, anti-HA, Roche). Sensory neurons from the labellum project through the labial nerve (arrow), mouthpart neurons project through the pharyngeal/accessory nerve, and neurons from thoracic ganglia project via the cervical connective (arrowhead). Scale bar represents 30 µM (C–D) A subset of Tdc2-positive neurons located in the SOG in a schematic (C) and with GFP expression driven by the Tdc2-LexA line (Tdc2-LexA;lexAop-rCD4:GFP. Cell bodies are visible (D) with extensive arborizations apparent in a series of optical sections ventral to the cell bodies (Figure S1). (E) GRASP-mediated GFP reconstitution is observed between Gr32a neurons expressing CD4::spGFP1-10 and UAS-syt:HA (red, anti-HA, Roche) and OA neurons expressing CD4::spGFP11. GRASP reconstitution is detected by immunofluorescence using a rabbit monoclonal GFP antibody (Life Technologies). Regions in the SOG with only syt:HA expression are indicated (arrows) in addition to GFP-reconstitution contacts that show co-localization with syt-HA expression (arrowhead). Scale bar is 50 µM. (F–H) Optical sections of the same brain at higher magnification showing GRASP-mediated GFP reconstituted expression (H), syt:HA localization (G) and clear overlap or close association at synaptic-like puncta in the merged channel (F). Scale bar represents 20 µM. (See also Figure S2.) (I) Schematic representation of the GRASP reporter lines combined with the Gr32a-Gal4 and Tdc2-lexA driver lines. To determine if Gr32a-expressing neurons directly contact OA neurons, we used the GFP Reconstitution Across Synaptic Partners (GRASP) method, which detects putative synaptic connections based on the reconstitution of two fragments of a split-GFP protein on the outer membrane of targeted neuronal populations [26], [27]. We observed GFP reconstitution in a reproducible, distinct pattern within the central SOG (Figure 1E–I) in flies containing one fragment of split-GFP under Tdc2 (OA/Tyramine) control (Tdc2-lexA; lexAop-CD4::spGFP11) and the second fragment driven by the promoter of Gr32a (Gr32a-Gal4; UAS-CD4::spGFP1-10). Little or no fluorescence was observed upon expression of either split-GFP fragment alone (Figure S2).
To confirm that at least a portion of the fluorescence seen in contact zones is likely synaptic, we added the UAS-syt:HA reporter [34] (Figure 1E–G, displayed as red puncta). The overall syt:HA pattern shows clear preferential localization of terminal regions of Gr32a neurons and an extensive overlap is seen between syt:HA localization and split-GFP reconstitution at both low and higher magnification (Figure 1E–H). In the merged channels (Figure 1E,F), regions of syt:HA expression where no GFP reconstitution is observed indicating that only specific neurons amongst the populations of Gr32a and OA neurons contact each other. In particular, the synaptic endings derived from Gr32a neurons that project directly to the ventrolateral protocerebrum region [15] do not express reconstituted GFP (Figure 1E, arrow) demonstrating specificity in the GFP reconstitution pattern and specificity in the Gr32a to OA neuronal connections. This anatomical data is consistent with a recent study suggesting a close, possibly synaptic, apposition of Gr32a-expressing axons with male mAL neurons [14].
Gr32a expression is seen in all bitter-sensing neurons within the sensilla of the labellum, usually accompanied by many additional gustatory receptors in most of the neurons [33], [35], [36]. In one subgroup of chemosensory neurons, the Gr22e (9 neurons) and Gr59b (4 neurons) receptors co-localize with Gr32a as has been reported previously [33], while in another distinct group Gr32a and Gr47a co-localize (3 neurons) [36]. Expressing Gr22e-Gal4 or Gr59b-Gal4 with Tdc2-lexA and the GRASP reporter transgenes resulted in split-GFP reconstitution in the SOG region as described above (Figure 1) albeit with reduced GRASP expression likely due to co-expression in only a subset of the population of Gr32a neurons (Figure S3). We also examined whether OA neurons might receive synaptic input from the Gr47a/Gr32a neurons, a different subgroup of bitter-responsive neurons [31], [37]. GFP reconstitution was not observed between the Gr47a-Gal4 labeled axons and OA neurons (Figure S4). Although definitive verification of the GRASP signals will require electron microscopy, our results suggest that a number of octopaminergic SOG neurons may serve as neuromodulatory links in the information pathways between specific Gr32a-expressing neurons and taste-related behavioral outputs.
Removing OA neurons changes Gr32a SOG axonal targeting
If a subset of Gr32a gustatory neurons are in synaptic contact with octopaminergic SOG interneurons, then removing the OA neurons might cause changes in the branching patterns of incoming Gr32a axonal projections. To test this hypothesis, we eliminated OA neurons by driving expression of the programmed cell death gene, head involution defective (hid, UAS-hid), coupled with the UAS-Red Stinger reporter transgene in OA/TA neurons. The Tdc2-Gal4/UAS-hid UAS-Red Stinger combination allowed us to identify transgenic brains that retained OA neurons (DsRed expression was observed) and brains that were devoid of OA neurons (DsRed and Tβh expression were absent) (Figure S5). Gr32a neuronal projections entering the SOG were visualized using the Gr32a-I-GFP reporter construct (Figure 2A–C) which drives GFP expression as a direct promoter fusion [33]. The resulting GFP fluorescence is weaker than when amplified through the Gal4/UAS system, however when all OA neurons were eliminated, we observed a range of axonal projection defects including an absence of Gr32a-I-GFP immunoreactivity in the SOG (data not shown, 31%) or a severe reduction and disorganization of Gr32a leg and labellum termini in 69% of preparations (n = 21, Figure 2D). Since the adult brains were dissected 1–5 days after eclosion, the differing severity of the Gr32a projection phenotypes could be due to increased axonal disorganization in the absence of OA neuronal targets as the flies age. No similar disorganization of Gr32a axonal projections is observed in control brains during the 1–5 day time frame.
Fig. 2. Removing OA neurons significantly alters Gr32a axonal projections. 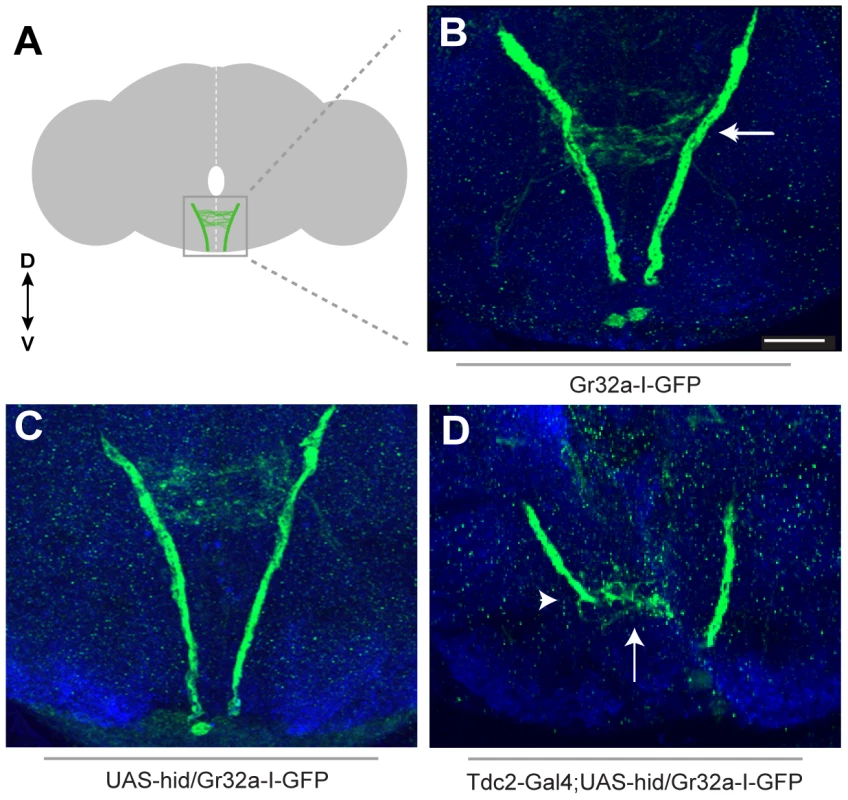
(A) Schematic representation of the adult brain with Gr32a-expressing axonal arborizations in the SOG. (B) Gr32a-I-GFP expression in a typical wildtype adult brain. The Gr32a-expressing neurons located in the tarsi, labellum, and mouthparts all terminate in the SOG (arrow). (C) Confocal sections of a UAS-hid UAS-Red Stinger control brain verifying wildtype organization of Gr32a-I-GFP projections (D) Confocal sections of transgenic Tdc2-Gal4/UAS-hid UAS-Red Stinger;Gr32a-I-GFP adult brains. When all OA neurons are eliminated, a range of axonal projection defects was observed including a severe reduction and disorganization of Gr32a leg and labellum termini (arrow, arrowhead). Scale bar represents 30 µM. We next asked if Gr32a axonal morphology is altered if OA neurons are present but lack OA due to a null mutation in Tyramine β-hydroxylase (tβhnM18). Using Gr32a-Gal4 to drive reporter GFP expression, the stereotypical projections of Gr32a-expressing neurons from control and OA deficient males were examined. Gr32a axons terminated in the SOG (Figure S6) in heterozygous control adult brains (tβhnM18/+;Tdc2-Gal4;20XUAS-6XGFP). Compiling the same number of confocal sections in controls and OA deficient male brains (tβhnM18;Tdc2-Gal4;20XUAS-6XGFP) indicates the majority of Gr32a projections reach the SOG as in controls. However, we observed aberrant termination of Gr32a axons in the antennal lobe region of OA deficient brains (Figure S6C–E) that is distinct from previously described projections into the ventrolateral protocerebrum [15]. The effects of eliminating production of OA on individual Gr32a-expressing neurons remains to be determined but results from these experiments suggest the correct differentiation of OA neurons is required for precise axon targeting by at least a subset of Gr32a chemosensory neurons.
Gr32a expressing neurons mediate onset of aggression via OA signaling
A previous study reported that the Gr32a receptor mediates aggression-inducing and courtship suppression effects of the male-enriched cuticular hydrocarbons, (z)-7-tricosene [16]. Results presented here indicate that Gr32a-expressing neurons contact OA neurons and suggest that octopaminergic signaling is one of the pathways through which Gr32a-mediated pheromonal information is conveyed to other brain or possibly ventral cord regions. To test this hypothesis, we first analyzed fighting defects in males with impaired Gr32a function in our aggression chambers. This data provides a baseline for calculating how removal of OA neuromodulation in addition to eliminating Gr32a-mediated pheromonal information may or may not further alter male aggression or courtship. We ablated Gr32a-expressing gustatory neurons through expression of Diphtheria Toxin (UAS-DTI) via the Gr32a-Gal4 driver line [38]. Pairs of UAS-DTI;Gr32a-Gal4 or transgenic control males were placed in an aggression chamber and latency to the first lunge (a key aggressive pattern essential for the establishment of hierarchical relationships) and total numbers of lunges were quantified. Consistent with a role of Gr32a-expressing neurons in perceiving pheromones utilized for sex and species recognition in males, the latency to first lunge was significantly longer in males without Gr32a neurons compared to parental controls (Figure 3A). Moreover, a significant reduction in the number of lunges was also observed (Figure 3B). Males without Gr32a neurons exhibited a reduction in aggressive behavior when paired with a single control male as demonstrated by few lunges per fight and a failure to initiate aggression (Figure S7A–C).
Fig. 3. Gr32a-expressing neurons promote aggression via OA signaling. 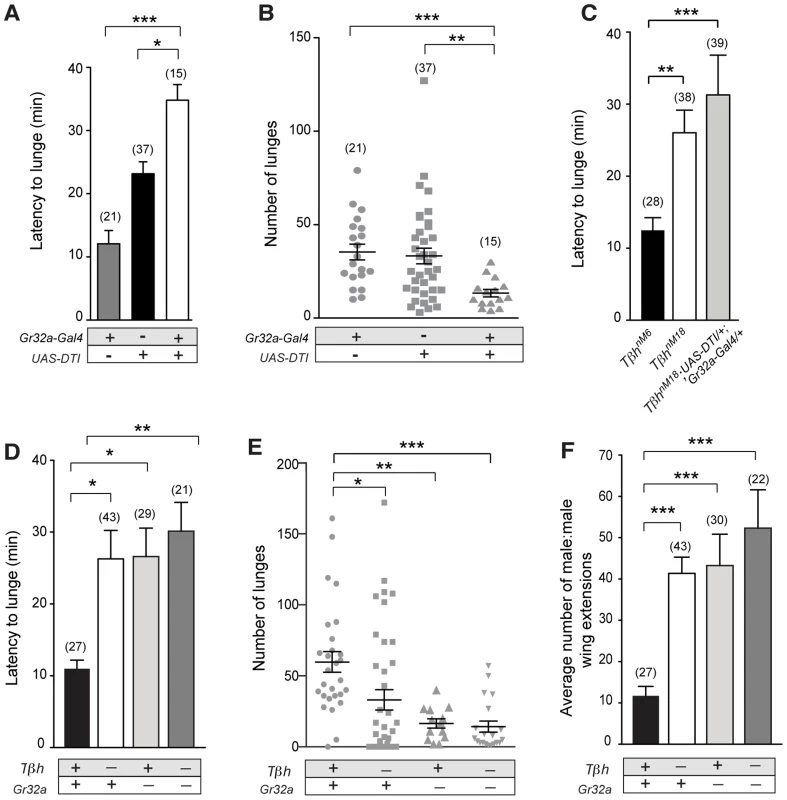
(A–B) Fights between males with Gr32a-expressing neurons removed by expressing Diptheria Toxin (UAS-DTI;Gr32a-Gal4) and individual transgenic controls, UAS-DTI or Gr32a-Gal4. (A) The latency to first lunge was significantly higher in UAS-DTI/+; Gr32a-Gal4/+ males as compared to controls (all statistical tests are Kruskal-Wallis with Dunn's multiple comparison test except where noted, ***p<0.001, *p<0.05). (B) Number of lunges (represented by each dot) performed in a 30 min period after the first lunge by any control or experimental male in a fighting pair. Males without Gr32a neurons exhibited a significant reduction in lunges as compared to controls (***p<0.001, **p<0.01). (C) Fights between control male pairs (revertant tβhM6 allele), experimental males without OA (revertant null mutation, tβhnM18), or experimental males without OA and without Gr32a-expressing neurons (tβhnM18;UAS-DTI/+; Gr32a-Gal4/+). The latency to first lunge was significantly higher in males without OA and in experimental males compared to control males (**p<0.01) and not statistically different between males without OA and experimental tβhnM18;UAS-DTI/+; Gr32a-Gal4/+ males. (D–F) Fights between control male pairs (revertant tβhM6 allele) and three groups of experimental males; without OA = tβhnM18, without Gr32a receptors = tβhM6;;Gr32a−/−, and without OA and Gr32a receptors = tβhnM18;;Gr32a−/−). (D) The latency to first lunge was significantly higher in males without OA (tβhnM18) and in experimental males without OA and the Gr32a receptor (tβhnM18; Gr32a−/−) or without only the Gr32 receptor (tβhM6; Gr32a−/−) males as compared to control tβhM6 males (One way ANOVA, post hoc Tukey's comparison, *p<0.05, **p<0.01). (E) The number of lunges by pairs of experimental males were significantly less than exhibited by control males but not when compared to each other (***p = 0.0002, **p = 0.002, *p = 0.01). (F) The average number of wing extensions directed toward the second male in each aggression assay. The number of wing extensions exhibited by males without the Gr32a receptor and without OA, and males without Gr32a receptors were significantly greater than control tβhM6 males (***p<0.001) but not males without OA (tβhnM18). Error bars denote s.e.m. To test the behavioral consequences of removing both Gr32a-expressing neurons and OA, we added the UAS-DTI;Gr32a-Gal4 transgenes to males with either the w+ tβhnM18 null recombinant chromosome) or the w+ tβhM6 recombinant control chromosome [23]. The resulting experimental males do not produce OA yet retain OA neurons and the Gr32a-expressing neurons are ablated. Similar to what was observed for flies without Gr32a neurons, flies without OA show a 2-fold increase in latency when compared to genetic control males (Figure 3C). If the function of Gr32a and OA neurons in setting the timely onset of an aggressive response were independent, the absence of both Gr32a receptors and OA function should result in an additive effect on aggression latency as compared to single mutants (flies lacking Gr32a-expressing neurons or OA only). Removing Gr32a signaling and OA via the tβhnM18 mutation did result in a small increase in the latency to the first lunge when compared to control males (Figure 3C). However, the increased latency was not significantly different from that observed in males without OA only (Figure 3C, (Mann-Whitney U test, p = 0.4). This equivalent aggression initiation delay exhibited by males without Gr32a neuronal function and tβhnM18;UAS-DTI;Gr32a-Gal4 males is the expected result if the aggression-promoting pheromonal signals transmitted by Gr32a neurons are at least partially conveyed via OA neurons. When males without OA and Gr32a neurons fight, the total lunges per fight are decreased (Figure 3D), though, the reduction in lunge number is not substantially different from UAS-DTI;Gr32a-Gal4 males (Figure 3B). Removing Gr32a neurons in males without OA significantly decreased lunge number (Figure S7D), however this additive value in lunge number reduction is not observed in males with only the Gr32a receptor eliminated (see below, Figure 3E).
Males with lowered levels of OA have been reported to exhibit lower numbers of lunges [24], [25]. Results in this study indicate that tβhnM18 mutant males take twice as long as controls to display their first lunges in fights (Figure 3C,D, Figure S7E). We previously demonstrated that males without detectable OA exhibited elevated courtship behavior towards other males [23]. One possible explanation of these results is that OA deficient males have difficulty recognizing the sex or species of a second fly. A similar delay in initiation observed in fights between males lacking Gr32a receptor neurons may be for this same reason (this study and [16]). Given such a large delay in the onset of aggression in OA mutant flies (Figure 3C,D and [25]), at least two factors can impact how lunge numbers are counted. First, counting lunges for a set period of time beginning when flies are first introduced to a chamber can yield very different results from counting at the start of lunging behavior (Figure S7E). A second consideration is the inclusion of male pairs that did not display lunges. If fights without lunges are scored as “zeros”, the numbers of lunges seen in fights between pairs of tβhnM18 males are significantly lower than the numbers seen in the genetic controls (Figure S7F), when fights that do not exhibit lunging are excluded, significant differences between tβh control and experimental are not found (Figure S7G). tβhnM18 males that exhibited low numbers of lunges also engaged in elevated levels of male-male courtship, which was not observed in tβhM6 controls while OA deficient males that exhibited high numbers of lunges engaged in male-male courtship at low levels. These results are displayed as a ratio of wing extensions (singing) divided by lunges (Figure S7H). Thus the affects of removing OA on the intensity of aggression also include a critical delay in the onset of aggression and an increase in male-male courtship.
To support the hypothesis that Gr32a receptor function itself is a key transducer of the aggression-enhancing stimuli regulated by OA, we tested males containing the Gr32a−/− mutation [15] in the tβhnM18 (null for OA) and tβhM6 (control) backgrounds. Males without the Gr32a receptor and males without OA and Gr32a exhibited a similar 2-fold increase in the latency to lunge (Figure 3D). The number of lunges displayed by males without OA (tβhnM18), without Gr32a (tβhM6;;Gr32a−/−), or without OA and the Gr32a receptor (tβhnM18;;Gr32a−/−) were each significantly reduced as compared to control males (tβhM6) (Figure 3E). Differences in lunge number between groups of experimental males were not observed (Figure 3E) providing further support that OA may be downstream of Gr32a sensory signaling processes.
As separately removing OA and Gr32a receptor function has been reported to increase male-male courtship toward intact males [23] and decapitated males [15], we quantified the occurrences of courtship to the second male within the aggression paradigm. Males without the Gr32a receptor, males without OA, and males without OA and Gr32a all displayed a significantly greater amount of male-male courtship to the second intact male compared to controls (Figure 3F). As with parameters of aggression, removing OA in the context of the Gr32a−/− mutation does not increase the already elevated levels of male-male courtship suggesting that OA may modulate Gr32a sensory input related to suppressing conspecific male courtship and promoting male aggression as these two processes have been suggested to reflect independent, parallel processes [39].
The intracellular Ca2+ response of OA SOG neurons to male CHCs requires Gr32a neurons
To determine if OA-expressing neurons modulate male aggression and courtship behavior by responding to sensory information concerning sexual recognition, we expressed the genetically encoded calcium indicator GCaMP6 [40], and assayed changes in intracellular Ca2+ responses evoked by application of CHC extracts to the male legs. Male CHC extracts evoked significant increases in GCaMP6s fluorescence in subsets of OA SOG neurons of Tdc2-LexA;20XLexAop2-IVS-GCaMP6s males (Figure 4A–B, E,G, n = 8). The response to male CHCs was abolished in males with Gr32a neurons eliminated via DTI expression (Tdc2-LexA/UAS-DTI;Gr32a-Gal4/20XLexAop2-IVS-GCaMP6s) (Figure 4C–D, F,G, n = 10) or through UAS-hid expression (Tdc2-LexA/UAS-hid UAS-RedStinger;Gr32a-Gal4/20XLexAop2-IVS-GCaMP6s, data not shown). Male CHC extracts were also applied to the forelegs of males expressing GCaMP3.0 in Gr32a neurons (UAS-GCaMP3.0/Gr32a-Gal4), however Ca2+ changes were not reliably detected in these foreleg neurons. As the cellular transduction mechanisms involved in Gr32a signaling are currently unknown, it is possible that Ca2+ changes may be near or below the detection threshold or that a response may not include a Ca2+ influx. Nevertheless, our physiological data support the hypothesis that sensory information received by Gr32a neurons is directly relayed to OA neurons in the SOG.
Fig. 4. Male CHCs evoke intracellular Ca2+ responses in OA neurons that are dependent on Gr32a neurons. 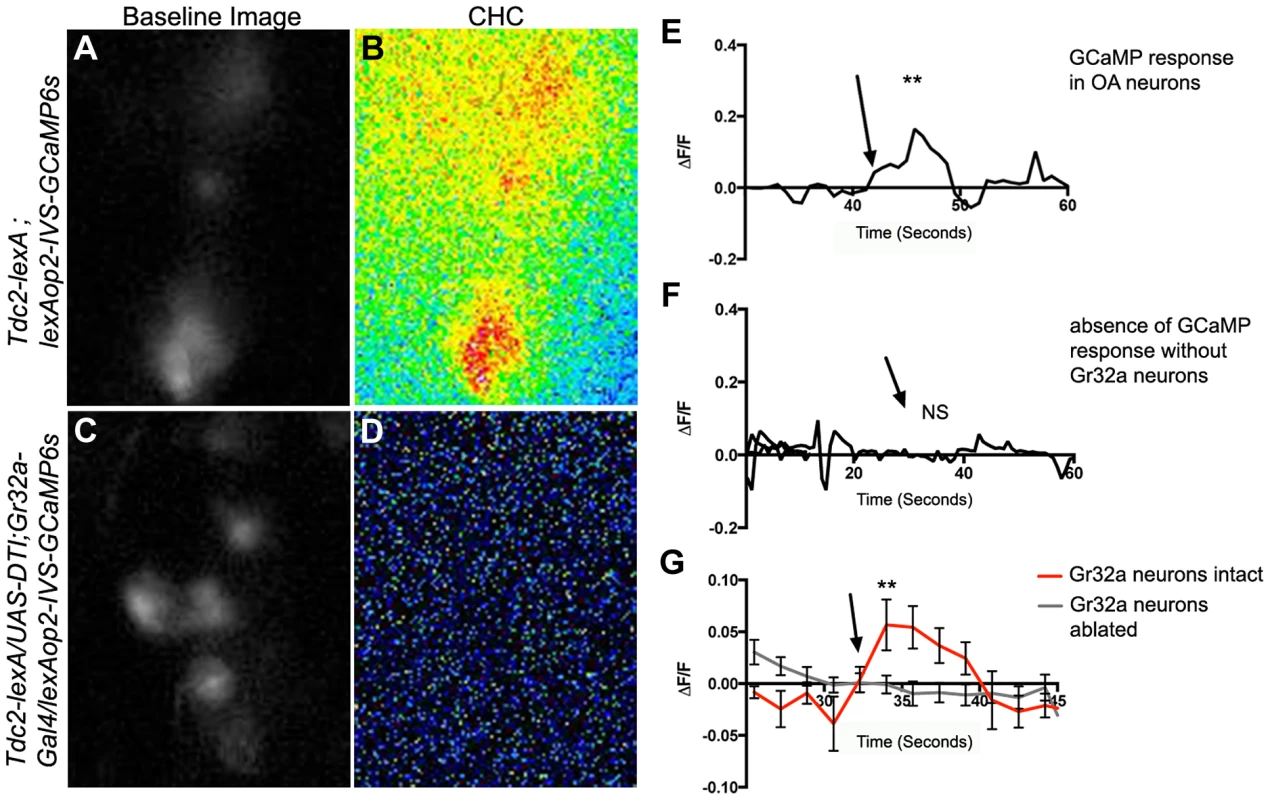
(A) Greyscale image (background subtracted) of GCaMP3 fluorescence in OA neurons located within the SOG in a Tdc2-lexA;lexAop2-IVS-GCaMP6s male. (B) Pseudocolored subtraction image demonstrating an increase in fluorescence in response to male CHC application. (C) Greyscale image (background subtracted) of baseline fluorescence in the SOG of a male with Gr32a neurons eliminated (Tdc2-lexA; UAS-DTI;Gr32a-Gal4/LexAop2-IVS-GCaMP6s). (D) No changes in fluorescence are observed in the pseudocolored subtraction image of OA SOG neurons when male CHC extract is administered to the legs of males lacking Gr32a neurons. (E) A representative calcium signal trace of OA neurons expressing GCaMP6s in panels A–B in response to male CHC extract application (arrow), unpaired t-test **p<0.006. (F) A representative trace demonstrating the lack of calcium response in OA neurons after male CHC extract application (arrow) to the legs of males without Gr32a neurons (Tdc2-LexA;UAS-DTI;Gr32a-LexA/20XLexAop2-IVS-GCaMP6s). (G) The average calcium response of eight regions of interest from five Tdc2-lexA;lexAop2-IVS-GCaMP6s males (red line) before and after male CHC administration (arrow, unpaired t-test, **p<0.0001). The gray line is the average calcium response of ten regions of interest from five Tdc2-LexA;UAS-DTI;Gr32a-LexA/20XLexAop2-IVS-GCaMP6s males in response to male CHC administration. No significant change in response was observed (unpaired t-test, p<0.0788). Error bars denote s.e.m. Subset-specific effects of Gr32a neuronal function on male aggression and courtship selection
Although a single receptor subtype, Gr32a, appears to mediate key pheromonal responses that inhibit interspecies courtship, promote male aggression, and suppress conspecific male-male courtship, different subpopulations of Gr32a-expressing neurons may be involved in each case. To test this idea, we selectively ablated Gr32a-expressing chemosensory neurons located in the mouth without removing the leg Gr32a neurons. For this purpose, we used the homeotic teashirt promoter driving Gal80 expression [41] to significantly block Gal4-mediated activation in regions outside of the head. Via this route Diphtheria Toxin expression (UAS-DTI) was prevented resulting in males lacking Gr32a-expressing neurons only in the labellum or mouth (Figure S8). As in experiments presented above, the latency to lunge was significantly longer in males without labellar Gr32a neurons (Figure 5A) and a significant reduction in lunge number was also observed (Figure 5B). As increased male-male courtship to a second intact male is exhibited by males without the Gr32a receptor and without OA (Figure 3F), we quantified the occurrences of courtship behavior (wing extensions and abdomen bending). The male-male courtship levels of UAS-DTI;teashirt(tsh)-Gal80/Gr32a-Gal4 male pairs are lower than control levels (Figure 5C) yet experimental males court females and successfully copulate in courtship assays (92%, n = 13) albeit with a longer latency to initiate courtship (Table S1). The ability of experimental males to successfully copulate is in agreement with a report indicating the ablation of the entire Gr32a neuron population does not alter the courtship of conspecific females [17]. Our results thereby indicate that there are functional differences on male social behavior served by the two separate populations of Gr32a-expressing chemosensory neurons and that the labellar Gr32a subpopulation is important for male aggression. Experiments in this study do not exclude a role for Gr32a leg neurons in male aggression, however the functional importance of the tarsal Gr32a subpopulation on male interspecies courtship behavior has recently been described [17].
Fig. 5. Gr32a chemosensory neurons located in the mouth promote aggression without an elevation in male-male courtship. 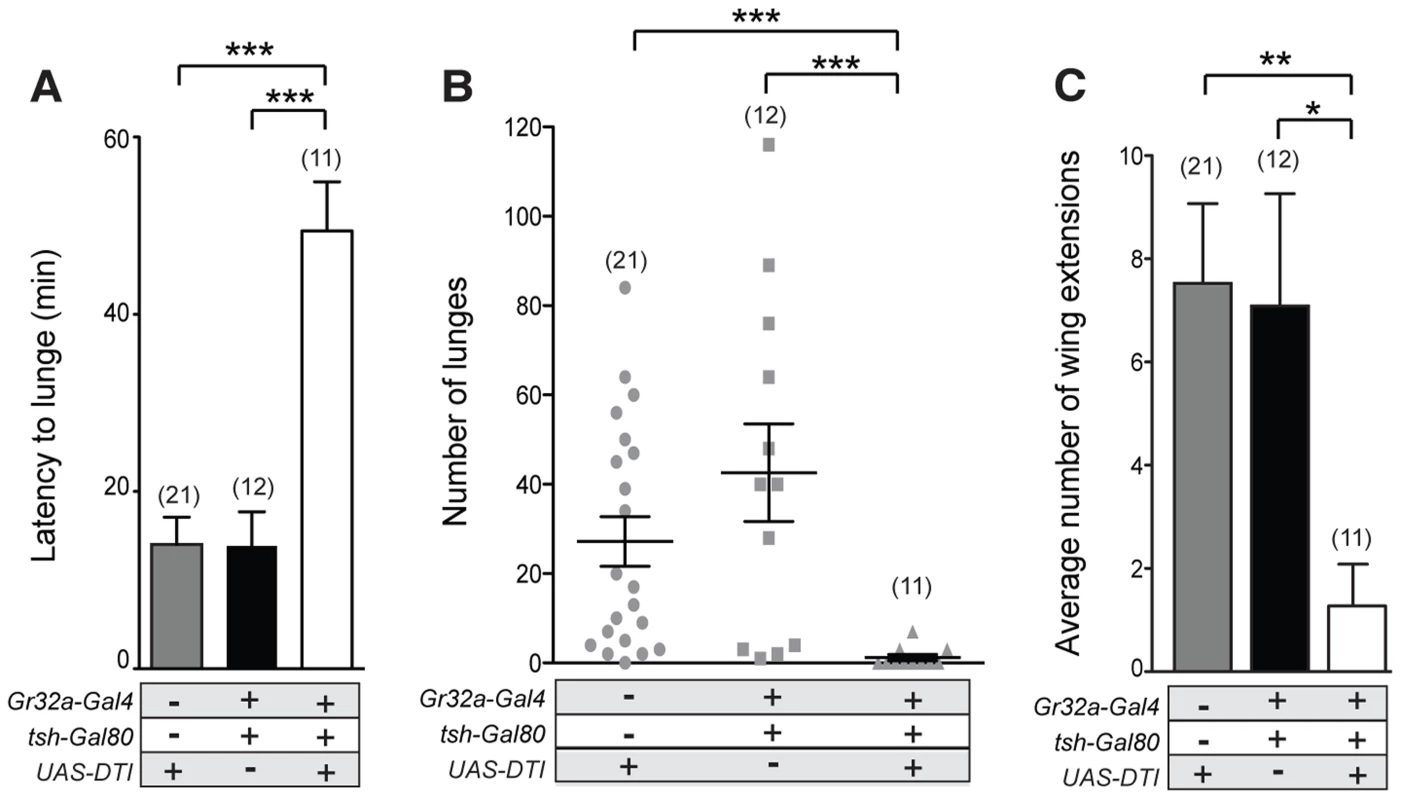
(A–B) Fights between males with the Gr32a-expressing mouth neuronal population removed by expressing Diptheria Toxin (UAS-DTI) through the Gr32a-Gal4 line with Gal4 activity in the legs blocked by tsh-Gal80. Separate transgenic controls, UAS-DTI/+ and tsh-Gal80/+; Gr32a-Gal4/+ were scored. (A) The latency to first lunge was significantly higher in UAS-DTI/tsh-Gal80; Gr32a-Gal4/+ males as compared to controls (Kruskal-Wallis with Dunn's multiple comparison test, ***p<0.001). (B) Number of lunges performed per 30 min period after the first lunge by controls or experimental UAS-DTI/tsh-Gal80; Gr32a-Gal4/+ males. Each dot represents the numbers of lunges performed by either male in a fighting pair. Males without Gr32a-expressing mouth neurons exhibited a significant reduction in lunges as compared to controls (Kruskal-Wallis test with Dunn's multiple comparison test, ***p<0.001). (C) The average number of wing extensions directed toward the second male in each aggression assay. The number of wing extensions exhibited by males without mouth Gr32a neurons were less than control males (Kruskal-Wallis with Dunn's multiple comparison test, *p<0.05, **p<0.01). Error bars denote s.e.m. Tissue-specific refinement of Gr32a to octopamine neuron synaptic contacts
To identify subpopulation-specific synaptic contacts between Gr32a and OA neurons, we used the teashirt-Gal80 line in combination with the GRASP system. Recent studies using the Gr32a-Gal4 driver to express GFP indicated at least 38 neurons in the mouth (19 neurons per labial palp) and 11 neurons located in the legs express the reporter [36], [38]. Adding the teashirt-Gal80 transgene significantly blocked Gal4-mediated activation in the thoracic region resulting in a reduction of GFP expression in the SOG. Thoracic ganglia neuronal projections via the cervical connective are reduced or absent (arrowhead in Fig. 1A, compare Figure 1A to Figure 6A). The reduction of GFP-expression in leg sensory neurons of UAS-nlsGFP; tsh-Gal80/Gr32a-Gal4 progeny (0.38 neurons per front leg, n = 8), versus males without Gal80 expression (5 neurons per front leg, n = 8) is shown in Figure 6F,G.
Fig. 6. Mouth-specific Gr32a neurons contact OA neurons in the suboesophageal ganglion. 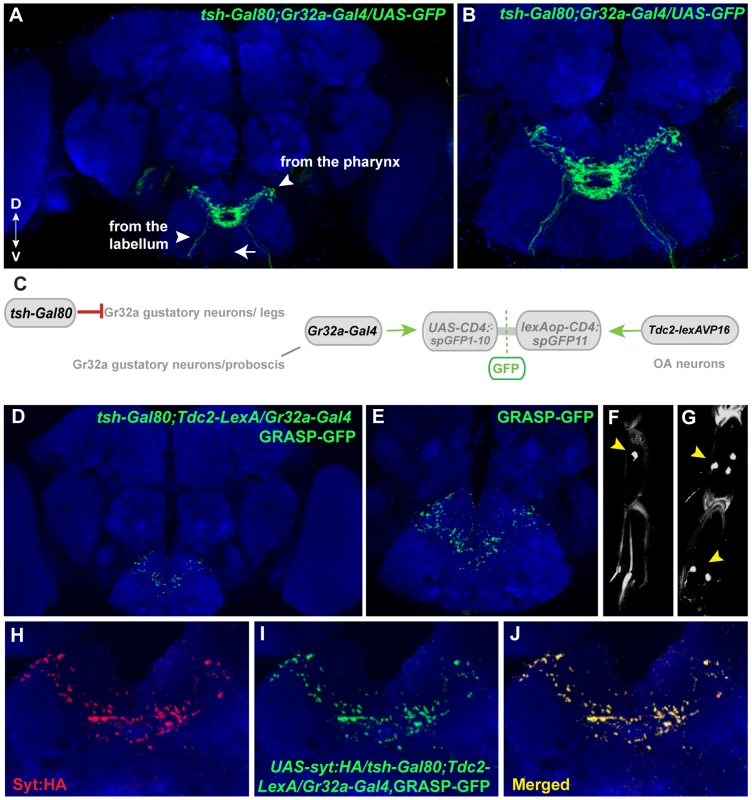
(A) Axons of Gr32a-expressing neurons located in the mouth identified by immunofluorescence to CD8:GFP in tsh-Gal80;UAS-CD8:GFP/Gr32a-Gal4 progeny (green, anti-CD8, Invitrogen). Note the absence of axonal projections from the legs via the thoracic ganglion (arrow, compare to Figure 1A). (B) Higher magnification of Gr32a mouth neurons expressing CD8:GFP. (C) Schematic representation of the GRASP reporter lines combined with the tsh-Gal80;Gr32a-Gal4 and Tdc2-lexA driver lines. Gal80 driven by the tsh-Gal80 line prevents Gal4 activity and subsequent expression of the UAS-CD4::spGFP1-10 GRASP reporter. (D–E) Two different confocal image magnifications of a male brain with the same number of optical sections as in panel A. A reduced amount of GRASP-mediated GFP reconstitution is observed reflecting Gr32a neurons located only in the mouth expressing CD4::spGFP1-10 and OA neurons expressing CD4::spGFP11. GRASP reconstitution is detected by immunofluorescence using rabbit monoclonal GFP antibody (green; Life Technologies). (F–G) tsh-Gal80 blocks GFP expression in Gr32a-expressing leg neurons. Less than one neuron per leg of UAS-nlsGFP; teashirt-Gal80/Gr32a-Gal4 progeny is observed (arrowhead, 0.38 neurons per front leg, n = 8), versus males without Gal80 expression (arrowhead, 5 neurons per front leg, n = 8). (H–J) Optical sections of a female brain (UAS-syt:HA; tsh-Gal80;UAS-CD8:GFP/Gr32a-Gal4) at higher magnification showing GRASP-mediated GFP reconstituted expression (I), syt:HA localization (H) and clear overlap or close association at synaptic-like puncta in the merged channel (J). With the addition of teashirt-Gal80 to restrict split-GFP expression to mouth Gr32a neurons, GFP reconstitution is visible in a highly reproducible pattern that appears to be part of the GRASP reconstituted pattern observed when the entire Gr32a-Gal4 expressing population is labeled (compare 6D with 1E). Furthermore, GFP reconstitution co-localizes with the UAS-syt:HA reporter added to visualize the presynaptic terminals of Gr32a-expressing neurons. (Figure 6H–J). As Gr32a and OA neuronal function strongly influence male-selective social behaviors, the GRASP patterns of male and female progeny were carefully examined. No apparent sex-specific differences were observed. Results from these experiments suggest that distinct behavioral responses to sex pheromone(s) are provided by separate subsets of Gr32a-expressing chemosensory neurons, in both cases involving potential direct reinforcement by OA.
Cell-specific refinement of octopamine neuron connections to Gr32a neurons
We previously demonstrated that three OA neurons express the male form of Fruitless (FruM), a neural sex determination factor that is a key determinant of male patterns of courtship and aggression (Figure 7A) [21], [42], [43]. The necessity of FruM expression in this small subset of OA neurons was evident as the absence of FruM resulted in an increase in male-male courtship in an aggression setting [21]. These results suggested that sexual specification of certain OA neurons might be involved in reliably establishing mate selection (or reliably suppressing conspecific male-male courtship). To determine if Gr32a-expressing neurons establish synaptic contacts with FruM-OA neurons, Tdc2-LexA was used in conjunction with the recently generated restrictable split-GFP component, lexAop2->stop>CD4::spGFP11 (María Paz Fernández, unpublished data). Selectively activating split-GFP11 expression in FruM neurons was achieved through the production of the FLP enzyme in Fruitless-expressing neurons via the fruFLP [44] line and putative synaptic connections were observed in male and female brains also expressing Gr32a-Gal4 driven UAS-CD4::spGFP1-10 (Figure 7B–D). At this time, we cannot simultaneously restrict Gr32a-expressing and OA neuronal populations or as yet quantify any sex-specific connection differences that may exist. However, our experiments indicate the FruM-OA neurons that account for increases in male-male courtship are anatomically connected to Gr32a neurons and these may form a microcircuit that contributes to the context-specificity of male courtship behavior.
Fig. 7. Gr32a neurons anatomically contact three FruM-OA neurons. 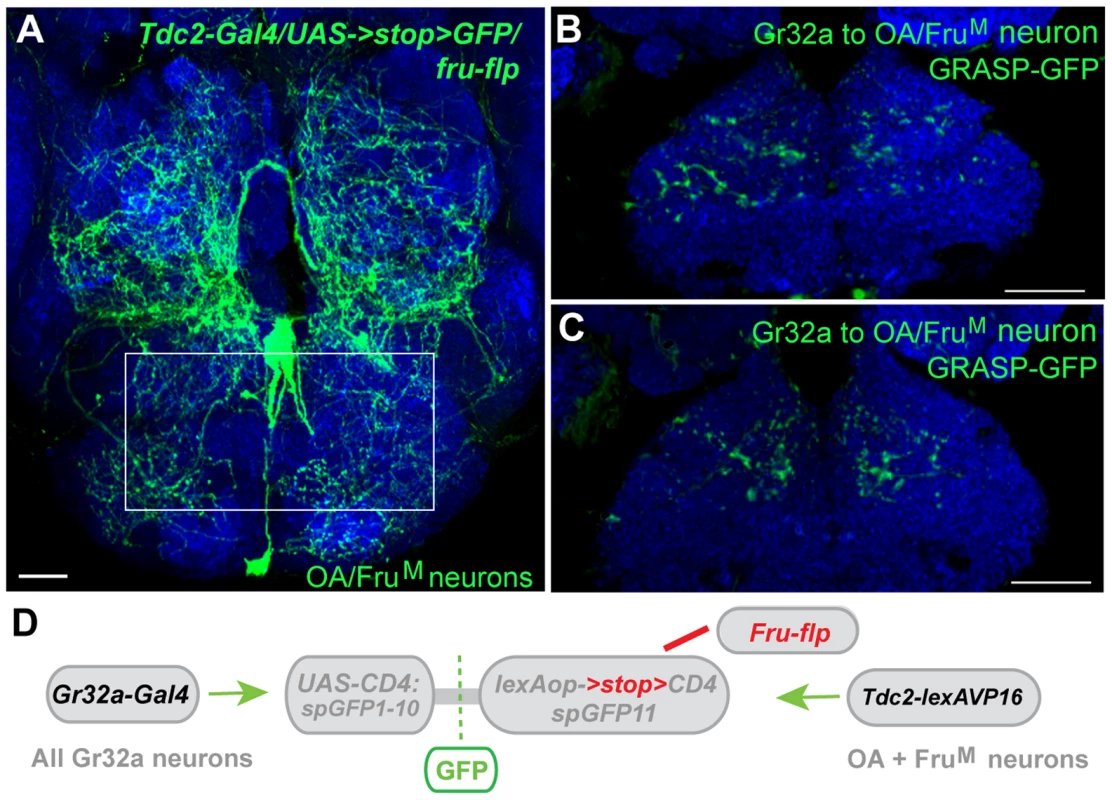
(A) The morphology of three FruM-OA neurons located in the suboesophageal ganglion identified by immunofluorescence to CD8:GFP in Tdc2-Gal4/UAS->stop>CD8:GFP;fruFLP progeny (green, anti-GFP, Life Technologies). The box outlines the area of putative synaptic connections observed in B and C. Scale bar represents 20 µM. (B, C) Two different optical sections of a male brain exhibiting GRASP-mediated GFP reconstitution as a result of FruM-OA neurons expressing CD4::spGFP11 and the entire Gr32a neuron population expressing CD4::spGFP1-10. GRASP reconstitution is detected by immunofluorescence using rabbit monoclonal GFP antibody (green; Life Technologies). Scale bar represents 30 µM. (D) Schematic representation outlining the GRASP reporter lines combined with fruFLP, Gr32aVP16-Gal4, and Tdc2-lexA. The FLP recombinase enzyme driven by fruFLP excises the stop codon and permits expression of the lexAop2>stop>::spGFP11 GRASP reporter. Scale bar represents 30 µM. Discussion
Studies on animal behavior have been ongoing for decades and these have resulted in identifying pheromones, hormones and neurohormones, neurons, circuits and more recently, genes, that cause or contribute to the expression of social behavior. Yet a broad gap still exists between the identification of neurons and circuits suspected of involvement in specific behaviors and an understanding of how these circuits orchestrate the many context-dependent complex decisions animals routinely make in their daily lives. In this study, we demonstrate a direct early sensory link to a neuromodulatory-signaling element concerned with male aggression and courtship behavior and show that the two are interconnected in the suboesophageal ganglion. Our results show that sensory neurons expressing Gr32a, a widely distributed gustatory receptor that plays a critical role in male social behaviors [14]–[17], relays primary sensory information to the SOG where octopaminergic interneurons are contacted. The high density of putative GRASP connections we observe between receptor neurons expressing Gr32a, 22e, and 59b, and OA neurons in the SOG (these are co-expressed in a subset of the labellar sensory receptor neuron pool) [36]), suggests that amine-dependent modulatory steps may serve as important second order components in connecting signals from taste receptor neuron subtypes to taste-evoked behavior in flies [31], [45] (in vertebrates and other invertebrate systems see [46], [47], [48]). A separate study also identified putative synaptic connections between Gr32a axons and the total population of FruM-expressing neurons [17]. Whether Gr32a-expressing neurons solely contact the OA-FruM neurons or whether they contact additional FruM neurons remains to be determined. We do observe regions of Gr32a-driven syt:HA expression without GFP reconstitution to OA neurons suggesting the Gr32a-expressing neuron population likely contacts additional neuron subsets.
The Gr32a receptor is categorized as a contact-based chemoreceptor and is required for physiological responses to caffeine and other aversive, bitter-tasting compounds [36], [49]–[51]. Gr32a is also reported to mediate the behavioral effects of the male pheromone (z)-7-tricosene and regulate interspecies courtship [16], [17]. (z)-7-tricosene application to male legs evoked an increase in Ca2+ signaling in OA neurons (Andrews and Certel, unpublished data), although we were unable to identify a reliable response to (z)-7-tricosene in Gr32a foreleg neurons at this time. Reconciling behavioral and physiological roles of Gr32a-expressing leg and labellar neurons to individual CHCs will require further investigation. Nevertheless, application of male CHCs to male legs evokes significant increases in Ca2+ signaling in OA neurons and this response is eliminated in males with ablated Gr32a neurons (Figure 4). These results support the behavioral data that indicates male aggression is promoted through the Gr32a receptor (this study and [16] and suggests that at least a portion of the sensory information mediated by Gr32a receptor-bearing sensory neurons and OA modulatory interneurons operate in a single circuit.
The manipulation of neuronal populations by altering the expression of single molecular products like the Gr32a gustatory receptor or one of the monoamines, commonly yields multiple behavioral phenotypes [14]–[16] indicating that such populations are heterogeneous in function. Separation of the grouped neurons into small subgroups can clarify the roles of these neurons in behavior and ultimately is essential in defining the circuitry involved. Recent findings indicate the tarsal Gr32a neurons are necessary to mediate species recognition [17]. Our data demonstrate that the foreleg tarsi and mouth populations of Gr32-expressing neurons may exert separable functional differences on male aggression and courtship behavior with both populations involving direct reinforcement by OA. Although Gr32a-expressing neurons do not exhibit any obvious sexual dimorphism, it has been postulated that their postsynaptic targets are sexually dimorphic [14]. With the increasing genetic capabilities of individual neuron manipulation, it will be interesting to determine if sexually dimorphic connectivity between single Gr32a and FruM-OA neurons regulate distinct differences in social behaviors. Results from further anatomical studies could provide insight into how potential sexual modification of OA signaling links chemosensory input to sex-specific behavioral output.
Neural networks mediating ever-changing environmental stimuli, context-specific social behavior, and internal states challenge us with the overwhelming structural and functional complexity of their interactions. To attempt to reduce network complexity, one common approach is to define network subunits and demonstrate their functional role by selective removal. It is well known that amine neurons can signal through hormonal volume transmission and act on targets at a distance [52], [53]. However, biogenic amines are also released synaptically and act on local targets [54]–[58]. Whether amine neurons function in separate modulatory circuits that run parallel to and interact with hard-wired circuitries directing behavior, or whether they are an integral part of such circuitry remains to be determined. However, understanding the presynaptic sources or postsynaptic targets of OA neurons should provide useful insight into the “structural” embeddedness of single cells within a network. An anatomical analysis of individual components will be necessary as proximity-based neuron groupings break down with the addition of cell-specific markers (like FruM) and within amine neuron populations [59]. Network anatomical characterization that includes neuromodulatory neurons may also provide insight into the reinforcing or opposing actions of amines through second amines or peptide modulators [60], [61]. For example, Burke et al., recently demonstrated plausible sites of synaptic contact between OA and DA neurons in the Drosophila mushroom body and a role for OA in providing appetitive reinforcement by OA receptor-mediated actions on DA neuron populations [29]. Our study offers a valuable framework in which to undertake the characterization of sensory-driven neural circuits and the underlying neuromodulation of sexually dimorphic patterns of social behavior.
Materials and Methods
The following strains were used in this study: Gr32a−/− [15], Gr32a-Gal4 [62], Gr32a-I-GFP [33], UAS-DTI (obtained from Leslie Stevens), UAS-transformer (BL 4590), UAS-synaptotagmin:hemagglutinin (UAS-syt:HA [34]), w+ tβhnM18 [23], w+ tβhM6 [23], dTdc2-Gal4 [28], tsh-Gal80 (provided by Julie Simpson) [63], UAS-mCD8:dsRed (obtained from Liz Gavis), lexAop-CD4::spGFP11 and UAS-CD4::spGFP1-10 [27], UAS-Red Stinger (BL 8545), UAS-hid UAS-Red Stinger, UAS-Denmark (BL 33063) [64], fruFLP [44], 20XUAS-6XGFP-Myc (a gift from Steve Stowers, BL 52262), UAS-GCaMP3.0 (BL-53742), 20XlexAop2-IVS-GCaMP6s (BL 44274) and the Canton-S strain from the Bloomington Stock Center, Bloomington, IN.
Generation of transgenic lines
The dTdc2-lexA:VP16 transgenic line was generated by cloning the same regulatory region as described previously [28] into the pBS_LexA::VP16_SV40 vector. In the previous construct, the GAL4 was inserted immediately before the coding start, and the entire construct (genomic segments interrupted by Gal4) was inserted into the polylinker of pCaSpeR4 [28]. To generate the dTdc2-lexA:VP16 construct, genomic DNA containing the region −3459 to +4530 was amplified with the Expand Long Template PCR system (Roche Applied Science). Fragment “A” of the dTdc2 genomic region was amplified using the following primers, Tdc2A - Forward: GTCGCGGCCGCAAAAGTTATTGCACATTG, Tdc2A-Reverse: GGCCGGCCGTTTCGGTAGGTTTTCCAAATC, and fragment “B” with the following primers, Tdc2B Forward: GTCGGGCCCATGGACAGCACCGAATTTC, Tdc2B-Reverse: GGCCGCGGCCGCTTAGAACATATCGAGTTG. The dTdc2 fragment A PCR product was inserted directly into the pBS-LexA::VP16_SV40 vector via the Eag1 site. Fragment B was first inserted into the TOPO vector and digested with Apa1, followed by ligation into to pBS-Tdc2fragmentA-LexA::VP16_SV40 using the Apa1 site on the vector. The fragment containing Tdc2 fragment A+ the LexA coding region+dTdc2 fragment B was subcloned into the Not1 site of pCaSpeR4.
The lexAop2-FRT-STOP-FRT-::spGFP11 line was generated by amplifying the spGF11 fragment through PCR from the previously described pLOT plasmid [27]. The FRT-STOP-FRT cassette was amplified from the pJFRC177 plasmid (#32149, AddGene) and both the STOP cassette and the spGFP11 fragment were cloned downstream of the 13XLexAop2 sequence in pJFRC19 (#26224, AddGene). The amplified fragments were verified by sequencing. Transgenic flies were raised by standard procedures and lines screened for appropriate expression.
Immunohistochemistry
Adult male and female dissected brains were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 25 minutes and labeled using a modification of protocols previously described [23]. The following primary antibodies were used: rabbit anti-GFP monoclonal (1∶200) (Life Technologies, G10362), mouse anti-GFP (1∶200) (Invitrogen, A-11120, Lot 764809), rabbit anti-FruM (1∶2000) [43], rat anti-CD8 (1∶100), rat anti-HA (Roche, 1∶1000), mAb nc82(anti-bruchpilot) (1∶30) [65], anti-Tβh (1∶400) [66]. Secondary antibodies include Alexa Fluor 488-conjugated goat anti-rabbit, Alexa Fluor 488-conjugated donkey anti-mouse, Alexa Fluor 594-conjugated donkey anti-mouse, Alexa Fluor 594-conjugated goat anti-rabbit, Alexa Fluor 647-conjugated donkey anti-mouse (Invitrogen). Cross-adsorbed goat anti-rabbit fluorescein-conjugated secondary antibodies were used in multi-labeling experiments. Images were collected on an Olympus Fluoview FV1000 laser scanning confocal mounted on an inverted IX81 microscope and processed using ImageJ (NIH) and Adobe Photoshop (Adobe, CA).
Behavioral assays
All fly strains were reared on standard fly food (medium containing agar, glucose, sucrose, yeast, cornmeal, propionic acid, and Tegosept). Flies were grown in temperature - and humidity-controlled incubators (25°C, 50% humidity) on a 12-h light/dark cycle. To collect socially naïve adults, pupae were isolated in individual 16×100-mm glass vials containing 1.5 ml of food medium. Upon eclosion, flies were anesthetized with CO2, painted on the thorax with acrylic paint for identification and returned to their isolation vials to allow for recovery from anesthesia a full 24 hours before testing.
Calcium imaging
Live brain preparations were made by anesthetizing a fly on ice followed by placement within a pipette tip with the head protruding. The pipette was then sealed with nail polish and allowed to dry. Flies thusly secured were placed in a 1 mL well for electrophysiology at an angle and the region containing the head was flooded with 400 µL of oxygenated HL3 solution. Removal of the proboscis and front of the head cuticle allowed for imaging. Each preparation was equilibrated for 5 min after proboscis and cuticle dissection. Male cuticular hydrocarbon extract (hexane extract from 150 male flies 3 days post eclosure), (z)-7-tricosene (Cayman Chemical #9000313 Lot# 0406404-32), or quinine (Sigma-Aldrich #6119-47-7 Lot # STBD3004V) dissolved in oxygenated HL3 solution was administered via syringe into the rear of the pipette tip. Administration of each compound occurred a minimum of 15 seconds apart. Flies received either male cuticular extract or (z)-7-tricosene first, followed by quinine. Analysis of ΔF/F values in regions of interest was calculated using Fiji and Prism 6.0.
Image analysis
Epifluorescene images were acquired at the rate of 1 image/.750s by Hamamatsu camera (ORCA ER series, model C4742-95-12ERG). Acquired images were registered (StackReg plugin, Fiji software) and regions of interest were selected within the suboesophageal ganglion. Image processing and analysis was accomplished with ImageJ version 1.44/Fiji version 1.43. Image subtraction was performed in Fiji using the image calculator. Intensity tables were exported to excel and (ΔF−F)/F calculated for each series of images. Traces were generated in Prism 6.0. Peak analysis was performed between regions no more than 5 seconds post compound administration (for post CHC) and no later than 4 seconds prior to compound administration (for pre-CHC).
Aggression and courtship paradigms
Aggression assays were performed in individual chambers of 12-well polystyrene plates containing a food cup in the center [67]. 4–5 day old males were transferred in pairs to assay chambers by aspiration. Experiments were performed at 25°C in a humidity controlled room (50%). Fights were videotaped for 90 minutes and lunges counted for 30 minutes from the first lunge unless otherwise specified. The time between introduction into the chamber and the onset of aggression (first lunge) was defined as the fighting latency. Lunging behavior was determined as previously described [68]. Courtship assays were performed in a 12 well polystyrene plate (VWR #82050-930) with one Canton S virgin female (aged 7–10 days) and one 4–5 day old male. The period between introduction into the courtship chamber and the first male wing extension (singing) was defined as courtship latency.
Supporting Information
Zdroje
1. DahanukarA, RayA (2010) Courtship, aggression and avoidance: Pheromones, receptors and neurons for social behaviors in Drosophila. Fly (Austin) 5 : 58–63.
2. FerreroDM, LiberlesSD (2010) The secret codes of mammalian scents. Wiley Interdiscip Rev Syst Biol Med 2 : 23–33.
3. MatsunamiH, AmreinH (2003) Taste and pheromone perception in mammals and flies. Genome Biol 4 : 220.
4. BirminghamJT, TauckDL (2003) Neuromodulation in invertebrate sensory systems: from biophysics to behavior. J Exp Biol 206 : 3541–3546.
5. FarooquiT (2007) Octopamine-mediated neuromodulation of insect senses. Neurochem Res 32 : 1511–1529.
6. MowreyWR, PortmanDS (2012) Sex differences in behavioral decision-making and the modulation of shared neural circuits. Biol Sex Differ 3 : 8.
7. Harris-WarrickRM (2011) Neuromodulation and flexibility in Central Pattern Generator networks. Curr Opin Neurobiol 21 : 685–692.
8. MarderE (2012) Neuromodulation of neuronal circuits: back to the future. Neuron 76 : 1–11.
9. StevensonPA, RillichJ (2012) The decision to fight or flee - insights into underlying mechanism in crickets. Front Neurosci 6 : 118.
10. YanowitchR, CoccaroEF (2011) The neurochemistry of human aggression. Adv Genet 75 : 151–169.
11. FernandezMP, KravitzEA (2013) Aggression and courtship in Drosophila: pheromonal communication and sex recognition. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 199 : 1065–1076.
12. FerveurJF (2005) Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet 35 : 279–295.
13. FerveurJF, JallonJM (1996) Genetic control of male cuticular hydrocarbons in Drosophila melanogaster. Genet Res 67 : 211–218.
14. KoganezawaM, HabaD, MatsuoT, YamamotoD (2010) The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr Biol 20 : 1–8.
15. MiyamotoT, AmreinH (2008) Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci 11 : 875–876.
16. WangL, HanX, MehrenJ, HiroiM, BilleterJC, et al. (2011) Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci 14 : 757–762.
17. FanP, ManoliDS, AhmedOM, ChenY, AgarwalN, et al. (2013) Genetic and Neural Mechanisms that Inhibit Drosophila from Mating with Other Species. Cell 154 : 89–102.
18. StockerRF (1994) The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res 275 : 3–26.
19. ParkJH, KwonJY (2011) A systematic analysis of Drosophila gustatory receptor gene expression in abdominal neurons which project to the central nervous system. Mol Cells 32 : 375–381.
20. BuschS, SelchoM, ItoK, TanimotoH (2009) A map of octopaminergic neurons in the Drosophila brain. J Comp Neurol 513 : 643–667.
21. CertelSJ, LeungA, LinCY, PerezP, ChiangAS, et al. (2010) Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS One 5: e13248.
22. ChiangAS, LinCY, ChuangCC, ChangHM, HsiehCH, et al. (2010) Three-Dimensional Reconstruction of Brain-wide Wiring Networks in Drosophila at Single-Cell Resolution. Curr Biol 21 : 1–11.
23. CertelSJ, SavellaMG, SchlegelDC, KravitzEA (2007) Modulation of Drosophila male behavioral choice. Proc Natl Acad Sci U S A 104 : 4706–4711.
24. HoyerSC, EckartA, HerrelA, ZarsT, FischerSA, et al. (2008) Octopamine in male aggression of Drosophila. Curr Biol 18 : 159–167.
25. ZhouC, RaoY (2008) A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci 11 : 1059–1067.
26. FeinbergEH, VanhovenMK, BendeskyA, WangG, FetterRD, et al. (2008) GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 57 : 353–363.
27. GordonMD, ScottK (2009) Motor control in a Drosophila taste circuit. Neuron 61 : 373–384.
28. ColeSH, CarneyGE, McClungCA, WillardSS, TaylorBJ, et al. (2005) Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem 280 : 14948–14955.
29. BurkeCJ, HuetterothW, OwaldD, PerisseE, KrashesMJ, et al. (2012) Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492 : 433–7.
30. DunipaceL, MeisterS, McNealyC, AmreinH (2001) Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol 11 : 822–835.
31. MiyazakiT, ItoK (2010) Neural architecture of the primary gustatory center of Drosophila melanogaster visualized with GAL4 and LexA enhancer-trap systems. J Comp Neurol 518 : 4147–4181.
32. StockerRF, SchorderetM (1981) Cobalt filling of sensory projections from internal and external mouthparts in Drosophila. Cell Tissue Res 216 : 513–523.
33. WangZ, SinghviA, KongP, ScottK (2004) Taste representations in the Drosophila brain. Cell 117 : 981–991.
34. RobinsonIM, RanjanR, SchwarzTL (2002) Synaptotagmins I and IV promote transmitter release independently of Ca(2+) binding in the C(2)A domain. Nature 418 : 336–340.
35. ThorneN, BrayS, AmreinH (2005) Function and expression of the Drosophila gr genes in the perception of sweet, bitter and pheromone compounds. Chem Senses 30 Suppl 1: i270–272.
36. WeissLA, DahanukarA, KwonJY, BanerjeeD, CarlsonJR (2011) The molecular and cellular basis of bitter taste in Drosophila. Neuron 69 : 258–272.
37. de Brito SanchezG, GiurfaM (2011) A comparative analysis of neural taste processing in animals. Philos Trans R Soc Lond B Biol Sci 366 : 2171–2180.
38. ThorneN, ChromeyC, BrayS, AmreinH (2004) Taste perception and coding in Drosophila. Curr Biol 14 : 1065–1079.
39. WangL, AndersonDJ (2010) Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463 : 227–231.
40. ChenTW, WardillTJ, SunY, PulverSR, RenningerSL, et al. (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499 : 295–300.
41. RoderL, VolaC, KerridgeS (1992) The role of the teashirt gene in trunk segmental identity in Drosophila. Development 115 : 1017–1033.
42. ManoliDS, FossM, VillellaA, TaylorBJ, HallJC, et al. (2005) Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436 : 395–400.
43. StockingerP, KvitsianiD, RotkopfS, TirianL, DicksonBJ (2005) Neural circuitry that governs Drosophila male courtship behavior. Cell 121 : 795–807.
44. YuJY, KanaiMI, DemirE, JefferisGS, DicksonBJ (2010) Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol 20 : 1602–1614.
45. SinakevitchI, MustardJA, SmithBH (2011) Distribution of the octopamine receptor AmOA1 in the honey bee brain. PLoS One 6: e14536.
46. BrezinaV (2010) Beyond the wiring diagram: signalling through complex neuromodulator networks. Philos Trans R Soc Lond B Biol Sci 365 : 2363–2374.
47. DelaneyAJ, CraneJW, SahP (2007) Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron 56 : 880–892.
48. MellonDJr (2000) Convergence of multimodal sensory input onto higher-level neurons of the crayfish olfactory pathway. J Neurophysiol 84 : 3043–3055.
49. LeeY, KimSH, MontellC (2010) Avoiding DEET through insect gustatory receptors. Neuron 67 : 555–561.
50. LeeY, MoonSJ, MontellC (2009) Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci U S A 106 : 4495–4500.
51. MoonSJ, LeeY, JiaoY, MontellC (2009) A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol 19 : 1623–1627.
52. AgnatiLF, GuidolinD, GuesciniM, GenedaniS, FuxeK (2010) Understanding wiring and volume transmission. Brain Res Rev 64 : 137–159.
53. FuxeK, Borroto-EscuelaDO, Romero-FernandezW, CiruelaF, MangerP, et al. (2012) On the role of volume transmission and receptor-receptor interactions in social behaviour: Focus on central catecholamine and oxytocin neurons. Brain Res 1476 : 119–31.
54. AgnatiLF, GuidolinD, LeoG, GuesciniM, PizziM, et al. (2011) Possible new targets for GPCR modulation: allosteric interactions, plasma membrane domains, intercellular transfer and epigenetic mechanisms. J Recept Signal Transduct Res 31 : 315–331.
55. De-MiguelFF, TruetaC (2005) Synaptic and extrasynaptic secretion of serotonin. Cell Mol Neurobiol 25 : 297–312.
56. HuoFQ, ChenT, LvBC, WangJ, ZhangT, et al. (2009) Synaptic connections between GABAergic elements and serotonergic terminals or projecting neurons in the ventrolateral orbital cortex. Cereb Cortex 19 : 1263–1272.
57. UmbriacoD, GarciaS, BeaulieuC, DescarriesL (1995) Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1). Hippocampus 5 : 605–620.
58. VargaV, LosonczyA, ZemelmanBV, BorhegyiZ, NyiriG, et al. (2009) Fast synaptic subcortical control of hippocampal circuits. Science 326 : 449–453.
59. MaoZ, DavisRL (2009) Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits 3 : 5.
60. BurkeCJ, HuetterothW, OwaldD, PerisseE, KrashesMJ, et al. (2012) Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492 : 433–437.
61. FlavellSW, PokalaN, MacoskoEZ, AlbrechtDR, LarschJ, et al. (2013) Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154 : 1023–1035.
62. DunipaceL, MeisterS, McNealyC, AmreinH (2001) Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol 11 : 822–835.
63. ClyneJD, MiesenbockG (2008) Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell 133 : 354–363.
64. NicolaiLJ, RamaekersA, RaemaekersT, DrozdzeckiA, MaussAS, et al. (2010) Genetically encoded dendritic marker sheds light on neuronal connectivity in Drosophila. Proc Natl Acad Sci U S A 107 : 20553–20558.
65. HofbauerA, EbelT, WaltenspielB, OswaldP, ChenYC, et al. (2009) The Wuerzburg hybridoma library against Drosophila brain. J Neurogenet 23 : 78–91.
66. KoonAC, AshleyJ, BarriaR, DasGuptaS, BrainR, et al. (2011) Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat Neurosci 14 : 190–199.
67. FernandezMP, ChanYB, YewJY, BilleterJC, DreisewerdK, et al. (2010) Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol 8: e1000541.
68. ChenS, LeeAY, BowensNM, HuberR, KravitzEA (2002) Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci U S A 99 : 5664–5668.
Štítky
Genetika Reprodukční medicína
Článek Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin PathwayČlánek Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human DiseasesČlánek G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify LongevityČlánek PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial MatrixČlánek Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus ConflictsČlánek The Impact of Population Demography and Selection on the Genetic Architecture of Complex TraitsČlánek Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar AcidificationČlánek The Case for Junk DNA
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 5- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
-
Všechny články tohoto čísla
- Genetic Interactions Involving Five or More Genes Contribute to a Complex Trait in Yeast
- A Mutation in the Gene in Dogs with Hereditary Footpad Hyperkeratosis (HFH)
- Loss of Function Mutation in the Palmitoyl-Transferase HHAT Leads to Syndromic 46,XY Disorder of Sex Development by Impeding Hedgehog Protein Palmitoylation and Signaling
- Heterogeneity in the Frequency and Characteristics of Homologous Recombination in Pneumococcal Evolution
- Genome-Wide Nucleosome Positioning Is Orchestrated by Genomic Regions Associated with DNase I Hypersensitivity in Rice
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Single Nucleotide Variants in Transcription Factors Associate More Tightly with Phenotype than with Gene Expression
- Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin Pathway
- Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human Diseases
- Epistatically Interacting Substitutions Are Enriched during Adaptive Protein Evolution
- Meiotic Drive Impacts Expression and Evolution of X-Linked Genes in Stalk-Eyed Flies
- G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify Longevity
- Population Genomic Analysis of Ancient and Modern Genomes Yields New Insights into the Genetic Ancestry of the Tyrolean Iceman and the Genetic Structure of Europe
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
- Whole Exome Re-Sequencing Implicates and Cilia Structure and Function in Resistance to Smoking Related Airflow Obstruction
- Allelic Expression of Deleterious Protein-Coding Variants across Human Tissues
- R-loops Associated with Triplet Repeat Expansions Promote Gene Silencing in Friedreich Ataxia and Fragile X Syndrome
- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- The Impairment of MAGMAS Function in Human Is Responsible for a Severe Skeletal Dysplasia
- Octopamine Neuromodulation Regulates Gr32a-Linked Aggression and Courtship Pathways in Males
- Mlh2 Is an Accessory Factor for DNA Mismatch Repair in
- Activating Transcription Factor 6 Is Necessary and Sufficient for Alcoholic Fatty Liver Disease in Zebrafish
- The Spatiotemporal Program of DNA Replication Is Associated with Specific Combinations of Chromatin Marks in Human Cells
- Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus Conflicts
- Genome-Wide Inference of Ancestral Recombination Graphs
- Mutations in Four Glycosyl Hydrolases Reveal a Highly Coordinated Pathway for Rhodopsin Biosynthesis and N-Glycan Trimming in
- SHP2 Regulates Chondrocyte Terminal Differentiation, Growth Plate Architecture and Skeletal Cell Fates
- The Impact of Population Demography and Selection on the Genetic Architecture of Complex Traits
- Retinoid-X-Receptors (α/β) in Melanocytes Modulate Innate Immune Responses and Differentially Regulate Cell Survival following UV Irradiation
- Genetic Dissection of the Female Head Transcriptome Reveals Widespread Allelic Heterogeneity
- Genome Sequencing and Comparative Genomics of the Broad Host-Range Pathogen AG8
- Copy Number Variation Is a Fundamental Aspect of the Placental Genome
- GOLPH3 Is Essential for Contractile Ring Formation and Rab11 Localization to the Cleavage Site during Cytokinesis in
- Hox Transcription Factors Access the RNA Polymerase II Machinery through Direct Homeodomain Binding to a Conserved Motif of Mediator Subunit Med19
- Drosha Promotes Splicing of a Pre-microRNA-like Alternative Exon
- Predicting the Minimal Translation Apparatus: Lessons from the Reductive Evolution of
- PAX6 Regulates Melanogenesis in the Retinal Pigmented Epithelium through Feed-Forward Regulatory Interactions with MITF
- Enhanced Interaction between Pseudokinase and Kinase Domains in Gcn2 stimulates eIF2α Phosphorylation in Starved Cells
- A HECT Ubiquitin-Protein Ligase as a Novel Candidate Gene for Altered Quinine and Quinidine Responses in
- dGTP Starvation in Provides New Insights into the Thymineless-Death Phenomenon
- Phosphorylation Modulates Clearance of Alpha-Synuclein Inclusions in a Yeast Model of Parkinson's Disease
- RPM-1 Uses Both Ubiquitin Ligase and Phosphatase-Based Mechanisms to Regulate DLK-1 during Neuronal Development
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- Heritable Transmission of Stress Resistance by High Dietary Glucose in
- Revertant Mutation Releases Confined Lethal Mutation, Opening Pandora's Box: A Novel Genetic Pathogenesis
- Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar Acidification
- A Genome-Wide Assessment of the Role of Untagged Copy Number Variants in Type 1 Diabetes
- Selectivity in Genetic Association with Sub-classified Migraine in Women
- A Lack of Parasitic Reduction in the Obligate Parasitic Green Alga
- The Proper Splicing of RNAi Factors Is Critical for Pericentric Heterochromatin Assembly in Fission Yeast
- Discovery and Functional Annotation of SIX6 Variants in Primary Open-Angle Glaucoma
- Six Homeoproteins and a linc-RNA at the Fast MYH Locus Lock Fast Myofiber Terminal Phenotype
- EDR1 Physically Interacts with MKK4/MKK5 and Negatively Regulates a MAP Kinase Cascade to Modulate Plant Innate Immunity
- Genes That Bias Mendelian Segregation
- The Case for Junk DNA
- An In Vivo EGF Receptor Localization Screen in Identifies the Ezrin Homolog ERM-1 as a Temporal Regulator of Signaling
- Mosaic Epigenetic Dysregulation of Ectodermal Cells in Autism Spectrum Disorder
- Hyperactivated Wnt Signaling Induces Synthetic Lethal Interaction with Rb Inactivation by Elevating TORC1 Activities
- Mutations in the Cholesterol Transporter Gene Are Associated with Excessive Hair Overgrowth
- Scribble Modulates the MAPK/Fra1 Pathway to Disrupt Luminal and Ductal Integrity and Suppress Tumour Formation in the Mammary Gland
- A Novel CH Transcription Factor that Regulates Expression Interdependently with GliZ in
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics
- Spermatid Cyst Polarization in Depends upon and the CPEB Family Translational Regulator
- Insights into the Genetic Structure and Diversity of 38 South Asian Indians from Deep Whole-Genome Sequencing
- Intron Retention in the 5′UTR of the Novel ZIF2 Transporter Enhances Translation to Promote Zinc Tolerance in
- A Dominant-Negative Mutation of Mouse Causes Glaucoma and Is Semi-lethal via LBD1-Mediated Dimerisation
- Biased, Non-equivalent Gene-Proximal and -Distal Binding Motifs of Orphan Nuclear Receptor TR4 in Primary Human Erythroid Cells
- Ras-Mediated Deregulation of the Circadian Clock in Cancer
- Retinoic Acid-Related Orphan Receptor γ (RORγ): A Novel Participant in the Diurnal Regulation of Hepatic Gluconeogenesis and Insulin Sensitivity
- Extensive Diversity of Prion Strains Is Defined by Differential Chaperone Interactions and Distinct Amyloidogenic Regions
- Fine Tuning of the UPR by the Ubiquitin Ligases Siah1/2
- Paternal Poly (ADP-ribose) Metabolism Modulates Retention of Inheritable Sperm Histones and Early Embryonic Gene Expression
- Allele-Specific Genome-wide Profiling in Human Primary Erythroblasts Reveal Replication Program Organization
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



