-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
Successful reproduction depends upon the receipt and processing of distinct chromatin packages from sperm and oocytes. This includes not only a unique complement of DNA, but information in the form of proteins, such as histones, that differentially package the DNA in each cell type. For example, histone variants and post-translationally modified histones can carry epigenetic information across generations. Such information is then reprogrammed in the new embryo to ensure proper development. However, it is unclear how many of these marks are established during gamete formation and reprogrammed after fertilization. We define a signature histone variant and post-translational modification profile of sperm chromatin from C. elegans. This profile is established during sperm formation in part by exchanging canonical histones with sperm-specific histone proteins. Histone variants and modifications passed from sperm and oocytes are differentially removed or retained, suggesting that the embryo can reprogram epigenetic information from each parent distinctly. These C. elegans studies reveal that both conserved and novel histone modification and exchange mechanisms are used across diverse species to establish and reprogram epigenetic information.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004588
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004588Summary
Successful reproduction depends upon the receipt and processing of distinct chromatin packages from sperm and oocytes. This includes not only a unique complement of DNA, but information in the form of proteins, such as histones, that differentially package the DNA in each cell type. For example, histone variants and post-translationally modified histones can carry epigenetic information across generations. Such information is then reprogrammed in the new embryo to ensure proper development. However, it is unclear how many of these marks are established during gamete formation and reprogrammed after fertilization. We define a signature histone variant and post-translational modification profile of sperm chromatin from C. elegans. This profile is established during sperm formation in part by exchanging canonical histones with sperm-specific histone proteins. Histone variants and modifications passed from sperm and oocytes are differentially removed or retained, suggesting that the embryo can reprogram epigenetic information from each parent distinctly. These C. elegans studies reveal that both conserved and novel histone modification and exchange mechanisms are used across diverse species to establish and reprogram epigenetic information.
Introduction
The totipotency of a new embryo depends upon the reprogramming of epigenetic information carried over from the sperm and oocyte, each of which distinctly packages its DNA. For example, canonical histones H2A, H2B, H3, H4 and the linker histone H1 within mammalian sperm are replaced by sperm nuclear basic proteins (SNBPs) including histone variants, transition proteins, and protamines [1]–[4]. This repackaging results in global transcriptional repression and tight compaction of sperm chromatin [4]. After fertilization, sperm and oocyte chromatin also undergo distinct processes [5], [6]. Oocyte chromosomes complete meiotic divisions. Then, while maternal and paternal chromatin decondense, SNBPs are removed from sperm chromatin. Thus, distinct programs package the chromatin from each gamete type during their development and after fertilization. However, still largely unknown are the epigenetic features that are inherited, how they are reprogrammed in the new embryo, and the extent to which these reprogramming steps are conserved across species.
During sperm formation, histone variants are incorporated to restructure chromatin. In human, mouse, and fly, testes-specific variants of H1, H2A, H2B, and H3 displace canonical histones prior to incorporation of transition proteins and protamines [7]–[9]. In vitro studies demonstrate that some of these variants cause nucleosome destabilization [8], [10]–[15]. Other histone variants mark specific chromatin domains. For example in mouse spermatids, H2AL1/2 are incorporated into heterochromatic pericentric chromatin [14], inherited by the embryo, and removed upon paternal chromatin decondensation [16]. The fate of other paternally contributed histone variants remains unclear.
Chromatin restructuring during spermatogenesis is also regulated by histone post-translational modifications (PTMs). In Drosophila and mammals, global acetylation of histones precedes their replacement after meiosis [7], [17]–[21]. In particular, H4K16 acetylation (H4K16ac) targets histones for polyubiquitin-independent degradation within sperm-specific proteasomes called spermatoproteasomes [18]. During spermatogenesis in Drosophila, mouse, and rat, histone H2A is mono-ubiquitinated (H2AK119ub) [21]–[24]. However, the extent to which histone removal requires H2A ubiquitination by the ubiquitin ligase RNF8 remains controversial [23], [25]. Many other histone PTMs have been identified in sperm or testes but roles in the histone-to-protamine transition for most of these PTMs are unknown [26], [27].
Other roles for histone PTMs are as paternal imprints. In human sperm, marks of active transcription, such as H3K4me2 and H3K4me3, localize to paternally-expressed imprinted loci and to promoters of genes required for development, suggesting these paternal marks play key roles in developmental programs in the embryo [28]. Conversely, a mark of repressed chromatin, H3K27me3, is enriched at paternal promoters that are repressed in gamete formation and early embryos [28], [29]. In mouse, H4K8ac and H4K12ac, which mark heterochromatin regions in elongating spermatids, are also carried over by sperm [30], [31]. In C. elegans, the exclusion of H3K4me2 at inactive chromatin domains during spermatogenesis is maintained at those domains within the embryonic genome [32]. Thus histone PTMs can serve as epigenetic marks to transmit the history of specific chromatin regions to the new embryo [5], [33]. With the array of possible histone PTMs, there is significant potential for other paternal chromatin PTMs to play directive roles in the embryo.
In this work, we define the unique complement of epigenetic information contributed by histones in sperm chromatin in C. elegans. Histones are hypothesized to be the major constituent of epigenetic information in C. elegans because this organism lacks DNA methylation, which is classically associated with genomic imprinting in some species [34]. Though three highly homologous putative protamines, called SPCH-1, SPCH-2, and SPCH-3 (SPCH-1, 2, 3), have been identified in C. elegans [35], histones and histone PTMs are retained in sperm chromatin and delivered to the new embryo. For example, previous studies have detected canonical and variant histones [32], [35], GFP-labeled histone H3.3, or specific post-translationally modified histones (such as H3K4me2) in C. elegans sperm [32], [36]. However, a global profile of histone variants and PTMs in paternal chromatin and an understanding of their fate during the reprogramming of the embryonic genome are lacking. To address this, we employ a combination of approaches. First, we use proteomic and western analysis to define the histone profile of isolated sperm and compare it to the profile of mixed-stage embryos. Immunostaining is then used to track the temporal and spatial dynamics of how histone marks are established during spermatogenesis and oogenesis and then processed in the early embryo. This combination of approaches identifies paternal-specific epigenetic features and shows how parent-specific information can be retained or erased before and after fertilization.
Results
Histones are retained in C. elegans sperm chromatin
To assess the extent of histone retention in sperm, we compared histone levels in sperm (a relatively uniform population of transcriptionally inactive, differentiated haploid cells [37]) to that of mixed-stage embryos (transcriptionally active and developing diploid cells). Western blot analysis shows canonical histone H3, H4, and the ubiquitous H2A variant HTZ-1 are present in both sperm and embryos, while the H2A variant HTAS-1 and the putative protamines SPCH-1, 2, 3 are found only in sperm (Figure 1A–E). Quantification revealed that lysates from sperm possess roughly 37% of canonical histones H3 and H4 compared to those of embryos that contain equivalent amounts of DNA (Figure 1A, B, Methods). HTZ-1 was found at 30% in sperm lysates compared to embryo lysates (Figure 1C, Methods). Thus, C. elegans retain a considerable portion of histones in sperm compared to humans (∼4–15%), mice (∼1%), and Drosophila (∼0%) [21], [28], [38], [39].
Fig. 1. Canonical histones are retained in C. elegans sperm. 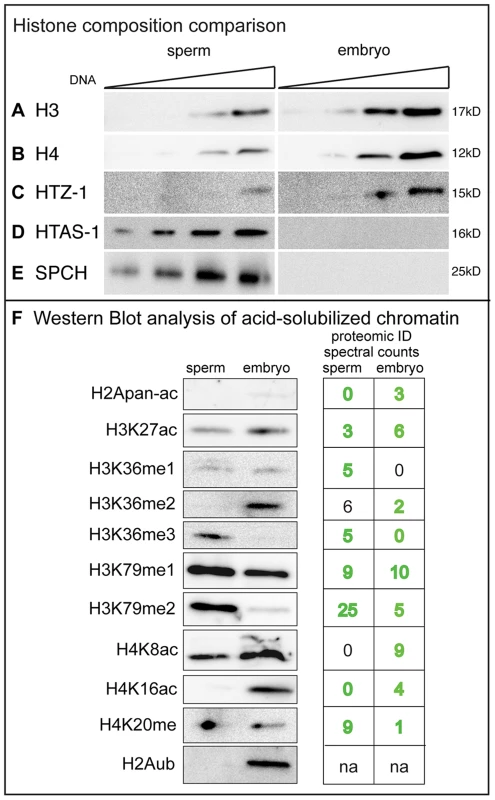
A–E) Western blot analysis of sperm and embryo lysates loaded with equivalent amounts of DNA. A) Histone H3: 12, 24, 60 and 120 ng; B) Histone H4: 24, 48, 120, and 240 ng; C) HTZ-1: 24, 48, 120, and 240 ng; D) HTAS-1: 12, 24, 60 and 120 ng; E) SPCH-1, 2, 3: 12, 24, 60 and 120 ng. Bands shown were quantified using ImageJ software (see Methods). F) Western analysis detection of histone post-translational modification (PTMs) in acid-solubilized sperm and embryo chromatin samples. The left panel shows western signals for the histone PTMs indicated. Right panels show spectral counts for histone PTMs identified by MuDPIT analysis (from Table 1). Green text indicates western and proteomic identification correspond. Black text indicates western and proteomic identification did not correspond. ‘na’ is not applicable because identification by proteomics of ubiquitination was not included in PTM counts (see Methods). The anti-H2AK119ub antibody (#87, see Table S7) recognizes the corresponding residue K120 in C. elegans (File S5). To further explore the histone content of chromatin from sperm and embryos we employed Multidimensional Protein Identification Technology (MudPIT), a sensitive approach that can detect low-abundance chromatin proteins and PTMs in complex mixtures of proteins [40]–[43]. We purified and acid solubilized chromatin from sperm and mixed-stage embryos [44]. SDS PAGE analysis revealed histones H2A, H2B, H3, and H4 were enriched in both chromatin samples (Figure S1A, B). These samples were digested with proteases and then analyzed by MudPIT. Spectra from each sample were matched by the ProLuCID algorithm to predicted peptides and assembled into corresponding C. elegans proteins by the DTASelect program [45]–[47]. Consistent with SDS PAGE and western analysis (Figure 1A–D, Figure S1A, B), histones were among the most highly represented from the 1889 spermatogenic and 2421 embryonic proteins identified as evaluated by either total spectral counts, normalized spectral abundance factor (NSAF) values, or Exponentially Modified Protein Abundance Index (EMPAI) values (Table S1, Table S2) [41], [48]–[50].
The global profile of histone variant and post-translational modifications in sperm and embryo chromatin is distinct
We looked for chromatin proteins enriched in embryo or sperm chromatin samples compared to one another utilizing the spectral count feature of MudPIT, which gives an idea of the relative levels of more abundant proteins [41], [51], [52]. Indeed, we found that the H2A variant HTAS-1 is highly enriched in sperm chromatin with 285 spectral IDs out of 3595 total H2A spectra IDs (which include modified and unmodified canonical H2A, HTZ-1, and HTAS-1 spectra) compared to embryo chromatin (0/4758 total H2A spectra IDs) (Table S3). Furthermore, MudPIT analysis was sensitive: we were able to detect 36 different histone PTMs including mono-methylated (me1), di-methylated (me2), tri-methylated (me3) and acetylated (ac) residues (Table 1, Tables S3–S6, Files S1–S4). Few phosphorylated residues were found, likely because phosphorylation is inherently labile and phospho-enrichment methods were not used. The coverage of the N - and C-termini of histones was also low because they are rich in lysine and arginine, the recognition sites of the proteases used.
Tab. 1. Post-translational modification sites of core histone proteins identified by MudPIT analysis in embryos and sperm. 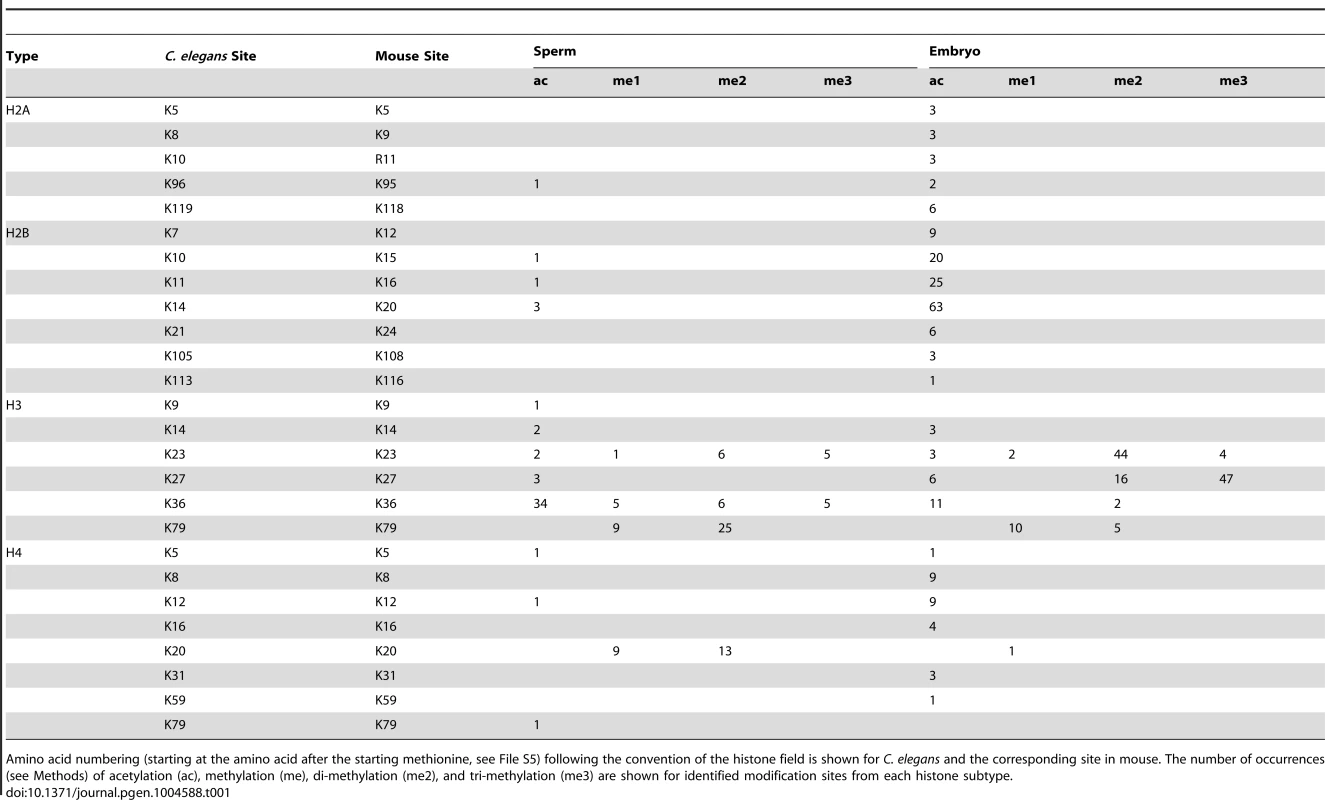
Amino acid numbering (starting at the amino acid after the starting methionine, see File S5) following the convention of the histone field is shown for C. elegans and the corresponding site in mouse. The number of occurrences (see Methods) of acetylation (ac), methylation (me), di-methylation (me2), and tri-methylation (me3) are shown for identified modification sites from each histone subtype. Overall, proteomic analysis found 31 histone PTM marks in embryos compared to 22 marks in sperm (Table 1), some of which would not have been identified by immunostaining or western analysis because antibodies that recognize them are not available. Our analysis also found ∼2.4 fold fewer modified peptides in sperm samples (127 PTM histone spectra IDs/7190 total histone spectra IDs = 1.8%) compared to embryo samples (253 PTM histone spectra IDs/5998 total histone spectra IDs = 4.2%) (Tables S3, S4, S5, S6, Methods). A comparison of histone PTMs between sperm and embryos revealed differences in the identification of some PTM classes in each histone subtype. In general, acetylation on histone H2A, H2B, and H4 were identified more frequently and on more sites in embryos compared to sperm, whereas methylation on histone H3 was detected at similar levels in both samples (Table 1).
To further investigate the presence of histone PTMs identified by proteomic analysis, we applied western analysis on sperm and mixed-stage embryo chromatin. One drawback of conducting westerns is the availability, reliability, and expense of suitable antibodies [53]. Indeed, of 27 available antibodies to modified and unmodified histones we screened (Table S7), 12 recognized bands at the expected molecular weight (Table S7, Figure 1F). A band was detected in 14/16 instances for sites where proteomic analysis had also identified the histone PTM (Figure 1F); therefore, proteomic identification is suitable for detecting both high and low abundance histone PTMs. However, in 2/4 instances western analysis detected a band for sites where proteomic analysis did not identify a modification (Figure 1F). Hence, the lack of detection by either proteomics or westerns is not in itself definitive evidence that the modification is absent. An antibody that recognizes ubiquitinated histone H2A (H2Aub) [(at lysine K119 in mammals, which corresponds to C. elegans K120 (File S5)] also detected H2Aub in embryos but not sperm (Figure 1F) [54]. Thus, both proteomic and western analysis support that the global histone variant and PTM profile of sperm is distinct from that of mixed-stage embryos.
The histone H2A variant HTAS-1 exclusively marks paternal chromatin
Because proteomic and western analysis on sperm and embryo chromatin indicated HTAS-1 is enriched in sperm (Table S3, Figure 1D), we verified it is sperm-specific. First, HTAS-1 is present in mutant strains that produce only sperm and not in those that produce only oocytes, similar to the sperm-specific Major Sperm Protein (MSP) (Figure S1C). In contrast, another H2A variant, HTZ-1, was detected in both (Figure S1C). Furthermore, immunolocalization on dissected males and hermaphrodites found that HTAS-1 is expressed only during spermatogenesis in both sexes (Figure 2A, Figure S2A) [35]. HTAS-1 incorporation into chromosomes starting from the late pachytene stage precedes incorporation of the putative protamines SPCH-1, 2, 3 (Figure 2D, F). HTAS-1 is strongly enriched on condensing DNA. It is also detectable on all post-meiotic spermatids when SPCH-1, 2, and 3 levels are high. In contrast, HTZ-1 and histone H1 are present at all stages (Figure 2A, F, Figure S2B). Thus, from proteomic, western, and cytological analysis, we conclude that HTAS-1 is a sperm-specific chromatin protein.
Fig. 2. HTAS-1 is incorporated as sperm chromosomes condense. 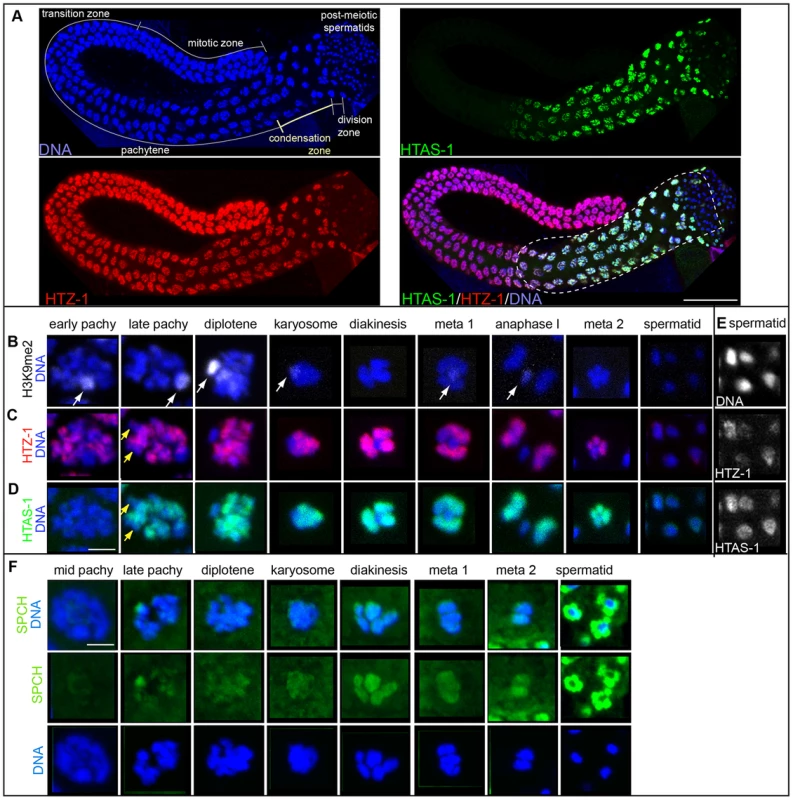
Immunolocalization of isolated and fixed C. elegans male gonads. A) DNA stained with DAPI (blue), HTAS-1 (green), HTZ-1 (red), merged image of HTAS-1 (green), HTZ-1 (red) and DAPI (blue). Scale bar represents 50 µm. B–E) Immunostaining of individual nuclei from late stages of sperm formation (as found in the proximal end of the gonad marked with the dotted line in A). The scale bar represents 2 µm and applies to all panels in B–F. B) Histone H3 dimethylated at lysine 9 (H3K9me2), which marks the X chromosome (white arrows). C) HTZ-1. D) HTAS-1. Yellow arrows mark regions of under-representation of HTAS-1 and HTZ-1 that are not the X chromosome. E) HTZ-1 and HTAS-1 are detectable immediately after meiotic divisions as sperm chromatin condenses. Contrast-adjusted black and white images of DNA, HTZ-1, and HTAS-1 staining of the early spermatid nuclei shown in panels C and D that show HTZ-1 and HTAS-1 are detectable. See Figure S2 for contrast-adjusted images of later spermatid nuclei. F) SPCH proteins (SPCH-1, 2, 3) (green) are detectable on DNA as sperm DNA condenses for meiotic divisions. Levels of SPCH proteins increase dramatically after meiosis, particularly around spermatid DNA. HTAS-1 incorporates into chromosomes as they condense and transcription becomes globally reduced (Figure 2A) [55], [56]. We were thus interested in determining the localization of HTAS-1 relative to HTZ-1, which incorporates at promoters and regulates transcription in embryos [57]–[59]. Though HTZ-1 is present in all cells, several chromosomal regions exhibit relatively low levels of HTZ-1, particularly the silenced and condensed X chromosome, which has been shown to be marked by dimethylation of lysine 9 on histone H3 (H3K9me2) in male germ lines (Figure 2B, C) [60]–[63]. Levels of HTZ-1 staining do not substantially diminish as HTAS-1 is incorporated, suggesting that the function of HTAS-1 is not solely to displace HTZ-1. Instead, HTAS-1 is integrated into similar chromosomal regions as HTZ-1 (Figure 2C and D) and both are retained on post-meiotic spermatids (Figure 2E). Staining of HTZ-1 and histone H1, which was previously found to be retained in sperm [64], decreases in later spermatids (Figure S2B); however, both are visible on paternal chromatin in newly fertilized embryos (Figure S3A), supporting the idea that they are retained in sperm and carried over at fertilization [55], [56].
In the new embryo, differences in histone variant dynamics were found by examining chromatin transitions unique to each gamete of origin (Figure 3A). Prior to fertilization, oocytes adjacent to the spermatheca mature, a process that includes nuclear envelope breakdown, nuclear migration, and cortical rearrangements [65]. After sperm entry, the oocyte pronucleus completes meiotic divisions to produce a haploid complement of maternal DNA and two polar bodies [6], [66]. Both the oocyte and sperm chromatin then decondense, become ensheathed within pronuclear envelopes, and undergo DNA replication [67]. The oocyte and sperm pronuclei migrate, transition to metaphase and undergo mitosis. Embryos in the 1 - to 2-cell stage rely on maternal stores of proteins and mRNAs because embryonic transcription does not initiate until the 4-cell stage [68], [69]. Embryos complete transfer from maternal to embryonic control of development around the 40-cell stage.
Fig. 3. Histone H2A variants have different fates in the new embryo. 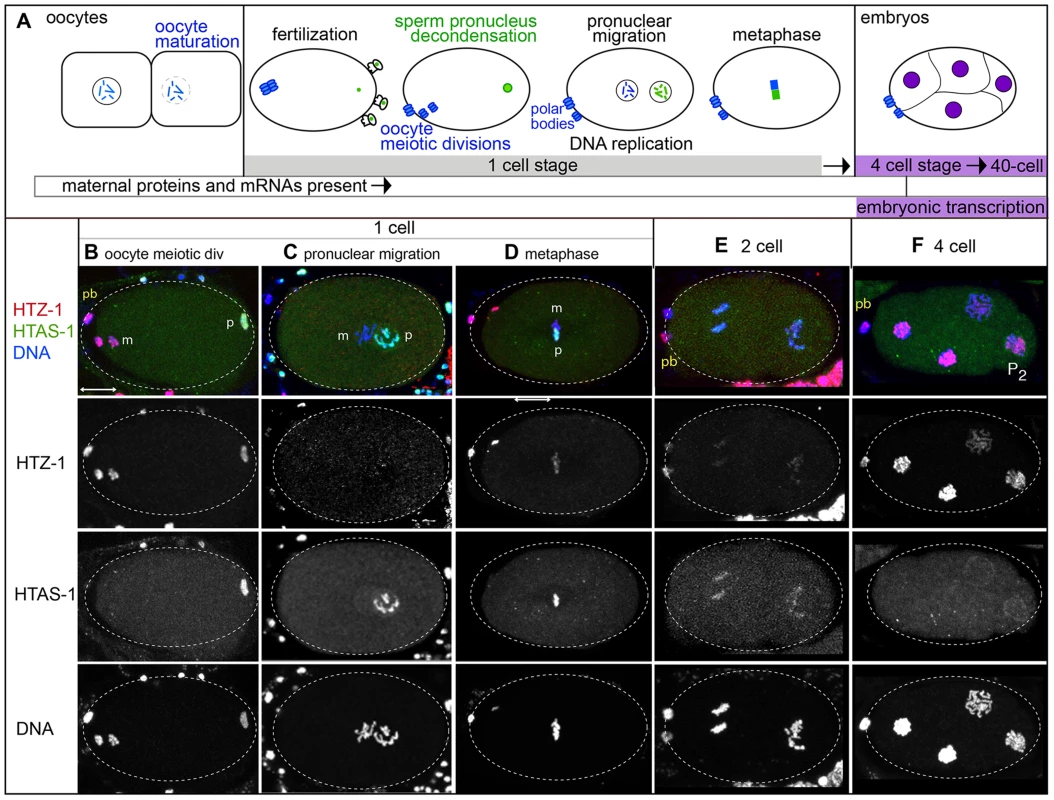
A) A schematic of paternal and maternal chromatin processing before and after fertilization. B–F) Immunostaining of embryos in different stages show that HTAS-1 (green in merged images) is retained in paternal (p) chromatin while HTZ-1 (red) is removed from paternal and maternal (m) chromatin in the 1-cell embryo. DNA is shown in blue. The scale bar represents 10 µm and applies to all panels. B) 1-cell embryos after fertilization. The oocyte pronucleus is undergoing meiotic divisions while the paternal pronucleus is decondensing. C) After DNA replication, the pronuclei migrate to the center of the embryo. D) Chromosomes align on the metaphase plate before segregation. E) 2-cell embryos F) 4-cell embryos. P2 marks the transcriptionally silent P2 germline precursor cell. After fertilization, paternally-delivered H2A variants have different fates
Interestingly, histone H2A variants have different fates in the embryo. HTZ-1 levels are high on both paternal and maternal chromatin just after fertilization (Figures 3B, Figure S3A), but levels drop on both after oocyte meiotic divisions (Figure 3C, D). Thus HTZ-1 is either removed by maternally loaded factors or displaced during DNA replication. HTZ-1 levels remain low in 1 - and 2-cell stage embryos (Figures 3C–E and S3A) then rise in the 4-cell stage on all nuclei (Figure 3F). This includes the transcriptionally silenced germline precursor P2 cell [70], [71], suggesting embryonic transcription is not necessary for HTZ-1 incorporation. In contrast, HTAS-1 is retained on paternal chromatin and absent on maternal chromatin throughout the 1-cell stage (Figure 3B–D, Figure S3B). HTAS-1 marks only the paternal half of the chromatin mass at metaphase, revealing the striking compartmentalization of chromosomes at this stage (Figure 3D). After division, HTAS-1 is detectable on chromosomes and levels subsequently decline with each cell division, becoming undetectable after the 4-cell stage (Figure 3E, F). Thus, HTZ-1 is removed after fertilization while HTAS-1 is retained. These results show that histone H2A variants are differentially processed in the early embryo and indicate distinct mechanisms recognize and either remove or retain each variant.
H2A ubiquitination correlates with histone exchange
The dynamics of HTZ-1 and HTAS-1 before and after fertilization suggests histone subunit exchange during these critical transitions. A clue about the mechanism of this process was revealed by western blot analysis, which detected H2Aub in chromatin from embryos but not sperm (Figure 1F) [54]. To investigate this difference further, we tested four antibodies via immunostaining in C. elegans that were previously shown to specifically recognize H2Aub in mammals (Table S7) [54], [72], [73]. Of these, one antibody, the monoclonal E6C5 antibody detected H2Aub in both male and hermaphrodite germ lines but not in later stages of spermatogenesis (Figure S4A). During the pachytene stage, H2Aub is found on all chromosomes (Figure S4A), which is in contrast to the heterochromatic sex body localization observed at that stage in mice [22], [74]. As H2Aub levels on chromosomes decrease in early pachytene, the E6C5 antibody also detected foci not associated with DNA, a pattern we subsequently refer to as “off-chromatin foci” (Figure S4A) that are distinct from germline P-granules [75]. The H2Aub off-chromatin foci do share partial overlap with poly-ubiquitin conjugates linked through either lysine 48 (Ub-K48), which target proteins to the proteasome, or lysine 63 (Ub-K63), which can act as a signal for autophagy (Figure S4B–D) [76]. The overlapping localization of Ub-K48 and the proteasome together with the presence of Ub-K63 foci suggest these pathways are active during late spermatogenesis, when chromatin is undergoing extensive remodeling. The lack of H2Aub on spermatid chromatin is consistent with western blot analysis and, together with the potential overlap of off-chromatin foci with ubiquitin conjugates, suggests H2A may be removed during late spermatogenesis.
Because spermatids lack the H2Aub mark before fertilization, we were surprised to observe high levels of H2Aub on paternal chromatin and in adjacent off-chromatin foci in newly fertilized embryos using either the E6C5 monoclonal or the polyclonal ABE569 and #308 anti-H2Aub antibodies (Figure S5, Figure 4A, B). Similar structures around paternal DNA were previously found to harbor sperm membranous organelles (MOs) that become poly-ubiquitinated on lysines 63 and 48 to target them for destruction by autophagy and possibly the proteasome after fertilization (Figure 4C–F) [76], [77]. H2Aub staining partially overlaps with Ub-K48 and Ub-K63 staining on paternal DNA and in some off-chromatin foci as oocyte chromosomes complete meiosis (Figure 4C–E) and is distinct from the localization of MOs both before and after fertilization (Figure 4F). The partially overlapping regions of staining with Ub-K48 and Ub-K63 suggest that paternally-delivered H2A may, like MOs, be targeted for destruction after fertilization [76]. Maturing and meiotically-dividing oocyte chromosomes also exhibit low H2Aub levels, where off-chromatin foci were also visible (Figure S5). After oocyte meiosis, levels of chromatin-associated H2Aub fall (Figure S5). By the 16-cell stage, H2Aub chromosomal staining rebounds (Figure S5) but Ub-K48 and Ub-K63 staining are absent [77]. Together, these results indicate that H2A may be removed and targeted for destruction by ubiquitination as paternal and maternal chromosomes decondense, suggesting that ubiquitination of H2A plays a role in H2A exchange in the newly fertilized embryo.
Fig. 4. Paternal histone H2A is ubiquitinated and removed after fertilization. 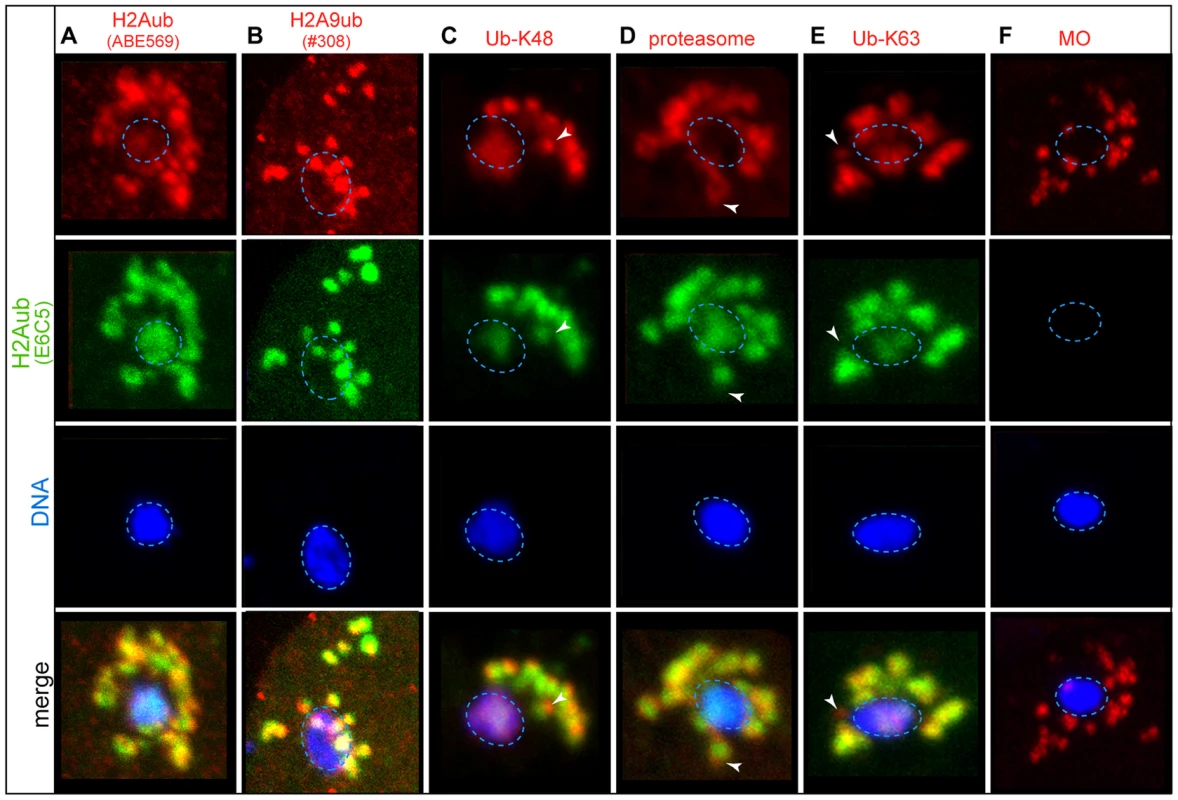
Immunostaining of fixed 1-cell embryos. The scale bar represents 10 µm and applies to all panels. The paternal DNA (blue dotted ovals) stained with the DNA dye DAPI (blue) and the monoclonal E6C5 antibody that recognizes H2Aub (green) [73] and the following (in red): A) H2Aub recognized by the ABE569 antibody [72]. B) H2Aub recognized by the polyclonal #308 antibody [54]. C) K48-linkage specific polyubiquitin (Ub-K48) that can target proteins for degradation via the D) proteasome. E) K63-linkage specific polyubiquitin (Ub-K63) tags targets, like F) Membranous Organelles (MOs), for autophagy [76]. The white arrowheads mark regions of staining that does not overlap with H2Aub staining. The overlapping staining suggests that H2Aub may be processed by either autophagy (like MOs) or by the proteasome after fertilization. Lack of bulk histone acetylation distinguishes the paternal from the maternal pronucleus in the newly fertilized embryo
The differential acetylation profile of sperm chromatin was next examined by testing commercially available antibodies to find those that recognized specific chromatin patterns in C. elegans (Table S7). We found that 8 acetylated histone marks [H4K16ac, H2BK12ac, H3K23ac, H3K27ac, H4K5ac, H4K8ac, H4K12ac, and H2A acetylated on sites K5, K9, K13, K15 (hereafter referred to as H2Apan-ac)] dramatically decrease during sperm chromosome condensation (Figure S6, Table S7, File S5). Six of the sites (H3K27ac, H4K5ac, H4K12ac, H4K16ac, H2Apan-ac, and H2BK12ac) showed no staining on nuclei undergoing the second meiotic division (Figure S6A–F), while 2 sites - H4K8ac and H3K23ac - were detectable at low levels on metaphase 2 nuclei (Figure S6G, H). Thus, overall acetylation levels are decreased on post-meiotic sperm, consistent with proteomic results (Table 1).
Bulk histone acetylation status becomes equalized before the first mitotic division
Immediately after fertilization, the lack of staining for 5 sites (H4K16ac, H3K27ac, H4K5ac, H4K12ac, H2Apan-ac) on paternally-derived chromatin distinguishes it from the maternal chromatin, where high levels of staining were observed (Figure 5A–E, Figure S3C–E). In the early embryo, acetylation dynamics reveal differences in the fate of different PTMs on each pronucleus and mechanisms of histone acetylation/deacetylation.
Fig. 5. Paternal and maternal chromatin differ in acetylation status after sperm entry. 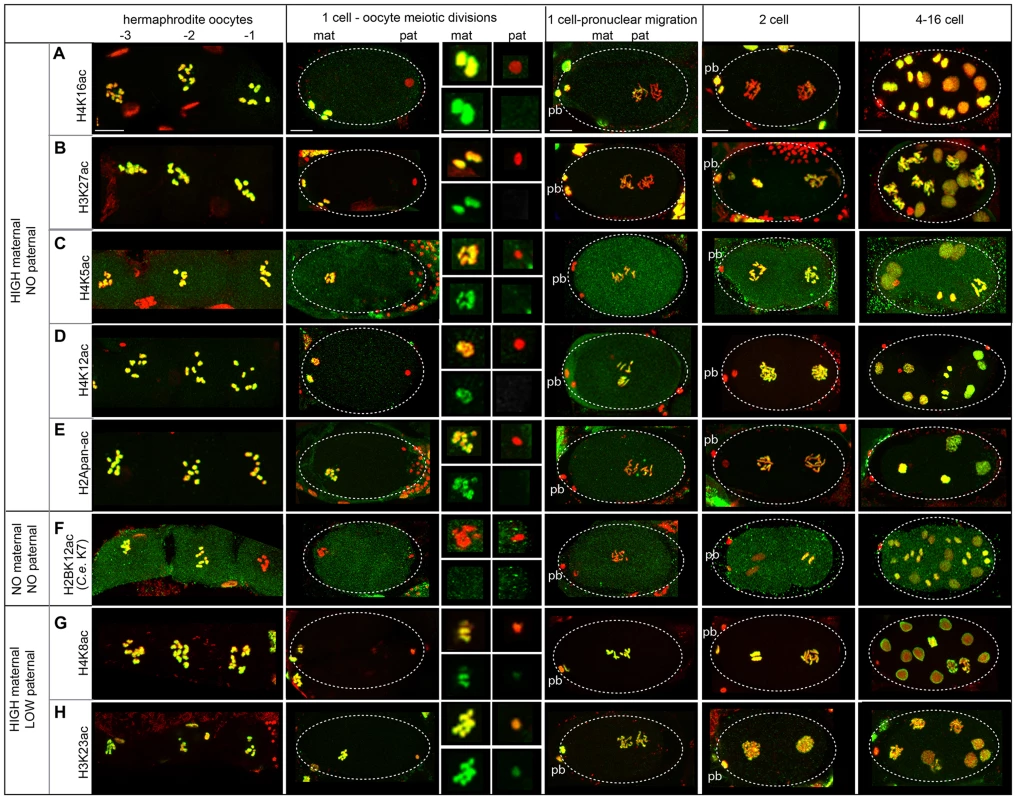
Immunostaining of dissected and fixed hermaphrodite gonads with antibodies against acetylated histones (green) and DNA stained with DAPI (red). Before fertilization, oocytes are numbered with the −1 oocyte adjacent to the spermatheca. Top insets from 1-cell embryos undergoing oocyte meiotic divisions show merged images of enlarged maternal and paternal pronuclei. Bottom insets show acetylated histone staining only. Polar bodies are denoted by ‘pb’, ‘mat’ is maternal, ‘pat’ is paternal. Scale bars represent 10 µm for all panels. A) H4K16ac levels are high in oocytes and the female pronucleus after fertilization, but absent on that paternal pronucleus. Levels are low until the 4-cell stage and are strong by the 16-cell stage. B) H3K27ac is present in oocytes and the female pronucleus but absent on the paternal pronucleus after fertilization. Levels increase gradually to become high in 2-cell embryos. C) H4K5ac D) H4K12ac and E) H2Apan-ac levels are strong in oocytes and the female pronucleus during meiotic divisions but are absent on sperm. Levels increase to become high in 2-cell embryos. F) H2BK12ac (corresponding to H2BK7 in C. elegans, see File S5) is not detectable in oocytes adjacent to the spermatheca or on maternal and paternal pronuclei after fertilization. Levels increase in 4-cell embryos. G) H4K8ac and H) H3K23ac are strong on maternal chromatin and weak, but present, on paternal pronuclei. Levels are high on both after meiotic divisions. In particular, H4K16ac staining is retained on the maternal chromosomes but excluded from the paternal chromosomes in 1-cell embryos (Figure 5A, Figure S3C). Levels of H4K16ac remain low in 1 - to 2-cell stage embryos but increase dramatically in 4-cell stage embryos in all cells, including the P2 cell, suggesting transcription is not necessary for H4K16 acetylation (Figure S7A). Overall, the sperm-specific loss of H4K16ac results in a maternal-specific H4K16 acetylation mark in 1-cell embryos.
Acetylation dynamics of H3K27 suggest that some histone acetylases are maternally loaded. H3K27ac levels are high on maternal chromatin after fertilization, but absent on paternal chromatin (Figure 5B). However, H3K27ac levels on paternal chromatin gradually rise until staining is strong in 2-cell embryos. This gradual rise in 1-cell embryos suggests maternal loading of the acetylase for this site, resulting in an “equalization” of H3K27ac levels between paternal and maternal pronuclei in the 1-cell embryo.
A general equalization of acetylation levels can occur via histone deposition during DNA replication. Histone H4 is marked by di-acetylation at K5 and K12 as it incorporates during DNA replication in Tetrahymena, Drosophila, and human cells [78]. Indeed in C. elegans, we also found H4K5ac and H4K12ac are present on the maternal pronucleus but absent on paternal chromatin after fertilization; however, levels of each are uniform on both pronuclei after DNA replication (Figure 5C, D, Figure S3D). The timing of this equalization suggests these marks may be added to the paternal chromatin via histone deposition during DNA replication. An antibody that recognizes multi-acetylated H2A (H2Apan-ac) on K5, K9, K13 and K15 (the only antibody we tested against H2A that produced positive immunostaining results, see Table S1 and File S5) exhibited similar dynamics (Figure 5E, Figure S3E). H2AK5 acetylation is influenced in the germline by XND-1, a protein involved in regulating meiotic crossovers in C. elegans [79]; however, little is known about acetylation of on these residues in any organism.
Deacetylation in oocytes before fertilization can also contribute to equalizing acetylation levels. Oocytes closest to the spermatheca, which are maturing [65], exhibit a dramatic drop in H2BK12ac levels on chromosomes during diakinesis (Figure 5F, Table S7, File S5), suggesting that H2BK12 deacetylation could be triggered by oocyte maturation signals. However, we also observe deacetylation of H2BK12 also occurs at diakinesis in spermatocytes (Figure S6F). H2BK12ac levels remain low on both sperm and oocyte chromatin until the 2–4 cell stage, when H2BK12ac then decorates all nuclei including the P2 cell (Figure S7B), suggesting that embryonic transcription is not directly correlated with its presence [71].
Epigenetic acetylation marks present at different levels in both pronuclei at sperm entry can become equalized. H4K8ac and H3K23ac exhibit high levels of staining in oocytes and low levels in late stages of sperm meiosis (Figure 5G, H, Figure S6G, H). Consistent with this, post-fertilization H4K8ac and H3K23ac staining is much weaker on paternal chromatin compared to maternal chromatin (Figure 5G, H). H4K8ac levels on both pronuclei increase dramatically during oocyte meiotic divisions, while H3K23ac levels remain modest in 1-cell embryos but become strong in 2-cell embryos. The increase in acetylation of both sites indicates that the acetylases that recognize each are likely to be maternally loaded.
Overall, we have identified features of paternal and maternal chromatin histone acetylation dynamics before and after fertilization in C. elegans that are both shared and distinct from that of other organisms. These observations show that 1) paternal chromatin undergoes erasure of many acetylation sites during sperm formation, 2) sperm and oocyte chromosomes carry parent-specific markings into the new embryo, 3) paternal and maternal pronuclei can undergo distinct histone remodeling events both before and after fertilization, 4) the processing of epigenetic information is dependent on maternal loading of histone modification enzymes.
Retention of histone methylation in sperm marks specific chromatin domains in embryos
Parent-specific imprinting in C. elegans can be specified by histone methylation that marks the transcriptional activity of chromatin domains by two different mechanisms [32], [61], [80]. Meiotic silencing of unpaired chromatin (MSUC) is implemented in part by excluding H3K4me, a mark of germline transcriptional activity, from the single X chromosome in XO males [61]. The silenced male X chromosome instead harbors H3K9 methylation marks [61]–[63], [81]. After fertilization, only the paternal X remains resistant to H3K4 methylation while autosomes and the maternal X exhibit this mark. In contrast, H3K36me2 is a different mark added by the MES-4 methyltransferase only on autosomes in hermaphrodites and males to transmit a memory of gene expression patterns from the parental germ line to germline precursors in offspring [80], [82]. Thus H3K36me2 is absent on both the paternal and maternal X chromosomes before and after fertilization. Our proteomic analysis identified other histone methylation marks on sperm chromatin (Table 1). We used immunostaining to investigate the retention of three methylation marks (H3K36me1, H4K20me1, and H3K79me2) with available commercial antibodies (Table S7).
We found H3K36me1 is likely specified by mechanisms that implement MSUC and not by MES-4. During spermatogenesis in males, H3K36me1 is absent from the single X chromosome but present on autosomes in nuclei at all stages, though low on post-meiotic sperm chromatin (Figure S8A). During oogenesis in hermaphrodites, all chromosomes, including the maternal X, stain with H3K36me1 at the diplotene stage (Figure 6A). In early embryos, only the paternal pronuclei lack one region of H3K36me1 staining, which, because it lacked staining before fertilization, is presumably the paternal X chromosome. This pattern is distinct from that of H3K36me2 [80], [82] and instead mirrors the staining pattern observed after fertilization for H3K4me [61], [62]. Thus, the dynamics of H3K36me1 we observe more closely correlates with the imprinting mechanisms of MSUC and not MES-4 [61], [80].
Fig. 6. Histone methylation dynamics on the paternal and maternal chromatin in the embryo. 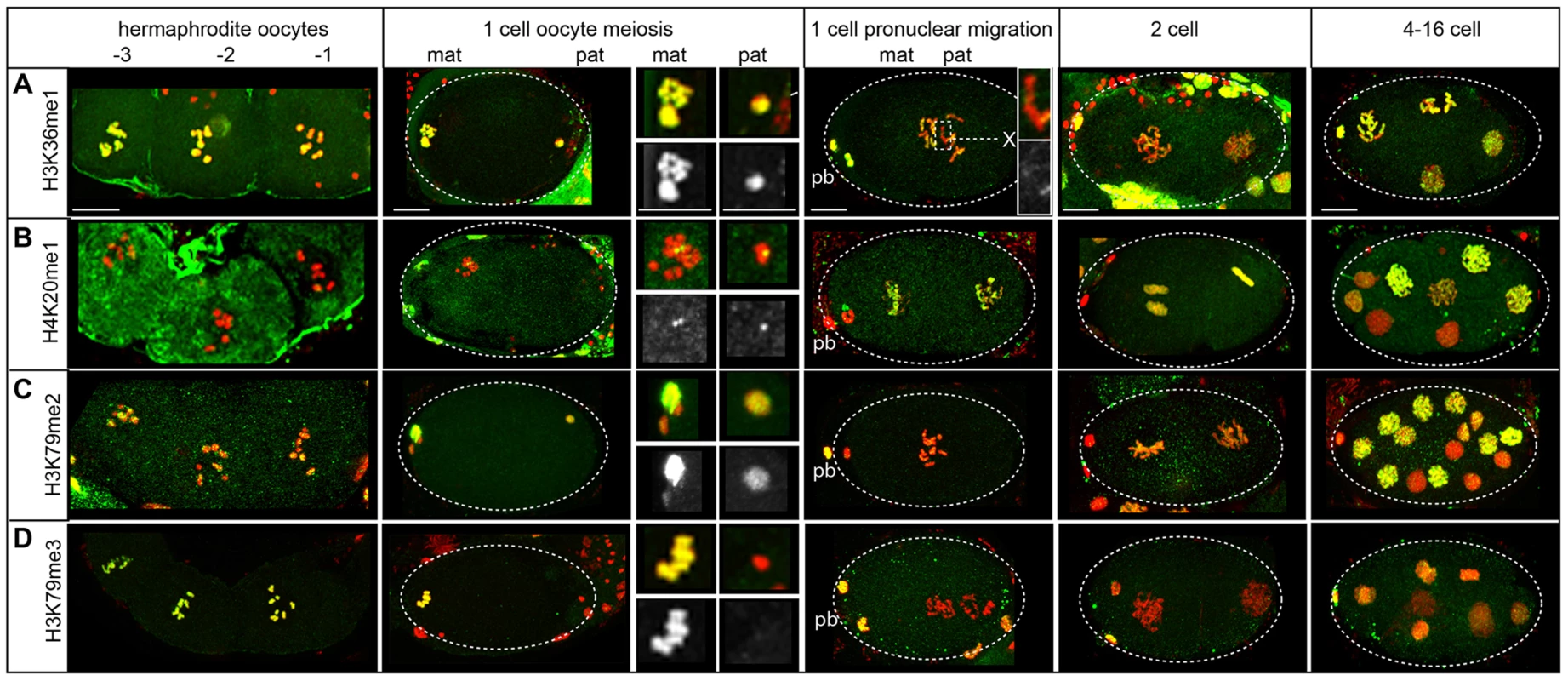
Immunostaining of dissected and fixed hermaphrodite gonads with antibodies specific to methylated histones (green) and DNA stained with DAPI (red). Before fertilization, oocytes are numbered with the −1 oocyte adjacent to the spermatheca. Polar bodies are denoted by ‘pb’, ‘mat’ is maternal, ‘pat’ is paternal. Scale bars represent 10 µm for all panels. A) H3K36me1 levels are high on both pronuclei after fertilization but absent on the paternal X chromosome, which is shown enlarged in the inset (top is merged image, bottom is H3K36me1 staining shown in grayscale). B) H4K20me1 is detectable as small foci on both pronuclei after fertilization with levels increasing on all chromosomes following oocyte meiosis. C) H3K79me2 levels are high on both pronuclei, low in 1 to 4-cell stage embryos, then high after the 16-cell stage. D) H3K79me3 is present on the maternal pronucleus but not the paternal after fertilization. Levels of H3K79me3 are very low after oocyte meiotic divisions and rise again after the 8-cell stage. H4K20me1 exhibits a distinctive localization pattern when carried over to the embryo. In C. elegans, though H4K20me1 is enriched on dosage-compensated X chromosomes in somatic cells, it is reduced on meiotically-silenced X chromosomes during the pachytene stage [83]. We observe that by diplotene/diakinesis, H4K20me1 becomes focused in one or two bright chromatin-associated spots (Figure 6B). In male sperm-producing germ lines, levels of H4K20me1 are also low on the single X chromosome during pachytene (Figure S8B) then fall; however, one or two bright chromatin-associated foci appear. Costaining with an antibody that recognizes H3K9me2, a marker for the X chromosome [61], [62], [81], show that these foci are not on X (Figure S8B). After fertilization, the paternal and maternal pronuclei are similarly decorated with small H4K20me1 foci. Levels then rise to cover all chromosomes in both pronuclei (Figure 6B), suggesting the H4K20 methylase is maternally loaded. These results show that H4K20me1 is retained on sperm chromatin in a novel localization pattern and demonstrate that paternal and maternal nuclei are equivalent in H4K20me1 status in the new embryo.
H3K79me2 is also carried over to the new embryo from both sperm and oocytes (Figure S8C, Figure 6C). H3K79me2 is found at high levels on oocyte chromosomes before and after fertilization. However, though H3K79me2 status is equivalent in paternal and maternal pronuclei after sperm entry; levels drop in 1-cell embryos (Figure 6C), suggesting a H3K79 demethylase is maternally loaded to reprogram paternally delivered marks in the new embryo. A similar demethylation of H3K79 also occurs in mouse [84]. H3K79me2 levels rise in 4-cell embryos, including the transcriptionally inactive P2 cell (Figure S7C), indicating its presence is not dependent on transcription [71].
Not all histone methylation marks are retained in sperm chromatin. H3K79me3 was not identified in sperm chromatin by proteomic analysis. In the male sperm-producing germ lines, H3K79me3 levels fall before meiotic divisions (Figure S8D). In contrast, H3K79me3 levels are high in oocyte-producing germ lines through diakinesis. Subsequently, lack of H3K79me3 distinguishes the paternal pronucleus from the maternal pronucleus after fertilization (Figure 6D). Levels of H3K79me3 become low in both maternal and paternal pronuclei then rise after the 16-cell stage. This suggests that the enzyme responsible for H3K79me3 is likely not maternally supplied. The removal of maternal H3K79me3 in early embryos was similarly observed in mouse early embryos [84].
Discussion
In this study, we define the epigenetic profile of C. elegans sperm chromatin. This distinctive profile includes (1) sperm-specific incorporation of the histone H2A variant HTAS-1, (2) a depletion of histone acetylation marks, and (3) the retention of specific histone marks, like H3K36me1, that convey the transcriptional history of chromatin domains. Overall, we find that sperm generally harbor less histone PTMs on fewer sites compared to embryos and tracked 13 sites via immunostaining before and after fertilization to define distinctive features of paternal and maternal chromatin (Tables 1, 2). An important product of these studies is a set of temporal markers of the dramatic chromatin restructuring and reprogramming events that occur during sperm formation and in early embryo development (Table 2, Figure 7).
Fig. 7. Summary of stages of chromatin remodeling events in C. elegans during spermatogenesis and post-fertilization. 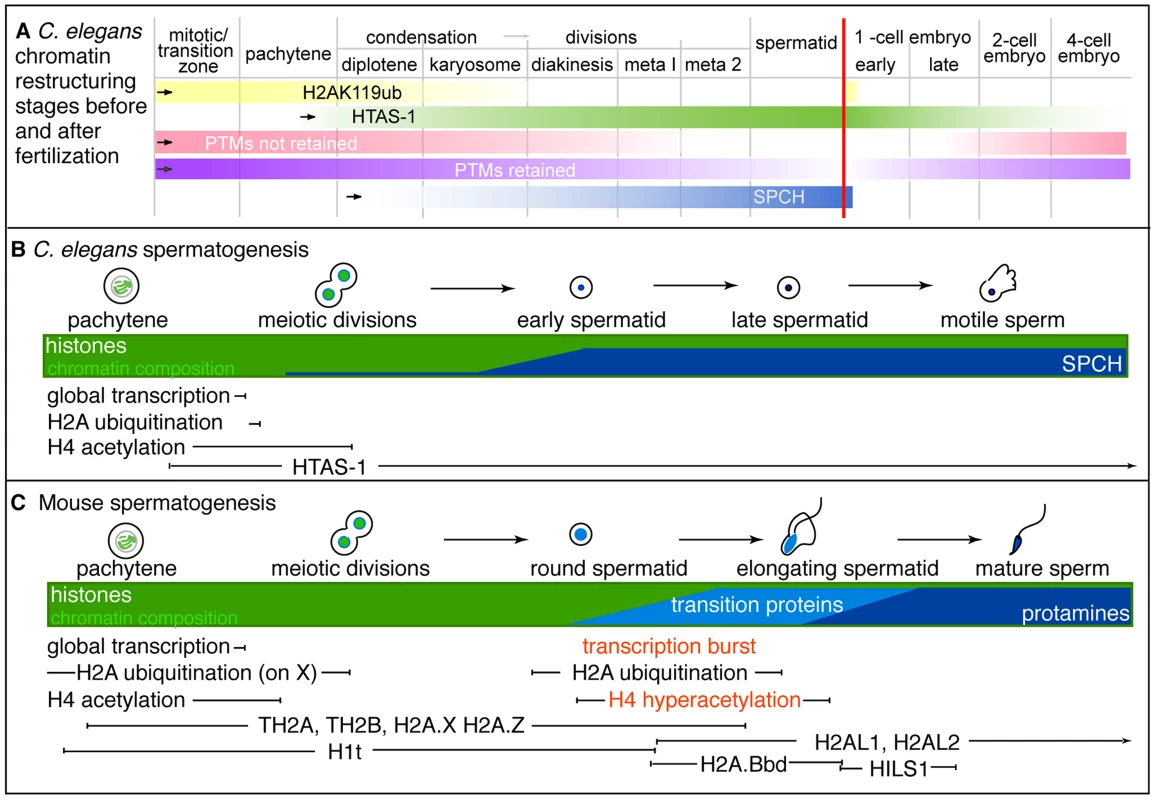
A) Schematic of overlapping stages that restructure sperm chromatin to establish paternal epigenetic information during spermatogenesis. Bars represent the presence and levels of key markers, the vertical red line represents the point fertilization occurs. The stages include: 1) H2Aub removal from chromatin (yellow) as HTAS-1 (green) is incorporated and retained in the new embryo. 2) Removal of many histone PTMs (pink) prior to meiotic divisions that result in the erasure of paternal epigenetic marks. Some histone PTMs are retained (purple). 3) Putative C. elegans protamines, SPCH-1, 2, 3 (SPCH, blue) begin incorporating during meiosis and are found at high levels in spermatids. B and C) Schematic comparison of key events during spermatogenesis between B) C. elegans and C) mouse (inspired by [92]). Notably, HTAS-1 is incorporated and retained in C. elegans while some histone variants are transiently incorporated to facilitate transition and protamine incorporation in mouse. Also, the post-meiotic transcriptional burst and histone hyperacetylation seen during mouse spermatogenesis are not observed during C. elegans spermatogenesis. Tab. 2. Summary of the profiles of histone post-translational modifications during spermatogenesis and after fertilization. 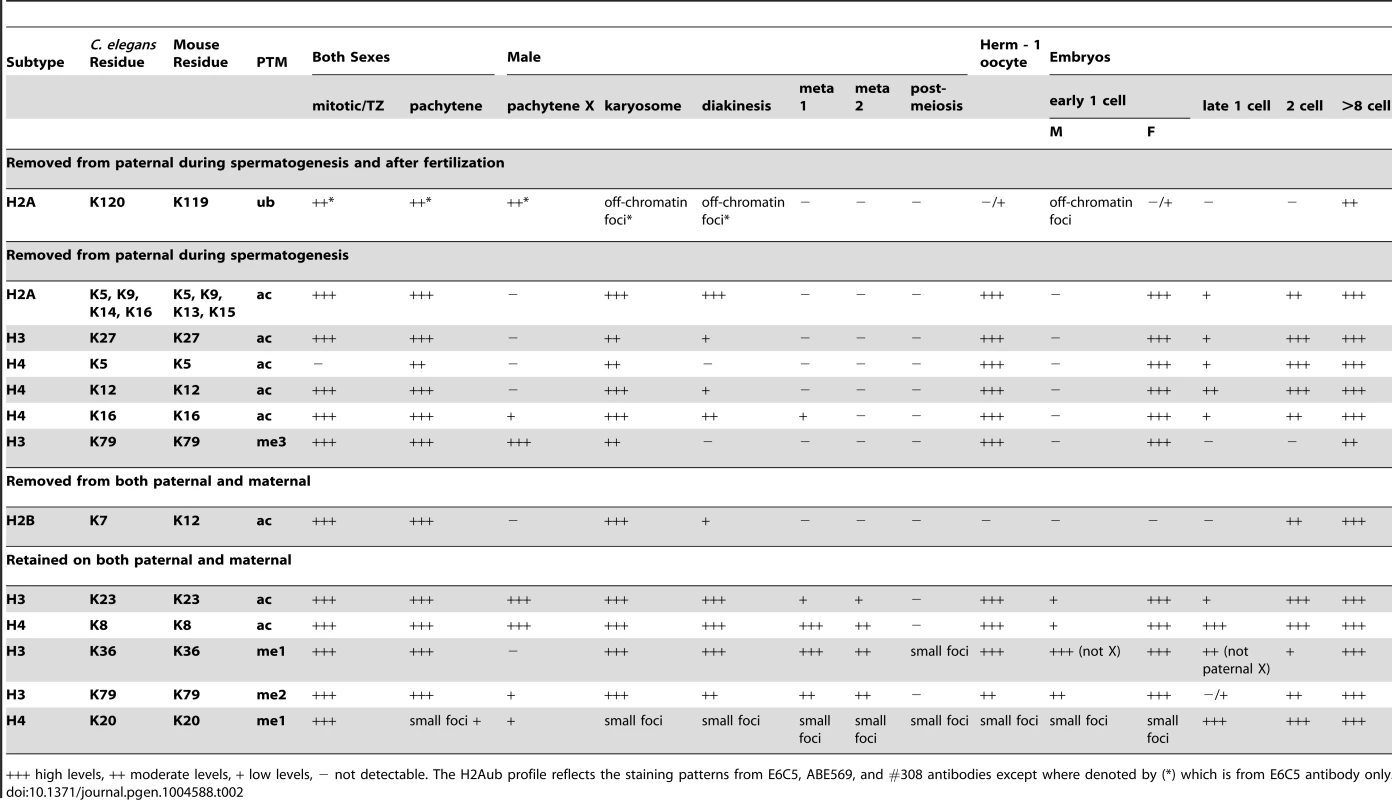
+++ high levels, ++ moderate levels, + low levels, − not detectable. The H2Aub profile reflects the staining patterns from E6C5, ABE569, and #308 antibodies except where denoted by (*) which is from E6C5 antibody only. The sperm epigenetic profile is established by four partially overlapping stages during sperm formation in C. elegans (Figure 7A, B) that are then processed in the embryo. The first involves H2A ubiquitination. H2A ubiquitination occurs in Drosophila, mouse, and rat spermatogenesis [21], [23], [24]; however, the role of this modification remains unclear because reports concerning how loss of the ubiquitin conjugating enzyme, RNF8, affects histone replacement during spermiogenesis vary in mouse [23], [25]. A recent report has instead shown histones may be targeted to sperm-specific proteasomes via H4K16 acetylation [18]. In our studies, chromatin-associated H4K16ac and H2Aub are removed as chromosomes condense for meiotic divisions. Additionally, we found evidence of H2Aub off-chromatin foci sharing partial overlap with Ub-K48 conjugates, which target proteins to proteasomes, and Ub-K63 conjugates, which are linked to the DNA damage response and autophagy (Figure S4), though only one (E6C5) of four antibodies we tested showed a clear signal in the male germ line [77]. However, the dynamics of ubiquitin conjugates and the proteasome localization defined here for the first time in the C. elegans germ line suggests that Ub-K48 and Ub-K63 pathways are active during late spermatogenesis when chromatin is remodeled (Figure S4). Furthermore, after fertilization, the appearance of H2Aub detected by three antibodies (E6C5, ABE569, and #308) as paternal chromatin decondenses suggests paternally contributed histones are targeted for destruction, just as other sperm-specific proteins, like membranous organelles, are targeted for autophagy [76], [85]. Thus, ubiquitination may play a general role in the immediate clearance of sperm proteins in the embryo after fertilization.
Incorporation of the sperm-specific H2A variant HTAS-1 is the next phase of chromatin remodeling (Figure 7A, B). Incorporation of histone variants before completion of meiosis occurs in other organisms (Figure 7C). For example, TH2B facilitates protamine incorporation in mouse and rat [9], [86]. Other histone variants, such as H2AL1/2 in mouse, are retained at pericentric regions, then delivered to the embryo and removed upon sperm decondensation [14], [16]. In contrast, HTAS-1 is carried over and not erased in the 1-cell embryo. This raises the intriguing possibility that HTAS-1 may be specifically retained at specific genomic sites for function in the embryo. In contrast, HTZ-1 is removed from paternal and maternal chromatin rapidly in the new embryo. Though it is not clear if HTZ-1 removal coincides with DNA replication, studies in mice show that maternally-contributed H2A.Z removal is DNA replication-independent [87]. Thus, it remains to be determined which structural differences between H2A variants may alter the stability of nucleosomes during DNA replication or are recognized by histone chaperones or chromatin modifiers [88], [89].
The third stage of chromatin remodeling during sperm formation is the bulk loss of many histone PTMs that results in the lack of histone acetylation marks on paternal chromatin in the embryo (Figure 5, Table 2). Levels of all histone PTMs fall as sperm chromosomes condense and divide. However, we found that removal prior to the second meiotic division correlates with erasure, indicating this cell cycle transition may be a critical juncture that coordinates histone PTM removal (Figure 7A, Table 2). After fertilization, many maternal and paternal chromatin histone PTM marks subsequently become equalized. Work from this study and others show this occurs via distinct mechanisms [5], [33], [84]. A notable mark that is not equalized is H4K16ac, which marks oocyte chromatin but remains absent from paternal chromatin. However, by the 4-cell stage, levels of all histone PTMs progressively rebound (Figure 7A). While embryonic transcription may be a factor, several marks are established on chromatin of the transcriptionally-silenced germline precursor cell (Figure S7). Thus the regulatory factors that temporally regulate histone modifications during the oocyte-to-embryo transition remain to be elucidated.
Histone PTMs that persist into the second meiotic division during spermatogenesis are retained (Figure 7A, Table 2). For example H4K20me1 forms small chromatin-associated foci detectable before and after fertilization. In later embryos, H4K20me1 levels rebound on all chromosomes then later become enriched on X chromosomes to function in dosage compensation [83], [90]. Further investigation will reveal whether H4K20me1 dynamics play roles in sperm or early embryos, as H4K20 methylation has a broad range of functions, including DNA damage response, DNA replication, mitotic condensation, and transcription [91]. Overall, we have found that many of the PTM marks in C. elegans share similar fates as those in mouse (Table S8). Importantly, our proteomic analysis has also identified both histone methylation and acetylation marks retained in sperm chromatin that also have the potential to pass on specific paternal epigenetic information to the embryo (Table 1, Table 2, Table S8).
The last stage of restructuring is the incorporation of sperm nuclear basic proteins (SNBPs), which exhibits differences compared with mammals (Figure 7B, C). Most strikingly, in C. elegans, incorporation of HTAS-1 and the putative protamines SPCH-1, 2, 3 overlaps with meiotic divisions (Figure 2, Figure 7A, B) [35], [55], in contrast to mammalian transition proteins and protamines that are incorporated after meiosis (Figure 7C) [92]. Furthermore, C. elegans sperm retain more histones compared to mammals. This may be due to the rapid progression of spermatogenesis as well as a lack of identifiable C. elegans transition proteins. Future studies, including assessing spch gene loss-of-function, are necessary to support the possibility that SPCH-1, 2, and 3 function as C. elegans protamines and to determine whether each plays a distinct role in sperm formation.
The overlap of events also results in a lack of a post-meiotic burst of transcription in C. elegans spermatogenesis, which prepares gametes for chromosome restructuring and spermiogenesis in mammals [4] (Figure 7B, C). In C. elegans, transcription is globally repressed before meiotic divisions and not reactivated after meiosis [55], [56], [93]. Further, C. elegans does not exhibit post-meiotic histone hyperacetylation, which plays roles in transcriptional activation and histone displacement in mammals and Drosophila [18]–[20], [94]. In C. elegans, histone acetylation levels remain high on nuclei until meiotic divisions, at which point levels rapidly drop (Figure 7B and Figure S6) [56]. The overlap, omission, or abbreviation of these events likely contributes to the rapid progression of sperm formation observed in C. elegans (∼24 hours) compared to mouse (∼30 days) or humans (∼60 days) [56], [95]–[97].
Historically, identifying epigenetic marks in sperm and embryos solely using cytology or western analysis in any organism has been challenging. Antibody availability is limited and specificity in different applications is variable [53]. For example, in this work, we tested four distinct antibodies used in previous studies to detect H2A ubiquitination: the monoclonal E6C5 and the highly purified polyclonal ABE569, #87, and #308 antibodies [54], [72]. Antibody #87 exhibited specificity in western analysis (Figure 1F), while E6C5, ABE569 and #308 showed distinct staining patterns in immunostaining experiments (Figure 4, Figure S4, Figure S5). While one possibility is that they may recognize off-target proteins or unmodified H2A in immunostaining experiments, it is also possible that they are specific for distinct epitopes that are accessible under specific conditions (i.e. denatured or partially folded protein conformations) because each was raised in individual animals against antigens with multiple possible epitopes. Another issue is whether lack of staining indicates the absence of a protein or the inaccessibility of antibodies to the tightly compacted paternal chromatin. Indeed, we observed the interior of the C. elegans spermatid nucleus is resistant to antibody staining regardless of the antibody or fixation condition used. Thus, the sole use of antibodies to identify histone PTMs should be used with caution [98].
Overall our study shows that despite the limitations of proteomics, western analysis and cytology, their combined use was an effective strategy to mine for modification sites and then define the spatial and temporal dynamics of their addition and removal. Further high-resolution studies of gametes and early embryos are necessary to distinguish specific genes associated with the epigenetic markers identified here. However, our studies provide key landmarks for understanding the dynamics of epigenetic erasure and reprogramming required for both sperm cell and early embryonic development.
Methods
Strains
C. elegans strains were maintained using standard conditions [99]. Strains used in this study are CB1489 him-8(e1489), DR466 him-5(e1490), TY0119 fem-1(hc17ts) and JK0816 fem-3(q20ts). Strains were cultured at 20°C, except for TY0119 fem-1(hc17ts) and JK0816 fem-3(q20ts), which were maintained at 15°C.
Isolation of C. elegans sperm and embryos
Large scale culturing of C. elegans hermaphrodites was conducted as in [35] except that fem-3(q20ts) animals were cultured at 15°C. Synchronous cultures of fem-3(q20ts) hermaphrodites were treated with a 1.2% sodium hypochlorite, 0.5 N NaOH solution with vigorous vortexing to release embryos. After washing in M9 buffer to remove hypochlorite, the embryos were either quick-frozen in liquid nitrogen and stored at −80°C degrees for chromatin isolation or were hatched overnight in M9 solution. The resulting L1 larvae were plated onto egg plates and cultured for 4 days at 25°C. Sperm was isolated as in [35] and frozen at −80°C degrees.
Histone quantitation
Approximately 600 µL of mixed-stage N2 embryos and 600 µL of fem-3(q20ts) sperm were collected and lysed by 6 rounds of sonication (3 seconds at 10% amplitude) and rest (1 minute on ice). Genomic DNA was isolated using phenol/chloroform (Koelle lab) from 200 µL of N2 embryo lysate or 200 µL of fem-3 (q20ts) sperm lysate, resuspended in 100 µL of dH2O, and quantified with the Qubit dsDNA BR Assay Kit on a Qubit 2.0 Fluorometer (Life Technologies). Titrations of sperm and embryo lysates representing equivalent amounts of DNA were subjected to 4–20% SDS PAGE (Bio-Rad) or 14% SDS PAGE (Fisher Scientific) and western analysis. Primary antibodies (and the dilutions) used include: anti-HTAS-1 (at 1∶1000, polyclonal rabbit antibodies 3656 and 3657 generated against the last 15 amino acids of the C-terminal, LPKKKAKEDDKENNS), anti-SPCH (at 1∶500, rabbit 2391 [35]), anti-HTZ-1 (a polyclonal rabbit antibody from the lab of Dr. Gyorgyi Csankovszki used at 5 ug/mL for the westerns) and anti-histone PTMs shown in Table S7. HRP-conjugated donkey anti-rabbit (abcam ab6802) was used at a 1∶1000 dilution. HRP signal was detected using SuperSignal Chemiluminescent Substrate (Pierce). Western blots were analyzed using a Kodak Imager. The Gel Analyzer tool from ImageJ was used to quantify bands from western blot analysis.
Isolation and acid solubilization of C. elegans spermatogenic and embryonic chromatin
For chromatin preparations, a minimum of 500 µL of frozen sperm or embryos was used per isolation. Cells were lysed as described above. Chromatin was isolated as in [35] except centrifugation through a sucrose gradient was conducted at 100,000×g for 1 hour at 4°C and 1 mM sodium orthovanadate (Na3VO4), 10 mM sodium butyrate (Na(C3H7COO)), and 0.02% protease inhibitors (Calbiochem) were added to all buffers prior to use. Nuclear basic proteins from sperm and embryo chromatin were isolated by resuspending samples in 0.4 N H2SO4 acid for 1 hour at 4°C [44]. Samples were subjected to centrifugation at 16,000×g for 10 minutes at 4°C. The resulting supernatant was resuspended in 500 µl of Buffer A [35].
Multidimensional Protein Identification Technology (MudPIT) identification of sperm and embryo acid-soluble chromatin-associated proteins
Trichloroacetic acid (TCA) precipitation
Two spermatogenic chromatin and two embryonic acid-solubilized chromatin protein samples were each precipitated by mixing 1 volume of the cold sample solution with 1/3 volume of 100% (w/v) TCA (6.1 N, Sigma) to give a final TCA concentration of 25%. Samples were left on ice for 3 hours then centrifuged for 30 minutes at 4°C. The resulting pellets were washed twice with ice-cold acetone (500 µL each). After each wash, the solution was centrifuged for 10 minutes. Samples were dried on a Speed-vac for 1–2 minutes or air-dried.
Protease digestion
Precipitated chromatin proteins were dissolved in 60 µL of 100 mM Tris-HCl, pH 8.5 containing 8M urea (Sigma). The protein was reduced by incubation in 5 mM tris(2 - carboxyethyl)phosphine (TCEP) for 20 minutes at room temperature followed with carboxyamidomethylation of cysteines by incubation at room temperature for 30 minutes in the dark in 10 mM iodoacetamide. The sample was diluted 2-fold (to 4M of the concentration of urea) by the addition of an equal volume of 100 mM Tris-HCl, pH 8.5 and then Lys-C (Promega) was added at ∼1∶100 enzyme to substrate ratio (wt∶wt) and incubated at 37°C for 4 hours in the dark. The sample was then further diluted to the concentration of 2M urea and trypsin (Promega) was added at ∼1∶100 enzyme to substrate ratio (wt∶wt) and incubated at 37°C overnight in the dark. The resulting peptides from the digests were dissolved using 90% formic acid to a final concentration of 2% formic acid. The sample was stored at −20°C prior to LC/LC-MS/MS analysis.
Multidimensional Protein Identification Technology (MudPIT)
Total peptide mixtures were pressure-loaded onto a biphasic fused-silica capillary column (250 µm i.d.) consisting of 2.5 cm long strong cation exchange (5 µm Partisphere SCX, Whatman, Clifton, NJ) and 2.5 cm long reversed phase (Aqua C18, Phenomenex, Torrance, CA) that was prepared by slurry packing using an in-house high pressure vessel. The column was washed with buffer A [water/acetonitrile/formic acid (95∶5∶0.1, v/v/v)] for more than 10 column volumes (100 µm i.d.) with a through-hole union (Upchurch Scientific, Oak Harbor, WA). The analytical columns were pulled beforehand by a laser puller (Sutter Instrument Co., Novato, CA) with a 5 µm opening. It was packed with 3 µm reversed phase (Aqua C18, Phenomenex, Torrance, CA) to 15 cm long. The entire column setting (biphasic column-union-analytical column) was placed in-line with an Agilent 1200 quaternary HPLC pump (Palo Alto, CA) for mass spectrometry analysis. The digested proteins were analyzed using a 12-step MudPIT separation method as described previously [100], [101]. The elution gradient of step 1 was as follows: 10 min of 100% buffer A, a 5 min gradient from 0 to 15% buffer B [water/acetonitrile/formic acid (20∶80∶0.1, v/v/v)], a 65 min gradient from 15 to 45% buffer B, a 15 min gradient from 45 to 100% buffer B, and 5 min of 100% buffer B. Steps 2–11 had the following profile: 1 min of 100% buffer A, 4 min of X% buffer C (500 mM ammonium acetate with 5% acetonitrile and 0.1% formic acid), followed by the same gradient as step 1. The 4 min buffer C percentages (X) were 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100%, respectively. The salt pulse for the final step (step 12) was 90% buffer C plus 10% buffer B (450 mM ammonium acetate in 12.5% acetonitrile).
Mass spectrometry conditions
Data-dependent tandem mass spectrometry (MS/MS) analysis was performed with an LTQ-Orbitrap mass spectrometer (ThermoFisher, San Jose, CA). Peptides eluted from the LC column were directly electrosprayed into the mass spectrometer with the application of a distal 2.5 kV spray voltage. A cycle of one full-scan MS spectrum (m/z 300–1800) was acquired followed by eight MS/MS events, sequentially generated on the first to the eighth most intense ions selected from the full MS spectrum at a 35% normalized collision energy. The number of microscans was one for both MS and MS/MS scans and the maximum ion injection time was 50 ms and 100 ms respectively. The dynamic exclusion settings used were as follows: repeat count, 1; repeat duration, 30 seconds; exclusion list size, 100; and exclusion duration, 180 seconds. MS scan functions and HPLC solvent gradients were controlled by the Xcalibur data system (ThermoFisher).
Data analysis
Full MS and tandem mass spectra were extracted from raw files, and the tandem mass spectra were searched against a Wormpep database (Ver. 04-25-12) [102]. In order to accurately estimate peptide probabilities and false discovery rates, we used a decoy database containing the reversed sequences of all the proteins appended to the original database. Tandem mass spectra were matched to sequences using the Sequest [47] or ProLuCID algorithm. Sequest and ProLuCID searches were done on an Intel Xeon 80-processor cluster running under the Linux operating system. The peptide mass search tolerance was set to 3 Da for spectra acquired on the LTQ instrument. The mass of the amino acid cysteine was statically modified by +57.02146 Da, to take into account the carboxyamidomethylation of the sample. Serine, threonine, and tyrosine were treated as differentially modified by +79.9663 Da for phosphorylation. Lysine and arginine were treated as differentially modified by +14.0157 Da for methylation, +28.0314 Da for di-methylation, and +42.0471 for tri-methylation. Lysine was treated as differentially modified by +42.0106 Da for acetylation, and +114.042927 Da for ubiquitination. No enzymatic cleavage conditions were imposed on the database search so the search space included all candidate peptides whose theoretical mass fell within the mass tolerance window, regardless of their tryptic status [43].
The validity of peptide/spectrum matches (PSMs) was assessed in DTASelect [45], [46] using two SEQUEST [47] defined parameters, the cross-correlation score (XCorr) and the normalized difference in cross-correlation scores (DeltaCN). The search results were grouped by charge state (+1, +2, +3, and greater than +3) and tryptic status (fully tryptic, half-tryptic, and non-tryptic) [103], resulting in 12 distinct sub-groups. In each one of these sub-groups, the distribution of Xcorr, DeltaCN, and DeltaMass values for (a) direct and (b) decoy database PSMs was obtained and then the direct and decoy subsets were separated by discriminant analysis. Full separation of the direct and decoy PSM subsets is not generally possible; therefore, peptide match probabilities were calculated based on a nonparametric fit of the direct and decoy score distributions. A peptide probability of 90% was set as the minimum threshold. The false discovery rate was calculated as the percentage of reverse decoy PSMs among all the PSMs that passed the 90% probability threshold. After this last filtering step, we estimate that both the protein and peptide false discovery rates were reduced to between 0.0% and 0.5%. PSMs with an absolute DeltaMass value of greater than 6 ppm were not accepted during filtering. Proteins identified are shown in Tables S1 and S2 and sorted by Total Spectral Counts. NSAF and EMPAI abundance values calculated from all peptides (including semi-tryptic fragments) identified are also presented and show histones are among the most abundant proteins in each sample [48]–[50]. Peptide match parameters for full tryptic peptides that match to histone proteins are shown in Tables S3–S6. NSAF and EMPAI abundance values are those calculated for all peptides as shown in Tables S2 and S3. Peptides that matched to multiple proteins were distributed as in [50]. Tables S3–S6 show all post-translational modifications identified. Annotated mass spectra for post-translationally modified peptides are shown in Files S1–S4. Table 1 shows the number of times (occurrences) a modification site was identified from peptides that bore both single and multiple PTMs. Thus, from 253 PTM histone peptides detected in acid-solubilized embryo chromatin, 330 modifications were identified. From 127 PTM histone peptides detected in acid-solubilized sperm chromatin, 135 modifications were identified (Tables S3–S6).
We searched for ubiquitination sites, however, because iodoacetamide was used during sample preparation, sites identified may be due to iodoacetamide-induced adducts [104]. Thus, we excluded these in our counts of identified PTMs. However, we note a possible enrichment of H2AK119ub (see C. elegans amino acid numbering, File S5) in embryo and not sperm chromatin samples. This could be valid because: (1) H2A ubiquitinated on lysine 119 (H2AK119ub) represents 132 spectral IDs in acid-solubilized embryo chromatin samples, compared to 0 spectral IDs in acid-solubilized sperm chromatin samples. This enrichment is unlikely due to non-specific adducts, which should be induced similarly in both samples, (2) H2AK119ub is the most abundant ubiquitination site in embryo chromatin samples, with the next most abundant ubiquitinated protein being PAR2, which is represented by only 11 spectral IDs. (3) The enrichment of H2AK119ub is also considerably higher compared to other modification sites identified on all histone proteins, which argues that H2AK119ub identification is not merely due the fact that histones are a major component of the sample. Ubiquitination of H2AK120 (which corresponds to mammalian K119) was also identified as shown in Table S3, though at lower levels. We have listed all histone ubiquitinated peptides identified (in gray text) in the total histone peptide lists (Tables S3–S6) but excluded them in the PTM counts (Table 1).
Western analysis
TCA precipitated sperm and embryo acid-solubilized chromatin proteins representing one-fifth of that analyzed by MudPIT were resuspended in 50 µL of 1×LSM. 5 µL were subjected to SDS PAGE and western blotting as described above. Primary antibodies and the dilutions used for westerns are described above (HTAS-1, HTZ-1, SPCH) or shown in Table S7. Mouse anti-MSP (from the lab of Dr. David Greenstein) was used at 1∶1000 dilution [105].
Immunostaining
Because sperm DNA is tightly compacted, we tested different fixation methods to achieve consistent and sensitive detection of proteins associated with spermatid chromatin. A methanol/acetone fixation (as detailed below) was the most effective for detecting integral chromatin proteins, such as histones, in comparison to methanol or ethanol/paraformaldehyde methods [35], [56], [106]. Using this method we detect staining on early spermatid chromatin for histone H1 (Figure S2), a protein previously shown to be present in sperm chromatin [64]. For most proteins tested, staining levels on sperm chromatin decrease gradually after completion of meiosis, suggesting that chromatin becomes progressively more compacted [56]. Each staining experiment was conducted a minimum of four times with at least three slides (each with 30–50 animals) for every experiment.
For methanol/acetone fixation, 30–60 adult him-8(e1489) males or hermaphrodites were dissected into sperm salts (50 mM Pipes, pH 7, 25 mM KCl, 1 mM MgSO4, 45 mM NaCl, and 2 mM CaCl2) on a slide. The slide was then freeze-cracked. For antibodies that recognize histones, slides were placed into 100% methanol for 10 minutes, then 100% acetone for 5 minutes and briefly air-dried. Slides were then washed 3 times with PBST (1× PBS, 0.5% Triton X-100, and 1 mM EDTA, pH 8) for 10 minutes each wash. For antibodies that recognize ubiquitin, MOs, and the proteasome, a methanol fixation procedure was also used, in which slides were placed in methanol for 30 minutes then processed as described. Primary and secondary antibodies were incubated overnight. Slides were washed with PBST as above then stained with DAPI (1 µg/ml) and mounted with Vectashield. Confocal images were obtained using a Zeiss LSM710 confocal microscope. Primary antibodies against commercially available post-translationally modified histones used in this study are listed in Table S7.
Supporting Information
Zdroje
1. MillerD, BrinkworthM, IlesD (2010) Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 139 : 287–301.
2. Eirin-LopezJM, AusioJ (2009) Origin and evolution of chromosomal sperm proteins. Bioessays 31 : 1062–1070.
3. BraunRE (2001) Packaging paternal chromosomes with protamine. Nat Genet 28 : 10–12.
4. Sassone-CorsiP (2002) Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science 296 : 2176–2178.
5. BurtonA, Torres-PadillaME (2010) Epigenetic reprogramming and development: a unique heterochromatin organization in the preimplantation mouse embryo. Brief Funct Genomics 9 : 444–454.
6. RobertsonS, LinR (2012) The oocyte-to-embryo transition. Adv Exp Med Biol 757 : 351–372.
7. GovinJ, CaronC, LestratC, RousseauxS, KhochbinS (2004) The role of histones in chromatin remodelling during mammalian spermiogenesis. Eur J Biochem 271 : 3459–3469.
8. GoddeJS, UraK (2009) Dynamic alterations of linker histone variants during development. Int J Dev Biol 53 : 215–224.
9. MontellierE, BoussouarF, RousseauxS, ZhangK, BuchouT, et al. (2013) Chromatin-to-nucleoprotamine transition is controlled by the histone H2B variant TH2B. Genes Dev 27 : 1680–1692.
10. BoulardM, GautierT, MbeleGO, GersonV, HamicheA, et al. (2006) The NH2 tail of the novel histone variant H2BFWT exhibits properties distinct from conventional H2B with respect to the assembly of mitotic chromosomes. Mol Cell Biol 26 : 1518–1526.
11. IshibashiT, LiA, Eirin-LopezJM, ZhaoM, MissiaenK, et al. (2010) H2A.Bbd: an X-chromosome-encoded histone involved in mammalian spermiogenesis. Nucleic Acids Res 38 : 1780–1789.
12. LiA, MaffeyAH, AbbottWD, Conde e SilvaN, PrunellA, et al. (2005) Characterization of nucleosomes consisting of the human testis/sperm-specific histone H2B variant (hTSH2B). Biochemistry 44 : 2529–2535.
13. BaoY, KoneskyK, ParkYJ, RosuS, DyerPN, et al. (2004) Nucleosomes containing the histone variant H2A.Bbd organize only 118 base pairs of DNA. Embo J 23 : 3314–3324.
14. GovinJ, EscoffierE, RousseauxS, KuhnL, FerroM, et al. (2007) Pericentric heterochromatin reprogramming by new histone variants during mouse spermiogenesis. J Cell Biol 176 : 283–294.
15. TachiwanaH, KagawaW, OsakabeA, KawaguchiK, ShigaT, et al. (2010) Structural basis of instability of the nucleosome containing a testis-specific histone variant, human H3T. Proc Natl Acad Sci U S A 107 : 10454–10459.
16. WuF, CaronC, De RobertisC, KhochbinS, RousseauxS (2008) Testis-specific histone variants H2AL1/2 rapidly disappear from paternal heterochromatin after fertilization. J Reprod Dev 54 : 413–417.
17. LewisJD, AbbottDW, AusioJ (2003) A haploid affair: core histone transitions during spermatogenesis. Biochem Cell Biol 81 : 131–140.
18. QianMX, PangY, LiuCH, HaratakeK, DuBY, et al. (2013) Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell 153 : 1012–1024.
19. AweS, Renkawitz-PohlR (2010) Histone H4 acetylation is essential to proceed from a histone - to a protamine-based chromatin structure in spermatid nuclei of Drosophila melanogaster. Syst Biol Reprod Med 56 : 44–61.
20. HazzouriM, Pivot-PajotC, FaureAK, UssonY, PelletierR, et al. (2000) Regulated hyperacetylation of core histones during mouse spermatogenesis: involvement of histone deacetylases. Eur J Cell Biol 79 : 950–960.
21. RathkeC, BaarendsWM, Jayaramaiah-RajaS, BartkuhnM, RenkawitzR, et al. (2007) Transition from a nucleosome-based to a protamine-based chromatin configuration during spermiogenesis in Drosophila. J Cell Sci 120 : 1689–1700.
22. BaarendsWM, HoogerbruggeJW, RoestHP, OomsM, VreeburgJ, et al. (1999) Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol 207 : 322–333.
23. LuLY, WuJ, YeL, GavrilinaGB, SaundersTL, et al. (2010) RNF8-dependent histone modifications regulate nucleosome removal during spermatogenesis. Dev Cell 18 : 371–384.
24. ChenHY, SunJM, ZhangY, DavieJR, MeistrichML (1998) Ubiquitination of histone H3 in elongating spermatids of rat testes. J Biol Chem 273 : 13165–13169.
25. SinHS, BarskiA, ZhangF, KartashovAV, NussenzweigA, et al. (2012) RNF8 regulates active epigenetic modifications and escape gene activation from inactive sex chromosomes in post-meiotic spermatids. Genes Dev 26 : 2737–2748.
26. BrunnerAM, NanniP, MansuyIM (2014) Epigenetic marking of sperm by post-translational modification of histones and protamines. Epigenetics Chromatin 7 : 2.
27. ShechterD, NicklayJJ, ChittaRK, ShabanowitzJ, HuntDF, et al. (2009) Analysis of histones in Xenopus laevis. I. A distinct index of enriched variants and modifications exists in each cell type and is remodeled during developmental transitions. J Biol Chem 284 1064 - Drosophila 4.
28. HammoudSS, NixDA, ZhangH, PurwarJ, CarrellDT, et al. (2009) Distinctive chromatin in human sperm packages genes for embryo development. Nature 460 : 473–478.
29. BrykczynskaU, HisanoM, ErkekS, RamosL, OakeleyEJ, et al. (2010) Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol 17 : 679–687.
30. van der HeijdenGW, DerijckAA, RamosL, GieleM, van der VlagJ, et al. (2006) Transmission of modified nucleosomes from the mouse male germline to the zygote and subsequent remodeling of paternal chromatin. Dev Biol 298 : 458–469.
31. ParadowskaAS, MillerD, SpiessAN, ViewegM, CernaM, et al. (2012) Genome wide identification of promoter binding sites for H4K12ac in human sperm and its relevance for early embryonic development. Epigenetics 7 : 1057–1070.
32. AricoJK, KatzDJ, van der VlagJ, KellyWG (2011) Epigenetic Patterns Maintained in Early Caenorhabditis elegans Embryos Can Be Established by Gene Activity in the Parental Germ Cells. PLoS Genet 7: e1001391.
33. HalesBF, GrenierL, LalancetteC, RobaireB (2011) Epigenetic programming: from gametes to blastocyst. Birth Defects Res A Clin Mol Teratol 91 : 652–665.
34. SimpsonVJ, JohnsonTE, HammenRF (1986) Caenorhabditis elegans DNA does not contain 5-methylcytosine at any time during development or aging. Nucleic Acids Res 14 : 6711–6719.
35. ChuD, LiuH, NixP, WuT, RalstonE, et al. (2006) Sperm chromatin proteomics identifies evolutionarily conserved fertility factors. Nature 443 : 101–105.
36. OoiSL, PriessJR, HenikoffS (2006) Histone H3.3 variant dynamics in the germline of Caenorhabditis elegans. PLoS Genet 2: e97.
37. L'HernaultSW, RobertsTM (1995) Cell biology of nematode sperm. Methods Cell Biol 48 : 273–301.
38. BalhornR, GledhillBL, WyrobekAJ (1977) Mouse sperm chromatin proteins: quantitative isolation and partial characterization. Biochemistry 16 : 4074–4080.
39. GatewoodJM, CookGR, BalhornR, SchmidCW, BradburyEM (1990) Isolation of four core histones from human sperm chromatin representing a minor subset of somatic histones. J Biol Chem 265 : 20662–20666.
40. LinD, TabbDL, YatesJR3rd (2003) Large-scale protein identification using mass spectrometry. Biochim Biophys Acta 1646 : 1–10.
41. LiuH, SadygovRG, YatesJR3rd (2004) A model for random sampling and estimation of relative protein abundance. Anal Chem 76 : 4193–4201.
42. CantinGT, YatesJR3rd (2004) Strategies for shotgun identification of post-translational modifications by mass spectrometry. J Chromatogr A 1053 : 7–14.
43. LuB, XuT, ParkSK, YatesJR3rd (2009) Shotgun protein identification and quantification by mass spectrometry. Methods Mol Biol 564 : 261–288.
44. ShechterD, DormannHL, AllisCD, HakeSB (2007) Extraction, purification and analysis of histones. Nat Protoc 2 : 1445–1457.
45. CociorvaD, DLT, YatesJR (2007) Validation of tandem mass spectrometry database search results using DTASelect. Curr Protoc Bioinformatics Chapter 13: Unit 13 14.
46. TabbDL, McDonaldWH, YatesJR3rd (2002) DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res 1 : 21–26.
47. EngJK, McCormackA, YatesJR3rd (1994) An Approach to Correlate Tandem Mass Spectral Data of Peptides with Amino Acid Sequences in a Protein Database. J Am Soc Mass Spectrom 5 : 976–989.
48. IshihamaY, OdaY, TabataT, SatoT, NagasuT, et al. (2005) Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics 4 : 1265–1272.
49. McIlwainS, MathewsM, BeremanMS, RubelEW, MacCossMJ, et al. (2012) Estimating relative abundances of proteins from shotgun proteomics data. BMC Bioinformatics 13 : 308.
50. ZhangY, WenZ, WashburnMP, FlorensL (2010) Refinements to label free proteome quantitation: how to deal with peptides shared by multiple proteins. Anal Chem 82 : 2272–2281.
51. ZybailovB, ColemanMK, FlorensL, WashburnMP (2005) Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem 77 : 6218–6224.
52. ZhangY, FonslowBR, ShanB, BaekMC, YatesJR3rd (2013) Protein analysis by shotgun/bottom-up proteomics. Chem Rev 113 : 2343–2394.
53. EgelhoferTA, MinodaA, KlugmanS, LeeK, Kolasinska-ZwierzP, et al. (2010) An assessment of histone-modification antibody quality. Nat Struct Mol Biol 18 : 91–93.
54. NakagawaT, KajitaniT, TogoS, MasukoN, OhdanH, et al. (2008) Deubiquitylation of histone H2A activates transcriptional initiation via trans-histone cross-talk with H3K4 di - and trimethylation. Genes Dev 22 : 37–49.
55. ChuDS, ShakesDC (2012) Spermatogenesis. Adv Exp Med Biol 757 : 171–203.
56. ShakesDC, WuJC, SadlerPL, LapradeK, MooreLL, et al. (2009) Spermatogenesis-specific features of the meiotic program in Caenorhabditis elegans. PLoS Genet 5: e1000611.
57. SantistebanMS, KalashnikovaT, SmithMM (2000) Histone H2A.Z regulats transcription and is partially redundant with nucleosome remodeling complexes. Cell 103 : 411–422.
58. WhittleCM, McClinicKN, ErcanS, ZhangX, GreenRD, et al. (2008) The genomic distribution and function of histone variant HTZ-1 during C. elegans embryogenesis. PLoS Genet 4: e1000187.
59. UpdikeDL, MangoSE (2006) Temporal regulation of foregut development by HTZ-1/H2A.Z and PHA-4/FoxA. PLoS Genet 2: e161.
60. KellyWG, SchanerCE, DernburgAF, LeeM-H, KimSK, et al. (2002) X chromosome silencing in the germline of C. elegans. Development 129 : 479–492.
61. BeanCJ, SchanerCE, KellyWG (2004) Meiotic pairing and imprinted X chromatin assembly in Caenorhabditis elegans. Nat Genet 36 : 100–105.
62. Schaner CE, Kelly WG (2006) Germline Chromatin; Community TCeR, editor.
63. Jaramillo-LambertA, EngebrechtJ (2010) A single unpaired and transcriptionally silenced X chromosome locally precludes checkpoint signaling in the Caenorhabditis elegans germ line. Genetics 184 : 613–628.
64. JedrusikMA, SchulzeE (2001) A single histone H1 isoform (H1.1) is essential for chromatin silencing and germline development in Caenorhabditis elegans. Development 128 : 1069–1080.
65. McCarterJ, BartlettB, DangT, SchedlT (1999) On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol 205 : 111–128.
66. SadlerPL, ShakesDC (2000) Anucleate Caenorhabditis elegans sperm can crawl, fertilize oocytes and direct anterior-posterior polarization of the 1-cell embryo. Development 127 : 355–366.
67. EdgarLG, McGheeJD (1988) DNA synthesis and the control of embryonic gene expression in C. elegans. Cell 53 : 589–599.
68. BaughLR, HillAA, SlonimDK, BrownEL, HunterCP (2003) Composition and dynamics of the Caenorhabditis elegans early embryonic transcriptome. Development 130 : 889–900.
69. SeydouxG, DunnMA (1997) Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development 124 : 2191–2201.
70. SeydouxG, MelloCC, PettittJ, WoodWB, PriessJR, et al. (1996) Repression of gene expression in the embryonic germ lineage of C. elegans. Nature 382 : 713–716.
71. WangJT, SeydouxG (2013) Germ cell specification. Adv Exp Med Biol 757 : 17–39.
72. FarcasAM, BlackledgeNP, SudberyI, LongHK, McGouranJF, et al. (2012) KDM2B links the Polycomb Repressive Complex 1 (PRC1) to recognition of CpG islands. Elife 1: e00205.
73. VassilevAP, RasmussenHH, ChristensenEI, NielsenS, CelisJE (1995) The levels of ubiquitinated histone H2A are highly upregulated in transformed human cells: partial colocalization of uH2A clusters and PCNA/cyclin foci in a fraction of cells in S-phase. J Cell Sci 108 (Pt 3) 1205–1215.
74. BaarendsWM, WassenaarE, van der LaanR, HoogerbruggeJ, Sleddens-LinkelsE, et al. (2005) Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol 25 : 1041–1053.
75. StromeS, WoodWB (1982) Immunofluorescence visualization of germ-line-specific cytoplasmic granules in embryos, larvae, and adults of Caenorhabditis elegans. Proc Natl Acad Sci U S A 79 : 1558–1562.
76. Al RawiS, Louvet-ValleeS, DjeddiA, SachseM, CulettoE, et al. (2011) Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science 334 : 1144–1147.
77. HajjarC, SampudaKM, BoydL (2014) Dual roles for ubiquitination in the processing of sperm organelles after fertilization. BMC Dev Biol 14 : 6.
78. SobelRE, CookRG, PerryCA, AnnunziatoAT, AllisCD (1995) Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc Natl Acad Sci U S A 92 : 1237–1241.
79. WagnerCR, KuerversL, BaillieDL, YanowitzJL (2010) xnd-1 regulates the global recombination landscape in Caenorhabditis elegans. Nature 467 : 839–843.
80. RechtsteinerA, ErcanS, TakasakiT, PhippenTM, EgelhoferTA, et al. (2010) The histone H3K36 methyltransferase MES-4 acts epigenetically to transmit the memory of germline gene expression to progeny. PLoS Genet 6.
81. ReubenM, LinR (2002) Germline X chromosomes exhibit contrasting patterns of histone H3 methylation in Caenorhabditis elegans. Dev Biol 245 : 71–82.
82. BenderLB, SuhJ, CarrollCR, FongY, FingermanIM, et al. (2006) MES-4: an autosome-associated histone methyltransferase that participates in silencing the X chromosomes in the C. elegans germ line. Development 133 : 3907–3917.
83. VielleA, LangJ, DongY, ErcanS, KotwaliwaleC, et al. (2012) H4K20me1 contributes to downregulation of X-linked genes for C. elegans dosage compensation. PLoS Genet 8: e1002933.
84. OogaM, InoueA, KageyamaS, AkiyamaT, NagataM, et al. (2008) Changes in H3K79 methylation during preimplantation development in mice. Biol Reprod 78 : 413–424.
85. SatoM, SatoK (2011) Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science 334 : 1141–1144.
86. MeistrichML, BucciLR, Trostle-WeigePK, BrockWA (1985) Histone variants in rat spermatogonia and primary spermatocytes. Dev Biol 112 : 230–240.
87. NashunB, YukawaM, LiuH, AkiyamaT, AokiF (2011) Changes in the nuclear deposition of histone H2A variants during pre-implantation development in mice. Development 137 : 3785–3794.
88. AusioJ (2006) Histone variants–the structure behind the function. Brief Funct Genomic Proteomic 5 : 228–243.
89. MillarCB (2013) Organizing the genome with H2A histone variants. Biochem J 449 : 567–579.
90. LiuT, RechtsteinerA, EgelhoferTA, VielleA, LatorreI, et al. (2011) Broad chromosomal domains of histone modification patterns in C. elegans. Genome Res 21 : 227–236.
91. BeckDB, OdaH, ShenSS, ReinbergD (2012) PR-Set7 and H4K20me1: at the crossroads of genome integrity, cell cycle, chromosome condensation, and transcription. Genes Dev 26 : 325–337.
92. RathkeC, BaarendsWM, AweS, Renkawitz-PohlR (2013) Chromatin dynamics during spermiogenesis. Biochim Biophys Acta 1839 : 155–168.
93. KulkarniM, ShakesDC, GuevelK, SmithHE (2013) SPE-44 implements sperm cell fate. PLoS Genet 8: e1002678.
94. GovinJ, LestratC, CaronC, Pivot-PajotC, RousseauxS, et al. (2006) Histone acetylation-mediated chromatin compaction during mouse spermatogenesis. Ernst Schering Res Found Workshop 155–172.
95. WardS, ArgonY, NelsonGA (1981) Sperm morphogenesis in wild-type and fertilization-defective mutants of Caenorhabditis elegans. J Cell Biol 91 : 26–44.
96. OakbergEF (1956) Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat 99 : 507–516.
97. Jaramillo-LambertA, EllefsonM, VilleneuveAM, EngebrechtJ (2007) Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev Biol 308 : 206–221.
98. FuchsSM, StrahlBD (2011) Antibody recognition of histone post-translational modifications: emerging issues and future prospects. Epigenomics 3 : 247–249.
99. BrennerS (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94.
100. LinkAJ, EngJ, SchieltzDM, CarmackE, MizeGJ, et al. (1999) Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol 17 : 676–682.
101. WashburnMP, WoltersD, YatesJR3rd (2001) Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol 19 : 242–247.
102. HarrisTW, BaranJ, BieriT, CabunocA, ChanJ, et al. (2014) WormBase 2014: new views of curated biology. Nucleic Acids Res 42: D789–793.
103. PicottiP, AebersoldR, DomonB (2007) The implications of proteolytic background for shotgun proteomics. Mol Cell Proteomics 6 : 1589–1598.
104. NielsenML, VermeulenM, BonaldiT, CoxJ, MoroderL, et al. (2008) Iodoacetamide-induced artifact mimics ubiquitination in mass spectrometry. Nat Methods 5 : 459–460.
105. KosinskiM, McDonaldK, SchwartzJ, YamamotoI, GreensteinD (2005) C. elegans sperm bud vesicles to deliver a meiotic maturation signal to distant oocytes. Development 132 : 3357–3369.
106. Shaham S (2006) Methods in Cell Biology; Community TCeR, editor.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Intrauterinní inseminace a její úspěšnost
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



