-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
We have examined gene duplication in a set of ancient genes used in patterning of animal embryos:
the Hox genes. These genes code for proteins that bind DNA and switch on or off other genes, and they are very similar between distantly related animal species. Butterflies and moths, however, have additional Hox genes whose origin and role has been unclear. We have sequenced the genomes of five species of butterfly and moth, and of a closely related caddisfly, to examine these issues. We found that one of the Hox genes, called zen, duplicated to generate four new genes in the evolution of the largest group of butterflies and moths. Further mutations greatly modified the DNA sequence of the new genes, although maintaining potential to encode stable protein folds. Gene expression also changed so that the new Hox-derived genes are deployed in egg and early embryonic stages marking the tissues that will later envelop, nourish and protect the embryo.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004698
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004698Summary
We have examined gene duplication in a set of ancient genes used in patterning of animal embryos:
the Hox genes. These genes code for proteins that bind DNA and switch on or off other genes, and they are very similar between distantly related animal species. Butterflies and moths, however, have additional Hox genes whose origin and role has been unclear. We have sequenced the genomes of five species of butterfly and moth, and of a closely related caddisfly, to examine these issues. We found that one of the Hox genes, called zen, duplicated to generate four new genes in the evolution of the largest group of butterflies and moths. Further mutations greatly modified the DNA sequence of the new genes, although maintaining potential to encode stable protein folds. Gene expression also changed so that the new Hox-derived genes are deployed in egg and early embryonic stages marking the tissues that will later envelop, nourish and protect the embryo.Introduction
The characterization of Hox genes in the 1980s awakened the idea that there may be similar processes controlling body patterning in divergent animals and gave the first opportunity to compare the control of developmental processes between taxa at a molecular level. In animals as evolutionarily divergent as insects, annelids and vertebrates, Hox genes encode transcription factors deployed in early development, most notably to control spatial identity along the anteroposterior axis of the developing embryo [1].
Conservation of Hox gene function is reflected in their constrained evolution. First, there is high conservation of encoded protein sequence, particularly within the 60-amino acid homeodomain motif (encoded by the homeobox) containing three alpha helices. Second, Hox genes are often arranged in a genomic cluster, which was generated by tandem gene duplication early in animal evolution [2], [3]. Gene order is generally constrained, partly through shared and long-range regulatory elements [1], [4], [5]. Third, after expansion of the Hox cluster in early animal evolution there has been relatively little variation in gene number. The ancestor of all Ecdysozoa, Lophotrochozoa and Deuterostomia possessed 7 to 10 Hox genes [3], and most bilaterian animals still have approximately this number despite hundreds of millions of years of subsequent evolution. The lack of expansion of the Hox gene cluster within Bilateria is intriguing and is in contrast to the pattern of evolution seen for many other sets of genes [6], [7]. Exceptions are Hox cluster expansion to 15 genes in amphioxus [8], [9] and duplication of the entire gene cluster in vertebrates [2], [5], [10].
There are few recorded cases of tandem duplication within the Hox gene cluster. The best characterised example relates to the Hox paralogy group 3 (PG3) gene of insects, called zerknullt (zen), which has duplicated in a beetle (Tribolium castaneum) to yield zen and zen2 [11], and in cyclorrhaphan flies to generate zen and the highly derived bicoid (bcd) [12]. A further duplication specific to the genus Drosophila generated zen2 [13]. Furthermore, early in insect evolution the zen/PG3 gene lost its ancestral function of providing positional identity along the anteroposterior axis, and acquired a novel role in extra-embryonic tissue formation [14], [15], [16].
There are indications that the Hox gene cluster also expanded in Lepidoptera. Analysis of the Domesticated Silkmoth Bombyx mori genome revealed a large array of divergent homeobox genes, named Shx (Special homeobox) genes, between pb and zen [17]. With 12 Shx loci described, in addition to zen, the canonical Hox genes and another divergent gene ftz, the Silkmoth has the largest Hox gene cluster described [17]. The Silkmoth Shx sequences are highly divergent; some loci have internal duplications manifest as two or three homeobox sequences per gene, and some have disruptive mutations and are probably pseudogenes. The Hox gene cluster has also been characterised in the nymphalid butterflies Heliconius melpomene and Danaus plexippus (Monarch) where four homeobox genes were found between pb and zen [18], [19]. To date, the timing of the gene duplications, the ancestral condition for the Lepidoptera, variation in Shx gene number and gene expression have not been addressed.
Here we investigate the origin and evolution of Shx genes through sequencing and assembly of genomes from six species representing successively diverging lepidopteran lineages as well as an outgroup from Trichoptera (caddisflies). We find that four distinct Shx genes arose from the zen gene in the ancestor of the Ditrysia, the clade encompassing most Lepidoptera, and that this complement, not the expanded number found in Bombyx, is the norm across lepidopteran evolution. By modelling tertiary structure, we show that Shx protein sequence is compatible with folding into helix-loop-helix-turn-helix homeodomains. Finally, we determine the expression of Shx genes in early developmental stages of the Speckled Wood butterfly Pararge aegeria. These data suggest that Shx genes encode homeodomain proteins with probable roles in extra-embryonic tissue specification and formation. The lepidopteran zen gene may play a more downstream role in extraembryonic membrane function following serosal closure.
Results
Genome sequencing of Lepidoptera
We generated low coverage genome sequences for six species chosen for their phylogenetic positions (Figure 1B). Shx sequence data were also extracted from genome projects of the Silkmoth [20], the Diamondback moth Plutella xylostella [21], and the butterflies H. melpomene [18] and the Monarch D. plexippus [19]. The last two species are members of the Nymphalidae, the largest butterfly family, which we elected to sample further using the Comma and Speckled Wood butterflies (Polygonia c-album and Pararge aegeria). To deduce the ancestral condition for the major ditrysian clade encompassing all butterflies and the majority of moths [22], [23], [24], we also selected the Scarlet Tiger moth Callimorpha dominula (family Arctiidae). To examine deeper in the evolutionary history of Lepidoptera, we chose the Horse Chestnut Leafminer moth Cameraria ohridella (family Gracillariidae) which, along with the Diamondback moth (Yponomeutoidea) represents one of the earliest evolutionary lineages of Ditrysia [21], [22], [23], [24]. As an outgroup to Ditrysia we selected the Orange Swift moth Hepialus sylvina (synonym Trioda sylvina, family Hepialidae), and for an outgroup to the Lepidoptera we used a caddisfly Glyphotaelius pellucidus (order Trichoptera). The Trichoptera and Lepidoptera together form the sister clade to the Diptera (flies).
Fig. 1. Shx genes originated as tandem duplications of zen within the Hox gene cluster. 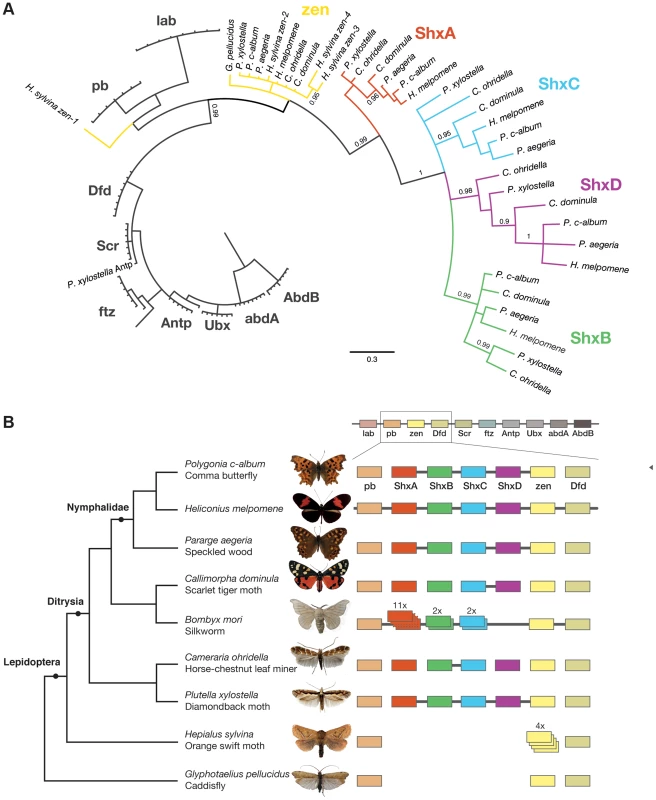
(A) Phylogenetic tree of Shx among lepidopteran Hox genes reconstructed using Phylobayes (C20), support values are posterior probabilies. (B) Shx complement of 8 lepidopteran species and the Trichoptera outgroup with available linkage information. Presence of multiple genes on the same genomic scaffolding is indicated by a plain line and gene duplication within a paralogy group as stacked boxes. Genomic DNA was sequenced using Illumina HiSeq technology, and multiple assemblies constructed using a range of k-mer sizes. For each species, we sequenced between 31.6 and 83.1 million paired-end reads granting coverage ranging from 6× to 17× as determined using a k-mer spectrum approach. We generated draft genome assemblies from 337 Mb to 1.4 Gb using de Bruijn approaches, yielding N50 values up to 5.3 kb. These datasets also provide the first estimates of genome size for these species (Table 1). Since our goal was gene and homeobox sequence hunting, rather than large-scale synteny analysis, relatively low N50 sizes are sufficient. To determine if the coverage generated was suitable, we searched the assemblies for the canonical Hox genes (lab, pb, Dfd, Scr, Antp, Ubx, abdA, AbdB) and ftz. All Hox genes were identified for all species, apart from the homeobox of Orange Swift Ubx, affording confidence in our sequencing approach to identify novel Hox genes in non-model lepidopteran species. In order to confirm that we did not lose genes during assembly of the raw read data, we also applied an alternative assembly strategy that maximally includes all sequence reads. This did not reveal any additional homeobox sequences.
Tab. 1. Summary statistics of genome sequencing and assembly. 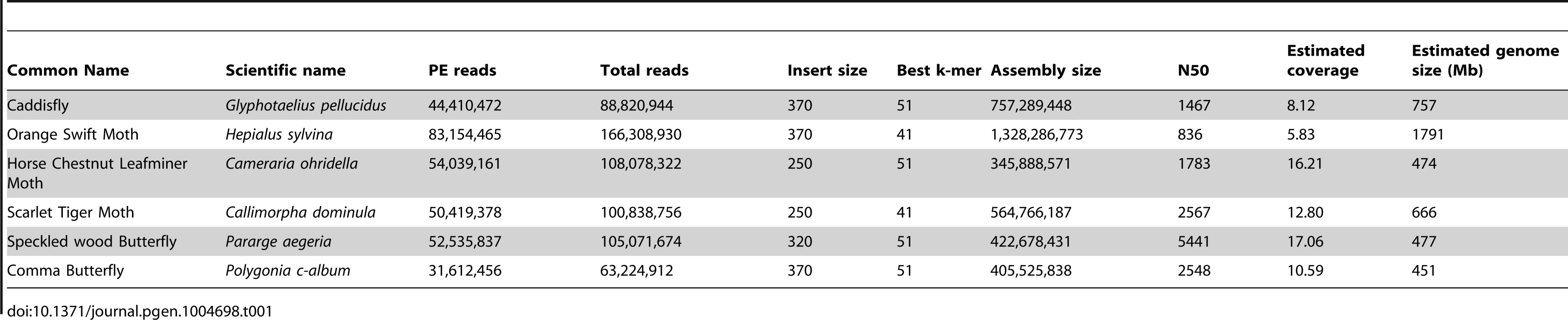
We were able to reconstruct genomic scaffolds around the Shx, zen, pb and Dfd genes by manually inspecting and aligning contigs from multiple assemblies, enabling the definition of gene models spanning multiple exons, as well as confirmation of linkage between adjacent genes in several species (Figure 1, Table S1).
Evolutionary origins of Shx genes
To examine the gene duplication events that generated Shx genes, we used molecular phylogenetic analysis and comparison of gene content between different species. Homeodomain phylogenetic trees demonstrate that the Shx genes form a monophyletic group (BP 86, PP 0.99) and are more closely related to zen than to any other Hox gene (Figure 1A, Figure S1). This suggests that Shx genes originated by tandem duplication from an ancestral zen gene, consistent with their genomic location between pb and zen (Figure 1B). Sequence alignments incorporating conserved domains outside the homeodomain confirmed this result (Figure S2).
In phylogenetic analyses, Shx genes divide into four distinct orthology groups each present in Speckled Wood, Comma, Scarlet Tiger moth, Horse Chestnut Leafminer and the Diamondback moth. The ShxA, ShxB, ShxC and ShxD groups identified in the butterflies H. melpomene and Monarch therefore originated in the clade Ditrysia, which radiated 100 to 140 Myr ago and encompasses the vast diversity of lepidopteran species [25], [26]. The identity of putative ShxC genes of the Diamondback moth and Horse Chestnut Leafminer is not clear when only the homeodomain is used, but the existence of conserved motifs outside the homeodomain strongly argues for orthology with ShxC, as does overall protein sequence similarity, gene linkage and phylogenetic analysis with an extended alignment (Figure 1B, Figures S2, S3). Our re-analysis of the Silkmoth genome identifies the previously reported Shx1 to Shx11 [17], plus four additional homeodomain-containing open reading frames which fall within the ShxA and B clades and lie between pb and zen, here named Shx13-16 (Figure 1, Figure S4). This observation contrasts with the stability of Shx genes through most of ditrysian evolution.
We also investigated the Hox complement in the Orange Swift Moth, an outgroup to Ditrysia but within Lepidoptera, and the caddisfly (order Trichoptera), the sister order to Lepidoptera. We find the Orange Swift moth has no bona fide Shx genes, but several copies of zen gene that do not branch within established Shx groups in our phylogenetic analysis (Figure 1, Figure S1 and S3). Three (zen2, zen3 and zen4) cluster with lepidopteran zen genes while zen1 has a more ambiguous affinity (Figure 1, Figures S1 and S3). Presence of diagnostic motifs C-terminal to the homeodomain suggests all are duplications of zen (Figure S2 and Figure S5G). It is less probable that they share a common origin with Shx, with extensive divergence causing ambiguity of orthology assignment. Exons coding for the homeodomains plus a single probable 5′ exon of a zen gene are located on separate scaffolds that could not be linked.
The absence of zen duplication before lepidopteran radiation was confirmed by recovery of only a single zen gene in the caddisfly genome. Duplication and divergence of zen is therefore independent in Lepidoptera and Diptera.
Evolution of lepidopteran zen and Shx gene sequence
Shx homeodomains have undergone faster sequence change than homeodomains encoded by zen or the canonical Hox genes. Homeodomain sequence of lab, pb, Dfd, Scr, Antp, Ubx, ftz, abdA and AbdB have 97% to 100% invariant sites across the four ditrysian Lepidoptera genomes sequenced in this study, canonical zen has 98% invariant sites and ShxA, ShxB, ShxC and ShxD have only 83%, 55%, 38% and 38% invariant sites respectively. Although lepidopteran zen and Shx genes are paralogues, and both descend from an ancestral zen, we retain the name Shx established in Bombyx [17] to reflect the more extreme sequence divergence in their homeodomains and to avoid confusion with earlier work. A number of conserved sites within the homeodomain are retained in Shx and zen, and S10 has been identified as unique to Hox3 orthologues (Figure S5I, red boxes) [15]; however, outside the homeodomain Shx proteins are radically different from each other and from zen (Figure S2, Figure S5C–F).
All lineages of ditryisian Lepidoptera (except Bombyx) have maintained a consistent complement of four different Shx genes, in addition to canonical zen, suggesting the genes have distinct functions. We examined whether gene-specific functions might be reflected in distinct protein motifs. Shx proteins have several short conserved motifs C-terminal to the homeodomain; these are different between the four proteins suggesting they may interact with different co-factors (Figure S2, Figure S5C–G). Lepidopteran zen shows more extensive protein conservation between species; these motifs are non-overlapping with those of the dipteran zen. Furthermore, analysis of caddisfly shows that motifs shared between basal Diptera and caddisfly have been lost in the Lepidoptera (Figure S2, Figure S5G, H). Rapid sequence evolution between closely related insect orders is consistent with a previous observation that outside the homeodomain there are no well conserved sequence motifs in zen genes of insects [27].
To investigate the dynamics underpinning diversification of Shx genes, we tested for signatures of selection by comparing synonymous (dS) and non-synonymous (dN) rates of substitutions in the homeobox region of Shx, zen and Hox genes in a maximum-likelihood framework. These analyses confirmed that there is strong purifying selection acting on the zen homeodomain in Lepidoptera (dN/dS or ω = 0.002) comparable to that inferred for canonical Hox genes (ω ratio of 0.001). However, the Shx genes show a marked increase in coding substitutions with a dN/dS ratio of 0.06; ShxB (ω = 0.1), ShxD (ω = 0.09) and ShxC (ω = 0.05) show more coding divergence than ShxA (ω 0.02). Accordingly, an excess of non-synonymous substitution is detected on the branch leading to the ShxB, ShxC and ShxD clade with an inferred ω ratio greater than 1 suggesting an episode of positive selection (Figure S6). We compared substitution ratios among codons within Shx proteins to determine whether some amino acids show evidence of positive selection. Using a site-model applied to Shx homeodomains only, we found an increased ω ratio at some sites but no statistical support (Table S2). However, taking the zen outgroup into account, the branch-site model found significant support (2Δℓ = 4.94, p<0.05) for positive selection at five sites (BEB pp>0.95). These sites are located between alpha helices and not known to be functionally involved in protein-DNA interaction (Table S2).
Predicted structure of Shx homeodomains
As the Shx homeodomains have diverged extensively from the ancestral zen sequence, we asked whether they had undergone disabling mutations that might prevent them forming stable tertiary folds compatible with binding DNA. We deployed homology modelling based on a well-resolved experimentally-determined tertiary structure of a related Hox protein: that of the Drosophila Antp homeodomain bound to a 13-mer DNA sequence. Using the Comma and Speckled Wood butterfly sequences of ShxA, ShxB, ShxC, ShxD and zen, we first computed the native energy of the deduced structures modelled on the known Antp protein structure. Each yielded a stable predicted helix-loop-helix-turn-helix structure typical of a homeodomain (Figure 2), although stability was lower when modelled in complex with the specified 13-mer DNA sequence (Note S1). This suggests that the DNA sequence used was not optimal for these homeodomains.
Fig. 2. Lowest energy structural models of deduced (A) ShxA, (B) ShxB, (C) ShxC, (D) ShxD and (E) zen homeodomains from Speckled Wood Pararge aegeria bound to DNA sequences predicted through in silico evolution. 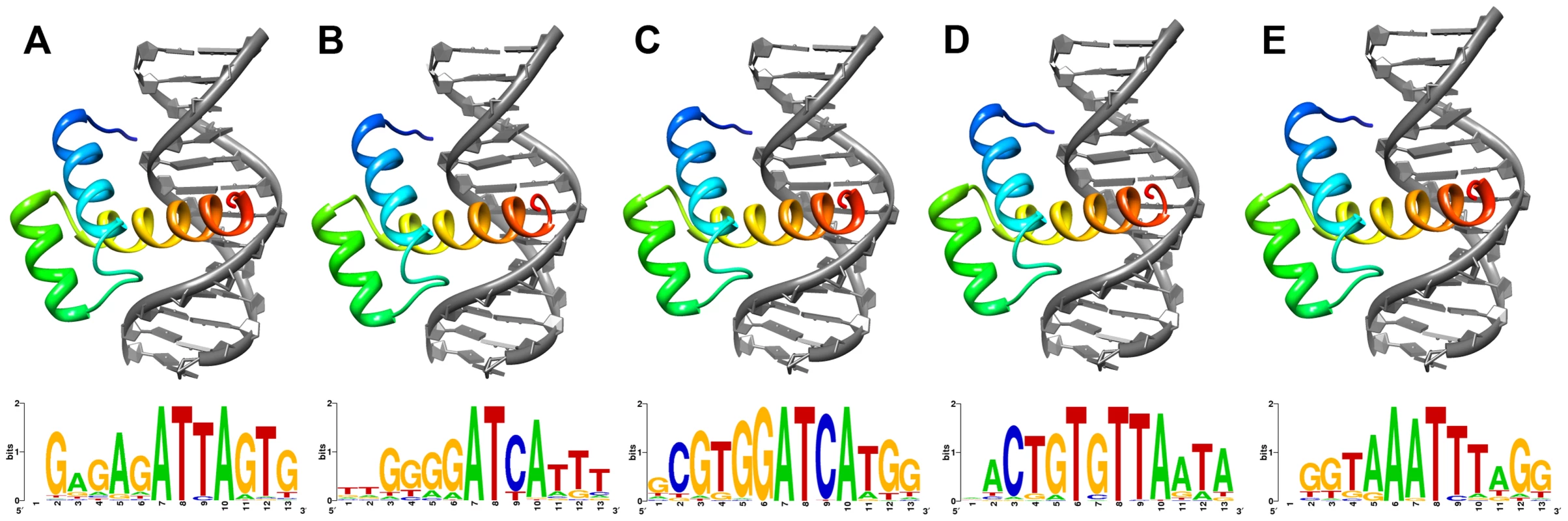
Sequence logos generated from DNA sequences of 50 lowest energy predicted protein-DNA complexes for each protein. To find more suitable DNA sequences, we used an in silico evolution approach and applied this to protein sequences of Comma, Speckled Wood and Horse Chestnut Leafminer, plus Drosophila Antp as a control. Starting with homopolymeric runs of either A, C, G or T, we ran 1000 cycles of ‘mutation’ and ‘selection’ to find the most energetically stable complexes, and generated consensus DNA sequences representing predicted optimal DNA binding sites for each homeodomain (Figure 2; Note S1). The evolved consensus sequence generated for Drosophila Antp was an approximation of the known DNA motif including the core ATTA which contacts with helix 3 of the homeodomain, plus a G residue immediately 5′.The evolved preferred DNA sequences for ShxA, ShxB and ShxC proteins included core ATTA or ATCA motifs, while the ShxD homeodomain showed more variation between the species preferring GTTA, ATTA or TTTA (Figure 2; Note S1). The zen proteins are somewhat different, tolerating a T in position 4 of the core. These results indicate that Shx and zen proteins have potential to fold into stable helix-loop-helix-turn-helix motifs compatible with sequence-specific DNA-binding. These analyses may not predict the exact in vivo binding sites [28], [29].
Expression of Shx genes
During insect oogenesis, localisation of RNA derived from maternal gene expression establishes the future positions of embryonic and extra-embryonic regions within the oocyte, as well as its body axes (for an overview of lepidopteran embryology, see Kobayashi et al. [30]). Maternal transcripts of zen and ShxC (and weakly ShxD) were detected by RT-PCR in ovarioles dissected from Speckled Wood female imagos (Figure 3A). Consistent with this, we also identified these transcripts in a maternal transcriptome dataset [31] (ShxC:PaContig23051, GB:GAIX01013843.1, GI:509161192; ShxD:PaContig8659, GB:GAIX01015570.1, GI:509158266). After egg-laying (AEL) each Shx gene has a distinct temporal expression profile (Figure 3A). Our observations and comparison with other lepidopteran species [32], [33] suggests the onset of blastoderm cellularization and major zygotic transcription commences around 8 h AEL; expression of all four Shx genes plus zen is clearly detected between 8 and 12 h AEL.
Fig. 3. (A) Expression of Shx genes throughout embryonic stages of P. aegeria. 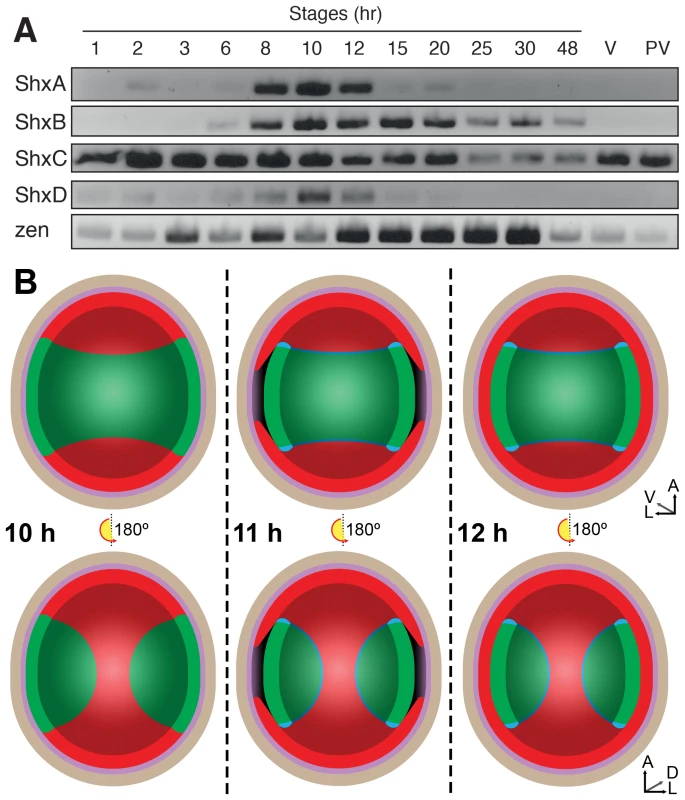
Agarose gel electrophoresis of RT-PCR products obtained using intron-crossing primers (Figure S10). (B) Schematic overview of serosa formation in P. aegeria. Diagrammatic cross section through a developing embryo and associated extra-embryonic cell layers inside a 10–12 h AEL egg. Chorion (brown), vitelline membrane (violet), extraembryonic region/serosa (red), germ anlage (green) and presumptive amniotic cells (blue) are illustrated during serosal specification, maturation and closure. Top row shows ventral half while bottom row shows dorsal half, anterior is top in both. Embryo-vitelline cavity following germ anlage sinking is shown in the middle panel. Orientation 3D axis indicates anterior (A), left (L) and ventral (V) or dorsal (D). AEL, after egg-laying (hours). In situ hybridisation to dissected ovarioles revealed that the spatial distribution of maternal ShxC and ShxD RNA is quite different to that of transcripts from their progenitor gene, zen (Figure 4). Pre-fertilisation transcripts from ShxC are detected in the nurse cells connected to the oocyte and are concentrated in a novel and striking asymmetrical ‘hourglass’ pattern which excludes the region later fated to become embryonic tissue, and corresponds to the presumptive serosal membranes (Figures 3B and 4C, Figure S7A–C). In contrast, transcripts of ShxD are faintly distributed throughout the developing oocyte without clear subcellular localisation (Figure 4D) and zen transcripts are specifically detected in the follicle cells surrounding the oocyte (Figure 4E).
Fig. 4. Spatiotemporal expression of Shx genes. 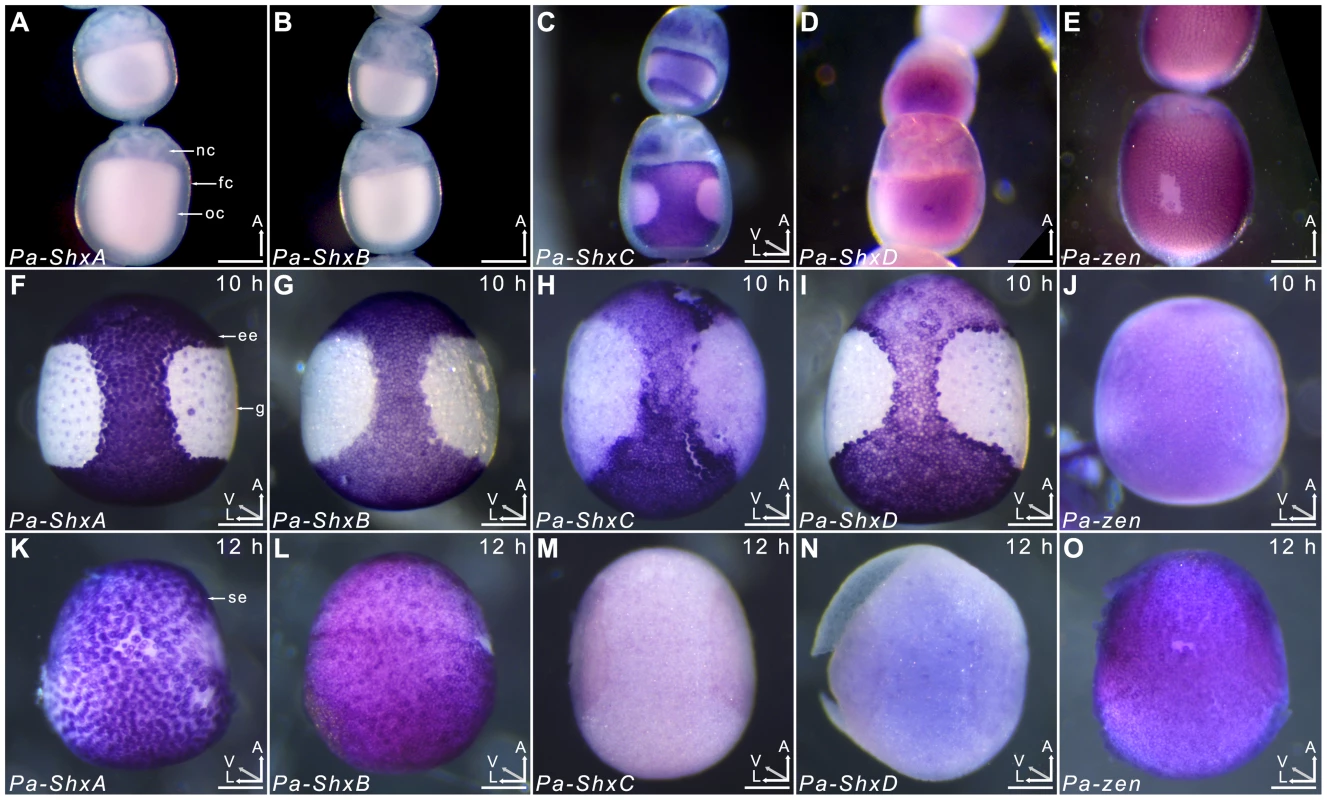
Localisation of ShxA, ShxB, ShxC, ShxD, and Pa-zen transcripts in P. aegeria ovarioles (A–E), 10 h embryos (F–J) and 12 h embryos (K–O). Embryos and oocytes are orientated with the anterior to the top. Embryos dorsal side facing while lower and upper oocytes in C show dorsal and ventral faces respectively. Note that in 12 h embryos the serosal cells have migrated over the germ anlage forming an enveloping layer. Some follicle cells in E are removed to show absence of staining in the oocyte. Labels indicate nurse cells (nc), follicle cells (fc), oocyte (oc), germ anlage (g), and extra embryonic anlage (ee) which differentiates into the serosa (s). Orientation for each panel is indicated in bottom right 3D axis indicating anterior (A), left (L) and ventral (V) when known. AEL, after egg-laying (hours). Scale bars 200 µm. In the embryo at 10 h AEL, transcripts of ShxA, ShxB, ShxC and ShxD are each detected in clear hourglass patterns in the cellularised blastoderm matching the earlier maternal ShxC RNA location in the oocyte (Figure 4F–I; Figure S7E–I). The location of Shx transcripts thus marks a clear distinction between the future embryonic regions (‘germ anlage’: small cells lacking Shx expression) and extraembryonic regions (larger cells expressing Shx genes). Within this latter domain, transcripts of ShxD are detected most strongly in the extraembryonic cells bordering the germ anlage (Figure 4I; Figure S7E–F, H–I). At the anterior pole of the egg near the micropyle, a cluster of cells with an increased concentration of ShxD transcripts correspond to a small region that previously lacked maternal ShxC transcripts (Figure S7D–F). In comparison, zen transcripts at 10 h AEL are very weakly detected throughout the blastoderm (Figure 4J).
Between 10 and 12 h AEL, the extraembryonic region expands over the germ anlage forming a protective serosal cell layer between the germ anlage and the vitelline membrane (Figure 3B). During this cell movement, ShxC and ShxD transcript levels, already lowered in the anterior (Figure S7E, F and I), reduce dramatically throughout the serosal layer (Figure 4M and N). However transcripts of ShxA and ShxB, which are only of zygotic origin, continue to be detected predominantly in the serosal layer, even after it has enveloped the germ anlage (Figure 4K,L). Transcripts of zen are detected in the serosa for the first time at this stage (Figure 4O) showing that expression patterns of zen and the Shx diverge dramatically in both time and space during butterfly embryogenesis. Significant zygotic transcription of the ShxA and ShxD genes was also detected in the large yolk cells beneath the blastoderm at 10–12 h AEL where transcripts were restricted to the nuclei suggesting either incipient transcription or RNA degradation in cytoplasm (Figure 4F,I; Figure S7H–J).
Discussion
The common ancestor of living arthropods most likely had 10 Hox genes arranged in a single genomic cluster: lab, pb, zen, Dfd, Scr, ftz, Antp, Ubx, abdA and AbdB [3]. The primary roles of Hox genes in bilaterian animals, including arthropods, are to encode positional information and to instruct position-specific cell fate along the anterior posterior axis of the embryo. Two clear exceptions are ftz, which evolved a role in parasegment formation in insects, and zen. The evolutionary history of insect zen has been well studied. In chelicerates and a crustacean the orthologous gene has a typical Hox gene expression pattern [34], [35], while during insect evolution the gene diverged in sequence and acquired a different expression pattern and developmental role [14]. In addition to loss of Hox-like function, the zen gene of insects has undergone independent tandem duplications in the Flour Beetle (to yield zen and zen2) and the cyclorrhaphan flies (to yield zen and bcd) [12], [14]. In the Drosophila clade, within the Cyclorrhapha, zen has duplicated again to yield zen and zen2 [36], [37].
Zen expression has been studied for a range of pterygote insects, including the Desert Locust Schistocerca gregaria, the Milkweed Bug Oncopeltus fasciatus [27], the Flour Beetle [38], and the flies [39]. Expression of the Hox3/zen precursor has also been analysed in an outgroup to the Pterygota, the apterygote Firebrat Thermobia domestica [40]. To some extent, inference of ancestral states within the insects is complicated by interspecific variation in the structure and function of the extraembryonic membranes and progression of embryogenesis [27]. In all pterygote insects studied however, zen expression is confined to the extraembryonic tissues with a dominant expression domain associated with early zygotic specification of the serosa, which in some species is accompanied by later, weaker expression in the amnion [14], [27], [38], [41].
Where zen duplication has occurred, both sub - and neofunctionalisation has occurred. Whereas zygotically expressed zen functions in extraembryonic membrane specification in Drosophila, maternally expressed bcd has radically diverged in sequence, and functions as an anterior determinant in the oocyte [12], [39]. A subsequent Drosophila zen duplication resulted in a putatively dispensable zen2 paralog [36], unlike in the Flour Beetle where early-acting zen-1 mainly specifies the serosal membranes and late-acting zen-2 coordinates the fusion of amnion and serosa, initiating dorsal closure [38].
In the present study, we demonstrate that the zen gene duplicated during evolution of the Lepidoptera, independently of its duplication in Diptera and Coleoptera. In the Ditrysia, a clade encompassing most of lepidopteran diversity, these duplications generated four distinct Shx genes located next to the ancestral zen gene. Lepidopteran zen and Shx genes are co-orthologues of the ancestral zen gene, hence ShxA to ShxD could logically be called zen2 to zen5. We retain the term Shx to avoid contradiction with earlier work, and to reflect their extensive sequence divergence and their shared ‘hourglass’ expression pattern in the blastoderm suggesting common functional roles. Additional Shx duplications occurred in the silkmoth lineage, but we find these are not typical of Lepidoptera. In the Orange Swift moth (Hepialidae), which diverged from a more basal node in lepidopteran phylogeny, Shx genes are not present but there is evidence of independent zen gene duplication. These data indicate that the generation of four recognisable Shx genes from an ancestral zen gene occurred after the Ditrysia had diverged; the common ancestor of Ditrysia and Hepialidae may have had multiple copies of zen but none had acquired sequence characters of Shx genes. The common ancestor of Lepidoptera and Trichoptera had just a single zen gene. The Shx genes are therefore an evolutionary novelty of ditrysian lepidopterans.
It is striking that all these examples of tandem gene duplication within insect Hox clusters can be traced to the same progenitor gene, zen. Indeed, we find no evidence of duplication of any other Hox gene within the Lepidoptera, and no such event has been reported in another insect. Why should the zen gene be prone to tandem gene duplication? The answer is likely to lie in the transition from an embryonic to extraembryonic function in the insects. If genomic clustering is important to Hox gene function, through shared enhancers or long-range chromatin effects, then tandem duplication of a canonical Hox gene would most likely disrupt regulation and generate a dominant effect mutation. Conversely, the expression of zen in extra-embryonic structures probably relies on a distinct regulatory mechanism less integrated with that of neighbouring genes; the immediate effect of duplication may therefore simply be increase of transcript dosage. The functional redundancy that is generated then offers potential for subsequent mutations to modify expression of either, or both, daughter genes.
After origin of the Shx genes, in an ancestor of the Ditrysia clade, the genes diverged radically in sequence, both within and outside the homeodomain. Within the Lepidoptera, the Shx genes also show an accumulation of coding substitutions, compared to other Hox genes, which likely reflects episodes of positive selection on some sites. In particular, we detect evidence of positive selection after the initial Shx gene duplicated to give ShxA and a progenitor of ShxB, ShxC and ShxD. We also find no Shx pseudogenes (except in the atypical Bombyx), but instead retention of the core set of these genes. Together these observations argue for functional constraints on Shx proteins and the acquisition of new essential roles for these genes in the biology of ditrysian lepidopterans.
Sequence divergence in the homeodomain raised the question of whether Shx proteins are still capable of functioning as DNA-binding proteins, potentially regulating the expression of other genes. Evidence that this biochemical role has most likely been retained comes from molecular modelling. We show that despite the extensive accumulation of amino acid substitutions in Shx homeodomains, they still have potential to fold into stable helix-loop-helix-turn-helix motifs with appropriate interaction surfaces for binding to DNA. An in silico evolution approach revealed that the Shx and zen proteins may have subtly different DNA sequence binding preferences, though these are not likely to be grossly dissimilar from target sequences recognised by canonical Hox proteins. We stress that these in silico approaches do not reveal definitive binding sites [28], [29]; however, they give confidence in the assertion that Shx proteins in Lepidoptera are likely to act as DNA-binding proteins.
What roles might Shx genes play in lepidopteran biology? Embryonic development is similar in the Silkmoth [42] and the Small White butterfly Pieris rapae suggesting conservation across the Ditrysia [30]. Following egg-laying the fertilised egg (zygote) undergoes continuous mitotic divisions and in the Silkmoth two regions can be distinguished in the cellular blastoderm based on cell density: the germ anlage which will become the embryo, and the remaining cells which will form the extraembryonic tissues notably the serosa [30], [42]. As observed for the Speckled Wood butterfly in the current study, in the Small White and Silkmoth, the presumptive serosa has a distinctive hourglass-shape [30]. At 10 h AEL in the Speckled Wood extraembryonic cells become polyploid, large and flat, and by 12 h this sheet of presumptive serosal cells moves over a region where more compact embryonic cells begin to sink into the yolk in the interior of the egg [32]. Serosal closure completes around 12 h AEL in the Speckled Wood butterfly (summarised in Figure 3B, Figure S8). As the embryonic germ anlage grows, cells at the edge of the anlage differentiate into a second extraembryonic membrane, the amnion, which extends around the ventral surface [30], [42].
The expression pattern of lepidopteran zen is intriguing because it differs from other insects. In Pterygota, except the Milkweed Bug, zen functions in early embryogenesis in the early specification of the extraembryonic membranes [14], [16], including in those species with a zen gene duplication. In the Lepidoptera, we find zen has largely lost this association and is instead expressed in follicle cells and then in the serosa following closure. Lepidopteran zen is therefore likely to have derived roles in the downstream functions of the serosal membrane. For example, we note that as the Speckled Wood zen expression intensifies, the maturing serosa takes on a glossy appearance indicative of cuticle secretion [43]. It has been suggested that the serosa plays roles in the innate immune system, processing environmental toxins, yolk catabolism, cuticle formation and desiccation resistance [44], [45].
The contrast between zen and Shx gene expression is striking. Our data reveal that Shx genes have a close association with development of the extraembryonic tissues of the Speckled Wood butterfly, but the zen gene does not. Indeed, all four Shx genes are expressed in the presumptive serosa well before zen expression is observed. We suggest that following zen gene duplication in Lepidoptera, the divergent Shx genes retained an ancestral association with extraembryonic membrane specification, while zen gene function diverged radically.
It would be a mistake, however, to consider all four lepidopteran Shx genes equivalent, as they have diverged from each other in sequence and in spatiotemporal expression patterns. Most strikingly, in the Speckled Wood there is maternal expression of ShxC and ShxD, but not ShxA and ShxB. It is notable that zen is maternally expressed in Locust and some basal fly species [39], [41], whilst in other pterygote insects zen transcripts are zygotically-derived. Maternal expression of ShxC and ShxD suggests that maternal expression may be an ancestral property of the zen gene [41]. However, in the flies and Locust zen transcripts are diffusely distributed within the oocyte, whereas in the Speckled Wood maternally-derived ShxC transcripts are tightly localised in a very distinctive hourglass shape, clearly prefiguring the region where extraembryonic tissues will later emerge after cellularisation. This hourglass pattern of ShxC transcripts within the single cell represents one of the most complex examples of RNA localisation ever reported in any species, and suggests that the Shx genes specify the future serosal tissue domain within the unfertilised oocyte. Differences between Shx gene expression domains are also seen in the embryonic stages: expression of ShxC and ShxD in serosal tissue is joined by expression of ShxA and ShxB, before these two genes become the dominant expressed Shx genes after serosal cell movements around the embryo.
The evolution of Shx genes provides some parallels to the evolution of bcd in Diptera. In both cases, the zen gene has undergone tandem duplication, daughter genes have diverged in sequence and there has been recruitment to patterning roles in the unfertilized oocyte.
Materials and Methods
Genome sequencing
DNA was extracted from individual adult specimens of the Comma butterfly (Polygonia c-album), the Speckled Wood butterfly (Pararge aegeria), the Scarlet Tiger moth (Callimorpha dominula), the Orange Swift moth (Hepialus sylvina) and a caddisfly (Glyphotaelius pellucidus), and from 75 pooled specimens of the Horse Chestnut Leafminer moth (Cameraria ohridella) using a phenol-chloroform method [46]. Sources of specimens are given in Table S3. Paired-end libraries were constructed and sequenced by Oxford Genomics Centre (http://www.well.ox.ac.uk) using standard Illumina procedures (http://www.illumina.com). Between 32 million and 83 million 101 bp paired-end reads were collected for each species (Table 1) using HiSeq2000 methodology. Low quality scoring bases were trimmed using sickle (https://github.com/najoshi/sickle.git). We assembled the reads using de Bruijn-based packages Velvet [47] and ABySS [48] with k-mers ranging from 31 to 61. Table 1 reports assemblies with the best combination of N50 and assembly length; these are available from the Oxford University Research Data Archive (DOI: 10.5287/bodleiandury.3). Alternative assemblies were also examined to assist with scaffolding around particular genes. As an additional method to identify homeodomain sequence contained in the reads, we also performed assembly using Fermi that implements an overlap-layout consensus approach using a FM-index and is designed to preserve all information in the raw reads [49]. Raw sequencing data are deposited in the NCBI BioProject database under accession number PRJNA241175. Genome size was determined using the k-mer spectrum approach: the frequency of all possible k-mers of a given length were calculated and plotted to reveal a peak representing the k-mer coverage (Ck), while low and high k-mer coverages correspond to sequencing errors and repeated regions respectively (Figure S9). K-mer coverage was converted to actual base coverage (C) using Ck = C×(L−k+1)/L where L is the read length and k the k-mer size. K-mers were counted and distributions calculated using Jellyfish [50] for a k-mer size of 17 (Table 1).
Hox gene identification
Analysis of the previously sequenced genomes of Bombyx mori, Heliconius melpomene and Plutella xylostella used data from Silkdb (http://silkworm.genomics.org.cn/), Butterflygenome (www.butterflygenome.org), KONAGAbase (http://dbm.dna.affrc.go.jp/px/) and the NCBI genome database. Scaffolds corresponding to the region pb-Dfd were downloaded and annotated according to conserved amino acid translations, sequence alignments and, where available, species-specific EST traces. Genome assemblies generated in this study were searched using the Hox homeodomains of H. melpomene, B. mori and P. xylostella using tBLASTn, scaffolds corresponding to significant hits (1e-6) were extracted and redundant scaffolds dismissed. Gene identification used a combination of phylogenetic analysis and amino acid signatures inside and outside the homeodomain (see below). Contigs containing homeoboxes were manually extended by generating a scaffold tilepath from assemblies obtained at multiple k-mer sizes. Conserved amino acid domains were also used to search for new contigs when scaffolds could not be extended. Gene annotation was carried out manually. Operations were carried out using Geneious V6 (Biomatters Ltd). Gene models were submitted to NCBI with accession numbers listed in Table S1.
Molecular evolution analysis
For phylogenetic analysis, translated sequences were cropped to either the homeodomain only or the homeodomain plus C-terminal sequence until the deduced stop codon (‘extended’ sequences) (for deduced translations see Figure S5). The extended sequences were aligned using Cobalt [51], and edited to exclude sites with a>50% missing data. Maximum-likelihood trees were built using RAxML [52] with an LG+Γ model and 500 bootstrap replicates. Bayesian analysis was performed using Phylobayes-MPI with a C20 pre-defined mixture of profile and a gamma distribution of among-site rate variation [53].
To evaluate the selective processes at play through the evolution of Shx genes, the dN/dS ratio (or ω ratio) of the synonymous and non-synonymous rates of substitution was estimated in a maximum likelihood framework using the codeml program of the PAML package [54]. The ‘branch’ model was employed to evaluate the selective effects along the branches leading to distinct groups of Shx genes (topology as in Figure 1) by assigning 2, 3 or 6 independent ω ratios. Alternatively, site models and branch-site models were employed to assess positive selection at the codon level and the significance of selective effect was assessed using a likelihood ratio test. The probability of sites being under positive selective was evaluated using Bayes Empirical Bayes criteria (posterior probability>0.95).
To search for additional motifs outside the homeodomain, deduced translations of genes from Diptera, Trichoptera and Lepidoptera were aligned using Cobalt. Dipteran analysis used the Mothfly (Clogmia albipunctata), Horsefly (Haematopota pluvialis), Dancefly (Empis livida), Scuttlefly (Megaselia abdita), Fruit fly (Drosophila melanogaster) and Marmalade Hoverfly (Episyrphus balteatus). Lepidopteran analysis used the five genomes sequenced in the current study plus H. melpomene and the Diamondback moth Plutella xylostella. Caddisfly (Trichoptera) sequences were from the present study, and were compared to the Diptera/Lepidoptera alignments (Figure S5). Conserved motifs were defined as three or more consecutive amino acids present in at least half the species examined and where each residue is shared between divergent lineages (for Lepidoptera one of Hepialus/Cameraria/Plutella vs. one of Heliconius/Polygonia/Pararge).
RT-PCR and in situ hybridisation in Speckled Wood butterfly
We examined the spatial and temporal expression patterns of the 4 Shx genes and zen in the Speckled Wood (Pararge aegeria) using RT-PCR and whole mount in situ hybridization (WMISH). Since zen is involved in extra-embryonic tissue formation in other winged (pterygote) insects, we paid particular attention to oogenesis and early embryonic development. For RT-PCR analysis, RNA was extracted from eggs and ovaries obtained from mated 4-day old females taken from a large outbred laboratory stock [31]. To examine zygotic expression, fifty embryos were pooled for time points 0, 1, 2, 3, 6, 8, 10, 12, 15, 20, 25, 30 and 48 hours after egg laying (AEL) in triplicate. In Lepidoptera, egg laying is nearly synchronous with fertilization, and time after egg laying (AEL) can therefore be taken as a proxy for time of development. To examine maternal expression, two mated 4-day old females were sacrificed, the abdomens removed and ovaries dissected in ice-cold PBS. Previtellogenic and vitellogenic regions were separated before RNA extraction [31]. RNA was purified using RNeasy Mini Kit (Qiagen) and cDNA synthesized using BioScript (Bioline). The expression of zen and Shx genes was assessed by 35 cycles of RT-PCR using GoTaq polymerase (Promega) and primer annealing temperatures of 55°C. All primers are given in Table S4 and their respective position and orientation in Figure S10.
Riboprobes were synthesized using a T7/SP6 DIG RNA labeling Kit (Roche Applied Science, Penzberg, Germany) either from linearized plasmids (ShxA, B, zen) or PCR amplified templates from Speckled Wood cDNA (ShxC, D) purified using QIAquick (Qiagen, Hilden, Germany). In the latter method, initial amplifications using gene-specific primers were followed by a second PCR implementing a modified reverse or forward primer with a T7 5′ tail (5′-TAATACGACTCACTATAGGG+Fw/Rev-3′) resulting in an antisense or sense template. Regions of genes targeted for RT-PCR and for WMISH are shown in Figure S10. In-situ hybridisation was carried out on 10 and 12 h AEL eggs which had been dechorionated prior to fixation using 4% sodium hypochlorite. Ovarioles and embryos were fixed in a 1∶1 mixture of heptane and 5.5% formaldehyde in PBS in glass vials (30 min at 25°C, then 4°C overnight, with gentle rotation) before gradual dehydration in methanol and storage at −20°C. Samples were hybridized with the riboprobes at 55°C and processed as detailed in Note S2, developed from Brakefield et al. (2009) [55]. After WMISH, samples were counter stained with SYTOX Green (Invitrogen; 450–490 nm) and imaged using a MZ FL III Stereo-Fluorescence Microscope (Leica, Wetzlar, Germany) using a ProgResC3 sensor (Jenoptik, Jena, Germany).
Protein modelling
The program MODELLER-9v7 [56] was used to model deduced Shx and zen homeodomain sequences onto a previously published crystal structure of the Drosophila Antp homeodomain bound to the DNA sequence AGAAAGCCATTAGAG (pdb code 9ant; [57]). Energetic stability of sequences was initially assessed using the sum of pairwise atomic interactions, estimated solvent interaction and overall combined energy (see Note S1). To assess stability of binding to the 9ant DNA sequence, the ROSETTA program was deployed (see Note S1) [58]. To identify energetically preferred DNA target sequences for each homeodomain, an in silico evolution approach was applied to Shx and zen homeodomains of Speckled Wood, Comma and Horse Chestnut Leafminer. Proteins were modelled in complex with homopolymer sequences using ROSETTA and then random changes introduced over the 11-core positions with elevated sampling in the inner 9 positions. After each round of mutation protein-DNA complexes were remodelled, side-chain and base-pair packing energies recalculated, and the lowest energy structure, as assessed using ROSETTA and dFIRE3, used as the next template for mutation [59]. After 1000 rounds of mutation, starting from each homopolymer run, the DNA sequences associated with the 50 lowest energy structures were used to build consensus sequences. Finally, substitution without in silico evolution was used to test for bias introduced from starting with homopolymer runs (see Note S1). Structures were displayed using CHIMERA [60] and consensus DNA sequences with WebLogo (http://weblogo.berkeley.edu).
Supporting Information
Zdroje
1. McGinnisW, KrumlaufR (1992) Homeobox genes and axial patterning. Cell 68 : 283–302.
2. KappenC, SchughartK, RuddleFH (1989) Two steps in the evolution of Antennapedia-class vertebrate homeobox genes. Proc Natl Acad Sci USA 86 : 5459–5463.
3. de RosaR, GrenierJK, AndreevaT, CookCE, AdoutteA, et al. (1999) Hox genes in brachiopods and priapulids and protostome evolution. Nature 399 : 772–776.
4. DubouleD (2007) The rise and fall of Hox gene clusters. Development 134 : 2549–2560.
5. LemonsD, McGinnisW (2006) Genomic evolution of Hox gene clusters. Science 313 : 1918–1922.
6. SimakovO, MarlétazF, ChoSJ, Edsinger-GonzalesE, HavlakP, et al. (2013) Insights into bilaterian evolution from three spiralian genomes. Nature 493 : 526–531.
7. ZhangG, FangX, GuoX, LiL, LuoR, et al. (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490 : 49–54.
8. PowersTP, AmemiyaCT (2004) Evidence for a Hox14 paralog group in vertebrates. Curr Biol 14: R183–184.
9. HollandLZ, AlbalatR, AzumiK, Benito-GutierrezE, BlowMJ, et al. (2008) The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res 18 : 1100–1111.
10. SchughartK, KappenC, RuddleFH (1989) Duplication of large genomic regions during the evolution of vertebrate homeobox genes. Proc Natl Acad Sci USA 86 : 7067–7071.
11. BrownSJ, FellersJP, ShippyTD, RichardsonEA, MaxwellM, et al. (2002) Sequence of the Tribolium castaneum homeotic complex: the region corresponding to the Drosophila melanogaster antennapedia complex. Genetics 160 : 1067–1074.
12. StauberM, JackleH, Schmidt-OttU (1999) The anterior determinant bicoid of Drosophila is a derived Hox class 3 gene. Proc Natl Acad Sci U S A 96 : 3786–3789.
13. NegreB, CasillasS, SuzanneM, Sánchez-HerreroE, AkamM, et al. (2005) Conservation of regulatory sequences and gene expression patterns in the disintegrating Drosophila Hox gene complex. Genome Res 15 : 692–700.
14. FalcianiF, HausdorfB, SchröderR, AkamM, TautzD, et al. (1996) Class 3 Hox genes in insects and the origin of zen. Proc Natl Acad Sci USA 93 : 8479–8484.
15. PanfilioKA, AkamM (2007) A comparison of Hox3 and Zen protein coding sequences in taxa that span the Hox3/zen divergence. Dev Genes Evol 217 : 323–329.
16. Schmidt-Ott U, Rafiqi AM, Lemke S (2010) Hox3/zen and the Evolution of Extraembryonic Epithelia in Insects. In: Deutsch J, editor. Hox Genes Studies from the 20th to 21st Century: Springer New York. pp. 133–144.
17. ChaiCL, ZhangZ, HuangFF, WangXY, YuQY (2008) A genomewide survey of homeobox genes and identification of novel structure of the Hox cluster in the silkworm Bombyx mori. Insect Biochem Mol Biol 38 : 1111–1120.
18. Heliconius GenomeC (2012) Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487 : 94–98.
19. ZhanS, MerlinC, BooreJL, ReppertSM (2011) The monarch butterfly genome yields insights into long-distance migration. Cell 147 : 1171–1185.
20. XiaQ, ZhouZ, LuC, ChengD, DaiF, et al. (2004) A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science. 306 : 1937–1940.
21. YouM, YueZ, HeW, YangX, YangG, et al. (2013) A heterozygous moth genome provides insights into herbivory and detoxification. Nat Genet 45 : 220–225.
22. RegierJC, MitterC, ZwickA, BazinetAL, CummingsMP, et al. (2013) A large-scale, higher-level, molecular phylogenetic study of the insect order Lepidoptera (moths and butterflies). PLoS ONE 8: e58568.
23. RegierJC, ZwickA, CummingsMP, KawaharaAY, ChoS, et al. (2009) Toward reconstructing the evolution of advanced moths and butterflies (Lepidoptera: Ditrysia): an initial molecular study. BMC Evol Biol 9 : 280.
24. KawaharaAY, BreinholtJW (2014) Phylogenomics provides strong evidence for relationships of butterflies and moths. P R Soc B 281 : 20140970–20140970.
25. Grimaldi D (2005) Evolution of the Insects: Cambridge University Press. 755 p.
26. WahlbergN, WheatCW, PeñaC (2013) Timing and Patterns in the Taxonomic Diversification of Lepidoptera (Butterflies and Moths). PLoS ONE 8: e80875.
27. PanfilioKA, LiuPZ, AkamM, KaufmanTC (2006) Oncopeltus fasciatus zen is essential for serosal tissue function in katatrepsis. Dev Biol 292 : 226–243.
28. SlatteryM, RileyT, LiuP, AbeN, Gomez-AlcalaP, et al. (2011) Cofactor Binding Evokes Latent Differences in DNA Binding Specificity between Hox Proteins. Cell 147 : 1270–1282.
29. MannRS, LelliKM, JoshiR (2009) Hox specificity unique roles for cofactors and collaborators. Curr Top Dev Biol 88 : 63–101.
30. KobayashiY, TanakaM, & AndoH (2003) Embryology. In Handbook of Zoology: Lepidoptera, Moths and Butterflies. Volume 2: Morphology, Physiology and Development 495–544. Kristensen, N.P. (Ed).
31. CarterJ-M, BakerSC, PinkR, CarterDR, CollinsA, et al. (2013) Unscrambling butterfly oogenesis. BMC Genomics 14 : 283.
32. Nagy LM (2006) A summary of lepidopteran embryogenesis and experimental embryology. In: Goldsmith MR, Wilkins AS, editors. Molecular Model Systems in the Lepidoptera: Cambridge University Press.
33. KeinoH, TakesueS (1982) Scanning Electron microscopic Study on the Early Development of Silkworm Eggs (Bombyx mori L.). Dev Growth Differ 24 : 287–294.
34. DamenWG, TautzD (1998) A Hox class 3 orthologue from the spider Cupiennius salei is expressed in a Hox-gene-like fashion. Dev Genes Evol 208 : 586–590.
35. PapillonD, TelfordMJ (2007) Evolution of Hox3 and ftz in arthropods: insights from the crustacean Daphnia pulex. Dev Genes Evol 217 : 315–322.
36. RushlowC, DoyleH, HoeyT, LevineM (1987) Molecular characterization of the zerknüllt region of the Antennapedia gene complex in Drosophila. Genes Dev 1 : 1268–1279.
37. RushlowC, FraschM, DoyleH, LevineM (1987) Maternal regulation of zerknüllt: a homoeobox gene controlling differentiation of dorsal tissues in Drosophila. Nature 330 : 583–586.
38. Van der ZeeM, BernsN, RothS (2005) Distinct functions of the Tribolium zerknüllt genes in serosa specification and dorsal closure. Curr Biol 15 : 624–636.
39. StauberM, PrellA, Schmidt-OttU (2002) A single Hox3 gene with composite bicoid and zerknullt expression characteristics in non-Cyclorrhaphan flies. Proc Natl Acad Sci U S A 99 : 274–279.
40. HughesCL, LiuPZ, KaufmanTC (2004) Expression patterns of the rogue Hox genes Hox3/zen and fushi tarazu in the apterygote insect Thermobia domestica. Evol Dev 6 : 393–401.
41. DeardenP, GrbicM, FalcianiF, AkamM (2000) Maternal expression and early zygotic regulation of the Hox3/zen gene in the grasshopper Schistocerca gregaria. Evol Dev 2 : 261–270.
42. Miya K (2003) The early embryonic development of Bombyx mori: an ultrastructural point of view. In: Yaginuma T, Suzuki K, editors: Gendaitosho. pp. 218.
43. LamerA, DornA (2001) The serosa of Manduca sexta (Insecta, Lepidoptera): ontogeny, secretory activity, structural changes, and functional considerations. Tissue and Cell 33 : 580–595.
44. JacobsCGC, RezendeGL, LamersGEM, van der ZeeM (2013) The extraembryonic serosa protects the insect egg against desiccation. P R Soc B 280 : 20131082–20131082.
45. PanfilioKA (2008) Extraembryonic development in insects and the acrobatics of blastokinesis. Dev Biol 313 : 471–491.
46. ZraketCA, BarthJL, HeckelDG, AbbottAG (1990) Genetic Linkage Mapping with Restriction Fragment Length Polymorphisms in the Tobacco Budworm, Heliothis virescens. Molecular Insect Science 13–20.
47. ZerbinoDR, BirneyE (2008) Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18 : 821–829.
48. SimpsonJT, WongK, JackmanSD, ScheinJE, JonesSJM, et al. (2009) ABySS: a parallel assembler for short read sequence data. Genome Res 19 : 1117–1123.
49. LiH (2012) Exploring single-sample SNP and INDEL calling with whole-genome de novo assembly. Bioinformatics 28 : 1838–1844.
50. MarçaisG, KingsfordC (2011) A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 27 : 764–770.
51. PapadopoulosJS, AgarwalaR (2007) COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics 23 : 1073–1079.
52. StamatakisA, AlachiotisN (2010) Time and memory efficient likelihood-based tree searches on phylogenomic alignments with missing data. Bioinformatics 26: i132–139.
53. LartillotN, LepageT, BlanquartS (2009) PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics 25 : 2286–2288.
54. YangZ (2007) PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol Biol Evol. 24 : 1586–1591.
55. BrakefieldPM, BeldadeP, ZwaanBJ (2009) Fixation and dissection of embryos from the African butterfly Bicyclus anynana. Cold Spring Harbor Protocols 2009: pdb.prot5206–pdb.prot5206.
56. EswarN, WebbB, Marti-RenomMA, MadhusudhanMS, EramianD, et al. (2007) Comparative protein structure modeling using MODELLER. Curr Protoc Protein Sci Chapter 2: Unit 2.9–2.9.31.
57. FraenkelE, PaboCO (1998) Comparison of X-ray and NMR structures for the Antennapedia homeodomain-DNA complex. Nat Struct Biol 5 : 692–697.
58. Leaver-FayA, TykaM, LewisSM, LangeOF, ThompsonJ, et al. (2011) ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Meth Enzymol 487 : 545–574.
59. XuB, SchonesDE, WangY, LiangH, LiG (2013) A structural-based strategy for recognition of transcription factor binding sites. PLoS ONE 8: e52460.
60. PettersenEF, GoddardTD, HuangCC, CouchGS, GreenblattDM, et al. (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25 : 1605–1612.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



