-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Coevolution within and between Regulatory Loci Can Preserve Promoter Function Despite Evolutionary Rate Acceleration
Phenotypes that appear to be conserved could be maintained not only by strong purifying selection on the underlying genetic systems, but also by stabilizing selection acting via compensatory mutations with balanced effects. Such coevolution has been invoked to explain experimental results, but has rarely been the focus of study. Conserved expression driven by the unc-47 promoters of Caenorhabditis elegans and C. briggsae persists despite divergence within a cis-regulatory element and between this element and the trans-regulatory environment. Compensatory changes in cis and trans are revealed when these promoters are used to drive expression in the other species. Functional changes in the C. briggsae promoter, which has experienced accelerated sequence evolution, did not lead to alteration of gene expression in its endogenous environment. Coevolution among promoter elements suggests that complex epistatic interactions within cis-regulatory elements may facilitate their divergence. Our results offer a detailed picture of regulatory evolution in which subtle, lineage-specific, and compensatory modifications of interacting cis and trans regulators together maintain conserved gene expression patterns.
Published in the journal: . PLoS Genet 8(9): e32767. doi:10.1371/journal.pgen.1002961
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002961Summary
Phenotypes that appear to be conserved could be maintained not only by strong purifying selection on the underlying genetic systems, but also by stabilizing selection acting via compensatory mutations with balanced effects. Such coevolution has been invoked to explain experimental results, but has rarely been the focus of study. Conserved expression driven by the unc-47 promoters of Caenorhabditis elegans and C. briggsae persists despite divergence within a cis-regulatory element and between this element and the trans-regulatory environment. Compensatory changes in cis and trans are revealed when these promoters are used to drive expression in the other species. Functional changes in the C. briggsae promoter, which has experienced accelerated sequence evolution, did not lead to alteration of gene expression in its endogenous environment. Coevolution among promoter elements suggests that complex epistatic interactions within cis-regulatory elements may facilitate their divergence. Our results offer a detailed picture of regulatory evolution in which subtle, lineage-specific, and compensatory modifications of interacting cis and trans regulators together maintain conserved gene expression patterns.
Introduction
Conserved patterns of gene expression, especially among closely related species, immediately suggest conservation of the regulatory mechanisms that bring them about. However, considerable sequence divergence has been documented in orthologous regulatory elements [1], [2] and turnover of experimentally validated transcription factor binding sites is known to occur [3], [4] and to be selected on [5]. Such cis-regulatory changes occur on their own, or coevolve with transcription factors that regulate them [6]–[8], and with chromatin modifiers [9]. Indeed, entire regulatory networks that are crucial for organismal survival nonetheless vary within [10], [11] and between species [12]–[14].
Since evolutionary biologists are interested in species divergence, most studies of regulatory evolution focus on gene expression differences between two species or strains [15]–[17], which is essential for understanding the molecular processes by which evolution occurs. However, the necessary counterpart to studies of differentially expressed genes are studies that address how, despite the inexorable evolution of genome sequence, some genes retain conserved expression. This second category comprises a substantial fraction of genes; for instance, only about a quarter of genes show expression differences between strains of yeast [18] or Drosophila [19], and over a third of orthologs show conserved expression even among distantly related vertebrates [20]. We want to understand how expression conservation is achieved.
One possibility is that purifying selection preserves functional elements, which are nestled within functionless and divergent sequences [21]. Another possibility is that regulatory functions can be carried out by degenerate sequences that can sustain substantial substitution without altering their conserved function [22]. Yet another possibility is that coevolutionary changes among the multiple regulators of a single gene compensate for one another to maintain a conserved output [23]–[25]. This scenario is only detectable in a comparative context—expression patterns must be conserved while the specific interactions among regulatory molecules diverge in one organism relative to another.
One way to document this phenomenon is to perform functional comparisons of orthologous cis-regulatory elements in a common trans background. This can be done in several ways. Diverged regulatory elements can be introduced into a hybrid trans environment on the genome-wide scale by interspecific crosses, after which allele-specific expression can be measured by microarray [26], [27], sequencing of individual genes [28], or high-throughput sequencing [29], [30]. Such methods have the advantage of assaying multiple loci at once and detecting genome-wide regulatory divergence. These approaches are useful for identifying the molecular underpinnings of hybrid incompatibility [31]. In some cases, QTL studies can be used to uncover genomic sequences associated with gene expression differences [32], [33]. Follow-up experiments can then determine the molecular effects of associated mutations on expression [34], [35].
Transgenic methods provide an approach to studying the loci of regulatory evolution in a controlled, experimentally manipulable way. Single regulatory elements from the genome of one species can be introduced into a host of a pure-species, rather than hybrid, background. While this method can only be used to dissect one regulatory element at a time, it has the advantages of being tractable in non-hybridizing species, isolating particular molecular interactions between cis and trans, and allowing experimental manipulations of regulatory sequence and genetic background to isolate mechanisms of action [36]. When a pair of organisms are both amenable to transgenesis, a highly controlled experiment of reciprocal cis-regulatory element swaps can be performed [37]–[43].
This necessary quality is found in Caenorhabditis elegans and C. briggsae, two nematodes with considerable sequence divergence [44] and morphological conservation. Here, we studied the cis-regulatory element of the unc-47 gene, which has a simple and quantifiable expression pattern [22], [45]. We examined the functions of regulatory sequences from one species in the other to discern whether lineage-specific cis-trans and cis-cis interactions have evolved.
Results
Divergent cis - and trans-regulatory information underlies a conserved expression pattern
The nervous system of C. elegans contains 26 GABAergic neurons ([46], Figure 1A), which are conserved with even distantly related nematodes [47]. The expression patterns of genes involved in defining the identity of GABAergic neurons, such as the vesicular GABA transporter unc-47 [48], are expected to be conserved as well. This expectation can readily be tested in C. elegans and C. briggsae, two closely related species that have nearly identical embryonic cell lineages [49], which allows for homology of individual cells to be unambiguously assigned. Indeed, cis-regulatory elements of C. elegans and C. briggsae unc-47 genes direct almost identical expression patterns in their respective trans-regulatory environments [45].
Fig. 1. The expression pattern of unc-47 is conserved despite divergent regulation. 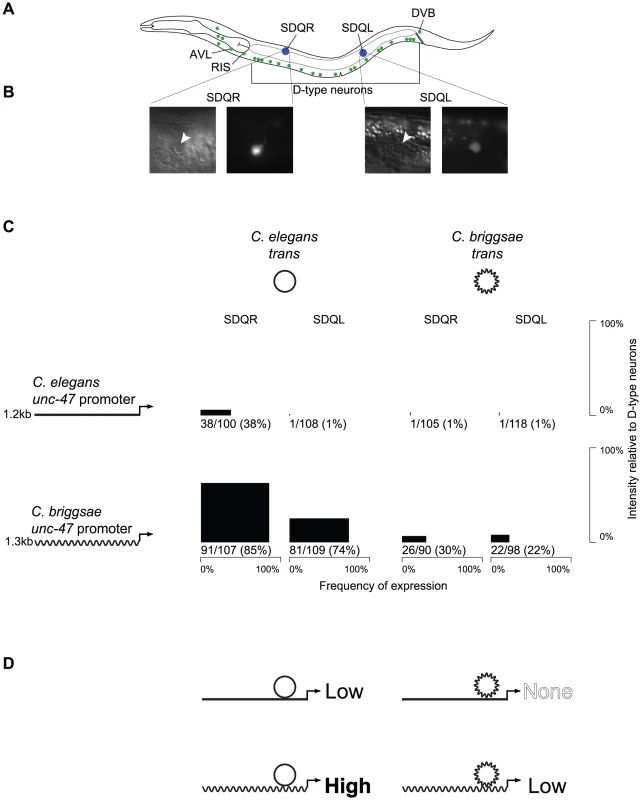
(A) Both C. elegans and C. briggsae promoters fused upstream of GFP in their endogenous trans-regulatory environments drive expression in all 26 GABAergic neurons (green). However, the C. briggsae promoter placed in the C. elegans trans environment additionally drives expression in SDQR and SDQL (blue). (B) Those cells were identified as SDQR/L based on their position and their characteristic projections. (C) For each combination of promoter and trans-regulatory environment, expression in SDQR and SDQL is presented. C. elegans is represented by straight lines, C. briggsae by wavy lines. Frequency of expression is represented by the width, and intensity of expression relative to D-type neurons by the height of black boxes. Number of individuals expressing and total number of individuals scored is indicated underneath. Measurements for independent strains are given in Figure S1. Differences in frequency of expression in SDQR are significant for all comparisons: C. elegans and C. briggsae promoters in C. elegans trans environment (p = 8.1×10−12), C. elegans and C. briggsae promoters in C. briggsae trans environment (p = 1.5×10−8), C. elegans promoter in C. elegans and C. briggsae trans environments (p = 4.8×10−11), C. briggsae promoter in C. elegans and C. briggsae trans environments (p = 2.4×10−14). (D) Interpretation of the results presented in panel C. Both C. elegans and C. briggsae promoters in their endogenous trans environments drive low levels of expression in SDQR and SDQL, while disruption of the endogenous interactions either drives high levels of expression (C. briggsae promoter in C. elegans) or abolishes expression (C. elegans promoter in C. briggsae). To test whether expression conservation results from conserved regulatory mechanisms, or from lineage-specific compensatory evolution, we performed reciprocal transgenic experiments using GFP reporters fused to regulatory elements of C. elegans and C. briggsae unc-47 genes. Although animals of all four possible cis by trans combinations expressed GFP in all GABAergic neurons, there were notable differences between them as well. In addition to expression in all 26 GABAergic neurons, GFP was also sometimes expressed in two other neurons, SDQR and SDQL (Figure 1A). Identity of these cells was definitively established based on their positions and the appearance of their projections (Figure 1B).
Both C. elegans and C. briggsae promoters fused to GFP and introduced as extrachromosomal arrays into their endogenous environments appear to drive very weak expression in SDQR/L (about 20-fold less intense than in GABAergic neurons) in around one-third of individuals (Figure 1C). While it is not thought that unc-47 is endogenously expressed in these non-GABAergic neurons, both promoters drive weak expression in SDQR/L in a minority of animals in a fashion that is consistent across independent strains (Figure S1).
However, when the cis element of one species is expressed in a transgenic host of the other species, expression in SDQR/L is very different. The C. elegans unc-47 promoter is almost never observed to drive expression in transgenic C. briggsae animals. On the other hand, the C. briggsae unc-47 promoter drives strong, consistent expression of GFP in most C. elegans animals carrying the transgene (Figure 1C and Figure S1). We infer that the strong expression in these cells results from mismatched cis-regulatory information of the C. briggsae promoter and trans-regulatory information in C. elegans.
To confirm that the expression differences we observed are due to different activities of the cis-regulatory elements in the different host backgrounds and are not artifacts of the extrachromosomal array method that we used, we performed additional experiments. First, we made sure that the patterns of expression we report are consistent across multiple strains bearing independently generated extrachromosomal arrays (Figure S1). Second, we integrated unc-47::GFP promoter fusions into the genomes of C. elegans and C. briggsae, and verified their expression consistency across multiple independent strains (Figure S2). Third, we utilized MosSCI technology [50], which is available only in C. elegans, to generate single-copy integrants of the C. elegans and C. briggsae unc-47::GFP transgenes into the same genomic locus (Figure S3). Both the direction and approximate magnitude of the expression differences between the four possible combinations of cis and trans are consistent among all of these transgenic methods, and between independent lines generated using the same method (Figure 1C; Figures S1, S2, and S3). These data overwhelmingly support the hypothesis that misexpression in SDQR/L is the result of interactions between divergent C. elegans and C. briggsae cis and trans regulators, and is not an experimental artifact.
In many cases, the effect of combining cis and trans elements from different species leads to misregulation of gene expression [36]. When such divergence occurs while preserving major characteristics of a phenotype (be it a morphological trait [51] or a gene expression pattern [52]), it is called Developmental Systems Drift [24]. Far from being meaningless experimental artifacts, these cases of misexpression reveal evolutionary divergence in regulatory components that would otherwise go undetected due to the conservation of their phenotypic output. This type of divergence [53], which leads to negative epistatic interactions, is evolutionarily significant, as it could create Dobzhansky-Muller Incompatibilities ([31], the genetic interactions that go awry in hybrids and keep species separate from one another [54]. In fact, the pattern that we observe in Figure 1C is reminiscent of the pattern that appears in cases of transgressive segregation [8], [55]–[57], in which hybrid phenotypes exceed parental values for a quantitative trait as a result of interactions between divergent elements in the parental genomes. As has been noted [58], there are compelling observations from both flies [8] and yeast [27] that the cis-trans coevolution often involves changes with the opposite effect on gene expression, perhaps as a result of balancing selection on gene expression favoring the fixation of compensatory mutations.
We therefore propose an explanation (Figure 1D) for our observations that is informed by the rich literature on misexpression of heterologous transgenes and hybrid dysregulation. We hypothesize that the conserved gene expression pattern of unc-47 in C. elegans and C. briggsae is the result of lineage-specific coevolution in which C. elegans balances the effects of a relatively weaker cis-regulatory element and a stronger trans-regulatory environment in SDQR/L to produce the conserved output, while C. briggsae balances a stronger cis-regulatory element with a weaker trans-regulatory environment in these cells. Therefore, in either host (compare down columns of Figure 1C), the C. briggsae cis element always drives stronger expression in SDQR/L than the C. elegans ortholog (Figure 1C; chi-squared test for difference in frequency of expression in SDQR between the two promoters in C. elegans p = 8.1×10−12; in C. briggsae p = 1.5×10−8). The trans environment of a C. elegans host animal always drives stronger expression in these cells than the trans environment of a C. briggsae host (compare across rows of Figure 1C; chi-squared test for difference in frequency of expression in SDQR between the two trans environments of the C. elegans promoter p = 4.8×10−11; of the C. briggsae promoter p = 2.4×10−14). Only when the cis and trans regulators of unc-47 from different species are combined experimentally can their different functions be observed.
A conserved regulatory motif is necessary for expression in SDQR/L and DVB
To identify the coevolved cis and trans regulators, we searched for transcription factors that are expressed in SDQR/L. The gene ahr-1, which encodes a bHLH transcription factor [59], is expressed in a number of neurons including SDQR/L [60]. It is known to regulate the fate of some GABAergic [61] as well as other [62] neurons. In C. elegans ahr-1 (ia03) mutants, expression of a C. briggsae unc-47 promoter in SDQR/L was completely abolished (Figure 2A), even though the cells were still present (Figure 2B and [62]). This experiment demonstrates that ahr-1 is necessary for expression in the cells in which ectopic expression is observed. To test whether ahr-1 is also the site of trans-regulatory divergence, we conducted several experiments. Our results find expression differences (via qRT-PCR and transgenic expression assays, data not shown) and coding sequence differences between the species that imply, but do not prove, that the function of ahr-1 has diverged between C. elegans and C. briggsae and could affect unc-47 regulation.
Fig. 2. Expression of the C. briggsae unc-47::GFP transgene in SDQR/L requires AHR-1. 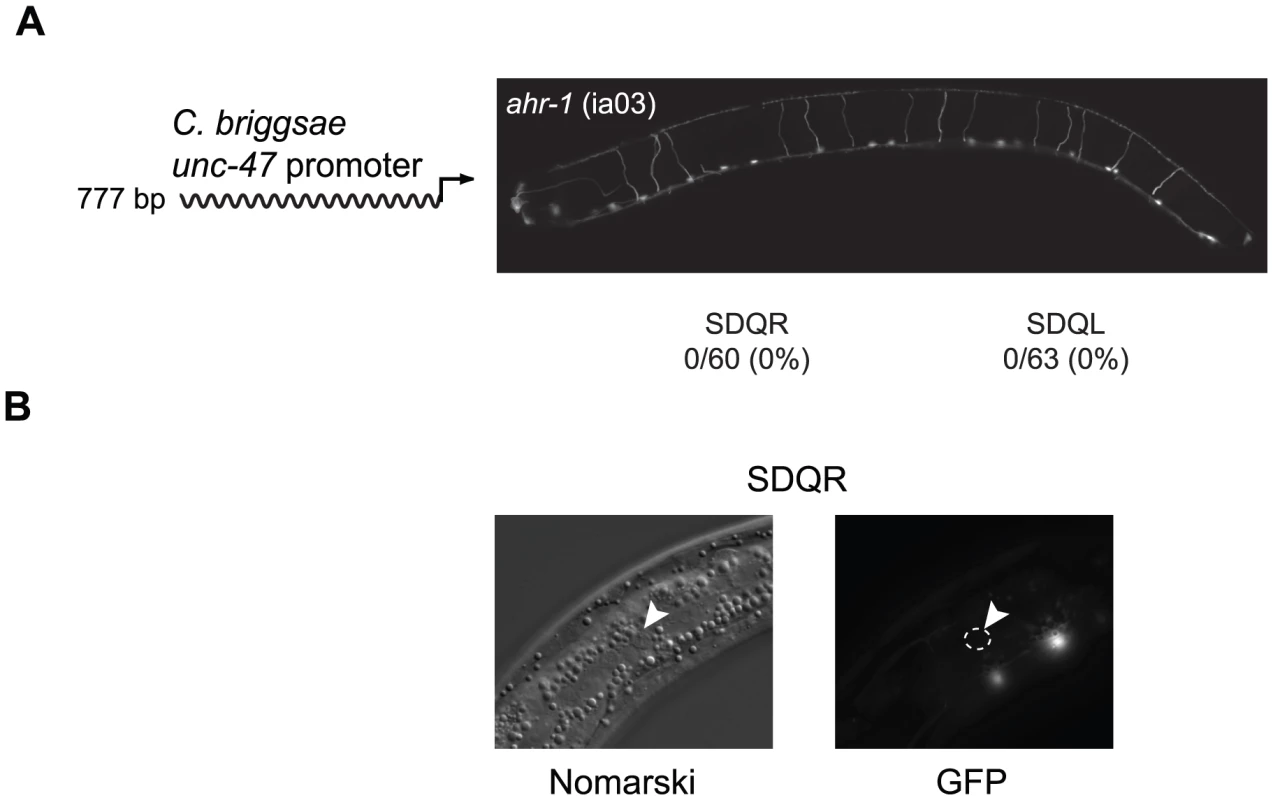
(A) In an ahr-1 loss-of-function mutant (ia03), GFP is not expressed in SDQR/L. Number of individuals expressing and total number of individuals scored is indicated. Expression of the 777 bp promoter in WT background is shown in Figure 3A. (B) SDQR/L are indeed present in the ahr-1 (ia03) mutant as can be seen by Nomarski, but are not expressing GFP. SDQR is shown. However, trans-regulatory differences are more difficult to identify than cis-regulatory differences. A causal cis-regulatory change must be located within the DNA that has divergent function (in this case, ∼1.3 kb of DNA upstream of unc-47). On the other hand, evidence for change in trans potentially implicates the entire genome. The causal nucleotide changes between C. elegans and C. briggsae could potentially reside in coding or regulatory sequence of ahr-1, an upstream regulator of ahr-1, a binding partner or antagonist of ahr-1, or in multiple interacting loci. However, we can use the ahr-1 clue to dissect the mechanism of cis-regulatory divergence.
The core binding sequence of AHR-1 has been experimentally defined as CACGC [63] or CACGCA [59]. There is a single occurrence of such a motif in a conserved block of sequence within the proximal promoter of unc-47 (Figure S4). Whereas a C. briggsae promoter with the AHR-1 core consensus site intact drove strong and consistent expression in C. elegans SDQR/L (Figure 3A and Table S1), a mutation of this putative binding site completely abrogated expression in these two, but not other neurons (Figure 3B and Table S1). Based on this evidence we concluded that the SDQR/L expression of the C. briggsae unc-47 promoter is regulated through this site. But does it have a regulatory function with respect to expression in GABAergic neurons?
Fig. 3. Expression in SDQR/L is mediated by a conserved motif, which also controls expression in DVB. 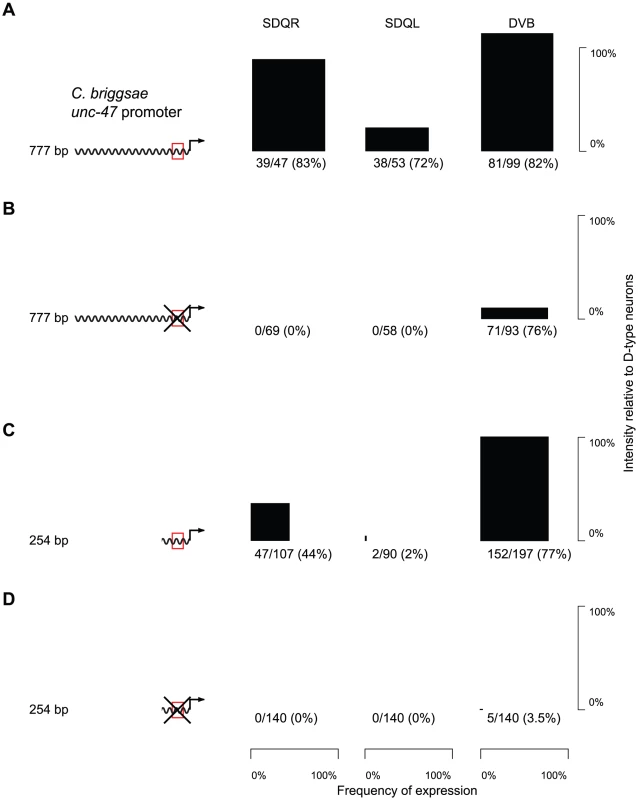
(A–D) GFP expression in SDQR, SDQL and DVB driven by cis elements, with an intact (red box; A, C) or mutated (crossed red box; B, D) AHR-1 core consensus motif. Frequency of expression is represented by the width, and intensity of expression relative to D-type neurons by the height of black boxes. Number of individuals expressing and total number of individuals scored is indicated underneath. The difference between distributions of DVB intensity in panels A and B is highly significant (p<2.2×10−16). Counts for multiple independent strains are given in Table S1. Worms carrying the mutated promoter showed significantly less intense expression in DVB (compare Figure 3A and 3B, Kolmogorov-Smirnov test p<2.2×10−16). We interpreted this to mean that endogenous expression of unc-47 in DVB is controlled via this motif as well as one or more additional sequences. The distal promoters of unc-47 are highly divergent in their sequences and contribute to the robustness of expression in DVB [22]. We excluded them as candidates for the sequence that directs expression in SDQR/L and DVB by examining expression of the proximal promoter alone. The proximal promoter with the intact motif drove expression in SDQR/L and DVB similarly to the full-length promoter, albeit less intensely (Figure 3C and Table S1). In contrast, the mutated proximal promoter showed essentially no SDQR/L or DVB expression (Figure 3D and Table S1). The proximal promoter must therefore be the site of cis-regulatory change that maintains expression in DVB via the conserved core consensus motif and has pleiotropic effects on expression in SDQR/L when the promoters are swapped between species.
Extensive epistasis within the proximal promoter of unc-47
The AHR-1 core consensus motif is conserved between C. elegans and C. briggsae, so it is clearly not the site of cis-regulatory divergence. Because nucleotides flanking transcription factor binding sites can substantially contribute to affinity and specificity of binding [64], [65], we next concentrated on sequences in the vicinity of this motif. Differences between C. elegans and C. briggsae in this region are particularly good candidates to mediate functional divergence. We designated as “Region A” approximately 30 bp containing two divergent sequences interrupted by 12 conserved nucleotides (Figure 4A). Because regulatory sequence divergence can be buffered [66], we tested the effects of Region A divergence experimentally.
Fig. 4. Lineage-specific coevolution within unc-47 promoters. 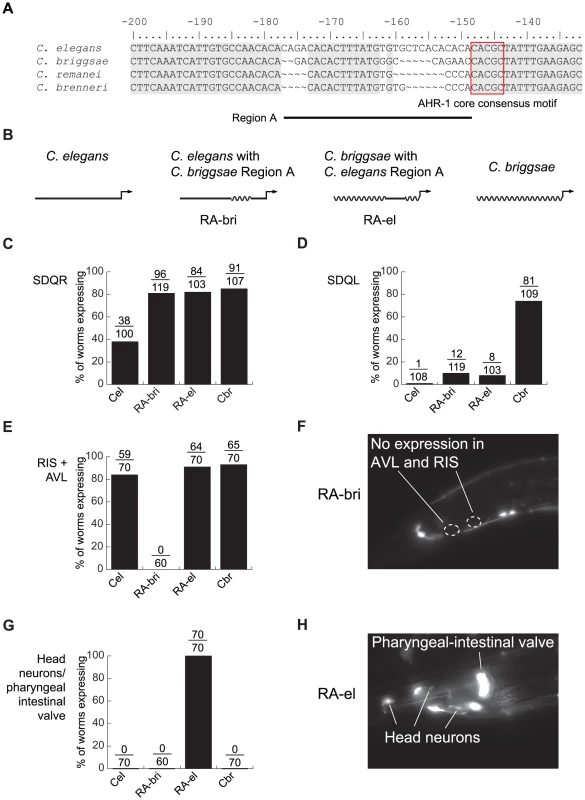
(A) An alignment of the sequences flanking the conserved AHR-1 core consensus motif (boxed in red). Regions conserved between all four species are shaded in gray. (B) Schematic representation of C. elegans and C. briggsae promoters and chimeric transgenes reciprocally exchanging Region A. (C–H) Expression driven by the four transgenes. Percentage of worms expressing GFP in (C) SDQR, (D) SDQL, (E) AVL and RIS, (G) head neurons and pharyngeal-intestinal valve. Representative pictures of (F) an individual carrying the RA-bri transgene, showing absence of expression in AVL and RIS, and (H) an individual carrying the RA-el transgene, showing strong expression in non-GABAergic head neurons and the pharyngeal-intestinal valve. Chimeric cis-regulatory elements that combine segments from orthologous promoters are powerful tools for detecting lineage-specific divergence that is difficult to reveal by other approaches [42], [67]. To test whether sequences within Region A have functionally diverged between C. elegans and C. briggsae, we generated reciprocal chimeric transgenes containing either most of the C. elegans promoter with Region A of C. briggsae (RA-bri) or vice versa (RA-el, Figure 4B; exact sequences shown in Figure S5). Because Region A is flanked by extended blocks of conservation, we can be sure that the swapped DNA in these chimeric promoters is indeed orthologous. We compared expression patterns of chimeric promoters to those of the intact full-length promoters from C. elegans and C. briggsae.
In SDQR, expression from both chimeric promoters was similar to that of the C. briggsae promoter (Figure 4C), suggesting that the C. briggsae promoter contains at least two elements that control expression in this cell—one in Region A and one outside of it. In SDQL, both chimeric transgenes drove expression similar to that of the C. elegans, not the C. briggsae, promoter (Figure 4D). This suggests that the C. briggsae-like expression is a consequence of a synergistic epistasis between two elements—one inside Region A, another outside of it. All functional differences between Regions A of C. elegans and C. briggsae reside in a shorter, approximately 15 bp region immediately upstream of the conserved motif (Figure S5).
Our results also suggest that the endogenous function of Region A is to control aspects of the conserved GABAergic expression of unc-47. Because both chimeric promoters directed expression different from this conserved pattern, we inferred that the promoters experienced lineage-specific cis-cis coevolution. Specifically, both C. elegans and C. briggsae promoters drove strong and consistent expression in RIS and AVL, two GABAergic neurons located near the posterior bulb of the pharynx (Figure 1A). RA-bri showed no detectable expression in RIS and very little in AVL (Figure 4E and 4F, and Figure S5), suggesting that a lineage-specific interaction between an element in Region A and another one outside of it is disrupted in this chimera. Disruption of a similar interaction in RA-el caused aberrant but strong expression in a group of 8–10 non-GABAergic head neurons and in the pharyngeal-intestinal valve, a non-neuronal cell type (Figure 4G and 4H, and Figure S5). No intact promoters drove expression in these cells.
Epistasis between cis-regulatory sites, such as we found in the unc-47 promoter, is not unprecedented. Intra-molecular epistatic interactions and evidence of coevolution have been observed in cis-regulatory elements [68] and proteins [69], [70]; they may have arisen via compensatory, pseudocompensatory, or other processes [71], [72]. Next, we sought to identify when these epistatic interactions evolved.
Functional divergence of unc-47 regulation occurred along the C. briggsae lineage
To determine when in the evolutionary history of these nematodes the C. briggsae-like function of the proximal promoter arose, we compared the function of the unc-47 promoter from two additional species, C. brenneri and C. remanei. These unc-47 promoters do not drive much expression in SDQR/L in transgenic C. elegans (Figure 5A–5D), meaning that the functional evolution we observed occurred specifically in the C. briggsae lineage. Is this functional divergence reflected in the evolution of the promoter's sequence?
Fig. 5. The C. briggsae unc-47 promoter has experienced lineage-specific sequence evolution and functional divergence. 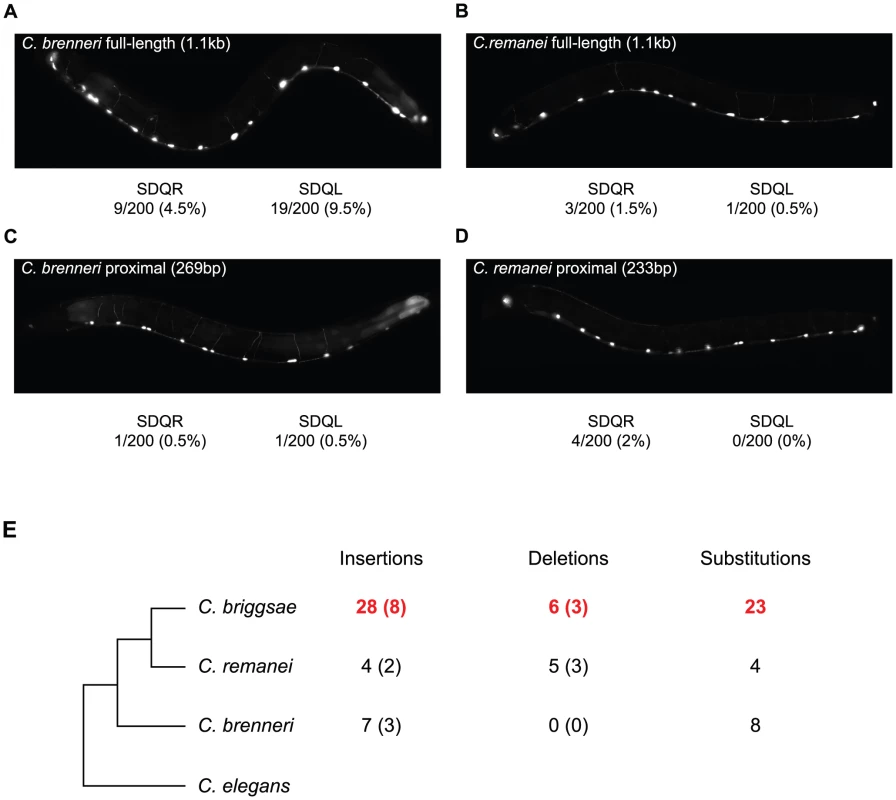
(A–D) Fluorescence images of C. elegans individuals carrying (A, C) C. brenneri, (B, D) C. remanei, (A, B) full-length, and (C, D) proximal promoter-GFP fusion transgenes. The percentage of individuals expressing GFP is given. In all cases when expression was visible it was weak compared to the GABAergic neurons. (E) The number of nucleotides inferred to be lineage-specific changes in the proximal promoter of unc-47. The number of indel events are shown in parentheses. Sites conserved between two species but divergent in the third were counted as lineage-specific. Details are shown in Figure S6. We compared the unc-47 proximal promoters in a phylogenetic context that includes the two additional species (Figure 5E) in order to assign changes in the promoter sequence to a particular lineage. We observed an excess of insertions and substitutions on the branch leading to C. briggsae (Figure 5E and Figure S6). This pattern is particularly striking in a region of ∼160 bp in which one fifth of C. briggsae positions are derived (Figure S6), while C. brenneri and C. remanei do not show any lineage-specific differences. Accelerated rate of sequence change is restricted to the promoter; the rates of divergence in the protein-coding portion of the gene are the same for all species. Compared to the 487aa C. elegans protein sequence, C. briggsae UNC-47 differs at 51 positions, C. remanei at 47, and C. brenneri at 50. Rates of nonsynonymous substitutions were also similar when C. elegans sequence was compared to the other three orthologs (Ka = 0.07, 0.07, 0.06, respectively). Whereas cis element evolutionary rate accelerations associated with phenotypic change are well-documented [68], [73], [74], in this case no overt phenotypic divergence seems to be linked to the acceleration of regulatory sequence evolution.
Discussion
Comparative functional and sequence data yielded a picture of evolution of unc-47 regulation in Caenorhabditis nematodes. Although the endogenous patterns of expression remain unchanged, the mechanisms responsible for maintaining them seem to have diverged in the C. briggsae lineage, possibly as a consequence of substantial divergence in the regulatory sequence. Because the C. briggsae promoter does not drive strong expression in SDQR/L in its endogenous trans environment despite its dramatic sequence evolution, a compensatory trans change must be inferred.
A simple model suggests the types of coevolutionary changes that were involved (Figure 1D). In their endogenous trans-regulatory environments, both C. elegans and C. briggsae promoters of unc-47 drive similar weak expression in SDQR and SDQL. This similarity cannot be due to conservation of the underlying regulatory system, given the difference in expression patterns of reciprocally swapped promoters. The C. elegans promoter directed virtually no expression in SDQR/L of C. briggsae, while the C. briggsae promoter was strongly expressed in these cells when placed in C. elegans. At least two lineage-specific changes must have occurred since the divergence of C. elegans and C. briggsae: one in the unc-47 promoter and another, possibly in a transcription factor that controls its expression. Similar cases have been documented in yeast [75] and animals [68], [76], [77].
The considerable pleiotropy (Figure 3) and epistasis (Figure 4) in the cis-regulatory elements of unc-47 revealed that the same sequences responsible for misexpression of the C. briggsae promoter in C. elegans also control expression in other cells, such as AVL, RIS, and DVB (Figure 3, Figure 4, Figure S5). Their pleiotropic effects, detectable only in our experimental paradigm, were to drive different levels of expression in SDQR/L in the C. elegans trans background. Ectopic expression that is mediated by the same regulatory elements that control endogenous expression has been reported before [78]. Far from being experimental artifacts, differences between heterologous transgene expression and endogenous expression reveal coevolution between interacting components of the regulatory machinery [36]. Simulations show that selection on one trait can affect the genetic basis of traits that share common regulation [79]. Our results highlight the utility of reciprocal transgenics in uncovering the likely ubiquitous coevolution between components of gene regulatory systems, underlying both divergent and apparently conserved traits. Because divergence between orthologous cis-regulatory elements is likely to be subtle [80], [81], detailed, focused, single-gene analyses will be required to understand this process.
Our findings contribute to a growing appreciation of the importance of cis-trans coevolution [8], [75], [82]–[85]. One manifestation of coevolution is promoter restructuring [86], [87] that is evident in functional comparisons of orthologous cis-regulatory elements [2], [67], [80], [88], [89]. Expression of C. elegans and C. briggsae promoters in heterologous trans environments showed differences (Figure 1C), implying that coevolved changes underlie their conserved endogenous patterns. Those expression differences resemble transgressive segregation [27], [30], [56], [90]–[92], which is observed for a considerable fraction of genes [55], and is commonly explained by antagonistic epistasis [90].
The importance of epistatic interactions in evolution is well established [93]. Epistasis has been documented not only between unlinked loci, but also within genes. Recent experimental data indicate that complex epistatic interactions between amino acid substitutions within proteins have played an important role in shaping protein evolution [69], [70], [94], [95], particularly by constraining the order of mutations [96], [97]. Because transcription involves orchestrated interactions of different molecules, epistasis is likely to be an important force in evolution of gene regulation [87]. This view is supported by theoretical considerations [98], [99] and empirical data [2], [67], [100]–[102]. Reciprocal swaps of Region A between C. elegans and C. briggsae (Figure 4) suggest that epistasis within cis-regulatory elements operates even on the scale of a few nucleotides. Redundancy in cis-regulatory architecture (Figure 3) may play a prominent role in mediating epistatic interactions [103], [104] perhaps by providing a permissive environment in which multiple compensatory changes can take place [84], [105]. While in some instances sequence turnover may be functionally silent, experimental [106] and theoretical [107] results suggest that this process can seed regulatory elements with novel interactions and lead to the origin of new expression patterns and potentially to adaptation.
We found remarkable acceleration of sequence divergence in the C. briggsae promoter of unc-47 that is concomitant with functional divergence (Figure 5 and Figure S6). Regular turnover of binding sites would be expected to lead to a clock-like evolution of regulatory sequences [108]. Instead, the pattern of accelerated sequence divergence resembles that seen in regulatory elements under strong artificial selection [109]. Whether the divergence in the C. briggsae promoter was adaptive, and what sort of selection pressure it might have been responding to, is not clear. Adaptive evolution in non-coding intergenic sequences may be more common than was previously thought [110].
Our results stress why functional tests are essential for meaningful comparisons between orthologous cis-regulatory elements. Accelerated lineage-specific evolution of regulatory sequences has been interpreted as evidence that divergent loci encode traits unique to a given species [111]–[113]. Not only did the sequence of the C. briggsae unc-47 promoter experience accelerated lineage-specific evolution, but when we tested it in C. elegans, it directed intense and consistent expression in SDQR/L. This could have suggested that the pattern of unc-47 had diverged between the two species, possibly reflecting a morphological or physiological adaptation. Analysis of reciprocal transgenics, however, showed that the expression pattern of unc-47 has been conserved in Caenorhabditis nematodes, and the accelerated divergence of the C. briggsae cis-regulatory element was compensated by changes in its trans-regulatory environment. It is therefore possible that at least some regions of accelerated sequence evolution are sites of cis-trans coevolution that do not correspond to phenotypic divergence.
Conserved expression patterns can be maintained between two species by bursts of lineage-specific coevolution in the components of regulatory pathways. These lineage-specific changes can be revealed when they are swapped out of the context in which they evolved. We have found that the relevant context of interacting molecules, as judged by the extent of coevolution we can detect, extends from the trans-regulatory milieu of a cell down to neighboring base pairs of DNA. Sequence change and functional change are no doubt related, but one should not be inferred on the basis of the other alone. Widespread conservation of gene expression patterns may conceal many instances of gene regulatory evolution.
Materials and Methods
Transgenes and strains
To generate reporter transgenes, promoter sequences were PCR amplified from genomic DNA and cloned upstream of GFP into the Fire lab vector pPD95.75. In all cases, the start codon of the unc-47 ortholog was included in the fusion. Prior to injection, all transgenes were sequenced to ensure accuracy. We injected a mixture (5 ng/µL promoter::GFP plasmid, 5 ng/µL pha-1 rescue transgene, 100 ng/µL salmon sperm DNA) into temperature-sensitive C. elegans pha-1 (e2123) strain [114]. Transformants were selected at 25°C. The C. briggsae strains carrying extrachromosomal arrays were produced by injecting a mixture (5 ng/µL promoter::GFP plasmid, 5 ng/µL Cbr-unc-119 rescue plasmid and 100 ng/µL salmon sperm DNA) into YR91 Cbr-unc-119 (nm67) strain. To examine the function of transcription factor ahr-1 we used ahr-1 (ia03), a loss-of-function allele [62]. Extrachromosomal arrays were integrated by UV integration [115]. The C. briggsae unc-47 promoter fusion was integrated into the YR91 strain of C. briggsae through bombardment [115]. MosSCI single copy integrated strains were generated following an established protocol [50].
Microscopy
Mixed-stage populations of C. elegans carrying transgenes were grown with abundant food and L4-stage worms were selected. These were immobilized on agar slides with 10 mM sodium azide in M9 buffer. The slides were examined on a Leica DM5000B compound microscope under 400-fold magnification. Presence/absence of GFP expression in a cell was recorded only if the cell was clearly visible, unobstructed by the intestine. Worms without any visible GFP expression were assumed to have lost the transgene. Fluorescence measurements were carried out as previously described [22]. Each photograph showing worms in figures is composed of several images of the same individual capturing anterior, middle, and posterior sections.
Site-directed mutagenesis
Two types of mutagenized promoters were generated using the QuickChangeII kit (Stratagene). To test the role of the AHR-1 core consensus motif in regulating expression in SDQR/L, we introduced two point mutations in the conserved AHR-1 consensus motif [59], changing the sequence from AACCACGCTATT to AACAACTCTATT (putative binding site underlined). To test the roles of the nonconserved regions upstream of the conserved motif, we swapped these sequences between the C. elegans and C. briggsae unc-47 promoters via a two-step site-directed mutagenesis.
Supporting Information
Zdroje
1. HareEE, PetersonBK, IyerVN, MeierR, EisenMB (2008) Sepsid even-skipped enhancers are functionally conserved in Drosophila despite lack of sequence conservation. PLoS Genet 4: e1000106 doi:10.1371/journal.pgen.1000106.
2. SwansonCI, EvansNC, BaroloS (2010) Structural rules and complex regulatory circuitry constrain expression of a Notch - and EGFR-regulated eye enhancer. Developmental Cell 18 : 359–370.
3. LudwigMZ, PatelNH, KreitmanM (1998) Functional analysis of eve stripe 2 enhancer evolution in Drosophila: Rules governing conservation and change. Development 125 : 949–958.
4. DonigerSW, FayJC (2007) Frequent gain and loss of functional transcription factor binding sites. PLoS Comput Biol 3: e99 doi:10.1371/journal.pcbi.0030099.
5. HeBZ, HollowayAK, MaerklSJ, KreitmanM (2011) Does positive selection drive transcription factor binding site turnover? A test with Drosophila cis-regulatory modules. PLoS Genet 7: e1002053 doi:10.1371/journal.pgen.1002053.
6. ShawPJ, WrattenNS, McGregorAP, DoverGA (2002) Coevolution in bicoid-dependent promoters and the inception of regulatory incompatibilities among species of higher Diptera. Evol Dev 4 : 265–277.
7. BakerCR, TuchBB, JohnsonAD (2011) Extensive DNA-binding specificity divergence of a conserved transcription regulator. Proc Natl Acad Sci U S A 108 : 7493–7498.
8. LandryCR, WittkoppPJ, TaubesCH, RanzJM, ClarkAG, et al. (2005) Compensatory cis-trans evolution and the dysregulation of gene expression in interspecific hybrids of Drosophila. Genetics 171 : 1813–1822.
9. TsankovAM, ThompsonDA, SochaA, RegevA, RandoOJ (2010) The role of nucleosome positioning in the evolution of gene regulation. PLoS Biol 8: e1000414 doi:10.1371/journal.pbio.1000414.
10. HittingerCT, GoncalvesP, SampaioJP, DoverJ, JohnstonM, et al. (2010) Remarkably ancient balanced polymorphisms in a multi-locus gene network. Nature 464 : 54–58.
11. GerkeJ, LorenzK, CohenB (2009) Genetic interactions between transcription factors cause natural variation in yeast. Science 323 : 498–501.
12. GaschAP, MosesAM, ChiangDY, FraserHB, BerardiniM, et al. (2004) Conservation and evolution of cis-regulatory systems in ascomycete fungi. PLoS Biol 2: e398 doi:10.1371/journal.pbio.0020398.
13. HittingerCT, CarrollSB (2007) Gene duplication and the adaptive evolution of a classic genetic switch. Nature 449 : 677–681.
14. TuchBB, GalgoczyDJ, HerndayAD, LiH, JohnsonAD (2008) The evolution of combinatorial gene regulation in fungi. PLoS Biol 6: e38 doi:10.1371/journal.pbio.0060038.
15. WangXD, ChamberlinHM (2004) Evolutionary innovation of the excretory system in Caenorhabditis elegans. Nat Genet 36 : 231–232.
16. SungH, WangT, WangD, HuangY, WuJ, et al. (2009) Roles of trans and cis variation in yeast intraspecies evolution of gene expression. Mol Biol Evol 26 : 2533–2538.
17. MarcelliniS, SimpsonP (2006) Two or four bristles: Functional evolution of an enhancer of scute in Drosophilidae. PLoS Biol 4: e386 doi:10.1371/journal.pbio.0040386.
18. EmersonJJ, HsiehL, SungH, WangT, HuangC, et al. (2010) Natural selection on cis and trans regulation in yeasts. Genome Res 20 : 826–836.
19. GibsonG, Riley-BergerR, HarshmanL, KoppA, VachaS, et al. (2004) Extensive sex-specific nonadditivity of gene expression in Drosophila melanogaster. Genetics 167 : 1791–1799.
20. ChanET, QuonGT, ChuaG, BabakT, TrochessetM, et al. (2009) Conservation of core gene expression in vertebrate tissues. Journal of Biology 8 : 33–33.
21. AparicioS, MorrisonA, GouldA, GilthorpeJ, ChaudhuriC, et al. (1995) Detecting conserved regulatory elements with the model genome of the Japanese puffer fish, Fugu rubripes. Proc Natl Acad Sci U S A 92 : 1684–1688.
22. BarriereA, GordonKL, RuvinskyI (2011) Distinct functional constraints partition sequence conservation in a cis-regulatory element. PLoS Genet 7: e1002095 doi:10.1371/journal.pgen.1002095.
23. DoverGA, FlavellRB (1984) Molecular coevolution: DNA divergence and the maintenance of function. Cell 38 : 622–623.
24. TrueJR, HaagES (2001) Developmental system drift and flexibility in evolutionary trajectories. Evol Dev 3 : 109–119.
25. TsongAE, TuchBB, LiH, JohnsonAD (2006) Evolution of alternative transcriptional circuits with identical logic. Nature 443 : 415–420.
26. ChangY, LiuFR, YuN, SungH, YangP, et al. (2008) Roles of cis - and trans-changes in the regulatory evolution of genes in the gluconeogenic pathway in yeast. Mol Biol Evol 25 : 1863–1875.
27. TiroshI, ReikhavS, LevyAA, BarkaiN (2009) A yeast hybrid provides insight into the evolution of gene expression regulation. Science 324 : 659–662.
28. WittkoppPJ, HaerumBK, ClarkAG (2008) Regulatory changes underlying expression differences within and between Drosophila species. Nat Genet 40 : 346–350.
29. McManusCJ, CoolonJD, DuffMO, Eipper-MainsJ, GraveleyBR, et al. (2010) Regulatory divergence in Drosophila revealed by mRNA-seq. Genome Res 20 : 816–825.
30. GrazeRM, McIntyreLM, MainBJ, WayneML, NuzhdinSV (2009) Regulatory divergence in Drosophila melanogaster and D. simulans, a genomewide analysis of allele-specific expression. Genetics 183 : 547–561.
31. Ortiz-BarrientosD, CountermanBA, NoorMAF (2007) Gene expression divergence and the origin of hybrid dysfunctions. Genetica 129 : 71–81.
32. SkellyDA, RonaldJ, AkeyJM (2009) Inherited variation in gene expression. Annual Review of Genomics and Human Genetics 10 : 313–332.
33. StrangerBE, ForrestMS, ClarkAG, MinichielloMJ, DeutschS, et al. (2005) Genome-wide associations of gene expression variation in humans. PLoS Genet 1: e78 doi:10.1371/journal.pgen.0010078.
34. TishkoffSA, ReedFA, RanciaroA, VoightBF, BabbittCC, et al. (2007) Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet 39 : 31–40.
35. BeallCM, CavalleriGL, DengL, ElstonRC, GaoY, et al. (2010) Natural selection on EPAS1 (HIF2 alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A 107 : 11459–11464.
36. GordonKL, RuvinskyI (2012) Tempo and mode in evolution of transcriptional regulation. PLoS Genet 8: e1002432 doi:10.1371/journal.pgen.1002432.
37. GilleardJS, BarryJD, JohnstoneIL (1997) Cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol Cell Biol 17 : 2301–2311.
38. KennedyBP, AamodtEJ, AllenFL, ChungMA, HeschlMFP, et al. (1993) The gut esterase gene (ges-1) from the nematodes Caenorhabditis elegans and Caenorhabditis briggsae. J Mol Biol 229 : 890–908.
39. KirouacM, SternbergPW (2003) Cis-regulatory control of three cell fate-specific genes in vulval organogenesis of Caenorhabditis elegans and C. briggsae. Dev Biol 257 : 85–103.
40. RomanoLA, WrayGA (2003) Conservation of Endo16 expression in sea urchins despite evolutionary divergence in both cis and trans-acting components of transcriptional regulation. Development 130 : 4187–4199.
41. WenickAS, HobertO (2004) Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Developmental Cell 6 : 757–770.
42. WangXD, ChamberlinHM (2002) Multiple regulatory changes contribute to the evolution of the Caenorhabditis lin-48 ovo gene. Genes Dev 16 : 2345–2349.
43. WittkoppPJ, VaccaroK, CarrollSB (2002) Evolution of yellow gene regulation and pigmentation in Drosophila. Current Biology 12 : 1547–1556.
44. KiontkeK, GavinNP, RaynesY, RoehrigC, PianoF, et al. (2004) Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc Natl Acad Sci U S A 101 : 9003–9008.
45. RuvinskyI, RuvkunG (2003) Functional tests of enhancer conservation between distantly related species. Development 130 : 5133–5142.
46. McintireSL, JorgensenE, KaplanJ, HorvitzHR (1993) The GABAergic nervous-system of Caenorhabditis elegans. Nature 364 : 337–341.
47. GuastellaJ, JohnsonCD, StrettonAOW (1991) GABA-immunoreactive neurons in the nematode Ascaris. J Comp Neurol 307 : 584–597.
48. McIntireSL, ReimerRJ, SchuskeK, EdwardsRH, JorgensenEM (1997) Identification and characterization of the vesicular GABA transporter. Nature 389 : 870–876.
49. ZhaoZ, BoyleTJ, BaoZ, MurrayJI, MericleB, et al. (2008) Comparative analysis of embryonic cell lineage between Caenorhabditis briggsae and Caenorhabditis elegans. Dev Biol 314 : 93–99.
50. Frokjaer-JensenC, DavisMW, HopkinsCE, NewmanBJ, ThummelJM, et al. (2008) Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet 40 : 1375–1383.
51. WangX, SommerRJ (2011) Antagonism of LIN-17/Frizzled and LIN-18/Ryk in nematode vulva induction reveals evolutionary alterations in core developmental pathways. PLoS Biol 9: e1001110 doi:10.1371/journal.pbio.1001110.
52. SwansonCI, SchwimmerDB, BaroloS (2011) Rapid evolutionary rewiring of a structurally constrained eye enhancer. Current Biology 21 : 1186–1196.
53. MaheshwariS, BarbashDA (2012) Cis-by-trans regulatory divergence causes the asymmetric lethal effects of an ancestral hybrid incompatibility gene. PLoS Genet 8: e1002597 doi:10.1371/journal.pgen.1002597.
54. OrrHA, TurelliM (2001) The evolution of postzygotic isolation: Accumulating Dobzhansky-Muller Incompatibilities. Evolution 55 : 1085–1094.
55. BremRB, KruglyakL (2005) The landscape of genetic complexity across 5,700 gene expression traits in yeast. Proc Natl Acad Sci U S A 102 : 1572–1577.
56. RanzJM, NamgyalK, GibsonG, HartlDL (2004) Anomalies in the expression profile of interspecific hybrids of Drosophila melanogaster and Drosophila simulans. Genome Res 14 : 373–379.
57. RiesebergLH, WidmerA, ArntzAM, BurkeJM (2003) The genetic architecture necessary for transgressive segregation is common in both natural and domesticated populations. Phil Trans R Soc B 358 : 1141–1147.
58. EmersonJJ, LiW (2010) The genetic basis of evolutionary change in gene expression levels. Phil Trans R Soc B 365 : 2581–2590.
59. Powell-CoffmanJA, BradfieldCA, WoodWB (1998) Caenorhabditis elegans orthologs of the aryl hydrocarbon receptor and its heterodimerization partner the aryl hydrocarbon receptor nuclear translocator. Proc Natl Acad Sci U S A 95 : 2844–2849.
60. QinH, ZhaiZ, Powell-CoffmanJA (2006) The Caenorhabditis elegans AHR-1 transcription complex controls expression of soluble guanylate cyclase genes in the URX neurons and regulates aggregation behavior. Dev Biol 298 : 606–615.
61. HuangX, Powell-CoffmanJA, JinYS (2004) The AHR-1 aryl hydrocarbon receptor and its co-factor the AHA-1 aryl hydrocarbon receptor nuclear translocator specify GABAergic neuron cell fate in C. elegans. Development 131 : 819–828.
62. QinHT, Powell-CoffmanJA (2004) The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev Biol 270 : 64–75.
63. LusskaA, ShenE, WhitlockJP (1993) Protein-DNA interactions at a dioxin-responsive enhancer. Analysis of 6 bona fide DNA-binding sites for the liganded Ah receptor. J Biol Chem 268 : 6575–6580.
64. GroveCA, De MasiF, BarrasaMI, NewburgerDE, AlkemaMJ, et al. (2009) A multiparameter network reveals extensive divergence between C. elegans bHLH transcription factors. Cell 138 : 314–327.
65. WalhoutAJM, van der VlietPC, TimmersHTM (1998) Sequences flanking the E-box contribute to cooperative binding by c-Myc/Max heterodimers to adjacent binding sites. Biochimica Et Biophysica Acta 139 : 189–201.
66. MauranoMT, WangH, KutyavinT, StamatoyannopoulosJA (2012) Widespread site-dependent buffering of human regulatory polymorphism. PLoS Genet 8: e1002599 doi:10.1371/journal.pgen.1002599.
67. LudwigMZ, BergmanC, PatelNH, KreitmanM (2000) Evidence for stabilizing selection in a eukaryotic enhancer element. Nature 403 : 564–567.
68. EmeraD, WagnerGP (2012) Transformation of a transposon into a derived prolactin promoter with function during human pregnancy. Proc Natl Acad Sci U S A 109 : 11246–11251.
69. KryazhimskiyS, DushoffJ, BazykinGA, PlotkinJB (2011) Prevalence of epistasis in the evolution of influenza A surface proteins. PLoS Genet 7: e1001301 doi:10.1371/journal.pgen.1001301.
70. OrtlundEA, BridghamJT, RedinboMR, ThorntonJW (2007) Crystal structure of an ancient protein: Evolution by conformational epistasis. Science 317 : 1544–1548.
71. HaagES (2007) Compensatory vs. pseudocompensatory evolution in molecular and developmental interactions. Genetica 129 : 45–55.
72. BullaugheyK (2012) Multidimensional adaptive evolution of a feed forward network and the illusion of compensation. Evolution In press.
73. RockmanMV, HahnMW, SoranzoN, ZimprichF, GoldsteinDB, et al. (2005) Ancient and recent positive selection transformed opioid cis-regulation in humans. PLoS Biol 3: e387 doi:10.1371/journal.pbio.0030387.
74. FrankelN, ErezyilmazD, McGregorAP, WangS, PayreF, et al. (2011) Morphological evolution caused by many subtle-effect substitutions in regulatory DNA. Nature 474 : 598–603.
75. KuoD, LiconK, BandyopadhyayS, ChuangR, LuoC, et al. (2010) Coevolution within a transcriptional network by compensatory trans and cis mutations. Genome Res 20 : 1672–1678.
76. WangXD, GreenbergJF, ChamberlinHM (2004) Evolution of regulatory elements producing a conserved gene expression pattern in Caenorhabditis. Evol Dev 6 : 237–245.
77. LynchVJ, TanzerA, WangY, LeungFC, GellersenB, et al. (2008) Adaptive changes in the transcription factor HoxA-11 are essential for the evolution of pregnancy in mammals. Proc Natl Acad Sci U S A 105 : 14928–14933.
78. BonnetonF, ShawPJ, FazakerleyC, ShiM, DoverGA (1997) Comparison of bicoid-dependent regulation of hunchback between Musca domestica and Drosophila melanogaster. Mech Dev 66 : 143–156.
79. JohnsonNA, PorterAH (2007) Evolution of branched regulatory genetic pathways: Directional selection on pleiotropic loci accelerates Developmental System Drift. Genetica 129 : 57–70.
80. LudwigMZ, PalssonA, AlekseevaE, BergmanCM, NathanJ, et al. (2005) Functional evolution of a cis-regulatory module. PLoS Biol 3: e93 doi:10.1371/journal.pbio.0030093.
81. CrockerJ, TamoriY, ErivesA (2008) Evolution acts on enhancer organization to fine-tune gradient threshold readouts. PLoS Biol 6: e263 doi:10.1371/journal.pbio.0060263.
82. ZillOA, ScannellD, TeytelmanL, RineJ (2010) Co-evolution of transcriptional silencing proteins and the DNA elements specifying their assembly. PLoS Biol 8: e1000550 doi:10.1371/journal.pbio.1000550.
83. XiaoW, PelcherLE, RankGH (1991) Evidence for cis-acting and trans-acting element coevolution of the 2-microns circle genome in Saccharomyces cerevisiae. J Mol Evol 32 : 145–152.
84. HershBM, CarrollSB (2005) Direct regulation of knot gene expression by ultrabithorax and the evolution of cis-regulatory elements in Drosophila. Development 132 : 1567–1577.
85. TakahasiKR, MatsuoT, Takano-Shimizu-KounoT (2011) Two types of cis-trans compensation in the evolution of transcriptional regulation. Proc Natl Acad Sci U S A 108 : 15276–15281.
86. McGregorAP, ShawPJ, HancockJM, BoppD, HedigerM, et al. (2001) Rapid restructuring of bicoid-dependent hunchback promoters within and between Dipteran species: Implications for molecular coevolution. Evol Dev 3 : 397–407.
87. BullaugheyK (2011) Changes in selective effects over time facilitate turnover of enhancer sequences. Genetics 187 : 567–U328.
88. TakahashiH, MitaniY, SatohG, SatohN (1999) Evolutionary alterations of the minimal promoter for notochord-specific brachyury expression in ascidian embryos. Development 126 : 3725–3734.
89. Oda-IshiiI, BertrandV, MatsuoI, LemaireP, SaigaH (2005) Making very similar embryos with divergent genomes: Conservation of regulatory mechanisms of Otx between the ascidians Halocynthia roretzi and Ciona intestinalis. Development 132 : 1663–1674.
90. RiesebergLH, ArcherMA, WayneRK (1999) Transgressive segregation, adaptation and speciation. Heredity 83 : 363–372.
91. BremRB, YvertG, ClintonR, KruglyakL (2002) Genetic dissection of transcriptional regulation in budding yeast. Science 296 : 752–755.
92. HaertyW, SinghRS (2006) Gene regulation divergence is a major contributor to the evolution of Dobzhansky-Muller Incompatibilities between species of Drosophila. Mol Biol Evol 23 : 1707–1714.
93. PhillipsPC (2008) Epistasis—the essential role of gene interactions in the structure and evolution of genetic systems. Nature Reviews Genetics 9 : 855–867.
94. BloomJD, GongLI, BaltimoreD (2010) Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 328 : 1272–1275.
95. LunzerM, GoldingGB, DeanAM (2010) Pervasive cryptic epistasis in molecular evolution. PLoS Genet 6: e1001162 doi:10.1371/journal.pgen.1001162.
96. WeinreichDM, DelaneyNF, DePristoMA, HartlDL (2006) Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312 : 111–114.
97. BridghamJT, OrtlundEA, ThorntonJW (2009) An epistatic ratchet constrains the direction of glucocorticoid receptor evolution. Nature 461 : 515–U78.
98. AzevedoRBR, LohausR, SrinivasanS, DangKK, BurchCL (2006) Sexual reproduction selects for robustness and negative epistasis in artificial gene networks. Nature 440 : 87–90.
99. GertzJ, GerkeJP, CohenBA (2010) Epistasis in a quantitative trait captured by a molecular model of transcription factor interactions. Theor Popul Biol 77 : 1–5.
100. GerkeJ, LorenzK, RamnarineS, CohenB (2010) Gene-environment interactions at nucleotide resolution. PLoS Genet 6: e1001144 doi:10.1371/journal.pgen.1001144.
101. BremRB, StoreyJD, WhittleJ, KruglyakL (2005) Genetic interactions between polymorphisms that affect gene expression in yeast. Nature 436 : 701–703.
102. SteinerCC, WeberJN, HoekstraHE (2007) Adaptive variation in beach mice produced by two interacting pigmentation genes. PLoS Biol 5: e219 doi:10.1371/journal.pbio.0050219.
103. WohlbachDJ, ThompsonDA, GaschAP, RegevA (2009) From elements to modules: Regulatory evolution in Ascomycota fungi. Curr Opin Genet Dev 19 : 571–578.
104. PaixaoT, AzevedoRBR (2010) Redundancy and the evolution of cis-regulatory element multiplicity. PLoS Comput Biol 6: e1000848 doi:10.1371/journal.pcbi.1000848.
105. LiH, JohnsonAD (2010) Evolution of transcription networks—lessons from yeasts. Current Biology 20: R746–R753.
106. RebeizM, JikomesN, KassnerVA, CarrollSB (2011) Evolutionary origin of a novel gene expression pattern through co-option of the latent activities of existing regulatory sequences. Proc Natl Acad Sci U S A 108 : 10036–10043.
107. DraghiJA, ParsonsTL, WagnerGP, PlotkinJB (2010) Mutational robustness can facilitate adaptation. Nature 463 : 353–355.
108. KimJ (2001) Macro-evolution of the hairy enhancer in Drosophila species. J Exp Zool 291 : 175–185.
109. WangRL, StecA, HeyJ, LukensL, DoebleyJ (1999) The limits of selection during maize domestication. Nature 398 : 236–239.
110. SellaG, PetrovDA, PrzeworskiM, AndolfattoP (2009) Pervasive natural selection in the Drosophila genome? PLoS Genet 5: e1000495 doi:10.1371/journal.pgen.1000495.
111. PrabhakarS, NoonanJP, PaeaeboS, RubinEM (2006) Accelerated evolution of conserved noncoding sequences in humans. Science 314 : 786–786.
112. PollardKS, SalamaSR, KingB, KernAD, DreszerT, et al. (2006) Forces shaping the fastest evolving regions in the human genome. PLoS Genet 2: e168 doi:10.1371/journal.pgen.0020168.
113. Lindblad-TohK, GarberM, ZukO, LinMF, ParkerBJ, et al. (2011) A high-resolution map of human evolutionary constraint using 29 mammals. Nature 478 : 476–482.
114. GranatoM, SchnabelH, SchnabelR (1994) pha-1, a selectable marker for gene-transfer in C. elegans. Nucleic Acids Res 22 : 1762–1763.
115. Evans TC (2006) Transformation and microinjection. In: WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.108.1, http://www.wormbook.org. Accessed 2012 Aug 14.
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese SubjectsČlánek Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in RiceČlánek Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System inČlánek An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural CrestČlánek A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of BacteriaČlánek A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 9- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
-
Všechny články tohoto čísla
- Heterozygous Mutations in DNA Repair Genes and Hereditary Breast Cancer: A Question of Power
- GWAS of Diabetic Nephropathy: Is the GENIE out of the Bottle?
- The Conflict within and the Escalating War between the Sex Chromosomes
- Proteome-Wide Analysis of Disease-Associated SNPs That Show Allele-Specific Transcription Factor Binding
- Exome Sequencing Identifies Rare Deleterious Mutations in DNA Repair Genes and as Potential Breast Cancer Susceptibility Alleles
- A Gene Family Derived from Transposable Elements during Early Angiosperm Evolution Has Reproductive Fitness Benefits in
- Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese Subjects
- Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in Rice
- Co-Evolution of Mitochondrial tRNA Import and Codon Usage Determines Translational Efficiency in the Green Alga
- SIRT6/7 Homolog SIR-2.4 Promotes DAF-16 Relocalization and Function during Stress
- CNV Formation in Mouse Embryonic Stem Cells Occurs in the Absence of Xrcc4-Dependent Nonhomologous End Joining
- Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System in
- Citrullination of Histone H3 Interferes with HP1-Mediated Transcriptional Repression
- Variation in Genes Related to Cochlear Biology Is Strongly Associated with Adult-Onset Deafness in Border Collies
- The Long Non-Coding RNA Affects Chromatin Conformation and Expression of , but Does Not Regulate Its Imprinting in the Developing Heart
- Rif2 Promotes a Telomere Fold-Back Structure through Rpd3L Recruitment in Budding Yeast
- Is a Metastasis Susceptibility Gene That Suppresses Metastasis by Modifying Tumor Interaction with the Cell-Mediated Immunity
- The p38/MK2-Driven Exchange between Tristetraprolin and HuR Regulates AU–Rich Element–Dependent Translation
- Rare Copy Number Variants Contribute to Congenital Left-Sided Heart Disease
- A Genetic Basis for a Postmeiotic X Versus Y Chromosome Intragenomic Conflict in the Mouse
- An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural Crest
- Characterization of Inducible Models of Tay-Sachs and Related Disease
- Hominoid-Specific Protein-Coding Genes Originating from Long Non-Coding RNAs
- Transcriptional Repression of Hox Genes by HP1/HPL and H1/HIS-24
- Integrative Genomic Analysis Identifies Isoleucine and CodY as Regulators of Virulence
- Convergence of the Transcriptional Responses to Heat Shock and Singlet Oxygen Stresses
- Genomics of Adaptation during Experimental Evolution of the Opportunistic Pathogen
- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Vsx2 Controls Eye Organogenesis and Retinal Progenitor Identity Via Homeodomain and Non-Homeodomain Residues Required for High Affinity DNA Binding
- The Long Path from QTL to Gene
- TCF7L2 Modulates Glucose Homeostasis by Regulating CREB- and FoxO1-Dependent Transcriptional Pathway in the Liver
- The Non-Flagellar Type III Secretion System Evolved from the Bacterial Flagellum and Diversified into Host-Cell Adapted Systems
- Complex Chromosomal Rearrangements Mediated by Break-Induced Replication Involve Structure-Selective Endonucleases
- Factors That Promote H3 Chromatin Integrity during Transcription Prevent Promiscuous Deposition of CENP-A in Fission Yeast
- A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of Bacteria
- Determinants of Human Adipose Tissue Gene Expression: Impact of Diet, Sex, Metabolic Status, and Genetic Regulation
- Genome-Wide Association Studies Identify Heavy Metal ATPase3 as the Primary Determinant of Natural Variation in Leaf Cadmium in
- Tethering of the Conserved piggyBac Transposase Fusion Protein CSB-PGBD3 to Chromosomal AP-1 Proteins Regulates Expression of Nearby Genes in Humans
- A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- H4K20me1 Contributes to Downregulation of X-Linked Genes for Dosage Compensation
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Coevolution within and between Regulatory Loci Can Preserve Promoter Function Despite Evolutionary Rate Acceleration
- New Susceptibility Loci Associated with Kidney Disease in Type 1 Diabetes
- SWI/SNF-Like Chromatin Remodeling Factor Fun30 Supports Point Centromere Function in
- A Response Regulator Interfaces between the Frz Chemosensory System and the MglA/MglB GTPase/GAP Module to Regulate Polarity in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
- Two Distinct Repressive Mechanisms for Histone 3 Lysine 4 Methylation through Promoting 3′-End Antisense Transcription
- Genetic Modifiers of Chromatin Acetylation Antagonize the Reprogramming of Epi-Polymorphisms
- UTX and UTY Demonstrate Histone Demethylase-Independent Function in Mouse Embryonic Development
- A Comparison of Brain Gene Expression Levels in Domesticated and Wild Animals
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



