-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Heterozygous Mutations in DNA Repair Genes and Hereditary Breast Cancer: A Question of Power
article has not abstract
Published in the journal: . PLoS Genet 8(9): e32767. doi:10.1371/journal.pgen.1003008
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003008Summary
article has not abstract
The emerging technology of massively parallel DNA sequencing has had a major impact on progress in genomics and personalized medicine [1]. Most recently, DNA sequencing of whole exomes (complete coding regions of the human genome) has revealed the genetic basis of many previously-not-localized Mendelian traits [2]. In diseases where the underlying genetic basis is more dilute and complex, old challenges reappear in new clothes [1], [3].
Both the promise and the limitations of these new technologies have been evident in the untangling of the polygenic basis of susceptibility to human breast cancer. The identification of single-gene defects in cancer susceptibility syndromes in the 1990s provided a deterministic model of genetic susceptibility to cancer. Discovery and genetic analysis of the hereditary breast and ovarian cancer genes BRCA1 and BRCA2 offered a preview of personalized genomics, improving medical management of a common form of inherited human neoplasia [4], [5]. Supporting the idea that new breast cancer genes (often referred to collectively as BRCA3) could be identified, analysis of the genetic variance remaining after BRCA1 and BRCA2 mutations had been excluded suggested that most of the excess genetic risk was concentrated in a small percentage of persons [6]. Yet, genetic linkage studies provided little encouragement for the existence of BRCA3 [7]. While genome-wide association studies (GWAS) uncovered new pathways in cancer biology, the GWAS results identified markers of very modest effect size [8]. The realization that a small proportion of excess genetic risk can be accounted for by common variants has resulted in a return to the study of multiplex breast cancer kindreds and the utilization of massively parallel DNA sequencing to uncover rare, disease-causing mutations in hereditary breast cancer.
The article by Thompson et al. published in this issue of PLOS Genetics [9], along with a similar article published recently in the American Journal of Human Genetics [10], provide a glimpse of the early applications of new sequencing technologies to the search for the “missing heritability” in hereditary breast cancer. In Thompson et al., the authors performed exome sequencing of multiple breast cancer cases from a small number of families (33 persons in 15 families) in whom BRCA1 and BRCA2 mutations had been excluded, and they focused on mutations that are predicted to ablate the function of the gene product, namely, mutations that cause premature termination of translation or that destroy splice-sites. After filtering out the overtly deleterious mutations that are polymorphic in the human population (under the assumption that the risk-causing variation in these breast cancer families should be rare), each sequenced individual harbored on average 35 overtly deleterious mutations. Thus, additional filtering criteria were needed to narrow the field: various strategies are possible, and here the authors focused on genes both hit by mutation in multiple individuals and participating in DNA repair through the homologous recombination pathway, a pathway that repairs double-strand breaks with high fidelity. Previous candidate-gene DNA sequencing studies have implicated homologous recombination in breast cancer susceptibility [11]–[16], and BRCA1 and BRCA2 themselves are players in this pathway [17]. Remarkably, two families carried overtly deleterious mutations in the Fanconi anemia (FA) gene FANCC and one family carried an overtly deleterious mutation in the Bloom's syndrome (BS) gene BLM.
FA and BS are rare, autosomal recessive conditions that are characterized by multiple developmental abnormalities (small size and congenital defects of the dermal, immune, skeletal, and reproductive systems), striking DNA repair defects and genomic instability in the somatic cells, and enormous predisposition to the development of various cancers (Figure 1) [18], [19]. Bi-allelic mutations in FANCC and BLM result in FA and BS, respectively, whereas in Thompson et al. heterozygous mutations in FANCC and BLM were identified in a few breast cancer families studied. The notion that heterozygous mutations in DNA repair genes might predispose carriers to incremental increases in cancer susceptibility is a long-standing and sometimes controversial hypothesis in cancer genetics that has increasingly gained traction. As noted above, heterozygous mutations in FA genes have been associated previously with increased breast cancer risk, and, conversely, bi-allelic mutations in breast cancer-associated genes BRCA2, BRIP1, PALB2, and RAD51C have been identified in persons with FA or FA–like syndromes [20]–[23]. Although the concept of increased cancer risk conferred by heterozygous mutations now seems unassailable, the evidence for specific associations between FANCC and BLM and breast cancer risk is not yet convincing [24]–[28]. The challenge of the “heterozygous-mutation” hypothesis is generally one of power. Because the allele frequency of FANCC and BLM mutations in most populations is very low (<0.001), large numbers of individuals are needed to test for differences in the allele frequency between cases and controls. Moreover, heterogeneity in the frequency of mutations across different populations could complicate interpretation of associations when populations are admixed and as investigators combine results from different populations to increase power. As an example of frequency heterogeneity, FANCC and BLM mutations are more frequent in Ashkenazi Jews (∼0.008), where a specific allele is present in most cases of FA and BS [29], [30].
Fig. 1. Fanconi anemia (FA) and Bloom's syndrome (BS) overlap at the clinical and molecular levels. 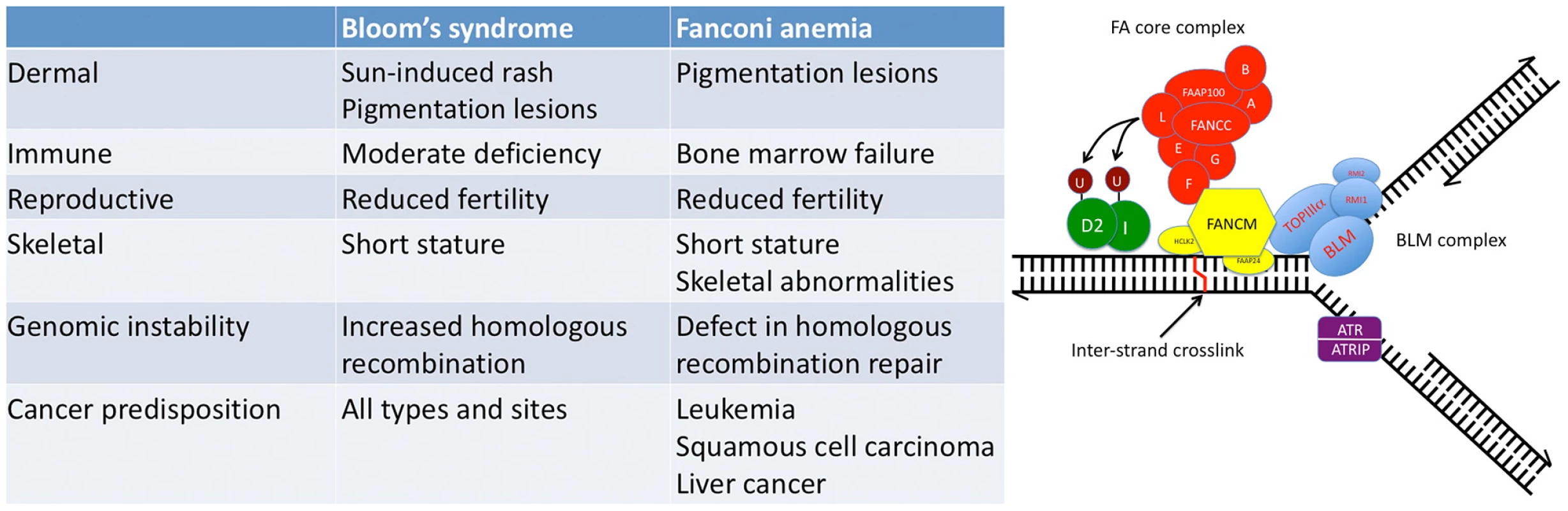
Left panel: Comparison of the clinical and cellular features present in FA and BS. FA and BS have features that are distinct to each syndrome; however, there are broad similarities. Some individuals with mutations in BLM have been diagnosed with FA, exhibiting classical FA features. Right panel: Depiction of a super-complex that is formed at sites of repair of replication forks that have been impeded by an inter-strand crosslink. The super-complex consists of two complexes that form independently in the nucleus. One complex consists of the interacting proteins identified through FA mutations and the other complex consists of proteins that interact with BLM, the gene mutated in BS [33]. These two complexes are brought together by mutual interactions with the FA gene product FANCM [34]. Molecular interactions between FANCJ and BLM at stalled forks have also been described [35]. Downstream signaling effects (FAND2 ubiquitylation and ATR activation) are also depicted. Figure was redrawn from Deans and West [34]. Selecting cases with strong family history of cancer is an enrichment strategy that can reduce the numbers of cases needed to find associations [31]. In Thompson et al., the authors sequenced FANCC and BLM, and in total they found FANCC mutations in four probands out of 1,395 BRCA-negative hereditary breast cancer families from Australia and BLM mutations in two probands out of 438 such families. No mutation carriers were found in either gene in 464 controls. No overtly deleterious FANC or BLM mutations have been reported in 1,192 completely sequenced persons in the 1000 genomes data [32]. On the other hand, in the NHLBI GO Exome Sequencing Project (http://evs.gs.washington.edu/EVS/), three FANCC and four BLM mutation carriers had been identified in 3,510 exomes. Persons from the 1000 genomes and EVS do not constitute a good control group; thus the authors refrained from calculating p-values, 95% confidence intervals, and effect sizes. Going forward it will be important to compare consecutive breast cancer cases and matched controls drawn from the same population to provide robust data to calculate these important parameters; it may take tens of thousands of cases and controls to quantify them! In addition, we will need to combine studies of hereditary breast cancer cases to increase power and drive segregation analysis, to conduct larger case-control studies in the Ashkenazi Jewish population where the increased frequency of specific alleles increases the power of the study group, and to continue to quantify cancer risk in the relatives of persons with FA and BS.
Thus, rare alleles identified by sequencing of multiplex kindreds pose significant challenges for the estimation of effect sizes in cancer susceptibility. In the end, the critical questions that grip rare alleles are how much increased risk do they confer and do they account for the missing heritability? The resolution of these questions is of paramount importance to genetic epidemiologists studying human populations as well as to clinicians caring for families at risk for hereditary breast cancer.
Zdroje
1. OffitK (2011) Personalized medicine: new genomics, old lessons. Hum Genet 130 : 3–14.
2. BamshadMJ, NgSB, BighamAW, TaborHK, EmondMJ, et al. (2011) Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet 12 : 745–755.
3. KiezunA, GarimellaK, DoR, StitzielNO, NealeBM, et al. (2012) Exome sequencing and the genetic basis of complex traits. Nat Genet 44 : 623–630.
4. RobsonM, OffitK (2007) Clinical Practice. Management of an inherited predisposition to breast cancer. N Engl J Med 357 : 154–62.
5. KauffND, SatagopanJM, RobsonME, ScheuerL, HensleyM, et al. (2002) Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 346 : 1609–1615.
6. AntoniouAC, PharoahPD, McMullanG, DayNE, PonderBA, et al. (2001) Evidence for further breast cancer susceptibility genes in addition to BRCA1 and BRCA2 in a population-based study. Genet Epidemiol 21 : 1–18.
7. ThompsonD, SzaboCI, MangionJ, OldenburgRA, OdefreyF, et al. (2001) Evaluation of linkage of breast cancer to the putative BRCA3 locus on chromosome 13q21 in 128 multiple case families from the Breast Cancer Linkage Consortium. Proc Natl Acad Sci U S A 99 : 827–831.
8. StadlerZK, ThomP, RobsonME, WeitzelJN, KauffND, et al. (2010) Genome-wide association studies of cancer. J Clin Oncol 28(27):4255–67.
9. ThompsonER, DoyleMA, RylandGL, RowleySM, ChoongDYH, et al. (2012) Exome sequencing identifies rare deleterious mutations in DNA repair genes FANCC and BLM as potential breast cancer susceptibility alleles. PLoS Genet e1002894 doi:10.1371/journal.pgen.1002894.
10. ParkDJ, LesueurF, Nguyen-DumontT, PertesiM, OdefreyF, et al. (2012) Rare mutations in XRCC2 increase the risk of breast cancer. Am J Hum Genet 90 : 734–739.
11. Meijers-HeijboerH, van den OuwelandA, KlijnJ, WasielewskiM, de SnooA, et al. (2002) Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 31 : 55–59.
12. LevranO, AttwoollC, HenryRT, MiltonKL, NevelingK, et al. (2005) The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat Genet 37 : 931–933.
13. RenwickA, ThompsonD, SealS, KellyP, ChagtaiT, et al. (2006) ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet 38 : 873–875.
14. SealS, ThompsonD, RenwickA, ElliottA, KellyP, et al. (2007) PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet 39 : 165–167.
15. MeindlA, HellebrandH, WiekC, ErvenV, WappenschmidtB, et al. (2010) Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet 42 : 410–414.
16. LovedayC, TurnbullC, RamsayE, HughesD, RuarkE, et al. (2011) Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet 43 : 879–882.
17. D'AndreaAD (2010) Susceptibility pathways in Fanconi's anemia and breast cancer. N Engl J Med 362 : 1909–1919.
18. Auerbach AD, Joenje H, Buchwald M (2011) Fanconi anemia. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarkis SE, et al.. Scriver's online metabolic and molecular bases of inherited disease. New York: McGraw Hill. Chapter 31.
19. German J, Ellis NA (2011) Bloom syndrome. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarkis SE, et al.. Scriver's online metabolic and molecular bases of inherited disease. New York: McGraw Hill. Chapter 30.
20. HowlettNG, TaniguchiT, OlsonS, CoxB, WaisfiszQ, et al. (2002) Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297 : 606–609.
21. LevitusM, WaisfiszQ, GodthelpBC, de VriesY, HussainS, et al. (2005) The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat Genet 37 : 934–935.
22. ReidS, SchindlerD, HanenbergH, BarkerK, HanksS, et al. (2007) Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet 39 : 162–164.
23. VazF, HanenbergH, SchusterB, BarkerK, WiekC, et al. (2010) Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat Genet 42 : 406–409.
24. GruberSB, EllisNA, RennertG, OffitK, et al. (2002) BLM heterozygosity and the risk of colorectal cancer. Science 297 : 2013.
25. ClearySP, ZhangW, Di NicolaN, AronsonM, AubeJ, et al. (2003) Heterozygosity for the BLM(Ash) mutation and cancer risk. Cancer Res 63 : 1769–1771.
26. BerwickM, SatagopanJM, Ben-PoratL, CarlsonA, MahK, et al. (2007) Genetic heterogeneity among Fanconi anemia heterozygotes and risk of cancer. Cancer Res 67 : 9591–9596.
27. BarisHN, KedarI, HalpernGJ, ShohatT, MagalN, et al. (2007) Prevalence of breast and colorectal cancer in Ashkenazi Jewish carriers of Fanconi anemia and Bloom syndrome. Isr Med Assoc J 9 : 847–850.
28. SokolenkoAP, IyevlevaAG, PreobrazhenskayaEV, MitiushkinaNV, AbyshevaSN, et al. (2012) High prevalence and breast cancer predisposing role of the BLM c.1642 C>T (Q548X) mutation in Russia. Int J Cancer 130 : 2867–2873.
29. WhitneyMA, SaitoH, JakobsPM, GibsonRA, MosesRE, et al. (1993) A common mutation in the FACC gene causes Fanconi anaemia in Ashkenazi Jews. Nat Genet 4 : 202–205.
30. EllisNA, CiocciS, ProytchevaM, LennonD, GrodenJ, et al. (1998) The Ashkenazic Jewish Bloom syndrome mutation blmAsh is present in non-Jewish Americans of Spanish ancestry. Am J Hum Genet 63 : 1685–1693.
31. HoulstonRS, PetoJ (2004) The search for low-penetrance cancer susceptibility alleles. Oncogene 23 : 6471–6476.
32. MacArthurDG, BalasubramanianS, FrankishA, HuangN, MorrisJ, et al. A systematic survey of loss-of-function variants in human protein-coding genes. Science 335 : 823–828.
33. MeeteiAR, SechiS, WallischM, YangD, YoungMK, et al. (2003) A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome. Mol Cell Biol 23 : 3417–3426.
34. DeansAJ, WestSC (2009) FANCM connects the genome instability disorders Bloom's syndrome and Fanconi anemia. Mol Cell 36 : 943–953.
35. SuhasiniAN, RawtaniNA, WuY, SommersJA, SharmaS, et al. (2011) Interaction between the helicases genetically linked to Fanconi anemia group J and Bloom's syndrome. EMBO J 30 : 692–705.
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese SubjectsČlánek Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in RiceČlánek Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System inČlánek An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural CrestČlánek A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of BacteriaČlánek A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 9- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
-
Všechny články tohoto čísla
- Heterozygous Mutations in DNA Repair Genes and Hereditary Breast Cancer: A Question of Power
- GWAS of Diabetic Nephropathy: Is the GENIE out of the Bottle?
- The Conflict within and the Escalating War between the Sex Chromosomes
- Proteome-Wide Analysis of Disease-Associated SNPs That Show Allele-Specific Transcription Factor Binding
- Exome Sequencing Identifies Rare Deleterious Mutations in DNA Repair Genes and as Potential Breast Cancer Susceptibility Alleles
- A Gene Family Derived from Transposable Elements during Early Angiosperm Evolution Has Reproductive Fitness Benefits in
- Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese Subjects
- Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in Rice
- Co-Evolution of Mitochondrial tRNA Import and Codon Usage Determines Translational Efficiency in the Green Alga
- SIRT6/7 Homolog SIR-2.4 Promotes DAF-16 Relocalization and Function during Stress
- CNV Formation in Mouse Embryonic Stem Cells Occurs in the Absence of Xrcc4-Dependent Nonhomologous End Joining
- Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System in
- Citrullination of Histone H3 Interferes with HP1-Mediated Transcriptional Repression
- Variation in Genes Related to Cochlear Biology Is Strongly Associated with Adult-Onset Deafness in Border Collies
- The Long Non-Coding RNA Affects Chromatin Conformation and Expression of , but Does Not Regulate Its Imprinting in the Developing Heart
- Rif2 Promotes a Telomere Fold-Back Structure through Rpd3L Recruitment in Budding Yeast
- Is a Metastasis Susceptibility Gene That Suppresses Metastasis by Modifying Tumor Interaction with the Cell-Mediated Immunity
- The p38/MK2-Driven Exchange between Tristetraprolin and HuR Regulates AU–Rich Element–Dependent Translation
- Rare Copy Number Variants Contribute to Congenital Left-Sided Heart Disease
- A Genetic Basis for a Postmeiotic X Versus Y Chromosome Intragenomic Conflict in the Mouse
- An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural Crest
- Characterization of Inducible Models of Tay-Sachs and Related Disease
- Hominoid-Specific Protein-Coding Genes Originating from Long Non-Coding RNAs
- Transcriptional Repression of Hox Genes by HP1/HPL and H1/HIS-24
- Integrative Genomic Analysis Identifies Isoleucine and CodY as Regulators of Virulence
- Convergence of the Transcriptional Responses to Heat Shock and Singlet Oxygen Stresses
- Genomics of Adaptation during Experimental Evolution of the Opportunistic Pathogen
- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Vsx2 Controls Eye Organogenesis and Retinal Progenitor Identity Via Homeodomain and Non-Homeodomain Residues Required for High Affinity DNA Binding
- The Long Path from QTL to Gene
- TCF7L2 Modulates Glucose Homeostasis by Regulating CREB- and FoxO1-Dependent Transcriptional Pathway in the Liver
- The Non-Flagellar Type III Secretion System Evolved from the Bacterial Flagellum and Diversified into Host-Cell Adapted Systems
- Complex Chromosomal Rearrangements Mediated by Break-Induced Replication Involve Structure-Selective Endonucleases
- Factors That Promote H3 Chromatin Integrity during Transcription Prevent Promiscuous Deposition of CENP-A in Fission Yeast
- A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of Bacteria
- Determinants of Human Adipose Tissue Gene Expression: Impact of Diet, Sex, Metabolic Status, and Genetic Regulation
- Genome-Wide Association Studies Identify Heavy Metal ATPase3 as the Primary Determinant of Natural Variation in Leaf Cadmium in
- Tethering of the Conserved piggyBac Transposase Fusion Protein CSB-PGBD3 to Chromosomal AP-1 Proteins Regulates Expression of Nearby Genes in Humans
- A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- H4K20me1 Contributes to Downregulation of X-Linked Genes for Dosage Compensation
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Coevolution within and between Regulatory Loci Can Preserve Promoter Function Despite Evolutionary Rate Acceleration
- New Susceptibility Loci Associated with Kidney Disease in Type 1 Diabetes
- SWI/SNF-Like Chromatin Remodeling Factor Fun30 Supports Point Centromere Function in
- A Response Regulator Interfaces between the Frz Chemosensory System and the MglA/MglB GTPase/GAP Module to Regulate Polarity in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
- Two Distinct Repressive Mechanisms for Histone 3 Lysine 4 Methylation through Promoting 3′-End Antisense Transcription
- Genetic Modifiers of Chromatin Acetylation Antagonize the Reprogramming of Epi-Polymorphisms
- UTX and UTY Demonstrate Histone Demethylase-Independent Function in Mouse Embryonic Development
- A Comparison of Brain Gene Expression Levels in Domesticated and Wild Animals
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



