-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
Base-excision repair and control of nucleotide pools safe-guard against permanent uracil accumulation in DNA relying on two key enzymes: uracil–DNA glycosylase and dUTPase. Lack of the major uracil–DNA glycosylase UNG gene from the fruit fly genome and dUTPase from fruit fly larvae prompted the hypotheses that i) uracil may accumulate in Drosophila genomic DNA where it may be well tolerated, and ii) this accumulation may affect development. Here we show that i) Drosophila melanogaster tolerates high levels of uracil in DNA; ii) such DNA is correctly interpreted in cell culture and embryo; and iii) under physiological spatio-temporal control, DNA from fruit fly larvae, pupae, and imago contain greatly elevated levels of uracil (200–2,000 uracil/million bases, quantified using a novel real-time PCR–based assay). Uracil is accumulated in genomic DNA of larval tissues during larval development, whereas DNA from imaginal tissues contains much less uracil. Upon pupation and metamorphosis, uracil content in DNA is significantly decreased. We propose that the observed developmental pattern of uracil–DNA is due to the lack of the key repair enzyme UNG from the Drosophila genome together with down-regulation of dUTPase in larval tissues. In agreement, we show that dUTPase silencing increases the uracil content in DNA of imaginal tissues and induces strong lethality at the early pupal stages, indicating that tolerance of highly uracil-substituted DNA is also stage-specific. Silencing of dUTPase perturbs the physiological pattern of uracil–DNA accumulation in Drosophila and leads to a strongly lethal phenotype in early pupal stages. These findings suggest a novel role of uracil-containing DNA in Drosophila development and metamorphosis and present a novel example for developmental effects of dUTPase silencing in multicellular eukaryotes. Importantly, we also show lack of the UNG gene in all available genomes of other Holometabola insects, indicating a potentially general tolerance and developmental role of uracil–DNA in this evolutionary clade.
Published in the journal: . PLoS Genet 8(6): e32767. doi:10.1371/journal.pgen.1002738
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002738Summary
Base-excision repair and control of nucleotide pools safe-guard against permanent uracil accumulation in DNA relying on two key enzymes: uracil–DNA glycosylase and dUTPase. Lack of the major uracil–DNA glycosylase UNG gene from the fruit fly genome and dUTPase from fruit fly larvae prompted the hypotheses that i) uracil may accumulate in Drosophila genomic DNA where it may be well tolerated, and ii) this accumulation may affect development. Here we show that i) Drosophila melanogaster tolerates high levels of uracil in DNA; ii) such DNA is correctly interpreted in cell culture and embryo; and iii) under physiological spatio-temporal control, DNA from fruit fly larvae, pupae, and imago contain greatly elevated levels of uracil (200–2,000 uracil/million bases, quantified using a novel real-time PCR–based assay). Uracil is accumulated in genomic DNA of larval tissues during larval development, whereas DNA from imaginal tissues contains much less uracil. Upon pupation and metamorphosis, uracil content in DNA is significantly decreased. We propose that the observed developmental pattern of uracil–DNA is due to the lack of the key repair enzyme UNG from the Drosophila genome together with down-regulation of dUTPase in larval tissues. In agreement, we show that dUTPase silencing increases the uracil content in DNA of imaginal tissues and induces strong lethality at the early pupal stages, indicating that tolerance of highly uracil-substituted DNA is also stage-specific. Silencing of dUTPase perturbs the physiological pattern of uracil–DNA accumulation in Drosophila and leads to a strongly lethal phenotype in early pupal stages. These findings suggest a novel role of uracil-containing DNA in Drosophila development and metamorphosis and present a novel example for developmental effects of dUTPase silencing in multicellular eukaryotes. Importantly, we also show lack of the UNG gene in all available genomes of other Holometabola insects, indicating a potentially general tolerance and developmental role of uracil–DNA in this evolutionary clade.
Introduction
In wild-type organisms, notably excepting some rare bacteriophages with thymineless DNA genomes [1], [2], uracil in DNA is thought to occur only transiently and at very low frequency (<20/million bases) as a damage product [3], [4]. Efficient base-excision DNA repair together with fine-tuned control of nucleotide pools safe-guard against uracil in DNA relying on two key enzymes: uracil–DNA glycosylase (UDG) [5] and dUTPase [6].
UDG deficiency, in combination with thymidylate synthase inhibition or depleted dUTPase activity, was reported to lead into notable uracil accumulation in DNA. However, deficiency in uracil–DNA glycosylase on its own contributes only slightly to the genomic uracil content [7]–[10]. Since dUTPase deficiency or silencing can be rescued by depleted activity of UDG [11]–[14], it can be argued that UDG is a major factor that renders uracil–DNA, formed in absence of dUTPase, intolerable for cells. Uracil–DNA glycosylases represent a superfamily that involves enzymes with specialized functions. Among the superfamily members, UNG is reported to be the most abundant one that also possesses the highest activity in removing uracils from any context of both single-stranded and double-stranded DNA [5]. SMUG has similar attributes to UNG but prefers uracil-containing ssDNA as substrate [15]. MBD4 and TDG recognize mismatched uracil or thymine bases that base-pair with guanine [16]. The latter enzyme is known to function within CpG islands, where thymine is formed after spontaneous methyl-cytosine deamination [17].
Through hydrolyzing dUTP to dUMP and pyrophosphate, dUTPase serves a dual role in cell physiology: it produces a precursor for thymidylate synthesis, and removes dUTP from the deoxynucleotide pool. Eukaryotic and bacterial DNA polymerases incorporate deoxyuridine into DNA with a rate that depends on the cellular dUTP concentration [6], [10]. Therefore dUTPase has a significant indirect impact in prevention of uracil incorporation into DNA. Lack of dUTPase is supposed to induce thymine to uracil substitution that does not alter the genetic code; however UDG still removes this kind of base modification supposedly resulting in genome instability. This process may serve as an explanation for overall lethality of dUTPase deficiency [6].
Here, we propose that a condition similar to simultaneous deficiency in both dUTPase and UDG is present in Drosophila larvae. The Drosophila genome does not encode the major uracil–DNA glycosylase UNG [18], therefore uracil–DNA tolerance may be expected. Drosophila larvae contain both proliferating primordial tissues of imago and differentiated tissues that degrade during metamorphosis. Our previous study reported Drosophila dUTPase [19], [20] expression only in the first one of the two kinds of tissues [21], [22]. We wished to investigate if the stage - and tissue-specific expression of dUTPase is translated into differences in the uracil content of genomic DNA and whether this putative pattern has developmental significance.
We now report high resistance of Drosophila cell lines to fluorodeoxyuridine (FdUR) that induced high uracil–DNA levels. We show that in addition to tolerance of uracil-substituted DNA, Drosophila cells correctly interpret this unusual DNA both in vitro and in vivo. Moreover, in wild type Drosophila we observe stage - and tissue-specific changes in uracil-content of DNA that are inversely correlated to dUTPase expression and cellular fate. We propose that the observed pattern is due to the lack of the ung gene from the Drosophila genome paralleled with developmental down-regulation of dUTPase in larval tissues. To check whether the absence of dUTPase may be causative for uracil accumulation in DNA, we show that silencing of dUTPase in larvae expands the uracil–DNA pattern to imaginal tissues, as well. Interestingly, dUTPase silencing results in abnormal DNA strand breaks, cell death and developmental arrest in early pupal stage. This may indicate that tolerance of highly uracil-containing DNA is also stage-specific, and other tolerance factors, in addition to the lack of UNG, may appear in this specific developmental phase. To our knowledge, our study is the first one that reports uracil–DNA appearance in absence of dUTPase in a wild type complex eukaryotic organism and demonstrates the developmental pattern of its tolerance and stability.
Results/Discussion
Since the Drosophila genome lacks UNG, we wished to test whether Drosophila cells show similar characteristics to UNG deficient organisms. The drug 5′-fluorodeoxyuridine (FdUR), frequently used as an inhibitor of thymidylate biosynthesis [23], [24], leads to perturbation of nucleotide levels and cell death. Loss of uracil–DNA glycosylase activity was found to lead to fluoropyrimidine resistance in E. coli [25], yeast [26] and C. elegans [27]. Deficient uracil–DNA glycosylase activity was also reported to be required for increased genomic uracil content after FdUR exposure in E. coli and mammalian cells [7]–[10]. Therefore, response to FdUR treatment may be an indicator for testing cellular uracil–DNA glycosylase activity. We observed that the Drosophila S2 cell line shows only small decrease in viability in the presence of 1 mM of FdUR, while HeLa cells, possessing the ung gene, show strong lethality at this drug concentration (Figure 1A). As a dose-dependent response to increasing concentrations of FdUR, uracil accumulation in genomic DNA of Drosophila S2 cells became highly elevated (up to approx. 450 uracil/million bases) (Figure 1B). Both the observed relatively high resistance for FdUR and the cellular response of genomic uracil incorporation may be explained by the fact that Drosophila cells lack significant uracil–DNA glycosylase activity.
Fig. 1. Tolerance and stability of uracil-containing DNA in D. melanogaster. 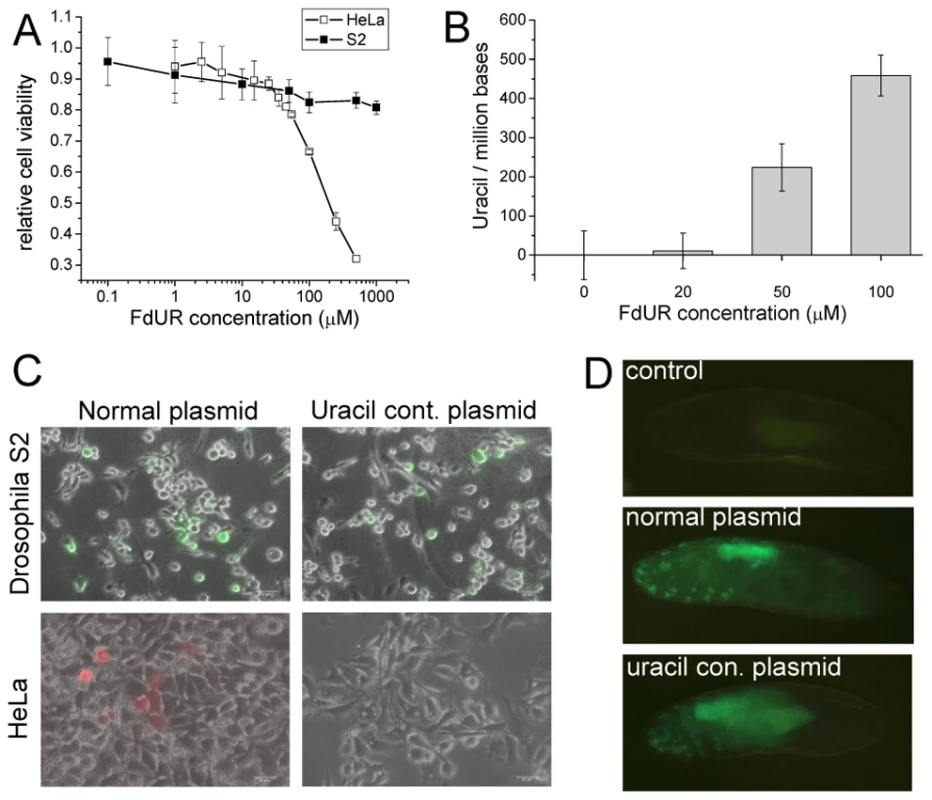
(A) Dose-response curve upon FdUR treatment followed by Alamar Blue assay. (B) FdUR leads to uracil accumulation in DNA of Drosophila S2 cells. Data indicate that increased level of uracil is well-tolerated in Drosophila, but not in human cells. Data are presented as mean ± s.e.m. (C) Drosophila S2 (top panels) and human HeLa cells (bottom panels) were transfected with normal plasmid (left panels) or uracil-containing plasmid (right panels). Expression of YFP in Drosophila S2 cells or dsRedMonomer in HeLa cells indicates stability of the DNA. (D) Microinjection of uracil-plasmid into Drosophila embryo. Non-injected embryos served as control sample. We then asked if uracil-containing chemically unusual DNA may be tolerated and interpreted in Drosophila cells. Uracil–DNA specific cell response was provoked by transfecting cells with exogenous plasmid uracil–DNA. The expression signal of the fluorescent reporters (eYFP or dsRedMonomer) encoded by plasmids produced in wild type (normal plasmid) or dut-1, ung-1 (uracil-plasmid, approx. 5500 uracil/million bases [8], [10]) E. coli were followed. The gene span for the eYFP or DSRed-monomer reporters included the promoter (CMV immediate early or metallothionein), the reporter protein gene (DSRed-monomer or eYFP), and the SV40 polyadenylation signal. The total length of these gene spans comprises 1515 or 1299 nucleotides. If synthesized in the double mutant dut-1, ung-1 E.coli strain, the average number of uracil substitutions on a single strand within these gene spans corresponds to 8.3 and 7.1, respectively. Human (HeLa) cells, possessing UNG, showed appreciable fluorescent signal only when transformed with normal plasmid, indicating that uracil-containing plasmid was not interpreted probably due to its degradation. On the contrary, Drosophila S2 cells could express reporter genes encoded either on normal or uracil containing plasmid at comparable level (Figure 1C and Figure S1).
Having established that Drosophila cells in in vitro culture may tolerate and do correctly interpret uracil-containing DNA, we wished to assess the fruit fly physiological response to uracil–DNA at the organism level. Therefore similarly to the cell culture reporter-assay, uracil–DNA plasmid was introduced to Drosophila embryo. Upon microinjection of pP{Gal4VP16} expression vector (where the 1000 nt segment includes the P-element promoter, the Gal4-VP16 fusion gene and the hsp70 terminator that may have an average number of 5.5 uracil substitutions if produced in double mutant E. coli) into P{mw+UASeGFP} transgenic animals, the strong eGFP signal indicated the stability of exogenous DNA (Figure 1D). In pre-hatching embryos eGFP signal was detected from both normal and uracil-containing DNA with commeasurable intensities. In both cases the expression pattern of the reporter construct is similar and eGFP is most pronounced in the gut. These results can be explained only by assuming that the genetic information stored in uracil-containing DNA serves as a cognate code for transcription in Drosophila cells. Such ability of the fruitfly cells is most probably due to lack of UNG under which condition uracil–DNA does not get rapidly degraded.
The other key factor responsible for keeping uracil out of DNA is the enzyme dUTPase, the importance of which is even more substantiated in D. melanogaster that lacks UNG. During development of D. melanogaster, high dUTPase protein levels can be observed only in embryonic stages (Figure 2A). As shown by Western blotting and immunohistochemistry, starting from late embryonic phase, dUTPase expression level is decreased drastically and remains low during postembryonic stages. Under normal physiological circumstances of larval development, dUTPase is predominantly expressed in imaginal discs, the central nervous system and in the testis (Figure 2) but not in most larval tissues, like salivary gland and gut. In the ventriculus and in the salivary gland, some cells are associated with dUTPase expression – these seem to correspond to the imaginal cells (Figure 2B). In the imago, dUTPase is present in the ovary of females. Cellular localization of dUTPase is usually nuclear [21], [28]–[31], however cytoplasmic occurrence is also evident in the nurse cells of mature follicles within ovaries, as described previously [21]. The present results are in agreement with our more limited earlier observations at the protein level [21]. We also quantified the dUTPase mRNA level by real-time PCR and found that protein and mRNA levels show similar tendencies during development (Figure 3A and 3B).
Fig. 2. Stage- and tissue-specific distribution of dUTPase protein levels in D. melanogaster. 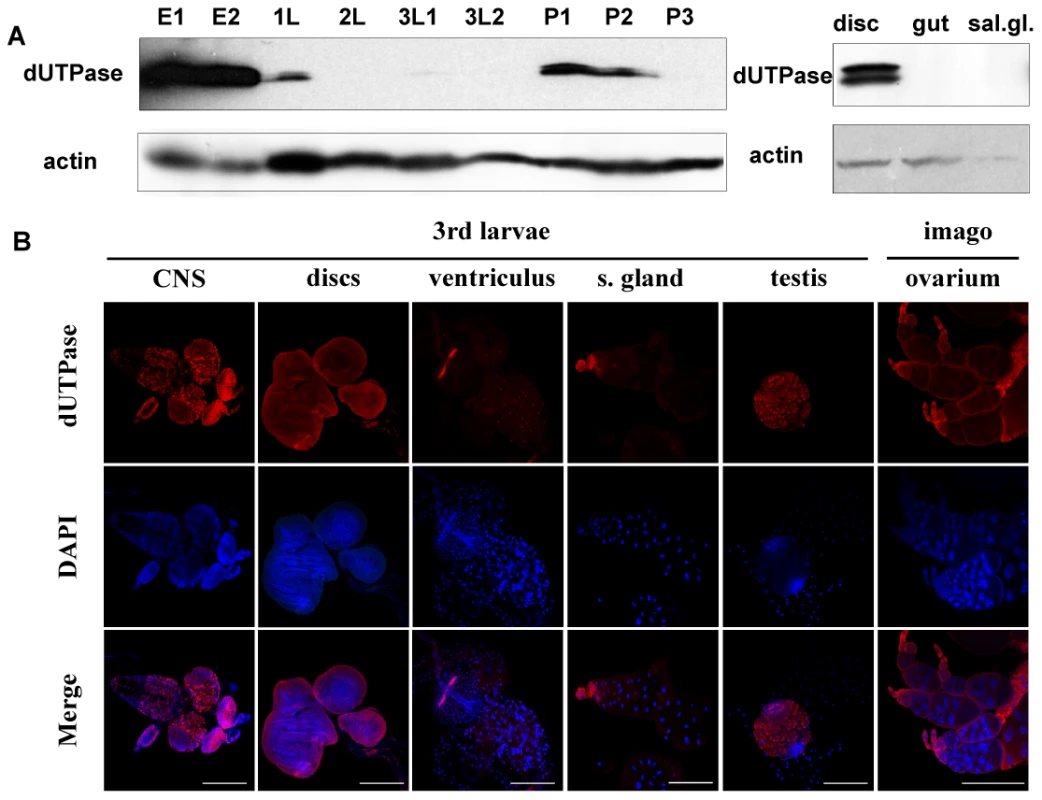
Western blotting (A) and immunohistochemistry (B) was performed on selected developmental stages and tissues. Embryo 0–6 h (E1), embryo 0–24 h (E2), 1st larvae (1L), 2nd larvae (2L), early 3rd larvae (3L1), wandering 3rd larvae (3L2), pupae before head eversion (P1), pupae after head eversion (P2) and pupae 50–60 h after puparium formation (P3). For Western blotting, actin was used as loading control. Note that dUTPase protein levels are down-regulated during larval stages and expression is confined to specific tissues. Fig. 3. D. melanogaster genomic DNA uracil content inversely correlates with dUTPase expression. 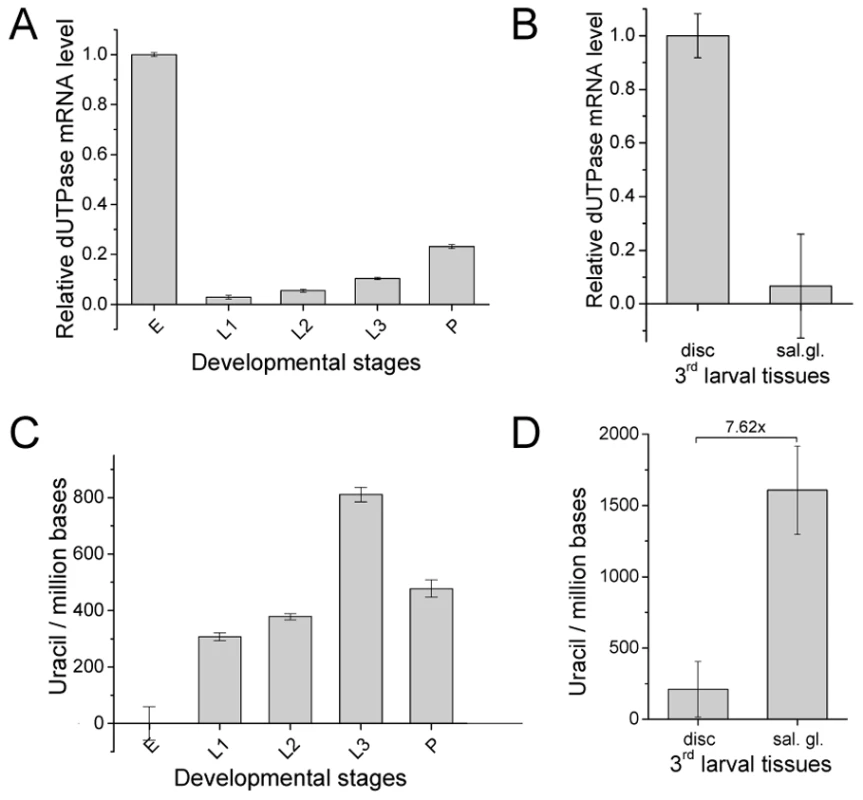
(A) Changes of dUTPase mRNA level throughout fruitfly development: embryo (E), 1st larvae (L1), 2nd larvae (L2), late 3rd larvae (L3) and pupae (P). Note that dUTPase is down-regulated in larvae. (B) Comparison of dUTPase RNA level in the larval tissues salivary gland and imaginal tissue. Data are presented as mean of triplicates ± s.e.m. mRNA level was measured by RT-qPCR and dUTPase mRNA level was normalized to Rp49 mRNA level. (C) Uracil content of D. melanogaster genome in different developmental stages: embryo (E), 1st larvae (L1), 2nd larvae (L2), late 3rd larvae (L3) and pupae (P). Embryonic sample was used as reference since it was shown to contain undetectable levels of uracil in DNA (cf. Figure S2). (D) Comparison of genomic uracil content in wild type imaginal disc and salivary gland of 3rd larvae. Data are presented as mean ± s.e.m. We were interested to learn whether coincidence of uracil–DNA tolerance and dUTPase down-regulation results in uracil accumulation in the genome of affected developmental stages and larval tissues. Determination of genomic uracil level was carried out by applying a recent quantitative real-time PCR-based method [10]. Data presented in Figure S2 and Figure 3C argue that DNA purified from embryo is relatively uracil-free, whereas in larval stages, uracil becomes much accumulated. The presence of uracil–DNA in larvae was further confirmed by using an independent method, the UNG-ARP assay (Figure S3) [8]. Uracil content also varies according to tissue type within the larvae: the salivary gland, a representative tissue of pupal degradation, accumulates high levels of uracil; while imaginal discs, which do not undergo abundant metamorphosis-coupled cell death, contain much less uracil (Figure 3D). Uracil level in larval tissues is comparable to that previously reported in the double mutant dut1-1, ung-1 E. coli strain [8], [10], amounting to several thousands of uracils per million bases. The above discussed pattern of uracil accumulation in different stages and tissues shows a clear negative correlation to the expression pattern of dUTPase (compare the respective developmental stages in Figure 2 and Figure 3).
In order to investigate if perturbation of the wild-type pattern of uracil levels in DNA may interfere with normal development, we aimed to silence dUTPase in transgenic D. melanogaster strains (Table S1). Efficient silencing could be successfully achieved in a setup resulting in well distinguishable F1 phenotypes: non-silenced animals were characterized by GFP expression and curly wings, whereas silenced animals had no markers [32] (Figure S4). Overall silencing resulted in efficiently decreased dUTPase protein levels in larvae and pupae (Figure 4A). RNAi silencing may not operate appropriately in early embryo due to maternal effects, but this stage is out of the scope of our present experiment with transgenic strains. We observed that dUTPase silencing did not perturb normal life and development of larvae. The silenced versus non-silenced larvae of F1 generation were selected based on GFP expression, and the time interval between the egg laying and puparium formation did not show any significant alteration (Figure 4B). Importantly, effective silencing of dUTPase in imaginal discs and larval brain did not cause any observable morphological changes in tissue morphology (Figure 5A). At pupal stage, however, dUTPase silencing induced 100% lethality, i.e. no silenced animals could develop into imago (observation is based on counting 2350 curly winged control imagos resulting from the first generation of crossings) (Figure 4C).
Fig. 4. Silencing of dUTPase in Drosophila larvae and pupae. 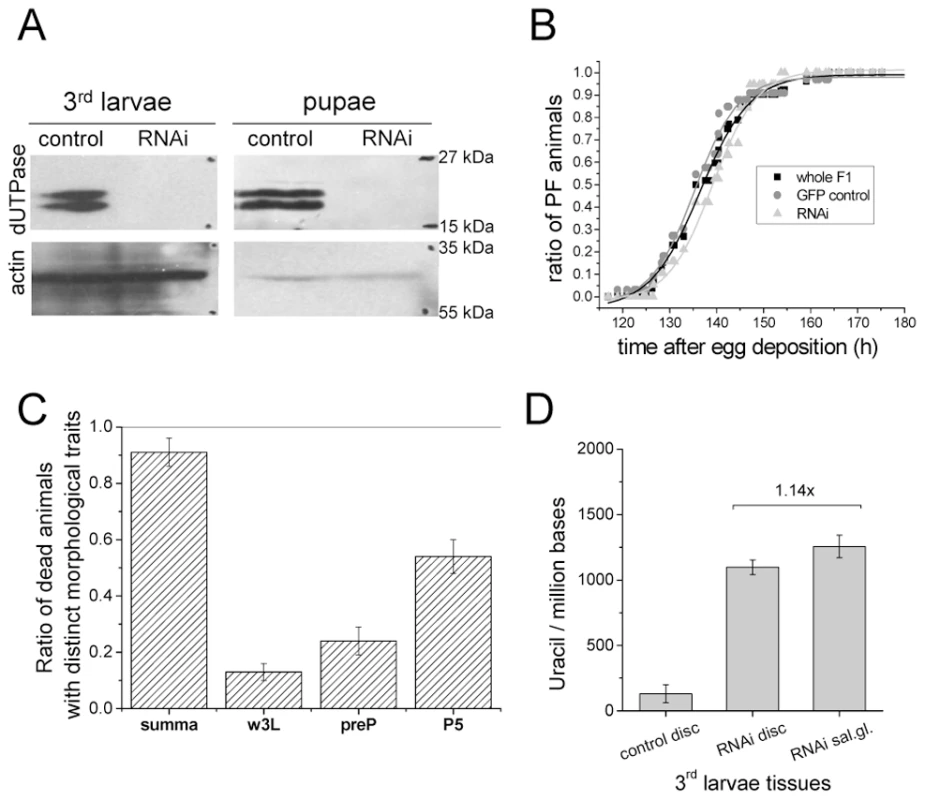
Western blots in (A) show that the protein level of dUTPase is under detection limit in silenced animals. Actin served as loading control. (B) Curves show the relative number of silenced and non-silenced animals that have undergone puparium formation at the given time point after egg deposition. Inflection points of the curves represent the mean time of puparium formation characteristic for the given population. dUTPase silencing did not perturb the time interval required for puparium formation. (C) Graph shows the number of counted dead animals relative to number of hatched curly winged control flies. Among these dead animals, three groups with distinct morphological traits characteristic for wandering larvae (w3L), prepupae (preP), and pupal stage P5 (P5) were identified and counted. (D) Genomic uracil content of dUTPase silenced and control tissues from 3rd larvae. One possible proof for RNAi specificity is a rescue by the corresponding complementary DNA [33], [34]. Therefore, to test the specificity of dUTPase RNAi, the RNAi construct was co-expressed with a dUTPase transgene that led to the expression of dUTPase protein, as detected in the rescued animals (cf. method described in Text S1 and shown on Figure S4C and S4D). We observed a full rescue of the lethality caused by the RNAi confirming that the RNAi phenotype was due to a reduction of dUTPase level (Table S2). Rescued animals underwent development and metamorphosis just like the wild type animals. In the larval, prepupal and imago stages, rescued animals were carefully investigated for morphology and no distinct traits were observed.
In the detailed phenotype analysis of dUTPase-silenced animals, morphologic observations indicated serious adverse effects and developmental arrest at or before the pupal stage P5 [35] (Figure 5B, Figures S5 and S6). Upon removing the puparium of the silenced animal, defects were identified in the inner texture of everted discs, and head sack, as well as in the development of adult eye. These defects are permanent, and are not simply due to slow down of normal development. Moreover, 3–4 days after puparium formation, tissues showing morphological traits associated with the larval stage were identified within dissected samples (Figure S7 versus Figure S8). Noteworthy, the typical organ structures of the wild type adult (legs, wings, Malpighian tubules, Yellow Body, eyes etc.) never appeared in the silenced animals. Darkened tissues that may result from cell death, necrosis or histolysis were also observed e.g. in the prothoracic region and organs (Figure 5B and Figure S8) [36]. This result argued that although dUTPase is dispensable in larval tissues [21], the presence of the enzyme at the developmental stage of early puparium formation is essential for normal development during metamorphosis.
Fig. 5. Morphological consequences of dUTPase silencing. 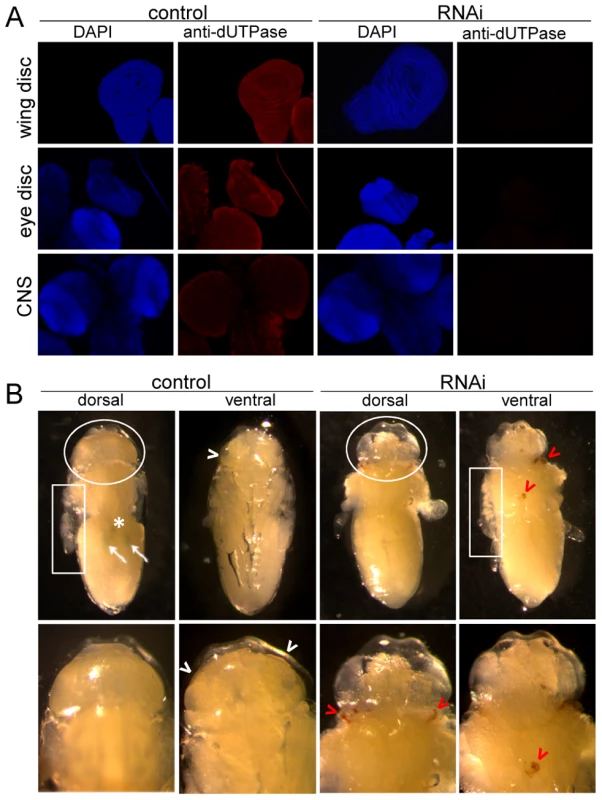
In larvae (A) and pupae (B). (A) Immunohistochemistry of wing and eye discs, and brain of non-silenced (control) and silenced larvae for dUTPase (red) and DAPI staining for DNA (blue) demonstrate on one hand highly effective silencing; and on the other hand no observable morphological changes within these tissues. (B) Wild type pupae (control) in stage P6 (cf. Figure S5) and dUTPase silenced pupae at corresponding time after puparium formation in dorsal and ventral view are shown, after puparium removal. Wild type traits, Malpighian tubules (white arrows), Yellow Body (white asterix), developing adult eye (white arrowheads) are not observable on silenced animals. Instead, darkened (apoptotic/necrotic or melanized) tissues (red arrowheads) can be visualized on these pupae. Note the basically different inner texture of the everted discs (white boxes) and head sack (white circles). Tissue specific silencing in the dorsal wing surface of imaginal discs was also developed as described in Figure S9 (cf. [37]). The great majority of silenced animals developed curly wings, and a significant portion of them even showed blisters on the wings. Curling and blistering of the wings was suggested to result from abnormal cell death in dorsal wing surface [38], [39]. These results suggest that dUTPase silencing within a well-defined tissue segment may be specifically and exclusively associated with phenotypic effects in the very same tissue segment.
To analyze whether dUTPase silencing changed the distribution of uracil–DNA within different larval tissues, we assayed genomic uracil content of imaginal discs from both non-silenced and silenced larvae of F1 generation. As Figure 4D shows, imaginal discs from dUTPase silenced larvae accumulated a high amount of uracil in their genome (1097+/−55 uracil/million bases, to be compared with 131+/−69 uracil/million bases in the non-silenced animals) that approximated the genomic uracil content of salivary gland. Further increase in uracil–DNA content of salivary gland DNA was not observed in dUTPase silenced larvae, indicating that silencing was effective in tissues that normally express dUTPase but had no effect on uracil–DNA in tissues that normally do not express dUTPase. This result confirms that uracil appearance in DNA depends on dUTPase expression, thus dUTPase activity is causative in maintaining DNA with low levels of uracil.
To analyze if the effects of dUTPase silencing may lead to DNA damage or cell death, we applied TUNEL and phospho-Histone H2Av assays [40], [41]. We observed that imaginal discs isolated from 3rd stage wandering larva of dUTPase-silenced animals show strong enrichment in TUNEL positive cells (Figure 6A and 6B). TUNEL staining in the tissues indicate primarily cell death in the phase of DNA fragmentation. To address the question whether dUTPase depletion violates genome integrity we stained nuclei for phospho-Histone H2Av. H2Av histone modification by the ATR/ATM kinases indicates DNA double strand breaks (DSBs) in the proximity of the foci [41]. We observed numerous phospho-H2Av foci in dUTPase silenced wing imaginal discs, while no such foci were visible in the wild type (Figure 6C). These results suggest a potential correlation between dUTPase activity and DNA integrity. The increased level of DNA damage observed in our experiments in the dUTPase-silenced animals may result from excessive processing of uracil-containing DNA that concludes to DNA fragmentation.
Fig. 6. dUTPase silencing results in cell death and DNA strand breaks in larval imaginal discs. 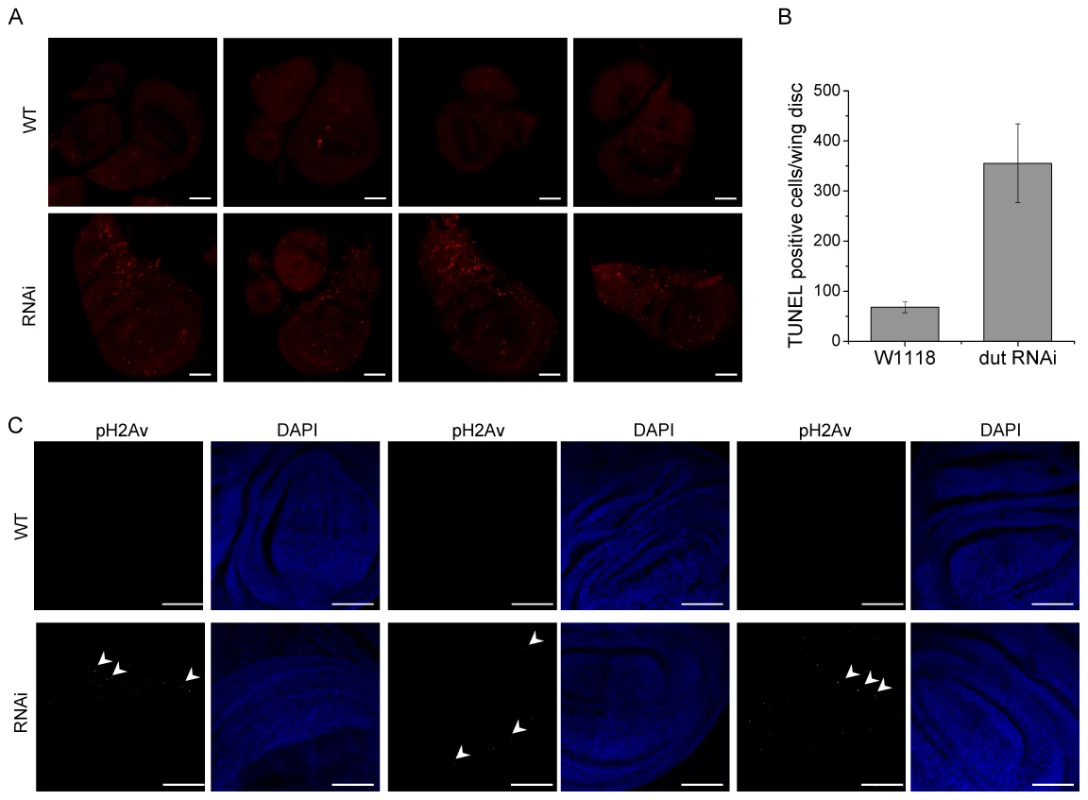
(A) Imaginal discs were isolated from wild type and dUTPase silenced wandering 3rd larvae and stained for TUNEL assay (shown as red dots). Discs from silenced animals showed highly increased TUNEL staining. (B) TUNEL positive cell counts in imaginal discs from wild type and dUTPase silenced wandering 3rd larvae. Error bars represent the standard error of mean. (C) Imaginal discs from wild type and dUTPase silenced 3rd wandering larvae stained against phospho-H2Av foci (white dots, some of these are appointed by white arrowheads). dUTPase depleted discs showed several nuclei with phospho-H2Av foci indicating DNA damage. Scale bar represents 50 µm. Uracil–DNA measurements provided direct evidence that larval tissues of D. melanogaster that undergo developmental degradation accumulate uracil in genomic DNA. Up to date, presence of highly uracil-substituted genomic DNA within wild type organisms was reported only in some viruses; e.g. in bacteriophages [1], [2] and recently in HIV. To our knowledge, the present study may present a first example for naturally occurring permanent existence of uracil–DNA in a free-living complex eukaryotic organism (cf. Figure 3C and 3D). This condition requires developmental down-regulation of dUTPase and absence of UNG in the case of D. melanogaster. Involvement of dUTPase in determining genomic uracil content by regulating dUTP levels was confirmed by dUTPase silencing that resulted in appearance of uracil–DNA in imaginal disc tissues (cf. Figure 4D). Silencing of dUTPase resulted in developmental defects and DNA strand breaks (cf. Figure 5 and Figure 6). We also reported that uracil–DNA is tolerated and interpreted at least from embryonic to 3rd larval stages.
The extraordinary situation of tolerance and stability of uracil–DNA may not be exclusively present in D. melanogaster as absence of ung is ubiquitous among Holometabola (Table 1). As uracil–DNA naturally occurs in larval tissues that are sentenced to death, we consider that uracil–DNA might be linked to metamorphosis and tissue degradation. Further investigations should be taken to describe the mechanism, its impact and its putative role.
Tab. 1. Occurrence of genes encoding dUTPase and UNG in different insects. 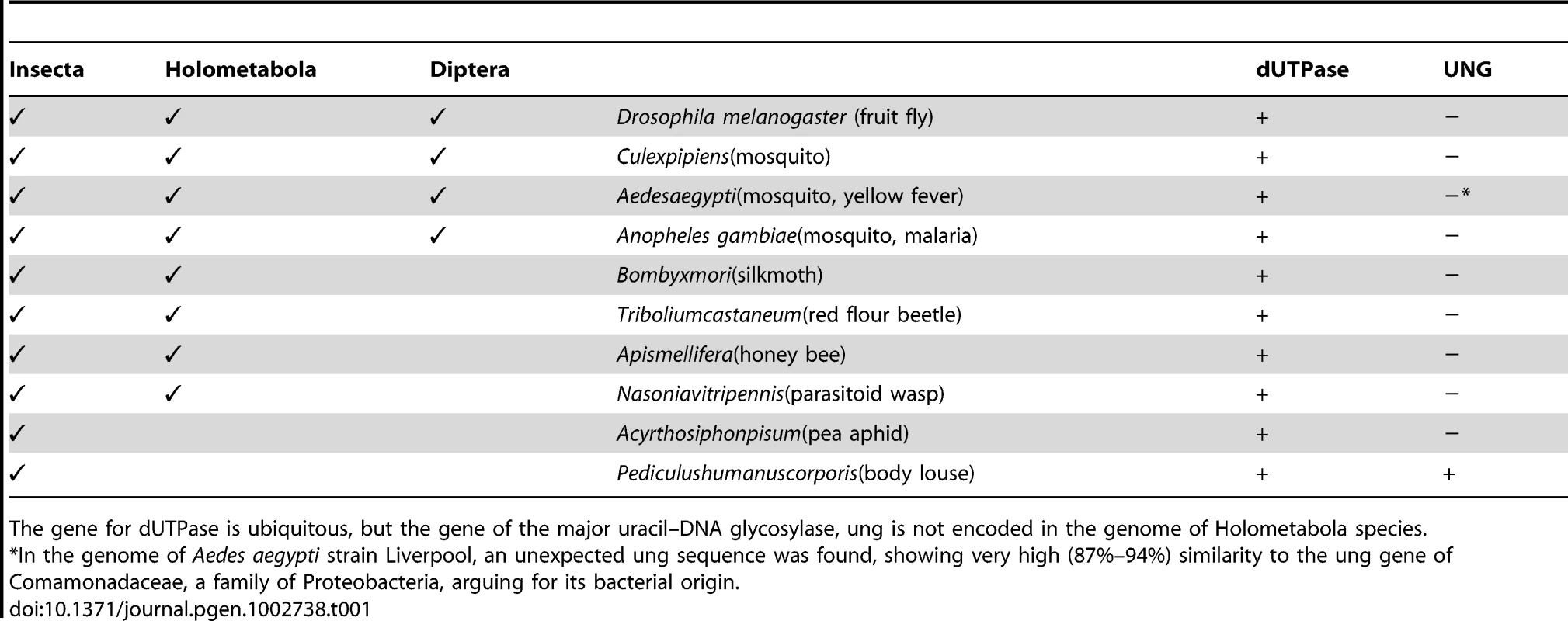
The gene for dUTPase is ubiquitous, but the gene of the major uracil–DNA glycosylase, ung is not encoded in the genome of Holometabola species. Pupal lethality was observed in flies affected by mutations or silencing of purine biosynthesis enzymes [42], [43]. These animals showed apoptosis in developing imaginal primordium during metamorphosis that were also observable as darkened tissues. In these studies, deficient deoxynucleotide biosynthesis may have resulted in imbalanced dNTP levels and increased ratio of mismatches in DNA [44]. Overrepresented DNA modifications like cytosine methylation also result in pupal lethality [45], [46]. In this case increased methylation pattern of DNA inhibits transcriptional activity and cell cycle progression.
We can suggest two hypotheses to explain pupal intolerance of uracil–DNA. First, similarly to the case of hypermethylated DNA, uracil–DNA may show a decreased response and interaction with transcriptional regulators, activators or other morphogenetic factors required specifically during pupal metamorphosis. Second, one or more factor(s), functional only in the pupal stage, may process uracil–DNA resulting in genome instability and defects in cell cycle progression or cell death [47], [48]. Beyond UNG that is missing from Drosophila, other uracil–DNA glycosylases would be suspect for this role. In agreement with this suggestion, expression patterns of other uracil–DNA glycosylases (SMUG and Thd1) and AP endonucleases (Rrp1, RpS3 and RpLP0) indicate relative upregulation during metamorphosis after their lowest expression in larval stages according to microarray data (Table S3) [49]. Developmental downregulation of these base-excision DNA repair pathway (BER) enzymes may contribute to uracil–DNA appearance and tolerance in larval stages, and their upregulation may initiate uracil–DNA processing, destabilization or degradation. The pupal uracil–DNA intolerance pathways hypothesized above are intended to be analyzed in further experiments.
Taken together, we suggest three different factors as being responsible for the stage-specific elevated level of uracil in larval DNA: 1) lack of ung gene, 2) absence of dUTPase and 3) decreased levels of enzymes involved in uracil removal. We also conclude that dUTPase is essential for the full completion of the Drosophila life cycle, although its absence may be tolerated in the larval stages. Although fruit flies, and perhaps other Holometabola insects, are unique in possessing a developmental period when uracil–DNA can be tolerated, preservation and transmission of genetic information for several generations still requires dUTPase.
Materials and Methods
Uracil–DNA stability assays
For the uracil–DNA stability assays, pRm-eYFP, pDsRedMonomer-N1, pP{Gal4VP16} (kind gift from László Sipos) were amplified in E.coli K12 XL1Blue strain and CJ236 dut-1, ung-1 strain (NEB). Plasmids were purified with Plasmid Midi Kit (QIAGEN). Uracil content of the plasmids was checked with UDG and AP endonuclease treatment followed by standard agarose gel electrophoresis [48].
Uracil-containing plasmid stability in cell culture
Transfection of pRm-eYFP-N-C* into Drosophila S2 cells was carried out in the presence of Cellfectine (Invitrogen) following the manufacturer's instructions and expression was induced from the metallothionein promoter at 25°C by addition of 700 µM CuSO4 and overnight incubation. pDsRedMonomer-N1 was transfected into HeLa cells by using Lipofectamine (Invitrogen). Samples were visualized with a Leica DMLS fluorescence microscope 48 hours after transfection.
Uracil-containing plasmid stability in Drosophila embryos
pP{Gal4VP16} plasmids were injected into 0–30 min pP{mw+UASeGFP} transgenic Drosophila embryos (kind gift of László Sipos). For each experiment, app. 40 dechorionated embryos were microinjected. They were aligned on a glass coverslip, dried for 30 min, than covered with 10S Voltalef oil before injection. Plasmid concentration was adjusted to 1 mg/ml, by dilution in standard injection buffer. GFP signal was detected in pre-hatching embryos, 22 h after injection. Embryos without injection served as a negative control.
Cell viability assay
Drosophila Schneider 2 (S2) cells and human HeLa cells were cultured in 96 well plates at 5×104cells/well or 2×103 cells/well, respectively. The culture media used were Serum Free Insect Medium (Sigma, S3777) supplemented with 1% penicillin–streptomycin solution for S2 cells; and DMEM-F12 (Sigma, D8437) supplemented with 10% FBS and 1% penicillin–streptomycin for HeLa cells. FdUR (Sigma) was added at a final concentration range of 0.1–1000 µM. After 96 hours in culture, cell viability was quantified by Alamar Blue assay (BioSource). The experiment was repeated in triplicates.
Quantitative measurement of the uracil content in DNA samples
In order to quantify uracil content of DNA, a real-time quantitative PCR-based assay was used [10]. Genomic DNA was isolated and digested with NheI. DNA fragments of 4–5 kb were purified from gel. Real-time PCR was performed on Mx3000P qPCR System (Agilent Technologies) using EvaGreen dye (Biotium) and PfuTurbo Hotstart DNA polymerase and PfuTurbo Cx Hotstart DNA polymerase (Stratagene). A segment with 963 base length defined by the primers (puBSd-Fw 5′-TCGGGATGACTTTTGGGTTCTG-3′ and puBSd985R 5′-CGCGGTTTAACACAGCGTCGG-3′) is amplified during the PCR reaction. Two-fold dilution series were prepared from DNA samples. Three or more parallel measurements were performed.
dUTPase silencing by RNA interference and rescue of RNAi
UAS-IR stocks were obtained from Vienna Drosophila RNAi Centre (VDRC) [32]. Strain #21883 and #21884 was used for dUTPase silencing. Rescue was performed by co-expression of the RNAi construct with a dUTPase transgene. For details see Text S1, Tables S1 and S2, Figures S4 and S9.
Immunohistochemistry and Western blot analysis
Western blot analysis of larval organs and stage specific dUTPase expression was performed as described previously [21]. For immunohistochemistry, applied primary antibodies were anti-dUTPase (1∶10000) or mouse anti-phospho-Histone H2A.X (Ser139) (Millipore) (1∶250). The latter was shown to recognize Drosophila phospho-Histone H2Av [50]. Tissues were fixed in 50% n-Heptane and 50% PEM-formaldehyde (100 mM PIPES, 1 mM MgCl2, 1 mM EGTA, 2.5% Tween-20, 4% PFA, pH = 6.9) for 30 minutes, with vigorous shacking at room temperature (RT). Samples were washed with inactivating buffer (50 mM TRIS, 150 mM NaCl, 0.5% Tween-20, pH = 7.4). Blocking was performed in the following: 5% goat serum, 1.5% BSA, 0.1% Tween-20, 1% Triton-X 100, 0,001% NaN3, in PBS, pH = 7.4 for 4 hours at RT. Tissues were incubated in primary antibody diluted in blocking buffer (1∶10000), at 4°C for 16 h. Samples were further washed with blocking buffer for 8 h at RT. Secondary antibody was applied to visualize dUTPase staining (Alexa 543 conjugated anti rabbit IgG, Molecular Probes) in blocking buffer (1∶1000) for 2 h, RT. DAPI was used for DNA staining. After further washing steps, samples were mounted in FluorSave (Calbiochem). Images were either acquired with a Zeiss LSCM 710 or a Leica DM IL LED Fluo microscope equipped with a Leica DFC345 FX monochrome camera.
TUNEL assay
TUNEL assay was carried out as described in [40]. After careful dissection in PBS, imaginal discs were fixed (0.1M PIPES, pH = 6.9, 1 mM EDTA, 1% Triton X-100, 2 mM MgSO4, 1% formaldehyde) for 30 min at RT, washed for times with PBS buffer also containing 0.1% Triton X-100 (PBT) and two times with PBT 5X (PBS, 0.5% Triton X-100) (10 min each), and transferred into permeabilization solution (0.1M sodium citrate in PBT) for 30 min at 65°C. Discs were washed twice with PBT 5X, three times in PBT and incubated in reaction buffer (30 mM Tris-HCl, pH = 7.2, 140 mM sodium cacodylate, 1 mM cobalt chloride) for 30 min RT. Reaction was carried out in reaction buffer containing 0.2 unit/microL terminal deoxynucleotidyl transferase (NEB) and 5 microM Cy5-dUTP (GE Healthcare) for 1 hour RT. The reaction was stopped with PBT 5X and washed three times with PBT and finally with PBS. Nuclei were stained by Hoechst. Discs were imaged by laser scanning confocal microscopy (Zeiss LSCM 710). The number of TUNEL positive cells was determined by ImageJ (Rasband, W.S., ImageJ, National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2004.)
Supporting Information
Zdroje
1. DuncanBKWarnerHR 1977 Metabolism of uracil-containing DNA: degradation of bacteriophage PBS2 DNA in Bacillus subtilis. J Virol 22 835 838
2. KiljunenSHakalaKPintaEHuttunenSPlutaP 2005 Yersiniophage phiR1-37 is a tailed bacteriophage having a 270 kb DNA genome with thymidine replaced by deoxyuridine. Microbiology 151 4093 4102
3. LindahlT 1993 Instability and decay of the primary structure of DNA [see comments]. Nature 362 709 715
4. KrokanHEDrablosFSlupphaugG 2002 Uracil in DNA–occurrence, consequences and repair. Oncogene 21 8935 8948
5. VisnesTDosethBPettersenHSHagenLSousaMM 2008 Review. Uracil in DNA and its processing by different DNA glycosylases. Philos Trans R Soc Lond B Biol Sci
6. VertessyBGTothJ 2009 Keeping uracil out of DNA: physiological role, structure and catalytic mechanism of dUTPases. Acc Chem Res 42 97 106
7. AndersenSHeineTSneveRKonigIKrokanHE 2005 Incorporation of dUMP into DNA is a major source of spontaneous DNA damage, while excision of uracil is not required for cytotoxicity of fluoropyrimidines in mouse embryonic fibroblasts. Carcinogenesis 26 547 555
8. LariSUChenCYVertessyBGMorreJBennettSE 2006 Quantitative determination of uracil residues in Escherichia coli DNA: Contribution of ung, dug, and dut genes to uracil avoidance. DNA Repair (Amst) 5 1407 1420
9. LuoYWallaMWyattMD 2008 Uracil incorporation into genomic DNA does not predict toxicity caused by chemotherapeutic inhibition of thymidylate synthase. DNA Repair (Amst) 7 162 169
10. HorvathAVertessyBG 2010 A one-step method for quantitative determination of uracil in DNA by real-time PCR. Nucleic Acids Res 38 e196
11. el-HajjHHWangLWeissB 1992 Multiple mutant of Escherichia coli synthesizing virtually thymineless DNA during limited growth. J Bacteriol 174 4450 4456
12. GadsdenMHMcIntoshEMGameJCWilsonPJHaynesRH 1993 dUtp pyrophosphatase is an essential enzyme in Saccharomyces cerevisiae. Embo J 12 4425 4431
13. DenggMGarcia-MuseTGillSGAshcroftNBoultonSJ 2006 Abrogation of the CLK-2 checkpoint leads to tolerance to base-excision repair intermediates. EMBO Rep 7 1046 1051
14. GuilletMVan Der KempPABoiteuxS 2006 dUTPase activity is critical to maintain genetic stability in Saccharomyces cerevisiae. Nucleic Acids Res 34 2056 2066
15. PettersenHSSundheimOGilljamKMSlupphaugGKrokanHE 2007 Uracil–DNA glycosylases SMUG1 and UNG2 coordinate the initial steps of base excision repair by distinct mechanisms. Nucleic Acids Res 35 3879 3892
16. HardelandUBenteleMJiricnyJScharP 2003 The versatile thymine DNA-glycosylase: a comparative characterization of the human, Drosophila and fission yeast orthologs. Nucleic Acids Res 31 2261 2271
17. SousaMMKrokanHESlupphaugG 2007 DNA-uracil and human pathology. Mol Aspects Med 28 276 306
18. AdamsMDCelnikerSEHoltRAEvansCAGocayneJD 2000 The genome sequence of Drosophila melanogaster. Science 287 2185 2195
19. TakacsEBarabasOPetoukhovMVSvergunDIVertessyBG 2009 Molecular shape and prominent role of beta-strand swapping in organization of dUTPase oligomers. FEBS Lett 583 865 871
20. FiserAVertessyBG 2000 Altered Subunit Communication in Subfamilies of Trimeric dUTPases. Biochem Biophys Res Commun 279 534 542
21. BekesiAZagyvaIHunyadi-GulyasEPongraczVKovariJ 2004 Developmental Regulation of dUTPase in Drosophila melanogaster. J Biol Chem 279 22362 22370
22. KovariJBarabasOTakacsEBekesiADubrovayZ 2004 Altered active site flexibility and a structural metal-binding site in eukaryotic dUTPase: kinetic characterization, folding, and crystallographic studies of the homotrimeric Drosophila enzyme. J Biol Chem 279 17932 17944
23. RichTAShepardRCMosleyST 2004 Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol 22 2214 2232
24. MerenyiGKovariJTothJTakacsEZagyvaI 2011 Cellular response to efficient dUTPase RNAi silencing in stable HeLa cell lines perturbs expression levels of genes involved in thymidylate metabolism. Nucleosides Nucleotides Nucleic Acids 30 369 390
25. WangZMosbaughDW 1988 Uracil–DNA glycosylase inhibitor of bacteriophage PBS2: cloning and effects of expression of the inhibitor gene in Escherichia coli. J Bacteriol 170 1082 1091
26. SeipleLJarugaPDizdarogluMStiversJT 2006 Linking uracil base excision repair and 5-fluorouracil toxicity in yeast. Nucleic Acids Res 34 140 151
27. KumarSAninatCMichauxGMorelF 2010 Anticancer drug 5-fluorouracil induces reproductive and developmental defects in Caenorhabditis elegans. Reprod Toxicol 29 415 420
28. TinkelenbergBAFazzoneWLynchFJLadnerRD 2003 Identification of sequence determinants of human nuclear dUTPase isoform localization. Exp Cell Res 287 39 46
29. BozokyZRonaGKlementEMedzihradszkyKFMerenyiG 2011 Calpain-catalyzed proteolysis of human dUTPase specifically removes the nuclear localization signal peptide. PLoS ONE 6 e19546 doi:10.1371/journal.pone.0019546
30. MerenyiGKonyaEVertessyBG 2010 Drosophila proteins involved in metabolism of uracil–DNA possess different types of nuclear localization signals. FEBS J 277 2142 2156
31. MuhaVZagyvaIVenkeiZSzabadJVertessyBG 2009 Nuclear localization signal-dependent and -independent movements of Drosophila melanogaster dUTPase isoforms during nuclear cleavage. Biochem Biophys Res Commun 381 271 275
32. DietzlGChenDSchnorrerFSuKCBarinovaY 2007 A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 151 156
33. HsuYCChernJJCaiYLiuMChoiKW 2007 Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature 445 785 788
34. LopezTVLappinTRMaxwellPShiZLopez-MarureR 2011 Autocrine/paracrine erythropoietin signalling promotes JAK/STAT-dependent proliferation of human cervical cancer cells. Int J Cancer 129 2566 2576
35. BainbridgeSPBownesM 1981 Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol 66 57 80
36. MinakhinaSStewardR 2006 Melanotic mutants in Drosophila: pathways and phenotypes. Genetics 174 253 263
37. CapdevilaJGuerreroI 1994 Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J 13 4459 4468
38. MorrisEJMichaudWAJiJYMoonNSRoccoJW 2006 Functional identification of Api5 as a suppressor of E2F-dependent apoptosis in vivo. PLoS Genet 2 e196 doi:10.1371/journal.pgen.0020196
39. LeeSBChoKSKimEChungJ 2003 blistery encodes Drosophila tensin protein and interacts with integrin and the JNK signaling pathway during wing development. Development 130 4001 4010
40. Vicente-CrespoMPascualMFernandez-CostaJMGarcia-LopezAMonferrerL 2008 Drosophila muscleblind is involved in troponin T alternative splicing and apoptosis. PLoS ONE 3 e1613 doi:10.1371/journal.pone.0001613
41. MadiganJPChotkowskiHLGlaserRL 2002 DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res 30 3698 3705
42. JiYClarkDV 2006 The purine synthesis gene Prat2 is required for Drosophila metamorphosis, as revealed by inverted-repeat-mediated RNA interference. Genetics 172 1621 1631
43. HollandCLipsettDBClarkDV 2011 A Link Between Impaired Purine Nucleotide Synthesis and Apoptosis in Drosophila melanogaster. Genetics 188 359 367
44. HoffmannJSCazauxC 1998 DNA synthesis, mismatch repair and cancer. Int J Oncol 12 377 382
45. LykoFRamsahoyeBHKashevskyHTudorMMastrangeloMA 1999 Mammalian (cytosine-5) methyltransferases cause genomic DNA methylation and lethality in Drosophila. Nat Genet 23 363 366
46. WeissmannFMuyrers-ChenIMuschTStachDWiesslerM 2003 DNA hypermethylation in Drosophila melanogaster causes irregular chromosome condensation and dysregulation of epigenetic histone modifications. Mol Cell Biol 23 2577 2586
47. BekesiAPukancsikMHaaszPFelfoldiLLevelesI 2011 Association of RNA with the uracil–DNA-degrading factor has major conformational effects and is potentially involved in protein folding. FEBS J 278 295 315
48. BekesiAPukancsikMMuhaVZagyvaILevelesI 2007 A novel fruitfly protein under developmental control degrades uracil–DNA. Biochem Biophys Res Commun 355 643 648
49. GelbartWEmmertD 2010 Flybase High Throughput Expression Pattern Data Beta Version. Flybase
50. WellsBSJohnstonLA 2012 Maintenance of imaginal disc plasticity and regenerative potential in Drosophila by p53. Dev Biol 361 263 276
51. MillardTHMartinP 2008 Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development 135 621 626
Štítky
Genetika Reprodukční medicína
Článek Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target GenesČlánek Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early EmbryosČlánek Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2Článek Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human CellsČlánek TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome EndsČlánek Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal CordČlánek Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth ofČlánek MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 6- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
- Prof. Petr Urbánek: Potřebujeme najít pacienty s nediagnostikovanou akutní intermitentní porfyrií
-
Všechny články tohoto čísla
- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- Mimetic Butterflies Introgress to Impress
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Selection-Driven Gene Loss in Bacteria
- Decreased Mitochondrial DNA Mutagenesis in Human Colorectal Cancer
- Parallel Evolution of Auditory Genes for Echolocation in Bats and Toothed Whales
- Diverse CRISPRs Evolving in Human Microbiomes
- The Rad4 ATR-Activation Domain Functions in G1/S Phase in a Chromatin-Dependent Manner
- Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains
- Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
- Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target Genes
- Adaptive Introgression across Species Boundaries in Butterflies
- G Protein Activation without a GEF in the Plant Kingdom
- Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes
- An Engineering Approach to Extending Lifespan in
- Incompatibility and Competitive Exclusion of Genomic Segments between Sibling Species
- Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in
- Patterns of Evolutionary Conservation of Essential Genes Correlate with Their Compensability
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
- Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early Embryos
- A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity
- Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2
- Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis
- Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells
- TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome Ends
- Stimulation of Host Immune Defenses by a Small Molecule Protects from Bacterial Infection
- A Broad Requirement for TLS Polymerases η and κ, and Interacting Sumoylation and Nuclear Pore Proteins, in Lesion Bypass during Embryogenesis
- Genome-Wide Identification of Ampicillin Resistance Determinants in
- The CCR4-NOT Complex Is Implicated in the Viability of Aneuploid Yeasts
- Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees
- Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
- Is a Key Regulator of Pancreaticobiliary Ductal System Development
- The NSL Complex Regulates Housekeeping Genes in
- Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord
- Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
- Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth of
- Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia
- MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
- Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family
- Found: The Elusive ANTAR Transcription Antiterminator
- The Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility
- Control of CpG and Non-CpG DNA Methylation by DNA Methyltransferases
- Polymorphisms in the Mitochondrial Ribosome Recycling Factor Compromise Cell Respiratory Function and Increase Atorvastatin Toxicity
- Gene Expression Profiles in Parkinson Disease Prefrontal Cortex Implicate and Genes under Its Transcriptional Regulation
- Global Regulatory Functions of the Endoribonuclease III in Gene Expression
- Extensive Evolutionary Changes in Regulatory Element Activity during Human Origins Are Associated with Altered Gene Expression and Positive Selection
- The Regulatory Network of Natural Competence and Transformation of
- Brain Expression Genome-Wide Association Study (eGWAS) Identifies Human Disease-Associated Variants
- Quantifying the Adaptive Potential of an Antibiotic Resistance Enzyme
- Divergence of the Yeast Transcription Factor Affects Sulfite Resistance
- The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis
- RNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
- Rare Copy Number Variants Observed in Hereditary Breast Cancer Cases Disrupt Genes in Estrogen Signaling and Tumor Suppression Network
- Genome-Wide Location Analysis Reveals Distinct Transcriptional Circuitry by Paralogous Regulators Foxa1 and Foxa2
- A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
- The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression
- Limits to the Rate of Adaptive Substitution in Sexual Populations
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- The NSL Complex Regulates Housekeeping Genes in
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



