-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Global Regulatory Functions of the Endoribonuclease III in Gene Expression
RNA turnover plays an important role in both virulence and adaptation to stress in the Gram-positive human pathogen Staphylococcus aureus. However, the molecular players and mechanisms involved in these processes are poorly understood. Here, we explored the functions of S. aureus endoribonuclease III (RNase III), a member of the ubiquitous family of double-strand-specific endoribonucleases. To define genomic transcripts that are bound and processed by RNase III, we performed deep sequencing on cDNA libraries generated from RNAs that were co-immunoprecipitated with wild-type RNase III or two different cleavage-defective mutant variants in vivo. Several newly identified RNase III targets were validated by independent experimental methods. We identified various classes of structured RNAs as RNase III substrates and demonstrated that this enzyme is involved in the maturation of rRNAs and tRNAs, regulates the turnover of mRNAs and non-coding RNAs, and autoregulates its synthesis by cleaving within the coding region of its own mRNA. Moreover, we identified a positive effect of RNase III on protein synthesis based on novel mechanisms. RNase III–mediated cleavage in the 5′ untranslated region (5′UTR) enhanced the stability and translation of cspA mRNA, which encodes the major cold-shock protein. Furthermore, RNase III cleaved overlapping 5′UTRs of divergently transcribed genes to generate leaderless mRNAs, which constitutes a novel way to co-regulate neighboring genes. In agreement with recent findings, low abundance antisense RNAs covering 44% of the annotated genes were captured by co-immunoprecipitation with RNase III mutant proteins. Thus, in addition to gene regulation, RNase III is associated with RNA quality control of pervasive transcription. Overall, this study illustrates the complexity of post-transcriptional regulation mediated by RNase III.
Published in the journal: . PLoS Genet 8(6): e32767. doi:10.1371/journal.pgen.1002782
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002782Summary
RNA turnover plays an important role in both virulence and adaptation to stress in the Gram-positive human pathogen Staphylococcus aureus. However, the molecular players and mechanisms involved in these processes are poorly understood. Here, we explored the functions of S. aureus endoribonuclease III (RNase III), a member of the ubiquitous family of double-strand-specific endoribonucleases. To define genomic transcripts that are bound and processed by RNase III, we performed deep sequencing on cDNA libraries generated from RNAs that were co-immunoprecipitated with wild-type RNase III or two different cleavage-defective mutant variants in vivo. Several newly identified RNase III targets were validated by independent experimental methods. We identified various classes of structured RNAs as RNase III substrates and demonstrated that this enzyme is involved in the maturation of rRNAs and tRNAs, regulates the turnover of mRNAs and non-coding RNAs, and autoregulates its synthesis by cleaving within the coding region of its own mRNA. Moreover, we identified a positive effect of RNase III on protein synthesis based on novel mechanisms. RNase III–mediated cleavage in the 5′ untranslated region (5′UTR) enhanced the stability and translation of cspA mRNA, which encodes the major cold-shock protein. Furthermore, RNase III cleaved overlapping 5′UTRs of divergently transcribed genes to generate leaderless mRNAs, which constitutes a novel way to co-regulate neighboring genes. In agreement with recent findings, low abundance antisense RNAs covering 44% of the annotated genes were captured by co-immunoprecipitation with RNase III mutant proteins. Thus, in addition to gene regulation, RNase III is associated with RNA quality control of pervasive transcription. Overall, this study illustrates the complexity of post-transcriptional regulation mediated by RNase III.
Introduction
Bacteria are highly adaptive organisms that are able to rapidly alter their gene expression in response to environmental changes. In addition to transcriptional control, regulation of RNA decay has emerged as a major pathway in fast adaptive processes. Changes in RNA turnover facilitate stress responses, growth phase transitions, and virulence factor production [1]–[3]. Over the past decades, the knowledge on key ribonucleases that act in processing and turnover of RNAs in Escherichia coli and Bacillus subtilis has increased considerably [1]–[3]. Degradation of mRNA can follow several pathways involving a combination of exo - and endoribonucleases, and differs substantially between Gram-negative and Gram-positive bacteria [3], [4]. For instance, E. coli uses the single-strand-specific RNase E to catalyze the initial rate-limiting cleavage of a large number of mRNAs [1], while mRNA decay in B. subtilis involves the action of the endoribonuclease RNase Y and the bi-functional RNases J1/J2, which are endowed with 5′ exoribonuclease and endoribonuclease activities [5], [6].
Among the endoribonucleases, ribonuclease III (RNase III) is a member of a highly conserved and universal family of double-stranded-RNA (dsRNA)-specific enzymes with essential roles in RNA processing and decay [1], [3], [7]. The discovery that RNase III-type enzymes generate eukaryotic microRNAs and short interfering RNAs has triggered interest in defining the mechanisms of action of this family [8], [9]. Crystal structures of Aquifex aeolicus RNase III in complex with different dsRNAs indicated that this protein contains a long RNA-binding surface cleft denoted the catalytic valley [9], [10]. Bacterial RNase III is a homodimer that forms a single processing center with each subunit contributing to the hydrolysis of one RNA strand. Each monomer contains four RNA binding motifs that make extensive contact with the ribose-phosphate of the dsRNA up to 10 base pairs from the cleavage site, while conserved acidic amino acids and Mg2+ are responsible for catalysis [9], [11]. Biochemical studies have identified the determinants of the dsRNA substrate and RNase III that are required for substrate specificity and catalytic activity. RNase III cleavage produces RNA fragments with 5′-phosphate and 3′-hydroxyl termini and a two-nucleotide 3′-overhang [11]–[14]. Aside from the universal function of RNase III in the maturation of ribosomal RNAs [15], E. coli RNase III plays a broad role in gene regulation. Not only does RNase III autoregulate its own synthesis [16], it also contributes to regulation by small RNAs [17], [18]. In addition, recent genomic analyses revealed that the absence of RNase III in E. coli [19] and B. subtilis [20] affects the abundance of numerous mRNAs and non-coding RNAs (ncRNAs).
Did the cellular functions and substrate specificity of the ubiquitous RNase III diverge in Gram-positive bacteria? In Streptococcus pyogenes, RNase III was identified as an essential host factor for the prokaryotic CRISPR/Cas immunity system [21]. In B. subtilis, the rnc gene is essential suggesting that RNase III-dependent maturation of one or several critical mRNAs is required for protein synthesis [20], [22]. In Staphylococccus aureus, an rnc mutant strain showed compromised virulence in a murine peritonitis model [23], while rnc deletion did not impair cell growth [23], [24]. Our previous studies in S. aureus have shown that RNase III coordinates the repression of mRNAs encoding virulence factors and a transcriptional regulator via the quorum-sensing-dependent regulatory RNA, RNAIII [24]–[26]. The RNAIII-target mRNA complexes adopt various topologies, such as imperfect duplexes and loop-loop interactions that are efficiently recognized and cleaved by RNase III, thus leading to irreversible repression [27]. In addition, a very recent study has shown an unprecedented role of RNase III in antisense regulation restricted to Gram-positive bacteria [28]. Deep sequencing of short S. aureus RNAs revealed numerous 22-nt RNA fragments generated by RNase III digestion of sense/antisense RNAs and almost 75% of the cleaved mRNAs had corresponding antisense RNAs [28]. These data are indicative of pervasive antisense regulation by RNase III. Collectively, the previous studies in E. coli [19], B. subtilis [20], and S. aureus [28] evaluated the role of RNase III at a genome-wide scale. However, these analyses of transcriptome changes by tiling array or RNA-seq are not per se suitable to identify direct RNase III substrates because they also score indirect regulatory effects. This prompted us to more precisely analyze the functions and direct targets of S. aureus RNase III in gene regulation.
We present here the first global map of direct RNase III targets in S. aureus. To this end, we used deep sequencing to identify RNAs associated with epitope-tagged wild-type RNase III and two catalytically impaired but binding-competent mutant proteins. Newly identified RNase III targets were validated by a combination of in vivo and in vitro approaches. Our analysis revealed an unexpected variety of structured RNA transcripts as novel RNase III substrates. In addition to rRNA operon maturation, autoregulation of rnc mRNA decay, degradation of structured RNA transcripts, and antisense regulation, we propose novel mechanisms by which RNase III activates the translation of mRNAs through cis - or trans-acting elements. Overall, our study explores the broad function of RNase III in gene regulation of S. aureus.
Results
Mutations in S. aureus RNase III uncouple binding and catalytic activities
Biochemical and structural studies performed on RNase III in A. aeolicus and E. coli demonstrated a stepwise hydrolysis mechanism of the phosphodiester bonds mediated by two Mg2+ ions, involving mutual conformational changes of the RNA and the enzyme [10], [29]. The nuclease domain of RNase III is characterized by two clusters of conserved acidic amino acids, in which the side chains of E41, D45, D114, and E117 (in E. coli) are coordinated to Mg2+ ions [13], [30], [31]. Two of these residues, E117 and D45, are essential for catalysis, as their substitution by alanine strongly compromised cleavage without affecting RNA binding [11], [14], [29], [31]. Although S. aureus RNase III (Sa-RNase III) shares only 33% amino acid identity with the E. coli enzyme, the acidic amino acids are strictly conserved (Figure 1A). To obtain catalytically inactive but binding-proficient variants of Sa-RNase III, amino acids E135 and D63 (corresponding to E117 and D45 in E. coli, respectively) were changed to alanine (Figure 1A). A histidine epitope tag was added to the N-terminus of the mutant and wild-type (WT) proteins, and the proteins were purified to homogeneity following expression in E. coli [27]. The activities of the mutant enzymes were compared to that of the WT protein using spa mRNA, a well-characterized Sa-RNase III substrate [24]. Terminally labeled spa mRNA was used to map the cleavage sites for WT and mutant S. aureus proteins and cleaved products were resolved by polyacrylamide gel electrophoresis under denaturing conditions (Figure 1B). As expected, WT RNase III cleaved both sides of a helix at U70, C98, and G110 in the coding sequence (CDS) of spa mRNA. The E135A mutation very strongly compromised the activity of the enzyme, while the effect of D63A was less pronounced (Figure 1B). Gel retardation assays were used to monitor the binding of the mutant enzymes to terminally labeled spa mRNA in a buffer containing Ca2+ instead of Mg2+ (Figure 1C). Ca2+ inhibits the catalytic activity of E. coli RNase III but does not affect RNA binding [32]. In our study, the mutant E135A (Figure 1C) and D63A (result not shown) enzymes bound spa mRNA in a manner similar to the WT RNase III. Hence, the two mutations uncoupled catalytic activity from RNA binding capacity in a manner similar to that described for E. coli RNase III [11], [14], [29]. These two mutant proteins were used to capture RNA substrates in vivo.
Fig. 1. Effect of mutations in the catalytic site of Staphylococcus aureus RNase III. 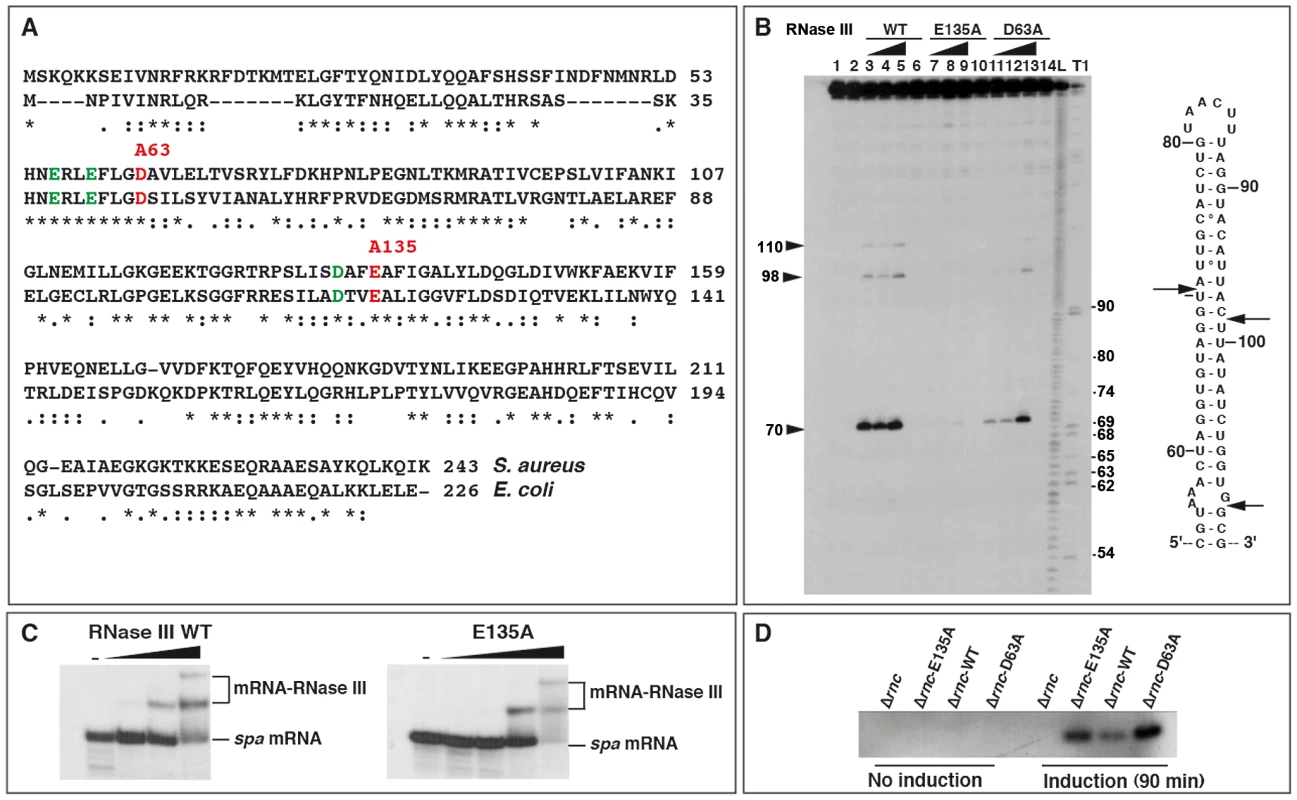
(A) Amino acid sequence alignment of RNase III from S. aureus and Escherichia coli. Acidic amino acids colored in green and red are conserved residues present in the catalytic site of E. coli RNase III. The two mutations (D63 to A and E135 to A) generated in S. aureus RNase III are shown in red. (B) RNase III cleavage assays were performed on 5′ end-labeled spa mRNA using the wild-type enzyme (RNase III-wt), the E135A, and the D63A RNase III mutant enzymes. Lanes 1, 2: incubation controls set in the absence of RNase III. Cleavage reactions were performed in the presence of Mg2+ with increasing concentrations of RNase III WT (lanes 3–5), the E135A (lanes 7–9) or D63A (lanes 11–13) mutant proteins: (lanes 3, 7, 11) 0.165 µM; (lanes 4, 8, 12) 0.33 µM; (lanes 5, 9, 13) 0.66 µM. Control reactions included Ca2+ and 0.66 µM of RNase III WT (lane 6), E135A (lane 10) or D63A (lane 14) mutants. Lanes T, L: RNase T1 and alkaline ladders, respectively, under denaturing conditions. Arrows denote the positions of RNase III cleavages which are shown in the secondary structure of the RNase III-binding site on spa mRNA. (C) Binding of the wild-type (RNase III-wt) and mutant (E135A) enzymes to 5′ end-labeled spa mRNA as visualized by gel retardation assays. Lane (−): incubation control of the free RNA in the absence of RNase III. Increasing concentrations of RNase III WT (0.05, 0.1 and 0.2 µM) or RNase III-E135A (0.08, 0.16, 0.32 and 0.49 µM) were added to 5′ end-labeled spa mRNA in a buffer containing Ca2+ instead of Mg2+. (D) RNase III-Flag protein levels at mid-logarithmic phase were monitored in various S. aureus strains: Δrnc mutant strain, Δrnc mutant strain complemented with the Flag-E135A mutant, the Flag-wt RNase III and the Flag-D63A mutant. Cells were collected before or after induction with CdCl2 for 90 min. 40 µg of total protein were resolved on an SDS-PAGE gel. The western blot was performed with an anti-Flag monoclonal antibody. Identification of several classes of structured RNAs associated with RNase III
To identify RNase III targets in vivo, co-immunoprecipitation (coIP) assays were carried out with Flag epitope-tagged WT and mutant proteins expressed from a plasmid-borne Cd2+-inducible promoter in a Δrnc S. aureus background. The Flag-epitope was added to the C-terminus of the proteins. A control coIP experiment was performed with the untagged WT RNase III expressed from the chromosome (strain RN6390). Bacteria were harvested at two time points (4 and 6 h of growth at 37°C) corresponding to exponential and late exponential phases of growth, respectively. The growth curves of RN6390 (WT strain), Δrnc mutant strain, and Δrnc complemented with WT RNase III, E135A, or D63A mutant proteins, were similar in the BHI medium (data not shown). Western blot analysis showed that the two mutant proteins accumulated at comparable levels while the WT protein was expressed at a lower level (Figure 1D), indicative of a possible autoregulatory event on the rnc mRNA.
RNAs isolated from the coIP experiments with the four strains were converted to cDNA libraries and analyzed by high-throughput pyrosequencing as previously described [33]. Recovered sequences ranged from 1 to 145 nt, but sequences below 18 nt were discarded in later analyses to increase the accuracy of mapping (Table S1). In agreement with the impaired catalytic activity of the mutants, we obtained more reads of ≥18 bp with the E135A (77% of the total reads) and D63A (86%) mutant proteins than with WT RNase III (51%). Mapping of the cDNA reads to the genome of S. aureus N315 revealed that the mutant enzymes primarily recovered RNA fragments arising from rRNA and tRNA operons (80 to 90% of the total number of mapped reads) (Table S2). However, a high number of reads were mapped to 58 different ncRNAs including the housekeeping ncRNAs tmRNA, RNase P, and the RNA component (4.5S RNA) of the signal recognition particle (SRP) (Tables S2, S3). Furthermore, in the coIPs of the mutant enzymes, reads were recovered for almost 1,500 individual mRNAs of the 2,653 annotated ORFs in the S. aureus genome, but considerably fewer were recovered for polycistronic mRNAs (mnhA-G, gapR, pdhA-D, and qox; Table S4). Moreover, a significant number of reads corresponded to antisense RNAs (asRNAs) that were assigned to the opposite strand of 1,175 mRNAs (Tables S1, S5). Given the limited number of sequenced cDNAs (∼1×105) (Table S1), the actual number of RNase III targets may be underestimated. Based on comparison to sequences from the control coIP (WT strain expressing untagged RNase III), which should represent RNAs that are unspecifically bound during the coIP, we only considered transcripts as potential RNase III substrates if they were significantly enriched in the coIP samples of the tagged variants (Tables S2, S3, S4, S5). Not surprisingly, the RNAs that were unspecifically bound in the control coIP reflect the abundance of the transcripts in the cell, i.e., most of these reads were derived from rRNAs that represent >90% of the transcripts in the cell (Table S2). We note that the co-immunoprecipitated RNAs identified with the E135A and D63A mutant proteins were very similar, supporting the reliability and reproducibility of the method (Tables S2, S3, S4, S5). Moreover, many of the target RNAs were detected at both time points of cell growth. Representatives of each RNA class were then selected for experimental validation using in vitro and in vivo approaches (Table 1).
Tab. 1. Validation of several RNA targets of RNase III. 
The annotation of the genome is taken from strain N315. We first performed gel retardation assays to validate a direct interaction between various classes of RNAs and the mutant E135A protein. The data showed that the E135A protein bound to a plethora of structured RNAs including cis-acting regulatory elements of mRNAs (e.g., the flavin mononucleotide (FMN) sensing riboswitch), ncRNAs, structured mRNAs, and small ORF-containing RNAs (Figure S1A). Competition binding assays were also performed to monitor the specificity of RNase III binding on cspA mRNA. Two forms of cspA mRNAs were analyzed: cspAL containing a long 5′UTR (113 nt), which was recovered with the two mutant proteins and cspAS containing a short 5′UTR (52 nt), which was pulled down with the WT enzyme. We also used SA2097 mRNA, which was not co-immunoprecipitated with the mutant and WT RNase III. The experiments were carried out with the 5′ end-labeled cspAL mRNA bound to E135A mutant protein in the presence of increasing concentrations of cold cspAL, cspAS or SA2097 mRNA (Figure S1B). The experiments showed that the concentrations of cspAS and SA2097 necessary to compete for binding were 10 times higher than that for cspAL suggesting that the interaction of RNase III with cspAL is specific.
Overall, the data strongly suggest that the immunoprecipitated RNAs resulted from a direct interaction with RNase III. The molecular mechanism of RNase III action on several target RNAs was then studied in more detail both in vivo and in vitro (Table 1).
RNase III initiates maturation of rRNA operons
A high number of reads were mapped to the five rRNA operons and several isolated tRNA operons. The most highly enriched RNA fragments, pulled down with the mutant proteins, corresponded to the intergenic regions of the rRNA operons (Figure 2A). This strongly suggests a role of RNase III in rRNA and tRNA processing as it was previously demonstrated in E. coli [34] and B. subtilis [22]. We probed one of the five rRNA operons in WT and mutant strains using antisense oligonucleotides complementary to different tRNA and rRNA intergenic sequences (Figure 2B). As expected in the case of impaired rRNA processing, 16S precursor transcripts were observed in the Δrnc strain and in the same strain complemented with either the E135A or D63A mutant enzyme, but not in the RN6390 (WT) strain or in the Δrnc strain complemented with WT RNase III. In addition, aberrant precursors from 5S rRNA and tRNAs were visible on Northern blots probed with a specific DIG-labeled riboprobe or the 5′ end-labeled oligonucleotide 278, respectively, in Δrnc cells and in the same strain expressing the mutant E135A protein (Figure 2B).
Fig. 2. Processing of the rRNA operon by RNase III. 
(A) Visualization of RNA fragments identified by deep sequencing in the S. aureus genome using the Integrated Genome Browser (IGB, Affymetrix). For mapping of cDNAs, the genome sequence of strain N315 was used. Sequenced cDNA reads of RNAs obtained from RN6390 strain (negative control) are shown in red, RNA fragments co-immunoprecipitated with the Flag-RNase III D63A mutant in blue, Flag-RNase III wild type (wt) in green, and Flag-RNase III E135A mutant in magenta. (+) Indicates the leading and (−) the lagging strand, respectively. The Y-axis indicates a relative score for the number of mapped reads per nucleotide normalized to the total number of reads. (B) Analysis of rRNA and tRNA precursors in the WT and mutant Δrnc strains. Northern blot analyses were performed with 5′ end-labeled oligonucleotides using RNAs isolated from RN6390 (WT), the Δrnc strain (Δrnc) or the same strain transformed with a plasmid expressing either the E135A mutant (Δrnc-E135A), the wild type (Δrnc-WT) or the mutant D63A (Δrnc-D63A) enzymes. 5S rRNA was detected with a specific DIG-labeled riboprobe, which was produced by in vitro T7 transcription from a PCR product amplified with the oligonucleotides 381/382 (Table S8). Arrows denote the different precursors. (C) Identification of RNase III cleavages in vitro and in vivo using primer extension. The cleavages were mapped in vitro on the 16S rRNA transcript carrying 134 nucleotides at its 5′ trailer region and 100 nucleotides at its 3′ trailer (lanes 1 to 4), and in vivo on total RNA extract (lane 5, WT strain; lane 6, Δrnc strain). RNase III cleavage sites were detected by reverse transcription using the 5′ end-labeled oligonucleotide 405. Lane 1: Incubation control; lanes 2–4: in vitro cleavage assays performed with RNase III WT on pre-16S rRNA using two concentrations of RNase III (lane 2, 0.1 µM; lanes 3–4, 0.2 µM) in the presence of Mg2+ (lanes 1–3) or of Ca2+ (lane 4); lanes U, C, G, A: sequencing ladders corresponded to the RNA sequence. Primer extension was performed on total RNAs prepared from exponential cultures of RN6390 (lane 5) and Δrnc strains (lane 6). (D) Secondary structure prediction of the corresponding pre-rRNA operon. Black arrows show positions of the RNase III cleavages, which were experimentally mapped in the 16S pre-rRNA. PE denotes the primer extension stop (red arrow) obtained from total RNA extract. Secondary structure analysis of the rRNA operon transcripts predicted that the termini of 16S rRNA and 23S rRNA might each base-pair within long helical domains, generating a typical RNase III substrate (Figure 2D). RNase III cleavage assays were performed on an in vitro transcribed 16S rRNA containing the 5′ and 3′ end trailing sequences (see Text S1). Cleavage sites were identified by primer extension on the cleaved rRNA with reverse transcriptase using either the 5′ end-labeled oligonucleotide 405 (Figure 2C) or the 5′ end-labeled oligonucleotide 279 (result not shown). Two specific RNase III cuts were identified at positions A-92 (Figure 2C) and U+64 (result not shown), respectively. These cleavages produced a two-nucleotide 3′ overhang, a hallmark of processing by RNase III (Figure 2D). Primer extension was also performed on total RNA extracted from the WT and Δrnc strains. Using the 5′ end-labeled oligonucleotide 405 that hybridizes within the 16S rRNA, a major reverse transcriptase (RT) stop at A-91 within the 5′ trailer of the 16S rRNA precursor was only seen in the WT strain. Thus, the in vivo and in vitro RNase III cleavages within the 16S rRNA precursor were congruent (Figure 2C). Interestingly, in the Δrnc strain, several RT stops were detected upstream of A-92 in vivo (Figure 2C, lane 6), suggesting that another ribonuclease might target the same region in the absence of RNase III. Such alternative rRNA processing that permits the production of functional ribosomes tentatively explains why the Δrnc mutation in S. aureus has only minor effects on cell viability [23], [24].
RNase III autoregulates its own synthesis at the post-transcriptional level
Reads, mapping to rnc mRNA, were consistently recovered with both the WT and the two RNase III mutants but not in the control coIP (Figure 3A). These data suggest that RNase III of S. aureus specifically recognizes its own mRNA. This hypothesis is supported by the Western blot of the E135A, D63A, and WT proteins expressed in the Δrnc strain because the two mutant proteins accumulated to higher levels than the fully catalytically active WT enzyme (Figure 1D). Prior to mapping the RNase III cleavage site, we determined the 5′ end of the rnc mRNA in vivo by primer extension (Figure S2A). Two major reverse transcriptase (RT) stops were found, one located at G+306 in the coding sequence and the other >70 nt upstream of the AUG start codon (Figure S2A). Several weaker stops were also observed, e.g. at position U+296, after longer exposure of the autoradiography (Figure S2A). Given the location in the CDS, the RT stop at G+306 represented an internal cleavage of rnc mRNA. We then mapped the RNase III cleavage sites on in vitro synthesized full-length rnc mRNA (843 nt) and a truncated version (752 nt) in which a large part of the 5′UTR had been deleted (Figure 3B). The unlabeled RNAs were subjected to RNase III hydrolysis, and the RNA fragments were separated on agarose gels under denaturing conditions followed by staining with ethidium bromide (for experimental details, see Text S1). RNase III specifically cleaved the in vitro transcribed rnc mRNA and generated at least two main fragments in a Mg2+-dependent manner (Figure 3B). Removal of the 5′UTR altered migration of the smaller fragment, identifying this fragment as 5′ proximal. This result suggests that RNase III recognizes and cleaves its own CDS.
Fig. 3. RNase III autoregulates its own expression. 
(A) Visualization of mapped cDNA reads on the S. aureus genome corresponding to rnc mRNA fragments using the Integrated Genome Browser (IGB, Affymetrix). Same legend as described in Figure 2A. The red arrow indicates the start of a cleaved fragment at position +U296. (B) In vitro RNase III cleavage assays on unlabeled full-length rnc mRNA (FL-rnc 843 nt, lanes 1–7) or the rnc mRNA lacking its 5′ untranslated region (Δ5′UTR-rnc 752 nt, lanes 8–12). The RNA fragments were separated using an agarose gel under denaturing conditions and visualized after ethidium bromide staining. Unlabeled rnc mRNA (200 nM) was incubated with purified wild type RNase III (WT) (lanes 2, 3, 9, 10) or the mutants E135A (lanes 4, 5, 11) or D63A (lanes 6, 7, 12). Lanes 1, 8: incubation controls of rnc mRNAs in the absence of enzyme. Cleavage assays were performed with WT-RNase III at 0.33 µM in a buffer containing Mg2+ (lanes 2, 9) or Ca2+ (lanes 3, 10), with the mutant E135A at 0.33 µM (lanes 4, 11) and 0.66 µM (lane 5), and with the mutant D63A at 0.33 µM (lane 6, 12) and 0.66 µM (lane 7). Lane M: Riboruler low range RNA marker (Fermentas). (C) RNase III cleavage sites were mapped on in vitro transcribed rnc mRNA using primer extension with the 5′ end-labeled oligonucleotide 69 (Table S8). Reactions were performed in the presence of increasing concentrations of wild type RNase III (0.33 and 0.66 µM) in a buffer containing either Mg2+ or Ca2+. Lanes U, C, G, A: DNA sequencing reactions but the labels correspond to the RNA sequence. The black arrow denotes the RNase III cleavage at U+296. (D) Secondary structure of the RNase III-binding site located in the coding region of rnc mRNA. A black triangle indicates the in vitro cleavage site at position U+296 while the red arrow represents the primer extension stop. The RNase III cleavage sites were then mapped more precisely by reverse transcription in vitro (Figure 3C). This experiment showed that position U+296, located within the CDS of rnc mRNA, is the site of the major RNase III-dependent cleavage. Notably, this cleavage coincided with the 5′ end of the RNA fragment recovered by coIP with WT RNase III (Figure 3A). It is surprising, however, that the primer extension performed on total RNA identified a potential RNase III-dependent cleavage at G+306 of rnc mRNA, 10 nucleotides downstream of U+296 (Figure 3C and 3D). Although we do not exclude that RNase III cleaves its own mRNA differently in vivo, additional trimming of the cleaved RNA by an unknown ribonuclease could tentatively explain this difference. Structure probing of the rnc mRNA was performed using the single-strand-specific RNases T2 and T1, and the double-strand-specific RNase V1 (Figure S2B and S2C). Enzymatic reactions were restricted to less than one cut per molecule, and cleavages were mapped by reverse transcription [35]. The structure probing supported the formation of three long hairpins in the CDS, as indicated by numerous RNase V1 cleavages located in the arms and strong RNase T2/T1 cuts occurring in the apical loops (I, II, and III) and the internal loop regions (Figure S2C). The long irregular helix III, in which the RNase III cleavage site at U+296 is located, appears to be the preferred RNase III binding site (Figure 3D).
Taken together, the data support a model wherein RNase III initiates decay of its own mRNA within the CDS, resulting in negative feedback regulation of its expression. The deep sequencing analysis additionally revealed several RNA fragments that were antisense to rnc mRNA (Figure 3A). However, expression of these asRNAs was not detectable by Northern blot experiments in the RN6390 strain, indicating a very low abundance and/or low stability of these transcripts.
The 5′ untranslated region of cspA mRNA is processed by RNase III
The cspA mRNA, which encodes the major cold-shock protein and RNA chaperone, was a candidate RNase III substrate because the entire transcript was represented by reads from the coIP with the E135A mutant protein (Figure 4A; Table 1 and Table S4). To validate this target, we used Northern blots to first compare cspA expression in RN6390 and the Δrnc strain, in the presence or absence of RNase III WT and mutant proteins (Figure 4B). Surprisingly, the absence of RNase III (Δrnc strain) led to the accumulation of a longer cspA mRNA (cspAL) than that observed in the WT strain (Figure 4B). While complementation of the Δrnc strain by functional RNase III partially restored the WT pattern, the two mutant variants did not (Figure 4B). Thus, RNase III appeared to process the cspA transcript into a shorter form (cspAS). Northern blot analysis was then performed on RNA samples collected throughout growth from WT or Δrnc strains at 37°C, after cold-shock at 15°C (at t0, Figure 4C), and after shifting cultures back to 37°C (at t3, Figure 4C). Under all of these conditions, cspAL mRNA only accumulated in the Δrnc strain, suggesting that the maturation is not regulated by cold-shock but rather is a step in the normal biogenesis of cspA mRNA. Next, we performed primer extension on total RNA extracts for a comparative mapping of the 5′ end of cspA mRNA in WT and Δrnc strains (Figure 4D). The 5′ end of the processed cspAS transcript (WT strain) mapped to U-52, while that of the unprocessed cspAL mRNA (in Δrnc) was found 60 nucleotides upstream, at U-113 (Figure 4D). Importantly, the 5′ end of the cspAL transcript precisely matched the 5′ boundary of RNA fragments recovered in the coIPs with the two mutant enzymes (Figure 4A). Thus, the comparison of WT and mutant enzymes pinpointed an RNase III-mediated processing event. We then precisely mapped the RNase III cleavage sites on an in vitro synthesized unlabeled cspAL by reverse transcription (Figure 4E), and by using 5′ end-labeled cspAL (Figure 5D). RNase III hydrolysis of unlabeled cspAL followed by primer extension, revealed a major cleavage site at G-53 and a minor one at A-88 (Figure 4E), generating the characteristic two-nucleotide 3′ overhang (Figure 4F). The cleavage at position A-88 was also clearly detected with the 5′ end-labeled cspAL (Figure 5D). Importantly, cleavage at G-53 matched the 5′ termini of cspAS in vivo (Figure 4D). Hence, the RNase III cleavage assay in vitro faithfully recapitulated a major step of cspAL mRNA processing in vivo.
Fig. 4. RNase III processes the 5′ untranslated region of cspA mRNA. 
(A) IGB representation of the cspA locus. Same legend as in Figure 2A is applied. The +1 site identified by primer extension in the Δrnc strain is indicated by a red arrow (U-113). (B) Expression of cspA mRNA forms after 4 h of growth at 37°C in strains RN6390 (WT), Δrnc and Δrnc expressing the E135A, WT or D63A Flag-tagged RNase III. CspAL corresponds to the longest form of the mRNA while cspAS corresponds to the processed form of the mRNA. (C) Growth curves of RN6390 (WT) and Δrnc mutant strains (Δrnc). Two cultures were grown at 37°C (black diamonds: RN6390; black triangles: Δrnc) while two other cultures (black squares: RN6390; black circles: Δrnc) were transferred to 15°C (t0) after 120 min of growth at 37°C, then incubated for an additional 120 min at 15°C, and retransferred to 37°C at time t3. In this experiment, a mild growth defect was observed for the Δrnc strain. However, this defect was not reproducible and could be attributed to a higher level of cell aggregation in this strain during the late exponential phase of growth. Northern blot analyses showing the expression of cspA mRNA at 37°C or at 15°C at the indicated time-points are depicted in the insets. Lanes 1, 2: RNA samples prepared from RN6390 or Δrnc mutant strains, respectively. Lanes t-1 to t5: incubation times of cell cultures as shown in the growth curves. A DIG-labeled DNA probe (amplified using the oligonucleotides 286 and 16) was used to detect cspA mRNA and the autoradiography was revealed after several seconds. (D) Primer extension analysis performed on total RNAs isolated from cells grown at 37°C for 3 h and 4 h. Lane 1: RN6390 strain; lane 2: Δrnc strain. The 5′ end detected in each strain is indicated. The nucleotides are numbered relatively to the AUG start codon. Lanes C, U, A, G: sequencing ladders. Primer extension was carried out using the 5′ end-labeled oligonucleotide 378 (Table S8). (E) RNase III cleavage of unlabeled cspAL mRNA. The reactions were done in the absence (−) and in the presence of RNase III (+, 0.33 µM; ++ 0.65 µM) in a buffer containing Mg2+ or Ca2+. Lanes C, U, G, A: sequencing reactions. The same oligonucleotide 378 was used for reverse transcription to analyze the cleavage sites. The RNase III cut at position A-88 appears as a faint band because the enzymatic reaction is too strong. Arrows denote the specific RNase III-induced cleavages at U-53 and A-88 (relatively to the AUG). Lane (−): incubation control in the absence of RNase III. The cleavages were assigned after primer extension using the 5′ end-labeled oligonucleotide 16. (F) Secondary structure of cspAL is deduced from structure probing experiments. The structure of the 5′UTR of cspAS is shown in the inset. The grey arrow corresponds to the RNase III cleavage sites obtained in vitro while the red arrow represents the reverse transcriptase stop, which was assigned by primer extension in RN6390 (WT). The Shine and Dalgarno (SD) sequence and the AUG strat codon are indicated in red. Fig. 5. RNase III–dependent processing stabilizes cspA mRNA and enhances translation. 
(A) Upper panel: cspA mRNA stability was assessed in RN6390 and Δrnc strains after rifampicin treatment. Expression of 5S rRNA was monitored in the same samples as a loading control. Molecular weight ladders are indicated on the left of the gel. The two forms of cspA mRNA were detected using a DIG-labeled riboprobe transcribed by T7 RNA polymerase from a PCR template amplified using the oligonucleotides 367–368 (Table S8). Lower panel: Quantification of cspA mRNA level and half-life determination in RN6390 (cspAS, diamonds) and in Δrnc strain (cspAL, squares) as a function of time. CspAL is the unprocessed mRNA and cspAS, the processed mRNA. The value corresponding to the percentage (%) of the remaining mRNA was normalized with the control experiment performed with 5S rRNA. The half-life was determined from a semi-logarithmic plot of the concentration of the mRNA over time. The slope of the best-fit line was then determined to calculate the half-life, which corresponded to the time-point where 50% of the initial mRNA amount remained. Three experiments provided reproducible results. (B) Formation of the ternary 30S initiation complex using the two forms of cspA mRNA (cspAL, and cspAS). Ternary complex formation was monitored in the presence of increasing concentrations of S. aureus 30S ribosomal subunit (5, 10, 50, 100, 200 and 300 nM), and the initiator tRNAfMet (1 µM). (−): Incubation controls without 30S. Lanes C, G, U, A: sequencing ladders of cspAL mRNA. The position of the toeprint at +16 and the +1 site corresponding to the AUG codon are indicated. Primer extension was done with the 5′ end-labeled oligonucleotide 16 (Table S8). Lower panel: Quantification of 30S ribosome binding on cspAL (green) and cspAS (red) mRNAs. Relative toeprints were calculated by relating the intensity of the band corresponding to the toeprint at +16 to the sum of the intensities of this band and the band corresponding to the full length RNA. (C) Schematic model summarizing the role of RNase III in cspA maturation. RNase III cleaves the long hairpin structure at the 5′ end of cspAL to produce an mRNA with a shorter 5′ untranslated region, which is more stable and translated with a higher efficiency. (D) Top panel: Northern blot analysis showing the expression of the antisense RNA as-cspA. Total RNAs were prepared from RN6390 (RN6390, WT) and Δrnc mutant (Δrnc) strains at 2, 4 and 6 h of growth at 37°C. Molecular weight ladders are indicated on the left of the gel. To detect as-cspA, a DIG-labeled riboprobe was transcribed in vitro with T7 RNA polymerase from a PCR template amplified with the oligonucleotides 286 and 16 (Table S8). The asRNA signal was detected after a long exposure of the autoradiography (30 min). Bottom panel: autoradiography showing the fractionation of RNase III cleavages of 5′ end-labeled cspAL mRNA alone or in the presence of the antisense RNA (as-cspA). Incubation controls of cspAL mRNA alone or with as-cspA in the absence of RNase III are shown respectively in lanes 1 and 4. The RNase III cleavage assays were done in the presence of Mg2+ (lanes 2, 5–8) or Ca2+ (lanes 3, 9) with cspAL mRNA alone (lanes 2–3) or with as-cspA (lanes 4–9). The cspA mRNA-as-cspA duplex was formed with denatured RNAs (denaturing conditions) or with RNAs, which were separately renatured (native conditions) (see Text S1). Increasing concentrations of asRNA were used: 10 nM (lane 5), 25 nM (lane 6), 50 nM (lane 7), and 100 nM (lanes 4, 8, 9). Lanes L, T: alkaline ladder and RNase T1 performed on cspAL mRNA under denaturing conditions, respectively. Lanes 3, 9 (native conditions): the experiments performed in the presence of Ca2+ under native conditions show a residual activity of RNase III due to the presence of Mg2+, which was used to fold the RNAs prior to complex formation. The arrow indicates the RNase III cleavage at position A-88 occurring in free cspAL mRNA, and the bar shows the strongest cleavages induced by the as-cspA binding. Processing of cspA mRNA by RNase III activates CspA synthesis
Having confirmed that RNase III processing occurs within the 5′UTR of cspA mRNA, we set out to define the functional consequences of this event. The secondary structures of cspAL and cspAS were compared using single-strand-specific RNases (RNases T2 and T1) and the double-strand-specific RNase V1 on in vitro synthesized mRNAs (Figure S3A). The enzymatic cleavages were mapped by primer extension (for experimental details, see Text S1). The derived secondary structure model supports that cspAL mRNA is highly structured and starts at the 5′ end with several unpaired nucleotides followed by an almost perfect 32-bp helix (Figure 4F and Figure S3B). This long 5′ hairpin structure resembles a typical RNase III binding site. Shortening of the 5′UTR led to the formation of a smaller but stable 5′ hairpin structure in cspAS (Figure 4F, inset). Paired nucleotides at the 5′ end of mRNAs are known to protect against pyrophosphate removal by RppH and degradation by the 5′-3′ exoribonuclease activity of RNase J1 in B. subtilis [3], [36]. To evaluate the effect of the short stable 5′ hairpin on transcript decay, we analyzed the in vivo RNA stability of cspA mRNA by Northern blot experiments after rifampicin treatment (Figure 5A). Quantification of the data showed that the processing significantly stabilized cspA, increasing transcript half-life from <2.5 min in the Δrnc strain to >16 min in the WT strain (Figure 5A).
We then used toeprinting assays to monitor the formation of translation initiation complexes comprised of S. aureus 30S subunits, initiator tRNAfMet and cspA mRNA variants (for experiment details, see Text S1). The experiment showed that ∼50% of ternary initiation complexes were formed at 30S concentrations of 120 nM with cspAS and of 300 nM with cspAL (Figure 5B). Thus, cspAS formed initiation complexes more readily than cspAL. Similarly, a differential proteomic analysis based on two-dimensional gel electrophoresis of cytoplasmic proteins prepared from WT and Δrnc bacteria showed that the synthesis of CspA protein was strongly reduced in the absence of RNase III-mediated processing (data not shown). The increased initiation complex formation of the processed cspAS mRNA most likely reflects higher accessibility of the RBS as suggested by the enzymatic structure probing of cspAS mRNA. Indeed, single-strand-specific RNase cleavages were significantly enhanced in the region encompassing the SD sequence in cspAS (Figure S3). Thus, in the WT strain, the RNase III processing event in the 5′UTR of cspAL stabilizes the mRNA and facilitates ribosome binding to increase CspA synthesis (Figure 5C). How the long 5′ hairpin of cspAL hampers ribosome binding remains to be studied. Interestingly, previous work showed that a stable hairpin structure located several nucleotides upstream of a SD sequence sterically interfered with translational initiation [37].
The deep sequencing data indicated the existence of an asRNA complementary to the entire 5′UTR of cspAL including the six first codons (Figure 4A). Northern blot and primer extension experiments confirmed the presence of this asRNA in both WT and Δrnc strains grown at 37°C (Figure 5D; Table S5). However, the Northern experiments performed with DIG-labeled riboprobes, covering the same region of the genome, suggested that the yield of this asRNA was very low compared to that of cspA mRNA (Figure 5D). We tested whether this asRNA guides RNase III cleavage of cspA. End-labeled cspAL mRNA was subjected to RNase III hydrolysis in vitro, in the absence or presence of the asRNA (Figure 5D). Two conditions were used to form the asRNA-mRNA complexes: both RNAs were either denatured together and directly hybridized (denaturing conditions), or were denatured and refolded separately before hybridization (native conditions). After RNase III hydrolysis, the labeled RNA fragments were separated on a sequencing gel (Figure 5D, lower panel). The results show that RNase III efficiently cleaved preformed mRNA-asRNA duplexes into short RNA fragments in vitro. Therefore, the asRNA suppressed rather than promoted the generation of stable cspAS mRNA. This regulation could contribute to fine-tuning of mRNA levels in vivo [28].
Overall, the RNase III-mediated processing step in the biogenesis of cspA mRNA is determined by the intrinsic structural properties of its 5′UTR alone. These data strongly suggest that RNase III cleavage activates the synthesis of the major cold-shock protein CspA at the post-transcriptional level.
Non-coding RNAs as RNase III targets
In addition to several mRNAs, the abundant housekeeping RNAs, tmRNA, RNase P, 4.5S RNAs, and the transcriptional regulator 6S RNA, were significantly enriched in the coIPs with the mutant proteins (Table S2). These ncRNAs are all processed from precursor transcripts by the concerted action of several endo - and exoribonucleases (e.g., [38], [39]). However, the frequent recovery of such abundant and highly structured RNAs does not strictly imply their maturation by RNase III. For example, although B. subtilis 4.5S RNA maturation involves RNase III [39], [40], we did neither observe an altered processing pattern or precursor accumulation in the Δrnc mutant strains in Northern blot experiments (Figure S4A), nor did we detect RNase III-dependent cleavages of 4.5S RNA in vitro (results not shown). Likewise, the mature 230 nt product of 6S RNA was recovered by coIP, and its irregular hairpin structure was recognized by the RNase III mutant E135A (Figure S1). Nevertheless, we failed to observe RNase III-dependent processing on Northern blots (Figure S4A) and in vitro cleavage assays (results not shown). As an aside, the 6S gene is located downstream of the aspS-hisS operon, which is controlled by a T-Box motif [41], [42]). Whether 6S RNA expression responds to decreased pools of amino acids or uncharged tRNAs remains to be investigated.
Many of the enriched RNA fragments recovered by coIP (listed in Table S3) originated from full-length and bona fide ncRNAs of S. aureus, such as RsaA, C, E, H, I, and J [43], [44], the pathogenicity island-encoded ncRNAs SprA, SprA3, SprB, SprC, and SprF3/SprG3 [42], [44], as well as RNAIII [45]. Several of these ncRNAs (RsaA, RsaE, RsaX29/X39, RsaI, RsaO, SprA) were enriched with the mutant proteins suggesting that they are substrates of RNase III (Figure S5). These RNAs carry stable stem-loop structures and typical Rho-independent terminator hairpins (Figure 6, Figure S6) [46]. Experimental validation was performed on RsaA (Figure 6). In addition to RsaA, a second larger RNA (RsaAL) was detected on Northern blots, which likely originated from read-through at the transcriptional terminator. RsaA and RsaAL share a similar 5′ end as determined by RACE experiments [43]. Half-live measurements revealed a significantly higher RsaA stability in the Δrnc strain (>60 min) compared to WT strain (∼25 min for RsaA; Figure 6A). The longer RsaAL RNA was also more stable in the Δrnc strain (10 min) than in the WT strain (∼2.5 min; Figure 6A). We also performed RNase III cleavage assays on in vitro transcribed RsaA followed by primer extension. Two main cleavages were identified in the bulged loop of the 5′ hairpin structure of RsaA (Figure 6B) and of RsaAL (data not shown). Notably, these RNase III-specific cleavages coincided with the 5′ ends of two RNA fragments recovered by coIP with WT RNase III (Figure S5). Thus, RNase III contributes to the turnover of RsaA and RsaAL.
Fig. 6. The ncRNA RsaA is a target of RNase III. 
(A) (Left) The half-life of RsaA was measured as a function of time after rifampicin treatment in the RN6390 and Δrnc strains. A strand specific DIG labeled riboprobe was used to monitor RsaA expression. The oligonucleotides 374 and 376 were used for PCR amplification and the RNA was transcribed in vitro with T7 RNA polymerase. (Right) Quantification of mRNA levels in the RN6390 (WT, diamonds) and Δrnc mutant strains (squares) as a function of time after rifampicin treatment. The value of the mRNA half-life was determined by representing a semi-logarithmic plot of the concentration of the mRNA over time. The value corresponding to the percentage (%) of the remaining mRNA was normalized with the control experiment performed with 5S rRNA. The slope of the best-fit line was then determined to calculate the half-life. Three experiments provided reproducible results. (B) RNase III cleavages of cold RsaA in vitro. The reactions were done in the absence (−) and in the presence of RNase III (+, 0.33 µM; ++ 0.65 µM) in a buffer containing Mg2+ or Ca2+. Lanes C, U, G, A: sequencing reactions performed on RsaAL. (C) The secondary structure of RsaA was experimentally determined [43]. The two RNase III cleavages are represented as follows: empty and black arrows denote weak and strong cleavages, respectively. The organization of the genes corresponds to the annotation of the N315 genome. The ncRNA genes are shown in red. This study identified novel ncRNAs such as RsaL, RsaN, and RsaO (Table S3; Figures S4B and S6). Northern blot analyses showed that RsaO was expressed in all strains tested (Figure S4B), while RsaN was only detectable in the Δrnc strain (Table 1 and Table S3, data not shown). Other novel transcripts mapped to loci with multiple copies in the genome. For instance, two of the transcripts with the most abundant sequence reads corresponded to homologous and redundant ncRNAs (RsaX29 and RsaX39) that originated from a partial duplication of the 5S rRNA genes. RsaX29 harbors a long helical structure that might be recognized by RNase III (Figure S6; Table S3).
According to the deep sequencing data, several ncRNAs have associated asRNAs. The abundance of these antisense transcripts varied considerably according to Northern blot experiments (Figure S4C and S4D). For instance, the putative asRNAs of RsaA or RsaH were solely detectable by deep sequencing (results not shown). Conversely, several sense-antisense RNA pairs (SAS028/teg102, SprF3/SprG3) gave strong signals on Northern blots in WT and Δrnc strains (Figure S4C, S4D). Teg102 has been previously identified as an asRNA complementary to SAS028 mRNA, which encodes a small hypothetical protein [44], [47]. Its 5′ half was found in two copies in the same intergenic region of the genome (Figure S4C). The levels of SAS028 mRNA were reproducibly lower in the Δrnc strain overexpressing the WT RNase III (Figure S4C). It remains to be seen whether this RNase III-dependent effect is a consequence of asRNA regulation. SprF3/SprG3, whose partial sequences are present in multiple copies in the genome [42], may belong to the group I toxin-antitoxin systems, with SprG being the putative toxin [48]. Whether SprG3 encodes a peptide is yet unknown. Measurement of the half-lives in vivo showed that SprG3 (>60 min) is more stable than SprF3 (<12 min) (Figure S4D). However, under the conditions of growth used in the experiment, the in vivo half-lives and the steady-state levels of SprF3 and SprG3 RNAs were similar in the WT and Δrnc strains (Figure S4D) even though RNase III efficiently cleaves the duplex formed in vitro (data not shown). These surprising results are reminiscent of a recent study of a B. subtilis class I toxin (bsrG)-antitoxin (SR4) system, which showed that the half-lives of bsrG and SR4 RNAs were increased only by 2-fold in a rnc mutant [49].
A possible function of RNase III in the decay of structured regions of mRNAs
Deep sequencing of RNase III-associated RNAs recovered several mRNAs that encode proteins of various functions, including regulatory proteins that control the expression of virulence factors (repressor of toxin Rot, transcriptional regulatory protein SarH, two component-system SrrA-SrrB), bona fide virulence factors (protein A, the exotoxin Geh), and enzymes involved in various metabolic pathways (Table S4). In many cases, certain mRNA fragments were strongly enriched. This observation might be due to fragmentation occurring during the purification procedure, or alternatively reflect RNase III binding to structured mRNA fragments as a step in promoting their subsequent degradation. Many coIP mRNA fragments contained long hairpin structures which are typical RNase III binding sites, as it is observed for secY mRNA (Figure 7A). In vitro RNase III cleavage assays were performed on in vitro transcribed and unlabeled secY mRNA followed by reverse transcription. Two cuts were located in a long hairpin structure within the CDS of one of the coIP fragments, generating typical RNA fragments with a two-nucleotide 3′ overhang (Figure 7A).
Fig. 7. Effect of RNase III on mRNA turnover. 
(A) RNase III cleavage assays on in vitro transcribed secY mRNA. The cleavages were assigned after primer extension using 5′ end labeled oligonucleotide 292 (Table S8). RNase III cleavage reactions were done in the absence (−) and in the presence of RNase III (+, 0.33 µM; ++ 0.65 µM) in a buffer containing Mg2+ or Ca2+. Lanes C, G, A: Sequencing reactions. The arrows denote the RNase III-induced cleavages, which are reported on the secondary structure of the RNase III binding site located in the coding sequence of secY mRNA. (B) Analysis of hu mRNA expression in RN6390 and the isogenic Δrnc mutant strain. Upper panel: measurements of the half-life of hu mRNA by monitoring mRNA levels after rifampicin treatment as a function of time (min). A strand specific labeled riboprobe was used to detect hu mRNA. The riboprobe was transcribed in vitro with T7 RNA polymerase using a PCR template amplified with the oligonucleotides 370 and 371 (Table S8). As an internal control, 5S RNA expression was detected on the same Northern blot experiment. Lower panel: quantification of hu mRNA stability in RN6390 (black diamond) and in Δrnc strain (black square) is given as a function of time. The half-life was calculated as described in Figure 5A. The dotted line represents the half-life for the fraction of hu mRNA, which appeared to be degraded in a manner dependent of RNase III. (C) Upper panel: Northern blot analysis of the expression of hu mRNA and the antisense RNA (as-hu) in various strains: RN6390 (wild type strain), Δrnc mutant strain (Δrnc), and the same strain transformed with plasmid expressing the mutant E135A RNase III (Δrnc-E135A), the wild type RNase III (Δrnc-wt), or the mutant D63A RNase III (Δrnc-D63A). A strand specific riboprobe was used to detect as-hu expression. The riboprobe was transcribed in vitro with T7 RNA polymerase using a PCR template amplified with oligonucleotides 270 and 71 (Table S8). Lower panel: Autoradiography showing RNase III cleavage products of 5′ end-labeled hu mRNA alone or associated with the as-hu mRNA. Incubation controls of hu mRNA alone or with hu-as in the absence of RNase III are shown in lanes 1 and 5, respectively. The RNase III cleavage assays were performed in the presence of Mg2+ (lanes 2–4 and 6–8) or Ca2+ (lane 9) on hu mRNA (lanes 2–4) or bound to hu-as (lanes 6–9). The hu mRNA-as-hu duplex was pre-formed with denatured RNAs (denaturing conditions). Increasing concentrations of RNase III were used: 0.165 µM (lanes 2, 6), 0.33 µM (lanes 3, 7) and 0.66 µM (lanes 4, 8, 9). Lanes L, T1: alkaline ladder and RNase T1 ladder of hu mRNA, respectively. The bar denotes the shortest hu mRNA fragments generated by RNase III cleavage upon the as-hu binding. Many mRNA fragments corresponded to highly structured 5′UTRs of mRNAs, e.g., ndrl and ptsG (Figure S7). These 5′UTRs were described as cis-acting regulatory elements of downstream genes with functions in the translational machinery or metabolic pathways (Table 1 and Table S4). They contain specific binding sites for diverse ligands such as metabolites, deacetylated tRNAs, or regulatory proteins (ribosomal proteins, antitermination regulatory proteins) [41], [46], [50]. A shared characteristic of most of these structured leaders is the presence of a long Rho-independent terminator structure, indicating that these RNA transcripts resulted from premature transcription termination (Figure S7). Other structured regions in the data sets corresponded to 3′UTRs of mRNAs that all carried stable Rho-independent terminators spanning at least one helical turn, i.e. the minimal substrate of E. coli RNase III [51] (Table 1 and Table S4; Figure S7). Several of these 3′UTRs are rather long (>100 nts) and two of them (RsaM, RsaL) correspond to ncRNAs (Table 1 and Table S3) [44], [47].
Overall, these examples illustrate that RNase III might affect the turnover of structured mRNAs, in addition to that of its own transcript and the cspA mRNA.
Identification of numerous antisense RNAs against mRNAs
The coIP strategy using two catalytically impaired RNase III mutant proteins facilitated the identification of asRNAs opposite to 44% of the annotated mRNA genes (Table S5). These asRNAs generally seem to be expressed at a very low level, or are rapidly degraded, since many of them were undetectable on Northern blots (Table 1). One example is hu mRNA and its asRNA (Figure 7B, 7C). The stability of hu mRNA was measured in vivo in WT and Δrnc strains after rifampicin treatment (Figure 7B). Quantification of the data showed that RNase III moderately affected the half-life of hu mRNA (Figure 7B). Northern blot experiments performed with DIG-labeled riboprobes, covering identical region of the genome, suggested that the levels of the asRNA were significantly below that of hu mRNA (Figure 7C). Moreover, in the Δrnc strain complemented with the WT RNase III, the signal of the asRNA was weaker than in the same strain complemented with the mutant enzymes (Figure 7C). To evaluate whether the asRNA can induce mRNA processing, RNase III cleavage assays were performed on in vitro synthesized and 5′ end-labeled full-length hu mRNA either free or bound to the asRNA. The cleaved products were resolved on sequencing gels. While the free hu mRNA was not efficiently cleaved by RNase III in vitro (Figure 7C), the pre-formed asRNA-hu mRNA duplexes were strongly cleaved into short RNA fragments (Figure 7C). Thus, hu mRNA may be subject to rapid degradation by the combined action of the asRNA and RNase III.
Several sense-antisense transcript pairs that were strongly enriched by coIP with the mutant proteins corresponded to overlapping UTRs of divergent genes, as illustrated with pdf1/SA0943 and tagG/tagH mRNAs (Figure 8A; Tables S4 and S5). While pdf1 encodes the essential peptide deformylase, the tagG/tagH genes encode the ABC transporter complex TagGH involved in the export of teichoic acids. Northern blot analysis was performed using specific labeled riboprobes complementary to the 5′UTR of SA0943 or to the CDS of pdf1 (Figure 8A). In addition to full-length SA0943 mRNA, we observed a weak but reproducible signal for a ∼350 nt long RNA fragment that was only detected in the Δrnc strain (Figure 8A). In contrast, among the three pdf1 mRNA species, the longest mRNA accumulated strongly in the Δrnc strain (Figure 8A). A very similar pattern was observed for the tagG/tagH mRNAs. An RNA probe complementary to tagG mRNA detected three forms of the mRNA, the longest of which strongly accumulated in the Δrnc strains expressing the mutant proteins (Figure 8A). Concomitantly, an RNA fragment (<300 nts) corresponding to the 5′UTR of tagG was detected in Δrnc cells, suggesting an additional RNase cleavage event. Mapping of the 5′ ends of the tagG/tagH mRNAs by primer extension and RACE confirmed that both mRNAs were processed by a mechanism that is partly dependent on RNase III (Figure 8B, Table S6). For tagG mRNA, RT stops mapped to positions −140 and −250 in both the RN6390 and Δrnc strains and to position −77 in the Δrnc strain. For tagH mRNA, two main RT stops were mapped at −25 and −279 in both strains, while the RT stop at −160 was only observed in the RN6390 WT strain (Figure 8B; Table 1 and Table S6). To assess a functional importance of the observed RNase processing, we further analyzed the RNase III cleavages on in vitro transcribed tagG/tagH mRNAs containing the long and overlapping 5′UTRs. Using the 5′ end-labeled oligonucleotide 410 complementary to tagH mRNA for primer extension, we observed short RNA fragments that were generated by RNase III hydrolysis only when tagG associated with tagH (Figure 8C). This processing resulted in the formation of a tagH mRNA with a shortened leader whose 5′-end lies several nucleotides upstream of the SD sequence (Figure 8D). Thus, RNase III likely targets the 5′ overlapping regions of divergent mRNAs to generate species with shorter or even leaderless 5′UTRs.
Fig. 8. RNase III cleaves mRNAs with overlapping 5′ untranslated regions (UTR). 
(A) Effect of RNase III on the overlapping 5′ UTRs of SA0943/pdf1 (top) and tagG/tagH (bottom). RNA levels were monitored on Northern blots in various strains: RN6390 (WT), Δrnc, and Δrnc transformed with plasmids expressing E135A RNase III mutant (Δrnc-E135A), the wild type RNase III (Δrnc-wt), or the D63A RNase III mutant (Δrnc-D63A). DIG labeled riboprobes were used to monitor the expression of mRNAs. The riboprobes were transcribed in vitro with T7 RNA polymerase using PCR templates amplified with the oligonucleotides 426/427 (SA0943), 429/433 (pdf1), 333/334 (5′UTR tagG) and 392/393 (tagG) (Table S8). Schematic drawings summarizing the data are given below the Northern blot experiments. They show the overlapping 5′UTRs that form a typical RNase III binding substrate. A second cleavage from an unknown RNase (?) generated RNA fragments of 300 nts corresponding to the size of the 5′UTRs of SA0943 or tagG, which only accumulated in the Δrnc strain. (B) Primer extension analysis was performed on total RNAs prepared from exponential phase of growth. Oligonucleotides 408 and 334 were used to probe the 5′ ends of tagG and tagH mRNAs, respectively. Lanes 1, 2: samples prepared from RN6390 and Δrnc mutant strains, respectively. The 5′ ends (−140 in tagG mRNA and −25 in tagH mRNA) were detected both by primer extension and by RACE experiments performed on circularized RNAs (Table S6). Additional 5′ ends as well as putative RNase cleavage sites are indicated. The nucleotides are numbered relatively to the AUG start codon. Lanes C, U, A, G: sequencing ladders performed on tagG and tagH mRNAs, which were transcribed from PCR templates amplified with primers 331/221 and 333/384, respectively. (C) RNase III cleavage assays performed with in vitro transcribed tagH mRNA. Cleavage sites were assigned by primer extension using the 5′ end-labeled oligonucleotide 410. Controls were done with free mRNA (lane 1) or bound to tagG mRNA (lane 3); RNase III cleavage assays were performed on the mRNA alone (lane 2) or bound to tagG mRNA in a buffer containing Mg2+ (lanes 4, 5) or Ca2+ (lane 6). Reactions were set with 0.6 µM (lane 2, 5, 6) and 0.8 µM (lane 4) of wild-type RNase III; lanes U, C, G, A: sequencing ladders. (D) Schematic representation of the tagG-tagH locus. The RNase III cleavage site in the 5′UTR of tagH mRNA at position −160 is shown. An additional cleavage site induced by an unknown RNase is designated by a small arrow at position −77 of tagG mRNA. The fragment detected by Northern blot in Δrnc mutant strain (Figure 8A, lower panel) and the hybridization sites of the primers are indicated. The 3′ ends of both mRNAs were identified by RACE on circularized mRNAs (+826 for tagH and +869 for tagG, respectively). Discussion
Studies of specific transcripts from S. aureus have indicated that the regulation of mRNA turnover by RNase III plays an important role in its virulence and adaptation to stress responses [23], [24], [26]. Furthermore, a recent genome-wide analysis revealed an unprecedented high number of asRNAs that are weakly expressed and specifically degraded by RNase III in S. aureus and other Gram-positive bacteria [28]. Here to gain better understanding of the broad action of S. aureus RNase III, we identified direct target RNAs of this enzyme by deep sequencing of RNAs that were recovered in vivo with epitope-tagged variants of the protein. This approach originally pioneered the genome-wide detection of ncRNA and mRNA targets of the RNA chaperone Hfq at single-nucleotide resolution [33], [52]. To facilitate the identification of nuclease targeted RNAs, which normally might be rapidly degraded, we included mutant proteins in which the catalytic activity was uncoupled from their RNA binding capacity (Figure 1A). Based on prior works on E. coli RNase III [11], [14], [31], we substituted residues E135 and D63 in S. aureus RNase III by alanines. The catalytic activity of the D63A mutant protein was indeed strongly decreased, and the activity of the E135A variant was almost abolished (Figure 1B, 1C). Both mutants retained full RNA binding capacity. Hence, the successful separation of catalytic and RNA binding activity by mutation of the S. aureus rnc gene provided independent proof for the contributions of E135 and D63 to the active site of RNase III [10], [14], [31].
Our analysis identified diverse structured transcripts of all gene classes as potential RNase III substrates (Table 1, Tables S2, S3, S4, S5). As expected, the longest RNA fragments were recovered with the two cleavage-impaired mutant enzymes (Table S1), and the highest fraction of mapped cDNA reads corresponded to rRNA and tRNA operons (Figure 2A, Table S2). However, other cDNA reads that mapped to mRNAs including 5′ and 3′UTRs, potential short ORF-containing mRNAs, RNAs from intergenic regions, and asRNAs were specifically enriched in the coIPs with the mutant and WT enzymes (Tables S2, S3, S4, S5). Many of these transcripts carry long hairpin structures, reminiscent of a specific RNase III binding motif (Figures S6 and S7). Thus, RNase III binds many different types of RNAs in the cell and, as discussed below, has a broad effect on RNA processing and turnover. This was recently demonstrated for the E. coli and B. subtilis RNase III and, interestingly, the steady-state levels of many transcripts showed overlapping effects of E. coli RNase III and RNase E [19] and of B. subtilis RNase III, RNase J1, and RNase Y [20]. Whether S. aureus RNase III acts in a coordinated manner with RNase Y or RNase J1, remains to be studied [53].
The catalytic activity of RNase III is involved in rRNA processing and rnc autoregulation
The requirement for the catalytic activity of RNase III was first demonstrated for the maturation of ribosomal RNA precursors in E. coli [34] and B. subtilis [22]. Under optimal growth conditions, the synthesis of ribosomes consumes a major fraction of available energy in cells. Thus, maturation of rRNA has to be efficient and accurate for fitness. As in E. coli and B. subtilis [22], [34], S. aureus rRNAs are synthesized as long 30S precursor transcripts containing the three rRNAs genes (16S, 23S, and 5S) interspersed by tRNA genes (Figure 2). Using specific probes that hybridized to the spacer regions of rRNA operons, precursor transcripts were detected in the Δrnc mutant strains (Figure 2B). The identification of RNase III-dependent cleavage in the processing stalk of S. aureus 16S rRNA precursors together with the conservation of the precursor structure strongly suggest that the initial processing of rRNAs is carried out by RNase III. Alternative pathways seem to substitute for 16S rRNA maturation in the absence of RNase III (Figure 2C), but the responsible enzymes are not yet known in S. aureus. In B. subtilis, the final maturation steps of 23S, 16S, and 5S rRNAs involve the mini-III enzyme, the 5′-3′ exoribonuclease RNase J1, and the double-strand-specific RNase M5, respectively [54]–[56]. Because these enzymes are present in S. aureus, we may speculate that these maturation pathways are generally conserved in Gram-positive bacteria. Notably, analysis of tRNA/rRNA precursors in the Δrnc strain (Figure 2B) strongly suggested that the maturation of tRNAs is initiated by RNase III cleavage of the large rRNA precursor stalk.
The present study also shows a role of RNase III in gene regulation in S. aureus. The enzyme autoregulates its own synthesis by a feedback mechanism similar to that identified in E. coli [16], [57] and Streptomyces coelicolor [58]. Autoregulation helps to adjust the intracellular amount of the protein to that of the RNA substrates and prevents a potential detrimental over-accumulation of RNase III [59], [60]. We show here that point mutations in the catalytic site of S. aureus RNase III cause a two to three-fold increase in the level of the mutant protein compared to the WT enzyme (Figure 1D), which argues that autoregulation depends on the catalytic activity of RNase III. Furthermore, S. aureus rnc mRNA is efficiently cleaved by RNase III both in vitro and in vivo at a specific position in a stem-loop structure located in the CDS (Figure 3D), which is conserved among Staphylococci. The ability of RNase III to cleave only one side of the helix is most likely due to the presence of bulged residues that interrupt the helix [27], [61]. We propose that cleavage at this site is responsible for rnc mRNA destabilization under conditions when RNase III is in excess over its other RNA substrates. Although the feedback mechanism is preserved in distantly related bacteria, the regulatory site varies. In E. coli, RNase III targets a 5′ terminal stem-loop of its own mRNA [57], while the S. aureus and Streptomyces [58] enzymes regulate themselves via the CDS of their respective gene. Such a structure within the rnc coding sequence might locally alter the speed of translation elongation thereby facilitating the access of RNase III.
RNase III cleavage has a positive effect on protein synthesis
Our results show for the first time that the abundance and translation efficiency of cspA mRNA, which encodes the major cold-shock protein CspA, is modulated by RNase III-cleavages within the 5′ leader (Figure 4 and Figure 5). This RNase III processing event generates a more stable mRNA with a shorter 5′ terminal hairpin, which results in strongly enhanced synthesis of the major cold-shock protein. CspA was also found to be involved in the susceptibility of S. aureus to an antimicrobial peptide of human cathepsin G thus linking a stress response system to host-pathogen interaction [62]. Interestingly, the 5′UTR of cspA is highly conserved in Staphylococcus species and Macrococcus species, and a similar long hairpin may form upstream of the SD sequence of cspB mRNA of Listeria monocytogenes (data not shown), suggesting that RNase III-dependent activation may be a conserved mechanism. The fact that RNase J1, a major 5′-3′ exo - and endoribonuclease in Gram-positive bacteria, is inhibited by a 5′ terminal hairpin [53], [63] may explain why the shorter stem-loop structure at the 5′ end stabilizes cspA mRNA. In addition, the RNase III-dependent processing of cspA mRNA promotes ribosome recruitment, most likely by resolving the inhibitory structure at the RBS.
There are other examples wherein perturbation at the 5′ end impacts the stability and translation of bacterial mRNAs. Binding of deacylated tRNAThr to the 5′ leader region of B. subtilis thrS mRNA induces transcriptional read-through and mRNA cleavage, causing mRNA stabilization due to the formation of a 5′ transcription attenuator hairpin structure [64]. More recently, Streptococcus pyogenes ska mRNA is stabilized by the regulatory RNA FasX through the formation of a 9 bp helix at the 5′ end [65]. Similarly, Clostridium perfringens collagenase mRNA is stabilized by VR-RNA-dependent cleavage in the 5′ UTR, which renders the SD sequence more accessible for ribosome binding [66]. In contrast with these examples wherein trans-acting RNAs are required, we have identified a new mechanism through which RNase III-processing alone confers mRNA stabilization and enhances translation (Figure 5C).
RNase III is associated with non-coding RNA regulation
We detected 58 ncRNAs that co-immunoprecipitated with RNase III (Table 1, Table S3). Most of these RNAs have been identified previously, and many of them carry hairpin motifs that could be specifically cleaved by RNase III (reviewed in [46]). For instance, RNase III-dependent cleavages were detected in the 5′ hairpin motif of RsaA in vitro, and the stability of this RNA was enhanced in the Δrnc strain (Figure 6). Similar to the quorum-sensing-dependent RNAIII, many of these ncRNAs presumably regulate gene expression by antisense mechanisms [27] and it is likely that they would be co-immunoprecipitated with their respective target mRNAs. For instance, RNAIII and two of its major target mRNAs, encoding Rot and protein A, were detected [24], [26], [67]. Likewise, we recovered the 5′UTRs of the sucC and folD mRNAs, which are known to base-pair with RsaE [43]. Thus, our coIP data sets should be useful to improve the prediction of ncRNA-mRNA interactions. Of note, E. coli and Salmonella RNase III were also found to affect the steady-state levels of several ncRNAs [19], [68]–[70], suggesting that a significant portion of the E. coli transcriptome was directly or indirectly affected by changes in the abundance of the ncRNAs. Thus, RNase III may play a more general role for trans-acting ncRNAs than it was previously appreciated.
A significant number of reads representing putative asRNAs complementary to all types of RNA species were found, namely ncRNAs, sORF, and mRNAs (Tables S2, S5). This antisense transcription was directed against 44% of the protein-coding genes. Most asRNAs were present at a low level, suggesting that they might arise from transcriptional noise (e.g., asRNAs against cspA and hu mRNAs; Figure 5D and Figure 7C). A recent study demonstrated that RNase III might rapidly remove low levels of asRNAs generated by pervasive transcription in S. aureus and other Gram-positive bacteria [28]. Interestingly, we observed that hu mRNA was more rapidly degraded in the WT strain than in the Δrnc strain (Figure 7B). Because hu mRNA was not efficiently cleaved by RNase III (Figure 7C), its rapid degradation might be mediated through asRNA regulation. It is tempting to propose that this RNA quality control mechanism may also contribute to fine-tune the final levels of mRNA in the cell. It is also conceivable that asRNA transcription is transiently enhanced until its concentration reaches a threshold that suffices to regulate the expression of the sense transcript. Indeed, the expression of several asRNAs was recently shown to be SigmaB-dependent, and their decreased expression levels in a ΔsigB mutant strain correlated with increasing expression of the sense transcripts [28]. Our data support the view that RNase III-dependent processing indeed contributes to regulate the level of sense mRNA.
Our study further reveals RNase III targets that are derived from long 5′UTRs of divergently transcribed genes. Two of the overlapping 5′UTRs (tagG/tagH and pdf1/SA0943) are processed by an unknown enzyme to generate mRNAs with shorter 5′ ends, while the processed 5′UTRs are rapidly degraded by RNase III (Figure 8). Shortening of the 5′ end of mRNAs could affect translation and mRNA stability, as illustrated for cspA mRNA (Figure 5). A coordinated regulation of TagG and TagH enzymes through overlapping 5′UTRs may be particularly important for the efficient synthesis of teichoic acids in S. aureus. Teichoic acids contribute to the structural integrity and shape of the bacteria by regulating the peptidoglycan cross-linking and metabolism during cell division. They are also required for virulence and biofilm formation (reviewed in [71]). Overlapping transcripts from divergently transcribed protein-coding genes with long and overlapping 5′ or 3′UTRs have also been described in Listeria [72]. This indicates a mechanism to regulate and coordinate gene expression between neighboring genes.
Impact of RNase III on gene regulation
In conclusion, this study unveiled the sophistication and complexity of post-transcriptional regulation mediated by RNase III in S. aureus. The use of catalytically inactive but binding-competent RNase III mutants allowed the identification of a large set of structured RNase III substrates in vivo. For instance, we demonstrated the involvement of the enzyme in rRNA and mRNA processing, in RNA turnover, in the activation of translation through cis - and trans-acting factors, as well as in antisense RNA-mediated regulation. All of these functions are mediated through the catalytic activity of RNase III. However, we predict that the enzyme may also regulate gene expression through its binding activity, as was shown for the cIII gene of bacteriophage lambda. In this system, RNase III stabilized a conformation of the mRNA that rendered the ribosome binding site accessible to the ribosome [73]. Combining our methodology with comparative proteomics and transcriptomics will help to address more comprehensively the roles of this universally conserved enzyme in gene regulation in response to stress and during host infection.
Materials and Methods
Strains and plasmids
Mutations E135A and D63A were introduced into the S. aureus RNase III enzyme following the Quickchange XL Site-directed mutagenesis procedure (Stratagene). Experimental details for the preparation of the biological materials and other detailed protocols on Northern blot analysis, RNA structure probing, and toeprinting are given in Text S1. The strains and plasmids used in this study are listed in Table S7.
Co-immunoprecipitation assays
Wild-type (WT) strain RN6390 or the isogenic Δrnc mutant strains alone or transformed with plasmids expressing either E135A, D63A or WT enzymes were grown in BHI medium at early exponential phase (OD 600 nm 0.2–0.3). Then, 10 µM of CdCl2 was added to the cultures, and after 2 h and 4 h of induction, the cells were pelleted and snap-frozen in liquid nitrogen. The bacterial cell pellet was suspended in lysis buffer (TBS, 1% Triton X-100 and protease inhibitor cocktail), transferred onto glass beads (provided by FastRNA Pro Blue Kit, Qbiogene) and processed in the FastPrep instrument (3×45 s at a setting of 6.0). Samples were centrifuged at 13,000 rpm for 5 min. The supernatants were mixed with mouse IgG-agarose (Sigma, A0919) to remove non-specifically binding proteins and incubated at 4°C for 50 min. The beads were spun down (1,500 g, 5 min) and the pre-cleared supernatants (3 ml) were kept separately. A fraction of the volume (0.2 ml) was removed for total RNA isolation and the rest of the sample was mixed with 40 µl (packed gel volume) of Anti-Flag M2 Affinity Gel (Sigma, A2220). Immunoprecipitation was performed according to the manufacturer's instructions. Briefly, the cleared lysates were incubated with the Anti-Flag M2 Affinity Gel for 2 h at 4°C, then the beads were washed three times with TBS. Elution was made with 0.2 ml of Flag Peptide (Sigma, F3290) prepared at the concentration recommended by the supplier. The sample was extracted with acidic phenol and then by chloroform: isoamylic alcohol. RNA was precipitated with ethanol, treated with DNase I, extracted with phenol and precipitated. The final RNA samples were dissolved in 50 µl of sterile water and lyophilized.
Deep-sequencing analysis
cDNA library construction, pyrosequencing and data analysis were done as previously described [33], [52]. In brief, cDNA-seq libraries were constructed with RNA samples from coIP experiments under exponential and late-exponential phase growth of the Flag-tagged wild-type and mutant enzymes expressed from the inducible plasmid. The resulting cDNA libraries were sequenced on a Roche 454 sequencer using FLX and Titanium chemistry. From the resulting cDNA reads, 5′-linker sequences and polyA-tails were clipped from the sequenced cDNA reads. Only reads of ≥18 nt were aligned to the reference genome, which was retrieved from the NCBI server (accession number of the chromosome: NC_002745.2; accession number of the plasmid: NC_003140.1), using the program segemehl [74]. Based on the resulting mapping data, read coverage files were generated in the GR format representing the number of mapped reads per nucleotide. The GR files were visualized in combination with FASTA and GFF files of the genome using the Integrated Genome Browser (IGB) [75]. Additionally, overlaps of mapped reads and gene annotation positions were identified and counted. The overlap between mapped read and a gene annotation had to be at least 10 nucleotides long to be taken into account. Each single overlap counting was normalized by the number of positions to which the overlapping read was mapped and the number of annotations that overlap with the read. For instance, if reads map to multiple regions with exactly the same score (e.g. this is the case for reads that map to the different multiple copies of the rRNA genes), only a relative fraction of one read is counted instead of a count of one read. For example, if a read maps twice, each location gets a score of 0.5 reads. Moreover, if a read overlaps two annotations, each annotation gets a score of 0.5 reads (Table S1).
Text S1 provided experimental details for all the experiments performed in this study.
Supporting Information
Zdroje
1. CarpousisAJLuisiBFMcDowallKJ 2009 Endonucleolytic initiation of mRNA decay in Escherichia coli. Prog Mol Biol Transl Sci 85 91 135
2. AndersonKLDunmanPM 2009 Messenger RNA Turnover Processes in Escherichia coli, Bacillus subtilis, and Emerging Studies in Staphylococcus aureus. Int J Microbiol 2009 525491
3. CondonCBechhoferDH 2011 Regulated RNA stability in the Gram positives. Curr Opin Microbiol 14 148 154
4. BelascoJG 2010 All things must pass: contrasts and commonalities in eukaryotic and bacterial mRNA decay. Nat Rev Mol Cell Biol 11 467 478
5. MaderUZigLKretschmerJHomuthGPutzerH 2008 mRNA processing by RNases J1 and J2 affects Bacillus subtilis gene expression on a global scale. Mol Microbiol 70 183 196
6. ShahbabianKJamalliAZigLPutzerH 2009 RNase Y, a novel endoribonuclease, initiates riboswitch turnover in Bacillus subtilis. EMBO J 28 3523 3533
7. BechhoferDH 2009 Messenger RNA decay and maturation in Bacillus subtilis. Prog Mol Biol Transl Sci 85 231 273
8. KimVNHanJSiomiMC 2009 Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10 126 139
9. JiX 2008 The mechanism of RNase III action: how dicer dices. Curr Top Microbiol Immunol 320 99 116
10. GanJTropeaJEAustinBPCourtDLWaughDS 2006 Structural insight into the mechanism of double-stranded RNA processing by ribonuclease III. Cell 124 355 366
11. ZhangHKolbFAJaskiewiczLWesthofEFilipowiczW 2004 Single processing center models for human Dicer and bacterial RNase III. Cell 118 57 68
12. PertzevAVNicholsonAW 2006 Characterization of RNA sequence determinants and antideterminants of processing reactivity for a minimal substrate of Escherichia coli ribonuclease III. Nucleic Acids Res 34 3708 3721
13. SunWLiGNicholsonAW 2004 Mutational analysis of the nuclease domain of Escherichia coli ribonuclease III. Identification of conserved acidic residues that are important for catalytic function in vitro. Biochemistry 43 13054 13062
14. LiHNicholsonAW 1996 Defining the enzyme binding domain of a ribonuclease III processing signal. Ethylation interference and hydroxyl radical footprinting using catalytically inactive RNase III mutants. EMBO J 15 1421 1433
15. SrivastavaRKMiczakAApirionD 1990 Maturation of precursor 10Sa RNA in Escherichia coli is a two-step process: the first reaction is catalyzed by RNase III in presence of Mn2+. Biochimie 72 791 802
16. BardwellJCRegnierPChenSMNakamuraYGrunberg-ManagoM 1989 Autoregulation of RNase III operon by mRNA processing. EMBO J 8 3401 3407
17. WagnerEGAltuviaSRombyP 2002 Antisense RNAs in bacteria and their genetic elements. Adv Genet 46 361 398
18. DarfeuilleFUnosonCVogelJWagnerEG 2007 An antisense RNA inhibits translation by competing with standby ribosomes. Mol Cell 26 381 392
19. SteadMBMarshburnSMohantyBKMitraJCastilloLP 2011 Analysis of Escherichia coli RNase E and RNase III activity in vivo using tiling microarrays. Nucleic Acids Res 39 3188 3203
20. DurandSGiletLBessièresPNicolasPCondonC 2012 Three essential ribonucleases - RNase Y, J1, and III-control the abundance of a majority of Bacillus subtilis mRNAs. PLoS Genet 8 e1002520 doi:10.1371/journal.pgen.1002520
21. EDChylinskiKSharmaCMGonzalesKChaoY CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 47 602 607
22. HerskovitzMABechhoferDH 2000 Endoribonuclease RNase III is essential in Bacillus subtilis. Mol Microbiol 38 1027 1033
23. LiuYDongJWuNGaoYZhangX 2011 The production of extracellular proteins is regulated by ribonuclease III via two different pathways in Staphylococcus aureus. PLoS ONE 6 e20554 doi:10.1371/journal.pone.0020554
24. HuntzingerEBoissetSSaveanuCBenitoYGeissmannT 2005 Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J 24 824 835
25. ChevalierCBoissetSRomillyCMasquidaBFechterP 2010 Staphylococcus aureus RNAIII binds to two distant regions of coa mRNA to arrest translation and promote mRNA degradation. PLoS Pathog 6 e1000809 doi:10.1371/journal.ppat.1000809
26. BoissetSGeissmannTHuntzingerEFechterPBendridiN 2007 Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev 21 1353 1366
27. ChevalierCHuntzingerEFechterPBoissetSVandeneschF 2008 Staphylococcus aureus endoribonuclease III purification and properties. Methods Enzymol 447 309 327
28. LasaIToledo-AranaADobinAVillanuevaMde los MozosIR 2011 Genome-wide antisense transcription drives mRNA processing in bacteria. Proc Natl Acad Sci U S A 108 20172 20177
29. GanJShawGTropeaJEWaughDSCourtDL 2008 A stepwise model for double-stranded RNA processing by ribonuclease III. Mol Microbiol 67 143 154
30. DasguptaSFernandezLKameyamaLInadaTNakamuraY 1998 Genetic uncoupling of the dsRNA-binding and RNA cleavage activities of the Escherichia coli endoribonuclease RNase III-the effect of dsRNA binding on gene expression. Mol Microbiol 28 629 640
31. SunWNicholsonAW 2001 Mechanism of action of Escherichia coli ribonuclease III. Stringent chemical requirement for the glutamic acid 117 side chain and Mn2+ rescue of the Glu117Asp mutant. Biochemistry 40 5102 5110
32. AmarasingheAKCalin-JagemanIHarmouchASunWNicholsonAW 2001 Escherichia coli ribonuclease III: affinity purification of hexahistidine-tagged enzyme and assays for substrate binding and cleavage. Methods Enzymol 342 143 158
33. SittkaALucchiniSPapenfortKSharmaCMRolleK 2008 Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator Hfq. PLoS Genet 4 e1000163 doi:10.1371/journal.pgen.1000163
34. NollerHFNomuraM 1987 Ribosomes in Escherichia coli and Salmonella typhimurium. NeidhardtFC Cellular and molecular biology Washington, DC American Society for Microbiology 104 125
35. ChevalierCGeissmannTHelferACRombyP 2009 Probing mRNA structure and sRNA-mRNA interactions in bacteria using enzymes and lead(II). Methods Mol Biol 540 215 232
36. RichardsJLiuQPellegriniOCelesnikHYoaS 2011 An RNA pyrophosphohydrolase triggers 5′-exonucleolytic degradation of mRNA in Bacillus subtilis. Mol Cell 43 940 949
37. MalmgrenCEngdahlHMRombyPWagnerEG 1996 An antisense/target RNA duplex or a strong intramolecular RNA structure 5′ of a translation initiation signal blocks ribosome binding: the case of plasmid R1. RNA 2 1022 1032
38. LiZPanditSDeutscherMP 1998 3′ exoribonucleolytic trimming is a common feature of the maturation of small, stable RNAs in Escherichia coli. Proc Natl Acad Sci USA 95 2856 2861
39. OguroAKakeshitaHNakamuraKYamaneKWangW 1998 Bacillus subtilis RNase III cleaves both 5′ - and 3′-sites of the small cytoplasmic RNA precursor. J Biol Chem 273 19542 19547
40. YaoSBlausteinJBBechhoferDH 2007 Processing of Bacillus subtilis small cytoplasmic RNA: evidence for an additional endonuclease cleavage site. Nucleic Acids Res 35 4464 4473
41. CaldelariIFechterPLioliouERomillyCChevalierC 2011 A current overview of regulatory RNAs in Staphylococcus aureus. MarchfelderAHessW Regulatory RNAs in prokaryotes Wien and New York Wiley Verlag
42. PichonCFeldenB 2005 Small RNA genes expressed from Staphylococcus aureus genomic and pathogenicity islands with specific expression among pathogenic strains. Proc Natl Acad Sci U S A 102 14249 14254
43. GeissmannTChevalierCCrosMJBoissetSFechterP 2009 A search for small noncoding RNAs in Staphylococcus aureus reveals a conserved sequence motif for regulation. Nucleic Acids Res 37 7239 7257
44. BeaumeMHernandezDFarinelliLDeluenCLinderP 2010 Cartography of methicillin-resistant S. aureus transcripts: detection, orientation and temporal expression during growth phase and stress conditions. PLoS ONE 5 e10725 doi:10.1371/journal.pone.0010725
45. NovickRPRossHFProjanSJKornblumJKreiswirthB 1993 Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J 12 3967 3975
46. FeldenBVandeneschFBoulocPRombyP 2011 The Staphylococcus aureus RNome and its commitment to virulence. PLoS Pathog 7 e1002006 doi:10.1371/journal.ppat.1002006
47. Abu-QatousehLFChinniSVSeggewissJProctorRABrosiusJ 2010 Identification of differentially expressed small non-protein-coding RNAs in Staphylococcus aureus displaying both the normal and the small-colony variant phenotype. J Mol Med (Berl) 88 565 575
48. FozoEMHemmMRStorzG 2008 Small toxic proteins and the antisense RNAs that repress them. Microbiol Mol Biol Rev 72 579 89, Table of Contents
49. JahnNPreisHWiedemannCBrantlS 2012 BsrG/SR4 from Bacillus subtilis - the first temperature-dependent type I toxin-antitoxin system. Mol Microbiol 83 579 598
50. BarrickJEBreakerRR 2007 The distributions, mechanisms, and structures of metabolite-binding riboswitches. Genome Biol 8 R239
51. NicholsonAW 1999 Function, mechanism and regulation of bacterial ribonucleases. FEMS Microbiol Rev 23 371 390
52. SittkaASharmaCMRolleKVogelJ 2009 Deep sequencing of Salmonella RNA associated with heterologous Hfq proteins in vivo reveals small RNAs as a major target class and identifies RNA processing phenotypes. RNA Biol 6 266 275
53. RouxCMDeMuthJPDunmanPM 2011 Characterization of components of the Staphylococcus aureus mRNA degradosome holoenzyme-like complex. J Bacteriol 193 5520 5526
54. BrittonRAWenTSchaeferLPellegriniOUickerWC 2007 Maturation of the 5′ end of Bacillus subtilis 16S rRNA by the essential ribonuclease YkqC/RNase J1. Mol Microbiol 63 127 138
55. RedkoYBechhoferDHCondonC 2008 Mini-III, an unusual member of the RNase III family of enzymes, catalyses 23S ribosomal RNA maturation in B. subtilis. Mol Microbiol 68 1096 1106
56. CondonCBrechemier-BaeyDBeltchevBGrunberg-ManagoMPutzerH 2001 Identification of the gene encoding the 5S ribosomal RNA maturase in Bacillus subtilis: mature 5S rRNA is dispensable for ribosome function. RNA 7 242 253
57. MatsunagaJSimonsELSimonsRW 1996 RNase III autoregulation: structure and function of rncO, the posttranscriptional “operator” RNA 2 1228 1240
58. XuWHuangJCohenSN 2008 Autoregulation of AbsB (RNase III) expression in Streptomyces coelicolor by endoribonucleolytic cleavage of absB operon transcripts. J Bacteriol 190 5526 5530
59. DeanaABelascoJG 2005 Lost in translation: the influence of ribosomes on bacterial mRNA decay. Genes Dev 19 2526 2533
60. RombyPSpringerM 2003 Bacterial translational control at atomic resolution. Trends Genet 19 155 161
61. Calin-JagemanINicholsonAW 2003 Mutational analysis of an RNA internal loop as a reactivity epitope for Escherichia coli ribonuclease III substrates. Biochemistry 42 5025 5034
62. KatzifSDanavallDBowersSBalthazarJTShaferWM 2003 The major cold shock gene, cspA, is involved in the susceptibility of Staphylococcus aureus to an antimicrobial peptide of human cathepsin G. Infect Immun 71 4304 4312
63. EvenSPellegriniOZigLLabasVVinhJ 2005 Ribonucleases J1 and J2: two novel endoribonucleases in B. subtilis with functional homology to E. coli RNase E. Nucleic Acids Res 33 2141 2152
64. PutzerHCondonCBrechemier-BaeyDBritoRGrunberg-ManagoM 2002 Transfer RNA-mediated antitermination in vitro. Nucleic Acids Res 30 3026 3033
65. Ramirez-PenaETrevinoJLiuZPerezNSumbyP 2010 The group A Streptococcus small regulatory RNA FasX enhances streptokinase activity by increasing the stability of the ska mRNA transcript. Mol Microbiol 78 1332 1347
66. ObanaNShirahamaYAbeKNakamuraK 2010 Stabilization of Clostridium perfringens collagenase mRNA by VR-RNA-dependent cleavage in 5′ leader sequence. Mol Microbiol 77 1416 1428
67. GeisingerEAdhikariRPJinRRossHFNovickRP 2006 Inhibition of rot translation by RNAIII, a key feature of agr function. Mol Microbiol 61 1038 1048
68. ViegasSCSilvaUSaramagoMDominguesSArraianoCM 2011 Regulation of the small regulatory RNA MicA by ribonuclease III: a target-dependent pathway. Nucleic Acids Res 39 2918 2930
69. ViegasSCPfeifferVSttkaASilvaIJVogelJ 2007 Characterization of the role of ribonucleases in Salmonella small RNA decay. Nucleic Acids Res 35 7651 7664
70. AfovnyushkinTVecerekBMollIBlasiUKaberdinVR 2005 Both RNase E and RNase III control the stability of sodB mRNA upon translational inhibition by the small regulatory RNA RyhB. Nucleic Acids Res 33 1678 1689
71. SwobodaJGCampbellJMeredithTCWalkerS 2010 Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem 11 35 45
72. Toledo-AranaADussurgetONikitasGSestoNGuet-RevilletH 2009 The Listeria transcriptional landscape from saprophytism to virulence. Nature 459 950 956
73. AltuviaSLocker-GiladiHKobySBen-NunOOppenheimAB 1987 RNase III stimulates the translation of the cIII gene of bacteriophage lambda. Proc Natl Acad Sci U S A 84 6511 6515
74. HoffmannSOttoCKurtzSSharmaCMKhaitovichP Fast mapping of short sequences with mismatches, insertions, and deletions using index structures. PLoS Comput Biol 5 e1000502 doi:10.1371/journal.pcbi.1000502
75. NicolJWHeltGAJrBlanchardSGRajaALoraineAE 2009 The Integrated Genome Browser: free software for distribution and exploration of genome-scale dataset. Bionformatics 25 2730 2731
Štítky
Genetika Reprodukční medicína
Článek Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental InvolvementČlánek Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target GenesČlánek Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early EmbryosČlánek Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2Článek Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human CellsČlánek TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome EndsČlánek Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal CordČlánek Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth ofČlánek MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- Mimetic Butterflies Introgress to Impress
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Selection-Driven Gene Loss in Bacteria
- Decreased Mitochondrial DNA Mutagenesis in Human Colorectal Cancer
- Parallel Evolution of Auditory Genes for Echolocation in Bats and Toothed Whales
- Diverse CRISPRs Evolving in Human Microbiomes
- The Rad4 ATR-Activation Domain Functions in G1/S Phase in a Chromatin-Dependent Manner
- Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains
- Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
- Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target Genes
- Adaptive Introgression across Species Boundaries in Butterflies
- G Protein Activation without a GEF in the Plant Kingdom
- Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes
- An Engineering Approach to Extending Lifespan in
- Incompatibility and Competitive Exclusion of Genomic Segments between Sibling Species
- Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in
- Patterns of Evolutionary Conservation of Essential Genes Correlate with Their Compensability
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
- Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early Embryos
- A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity
- Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2
- Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis
- Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells
- TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome Ends
- Stimulation of Host Immune Defenses by a Small Molecule Protects from Bacterial Infection
- A Broad Requirement for TLS Polymerases η and κ, and Interacting Sumoylation and Nuclear Pore Proteins, in Lesion Bypass during Embryogenesis
- Genome-Wide Identification of Ampicillin Resistance Determinants in
- The CCR4-NOT Complex Is Implicated in the Viability of Aneuploid Yeasts
- Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees
- Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
- Is a Key Regulator of Pancreaticobiliary Ductal System Development
- The NSL Complex Regulates Housekeeping Genes in
- Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord
- Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
- Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth of
- Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia
- MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
- Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family
- Found: The Elusive ANTAR Transcription Antiterminator
- The Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility
- Control of CpG and Non-CpG DNA Methylation by DNA Methyltransferases
- Polymorphisms in the Mitochondrial Ribosome Recycling Factor Compromise Cell Respiratory Function and Increase Atorvastatin Toxicity
- Gene Expression Profiles in Parkinson Disease Prefrontal Cortex Implicate and Genes under Its Transcriptional Regulation
- Global Regulatory Functions of the Endoribonuclease III in Gene Expression
- Extensive Evolutionary Changes in Regulatory Element Activity during Human Origins Are Associated with Altered Gene Expression and Positive Selection
- The Regulatory Network of Natural Competence and Transformation of
- Brain Expression Genome-Wide Association Study (eGWAS) Identifies Human Disease-Associated Variants
- Quantifying the Adaptive Potential of an Antibiotic Resistance Enzyme
- Divergence of the Yeast Transcription Factor Affects Sulfite Resistance
- The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis
- RNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
- Rare Copy Number Variants Observed in Hereditary Breast Cancer Cases Disrupt Genes in Estrogen Signaling and Tumor Suppression Network
- Genome-Wide Location Analysis Reveals Distinct Transcriptional Circuitry by Paralogous Regulators Foxa1 and Foxa2
- A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
- The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression
- Limits to the Rate of Adaptive Substitution in Sexual Populations
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- The NSL Complex Regulates Housekeeping Genes in
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



