-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mimetic Butterflies Introgress to Impress
article has not abstract
Published in the journal: . PLoS Genet 8(6): e32767. doi:10.1371/journal.pgen.1002802
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002802Summary
article has not abstract
The extent to which hybridization and the resulting interspecific gene flow (introgression) contribute to adaptation is a matter of great debate. On the one hand, fertile hybrids have the potential to transfer beneficial alleles between species [1] or even to spawn new species [2]. On the other hand, hybrids tend to have reduced fitness relative to parental species [3], which will often make them an evolutionary dead end. Only a handful of examples of adaptive introgression are known, such as recent evidence for the transfer of warfarin resistance between mouse species [4]. However, one place where introgression of beneficial alleles could be common is in adaptive radiations [5]. These explosions of phenotypic and species diversity may be just the place to look for adaptive introgression because they often contain closely related, hybridizing species, and introgression could provide the raw genetic material for their exceptional rates of diversification. Two new papers, one in this issue of PLoS Genetics [6] and another in Nature [7], provide long-awaited evidence for a direct role of introgression in fueling a particularly striking adaptive radiation, the mimetic wing pattern radiation of Heliconius butterflies.
The Neotropical genus Heliconius is a diverse clade of brightly colored and chemically defended butterflies. This group is well-known for mimicry, in which different species evolve nearly identical wing patterns as a means of protection from predators [8]. Rapid evolution of wing pattern diversity in Heliconius, combined with convergence due to mimicry, has resulted in a group of closely related and hybridizing species, some of which look very different and others that look nearly identical. These two new papers show that alleles for wing patterning have moved across species boundaries multiple times, effectively transferring mimicry from one species to another.
This discovery of adaptive introgression in Heliconius builds upon five important prior advances. First, close to a decade ago, Larry Gilbert used results from a multitude of interspecific crosses to propose a model whereby Heliconius mimicry evolved by repeated interspecific transfer of color patterning alleles [9]. Second, surveys of wild-caught specimens have revealed many instances of natural hybridization in Heliconius [10], and molecular analyses based on neutral markers detected signatures of relatively widespread introgression among closely related species [11], [12]. Third, recent discoveries of cryptic species have provided multiple examples of sympatric, co-mimetic, and potentially hybridizing species [13]; species among which mimicry transfer might be particularly likely. Fourth, there has been a concerted effort by those studying Heliconius to map and characterize their mimicry loci [14]. This recently culminated in the identification of optix as the red patterning gene [15], with high-resolution SNP data revealing strong associations just upstream of the gene [16]. Finally, new population genetic data from optix itself revealed that, within polymorphic species like H. erato and H. melpomene, similar wing patterns in distinct subspecies share a common origin [17]. This result, while focused on within-species variation, showed that apparent convergence can result from shared ancestry of mimicry alleles.
The two new studies build on this foundation to explore adaptive introgression of mimicry using different but highly complementary approaches. Pardo-Diaz et al. [6] use amplicon sequencing from targeted portions around optix, combined with phylogenetic - and coalescent-based tests for gene flow. The Heliconius Genome Consortium [7] present a reference genome sequence for H. melpomene, and then use RAD markers, targeted resequencing, and an ABBA-BABA statistical approach to examine introgression genome-wide, with a special emphasis on the optix interval, as well as a second region that controls yellow color patterning. The results of the two studies are highly congruent—genetic variation from a narrow genomic interval just upstream of optix groups populations and species by red wing patterning rather than known phylogenetic relationships (Figure 1). Amazingly, this even applies to H. elevatus, a “rayed” pattern species from the silvaniform clade, a group that generally displays “tiger-stripe” patterns. Importantly, The Heliconius Genome Consortium [7] show that these signatures of introgression ultimately encompass hundreds of SNPs, ruling out the possibility that these groupings are the result of convergent molecular evolution.
Fig. 1. Two new papers show that wing patterning has been swapped among Heliconius butterfly species via introgressive hybridization. 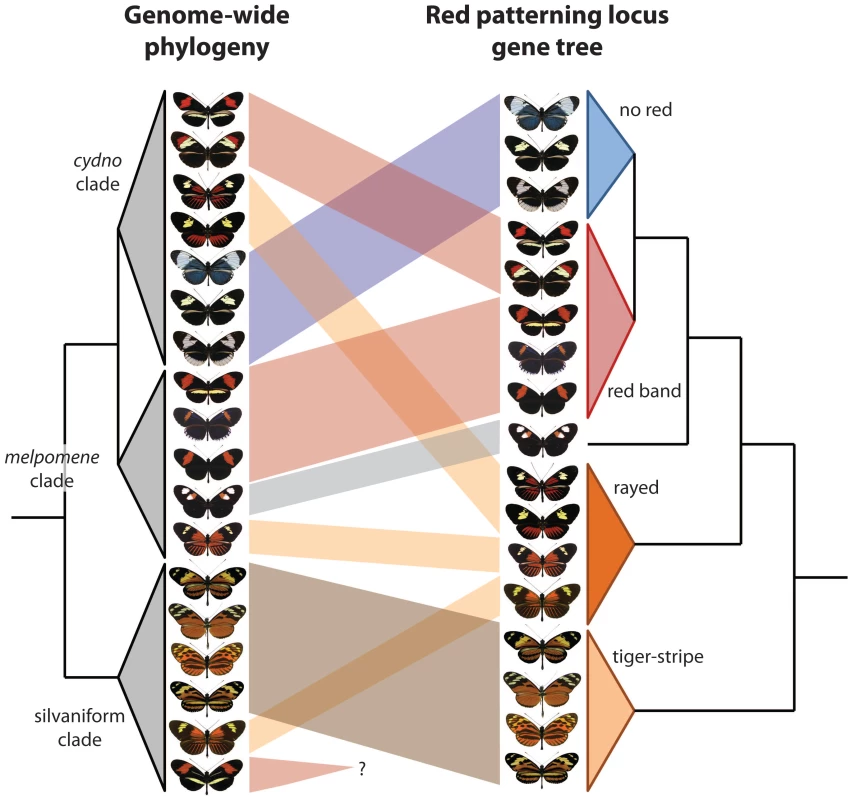
One clear signature of this history is the discordance between a phylogeny based on the entire genome and one based on a single portion of the genome that controls wing patterning, in this case an intergenic region near optix that is strongly associated with red patterning [15], [16]. The genome-based tree (left) reflects the known organismal phylogeny, while the optix-based tree groups individuals by phenotype. This figure, which consolidates the findings of Pardo-Diaz et al. [6] and The Heliconius Genome Consortium [7], depicts only a subset of the species and wing pattern phenotypes contained in the melpomene/cydno/silvaniform clade of Heliconius. Furthermore, this clade is but one of four major clades within the genus. This evidence for adaptive introgression is striking, yet there is much that remains unknown. For instance, H. besckei is the only silvaniform species with a “red band” phenotype, but it remains unknown if it swapped mimicry genes with other red banded species (Figure 1). Furthermore, there are potential instances of more subtle introgression that have yet to be explored; cases in which a single pattern element, as opposed to an entire phenotype, appears to be shared between species [9]. Other important questions include: In which species did mimicry alleles originally arise and when did they spread? How frequently does mimicry introgression precipitate speciation? How do these closely related, sympatric, co-mimetic taxa remain distinct given color pattern's important role in generating reproductive isolation [18]? What about adaptive introgression of other traits and genes not related to mimicry? Has introgression contributed to diversity and mimicry in other Heliconius clades?
Beyond Heliconius, it is important that we better understand the frequency and taxonomic distribution of adaptive introgression, as well as its potential link to adaptive radiation. These new results from Heliconius reveal that introgression has played an essential role in driving adaptive evolution across an entire clade. Whether we find a more general role for introgression in facilitating adaptive radiation remains to be seen. However, the fact that the first comprehensive examination revealed widespread adaptive introgression certainly makes this a very real possibility.
Zdroje
1. ArnoldMLMartinNH 2009 Adaptation by introgression. J Biol 8 82
2. KunteKSheaCAardemaMLScriberJMJuengerTE 2011 Sex chromosome mosaicism and hybrid speciation among tiger swallowtail butterflies. PLoS Genet 7 e1002274 doi:10.1371/journal.pgen.1002274
3. BurkeJMArnoldML 2001 Genetics and the fitness of hybrids. Annu Rev Genet 35 31 52
4. SongYEndepolsSKlemannNRichterDMatuschkaFR 2011 Adaptive introgression of anticoagulant rodent poison resistance by hybridization between old world mice. Curr Biol 21 1296 1301
5. SeehausenO 2004 Hybridization and adaptive radiation. Trends Ecol Evol 19 198 207
6. Pardo-DiazCSalazarCBaxterSWMerotCFigueiredo-ReadyW 2012 Adaptive introgression across species boundaries in Heliconius butterflies. PLoS Genet 8 e1002752 doi:10.1371/journal.pgen.1002752
7. The Heliconius Genome Consortium 2012 Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature E-pub ahead of print 16 May 2012
8. MüllerF 1879 Ituna and Thyridia; a remarkable case of mimicry in butterflies. Trans Entomol Soc Lond 1879 xx xxix
9. GilbertLE 2003 Adaptive novelty through introgression in Heliconius wing patterns: evidence for shared genetic “tool box” from synthetic hybrid zones and a theory of diversification. BoggsCLWattWBEhrlichPR Ecology and evolution taking flight: butterflies as model systems Chicago University of Chicago Press 281 318
10. MalletJBeltranMNeukirchenWLinaresM 2007 Natural hybridization in heliconiine butterflies: the species boundary as a continuum. BMC Evol Biol 7 28
11. BullVBeltranMJigginsCDMcMillanWOBerminghamE 2006 Polyphyly and gene flow between non-sibling Heliconius species. BMC Biol 4 11
12. KronforstMRYoungLGBlumeLMGilbertLE 2006 Multilocus analyses of admixture and introgression among hybridizing Heliconius butterflies. Evolution 60 1254 1268
13. GiraldoNSalazarCJigginsCDBerminghamELinaresM 2008 Two sisters in the same dress: Heliconius cryptic species. BMC Evol Biol 8 324
14. PapaRMartinAReedRD 2008 Genomic hotspots of adaptation in butterfly wing pattern evolution. Current Opin Gen Dev 18 559 564
15. ReedRDPapaRMartinAHinesHMCountermanBA 2011 optix drives the repeated convergent evolution of butterfly wing pattern mimicry. Science 333 1137 1141
16. NadeauNJWhibleyAJonesRTDaveyJWDasmahapatraKK 2012 Genomic islands of divergence in hybridizing Heliconius butterflies identified by large-scale targeted sequencing. Phil Trans R Soc B 367 343 353
17. HinesHMCountermanBAPapaRAlbuquerque de MouraPCardosoMZ 2011 Wing patterning gene redefines the mimetic history of Heliconius butterflies. Proc Natl Acad Sci U S A 108 19666 19671
18. JigginsCDNaisbitRECoeRLMalletJ 2001 Reproductive isolation caused by colour pattern mimicry. Nature 411 302 305
Štítky
Genetika Reprodukční medicína
Článek Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental InvolvementČlánek Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target GenesČlánek Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early EmbryosČlánek Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2Článek Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human CellsČlánek TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome EndsČlánek Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal CordČlánek Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth ofČlánek MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- Mimetic Butterflies Introgress to Impress
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Selection-Driven Gene Loss in Bacteria
- Decreased Mitochondrial DNA Mutagenesis in Human Colorectal Cancer
- Parallel Evolution of Auditory Genes for Echolocation in Bats and Toothed Whales
- Diverse CRISPRs Evolving in Human Microbiomes
- The Rad4 ATR-Activation Domain Functions in G1/S Phase in a Chromatin-Dependent Manner
- Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains
- Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
- Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target Genes
- Adaptive Introgression across Species Boundaries in Butterflies
- G Protein Activation without a GEF in the Plant Kingdom
- Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes
- An Engineering Approach to Extending Lifespan in
- Incompatibility and Competitive Exclusion of Genomic Segments between Sibling Species
- Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in
- Patterns of Evolutionary Conservation of Essential Genes Correlate with Their Compensability
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
- Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early Embryos
- A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity
- Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2
- Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis
- Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells
- TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome Ends
- Stimulation of Host Immune Defenses by a Small Molecule Protects from Bacterial Infection
- A Broad Requirement for TLS Polymerases η and κ, and Interacting Sumoylation and Nuclear Pore Proteins, in Lesion Bypass during Embryogenesis
- Genome-Wide Identification of Ampicillin Resistance Determinants in
- The CCR4-NOT Complex Is Implicated in the Viability of Aneuploid Yeasts
- Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees
- Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
- Is a Key Regulator of Pancreaticobiliary Ductal System Development
- The NSL Complex Regulates Housekeeping Genes in
- Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord
- Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
- Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth of
- Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia
- MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
- Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family
- Found: The Elusive ANTAR Transcription Antiterminator
- The Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility
- Control of CpG and Non-CpG DNA Methylation by DNA Methyltransferases
- Polymorphisms in the Mitochondrial Ribosome Recycling Factor Compromise Cell Respiratory Function and Increase Atorvastatin Toxicity
- Gene Expression Profiles in Parkinson Disease Prefrontal Cortex Implicate and Genes under Its Transcriptional Regulation
- Global Regulatory Functions of the Endoribonuclease III in Gene Expression
- Extensive Evolutionary Changes in Regulatory Element Activity during Human Origins Are Associated with Altered Gene Expression and Positive Selection
- The Regulatory Network of Natural Competence and Transformation of
- Brain Expression Genome-Wide Association Study (eGWAS) Identifies Human Disease-Associated Variants
- Quantifying the Adaptive Potential of an Antibiotic Resistance Enzyme
- Divergence of the Yeast Transcription Factor Affects Sulfite Resistance
- The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis
- RNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
- Rare Copy Number Variants Observed in Hereditary Breast Cancer Cases Disrupt Genes in Estrogen Signaling and Tumor Suppression Network
- Genome-Wide Location Analysis Reveals Distinct Transcriptional Circuitry by Paralogous Regulators Foxa1 and Foxa2
- A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
- The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression
- Limits to the Rate of Adaptive Substitution in Sexual Populations
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- The NSL Complex Regulates Housekeeping Genes in
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



