-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
article has not abstract
Published in the journal: . PLoS Genet 8(6): e32767. doi:10.1371/journal.pgen.1002758
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002758Summary
article has not abstract
Mitochondria are the bioenergetic centers of eukaryotic cells that produce ATP through oxidative phosphorylation. A byproduct of oxidative phosphorylation is the generation of reactive oxygen species (ROS). Cancer cells exhibit high basal levels of oxidative stress due to activation of oncogenes, loss of tumor suppressors, and the effects of the tumor microenvironment [1]. A large body of evidence suggests important roles for oxygen free radicals in the mutagenesis that drives carcinogenesis [2], the expansion of tumor clones, and the acquisition of malignant properties [3].
Several studies have reported that a high frequency of clonal and, therefore, selected mitochondrial mutations are present in a variety of human tumors [4]–[15]. Mitochondrial DNA (mtDNA) mutations in cancer cells include intragenic deletions, missense and chain-termination point mutations, and alterations of homopolymeric sequences that result in frameshift mutations [16], [17]. The biological impact of a given mutation may vary, depending on the proportion of mutant mtDNAs carried by the cell. The assumption from these studies is that this high frequency of clonal mutations arises from the ROS produced in mitochondria by the escape of oxygen free radicals during oxidative phosphorylation, and that these mutations play a role in driving cancer (Figure 1). Therefore, genomic instability of mitochondria was thought to be a hallmark of cancer.
Fig. 1. Network modeling of the interconnections among the crucial factors involved in metabolic flow and signaling pathways of the Warburg effect to “protect” the cancer cells. 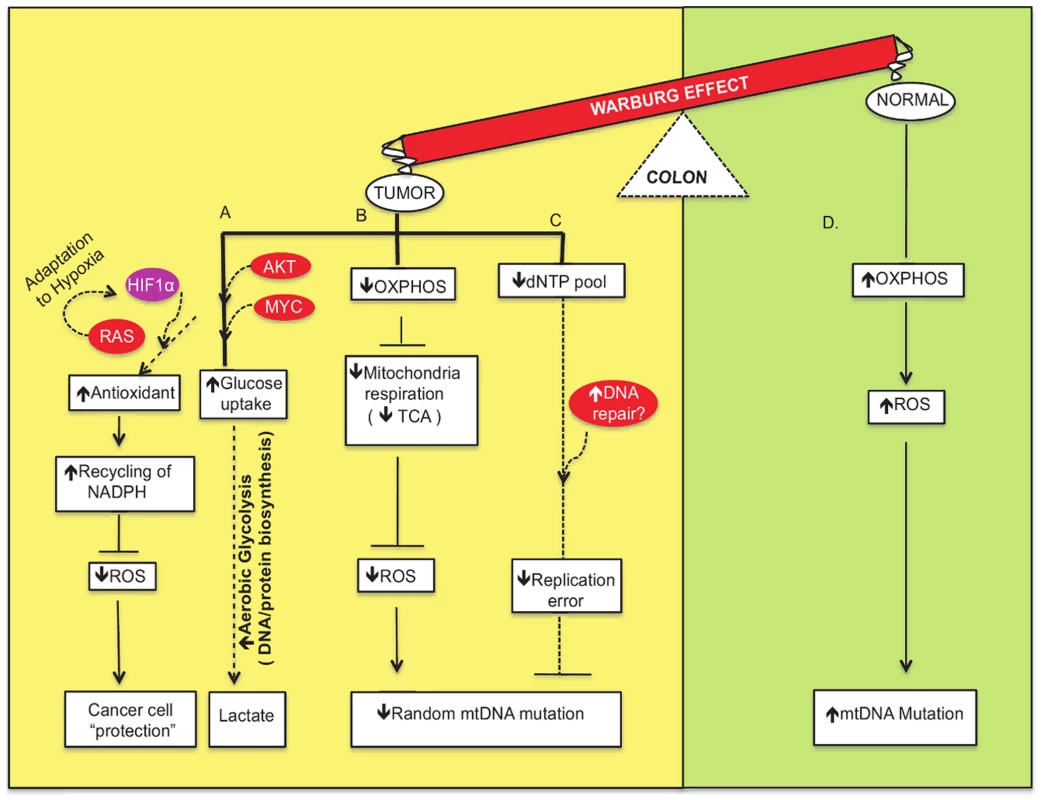
(A) Cancer cells make use of their nutrient-rich environment by taking in glucose and converting it into molecular precursors by aerobic glycolysis (shown in yellow). This is mediated by activation of proto-oncogenes such as AKT and MYC and other genes in important growth factor signaling pathways. Moreover, RAS-mediated activation of HIF1 induces adaptation to hypoxic environments and promotes “niches” that are conducive to cancer cells. In addition, the use of antioxidants and recycling of NADPH as defense mechanisms to sequester ROS favor the survival of cancer cells. (B) Oxidative phosphorylation (OXPHOS) impairment leads to crippled mitochondrial respiration. The dysfunctional TCA cycle generates fewer reactive oxygen species that may or may not induce DNA damage, and this subsequently leads to fewer mitochondrial DNA mutations. (C) Efficient repair of mtDNA leads to fewer mutations and less mitochondrial dysfunction. (D) In contrast, normal, low proliferative cells utilize OXPHOS and generate ROS that induce mtDNA damage and increase mutation frequency (shown in green). Cancer arises from a mutator phenotype and it has been established that the random mutation rate of the nuclear DNA of tumors is quite high [18]. However, little is known about the random mutation rates of mitochondria derived from tumors. In this issue of PLoS Genetics, Ericson and colleagues [19] report their striking finding that the random mutation frequency of colorectal tumor mtDNA is significantly lower than that of nuclear DNA or of mtDNA in the surrounding normal tissue. These results were obtained using the random mutation capture assay that was developed to measure random, and not clonal, mutations [19]. This group also shows that the mitochondria from the colon tumors use aerobic glycolysis rather than oxidative phosphorylation to generate energy for the cells. Importantly, this metabolic shift from oxidative phosphorylation to aerobic glycolysis is likely to produce fewer ROS that damage mtDNA and lead to mutagenesis, mitochondrial dysfunction, and cell death.
Seven decades ago, Warburg discovered that mitochondria in cancer cells metabolize glucose by aerobic glycolysis and suggested that this was a result of impaired mitochondrial function that contributes to tumorigenesis [20], [21]. Recently, Thompson and colleagues suggested that mitochondrial function itself is not impaired in cancer cells that metabolize glucose by aerobic glycolysis, and that this type of anabolic metabolism is critical for the production of essential cellular building blocks including dNTPs, amino acids, and lipids [22] (Figure 1). Growth factor signaling by activated AKT, Myc, and other proto-oncogenes results in altered mitochondrial metabolism [22]. For example, a majority of human tumors harbor mutations in the AKT gene, and activated AKT enhances glucose uptake, allowing cells to maintain a higher than adequate level of ATP [23]. What is unknown is whether the colon tumors that were characterized by the Bielas group [19] harbor activated proto-oncogenes. Nevertheless, the picture that now emerges is that aerobic glycolysis enhances growth of cancer cells by anabolic metabolism without producing high levels of ROS that could inactivate the power supply of the cell.
Other explanations for the low frequency of random mitochondrial mutations in colon tumors include highly efficient DNA repair (for excellent review see [24]) and the coupling of antioxidant proteins to the NADPH/NADP balance (Figure 1). Recent studies have demonstrated that mtDNA is repaired by a variety of mechanisms, including short - and long-patch base excision repair, mismatch repair, and homologous recombination [24]–[27]. The sanitation of dNTPs is also likely to result in fewer mutations. Antioxidant proteins may also play a role in the low random mutation rate observed in mitochondria. Proteins such as reduced glutathione (GSH) or thioredoxin that are closely coupled to the NADPH/NADP balance can inactivate ROS [28], [29]. The presence of NADPH and other free radical scavengers may be more pronounced in the colorectal tumor environment to neutralize the impact of oxidative stress (Figure 1).
What are the implications of the findings of Ericson et al. [19] for future cancer therapy strategy or biomarker discovery? Cancer cells exhibit increased uptake of glucose, which increases the bioenergetic competence required of malignant cells. This metabolic feature has led to the hypothesis that inhibition of glycolysis may abolish ATP and important precursor generation in cancer cells and thus may preferentially kill the malignant cells [30], [31]. Nuclear genetic and epigenetic changes have been the cornerstone of such studies. In addition to these strategies, it is likely that mutated genes that function to alter mitochondrial metabolic competence in tumors, including HIF-1 and MYC, will emerge as new drug targets and molecular markers of prognosis and responses to therapy. In addition, targeting mtDNA repair proteins could serve as a potential alternative approach to kill cancer cells. The work of the Ericson et al. [19] adds significantly to our understanding of the roles of mitochondria in supporting the growth of tumors. In combination with recent findings regarding mitochondrial metabolism in cancer cells, this groundbreaking finding suggests that novel drugs that target the powerhouse of cancer cells are likely to make a significant impact on cancer treatment.
Zdroje
1. CairnsRAHarrisIMcCrackenSMakTW 2011 Cancer cell metabolism. Cold Spring Harb Symp Quant Biol E-pub ahead of print 12 December 2011
2. LoebLASpringgateCFBattulaN 1974 Errors in DNA replication as a basis of malignant changes. Cancer Res 34 2311 2321
3. MoolgavkarSHLuebeckEG 2003 Multistage carcinogenesis and the incidence of human cancer. Genes Chromosomes Cancer 38 302 306
4. Abu-AmeroKKAlzahraniASZouMShiY 2005 High frequency of somatic mitochondrial DNA mutations in human thyroid carcinomas and complex I respiratory defect in thyroid cancer cell lines. Oncogene 24 1455 1460
5. AlonsoAMartinPAlbarranCAquileraBGarciaO 1997 Detection of somatic mutations in the mitochondrial DNA control region of colorectal and gastric tumors by heteroduplex and single-strand conformation analysis. Electrophoresis 18 682 685
6. LiuVWShiHHCheungANChiuPMLeungTW 2001 High incidence of somatic mitochondrial DNA mutations in human ovarian carcinomas. Cancer Res 61 5998 6001
7. MontaniniLRegna-GladinCEoliMAlbarosaRCarraraF 2005 Instability of mitochondrial DNA and MRI and clinical correlations in malignant gliomas. J Neurooncol 74 87 89
8. NagyAWilhelmMKovacsG 2003 Mutations of mtDNA in renal cell tumours arising in end-stage renal disease. J Pathol 199 237 242
9. NomotoSYamashitaKKoshikawaKNakaoASidranskyD 2002 Mitochondrial D-loop mutations as clonal markers in multicentric hepatocellular carcinoma and plasma. Clin Cancer Res 8 481 487
10. PetrosJABaumannAKRuiz-PesiniEAminMBSunCQ 2005 mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci U S A 102 719 724
11. Sanchez-CespedesMAhrendtSAPiantadosiSRosellRMonzoM 2001 Chromosomal alterations in lung adenocarcinoma from smokers and nonsmokers. Cancer Res 61 1309 1313
12. TanDJBaiRKWongLJ 2002 Comprehensive scanning of somatic mitochondrial DNA mutations in breast cancer. Cancer Res 62 972 976
13. WuCWYinPHHungWYLiAFLiSH 2005 Mitochondrial DNA mutations and mitochondrial DNA depletion in gastric cancer. Genes Chromosomes Cancer 44 19 28
14. Modica-NapolitanoJSSinghKK 2004 Mitochondrial dysfunction in cancer. Mitochondrion 4 755 762
15. SinghKKKulawiecMStillIDesoukiMMGeradtsJ 2005 Inter-genomic cross talk between mitochondria and the nucleus plays an important role in tumorigenesis. Gene 354 140 146
16. CopelandWCWachsmanJTJohnsonFMPentaJS 2002 Mitochondrial DNA alterations in cancer. Cancer Invest 20 557 569
17. CarewJSHuangP 2002 Mitochondrial defects in cancer. Mol Cancer 1 9
18. BielasJHLoebLA 2005 Quantification of random genomic mutations. Nat Methods 2 285 290
19. EricsonNGKulawiecMVermulstMSheahanKO'SullivanJ 2012 Decreased mitochondrial DNA mutagenesis in human colorectal cancer. PLoS Genet 8 e1002689 doi:10.1371/journal.pgen.1002689
20. WarburgO 1956 On the origin of cancer cells. Science 123 309 314
21. WarburgO 1956 On respiratory impairment in cancer cells. Science 124 269 270
22. WardPSThompsonCB 2012 Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21 297 308
23. ElstromRLBauerDEBuzzaiMKarnauskasRHarrisMH 2004 Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64 3892 3899
24. LiuPDempleB 2010 DNA repair in mammalian mitochondria: Much more than we thought? Environ Mol Mutagen 51 417 426
25. AkbariMVisnesTKrokanHEOtterleiM 2008 Mitochondrial base excision repair of uracil and AP sites takes place by single-nucleotide insertion and long-patch DNA synthesis. DNA Repair (Amst) 7 605 616
26. FukuiHMoraesCT 2009 Mechanisms of formation and accumulation of mitochondrial DNA deletions in aging neurons. Hum Mol Genet 18 1028 1036
27. KraytsbergYSchwartzMBrownTAEbralidseKKunzWS 2004 Recombination of human mitochondrial DNA. Science 304 981
28. HamanakaRBChandelNS 2011 Cell biology. Warburg effect and redox balance. Science 334 1219 1220
29. SattlerUGMueller-KlieserW 2009 The anti-oxidant capacity of tumour glycolysis. Int J Radiat Biol 85 963 971
30. IzyumovDSAvetisyanAVPletjushkinaOYSakharovDVWirtzKW 2004 “Wages of fear”: transient threefold decrease in intracellular ATP level imposes apoptosis. Biochim Biophys Acta 1658 141 147
31. Munoz-PinedoCRuiz-RuizCRuiz de AlmodovarCPalaciosCLopez-RivasA 2003 Inhibition of glucose metabolism sensitizes tumor cells to death receptor-triggered apoptosis through enhancement of death-inducing signaling complex formation and apical procaspase-8 processing. J Biol Chem 278 12759 12768
Štítky
Genetika Reprodukční medicína
Článek Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental InvolvementČlánek Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target GenesČlánek Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early EmbryosČlánek Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2Článek Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human CellsČlánek TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome EndsČlánek Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal CordČlánek Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth ofČlánek MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 6- Délka menstruačního cyklu jako marker ženské plodnosti
- Srdeční frekvence embrya může být faktorem užitečným v předpovídání výsledku IVF
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
-
Všechny články tohoto čísla
- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- Mimetic Butterflies Introgress to Impress
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Selection-Driven Gene Loss in Bacteria
- Decreased Mitochondrial DNA Mutagenesis in Human Colorectal Cancer
- Parallel Evolution of Auditory Genes for Echolocation in Bats and Toothed Whales
- Diverse CRISPRs Evolving in Human Microbiomes
- The Rad4 ATR-Activation Domain Functions in G1/S Phase in a Chromatin-Dependent Manner
- Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains
- Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
- Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target Genes
- Adaptive Introgression across Species Boundaries in Butterflies
- G Protein Activation without a GEF in the Plant Kingdom
- Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes
- An Engineering Approach to Extending Lifespan in
- Incompatibility and Competitive Exclusion of Genomic Segments between Sibling Species
- Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in
- Patterns of Evolutionary Conservation of Essential Genes Correlate with Their Compensability
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
- Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early Embryos
- A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity
- Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2
- Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis
- Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells
- TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome Ends
- Stimulation of Host Immune Defenses by a Small Molecule Protects from Bacterial Infection
- A Broad Requirement for TLS Polymerases η and κ, and Interacting Sumoylation and Nuclear Pore Proteins, in Lesion Bypass during Embryogenesis
- Genome-Wide Identification of Ampicillin Resistance Determinants in
- The CCR4-NOT Complex Is Implicated in the Viability of Aneuploid Yeasts
- Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees
- Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
- Is a Key Regulator of Pancreaticobiliary Ductal System Development
- The NSL Complex Regulates Housekeeping Genes in
- Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord
- Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
- Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth of
- Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia
- MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
- Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family
- Found: The Elusive ANTAR Transcription Antiterminator
- The Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility
- Control of CpG and Non-CpG DNA Methylation by DNA Methyltransferases
- Polymorphisms in the Mitochondrial Ribosome Recycling Factor Compromise Cell Respiratory Function and Increase Atorvastatin Toxicity
- Gene Expression Profiles in Parkinson Disease Prefrontal Cortex Implicate and Genes under Its Transcriptional Regulation
- Global Regulatory Functions of the Endoribonuclease III in Gene Expression
- Extensive Evolutionary Changes in Regulatory Element Activity during Human Origins Are Associated with Altered Gene Expression and Positive Selection
- The Regulatory Network of Natural Competence and Transformation of
- Brain Expression Genome-Wide Association Study (eGWAS) Identifies Human Disease-Associated Variants
- Quantifying the Adaptive Potential of an Antibiotic Resistance Enzyme
- Divergence of the Yeast Transcription Factor Affects Sulfite Resistance
- The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis
- RNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
- Rare Copy Number Variants Observed in Hereditary Breast Cancer Cases Disrupt Genes in Estrogen Signaling and Tumor Suppression Network
- Genome-Wide Location Analysis Reveals Distinct Transcriptional Circuitry by Paralogous Regulators Foxa1 and Foxa2
- A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
- The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression
- Limits to the Rate of Adaptive Substitution in Sexual Populations
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- The NSL Complex Regulates Housekeeping Genes in
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



