-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
As one of the leading causes of visual impairment and blindness, myopia poses a significant public health burden in Asia. The primary determinant of myopia is an elongated ocular axial length (AL). Here we report a meta-analysis of three genome-wide association studies on AL conducted in 1,860 Chinese adults, 929 Chinese children, and 2,155 Malay adults. We identified a genetic locus on chromosome 1q41 harboring the zinc-finger 11B pseudogene ZC3H11B showing genome-wide significant association with AL variation (rs4373767, β = −0.16 mm per minor allele, Pmeta = 2.69×10−10). The minor C allele of rs4373767 was also observed to significantly associate with decreased susceptibility to high myopia (per-allele odds ratio (OR) = 0.75, 95% CI: 0.68–0.84, Pmeta = 4.38×10−7) in 1,118 highly myopic cases and 5,433 controls. ZC3H11B and two neighboring genes SLC30A10 and LYPLAL1 were expressed in the human neural retina, retinal pigment epithelium, and sclera. In an experimental myopia mouse model, we observed significant alterations to gene and protein expression in the retina and sclera of the unilateral induced myopic eyes for the murine genes ZC3H11A, SLC30A10, and LYPLAL1. This supports the likely role of genetic variants at chromosome 1q41 in influencing AL variation and high myopia.
Published in the journal: . PLoS Genet 8(6): e32767. doi:10.1371/journal.pgen.1002753
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002753Summary
As one of the leading causes of visual impairment and blindness, myopia poses a significant public health burden in Asia. The primary determinant of myopia is an elongated ocular axial length (AL). Here we report a meta-analysis of three genome-wide association studies on AL conducted in 1,860 Chinese adults, 929 Chinese children, and 2,155 Malay adults. We identified a genetic locus on chromosome 1q41 harboring the zinc-finger 11B pseudogene ZC3H11B showing genome-wide significant association with AL variation (rs4373767, β = −0.16 mm per minor allele, Pmeta = 2.69×10−10). The minor C allele of rs4373767 was also observed to significantly associate with decreased susceptibility to high myopia (per-allele odds ratio (OR) = 0.75, 95% CI: 0.68–0.84, Pmeta = 4.38×10−7) in 1,118 highly myopic cases and 5,433 controls. ZC3H11B and two neighboring genes SLC30A10 and LYPLAL1 were expressed in the human neural retina, retinal pigment epithelium, and sclera. In an experimental myopia mouse model, we observed significant alterations to gene and protein expression in the retina and sclera of the unilateral induced myopic eyes for the murine genes ZC3H11A, SLC30A10, and LYPLAL1. This supports the likely role of genetic variants at chromosome 1q41 in influencing AL variation and high myopia.
Introduction
Myopia increases the risk of visual morbidity and poses a considerable public health and economic burden globally, especially in Asia, where the prevalence is significantly higher than other parts of the world [1]. Human myopia primarily results from an abnormal increase in ocular axial length (AL), the distance between the anterior and posterior poles of the eye globe, whereas the role of corneal curvature and lens thickness is minimal [2]. A 1 millimeter (mm) increase in AL is equivalent to a myopic shift of −2.00 to −3.00 diopters (D) with no corresponding changes in the optical power of the cornea and lens. High myopia, often defined as ocular spherical equivalent (SE) refraction below −6.00 D, is associated with an abnormally long AL, and this affects between 1% to 10% of the general population [3]. The degenerative changes in the retina and the choroid due to the excessive elongation of the globe are not prevented by optical correction and this subsequently increases the risk of visual morbidity through myopic maculopathy, choroidal neovascularization, retinal detachment and macular holes [4]. The active remodeling of the sclera, mediated by the signaling cascade initiated in the retina under visual input, has also been found to be critical in determining axial growth, and thus the refractive state of the eye [5].
Environmental factors such as the extent of near work, level of educational attainment and amount of outdoor activities have been documented to affect myopia development [6]. Evidence from family and twin studies has also supported a substantial genetic component in spherical refractive error and AL [7]–[9]. The heritability of the quantitative trait AL has been estimated to be as high as 94% comparable to that for SE (for a review, see [10]). Although linkage scans on pedigrees (myopia loci MYP1 to MYP18; see http://www.omim.org) and genome-wide association studies (GWAS) [11]–[16] have implicated several regions in the human genome as being significant for refractive error and myopia, no myopia genes have been consistently identified within or across different population groups. This scenario reflects the complexity in the disease architecture of myopia pathogenesis.
Genetic factors influencing AL and refraction appear to be at least partly shared, given previous literature from twin studies illustrating that at least half of the covariance between AL and refraction are due to common genetic factors [18]. The measurement of AL is more precise and less prone to errors compared to cycloplegic or non-cycloplegic assessments of refraction. As AL is an endophenotype for spherical refractive error, identifying genes that are responsible for AL variation provides insight into myopia predisposition and development. Presently there are only two genome-wide linkage studies performed in European descent populations that suggest the presence of AL quantitative trait loci (QTLs) on chromosomes 2p24 [19] and 5q (at 98 centimorgans) along with two classical myopia loci (MYP3 at 12q21 and MYP9 at 4q12) [20], and there are no reports of any genes that are indisputably confirmed to be associated with AL.
We thus performed a meta-analysis of three genome-wide surveys of AL in a total of 4,944 individuals in Asian populations comprising (i) Chinese adults from the Singapore Chinese Eye Study (SCES); (ii) Chinese children from the Singapore cohort Study of the Risk factors for Myopia (SCORM); and (iii) Malay adults from the Singapore Malay Eye Study (SiMES). SNPs that have been identified from this meta-analysis to be significantly associated with AL were further assessed for association with high myopia in an additional two independent case-control studies from Japan. We also examined the expression patterns of the candidate genes located in the vicinity of the identified SNPs in human ocular tissues and in the eyes of myopic mice.
Results
A genome-wide meta-analysis of three GWAS on AL was performed in the post quality control samples from SCES (n = 1,860), SCORM (n = 929) and SiMES (n = 2,155). Principal component analysis (PCA) of these samples with reference to the HapMap Phase 2 individuals showed that the two Chinese cohorts (SCES and SCORM) are indistinguishable with respect to samples of Han Chinese descent, and the differentiation from samples of Japanese descent is evident only on the fourth principal component (Figure S1). The SiMES Malays are genetically similar to the Chinese-descent samples relative to individuals with European or African ancestries. The distributions of AL measurements in the three cohorts were approximately Gaussian and the baseline characteristics are summarized in Table 1. The mean AL were 23.98 mm (SD = 1.39 mm), 24.10 mm (SD = 1.18 mm) and 23.57 mm (SD = 1.04 mm) for SCES, SCORM and SiMES respectively. Moderate to high correlations between AL and SE were observed (SCES/SCORM/SiMES; Pearson correlation coefficient r = −0.75, −0.76 and −0.62 respectively). The meta-analysis was performed on 456,634 SNPs present in all three studies, and the quantile-quantile (QQ) plots of the P-values showed only modest inflation of the test statistics in SCES and in the meta-analysis (genomic control inflation factor: λmeta = 1.03; λSCES = 1.05; λSCORM = 1.00; λSiMES = 1.00, Figure S2).
Tab. 1. Characteristics of study participants in the five Asian cohorts. 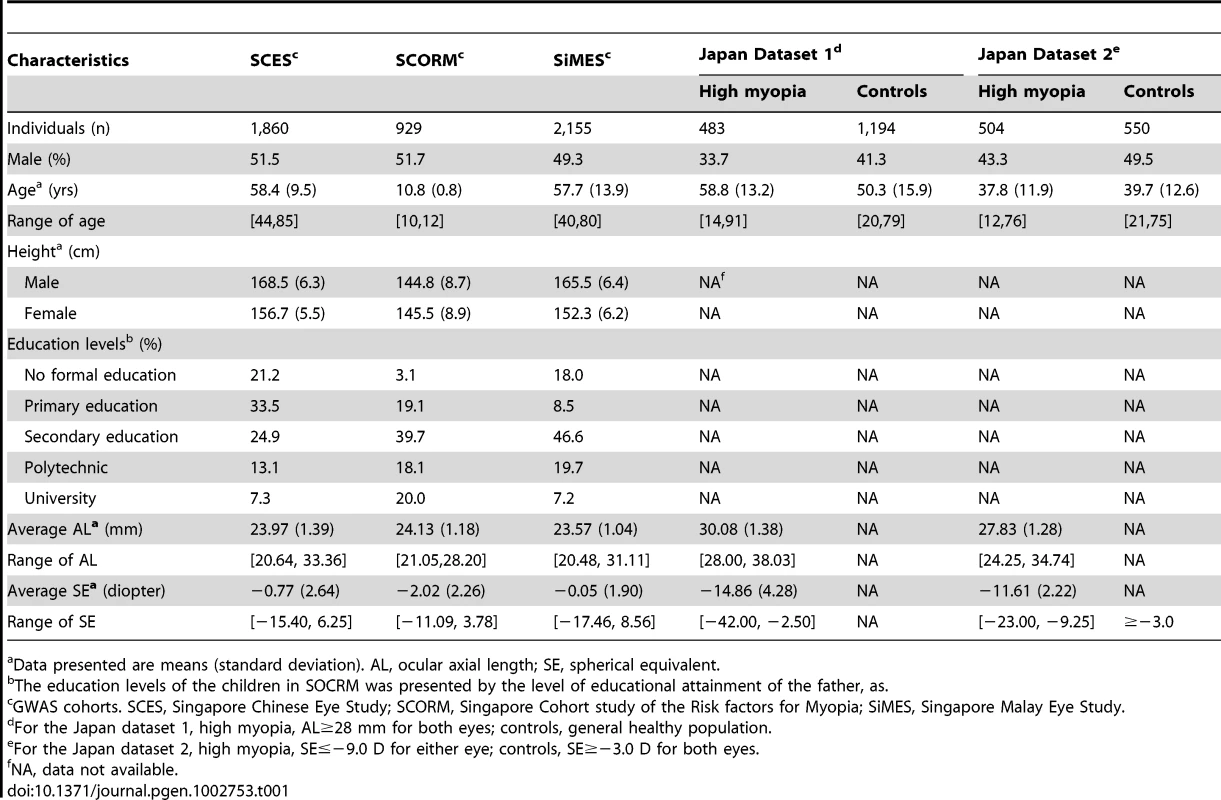
Data presented are means (standard deviation). AL, ocular axial length; SE, spherical equivalent. A cluster of four SNPs on chromosome 1q41 (rs4373767, rs10779363, rs7544369 and rs4428898) attained genome-wide significance on meta-analysis for AL, adjusting for age, gender, height and education level (Figure 1). Analyses conducted without adjustment for height or education level yielded the same pattern of results. The most significant SNP rs4373767 (Pmeta = 2.69×10−10) explained 0.98% of AL variance in SCES, 0.86% in SCORM and 0.73% in SiMES, and each copy of the minor allele (cytosine) decreased AL by 0.16 mm on average (Table 2). These top associated SNPs at chromosome 1q41 remained significant after adjustment for genomic control (Pmeta≤1.85×10−8). Table 2 also lists three genetic loci at chromosome 2p13.1 (SEMA4F), 2p21 (SPTBN1) and 5q11.1 (PARP8) exhibiting suggestive evidence of association with AL that were seen in at least one SNP with P-values<1×10−5.
Fig. 1. Manhattan plot of -log10(P) for the association on axial length from the meta-analysis in the combined cohorts of SCES, SCORM, and SiMES. 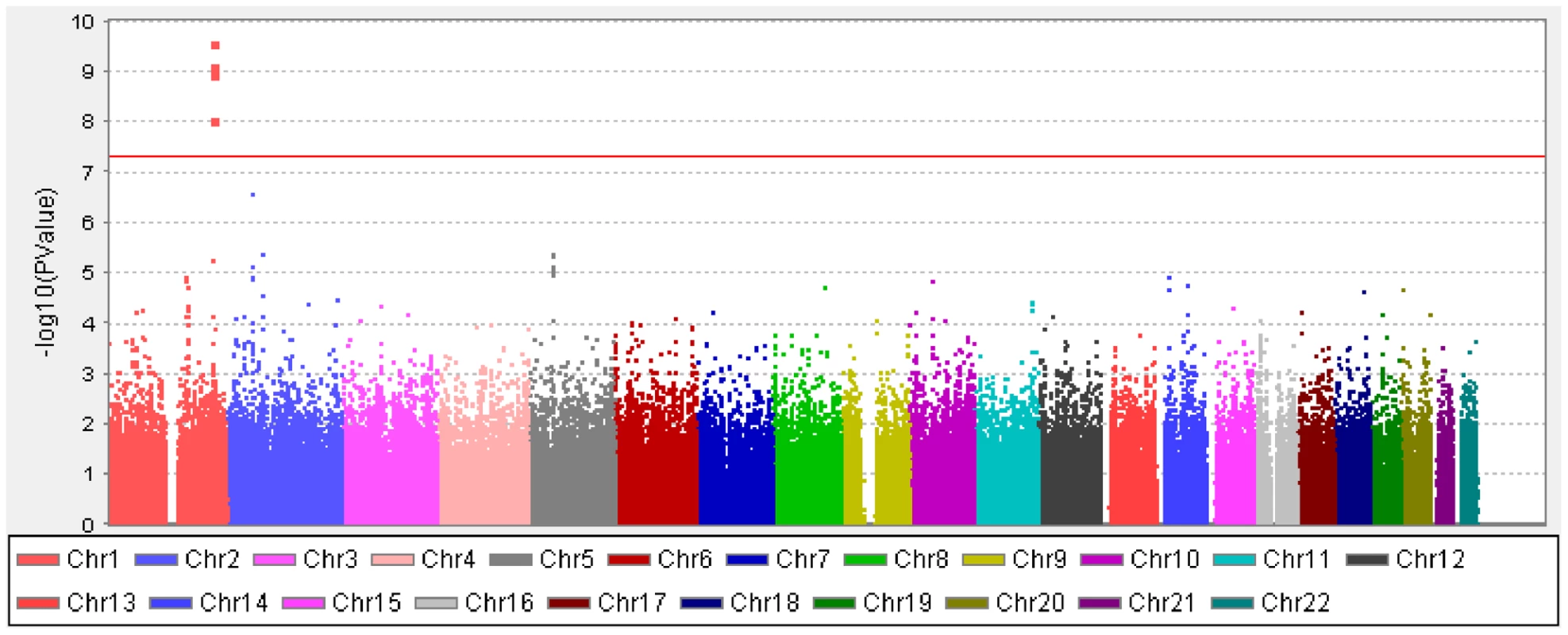
The red horizontal line denotes genome-wide significance (P = 5×10−8). Tab. 2. Top SNPs (Pmeta-value≤1×10−5) associated with AL from the meta-analysis in the three Asian cohorts. 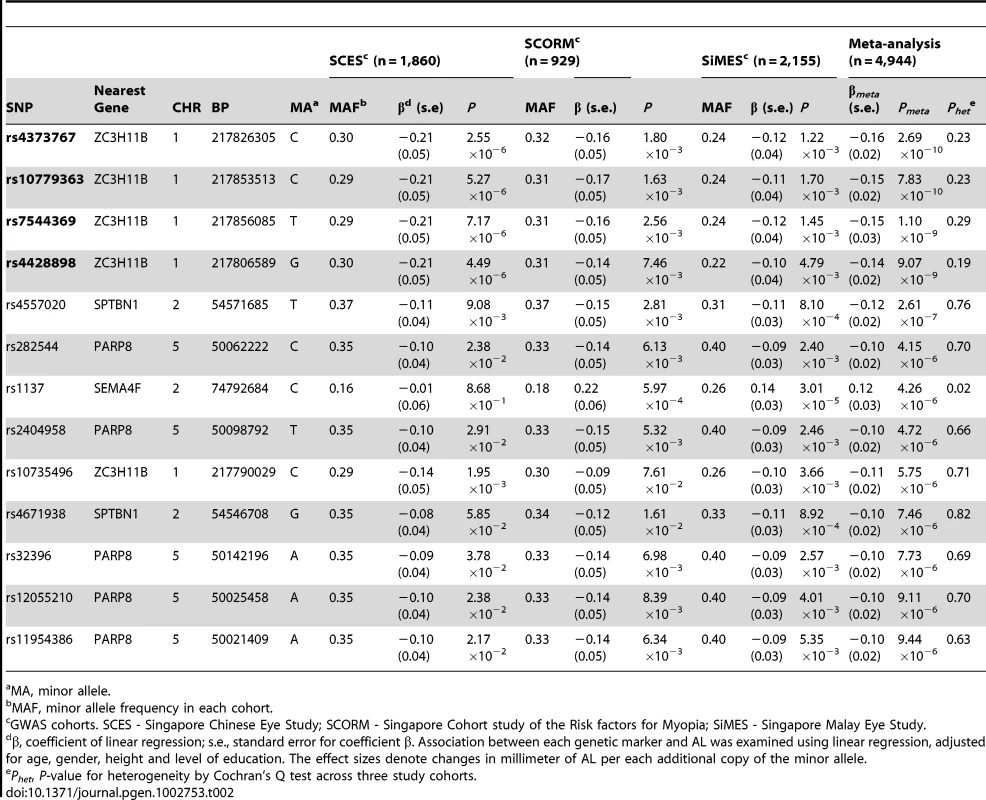
MA, minor allele. To assess whether these four SNPs at chromosome 1q41 have any role in high myopia predisposition, we performed association testing of these SNPs with high myopia in two independent case-control studies from Japan consisting of 987 high myopes and 1,744 controls. High myopes were defined as individuals with SE≤−9.00 D or AL≥28 mm (see Materials and Methods). All four SNPs exhibited consistent evidence of association (P<0.05) in both Japanese studies, suggesting a potential role of these SNPs for high myopia (Table 3).
Tab. 3. Association between genetic variants at chromosome 1q41 and high myopia in the five Asian cohorts. 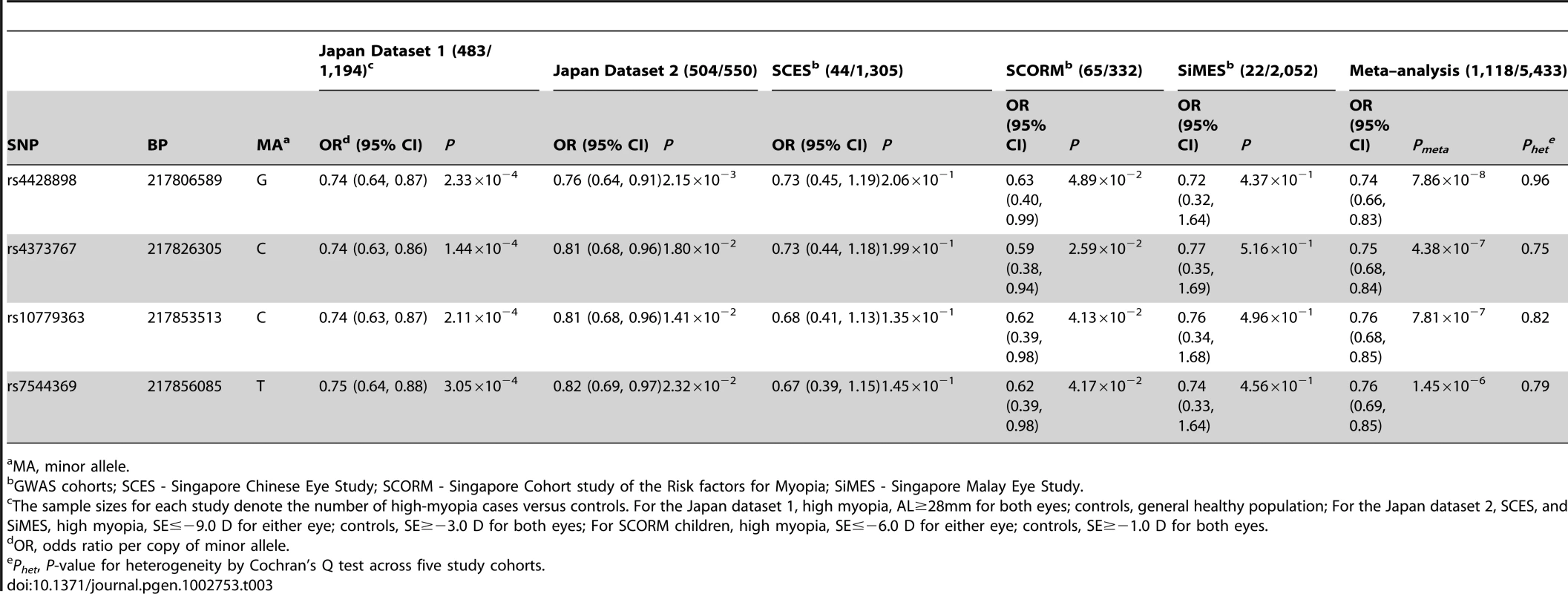
MA, minor allele. We further dichotomized the quantitative refraction from our three population-based studies (SCORM, SCES, and SIMES) to define samples as high myopes and controls according to similar criteria from the Japanese datasets. High myopes in SCES and SiMES were younger and more highly educated than controls (Table S1). While the case-control associations of these 4 SNPs with high myopia did not achieve statistical significance in SCES and SiMES, this is likely a consequence of the small sample sizes since the direction and magnitude of the odds ratios were highly similar across all cohorts. The meta-analysis of 1,118 high myopia cases and 5,433 controls from all the five cohorts yielded strong evidence of association with high myopia at these SNPs (Pmeta between 1.45×10−6 to 7.86×10−8, Table 3), with no evidence of inter-study heterogeneity (P≥0.75 for heterogeneity). The minor allele cytosine at rs4373767 lowered the odds of high myopia by 25% with respect to the thymidine allele (ORmeta = 0.75, 95% CI: 0.68–0.84, Pmeta = 4.38×10−7). The stringent definition of high myopia (SE≤−9.00D) used here only considered between 1.0% to 2.4% of our samples as cases, and relaxing this criterion to the commonly adopted threshold of SE≤−6.00D identified more myopia cases and increased the statistical support of all four SNPs (Pmeta between 1.47×10−7 to 9.13×10−9, Table S2).
This associated interval spans approximately 70 kb in the extended linkage disequilibrium (LD) block within an intergenic region on chromosome 1q41 (pairwise r2>0.5 with the most significant SNP rs4373767, Figure 2A). Zinc finger family CCCH-type 11B pseudogene ZC3H11B (RefSeq NG_007367.2) is embedded between the associated top SNPs rs4373767 and rs10779363 (Figure 2B). The most significant SNP rs4373767 is located 223 kb downstream from SLC30A10 (RefSeq NM_018713.2), which is a member of solute carrier family 30, and 354 kb downstream of LYPLAL1 (RefSeq NM_138794.3), encoding a lysophospholipase-like protein.
Fig. 2. The chromosome 1q41 region and its association with axial length in the Asian cohorts. 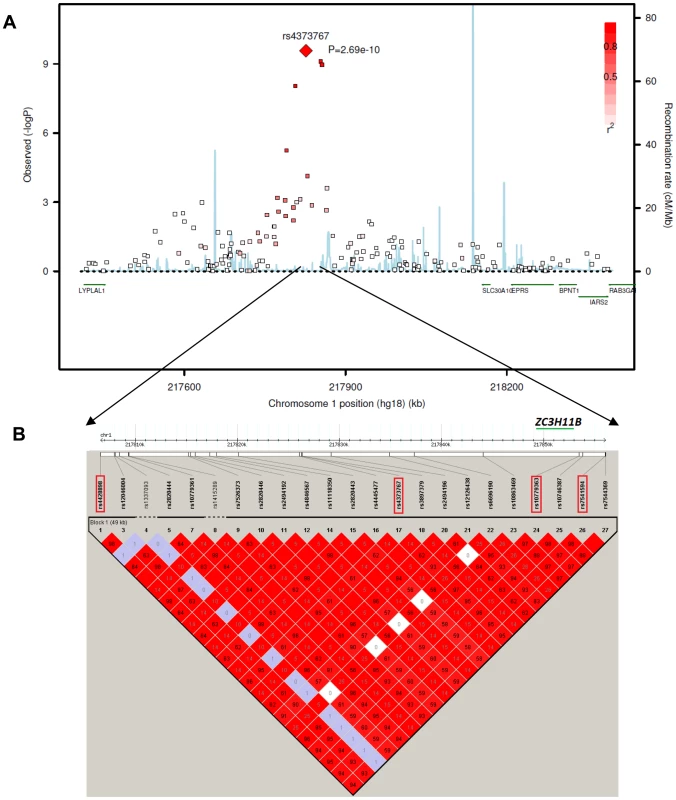
A) Regional plots for AL from the meta-analysis of three Asian GWAS cohorts: SCES, SCORM and SiMES. The association signals in a 1 megabase (Mb) region at chromosome 1q41 from 217,400 kb to 218,400 kb around the top SNP rs4373767 (red diamond) are plotted. The degree of pair-wise LD between the rs4373767 and any genotyped SNPs in this region is indicated by red shading, measured by r2. Superimposed on the plots are gene locations and recombination rates in HapMap Chinese and Japanese populations (blue lines). B) LD plot showing pair-wise r2 for all the SNPs genotyped in HapMap database residing between rs4428898 and rs7544369, inclusively, at chromosome 1q41. The four identified top SNPs are in red rectangles. The LD plot is generated by Haploview using SNPs (MAF>1%) genotyped on Han Chinese and Japanese samples in the HapMap database. All coordinates are in Build hg18. The mRNA expression levels of ZC3H11B, SLC30A10 and LYPLAL1 were surveyed in 24-week human fetal and adult tissues using reverse-transcriptase polymerase chain reaction (RT-PCR). Whilst ZC3H11B and LYPLAL1 were found to be expressed across all the tissues including brain, placenta, neural retina, retina pigment epithelium (RPE) and sclera, the expression of ZC3H11B was more abundant compared to LYPLAL1 (Figure 3). SLC30A10 was expressed in all tissues but the adult sclera, analogous to observations made in other zinc transporters [21].
Fig. 3. mRNA expression of ZC3H11B, SLC30A10, and LYPLAL1 in human tissues. 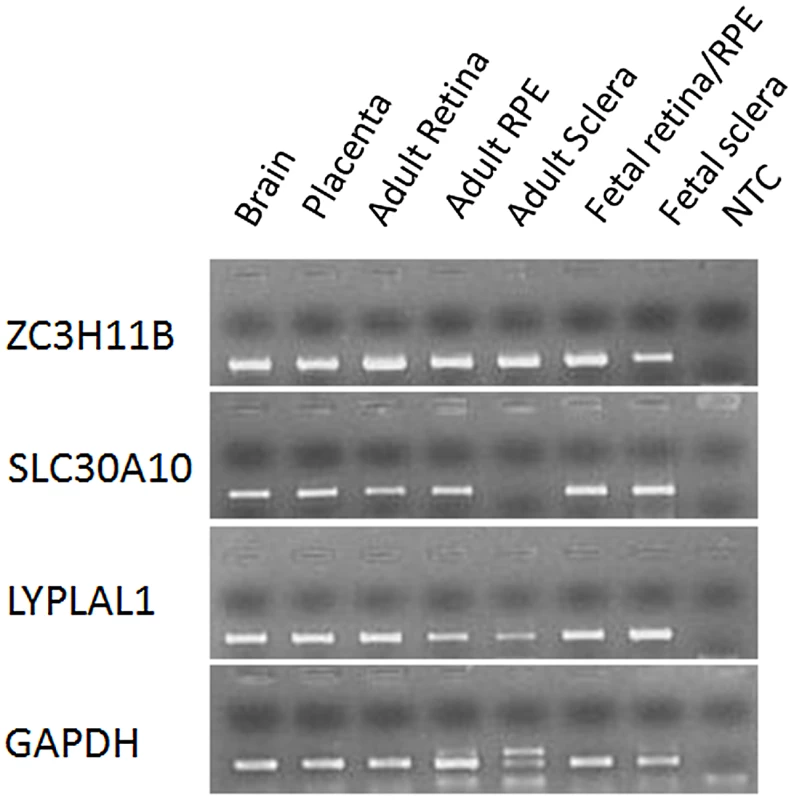
Expression of mRNA for the three genes was examined in human brain, placenta, neural retina (retina), retinal pigment epithelium (RPE) and sclera from adult tissues, and retina/RPE and sclera from 24-week gestation fetal tissues using reverse transcription polymerase chain reaction (RT-PCR). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is a housekeeping gene and was used as an internal control for the quantification of mRNA expression. NTC (No template control) served as a negative control with the use of water rather than cDNA during PCR. Gene expressions for ZC3H11A, SLC30A10 and LYPLAL1 from the tissues of myopic (with SE<−5.0 D) and fellow non-occluded eyes of the experimental mice were compared with age-matched control tissues (Figure 4). The mRNA levels of ZC3H11A, a gene that is conserved with respect to ZC3H11B in human, were significantly down-regulated in myopic eyes compared to naive controls (retina/RPE/sclera, Fold change = −2.88, −3.24 and −2.07; P = 2.60×10−5, 2.62×10−6 and 1.08×10−4, respectively). At the neighboring gene SLC30A10, there was a similarly significant reduction in the expression of mRNA in the retina tissue of myopic eyes in contrast to independent controls (retina/RPE, Fold change = −2.02, −2.69; P = 2.00×10−4, 2.00×10−4, respectively), with elevated expression in the sclera (Fold change = 4.58; P = 4.02×10−4). Another neighboring gene LYPLAL1 exhibited up-regulation of transcription levels in retina tissue but was down-regulated in the sclera (retina/RPE/sclera, Fold change = 2.71, 3.45 and −2.36; P = 1.50×10−4, 1.50×10−4 and 1.54×10−4, respectively).
Fig. 4. Transcription quantification of ZC3H11A, SLC30A10, and LYPLAL1 in mouse retina, retinal pigment epithelium, and sclera in induced myopic eyes, fellow eyes, and independent control eyes. 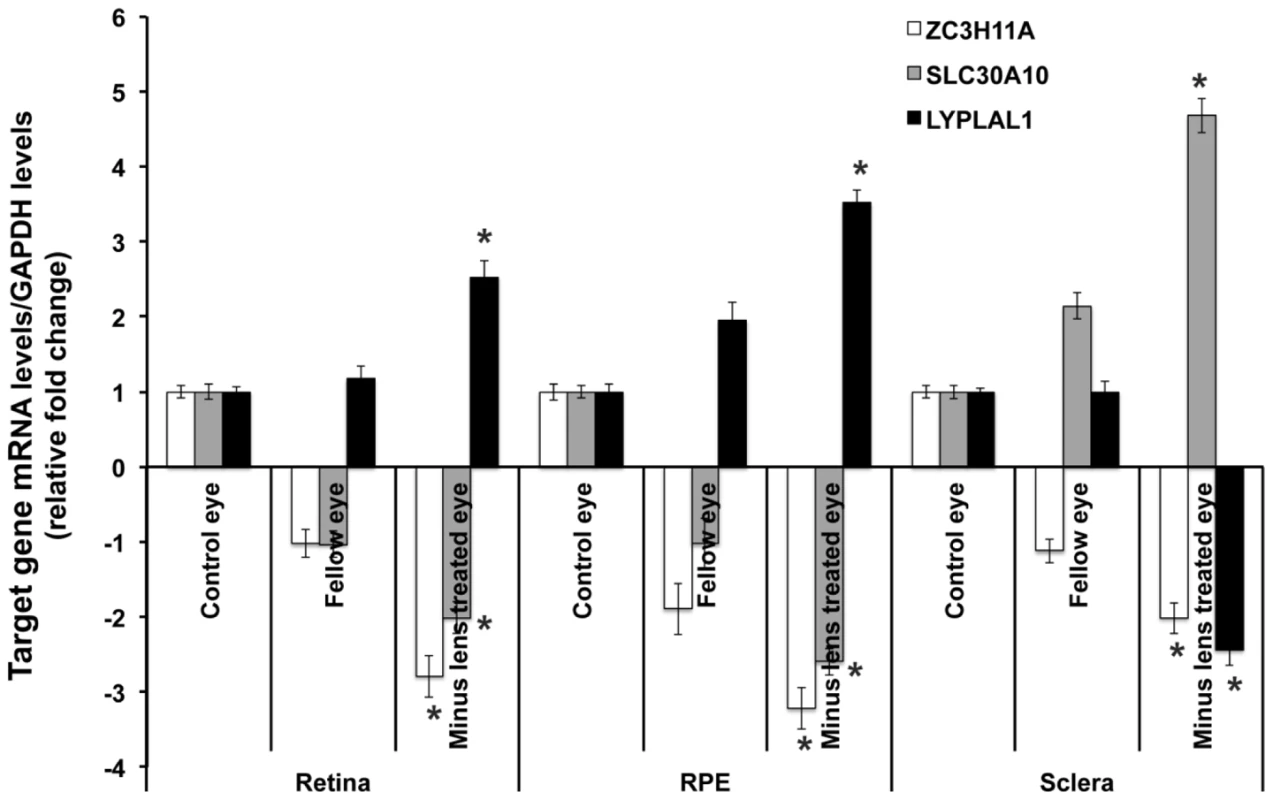
Myopia was induced using −15 diopter negative lenses in the right eye of mice for 6 weeks. Uncovered left eyes were served as fellow eyes and age-matched naive mice eyes were controls. Quantification of mRNA expression in mice neural retina (retina), retinal pigment epithelium (RPE) and sclera using quantitative real-time PCR. The bar represents the fold changes of mRNA for each gene after normalization using GAPDH as reference. The mRNA levels of murine ZC3H11A, a gene that is conserved with respect to ZC3H11B in human, SLC30A10 and LYPLAL1 in myopic and fellow retina, RPE and sclera are compared with independent controls with P-values as follows: ZC3H11A (retina/RPE/sclera, P = 2.60×10−5, 2.62×10−6 and 1.08×10−4 respectively), SLC30A10 (P = 2.00×10−4, 2.00×10−4 and 4.02×10−4 respectively) and LYPLAL1 (P = 1.50×10−4, 1.50×10−4, 1.54×10−4 respectively). *P<0.0001. Immunohistochemical results confirmed the localization of ZC3H11A, SLC30A10 and LYPLAL1 proteins in the neural retina, RPE and sclera (Figure 5). For ZC3H11A, positive immunostaining intensity was reduced significantly in the myopic tissues of experimental mice compared to the non-myopic independent controls (Figure 5A). This is consistent with the differential expression patterns at the transcription level. For SLC30A10 and LYPLAL1, there were also similarly noticeable changes in the expression of proteins to that of their mRNA levels (Figure 5B and 5C).
Fig. 5. Immunofluorescent labeling. 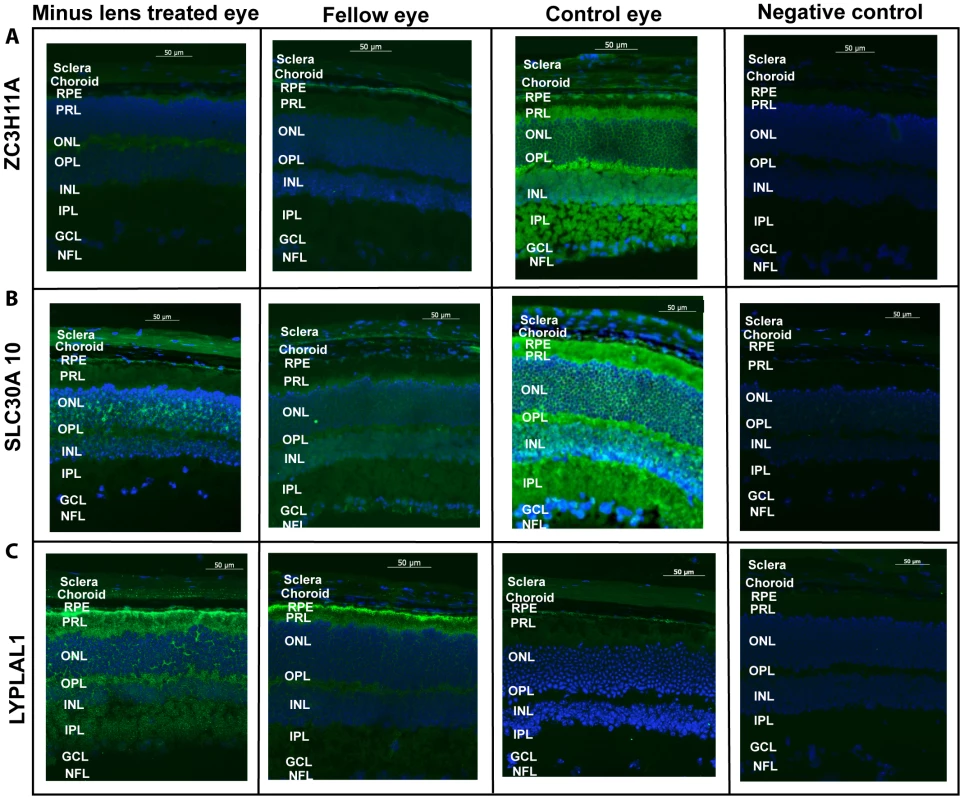
Immunofluorescent labeling of (A) ZC3H11A (B) SLC30A10 and (C) LYPLAL1 in mouse retina, retinal pigment epithelium and sclera in induced myopic eyes, fellow eyes and independent control eyes. The neural retina (retina), retinal pigment epithelium (PRE) and scleral cells were immunolabeled with the polycolonal antibodies against ZC3H11A, SLC30A10 and LYPLAL1 and were co-labeled with 4′,6-diamidino-2-phenylindole (DAPI). Negative controls were devoid of a fluorescence signal, treated with the secondary antibody alone and DAPI. No immunostaining was observed in the negative controls. Scale bar represents 50 µM and magnification is 200×. The florescence intensity labeled of the green color shows the localization of proteins and blue color indicates the nuclei that were stained with DAPI. Expression of the proteins had a trend in abundance similarly to that of their mRNA levels as depicted in Figure 4. Lower level of expression was determined for ZC3H11A in all tissues for myopic mice. Similarly significant reduction was shown in the expression of SLC30A10 in retina and RPE while higher level of expression was found in myopic sclera. LYPLAL1 showed higher level of expression in the retina and RPE tissue but reduced expression in the sclera in myopic mice. The following abbreviations represent the retinal layers: nerve fibre layer (NFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), photo receptor layer (PRL) and retinal pigment epithelium (RPE). Discussion
We report that the chromosome 1q41 locus (most significant SNP rs4373767) is associated with AL in a meta-analysis of three GWAS performed in the study cohorts consisting of Chinese adults, Chinese children, and Malay adults. The discovery of chromosome 1q41 as a locus for high myopia in our data is further supported by validation in two independent Japanese cohorts, and the observed genetic effects are highly consistent across all five studies. The pseudogene ZC3H11B and two nearby genes SLC30A10 and LYPLAL1 were found to be expressed in the human retina and sclera. The potential roles in regulating myopia at three candidate genes were further implicated by the concordant changes in the pattern of transcription and protein expression in the mouse model.
The ZC3H11B pseudogene belongs to the CCCH-type zinc finger family, whereas such type of zinc finger protein has been shown as a RNA-binding motif to facilitate the mRNA processing at transcription [22]. Emerging evidence suggests that pseudogenes, resembling known genes but not producing proteins, play a significant role in pathological conditions by competing for binding sites to regulate the transcription of its protein-coding counterpart [23]–[25]. Although the function of the ZC3H11B in humans is presently unknown, the implicated role of the murine gene ZC3H11A (conserved gene of ZC3H11B in mouse) in myopia development is in keeping with previous findings that several zinc finger proteins are involved in myopia [26], [27]. Given their role as transcription factors [28], zinc finger protein ZENK has been proposed to function as a messenger in modulating the visual signaling cascade in the chicken retina, where the expression of the ZENK was suppressed by the condition of minus defocus (induced myopic eye growth) and enhanced by positive defocus (induced hyperopic eye growth) [29]–[31]. Similarly, it has been reported that ZENK knockout mice had elongated AL and a myopic shift in refraction [27]. Moreover, early growth response gene type1 EGR-1 (the human homologue of ZENK) has been shown to activate transforming growth factor beta 1 gene TGFB1 by binding its promoter [32], [33], a gene that is implicated to be associated with myopia [34], [35]. Another zinc protein finger protein 644 isoform ZNF644 has recently been identified to be responsible for high myopia using whole genome exome sequencing in a Han Chinese family [26], whereas its influence on “myopia genes” remains to be elucidated. In light of this, the observation that ZC3H11B is abundantly expressed in retina and sclera, together with the significant down-regulation of the coding counterpart ZC3H11A in myopic mice eyes, suggests it may promote or inhibit the transcription of ocular growth genes vital in myopia development.
One of the two neighboring genes SLC30A10 is an efflux transporter that reduces cytoplasmic zinc concentrations [36]. The SLC30 zinc transporters are expressed abundantly in human RPE cells, and the retina has been observed to possess the highest concentration of zinc in the human body [21]. Zinc deficiency in the intracellular retina has thus been implicated in the pathogenesis of age-related macular degeneration (AMD) [37], [38], and in RPE-photoreceptor complex deficits, which can affect visual signal transduction from retina to sclera and lead to visual impairment [39]. LYPLAL1 functions as a triglyceride lipase and this gene has been shown to be up-regulated in subcutaneous adipose tissue in obese individuals [40]–[42]. While the relationship between LYPLAL1 and myopia is unknown, elevated saturated-fat intake has been proposed to influence myopia development through the retinoid receptor pathway [43]–[45]. Interestingly, the SNPs pinpointing chromosome 1q41 in our study are 1 Mb away from the transforming growth factor beta 2 gene (TGFβ2) which has been implicated in the down-regulation of mRNA levels in myopia progression of an induced tree shrew myopia model [46]. None of these nearby genes, however, are within the LD block containing our identified SNPs.
Chromosome 1q41 is a previously reported locus for refraction from a linkage analysis of 486 pedigrees in the Beaver Dam Eye Study, US [47]. Using microsatellite markers, Klein et al identified novel regions of linkage to SE on chromosome 1q41, whereas the peak spanned a broad region near Marker D1S2141 (multipoint P<1.9×10−4). This result however was not replicated in a subsequent genome-wide linkage scan for SE with denser SNP markers, partially due to varying information of linkage conveyed by SNPs versus microsatellites [48]. The identified variants at chromosome 1q41 in our study were noted to exhibit weaker, albeit still significant, association with SE in SCES and SCORM (rs4373767, SCES/SCORM: P = 3.54×10−3, 3.49×10−2, respectively; Table S3), but not in SiMES (3.51×10−1), which is consistent with the lower correlation of AL and SE seen in the SiMES data, partially from increasing lens opalescence in the Malay population [49], [50].
Our data have shown that genetic variants on chromosome 1q41 influence the physiological attribute of AL and are also associated with high myopia. Elongation of AL is the major underlying structural determinant of high myopia, mostly accompanied with prolate eyeballs and thinning of the sclera, macula and retina [4]. Thus, high myopia is also defined as AL of >26 mm in some studies [13], [51]. It is possible that genes involved in a quantitative trait (refraction or underlying AL) also play a role in the extreme forms of the trait (high myopia) [52]. Two recent GWAS performed in general Caucasians population have identified genetic variants for quantitative refraction at chromosome 15q14 [11] and 15q25 [12], of which the locus on 15q14 was subsequently confirmed to be associated with high myopia in the Japanese [53]. Our GWAS results herein highlight AL QTLs relevant for high myopia predisposition, which advances our understanding of the genetic etiology of myopia at different levels of severity.
The meta-analysis of three GWAS in our discovery suggests that the quantitative trait locus at chromosome 1q41 accounts for variation in AL in both school children and adults, regardless of age differences. Notably, the early-onset of myopia in childhood may continuously progress toward high myopia in later life, while adult-onset of myopia is usually in the low or moderate form [54],[55]. The significant association on chromosome 1q41 for high myopia in adults and children thus also implicates this locus identified for AL is likely to be associated with early-onset myopia.
The prevalence of myopia among Asian population is considerably higher than in Caucasians [1]. Although distinct genetic mechanisms governing myopia may exist for populations with different genetic backgrounds, we believe there are polymorphisms involved in refractive variation that are shared across populations. However, the allele frequencies of these identified SNPs vary across populations. For instance, the minor C allele of rs4373767 was a major allele in the HapMap Africans and Europeans with frequency of 0.92 and 0.62 respectively. Four distinct linkage disequilibrium (LD) blocks existed in 50 kb region encapsulating our top SNPs in the HapMap Africans, whereas high LD was observed for the Chinese, Malays and Japanese populations. Such heterogeneity may confer different statistical power and confound the transferability of the same variants across populations [56], [57]. In addition, we note that the variability in refraction attributed to AL may vary in different ethnic groups. For example, AL has been reported to account for a larger proportion of the variation in refraction in East-Asian children compared to their Caucasian counterparts [58], therefore the increased power of refraction may reflect more variation in factors other than pure elongation of AL in certain ethnic groups.
In conclusion, our findings suggest that common variants at chromosome 1q41 are associated with AL and high myopia in a pediatric and an adult cohort, the latter incorporating Chinese, Malay and Japanese populations. Further evaluation of causal variants and underlying pathway mechanisms may contribute to early identification of children at highest risk of developing myopia, and eventually lead to appropriate interventions to retard the progression of myopia.
Materials and Methods
Discovery cohorts
Singapore Chinese Eye Study (SCES)
SCES is an ongoing population-based cross-sectional survey of eye diseases in Chinese adults aged 40 to 80 years residing in the Southwestern part of Singapore. The study began in 2007 and a detailed description was published elsewhere [59]. In brief, a total of 2,226 residents in the Southwestern area of Singapore completed comprehensive ophthalmologic examinations, including visual acuity assessments, refraction, lens and retinal imaging, and slit lamp examinations. Genome-wide genotyping was performed in 1,952 individuals. Completed post quality control (QC) data for GWAS were available for 1,860 adults with AL measurements.
Singapore Cohort study of the Risk factors for Myopia (SCORM)
A total of 1,979 children in grades 1, 2, and 3 from three schools in Singapore were recruited from 1999 to 2001 [17]. The children were examined on their respective school premises annually by a team of eye care professionals. The GWAS was conducted in a subset of 1,116 Chinese children [14], [60]. The phenotype used in this study was based on the AL measured on the 4th annual examination of the study (children at age 10 to 12 years). Complete post-filtering data on AL measurements and SNP data were available in 929 children.
Singapore Malay Eye Study (SiMES)
SiMES is a population-based cross-sectional survey of eye diseases in Malay adults aged 40 to 80 years living in Singapore. It was conducted between August of 2004 and June of 2006 [61]. A total of 4,168 Malay residents in the Southwestern area of Singapore were identified and invited for a detailed ocular examination where 3,280 (78.7%) participated. Genome-wide genotyping was performed in 3,072 individuals [62], [63]. Complete post-filtering data for GWAS with AL measurements were available for 2,155 subjects.
Validation cohorts for high myopia
Japan dataset 1
The Japan dataset 1 consisted of 483 high myopia cases and 1,194 general healthy population controls. High myopia status was determined primarily on the basis of AL≥28 mm for both eyes, which corresponded to the spherical equivalent (SE) cut-off of at least −9.00 D [64]. Cases were recruited at the Center for Macular Disease of Kyoto University Hospital, the High Myopia Clinic of Tokyo Medical and Dental University, and the Fukushima Medical University Hospital. Details of the data have been reported elsewhere [13]. The population controls were recruited at the Aichi Cancer Center Research Institute.
Japan dataset 2
The Japan dataset 2 was comprised of 504 high myopia cases (SE≤−9.00 D in either eye) and 550 non-highly myopic controls (SE≥−3.00 D in both eyes). Less stringent thresholds were adopted for controls for the purpose of ease of recruitment from the clinics. Given the large phenotypic separation between the cases and controls, and assumption of homoscedasticity across genotype categories, such a study design using the extreme on one end (i.e. SE≤−9.00 D) but sampling less extreme controls (i.e. SE≥−3.00 D) still provides sufficient statistical power to detect the true positive signals in the association study [65]. Cases were recruited at the Yokohama City University and Okada Eye Clinic. Controls were obtained from the Yokohama City University and Tokai University Hospital.
Measurements of AL, refractive error, and covariates
All the studies used a similar protocol for ocular phenotype measurements. For subjects in SCES and SiMES, AL for both eyes were measured using optical laser interferometry (IOLMaster V3.01, Carl Zeiss; Meditec AG Jena, Germany) [59], [61]. Children in the SCORM study underwent AL measurements using the A-scan ultrasound biometry machine (Echoscan US-800; Nidek Co, Tokyo, Japan) [17]. For subjects in the Japan dataset 1, applanation A-scan ultrasongraphy (UD-6000, Tomey, Nagoya, Japan) or partial coherence interferometry (IOLMaster, Carl Zeiss Meditec, Dublin, CA) were used to measure AL. AL was assessed using a portable A-scan Biometer/pachymeter (AL-2000, Tomey, Negoya, Japan) for the participants in the Japan dataset 2.
Non-cycloplegic refraction in SCES and SiMES as well as cycloplegic refraction in SCORM (three drops of 1% cyclopentolate at 5 minutes apart) were measured by autorefractor (Canon RK-5, Tokyo, Japan) [66]. For subjects in the Japan dataset 2, refraction was measured using auto-refraction ARK-730A (NIDEK), ARK-700A (NIDEK) and KR-8100P (TOPCON). SE was calculated as the sphere power plus half of the cylinder power for each eye.
To perform the genetic association of high myopia in SCES and SiMES, we used the definition adopted by the Japan case-control studies and defined high myopia cases as subjects having SE≤−9.0 D in at least one eye, and non high-myopia controls as samples with SE≥−3.0 D in both eyes. For children from SCORM aged 10 to 12 years, cases were defined as SE≤−6.0 D for at least one eye, while controls were defined as SE≥−1.0 D for both eyes; this is approximately equivalent to the projected SE of −9.0 and −3.0 respectively at university age based on the estimated annual progression rate in SE of −0.6 D for Chinese myopic children and −0.3 D in the controls [67]. Given the small sample sizes of high myopia cases identified in our population-based cohorts, in the supplementary analysis, we further applied the commonly adopted criteria of SE≤−6.0 D in either eye as cases. Controls were defined as SE≥−1.0 D in both eyes. For SCORM children, we retained the same criteria in both analyses. The detailed definitions of cases and controls are described in Table S4.
Age, gender, height and level of education were obtained from all Singapore participants who underwent ophthalmologic examination. Education was measured on an ordinal scale from no formal education to the highest educational level. For participants in SCORM, the education of the child was defined by the level of educational attainment of the father, as a marker of socioeconomic status.
Ethics
All studies followed the principle of the Declaration of Helsinki. Study procedures and protocols were approved by the Institutional Review Board of each local institution involved in the study. In all cohorts, participants provided written, informed consent at the recruitment into the studies. Informed written consent was obtained from adult participants, and from the parents of the SCORM children.
Animal study approval was obtained from the SingHealth IACUC (AAALAC accredited). All procedures performed in this study complied with the Association of Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmology and Vision Research.
Genotyping and data quality control in discovery cohorts
For SCES, a total of 1,952 venous blood-derived samples were genotyped using Illumina Human 610 Quad Beadchips (Illumina Inc., San Diego, US) according to the manufacturer's protocols. Samples which failed genotyping or with low call rate (<95%, n = 11), with excessive heterozygosity (defined as sample heterozygosity exceeding 3 standard deviations from the mean sample heterogzygosity; n = 3), with gender discrepancies (n = 2) were excluded, as were cryptically related samples identified by the identity-by-state (IBS) (n = 41) and population structure in the principal components analyses (PCA) (n = 6). The criteria to define cryptically related samples and outliers with population structure in the discovery cohorts are described in the following paragraph. After the removal of the samples, SNP QC was then applied on a total of 579,999 autosomal SNPs for the 1,889 post-QC samples. SNPs were excluded based on (i) high rates of missingness (>5%) (n = 26,437); (ii) monomorphism or minor allele frequency (MAF)<1% (n = 59,633); or (iii) genotype frequencies deviating from Hardy-Weinberg Equilibrium (HWE) defined as HWE P-value<10−6 (n = 1,821). This yielded 492,108 autosomal SNPs. Those individuals with missing data on phenotypes were further removed (n = 29). Finally, 492,108 SNPs in 1,860 samples were available for analyses.
For SCORM, 1,116 DNA samples (1,037 from buccal swab and 79 from saliva) were genotyped on the Illumina HumanHap 550 Beadchips and 550 Duo Beadarrays. A total of 108 samples were excluded, comprising (i) 70 samples with call rates below 98%; (ii) 6 with poor genotyping quality; (iii) 11 samples identified from sib-ships; (iv) 18 with inconsistent gender information; and (v) 3 due to population structure. This left a total of 1,008 samples for further SNP QC. Based on 514,849 autosomal SNPs, we excluded 32,669 markers if they had missing genotype calls >5%, MAF<1%, or significantly deviated from HWE (P<10−6) [14]. A final set of 929 samples with 482,180 post-QC SNPs and completed AL measurement were included in analyses.
For SiMES, 3,072 DNA samples were genotyped using the Illumina Human 610 Quad Beadchips. The detailed QC procedures were provided elsewhere [68]. In brief, we omitted a total of 530 individuals due to: (i) subpopulation structure (n = 170); (ii) cryptic relatedness (n = 279); (iii) excessive heterozygosity or high missingness rate >5% (n = 37); and (iv) gender discrepancy (n = 44). After the removal of the samples, SNP QC was then applied on a total of 579,999 autosomal SNPs for the 2,542 post-QC samples. SNPs were excluded based on: (i) high rates of missingness (>5%) (n = 26,343); (ii) monomorphism or MAF<1% (n = 34,891); or (iii) genotype frequencies deviating from HWE (P<10−6) (n = 3,645). This yielded 515,120 SNPs after the same SNP QC criteria. Individuals without valid measurements for AL were further removed (n = 387). After the above filtering criteria, 515,120 SNPs in 2,155 samples were available for association analyses.
In our discovery cohorts, IBS was estimated with the genome-wide SNP data using PLINK software to assess the degree of recent shared ancestry for a pair of individuals [69]. For a pair of putatively-related samples defined as an identity by descent (IBD) value greater than 0.185 [70], we removed one individual from each pair of monzygotic twins/duplicates, parent-offspring or full-siblings etc. Population structure was ascertained using PCA with the EIGENSTRAT program and genetic outliers were defined as individuals whose ancestry was at least 6 standard deviations from the mean on one of the top ten inferred axes of variation [71].
For SiMES Malays, we also excluded the samples falling in the main clusters of PCA plots of the Chinese and Indians ethnic groups, as described in the previous study [68]. In SiMES, we noticed some degree of admixture in genetic ancestry of Malays and thus adjusted for ancestry along the top five axes of variation, as the spread of principal component scores was greater for the top five eigenvectors in the bivariate plots of PCA (Figure S3), The top ten principal components explained a small percentage of the global genetic variability of 1.3% while top five explained 1.0%, suggesting, all together, they had minimal effects on our association analyses.
Validation cohorts for high myopia
High myopia cases in the Japan dataset 1 were genotyped using Illumina Human-Hap550 and 660 chips [13], while controls in the Japan dataset 1 were genotyped on Illumina Human-Hap610 chips. Subjects in the Japan dataset 2 were genotyped on the Affymetrix GeneChip Human Mapping 500 K Array Set (Affymetrix Inc., Santa Clara, US). For SNPs not available on the Affymetric chips (rs43737678, rs10779363 and rs7544369), genotyping was performed with TaqMan 5′ exonuclease assays using primers supplied by Applied Biosystems (Foster City, US). The probe fluorescence signal was detected using the TaqMan Assay for Real-Time PCR (7500 Fast Real-Time PCR System, Applied Biosystems).
Gene expression in a mouse model of myopia
Experimental myopia was induced in B6 wild-type (WT) mice (n = 36) by applying a −15.00 D spectacle lens on the right eye (experimental eye) for 6 weeks since post-natal day 10. The left eyes were uncovered and served as contra-lateral fellow eyes. Age matched naive mice eyes were used as independent control eyes (n = 36). Each eye was refracted weekly using the automated infrared photorefractor as described previously [72]. AL was measured by AC - Master, Optic low coherence interferometry (Carl-Zeiss), in-vivo at 2, 4 and 6 weeks after the induction of myopia [73]. The minus-lens-induced eyes after six weeks were significantly associated with increased AL and myopic shift in refraction of <−5.00 D as compared to independent control eyes (n = 36, P = 3.00×10−6 for AL, and 2.05×10−4 for refraction). Eye tissues were collected at 6 weeks post myopia induction for further analyses.
Total RNA was isolated from pooled cryogenically ground mouse neural retina (retina), retinal pigment epithelium (RPE) and sclera for three batches using TRIzol Reagent (Invitrogen, Carlsbad, CA) with each batch (n = 6) comprising the myopic eye, fellow eye and control eye. RNA concentration and quality were assessed by the absorbance at 260 nm and the ratio of absorbance ratio at 260 and 280 nm respectively, using Nanodrop ND-1000 Spectrophotometer (Nanodrop Technologies, Wilmington, DE). RNA was purified using the RNeasy Mini kit (Qiagen, GmbH).
500 ng of purifed RNA was reverse-transcribed into cDNA using random primers and reagents from iScriptTM select cDNA synthesis kit (Bio-rad Laboratories, Hercules, CA). The pseudogene ZC3H11B (zinc finger CCCH type containing 11B) is not characterized in the mouse genome, therefore we examined a similar gene ZC3H11A (zinc finger CCCH type containing 11A) in mice. ZC3H11A in mice and ZC3H11B in humans are highly conserved with 79% nucleotide similarity by BLAST alignment analysis (http://blast.ncbi.nlm.nih.gov). We used quantitative Real-Time PCR (qRT-PCR) to validate the gene expression. qRT-PCR primers (Table S5) were designed using ProbeFinder 2.45 (Roche Applied Science, Indianapolis, IN) and this was performed using a Lightcycler 480 Probe Master (Roche Applied Science, Indianapolis, IN). The reaction was run in a Lightcycler 480 for 45 cycles under the following conditions: 95°C for 10 s, 56°C for 10 s and 72°C for 30 s. Gene expressions in the retina, RPE and sclera after six weeks of myopic eyes and the fellow eyes were compared to the control eyes. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous internal control.
Immunohistochemistry
Whole mouse eyes (6 weeks minus lens treated myopic, contra-lateral fellow and independent control eyes, n = 6 per type) were embedded in frozen tissue matrix compound at −20°C for 1 hour. Prepared tissue blocks were sectioned with a cryostat at 6 microns thicknesses and collected on clean polysine™ glass slides. Slides with the sections were air dried at room temperature (RT) for 1 hour and fixed with 4% para-formaldehyde for 10 min. After washing 3X with 1x PBS for 5 minutes, 4% bovine serum albumin (BSA) diluted with 1x PBS was added as a blocking buffer. The slides were then covered and incubated for 1 hour at RT in a humid chamber. After rinsing with 1x PBS, a specific primary antibody raised in rabbit against ZC3H11A, SLC30A10 and raised in goat against LYPLAL1 (Abcam, Cambridge, UK) diluted (1∶200) with 4% BSA was added and incubated further at 4°C in a humid chamber overnight. After washing 3X with 1x PBS for 10 min, fluorescein-labeled goat anti-rabbit secondary antibody (1∶800, Invitrogen-Molecular Probes, Eugene, OR) and fluorescein-labeled rabbit anti-goat secondary antibody (1∶800, Santa Cruz Biotechnology, Inc. CA, USA) was applied respectively and incubated for 90 min at RT. After washing and air-drying, slides were mounted with antifade medium containing DAPI (4,6-diamidino-2-phenylindole; Vectashield, Vector Laboratories, Burlingame, CA) to visualize the cell nuclei. Sections incubated with 4% BSA and omitted primary antibody were used as a negative control. A fluorescence microscope (Axioplan 2; Carl Zeiss Meditec GmbH, Oberkochen, Germany) was used to examine the slides and capture images. Experiments were repeated in duplicates from three different samples.
Gene expression in human tissues
GAPDH, ZC3H11B, SLC30A10, and LYLPLAL1 were run using 10 ul reactions with Qiagen's PCR products consisting of 1.26 ul H2O, 1.0 ul 10X buffer, 1.0 ul dNTPs, 0.3 ul MgCl, 2.0 ul Q - Solution, 0.06 ul taq polymerase, 1.0 ul forward primer, 1.0 ul reverse primer and 1.5.0 ul cDNA. The reactions were run on a Eppendorf Mastercycler Pro S thermocycler with touchdown PCR ramping down 1°C per cycle from 72°C to 55°C followed by 50 cycles of 94°C for 0 : 30, 55°C for 0 : 30 and 72°C for 0 : 30 with a final elongation of 7 : 00 at 72°C. All primer sets were designed using Primer3 [74]. The gel electrophoresis was run on a 2% agarose gel at 70 volts for 35 minutes. The primers were run on a custom tissue panel including Clontech's Human MTC Panel I, Fetal MTC Panel I and an ocular tissue panel. The adult ocular samples were obtained from normal eyes of an 82-year-old Caucasian female from the North Carolina Eye Bank, Winston-Salem, North Carolina, USA. The fetal ocular samples were from 24-week fetal eyes obtained by Advanced Bioscience Resources Inc., Alameda, California, USA. All adult ocular samples were stored in Qiagen's RNAlater within 6.5 hours of collection and shipped on ice overnight to the lab. Fetal eyes were preserved in RNAlater within minutes of harvesting and shipped over night on ice. Whole globes were dissected on the arrival day. Isolated tissues were snap-frozen and stored at −80°C until RNA extraction. RNA was extracted from each tissue sample independently using the Ambion mirVana total RNA extraction kit. The tissue samples were homogenized in Ambion lysis buffer using an Omni Bead Ruptor Tissue Homogenizer per protocol. Reverse transcription reactions were performed with Invitrogen SuperScript III First-Strand Synthesis kit.
Statistical analysis
The primary analysis was performed on the AL quantitative trait. As a strong correlation exists in AL measurements from both eyes (r>0.9), we used the mean AL across both eyes in the GWAS analysis, as was recommended in a review [75]. Linear regression was used to interrogate the association of each SNP with AL after adjusting for age, gender, height and level of education, under the assumption of an additive genetic effect where the genotypes of each SNP are coded numerically as 0, 1 and 2 for the number of minor alleles carried. In addition, for SiMES, the top five principal components of genetic ancestry from the EIGENSTRAT PCA were also included as covariates to account for the effects of population substructure as described in genotype QC section [60]. Association tests between each genetic marker and phenotype were carried out using PLINK software [69] (version 1.07). Analyses were also repeated without adjustment for education level or height for the purpose of comparison.
In the discovery phase, we conducted a meta-analysis of GWAS results from 3 cohorts for AL using a weighted-inverse variance approach by fixed-effect modeling in METAL (http://www.sph.umich.edu/csg/abecasis/metal). In the secondary analyses, SNPs that have been identified from the primary analyses were tested for association with high myopia onset (as a binary trait) and SE (as a quantitative trait). For Singapore cohorts, the association analyses adjusted for the same covariates as the primary analyses within a linear regression and logistic regression framework respectively. For Japan case-control datasets, only age and gender were included as covariates in the model for high myopia, as the other covariates were not available.
The regional association plots were constructed by SNAP (http://www.broadinstitute.org/mpg/snap). Haploview 4.1 (http://www.broad.mit.edu/mpg/haploview) was used to visualize the LD of the genomic regions. Genotyping quality of all reported SNPs has been visually evaluated by the intensity clusterplots. The coordinates reported in this paper are on NCB136 (hg18).
For functional studies in the myopic mouse model, gene expression of all three identified genes in control and experimental groups was quantified using the 2−ΔΔCt method [76]. The standard student's t-test was performed to determine the significance of the relative fold change of mRNA between the myopic eyes of the experimental mice with the independent age-matched controls.
Supporting Information
Zdroje
1. PanCWRamamurthyDSawSM 2012 Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt 32 3 16
2. SawSMChuaWHGazzardGKohDTanDT 2005 Eye growth changes in myopic children in Singapore. Br J Ophthalmol 89 1489 1494
3. WongTYFosterPJHeeJNgTPTielschJM 2000 Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci 41 2486 2494
4. SawSMGazzardGShih-YenECChuaWH 2005 Myopia and associated pathological complications. Ophthalmic Physiol Opt 25 381 391
5. McBrienNAGentleA 2003 Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res 22 307 338
6. SawSMKatzJScheinODChewSJChanTK 1996 Epidemiology of myopia. Epidemiol Rev 18 175 187
7. HammondCJSniederHGilbertCESpectorTD 2001 Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci 42 1232 1236
8. KleinAPSuktitipatBDuggalPLeeKEKleinR 2009 Heritability analysis of spherical equivalent, axial length, corneal curvature, and anterior chamber depth in the Beaver Dam Eye Study. Arch Ophthalmol 127 649 655
9. LyhneNSjolieAKKyvikKOGreenA 2001 The importance of genes and environment for ocular refraction and its determiners: a population based study among 20–45 year old twins. Br J Ophthalmol 85 1470 1476
10. SanfilippoPGHewittAWHammondCJMackeyDA 2010 The heritability of ocular traits. Surv Ophthalmol 55 561 583
11. SoloukiAMVerhoevenVJvan DuijnCMVerkerkAJIkramMK 2010 A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat Genet 42 897 901
12. HysiPGYoungTLMackeyDAAndrewTFernandez-MedardeA 2010 A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet 42 902 905
13. NakanishiHYamadaRGotohNHayashiHYamashiroK 2009 A genome-wide association analysis identified a novel susceptible locus for pathological myopia at 11q24.1. PLoS Genet 5 e1000660 doi:10.1371/journal.pgen.1000660
14. LiYJGohLKhorCCFanQYuM 2011 Genome-wide association studies reveal genetic variants in CTNND2 for high myopia in Singapore Chinese. Ophthalmology 118 368 375
15. LiZQuJXuXZhouXZouH 2011 A genome-wide association study reveals association between common variants in an intergenic region of 4q25 and high-grade myopia in the Chinese Han population. Hum Mol Genet 20 2861 2868
16. ShiYQuJZhangDZhaoPZhangQ 2011 Genetic variants at 13q12.12 are associated with high myopia in the han chinese population. Am J Hum Genet 88 805 813
17. SawSMShankarATanSBTaylorHTanDT 2006 A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci 47 1839 1844
18. DiraniMShekarSNBairdPN 2008 Evidence of shared genes in refraction and axial length: the Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci 49 4336 4339
19. BiinoGPalmasMACoronaCProdiDFanciulliM 2005 Ocular refraction: heritability and genome-wide search for eye morphometry traits in an isolated Sardinian population. Hum Genet 116 152 159
20. ZhuGHewittAWRuddleJBKearnsLSBrownSA 2008 Genetic dissection of myopia: evidence for linkage of ocular axial length to chromosome 5q. Ophthalmology 115 1053 1057 e1052
21. LeungKWLiuMXuXSeilerMJBarnstableCJ 2008 Expression of ZnT and ZIP zinc transporters in the human RPE and their regulation by neurotrophic factors. Invest Ophthalmol Vis Sci 49 1221 1231
22. LiangJSongWTrompGKolattukudyPEFuM 2008 Genome-wide survey and expression profiling of CCCH-zinc finger family reveals a functional module in macrophage activation. PLoS ONE 3 e2880 doi:10.1371/journal.pone.0002880
23. PolisenoLSalmenaLZhangJCarverBHavemanWJ 2010 A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465 1033 1038
24. SalmenaLPolisenoLTayYKatsLPandolfiPP 2011 A ceRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 146 353 358
25. D'ErricoIGadaletaGSacconeC 2004 Pseudogenes in metazoa: origin and features. Brief Funct Genomic Proteomic 3 157 167
26. ShiYLiYZhangDZhangHLuF 2011 Exome sequencing identifies ZNF644 mutations in high myopia. PLoS Genet 7 e1002084 doi:10.1371/journal.pgen.1002084
27. SchippertRBurkhardtEFeldkaemperMSchaeffelF 2007 Relative axial myopia in Egr-1 (ZENK) knockout mice. Invest Ophthalmol Vis Sci 48 11 17
28. LaityJHLeeBMWrightPE 2001 Zinc finger proteins: new insights into structural and functional diversity. Curr Opin Struct Biol 11 39 46
29. FischerAJMcGuireJJSchaeffelFStellWK 1999 Light - and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci 2 706 712
30. BitzerMSchaeffelF 2002 Defocus-induced changes in ZENK expression in the chicken retina. Invest Ophthalmol Vis Sci 43 246 252
31. SimonPFeldkaemperMBitzerMOhngemachSSchaeffelF 2004 Early transcriptional changes of retinal and choroidal TGFbeta-2, RALDH-2, and ZENK following imposed positive and negative defocus in chickens. Mol Vis 10 588 597
32. LiuCAdamsonEMercolaD 1996 Transcription factor EGR-1 suppresses the growth and transformation of human HT-1080 fibrosarcoma cells by induction of transforming growth factor beta 1. Proc Natl Acad Sci U S A 93 11831 11836
33. BaronVAdamsonEDCalogeroARagonaGMercolaD 2006 The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther 13 115 124
34. KhorCCFanQGohLTanDYoungTL 2010 Support for TGFB1 as a susceptibility gene for high myopia in individuals of Chinese descent. Arch Ophthalmol 128 1081 1084
35. ZhaYLeungKHLoKKFungWYNgPW 2009 TGFB1 as a susceptibility gene for high myopia: a replication study with new findings. Arch Ophthalmol 127 541 548
36. SeveMChimientiFDevergnasSFavierA 2004 In silico identification and expression of SLC30 family genes: an expressed sequence tag data mining strategy for the characterization of zinc transporters' tissue expression. BMC Genomics 5 32
37. van LeeuwenRBoekhoornSVingerlingJRWittemanJCKlaverCC 2005 Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 294 3101 3107
38. UgarteMOsborneNN 2001 Zinc in the retina. Prog Neurobiol 64 219 249
39. HuibiXKaixunHQiuhuaGYushanZXiuxianH 2001 Prevention of axial elongation in myopia by the trace element zinc. Biol Trace Elem Res 79 39 47
40. SteinbergGRKempBEWattMJ 2007 Adipocyte triglyceride lipase expression in human obesity. Am J Physiol Endocrinol Metab 293 E958 964
41. HeidIMJacksonAURandallJCWinklerTWQiL 2010 Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 42 949 960
42. LindgrenCMHeidIMRandallJCLaminaCSteinthorsdottirV 2009 Genome-wide association scan meta-analysis identifies three Loci influencing adiposity and fat distribution. PLoS Genet 5 e1000508 doi:10.1371/journal.pgen.1000508
43. CordainLEatonSBBrand MillerJLindebergSJensenC 2002 An evolutionary analysis of the aetiology and pathogenesis of juvenile-onset myopia. Acta Ophthalmol Scand 80 125 135
44. CordainLEadesMREadesMD 2003 Hyperinsulinemic diseases of civilization: more than just Syndrome X. Comp Biochem Physiol A Mol Integr Physiol 136 95 112
45. LimLSGazzardGLowYLChooRTanDT 2010 Dietary factors, myopia, and axial dimensions in children. Ophthalmology 117 993 997 e994
46. GaoHFrostMRSiegwartJTJrNortonTT 2011 Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis 17 903 919
47. KleinAPDuggalPLeeKEKleinRBailey-WilsonJE 2007 Confirmation of linkage to ocular refraction on chromosome 22q and identification of a novel linkage region on 1q. Arch Ophthalmol 125 80 85
48. KleinAPDuggalPLeeKEChengCYKleinR 2011 Linkage analysis of quantitative refraction and refractive errors in the beaver dam eye study. Invest Ophthalmol Vis Sci 52 5220 5225
49. WongTYFosterPJJohnsonGJSeahSK 2003 Refractive errors, axial ocular dimensions, and age-related cataracts: the Tanjong Pagar survey. Invest Ophthalmol Vis Sci 44 1479 1485
50. WuRWangJJMitchellPLamoureuxELZhengY 2010 Smoking, socioeconomic factors, and age-related cataract: The Singapore Malay Eye study. Arch Ophthalmol 128 1029 1035
51. TokoroT 1988 On the definition of pathologic myopia in group studies. Acta Ophthalmol Suppl 185 107 108
52. PlominRHaworthCMDavisOS 2009 Common disorders are quantitative traits. Nat Rev Genet 10 872 878
53. HayashiHYamashiroKNakanishiHNakataIKurashigeY 2011 Association of 15q14 and 15q25 with High Myopia in Japanese. Invest Ophthalmol Vis Sci
54. DiraniMShekarSNBairdPN 2008 Adult-onset myopia: the Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci 49 3324 3327
55. JensenH 1995 Myopia in teenagers. An eight-year follow-up study on myopia progression and risk factors. Acta Ophthalmol Scand 73 389 393
56. McCarthyMIHirschhornJN 2008 Genome-wide association studies: potential next steps on a genetic journey. Hum Mol Genet 17 R156 165
57. TeoYYSmallKSFryAEWuYKwiatkowskiDP 2009 Power consequences of linkage disequilibrium variation between populations. Genet Epidemiol 33 128 135
58. IpJMHuynhSCKifleyARoseKAMorganIG 2007 Variation of the contribution from axial length and other oculometric parameters to refraction by age and ethnicity. Invest Ophthalmol Vis Sci 48 4846 4853
59. LavanyaRJeganathanVSZhengYRajuPCheungN 2009 Methodology of the Singapore Indian Chinese Cohort (SICC) eye study: quantifying ethnic variations in the epidemiology of eye diseases in Asians. Ophthalmic Epidemiol 16 325 336
60. FanQZhouXKhorCCChengCYGohLK 2011 Genome-wide meta-analysis of five Asian cohorts identifies PDGFRA as a susceptibility locus for corneal astigmatism. PLoS Genet 7 e1002402 doi:10.1371/journal.pgen.1002402
61. FoongAWSawSMLooJLShenSLoonSC 2007 Rationale and methodology for a population-based study of eye diseases in Malay people: The Singapore Malay eye study (SiMES). Ophthalmic Epidemiol 14 25 35
62. VithanaENAungTKhorCCCornesBKTayWT 2011 Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum Mol Genet 20 649 658
63. KhorCCRamdasWDVithanaENCornesBKSimX 2011 Genome-wide association studies in Asians confirm the involvement of ATOH7 and TGFBR3, and further identify CARD10 as a novel locus influencing optic disc area. Hum Mol Genet 20 1864 1872
64. GrosvenorTP 2007 Primary care optometry St. Louis, Mo Butterworth-Heinemann/Elsevier xiii, 510 p.
65. SchorkNJNathSKFallinDChakravartiA 2000 Linkage disequilibrium analysis of biallelic DNA markers, human quantitative trait loci, and threshold-defined case and control subjects. Am J Hum Genet 67 1208 1218
66. SawSMChanYHWongWLShankarASandarM 2008 Prevalence and risk factors for refractive errors in the Singapore Malay Eye Survey. Ophthalmology 115 1713 1719
67. FanDSLamDSLamRFLauJTChongKS 2004 Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest Ophthalmol Vis Sci 45 1071 1075
68. SimXOngRTSuoCTayWTLiuJ 2011 Transferability of type 2 diabetes implicated loci in multi-ethnic cohorts from Southeast Asia. PLoS Genet 7 e1001363 doi:10.1371/journal.pgen.1001363
69. PurcellSNealeBTodd-BrownKThomasLFerreiraMA 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
70. AndersonCAPetterssonFHClarkeGMCardonLRMorrisAP 2010 Data quality control in genetic case-control association studies. Nat Protoc 5 1564 1573
71. PriceALPattersonNJPlengeRMWeinblattMEShadickNA 2006 Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 904 909
72. SchaeffelFBurkhardtEHowlandHCWilliamsRW 2004 Measurement of refractive state and deprivation myopia in two strains of mice. Optom Vis Sci 81 99 110
73. BarathiVABoopathiVGYapEPBeuermanRW 2008 Two models of experimental myopia in the mouse. Vision Res 48 904 916
74. RozenSSkaletskyH 2000 Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132 365 386
75. FanQTeoYYSawSM 2011 Application of advanced statistics in ophthalmology. Invest Ophthalmol Vis Sci 52 6059 6065
76. BrinkNSzamelMYoungARWitternKPBergemannJ 2000 Comparative quantification of IL-1beta, IL-10, IL-10r, TNFalpha and IL-7 mRNA levels in UV-irradiated human skin in vivo. Inflamm Res 49 290 296
Štítky
Genetika Reprodukční medicína
Článek Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental InvolvementČlánek Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target GenesČlánek Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early EmbryosČlánek Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2Článek Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human CellsČlánek TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome EndsČlánek Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal CordČlánek Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth ofČlánek MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- Mimetic Butterflies Introgress to Impress
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Selection-Driven Gene Loss in Bacteria
- Decreased Mitochondrial DNA Mutagenesis in Human Colorectal Cancer
- Parallel Evolution of Auditory Genes for Echolocation in Bats and Toothed Whales
- Diverse CRISPRs Evolving in Human Microbiomes
- The Rad4 ATR-Activation Domain Functions in G1/S Phase in a Chromatin-Dependent Manner
- Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains
- Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
- Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target Genes
- Adaptive Introgression across Species Boundaries in Butterflies
- G Protein Activation without a GEF in the Plant Kingdom
- Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes
- An Engineering Approach to Extending Lifespan in
- Incompatibility and Competitive Exclusion of Genomic Segments between Sibling Species
- Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in
- Patterns of Evolutionary Conservation of Essential Genes Correlate with Their Compensability
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
- Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early Embryos
- A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity
- Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2
- Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis
- Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells
- TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome Ends
- Stimulation of Host Immune Defenses by a Small Molecule Protects from Bacterial Infection
- A Broad Requirement for TLS Polymerases η and κ, and Interacting Sumoylation and Nuclear Pore Proteins, in Lesion Bypass during Embryogenesis
- Genome-Wide Identification of Ampicillin Resistance Determinants in
- The CCR4-NOT Complex Is Implicated in the Viability of Aneuploid Yeasts
- Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees
- Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
- Is a Key Regulator of Pancreaticobiliary Ductal System Development
- The NSL Complex Regulates Housekeeping Genes in
- Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord
- Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
- Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth of
- Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia
- MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
- Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family
- Found: The Elusive ANTAR Transcription Antiterminator
- The Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility
- Control of CpG and Non-CpG DNA Methylation by DNA Methyltransferases
- Polymorphisms in the Mitochondrial Ribosome Recycling Factor Compromise Cell Respiratory Function and Increase Atorvastatin Toxicity
- Gene Expression Profiles in Parkinson Disease Prefrontal Cortex Implicate and Genes under Its Transcriptional Regulation
- Global Regulatory Functions of the Endoribonuclease III in Gene Expression
- Extensive Evolutionary Changes in Regulatory Element Activity during Human Origins Are Associated with Altered Gene Expression and Positive Selection
- The Regulatory Network of Natural Competence and Transformation of
- Brain Expression Genome-Wide Association Study (eGWAS) Identifies Human Disease-Associated Variants
- Quantifying the Adaptive Potential of an Antibiotic Resistance Enzyme
- Divergence of the Yeast Transcription Factor Affects Sulfite Resistance
- The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis
- RNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
- Rare Copy Number Variants Observed in Hereditary Breast Cancer Cases Disrupt Genes in Estrogen Signaling and Tumor Suppression Network
- Genome-Wide Location Analysis Reveals Distinct Transcriptional Circuitry by Paralogous Regulators Foxa1 and Foxa2
- A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
- The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression
- Limits to the Rate of Adaptive Substitution in Sexual Populations
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- The NSL Complex Regulates Housekeeping Genes in
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



