-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
Inherited ataxias are characterized by degeneration of the cerebellar structures, which results in progressive motor incoordination. Hereditary ataxias occur in many species, including humans and dogs. Several mutations have been found in humans, but the genetic background has remained elusive in dogs. The Finnish Hound suffers from an early-onset progressive cerebellar ataxia. We have performed clinical, pathological, and genetic studies to describe the disease phenotype and to identify its genetic cause. Neurological examinations on ten affected dogs revealed rapidly progressing generalized cerebellar ataxia, tremors, and failure to thrive. Clinical signs were present by the age of 3 months, and cerebellar shrinkage was detectable through MRI. Pathological and histological examinations indicated cerebellum-restricted neurodegeneration. Marked loss of Purkinje cells was detected in the cerebellar cortex with secondary changes in other cortical layers. A genome-wide association study in a cohort of 31 dogs mapped the ataxia gene to a 1.5 Mb locus on canine chromosome 8 (praw = 1.1×10−7, pgenome = 7.5×10−4). Sequencing of a functional candidate gene, sel-1 suppressor of lin-12-like (SEL1L), revealed a homozygous missense mutation, c.1972T>C; p.Ser658Pro, in a highly conserved protein domain. The mutation segregated fully in the recessive pedigree, and a 10% carrier frequency was indicated in a population cohort. SEL1L is a component of the endoplasmic reticulum (ER)–associated protein degradation (ERAD) machinery and has not been previously associated to inherited ataxias. Dysfunctional protein degradation is known to cause ER stress, and we found a significant increase in expression of nine ER stress responsive genes in the cerebellar cortex of affected dogs, supporting the pathogenicity of the mutation. Our study describes the first early-onset neurodegenerative ataxia mutation in dogs, establishes an ERAD–mediated neurodegenerative disease model, and proposes SEL1L as a new candidate gene in progressive childhood ataxias. Furthermore, our results have enabled the development of a genetic test for breeders.
Published in the journal: . PLoS Genet 8(6): e32767. doi:10.1371/journal.pgen.1002759
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002759Summary
Inherited ataxias are characterized by degeneration of the cerebellar structures, which results in progressive motor incoordination. Hereditary ataxias occur in many species, including humans and dogs. Several mutations have been found in humans, but the genetic background has remained elusive in dogs. The Finnish Hound suffers from an early-onset progressive cerebellar ataxia. We have performed clinical, pathological, and genetic studies to describe the disease phenotype and to identify its genetic cause. Neurological examinations on ten affected dogs revealed rapidly progressing generalized cerebellar ataxia, tremors, and failure to thrive. Clinical signs were present by the age of 3 months, and cerebellar shrinkage was detectable through MRI. Pathological and histological examinations indicated cerebellum-restricted neurodegeneration. Marked loss of Purkinje cells was detected in the cerebellar cortex with secondary changes in other cortical layers. A genome-wide association study in a cohort of 31 dogs mapped the ataxia gene to a 1.5 Mb locus on canine chromosome 8 (praw = 1.1×10−7, pgenome = 7.5×10−4). Sequencing of a functional candidate gene, sel-1 suppressor of lin-12-like (SEL1L), revealed a homozygous missense mutation, c.1972T>C; p.Ser658Pro, in a highly conserved protein domain. The mutation segregated fully in the recessive pedigree, and a 10% carrier frequency was indicated in a population cohort. SEL1L is a component of the endoplasmic reticulum (ER)–associated protein degradation (ERAD) machinery and has not been previously associated to inherited ataxias. Dysfunctional protein degradation is known to cause ER stress, and we found a significant increase in expression of nine ER stress responsive genes in the cerebellar cortex of affected dogs, supporting the pathogenicity of the mutation. Our study describes the first early-onset neurodegenerative ataxia mutation in dogs, establishes an ERAD–mediated neurodegenerative disease model, and proposes SEL1L as a new candidate gene in progressive childhood ataxias. Furthermore, our results have enabled the development of a genetic test for breeders.
Introduction
Ataxia is a neurological symptom of defective motor coordination that can affect gait, balance, speech and gaze [1]. Human hereditary ataxias are rare heterogeneous disorders characterized by progressive degeneration of the cerebellum and cerebellar connections, with a variable degree of involvement from extra-cerebellar structures [2]. The predominant inheritance patterns are autosomal dominant and autosomal recessive [1]. Unlike the autosomal dominant spinocerebellar ataxias (SCAs), which usually affect the central nervous system (CNS), the recessive disorders involve more often other organs [1]. Typical age of onset for dominant ataxias is between 30 to 50 years of age [3], whereas the recessive forms tend to have an onset before the age of 20 years [4].
Causative mutations have been identified for at least 19 different dominant SCAs, most of which are caused by repeat expansions [5], [6]. In recessive human ataxias, the number of known disease genes is somewhere around 20, depending on the classification criteria [2], [7]–[10]. Described pathological mechanisms are diverse but include some common themes, such as accumulation of protein aggregates, defects in the DNA-repair system, mitochondrial dysfunction and oxidative stress [1], [2], [9], [11]. In addition to the known human ataxia genes, several spontaneous mutations that cause cerebellar degeneration have been recognized in mice [12]–[14].
Cerebellar degeneration has also been described in several dog breeds [15]–[30]. In veterinary medicine, the disease group is referred to as cerebellar cortical abiotrophies (CCAs), where abiotrophy describes the idiopathic premature neuronal degeneration [31]. Clinical signs in canine CCAs include ataxia, dysmetria, tremors, broad-based stance and loss of balance, all of which contribute to the often significant ambulatory difficulties [32], [33]. Majority of the described canine phenotypes are early-onset and manifest by the age of 3 to 4 months [16], [17], [19]–[23], [25], [27], [28]. Later-onset and slowly progressing CCAs are less common but occur in some breeds [18], [24], [26]. In a classical CCA, pathological findings are focused on the cerebellar cortex where the primary degenerative change is the loss of cortical Purkinje cells (PCs), followed by secondary changes in granular and molecular cell layers [32], [33]. Primary degeneration of cortical granule cells is seen more rarely [27], [29]. Involvement of CNS structures other than the cerebellum has been reported in some breeds, for instance in Kerry Blue Terriers [16] and Brittany Spaniels [24]. A more systemic phenotype is seen in the Bernese Mountain Dog, where cerebellar degeneration is accompanied by a hepatic degeneration [23]. In Rhodesian Ridgebacks, affected dogs present with a diluted coat color [22]. Collectively, the variability in disease onset, severity and histopathological details indicate a heterogeneous genetic etiology across different breeds. Although autosomal recessive inheritance has been proposed in several breeds [16], [18], [26], [30], the underlying genetic causes of canine primary ataxias have remained largely unidentified. Thus far, a molecular characterization has been reported only in a rare type of neonatal ataxia in Coton De Tulear dogs that have a mutation in the GRM1 glutamate receptor gene [34]. Additionally, a putative CCA locus has been recently mapped to canine chromosome 3 (CFA3) in Australian Kelpies [35].
In the present study, we have examined clinical and genetic characteristics of hereditary ataxia that affects the Finnish Hound (FH) dog breed. A previous case report has indicated an early-onset progressive cerebellar neurodegeneration in a FH puppy [15]. We provide a more comprehensive clinical picture in a larger sample cohort and identify a recessive mutation in a novel ataxia gene.
Results
Clinical examinations indicate generalized cerebellar ataxia
Ten affected FH puppies from six different litters were referred to a veterinary neurology clinic for general clinical, orthopedical and neurological examinations (Table 1). One healthy littermate was examined as a control dog. At the time of examination, affected puppies were from 3 to 4 months old. The clinical signs were first noticed at a mean age of 9 weeks, ranging from 4 to 12 weeks (Table 1). General clinical and orthopedical examination did not reveal any significant changes. Neurological examinations were strongly indicative of cerebellar dysfunction by revealing generalized cerebellar ataxia with dysmetria (Video S1), postural reaction deficits (Video S2) and intention tremor (Video S3). Cranial and spinal nerve reflexes were normal, and all affected dogs had normal cognition. Serum biochemistry profiles, complete blood cell count (CBC) and cerebrospinal fluid (CSF) cell count were within normal limits in all affected dogs. For an undefined reason, protein concentration was mildly elevated in one affected puppy. All ten affected puppies were euthanized because of a rapid disease progression and poor prognosis.
Tab. 1. A summary of clinical and pathological examinations in Finnish Hound puppies. 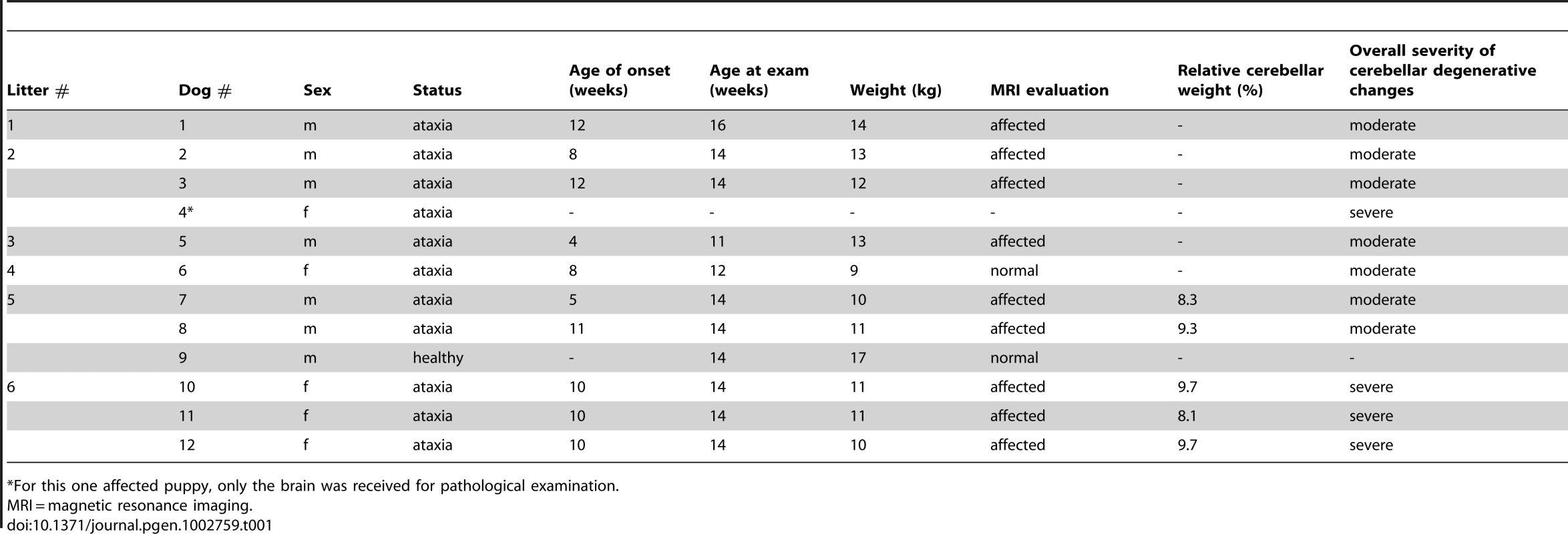
For this one affected puppy, only the brain was received for pathological examination. MRI and histopathological examinations reveal cerebellar neurodegeneration
Cerebellar pathology was supported by magnetic resonance imaging (MRI) and post-mortem examinations. Nine out of ten affected puppies showed reduced cerebellar size on T1 - and T2-weighted (T1W and T2W) midsagittal brain MRI scans (Figure 1). No changes were detected in the cerebrum or brainstem. General pathological examination did not reveal any significant gross changes outside the CNS. The weight of the cerebellum relative to the total brain mass was measured in five dogs and ranged from 8.1 to 9.7%. This indicated a loss of cerebellar mass as the normal proportion of the cerebellum is ≥10% [32]. A few disease nonspecific histological findings were made; a mild interstitial pneumonia was seen in four dogs and mild follicular hyperplasia in the spleen of three dogs.
Fig. 1. Brain MRI scans. 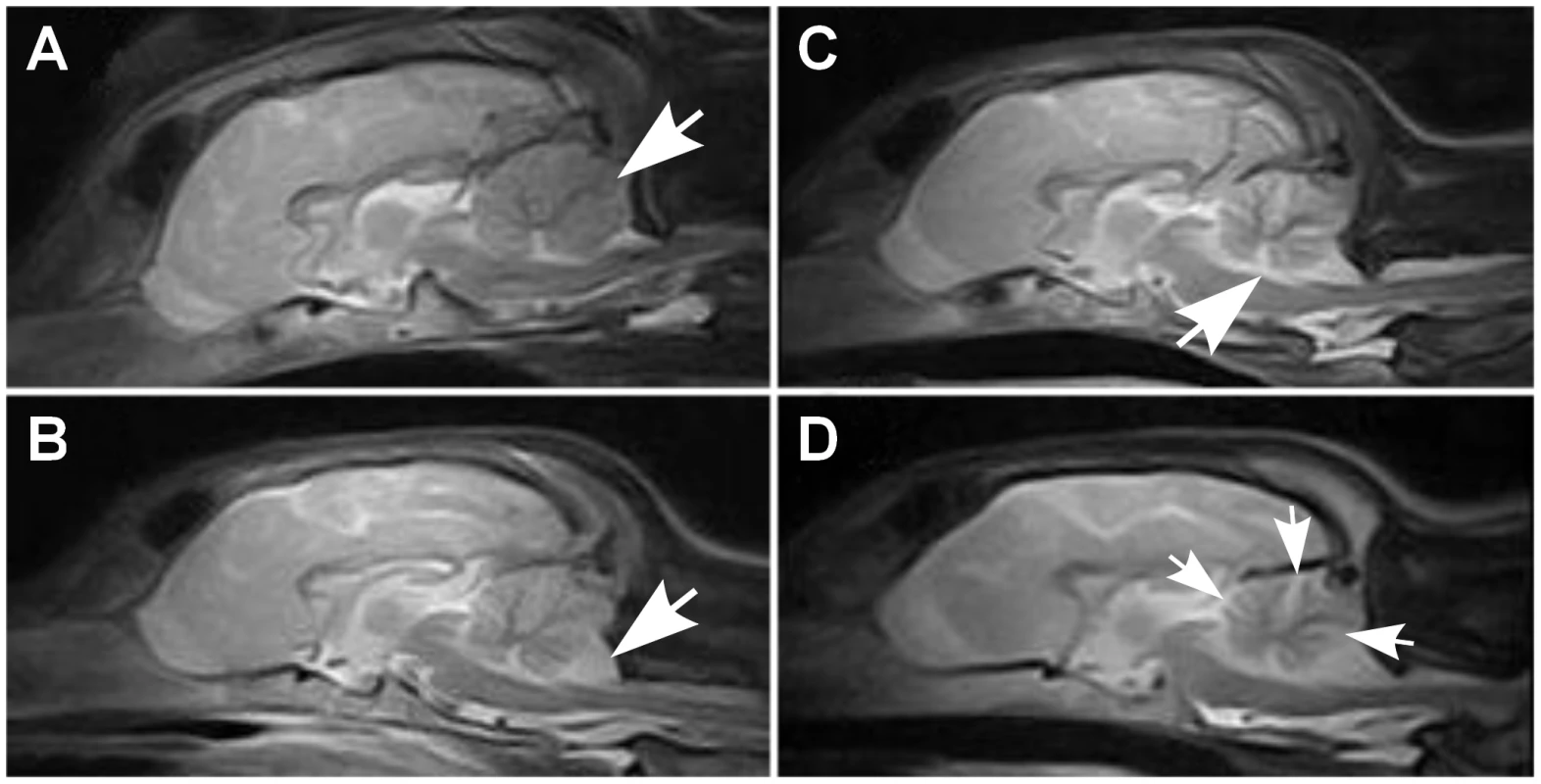
Sagittal midline T2W brain MRI images of a normal and affected Finnish Hounds. (A) A control dog with normal cerebellum (arrow). (B) An affected dog that has smaller cerebellar size and increased cerebrospinal fluid space (white) between the cerebellum and the occipital bone ventrally (arrow). (C) An affected dog that shows reduced cerebellar size and increase in the fluid filled space in the area of the fourth ventricle (arrow). (D) Another affected dog that has reduced cerebellar size and increased fluid filled spaces between the cerebellar folia (arrows). Histological changes of the nervous system were restricted to the cerebellum in all examined puppies. The cerebellar cortex showed marked premature degeneration and loss of PCs with consequent neuronal depletion in the granular cell layer (Figure 2B and 2D). The cerebellar vermis and the paramedian lobule were consistently the most severely affected areas. The cranial regions of the cerebellar cortex were more affected than the caudal regions. The ventrolateral parts, including paraflocculi and flocculus, were spared and partially normal (Figure 2A and 2C). In the cortical areas, where severe PC loss was present, glial cells (Bergmans glia) were proliferating between the molecular and the granular cell layers (Figure 2D). The remaining PCs were shrunken and eosinophilic with marginated nuclear chromatin or showed total loss of cytoplasmic basophilic Nissl substance (chromatolysis) (Figure 2E and 2F). The granular cell layer was markedly depleted of neurons and showed mild astrocytosis in areas of profound PCs loss (Figure 2G). Occasional degenerated and vacuolated axons were detected in the granular layer. Mild to moderate ongoing degeneration and myelinophagia was seen within the cerebellar white matter of the severely affected areas (Figure 2H). Neither transsynaptic degeneration in the cerebellar nuclei nor retrograde degeneration of the olivary nucleus was found. Immunohistochemical (IHC) staining for canine distemper virus and parvovirus showed no positivity. The overall severity of the histopathological findings, including active PC degeneration, total granule cell and PC loss, consecutive white matter lesions and the extent of the lesions, are summarized in Table 1.
Fig. 2. Histological findings within the cerebellar cortex of affected dogs. 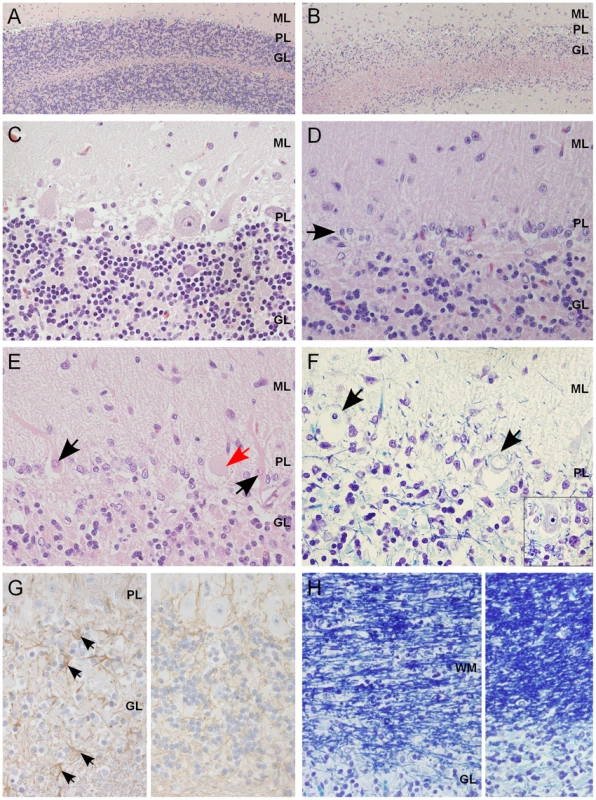
(A) Normal cerebellar cortex in the ventrolateral parts of the cerebellum, with densely cellular granular cell layer (HE 100×). (B) Affected cerebellar cortex in the vermal region with marked loss of granular cells (HE 100×). (C) A higher magnification of unaffected cortex with viable Purkinje cells (PCs) and a normal granular cell density (HE 400×). (D) A higher magnification of affected cortex, with severe loss of PCs and linear reactive gliosis between the molecular and the granular layers (arrow) (HE 400×). (E) Degenerating, shrunken, eosinophilic PCs with margination of the nuclear chromatin (black arrow) or central chromatolysis of the cytoplasma (red arrow) (HE 400×). (F) PCs that show total loss of cytoplasmic basophilia (Nissl substance) and pyknotic or karyorhektic nuclei (arrows). Inset: viable PC with intact Nissl substance seen as basophilic cytoplasmic granulation (LFB-CEV, 400×). (G) Left: mild astrogliosis (arrows) within the granular cell layer and the white matter of the cerebellar folia. Right: unaffected part of same dog (IHC GFAP, 400×). (H) Left: secondary degeneration and myelinophagia in the affected cerebellar white matter. Right: unaffected parts of same dog (LFB-CEV, 400×). ML = molecular layer, PL = Purkinje cell layer, GL = granular cell layer, WM = white matter. Genetic studies map a novel recessive ataxia locus
A pedigree was established around the known affected FH puppies to determine the most likely mode of inheritance (Figure 3). According to the pedigree data, all affected dogs were born from healthy parents, both sexes were affected and the proportion of affected puppies across litters was 29%, near the expected 25%. These observations were consistent with an autosomal recessive mode of inheritance.
Fig. 3. Finnish Hound ataxia pedigree. 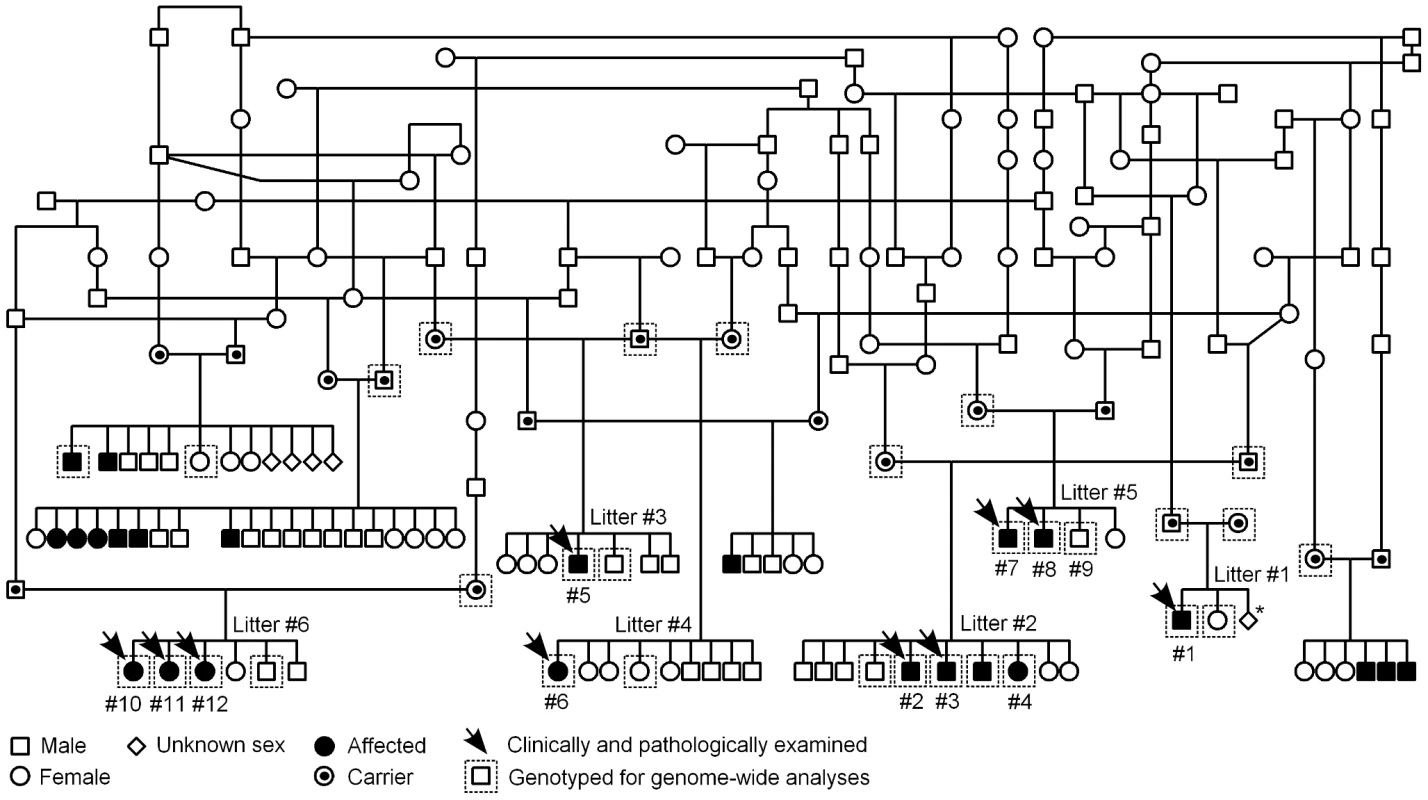
Pedigree shows those affected litters that were used in the study. Disease segregation is consistent with autosomal recessive mode of inheritance as all affected dogs are born from healthy parents and both sexes are affected. The proportion of affected puppies is 29%, which is close to expected 25%. Denoted are the clinically and pathologically examined cases and the dogs that were genotyped for genome-wide analyses. The numbering of litters and puppies refers to the numbering used in Table 1. *In this one litter, the total number of offspring was unknown. We first used a candidate gene approach to try to identify the causative gene. Altogether 24 known human and murine ataxia genes were selected (Table S1), and the segregation of microsatellite markers, within or adjacent to the candidate genes, was studied in three nuclear families. Each family included both parent dogs and at least one affected and one healthy offspring. No co-segregation was observed between any of the markers and the disease.
We subsequently proceeded to map the FH ataxia locus by using a genome-wide approach. A cohort of 31 dogs, comprising 13 cases, 11 obligate carrier parents and seven non-affected siblings, were genotyped using Illumina's 22K canine SNP chip. A standard case-control association test was carried out on the 13 cases and seven full-sibling controls by using PLINK software [36]. This revealed a genome-wide significant association on CFA8 with two SNPs, BICF2P948919 and BICF2P754995, that had the best nominal and corrected p-values of praw = 1.1×10−7 and pgenome = 7.5×10−4 (Figure 4A). The association results were confirmed by utilizing a joint family-based linkage and association analysis program PSEUDOMARKER [37]. The joint analysis, which was carried out on the associated CFA8 using the entire genotyped sample cohort, identified the same locus and a single most significant SNP, BICF2P948919 (LOD score = 3.3, association p = 2.2×10−7 and joint analysis p = 4.0×10−10) (Figure 4B).
Fig. 4. Genetic studies. 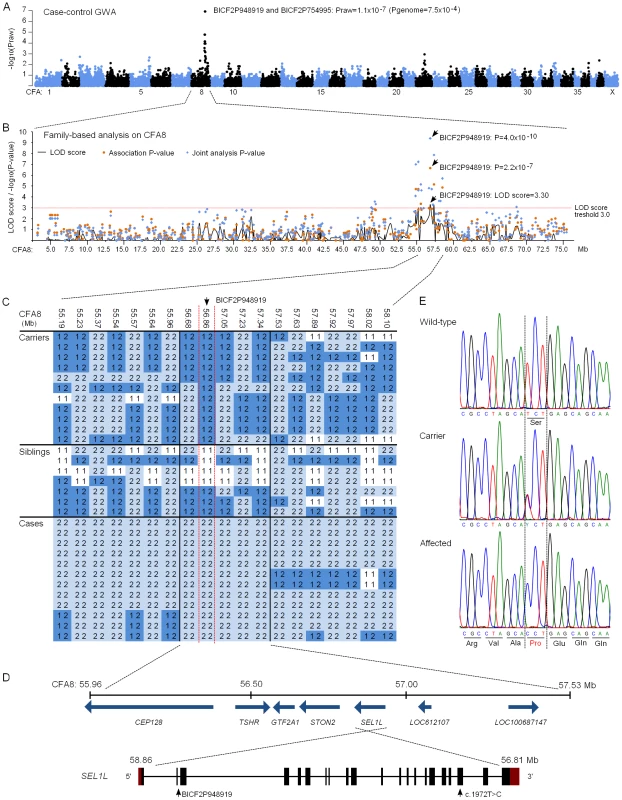
(A) A Manhattan plot of case-control genome-wide association test performed using 13 cases and 7 unaffected sibling-controls. (B) Results of the family-based testing on the disease associated chromosome 8. Plotted are single-point linkage analysis LOD scores, association test p-values and joint analysis p-values. (C) Genotypes at the disease associated locus on CFA8. All cases share a 1.5 Mb homozygous block, and within this block BICF2P948919 shows complete segregation with the disease. (D) A schematic representation of the seven genes found on the 1.5 Mb block and of the SEL1L gene structure. SEL1L exons are marked with black boxes and red denotes the untranslated regions (UTRs). BICF2P948919 is located on second SEL1L intron and the c.1972T>C mutation on exon 19. (E) Chromatograms of the c.1972T>C mutation in an affected, a carrier and a wild-type dog. Assessment of genotypes at the associated CFA8 locus revealed a shared 1.5 Mb homozygous haplotype block in affected dogs, spanning from 56.0 to 57.5 Mb (Figure 4C). All genotyped parent dogs were carriers of this disease haplotype. Within the 1.5 Mb block, only the most significant SNP, BICF2P948919, showed complete segregation with the disease, indicating that the causative variant probably lies in its vicinity. The 1.5 Mb haplotype contained seven genes. Two of these, LOC100687147 (serine/threonine-protein kinase Nek6-like) and LOC612107 (uncharacterized hypothetical gene) were likely pseudogenes. The other five were known protein-coding genes, centrosomal protein 128kDa (CEP128), thyroid stimulating hormone receptor (TSHR), general transcription factor IIA (GTF2A1), stoning 2 (STON2) and sel-1 suppressor of lin-12-like (SEL1L) (Figure 4D). We ranked SEL1L as the best candidate gene for mutation screening due to its neuronal expression, and function in a protein degradation pathway in the endoplasmic reticulum (ER) [38]–[41]. Impaired protein degradation is a common feature in several neurodegenerative diseases [42], [43]. In addition, the fully segregating SNP, BICF2P948919, was located on the second intron of the SEL1L gene. Besides SEL1L, the STON2 gene was considered a plausible causative gene because of its neuronal function in synaptic vesicle recycling at presynaptic nerve terminals [44]–[47].
Exonic sequencing identifies a missense mutation in the SEL1L gene
The coding regions of the five known protein coding genes were sequenced in two affected puppies and in two obligate carrier parents in order to identify possible disease-causing mutations. No sequence variants that segregated with the phenotype were found in TSHR or GTF2A1. Sequencing of STON2 and CEP128 revealed a few segregating variants but none of them were in the coding regions (Table S2). In SEL1L, we identified altogether four coding and 19 non-coding variants, all of which segregated with the disease (Table S2). Three of the exonic variants were synonymous but one was a non-synonymous cytosine to thymine change on exon 19 (c.1972T>C) that results in a serine to proline alteration at position 658 of the encoded protein (p.Ser658Pro) (Figure 4E). The c.1972T>C variant was genotyped in the full sample cohort and showed complete segregation and a 100% penetrance with the disease. All 13 affected puppies were homozygous for the T>C change, all 13 parents heterozygous and 20 littermates either heterozygous (12 out of 20) or wild-type (8 out of 20). Segregation of the variant was further validated by genotyping altogether 241 randomly selected unaffected FHs. None of these population controls were homozygous for the C allele but a 10% carrier frequency (24/241) was indicated. Segregation analysis gave a highly significant association between the C allele and disease (p = 1.8×10−42). Moreover, the c.1972T>C allele was completely absent in 349 dogs from 51 other breeds (Table S3), including a Russian hound breed, which is related to FHs. We later received a sample from a newly affected FH puppy from Finland that was found homozygous for the CC genotype. Another suspected FH ataxia puppy from Sweden was tested free of the mutation. However, further inquiries of this puppy's phenotype revealed that it had not presented with clinical signs typical for FH ataxia but had in reality suffered from an episodic disorder. The puppy could not be examined further as it had been euthanized at 6 weeks of age.
The probability of the SEL1L p.Ser658Pro change having a pathogenic effect was evaluated by utilizing bioinformatics tools. SEL1L is a transmembrane glycoprotein that resides in the endoplasmic reticulum (ER) [48], [49]. The carboxy-terminus of the protein harbors the transmembrane domain and amino-terminal protein body is exposed to the ER lumen. The lumenal protein body is composed of a single fibronectin type II domain and three clusters of tetratricopeptide repeat (TPR)-like motifs, the Sel1-repeats (Figure 5A) [39], [40], [49], [50]. The p.Ser658Pro amino acid change is positioned in one out of the several Sel1-repeat motifs of the protein (Figure 5A). We found Ser658 to be fully conserved in all aligned vertebrates, insects and in the hemichordate acorn worm. Only C. elegans's orthologue Sel1 and yeast's orthologue Hrd3p differed at the position (Figure 5B). Moreover, three sequence homology-based prediction tools, PANTHER, PolyPhen and SIFT, all predicted the Ser658Pro change to have a probably damaging effect on protein function (PANTHER SubPSEC score = −5.4, PolyPhen-2 (HumVar-trained) score = 0.98 and the SIFT score = 0.02).
Fig. 5. SEL1L protein structure and partial sequence alignment. 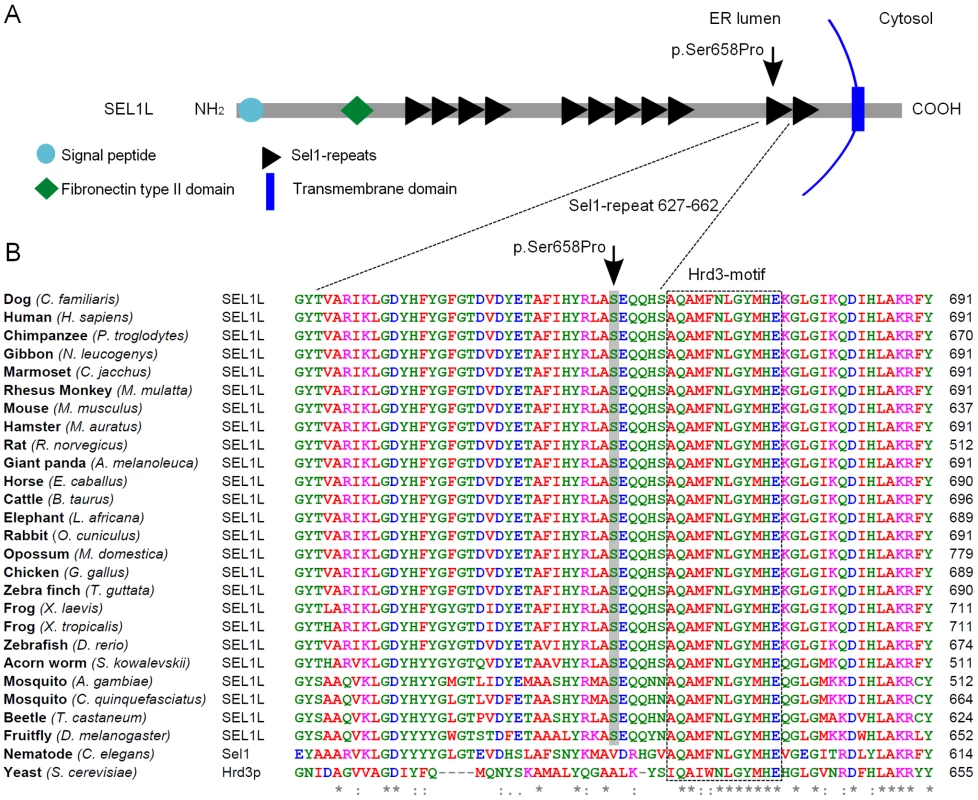
(A) A Schematic representation of the SEL1L protein, which shows the multi-modular protein structure. The p.Ser658Pro mutation hits one of the C-terminal Sel1-repeats. (B) SEL1L amino acid sequence alignment. Position of the mutation is conserved in all aligned species except for C. elegans and yeast. The Hrd3-motif, which is positioned five residues downstream from the Ser658, shows conservation across all aligned species. According to various expression databases, mammalian SEL1L is ubiquitously expressed. However, we wanted to specifically confirm SEL1L cerebellar expression and also to see, if the non-synonymous c.1972T>C variant has an effect on SEL1L mRNA stability. Amplification and sequencing of the entire SEL1L transcript confirmed cerebellar expression, the presence of the mutation at mRNA level and the predicted exon/intron boundaries, and finally, excluded the possible splicing effects of the several identified intronic variants (Table S2). Real-time quantification of the SEL1L transcript in cerebellar cortical tissue samples of five affected dogs and a control puppy revealed a 2.8-fold increase in the affected dogs (Figure 6A). We also studied the cerebellar expression of the two other genes with segregating non-coding variants, STON2 and CEP12, but did not find differences in their transcript levels between the control and affected dogs (Figure 6A).
Fig. 6. Gene expression analysis in the affected cerebellum. 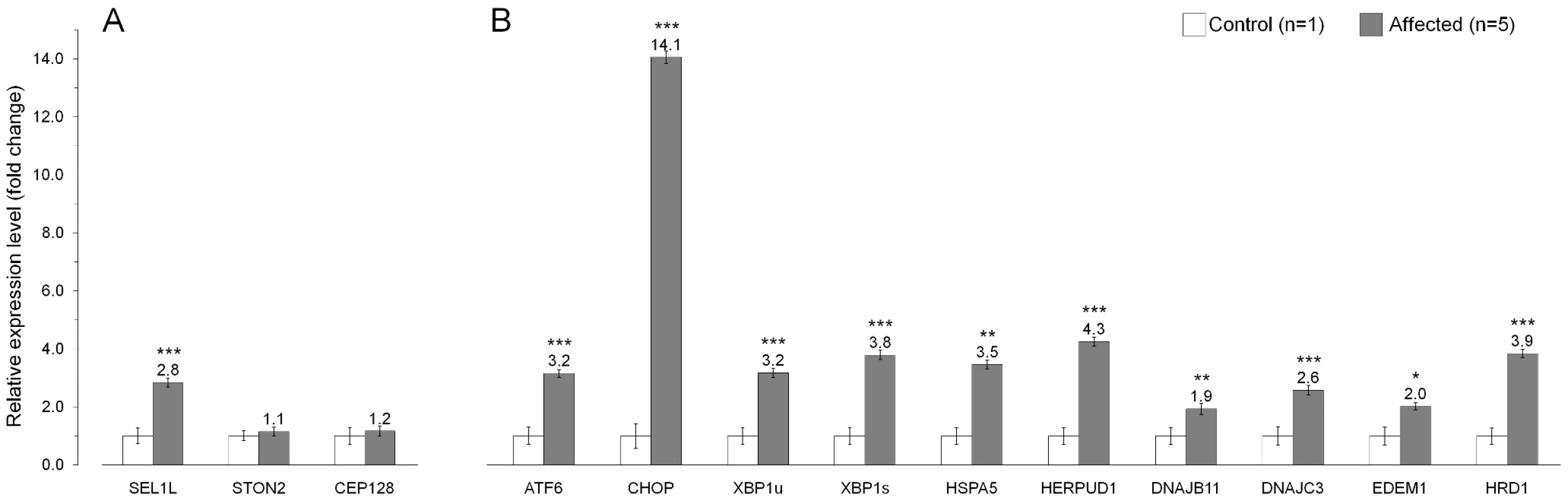
(A) The mRNA levels of SEL1L, STON2 and CEP128 in the cerebellar cortex of five affected dogs compared to a control puppy. These three genes had sequence variants that segregated with the ataxia phenotype. (B) Endoplasmic reticulum (ER) stress markers are upregulated in the cerebellar cortex of affected animals. The relative mRNA expression levels are represented as a fold change. Error bars denote the standard error of normalized Ct-values. *p≤0.05 **p≤0.001 ***p≤0.000 (two-tailed t-test p-values). Upregulation of ER stress genes in affected cerebellar cortex
SEL1L is a component of an ER-associated protein complex that functions in protein degradation [38]–[41]. Impaired protein degradation is known to affect ER homeostasis and result in ER stress [51], [52] We therefore hypothesized that the c.1972T>C change in SEL1L could lead to dysfunction of the protein complex and cause ER stress. Disruption of ER homeostasis activates the unfolded protein response (UPR), which upregulates genes that are required for cell survival during ER stress [53]–[57]. We measured the transcript levels of nine known ER stress responsive genes, ATF6, CHOP, XBP1, HSPA5, HERPUD1, DNAJB11 (ERdj3), DNAJC (p58IPK), EDEM1 and HRD1 in cerebellar cortical tissue of five affected FHs and a control dog. For XBP1, we measured both the unspliced (XBP1u) and the spliced (XBP1s) transcripts [58]–[60]. All tested UPR genes showed increased expression levels in the affected dogs (Figure 6B). The highest, 14-fold increase was observed for CHOP, whereas 1.9 to 4.2-fold increases were observed for the other eight UPR genes. These results are indicative of ER stress in the affected cerebellar cortex and support the pathogenicity of the identified SEL1L mutation.
Discussion
We describe here the clinical and histopathological phenotype of a progressive early-onset cerebellar ataxia in FHs and identify a missense mutation in the SEL1L gene that segregates with the recessive disease. The clinical course in FH ataxia is compatible to the classical canine cerebellar abiotrophy, which has an early-onset, rapid progression and poor prognosis [16], [17], [19]–[22], [25], [27], [28]. Affected FHs present with a progressive ataxia that causes significant ambulatory difficulties. The symptoms worsen rapidly and the affected puppies are euthanized soon after diagnosis. Cerebellar degeneration is visible in MRI at the age of 3 months. Histopathological features are consistent with a premature degeneration of cerebellar cortical PCs, where the PCs are the primary target of a degenerative pathological process, followed by secondary changes in other cortical cell layers. We suggest that the now identified SEL1L mutation (c.1972T>C, p.Ser658Pro) is the most likely underlying cause in FH ataxia. Several lines of evidence support SEL1L as the causative gene.
First, SEL1L is the best positional and functional candidate in the 1.5 Mb disease associated haplotype block. The haplotype contained five known protein coding genes, which were screened for mutations. The only coding variant that causes a protein level change was found in the SEL1L gene. SEL1L shows ubiquitous expression in adult tissues and is widely expressed during embryonic development, with intense expression in developing neural tissue and pancreas [49], [61], [62]. We confirmed SEL1L expression in the cerebellar cortex, the affected organ in FH ataxia.
Second, the SEL1L protein belongs to a disease-relevant pathway. SEL1L functions in a large protein complex in the ER, in a cellular process referred to as ER-associated degradation (ERAD) [38]–[41]. The ERAD machinery targets terminally misfolded and unassembled polypeptides which are recognized, dislocated to the cytoplasm and marked for proteasome-dependent degradation [55], [63], [64]. SEL1L is a component in an ERAD complex that is organized around an E3 ubiquitine ligase, HRD1 [38], [40], [41], [65]–[67]. Although the precise function of SEL1L is not known, it is proposed to have an adaptor role and to be involved in recruiting ERAD substrates to the HRD1 ligase complex, either directly or via other proteins [39], [40], [65]–[67]. ER stress and impaired protein degradation have been implicated in several neurodegenerative diseases [52], [68], [69]. As an indication of acute ER stress in the cerebellar cortex of affected FHs, we show increased expression of several transcription factors and chaperone proteins that belong to the ER stress response pathway, UPR. Increased transcription levels were also found for SEL1L and HRD1, which are both known to be upregulated in ER stress [70]. However, the highest increase was detected in the expression of a proapoptotic transcription factor CHOP [71]–[73]. CHOP is normally expressed at very low levels but is highly induced in ER stress [74], [75]. Overall, our expression data supports the pathogenic role of the SEL1L mutation in FH ataxia. There is one previous report that connects SEL1L to a neurodegenerative phenotype. A tentative association was found between an intronic SEL1L SNP and Alzheimer's disease [76]. However, given the early-onset and rapid progression of the disease in FHs, our study indicates that SEL1L has crucial role in the developing brain and suggests that SEL1L is an unlikely candidate gene for late-onset neurodegenerative disorders. This is further supported by a prenatal lethality of Sel1l-deficient mice [77].
Third, the p.Ser658Pro amino acid change hits a highly conserved residue in an evolutionary conserved repeat motif in the SEL1L protein. The affected domain is the next to last carboxy-terminal Sel1-repeat. Unlike the amino-terminal fibronectin type II domain, which is absent from invertebrate SEL1L orthologues, the Sel1-repeats are highly conserved across species [49]. The α/α-helical Sel1-repeats are found in several proteins that function as adaptors in protein complex formation [78]. Deletion of the two most carboxy-terminal Sel1-repeat clusters in mammalian SEL1L have been reported to abolish interactions with HRD1 and other proteins [40], and recent data suggests that the stabilization of the mammalian SEL1L depends on HRD1 [79]. Sequence homology-based prediction programs all indicated a likely damaging effect of the p.Ser658Pro change. Moreover, there is a considerable difference in structure between the two amino acids, serine and proline. The latter possesses a conformationally restricted cyclic molecular structure, which could interfere with proper protein folding. Given the role of the Sel1-repeats in protein interactions, it is possible that the p.Ser658Pro change disrupts SEL1L protein-protein interactions, for instance with HRD1, compromising the function of the ERAD complex.
Forth, a mouse model connects Sel1l deficiency to impaired ER-mediated protein quality control. A recent paper reported embryonic lethality and signs of systemic ER stress in Sel1l-deficient mice [77]. Heterozygous mice were normal and fertile but those that were homozygous for a gene trap mutation between Sel1l exons 14 and 15, died at mid-gestation, and suffered from growth retardation and morphological brain abnormalities [77]. Cell lines derived from the mutant mice revealed changes in ER morphology, hypersensitivity to ER stress inducers, defects in degradation of unfolded proteins and an impaired protein secretory pathway [77]. In accordance with our results, several UPR genes were upregulated in the mutant embryos [77]. The embryonic lethality of the Sel1l-deficient mice suggests that the canine mutation is milder and does not abolish all SEL1L function. The affected FH puppies did not show any gross morphological abnormalities and seemed to develop normally over the first few weeks of life. Furthermore, some SEL1L functions might be complemented by protein isoforms [49], [62], [80]–[82]. Amino-terminal SEL1L isoforms that contain a variable number of Sel1-repeats but lack the carboxy-terminal region, have been indicated to function in parallel or complementary to the full length SEL1L in ER stress [81], [82].
Although SEL1L is ubiquitously expressed, another explanation for the cerebellum-restricted pathology may come from the vulnerability of the cerebellar PCs to ER stress. Various disease phenotypes indicate that cerebellar PCs are susceptible to undergo premature degeneration [13], [14], [33], [83], [84] and accumulating evidence indicate ER stress specifically as causative in PC pathology [85]–[92]. These observations could account for the primary PC degeneration in affected FHs. In addition to our results on SEL1L cerebellar expression, a previous study found HRD1 in murine PCs [93]. It is plausible that a compromised function of the HRD1-ligase pathway would not be tolerated by ER stress sensitive PC and would lead to excessive or prolonged ER stress, triggering apoptosis and neurodegeneration. Whether any pathological changes would be seen in other tissues if the affected dogs were kept alive longer, is not known.
In conclusion, we identify a novel progressive ataxia gene and link a defective ERAD pathway to an early-onset cerebellar neurodegeneration. Our study implicates a critical function for the HRD1-SEL1L-mediated ERAD pathway in the postnatal PC survival and provides a novel model to investigate the role of the ER stress in neurodegeneration. SEL1L represents a novel candidate gene for human ataxias and we have initiated mutation screenings in childhood phenotypes. Meanwhile, a genetic test has been offered for FH breeders to identify carriers and to eradicate the disease from the breed.
Materials and Methods
Ethics statement
All the dogs used in this study were privately-owned pets, and the genetic and clinical examinations were approved by the Animal Ethics Committee at the State Provincial Office of Southern Finland (permits: ESLH-2006-08207/Ym-23 and ESHL-2009-07827).
Animals and blood samples
EDTA-blood samples were collected from 13 affected FH puppies that belonged to seven different litters, and from 33 unaffected first-degree relatives. The unaffected relative sample cohort comprised 13 parent dogs and 20 unaffected full-siblings. A pedigree was constructed around the affected dogs by using GenoPro genealogy software (http://www.genopro.com/). FH population controls (n = 241) and an additional control sample cohort of 349 dogs from 51 other dog breeds were selected among samples stored at the Canine DNA Bank located at Biomedicum Helsinki, Finland. The FH population cohort included random samples from unaffected FHs that had been collected for instance at dog shows. Full-siblings were excluded from the population cohort to obtain a reliable estimate a carrier frequency. The control cohort from other breeds comprised one dog from two breeds, two dogs from 38 breeds and 10 to 32 dogs from 11 breeds (Table S3). Puregene DNA Purification Kit (Gentra Systems) was used to extract genomic DNA from affected dogs and their relatives. A semi-automated Chemagen extraction robot (Chemagen Biopolymer-Technologie AG) was used for the control samples. Concentration of DNA samples was determined using a ND-1000 UV/Vis Spectrophotometer (NanoDrop Technologies).
Clinical examinations
General clinical, orthopedical and neurological examinations were performed on ten ataxic FH puppies that were referred to a veterinary neurology clinic during December 2006 and September 2008. The examined puppies came from six different litters. One healthy sibling was examined as a control dog. Neurological examinations were filmed and are available for retrospective evaluation. CBC and serum biochemistry profiles (sodium, potassium, calcium, phosphorus, magnesium, glucose, total protein, albumin, globulin, blood urea nitrogen, creatinine, total bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and creatine kinase) were examined on admission. Brain MRI scanning and CSF sample collection were performed under general anesthesia. Intramuscular anaesthesia premedication was carried out with the combination of Medetomidine (Domitor, Orion Pharma) 0.02 mg/kg and Butorphanol (Torbugesic, Scan-Vet) 0.1 mg/kg. Anaesthesia was induced through intravenous bolus of Propofol (Propofolum, Abbott Laboratories) 6–8 mg/kg and maintained through inhalation of Isoflurane (IsoFlo vet, Orion Pharma) and oxygen.
A 0.2 Tesla MRI scanner (VetMR, Esaote) was used to record T1W and T2W images in transversal and sagittal planes on animals placed in sternal recumbency. TW1 images were recorded using 750.0 ms repetition time (TR), 26.0 ms echo time (TE) and a field of view (FoV) of 150×150, 160×160 and 170×170 mm and T2W images with 3000.0 ms TR, 90.0 ms TE and 160×160 or 170×170 mm FoV. In all images, slice thickness was 4.0, 4.5 or 5.0 mm and interslice gap 0.4–0.5 mm. T1W images were repeated immediately after intravenous injection of contrast agent, gadolinium-diethylenetriaminepenta-acetate (-DTPA) dimeglumine 0.2 mL/kg (0.1 mmol/kg). Two blinded examiners (SC and JJ) rated the dogs subjectively as affected or healthy based on cerebellar size, amount of CSF between the cerebellar folia, size of the fourth ventricle and distance between caudoventral edge of the cerebellum and foramen magnum. CSF samples were collected from the cerebellomedullary cistern after the MRI examination. Total cell count and protein concentration were evaluated from the CSF samples and considered normal if there were less than five nucleated cells per microliter and if Pandy reaction was negative. Affected dogs with compatible clinical signs and MRI findings were euthanized with owner's agreement.
Pathological and histological examinations
A complete autopsy was performed on the ten clinically examined dogs. For one additional puppy, only the brain was received for examination. The weight of the cerebellum relative to the total weight of the brain was determined in five dogs. Samples from the CNS, liver, lungs, spleen, kidney and heart were collected and fixed in neutral buffered 10% formalin. Sections from the fixed tissues were embedded in paraffin and processed for light microscopic examination, using the haematoxylin-eosin (HE) stain. The CNS was sectioned at nucleus caudatus with cerebral cortex, hippocampus with temporal cortex, mesencephalon at the height of the cranial colliculi, cerebellum transversally and longitudinally with pons and medulla oblongata. Sections from the CNS were stained with luxol fast blue/cresyl echt violet (LFB-CEV) to evaluate chromatolysis and myelin loss. An IHC stain for glial fibrillary acidic protein (GFAP, MCA 1909, Serotec) was used to assess astrogliosis. IHC staining of spleen, lung and kidney sections were performed for canine distemper virus (CDV, MCA 1893, Serotec), and sections of spleen were stained for canine parvovirus (CPV, MCA2064, Serotec).
Candidate gene analysis
Altogether 24 known human and murine ataxia genes were selected for a microsatellite marker-based candidate gene analysis (Table S1). Segregation of microsatellite markers was examined in three nuclear families, comprising six parents, five affected dogs and three healthy siblings. Allele sizes were determined by fragment analysis. Human and murine mRNA sequences for the candidate genes were obtained from the GeneBank database (http://www.ncbi.nlm.nih.gov/) and the corresponding canine sequences were identified from the CanFam2.0 annotation using the BLAT search tool [94]. Microsatellite primers (Table S4) were designed using Primer 3 (http://frodo.wi.mit.edu/primer3/). Forward primers were either directly labeled with a fluorescence dye (HEX or FAM) or alternatively, an M13-tail sequence was added to the 5′-end and used together with a third, FAM-labeled M13-primer [95]. PCR amplifications were performed using a PTC-225 Peltier Thermal Tetrad Cycler (Bio-Rad) and a standard PCR protocol. Reactions that included directly labeled forward-primers were performed in a reaction volume of 10 µl with 20 ng of genomic DNA, 1 X PCR buffer, 2.5 mM MgCl2, 0.2 mM dNTPs, 0.5 µM of forward - and reverse primers and 0.375 units of AmpliTaq Gold Polymerase (Applied Biosystems). Amplifications that were performed using the M13-primers were carried out in a 12 µl volume containing 15 ng of genomic DNA, 1 X PCR buffer, 2.1 mM MgCl2, 0.33 mM dNTPs, 0.05 µM M13-tailed forward primer, 0.25 µM reverse primer, 0.2 µM M13 primer and 1.2 units of Biotools DNA polymerase. Intensity of PCR products was evaluated from 1% agarose gel stained with 0.5 µg/ml ethidium bromides (Amresco). Fragment analysis runs were performed on a 3730xl DNA Analyzer (Applied Biosystems). The Peak Scanner software (Applied Biosystems) was used to determine allele sizes.
Genotype data analysis
Thirteen affected dogs from seven nuclear families and 18 related control dogs were genotyped using Illumina's CanineSNP20 BeadChip of 22,362 validated SNPs. Healthy control dogs included 11 obligate disease carrier parents and seven non-affected siblings (Figure 3). Genotype data was filtered using a SNP call rate of >95%, an array call rate of >95% and minor allele frequency of >5%. Based on these criteria, 289 SNPs were removed for low genotyping efficiency and 6739 SNPs for low minor allele frequency. Mendel errors were detected in 35 SNPs, which were removed from analyses. No samples were removed for low genotyping and no SNPs for significant deviations from the Hardy-Weinberg equilibrium (p≤0.0001). After the filtering steps, 15,299 SNPs remained for analyses. A basic case-control association test was performed by using the software package PLINK [36]. Obligate carrier parents were excluded from the case-control association test and the remaining seven healthy siblings were used as controls. Genome-wide significance was ascertained through phenotype permutation testing (n = 100,000). Pseudomarker program was used to perform family-based testing on CFA8, which showed genome-wide significant association in the PLINK analysis [37]. The family-based analyses were performed under a recessive inheritance model, and included parametric single-point linkage test, association analysis (LD|Linkage) and joint analysis (LD+Linkage).
Mutation screening
Mutation screening of CEP128, TSHR, GTF2A1, STON2 and SEL1L exons, exon-intron junctions and 5′ and 3′ UTRs was performed using samples from two affected dogs and two obligate disease carriers. Primers (Table S5) were designed by using Primer 3 (http://frodo.wi.mit.edu/primer3/). PCR reactions were carried out in a total reaction volume of 20 µl with 20 ng of genomic DNA, 1 X PCR buffer, 2 mM MgCl2, 0.2 mM dNTPs, 0.5 µM of forward - and reverse primers and 0.5 units of Biotools DNA Polymerase. PCR amplification was performed using a PTC-225 Peltier Thermal Tetrad Cycler (Bio-Rad) and a standard PCR protocol. The reaction products were run on a 1% agarose gel stained with 0.5 µg/ml ethidium bromide (Amresco). PCR products were purified with ExoSAP-IT (GE Healthcare) and sequenced using an Applied Biosystems' 3730xl DNA Analyzer. Sequence Scanner v1.0 and Variant Reporter v1.0 (Applied Biosystems) were used to assess sequence quality and identify polymorphisms. Control sample cohorts were screened by using Applied Biosystems' TaqMan chemistry and 7500 Fast Real-Time PCR instrumentation. The genotyping reactions were carried out in a 10 µl reaction volume with 1 X TaqMan genotyping assay (Applied Biosystems), 1 X Taqman Genotyping Master Mix (Applied Biosystems) and 10 ng of genomic DNA. Primer sequences for the Taqman assay were 5′ - CGTAGACTACGAGACTGCATTTATTCA -3′ for the forward, and 5′ - GATTAAACATAGCTTGTGCACTGTGT - 3′ for the reverse primer. Probe sequences were 5′ - TGCTGCTCAGATGCTA - 3′ and 5′ - TGCTGCTCAGGTGCTA - 3′, labeled with VIC and FAM, respectively.
Bioinformatic analysis
Pfam protein families database (http://pfam.sanger.ac.uk/) [96] and the SMART tool (http://smart.embl-heidelberg.de/) [97], [98] were used to confirm the protein domain structure of canine SEL1L. The ClustalW2 algorithm was used to compose a multiple sequence alignment to examine cross-species conservation (http://www.ebi.ac.uk/Tools/clustalw2/). Three sequence homology-based software tools, PANTHER (http://www.pantherdb.org/tools/) [99], [100], PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) [101] and SIFT (http://sift.jcvi.org/) [102]–[104], were used to predict the potential functional impact of the identified non-synonymous variant. The PANTHER tool has a score range from 0 to about −10, with a cutoff for functional significance at ≤−3. The PolyPhen-2 score ranges from 0 to 1, with the threshold for probably damaging at 0.85. The SIFT score ranges from 0 to 1, and substitutions are predicted to affect function if the score is ≤0.05.
Tissue samples and mRNA experiments
Tissue samples were collected from the cerebellar cortex of five affected puppies immediately after euthanasia. Samples were stabilized in RNAlater reagent (Ambion, Inc) and stored in −80°C. Total mRNA extraction was performed using the RNeasy Mini Kit (Qiagen) with a DNase I digestion step included (RNase-Free DNase Set, Qiagen). Concentration of total RNA was measured using a ND-1000 UV/Vis Spectrophotometer (NanoDrop Technologies). Reverse-transcriptase (RT) -PCR was carried out on equal amounts of RNA in each sample by using the High Capacity RNA-to-cDNA Kit (Applied Biosystems). A cerebellar control sample was obtained from an 11 days old Saluki puppy that was put down due to a peritoneo-pericardial hernia.
SEL1L mRNA sequencing primers (Table S5) and all qPCR primers (Table S6) were designed using Primer 3 (http://frodo.wi.mit.edu/primer3/). If possible, forward and reverse primers were positioned in different exons to help control for genomic DNA contamination. SEL1L mRNA amplification reactions and sequence analysis were performed as described for mutation screening. Real-time quantitative PCR was performed by using the Applied Biosystems' 7500 Fast Real-Time PCR instrumentation and Roche's FastStart Universal SYBR Green Master. A total reaction volume of 20 µl was used, together with a 0.25 µM concentration of forward - and reverse primers. Two house-keeping genes, GAPDH and YWHAZ, were used as normalization controls, and triplicate samples were used for all reactions. The efficiency of the qPCR reactions was calculated from a five-point dilutions series. No significant differences were detected in the efficiencies between the house-keeping and target reactions, and the comparative ΔΔCt-method could be used to determine relative expression differences [105]. Statistical significance of the expression differences was calculated by using the Student's t-test on normalized mean cycle threshold (Ct) -values. PASW Statistics 18 software (IBM) was used to perform the statistical tests.
Supporting Information
Zdroje
1. TaroniFDiDonatoS 2004 Pathways to motor incoordination: The inherited ataxias. Nat Rev Neurosci 5 641 655
2. MantoMMarmolinoD 2009 Cerebellar ataxias. Curr Opin Neurol 22 419 29
3. Matilla-DuenasASanchezICorral-JuanMDavalosAAlvarezR 2010 Cellular and molecular pathways triggering neurodegeneration in the spinocerebellar ataxias. Cerebellum 9 148 66
4. HardingAE 1983 Classification of the hereditary ataxias and paraplegias. Lancet 1 1151 1155
5. DurrA 2010 Autosomal dominant cerebellar ataxias: Polyglutamine expansions and beyond. Lancet Neurol 9 885 894
6. KlockgetherT 2011 Update on degenerative ataxias. Curr Opin Neurol 24 339 345
7. PalauFEspinosC 2006 Autosomal recessive cerebellar ataxias. Orphanet J Rare Dis 1 47
8. FogelBLPerlmanS 2007 Clinical features and molecular genetics of autosomal recessive cerebellar ataxias. Lancet Neurol 6 245 257
9. KlockgetherTPaulsonH 2011 Milestones in ataxia. Mov Disord 26 1134 1141
10. VermeerSvan de WarrenburgBPWillemsenMACluitmansMSchefferH 2011 Autosomal recessive cerebellar ataxias: The current state of affairs. J Med Genet 48 651 659
11. De MicheleGCoppolaGCocozzaSFillaA 2004 A pathogenetic classification of hereditary ataxias: Is the time ripe? J Neurol 251 913 922
12. Grüsser-CornehlsUBäurleJ 2001 Mutant mice as a model for cerebellar ataxia. Progress in Neurobiology 63 489 540
13. DusartIGuenetJLSoteloC 2006 Purkinje cell death: Differences between developmental cell death and neurodegenerative death in mutant mice. Cerebellum 5 163 173
14. LalondeRStrazielleC 2007 Spontaneous and induced mouse mutations with cerebellar dysfunctions: Behavior and neurochemistry. Brain Res 1140 51 74
15. TonttilaPLindbergLA 1971 [Cerebellar ataxia in a Finnish hurrier] Ett fall av cerebellar ataxi hos finsk stövare (Swedish). Suomen Eläinlääkärilehti 77 135 138
16. deLahuntaAAverillDRJr 1976 Hereditary cerebellar cortical and extrapyramidal nuclear abiotrophy in Kerry Blue Terriers. J Am Vet Med Assoc 168 1119 1124
17. GillJMHewlandM 1980 Cerebellar degeneration in the Border Collie. N Z Vet J 28 170
18. SteinbergHSTroncosoJCCorkLCPriceDL 1981 Clinical features of inherited cerebellar degeneration in Gordon Setters. J Am Vet Med Assoc 179 886 890
19. YasubaMOkimotoKIidaMItakuraC 1988 Cerebellar cortical degeneration in Beagle dogs. Vet Pathol 25 315 317
20. ThomasJBRobertsonD 1989 Hereditary cerebellar abiotrophy in Australian Kelpie dogs. Aust Vet J 66 301 302
21. PerilleALBaerKJosephRJCarrilloJMAverillDR 1991 Postnatal cerebellar cortical degeneration in Labrador Retriever puppies. Can Vet J 32 619 621
22. ChieffoCStalisIHVan WinkleTJHaskinsMEPattersonDF 1994 Cerebellar Purkinje's cell degeneration and coat color dilution in a family of Rhodesian Ridgeback dogs. J Vet Intern Med 8 112 116
23. CarmichaelKPMillerMRawlingsCAFischerAOliverJE 1996 Clinical, hematologic, and biochemical features of a syndrome in Bernese Mountain Dogs characterized by hepatocerebellar degeneration. J Am Vet Med Assoc 208 1277 1279
24. HigginsRJLeCouteurRAKornegayJNCoatesJR 1998 Late-onset progressive spinocerebellar degeneration in Brittany Spaniel dogs. Acta Neuropathol 96 97 101
25. van TongernSEvan VonderenIKvan NesJJvan den InghTS 2000 Cerebellar cortical abiotrophy in two Portuguese Podenco littermates. Vet Q 22 172 174
26. SteinbergHSVan WinkleTBellJSde LahuntaA 2000 Cerebellar degeneration in Old English Sheepdogs. J Am Vet Med Assoc 217 1162 1165
27. SandyJRSlocombeRFMittenRWJedwabD 2002 Cerebellar abiotrophy in a family of Border Collie dogs. Vet Pathol 39 736 738
28. GandiniGBotteronCBriniEFatzerRDianaA 2005 Cerebellar cortical degeneration in three English Bulldogs: Clinical and neuropathological findings. J Small Anim Pract 46 291 294
29. FlegelTMatiasekKHenkeDGrevelV 2007 Cerebellar cortical degeneration with selective granule cell loss in Bavarian Mountain Dogs. J Small Anim Pract 48 462 465
30. UrkasemsinGLinderKEBellJSde LahuntaAOlbyNJ 2010 Hereditary cerebellar degeneration in Scottish Terriers. J Vet Intern Med 24 565 570
31. de LahuntaA 1990 Abiotrophy in domestic animals: A review. Can J Vet Res 54 65 76
32. SummersBACummingsJFDe LahuntaA 1995 Veterinary neuropathology St. Louis Mosby 300 305
33. SisóSHanzlíc˘ekDFluehmannGKathmannITomekA 2006 Neurodegenerative diseases in domestic animals: A comparative review. The Veterinary Journal 171 20 38
34. ZengRFariasFHJohnsonGSMcKaySDSchnabelRD 2011 A truncated retrotransposon disrupts the GRM1 coding sequence in Coton de Tulear dogs with Bandera's Neonatal Ataxia. J Vet Intern Med 25 267 272
35. ShearmanJRCookRWMcCowanCFletcherJLTaylorRM 2011 Mapping cerebellar abiotrophy in australian kelpies. Anim Genet 42 675 678
36. PurcellSNealeBTodd-BrownKThomasLFerreiraMAR 2007 PLINK: A tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics 81 559 575
37. HiekkalinnaTSchafferAALambertBNorrgrannPGoringHH 2011 PSEUDOMARKER: A powerful program for joint linkage and/or linkage disequilibrium analysis on mixtures of singletons and related individuals. Hum Hered 71 256 266
38. LilleyBNPloeghHL 2005 Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proc Natl Acad Sci U S A 102 14296 14301
39. MuellerBLilleyBNPloeghHL 2006 SEL1L, the homologue of yeast Hrd3p, is involved in protein dislocation from the mammalian ER. J Cell Biol 175 261 270
40. ChristiansonJCShalerTATylerREKopitoRR 2008 OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat Cell Biol 10 272 282
41. MuellerBKlemmEJSpoonerEClaessenJHPloeghHL 2008 SEL1L nucleates a protein complex required for dislocation of misfolded glycoproteins. Proc Natl Acad Sci U S A 105 12325 12330
42. RossCAPoirierMA 2004 Protein aggregation and neurodegenerative disease. Nat Med 10 Suppl S10 7
43. TaiHCSchumanEM 2008 Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci 9 826 838
44. MartinaJABonangelinoCJAguilarRCBonifacinoJS 2001 Stonin 2: An adaptor-like protein that interacts with components of the endocytic machinery. J Cell Biol 153 1111 1120
45. WaltherKDirilMKJungNHauckeV 2004 Functional dissection of the interactions of stonin 2 with the adaptor complex AP-2 and synaptotagmin. Proc Natl Acad Sci U S A 101 964 969
46. DirilMKWienischMJungNKlingaufJHauckeV 2006 Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell 10 233 244
47. JungNWienischMGuMRandJBMullerSL 2007 Molecular basis of synaptic vesicle cargo recognition by the endocytic sorting adaptor stonin 2. J Cell Biol 179 1497 1510
48. BiunnoICastiglioniBRogozinIBDeBellisGMalferrariG 2002 Cross-species conservation of SEL1L, a human pancreas-specific expressing gene. OMICS 6 187 198
49. BiunnoICattaneoMOrlandiRCantonCBiagiottiL 2006 SEL1L a multifaceted protein playing a role in tumor progression. J Cell Physiol 208 23 38
50. BiunnoIBernardLDearPCattaneoMVolorioS 2000 SEL1L, the human homolog of C. elegans sel-1: Refined physical mapping, gene structure and identification of polymorphic markers. Hum Genet 106 227 235
51. ZhaoLAckermanSL 2006 Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol 18 444 452
52. MatusSGlimcherLHHetzC 2011 Protein folding stress in neurodegenerative diseases: A glimpse into the ER. Curr Opin Cell Biol 23 239 252
53. ZhangKKaufmanRJ 2006 The unfolded protein response: A stress signaling pathway critical for health and disease. Neurology 66 S102 9
54. MalhotraJDKaufmanRJ 2007 The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18 716 731
55. VembarSSBrodskyJL 2008 One step at a time: Endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 9 944 957
56. WalterPRonD 2011 The unfolded protein response: From stress pathway to homeostatic regulation. Science 334 1081 1086
57. HetzC 2012 The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13 89 102
58. CalfonMZengHUranoFTillJHHubbardSR 2002 IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415 92 96
59. Acosta-AlvearDZhouYBlaisATsikitisMLentsNH 2007 XBP1 controls diverse cell type - and condition-specific transcriptional regulatory networks. Mol Cell 27 53 66
60. YamamotoKSatoTMatsuiTSatoMOkadaT 2007 Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13 365 376
61. BiunnoIAppiertoVCattaneoMLeoneBEBalzanoG 1997 Isolation of a pancreas-specific gene located on human chromosome 14q31: Expression analysis in human pancreatic ductal carcinomas. Genomics 46 284 286
62. DonovielDBDonovielMSFanEHadjantonakisA-BernsteinA 1998 Cloning and characterization of sel-1l, a murine homolog of the C. elegans sel-1 gene. Mech Dev 78 203 207
63. MeusserBHirschCJaroschESommerT 2005 ERAD: The long road to destruction. Nat Cell Biol 7 766 772
64. HirschCGaussRHornSCNeuberOSommerT 2009 The ubiquitylation machinery of the endoplasmic reticulum. Nature 458 453 460
65. HosokawaNWadaINagasawaKMoriyamaTOkawaK 2008 Human XTP3-B forms an endoplasmic reticulum quality control scaffold with the HRD1-SEL1L ubiquitin ligase complex and BiP. J Biol Chem 283 20914 20924
66. CormierJHTamuraTSunrydJCHebertDN 2009 EDEM1 recognition and delivery of misfolded proteins to the SEL1L-containing ERAD complex. Mol Cell 34 627 633
67. RiemerJAppenzeller-HerzogCJohanssonLBodenmillerBHartmann-PetersenR 2009 A luminal flavoprotein in endoplasmic reticulum-associated degradation. Proc Natl Acad Sci U S A 106 14831 14836
68. LindholmDWootzHKorhonenL 2006 ER stress and neurodegenerative diseases. Cell Death Differ 13 385 392
69. KimIXuWReedJC 2008 Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7 1013 1030
70. KanekoMNomuraY 2003 ER signaling in unfolded protein response. Life Sci 74 199 205
71. MatsumotoMMinamiMTakedaKSakaoYAkiraS 1996 Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett 395 143 147
72. ZinsznerHKurodaMWangXBatchvarovaNLightfootRT 1998 CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12 982 995
73. OyadomariSMoriM 2004 Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11 381 389
74. RonDHabenerJF 1992 CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev 6 439 453
75. OkadaTYoshidaHAkazawaRNegishiMMoriK 2002 Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366 585 594
76. SaltiniGDominiciRLovatiCCattaneoMMicheliniS 2006 A novel polymorphism in SEL1L confers susceptibility to alzheimer's disease. Neurosci Lett 398 53 58
77. FranciscoABSinghRLiSVaniAKYangL 2010 Deficiency of suppressor enhancer Lin12 1 like (SEL1L) in mice leads to systemic endoplasmic reticulum stress and embryonic lethality. J Biol Chem 285 13694 13703
78. MittlPRESchneider-BrachertW 2007 Sel1-like repeat proteins in signal transduction. Cell Signal 19 20 31
79. IidaYFujimoriTOkawaKNagataKWadaI 2011 SEL1L protein critically determines the stability of the HRD1-SEL1L endoplasmic reticulum-associated degradation (ERAD) complex to optimize the degradation kinetics of ERAD substrates. J Biol Chem 286 16929 16939
80. HaradaYOzakiKSuzukiMFujiwaraTTakahashiE 1999 Complete cDNA sequence and genomic organization of a human pancreas-specific gene homologous to caenorhabditis elegans sel-1. J Hum Genet 44 330 336
81. CattaneoMLottiLVMartinoSCardanoMOrlandiR 2009 Functional characterization of two secreted SEL1L isoforms capable of exporting unassembled substrate. J Biol Chem 284 11405 11415
82. CattaneoMLottiLVMartinoSAlessioMContiA 2011 Secretion of novel SEL1L endogenous variants is promoted by ER stress/UPR via endosomes and shed vesicles in human cancer cells. PLoS ONE 6 e17206 10.1371/journal.pone.0017206
83. KlockgetherTEvertB 1998 Genes involved in hereditary ataxias. Trends Neurosci 21 413 418
84. LimJHaoTShawCPatelAJSzabóG 2006 A Protein–Protein interaction network for human inherited ataxias and disorders of purkinje cell degeneration. Cell 125 801 814
85. AnttonenAKMahjnehIHamalainenRHLagier-TourenneCKopraO 2005 The gene disrupted in marinesco-sjogren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet 37 1309 1311
86. SenderekJKriegerMStendelCBergmannCMoserM 2005 Mutations in SIL1 cause marinesco-sjogren syndrome, a cerebellar ataxia with cataract and myopathy. Nat Genet 37 1312 1314
87. ZhaoLLongo-GuessCHarrisBSLeeJWAckermanSL 2005 Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet 37 974 979
88. ZhaoLRosalesCSeburnKRonDAckermanSL 2010 Alteration of the unfolded protein response modifies neurodegeneration in a mouse model of marinesco-sjogren syndrome. Hum Mol Genet 19 25 35
89. LeeJWBeebeKNangleLAJangJLongo-GuessCM 2006 Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature 443 50 55
90. KyuhouSKatoNGembaH 2006 Emergence of endoplasmic reticulum stress and activated microglia in purkinje cell degeneration mice. Neurosci Lett 396 91 96
91. KitaoYHashimotoKMatsuyamaTIsoHTamataniT 2004 ORP150/HSP12A regulates purkinje cell survival: A role for endoplasmic reticulum stress in cerebellar development. J Neurosci 24 1486 1496
92. WangMYeRBarronEBaumeisterPMaoC 2010 Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ 17 488 498
93. OmuraTKanekoMTabeiNOkumaYNomuraY 2008 Immunohistochemical localization of a ubiquitin ligase HRD1 in murine brain. J Neurosci Res 86 1577 1587
94. KentWJ 2002 BLAT—the BLAST-like alignment tool. Genome Res 12 656 664
95. OettingWSLeeHKFlandersDJWiesnerGLSellersTA 1995 Linkage analysis with multiplexed short tandem repeat polymorphisms using infrared fluorescence and M13 tailed primers. Genomics 30 450 458
96. FinnRDMistryJTateJCoggillPHegerA 2010 The pfam protein families database. Nucleic Acids Res 38 D211 22
97. SchultzJMilpetzFBorkPPontingCP 1998 SMART, a simple modular architecture research tool: Identification of signaling domains. Proc Natl Acad Sci U S A 95 5857 5864
98. LetunicIDoerksTBorkP 2009 SMART 6: Recent updates and new developments. Nucleic Acids Res 37 D229 32
99. ThomasPDCampbellMJKejariwalAMiHKarlakB 2003 PANTHER: A library of protein families and subfamilies indexed by function. Genome Res 13 2129 2141
100. ThomasPDKejariwalA 2004 Coding single-nucleotide polymorphisms associated with complex vs. mendelian disease: Evolutionary evidence for differences in molecular effects. Proc Natl Acad Sci U S A 101 15398 15403
101. AdzhubeiIASchmidtSPeshkinLRamenskyVEGerasimovaA 2010 A method and server for predicting damaging missense mutations. Nat Methods 7 248 249
102. NgPCHenikoffS 2001 Predicting deleterious amino acid substitutions. Genome Res 11 863 874
103. NgPCHenikoffS 2003 SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 31 3812 3814
104. KumarPHenikoffSNgPC 2009 Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4 1073 1081
105. LivakKJSchmittgenTD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25 402 408
Štítky
Genetika Reprodukční medicína
Článek Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental InvolvementČlánek Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target GenesČlánek Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early EmbryosČlánek Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2Článek Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human CellsČlánek TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome EndsČlánek Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal CordČlánek Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth ofČlánek MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 6- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- Mimetic Butterflies Introgress to Impress
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Selection-Driven Gene Loss in Bacteria
- Decreased Mitochondrial DNA Mutagenesis in Human Colorectal Cancer
- Parallel Evolution of Auditory Genes for Echolocation in Bats and Toothed Whales
- Diverse CRISPRs Evolving in Human Microbiomes
- The Rad4 ATR-Activation Domain Functions in G1/S Phase in a Chromatin-Dependent Manner
- Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains
- Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
- Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target Genes
- Adaptive Introgression across Species Boundaries in Butterflies
- G Protein Activation without a GEF in the Plant Kingdom
- Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes
- An Engineering Approach to Extending Lifespan in
- Incompatibility and Competitive Exclusion of Genomic Segments between Sibling Species
- Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in
- Patterns of Evolutionary Conservation of Essential Genes Correlate with Their Compensability
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
- Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early Embryos
- A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity
- Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2
- Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis
- Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells
- TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome Ends
- Stimulation of Host Immune Defenses by a Small Molecule Protects from Bacterial Infection
- A Broad Requirement for TLS Polymerases η and κ, and Interacting Sumoylation and Nuclear Pore Proteins, in Lesion Bypass during Embryogenesis
- Genome-Wide Identification of Ampicillin Resistance Determinants in
- The CCR4-NOT Complex Is Implicated in the Viability of Aneuploid Yeasts
- Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees
- Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
- Is a Key Regulator of Pancreaticobiliary Ductal System Development
- The NSL Complex Regulates Housekeeping Genes in
- Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord
- Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
- Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth of
- Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia
- MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
- Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family
- Found: The Elusive ANTAR Transcription Antiterminator
- The Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility
- Control of CpG and Non-CpG DNA Methylation by DNA Methyltransferases
- Polymorphisms in the Mitochondrial Ribosome Recycling Factor Compromise Cell Respiratory Function and Increase Atorvastatin Toxicity
- Gene Expression Profiles in Parkinson Disease Prefrontal Cortex Implicate and Genes under Its Transcriptional Regulation
- Global Regulatory Functions of the Endoribonuclease III in Gene Expression
- Extensive Evolutionary Changes in Regulatory Element Activity during Human Origins Are Associated with Altered Gene Expression and Positive Selection
- The Regulatory Network of Natural Competence and Transformation of
- Brain Expression Genome-Wide Association Study (eGWAS) Identifies Human Disease-Associated Variants
- Quantifying the Adaptive Potential of an Antibiotic Resistance Enzyme
- Divergence of the Yeast Transcription Factor Affects Sulfite Resistance
- The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis
- RNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
- Rare Copy Number Variants Observed in Hereditary Breast Cancer Cases Disrupt Genes in Estrogen Signaling and Tumor Suppression Network
- Genome-Wide Location Analysis Reveals Distinct Transcriptional Circuitry by Paralogous Regulators Foxa1 and Foxa2
- A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
- The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression
- Limits to the Rate of Adaptive Substitution in Sexual Populations
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- The NSL Complex Regulates Housekeeping Genes in
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



