-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells in
Clearance of apoptotic cells by engulfment plays an important role in the homeostasis and development of multicellular organisms. Despite the fact that the recognition of apoptotic cells by engulfment receptors is critical in inducing the engulfment process, the molecular mechanisms are still poorly understood. Here, we characterize a novel cell corpse engulfment pathway mediated by the integrin α subunit PAT-2 in Caenorhabditis elegans and show that it specifically functions in muscle-mediated engulfment during embryogenesis. Inactivation of pat-2 results in a defect in apoptotic cell internalization. The PAT-2 extracellular region binds to the surface of apoptotic cells in vivo, and the intracellular region may mediate signaling for engulfment. We identify essential roles of small GTPase CDC-42 and its activator UIG-1, a guanine-nucleotide exchange factor, in PAT-2–mediated cell corpse removal. PAT-2 and CDC-42 both function in muscle cells for apoptotic cell removal and are co-localized in growing muscle pseudopods around apoptotic cells. Our data suggest that PAT-2 functions through UIG-1 for CDC-42 activation, which in turn leads to cytoskeletal rearrangement and apoptotic cell internalization by muscle cells. Moreover, in contrast to PAT-2, the other integrin α subunit INA-1 and the engulfment receptor CED-1, which signal through the conserved signaling molecules CED-5 (DOCK180)/CED-12 (ELMO) or CED-6 (GULP) respectively, preferentially act in epithelial cells to mediate cell corpse removal during mid-embryogenesis. Our results show that different engulfing cells utilize distinct repertoires of receptors for engulfment at the whole organism level.
Published in the journal: . PLoS Genet 8(5): e32767. doi:10.1371/journal.pgen.1002663
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002663Summary
Clearance of apoptotic cells by engulfment plays an important role in the homeostasis and development of multicellular organisms. Despite the fact that the recognition of apoptotic cells by engulfment receptors is critical in inducing the engulfment process, the molecular mechanisms are still poorly understood. Here, we characterize a novel cell corpse engulfment pathway mediated by the integrin α subunit PAT-2 in Caenorhabditis elegans and show that it specifically functions in muscle-mediated engulfment during embryogenesis. Inactivation of pat-2 results in a defect in apoptotic cell internalization. The PAT-2 extracellular region binds to the surface of apoptotic cells in vivo, and the intracellular region may mediate signaling for engulfment. We identify essential roles of small GTPase CDC-42 and its activator UIG-1, a guanine-nucleotide exchange factor, in PAT-2–mediated cell corpse removal. PAT-2 and CDC-42 both function in muscle cells for apoptotic cell removal and are co-localized in growing muscle pseudopods around apoptotic cells. Our data suggest that PAT-2 functions through UIG-1 for CDC-42 activation, which in turn leads to cytoskeletal rearrangement and apoptotic cell internalization by muscle cells. Moreover, in contrast to PAT-2, the other integrin α subunit INA-1 and the engulfment receptor CED-1, which signal through the conserved signaling molecules CED-5 (DOCK180)/CED-12 (ELMO) or CED-6 (GULP) respectively, preferentially act in epithelial cells to mediate cell corpse removal during mid-embryogenesis. Our results show that different engulfing cells utilize distinct repertoires of receptors for engulfment at the whole organism level.
Introduction
During the development of multicellular organisms, cells that are unnecessary, damaged, or harmful undergo programmed cell death (apoptosis) [1]. Apoptotic cells are recognized and subsequently internalized by engulfing cells [2], [3]. Improper engulfment of apoptotic cells has been linked to diseases: too little engulfment may cause inflammation, autoimmune diseases, and cancers [4]–[6], whereas too much engulfment has been implicated in degenerative diseases [7]–[9].
In flies and mammals, engulfment of apoptotic cells is mediated by “professional” phagocytes, such as mobile macrophages and dendritic cells, or by “amateur” phagocytes, such as muscle cells, epithelial cells, and endothelial cells [10]–[14]. Several mammalian receptors involved in apoptotic cell engulfment have been identified and characterized. Receptors such as BAI1 [15], stabilin-1 [16], stabilin-2 [17], TIM-1 [18], TIM-3 [19], TIM-4 [18], integrins [20], [21], and receptor tyrosine kinase Mer [22] bind to the “eat me” signal, externalized phosphatidylserine (PS) [23], [24], on the surface of apoptotic cells either directly [25], [26] or through bridging molecules [27], [28]. BAI1, integrins, and Mer then signal through the conserved DOCK180/ELMO1/RAC GTPase signaling module to promote the internalization of apoptotic cells [15], [29]–[31], whereas stabilin-1 and stabilin-2 do so through the intracellular adaptor GULP [16]. Other “eat me” signal and receptor pairs for engulfment have been reported. For example, lectin receptors bind to altered sugars on apoptotic cells [32], scavenger receptors to oxidized LDL-like moieties [33], and CD14 to ICAM3 [34]. The in vivo role of most of these receptors in the clearance of apoptotic cells and the tissues in which they act at the whole organism level have not been defined.
During the development of a C. elegans adult hermaphrodite, 1090 somatic cells are generated, 131 of which undergo apoptosis [35]–[37]. The apoptotic cells are removed by their neighboring cells [35], [38]. Cell types such as hypodermal cells (which constitute the external epithelium), pharyngeal muscle cells, and intestinal cells have been shown to function as engulfing cells [37], [38]. Three partially redundant pathways that regulate the engulfment process have been identified. The first pathway is mediated by two cell-surface proteins CED-1 (mammalian homologue MEGF10) and CED-7 (ABCA1) [39], [40]. CED-1 binds to an apoptotic cell through secreted molecule TTR-52 (transthyretin) and transduces the engulfment signal through the adaptor protein CED-6 (GULP) and DYN-1 (dynamin) to promote the engulfment and degradation of apoptotic cells [41]–[43]. The second pathway is regulated by at least three engulfment receptors, phosphatidylserine receptor PSR-1 [44], Frizzled MOM-5 [45], and integrin INA-1/PAT-3 [46], all of which signal through the adaptor protein CED-2 (CRKII) and the bipartite GEF complex CED-5 (DOCK180)/CED-12 (ELMO) for CED-10 (RAC1) GTPase activation [47]–[52]. Phosphoinositide phosphatase MTM-1 (myotubularin) negatively regulates this pathway by inhibiting the recruitment of CED-12 to the plasma membrane [53], [54]. These two engulfment pathways may converge at CED-10 GTPase, which promotes the actin-based cytoskeleton rearrangement required for phagocytosis of apoptotic cells in engulfing cells [55]. CED-10 activity is negatively regulated by GTPase activating protein SRGP-1 during the engulfment process [56]. Compared to these two major pathways, little is known about the third pathway, which is negatively regulated by the cytoskeletal regulator ABL-1 (Abl), which inhibits the engulfment of apoptotic cells by inhibiting ABI-1 (Abl-interacting protein) and acts independently of CED-10 [57].
Integrins are transmembrane αβ heterodimers that make connections to the extracellular matrix and cytoskeleton and activate several signaling pathways required for multiple cellular processes, including cell adhesion, cell migration, and cell survival [58], [59]. C. elegans has two integrin α subunits, INA-1 and PAT-2, and a single β subunit, PAT-3 [60]–[62]. Integrin PAT-2/PAT-3 is a component of muscle attachment complexes and is essential for sarcomere assembly [63], [65] and also acts to direct muscle arm extension [66] and distal tip cell migration [67]. We have recently shown that integrin INA-1/PAT-3 functions as an engulfment receptor for apoptotic cells [46]. Intriguingly, the pat-3 knockout mutant has a stronger defect in cell corpse engulfment than the ina-1 mutant [46], raising the possibility that pat-2 may also mediate the removal of apoptotic cells. In this study, we examined and characterized the role of pat-2 in cell corpse engulfment and showed that it functions in the muscle-mediated internalization of apoptotic cells and acts through a pathway distinct from the previously known pathways.
Results
pat-2 loss-of-function results in an increased number of embryonic cell corpses
pat-2(st567) mutants [64] and worms treated with pat-2 RNAi are embryonic lethal and show a phenotype of paralyzed arrest at the two-fold stage (Pat), as PAT-2 plays an essential role in body wall muscle assembly and function during embryogenesis [63]–[65]. We tested the involvement of pat-2 in apoptosis by counting the number of apoptotic cells at the comma and 1.5-fold stages, the two stages at which the majority of embryonic apoptosis occurs [37] and pat-2 mutant embryos are still developing normally, and found that both pat-2(st567) and pat-2(RNAi) embryos had a Ced (cell death abnormal) phenotype with increased numbers of apoptotic cells (Table 1). The Ced phenotype of the pat-2(st567) mutant was rescued by the transgene Ppat-2pat-2::gfp, in which the pat-2::gfp translational fusion construct is expressed under the control of the endogenous pat-2 promoter Ppat-2 (Table 2), confirming that the Ced phenotype of the pat-2(st567) mutant was specifically caused by pat-2 loss of function. The Ppat-2pat-2::gfp transgene also rescued the Pat phenotype of the pat-2(st567) mutant (Table 3).
Tab. 1. pat-2 mutants contain more apoptotic cells during mid-embryogenesis than the wild-type. 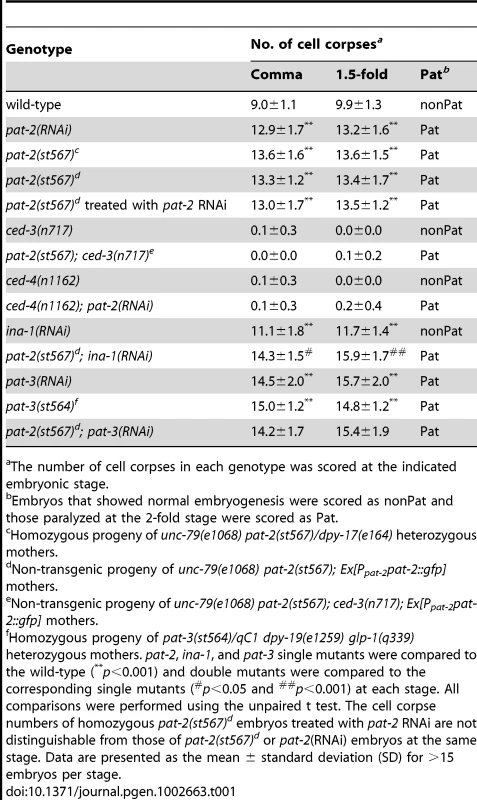
The number of cell corpses in each genotype was scored at the indicated embryonic stage. Tab. 2. pat-2 acts in muscle cells to mediate apoptotic cell engulfment. 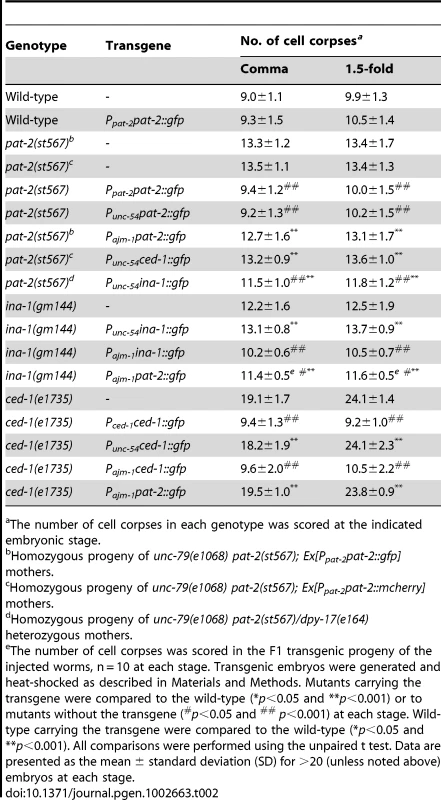
The number of cell corpses in each genotype was scored at the indicated embryonic stage. Tab. 3. Effects of mutant pat-2 transgenes on the Ced and Pat phenotypes. 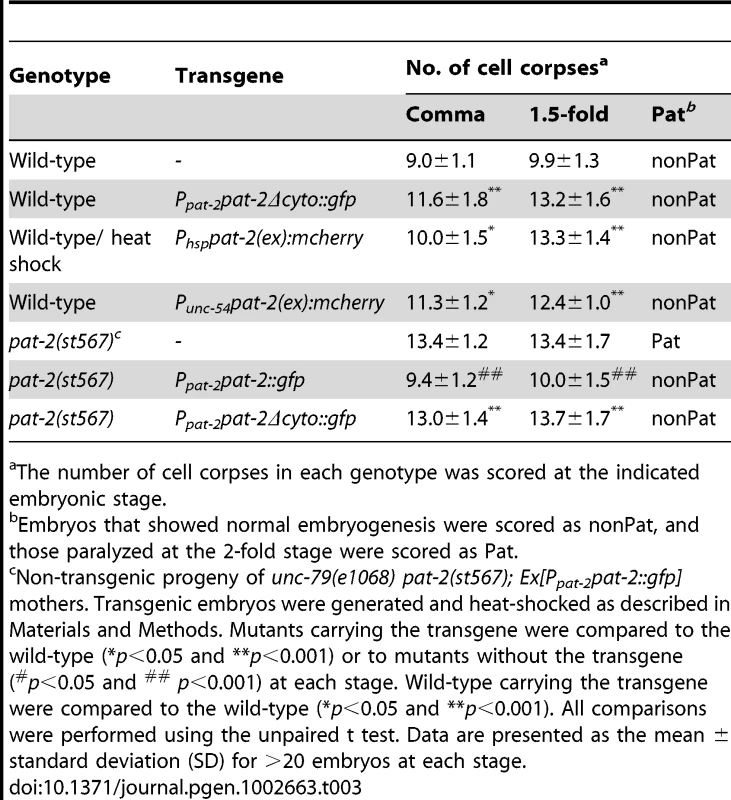
The number of cell corpses in each genotype was scored at the indicated embryonic stage. ced-3(n717) and ced-4(n1162), strong mutations in the pro-apoptotic genes ced-3 and ced-4 that block almost all programmed cell death [68], suppressed the phenotype of an increased number of cell corpses in pat-2(st567) or pat-2(RNAi) embryos (Table 1), showing that the extra cell corpses observed in the pat-2 mutants were generated by programmed cell death. In contrast, the Pat phenotype of the pat-2(st567) or pat-2(RNAi) mutants was not suppressed by either the ced-3 or ced-4 mutation (Table 1). The fact that the Pat and Ced phenotypes can be uncoupled shows they are probably due to the loss of different pat-2 functions.
pat-2 is required for the removal of embryonic cell corpses
To determine the cause of the Ced phenotype of the pat-2 mutant, we performed a time-lapse differential interference contrast (DIC) microscopy analysis of cell corpses in the wild-type and pat-2(st567) mutant during embryogenesis prior to the 2-fold stage. We found that, although the timing and number of cell death events were similar in the two types of embryo (Figure 1A), the length of time that the cell corpses persisted was significantly different (Figure 1B). In the wild-type, approximately 97% of cell corpses disappeared within 40 minutes and no cell corpses persisted longer than 60 minutes, whereas, in the pat-2 embryo, more than 50% of the cell corpses persisted longer than 40 minutes and about 25% longer than 100 minutes. These results demonstrate that pat-2 functions in the clearance of cell corpses.
Fig. 1. pat-2 and cdc-42 mutants are defective in apoptotic cell removal. 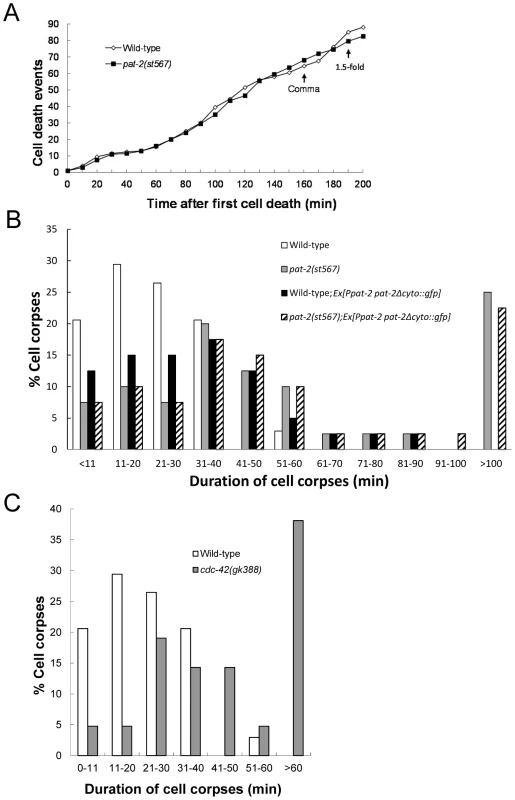
(A) pat-2 loss of function does not affect the number of cell death events. Embryonic cell deaths that occurred in the 200 min following the first cell death (up to about the 1.5-fold stage) were followed in wild-type (white rhombi) and pat-2(st567) (black squares) embryos. The y axis shows the total number of cell death events at the different time points shown on the x axis. The data shown are the average for two embryos for each genotype. The times corresponding to the comma and 1.5-fold stages are indicated. (B–C) Cell corpses in pat-2(st567) (B) and cdc-42 (gk388) (C) mutants persist longer than in the wild-type. The persistence of cell corpses that appeared 360–410 min after the first cleavage was recorded using four-dimensional Nomarski microscopy. The y axis shows the percentage of cell corpses that persisted for the time indicated on the x axis. Forty corpses were analyzed for each genotype. The data of wild-type (white bars), pat-2(st567) (gray bars); wild-type; Ex[Ppat-2pat-2Δcyto::gfp] (black bars) and pat-2(st567); Ex[Ppat-2pat-2Δcyto::gfp] (slashed bars) are shown in (B) and those of the wild-type (white bars) and cdc-42(gk388) (gray bars) in (C). pat-2 does not function in any previously known pathway for apoptotic cell removal
In addition to PAT-2, C. elegans has another integrin α subunit, INA-1, which forms a complex with the single integrin β subunit, PAT-3, on the cell surface [62] and is also required for the clearance of embryonic cell corpses [46]. RNAi inactivation of either ina-1 or pat-3 results in increased numbers of apoptotic cells at the comma and 1.5-fold stages [46] (Table 1). The pat-2(st567) mutation further increased the number of cell corpses in the ina-1(RNAi) mutant, but not in the pat-3 mutant (Table 1). These results support the notions that PAT-2, like INA-1, acts together with PAT-3 during cell corpse clearance and that the two integrins, PAT-2/PAT-3 and INA-1/PAT-3, function in a partially redundant fashion during the clearance process.
We next determined whether pat-2 functioned together with previously identified genes to promote cell corpse removal. The two major pathways that regulate cell corpse removal are mediated, respectively, by ced-1, ced-6, and ced-7 or ced-2, ced-5, and ced-12 [2]. We therefore generated and analyzed double mutants containing either the pat-2(st567) or pat-2(RNAi) mutation and strong loss-of-function or null alleles of the engulfment ced genes for the two pathways. Interestingly, the pat-2(st567) or pat-2(RNAi) mutation further enhanced the engulfment defect in mutants defective in either pathway (Table 4). This suggests two possibilities. First, pat-2 could act in both pathways, with the combination of a pat-2 mutation and a defect in either pathway having an additive effect. Alternatively, pat-2 could act in a separate pathway that is partially redundant with these two pathways. To distinguish between these two possibilities, we tested whether the pat-2(st567) or pat-2(RNAi) mutation could increase the engulfment defect in double mutants between the two pathways, such as the ced-1(e1735); ced-5(n1812) and ced-12(tp2); ced-7(n1892) double mutants. In the first case in which pat-2 would act in both pathways, we would expect that pat-2(st567) or pat-2(RNAi) would not enhance the engulfment defect of the double mutants. However, we found that the pat-2(st567) or pat-2(RNAi) mutation significantly increased the number of cell corpses in both double mutants (Table 4). We therefore conclude that pat-2 probably functions in a pathway distinct from these two pathways to promote the engulfment of cell corpses.
Tab. 4. pat-2 promotes apoptotic cell engulfment in a pathway distinct from previously known pathways. 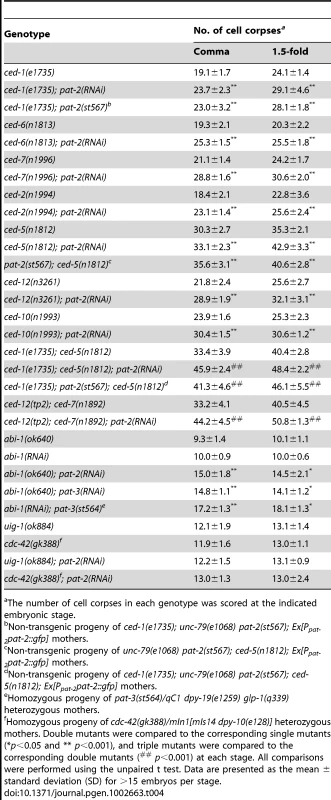
The number of cell corpses in each genotype was scored at the indicated embryonic stage. A recent study has shown that abi-1 (abl-1 interactor 1) acts in parallel to the two major engulfment pathways during cell corpse removal [57]. Our analysis showed that pat-2(RNAi) or pat-3(st564) enhanced the engulfment defect in the abi-1(null) or abi-1(RNAi) embryo (Table 4), showing that pat-2 and pat-3 function independently of abi-1 to mediate the removal of apoptotic cells.
pat-2 functions in muscle cells to mediate the removal of apoptotic cells
We next examined the localization of PAT-2 using the Ppat-2pat-2::gfp or Ppat-2pat-2::mcherry transgene, which rescued the Ced and Pat phenotypes of the pat-2 mutant (Table 3 and data not shown). PAT-2::GFP or PAT-2::mCherry was found to be expressed in body wall muscles and hypodermal cells during embryogenesis (Figure 2A, 2B and Figure S2A). Notably, a strong GFP signal was observed along the surface of apoptotic cells adjacent to muscle cells (Figure 2A and 2E), whereas a relatively weak GFP signal was seen around apoptotic cells engulfed by hypodermal cells (Figure 2B and 2F). Our analysis of embryos expressing the transgene Ppat-2nls::gfp, in which GFP was expressed under the control of Ppat-2 and was predominantly localized to the nucleus, indicated that Ppat-2 expression was absent from cell corpses during embryogenesis (Figure 2I, 2K, Figure S1 and Text S1). Thus, the PAT-2::GFP signal surrounding apoptotic cells likely originated from the surface of the engulfing cells. The localization of PAT-2::GFP around the surface of apoptotic cells near body wall muscles was not affected by the ced-1;ced-5 double mutation (Figure S3), consistent with our genetic data showing that pat-2 acts in parallel to ced-1 and ced-5 to mediate cell corpse engulfment. When PAT-2::mCherry and PAT-3::GFP were co-expressed under the control of their respective endogenous promoters, PAT-2::mCherry and PAT-3::GFP were co-localized on apoptotic cells and to the dense bodies (Z-disks) and M-lines of muscle cells (Figure S2A), in agreement with the idea that PAT-2 and PAT-3 form a complex.
Fig. 2. PAT-2 is strongly expressed in muscle cells and clusters around apoptotic cells. 
(A–H) PAT-2::GFP, MOESIN::GFP, and GFP::CDC-42 are localized to pseudopods around apoptotic cells. GFP (A–D) and DIC (E–H) images of wild-type embryos expressing PAT-2::GFP (A–B), MOESIN::GFP (C), or GFP::CDC-42 (D) under the control of the indicated promoter. Apoptotic cells are indicated by arrows and shown enlarged in the insets. The arrowheads indicate hypodermal cells. (I–K) Ppat-2nls::gfp is not expressed in apoptotic MSpppaaa cells. GFP (I), membrane-bound mRFP (J), and DIC (K) images of an embryo co-expressing Punc-54ced-1::mrfp and Ppat-2nls::gfp. MSpppaaa cell corpses are indicated by the arrows and shown enlarged in the insets. The arrowheads indicate the nucleus of the engulfing muscle cell. (L–M) The extracellular region of PAT-2 binds to the surface of apoptotic cells. DIC (L) and PAT-2(ex)::mCherry (M) images of a ced-1(e1735); ced-5(n1812) double mutant embryo expressing PAT-2(ex)::mCherry under the control of the heat-shock promoter. Apoptotic cells are indicated by arrows. (N–Q) GFP::CDC-42 and PAT-2::mCherry are co-localized to the pseudopods around apoptotic MSpppaaa cells. DIC (N), GFP::CDC-42 (O), PAT-2::mCherry (P), and merged (Q) images of a wild-type embryo co-expressing the transgenes Punc-54 gfp:cdc-42 and Ppat-2pat-2::mcherry. The MSpppaaa cells are indicated by arrows and shown in the insets. All scale bars represent 5 µm. In contrast to flies and mammals, C. elegans does not have macrophage-like motile phagocytes; instead, apoptotic cells are removed by their neighboring cells [36], [38]. Hypodermal cells, pharyngeal muscle cells, and intestinal cells have been shown to function as engulfing cells [37], [38]. Since pat-2 was expressed in both hypodermal cells and body wall muscles, we tested whether it functioned in muscle cells and/or hypodermal cells for the removal of apoptotic cells. To this end, we expressed pat-2 cDNA under the control of the Pajm-1 or Punc-54 promoter and examined the ability of each transgene to rescue the Ced phenotype of the pat-2 mutant. Pajm-1 is expressed in all epithelia including hypodermal cells [69], whereas Punc-54 is expressed in body wall muscles [70]. We found that expression of pat-2 by Punc-54, but not by Pajm-1, fully rescued the Ced phenotype of the pat-2(st567) embryo (Table 2). Punc-54 did not appear to express in apoptotic cells (Figure S4A). These results support the notion that pat-2 acts in muscle cells to mediate cell corpse removal during embryogenesis.
We next co-expressed PAT-2::mCherry and PAT-2::GFP by the Ppat-2 and Punc-54 promoters, respectively, to monitor the PAT-2-mediated and muscle-mediated engulfment processes simultaneously in the 1.5-fold embryos. Approximately 22.7% and 18.1% of the cell corpses were enclosed by the PAT-2::mCherry and PAT-2::GFP circles, respectively, and most of the mCherry and GFP circles were co-localized, suggesting that most, if not all, PAT-2-mediated engulfment involves muscle cells (Table S1).
CED-1 and INA-1 may function in the hypodermal cell-mediated engulfment of apoptotic cells
CED-1 and INA-1/PAT-3 are engulfment receptors that are expressed in multiple cell types, including hypodermis and body wall muscles [40], [46], but act in pathways in parallel to that involving PAT-2 (Table 1 and Table 4). We therefore tested whether CED-1 and INA-1 acted in specific cell types to mediate the engulfment of apoptotic cells. Expression of ced-1 in hypodermal cells using the Pajm-1 promoter fully rescued the ced-1 engulfment defect, whereas expression of ced-1 in body wall muscles using the Punc-54 promoter did not (Table 2). Similarly, expression of ina-1 in hypodermal cells, but not muscle cells, rescued the ina-1 engulfment defect (Table 2). These data suggest that CED-1 and INA-1 preferentially act in hypodermal cells, at least during the comma and 1.5-fold stages. This observation, together with our result that PAT-2 predominantly functions in muscle cells, indicates that different engulfing cells may utilize different engulfment receptors to mediate cell corpse removal.
Removal of apoptotic MSpppaaa cells by muscle cells requires PAT-2
The mesodermal MSpppaaa cell is generated in the head region about 250 minutes after the first cleavage of a zygote [37]. After circumferential migration to the dorsal midline, the MSpppaaa cell is located near the anterior dorsal muscle cells. Approximately 400 minutes after its generation, the MSpppaaa cell undergoes apoptosis at the four-fold stage [37]. To examine whether the apoptotic MSpppaaa cell was removed by a muscle cell, we expressed membrane-bound monomeric red fluorescent protein (mRFP) in muscle cells using Punc-54. MSpppaaa cell corpses were found inside the adjacent muscle cells (Figure 2J and 2K), showing that MSpppaaa cell corpses were engulfed by muscle cells. To confirm this result, we tagged PAT-2 with mCherry and GFP and co-expressed PAT-2::mCherry in body wall muscles using Punc-54 and PAT-2::GFP in hypodermal cells using Pajm-1 in wild-type embryos. The PAT-2::mCherry, but not PAT-2::GFP, signal was observed around apoptotic MSpppaaa cells (Figure S5), confirming that apoptotic MSpppaaa cells are engulfed by muscle cells, but not hypodermal cells. The basement membrane between muscle and hypodermis may limit the access of hypodermal cells to the apoptotic MSpppaaa cell for engulfment.
We next examined whether the clearance of apoptotic MSpppaaa cells is defective in pat-2 embryos by scoring apoptotic MSpppaaa cells in wild-type and pat-2 embryos at specific embryonic stages. The two stages during which the pharyngeal grinder has just formed and the pharynx is pumping were chosen. The MSpppaaa cell undergoes apoptosis during pharyngeal grinder formation. Pharyngeal pumping begins about 1.5 hours after grinder formation is complete. At the time when pharyngeal grinder formation had just finished, only 19.6% of wild-type embryos contained the MSpppaaa cell corpse and none remained at the time when pharyngeal pumping occurred (Table 5). However, 74.6% of pat-2 embryos contained the MSpppaaa cell corpse at the time when pharyngeal grinder formation had just finished, and 52.4% still contained a corpse when pharyngeal pumping had started (Table 5). This shows that pat-2 is important for the removal of apoptotic MSpppaaa cells. In addition, the muscle-specific expression of pat-2 by Punc-54 significantly reduced the percentage of pat-2 mutant embryos containing the MSpppaaa cell corpse at the time when pharyngeal grinder formation had just finished or pharyngeal pumping had started (Table 5). In contrast, the hypodermal cell-specific expression of pat-2 by Pajm-1 failed to do so (Table 5). These results show that pat-2 functions in muscle cells to mediate the removal of the MSpppaaa cell corpse.
Tab. 5. pat-2 functions in the muscle-mediated clearance of apoptotic MSpppaaa cells. 
Percentage of embryos with the MSpppaaa cell corpse at the indicated developmental stage, with the number of embryos scored in parentheses. Three cells C1, C2 and C3, called as in reference [42], are generated in the ventral side of embryos at approximately 300–350 min after first cleavage and subsequently undergo apoptosis. Their cell corpses are engulfed by the ventral hypodermal cells ABplaapppp, ABpraapppa and ABplaapppa, respectively [42]. The duration time of these apoptotic cells appeared normal in the pat-2 mutants (Table S2). This result further supports the notion that pat-2 preferentially acts in muscle cells but not hypodermal cells for cell-corpse removal. In contrast to pat-2, ced-1 has been previously shown to be important for the hypodermal cell-mediated removal of C1, C2 and C3 cell corpses [42]. In the ced-1 mutants, the duration time of MSpppaaa cell corpses was slightly longer than that of the wild-types: approximately 19% of MSpppaaa cell corpses still persisted in the ced-1 mutants during pharyngeal pumping, whereas none remained in the wild-type embryos at this stage (Table 5). Thus, ced-1 also plays a minor role in the muscle-mediated removal of apoptotic MSpppaaa cells in late embryogenesis.
Muscle cell-mediated internalization of apoptotic cells is defective in pat-2 mutants
The persistence of cell corpses in the pat-2 mutant could be due to a defect in either the internalization or the degradation of the corpse. We used the Punc-54myri::mrfp translational reporter to express the MYRI::mRFP fusion protein on the surface of muscle cells and followed the membrane processes of a muscle cell around an apoptotic cell using the time-lapse fluorescence microscopy analysis. The comma stage was chosen because the pat-2 embryos at this stage show an increased number of cell corpses and the embryos at this stage do not move around. In wild-type embryos, MYRI::mRFP fusion protein appeared to localize to the growing pseudopods, which eventually formed a circle around an apoptotic cell upon the completion of the internalization process (Figure 3A). The MYRI::mRFP circle formed around an apoptotic cell in approximately 6 minutes. However, in the pat-2(st567) mutant embryos, the MYRI::mRFP circle formation took approximately 21 minutes to complete, more than three times longer than that in the wild-type, indicating that the internalization process was compromised.
Fig. 3. PAT-2 is required for the internalization of apoptotic cells. 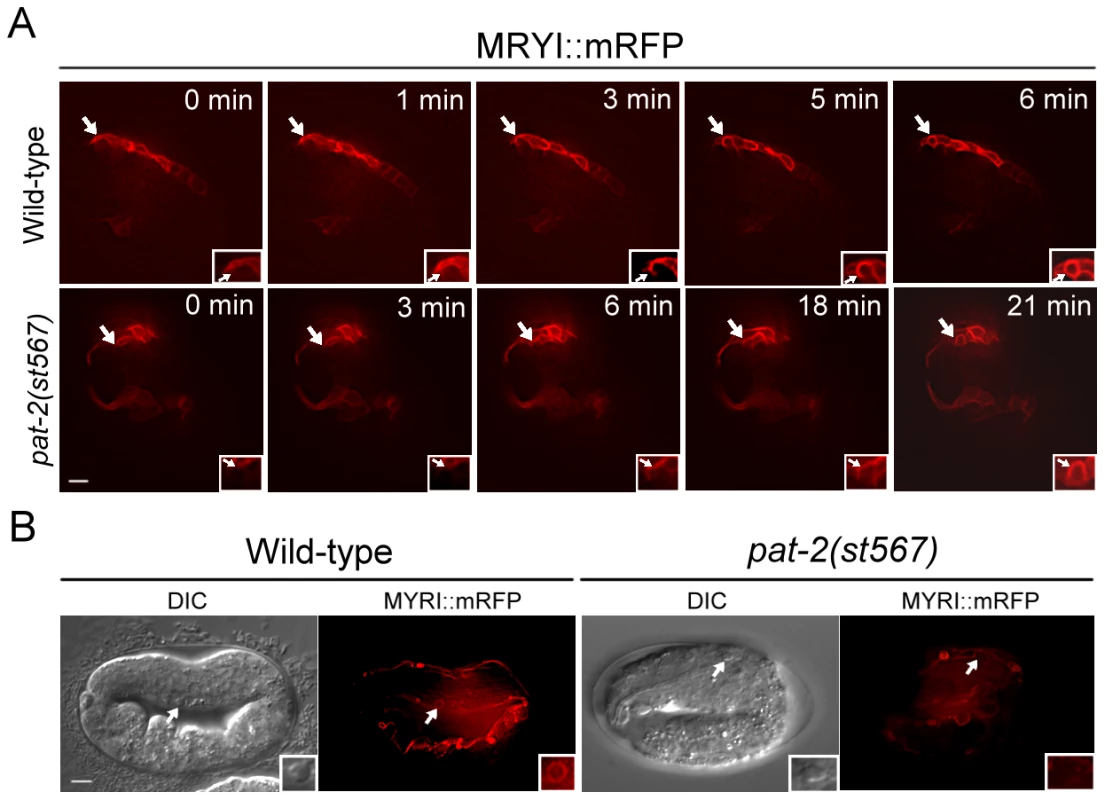
(A) The formation of MYRI::mRFP circles around cell corpses was followed and time-lapse MYRI::mRFP images of wild-type and pat-2(st567) embryos expressing Punc-54myri::mrfp were shown. The time point immediately prior to the appearance of trace amounts of MYRI::mRFP adjacent to cell corpses was set as 0 min. Apoptotic cells are indicated by arrows and shown enlarged in the insets. (B) The DIC and MYRI::mRFP images of the apoptotic MSpppaaa cells in the wild-type and pat-2(st567) embryos expressing Punc-54myri::mrfp. The MYRI::mRFP circle around the apoptotic MSpppaaa cell was observed in the wild-type but not pat-2(st567) embryos. MSpppaaa cell corpses are indicated by arrows and shown in enlarged insets. Both scale bars represent 5 µm. We further examined the internalization of MSpppaaa cell corpses in the pat-2 mutant using the Punc-54myri::mrfp transgene. As shown in Table 5 and Figure 3B, in wild-type embryos, all apoptotic MSpppaaa cells showed the MYRI::mRFP circle at the time when grinder formation had just finished and their cell corpses were cleared at the time of pharyngeal pumping. However, in pat-2 mutants, no apoptotic MSpppaaa cells had the MYRI::mRFP circle at the time when grinder formation had just finished, and nearly half of MSpppaaa cell corpses still persisted and showed no MYRI::mRFP circle at the time of pharyngeal pumping (Table 5). These data indicate that pat-2 is required for the internalization of an apoptotic MSpppaaa cell by a muscle cell.
The extracellular domain of PAT-2 recognizes and binds to apoptotic cells in vivo
We then examined whether PAT-2 recognized apoptotic cells to trigger their internalization. To this end, we generated the transgene Phsppat-2(ex)::mcherry, in which the coding sequence for the PAT-2 extracellular domain with a signal sequence [PAT-2(ex)] was fused to that of mCherry under the control of the heat-shock promoter Phsp. The transgene was then introduced into the wild-type and ced-1(e1735); ced-5(n1812) double mutant embryos. The ced-1(e1735); ced-5(n1812) double mutant embryos contain many persistent apoptotic cells, resulting in a greater chance of seeing PAT-2(ex)::mCherry binding, especially during late embryogenesis when very few cell corpses are present in the wild-type embryos. We found that secreted PAT-2(ex)::mCherry clustered on the surface of apoptotic cells (Figure 2M), albeit with a weaker fluorescence intensity compared to that of PAT-2::GFP (Figure 2A). Approximately 15.2% of apoptotic cells displayed the PAT-2(ex)::mCherry circle in the ced-1(e1735); ced-5(n1812) double mutant embryos at the 4-fold stage. A similar percentage (16.6%) was observed in the wild-type embryos at the 1.5-fold stage (Table S1). Thus, PAT-2(ex) recognizes and binds to the surface of apoptotic cells. However, we do not know if binding is direct or indirect.
We further examined whether PAT-2(ex)::mCherry bound to specific apoptotic cells. Approximately 20% of the MSpppaaa cell corpses had a PAT-2(ex)::mCherry circle (Table S1 and Figure S6A), but none of C1, C2 and C3 cell corpses, which are engulfed by hypodermal cells, had a PAT-2(ex)::mCherry circle (Table S1 and Figure S6C and S6D). This result indicates that PAT-2(ex) binds to specific apoptotic cells and may explain why PAT-2 is required for the removal of MSpppaaa (Table 5), but not C1, C2 or C3, cell corpses (Table S2) and why only a subset, but not all, of apoptotic cells are labeled with PAT-2(ex)::mCherry (Figure 2L and 2M). Furthermore, we used the Punc-54 promoter to express PAT-2(ex)::mCherry and observed PAT-2(ex)::mCherry clustering around apoptotic cells including the apoptotic MSpppaaa cell (Figure S4B), despite the fact that the PAT-2(ex)::mCherry signal was not as strong as that expressed by the Phsp promoter. This result shows that PAT-2(ex)::mCherry expressed in and secreted from muscle cells recognizes, and binds to, the surface of apoptotic cells. Embryos with PAT-2(ex)::mCherry overexpression by either Phsp or Punc-54 resulted in increased numbers of apoptotic cells at the comma and 1.5-fold stages (Table 3) and delayed the removal of MSpppaaa cell corpses (Figure S6A), indicating that PAT-2(ex)::mCherry overexpression interferes with the normal process of cell corpse engulfment.
The INA-1 extracellular domain, termed INA-1(N) as in reference [46], has been shown to recognize apoptotic cells [46]. To test whether PAT-2 and INA-1 may recognize the same apoptotic cells, we co-expressed PAT-2(ex)::mCherry and INA-1(N)::GFP in embryos using the transgenes Phsppat-2(ex)::mcherry and Phsp ina-1(N)::gfp. We found that PAT-2(ex)::mCherry was co-localized with INA-1(N)::GFP on some apoptotic cells (Figure S6E). Thus, the extracellular domains of PAT-2 and INA-1 can recognize identical apoptotic cells, although they preferentially function in different cell types for apoptotic cell removal.
The cytoplasmic domain of PAT-2 is required for cell-corpse engulfment, but not for muscle assembly or contraction
As shown above, PAT-2 binds to apoptotic cells and may serve simply to tether the corpse to an engulfing cell or also initiate a signaling pathway for engulfment. To distinguish between these two possibilities, we tested whether the cytoplasmic domain of PAT-2 was essential for signaling by deleting it and examining the ability of truncated PAT-2Δcyto to rescue pat-2(st567) mutants. We generated pat-2(st567); Ppat-2pat-2Δcyto::gfp transgenic animals that expressed the PAT-2Δcyto::GFP fusion protein under the control of the pat-2 promoter. To our surprise, PAT-2Δcyto::GFP fully rescued the Pat phenotype of the pat-2(st567) mutant, but failed to rescue the Ced phenotype (Table 3). The pat-2(st567); Ppat-2pat-2Δcyto::gfp transgenic embryos contained increased numbers of cell corpses (Table 3) at comma and 1.5-fold stages, and some of the cell corpses persisted longer than those of the wild-type embryos (Figure 1B), showing an engulfment defect. The non-Pat phenotype of the transgenic embryos allowed us to count cell corpses at and beyond the 2-fold stage, which is impossible for homozygous pat-2(st567) embryos because of developmental arrest. The pat-2(st567) embryos carrying the Ppat-2 pat-2Δcyto::gfp transgene also had increased numbers of cell corpses at the 2-, 3 - and 4-fold stages (Table S3). Thus, pat-2 is required for the engulfment of apoptotic cells throughout embryogenesis. In addition, MSpppaaa cell corpses also persisted longer in the pat-2(st567); Ppat-2 pat-2Δcyto::gfp transgenic embryos than those in wild-type embryos. Approximately 61% of the MSpppaaa cell corpses still persisted at the stage of pharyngeal pumping, while none remained in the wild-type embryos at this stage (Table 5).
PAT-2Δcyto::GFP was localized to the surfaces, dense bodies and M-lines of muscle cells in the wild-type and pat-2 mutants (Figure S7), similar to PAT-2::GFP or PAT-2:: mCherry (Figure S2, S7 and data not shown), suggesting that the cytoplasmic domain is not required for PAT-2 localization. We then used the PAT-2Δcyto::GFP signal to monitor the internalization of MSpppaaa cell corpses by muscle cells. In the wild-type embryos, approximately 66.6% of MSpppaaa cell corpses were enclosed by the PAT-2Δcyto::GFP circle at the time when grinder formation had just finished, whereas no MSpppaaa cell corpses were enclosed by the PAT-2Δcyto::GFP circle in the pat-2(st567) embryos at this stage (Table 5 and Figure S8). Thus, the internalization of MSpppaaa cell corpses is defective in the pat-2(st567); Ppat-2pat-2Δcyto::gfp embryos. This result and the aforementioned data together indicate that the PAT-2 cytoplasmic domain is required for the muscle-mediated engulfment of apoptotic cells, but is dispensable for its subcellular localization in muscle cells and function during muscle development.
Expression of the PAT-2Δcyto::GFP fusion protein in the wild-type embryos resulted in increased numbers of cell corpses at the comma and 1.5-fold stages comparable to those seen in pat-2 mutants, but failed to induce the Pat phenotype (Table 3). In addition, PAT-2Δcyto::GFP also prolonged the duration time of the MSpppaaa cell corpses and interfered with the internalization of the MSpppaaa cell corpses (Table 5). For example, in the wild-type embryos expressing PAT-2Δcyto::GFP, 39% of the MSpppaaa cell corpses persisted at the time when grinder formation had just finished, and 66.6% of these cell corpses were internalized by engulfing muscle cells. However, in the control wild-type embryos, only 16.6% of the MSpppaaa cell corpses were present at this stage, and all the remaining cell corpses were internalized by engulfing muscle cells (Table 5). This result reinforces the specific function of the PAT-2 cytoplasmic domain in the engulfment of apoptotic cells. PAT-2Δcyto::GFP may compete with the endogenous PAT-2 for binding of PAT-3 or apoptotic cells, but fail to initiate signaling for the internalization of cell corpses.
PAT-2 functions through UIG-1 and CDC-42 to promote the engulfment process
During the engulfment of apoptotic cells, cytoskeletal rearrangement occurs as an engulfing cell extends pseudopods around an apoptotic cell [71]. Rho-family GTPases are important regulators of the actin cytoskeleton [72]. Although CED-10 (RAC1) GTPase is required for cell corpse engulfment [55], [72], our data showing that a ced-10 mutation enhanced the engulfment defect in the pat-2(RNAi) mutant (Table 4) and that ced-10 overexpression failed to rescue the engulfment defect of the pat-2(RNAi) mutant (Table 6) suggest that pat-2 may act independently of ced-10 in promoting the cytoskeletal rearrangement required for the internalization process.
Tab. 6. cdc-42 acts downstream of pat-2 during cell-corpse engulfment. 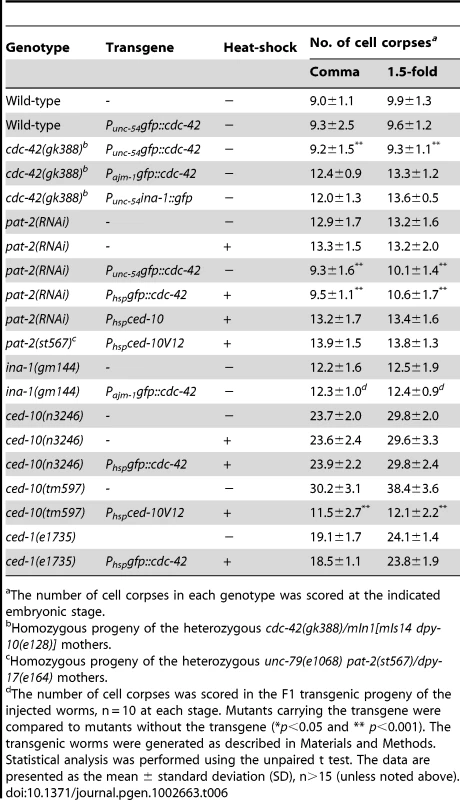
The number of cell corpses in each genotype was scored at the indicated embryonic stage. We then examined whether actin filament assembly occurred during the muscle-mediated internalization of apoptotic cells. MOESIN has been used in Drosophila and C. elegans to specifically mark the filamentous form of actin [73], [74]. Analysis of embryos expressing Punc-54moesin::gfp in which MOESIN::GFP was expressed in muscle cells using Punc-54 showed a MOESIN::GFP circle around apoptotic cells (Figure 2C), demonstrating that filamentous actin assembly occurs as an engulfing muscle cell extends pseudopods along the surface of an apoptotic cell.
A previous study showed that the Rho-family GTPase CDC-42 and UIG-1, a guanine nucleotide exchange factor (GEF) specific for CDC-42, function downstream of PAT-2/PAT-3 signaling for muscle assembly [75]. We next tested the involvement of uig-1 and cdc-42 in PAT-2-mediated cell corpse engulfment. The cdc-42(gk388) allele has a deletion that eliminates part of the 5′ regulatory sequence, the entire first exon, and part of the first intron of the cdc-42 gene (C. elegans Gene Knockout Consortium), while the uig-1(ok884) mutation deletes the region coding for the DH/PH domain, which has the GEF activity [76]. Both alleles are likely to be null. We found that uig-1 or cdc-42 embryos contained increased numbers of cell corpses at the comma and 1.5-fold stages (Table 4), similar to those observed in the pat-2 mutants. A four-dimensional DIC analysis of cell corpse persistence in the cdc-42(gk388) embryos revealed a cell-corpse engulfment defect. In the wild-type, 97% of cell corpses disappeared within 40 minutes and none lasted longer than 60 minutes (Figure 1C). However, in the cdc-42(gk388) mutants, only 40% of the cell corpses disappeared within 40 minutes and almost 40% persisted longer than 60 minutes (Figure 1C).
We next tested whether cdc-42 acted in the same pathway as pat-2 to promote apoptotic cell engulfment. We found that neither cdc-42 nor uig-1 increased the number of cell corpses in pat-2 mutants at the comma and 1.5-fold stages (Table 4), suggesting that cdc-42 and uig-1 both act in the same genetic pathway as pat-2. Like PAT-2::GFP, the GFP::CDC-42 fusion protein expressed using the transgene Punc-54gfp::cdc-42 was localized in muscle pseudopods around apoptotic cells (Figure 2D). In addition, the transgene Punc-54gfp::cdc-42 rescued the engulfment defect of the cdc-42(gk388) mutants, whereas Pajm-1gfp::cdc-42, which expressed GFP::CDC-42 in hypodermal cells, did not (Table 6). This result suggests that cdc-42 acts in muscle cells to mediate the engulfment of apoptotic cells. This muscle-specific function of cdc-42 is further supported by the observation that loss of cdc-42 results in a defect in the engulfment of apoptotic MSpppaaa cells (Table 5), but not C1, C2 or C3 cells (Table S2). When Punc-54gfp::cdc-42 and Ppat-2pat-2::mcherry were co-expressed, GFP::CDC-42 was co-localized with PAT-2::mCherry along the surface of apoptotic MSpppaaa cells (Figure 2N–2Q). Furthermore, the Punc-54gfp::cdc-42 transgene rescued the engulfment defect of the pat-2(RNAi) embryos (Table 6). These results support the model that cdc-42 functions downstream of pat-2 in the muscle-mediated engulfment of apoptotic cells.
CED-10 and CDC-42 are not exchangeable in function during cell-corpse engulfment
Our genetic data suggested that cdc-42 acts with pat-2 in the same pathway, whereas ced-10 functions in a partially redundant manner with pat-2 to mediate cell corpse engulfment (Table 4). We then tested whether the functions of ced-10 and cdc-42 in engulfment were exchangeable when ubiquitously overexpressed. We found that overexpression of ced-10 or constitutively active ced-10 V12 by the heat-shock promoter Phsp, which rescued the ced-10(n1993) or ced-10(tm597) mutant ([50], Table 6), failed to rescue the engulfment defect of the pat-2(RNAi) embryos (Table 6). Reciprocally, overexpression of cdc-42 by Phsp, which rescued the pat-2 mutant (Table 6), did not rescue the engulfment defect of the ced-10(n3246) mutants (Table 6). Moreover, overexpression of cdc-42 by the Phsp or Pajm-1 promoter also failed to rescue the defective engulfment of the ced-1(e1735) or ina-1(gm144) mutants, respectively (Table 6). These data suggest that the mechanisms by which cdc-42 and ced-10 mediate the actin-based cytoskeletal rearrangement during cell-corpse engulfment are distinct and that their functions are not exchangeable.
INA-1 and PAT-2 can partially substitute for each other in cell-corpse engulfment
As shown in Figure S6E, the INA-1 and PAT-2 extracellular domains can recognize the same apoptotic cells. We found that overexpression of pat-2 by the Pajm-1 promoter in hypodermal cells partially rescued the defective engulfment of the ina-1 mutants and, reciprocally, overexpression of ina-1 by the Punc-54 promoter in muscle cells also partially rescued the engulfment defect of the pat-2 mutants (Table 2). This result indicates that ina-1 and pat-2 can partially substitute for each other in cell corpse engulfment. Interestingly, overexpression of ina-1 by the Punc-54 promoter, which partially rescued the engulfment defect of the pat-2 mutants, did not rescue that of the cdc-42(gk388) mutants (Table 6) suggests that cdc-42 may be necessary for ina-1 overexpression-induced engulfment in muscle cells. If so, ina-1 overexpression in muscle cells may promiscuously activate cdc-42 signaling, which, in turn, leads to the engulfment of apoptotic cells. Nevertheless, ina-1 overexpression does not efficiently activate the phagocytosis machinery for cell corpse removal in muscle cells since only partial rescue of the pat-2 engulfment defect by ina-1 overexpression was observed. Similarly, overexpression of pat-2 dose not efficiently induce the phagocytosis machinery in hypodermal cells. This may, in part, explain why pat-2 and ina-1 preferentially function in different cell types for cell corpse removal, despite that they are expressed in both cell types.
In contrast, overexpression of ced-1 under the control of the Punc-54 promoter failed to rescue the defective engulfment of the pat-2 mutants (Table 2). In a reciprocal experiment, overexpression of pat-2 by the Pajm-1 promoter also failed to rescue the defective engulfment of the ced-1 mutants (Table 2). These results indicate that ced-1 and pat-2 functions are distinct and not exchangeable in cell corpse engulfment.
Discussion
The engulfment of apoptotic cells requires the recognition and subsequent internalization of apoptotic cells by the engulfing cells. Here, we showed that, in C. elegans, the integrin α subunit PAT-2 functions in both the recognition and internalization steps. pat-2 loss of function resulted in an increased number of embryonic cell corpses due to a defect in cell corpse removal. Our data showed that PAT-2 bound to apoptotic cells through its extracellular domain and initiated downstream signaling via its cytoplasmic domain. We characterized the pat-2 signaling pathway and identified a previously unassigned function of cdc-42 and uig-1 in cell corpse engulfment. We further showed that PAT-2 predominantly functioned in muscle cells to mediate the engulfment process. We propose that binding of PAT-2 to an apoptotic cell results in the recruitment of UIG-1 and the subsequent activation of CDC-42 GTPase, which, in turn, regulates cytoskeletal rearrangement as a muscle cell extends pseudopods around an apoptotic cell.
The finding that truncated PAT-2 lacking the cytoplasmic domain (PAT-2Δcyto::GFP ) fully rescued the Pat phenotype of the pat-2 mutants, but failed to rescue the engulfment defect (Table 3) argues against the possibility that the engulfment defect of the pat-2 mutant is a secondary effect caused by abnormality of muscle assembly or organization during embryogenesis. PAT-2 is co-localized with PAT-3 to the dense bodies and M-lines (Figure S2A), which are platforms serving as anchoring sites for signaling/adapter proteins in muscle attachment and organization [65]. Because the deletion of the cytoplasmic domain of PAT-2 affects neither its localization pattern (Figure S7) nor its function in muscle development (Table 3), muscle development probably requires only the transmembrane and extracellular domains, which are likely sufficient for the interaction of PAT-2 with PAT-3 and/or the extracellular matrix. Thus, the PAT-2/PAT-3 integrin likely mediates the intracellular signaling and/or adaptor protein binding through the cytoplasmic domain of PAT-3, but not that of PAT-2, for muscle development. In contrast, the requirement of the cytoplasmic domain of PAT-2 for cell-corpse removal suggests that this domain is important for signaling and/or serves as an anchorage site for adapter proteins during cell-corpse internalization.
The pat-2(st567) mutants show a weak engulfment phenotype compared with that of the ced-1 mutants during embryogenesis (Table 2). One possible explanation for the weak engulfment defect is that pat-2 predominantly functions in muscle cells, while only a small fraction of cell corpses (e.g. ∼20% of cell corpses in the 1.5-fold stage embryos, as shown in Table S1) are removed by muscle cells. Secondly, other engulfment receptor(s) may act redundantly with pat-2 in muscle-mediated engulfment. For instance, both PAT-2 and CED-1 are involved in the muscle-mediated internalization of MSpppaaa cells, despite that PAT-2 plays a bigger role than CED-1 (Table 5).
The PAT-2 extracellular region bound to apoptotic cells when PAT-2(ex) was fused to mCherry and overexpressed using the heat shock promoter (Figure 2M). Because exposed PS is detected on the surface of apoptotic MSpppaaa cells (Figure S1B), which are then removed by the PAT-2-mediated engulfment process (Table 5), PAT-2 might recognize exposed PS on apoptotic MSpppaaa cells. Mammalian integrins αvβ3 and αvβ5 have been shown to bind to apoptotic cells via the secreted bridging molecule MFG-E8 [25], [48]. In addition, integrin αvβ3 binds synergistically with the cell-surface protein CD36 to apoptotic cells through the bridging molecule thrombospondin, an extracellular matrix glycoprotein [50]. MFG-E8 binds to integrin αv through its RGD domain. On the basis of amino acid sequence, PAT-2 is more closely related to the RGD-binding integrins than to the laminin-binding integrins. However, C. elegans does not appear to have an MFG-E8 homolog. A screen for RGD-containing molecules may be helpful in testing the involvement of RGD-containing molecules in the binding of PAT-2(ex)::mCherry to apoptotic cells.
Like PAT-2, CED-1 and INA-1 are expressed in muscle and hypodermal cells [40], [46]. It is intriguing that different receptors are preferentially used in different cell types when they are all present in these cells. This may be, in part, because some receptors preferentially bind to a subset, but not all, apoptotic cells. For example, PAT-2(ex)::mCherry binds to MSpppaaa, but not C1, C2 or C3, cell corpses (Figure S6A, S6C and S6D) and PAT-2 is required for the removal of MSpppaaa, but not C1, C2 or C3, cell corpses. In contrast, INA-1 and CED-1 receptors recognize apoptotic C1, C2 and C3 cells and mediate the engulfment of these cells [42], [46]. However, some cell corpses can be recognized by multiple receptors. For example, PAT-2(ex)::mCherry and INA-1(N)::GFP are co-localized on some apoptotic cells (Figure S6E). Therefore, additional factor(s) other than the receptor-cell corpse interaction determines the cell-type specificity of engulfment receptors. The observation that CDC-42 preferentially functions in muscle cells but not hypodermal cells for engulfment suggests that the downstream molecule(s) are important for the cell-type specificity of engulfment receptors. CED-10 and CDC-42 are important for the actin-based cytoskeleton rearrangement [72], which occurs during engulfment of apoptotic cells ([55], Figure 2C). Hypodermal cells and muscle cells appear to have different requirement for CED-10 and CDC-42 in cell corpse removal, although the two GTP ases are expressed in both cell types [76]. Hypodermal cells and muscle cells may utilize different actin-based phagocytosis mechanisms or employ different ways to activate the phagocytosis machinery for cell corpse removal.
We showed that the muscle-mediated internalization of cell corpses took apporximately 6 mintues (Figure 3A). Similarly, the internalization of the C3 cell corpse by the hypodermal cell ABplaapppp took about 6 minutes [42], [46]. However, previous studies by Wang et al. and Zou et al. [43], [53] showed that the time for the CED-1::GFP-mediated internalization of apoptotic cells may take about 18 or 25 minutes, respectively, although the identities of the engulfing cells are unclear. Therefore, the time necessary for the entire internalization process to occur from initiation to completion appears different for different engulfing cells. Nonetheless, at least some engulfment processes mediated by the muscle cell and hypodermal cell proceeds with similar kinetics.
Recently, conditional deletion of integrin αv in the mouse immune system revealed that this protein is essential for the engulfment of apoptotic cells by gut-associated macrophages and dendritic cells [4]. In addition, mice lacking MFG-E8, which mediates apoptotic cell clearance through integrin αv, are defective in the removal of apoptotic B cells by tingible body macrophages in the spleen germinal centers [77]. However, little was previously known about whether integrin α or other engulfment receptors were involved in apoptotic cell removal mediated by amateur phagocytes, such as muscle cells. C. elegans provides a good system for studying the amateur phagocyte-mediated engulfment of apoptotic cells, as it does not have professional phagocytes. Our data showed that PAT-2 acts in muscle cells and transduces the engulfment signal through a novel signaling pathway for apoptotic cell removal. Recently, a mouse lacking ELMO1 showed a defect in Sertoli cell-mediated engulfment of apoptotic germ cells, but no engulfment defect was detected in macrophages or fibroblasts [31]. This result, together with our data, suggest that, like professional phagocytes [78], amateur phagocytes in different tissues utilize different sets of engulfment receptors and signaling molecules for apoptotic cell engulfment at the whole organism level.
Materials and Methods
Strains and alleles
The N2 Bristol strain was used as the reference wild-type strain. All strains were maintained on nematode growth medium (NGM) plates with Escherichia coli OP50 as the food source at 20°C unless otherwise noted [79]. The following mutations were used: linkage group I (LGI), ced-1(e1735), ced-12 (n3261, tp2); LGII, cdc-42(gk388), mIn1[mIs14 dpy-10(e128)]; LGIII, abi-1(ok640), ced-4(n1162), ced-6(n1813), ced-7(n1892, n1996), unc-79(e1068), pat-2(st567), dpy-17(e164), pat-3(st564), qC1 dpy-19(e1259) glp-1(q339); LGIV, ced-2(n1994), ced-3(n717), ced-5(n1812), ced-10(n1993, n3246, tm597); LGIV, uig-1(ok884). dpy-17(e164) was used to balance unc-79(e1068) pat-2(st567). The homozygous unc-79(e1068) pat-2(st567) mutants were also maintained using the extrachromosomal Ppat-2pat-2::gfp or Ppat-2pat-2::mcherry transgene. In either of the transgenic lines, the unc-79(e1068) pat-2(st567) mutant embryos that lost the transgene were both Pat and Ced. The numbers of cell corpses in the unc-79(e1068) mutant at the comma and 1.5-fold stages were similar to those of the wild-type at the same stage, indicating that unc-79(e1068) does not affect apoptosis. qC1 dpy-19(e1259) glp-1(q339) was used to balance pat-3(st564). mIn1 was used to balance cdc-42(gk388). The pat-2(st567) allele has a G441D alternation in the extracellular domain. The information of the transgenic strains used in this work is listed in Table S4.
Plasmid construction
Three vectors were used to generate constructs expressing fluorescent proteins, the gfp vector pPD95.75, the mcherry vector pYW806, and the mrfp vector pYW897. pYW806 and pYW897 were respectively generated by replacing gfp in pPD95.75 with mcherry or mrfp via the KpnI/EcoRI sites. To generate Ppat-2pat-2::gfp (pYW903) or Ppat-2pat-2::mCherry (pYW950), the 10 kb fragment containing the 4 kb upstream sequence and the full-length pat-2 coding region without the stop codon was PCR-amplified from the cosmid F54F2 (Sanger Institute, Cambridge, United Kingdom) using the primers TCCCCCCGGGTTTATGACTCACAGAC and GGGTACCGATGCATTTGTC CGTGACGT, and cloned into pPD95.75 or pYW806, respectively, via the KpnI site. The full-length pat-2 cDNA construct (pYW901) was generated by inserting into yk616b4 (Dr. Yuji Kohara) via the PstI site a 0.6 kb pat-2 cDNA which was amplified by RT-PCR using the primers AACTGCAGATGCGAGAGGGTAGTTTTCC and GATTCTTCTTTCCTGGAACTGCAGC. To generate Ppat-2nls::gfp (pYW949) or Ppat-2gfp (pYW903), the 4 kb upstream sequence of pat-2 was first amplified by PCR from the cosmid F54F2 using the primers TCCCCCCGGGTTTATGACTCACAGAC and TCCCCCCGGGATCTACTGG AAATTTG and inserted into pPD95.67 or pPD95.75, respectively. The Punc-54gfp (pYW899) or Punc-54mcherry (pYW900) construct was generated by inserting the 1 kb HindIII/KpnI fragment of pPD30.38 containing Punc-54 into pPD95.75 or pYW806, respectively. The Pajm-1gfp (pYW902) was generated by inserting into pPD95.75 via the SalI/BamHI sites a 5.5 kb Pajm-1 fragment which was amplified by PCR from pBR980 [69] using the primers CGTCGACCGATTTGACCGTTCGATAAG and CGGATCCTCGTCGGTA GTACTCGTCC. Pced-1gfp (pYW898) was generated by inserting into pPD95.75 via the SphI site a 5 kb Pced-1 fragment which was PCR-amplified from genomic DNA using the primers GGCATGCATACCTCCTGATATG GGGTGA and GCATGCTTGC GGCTGCAAAAAAACAGGG. Pced-1ced-1::gfp (pYW904) was generated by three-piece ligation. The 6 kb PCR-amplified fragment from Pced-1ced-1::gfp [40] using the primers AGGTACCATGCGTCTCATTCTCCTTGTGC and GGTCGA CGTGATTGTTCAGATGA and the 2.4 kb fragment PCR-amplified from genomic DNA using the primers CGTCGACCTCTATTAGAAGAGCATGACG and TGGTACCGAGGTGTACAAATTGTCCTGAGC were inserted into pYW898 via the SalI and KpnI sites. Pced-1ced-1::gfp fully rescued the engulfment defect of the ced-1(e1735) mutant (data not shown). To generate pYW901 containing pat-2 cDNA without the stop codon, pat-2 cDNA was PCR-amplified from the full-length pat-2 cDNA clone using the primers CGGTACCATGCGAGAGGGTAGTTTTCC and GGGTACCGATAGCATTTGTC CGTGACGT and inserted into the pGEM-T Easy vector (Promega) via the KpnI site. To generate Punc-54pat-2::gfp (pYW913) or Punc-54pat-2::mCherry (pYW916), the 3.7 kb KpnI pat-2 fragment from pYW901 was inserted into pYW899 or pYW900, respectively, via the KpnI site. To generate Punc-54ced-1::gfp (pYW905), the 8.5 kb KpnI ced-1 fragment from pYW904 was inserted into pYW899 via the KpnI site. To generate Punc-54 ced-1::mrfp (pYW941), a 8.6 kb SpeI/BamHI fragment, containing Punc-54 and the first 4.9 Kb of ced-1, and a 3.6 kb BamHI/KpnI fragment, corresponding to the rest ced-1 sequence, of pYW905 were fused to a 1.5 kb KpnI/SpeI fragment, containing the mrfp sequence, of pYW897 by three-piece-ligation. To generate Punc-54moesin::gfp (pYW940), the 0.6 kb SmaI/NcoI fragment containing the moesin actin-binding sequence was inserted into pYW899 via the NheI/NcoI sites. The moesin plasmid was a gift from Dr. Fabio Piano [75]. To generate Punc-54 gfp::cdc-42 (pYW906), two plasmids gfp_Ntag_TA and cdc-42_Ntag_TA were generated first. To generate the gfp_Ntag_TA plasmid, PCR-amplified gfp, using oligonucleotides CGGTACCATGAGTAAAGGAG AAGAACT and GTCTAGATTTGTATAGTTCATCCATGCC as primers and pPD95.75 as template was inserted to the vector pGEM T-Easy. To generate the cdc-42_Ntag_TA plasmid, PCR-amplified cdc-42 from k1101h01(Y. Kohara) using primers GTCTAGAATGCAGACGATCAAGTGCG and CGTTAACCTAGAGAATATTGCACTTCTTC was inserted into the pGEM T-Easy vector. The 1 kb HindIII/KpnI fragment of pPD30.38 containing Punc-54, the KpnI /XbaI gfp fragment from the gfp_Ntag_TA plasmid, and the XbaI/EcoR1 cdc-42 fragment from the cdc-42_Ntag_TA plasmid were inserted to the pPD95.75 vector previously digested with HindIII and EcoRI in a four-piece-ligation reaction to generate pYW906. To generate Pajm-1pat-2::gfp (pYW948) or Pajm-1ced-1::gfp (pYW914), the 3.7 kb KpnI pat-2 sequence from pYW901 or the 8.5 kb KpnI ced-1 sequence from pYW904 was inserted, respectively, into pYW902. To generate Pced-1pat-2::gfp (pYW942), the 3.7 kb KpnI pat-2 sequence from pYW901 was inserted into pYW898 via the KpnI site. To generate Ppat-2pat-2Δcyto::gfp (pYW964), pat-2Δcyto was PCR-amplified from pYW901 using the primers CGGTACCATGCGA GAGGGTAGTTTTCC and TTGGT ACCGTC CTATAGAATAATGCAA and cloned into pYW903 via the KpnI site. To generate Pemphtypepat-2(ex)::mcherry (pYW917), the DNA fragment corresponding to pat-2(ex) was PCR-amplified from pYW901 using the primers CGGTACCATGCGAGAGG GTAGTTTTCC and CGGTACCAGATCTCTTCC TTCTTCAGA and cloned into pYW900 via the KpnI site. To generate Phsppat-2(ex)::mcherry (pYW966), the 4.2 kb NheI/PvuI fragment of the Punc-54 pat-2(ex)::mCherry plasmid containing pat-2(ex)::mCherry was inserted into pPD49.78 via the NheI/PvuI sites. To generate Phspgfp::cdc-42 (pYW959), the 1.5 kb KpnI/HpaI fragment of pYW906 corresponding to gfp::cdc-42 was inserted into pPD49.78 and pPD49.83 (different tissue specificity) via the KpnI/EcoRV sites. To generate the Punc-54ina-1::gfp plasmid, Punc-54 was PCR-amplified using pYW913 as template and oligonucleotides GCATCCGCCAAGCTTGTCTTCTTC and GGATCCGGTACCGT CGACGCTAC as primers and used to replace the Pced-1 region of Pced-1ina-1::gfp via the SphI/BamHI sites. To generate the Ppat-3pat-3::gfp (pYW1091) plasmid, the 10.2 kb pat-3 genomic DNA was amplified by PCR and subsequently cloned into the pPD95.75 vector via the XmaI/KpnI sites. To generate the Punc-54myri::mrfp (pYW1092) construct, the myri::mrfp cDNA of Punc-86myri::gfp (from C. Bargmann) was inserted into the Punc-54mrfp vector via the KpnI site. To generate the Pajm-1gfp::cdc-42 (pYW1093) plasmid, the gfp region of the pPD95.75 plasmid was replaced by gfp::cdc-42 from the Phspgfp::cdc-42 construct via the KpnI site, and the resulting construct was then digested with BamHI and SalI and subsequently inserted with the BamHI and SalI fragment containing the Pajm-1 promoter from pYW902.
RNAi experiments
To generate the pat-2 RNAi clone, the 1.3 kb PstI/HindIII fragment of pat-2 cDNA was inserted into the pPD129.36 vector. The pat-3 RNAi plasmid was obtained from the J. Ahringer RNAi library. All RNAi experiments were carried out using a bacterial feeding protocol [80]. In brief, L4 larvae were laid out on control plates (pPD129.36) or the indicated RNAi plates and cultured at 20°C for 48 h, then the F1 embryos were picked for phenotypic analysis.
Transgenic animals
Transgenic animals were generated by microinjection of the indicated plasmid(s) (3–50 ng/µL), using the pRF4[rol-6 (su1006)], pTG96[sur-5::gfp] or Psur-5rfp plasmids (50 ng/µL) as coinjection markers [81], [82]. The injection procedure was performed as described previously [8]. The resulting transgenes and genetic backgrounds of the strains were listed in Table S4. Phspced-10V12 was injected with the coinjection marker pTG96[sur-5::gfp] to unc-79(e1068) pat-2(st567)/dpy-17(e164), and no rescue of the Pat phenotype was observed. To score cell corpses, transgenic embryos carrying the transgene Phspced-10V12 and pTG96[sur-5::gfp] were scored and only the data for those which later exhibited the Pat phenotype were used.
Microscopy
Embryos were mounted on a 4% agar pad in M9 buffer in the presence (four-fold stage embryos) or absence (comma and 1.5-fold stage embryos) of 30 mM sodium azide at 20°C. Cell corpses were analyzed using DIC microscopy, as previously described [46]. A Zeiss Axioplan 2 microscope equipped with a digital camera (AxioCam; Carl Zeiss, Inc.) and 4.7 AxioVision imaging software was used. To obtain the cell corpse data of homozygous pat-2(st567) embryos derived from the heterozygous unc-79(e1068) pat-2(st567)/dpy-17(e164) mothers, embryos at the indicated developmental stages were analyzed and only the data for those which later exhibited the Pat phenotype were used. To obtain the cell corpse data of homozygous pat-2(st567) embryos derived from unc-79(e1068) pat-2(st567) mothers carrying either Ppat-2pat-2::gfp or Ppat-2pat-2::mcherry transgene, non-fluorescent embryos at the indicated developmental stages were analyzed. To obtain the cell corpse data of homozygous cdc-42(gk388) embryos derived from the heterozygous cdc-42(gk388)/mIn1[mIs14 dpy-10(e128)] mothers, GFP-negative embryos at the indicated developmental stages were analyzed. To analyze cell death events during embryogenesis, wild-type or mutant embryos were filmed during the period 200–460 min after the first cleavage and the time point at which each cell corpse appeared was noted and was reported relative to the first cell death for comparison between the wild-type and mutants. About 40–50 serial Z-sections were recorded at 0.4 µm intervals every 1 min. To measure the duration of cell corpses in the wild-type and mutants, cell corpses appearing between 360 to 410 min after the first cleavage during embryogenesis were followed, and about 50–60 serial Z-sections were recorded at 0.3 or 0.4 µm intervals every 1 min. To monitor the muscle-mediated internalization of apoptotic cells, the fluorescence images of wild-type or mutant embryos carrying the Punc-54myri::mrfp transgene were recorded using the DeltaVision microscope (GE Healthcare company) equipped with a digital camera (Photometrics Cascade II 512 EMCCD) at 1 (for wild-type) or 3 (for mutant)-minute intervals for about 120 minutes.
Heat shock experiments
For the heat shock rescue experiments, transgenic embryos were subjected to heat shock at 33°C for 30 min and transferred to 20°C to recover for 2 hours, then cell corpses in embryos at the indicated stages were counted. To test the binding of PAT-2(ex)::mCherry to apoptotic cells and its effect on cell-corpse engulfment when overexpressed, embryos carrying the transgene Phsppat-2(ex)::mcherry or embryos carrying the transgenes Phsppat-2(ex)::mcherry and Phspina-1(N)::gfp were subjected to heat shock at 33°C for 60 min and transferred to 20°C to recover for 4–5 hours, then embryos were examined using fluorescence microscopy (for mCherry and/or INA-1(N)::GFP signal) or DIC microscopy (for cell corpses). To overexpress GFP::CDC-42, CED-10V12 or Annexin V::mRFP by heat-shock, the embryos carrying the respective transgene were subjected to heat shock at 33°C for 30 min and transferred to 20°C to recover for 2 hours, then embryos were examined using fluorescence microscopy (mCherry signal) or DIC microscopy (cell corpses).
Supporting Information
Zdroje
1. BaehreckeEH 2002 How death shapes life during development. Nat Rev Mol Cell Biol 3 779 787
2. ReddienPWHorvitzHR 2004 The engulfment process of programmed cell death in caenorhabditis elegans. Annu Rev Cell Dev Biol 20 193 221
3. ErwigLPHensonPM 2008 Clearance of apoptotic cells by phagocytes. Cell Death Differ 15 243 250
4. Lacy-HulbertASmithAMTissireHBarryMCrowleyD 2007 Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A 104 15823 15828
5. ElliottMRRavichandranKS 2010 Clearance of apoptotic cells: implications in health and disease. J Cell Biol 189 1059 1070
6. NagataSHanayamaRKawaneK 2010 Autoimmunity and the clearance of dead cells. Cell 140 619 630
7. Darland-RansomMWangXSunCLMapesJGengyo-AndoK 2008 Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science 320 528 531
8. GalvinBDKimSHorvitzHR 2008 Caenorhabditis elegans genes required for the engulfment of apoptotic corpses function in the cytotoxic cell deaths induced by mutations in lin-24 and lin-33. Genetics 179 403 417
9. KaoAWEisenhutRJMartensLHNakamuraAHuangA 2011 A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Proc Natl Acad Sci U S A 108 4441 4446
10. AbramsJMWhiteKFesslerLIStellerH 1993 Programmed cell death during Drosophila embryogenesis. Development 117 29 43
11. BennettMRGibsonDFSchwartzSMTaitJF 1995 Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phosphatidylserine. Circ Res 77 1136 1142
12. DiniL 1998 Endothelial liver cell recognition of apoptotic peripheral blood lymphocytes. Biochem Soc Trans 26 635 639
13. WalshGMSextonDWBlaylockMGConveryCM 1999 Resting and cytokine-stimulated human small airway epithelial cells recognize and engulf apoptotic eosinophils. Blood 94 2827 2835
14. SavillJFadokV 2000 Corpse clearance defines the meaning of cell death. Nature 407 784 788
15. ParkDTosello-TrampontACElliottMRLuMHaneyLB 2007 BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450 430 434
16. ParkSYJungMYLeeSJKangKBGratchevA 2009 Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J Cell Sci 122 3365 3373
17. ParkSYJungMYKimHJLeeSJKimSY 2008 Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ 15 192 201
18. KobayashiNKarisolaPPena-CruzVDorfmanDMJinushiM 2007 TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27 927 940
19. NakayamaMAkibaHTakedaKKojimaYHashiguchiM 2009 Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 113 3821 3830
20. SavillJDransfieldIHoggNHaslettC 1990 Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature 343 170 173
21. AlbertMLPearceSFFranciscoLMSauterBRoyP 1998 Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 188 1359 1368
22. ScottRSMcMahonEJPopSMReapEACaricchioR 2001 Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411 207 211
23. FadokVAVoelkerDRCampbellPACohenJJBrattonDL 1992 Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148 2207 2216
24. FadeelBXueD 2009 The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit Rev Biochem Mol Biol 44 264 277
25. MiyanishiMTadaKKoikeMUchiyamaYKitamuraT 2007 Identification of Tim4 as a phosphatidylserine receptor. Nature 450 435 439
26. ParkSYKimSYJungMYBaeDJKimIS 2008 Epidermal growth factor-like domain repeat of stabilin-2 recognizes phosphatidylserine during cell corpse clearance. Mol Cell Biol 28 5288 5298
27. NagataKOhashiKNakanoTAritaHZongC 1996 Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 271 30022 30027
28. AkakuraSSinghSSpataroMAkakuraRKimJI 2004 The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res 292 403 416
29. AlbertMLKimJIBirgeRB 2000 alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol 2 899 905
30. WuYSinghSGeorgescuMMBirgeRB 2005 A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci 118 539 553
31. ElliottMRZhengSParkDWoodsonRIReardonMA 2010 Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature 467 333 337
32. StuartLMTakahashiKShiLSavillJEzekowitzRA 2005 Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol 174 3220 3226
33. KodamaTReddyPKishimotoCKriegerM 1988 Purification and characterization of a bovine acetyl low density lipoprotein receptor. Proc Natl Acad Sci U S A 85 9238 9242
34. GregoryCDDevittAMoffattO 1998 Roles of ICAM-3 and CD14 in the recognition and phagocytosis of apoptotic cells by macrophages. Biochem Soc Trans 26 644 649
35. SulstonJEHorvitzHR 1977 Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56 110 156
36. KimbleJHirshD 1979 The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol 70 396 417
37. SulstonJESchierenbergEWhiteJGThomsonJN 1983 The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100 64 119
38. RobertsonAThomsonN 1982 Morphology of programmed cell death in the ventral nerve cord of Caenorhabditis elegans larvae. J Embryol Exp Morphol 67 89 100
39. WuYCHorvitzHR 1998 The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell 93 951 960
40. ZhouZHartwiegEHorvitzHR 2001 CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104 43 56
41. LiuQAHengartnerMO 1999 Human CED-6 encodes a functional homologue of the Caenorhabditis elegans engulfment protein CED-6. Curr Biol 9 1347 1350
42. YuXOderaSChuangCHLuNZhouZ 2006 C. elegans Dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev Cell 10 743 757
43. WangXLiWZhaoDLiuBShiY 2010 Caenorhabditis elegans transthyretin-like protein TTR-52 mediates recognition of apoptotic cells by the CED-1 phagocyte receptor. Nat Cell Biol 12 655 664
44. WangXWuYCFadokVALeeMCGengyo-AndoK 2003 Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science 302 1563 1566
45. CabelloJNeukommLJGunesdoganUBurkartKCharetteSJ 2010 The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. PLoS Biol 8 e1000297 doi:10.1371/journal.pbio.1000297
46. HsuTYWuYC 2010 Engulfment of apoptotic cells in C. elegans is mediated by integrin alpha/SRC signaling. Curr Biol 20 477 486
47. WuYCHorvitzHR 1998 C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 392 501 504
48. ReddienPWHorvitzHR 2000 CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol 2 131 136
49. GumiennyTLBrugneraETosello-TrampontACKinchenJMHaneyLB 2001 CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 107 27 41
50. WuYCTsaiMCChengLCChouCJWengNY 2001 C. elegans CED-12 acts in the conserved CrkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev Cell 1 491 502
51. ZhouZCaronEHartwiegEHallAHorvitzHR 2001 The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev Cell 1 477 489
52. BrugneraEHaneyLGrimsleyCLuMWalkSF 2002 Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol 4 574 582
53. ZouWLuQZhaoDLiWMapesJ 2009 Caenorhabditis elegans myotubularin MTM-1 negatively regulates the engulfment of apoptotic cells. PLoS Genet 5 e1000679 doi:10.1371/journal.pgen.1000679
54. NeukommLJNicotASKinchenJMAlmendingerJPintoSM 2011 The phosphoinositide phosphatase MTM-1 regulates apoptotic cell corpse clearance through CED-5-CED-12 in C. elegans. Development 138 2003 2014
55. KinchenJMCabelloJKlingeleDWongKFeichtingerR 2005 Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 434 93 99
56. NeukommLJFreiAPCabelloJKinchenJMZaidel-BarR 2011 Loss of the RhoGAP SRGP-1 promotes the clearance of dead and injured cells in Caenorhabditis elegans. Nat Cell Biol 13 79 86
57. HurwitzMEVanderzalmPJBloomLGoldmanJGarrigaG 2009 Abl kinase inhibits the engulfment of apoptotic [corrected] cells in Caenorhabditis elegans. PLoS Biol 7 e99 doi:10.1371/journal.pbio.1000099
58. HynesRO 1987 Integrins: a family of cell surface receptors. Cell 48 549 554
59. HynesRO 1992 Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69 11 25
60. WilliamsBDWaterstonRH 1994 Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. J Cell Biol 124 475 490
61. GettnerSNKenyonCReichardtLF 1995 Characterization of beta pat-3 heterodimers, a family of essential integrin receptors in C. elegans. J Cell Biol 129 1127 1141
62. BaumPDGarrigaG 1997 Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron 19 51 62
63. HreskoMCWilliamsBDWaterstonRH 1994 Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. J Cell Biol 124 491 506
64. WilliamsBDWaterstonRH 1994 Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. J Cell Biol 124 4 475 90
65. MoermanDGWilliamsBD Sarcomere assembly in C. elegans muscle (January 16, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.81.1, http://www.wormbook.org
66. DixonSJAlexanderMFernandesRRickerNRoyPJ 2006 FGF negatively regulates muscle membrane extension in Caenorhabditis elegans. Development 133 1263 1275
67. MeighanCMSchwarzbauerJE 2007 Control of C. elegans hermaphrodite gonad size and shape by vab-3/Pax6-mediated regulation of integrin receptors. Genes Dev 21 1615 1620
68. EllisHMHorvitzHR 1986 Genetic control of programmed cell death in the nematode C. elegans. Cell 44 817 829
69. KoppenMSimskeJSSimsPAFiresteinBLHallDH 2001 Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol 3 983 991
70. OkkemaPGHarrisonSWPlungerVAryanaAFireA 1993 Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135 385 404
71. MayRCMacheskyLM 2001 Phagocytosis and the actin cytoskeleton. J Cell Sci 114 1061 1077
72. HallANobesCD 2000 Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci 355 965 970
73. EdwardsKADemskyMMontagueRAWeymouthNKiehartDP 1997 GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev Biol 191 103 117
74. MotegiFVelardeNVPianoFSugimotoA 2006 Two phases of astral microtubule activity during cytokinesis in C. elegans embryos. Dev Cell 10 509 520
75. HikitaTQadotaHTsuboiDTayaSMoermanDG 2005 Identification of a novel Cdc42 GEF that is localized to the PAT-3-mediated adhesive structure. Biochem Biophys Res Commun 335 139 145
76. LundquistEAReddienPWHartwiegEHorvitzHRBargmannCI 2001 Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development 128 22 4475 88
77. HanayamaRTanakaMMiyasakaKAozasaKKoikeM 2004 Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304 1147 1150
78. FadokVASavillJSHaslettCBrattonDLDohertyDE 1992 Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol 149 4029 4035
79. BrennerS 1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
80. FraserAGKamathRSZipperlenPMartinez-CamposMSohrmannM 2000 Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408 325 330
81. MelloCCKramerJMStinchcombDAmbrosV 1991 Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10 3959 3970
82. GuTOritaSHanM 1998 Caenorhabditis elegans SUR-5, a novel but conserved protein, negatively regulates LET-60 Ras activity during vulval induction. Mol Cell Biol 18 4556 4564
Štítky
Genetika Reprodukční medicína
Článek Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication inČlánek Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the TranscriptomeČlánek Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing BoneČlánek Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 5- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- Slowing Replication in Preparation for Reduction
- Chromosome Pairing: A Hidden Treasure No More
- Loss of Imprinting Differentially Affects REM/NREM Sleep and Cognition in Mice
- Six Novel Susceptibility Loci for Early-Onset Androgenetic Alopecia and Their Unexpected Association with Common Diseases
- Regulation by the Noncoding RNA
- UDP-Galactose 4′-Epimerase Activities toward UDP-Gal and UDP-GalNAc Play Different Roles in the Development of
- Deletion of PTH Rescues Skeletal Abnormalities and High Osteopontin Levels in Mice
- Karyotypic Determinants of Chromosome Instability in Aneuploid Budding Yeast
- Genome-Wide Copy Number Analysis Uncovers a New HSCR Gene:
- MicroRNA-277 Modulates the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication in
- Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the Transcriptome
- Scientist Citizen: An Interview with Bruce Alberts
- YY1 Regulates Melanocyte Development and Function by Cooperating with MITF
- Congenital Heart Disease–Causing Gata4 Mutation Displays Functional Deficits
- Recombination Drives Vertebrate Genome Contraction
- KATNAL1 Regulation of Sertoli Cell Microtubule Dynamics Is Essential for Spermiogenesis and Male Fertility
- Re-Patterning Sleep Architecture in through Gustatory Perception and Nutritional Quality
- Using Whole-Genome Sequence Data to Predict Quantitative Trait Phenotypes in
- Genome-Wide Analysis of GLD-1–Mediated mRNA Regulation Suggests a Role in mRNA Storage
- Meiotic Chromosome Pairing Is Promoted by Telomere-Led Chromosome Movements Independent of Bouquet Formation
- LINT, a Novel dL(3)mbt-Containing Complex, Represses Malignant Brain Tumour Signature Genes
- The H3K27 Demethylase UTX-1 Is Essential for Normal Development, Independent of Its Enzymatic Activity
- Suppresses Senescence Programs and Thereby Accelerates and Maintains Mutant -Induced Lung Tumorigenesis
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
- Identification of Genes That Promote or Antagonize Somatic Homolog Pairing Using a High-Throughput FISH–Based Screen
- Principles of Carbon Catabolite Repression in the Rice Blast Fungus: Tps1, Nmr1-3, and a MATE–Family Pump Regulate Glucose Metabolism during Infection
- Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells in
- Histone H3 Localizes to the Centromeric DNA in Budding Yeast
- Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes
- Hypersensitive to Red and Blue 1 and Its Modification by Protein Phosphatase 7 Are Implicated in the Control of Arabidopsis Stomatal Aperture
- Extent, Causes, and Consequences of Small RNA Expression Variation in Human Adipose Tissue
- TBC-8, a Putative RAB-2 GAP, Regulates Dense Core Vesicle Maturation in
- Regulating Repression: Roles for the Sir4 N-Terminus in Linker DNA Protection and Stabilization of Epigenetic States
- Common Genetic Determinants of Intraocular Pressure and Primary Open-Angle Glaucoma
- Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing Bone
- Fitness Landscape Transformation through a Single Amino Acid Change in the Rho Terminator
- Repeated, Selection-Driven Genome Reduction of Accessory Genes in Experimental Populations
- Allelic Variation and Differential Expression of the mSIN3A Histone Deacetylase Complex Gene Promote Mammary Tumor Growth and Metastasis
- DNA Demethylation and USF Regulate the Meiosis-Specific Expression of the Mouse
- Knowledge-Driven Analysis Identifies a Gene–Gene Interaction Affecting High-Density Lipoprotein Cholesterol Levels in Multi-Ethnic Populations
- A Duplication CNV That Conveys Traits Reciprocal to Metabolic Syndrome and Protects against Diet-Induced Obesity in Mice and Men
- EMT Inducers Catalyze Malignant Transformation of Mammary Epithelial Cells and Drive Tumorigenesis towards Claudin-Low Tumors in Transgenic Mice
- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association for Abdominal Subcutaneous and Visceral Adipose Reveals a Novel Locus for Visceral Fat in Women
- Stratifying Type 2 Diabetes Cases by BMI Identifies Genetic Risk Variants in and Enrichment for Risk Variants in Lean Compared to Obese Cases
- New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties
- Activated Cdc42 Kinase Has an Anti-Apoptotic Function
- The Region Is Critical for Birth Defects and Electrocardiographic Dysfunctions Observed in a Down Syndrome Mouse Model
- COP9 Signalosome Integrity Plays Major Roles for Hyphal Growth, Conidial Development, and Circadian Function
- Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest
- Psip1/Ledgf p52 Binds Methylated Histone H3K36 and Splicing Factors and Contributes to the Regulation of Alternative Splicing
- The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice
- Target Gene Analysis by Microarrays and Chromatin Immunoprecipitation Identifies HEY Proteins as Highly Redundant bHLH Repressors
- Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
- ELK1 Uses Different DNA Binding Modes to Regulate Functionally Distinct Classes of Target Genes
- Histone H1 Depletion Impairs Embryonic Stem Cell Differentiation
- IDN2 and Its Paralogs Form a Complex Required for RNA–Directed DNA Methylation
- Separation of DNA Replication from the Assembly of Break-Competent Meiotic Chromosomes
- Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- Slowing Replication in Preparation for Reduction
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



