-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
Katanin is an evolutionarily conserved microtubule-severing complex implicated in multiple aspects of microtubule dynamics. Katanin consists of a p60 severing enzyme and a p80 regulatory subunit. The p80 subunit is thought to regulate complex targeting and severing activity, but its precise role remains elusive. In lower-order species, the katanin complex has been shown to modulate mitotic and female meiotic spindle dynamics and flagella development. The in vivo function of katanin p80 in mammals is unknown. Here we show that katanin p80 is essential for male fertility. Specifically, through an analysis of a mouse loss-of-function allele (the Taily line), we demonstrate that katanin p80, most likely in association with p60, has an essential role in male meiotic spindle assembly and dissolution and the removal of midbody microtubules and, thus, cytokinesis. Katanin p80 also controls the formation, function, and dissolution of a microtubule structure intimately involved in defining sperm head shaping and sperm tail formation, the manchette, and plays a role in the formation of axoneme microtubules. Perturbed katanin p80 function, as evidenced in the Taily mouse, results in male sterility characterized by decreased sperm production, sperm with abnormal head shape, and a virtual absence of progressive motility. Collectively these data demonstrate that katanin p80 serves an essential and evolutionarily conserved role in several aspects of male germ cell development.
Published in the journal: . PLoS Genet 8(5): e32767. doi:10.1371/journal.pgen.1002698
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002698Summary
Katanin is an evolutionarily conserved microtubule-severing complex implicated in multiple aspects of microtubule dynamics. Katanin consists of a p60 severing enzyme and a p80 regulatory subunit. The p80 subunit is thought to regulate complex targeting and severing activity, but its precise role remains elusive. In lower-order species, the katanin complex has been shown to modulate mitotic and female meiotic spindle dynamics and flagella development. The in vivo function of katanin p80 in mammals is unknown. Here we show that katanin p80 is essential for male fertility. Specifically, through an analysis of a mouse loss-of-function allele (the Taily line), we demonstrate that katanin p80, most likely in association with p60, has an essential role in male meiotic spindle assembly and dissolution and the removal of midbody microtubules and, thus, cytokinesis. Katanin p80 also controls the formation, function, and dissolution of a microtubule structure intimately involved in defining sperm head shaping and sperm tail formation, the manchette, and plays a role in the formation of axoneme microtubules. Perturbed katanin p80 function, as evidenced in the Taily mouse, results in male sterility characterized by decreased sperm production, sperm with abnormal head shape, and a virtual absence of progressive motility. Collectively these data demonstrate that katanin p80 serves an essential and evolutionarily conserved role in several aspects of male germ cell development.
Introduction
The regulation of microtubule dynamics is an essential requirement for all cells and in many aspects of their daily function. The ability to precisely regulate microtubule number, the assembly of networks, and the rate of microtubule assembly and disassembly underpins cellular processes including division, differentiation and migration. Male gamete development in particular relies upon the co-ordinated development and rapid remodelling of complex microtubule structures, such as the mitotic (spermatogonia) and meiotic (spermatocyte) spindle; flagella formation needed for sperm motility; and the manchette, which determines sperm head shape and contributes to tail structure. Approximately one in 20 men of reproductive age is sub-fertile or sterile, of which 60% of cases are due to intrinsic defects in spermatogenesis. This heterogeneous disorder manifests clinically as diminished sperm number, or abnormal motility or morphology, or commonly combinations thereof, in the ejaculate [1]. All of these clinical presentations may be underpinned by defective microtubule dynamics.
Microtubule severing is emerging as a key regulator of microtubule dynamics [2], [3], [4], [5], . The most well characterized microtubule severing enzyme is the katanin complex [8], the severing function of which is carried out by an ATPase enzymatic subunit, named p60, encoded by the Katna1 gene. Katanin p60 is a member of the AAA domain (ATPases Associated with diverse cellular Activities) protein family. Upon binding ATP, katanin p60 oligomerizes onto the tail of an individual tubulin subunit within a microtubule to form a 14–16 nm ring structure [9]. ATP hydrolysis confers a conformational change in the oligomer and ‘tugs’ upon the tail of the tubulin subunit. This leads to destabilization, and ultimately severing, of the polymer [2]. Other AAA microtubule-severing proteins include spastin and fidgetin [8]. Mutations in the gene encoding spastin cause progressive axon degeneration and underlie ∼40% of autosomal dominant cases of hereditary spastic paraplegia [10] and deletion of the fidgetin gene in mice results in a severe behavioural and developmental phenotype [11], illustrating the importance of the family in neuronal development.
The regulation and compartmentalization of microtubule severing is essential for normal cell function and survival. Katanin p60-mediated severing can be modulated by a p80 regulatory subunit, encoded by the Katnb1 gene [9]. The p80 subunit of katanin binds to p60 and targets it to the centrosome in transfected mammalian cell lines [9], [12], and generally enhances severing, but can also inhibit it depending on the cellular context [4], [12]. In Tetrahymena thermophila [13] and Caenorhabditis elegans [14], p80 null mutants phenocopy p60 null mutants. However the in vivo role of the p80 subunit in mammals remains enigmatic. Katanin was identified as a heterodimer of p60 and p80 subunits in sea urchins [8], however the ratio of the two subunits shows developmental and regional variation in rat neurons [15] and in mouse testis (the current study), suggesting that the expression of the p80 regulatory subunit may be one way in which p60-mediated severing is controlled in mammals [4]. The Katnb1 gene contains a C-terminal WD40 domain predicted to be involved in protein-protein interactions, and as such is a strong candidate for targeting p60-mediated severing to particular locations within a cell and for targeting p60-mediated severing to post-translationally modified or microtubule-associated protein (MAP)-associated tubulin polymers [16], [17]. Katanin p80 also binds to the molecular motor protein dynein [18] and to dynein-regulating proteins [18], [19] and could thus be involved in the transport of the katanin complex to specific sites.
Katanin function is evolutionarily conserved, with p60 and p80 orthologues identified in species from all 5 kingdoms, including in C. elegans, Drosophila melanogaster, Arabidopsis thaliana, Chlamydomonas reinhardtii, mice and humans. Katanin localizes to mitotic spindle poles in mammalian cell lines, where it regulates spindle structure and chromosome movement [2], [3], [20], [21]. Mutations in C. elegans orthologues of katanin p60 and p80 reveal roles for katanin in oocyte meiotic spindle assembly and chromosome movement [20], [22], and katanin regulates different microtubule populations, including kinetochore-associated bundles, to control oocyte meiotic spindle length in Xenopus [23]. Of note, katanin regulates mitotic chromosome movement in D. melanogaster by participating in the so-called “Pacman-mediated” shortening of spindle microtubule plus-ends which results in the poleward movement of the chromosomes [24]. In addition, mutations in katanin orthologues in two distantly related organisms Tetrahymena and Chlamydomonas result in the absence of the central axoneme microtubule pair and cilia/flagella defects, indicating a role in ciliogenesis [13], [25], [26]. To date there have been no in vivo models of katanin dysfunction in mammals.
Here we show that a missense mutation in the highly conserved WD40 domain of the Katnb1 gene, encoding the p80 regulatory subunit of katanin, causes male sterility in mice characterized by oligoasthenoteratozoospermia. Katnb1 mutant mice, denoted as Taily, show frequent failure of meiotic spindle resolution, defective manchette function and abnormal axoneme development. Our findings highlight the critical role for katanin p80 in the regulation of microtubules dynamics in many aspects of male germ cell development. These data raise the possibility that defective katanin function may also contribute to human infertility, specifically defective sperm number, morphology and/or sperm motility.
Results
Infertility in Taily mice is caused by a point mutation in the Katnb1 gene
Mouse lines carrying ENU-induced mutations causing male sterility were identified using breeding trials as described previously [27]. Lines with G3 male sterility at a frequency of one in four, but with normal mating behaviour, were chosen for further analysis. They included the ‘Taily’ line. The causal mutation was mapped using SNP-based methods and ultimately narrowed to a linkage interval on chromosome 8 (between SNP markers rs3089148 and rs3710112) containing 74 genes. Candidate genes were chosen on the basis of testis expression and proposed function. The protein-coding region and intron-exon boundaries of 30 genes were sequenced. The causative mutation in Taily mice was identified as a recessive G to T substitution in exon 9 of the Katnb1 gene. No other mutations were found. Unaffected males possessed either homozygous wild type alleles (Katnb1WT/WT) or were heterozygous for the wild type and Taily allele (Katnb1WT/Taily). Greater than 50 mice of each genotype were assessed and the genotype-phenotype correlation was absolute. The Taily mutation resulted in the conversion of a valine (GTC) to a phenylalanine (TTC) in the WD40 repeat region of the katanin p80 protein (Figure 1). The presence of an aliphatic amino acid (e.g. V or I) at amino 234, relative to the mouse sequence, is absolutely conserved across all species, and is strongly suggestive of a functionally important role (Figure 1B). Western blot analysis revealed that haploid germ cells from Katnb1Taily/Taily mice contained markedly less p80 protein than those from Katnb1WT/WT mice (Figure 2), demonstrating that the ‘Taily’ allele likely results in a loss-of-function.
Fig. 1. The Taily mutation within the Katnb1 gene and protein. 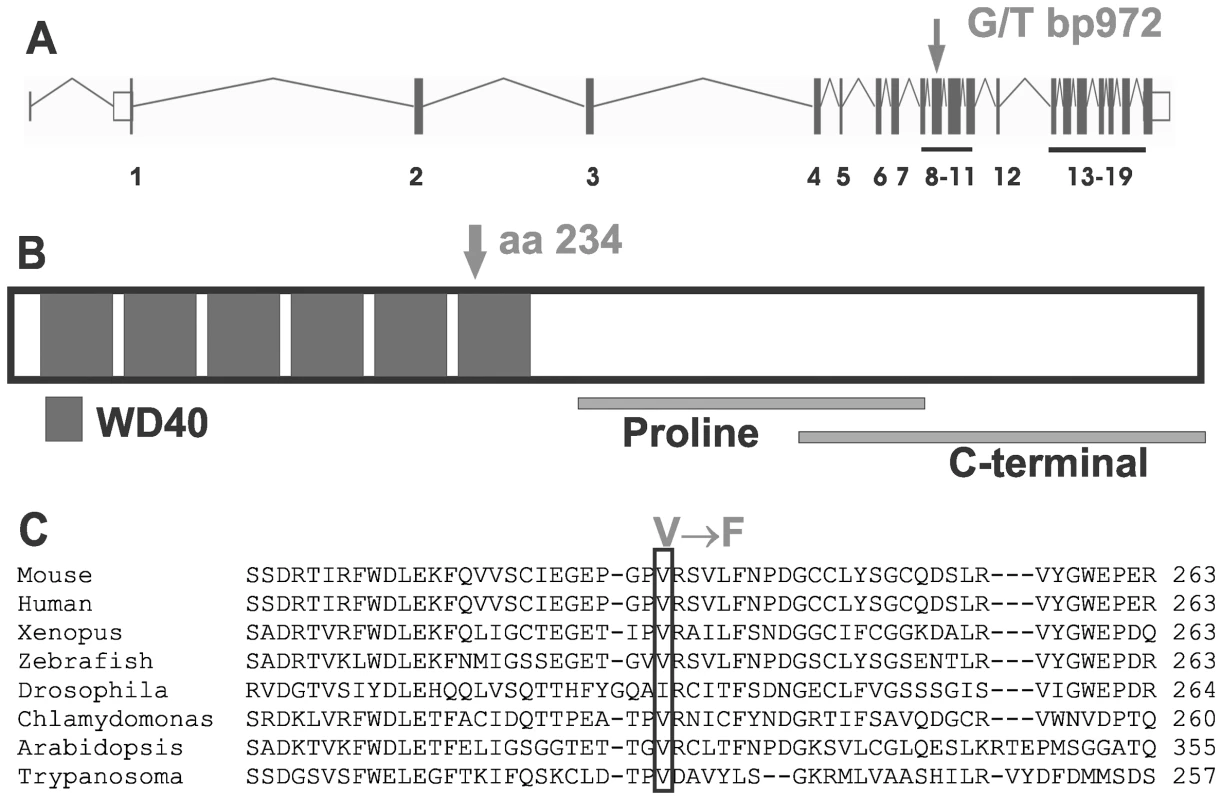
A. The position of the mutation in the WD40 region of the Katnb1 gene (arrow) and protein (B). The Taily mutation (arrow) results in the conversion of a valine (GTC) to a phenylalanine (TTC) in the WD40 repeat region of the katanin p80 protein. C. A cross-phyla comparison of the region of the Katnb1 gene containing the Taily mutation. Fig. 2. Reduction in katanin p80 protein in haploid germ cells from Katnb1Taily/Taily mice. 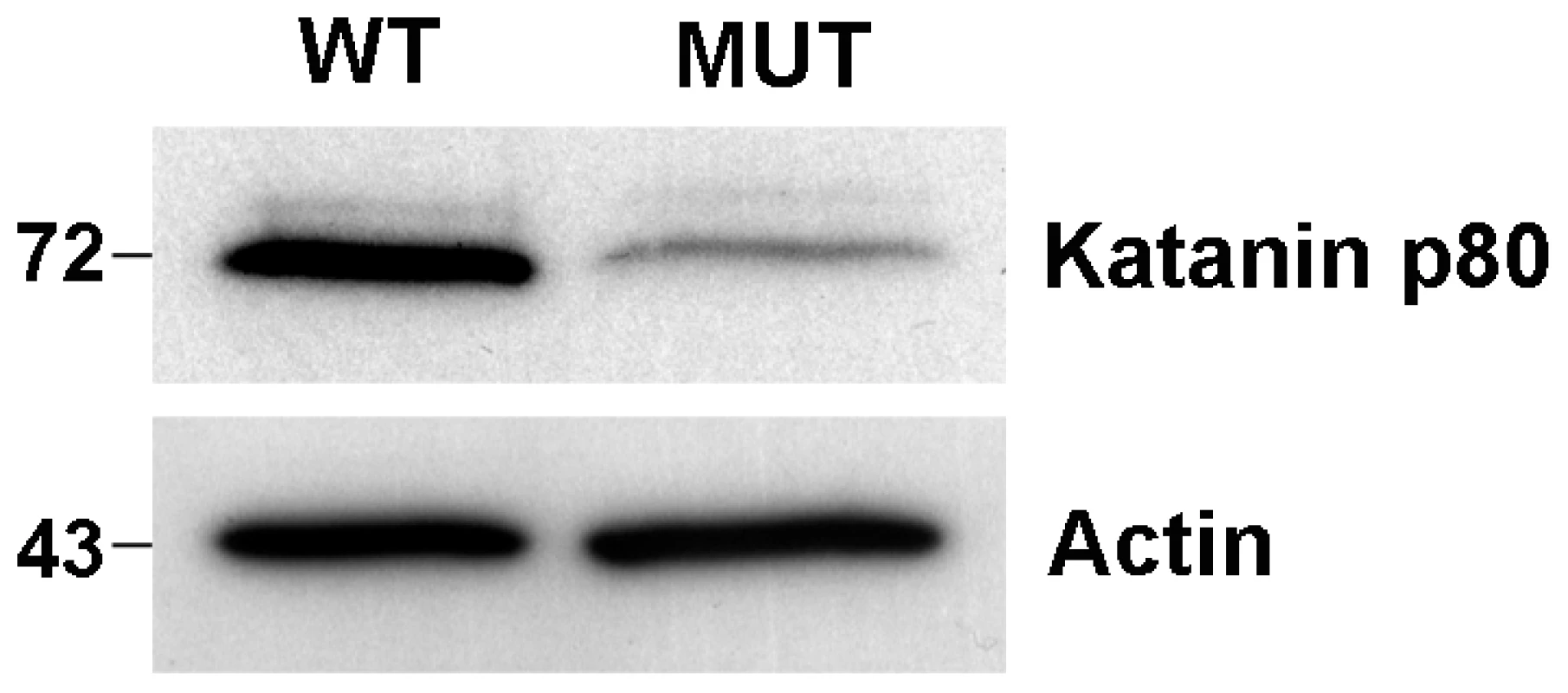
Round spermatids were isolated from Katnb1WT/WT (WT) and Katnb1Taily/Taily (MUT) mice. 10 µg of protein from each cell preparation was immunoblotted for katanin p80, and actin as a protein loading control. Molecular weights are shown on the left. Of note, the molecular weight of the p80 subunit in mice is 72 kDa as predicted by Ensembl (www.ensembl.org). The katanin p80 regulatory subunit is essential for male fertility
Katnb1Taily/Taily males showed no overt behavioural abnormalities, were morphologically identical to wild type littermates, were of normal weight (Figure S1), but were uniformly sterile when mated with wild type females (n≥10, Katnb1Taily/Taily males aged ≥8 weeks of age). Katnb1Taily/Taily females had apparently normal fertility.
Testes from adult (8–12 weeks) Katnb1Taily/Taily mice were 18.7% smaller than those from wild type littermates (p<0.0001, Figure 3A). Seminiferous tubules contained all germ cells types. Two major discordant features were apparent within the seminiferous epithelium from Katnb1Taily/Taily mice: 1) abnormally shaped spermatid heads (Figure 3C and 3D) and 2) abnormal meiotic cells at metaphase-anaphase (Figure 3E and 3F). Stereological analysis revealed that the number of Sertoli cells, spermatogonia and spermatocytes per testis was not different between genotypes (Figure 3B and Table S1), indicating that the initiation of spermatogenesis and entry into meiosis were unaffected by the Taily mutation. The latter finding suggested that the function of the Sertoli cell blood-testis-barrier was normal, a proposition supported by the appearance of normal inter-Sertoli cell junctions by electron microscopy (not shown). By contrast, testes from Katnb1Taily/Taily mice contained ∼30% fewer spermatids (round and elongating) compared to wild type (Figure 3B). The reduction in spermatid populations was due to a decrease in the number of cells exiting meiosis, specifically during the final meiotic division in stage XII (Figure 3B and Table S1). TUNEL-labelling revealed that apoptotic cells were predominantly present in stage XII and stage I tubules which is when the final events of meiosis occur (Figure 3G). Apoptotic spermatocytes in the process of meiotic division were observed (Figure 3H). Collectively these results indicate that katanin p80 function is required for the final phases of male meiotic cell division.
Fig. 3. Spermatogenic defects in Taily mice. 
A. Testis weight in wild type (WT) (n = 14) and Katnb1Taily/Taily mice (n = 22). B. Stereological analysis of germ cell numbers per testis in Katnb1Taily/Taily versus WT mice (n = 5–6). Numbers of germ cells per Sertoli cell were expressed as %WT (left hand axis). The number of spermatids retained within Sertoli cells after spermiation was expressed as fold WT (right hand axis). Abbreviations: SG, spermatogonia; eSc, early spermatocytes; lSc, late spermatocytes; rST, round spermatids; eST, elongating and elongated spermatids; ret, retained spermatids. In A, B, data are mean ± SD, * denotes p<0.05, *** denotes p<0.001 Katnb1Taily/Taily versus WT. C, D. Electron microscopy of sperm nuclear shape in elongating spermatids from WT (C) and Katnb1Taily/Taily (D) mice. White arrowheads indicate the perinuclear ring, which shows an abnormal constriction in spermatids from Katnb1Taily/Taily mice (D). White arrows indicate the manchette microtubules. Scale bar in C,D = 5 µm. E, F. Chromosome segregation in WT (E) and Katnb1Taily/Taily (F) mice. Arrow heads indicate cells stalled in anaphase of meiosis I. Scale bar in E, F = 10 µm. G, H. TUNEL analysis of apoptotic cells in Katnb1Taily/Taily mice. Apoptotic cells were predominantly seen in stage XII and I tubules (asterix in G). Scale bar in G = 200 µm, H = 10 µm. I, J. Spermiation in WT (I) and Katnb1Taily/Taily (J) mice. Retained elongated spermatids (arrowheads) were frequently observed being phagocytosed by Sertoli cells in stage IX tubules from Katnb1Taily/Taily (J) but were rarely observed in WT (I) mice. Bar in I, J = 10 µm. K, L. Cauda epididymal sperm morphology from WT (K) and Katnb1Taily/Taily (L) mice. Taily sperm frequently displayed a hammer-head shape (arrowheads). Sperm heads were often bent back towards the tail (asterisk). Scale bar in K, L = 10 µm. Roman numerals indicate the stage of the spermatogenic cycle. Stereology also showed that additional germ cells were not lost as they progressed through spermiogenesis (Table S1), however there was a 36 fold increase in the number of spermatozoa being phagocytosed by Sertoli cells (Figure 3B) in stages IX–XI tubules (Figure 3I and 3J). These data indicate a failure in spermiation, the process by which sperm are released by the Sertoli cell at the end of their development, prior to their passage to the epididymis [28]. The data show that a significant proportion of spermatozoa failed to be released from the Sertoli cell and were instead phagocytosed, thus leading to a reduced number of sperm entering the epididymis. As a consequence, the epididymides from Katnb1Taily/Taily males contained a lower total number of sperm than would be anticipated from the testicular daily sperm output, i.e. 11% of wild type in the epididymis compared to 57% in the testis (Figure 4A). Of the sperm found in the cauda epididymis of Taily mice, when compared to wildtype (Figure 3K), all had abnormally shaped heads (Figure 3L) and displayed compromised total motility as assessed by computer assisted sperm analysis (80.3% in Katnb1WT/WT versus 37.2% in Katnb1Taily/Taily) (Figure 4B). Very few sperm were capable of forward (progressive) motility (52.4% in Katnb1WT/WT versus 11.1% in Katnb1Taily/Tail) (Figure 4B).
Fig. 4. Sperm output and functional characteristics. 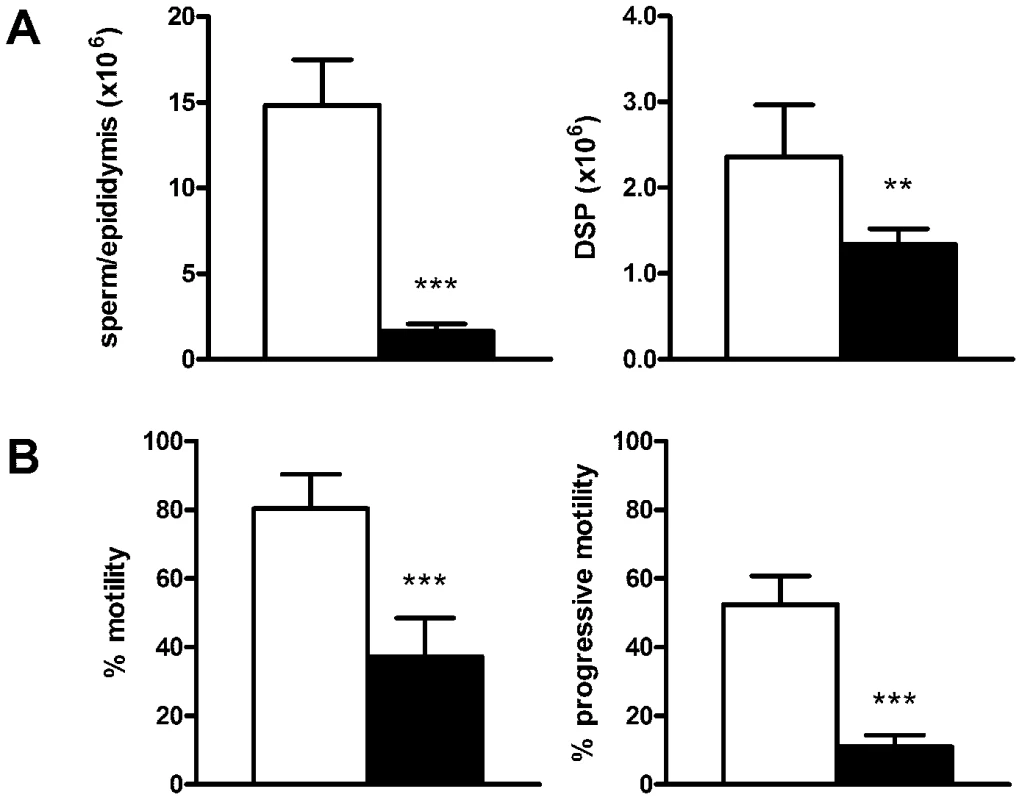
A. Total epididymal sperm content (n = 7/group) (left hand panel) and daily sperm output (DSP, right hand panel) by the testes (n = 6/group) from wild type (white bars) and Katnb1Taily/Taily (black bars) mice. B. The percentage of sperm showing any form of motility (left hand panel) and those showing progressive motility (right hand panel) in wild type (white bars) and Katnb1Taily/Taily (black bars) mice (n = 6–7/group). Data are mean ± SD, ** denotes p<0.01, *** p<0.001 compared to WT. Collectively these data indicate that katanin p80 has a role in germ cell exit from meiosis, in the establishment of structures or pathways within the sperm tail involved in motility and in the shaping of the sperm head. Katnb1Taily/Taily males were sterile as a consequence of decreased sperm production, abnormal sperm morphology and sperm being unable to ascend the female reproductive tract following mating. The analogous human phenotype is referred to as oligoasthenoteratozoospermia (low sperm count, poor motility and abnormal shape).
Expression of katanin p80 and p60 subunits in the mouse testis
Both katanin p80 and p60 mRNAs were expressed in the testis during the post-natal establishment of the spermatogenic cycle (Figure 5). The katanin p60 microtubule severing enzyme was expressed at relatively similar levels in all ages examined, suggesting expression in multiple cell types (Figure 5A). Katanin p80 regulatory subunit expression, however, peaked at day 30, suggesting predominant expression in post-meiotic haploid spermatids (Figure 5A). These data are consistent with previous microarray data (germonline.org) indicating that the katanin p60 catalytic subunit is expressed in Sertoli cells and germ cells to a similar degree, whereas the p80 regulatory subunit while detectable in Sertoli cells and spermatogonia, is more highly expressed (5-fold) in spermatocytes and spermatids [29]. In accordance with the mRNA data, katanin p80 protein was most strongly localized within round through to elongating spermatids (Figure 5B). Katanin p60 was also prominent in spermatids and was visible in Sertoli cells (Figure 5B).
Fig. 5. Katanin p60 and p80 expression in the mouse testis. 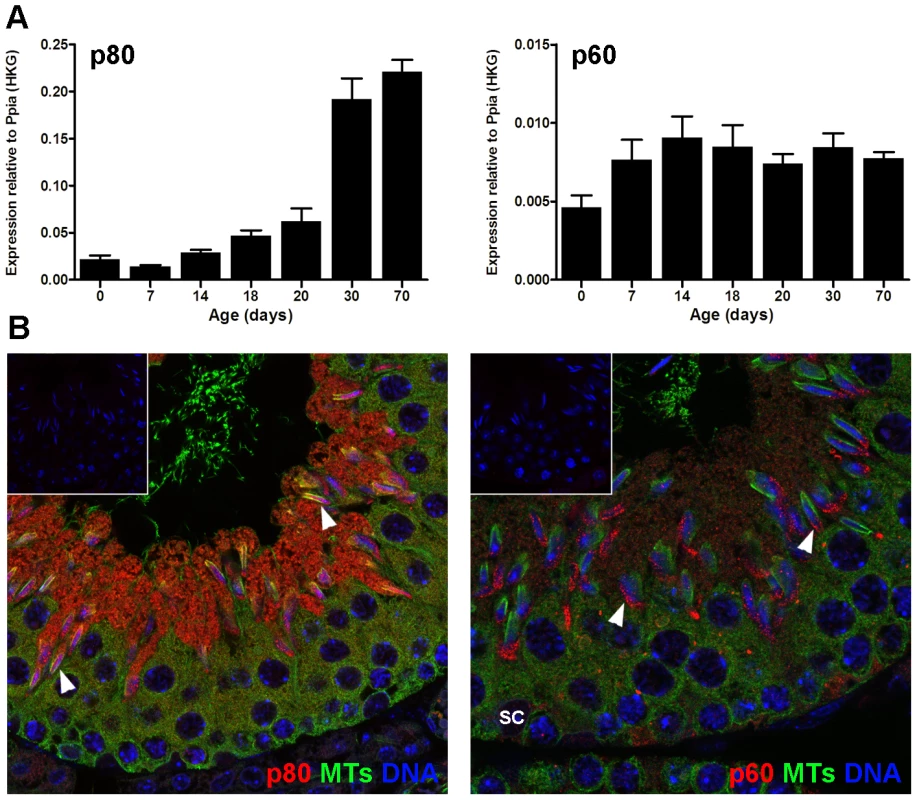
A. Quantitative RT-PCR expression data for katanin p80 (left hand panel) and p60 (right hand panel) during post-natal testis development. Expression data was normalized against Ppia (housekeeper gene expression) and shown as mean ± SEM, n = 3. B. Immunohistochemical localization of katanin p60 (right) and p80 (left) in the mouse seminiferous epithelium. Katanin subunits = red, α-tubulin = green, nuclei = blue. Both katanin subunits were prominently localized to elongating spermatids (arrowheads). Katanin p60 was also visible in Sertoli cell nuclei (SC). The inset in each micrograph shows a control for the secondary antibody (primary antibody substituted with buffer). In addition, the katanin p60 orthologues Katnal1 (p60-like 1) and Katnal2 (p60-like 2) were also expressed within the developing post-natal testis with a timing similar to that observed for katanin p80 (Figure S2). Absence of KATNAL1 immunolocalization in germ cells (Smith et al, submitted for publication) and immunolocalization of KATNAL2 predominantly to the sperm tail and cytoplasm, but not associated with the sperm head, (Figure S2), suggests that the meiotic and spermatid head-shape phenotypes reported herein were primarily mediated by katanin p80 regulation of the eponymous p60 subunit.
Katanin p80 regulates microtubule dynamics during male meiotic division
The loss of germ cells during meiotic division prompted an investigation of the microtubule-based meiotic spindle in Katnb1Taily/Taily males. Compared to Katnb1WT/WT littermates, all Katnb1Taily/Taily meiotic spindles were abnormal (Figure 6A and 6B). Specifically, metaphase spindles appeared to be more densely populated with microtubules, and projected from the poles at a wider angle than those from wild type animals (Figure 6A and 6B and Videos S1 and S2). Pole-to-pole measurements in metaphase spindles were longer in Katnb1Taily/Taily compared to Katnb1WT/WT (12.15±0.16, n = 58, versus 10.52±0.17 µm, n = 78, mean ± SEM, p<0.0001).
Fig. 6. Defective meiotic division in Katnb1Taily/Taily male gametes. 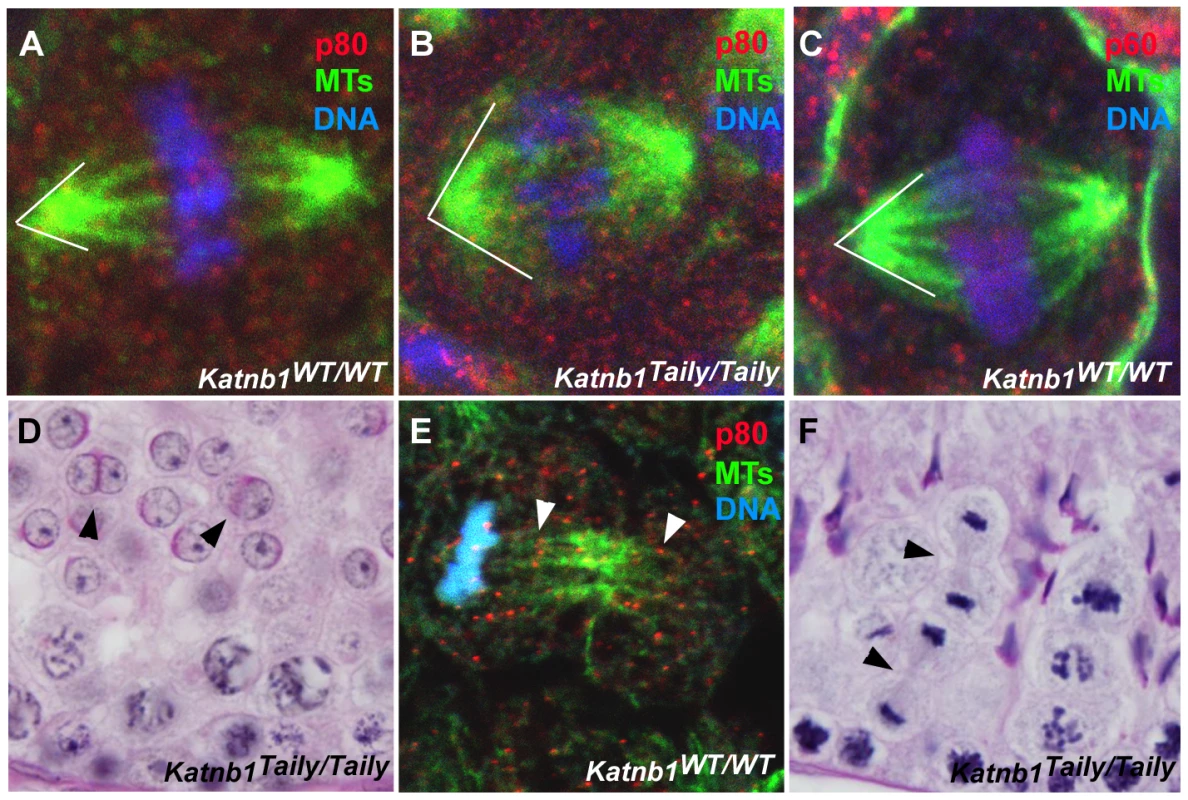
A, B. Katanin p80 localization and meiotic spindle structure in wild type (WT) (A) and Katnb1Taily/Taily (B) testes. White lines indicate the angle at which microtubules project from spindle poles. C. Katanin p60 localization to meiotic spindles in WT mice. D. Binucleated haploid spermatids (arrowheads) in Katnb1Taily/Taily testes. E. Katanin p80 localization to mid-bodies in meiotic telophase. Arrowheads indicate p80 localization at midbody microtubules. F. Late telophase cells with prominent midbody structures (arrowheads) were observed in Katnb1Taily/Taily but not WT testes. In A, B, C and E red represents katanin subunits, green represents microtubules (MTs) as labelled by α-tubulin immunostaining, and blue represents DNA as labelled by DAPI. Within metaphase and anaphase cells, p60 (Figure 6C and Videos S1 and S2) and p80 (Figure 6A and 6B) proteins localized to microtubules of meiotic spindles. The Katnb1Taily/Taily mutation did not overtly alter this localization. Both p60 and p80 were observed along the microtubules of the spindle and at the microtubule-chromosome interface. The latter localization is consistent with katanin involvement in the poleward movement of chromosomes in Drosophila mitotic cells [24]. Specifically within Drosophila, katanin is believed to participate in the depolymerization of microtubule plus-ends in the midzone at anaphase during Drosophila mitosis, effectively “chewing away” the microtubule ends to facilitate spindle shortening via a process known as “Pacman” [24]. A role for katanin p80 in the Pacman-mediated poleward movement of chromosomes in mammalian meiotic anaphase is further supported by the appearance of multiple cells stalled in late anaphase in Katnb1Taily/Taily mice (Figure 3F).
Disordered meiosis is further evidenced by the frequent occurrence of binucleated haploid spermatids in Katnb1Taily/Taily testis sections (Figure 6D). Binucleated spermatids were never observed in wild type animals. These data strongly suggest a role for katanin p80 in cytokinesis and midbody resolution. This hypothesis is supported by the localization of katanin p80 (Figure 6E) and p60 (Figure S3) to the microtubules of the midbody in late telophase cells in both Katnb1WT/WT (Figure 6E) and Katnb1Taily/Taily germ cells (Figures S3 and data not shown). In addition, telophase cells with prominent midbody microtubules were observed in testes from Katnb1Taily/Taily, but not in wild type animals (Figure 6F). Taken together, the results suggest that katanin p80, most likely in association with p60, has a prominent role in midbody dissolution in male meiotic cells, and that this function is disrupted in Taily mice.
Katanin p80 regulates microtubule-based structures essential for normal sperm morphology and motility
Abnormal sperm head shape (Figure 3D and 3L) is frequently associated with defects in the function of the manchette [30], [31], [32]. The manchette is a transient microtubule structure assembled in elongating spermatids with proposed roles in both the sculpting of the sperm head and in the movement of proteins destined for the sperm tail, via a process referred to as intra-manchette transport (IMT) [33]. The manchette is comprised of large, parallel arrays of microtubule bundles that extend from beneath the acrosome/acroplaxome region of the spermatid head and project into the spermatid cytoplasmic lobe containing the growing sperm tail ([34] and Figure S4). The manchette is first seen at step 8 of spermiogenesis, when the round spermatid nucleus polarizes to one side of the cytoplasm, and the spermatid commences elongation. Nucleation of microtubules in the manchette is thought to occur on the perinuclear ring region of the spermatid head (Figure S4), and large parallel bundles are assembled as the spermatid nucleus starts to change shape in step 9.
In order to investigate the hypothesis that head abnormalities in sperm from Katnb1Taily/Taily mice were the consequence of abnormal manchette structure or function, testis sections were examined using electron microscopy. Manchettes in wild type elongating spermatids displayed the characteristic perinuclear ring and microtubule array structure (Figure 3C). Those from Katnb1Taily/Taily elongating spermatids, however, displayed several abnormalities including constricted perinuclear rings, nuclear distortion and abnormally long microtubules extending into the distal cytoplasm (Figure 3D).
A stage-by-stage comparison of sections from wild type and Katnb1Taily/Taily males suggested defective manchette resolution in mutant animals. Although manchettes eventually resolved, the removal of manchettes in Katnb1Taily/Taily males was delayed. In wild type mice, manchettes normally reduce in size and then disappear in step 13 spermatids. In contrast, and when compared with wild type mice (Figure 7A), in step 13 spermatids from Katnb1Taily/Taily mice, abnormally long manchette microtubules extended into the cytoplasm and were associated with tubulin-labelled ‘clouds’ (Figure 7B). The timing and location of the ‘clouds’ is suggestive of abnormal microtubule disassembly.
Fig. 7. Katanin localization to manchettes and abnormalities in manchette resolution and flagella structure in Katnb1Taily/Taily spermatids. 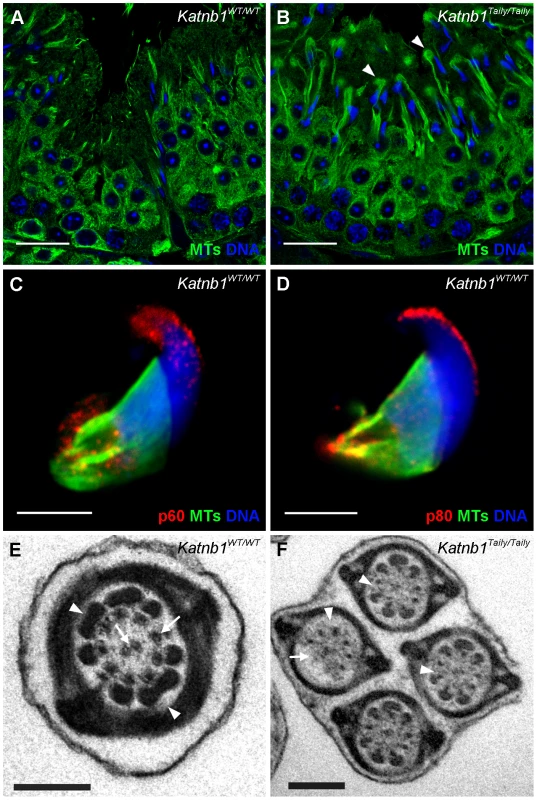
A. Immunolocalization of α-tubulin (microtubules, MTs) in stage I, during which time manchette resolution occurs in wild type mice. B. The localization of α-tubulin in a stage I tubule from a Katnb1Taily/Taily mouse. Arrowheads indicate abnormal “clouds” of tubulin in the spermatid cytoplasm at the time of manchette disassembly. In A and B, green = microtubules (MTs), blue = DNA (DAPI), scale bar in B = 20 µm. C, D. Immunolocalization of katanin p60 (C) and katanin p80 (D) in wild type spermatids. Scale bar = 5 µm. Red = katanin subunits, green = α-tubulin (microtubules, MTs), blue = DNA (TOPRO). E, F. Structure of sperm flagella in Katnb1WT/WT (E) and Katnb1Taily/Taily (F) sperm. Scale bar = 0.5 µm. In E, arrowheads indicate normal outer dense fiber structures and arrows indicate microtubules of the axoneme. In F, arrowheads indicate abnormal or missing outer dense fibers, and arrows indicate missing axonemal microtubules. The above defects were confirmed and more dynamically visualized in elongating spermatids isolated from wild type and Katnb1Taily/Taily males labelled with α-tubulin and TOPRO to visualize microtubules and the nucleus, respectively (Figure 8 and Videos S3 and S4). An analysis of progressively more mature elongating spermatids revealed that while manchettes in Katnb1Taily/Taily mice appeared to form at the correct time and initially began to move distally as spermiogenesis proceeded, movement stalled at approximately step 10 (Figure 8). By contrast, the progressive constriction of the peri-nuclear ring that normally occurs as the manchette moves over the caudal half of the spermatid, continued to occur (Figure 8). This resulted in a bulbous nuclear shape forward of the stalled peri-nuclear ring, and an abnormally elongated nucleus distally, resulting in the unusual ‘knob-like’ head structure also visible at an electron microscopic level (Figure 3D). As observed by electron microscopy (Figure 3D) and in testis sections (Figure 7B) abnormal elongated manchette microtubules were easily observed in isolated late step spermatids (Figure 8).
Fig. 8. Manchette structure in wild-type and Katnb1Taily/Taily spermatids. 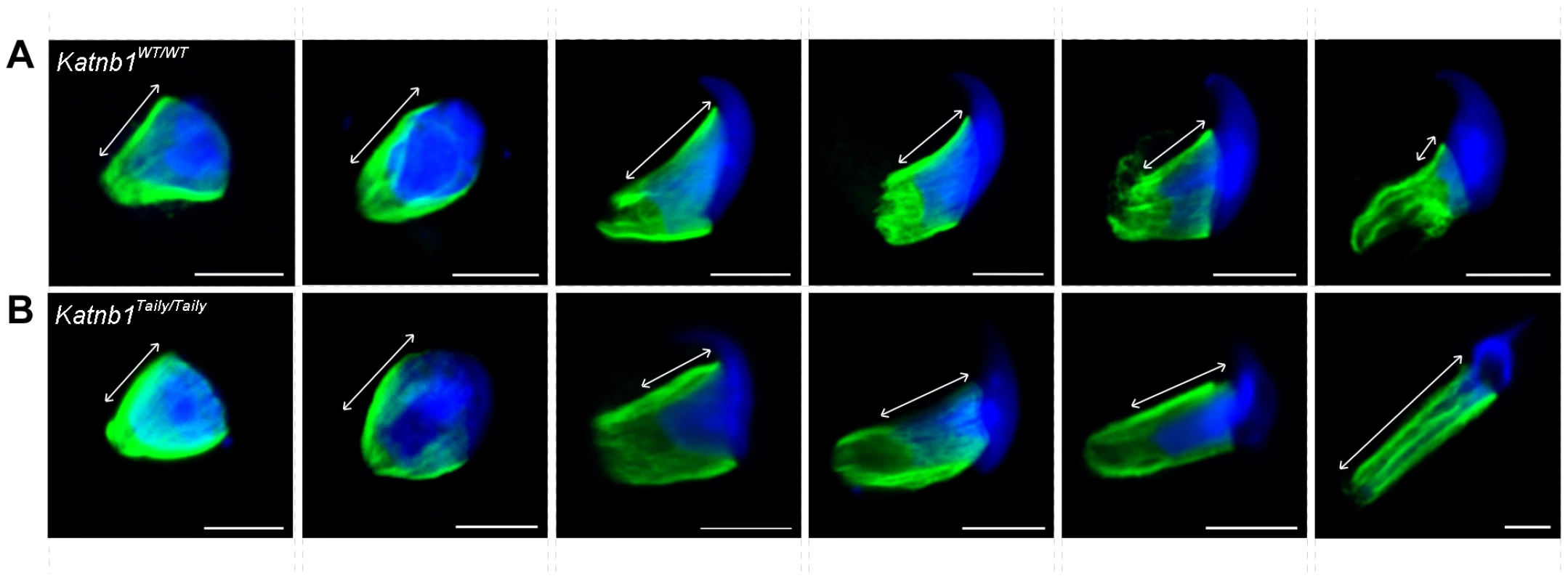
A. α-Tubulin staining (green) of manchette microtubules in isolated spermatids from WT mice. Elongating spermatids are shown in progressive steps of manchette development from left to right. Arrows indicate the distance between perinuclear ring of the manchette and the posterior portion of the nucleus. B. α-Tubulin staining (green) of manchette microtubules in isolated spermatids from Katnb1Taily/Taily mice. Arrows indicate the distance of the peri-nuclear ring to the posterior portion of the nucleus. In all images, blue staining = DNA (TOPRO). The scale bar in each micrograph = 5 µm. Consistent with the defects seen in Katnb1Taily/Taily mice, both the katanin p80 regulatory subunit and the p60 catalytic subunit localized to microtubules of the manchette (Figure 7C and 7D). Focal labelling of katanin subunits was observed along the manchette microtubules and particularly at the microtubule ends projecting into the cytoplasm (Figure 7C and 7D). This localization is consistent with a role for katanin-mediated severing in regulating manchette length. Localization was not obviously affected in the manchette of Katnb1Taily/Taily mice (not shown). Both subunits also localized to the acrosome/acroplaxome region, in a manner reminiscent of proteins that undergo trafficking in an acrosome/acroplaxome-manchette-flagella pathway [35].
Collectively these results reveal that katanin p80, most likely in association with p60, has an essential role in both the formation of the manchette and in the dynamics of its movement and resolution. Perturbed katanin p80 function results in the failure of manchette migration, abnormally long and mal-orientated manchette microtubules and abnormal dissolution. Such abnormalities are entirely consistent with the observed defects in sperm head shape (teratozoospermia) in sperm from Katnb1Taily/Taily animals.
The manchette also plays a critical role in the development of sperm flagella via a process known as intra-manchette transport, or IMT [33]. IMT is thought to be involved in sperm tail development in a manner analogous to intra-flagella transport in somatic cilia and in flagella in organisms including Chlamydomas and Trypanosomatids [36]. Defects in manchettes and sperm motility in Katnb1Taily/Taily mice, and a previously demonstrated role for katanin p80-mediated (PF15p) severing in the formation of axoneme central microtubules in Chlamydomonas [26], prompted us to investigate flagella/tail structure in Katnb1WT/WT and Katnb1Taily/Taily sperm. When compared to controls (Figure 7E), electron microscopic analysis revealed a variety of axoneme defects, including missing central microtubules and hemi-axonemes (Figure 7F). Of note, the majority of Katnb1Taily/Taily sperm also contained flimsy or missing outer dense fibers (Figure 7F) consistent with previously proposed roles for the manchette in the transport of proteins into the developing sperm tail and formation of accessory tail structures [33]. The outer dense fibers are rod-like structures running parallel to, and connected to, the microtubules of the axoneme that are believed to protect sperm against shearing forces and to provide directionality to tail bending (reviewed in [37]). Collectively, these defects demonstrate an essential role for katanin p80 in the development of the sperm flagellum, including the axoneme and the formation of the accessory structures.
Discussion
Analysis of male mice with a mutation in the regulatory subunit of the katanin microtubule severing enzyme complex revealed several roles for katanin p80 in mammalian microtubule dynamics. These studies reveal an essential requirement for katanin p80 in male fertility, and in multiple aspects of mammalian male gamete development, including in meiotic spindle dynamics, cytokinesis, flagella development and sperm head shaping.
Together with in vitro data and evidence of disordered microtubule structure and function in lower order species, this novel mouse mutation reveals that katanin has roles in controlling microtubule dynamics. While severing can result in microtubule destruction, it is also important for the creation of new microtubules, via the severing of longer stable microtubules into shorter segments that can then be used as “seeds” for further microtubule polymerization [4], [7]. For example, katanin severs newly created microtubules at the neuronal centrosome, facilitating their transport to other sites, such as within developing axons [38]. Katanin can sever microtubule lattice defects in a quality control mechanism [39], and in neurons can create branched microtubule networks [4], [40]. Recent data also revealed that katanin can depolymerize microtubules at their plus-ends [5], [16], [39] and finally, the p60-p80 katanin complex has a severing-independent, microtubule cross-linking function at C.elegans oocyte meiotic spindle poles [23].
The p80 protein contains a WD40 domain that likely mediates protein-protein interactions [9], [12]. The C-terminal region interacts with the p60 enzyme [12] and contains binding sites for the molecular motor protein dynein. The C-terminal region also binds to the dynein-associated proteins LIS1 and NDE1 in neurons [18]. While p80 is thought to modulate p60 targeting and activity [3], [9], [12], the precise in vivo roles of p80 are not well understood. The Katnb1Taily/Taily mutation in the WD40 domain results in decreased p80 protein within germ cells and defects in microtubule-based processes. Based on the position of the mutation, and on the fact that less p80 protein is produced in mutant mice, we predict that the mutation influences the ability of p60 to sever, as well as the targeting of this severing activity to specific sites within the cell. This mouse model recapitulates many of the proposed functions of katanin observed in lower order species. It is of note, that the manchette defects observed in Taily mice phenocopy many of the defects observed in a Lis1 null mice [31]. This observation and previous studies showing the localization of LIS1 and NDE1 in the manchette [41], suggests that similar to the proposed role for these proteins in neurons, the katanin complex co-operates with LIS1 and NDE1 during sperm head shaping. This interaction will be the subject of future investigations.
The data demonstrate a role for katanin p80 in mammalian male meiotic cell division. Null mutations in the C.elegans p80 ortholog mei2 are associated with meiotic defects in oocytes, including an inability to assemble a meiotic spindle [14]. Katanin function in male germ cells has not previously been studied to the best of our knowledge. Our observations on male metaphase spindles in Katnb1Taily/Taily mice are consistent with the longer metaphase meiotic spindles produced in C.elegans oocytes with a partial loss-of-function mei2 mutation [20] and with the recent demonstration of a conserved role for katanin in controlling the length of meiotic metaphase spindles in Xenopus oocytes [23].
Within C.elegans, the p80 protein targets the p60 severing enzyme to the spindle poles in meiotic oocytes [20], [42]. We did not observe either p60 or p80 at spindle poles in male meiotic germ cells. All meiotic spindles were, however, abnormal, indicating a role for katanin in the assembly of spindles, as supported by various studies [14], [20], [21], [23]. In meiotic male germ cells, the most obvious localization of katanin subunits was to the microtubule ends near the chromosomes in metaphase-anaphase cells, suggesting a role for katanin in the shortening of microtubule plus ends during anaphase. The “Pacman-mediated” shortening of microtubule plus-ends within the spindle midzone is important for the poleward movement of chromosomes in mitotic anaphase [24], but has not been studied in meiotic cells. Our observation of cells apparently stalled in anaphase, together with the finding that 30% of cells die during the later phases of meiosis, supports the hypothesis that disturbed p80 function causes defects in the poleward movement of chromosomes during anaphase. Such defects result in disturbed spindle resolution and, often, cell death. Finally, the appearance of binucleated spermatids and the localization of katanin subunits to the midbody in meiotic cells in male mice supports the hypothesis that p80, and potentially the katanin complex, has a conserved role in modulating microtubule dynamics at the midbody during meiotic cytokinesis. In support, katanin p80 dysfunction or mislocalization is associated with defective mitotic cytokinesis in Trypanosomes [25] and in sarcoma cells in vitro [43].
Katanin p80 is essential for sperm head shaping via the regulation of the manchette, which is in itself a complex microtubule network. An analysis of manchette position during spermiogenesis indicated that manchette movement is defective in Katnb1Taily/Taily mice, suggesting katanin is involved in the organization and remodelling of this microtubule network as it moves over the nucleus. The localization of p60 and p80 within the manchette, together with the Taily phenotype is consistent with a role for katanin action at multiple sites. These include 1) the severing of microtubules at the perinuclear ring, thereby facilitating the release of microtubules from the nucleating center, and the production of the microtubule lattice, as has been proposed in other systems [4]; 2) the severing of microtubules near the nucleus to permit movement of the manchette perinuclear ring as it shapes the nucleus; 3) within the microtubule lattice to facilitate remodelling of this complex structure; and 4) the severing of microtubules at the caudal end of the manchette to control manchette length and dissolution. Katanin activity also regulates the dynamics of large microtubule-based array structures in neurons [18], [38], [40]. Taken together, the data support the hypothesis that p80, and katanin function, is important for the movement and remodelling of large microtubule arrays in mammalian cells. This role is essential for the normal development and shaping of sperm, which in turn is critical for normal sperm function and male fertility.
Finally, we demonstrate for the first time that katanin p80 is required for mammalian sperm flagella development and subsequent motility. A conserved role for katanin in the assembly and disassembly of cilia and flagella has been revealed in two distantly related lower order species Chlamydomonas [26] and Tetrahymena [13] (reviewed in [6]). Katanin activity controls flagellum length in Trypanosomatids [25] and severs axonemal microtubules during the deflagellation process in Chlamyodmonas [44], [45]. In Chlamydomonas, katanin p80 is also specifically required for the assembly of the central microtubule doublet of the flagellum axoneme [26]. Defects in axonemal structures (including missing central microtubule doublets) and outer-dense fibers in Katnb1Taily/Taily mice suggest that katanin p80 regulates sperm motility by acting at multiple sites in sperm development. Specifically, p80 function is required in the regulation of axonemal assembly and in the delivery of proteins to the developing flagellum via IMT.
Given the high level of expression of katanin p80 in other tissues, and the proven role for katanin in C.elegans oogenesis, it is surprising that other overt phenotypes were not noted in mutant mice. We hypothesize that other phenotypes will be revealed when mice are exposed to environmental insults. Studies into the role of katanin in oocyte function are ongoing.
In conclusion, the p80 subunit of the katanin microtubule severing enzyme complex is required for male fertility in mice. This is the first in vivo mammalian model of katanin p80 dysfunction, and it presents with a phenotype reminiscent of a commonly observed clinical phenotype of male infertility characterized by low sperm counts, poor motility and abnormal sperm morphology (referred to as oligoasthenoteratospermia or OAT). We conclude that p80 katanin is required for male meiotic spindle development and dynamics, and for the shaping of the sperm head via the regulation of manchette development and movement. Katanin p80 also participates in meiotic cytokinesis, likely via the regulation of the microtubules within the midbody, and controls the development and function of sperm flagella.
Materials and Methods
Ethics statement
All animal experimentation was approved by the Australian National University and Monash University Animal Experimentation Ethics Committees and performed in accordance with Australian NHMRC Guidelines on Ethics in Animal Experimentation.
Identification of the Taily mouse line and the causal mutation
Point mutant mice were generated as described previously on a C57BL/6 background and outbred to CBA [27]. Mouse lines containing sterility causing mutations were identified by breeding trials wherein eight G3 brother-sister pairs per line were co-housed and the presence of pups monitored. Lines where male sterility was observed in a ratio of approximately one in four in the G3 generation with apparently normal mating behaviour were selected for further analysis.
Affymetrix 5K mouse SNP Chip arrays were used to map the sterility causing mutation. Genomic DNA from five affected males was hybridized onto the array at the Australian Genome Research Facility and compared to wild type C57BL/6 and CBA sequences. The linkage interval was subsequently narrowed using additional mice and SNPs (www.well.ox.ac.uk/mouse/INBREDS/) using the Amplifluor SNP Genotyping System (Chemicon). Plates were read in a BMG Fluostar optima fluorescent microplate reader.
Following the identification of the causal mutation, mice were specifically genotyped using the Amplifluor SNPs HT genotyping system using a wild type-specific antisense primer 5′-GAAGGTCGGAGTCAACGGATTAAGAGCACCCGTACCTGAC-3′, a mutant allele antisense primer 5′-GAAGGTGACCAAGTTCATGCTGAAGAGCACCCGTACCTGAA-3′, a sense primer, 5′-GGTGGTGAGCTGCATTGAA-3′ and Platinum Taq DNA Polymerase (Invitrogen). Conditions for amplification were as follows: 4 minute denaturation at 95°C, 35 cycles of denaturation at 95°C for 10 seconds, annealing at 60°C for 20 seconds and elongation at 72°C for 40 seconds, followed by a final 3 minute elongation at 72°C. Following the reaction, plates were read in a BMG Fluostar optima fluorescent plate reader.
Infertility characterization
Infertility in the Taily mouse line was classified using the scheme outlined in Borg et al [46]. Daily sperm output and total epididymal sperm content were determined as described previously [47]. Sperm motility was assessed using computer assisted sperm analysis [48] and ultra-structure using electron microscopy [49]. Cauda epididymal sperm morphology was assessed following staining with hematoxylin. Cells undergoing apoptosis were visualized using the Apoptag kit (Millipore) as recommended by the manufacturer.
Stereological analysis
The number of germ cells per Sertoli cell were enumerated in 25 µm thick, periodic acid Schiffs (PAS) stained methacrylate sections using the optical disector as previously described [50]. Retained elongated spermatids were counted in stage XI-XI [50] and expressed as fold wild type.
Katanin subunit expression
RNA was extracted from testes at defined periods throughout post-natal development using TRIzol regents (Life Technologies), treated with DNase I (Ambion) and cDNA sythnesized using oligo-dT primers and SuperScript III reverse transcriptase (Life Technologies). The relative expression of Katnb1, Katna1, Katnal1 and Katnal2 were defined using quantitative PCR using TaqMan assays (Applied Biosystems) Mm01244795_m1, Mm00496172_m1, Mm00463780_m1 and Mm00510701_m1 respectively. Expression of these was normalized against peptidylprolyl isomerase A (Mm002342429_g1).
Germ cell isolation
Germ cell sub-populations were purified using the Staput method as previously described [51]. Single cell suspensions were loaded onto a 2–4% continuous BSA gradient and elongated spermatids and round spermatids collected after a 3 hour and 3.5 hour sedimentation period, respectively. For immunofluorescent staining, gradient fractions were pelleted and resuspended in 4% paraformaldehyde (PFA) fixative for 2 hours on ice. Cells were then washed with PBS and spread onto slides.
Western blotting
Protein was extracted from round spermatid fractions (>90% purity) using 20 µL M-PER buffer (Thermo Scientific). 10 µg of protein was separated on a 10% SDS-PAGE gel and probed for rabbit katanin p80 (HPA041165, which recognizes a C-terminal region of p80, Sigma Aldrich) and actin (A2066, Sigma Aldrich). Bound antibody was detected using a goat anti-rabbit IgG HRP (P0488, Dako) secondary antibody and an enhanced chemiluminescence (ECL Plus) detection kit (Amersham Biosciences).
Immunofluorescent microscopy
Katanin subunits and α-tubulin were localized in testis sections as described [52]. Primary antibodies included: anti-α-tubulin (T5168, Sigma, diluted 1 in 5000), anti-katanin p60-like 2 (p60AL2, #sc-84855, Santa Cruz, diluted 1 in 100), anti-katanin p80 (diluted 1 in 200) [9] and anti-katanin p60 (diluted 1 in 200) [53]. Both p60 and p80 antibodies were affinity-purified from rabbits immunised against full length recombinant human proteins. These antibodies have been validated extensively and have been shown to recognize a single polypeptide in HeLa cells and a range of human tissues [9], [53]. Given the sequence homology between the p60 and p60L1 subunits, and between p80 and the uncharacterized c15orf29 subunit, there remains the possibility of partial cross-reactivity. Secondary antibodies included: Alexa Fluor 555 donkey anti-rabbit IgG (A-31572) and Alexa Fluor 488 donkey anti-mouse IgG (A-21202) (diluted 1 in 500). DNA was labelled using DAPI (Invitrogen). To define the localization of proteins within isolated elongating spermatids, cells were permeabilized in 0.2% Triton X-100 diluted in 10% normal horse serum (NHS) in PBS for one hour at room temperature. Non-specific labelling was minimized by blocking in 10% NHS in PBS for 30 minutes. Primary antibodies were diluted in 10% NHS in PBS and incubated overnight at 4°C. Secondary antibodies were diluted 1 in 200 and incubated at room temperature for 2 hours. DNA was labelled using TOPRO3 (Invitrogen, 1 in 200) or DAPI. Images were taken with an SP5 5-channel (Leica Microsystems) confocal microscope in the Monash University Microimaging facility. Metaphase spindle lengths were measured on α-tubulin and DAPI-stained sections from Katnb1WT/WT and Katnb1Taily/Taily mice using LAS AF (Leica Application Suite Advanced Fluorescence) software. Z-stacks of spindles and manchettes were collected at 0.5 µm intervals. Images were assembled using Adobe Photoshop. Test and subject images were adjusted uniformly across the image and between groups.
Statistics
Differences between Katnb1WT/WT and Katnb1Taily/Taily mice were determined using unpaired t tests in GraphPad Prism 5.0.
Supporting Information
Zdroje
1. McLachlanRIRajpert-De MeytsEHoei-HansenCEde KretserDMSkakkebaekNE 2007 Histological evaluation of the human testis–approaches to optimizing the clinical value of the assessment: mini review. Hum Reprod 22 2 16
2. McNallyF 2000 Capturing a ring of samurai. Nat Cell Biol 2 E4 7
3. QuarmbyL 2000 Cellular Samurai: katanin and the severing of microtubules. J Cell Sci 113 Pt 16 2821 2827
4. BaasPWKarabayAQiangL 2005 Microtubules cut and run. Trends Cell Biol 15 518 524
5. BaasPWSharmaV 2011 Cell migration: katanin gives microtubules a trim. Curr Biol 21 R302 304
6. Roll-MecakAMcNallyFJ 2010 Microtubule-severing enzymes. Curr Opin Cell Biol 22 96 103
7. Roll-MecakAValeRD 2006 Making more microtubules by severing: a common theme of noncentrosomal microtubule arrays? J Cell Biol 175 849 851
8. McNallyFJValeRD 1993 Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell 75 419 429
9. HartmanJJMahrJMcNallyKOkawaKIwamatsuA 1998 Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell 93 277 287
10. SalinasSCarazo-SalasREProukakisCSchiavoGWarnerTT 2007 Spastin and microtubules: Functions in health and disease. J Neurosci Res 85 2778 2782
11. CoxGAMahaffeyCLNystuenALettsVAFrankelWN 2000 The mouse fidgetin gene defines a new role for AAA family proteins in mammalian development. Nat Genet 26 198 202
12. McNallyKPBazirganOAMcNallyFJ 2000 Two domains of p80 katanin regulate microtubule severing and spindle pole targeting by p60 katanin. J Cell Sci 113 Pt 9 1623 1633
13. SharmaNBryantJWlogaDDonaldsonRDavisRC 2007 Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J Cell Biol 178 1065 1079
14. MainsPEKemphuesKJSprungerSASulstonIAWoodWB 1990 Mutations affecting the meiotic and mitotic divisions of the early Caenorhabditis elegans embryo. Genetics 126 593 605
15. YuWSolowskaJMQiangLKarabayABairdD 2005 Regulation of microtubule severing by katanin subunits during neuronal development. J Neurosci 25 5573 5583
16. ZhangDGrodeKDStewmanSFDiaz-ValenciaJDLieblingE 2011 Drosophila katanin is a microtubule depolymerase that regulates cortical-microtubule plus-end interactions and cell migration. Nat Cell Biol 13 361 370
17. SudoHBaasPW 2010 Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J Neurosci 30 7215 7226
18. Toyo-OkaKSasakiSYanoYMoriDKobayashiT 2005 Recruitment of katanin p60 by phosphorylated NDEL1, an LIS1 interacting protein, is essential for mitotic cell division and neuronal migration. Hum Mol Genet 14 3113 3128
19. McKenneyRJVershininMKunwarAValleeRBGrossSP 2010 LIS1 and NudE induce a persistent dynein force-producing state. Cell 141 304 314
20. McNallyKAudhyaAOegemaKMcNallyFJ 2006 Katanin controls mitotic and meiotic spindle length. J Cell Biol 175 881 891
21. BusterDMcNallyKMcNallyFJ 2002 Katanin inhibition prevents the redistribution of gamma-tubulin at mitosis. J Cell Sci 115 1083 1092
22. McNallyKPMcNallyFJ 2011 The spindle assembly function of Caenorhabditis elegans katanin does not require microtubule-severing activity. Mol Biol Cell 22 1550 1560
23. LoughlinRWilburJDMcNallyFJNedelecFJHealdR 2011 Katanin contributes to interspecies spindle length scaling in Xenopus. Cell 147 1397 1407
24. ZhangDRogersGCBusterDWSharpDJ 2007 Three microtubule severing enzymes contribute to the “Pacman-flux” machinery that moves chromosomes. J Cell Biol 177 231 242
25. CasanovaMCrobuLBlaineauCBourgeoisNBastienP 2009 Microtubule-severing proteins are involved in flagellar length control and mitosis in Trypanosomatids. Mol Microbiol 71 1353 1370
26. DymekEELefebvrePASmithEF 2004 PF15p is the chlamydomonas homologue of the Katanin p80 subunit and is required for assembly of flagellar central microtubules. Eukaryot Cell 3 870 879
27. JamsaiDO'BryanMK 2010 Genome-wide ENU mutagenesis for the discovery of novel male fertility regulators. Syst Biol Reprod Med 56 246 259
28. O'DonnellLNichollsPKO'BryanMKMcLachlanRIStantonPG 2011 Spermiation: The process of sperm release. Spermatogenesis 1 14 35
29. ChalmelFRollandADNiederhauser-WiederkehrCChungSSDemouginP 2007 The conserved transcriptome in human and rodent male gametogenesis. Proc Natl Acad Sci U S A 104 8346 8351
30. RussellLDRussellJAMacGregorGRMeistrichML 1991 Linkage of manchette microtubules to the nuclear envelope and observations of the role of the manchette in nuclear shaping during spermiogenesis in rodents. Am J Anat 192 97 120
31. NayerniaKVautiFMeinhardtACadenasCSchweyerS 2003 Inactivation of a testis-specific Lis1 transcript in mice prevents spermatid differentiation and causes male infertility. J Biol Chem 278 48377 48385
32. AkhmanovaAMausset-BonnefontALvan CappellenWKeijzerNHoogenraadCC 2005 The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis. Genes Dev 19 2501 2515
33. KierszenbaumAL 2002 Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail. Mol Reprod Dev 63 1 4
34. KierszenbaumALTresLL 2004 The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol 67 271 284
35. KierszenbaumALRivkinETresLLYoderBKHaycraftCJ 2011 GMAP210 and IFT88 are present in the spermatid golgi apparatus and participate in the development of the acrosome-acroplaxome complex, head-tail coupling apparatus and tail. Dev Dyn 240 723 736
36. IshikawaHMarshallWF 2011 Ciliogenesis: building the cell's antenna. Nat Rev Mol Cell Biol 12 222 234
37. HermoLPelletierRMCyrDGSmithCE 2010 Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: changes in spermatid organelles associated with development of spermatozoa. Microsc Res Tech 73 279 319
38. AhmadFJYuWMcNallyFJBaasPW 1999 An essential role for katanin in severing microtubules in the neuron. J Cell Biol 145 305 315
39. Diaz-ValenciaJDMorelliMMBaileyMZhangDSharpDJ 2011 Drosophila katanin-60 depolymerizes and severs at microtubule defects. Biophys J 100 2440 2449
40. CondeCCaceresA 2009 Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 10 319 332
41. YamaguchiNTakanezawaYKoizumiHUmezu-GotoMAokiJ 2004 Expression of NUDEL in manchette and its implication in spermatogenesis. FEBS Lett 566 71 76
42. SraykoMBusterDWBazirganOAMcNallyFJMainsPE 2000 MEI-1/MEI-2 katanin-like microtubule severing activity is required for Caenorhabditis elegans meiosis. Genes Dev 14 1072 1084
43. SudoHMaruY 2007 LAPSER1 is a putative cytokinetic tumor suppressor that shows the same centrosome and midbody subcellular localization pattern as p80 katanin. FASEB J 21 2086 2100
44. LohretTAMcNallyFJQuarmbyLM 1998 A role for katanin-mediated axonemal severing during Chlamydomonas deflagellation. Mol Biol Cell 9 1195 1207
45. RasiMQParkerJDFeldmanJLMarshallWFQuarmbyLM 2009 Katanin knockdown supports a role for microtubule severing in release of basal bodies before mitosis in Chlamydomonas. Mol Biol Cell 20 379 388
46. BorgCLWolskiKMGibbsGMO'BryanMK Phenotyping male infertility in the mouse: how to get the most out of a ‘non-performer’. Hum Reprod Update 16 205 224
47. CottonLGibbsGMSanchez-PartidaLGMorrisonJRde KretserDM 2006 FGFR-1 [corrected] signaling is involved in spermiogenesis and sperm capacitation. J Cell Sci 119 75 84
48. GibbsGMOrtaGReddyTKoppersAJMartinez-LopezP Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function. Proc Natl Acad Sci U S A 108 7034 7039
49. ArsovTSilvaDGO'BryanMKSainsburyALeeNJ 2006 Fat aussie–a new Alstrom syndrome mouse showing a critical role for ALMS1 in obesity, diabetes, and spermatogenesis. Mol Endocrinol 20 1610 1622
50. SaitoKO'DonnellLMcLachlanRIRobertsonDM 2000 Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology 141 2779 2785
51. RomrellLJBellveARFawcettDW 1976 Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol 49 119 131
52. GibbsGMLoJCNixonBJamsaiDO'ConnorAE Glioma pathogenesis-related 1-like 1 is testis enriched, dynamically modified, and redistributed during male germ cell maturation and has a potential role in sperm-oocyte binding. Endocrinology 151 2331 2342
53. McNallyFJThomasS 1998 Katanin is responsible for the M-phase microtubule-severing activity in Xenopus eggs. Mol Biol Cell 9 1847 1861
Štítky
Genetika Reprodukční medicína
Článek Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication inČlánek Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the TranscriptomeČlánek Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells inČlánek Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing BoneČlánek Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 5- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Slowing Replication in Preparation for Reduction
- Chromosome Pairing: A Hidden Treasure No More
- Loss of Imprinting Differentially Affects REM/NREM Sleep and Cognition in Mice
- Six Novel Susceptibility Loci for Early-Onset Androgenetic Alopecia and Their Unexpected Association with Common Diseases
- Regulation by the Noncoding RNA
- UDP-Galactose 4′-Epimerase Activities toward UDP-Gal and UDP-GalNAc Play Different Roles in the Development of
- Deletion of PTH Rescues Skeletal Abnormalities and High Osteopontin Levels in Mice
- Karyotypic Determinants of Chromosome Instability in Aneuploid Budding Yeast
- Genome-Wide Copy Number Analysis Uncovers a New HSCR Gene:
- MicroRNA-277 Modulates the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication in
- Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the Transcriptome
- Scientist Citizen: An Interview with Bruce Alberts
- YY1 Regulates Melanocyte Development and Function by Cooperating with MITF
- Congenital Heart Disease–Causing Gata4 Mutation Displays Functional Deficits
- Recombination Drives Vertebrate Genome Contraction
- KATNAL1 Regulation of Sertoli Cell Microtubule Dynamics Is Essential for Spermiogenesis and Male Fertility
- Re-Patterning Sleep Architecture in through Gustatory Perception and Nutritional Quality
- Using Whole-Genome Sequence Data to Predict Quantitative Trait Phenotypes in
- Genome-Wide Analysis of GLD-1–Mediated mRNA Regulation Suggests a Role in mRNA Storage
- Meiotic Chromosome Pairing Is Promoted by Telomere-Led Chromosome Movements Independent of Bouquet Formation
- LINT, a Novel dL(3)mbt-Containing Complex, Represses Malignant Brain Tumour Signature Genes
- The H3K27 Demethylase UTX-1 Is Essential for Normal Development, Independent of Its Enzymatic Activity
- Suppresses Senescence Programs and Thereby Accelerates and Maintains Mutant -Induced Lung Tumorigenesis
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
- Identification of Genes That Promote or Antagonize Somatic Homolog Pairing Using a High-Throughput FISH–Based Screen
- Principles of Carbon Catabolite Repression in the Rice Blast Fungus: Tps1, Nmr1-3, and a MATE–Family Pump Regulate Glucose Metabolism during Infection
- Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells in
- Histone H3 Localizes to the Centromeric DNA in Budding Yeast
- Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes
- Hypersensitive to Red and Blue 1 and Its Modification by Protein Phosphatase 7 Are Implicated in the Control of Arabidopsis Stomatal Aperture
- Extent, Causes, and Consequences of Small RNA Expression Variation in Human Adipose Tissue
- TBC-8, a Putative RAB-2 GAP, Regulates Dense Core Vesicle Maturation in
- Regulating Repression: Roles for the Sir4 N-Terminus in Linker DNA Protection and Stabilization of Epigenetic States
- Common Genetic Determinants of Intraocular Pressure and Primary Open-Angle Glaucoma
- Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing Bone
- Fitness Landscape Transformation through a Single Amino Acid Change in the Rho Terminator
- Repeated, Selection-Driven Genome Reduction of Accessory Genes in Experimental Populations
- Allelic Variation and Differential Expression of the mSIN3A Histone Deacetylase Complex Gene Promote Mammary Tumor Growth and Metastasis
- DNA Demethylation and USF Regulate the Meiosis-Specific Expression of the Mouse
- Knowledge-Driven Analysis Identifies a Gene–Gene Interaction Affecting High-Density Lipoprotein Cholesterol Levels in Multi-Ethnic Populations
- A Duplication CNV That Conveys Traits Reciprocal to Metabolic Syndrome and Protects against Diet-Induced Obesity in Mice and Men
- EMT Inducers Catalyze Malignant Transformation of Mammary Epithelial Cells and Drive Tumorigenesis towards Claudin-Low Tumors in Transgenic Mice
- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association for Abdominal Subcutaneous and Visceral Adipose Reveals a Novel Locus for Visceral Fat in Women
- Stratifying Type 2 Diabetes Cases by BMI Identifies Genetic Risk Variants in and Enrichment for Risk Variants in Lean Compared to Obese Cases
- New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties
- Activated Cdc42 Kinase Has an Anti-Apoptotic Function
- The Region Is Critical for Birth Defects and Electrocardiographic Dysfunctions Observed in a Down Syndrome Mouse Model
- COP9 Signalosome Integrity Plays Major Roles for Hyphal Growth, Conidial Development, and Circadian Function
- Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest
- Psip1/Ledgf p52 Binds Methylated Histone H3K36 and Splicing Factors and Contributes to the Regulation of Alternative Splicing
- The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice
- Target Gene Analysis by Microarrays and Chromatin Immunoprecipitation Identifies HEY Proteins as Highly Redundant bHLH Repressors
- Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
- ELK1 Uses Different DNA Binding Modes to Regulate Functionally Distinct Classes of Target Genes
- Histone H1 Depletion Impairs Embryonic Stem Cell Differentiation
- IDN2 and Its Paralogs Form a Complex Required for RNA–Directed DNA Methylation
- Separation of DNA Replication from the Assembly of Break-Competent Meiotic Chromosomes
- Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- Slowing Replication in Preparation for Reduction
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



