-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genome-Wide Association Studies of Serum Magnesium, Potassium, and Sodium Concentrations Identify Six Loci Influencing Serum Magnesium Levels
Magnesium, potassium, and sodium, cations commonly measured in serum, are involved in many physiological processes including energy metabolism, nerve and muscle function, signal transduction, and fluid and blood pressure regulation. To evaluate the contribution of common genetic variation to normal physiologic variation in serum concentrations of these cations, we conducted genome-wide association studies of serum magnesium, potassium, and sodium concentrations using ∼2.5 million genotyped and imputed common single nucleotide polymorphisms (SNPs) in 15,366 participants of European descent from the international CHARGE Consortium. Study-specific results were combined using fixed-effects inverse-variance weighted meta-analysis. SNPs demonstrating genome-wide significant (p<5×10−8) or suggestive associations (p<4×10−7) were evaluated for replication in an additional 8,463 subjects of European descent. The association of common variants at six genomic regions (in or near MUC1, ATP2B1, DCDC5, TRPM6, SHROOM3, and MDS1) with serum magnesium levels was genome-wide significant when meta-analyzed with the replication dataset. All initially significant SNPs from the CHARGE Consortium showed nominal association with clinically defined hypomagnesemia, two showed association with kidney function, two with bone mineral density, and one of these also associated with fasting glucose levels. Common variants in CNNM2, a magnesium transporter studied only in model systems to date, as well as in CNNM3 and CNNM4, were also associated with magnesium concentrations in this study. We observed no associations with serum sodium or potassium levels exceeding p<4×10−7. Follow-up studies of newly implicated genomic loci may provide additional insights into the regulation and homeostasis of human serum magnesium levels.
Published in the journal: . PLoS Genet 6(8): e32767. doi:10.1371/journal.pgen.1001045
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001045Summary
Magnesium, potassium, and sodium, cations commonly measured in serum, are involved in many physiological processes including energy metabolism, nerve and muscle function, signal transduction, and fluid and blood pressure regulation. To evaluate the contribution of common genetic variation to normal physiologic variation in serum concentrations of these cations, we conducted genome-wide association studies of serum magnesium, potassium, and sodium concentrations using ∼2.5 million genotyped and imputed common single nucleotide polymorphisms (SNPs) in 15,366 participants of European descent from the international CHARGE Consortium. Study-specific results were combined using fixed-effects inverse-variance weighted meta-analysis. SNPs demonstrating genome-wide significant (p<5×10−8) or suggestive associations (p<4×10−7) were evaluated for replication in an additional 8,463 subjects of European descent. The association of common variants at six genomic regions (in or near MUC1, ATP2B1, DCDC5, TRPM6, SHROOM3, and MDS1) with serum magnesium levels was genome-wide significant when meta-analyzed with the replication dataset. All initially significant SNPs from the CHARGE Consortium showed nominal association with clinically defined hypomagnesemia, two showed association with kidney function, two with bone mineral density, and one of these also associated with fasting glucose levels. Common variants in CNNM2, a magnesium transporter studied only in model systems to date, as well as in CNNM3 and CNNM4, were also associated with magnesium concentrations in this study. We observed no associations with serum sodium or potassium levels exceeding p<4×10−7. Follow-up studies of newly implicated genomic loci may provide additional insights into the regulation and homeostasis of human serum magnesium levels.
Introduction
Magnesium is the second most abundant intra-cellular cation and is a co-factor in several important reactions, including nucleic acid synthesis and many enzymatic reactions [1]. Nearly 60% of magnesium in the human body resides in bone, 20% in skeletal muscle, and 20% in soft tissue. Although only a fraction of total magnesium is present in blood, serum magnesium concentrations are reported to associate with several common and chronic diseases, including diabetes [2], hypertension [3], and osteoporosis [4]. Sodium and potassium are the most abundant cations in extra - and intracellular fluids, respectively [5], and are also commonly measured in serum. They have important roles in the maintenance of fluid and electrolyte balance as well as cell excitability.
Although most magnesium deficiencies are acquired [6], serum magnesium concentrations have been shown to have a heritable component with heritability estimates of ∼30% [7], [8]. In addition, several rare monogenic disorders have been identified that are characterized by abnormalities in magnesium homeostasis [1], [6], [9], including Gitelman syndrome (OMIM #263800), Bartter syndrome (OMIM #601678, #241200, #607364), and several hypomagnesemia syndromes (OMIM #602014, #154020, #248250, #611718, and #248190). Heritability estimates for serum sodium and potassium concentrations were comparable to the ones for magnesium in previous studies [10]–[13], and several monogenic diseases with disturbances in serum potassium or sodium concentrations exist [14].
Information on common genomic variants that are associated with serum cation concentrations in the general population may provide insights into physiologic regulators of electrolyte homeostasis. Thus, we undertook genome-wide association studies (GWAS) of serum magnesium, potassium and sodium concentrations in 15,366 subjects in the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium. Since the kidney has an essential role in maintaining serum concentrations of these cations, and since magnesium, sodium, and potassium have been implicated in blood pressure regulation, we also assessed whether our newly identified variants were associated with glomerular filtration rate (eGFR) estimated from serum creatinine levels as a measure of kidney function as well as with systolic and diastolic blood pressure (SBP and DBP) in the CHARGE Consortium. We further evaluated the identified variants in association with fasting glucose in the Meta-Analyses of Glucose and Insulin Related Traits Consortium (MAGIC) [15] and bone mineral density (BMD) in the Genetic Factors for Osteoporosis (GEFOS) Consortium [16]; two continuous traits used to identify the presence of diabetes and osteoporosis.
Results
Overall, 15,366 individuals of European descent from the Atherosclerosis Risk in Communities (ARIC) Study (N = 8,122), the Framingham Heart Study (FHS; N = 2,866), and the Rotterdam Study (RS; N = 4,378) contributed data to the discovery analyses of common variants associated with serum magnesium concentrations. Meta-analysis of serum sodium concentrations included information from 11,552 individuals from the three cohorts, and 13,683 individuals contributed information to the meta-analysis of serum potassium concentrations (including 3,370 participants from the Cardiovascular Health Study [CHS]). Selected characteristics for these four study samples as well as an additional CHARGE cohort that contributed information to secondary analyses of kidney function and blood pressure [The Age, Gene/Environment Susceptibility (AGES) —Reykjavik Study (N = 3,219)] are reported in Table 1.
Tab. 1. Baseline characteristics of the CHARGE Consortium discovery cohorts.* 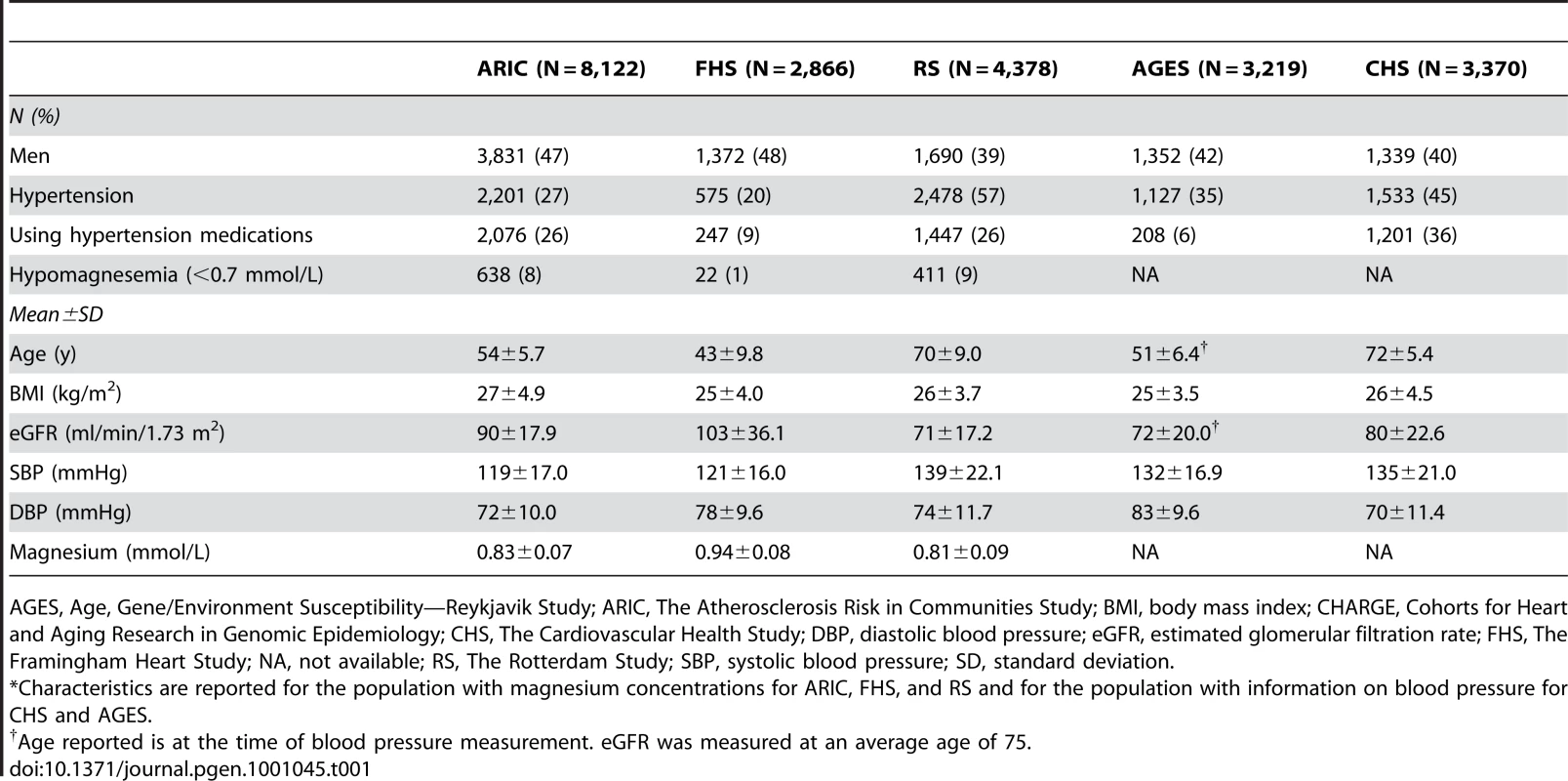
AGES, Age, Gene/Environment Susceptibility—Reykjavik Study; ARIC, The Atherosclerosis Risk in Communities Study; BMI, body mass index; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; CHS, The Cardiovascular Health Study; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FHS, The Framingham Heart Study; NA, not available; RS, The Rotterdam Study; SBP, systolic blood pressure; SD, standard deviation. In total, 2,585,820 common single nucleotide polymorphisms (SNPs) were examined in association with serum magnesium, sodium, and potassium within each study, and the findings were meta-analyzed across studies using inverse-variance weighted fixed-effects models. SNPs were imputed in the individual studies as described in Table S1. No genome-wide significant (p<5×10−8) or suggestive (p<4×10−7) results were observed for serum sodium or potassium concentrations after adjustment for age, sex, and study center (where applicable). SNPs that showed evidence for association at p<1×10−5 after correction for genomic control are provided in Table S2 (sodium) and Table S3 (potassium). Q-Q plots of the observed versus expected p-value distributions for associations between the ∼2.5 million SNPs and magnesium, sodium and potassium levels are provided in Figure S1A, S1B, S1C. Heritability of serum magnesium, sodium, and potassium was estimated in the family-based FHS. Heritability was significant for serum magnesium (0.45; SE = 0.06; p = 1×10−13, N = 2,657) but not for serum sodium (0.04; SE = 0.06; p = 0.27, N = 2,416) or potassium (0.03; SE = 0.06; p = 0.29, N = 2,418) after excluding individuals on hypertension treatment. The traits were only weakly correlated in the ARIC study, the largest cohort in CHARGE (r2≤0.15).
Figure 1 shows the Manhattan plot for associations between SNPs and magnesium levels in the discovery cohorts after adjustment for age, sex, and center (where applicable). There were six regions with variants associated with serum magnesium concentrations at a genome-wide significance level of p<5×10−8. Information about the SNP with the lowest p-value within each region (lead SNP) is presented in Table 2; the lead SNPs were located in or near MUC1 (91 kb region, chr 1), SHROOM3 (175 kb region, chr 4), TRPM6 (77 kb region, chr 9), DCDC5 (25 kb region, chr 11), ATP2B1 (233 kb region, chr 12), and PRMT7 (395 kb region, chr 16). Individually, the six genome-wide significant SNPs in the combined discovery and replication cohorts explained between 0.1 and 0.6% of the variance in serum magnesium concentrations; jointly, they explained about 1.6% of the variance (1.9% in the discovery cohorts and 1.2% in the replication cohorts). Three additional regions showed evidence of suggestive association (p<4×10−7) with serum magnesium concentrations (Table 2). Associations between the lead SNPs and serum magnesium within each of the discovery cohorts as well as their combined effect are presented in Table S4. Summary information for all SNPs associated with serum magnesium at p<10−6 is included in Table S5. Regional association plots for the six genomic regions with evidence for genome-wide association in the discovery cohorts are provided in Figure S2A, S2B, S2C, S2D, S2E, S2F. Results were similar when individuals on hypertension medications were excluded from the discovery analysis.
Fig. 1. Genome-wide –log10(p-value) plot from association analyses with serum magnesium concentrations in 15,366 participants of European ancestry from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium. 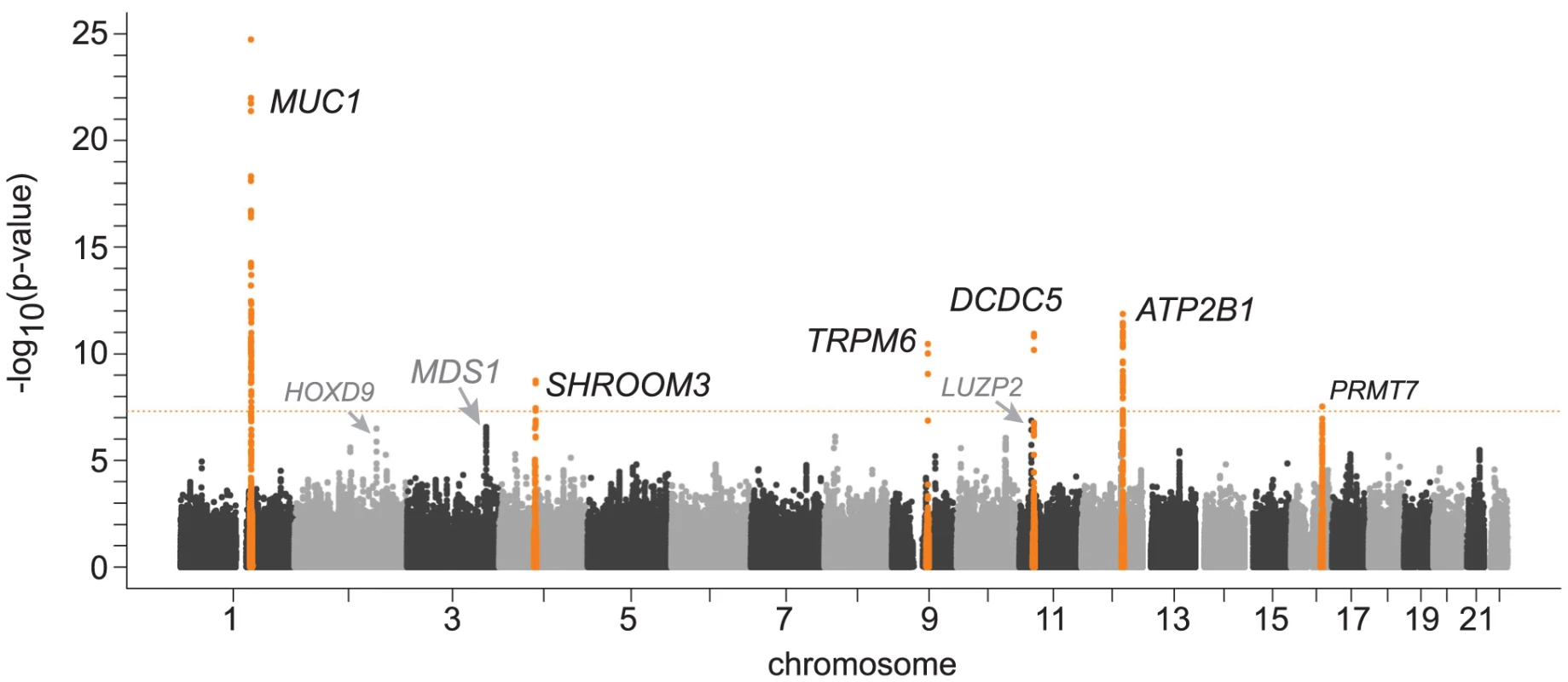
Adjusted for age, sex, and center. Tab. 2. Associations between serum magnesium levels and the lead regional genome-wide significant SNPs in the combined discovery (N = 15,366) and replication (N = 8,463) samples. 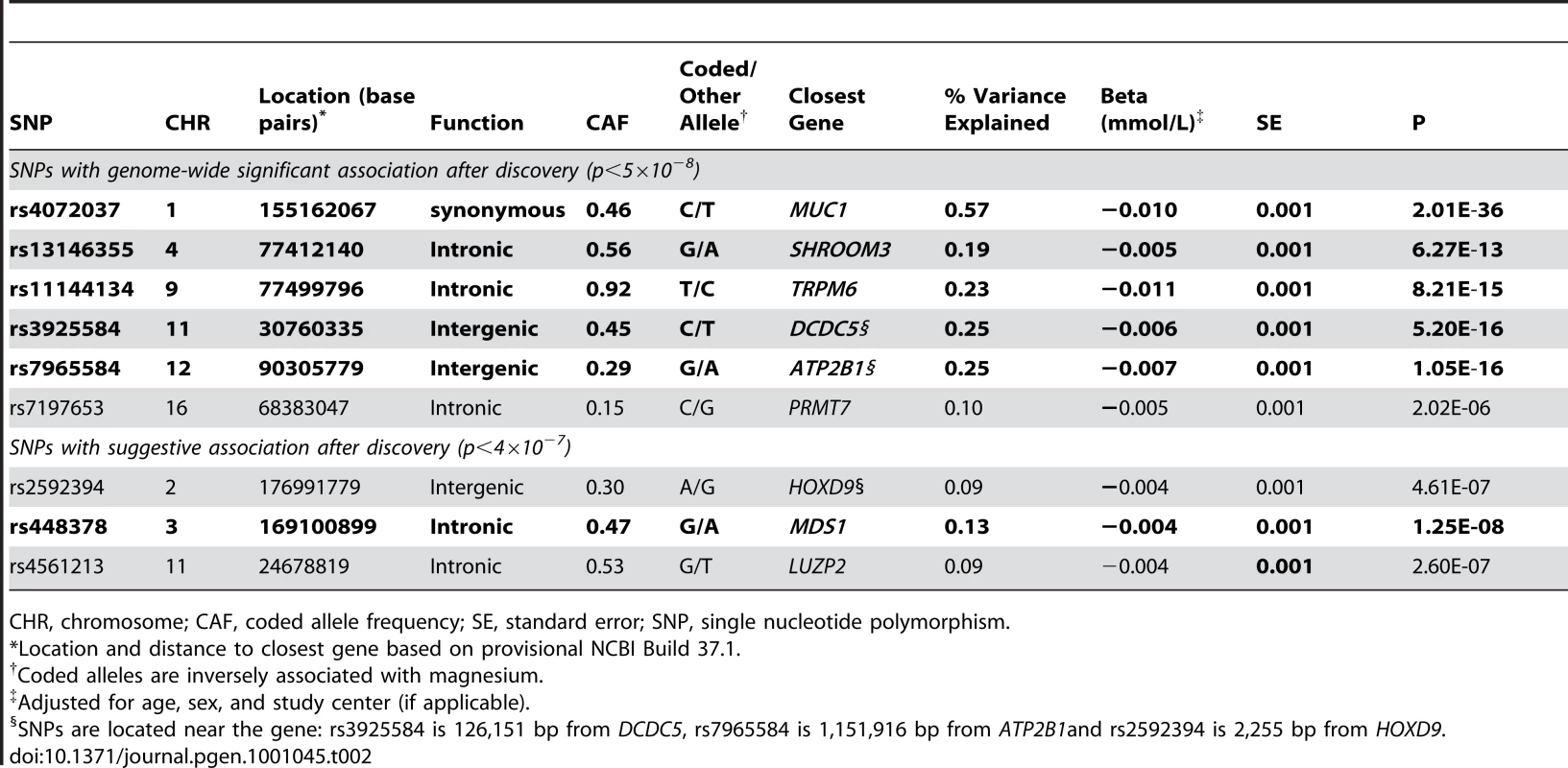
CHR, chromosome; CAF, coded allele frequency; SE, standard error; SNP, single nucleotide polymorphism. Replication of the lead SNPs with evidence of significant or suggestive association in the discovery cohorts was attempted in an additional 8,463 independent individuals of European descent (N = 1,641, KORA F3 Study; N = 1,809, KORA F4 Study; N = 4,065, SHIP Study; N = 948, ARIC Study). Mean serum magnesium levels in the replication cohorts were 0.83±0.07 (ARIC), 0.86±0.07 (KORA F3), 0.91±0.06 (KORA F4), and 0.78±0.09 (SHIP) mmol/L. At a Bonferroni-corrected significance level of 5.5×10−3 (0.05/9), five of the six SNPs with evidence of genome-wide significant association in the discovery samples showed evidence for replication in the independent replication cohorts (Table 2). Of the three SNPs with suggestive evidence for association in the discovery cohorts, the SNP at the MDS1 locus showed evidence for independent replication, and combined with the discovery samples, reached a genome-wide level of significance (Table 2). Cohort-specific associations for the replication cohorts along with summary associations are presented in Table S6. Information about the quality of imputation for the lead SNPs within each cohort is reported in Table S7.
Replicated SNPs with evidence for genome-wide association in the discovery cohorts were related to clinically relevant hypomagnesemia, using a 0.7 mmol/L cutpoint [17]. All SNPs showed nominally significant p-values, and the odds ratios ranged from 1.11 (SHROOM3) to 1.27 (MUC1) per copy of the magnesium-lowering allele (Table 3).
Tab. 3. Association between hypomagnesemia, estimated glomerular filtration rate, fasting glucose, and bone mineral density with the lead replicated SNPs showing genome-wide significant associations with serum magnesium concentrations. 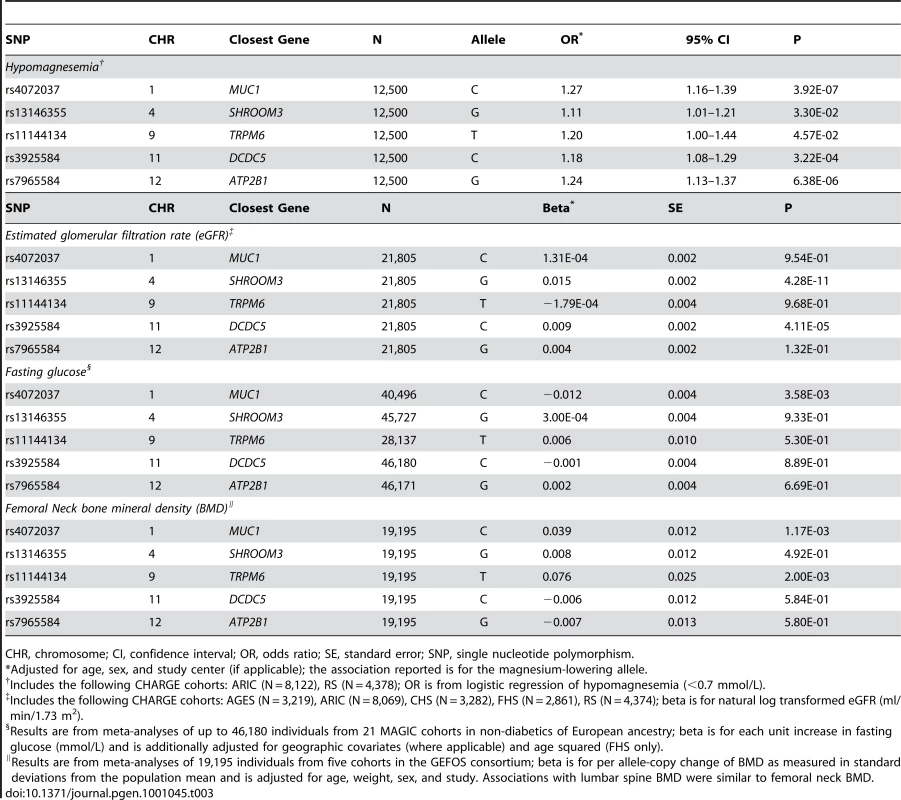
CHR, chromosome; CI, confidence interval; OR, odds ratio; SE, standard error; SNP, single nucleotide polymorphism. As the kidney is one of the primary regulators of serum magnesium concentrations, we also examined these SNPs in association with the kidney function measure, eGFR. The allele associated with lower magnesium levels at two of the SNPs showed evidence for association with higher eGFR: rs3925584 near DCDC5 (p = 4.1×10−5) and rs77412140 in SHROOM3 (p = 4.3×10−11; Table 3). Adjusting the magnesium-SNP associations for eGFR did not materially change the association with serum magnesium levels (eGFR-adjusted beta = −0.006, p = 6.3×10−12 for the DCDC5 and beta = −0.005, p = 9.5×10−9 for the SHROOM3 SNP).
Since magnesium levels associate with hypertension [3], diabetes [2], and osteoporosis [4] in observational studies, we further evaluated the lead replicated SNPs in association with the continuous traits used to define these chronic conditions: SBP and DBP (CHARGE cohorts), fasting glucose (MAGIC Consortium [15]) and BMD (GEFOS Consortium [16]). None of the SNPs was associated with SBP or DBP in our study (Table S8), but the allele associated with lower magnesium levels at the MUC1 SNP showed nominally-significant evidence of association with lower fasting glucose after correcting for the number of SNPs investigated (Bonferroni-corrected significance level = 0.05/5 = 0.01; Table 3). The same allele showed association with higher BMD, as did the magnesium-lowering allele of the TRPM6 SNP (Table 3).
Finally, in the CHARGE discovery cohorts, we evaluated genes that contain rare variants known to cause monogenic syndromes of abnormal magnesium metabolism [6] for common susceptibility variants that associate with normal magnesium levels. We also evaluated common SNPs in genes that have been implicated as magnesium transporters in model systems [18] but, to date, have an unknown functional role in humans from the general population. The number of SNPs per gene examined as well as summary information for the SNP with the lowest p-value from each gene are provided in Table 4. Common variants in CNNM2 (rs3740393, p = 8.6×10−7), CNNM3 (rs994430, p = 1.5×10−4) and CNNM4 (rs6746896, p = 7.0×10−5) were associated with magnesium concentrations after applying a Bonferroni-correction for the number of SNPs examined in each region.
Tab. 4. SNPs in or near genes known to cause monogenic syndromes with abnormal magnesium metabolism or near known magnesium transport genes in association with serum magnesium in 15,366 participants from the CHARGE Consortium. 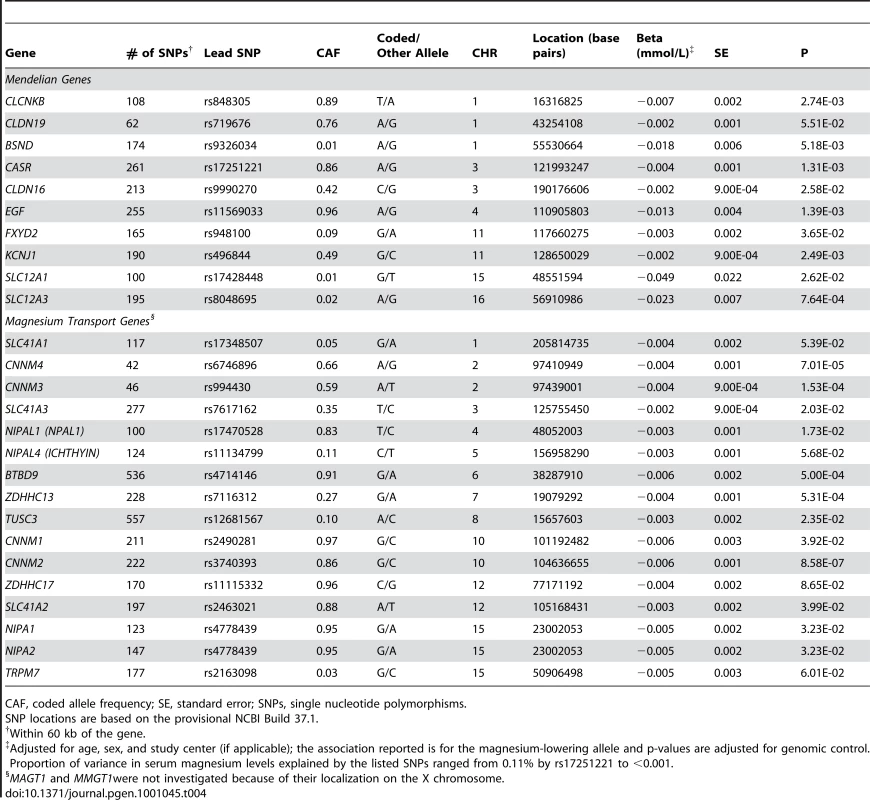
CAF, coded allele frequency; SE, standard error; SNPs, single nucleotide polymorphisms. Discussion
We report a large genome-wide association study of serum magnesium, potassium and sodium levels in 15,366 community-dwelling subjects of European ancestry from the CHARGE Consortium. Associations with serum potassium and sodium did not reach the level of genome-wide significance in our study, but common genetic variants in six genomic regions in or near the MUC1, MDS1, SHROOM3, TRPM6, DCDC5, and ATP2B1 genes were significantly and reproducibly associated with serum magnesium levels and clinically defined hypomagnesemia. Together, these SNPs explained about 1.6% of variation in serum magnesium levels. Variation at the DCDC5 SNP (rs3925584) on chromosome 11 and the SHROOM3 SNP (rs13146355) on chromosome 4 was also independently associated with eGFR, a measure of kidney function, while the MUC1 SNP (rs4072037) was associated with fasting glucose as well as BMD and the TRPM6 (rs11144134) SNP was associated with BMD. Finally, we provide evidence for a role of the magnesium transporters encoded by CNNM2 as well as CNNM3 and CNNM4 in the regulation of physiological magnesium homeostasis in humans.
Magnesium homeostasis is maintained as a balance between intestinal magnesium absorption and renal magnesium excretion [7]. Magnesium transport in the kidney occurs both by passive paracellular reabsorption in the loop of Henle and by active transcellular reabsorption in the distal convoluted tubule [7].
Of the loci discovered here, only TRPM6 on chromosome 9 had a previously known role in magnesium homeostasis. TRPM6 encodes a TRP ion channel subunit, which is abundantly expressed in the gut and the kidney [19], [20], where it is responsible for transcellular magnesium transport by mediating magnesium reuptake at the apical membrane of renal epithelial cells in the distal tubule [9]. Rare mutations in TRPM6 are a cause of autosomal recessive hypomagnesemia with secondary hypocalcemia (OMIM #602014) [19], [20]. The common T allele of rs11144134 in TRPM6 that associated with lower serum magnesium levels in our study was also associated with higher femoral neck and lumbar spine BMD. Although magnesium-deficiency has been linked to osteoporosis and low BMD in observational and animal studies [9], our observations are in line with the higher BMD observed in patients with low plasma magnesium levels as a result of Gitelman's syndrome [21].
On chromosome 12, we identified variants in the ATP2B1 gene region as associated with serum magnesium concentrations. This gene encodes plasma-membrane calcium ATPase 1 (PMCA1) [22], responsible for the removal of calcium ions from cells. One previous study reported that the phosphatase activity of PMCA1 is dependent on magnesium ions [23]. While magnesium uptake via TRPM6 at the apical membrane of epithelial cells has been demonstrated as the mechanism for magnesium entry, the mechanism by which magnesium ions exit the cells at the basolateral membrane is hitherto unknown [9]. Our epidemiologic findings relating variation in ATP2B1 to serum magnesium concentrations, combined with the localization of PMCA1 in the basolateral membrane of epithelial cells in the distal renal tubule, makes PMCA1 an interesting candidate for further functional studies of renal magnesium transport. The genomic region containing the ATP2B1 gene was previously identified in a genome-wide association study of blood pressure and hypertension [24]. Linkage disequilibrium (LD) between the blood pressure associated variant and the one reported in our study is low (r2 = 0.013 in HapMap CEU), supporting the independent effects of the two variants on blood pressure and magnesium homeostasis.
The genomic region containing the SHROOM3 gene on chromosome 4 has been associated with eGFR in a previous GWAS [25] and with serum creatinine in another large consortium study [26]. Previously described eGFR/creatinine-associated variants are in strong LD with the magnesium-associated one reported in our study (r2>0.8 in HapMap CEU), in agreement with the significant association with eGFR detected in our study (p = 4.3×10−11). The magnitude of the association between the SNP and magnesium levels remained unchanged after adjustment for eGFR, which may suggest a pleiotropic effect of the same underlying causal variant.
The region on chromosome 1 spans about 100 kb and contains many genes. The SNP with the strongest association within this region was located in the gene MUC1. MUC1 encodes mucin 1, a membrane bound, glycosylated phosphoprotein. It is attached to the apical surface of many epithelia, where it binds pathogens and functions in a cell signaling capacity. Aberrant forms of the protein have been associated with carcinomas. In addition to the observed association with lower serum magnesium levels, the C allele at rs4072037 in MUC1 was also associated with higher femoral neck and lumbar spine BMD as well as with lower fasting glucose levels in two large consortia. The direction of association with BMD is consistent with the one we observed for the magnesium-lowering allele of rs11144134 in TRPM6.
The closest gene to the associated SNP on chromosome 11, rs3925584, is doublecortin domain containing 5 (DCDC5) of currently unknown physiological function. LD in the region also extends to the neighboring MPPED2 gene, which encodes for a metallophosphoesterase that needs divalent metal ions for its catalytic activity [27]. Variants in the DCDC5 genetic region were identified in a large GWAS as associated with lumbar spine BMD [16]. Although the reported BMD-associated variant and the variant associated with serum magnesium in our study are only in low LD (r2 = 0.04 in HapMap CEU), it is of interest that variation in three regions identified in our study (MUC1, TRPM6, DCDC5) can be linked to measures of BMD. In addition, the SNP we identified near DCDC5 also showed some association with eGFR, and the association with magnesium levels remained unchanged upon adjustment for eGFR.
Finally, the rs448378 SNP on chromosome 3 is located in the myelodysplasia syndrome 1 (MDS1) gene. Like MUC1 and DCDC5, MDS1 is not an obvious candidate for magnesium homeostasis based on prior biological knowledge.
Previous studies in model systems have identified several genes coding for magnesium transport proteins [7], [28]–[34], but the contribution of common genetic variation in these genes to magnesium homeostasis in humans is unclear. Common variants in CNNM2, CNNM3, and CNNM4 showed significant association with serum magnesium concentrations in our study after applying a conservative Bonferroni correction for the number of regional SNPs investigated, supporting the role of these proteins in human magnesium homeostasis under physiological conditions. The CNNM2-encoded magnesium transporter, ACDP2, belongs to the ancient conserved domain proteins (ACDP) family [35]. It is widely expressed in human tissues with strongest levels in brain, kidney and placenta [35], and experimental studies provide evidence for its involvement in magnesium transport [29], [36]. Rare mutations in CNNM4 have recently been reported as a cause of autosomal-recessive cone-rod dystrophy with amelogenesis imperfecta [37], [38]. A magnesium transport function of the encoded ACDP4 has not yet been shown. Little is known about ACDP3 encoded by CNNM3; due to the close physical proximity of CNNM3 and CNNM4, common variants associated with magnesium concentrations in our study may not represent independent signals. We further identify several common variants in genes responsible for monogenic disorders of magnesium metabolism that show some degree of association with serum magnesium concentrations in our study. As true associations may be missed at the stringent significance levels applied in genome-wide association studies, we noted the SNP with the lowest p-value in each of the genetic regions although they did not show evidence of genome-wide significant association. These results should therefore be interpreted with caution; on the other hand, applying a region-wide Bonferroni-correction as we did for these candidate regions may be overly conservative due to the presence of linkage disequilibrium.
There are several potential explanations for the observed lack of genome-wide evidence of associations with serum potassium or sodium levels in our study. While we had based our decision to conduct GWAS of serum sodium and potassium levels on earlier point estimates for heritability on the order of 25–30%, we only observed significant heritability for serum magnesium levels but not for serum levels of sodium or potassium. These findings are not necessarily inconsistent with previous estimates since earlier studies were mostly small and the 95% confidence intervals of heritability estimates for either serum sodium or potassium concentrations or both included 0. Other potential explanations for the difference in heritability estimates include our exclusion of individuals on hypertension medication, differences in the statistical model, and differences in study sample characteristics. Another reason for the lack of findings could be that genetic variants other than common SNPs could be of importance, which could not be detected in our study. Finally, fewer individuals were available for the analyses of serum sodium and potassium concentrations compared to magnesium concentrations thus impacting statistical power. The weak correlation of serum magnesium with serum sodium and potassium levels we observed (r2≤0.15) is consistent with the identification of genomic regions specific to serum magnesium.
Several limitations of this study should be considered when interpreting the results. Serum magnesium concentrations represent only a small portion of the total magnesium stores in the body, and markers associated with serum magnesium concentrations are therefore not necessarily markers of total magnesium stores. Second, results from our study are based on individuals of European descent only and should be replicated in other ethnicities. Third, our study likely did not have sufficient power to detect common variants in association with serum sodium or potassium concentrations. Finally, the functional significance of the lead SNPs identified in this study is unknown, and true causal variants likely remain to be determined. The proportion of serum magnesium variance explained by the SNPs identified here is modest, as has been observed from GWAS of other traits [39]. However, the genes discovered in our study provide a basis for future studies of magnesium homeostasis and for the targeted investigation of the presence of rare genetic variants of larger effect.
In conclusion, we identified six genomic regions that contained common variants reproducibly associated with serum magnesium levels in a genome-wide meta-analysis of CHARGE cohorts and four independent replication cohorts. All of the variants were nominally associated with clinically defined hypomagnesemia, and lead SNPs in four of the regions were also associated with measures of kidney function, fasting glucose, and BMD. As only the TRPM6 gene was previously known to be involved in magnesium homeostasis, follow-up of the other associated regions may provide additional clues to the regulation of magnesium homeostasis in humans.
Materials and Methods
Ethics statement
Each of the cohorts collected written informed consent from study participants and received approval from their respective Institutional Review Boards.
Discovery study samples
The CHARGE Consortium was established to facilitate meta-analysis of GWAS for traits related to cardiovascular disease (CVD) and aging [40]. Briefly, five large, population-based cohort studies from the United States and Europe with genome-wide genotyping information available in 2007 to 2008 were included: AGES—Reykjavik, ARIC, CHS, FHS, and RS. Detailed information about each cohort is provided in other references (AGES—Reykjavik [41]; ARIC [42]; CHS [43]; FHS [44]–[47]; RS [48]) and is summarized below.
The AGES—Reykjavik Study includes a sample of 5,764 survivors from the Reykjavik Study of 30,795 men and women born between 1907 and 1935. The ARIC study includes 15,792 men and women aged 45 to 64 who were enrolled in a prospective follow-up study from four US communities from 1987 to 1989. The CHS includes 5,201 mostly Caucasian participants aged 65 years or older that were randomly sampled from Medicare lists in four US communities from 1989 to 1990. The FHS recruited 5,209 participants aged 28 to 62 from Framingham, Massachusetts beginning in 1948. Beginning in 1971, 5,124 offspring of the original cohort members and the offspring's spouses were also recruited as part of the Offspring Cohort. FHS subjects in this study are from the Offspring Cohort who attended the second examination in 1971–1973. Finally, the RS recruited 7,983 subjects aged 55 years or older from Ommoord, a suburb of Rotterdam, between 1990 and 1993. Only subjects self-reporting European ancestry from each cohort are included as a part of this study.
Replication study samples
ARIC Study
After conducting the meta-analysis within the CHARGE Study, genotype data on an additional 948 study participants of European ancestry became available within ARIC. These individuals were independent from the ones included in the discovery sample: they were not part of the discovery, had no first-degree relationship with any individual in the discovery sample, and would not have been classified as an outlier based on allele sharing measures generated during quality control procedures of the discovery sample.
KORA F3 and F4
The KORA Study is a series of independent population-based epidemiological surveys of participants living in the region of Augsburg, Southern Germany [49]. All survey participants were residents of German nationality identified through the registration office and were examined in 1994/95 (KORA S3) and 1999/2001 (KORA S4). In 2004/05, 3,006 subjects participated in a 10-year follow-up examination of S3 (KORA F3) and in 2006/08, 3,080 subjects participated in a 7-year follow-up examination of S4 (KORA F4). Individuals for genotyping in KORA F3 and KORA F4 were randomly selected. The age range of the participants was 25 to 74 years at recruitment.
SHIP
The Study of Health in Pomerania (SHIP) is a cross-sectional survey in West Pomerania, the north-east area of Germany [50]. A sample from the population aged 20 to 79 years was drawn from population registries. Only individuals with German citizenship and main residency in the study area were included. Of 7,008 subjects sampled, 4,310 participants comprised the final SHIP population.
Genotyping and imputation
Details of genotyping methods, exclusion criteria, and imputation methods for the discovery and replication samples can be found in Table S1. Briefly, SNPs were genotyped within each cohort from 2006–2008 using commercially available whole-genome platforms, and each cohort imputed genotypes to a common set of about 2.5 million autosomal SNPs. Imputation was carried out using MACH version 1.09/.15/.16 (AGES—Reykjavik, ARIC, FHS, KORA and RS) (accessed from http://www.sph.umich.edu/csg/abecasis/MACH/), BimBam version 0.99 [51] software (CHS), or IMPUTEv0.5.0 [52] (SHIP). For the imputation, genotype data from the individual studies was combined with genotype data from HapMap CEU samples to probabilistically infer the allelic dosage for each SNP (a fractional value from 0.0 to 2.0) based on the HapMap CEU haplotype structure. Imputation quality scores were calculated for each SNP as the ratio of observed dosage-variance to the expected binomial variance.
Study variables
The primary outcomes for this study were serum concentrations of magnesium, potassium and sodium. We additionally evaluated the lead SNPs identified in association with other clinically-relevant phenotypes, including hypomagnesemia (defined as serum magnesium <0.7 mmol/L; CHARGE), blood pressure (CHARGE), eGFR (CHARGE) fasting glucose (MAGIC), and BMD (GEFOS). Serum magnesium (discovery: ARIC, FHS, RS; replication: ARIC, KORA F3, KORA F4, SHIP), sodium (ARIC, FHS, RS), and potassium (ARIC, CHS, FHS, RS) concentrations were measured using standard protocols from fasting blood, where possible. Serum magnesium levels were determined using the method described by Gindler and Heth with metallochromic dye, Calmigate [1,-[1-hydroxy-4-methyl-2-phenylazo)-2-napthol-4-sulfonic acid] in the ARIC Study, by METPATH in FHS, with a Merck Diagnostica kit (method Xylidyl blue) on an Elan Autoanalyzer (Merk) in RS, with a Xylidylblue kit on a Modular analyzer (Roche) in the KORA Study, or using a commercial colorimetric test (Roche Diagnostics, Mannheim, Germany) with a Hitachi 717 autoanalyzer in the SHIP Study. Sodium and potassium levels were measured using standard ion electrode devices in all cohorts.
Detailed descriptions of blood pressure and eGFR traits are given in other references [24], [25] and are described in brief here. Serum creatinine, used to calculate eGFR, was measured using a modified kinetic Jaffe method (ARIC, CHS, FHS, RS) or an enzymatic method (AGES—Reykjavik). Creatinine values were calibrated to age - and sex-adjusted mean values from a nationally representative study as described previously [53], and eGFR (ml/min/1.73 m2) was calculated using the 4-variable MDRD Study formula [54]. Due to the skewed distribution, a natural log transformation was applied before the association analyses. Repeated resting SBP and DBP measures were recorded by trained staff in all studies, and the average of multiple readings was used. Height and weight were measured by trained study personnel in all studies and were used to calculate BMI (kg/m2). Use of blood pressure medications was defined differently in the different cohorts, but for all cohorts, hypertension medication use was determined at the time of serum electrolyte determination and included all classes of anti-hypertension medications commonly prescribed at the time, including beta-blockers, diuretics, ACE-inhibitors, angiotensin type-2 antagonists, calcium-channel blockers, as well as combination therapies.
Statistical analysis
SNPs were modeled as allelic dosages in all analyses. Genome-wide analyses of electrolyte concentrations (magnesium, potassium, and sodium) were conducted within the R package ProbABEL (http://mga.bionet.nsc.ru/~yurii/ABEL/) [55] for ARIC, CHS and RS, or using linear mixed effects regression models in the R kinship package to account for pedigree structure in FHS. SNP-electrolyte associations were adjusted for age, sex, and study center, where applicable. For analyses of sodium and potassium concentrations subjects using any hypertension medications at the time of electrolyte assessment were excluded to avoid a possible influence of the medications on serum concentrations of sodium and potassium. Genomic control correction based on median chi-square was used within each study to adjust for inflation of the test statistics prior to meta-analysis, as well as applied to the combined results after the meta-analysis. Inverse-variance weighted fixed-effects meta-analyses were carried out by two independent analysts using the software METAL (www.sph.umich.edu/csg/abecasis/metal/) for the ∼2.5 million SNPs across ARIC, FHS, RS, and CHS (potassium only). After meta-analysis, results were filtered to remove SNPs with low minor allele frequency (<0.01). Statistical heterogeneity was evaluated using Cochrane's χ2 test (Q-test). P-values <5×10−8 were used to indicate genome-wide significant results. The size of the associated regions was determined using the positions of the most upstream and downstream regional SNPs with p-values<5×10−5. Manhattan and Q-Q plots were generated for the meta-analyzed data using the R statistical software package (http://www.R-project.org). Plots of the –log10(p-values) by genomic position for associations within regions of statistical significance were generated using the SNAP program (http://www.broad.mit.edu/mpg/snap/ldsearch.php). In SNAP, the HapMap CEU population was used as the reference group to map LD patterns. In the family-based FHS, heritability of serum magnesium, sodium, and potassium was estimated using age and sex-adjusted residuals in a variance components model that estimated additive genetic heritability and a random environmental component using SOLAR v.1.4 [56].
The six lead SNPs with evidence of genome-wide significant association in discovery plus an additional three SNPs with suggestive evidence of association were evaluated for independent replication. In the replication studies, SNP-magnesium associations were determined in linear regression models as described for the discovery cohorts. Inverse-variance weighted fixed effects meta-analysis was used to determine associations across the replication samples and to calculate the overall combined associations for the discovery and replication cohorts.
For the lead SNPs, we calculated the percent of magnesium variance attributable to the SNP as the difference in the adjusted r2 value for a model containing the SNP, age, sex, and study center, where applicable, to a model containing only age, sex and study center, expressed as a percent. Assuming independent effects of the SNPs, we added the individual variance across the SNPs to calculate the total variance explained by the set of SNPs. The independence assumption was verified by simultaneous inclusion of all SNPs into a regression model. We also evaluated the five lead SNPs from CHARGE with evidence for replication in logistic models of hypomagnesemia (in ARIC and RS only because of small numbers of subjects with hypomagnesemia in FHS) or linear models of eGFR (ml/min/1.73 m2) adjusted for age, sex, and study center. Results for blood pressure (mm Hg) traits in association with the SNPs were adjusted for age, age squared, sex, and BMI to be consistent with the published data from a GWAS of blood pressure in the CHARGE Consortium, and blood pressure among treated and untreated individuals was modeled as described in this publication [24], [57]. Inverse-variance weighted fixed effects meta-analysis was used to determine summary effect estimates for these additional traits.
We further evaluated these SNPs in association with fasting glucose and BMD as an in silico lookup in large available datasets from two consortia. Fasting glucose associations were available from up to 46,180 subjects of European descent from the MAGIC Consortium [15], and BMD associations (femoral neck and lumbar spine) in 19,195 subjects of Northern European descent from the GEFOS Consortium [16].
To examine the association between serum magnesium levels and common variation in previously identified magnesium transporter proteins from model systems [7], [28]–[34] or in genes with rare variants responsible for monogenic disorders of magnesium metabolism, we examined associations with SNPs within 60 kb of the genes [58] and reported the association and annotation for the lead SNP within each gene region.
Supporting Information
Zdroje
1. SwaminathanR
2003 Magnesium metabolism and its disorders. Clin Biochem Rev 24 47 66
2. KaoWHL
FolsomAR
NietoFJ
MoJ-P
WatsonRL
1999 Serum and dietary magnesium and the risk for type 2 diabetes mellitus: The Atherosclerosis Risk in Communities Study. Arch Intern Med 159 2151 2159
3. WittemanJ
GrobbeeD
DerkxF
BouillonR
de BruijnA
1994 Reduction of blood pressure with oral magnesium supplementation in women with mild to moderate hypertension. Am J Clin Nutr 60 129 135
4. WallachS
1990 Effects of magnesium on skeletal metabolism. Magnes Trace Elem 9 1 14
5. AaronsonPS
BoronWF
BoulpaepEL
2005 The Physiology of Membranes.
BoronWF
BoulpaepEL
Medical Physiology. Updated ed Philadelphia Elsevier Saunders 50 86
6. NaderiASA
ReillyRF
2008 Hereditary etiologies of hypomagnesemia. Nat Clin Pract Neph 4 80 89
7. ColeDE
QuammeGA
2000 Inherited disorders of renal magnesium handling. J Am Soc Nephrol 11 1937 1947
8. HunterDJ
LangeMd
SniederH
MacGregorAJ
SwaminathanR
2002 Genetic contribution to renal function and electrolyte balance: a twin study. Clin Sci 103 259 265
9. AlexanderRT
HoenderopJG
BindelsRJ
2008 Molecular determinants of magnesium homeostasis: insights from human disease. J Am Soc Nephrol 19 1451 1458
10. BathumL
FagnaniC
ChristiansenL
ChristensenK
2004 Heritability of biochemical kidney markers and relation to survival in the elderly—results from a Danish population-based twin study. Clinica Chimica Acta 349 143 150
11. MarroniF
GrazioD
PattaroC
DevotoM
PramstallerP
2008 Estimates of Genetic and environmental contribution to 43 quantitative traits support sharing of a homogeneous environment in an isolated population from South Tyrol, Italy. Hum Hered 65 175 182
12. NilssonSE
ReadS
BergS
JohanssonB
2009 Heritabilities for fifteen routine biochemical values: findings in 215 Swedish twin pairs 82 years of age or older. Scand J Clin Lab Invest 69 562 569
13. PiliaG
ChenW-M
ScuteriA
OrrúM
AlbaiG
2006 Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet 2 e132 doi:10.1371/journal.pgen.0020132
14. KletaR
BockenhauerD
2006 Bartter Syndromes and other salt-losing Tubulopathies. Nephron Physiol 104 73 80
15. DupuisJ
LangenbergC
ProkopenkoI
SaxenaR
SoranzoN
2010 New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42 105 116
16. RivadeneiraF
StyrkársdottirU
EstradaK
HalldórssonBV
HsuY-H
2009 Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41 1199 1206
17. LandahlS
GraffnerC
JagenburgR
LundborgP
SteenB
1980 Prevalence and treatment of hypomagnesemia in the elderly studies in a representative in 70-year-old population and in geriatric patients. Aktuelle Gerontol 10 397 403
18. QuammeGA
2010 Molecular identification of ancient and modern mammalian magnesium transporters. Am J Physiol Cell Physiol 298 C407 429
19. SchlingmannKP
WeberS
PetersM
Niemann NejsumL
VitzthumH
2002 Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31 166 170
20. WalderRY
LandauD
MeyerP
ShalevH
TsoliaM
2002 Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31 171 174
21. Nicolet-BarousseL
BlanchardA
RouxC
PietriL
Bloch-FaureM
2005 Inactivation of the Na-Cl co-transporter gene is associated with high BMD through both renal and bone mechanisms: analysis of patients with Gitelman Syndrome and Ncc null mice. J Bone Miner Res 20 799 808
22. VermaAK
FiloteoAG
StanfordDR
WiebenED
PennistonJT
1988 Complete primary structure of a human plasma membrane Ca2+ pump. J Biol Chem 263 14152 14159
23. MazzitelliLR
AdamoHP
2007 The phosphatase activity of the plasma membrane Ca2+ pump. Activation by acidic lipids in the absence of Ca2+ increases the apparent affinity for Mg2+. Biochim Biophys Acta 1768 1777 1783
24. LevyD
EhretGB
RiceK
VerwoertGC
LaunerLJ
2009 Genome-wide association study of blood pressure and hypertension. Nat Genet 41 677 687
25. KottgenA
GlazerNL
DehghanA
HwangS-J
KatzR
2009 Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41 712 717
26. PattaroC
De GrandiA
VitartV
HaywardC
FrankeA
2010 A meta-analysis of genome-wide data from five European isolates reveals an association of COL22A1, SYT1, and GABRR2 with serum creatinine level. BMC Med Genet 11 41
27. TyagiR
ShenoyAR
VisweswariahSS
2009 Characterization of an evolutionarily conserved metallophosphoesterase that is expressed in the fetal brain and associated with the WAGR Syndrome. J Biol Chemy 284 5217 5228
28. GoytainA
HinesRM
QuammeGA
2008 Functional characterization of NIPA2, a selective Mg2+ transporter. Am J Physiol Cell Physiol 295 C944 953
29. GoytainA
QuammeGA
2005 Functional characterization of ACDP2 (ancient conserved domain protein), a divalent metal transporter. Physiol Genomics 22 382 389
30. GoytainA
QuammeGA
2005 Functional characterization of human SLC41A1, a Mg2+ transporter with similarity to prokaryotic MgtE Mg2+ transporters. Physiol Genomics 21 337 342
31. GoytainA
QuammeGA
2008 Identification and characterization of a novel family of membrane magnesium transporters, MMgT1 and MMgT2. Am J Physiol Cell Physiol 294 C495 502
32. KolisekM
ZsurkaG
SamajJ
WeghuberJ
SchweyenRJ
2003 Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. Embo J 22 1235 1244
33. SchlingmannKP
WaldeggerS
KonradM
ChubanovV
GudermannT
2007 TRPM6 and TRPM7—Gatekeepers of human magnesium metabolism. Biochim Biophys Acta 1772 813 821
34. ShuenAY
WongBY
WeiC
LiuZ
LiM
2009 Genetic determinants of extracellular magnesium concentration: analysis of multiple candidate genes, and evidence for association with the estrogen receptor alpha (ESR1) locus. Clin Chim Acta 409 28 32
35. WangC-Y
ShiJ-D
YangP
KumarPG
LiQ-Z
2003 Molecular cloning and characterization of a novel gene family of four ancient conserved domain proteins (ACDP). Gene 306 37 44
36. WillC
BreiderhoffT
ThumfartJ
StuiverM
KopplinK
2010 Targeted deletion of murine Cldn16 identifies extra - and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am J Physiol Renal Physiol doi:10.1152/ajprenal.00499.02009
37. ParryDA
MighellAJ
El-SayedW
ShoreRC
JaliliIK
2009 Mutations in CNNM4 cause Jalili Syndrome, consisting of autosomal-recessive cone-rod dystrophy and amelogenesis imperfecta. Am J Hum Genet 84 266 273
38. PolokB
EscherP
AmbresinA
ChoueryE
BolayS
2009 Mutations in CNNM4 cause recessive cone-rod dystrophy with amelogenesis imperfecta. Am J Hum Genet 84 259 265
39. ManolioTA
CollinsFS
CoxNJ
GoldsteinDB
HindorffLA
2009 Finding the missing heritability of complex diseases. Nature 461 747 753
40. PsatyBM
O'DonnellCJ
GudnasonV
LunettaKL
FolsomAR
2009 Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet 2 73 80
41. HarrisTB
LaunerLJ
EiriksdottirG
KjartanssonO
JonssonPV
2007 Age, Gene/Environment Susceptibility-Reykjavik Study: Multidisciplinary Applied Phenomics. Am J Epidemiol 165 1076 1087
42. The ARIC Investigators 1989 The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 129 687 702
43. FriedL
BorhaniN
EnrightP
FurbergC
GardinJ
1991 The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1 263 276
44. DawberTR
KannelWB
LyellLP
1963 An approach to longitudinal studies in a community: The Framingham Study. Ann N Y Acad Sci 107 539 556
45. FeinleibM
KannelWB
GarrisonR
McNamaraP
CastelliW
1975 The Framingham Offspring Study. Design and preliminary data. Prev Med 4 518 525
46. GarrisonRJ
CastelliWP
FeinleibM
KannelWB
HavlikRJ
1979 The association of total cholesterol, triglycerides and plasma lipoprotein cholesterol levels in first degree relatives and spouse pairs. Am J Epidemiol 110 313 321
47. SplanskyGL
CoreyD
YangQ
AtwoodLD
CupplesLA
2007 The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 165 1328 1335
48. HofmanA
BretelerM
van DuijnC
JanssenH
KrestinG
2009 The Rotterdam Study: 2010 objectives and design update. Eur J Epidemiol 24 553 572
49. WichmannHE
GiegerC
IlligT
for the MKSG 2005 KORA-gen - Resource for population genetics, controls and a broad spectrum of disease phenotypes. KORA-gen - Ressource für Bevölkerungsgenetik, Kontrolle und ein breites Spektrum an Krankheitsphänotypen 67 26 30
50. JohnU
GreinerB
HenselE
LudemannJ
PiekM
2001 Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Soz Praventivmed 46 186 94
51. ServinB
StephensM
2007 Imputation-based analysis of association studies: candidate regions and quantitative traits. PLoS Genet 3 e114 doi:10.1371/journal.pgen.0030114
52. MarchiniJ
HowieB
MyersS
McVeanG
DonnellyP
2007 A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 39 906 913
53. CoreshJ
AstorBC
McQuillanG
KusekJ
GreeneT
2002 Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 39 920 929
54. LeveyAS
BoschJP
LewisJB
GreeneT
RogersN
1999 A more accurate method to estimate glomerular filtration rate from serum creatinine: A New Prediction Equation. Ann Intern Med 130 461 470
55. AulchenkoYS
RipkeS
IsaacsA
van DuijnCM
2007 GenABEL: an R library for genome-wide association analysis. Bioinformatics 23 1294 1296
56. AlmasyL
BlangeroJ
1998 Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 62 1198 1211
57. CuiJS
HopperJL
HarrapSB
2003 Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension 41 207 210
58. DehghanA
KöttgenA
YangQ
HwangS-J
KaoWHL
2008 Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. The Lancet 372 1953 1961
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 8- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
-
Všechny články tohoto čísla
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Mutation in the Gene Encoding Ubiquitin Ligase LRSAM1 in Patients with Charcot-Marie-Tooth Disease
- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Did Genetic Drift Drive Increases in Genome Complexity?
- The 5p15.33 Locus Is Associated with Risk of Lung Adenocarcinoma in Never-Smoking Females in Asia
- An Alpha-Catulin Homologue Controls Neuromuscular Function through Localization of the Dystrophin Complex and BK Channels in
- Epigenetically-Inherited Centromere and Neocentromere DNA Replicates Earliest in S-Phase
- Survival and Growth of Yeast without Telomere Capping by Cdc13 in the Absence of Sgs1, Exo1, and Rad9
- Tuberous Sclerosis Complex 1 Regulates dE2F1 Expression during Development and Cooperates with RBF1 to Control Proliferation and Survival
- Disease-Associated Mutations That Alter the RNA Structural Ensemble
- The Transcriptomes of Two Heritable Cell Types Illuminate the Circuit Governing Their Differentiation
- Inactivation of VCP/ter94 Suppresses Retinal Pathology Caused by Misfolded Rhodopsin in
- Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: a Meta-Analysis and Comparison with Lung Cancer and COPD
- Transcriptional Regulation by CHIP/LDB Complexes
- Conserved Role of in Ethanol Responses in Mutant Mice
- A Global Overview of the Genetic and Functional Diversity in the Pathogenicity Island
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3–Dependent Manner in
- Genetic Analysis of Baker's Yeast Msh4-Msh5 Reveals a Threshold Crossover Level for Meiotic Viability
- Genome-Wide Association Studies of Serum Magnesium, Potassium, and Sodium Concentrations Identify Six Loci Influencing Serum Magnesium Levels
- Something New: An Interview with Radoje Drmanac
- The Extinction Dynamics of Bacterial Pseudogenes
- Microtubule Actin Crosslinking Factor 1 Regulates the Balbiani Body and Animal-Vegetal Polarity of the Zebrafish Oocyte
- Consistent Association of Type 2 Diabetes Risk Variants Found in Europeans in Diverse Racial and Ethnic Groups
- Transmission of Mitochondrial DNA Diseases and Ways to Prevent Them
- Telomere Disruption Results in Non-Random Formation of Dicentric Chromosomes Involving Acrocentric Human Chromosomes
- Chromosome Axis Defects Induce a Checkpoint-Mediated Delay and Interchromosomal Effect on Crossing Over during Drosophila Meiosis
- Dynamic Chromatin Organization during Foregut Development Mediated by the Organ Selector Gene PHA-4/FoxA
- Ancient Protostome Origin of Chemosensory Ionotropic Glutamate Receptors and the Evolution of Insect Taste and Olfaction
- A Wnt-Frz/Ror-Dsh Pathway Regulates Neurite Outgrowth in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Did Genetic Drift Drive Increases in Genome Complexity?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



