-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDynamic Chromatin Organization during Foregut Development Mediated by the Organ Selector Gene PHA-4/FoxA
Central regulators of cell fate, or selector genes, establish the identity of cells by direct regulation of large cohorts of genes. In Caenorhabditis elegans, foregut (or pharynx) identity relies on the FoxA transcription factor PHA-4, which activates different sets of target genes at various times and in diverse cellular environments. An outstanding question is how PHA-4 distinguishes between target genes for appropriate transcriptional control. We have used the Nuclear Spot Assay and GFP reporters to examine PHA-4 interactions with target promoters in living embryos and with single cell resolution. While PHA-4 was found throughout the digestive tract, binding and activation of pharyngeally expressed promoters was restricted to a subset of pharyngeal cells and excluded from the intestine. An RNAi screen of candidate nuclear factors identified emerin (emr-1) as a negative regulator of PHA-4 binding within the pharynx, but emr-1 did not modulate PHA-4 binding in the intestine. Upon promoter association, PHA-4 induced large-scale chromatin de-compaction, which, we hypothesize, may facilitate promoter access and productive transcription. Our results reveal two tiers of PHA-4 regulation. PHA-4 binding is prohibited in intestinal cells, preventing target gene expression in that organ. PHA-4 binding within the pharynx is limited by the nuclear lamina component EMR-1/emerin. The data suggest that association of PHA-4 with its targets is a regulated step that contributes to promoter selectivity during organ formation. We speculate that global re-organization of chromatin architecture upon PHA-4 binding promotes competence of pharyngeal gene transcription and, by extension, foregut development.
Published in the journal: . PLoS Genet 6(8): e32767. doi:10.1371/journal.pgen.1001060
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001060Summary
Central regulators of cell fate, or selector genes, establish the identity of cells by direct regulation of large cohorts of genes. In Caenorhabditis elegans, foregut (or pharynx) identity relies on the FoxA transcription factor PHA-4, which activates different sets of target genes at various times and in diverse cellular environments. An outstanding question is how PHA-4 distinguishes between target genes for appropriate transcriptional control. We have used the Nuclear Spot Assay and GFP reporters to examine PHA-4 interactions with target promoters in living embryos and with single cell resolution. While PHA-4 was found throughout the digestive tract, binding and activation of pharyngeally expressed promoters was restricted to a subset of pharyngeal cells and excluded from the intestine. An RNAi screen of candidate nuclear factors identified emerin (emr-1) as a negative regulator of PHA-4 binding within the pharynx, but emr-1 did not modulate PHA-4 binding in the intestine. Upon promoter association, PHA-4 induced large-scale chromatin de-compaction, which, we hypothesize, may facilitate promoter access and productive transcription. Our results reveal two tiers of PHA-4 regulation. PHA-4 binding is prohibited in intestinal cells, preventing target gene expression in that organ. PHA-4 binding within the pharynx is limited by the nuclear lamina component EMR-1/emerin. The data suggest that association of PHA-4 with its targets is a regulated step that contributes to promoter selectivity during organ formation. We speculate that global re-organization of chromatin architecture upon PHA-4 binding promotes competence of pharyngeal gene transcription and, by extension, foregut development.
Introduction
Selector genes govern the fates of groups of cells related to each other by virtue of their cell type, position or affiliation to an organ [1]. Genomic methods have revealed that selector genes directly control hundreds, even thousands, of target genes, which define the characteristics of a particular cell type [2]–[6]. For example, the mesodermal factor Twist regulates genes that control mesodermal behaviors including gastrulation, migration and proliferation [7]. The myogenic regulatory factor MyoD directly activates skeletal muscle genes during both early cell-fate specification and later differentiation [4], [8]. The global regulatory strategy of selector genes raises the question of how targets of broadly active selector genes are expressed selectively at the appropriate times and places.
The selector gene pha-4/FoxA plays a broad role in the development and physiology of the C. elegans digestive tract. PHA-4 establishes the diverse cell types of the C. elegans pharynx during early embryogenesis, and drives differentiation and morphogenesis at later stages [9]–[12]. After birth, PHA-4 is required for growth and gonadogenesis in larvae [2], [13]–[15] and promotes longevity in adults [16], [17]. The targets of PHA-4 are likely distinct in different tissues and at different developmental stages. For example, numerous PHA-4 target genes have been identified within the pharynx, but most of these are not active in the intestine or gonad [2], [11], [18]. Recent chromatin immunoprecipitation data with tagged PHA-4 suggest different genes are bound by PHA-4 at different developmental stages [19]. How is appropriate regulation of PHA-4 target genes achieved? One mechanism is combinatorial control by PHA-4 with other transcription factors. A single PHA-4 binding site is not sufficient for transcriptional activation, and most foregut promoters carry four or more cis-regulatory elements that contribute towards appropriate spatial and temporal expression [13], [18], [20]–[25]. In addition, DNA binding affinity of PHA-4 for target genes modulates the timing of activation [2], [18]. High affinity sites promote earlier transcriptional onset compared to lower affinity sites, within the context of the intact cis regulatory region [2]. These studies suggest that binding affinity, feed-forward loops, positive feedback and combinatorial control, are necessary to achieve accurate temporal gene expression. However, it is still largely unknown how spatial regulation is accomplished. For example, why are pharyngeal genes active in the pharynx but not in the intestine, despite the widespread expression of PHA-4 in both organs?
Studies have implicated the nuclear periphery for modulation of gene transcription. Active and inducible genes are recruited to nuclear pores [26]–[30]. Conversely, nuclear lamins and their associated proteins have been associated with transcriptional repression and chromatin organization [31]–[36]. Inactive genes are often positioned at the nuclear lamina [37], and tethering of genes to the nuclear lamina can reduce expression levels [38], [39]. This effect is not comprehensive, however, as some peripherally-located genes are active [38]–[41]. These results indicate that the nuclear lamina is transcriptionally competent, and raise the question of the nature and degree of lamina-mediated repression.
The nuclear lamina of C. elegans is composed of a single B-family lamin (lmn-1; [34], [42], three associated LEM proteins [43] and additional factors [44]. Loss of LMN-1 leads to embryonic arrest by the 300-cell stage, with chromosome bridges between sister cells [34]. Inactivation of the LEM protein emr-1/Ce-emerin has no obvious phenotype on its own and produces viable animals, but inactivation of both emr-1 and a second LEM protein man-1/Ce-MAN1, causes lethality at around the 100 cell stage with phenotypes similar to those of lmn-1 [43], [45]. Barrier to autointegration factor BAF-1 is a fourth lamina protein required for chromosome segregation and integrity of the lamina [46], [47]. BAF-1 associates with cis-regulatory sites within the promoters of eff-1 and aff-1, and is required to repress eff-1 expression in epidermal seam cells [31]. These data implicate the C. elegans nuclear lamina for transcriptional repression, but the mechanism is unknown.
In this study, we probe the role of PHA-4 for pharyngeal gene activation, using artificial chromosomes to monitor PHA-4 binding and activity in living embryos [48]–[52]. We find that PHA-4 associates with its targets long before their activation. This association is restricted to a subset of pharyngeal cells, despite the ubiquitous expression of PHA-4 throughout the digestive tract, and is modulated by the nuclear lamina protein EMR-1/Emerin. Binding of PHA-4 leads to extensive chromatin decompaction and repositioning, in a process that precedes transcription. Previous studies implicated mammalian FoxA factors for local opening of chromatin and inhibition of linker histones [53]. Our data suggest that, in addition to local alterations, FoxA factors can induce large-scale changes in chromatin architecture, which may contribute to the long-range effects of FoxA proteins on transcription and recombination [54], [55]. These studies provide a framework for understanding the cell-type biases of selector genes for their targets.
Results
pax-1 is expressed in a subset of pharyngeal cells, and its expression is regulated by cis-regulatory elements that cooperate with PHA-4/FoxA
Our goal was to explore PHA-4 association with its target genes in living embryos. We chose to analyze myo-2, which is a well-characterized gene expressed exclusively in pharyngeal muscles [56], [57], and pax-1, which we show below is a PHA-4 target expressed in the pharyngeal marginal cells and some other pharyngeal cell types. To initiate the study, we characterized pax-1 cis-regulatory sites for pharyngeal expression.
To analyze pax-1, we constructed two GFP reporters: a translational fusion within the second exon of pax-1 (PAX-1::GFP; Table S1A) and a transcriptional fusion between GFP and the pax-1 translation initiation site (pax-1::GFP). These constructs revealed that pax-1 was expressed in 14 pharyngeal cells, which included nine marginal cells, the e2 epithelial cells and the pm8 muscle, based on morphology, position and co-staining for marginal cell filaments (Figure 1B, Figure S1). We focus on the marginal cells here. Expression of pax-1::GFP in marginal cells was first detectable in two rows of pharyngeal nuclei shortly after embryonic cell division ceased, at the late-bean to early-comma stages of development (). Expression gradually faded during later embryogenesis and was undetectable in larvae or adult worms.
Fig. 1. Scanning mutagenesis of the pax-1 promoter. 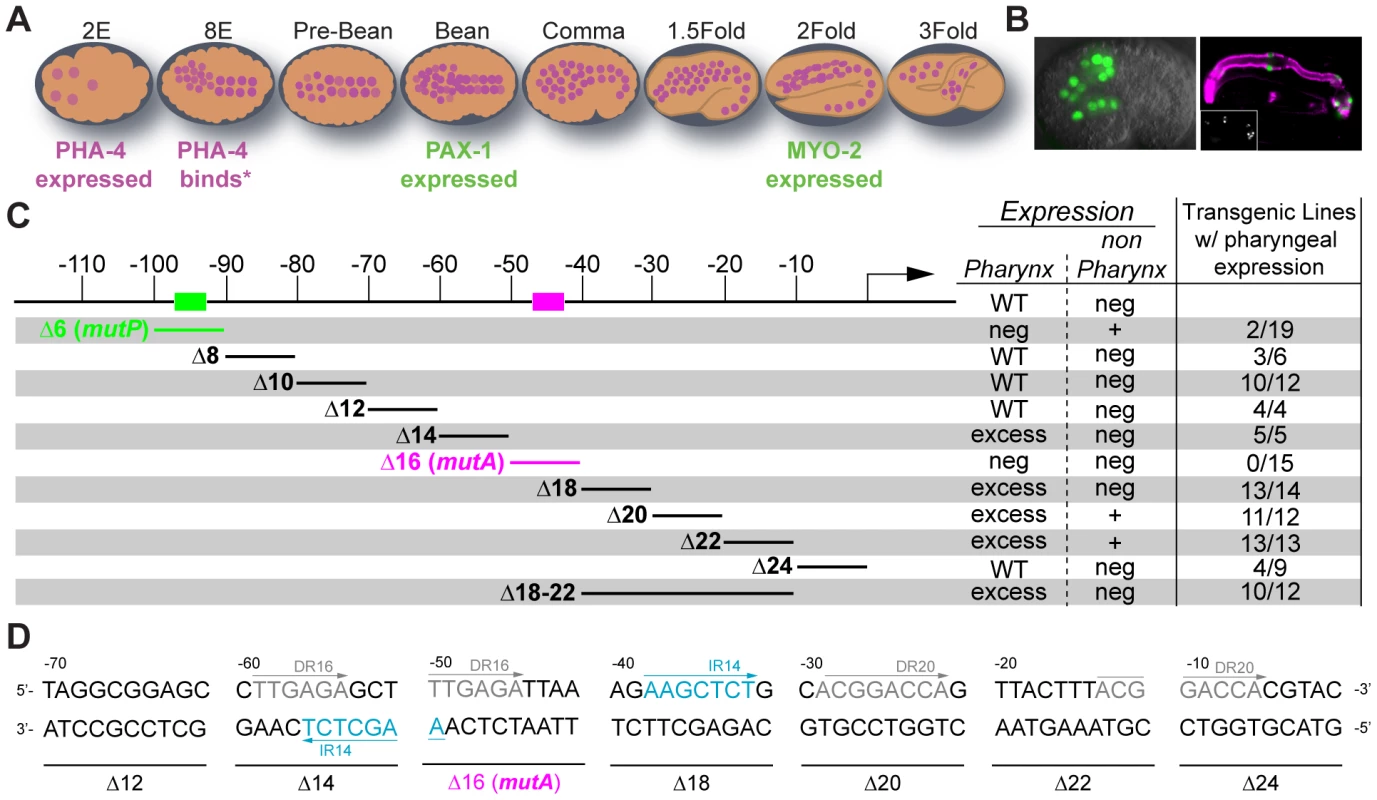
(A) A cartoon depicting the pattern of PHA-4 expression during different stages of embryogenesis. Embryonic events that occur at specific developmental stages are annotated. PHA-4 expression from [9][10], myo-2 expression from [99] and pax-1 expression from this study. (B) pax-1::GFP expression in 14 pharyngeal cells in a comma stage embryo (left) and GFP (green; anti-GFP Molecular Probes) co-stained with anti-intermediate filament antibody (magenta, right, [95]). Marginal cells and pm8 are visible, but e2 cells are faint in this image. GFP alone shown in the inset. (C) Linker scanning mutagenesis reveals two positive cis-regulatory sites: mutP (green) and mutA (magenta).% pharyngeal expression: number of independent lines with pharyngeal expression/total number of independent lines analyzed. Neg: negative. WT: wild type. (D) The architecture of the pax-1 promoter 70 bp upstream to the TSS revealing direct repeats (DR in grey) and inverted repeats (IR in blue). Examination of the pax-1 promoter revealed a consensus PHA-4 binding site between −92 and −98 base pairs (bp) upstream of the transcriptional start site (Figure 1C). Gaudet et al. previously showed that three copies of this site were sufficient to activate expression of a heterologous promoter within pharyngeal cells [18]. Conversely, we found that deletion () or mutation (MutP Figure 1C) of the predicted PHA-4-binding site eliminated pax-1::GFP expression in 17/19 transgenic lines (Figure 1C). We speculate that the 2/19 lines with residual pax-1::GFP expression in pharyngeal cells may be activated by cryptic enhancers originating from nearby sequences in the array. Interestingly, in addition to loss of pharyngeal expression, the mutant reporters exhibited significant ectopic GFP in the epidermis (Figure 1C and Figure S3). Together, these results suggest the PHA-4 binding site is required to activate expression in the pharynx and repress expression in epidermal cells.
To identify additional cis-regulatory sites within the pax-1 promoter, we performed linker-scanning mutational analysis beginning −115 bp upstream of the pax-1 transcriptional start site (Figure 1C). This survey revealed a second activation site within Delta16, which we will refer to as mutA: replacement of 10 bp from mutA abolished all GFP reporter expression (TTGAGATTAA; Figure 1D). Scanning mutagenesis also uncovered two negative regulatory regions. First, mutations in either Delta14 or Delta18 generated a high proportion of transgenic lines that expressed pax-1::GFP in additional pharyngeal cells, to approximately 20 cells (Figure S4A). Second, mutations in Delta20, and to a lesser degree Delta22, lead to GFP+ cells outside of the pharynx (Figure S4B). In sum, mutational analysis revealed both positive and negative cis-regulatory sites that cooperated with PHA-4 to activate pax-1 within pharyngeal marginal cells. A direct repeat (TTGAGA) lies within Delta14 and Delta16, and an inverted repeat (AAGCTCT) lies within Delta14 and Delta18, suggesting one or both of these may be recognition sites for transcription factors (Figure 1D). These cis-regulatory sites provided a means to examine the role of PHA-4 for pharyngeal gene activation, described below.
PHA-4 binds to its pharyngeal targets hours before the onset of gene expression
The mutational analysis suggested that both myo-2 and pax-1 were direct PHA-4 target genes. To test this idea further, we used the Nuclear Spot Assay (NSA) to examine association of PHA-4 with pharyngeal promoters in vivo. The NSA allowed us to track PHA-4 binding to promoters in living embryos, with precise spatial and temporal resolution. For this assay, we constructed a transgene array or “pseudo-chromosome” that carried multiple copies of a target promoter and the Lac operator [48]–[52], [58]. A co-selectable marker (to identify transgenic animals) and herring sperm genomic DNA (to provide sequence complexity without added C. elegans' sequences [59]) were also included. The pseudo-chromosome arrays carried fusions of CFP::LacI and PHA-4::YFP; CFP::LacI bound to LacO sequences on the arrays and revealed their position and morphology in the nucleus. PHA-4::YFP bound to its promoter appeared as a dense magenta “dot” that colocalized with CFP::LacI. Diffuse PHA-4::YFP in the background indicated binding of PHA-4::YFP to genomic loci. It was previously shown that the NSA method accurately reflected transcriptional regulation, as detected by other methods such as chromatin immunoprecipitation [50], [52]. C. elegans arrays are relatively stable through mitosis and meiosis, and are incorporated into chromatin [59], [60]. However, we recognize that pseudo-chromosomes are not replicas of C. elegans chromosomes, and they likely differ from endogenous chromosomes in some regards.
We observed multiple pharyngeal cells with PHA-4::YFP enriched on pseudo-chromosome arrays, supporting the notion that myo-2 and pax-1 are direct PHA-4 targets (Figure 2 and an additional third target C44H4.1: Figure S5A). The association was detected by the 8E (endodermal) stage, which was the earliest stage we could visualize PHA-4::YFP (∼100 cells). The proportion of embryos with associated PHA-4::YFP remained relatively constant until the two-fold (pax-1) or three-fold (myo-2) stages. The robust association of PHA-4 to its target promoters required a consensus PHA-4 binding site since pseudo-chromosome arrays that carried a promoter with mutated PHA-4 binding sites [2] failed to recruit PHA-4::YFP (Figure 2). These data reveal that PHA-4 bound target promoters long before they were transcriptionally active, indicating that PHA-4 occupancy did not correlate with transcriptional activity per se, but rather with transcriptional potential. Similarly, vertebrate FoxA2, which is orthologous to PHA-4, binds the albumin promoter in mouse endodermal cells long before the gene is active [61].
Fig. 2. PHA-4 associates with pharyngeal target promoters by the 8E (∼100 cell) stage. 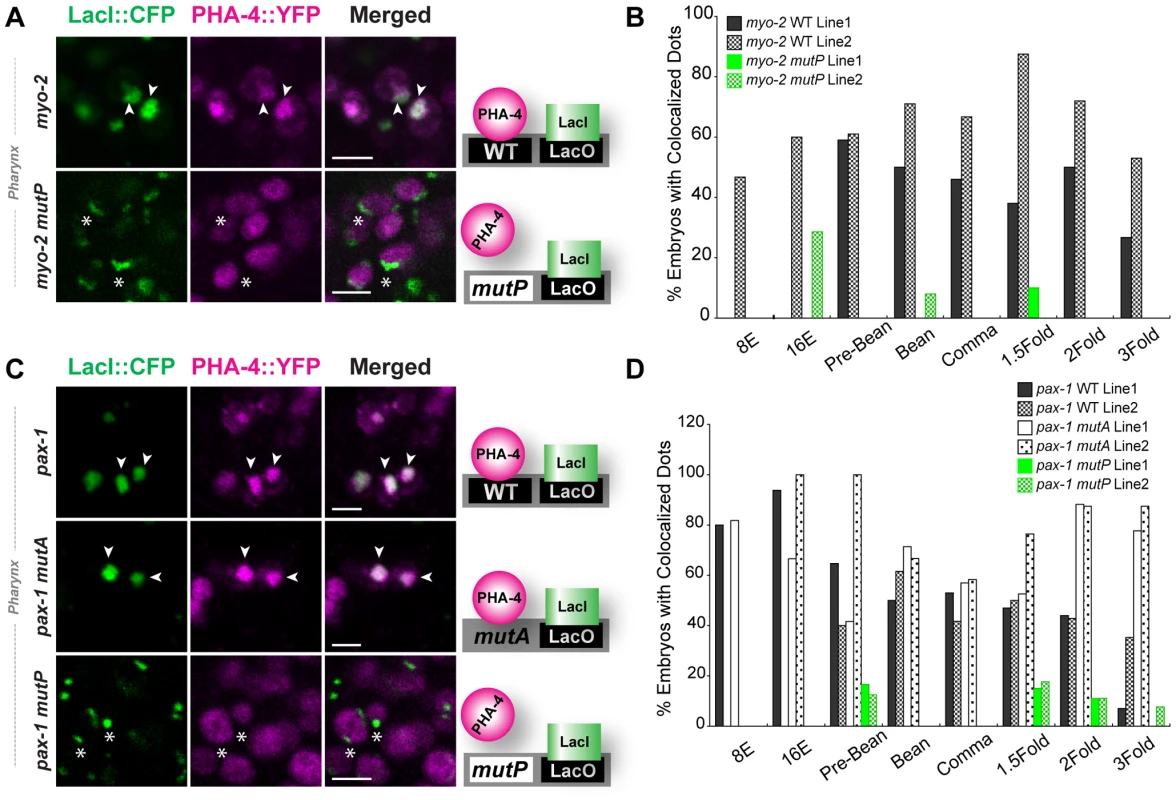
CFP::LacI (depicted in green) and PHA-4::YFP (depicted in magenta) co-localization on pseudo-chromosomes bearing (A) myo-2 or (C) pax-1 promoters. Merge is white. Binding to pseudo-chromosomes is abolished by mutating the PHA-4 binding sites in myo-2 mutP or pax-1 mutP but is not affected when an unrelated activation site is mutated (pax-1 mutA). The cartoon illustrates the interpretation of the data. (B, D) Quantitation of embryos with co-localized CFP::LacI and PHA-4::YFP in transgenic lines bearing (B) a wild-type myo-2 promoter (solid and hatched black, WT) or one with mutated PHA-4 sites (solid and hatched green, mutP) and (D) a wild-type pax-1 promoter, a mutant promoter lacking PHA-4 binding sites (pax-1 mutP) or a mutant promoter inactivated for an unrelated activation site (pax-1 mutA) (white and dotted, mutA1). Numbers of embryos scored per stage shown in Figure S5C. Scale bar, 3 microns. Arrowheads indicate PHA-4 bound (co-localized) pseudo-chromosomes. Asterisks indicate arrays that lack associated PHA-4::YFP. PHA-4 binding leads to large-scale chromatin decompaction that is largely independent of transcription
What are the repercussions of PHA-4 association to target genes? Previous genetic studies suggested that PHA-4 and its orthologues influence the chromatin environment [11], [53], [62]–[64]. For example, PHA-4 recruits the histone variant HTA.Z/HTZ-1 to a subset of pharyngeal promoters, including that of myo-2 [63], and it interacts genetically with predicted chromatin regulators [11], [63]. Vertebrate orthologues of PHA-4 associate with chromatin and can block compaction by H1 histones [53], [54], [65]. These observations prompted us to examine the chromatin morphology of the pseudo-chromosome arrays.
We observed progressive decompaction of pseudo-chromosomes bearing wild-type myo-2 or pax-1 promoters, detectable as a large, diffuse dot (Figure 3A). We quantified the changes by measuring the areas of individual pseudo-chromosomes and analyzing the areas with Cox regression models (Materials and Methods). This analysis revealed that both the number of decompacted pseudo-chromosomes and the degree of decompaction increased over time (Figure 3B, Table S2, Table S3). This effect was observed within pharyngeal cells, the eventual site of myo-2 and pax-1 expression, but not in non-pharyngeal cells (Figure 3). Cumulative areas were larger in the pharynx compared to “outside” the pharynx as early as the pre-bean stage for myo-2 (p = 7×10−11) and the bean stage for pax-1 (p = 0.007). For myo-2, many pseudo-chromosomes became decompacted prior to transcription at the 2-fold stage and remained decompacted. For pax-1, decompaction began at the comma stage and was maximal at the 1.5 and 2-fold stages, when pax-1 is transcribed (Figure 3B). In sum, pax-1 and myo-2 pseudo-chromosomes underwent decompaction preceding and during transcription within the pharynx. In contrast, pseudo-chromosomes in which PHA-4 binding sites had been mutated behaved similarly in pharyngeal and non-pharyngeal cells, with little increase in size over time (Figure 4). These observations indicate that PHA-4 is required for large-scale decompaction of chromatin in extragenic arrays.
Fig. 3. Decompaction of pseudo-chromosomes during pharyngeal differentiation. 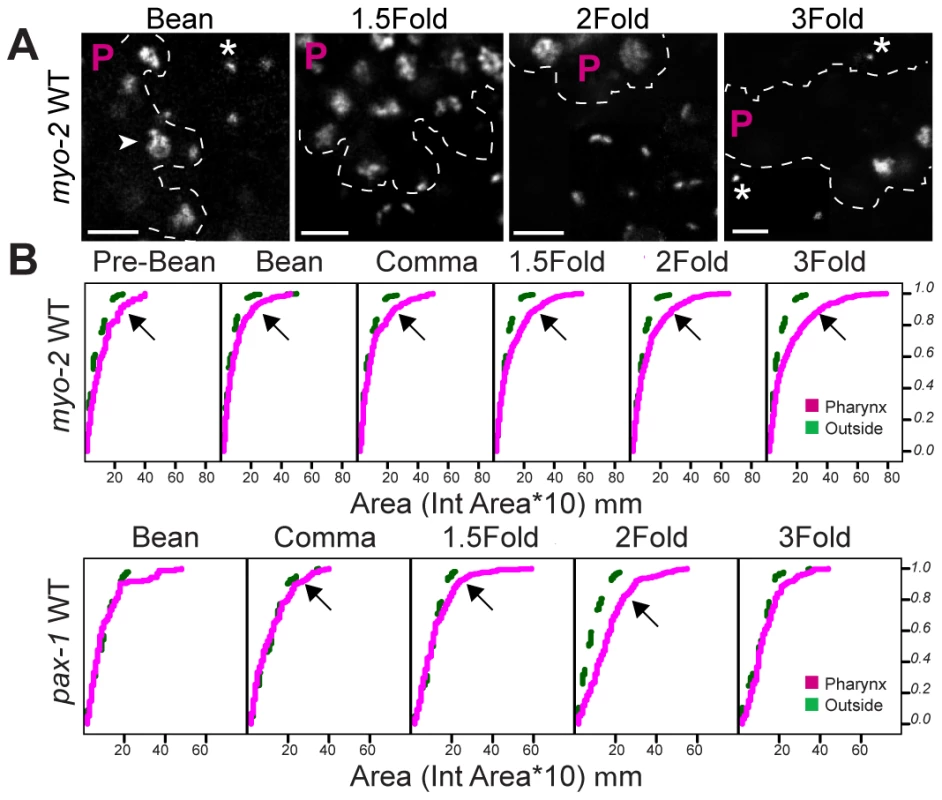
(A) Pseudo-chromosomes bearing wild-type myo-2 promoters within the pharynx (P region) or outside, at the indicated stages. Decompacted (arrow) and compacted (asterisk) pseudo-chromosomes are noted. PHA-4::YFP was used to identify pharyngeal cells (not shown). Scale bar, 3 microns. (B) Cumulative distributions of areas for pseudo-chromosomes bearing wild-type myo-2 or pax-1 promoters at the indicated developmental stages. The horizontal axis represents the area of individual pseudo-chromosomes multiplied by 10. The vertical axis represents the cumulative proportion of pseudo-chromosomes with an equal or smaller area. Curves shifted to the right, indicate a greater proportion of pseudo-chromosomes with large areas, for pharyngeal cells (magenta) relative to cells outside of the pharynx (green). Areas of pseudo-chromosomes increased as embryos developed (p = 0.00003 for myo-2, p = 0.0002 for pax-1). For myo-2, n = 2 lines, 10 embryos per stage per line. For pax-1, n = 1 line, 5 embryos per stage. Fig. 4. PHA-4 is required for chromatin decompaction. 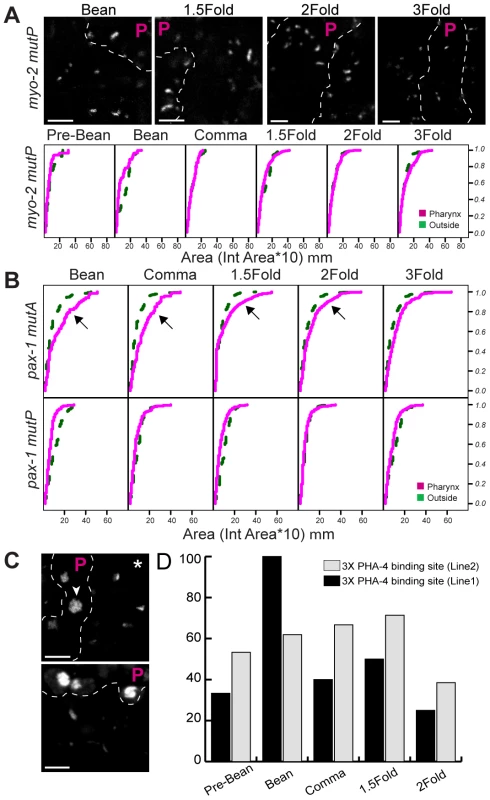
(A) Pseudo-chromosomes bearing mutated PHA-4 binding sites within myo-2 either within the pharynx (P region) or outside, at the indicated stages. PHA-4::YFP was used to identify pharyngeal cells (not shown). Scale bar, 3 microns. Cumulative distributions of pseudo-chromosome areas for (A) mutant myo-2 or (B) mutant pax-1 pseudo-chromosomes. Lines analyzed were mutated for PHA-4 binding sites within myo-2 (MutP), the PHA-4 binding site within pax-1 (MutP) or an alternative activation site within pax-1 (mutA). The horizontal axis represents the area of individual pseudo-chromosomes multiplied by 10. The vertical axis represents the cumulative proportion of pseudo-chromosomes with an equal or smaller area. Note the overlap of pseudo-chromosome areas for PHA-4-binding mutations within the pharynx (magenta) and outside of the pharynx (green), indicating no induced decompaction. For myo-2, n = 2 lines, 10 embryos per stage, per line. For pax-1, n = 1 line each mutant, 5 embryos per stage. (C) Pseudo-chromosomes bearing 3X PHA-4 binding site repeats within the pharynx (P region) or outside, at the bean stage (upper) and 2-Fold stage (lower). PHA-4::YFP was used to identify pharyngeal cells (not shown). Scale bar, 3 mm. Note the decompaction with pharyngeal cells (arrowheads) relative to non-pharyngeal cells (asterisk). (D) Quantitation of embryos with co-localized CFP::LacI and PHA-4::YFP in transgenic lines bearing 3X repeats. We considered three spurious reasons for changing pseudo-chromosome areas, independent of PHA-4. First, we examined whether array sizes were a consequence of expanding nuclear size. However, nuclear size remained relatively constant at the stages assayed in this study, and no normalization to nuclear size was necessary (Figure S5D). Second, we tested whether decompaction reflected an artificial interaction between LacI and PHA-4. However, PHA-4 binding and consequent decompaction of pseudo-chromosomes was observed in transgenic lines lacking LacI protein (Figure S5B). Third, we wondered if 3D volumetric measurements would be more accurate than the 2D area measurements used here. 3D analysis was subject to photobleaching of the YFP signal while collecting Z-stacks, which hindered 3D reconstruction. A comparison of area versus volume measurements in embryos with minimal photobleaching revealed a similar trend in array expansion (Figure S7). These controls suggest that array decompaction reflects PHA-4 interactions with target chromatin.
PHA-4 is a critical regulator of pharyngeal gene transcription, and transcription is often associated with chromatin decondensation [66]–[69]. We therefore tested whether decompaction of pseudo-chromosome arrays in pharyngeal cells reflected PHA-4 binding or transcriptional activity. When the PHA-4 binding site is mutated in the myo-2 promoter, myo-2 is still transcribed, but at a later developmental time [2]. We observed little pseudo-chromosome decompaction for arrays bearing this mutant promoter (Figure 4). Residual decompaction was observed at the 3-fold stage, which may reflect transcriptional activity. This result suggests that PHA-4 is a critical contributor to large-scale decompaction of myo-2, especially at early developmental stages. Conversely, we examined pseudo-chromosome arrays bearing mutant pax-1 mutA promoters, which were no longer transcribed but which bound PHA-4. These arrays became decompacted despite the absence of productive transcription (inside versus outside the pharynx (p<0.0001)), whereas pax-1 mutP arrays bearing a mutated PHA-4 binding site did not (Figure 4B) (smaller inside vs. outside the pharynx p = 2×10−14). This result suggests that productive transcription is not essential for decompaction, and that PHA-4 association is sufficient. To test this idea more stringently, we created arrays bearing three repeats (3X) of a PHA-4 binding site derived from pax-1, but lacking additional promoter sequences. The 3X repeats were sufficient for PHA-4::YFP recruitment to the pseudo-chromosome and caused large-scale decompaction (Figure 4C). These data reveal that PHA-4 binding, more than ongoing transcription, induces large-scale reorganization of chromatin in developing C. elegans embryos.
PHA-4 binding is spatially regulated
PHA-4 is expressed broadly, including the pharynx, intestine, rectum, somatic gonad and some neurons [9], [10], [14], [16], [70], yet PHA-4 targets are activated in discrete cell-types. For example, pax-1 is expressed in marginal cells but not in the intestine (Figure 1). We wondered if the discriminate activation of downstream targets could be explained by regulated binding of PHA-4. PHA-4 binding was surveyed in a transgenic line carrying the pax-1 mutA promoter at three developmental stages (bean, comma and 1.5-fold) in one mid-section focal plane. Pharyngeal binding was detected in ∼67% of embryos at the bean stage (10/15), ∼58% at the comma stage (7/12), and ∼76% at the 1.5-fold stage (13/17; average 68%). By contrast, binding was almost never detected in the intestine at any stage (<1%; 0/44 embryos counted; additional embryos surveyed but not counted; Figure 5A). Similar results were observed with arrays bearing myo-2 (data not shown). An optical section through a 1.5-fold embryo sampled approximately 10 pharyngeal nuclei and 10 intestinal nuclei, indicating that the differential association of PHA-4 did not reflect different numbers of nuclei in each organ.
Fig. 5. PHA-4 binding and activity is limited in the intestine. 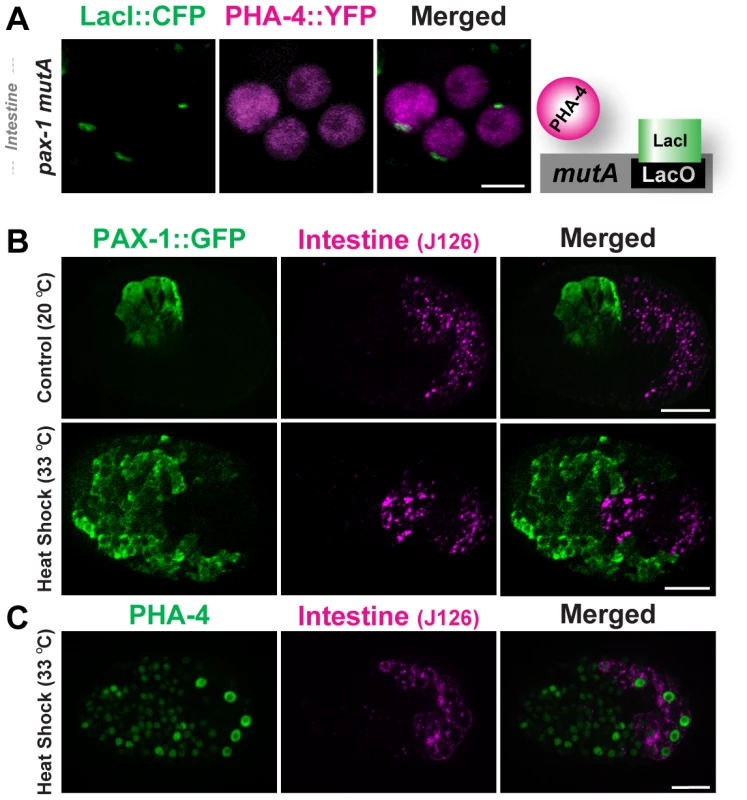
(A) PHA-4::YFP (depicted in magenta) does not associate with pseudo-chromosomes (marked with CFP::LacI, green) bearing the pax-1 mutA promoter in the intestine. The cartoon illustrates the interpretation of the data. (B) Over-expression of PHA-4 under a heat-shock promoter leads to widespread expression of PAX-1::GFP (green) in multiple tissues but not in the intestine (magenta; J126 (lower panel). Control embryos that did not receive heat-shock express PAX-1::GFP only in marginal cells (upper panel). (C) PHA-4 is expressed in all tissues after heat shock, including the intestine (magenta; J126). Scale bar, 10 microns. Does regulated binding lead to differential PHA-4 activity in disparate tissues? To answer this question we induced ectopic PHA-4 using a heat-shock promoter in transgenic lines bearing pax-1::GFP. HS::PHA-4 induced widespread expression of pax-1::GFP in many cells. However, we did not observe pax-1::GFP in the developing intestine (0/50) (Figure 5B). This absence did not reflect variable PHA-4 expression, since antibody staining demonstrated that PHA-4 was expressed in intestinal cells equivalently to other tissues after heat shock (Figure 5C). We detected no ectopic expression of the GFP reporter in non-heat shocked embryos (Figure 5B), nor did we observe ectopic expression when we tested HS::pha-4DeltaDBD [9], which lacked the DNA binding domain (data not shown). These findings indicate that PHA-4 binding to pseudo-chromosome arrays limits PHA-4 activity, and that both binding and activity are sensitive to the cellular environment. This conclusion agrees with previous observations that HS::PHA-4 can induce embryonic cells to convert to a pharyngeal fate, but that the intestine is immune to ectopic PHA-4 [9].
The integral nuclear membrane protein emr-1 regulates PHA-4 binding to targets in the pharynx
To begin to understand the selective binding of PHA-4 in different cell types, we conducted a small RNAi screen for nuclear factors that modulate PHA-4 binding to target promoters. We used SM1634 carrying a mutant pax-1 promoter because pax-1-containing arrays typically bound PHA-4::YFP in fewer pharyngeal cells than myo-2-containing arrays (data not shown). We surveyed genes involved in chromatin modification such as histone demethylation, methylation, acetylation and RNA interference. Given the proximity of the pseudo-chromosomes to the nuclear lamina, we also tested genes involved in nuclear envelope structure and function. We counted the number of nuclei with bound PHA-4::YFP in a section that passed through the pharynx and intestine of comma to 1.5 fold embryos.
Of 28 genes surveyed, emr-1/Emerin had the most dramatic effect on PHA-4 binding (Figure 6). In the control, almost half of embryos had at least one nucleus with PHA-4::YFP bound to the pax-1 promoter, with an average of 17% pharyngeal nuclei bound within an embryo (Figure 6B and 6C). Inactivation of emerin lead to a large increase in the number of pharyngeal nuclei with bound PHA-4::YFP, to ∼60% (Figure 6A and 6B). Although EMR-1 is widely expressed in all embryonic tissues [36], we observed binding only in the pharynx and not in the intestine of emr-1(RNAi) embryos (1 of 88 embryos (1.25%) in three experiments). These results reveal that the nuclear lamina interferes with binding of PHA-4::YFP to its targets within pharyngeal cells, but that additional processes function in the intestine.
Fig. 6. Emerin inhibits PHA-4 binding in the pharynx. 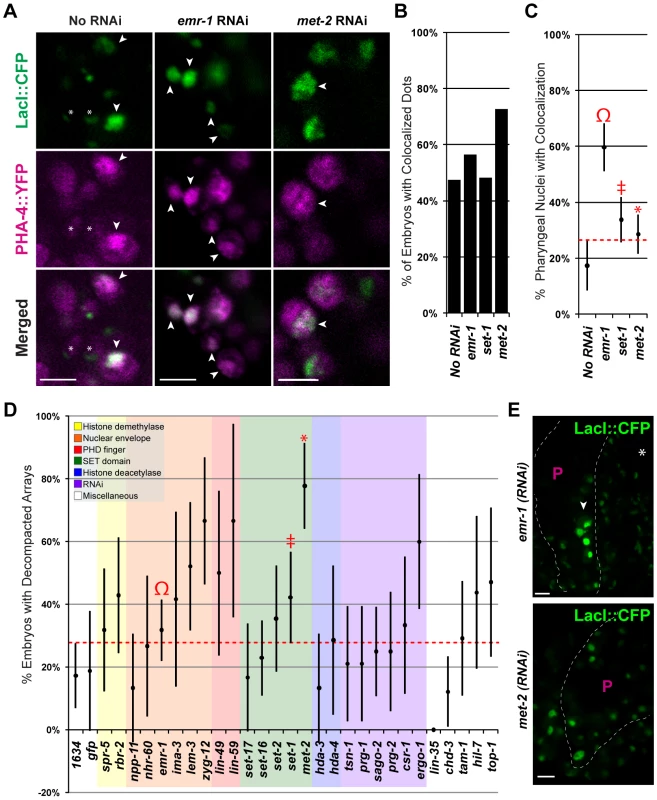
(A) CFP::LacI (depicted in green) and PHA-4::YFP (depicted in magenta) co-localization on pseudo-chromosomes bearing pax-1MutA after No RNAi, emr-1(RNAi) or met-2(RNAi). (B) Percentage of embryos with at least one co-localized dot (No RNAi 9/19, emr-1(RNAi) 13/23, set-1(RNAi) 14/29, and met-2(RNAi) 16/22) (C) Percentage of pharyngeal nuclei with bound PHA-4::YFP among embryos with co-localization. After emr-1 reduction (Ω, n = 13), embryos had a ∼3 fold increase in PHA-4::YFP binding compared to No RNAi controls (n = 9). For met-2 and set-1 n = 16 and 14, respectively D) The proportion of embryos bearing de-condensed arrays for each RNAi treatment is graphed. See Table S2 for the number of embryos assayed. (E). Example of pseudo-chromosomes after emr-1(RNAi) or met-2(RNAi). Decompaction within the pharynx (arrowhead) presumably reflects PHA-4 association. Scale bar, 3 microns. emr-1(RNAi) strongly lowered expression of EMR-1 protein (Figure S6), and promoted pseudo-chromosome decompaction compared to wild-type embryos, raising the possibility that increased binding of PHA-4 in emr-1(RNAi) embryos could be a consequence of increased accessibility. 32% (28/88) of emr-1(RNAi) embryos had decondensed arrays compared to 17% (9/52) for wild-type (Figure 6A and 6D). To explore the role of decompaction, we examined other genes for effects on pseudo-chromosome morphology and PHA-4::YFP binding. RNAi against 6 additional genes caused a global de-condensation of pseudo-chromosomal arrays at the comma to 1.5-fold stages of embryogenesis (lem-3, zyg-12, lin-59, set-1, met-2, ergo-1 Figure 6A and 6D). The arrays in these embryos appeared more distended and were brighter than wild-type embryos suggesting increased CFP::LacI expression. The de-condensation of the pseudo-chromosomes was not restricted to a specific tissue, but was observed in most nuclei in an optical section across the embryo. RNAi against set-1, a gene encoding a potential SET-domain methyltransferase [71], caused global decompaction in 19 of 45 embryos (40%, Figure 6D). The decompaction observed in set-1(RNAi) embryos was not surprising given that set-1 has been implicated in transgene silencing [72]. However, set-1(RNAi) embryos did not lead to increased PHA-4::YFP association (Figure 6B and 6C). The most dramatic effect was observed for met-2, a histone H3 lysine 9 dimethyltransferase that is homologous to human SETDB1 [73][74]. Arrays appeared more decompacted in met-2(RNAi) nuclei, and a greater proportion of arrays were decondensed compared to those in wild-type embryos (77%, 28/36, Figure 6A, 6D, and 6E). Decompaction by reduced met-2 had some effect on PHA-4 binding since a greater proportion of met-2(RNAi) embryos had PHA-4::YFP localized to pseudo-chromosomes (Figure 6C). However, within those embryos, only 29% of pharyngeal nuclei had PHA-4::YFP bound to pseudo-chromosomes, and this difference was not statistically significant from control. (Figure 6A and 6C). These data indicate that general decompaction may influence PHA-4::YFP association, but that Emerin likely modulates PHA-4::YFP binding by additional mechanisms as well.
Discussion
This study provides three insights towards understanding the regulatory strategies that drive foregut organogenesis by a selector gene. First, we have probed the association and activity of PHA-4 with its target genes in living embryos. PHA-4 binds foregut genes selectively, within the pharynx but not within a neighboring organ. This association promotes large-scale chromatin decompaction of target genes and surrounding sequences, prior to the onset of transcription. We hypothesize that opening of chromatin may facilitate productive transcription at later stages. Second, binding of PHA-4 to pseudo-chromosomes in the pharynx is restricted by the lamina-associated protein EMR-1/Emerin. The nuclear lamina induces transcriptional silencing in many organisms, but the mechanism is unclear [75]. Our study reveals that EMR-1-mediated silencing can occur by blocking transcription factor association. Third, we have defined the promoter architecture that establishes expression of the pax-1 gene within a subset of pharyngeal cells. Expression is promoted by broadly-acting enhancer regions, which include a PHA-4 site, and limited to fourteen pharyngeal cells by repressive elements.
PHA-4 binding leads to large-scale chromatin decompaction in living embryos
The transparency of C. elegans enables analysis of selector gene behavior in living embryos. Our characterization of PHA-4 and its target genes revealed regulated association with target promoters, which induced extensive chromatin decompaction in selected cells. We note that these events were visualized by single-cell analysis and would not have been detected by biochemical approaches such as chromatin immunoprecipitation. Importantly, the expression of pharyngeal reporter constructs embedded within complex DNA sequences, with few exceptions, mimics expression of the endogenous cognates, as detected by in situ stains [66], [76]. Thus, the bulk of gene regulatory processes are preserved in the arrays.
Large-scale decompaction of chromatin by a selector gene, which, to our knowledge, has not been observed previously, is consistent with observations regarding pha-4/Fox orthologues in other organisms. In breast cancer cells, global location analysis previously revealed that FoxA1 bound many regions located >50 Kb from a transcription start site [77]. FoxA1 induced both local effects, such as chromatin remodeling and transcription factor recruitment [54], but also long-range effects, such as physical interactions between enhancers and promoters [77]. In S. cerevisiae, Fkh1/Fox controls donor preference during mating-type switching [55]. Fkh1 promotes recombination for loci separated by 50 Kb and does so without altering transcription or local chromatin [78]. These observations suggest Fox factors in diverse organisms contribute to long-range interactions between distant loci. In our system, we estimate roughly one PHA-4 target promoter per 25 Kb of DNA within the pseudo-chromosomes. This number derives from ∼200 copies of target promoter (qPCR, data not shown) embedded in arrays of ∼5–7 Mb [79]. At endogenous loci, PHA-4::GFP associates with 4350 sites in the embryonic genome, a surprisingly high density of binding sites [19]. The distance observed in C. elegans is comparable to those in the yeast and mammalian studies, suggesting that large-scale, Fox-mediated chromatin re-organization might operate in all three organisms.
The association of PHA-4::YFP with pseudo-chromosomes was not constitutive, but responded to the cellular milieu in two ways. First, within the pharynx, PHA-4::YFP binding was restricted by EMR-1/emerin. EMR-1/emerin resides at the nuclear lamina, which suggests that tethering of pharyngeal genes or trans-acting factors at the nuclear periphery may modulate PHA-4 binding. We note that the nuclear lamina appears normal after emr-1(RNAi), and affected embryos are healthy and viable (This study and [43]). We speculate that the loss of emerin may have subtle effects on tethering or formation of heterochromatin, signaling pathways or lamina-associated proteins that alter gene activity [80]. Second, in the intestine, PHA-4 binding to pax-1 and myo-2 was inhibited completely, and inhibition was not relieved by emr-1(RNAi). HS::pha-4 cannot activate pax-1::GFP within intestinal cells (this study) or convert nascent intestinal cells to a pharyngeal fate [9], indicating PHA-4 functions poorly in this embryonic tissue. By contrast, ubiquitous expression of the C. elegans MyoD homolog hlh-1 induces the body-wall muscle program throughout the embryo, including developing intestinal cells [48], [81]. We suggest that the limited activity of PHA-4 within the intestine may reflect the inability of PHA-4 to associate with its pharyngeal target genes. This lack of association in the intestine may reflect the presence of gut-specific repressive systems that block pharyngeal gene activation in the intestine, or the absence of appropriate cofactors and coactivators.
What is the nature of PHA-4-induced chromatin restructuring? The global decompaction we observe is consistent with a disordered structure, such as decondensation by loss of nucleosomes and/or reconfiguring of chromatin into loops or coils [82]. Although nucleosome loss can be associated with transcription [83], our data suggest that the effect of PHA-4 is independent of productive transcription. Arrays bearing the mutA promoter or 3X PHA-4 binding site repeats recruit PHA-4::YFP and undergo decompaction, in the absence of GFP production. In Drosophila, nucleosomes are lost rapidly at heat-shock loci prior to transcription, and this loss extends across several kilobases upstream and downstream of the activated gene [84]. Transcription-independent decondensation of chromatin might be required to “clear the way” for RNA Pol II, enabling cells to activate gene expression rapidly and respond promptly to developmental and environmental cues.
Cis-regulatory architecture of the pax-1 gene
An interesting feature of pax-1 expressing cells is that they share a lineage relationship. We identified 11 of the 14 pax-1::GFP+ cells unambiguously, and found that each of these cells derived from the posterior daughter of the penultimate cell division (“px” cells; Figure S1). For example, ABaraaapapa generates a marginal cell that expresses pax-1. Previous studies have shown that C. elegans embryos are patterned according to antero-posterior (A-P) cell divisions in which pairs of A-P siblings are distinguished by high (anterior) or low (posterior) levels of nuclear POP-1, a TCF transcription factor ([85], [86] reviewed in [66]). Loss of POP-1 asymmetry alters cell fate decisions, suggesting transcriptional regulation by POP-1 confers anterior or posterior identity after each cell division [85], [86]. However, few transcriptional targets of POP-1 are known. We considered an appealing model that POP-1 might regulate pax-1 transcription directly during the penultimate cell division and thereby contribute to A–P fate distinctions. However, none of the cis-regulatory sites we identified are a good fit with the canonical TCF binding site G(A/T)(A/T)CAAAG [87]. Thus, the relationship between pax-1 and A-P specification remains a mystery.
Our promoter analysis identified four regulatory elements that establish pax-1 expression in fourteen pharyngeal cells. The first was an enhancer element likely recognized by PHA-4 and defined by D6. PHA-4 can bind this sequence in vitro [2] and in vivo (this study). Moreover, this site is required for pharyngeal expression (this study), and multimers of this sequence respond to PHA-4 in vivo [18]. This result supports the notion that many genes expressed within the pharynx are direct targets of PHA-4 [2]. Surprisingly, while mutation of the predicted PHA-4 binding site eliminated pax-1 expression within the pharynx, it also led to ectopic expression in non-pharyngeal cells such as epidermis. M05B5.2 and T05E11.3 are two additional PHA-4 target genes [2], and these also exhibited epidermal expression when the PHA-4 site was mutated to random sequences (J. Gaudet, pers. comm.). A likely possibility is that this site functions as a repression element in non-pharyngeal epithelia. PHA-4 is not expressed in the epidermis, leaving open the identity of the factor that represses epidermal expression. RNAi of the other C. elegans Fox genes did not result in ectopic expression in lines carrying the wildtype M05B5.2 reporter (J. Gaudet, unpublished). This result suggests that multiple Fox proteins function redundantly to repress epidermal expression, or alternatively, that an unrelated protein acts through the predicted PHA-4 binding site.
A second enhancer element defined by Delta16 contributes to pax-1 activation. The Delta16 region contains a match to a GATA-2,3 binding site (AGATTA; [88], [89]. However, mutation of AGATTA to CTGCAG does not inactivate pax-1 expression, suggesting this site is not recognized by a GATA factor (J.S., data not shown). We note that the sequence TTGAGA lost in Delta16 is half of a direct repeat, with a second copy located within Delta14 (Figure 1). Abutting Delta16 sequences, mutations Delta14 and Delta18 each lead to pax-1 expression in extra pharyngeal cells. These sequences carry an inverted repeat AGAGCT that is lost in Delta14 or Delta18 (Figure 1). Two additional elements, defined by Delta20 and Delta22/Delta24, functioned negatively to restrict pax-1 expression. A direct repeat (ACGGACCA) lies within these sequences, with one copy entirely within Delta20 and a second spanning Delta22 and Delta24. An appealing model is that PHA-4 promotes expression within the pharynx in combination with Delta16 sequences. The broad activation is refined by the repression elements embedded in Delta14/Delta18 and Delta20/Delta22/Delta24. The combination of four cis-regulatory sites explains why pan-pharyngeal PHA-4::YFP can bind its target promoters, yet those targets become transcriptionally active in only a subset of pharyngeal cells and after PHA-4 is first expressed.
We have demonstrated that the master regulator PHA-4 binds to its pharyngeal targets hours before the onset of gene expression. PHA-4 binding and activity is restricted in the intestine and negatively regulated by EMR-1/emerin in the pharynx. The association of PHA-4 with target promoters led to large-scale chromatin decompaction, which may facilitate chromatin-associated processes such as transcription. These in vivo results expand our understanding of PHA-4/FoxA function in driving pharyngeal transcriptional programs. Moving beyond the Nuclear Spot Assay, it will be interesting to investigate the binding and down-stream consequences of PHA-4 in its native environment, at endogenous loci.
Materials and Methods
Strains and growth conditions
Strains were maintained as described in [90], at 20°C, and were provided by Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR), unless stated otherwise. Bristol N2 was used as the wild-type strain. The following mutation was used LGIV: cha-1(p1182). For pax-1::GFP analysis the following transgenic strains were used: SM202 pxls2(pax-1::GFP + pRF4), SM699 N2(pax-1::GFP + pRF4), SM707 N2(pax-1 mutP-pro::GFP + pRF4), SM658 N2(pax-1 mutA-pro::GFP + pRF4), SM660 N2(pax-1 Delta14-pro::GFP), SM700 N2(pax-1Delta18-pro::GFP). For Heat Shock: SM259 pxEx(HS::PHA-4 + pax-1::GFP + UL8::lacZ + pRF4 + 1 KB ladder + Herring Sperm DNA)[9]. For the Nuclear Spot Assay (NSA), the following strains were used: SM1560 cha-1(p1182); pxEx(cha-1 + his-24pro::CFP::LacI + pha-4::yfp+ lacO + Herring Sperm DNA), SM1476 cha-1(p1182); pxEx(cha-1 + htz-1pro::CFP::LacI + PHA-4::YFP +myo-2proWT + lacO + Herring Sperm DNA), SM1429 cha-1(p1182); pxEx(cha-1 + htz-1pro::CFP::LacI + PHA-4::YFP +myo-2proWT + lacO + Herring Sperm DNA), SM1443 cha-1(p1182); pxEx(cha-1 + htz-1pro::CFP::LacI + PHA-4::YFP +myo-2 mutP + lacO + Herring Sperm DNA), SM1444 cha-1(p1182); pxEx(cha-1 + htz-1pro::CFP::LacI + PHA-4::YFP +myo-2 mutP + lacO + Herring Sperm DNA), SM1432 cha-1(p1182); pxEx(cha-1 + htz-1pro::CFP::LacI + PHA-4::YFP + pax-1proWT + lacO + Herring Sperm DNA), SM1434 cha-1(p1182); pxEx(cha-1 + htz-1pro::CFP::LacI + PHA-4::YFP + pax-1proWT + lacO + Herring Sperm DNA), SM1463 cha-1(p1182); pxEx(cha-1 + htz-1pro::CFP::LacI + PHA-4::YFP + pax-1 mutP + lacO + Herring Sperm DNA), SM1628 cha-1(p1182); pxEx(cha-1 + his-24pro::CFP::LacI + PHA-4::citrineYFP + pax-1Delta6proMut + lacO + Herring Sperm DNA), SM1564 cha-1(p1182); pxEx(cha-1 + his-24pro::CFP::LacI + PHA-4::citrineYFP + pax-1 mutA + lacO + Herring Sperm DNA), SM1634 cha-1(p1182); pxEx(cha-1 + his-24pro::CFP::LacI + PHA-4::citrineYFP + pax-1 mutA + lacO + Herring Sperm DNA), SM1523 cha-1(p1182); pxEx(cha-1 + his-24pro::CFP::LacI + PHA-4::citrineYFP + 3X low affinity pha-4 site + lacO + Herring Sperm DNA). SM1876 cha-1(p1182); stIs10389 (pha-4::gfp::3xFLAG); pxEx(cha-1 + htz-1pro::mCherry::LacI + M05B5.2 + lacO + Salmon testes DNA). Transgenic worms used for the Nuclear Spot Assay (NSA) were grown at 24°C on an E. coli OP50 lawn or on RNAi plates (see below).
DNA constructs
For the pax-1::GFP cytoplasmic translational fusion construct (BSEM74): a 4.6 kb genomic SacI DNA fragment was cloned from K07C11.1 into pBluescriptIISK+. The resulting plasmid was digested with NsiI, which is located within the pax-1 locus, and XbaI from the polylinker, to generate a 3 kb pax-1 fragment that was inserted into PstI/XbaI-digested pPD95.77 (A gift from Dr. Andrew Fire). The resulting pax-1::GFP reporter contained approximately 2.4 KB upstream sequences, with GFP fused to pax-1 within the second predicted exon of pax-1. The transcriptional pax-1 nuclear construct (BSEM274) was made starting with the cytoplasmically-expressed pax-1::GFP translational construct, we created a transcriptional fusion by removing all coding sequence. We performed inverse PCR using primers containing BglII tails that flanked the region to be deleted. The linear PCR product was then digested with BglII and re-ligated, placing the GFP translational start site at the same position as the one removed for pax-1. PCR products for injection were generated using pax-1 5′ as the forward primer and pax-1 3′ as the reverse primer.
The transcriptional fusion construct was modified for use in the scanning mutagenesis. A 1.2 kB Bst1071I fragment was removed from BSEM274, and replaced with a 1.9 kb Bst1107I/ApaI fragment from pAP.10 that extends from within the GFP coding region through the unc-54 poly A addition site was removed. This generates a pax-1::GFP transcriptional fusion with the coding sequence for histone H2B fused to the 3′ end of GFP. 276, 277,279,280.
pha-4::citrineYFP for the nuclear spot assay was created using QuickChange site directed mutagenesis (Stratagene, #200519). Two mutations, V68L and Q69M (5′-GTT-CAA-3′ mutated to 5′-CTT-ATG-3′), were introduced into the YFP sequence of the pha-4::YFP (SEM962) [11] to convert YFP into citrineYFP [91]. The following primers were used: YFP FW Citrine 5′-GTCAC TACTTTCGGTTATGGTCTTATGTGCTTCGCCAGATACCCAGATC-3′ and YFP RV Citrine 5′GATCTGGGTATCTGGCGAAGCACATAAGACCATAACCGAAAGT AGTGAC-3′.
3X low affinity PHA-4 binding site oligos were designed as in [18] but without restriction sites flanking the 3 tandem binding sites and were created to have an overhang to facilitate repeat formation in the array. The oligos are: Top 5′-CTACTATTTGTCCCTACTATTTGTCCCTACTATTTGTCC-3′ Bottom 5′ GGGACAAATAGTAGGGACAAATAGTAGGGACAAATAGTA-3′ (underlined are the PHA-4 binding sites). Oligos were diluted to a concentration of 2 mg/ml and heated to 95°C for 3 minutes. The temperature was dropped 0.10/sec until 20°C was reached to hybridize the oligos.
Injections for pax-1 promoter analysis
Reporter constructs were injected into the germ line of hermaphrodites and stable F2 transgenic roller lines examined. All reporter constructs were injected at 0.5 ng/ml as PCR fragments. Low concentrations of PCR products circumvented artificial expression in pharyngeal cells that has been observed with plasmid constructs [92]. We used pRF4 (rol-6(su1006)) as a co-injection marker [93]. The injection mix also included complex DNA (salmon sperm DNA, 1 kb ladder) up to 100 ng/ml, to prevent silencing [59].
Antibody stains
Antibody staining was performed as described previously [11]. The primary antibodies used were anti-GFP rabbit IgG fraction at 1∶1000 (Molecular Probes), anti-PHA-4 PAb at 1∶1000 [94], the anti-intermediate filament (a-IF) at 1∶3 that recognizes pharyngeal marginal cells [95], and the monoclonal antibody J126, from Dr. Susan Strome, was used at 1∶30 to detect intestinal cells.
Scanning mutagenesis
All constructs for the scanning mutagenesis were constructed using an inverse PCR strategy. For each mutant, we used a specific pair of primers that flank the 10 bp region to be altered. These primers each carry 5′ tails that contain a restriction site (PstI or ClaI). Following inverse PCR (BSEM279 as template), the linear PCR product was digested with the appropriate restriction enzyme (PstI or ClaI) and re-ligated. Each resulting reporter plasmid contains the restriction site plus a variable number of base pairs in place of the 10 bp of wild-type sequence. For injection, PCR products were generated from each mutant plasmid. All constructs were sequenced to confirm the predicted sequence.
Nuclear spot assay
Transgenic lines for the Nuclear Spot Assay were as follows: no target control (SM1560), 3X low affinity pha-4 binding sites (SM1523), myo-2 wild-type promoter (SM1476, SM1429) bearing two high affinity PHA-4 binding sites [2], myo-2 promoter bearing mutagenized FoxA sites (SM1443, SM1444) [2], pax-1 wild-type promoter (SM1432, SM1434), pax-1 promoter with a mutagenized FoxA site (SM1463, SM1628) and pax-1 promoter with a mutagenized positive regulator site (SM1564, SM1634) (see below). SM1560 was created by injecting cha-1(p1182) worms with Xho1-linearized pha-4::citrineyfp plasmid (bSEM1045) (1 ng/µl), his24promoter::CFP::LacI PCR product [63] (2.5 ng/µl), a 10 kb Sph1/Kpn1 fragment from lacO multimeric plasmid pSV2-dhfr-8.23 (3 ng/µl) [96], cha-1 plasmid (RM527P, a gift from J. Rand) linearized with Apa1 (2 ng/µl) for rescue, and sheared herring sperm DNA to make 100 ng/µl total DNA. For SM1476 and SM1429, 499 bp of the endogenous myo-2 promoter upstream of the start codon was used in addition to the components listed for SM1560 with one difference, CFP::lacI expression was driven by the htz-1 promoter (BSEM995) [63]. SM1443 and SM1444 were created similar to SM1476 and SM1429 but with a myo-2 promoter bearing two mutated PHA-4 binding sites [2]. For SM1434 a 240 (bp) fragment of the pax-1 promoter upstream of the start codon was used (the fragment contains one PHA-4 binding site (TGTTTGC)). SM1463 carried an altered version of the 240 (bp) pax-1 promoter in which the PHA-4 binding site was mutated from TGTTTGC to ATCGATT (MutP). Both SM1463 and SM1434 were injected with htz-1pro::CFP::LacI. For SM1564 and SM1634 a positive regulator site −40 to −50 upstream of the TSS was mutated from TTGAGATTAA to CAATCGATTG. SM1876 was created by injecting SM1754 cha-1(p1182); stIs10389 (pha-4::gfp::3xFLAG) worms with cha-1, a 440 (bp) M05B5.2 promoter fragment [2], and htz-1pro::mCherry::LacI that has a premature stop codon at the end of the mCherry sequence, thus failing to make any mCherry::LacI. SM1876 was used to examine whether decompaction reflected an artificial interaction between LacI and PHA-4. Nuclear spot assays were performed as described previously [11], [50], [51], [63], with the following modifications: sequential scan images were acquired using the Andor Revolution XD microscopy system (≤16E stages). For later stages, images were acquired using an Olympus FluoView FV1000 confocal microscope (for pax-1 mutA) or a Leica DM RXE confocal (for everything else).
To determine copy number, worms were grown at restrictive temperature for cha-1(p1182) (25°), and treated with bleach to synchronize embryos. Four 10 cm OP50 plates of moving, nonCha-1 L3 animals were harvested for DNA isolation by phenol chloroform extraction and ethanol precipitation. qPCR was performed for promoter regions and normalized to act-1 using a LightCycler PCR machine with LightCycler FastStart DNA MasterPlus SYBR Green 1 kit (Roche) for quantitation. qPCR indicated a copy number of ≤200 for each promoter.
Heat shock
Gravid mothers were dissected and embryos collected in a PCR tube. Heat-shock was administered in a PCR machine. Embryos were initially incubated at 20°C for 75 min. After the initial incubation, the temperature was raised gradually to 33°C at a rate of 0.1°C/second. Embryos were then incubated at 33°C for 30 minutes. Following heat shock, the temperature was gradually lowered to 20°C at a rate of 0.1°C/second, and embryos incubated at 20°C for 5 hours.
Image analysis
Perkin Elmer Volocity was used to calibrate images for true X and Y pixel dimensions to ensure accurate spatial measurements. Classifiers were designed to select CFP::LacI areas and PHA-4::YFP+ cells using an intensity threshold. The CFP::LacI classifier included separation of touching objects, removal of noise and exclusion of objects smaller than 0.25 micron2. The PHA-4::YFP classifier was modified to remove noise. CFP::LacI areas within PHA-4::YFP+ cells were considered “inside the pharynx” and the remainder as “outside the pharynx.” Proofreading of selections was performed blind by comparing measurements with images. Area measurements (INT Area (micron2)*10) were analyzed using Cox regression models to evaluate differences in chromosome area with location (inside the pharynx versus outside), developmental stage, or transgenic line. While Cox regression models were originally developed for analyzing survival data, their semi-parametric nature made them suitable for analyzing data following a non-standard distribution that was difficult to capture parametrically.
RNA interference screen and analysis of general decompaction and PHA-4 binding
RNAi by bacterial feeding was performed similarly to [15]. HT115 bacteria [97] expressing dsRNA for gfp, spr-5, rbr-2, npp-11, emr-1, nhr-60, ima-3, lem-3, zyg-12, lmn-1, lin-49, lin-59, set-17, set-16, set-2, set-1, met-2, hda-3, hda-4, tsn-1, prg-1, sago-2, prg-2, csr-1, top-1, ergo-1, chd-3, tam-1, lin-35, or hil-7 were grown in liquid cultures for 8 hours and seeded onto plates containing 8 mM IPTG (Sigma) and 50 g/ml Carbenicillin (Sigma). All RNAi clones were derived from the Ahringer library [98]. The emr-1 clone was validated by sequencing using pPD129_for 5′-GAGTGAGCTGATACCGCTCG-3′ and pPD129_rev 5′-CACGACGGTGTATTTCGACGGC-3′ primers at the Dana-Farber/Harvard Cancer Center DNA Resource Core. Adult SM1634 worms were bleached and ∼50 embryos were placed on RNAi plates (Po). For every experiment, F1 progeny embryos from 5 6 cm plates were collected by bleaching and analyzed, and each experiment was repeated at least twice. Images were acquired from live embryos using an Olympus FluoView FV1000 confocal microscope and DeltaVision RT Deconvolution System and SoftWoRx software (Applied Precision). A multitrack setting was used to acquire separate CFP and YFP images from slices through the pharynges of comma to 1.5fold-stage embryos. Embryos were scored for general non-tissue specific extrachromosomal array decompaction and for the number of nuclei containing PHA-4-::YFP colocalized with CFP::LacI.
Supporting Information
Zdroje
1. MannRS
CarrollSB
2002 Molecular mechanisms of selector gene function and evolution. Curr Opin Genet Dev 12 592 600
2. GaudetJ
MangoSE
2002 Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science 295 821 825
3. FurlongEE
AndersenEC
NullB
WhiteKP
ScottMP
2001 Patterns of gene expression during Drosophila mesoderm development. Science 293 1629 1633
4. TapscottSJ
2005 The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 132 2685 2695
5. LiangZ
BigginMD
1998 Eve and ftz regulate a wide array of genes in blastoderm embryos: the selector homeoproteins directly or indirectly regulate most genes in Drosophila. Development 125 4471 4482
6. ZeitlingerJ
ZinzenRP
StarkA
KellisM
ZhangH
2007 Whole-genome ChIP-chip analysis of Dorsal, Twist, and Snail suggests integration of diverse patterning processes in the Drosophila embryo. Genes Dev 21 385 390
7. SandmannT
GirardotC
BrehmeM
TongprasitW
StolcV
2007 A core transcriptional network for early mesoderm development in Drosophila melanogaster. Genes Dev 21 436 449
8. CaoY
YaoZ
SarkarD
LawrenceM
SanchezGJ
2010 Genome-wide MyoD binding in skeletal muscle cells: a potential for broad cellular reprogramming. Dev Cell 18 662 674
9. HornerMA
QuintinS
DomeierME
KimbleJ
LabouesseM
1998 pha-4, an HNF-3 homologue, specifies pharyngeal organ identity in Caenorhabditis elegans. Genes Dev 12 1947 1952
10. KalbJM
LauKK
GoszczynskiB
FukushigeT
MoonsD
1998 pha-4 is Ce-fkh-1, a fork head/HNF-3alpha,beta,gamma homolog that functions in organogenesis of the C. elegans pharynx. Development 125 2171 2180
11. KieferJC
SmithPA
MangoSE
2007 PHA-4/FoxA cooperates with TAM-1/TRIM to regulate cell fate restriction in the C. elegans foregut. Dev Biol 303 611 624
12. MangoSE
LambieEJ
KimbleJ
1994 The pha-4 gene is required to generate the pharyngeal primordium of Caenorhabditis elegans. Development 120 3019 3031
13. AoW
GaudetJ
KentWJ
MuttumuS
MangoSE
2004 Environmentally induced foregut remodeling by PHA-4/FoxA and DAF-12/NHR. Science 305 1743 1746
14. ChenD
RiddleDL
2008 Function of the PHA-4/FOXA transcription factor during C. elegans post-embryonic development. BMC Dev Biol 8 26
15. UpdikeDL
MangoSE
2007 Genetic suppressors of Caenorhabditis elegans pha-4/FoxA identify the predicted AAA helicase ruvb-1/RuvB. Genetics 177 819 833
16. PanowskiSH
WolffS
AguilaniuH
DurieuxJ
DillinA
2007 PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 447 550 555
17. SheafferKL
UpdikeDL
MangoSE
2008 The Target of Rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr Biol 18 1355 1364
18. GaudetJ
MuttumuS
HornerM
MangoSE
2004 Whole-genome analysis of temporal gene expression during foregut development. PLoS Biol 2 e352 doi:10.1371/journal.pbio.0020352
19. ZhongM
NiuW
LuZJ
SarovM
MurrayJI
2010 Genome-wide identification of binding sites defines distinct functions for Caenorhabditis elegans PHA-4/FOXA in development and environmental response. PLoS Genet 6 e1000848 doi:10.1371/journal.pgen.1000848
20. ThatcherJD
HaunC
OkkemaPG
1999 The DAF-3 Smad binds DNA and represses gene expression in the Caenorhabditis elegans pharynx. Development 126 97 107
21. ThatcherJD
FernandezAP
Beaster-JonesL
HaunC
OkkemaPG
2001 The Caenorhabditis elegans peb-1 gene encodes a novel DNA-binding protein involved in morphogenesis of the pharynx, vulva, and hindgut. Dev Biol 229 480 493
22. DeplanckeB
MukhopadhyayA
AoW
ElewaAM
GroveCA
2006 A gene-centered C. elegans protein-DNA interaction network. Cell 125 1193 1205
23. OkkemaPG
HaE
HaunC
ChenW
FireA
1997 The Caenorhabditis elegans NK-2 homeobox gene ceh-22 activates pharyngeal muscle gene expression in combination with pha-1 and is required for normal pharyngeal development. Development 124 3965 3973
24. RaharjoI
GaudetJ
2007 Gland-specific expression of C. elegans hlh-6 requires the combinatorial action of three distinct promoter elements. Dev Biol 302 295 308
25. RasmussenJP
EnglishK
TenlenJR
PriessJR
2008 Notch signaling and morphogenesis of single-cell tubes in the C. elegans digestive tract. Dev Cell 14 559 569
26. CasolariJM
BrownCR
KomiliS
WestJ
HieronymusH
2004 Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell 117 427 439
27. TaddeiA
Van HouweG
HedigerF
KalckV
CubizollesF
2006 Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature 441 774 778
28. BricknerJH
WalterP
2004 Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol 2 e342 doi:10.1371/journal.pbio.0020342
29. CabalGG
GenovesioA
Rodriguez-NavarroS
ZimmerC
GadalO
2006 SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature 441 770 773
30. DieppoisG
IglesiasN
StutzF
2006 Cotranscriptional recruitment to the mRNA export receptor Mex67p contributes to nuclear pore anchoring of activated genes. Mol Cell Biol 26 7858 7870
31. MargalitA
NeufeldE
FeinsteinN
WilsonKL
PodbilewiczB
2007 Barrier to autointegration factor blocks premature cell fusion and maintains adult muscle integrity in C. elegans. J Cell Biol 178 661 673
32. NiliE
CojocaruGS
KalmaY
GinsbergD
CopelandNG
2001 Nuclear membrane protein LAP2beta mediates transcriptional repression alone and together with its binding partner GCL (germ-cell-less). J Cell Sci 114 3297 3307
33. SomechR
ShaklaiS
GellerO
AmariglioN
SimonAJ
2005 The nuclear-envelope protein and transcriptional repressor LAP2beta interacts with HDAC3 at the nuclear periphery, and induces histone H4 deacetylation. J Cell Sci 118 4017 4025
34. LiuJ
Rolef Ben-ShaharT
RiemerD
TreininM
SpannP
2000 Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell 11 3937 3947
35. DechatT
PfleghaarK
SenguptaK
ShimiT
ShumakerDK
2008 Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 22 832 853
36. GruenbaumY
LeeKK
LiuJ
CohenM
WilsonKL
2002 The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans. J Cell Sci 115 923 929
37. SproulD
GilbertN
BickmoreWA
2005 The role of chromatin structure in regulating the expression of clustered genes. Nat Rev Genet 6 775 781
38. FinlanLE
SproulD
ThomsonI
BoyleS
KerrE
2008 Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet 4 e1000039 doi:10.1371/journal.pgen.1000039
39. ReddyKL
ZulloJM
BertolinoE
SinghH
2008 Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 452 243 247
40. KumaranRI
SpectorDL
2008 A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J Cell Biol 180 51 65
41. RagoczyT
BenderMA
TellingA
ByronR
GroudineM
2006 The locus control region is required for association of the murine beta-globin locus with engaged transcription factories during erythroid maturation. Genes Dev 20 1447 1457
42. RiemerD
DodemontH
WeberK
1993 A nuclear lamin of the nematode Caenorhabditis elegans with unusual structural features; cDNA cloning and gene organization. Eur J Cell Biol 62 214 223
43. LiuJ
LeeKK
Segura-TottenM
NeufeldE
WilsonKL
2003 MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc Natl Acad Sci U S A 100 4598 4603
44. OegemaK
HymanAA
2006 Cell division. WormBook 1 40
45. LeeKK
GruenbaumY
SpannP
LiuJ
WilsonKL
2000 C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol Biol Cell 11 3089 3099
46. MargalitA
Segura-TottenM
GruenbaumY
WilsonKL
2005 Barrier-to-autointegration factor is required to segregate and enclose chromosomes within the nuclear envelope and assemble the nuclear lamina. Proc Natl Acad Sci U S A 102 3290 3295
47. ZhengR
GhirlandoR
LeeMS
MizuuchiK
KrauseM
2000 Barrier-to-autointegration factor (BAF) bridges DNA in a discrete, higher-order nucleoprotein complex. Proc Natl Acad Sci U S A 97 8997 9002
48. YuzyukT
FakhouriTH
KieferJ
MangoSE
2009 The polycomb complex protein mes-2/E(z) promotes the transition from developmental plasticity to differentiation in C. elegans embryos. Dev Cell 16 699 710
49. BelmontAS
StraightAF
1998 In vivo visualization of chromosomes using lac operator-repressor binding. Trends Cell Biol 8 121 124
50. CarmiI
KopczynskiJB
MeyerBJ
1998 The nuclear hormone receptor SEX-1 is an X-chromosome signal that determines nematode sex. Nature 396 168 173
51. Gonzalez-SerricchioAS
SternbergPW
2006 Visualization of C. elegans transgenic arrays by green fluorescent protein (GFP). BMC Genet 7 36
52. FukushigeT
HendzelMJ
Bazett-JonesDP
McGheeJD
1999 Direct visualization of the elt-2 gut-specific GATA factor binding to a target promoter inside the living Caenorhabditis elegans embryo. Proc Natl Acad Sci U S A 96 11883 11888
53. CirilloLA
LinFR
CuestaI
FriedmanD
JarnikM
2002 Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell 9 279 289
54. LupienM
EeckhouteJ
MeyerCA
WangQ
ZhangY
2008 FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132 958 970
55. SunK
CoicE
ZhouZ
DurrensP
HaberJE
2002 Saccharomyces forkhead protein Fkh1 regulates donor preference during mating-type switching through the recombination enhancer. Genes Dev 16 2085 2096
56. OkkemaPG
KrauseM
2005 Transcriptional regulation. WormBook 1 40
57. EpsteinHF
WaterstonRH
BrennerS
1974 A mutant affecting the heavy chain of myosin in Caenorhabditis elegans. J Mol Biol 90 291 300
58. KaltenbachL
HornerMA
RothmanJH
MangoSE
2000 The TBP-like factor CeTLF is required to activate RNA polymerase II transcription during C. elegans embryogenesis. Mol Cell 6 705 713
59. KellyWG
XuS
MontgomeryMK
FireA
1997 Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146 227 238
60. EvansTC
2006 Transformation and microinjection. WormBook doi/10.1895/wormbook.1.108.1
61. GualdiR
BossardP
ZhengM
HamadaY
ColemanJR
1996 Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev 10 1670 1682
62. MangoSE
2007 The C. elegans pharynx: a model for organogenesis. WormBook doi/10.1895/wormbook.1.7.1
63. UpdikeDL
MangoSE
2006 Temporal regulation of foregut development by HTZ-1/H2A.Z and PHA-4/FoxA. PLoS Genet 2 e161 doi:10.1371/journal.pgen.0020161
64. ZaretKS
2002 Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet 3 499 512
65. ZaretK
1999 Developmental competence of the gut endoderm: genetic potentiation by GATA and HNF3/fork head proteins. Dev Biol 209 1 10
66. MangoSE
2009 The molecular basis of organ formation: insights from the C. elegans foregut. Annu Rev Cell Dev Biol 25 597 628
67. ChambeyronS
BickmoreWA
2004 Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev 18 1119 1130
68. WilliamsSK
TylerJK
2007 Transcriptional regulation by chromatin disassembly and reassembly. Curr Opin Genet Dev 17 88 93
69. MullerWG
WalkerD
HagerGL
McNallyJG
2001 Large-scale chromatin decondensation and recondensation regulated by transcription from a natural promoter. J Cell Biol 154 33 48
70. AzzariaM
GoszczynskiB
ChungMA
KalbJM
McGheeJD
1996 A fork head/HNF-3 homolog expressed in the pharynx and intestine of the Caenorhabditis elegans embryo. Developmental Biology 178 289 303
71. TerranovaR
PujolN
FasanoL
DjabaliM
2002 Characterisation of set-1, a conserved PR/SET domain gene in Caenorhabditis elegans. Gene 292 33 41
72. GrishokA
SinskeyJL
SharpPA
2005 Transcriptional silencing of a transgene by RNAi in the soma of C. elegans. Genes Dev 19 683 696
73. AndersenEC
HorvitzHR
2007 Two C. elegans histone methyltransferases repress lin-3 EGF transcription to inhibit vulval development. Development 134 2991 2999
74. BesslerJB
AndersenEC
VilleneuveAM
2010 Differential localization and independent acquisition of the H3K9me2 and H3K9me3 chromatin modifications in the Caenorhabditis elegans adult germ line. PLoS Genet 6 e1000830 doi:10.1371/journal.pgen.1000830
75. GruenbaumY
WilsonKL
HarelA
GoldbergM
CohenM
2000 Review: nuclear lamins—structural proteins with fundamental functions. J Struct Biol 129 313 323
76. SchanerCE
KellyWG
2006 Germline chromatin. WormBook 1 14
77. CarrollJS
LiuXS
BrodskyAS
LiW
MeyerCA
2005 Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122 33 43
78. ErcanS
ReeseJC
WorkmanJL
SimpsonRT
2005 Yeast recombination enhancer is stimulated by transcription activation. Mol Cell Biol 25 7976 7987
79. StinchcombDT
ShawJE
CarrSH
HirshD
1985 Extrachromosomal DNA transformation of Caenorhabditis elegans. Mol Cell Biol 5 3484 3496
80. GruenbaumY
MargalitA
GoldmanRD
ShumakerDK
WilsonKL
2005 The nuclear lamina comes of age. Nat Rev Mol Cell Biol 6 21 31
81. FukushigeT
KrauseM
2005 The myogenic potency of HLH-1 reveals wide-spread developmental plasticity in early C. elegans embryos. Development 132 1795 1805
82. CairnsBR
2007 Chromatin remodeling: insights and intrigue from single-molecule studies. Nat Struct Mol Biol 14 989 996
83. BoegerH
GriesenbeckJ
KornbergRD
2008 Nucleosome retention and the stochastic nature of promoter chromatin remodeling for transcription. Cell 133 716 726
84. PeteschSJ
LisJT
2008 Rapid, transcription-independent loss of nucleosomes over a large chromatin domain at Hsp70 loci. Cell 134 74 84
85. LinR
HillRJ
PriessJR
1998 POP-1 and anterior-posterior fate decisions in C. elegans embryos. Cell 92 229 239
86. KalettaT
SchnabelH
SchnabelR
1997 Binary specification of the embryonic lineage in Caenorhabditis elegans. Nature 390 294 298
87. KorswagenHC
HermanMA
CleversHC
2000 Distinct beta-catenins mediate adhesion and signalling functions in C. elegans. Nature 406 527 532
88. OrkinSH
1992 GATA-binding transcription factors in hematopoietic cells. Blood 80 575 581
89. PedonePV
OmichinskiJG
NonyP
TrainorC
GronenbornAM
1997 The N-terminal fingers of chicken GATA-2 and GATA-3 are independent sequence-specific DNA binding domains. EMBO J 16 2874 2882
90. BrennerS
1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
91. GriesbeckO
BairdGS
CampbellRE
ZachariasDA
TsienRY
2001 Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem 276 29188 29194
92. HopeIA
1991 “Promoter trapping” in Caenorhabditis elegans. Development 113 399 408
93. MelloCC
KramerJM
StinchcombD
AmbrosV
1991 Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10 3959 3970
94. KaltenbachLS
UpdikeDL
MangoSE
2005 Contribution of the amino and carboxyl termini for PHA-4/FoxA function in Caenorhabditis elegans. Dev Dyn 234 346 354
95. PrussRM
MirskyR
RaffMC
ThorpeR
DowdingAJ
1981 All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell 27 419 428
96. StraightAF
BelmontAS
RobinettCC
MurrayAW
1996 GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr Biol 6 1599 1608
97. TimmonsL
CourtDL
FireA
2001 Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263 103 112
98. KamathRS
FraserAG
DongY
PoulinG
DurbinR
2003 Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 231 237
99. TabaraH
MotohashiT
KoharaY
1996 A multi-well version of in situ hybridization on whole mount embryos of Caenorhabditis elegans. Nucleic Acids Res 24 2119 2124
100. AltunZF
HerndonL.A.
CrockerC.
LintsR.
HallD.H.
2002–2009 WormAtlas http://wwwwormatlasorg
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 8- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
-
Všechny články tohoto čísla
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Mutation in the Gene Encoding Ubiquitin Ligase LRSAM1 in Patients with Charcot-Marie-Tooth Disease
- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Did Genetic Drift Drive Increases in Genome Complexity?
- The 5p15.33 Locus Is Associated with Risk of Lung Adenocarcinoma in Never-Smoking Females in Asia
- An Alpha-Catulin Homologue Controls Neuromuscular Function through Localization of the Dystrophin Complex and BK Channels in
- Epigenetically-Inherited Centromere and Neocentromere DNA Replicates Earliest in S-Phase
- Survival and Growth of Yeast without Telomere Capping by Cdc13 in the Absence of Sgs1, Exo1, and Rad9
- Tuberous Sclerosis Complex 1 Regulates dE2F1 Expression during Development and Cooperates with RBF1 to Control Proliferation and Survival
- Disease-Associated Mutations That Alter the RNA Structural Ensemble
- The Transcriptomes of Two Heritable Cell Types Illuminate the Circuit Governing Their Differentiation
- Inactivation of VCP/ter94 Suppresses Retinal Pathology Caused by Misfolded Rhodopsin in
- Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: a Meta-Analysis and Comparison with Lung Cancer and COPD
- Transcriptional Regulation by CHIP/LDB Complexes
- Conserved Role of in Ethanol Responses in Mutant Mice
- A Global Overview of the Genetic and Functional Diversity in the Pathogenicity Island
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3–Dependent Manner in
- Genetic Analysis of Baker's Yeast Msh4-Msh5 Reveals a Threshold Crossover Level for Meiotic Viability
- Genome-Wide Association Studies of Serum Magnesium, Potassium, and Sodium Concentrations Identify Six Loci Influencing Serum Magnesium Levels
- Something New: An Interview with Radoje Drmanac
- The Extinction Dynamics of Bacterial Pseudogenes
- Microtubule Actin Crosslinking Factor 1 Regulates the Balbiani Body and Animal-Vegetal Polarity of the Zebrafish Oocyte
- Consistent Association of Type 2 Diabetes Risk Variants Found in Europeans in Diverse Racial and Ethnic Groups
- Transmission of Mitochondrial DNA Diseases and Ways to Prevent Them
- Telomere Disruption Results in Non-Random Formation of Dicentric Chromosomes Involving Acrocentric Human Chromosomes
- Chromosome Axis Defects Induce a Checkpoint-Mediated Delay and Interchromosomal Effect on Crossing Over during Drosophila Meiosis
- Dynamic Chromatin Organization during Foregut Development Mediated by the Organ Selector Gene PHA-4/FoxA
- Ancient Protostome Origin of Chemosensory Ionotropic Glutamate Receptors and the Evolution of Insect Taste and Olfaction
- A Wnt-Frz/Ror-Dsh Pathway Regulates Neurite Outgrowth in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Did Genetic Drift Drive Increases in Genome Complexity?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



