-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The 5p15.33 Locus Is Associated with Risk of Lung Adenocarcinoma in Never-Smoking Females in Asia
Genome-wide association studies of lung cancer reported in populations of European background have identified three regions on chromosomes 5p15.33, 6p21.33, and 15q25 that have achieved genome-wide significance with p-values of 10−7 or lower. These studies have been performed primarily in cigarette smokers, raising the possibility that the observed associations could be related to tobacco use, lung carcinogenesis, or both. Since most women in Asia do not smoke, we conducted a genome-wide association study of lung adenocarcinoma in never-smoking females (584 cases, 585 controls) among Han Chinese in Taiwan and found that the most significant association was for rs2736100 on chromosome 5p15.33 (p = 1.30×10−11). This finding was independently replicated in seven studies from East Asia totaling 1,164 lung adenocarcinomas and 1,736 controls (p = 5.38×10−11). A pooled analysis achieved genome-wide significance for rs2736100. This SNP marker localizes to the CLPTM1L-TERT locus on chromosome 5p15.33 (p = 2.60×10−20, allelic risk = 1.54, 95% Confidence Interval (CI) 1.41–1.68). Risks for heterozygote and homozygote carriers of the minor allele were 1.62 (95% CI; 1.40–1.87), and 2.35 (95% CI: 1.95–2.83), respectively. In summary, our results show that genetic variation in the CLPTM1L-TERT locus of chromosome 5p15.33 is directly associated with the risk of lung cancer, most notably adenocarcinoma.
Published in the journal: . PLoS Genet 6(8): e32767. doi:10.1371/journal.pgen.1001051
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001051Summary
Genome-wide association studies of lung cancer reported in populations of European background have identified three regions on chromosomes 5p15.33, 6p21.33, and 15q25 that have achieved genome-wide significance with p-values of 10−7 or lower. These studies have been performed primarily in cigarette smokers, raising the possibility that the observed associations could be related to tobacco use, lung carcinogenesis, or both. Since most women in Asia do not smoke, we conducted a genome-wide association study of lung adenocarcinoma in never-smoking females (584 cases, 585 controls) among Han Chinese in Taiwan and found that the most significant association was for rs2736100 on chromosome 5p15.33 (p = 1.30×10−11). This finding was independently replicated in seven studies from East Asia totaling 1,164 lung adenocarcinomas and 1,736 controls (p = 5.38×10−11). A pooled analysis achieved genome-wide significance for rs2736100. This SNP marker localizes to the CLPTM1L-TERT locus on chromosome 5p15.33 (p = 2.60×10−20, allelic risk = 1.54, 95% Confidence Interval (CI) 1.41–1.68). Risks for heterozygote and homozygote carriers of the minor allele were 1.62 (95% CI; 1.40–1.87), and 2.35 (95% CI: 1.95–2.83), respectively. In summary, our results show that genetic variation in the CLPTM1L-TERT locus of chromosome 5p15.33 is directly associated with the risk of lung cancer, most notably adenocarcinoma.
Introduction
To date, several large genome-wide association studies (GWAS) of lung cancer conducted in subjects of European background have identified susceptibility alleles on chromosomes 5p15.33, 6p21.33 and 15q25 [1]–[8]. These studies have shown that statistical evidence that exceeds the threshold of genome wide significance, defined as a p value less than 5×10−7 [9] or 1×10−8 [10]. In each study, the majority of cases and controls were cigarette smokers, making it difficult to determine whether these loci are associated with lung carcinogenesis or tobacco use, or perhaps both [11]. It has been difficult to accrue a sufficiently large set of lung cancer cases with no history of smoking because a high proportion of lung cancer in women as well as men in North America and Europe is directly related to tobacco use. In contrast, a substantial proportion of lung cancer in East Asian women occurs among non-smokers, who interestingly have a relatively high rate of lung cancer [12]. This suggests that genetic and/or environmental factors could account for the observed differences. To investigate this further, we conducted a genome-wide association study with follow-up of notable SNPs in never-smoking women in East Asia. In addition, we genotyped tag SNPs optimized for East Asians for the three regions previously identified by GWAS in European populations.
Results
Genome-wide association scan
We conducted an initial GWAS of 584 lung cancer cases and 585 controls drawn from a case-control study in Taiwan, the Genetic Epidemiological Study of Lung Adenocarcinoma (GELAC) [13] (Table 1). Cases were restricted to those never-smoking females with a confirmed diagnosis of adenocarcinoma of the lung in GELAC. Controls were drawn from never-smoking female controls in GELAC and frequency-matched by age with cases (see Text S1 for more details). We began with a pilot study in which 54 cases and 54 controls were genotyped with the Illumina HumanCNV370-Duo BeadChip and, based on its success, 550 cases and 549 controls were genotyped on the Illumina HumanHap 610 Quad BeadChip. After quality control metrics were applied to both data sets (see Materials and Methods), the variance inflation factor λ in the genomic control model was found to be 1.013 and the inflation factor λ1000 for an equivalent study of 1000 cases and controls [14] was 1.022; together with the comparison of the observed and expected p-values in the quantile-quantile plot, shown in Figure 1, there is no evidence of a substantial issue related to population substructure but instead, several promising regions in the tail of the distribution are apparent, suitable for follow-up analysis. In fact, the distribution of the bottom 90% of p-values is similar to the expected distribution (Figure 1A) whereas the top 10% p-values displayed a deviation consistent with possible new signals (Figure 1B).
Fig. 1. Genome-wide association results in the GELAC study. 
(A) Quantile-quantile plot for lower 90% of −log P-values, (B) upper 10% of −log P-values, and (C) scatter plot of P-values in−log scale from the trend test for 457,504 genotyped variants comparing 584 cases and 585 controls. Tab. 1. Number of cases and controls, and study characteristics, for each participating study center. 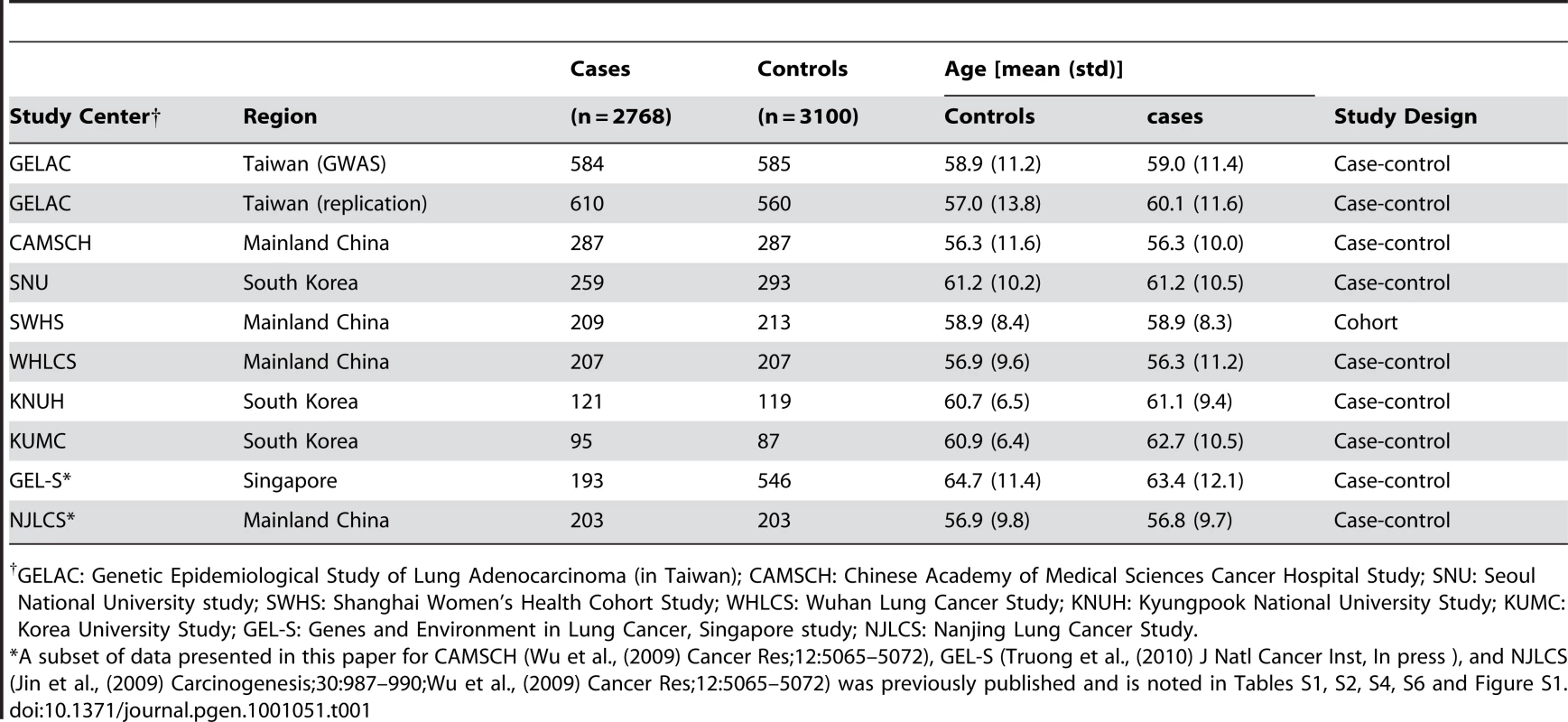
GELAC: Genetic Epidemiological Study of Lung Adenocarcinoma (in Taiwan); CAMSCH: Chinese Academy of Medical Sciences Cancer Hospital Study; SNU: Seoul National University study; SWHS: Shanghai Women's Health Cohort Study; WHLCS: Wuhan Lung Cancer Study; KNUH: Kyungpook National University Study; KUMC: Korea University Study; GEL-S: Genes and Environment in Lung Cancer, Singapore study; NJLCS: Nanjing Lung Cancer Study. As shown in Figure 1C, the scatter plot of p-values on a −log scale for the trend test conducted for 457,504 SNPs used after quality control metrics were applied, only one SNP, rs2736100, was highly associated with lung cancer (p = 1.30 * 10−11) below the threshold of genome-wide significance, namely, p less than 1×10−7 (Figure 2). In an analysis for trend adjusted for age, the allelic odds ratio was 1.83 (1.54–2.18), which is notably higher than the estimates reported in the European studies (Figure 2) [4]. It is remarkable that our finding suggests a higher estimated effect size compared to that which was reported in Europeans, who were primarily smokers. To confirm the signal at rs2736100, the samples in the GWAS were genotyped using an optimized TaqMan assay (ABI, Foster City, CA), which had a concordance of 99.7% between the two platforms [15].
Fig. 2. Risk of lung adenocarcinoma associated with rs2736100 for never-smoking female cases and never-smoking female controls from East Asia. 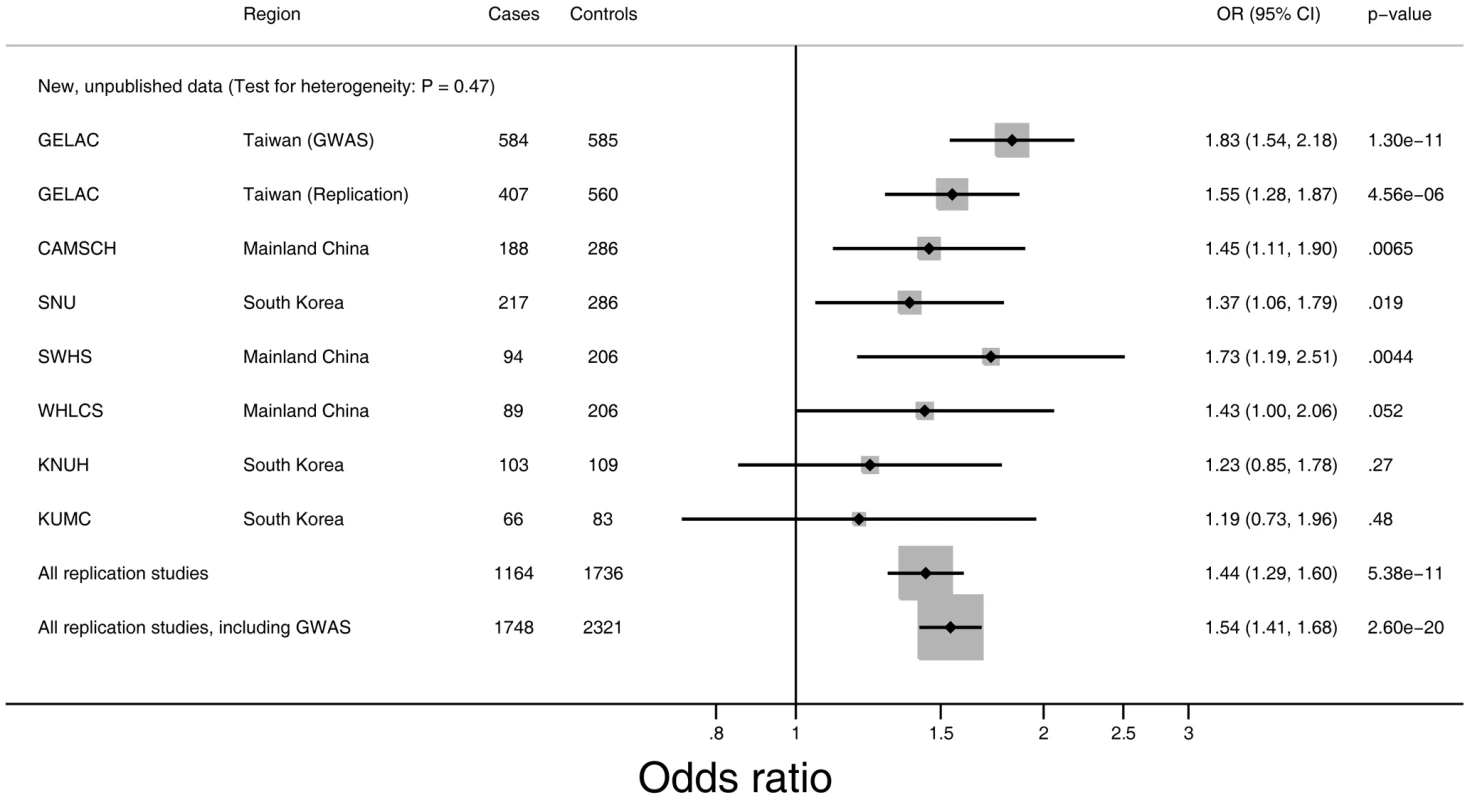
Forest plot representing lung adenocarcinoma risk and the rs2736100 genotype. Odds ratios (OR) and 95% confidence intervals (CI) for lung adenocarcinoma are derived from the per-allele model. All models are adjusted for age and study center. GELAC: Genetic Epidemiological Study of Lung Adenocarcinoma (in Taiwan); CAMSCH: Chinese Academy of Medical Sciences Cancer Hospital Study; SNU: Seoul National University study; SWHS: Shanghai Women's Health Cohort Study; WHLCS: Wuhan Lung Cancer Study; KNUH: Kyungpook National University Study; KUMC: Korea University Study. Replication of the association of rs2736100 with lung cancer risk
Replication of the strongest signal, rs2736100 was performed in the remaining subjects of the GELAC study [13] as well as six studies of never-smoking Asian women with lung adenocarcinoma in East Asia. A total of 1164 cases with lung adenocarcinoma and 1736 controls were genotyped using an optimized TaqMan assay (shown to have high concordance with the Illumina results as described above). The additional replication studies included the Chinese Academy of Medical Sciences Cancer Hospital study (CAMSCH) [16], Wuhan lung cancer study (WHLCS) [17], Seoul National University study (SNU) [18], Korea University Medical Center study (KUMC) [19], Kyungpook National University Hospital study (KNUH) [20] and Shanghai Women's Health Cohort Study (SWHS) [21], [22] (Table 1). Characteristics of the study subjects from the GWAS and the replication studies are presented in Table 1.
The combined replication study confirmed that rs2736100 is associated with risk for lung adenocarcinoma in never-smoking women in East Asia (p = 5.38 * 10−11; allelic OR = 1.44; 95% CI 1.29–1.60) (Figure 2). In a pooled analysis of the GWAS and replication studies, rs2736100 was conclusively associated with the risk for lung adenocarcinoma in never-smoking females in East Asian populations; the allelic OR is 1.54 (95% CI 1.41–1.68; p = 2.60 * 10−20) (Figure 2 and Table 2). The estimated odds ratios for the heterozygous and homozygous carriers are 1.62 (95% CI 1.40–1.87) and 2.35 (95% CI 1.95–2.83). There was no evidence of heterogeneity between the results of the one cohort study (SWHS) and the pooled analysis of the 6 case-control studies (p = 0.36). Further pooling with two previously published studies, the Nanjing lung cancer study (NJLCS) [23] and the Genes and Environment in Lung Cancer, Singapore study (GEL-S) [24], [25] (Table 1, Table S1), yielded comparable results (p = 1.16 * 10−21) (Table S2, Figure S1). Across all studies, we observed consistently increased risk associated with rs2736100 with no evidence for heterogeneity between studies, measured by the I2 test for heterogeneity (Figure S1). In a subsequent analysis combining all lung cancer cases, rs2736100 was also significantly associated with lung cancer susceptibility (p = 5.50 * 10−20; allelic OR = 1.48; 95% CI 1.36–1.62) (Table 2). This observation is comparable to what is being reported for adenocarcinoma alone, which is not surprising because adenocarcinomas constitute 76% of cases (Table 2).
Tab. 2. Lung cancer risk associated with rs2736100, among never-smoking females from East Asia, by histology. 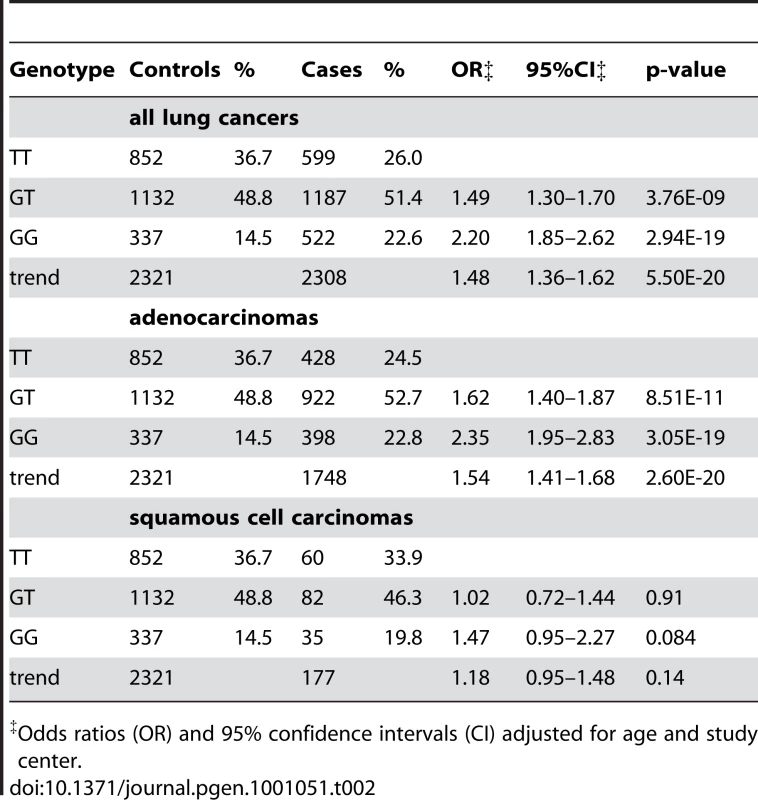
Odds ratios (OR) and 95% confidence intervals (CI) adjusted for age and study center. We conducted a first generation fine mapping of this region of chromosome 5p15.33 using 15 tag SNPs optimized in the East Asian studies in HapMap phase 2; the 15 SNPs were chosen using an r2≥0.8 as a threshold and estimated to cover approximately 85% of the known SNPs in HapMap phase 2 (Table S3). We did not identify stronger signals for association with lung adenocarcinoma in the 15 SNPs, as shown in Figure 3 and Table S4. Notably, rs402710, previously reported in GWAS of European ancestry [4], was the second most significant SNP tested in this region but did not achieve genome-wide significance (p = 0.0046) (Table S4). When rs402710 and rs2736100 were analyzed in a multivariable model, the former became non-significant (p = 0.33).
Fig. 3. LD structure and association results for the chr5p15 region and lung adenocarcinoma. 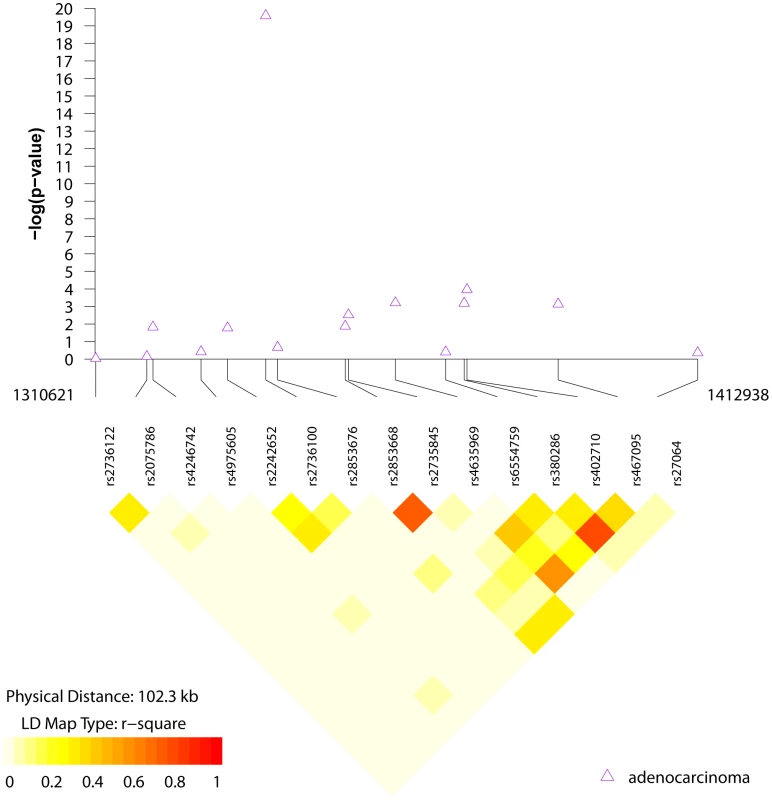
LD structure and regional association results for 15 SNPs genotyped in the 5p15 region. SNPs included were tagged with r2≥0.8 in HapMap CHB [37]. Discussion
In this study of lung adenocarcinoma in East Asian never-smoking women, we report a highly significant association with the common SNP, rs2736100, which localizes to the TERT-CLPTM1L locus on chromosome 5p15.33. Our study is notable because the sample size for never-smoking female cases is substantially larger than previous reports. Moreover, the estimated effect size observed for rs2736100 and adenocarcinoma of the lung (OR = 1.54) is greater than the associations previously reported in European populations (e.g., OR = 1.24 from the largest meta-analysis reported to date [4], [8], p = 0.000046 for difference). Our study provides strong evidence that this locus on chromosome 5p15.33 is directly related to lung carcinogenesis because it has been conclusively shown in non-smoking women.
The SNP marker, rs2736100, is mapped to a region of chromosome 5p15.33 in which common and rare genetic variants have been linked to a spectrum of cancers and related conditions. rs2736100 is localized to intron 2 of the telomerase gene TERT, a reverse transcriptase that is critical for telomere replication and stabilization by controlling telomere length. Variants in the TERT-CLPTM1L locus have been identified by GWAS to harbor susceptibility alleles for cancer of the brain, pancreas and lung [8], [26], [27]. For the latter, a large meta-analysis combined with a new scan indicates that the signal in this locus is most strongly associated with one histology, adenocarcinoma in studies of European subjects [8].
There is further evidence for association of this locus with additional cancers, though the reported results have not yet achieved the genome-wide association threshold; these include cancer of the bladder, prostate, uterine cervix, and skin including basal cell carcinoma and melanoma [4], [5], [7], [27]. Rare variations/mutations in the TERT gene have been described as a risk factor for acute myelogenous leukemia and also explain a proportion of the inherited bone marrow failure family pedigrees with dyskeratosis congenita, a cancer predisposition syndrome [28], [29]. Mutations in the TERT gene have also been described in patients with idiopathic pulmonary fibrosis [30], [31]. Together these findings suggest that the TERT-CLPTM1L 5p15.33 region could be important in the development of a spectrum of cancers. Still, at this time, further studies are needed to fine map the region, based on comprehensive re-sequence analysis in East Asian populations, to narrow the set of genetic variants worthy of functional studies to establish the mechanism underpinning the association marked by the SNP rs2736100 and subsequently compare these findings with comparable analyses in the other diseases.
The plausible mechanisms underlying the association signals across this region of chromosome 5p15.33 are currently under active investigation by many groups. Our findings are particularly interesting because we have identified variants that appear to be directly related to primary carcinogenesis. In this regard, it is critical that future studies evaluate environmental risk factors that may contribute, particularly since there is preliminary data suggesting that smoking as well as other exposures could directly influence telomere lengths [32]. It is noteworthy that lung cancer risk among non-smoking women in East Asia has been linked to indoor air pollution from environmental tobacco smoke [12], fumes produced by high temperature cooking [33], and coal combustion products [34].
Based on the discovery of susceptibility loci on chromosomal regions 6p21.33 and 15q25 first observed in European populations [1]–[3], [35], we attempted to replicate the findings in never-smoking women in East Asia. The strongest SNPs reported in each region plus additional tag SNPs, chosen on the basis of HapMap Phase 2, were genotyped in seven studies. 15 SNPs were selected for 6p21.33, covering an estimated 93% of known SNPs in HapMap phase 2 in the East Asian populations, whereas 24 SNPs were genotyped across 15q25, covering an estimated 83% of known common SNPs in the region (Table S3). In these East Asian never-smoking women, there was no convincing evidence for association at chromosome 6p21.33 or for 15q25 for lung cancer overall or for the adenocarcinoma subtype (Tables S5 and S6).
We report conclusive evidence that common genetic variants in the TERT-CLPTM1L locus on chromosome 5p15.33 are associated with risk for lung adenocarcinoma in non-smoking Asian women. We observed estimated effect sizes that are substantially higher than those previously reported in European smokers, which bears follow-up investigation into the biology of the underlying mechanism of the contribution of this region to primary lung carcinogenesis. Since this region on chromosome 5p15.33 has been implicated in many cancers, our observations should stimulate further investigation of the region that could lead to new insights into carcinogenesis.
Materials and Methods
Studies
A description of each study is provided in Table 1 and Text S1. Lung cancer cases and controls for the GWAS were drawn from the Genetic Epidemiological Study of Lung Adenocarcinoma (GELAC) in Taiwan. A total of 584 never-smoking incident cases and 585 never-smoking controls were included in the GWAS. The replication studies were drawn from seven studies, including additional subjects from the GELAC study [13], the Chinese Academy of Medical Sciences Cancer Hospital study (CAMSCH) [16], the Wuhan lung cancer study (WHLCS) [17], the Seoul National University study (SNU) [18], the Korea University Medical Center study (KUMC) [19], the Kyungpook National University Hospital study (KNUH) [20], and the Shanghai Women's Health Cohort Study (SWHS) [21], [22]. In addition, data were pooled with previously published findings from the Nanjing lung cancer study (NJLCS) [23] and the Genes and Environment in Lung Cancer, Singapore study (GEL-S) [24] (Table 1). All studies are case-control studies with the exception of the SWHS, which is a prospective cohort study. The range of ages is similar in cases and controls across all studies (Table 1).
Ethics statement
All study subjects provided informed consent and each study was approved by its respective institution's IRB.
Genotyping and quality control
Genome-wide association study genotyping and quality control
GWAS genotyping of the GELAC samples was performed in two separate phases. In the pilot phase, 54 cases and 54 controls were genotyped by GeneTech Biotech Co., (Taiwan), using the Illumina HumanCNV370-Duo BeadChip. The cases were never-smoking females diagnosed with lung adenocarcinoma at age ≤51 who had questionnaire data and DNA that passed quality control criteria for scanning. The controls were never-smoking females matched by age (±2 years) to cases.
Cluster definitions were determined using Illumina BeadStudio Genotyping Module v.3.3.4. Genotype calls were based on a quality score (Gene call value) of 0.25 or higher. Four blind duplicate pairs were included, and the concordance of SNP genotype calls between each pair is greater than 99.997%. Quality control metrics for data from the first phase are similar to those for data from the second phase, detailed below.
In the second phase of the GWAS, 550 cases and 549 controls were genotyped with the Illumina HumanHap610 Quad BeadChip on contract at deCODE Genetics, Iceland. The cases were the first never-smoking female lung adenocarcinoma subjects to be enrolled in the study with questionnaire data and DNA that passed quality control for scanning. Cluster definitions were determined using the Illumina BeadStudio Genotyping Module. The median genotype call rate for samples was 99.78%. 95% overall displayed call rates larger than 99.49%; the median call rate for variants is 99.91%, with 95% of variants with call rates above 99.55%. 21 blind duplicate pairs displayed an average concordance greater than 99.99%.
After quality control metrics were applied, 457,504 SNPs were used for the association analysis. SNPs (n = 1,705) were excluded if the call rate was below 90%, (i.e., a missing rate larger than 0.1); SNPs with a minor allele frequency below 0.05 (n = 131,558); SNPs with missing rate between 0.02 and 0.1 and non-random genotype failure with p<0.02 (n = 1,046); and, significant deviation from fitness for Hardy-Weinberg equilibrium (p<0.0001 in controls) (n = 718). 1064 unique samples from phase 2 were used in the association analysis, after two exclusion steps. The first set of exclusions was based on the quality control metrics described above and relatedness among individuals: call rates less than 90% (n = 3); sex discrepancies based on the X chromosome heterozygosity (n = 7); contaminated samples with high heterozygosity scores (n = 4), first or second degree relatives identified using genome-wide pairwise identical by descent (IBD) estimates (n = 9).
We further excluded 12 individuals from phase 2, based on population substructure analysis. In fact, to detect differences in population substructure, pairwise population concordance (PPC) test in PLINK (http://pngu.mgh.harvard.edu/purcell/plink/) [36] were performed with a threshold of 10−20 on two data sets using all autosomal SNPs that had passed the quality control metrics described above. The first data set consists of the 1184 unrelated individuals with high quality genotype data (108 from phase 1 and 1076 from phase 2). The PPC test identified 15 outliers who were distinct from the remaining 1169 (105 from phase 1 and 1064 from phase 2). The eight self-described aborigines (2 in Phase 1 and 6 in Phase 2) were among the outliers. Based on the PPC analysis, the final genome-wide association analysis was conducted using 1169 samples.
To further assess the population homogeneity in our study sample, we conducted additional analyses in our 1184 individuals with HapMap3 release 2 data [37]. The results indicate that for the 1184 unrelated individuals with high quality genotype data, 15 outliers were detected, thus yielding 1169 individuals with homogeneous genetic structure available for follow-up analyses. We seeded the study population with genotype data from hapmap 3 as well as hapmap 2; this included 85 CHD (Chinese in Metropolitan Denver, Colorado), in addition to our 1184 individuals and the hapmap 2 (84 CHB (Han Chinese in Beijing, China) and 86 JPT (Japanese in Tokyo, Japan)). A second analysis included our 1184 study individuals and a larger sample of HapMap3 release 2, namely the CHB, CHD, GIH (Gujarati Indians in Houston, Texas), JPT, LWK (Luhya in Webuye, Kenya), MKK (Maasai in Kinyawa, Kenya), and TSI (Toscani in Italia): the results confirmed that 15 outliers were detected whereas the 1164 represented a homogeneous population.
Although the above PPC tests seem to suggest little population substructure in our 1169 samples, we still used EIGENSTRAT [38] to conduct GWAS analysis to correct possible population stratification. We found that for the SNP rs2736100, the P-value is 1.239×10−11 based on the Armitage trend Chi-square statistic with no stratification correction and the P-value is 2.764×10−11 based on EIGENSTRAT using 10 principal components (the default value) for stratification correction. There was a negligible difference in p-values with and without this correction.
The genotyping cluster plot generated by the Illumina platform for rs2736100 is presented in Figure S2. The adjusted intensities for each allele are plotted, where each color represents a different genotype in the cluster plots. As shown in the figure, clusters of different genotypes are well separated from each other, indicating a high confidence in genotype calling in our study. The genotype call at this locus was confirmed with TaqMan genotyping (concordance of 99.7%).
Replication SNP selection and genotyping
DNA was extracted from blood samples and genotyped at the National Cancer Institute Core Genotyping Facility (CGF) (Http://cgf.nci.nih.gov) for four studies, SNU, KUMC, KNUH, and SWHS. TaqMan genotyping for the GELAC study (including all previously scanned cases and controls plus remaining never-smoking female cases and their matched controls) and the GEL-S studies was conducted in Taiwan and Singapore, respectively. Genotyping for the CAMSCH, WHLCS, and NJLCS studies were conducted at the Cancer Institute and Hospital, Chinese Academy of Medical Science, using TaqMan assays designed and optimized by the CGF (http://snp500cancer.nci.nih.gov).
We selected 54 SNPs optimized for Eastern Asian populations to cover the three chromosomal regions previously reported to show association for lung cancer (i.e., 15 SNPs in 5p15, 15 SNPs in 6p, and 24 SNPs in 15q25) (Table S3). The coordinates for selecting the tag SNPs were based on an r2<0.8 using the CHB samples of HapMap phase 2. The boundaries for the tag SNP selection were as follows: 5p15.33 from 1310620 to 1412939, 6p21.33 from 28782776 to 29018856 and 15q25 from 76593077 to 76702301 (Build 37). We computed genomic coverage using the GLU software package (http://code.google.com/p/glu-genetics/) for common SNPs (MAF≥0.05) based on the most recent build (Build 37) of the HapMap CHB [37] genotype data.
All TaqMan assays (Applied Biosystems Inc., Foster City, CA) for this study were optimized on the ABI 7900HT detection system with high concordance with sequence analysis of 102 individuals as listed on the SNP500Cancer website (http://snp500cancer.nci.nih.gov). All of the genotype frequencies were consistent with Hardy-Weinberg equilibrium except three SNPs (rs402710, rs9368570, and rs9257280) using a chi-square test (P<0.0001, Table S3). All reported genotyped results are based on completion rates of greater than 94% across all studies.
Statistical analysis
Genome-wide association tests
The program PLINK [36] was used to conduct primary statistical tests for association in the discovery phase. Association analyses between individual SNP and the lung cancer risk were carried out using computer packages in PLINK. Q-Q plots analyzed by the trend test are shown in Figure 1. We note that for Phase 1, we imputed the genotypes at the SNPs contained in HumanHap 610 Quad BeadChip but not in HumanCNV370-Duo BeadChip by using IMPUTE developed by Marchini et al. [39] and haplotypes of CHB in HapMap as the reference.
Replication and pooled analyses
Unconditional logistic regression was used to estimate the ORs and 95% CIs, adjusting for age and study center. All p values are two-sided. The most prevalent homozygous genotype was used as the reference group. Tests for trend were conducted by assigning the ordinal values 1, 2, and 3 to the most prevalent genotypes in rank order of wild type, heterozygous, and variant homozygous genotypes, respectively.
Supporting Information
Zdroje
1. AmosCI
WuX
BroderickP
GorlovIP
GuJ
2008 Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet 40 616 622
2. HungRJ
McKayJD
GaborieauV
BoffettaP
HashibeM
2008 A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452 633 637
3. LiuP
VikisHG
WangD
LuY
WangY
2008 Familial aggregation of common sequence variants on 15q24–25.1 in lung cancer. J Natl Cancer Inst 100 1326 1330
4. McKayJD
HungRJ
GaborieauV
BoffettaP
ChabrierA
2008 Lung cancer susceptibility locus at 5p15.33. Nat Genet 40 1404 1406
5. RafnarT
SulemP
StaceySN
GellerF
GudmundssonJ
2009 Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet 41 221 227
6. SpitzMR
AmosCI
DongQ
LinJ
WuX
2008 The CHRNA5-A3 region on chromosome 15q24–25.1 is a risk factor both for nicotine dependence and for lung cancer. J Natl Cancer Inst 100 1552 1556
7. WangY
BroderickP
WebbE
WuX
VijayakrishnanJ
2008 Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet 40 1407 1409
8. LandiMT
ChatterjeeN
YuK
GoldinLR
GoldsteinAM
2009 A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet 85 679 691
9. The Wellcome Trust Case Control Consortium 2007 Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 661 678
10. DonnellyP
2008 Progress and challenges in genome-wide association studies in humans. Nature 456 728 731
11. ChanockSJ
HunterDJ
2008 Genomics: when the smoke clears. Nature 452 537 538
12. LamWK
2005 Lung cancer in Asian women-the environment and genes. Respirology 10 408 417
13. JouYS
LoYL
HsiaoCF
ChangGC
TsaiYH
2009 Association of an EGFR intron 1 SNP with never-smoking female lung adenocarcinoma patients. Lung Cancer 64 251 256
14. de BakkerPI
FerreiraMA
JiaX
NealeBM
RaychaudhuriS
2008 Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum Mol Genet 17 R122 R128
15. ChanockSJ
ManolioT
BoehnkeM
BoerwinkleE
HunterDJ
2007 Replicating genotype-phenotype associations. Nature 447 655 660
16. WuC
HuZ
YuD
HuangL
JinG
2009 Genetic variants on chromosome 15q25 associated with lung cancer risk in Chinese populations. Cancer Res 69 5065 5072
17. BaiY
XuL
YangX
HuZ
YuanJ
2007 Sequence variations in DNA repair gene XPC is associated with lung cancer risk in a Chinese population: a case-control study. BMC Cancer 7 81
18. KimJH
KimH
LeeKY
ChoeKH
RyuJS
2006 Genetic polymorphisms of ataxia telangiectasia mutated affect lung cancer risk. Hum Mol Genet 15 1181 1186
19. JungHY
WhangYM
SungJS
ShinHD
ParkBL
2008 Association study of TP53 polymorphisms with lung cancer in a Korean population. J Hum Genet 53 508 514
20. ParkJY
ParkSH
ChoiJE
LeeSY
JeonHS
2002 Polymorphisms of the DNA repair gene xeroderma pigmentosum group A and risk of primary lung cancer. Cancer Epidemiol Biomarkers Prev 11 993 997
21. ZhangY
ShuXO
GaoYT
JiBT
YangG
2007 Family history of cancer and risk of lung cancer among nonsmoking Chinese women. Cancer Epidemiol Biomarkers Prev 16 2432 2435
22. ZhengW
ChowWH
YangG
JinF
RothmanN
2005 The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol 162 1123 1131
23. JinG
XuL
ShuY
TianT
LiangJ
2009 Common genetic variants on 5p15.33 contribute to risk of lung adenocarcinoma in a Chinese population. Carcinogenesis 30 987 990
24. TruongT
HungRJ
AmosCI
WuX
BickeböllerH
2010 Replication of lung cancer susceptibility loci at chromosomes 15q25, 5p15, and 6p21: a pooled analysis from the International Lung Cancer Consortium. J Natl Cancer Inst In press
25. TangL
LimW
EngP
LeongSS
LimTK
2010 Lung cancer in Chinese women: evidence for an interaction between tobacco smoking and exposure to inhalants in the indoor environment. Environ Health Perspect Environ Health Perspect In press
26. PetersenGM
AmundadottirL
FuchsCS
KraftP
Stolzenberg-SolomonRZ
2010 A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet 42 224 228
27. SheteS
HoskingFJ
RobertsonLB
DobbinsSE
SansonM
2009 Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet 41 899 904
28. YamaguchiH
CaladoRT
LyH
KajigayaS
BaerlocherGM
2005 Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med 352 1413 1424
29. CaladoRT
RegalJA
HillsM
YewdellWT
DalmazzoLF
2009 Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A 106 1187 1192
30. MushirodaT
WattanapokayakitS
TakahashiA
NukiwaT
KudohS
2008 A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet 45 654 656
31. TsakiriKD
CronkhiteJT
KuanPJ
XingC
RaghuG
2007 Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A 104 7552 7557
32. ValdesAM
AndrewT
GardnerJP
KimuraM
OelsnerE
2005 Obesity, cigarette smoking, and telomere length in women. Lancet 366 662 664
33. WakeleeHA
ChangET
GomezSL
KeeganTH
FeskanichD
2007 Lung cancer incidence in never smokers. J Clin Oncol 25 472 478
34. LanQ
ChapmanRS
SchreinemachersDM
TianL
HeX
2002 Household stove improvement and risk of lung cancer in Xuanwei, China. J Natl Cancer Inst 94 826 835
35. ThorgeirssonTE
GellerF
SulemP
RafnarT
WisteA
2008 A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452 638 642
36. PurcellS
NealeB
Todd-BrownK
ThomasL
FerreiraMA
2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
37. The International HapMap Consortium 2005 A haplotype map of the human genome. Nature 437 1299 1320
38. PriceAL
PattersonNJ
PlengeRM
WeinblattME
ShadickNA
2006 Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 904 909
39. MarchiniJ
HowieB
MyersS
McVeanG
DonnellyP
2007 A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 39 906 913
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 8- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
-
Všechny články tohoto čísla
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Mutation in the Gene Encoding Ubiquitin Ligase LRSAM1 in Patients with Charcot-Marie-Tooth Disease
- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Did Genetic Drift Drive Increases in Genome Complexity?
- The 5p15.33 Locus Is Associated with Risk of Lung Adenocarcinoma in Never-Smoking Females in Asia
- An Alpha-Catulin Homologue Controls Neuromuscular Function through Localization of the Dystrophin Complex and BK Channels in
- Epigenetically-Inherited Centromere and Neocentromere DNA Replicates Earliest in S-Phase
- Survival and Growth of Yeast without Telomere Capping by Cdc13 in the Absence of Sgs1, Exo1, and Rad9
- Tuberous Sclerosis Complex 1 Regulates dE2F1 Expression during Development and Cooperates with RBF1 to Control Proliferation and Survival
- Disease-Associated Mutations That Alter the RNA Structural Ensemble
- The Transcriptomes of Two Heritable Cell Types Illuminate the Circuit Governing Their Differentiation
- Inactivation of VCP/ter94 Suppresses Retinal Pathology Caused by Misfolded Rhodopsin in
- Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: a Meta-Analysis and Comparison with Lung Cancer and COPD
- Transcriptional Regulation by CHIP/LDB Complexes
- Conserved Role of in Ethanol Responses in Mutant Mice
- A Global Overview of the Genetic and Functional Diversity in the Pathogenicity Island
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3–Dependent Manner in
- Genetic Analysis of Baker's Yeast Msh4-Msh5 Reveals a Threshold Crossover Level for Meiotic Viability
- Genome-Wide Association Studies of Serum Magnesium, Potassium, and Sodium Concentrations Identify Six Loci Influencing Serum Magnesium Levels
- Something New: An Interview with Radoje Drmanac
- The Extinction Dynamics of Bacterial Pseudogenes
- Microtubule Actin Crosslinking Factor 1 Regulates the Balbiani Body and Animal-Vegetal Polarity of the Zebrafish Oocyte
- Consistent Association of Type 2 Diabetes Risk Variants Found in Europeans in Diverse Racial and Ethnic Groups
- Transmission of Mitochondrial DNA Diseases and Ways to Prevent Them
- Telomere Disruption Results in Non-Random Formation of Dicentric Chromosomes Involving Acrocentric Human Chromosomes
- Chromosome Axis Defects Induce a Checkpoint-Mediated Delay and Interchromosomal Effect on Crossing Over during Drosophila Meiosis
- Dynamic Chromatin Organization during Foregut Development Mediated by the Organ Selector Gene PHA-4/FoxA
- Ancient Protostome Origin of Chemosensory Ionotropic Glutamate Receptors and the Evolution of Insect Taste and Olfaction
- A Wnt-Frz/Ror-Dsh Pathway Regulates Neurite Outgrowth in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Did Genetic Drift Drive Increases in Genome Complexity?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



