-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetic Analysis of Baker's Yeast Msh4-Msh5 Reveals a Threshold Crossover Level for Meiotic Viability
During meiosis, the Msh4-Msh5 complex is thought to stabilize single-end invasion intermediates that form during early stages of recombination and subsequently bind to Holliday junctions to facilitate crossover formation. To analyze Msh4-Msh5 function, we mutagenized 57 residues in Saccharomyces cerevisiae Msh4 and Msh5 that are either conserved across all Msh4/5 family members or are specific to Msh4 and Msh5. The Msh5 subunit appeared more sensitive to mutagenesis. We identified msh4 and msh5 threshold (msh4/5-t) mutants that showed wild-type spore viability and crossover interference but displayed, compared to wild-type, up to a two-fold decrease in crossing over on large and medium sized chromosomes (XV, VII, VIII). Crossing over on a small chromosome, however, approached wild-type levels. The msh4/5-t mutants also displayed synaptonemal complex assembly defects. A triple mutant containing a msh4/5-t allele and mutations that decreased meiotic double-strand break levels (spo11-HA) and crossover interference (pch2Δ) showed synergistic defects in spore viability. Together these results indicate that the baker's yeast meiotic cell does not require the ∼90 crossovers maintained by crossover homeostasis to form viable spores. They also show that Pch2-mediated crossover interference is important to maintain meiotic viability when crossovers become limiting.
Published in the journal: . PLoS Genet 6(8): e32767. doi:10.1371/journal.pgen.1001083
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001083Summary
During meiosis, the Msh4-Msh5 complex is thought to stabilize single-end invasion intermediates that form during early stages of recombination and subsequently bind to Holliday junctions to facilitate crossover formation. To analyze Msh4-Msh5 function, we mutagenized 57 residues in Saccharomyces cerevisiae Msh4 and Msh5 that are either conserved across all Msh4/5 family members or are specific to Msh4 and Msh5. The Msh5 subunit appeared more sensitive to mutagenesis. We identified msh4 and msh5 threshold (msh4/5-t) mutants that showed wild-type spore viability and crossover interference but displayed, compared to wild-type, up to a two-fold decrease in crossing over on large and medium sized chromosomes (XV, VII, VIII). Crossing over on a small chromosome, however, approached wild-type levels. The msh4/5-t mutants also displayed synaptonemal complex assembly defects. A triple mutant containing a msh4/5-t allele and mutations that decreased meiotic double-strand break levels (spo11-HA) and crossover interference (pch2Δ) showed synergistic defects in spore viability. Together these results indicate that the baker's yeast meiotic cell does not require the ∼90 crossovers maintained by crossover homeostasis to form viable spores. They also show that Pch2-mediated crossover interference is important to maintain meiotic viability when crossovers become limiting.
Introduction
Meiosis produces haploid gametes from diploid progenitor cells. This reduction in ploidy results from the segregation of homologous chromosomes at the first meiotic division (Meiosis I). In most organisms, the accurate segregation of chromosomes during Meiosis I requires crossing over between homologs. These crossovers provide physical linkages between homologs that enable their proper positioning at metaphase I through spindle microtubule generated forces [1]. Disruption of these forces by the loss of chromosome arm cohesion facilitates the Meiosis I division [2]. Failure to achieve at least one crossover per homolog pair results in non-disjunction of the homolog pair, leading to the production of aneuploid gametes (reviewed in [3]).
Meiotic crossing over is initiated in meiotic prophase by the formation of Spo11-dependent DNA double strand breaks (DSBs; [4]). Meiotic DSBs can be repaired as either crossovers or non-crossovers through distinct repair pathways [5], [6]. In Saccharomyces cerevisiae, approximately 60% of the 140–170 DSBs that form in meiosis (estimated from a whole genome microarray analysis of dmc1Δ and dmc1Δ rad51Δ mutants) are processed as crossovers [7], [8]. A single S. cerevisiae cell in meiosis forms approximately 90 crossovers distributed over sixteen homolog pairs [9]–[11]. In contrast, in C. elegans meiosis, only a single crossover forms between each homolog pair that ensures Meiosis I disjunction [12].
The majority of meiotic crossovers in baker's yeast display interference. Interference ensures that a crossover designation for one DSB site makes a non-crossover fate more likely at adjacent sites, and leads to the formation of widely and evenly spaced crossovers [13]–[15]. In the interference-dependent crossover pathway, DSBs are processed to form single end invasion intermediates (SEIs) that result from the invasion of a DSB end into an intact homolog. These intermediates are then thought to undergo second-end capture with the intact homolog to form double Holliday junctions (dHJs) that are ultimately resolved to form crossovers [16]–[19]. A crossover homeostasis mechanism was identified in baker's yeast that ensures crossovers are preferentially formed at the expense of non-crossovers when the number of initiating DSBs is reduced [20]. Thus crossover interference and homeostasis ensure formation of at least one crossover on all homolog pairs [20], [21]. The presence of at least one crossover per homolog pair is known as the obligate crossover. Barchi et al. [22] further define the obligate crossover “as one of the outcomes of the process(es) through which most crossovers form, not as a special type of crossover.” Control mechanisms that ensure the obligate crossover are likely to act during the crossover/non-crossover decision, an event that takes place at or just prior to SEI formation [5], [6]. It is important to note that previous work in baker's yeast suggested that ∼20% of crossovers on a large chromosome and ∼50% of crossovers on a small chromosome involved interference-independent crossovers that occurred through a distinct Mms4-Mus81 pathway [23], [24].
The ZMM proteins (Zip1-4, Spo16, Mer3, Msh4-Msh5) act as pro-crossover factors in the interference-dependent crossover pathway by coordinating crossing over with formation of the synaptonemal complex, a zipper-like structure that connects homologous chromosomes in late stages of meiotic prophase [24]–[34]. Msh4-Msh5 attracted our attention because strains defective in this complex show strong defects in Zip1 polymerization during synaptonemal complex formation [27], [29]. Msh4 and Msh5 each contain domains II–V found in the bacterial MutS family of mismatch repair proteins, but lack the N - terminal domain I that is required to interact with domain IV for mismatch DNA binding (Figure 1A; [25], [26], [35], [36]). S. cerevisiae msh4Δ and msh5Δ mutants display reduced crossing over (∼2.5 fold decreased) and spore viability (30–40%). Tetrads obtained from these mutants display an excess of zero and two viable spores compared to wild-type. This phenotype is consistent with a Meiosis I disjunction defect [24]–[27]. The equivalent mutations in male and female mice result in sterility as a consequence of chromosome pairing and synapsis defects [37]–[39]. The residual crossovers seen in yeast msh4/5Δ mutants lack genetic interference [24], [27]; however in msh4Δ mutants, Zip2 foci, which mark crossover designation sites, still display a pattern indicating that they are subject to interference [40]. These and other data suggest that Msh4-Msh5 acts after the crossover/noncrossover decision [16], [40]. Consistent with the above data, biochemical and molecular studies showed that Msh4-Msh5 is required to stabilize SEIs and is capable of specifically binding to Holliday junctions as multiple sliding clamps [16], [41].
Fig. 1. Structure-function analysis of msh4, msh5 alleles. 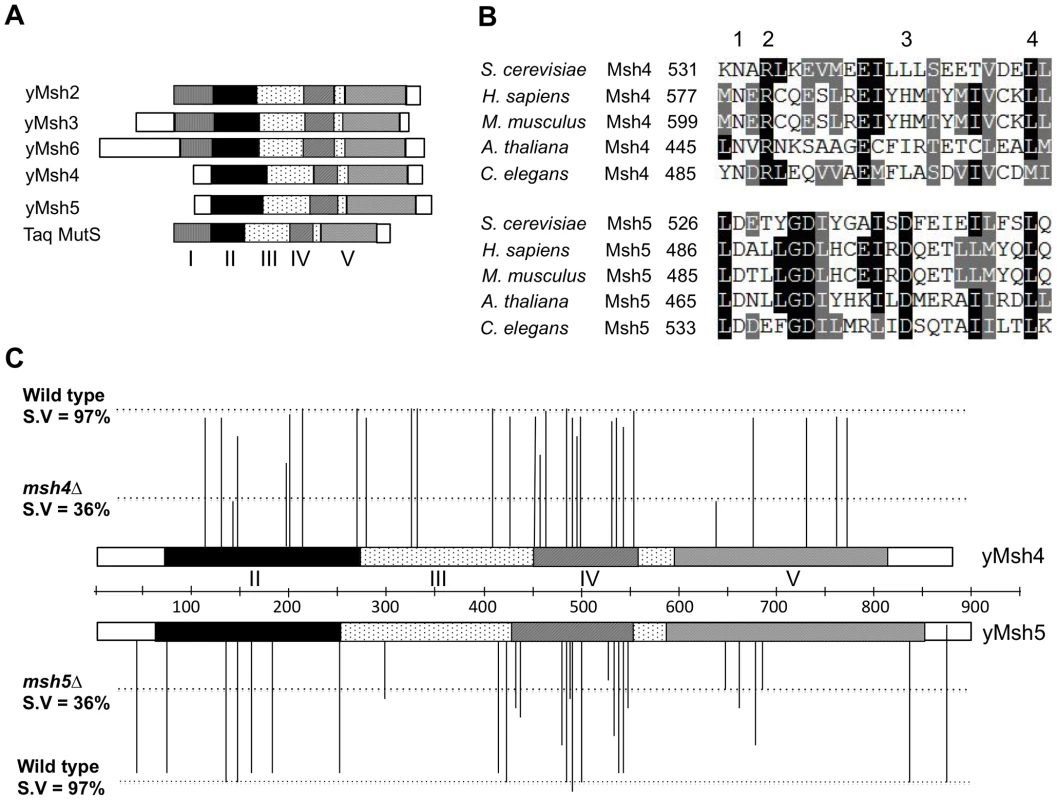
(A) Comparison of domain organization of yeast Msh proteins with the Thermus aquaticus (Taq) MutS protein. The five domains (I–V) identified in yeast Msh proteins based on structural homology to Taq MutS are shown to scale [35]. (B) Sequence alignment of Msh4 protein sequences from S. cerevisiae (YFL003C), H. sapiens (NM_002440), M. musculus (BC145838), A. thaliana (NM_117842) and C. elegans (AF178755) and Msh5 protein sequences from S. cerevisiae (YDL154W), H. sapiens (BC002498), M. musculus (NM_013600), A. thaliana (EF471448) and C. elegans (NM_070130). Representative residues from four different classes used for structure-function analysis are shown; Class 1 (Msh4, Msh5 specific); Class 2 (Msh4 specific); Class 3 (Msh5 specific) and Class 4 (Msh family specific). (C) Spore viability profiles of 57 msh4, msh5 mutations in the EAY background are shown with reference to the domain organization of the Msh4, Msh5 proteins. The height of each line corresponds to the spore viability of each mutant relative to wild-type and null. Four domains (II–V) in the Msh4, Msh5 proteins based on structural homology to MutS are shown [35]. Additional cell biological observations, primarily in the mouse, have led to a model in which Msh4-Msh5 interacts with the MutL mismatch repair homologs Mlh1-Mlh3 to resolve Holliday junctions [25], [41]–[47]. In mouse spermatocytes in zygotene, Msh4/5 foci are present at high levels (∼140 per nucleus) but decrease until mid pachytene, where they are present at roughly twice the number of crossover sites. At this stage, roughly half of Msh4/5 foci interact with Mlh1/3 foci, which localize to sites of crossing over [48]–[50]. The presence of a large number of Msh4/5 foci in zygotene suggest the possibility of early roles for Msh4/5 in meiosis; consistent with this idea is work in Sordaria which show an early role for Msh4-Msh5 during interhomolog interactions, at a time prior to when it is required for recombination progression [51].
The above information encouraged us to systematically mutagenize Msh4-Msh5 to study its role in implementing the crossover decision. We identified a class of msh4/5 threshold (msh4/5-t) mutants that displayed high spore viability despite 1.5 to 2 fold reductions in crossing over that occurred primarily on large (XV, VII) and medium (VIII) sized chromosomes. msh4/5-t mutants displayed Msh5 foci similar to wild-type; however, they showed defects in Zip1 polymerization during synaptonemal complex formation. This phenotype is consistent with defects in a crossover maturation process that occurs after Msh4-Msh5 loading onto chromosomes. A triple mutant containing a msh4/5-t allele and mutations that decreased DSB levels (spo11-HA) and crossover interference (pch2Δ) showed preferential loss of crossovers on the small chromosome III and a synthetic spore viability defect, suggesting that crossover interference is critical to maintain meiotic viability when crossovers become limiting.
Results
Rationale for structure-function analysis of Msh4 and Msh5
Msh4 and Msh5 amino acid sequences from S. cerevisiae, H. sapiens, M. musculus, A. thaliana, and C. elegans were aligned using clustalW and CLC free Workbench software (Figure 1, Figure S1; data not shown). We selected four different classes of conserved residues to alter by site-specific mutagenesis (Figure 1B). Class 1 (Msh4/5-specific) residues were conserved in Msh4 and Msh5 but were not conserved in other Msh family members such as Msh2, Msh3, and Msh6. Class 2 (Msh4-specific) and Class 3 (Msh5-specific) were conserved only in Msh4 and Msh5, respectively (Figure 1B; Table 1). Previous work by Pochart et al. [52] showed that mutations in the ATP binding domain of Msh5 conferred a null phenotype. Based on these observations, we also mutagenized ATP and DNA binding residues conserved among all Msh family members (Class 4). Eight of these Class 4 mutations were in homologous positions in Msh4 and Msh5 (Figure S1). In total 57 residues were mutated, 29 from Msh4 and 28 from Msh5 (Table 1). All residues were mutated to alanine, with the exception of one residue in the Msh4/5 ATP binding domain that was mutated to tryptophan to allow comparison with an amino acid substitution in a homologous position in Msh2 that affected Msh2-Msh6 ATP hydrolysis [53]. All alleles were integrated into the congenic SK1 strain EAY1108 (EAY background, [24]).
Tab. 1. Spore viability and genetic map distances in EAY1108/EAY1112 strains bearing the indicated msh4 and msh5 mutations. 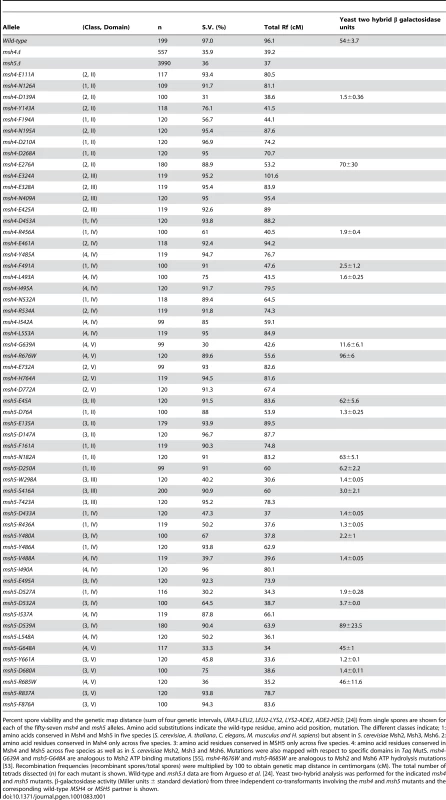
Percent spore viability and the genetic map distance (sum of four genetic intervals, URA3-LEU2, LEU2-LYS2, LYS2-ADE2, ADE2-HIS3; [24]) from single spores are shown for each of the fifty-seven msh4 and msh5 alleles. Amino acid substitutions indicate the wild-type residue, amino acid position, mutation. The different classes indicate; 1: amino acids conserved in Msh4 and Msh5 in five species (S. cerevisiae, A. thaliana, C. elegans, M. musculus and H. sapiens) but absent in S. cerevisiae Msh2, Msh3, Msh6. 2: amino acid residues conserved in Msh4 only across five species. 3: amino acid residues conserved in MSH5 only across five species. 4: amino acid residues conserved in Msh4 and Msh5 across five species as well as in S. cerevisiae Msh2, Msh3 and Msh6. Mutations were also mapped with respect to specific domains in Taq MutS. msh4-G639A and msh5-G648A are analogous to Msh2 ATP binding mutations [55]. msh4-R676W and msh5-R685W are analogous to Msh2 and Msh6 ATP hydrolysis mutations [53]. Recombination frequencies (recombinant spores/total spores) were multiplied by 100 to obtain genetic map distance in centimorgans (cM). The total number of tetrads dissected (n) for each mutant is shown. Wild-type and msh5Δ data are from Argueso et al. [24]. Yeast two-hybrid analysis was performed for the indicated msh4 and msh5 mutants. β-galactosidase activity (Miller units ± standard deviation) from three independent co-transformants involving the msh4 and msh5 mutants and the corresponding wild-type MSH4 or MSH5 partner is shown. Msh5 appears more sensitive to mutagenesis than Msh4
msh4 and msh5 alleles were analyzed as heterozygotes over their respective deletion mutations in the SK1 congenic strain EAY1112 [24]. The mutant diploid strains were sporulated and assessed for spore viability and genetic map distances on chromosome XV (Table 1; Figure 1C). The mutations are presented relative to Thermus aquaticus MutS domains II, III (linker), IV (DNA binding) and V (ATPase) [35]. The spore viability profiles of msh4 and msh5 mutants indicated that the Msh5 subunit was more sensitive to mutagenesis (Figure 2A). A larger proportion of msh5 mutants showed ≤50% spore viability compared to msh4 (9 of 28 for msh5 versus 2 of 29 of msh4; p = 0.02, Fisher's exact test). This difference was also seen in an analysis of mutations in domain IV (DNA binding); 5 of 12 msh5 mutations conferred ≤50% spore viability compared to 0 of 11 msh4 mutations (p = 0.03, Fisher's exact test).
Fig. 2. The Msh5 subunit is more sensitive to mutagenesis. 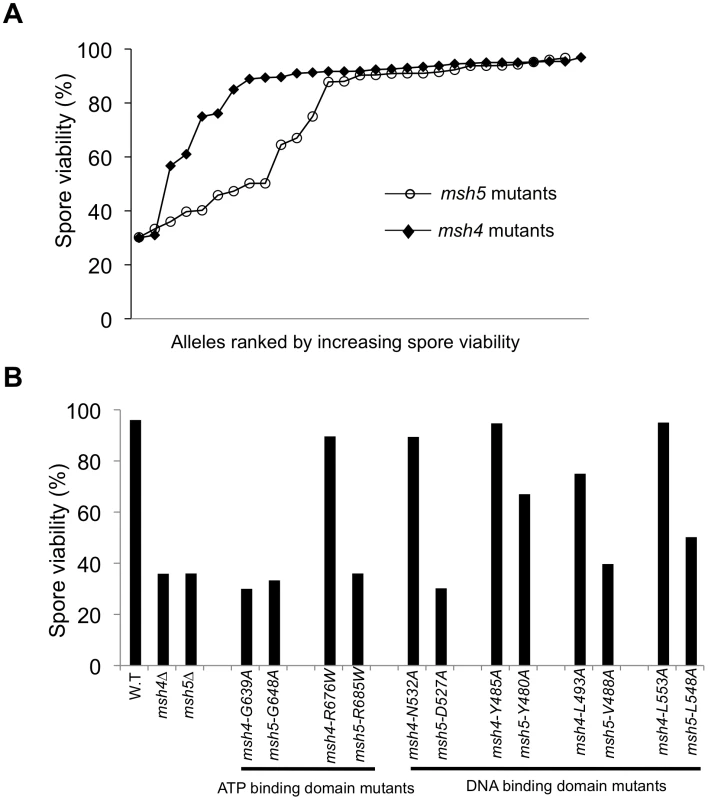
(A) Comparison of spore viability of 29 msh4 and 28 msh5 mutants in ascending order in the EAY background. (B) Spore viability of conserved pairs of residues in Msh4, Msh5 ATP binding domain and DNA binding domain. msh4-G639A and msh5-G648A contain mutations analogous to ATP binding mutations in Msh2 while msh4-R676W and msh5-R685W contain mutations analogous to ATP hydrolysis mutations in Msh2. msh4-N532A, msh4-Y485A, msh4-L493A, msh4-L553A, and their matched mutations in Msh5 (msh5-D527A, msh5-Y480A, msh5-V488A, msh5-L548A) are conserved within the DNA binding domain (IV). The number of tetrads dissected for each strain is presented in Table 1. Five of the eight mutations in homologous positions in Msh4 and Msh5 conferred subunit-specific phenotypes. Both msh4-G639A and msh5-G648A strains contain mutations (Walker motif A) predicted to disrupt ATP binding; both of these strains displayed null phenotypes [35]–[36], [52]–[55]. In contrast, a predicted ATP hydrolysis mutation in Msh4, msh4-R676W, conferred wild-type spore viability but the corresponding mutation in Msh5, msh5-R685W, conferred a null phenotype (Figure 2B; Table 1). Similar asymmetries between Msh4 and Msh5 were observed at four residues in the DNA binding domain IV (Figure 2B; Table 1). msh4-N532A, msh4-Y485A, msh4-L493A, and msh4-L553A had spore viabilities of 89, 95, 75, and 95%, respectively; corresponding mutants msh5-D527A, msh5-Y480A, msh5V-488A, and msh5-L548A had significantly lower spore viabilities (30, 67, 40, and 50%, respectively).
Most msh4 and msh5 mutants with significant spore viability and/or crossover defects could not form stable Msh4-Msh5 complexes as assessed in the two-hybrid assay (Table 1). The only exceptions were msh4-E276A (domain II), msh4-R676W (ATP hydrolysis), msh5-D539A (domain IV), msh5-G648A (ATP binding), and msh5-R685W (ATP hydrolysis) mutants that displayed poor spore viability or crossover defects but formed stable complexes with a wild-type partner. Inability to form a stable complex in the two-hybrid assay can be explained by the disruption of an interaction domain or a loss in protein stability. Because most mutations were created in highly conserved residues that lie outside of putative interaction domains in Msh proteins [35], [36], [54], a defect in the two-hybrid assay is likely to reflect a disruption of protein structure.
A threshold level of crossing over is sufficient to ensure spore viability
Spore viability was plotted as a function of genetic map distance for all msh4 and msh5 mutants (Figure 3). This plot shows that crossing over could be reduced by up to two-fold on the large chromosome XV without affecting spore viability. msh4/5 mutations (msh4/5-t) near the threshold limit for crossovers included msh4-E276A, msh4-F491A, msh4-N532A, msh4-R676W, msh5-D76A, msh5-D250A, msh5-S416A, msh5-Y486A, and msh5-D539A (Table 1). The phenotypes conferred by these mutations were independent of their ability to disrupt the Msh4-Msh5 complex as measured in the two-hybrid assay (Table 1). A second class of msh4/5 mutants showed greater than two-fold decreases in crossing over on chromosome XV. This below-threshold class (msh4/5-bt; msh4-Y143A, msh4-F194A, msh4-R456A, msh4-L493A, msh5-R436A, msh5-Y480A, msh5-D532A, msh5-L548A, msh5-D680A) showed spore viabilities between 50 and 76%. These mutants were all defective in their ability to form stable Msh4-Msh5 complexes in the two-hybrid assay (Table 1).
Fig. 3. Crossovers can be reduced to a threshold level without affecting spore viability. 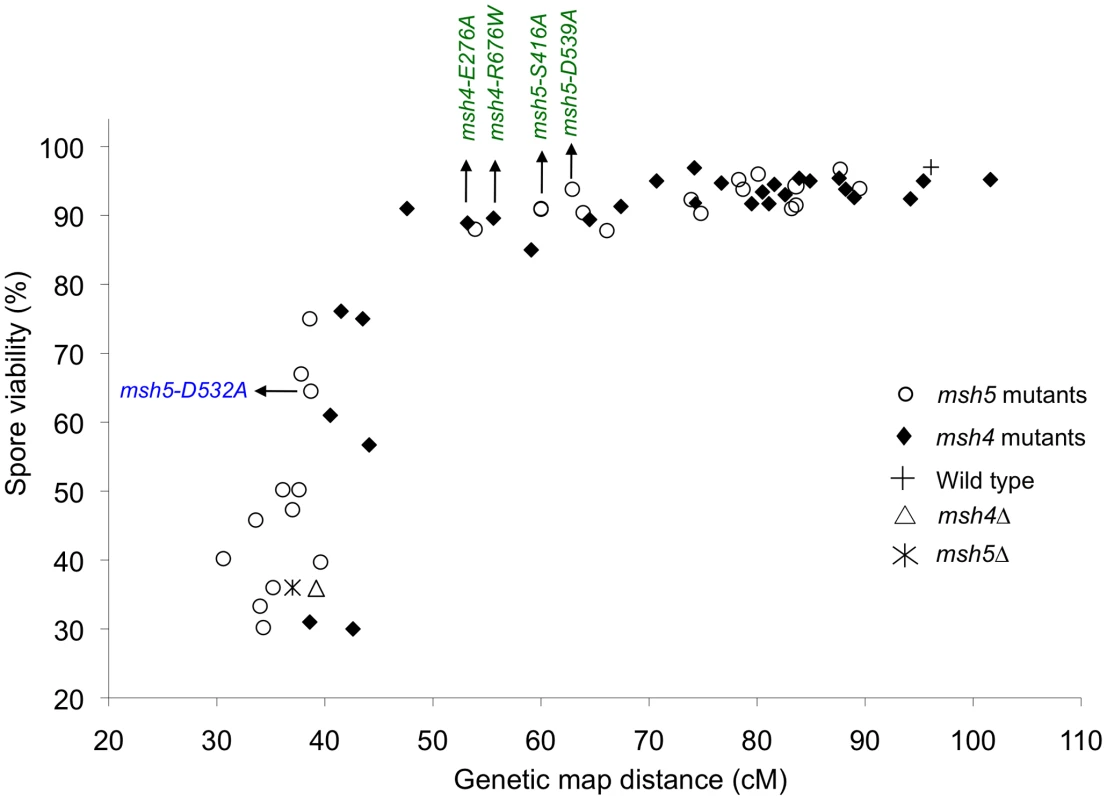
Plot of spore viability versus genetic map distance on chromosome XV in 57 msh4, msh5 mutants in the EAY strain background. Wild-type, msh4Δ, and msh5Δ data were also plotted. The msh4/5-t (green font) and msh4/5-bt (blue font) alleles analyzed in greater depth are shown. Raw data are shown in Table 1. msh4/5-t mutants display a preferential loss of crossing over on large chromosomes
The wild-type spore viability profile for the msh4/5-t mutants suggested they were able to properly segregate all sixteen homolog pairs in Meiosis I (Table 1; Figure 3, Figure 4). We further examined the phenotype of a subset of msh4/5-t mutants (msh4-E276A, msh4-R676W, msh5-S416A, msh5-D539A; all but msh5-S416A showed wild-type two-hybrid interactions) in the SK1 isogenic NHY strain background. msh4 and msh5 alleles were analyzed as heterozygotes over their respective deletion mutations. The NHY diploid strains allowed us to measure genetic map distances in large (VII), medium (VIII), and small (III) chromosomes (Figure 5A; [23]). Smaller chromosomes have higher map distances per physical distance and weaker interference relative to larger chromosomes ([40], [56], [57] but see [58]). Thus we used this strain set to determine if msh4/5-t mutations altered crossover patterns on representative small, medium, and large chromosomes.
Fig. 4. Spore viability profile of wild-type and mutant strains in the NHY942/943 strain background. 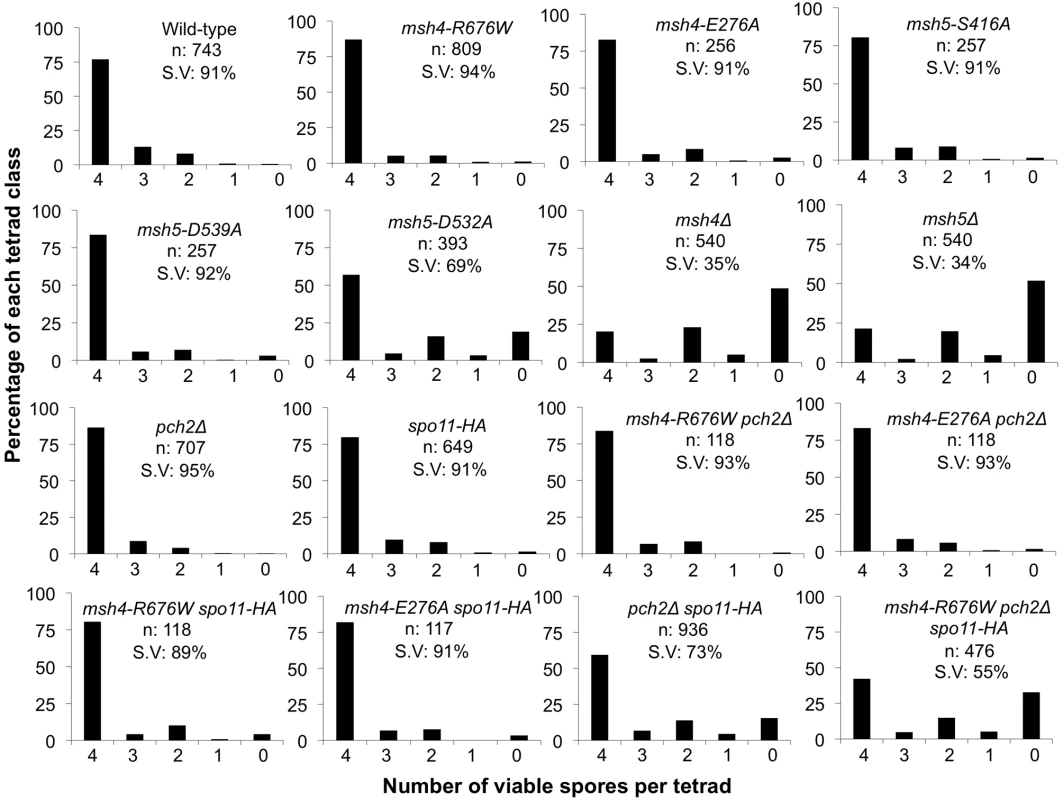
The vertical axis shows the percentage of each tetrad class and the horizontal axis represents the number of viable spores in a tetrad. n: total number of tetrads dissected, SV: percentage spore viability. Data for wild-type, pch2Δ, spo11-HA and pch2Δ spo11-HA are from Zanders and Alani [21]. Fig. 5. Cumulative genetic map distance in msh4/5 hypomorphs and double and triple mutations with pch2Δ and spo11-HA. 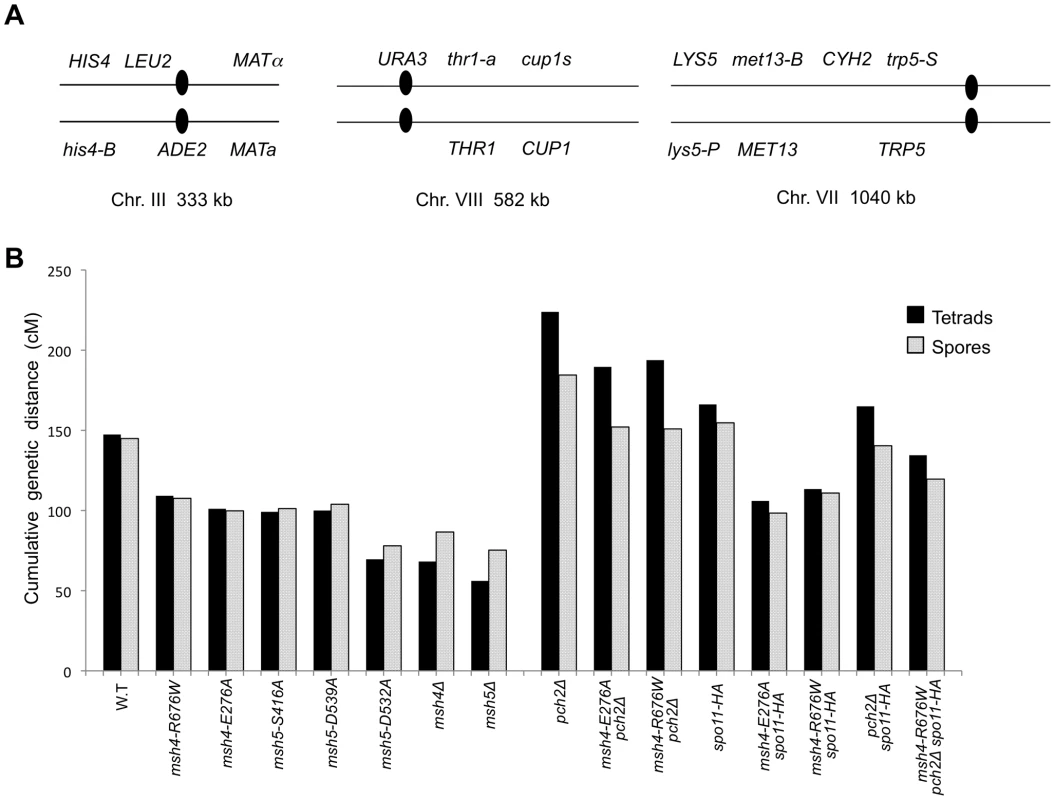
(A) Location of genetic markers assayed on chromosomes III, VII and VIII in the NHY strain background. Solid circle indicates the centromere. (B) Sum of the genetic map distance (from total spores and complete tetrads) over chromosomes III, VII and VIII in the NHY942/NHY943 strain background. Raw data are shown in Table 2. Data for wild-type, pch2Δ, spo11-HA, and pch2Δ spo11-HA are from Zanders and Alani [21]. All four msh4/5-t mutants displayed wild-type spore viability but decreased crossing over (∼1.5-fold for the sum of map distances in three chromosomes; Figure 4, Figure 5B; Table 2). The spore viabilities of wild-type and one msh4/5-t mutant, msh4-R676W, were unaffected by raising the sporulation temperature to 33°C, a condition shown previously in the SK1 background to cause coordinated defects in the formation of recombination intermediates and crossover products in msh5Δ (data not shown; [16]). This observation provides another indication that msh4/5-t alleles confer sufficient Msh4-Msh5 function in meiosis. The sum of genetic map distances calculated from tetrads (similar values were obtained from total spores) in wild-type was 147 cM; map distances for msh4-E276A, msh4-R676W, msh5-S416A and msh5-D539A were 101, 109, 99, and 100 cM, respectively.
Tab. 2. Genetic map distances and distribution of parental and recombinant progeny in msh4, msh5 mutants in the NHY942/NHY943 strain background. 
All mutants are isogenic derivatives of NHY942/NHY943 (Materials and Methods). For single spores, recombination frequencies (recombinant spores/total spores) were multiplied by 100 to yield genetic map distances (cM). 95% confidence intervals for genetic map distance in the single spores were determined using VassarStats (http://faculty.vassar.edu/lowry/VassarStats.html). For tetrads, genetic distance in centimorgans (cM) was calculated using the RANA software without considering aberrant segregants [24]. The Stahl Laboratory Online Tools website (http://groik.com/stahl/) was used to calculate standard error around the genetic distance for tetrads. n; number of single spores, N; four spore viable tetrads analyzed; Par, parental single spores; Rec, recombinant single spores; S.E; standard error. Wild-type, pch2Δ, spo11-HA and pch2Δ spo11-HA data are from Zanders and Alani [21]. As shown in Figure 6, msh4/5-t mutants displayed a chromosome size-dependent loss of crossovers. For three intervals on the smallest chromosome III, the four msh4/5-t mutants showed 73 to 92% of wild-type crossover levels (determined from tetrad data). In contrast these mutants showed 63 to 76% of wild-type levels for the two intervals on a medium sized chromosome VIII, and 61 to 66% of wild-type levels for the three intervals on a large chromosome (Chromosome VII). The loss of crossovers on the large chromosome VII approached that seen in msh4/5Δ strains. For the msh4Δ and msh5Δ mutants, the sum of genetic map distances calculated from tetrads was 68 and 56 cM, respectively (2.2 to 2.6-fold drop in crossovers over three chromosomes, Figure 5; Table 2). The values from total spores were 87 and 75 cM for msh4Δ and msh5Δ, respectively. The differences in map distance calculated by spore and tetrad data were likely due to the high rate of gene conversion seen in msh4Δ and msh5Δ mutants (see below). Based on tetrad data msh4Δ crossovers levels were 36, 42 and 54% of wild-type on chromosomes III, VIII, and VII, respectively. For msh5Δ crossover levels were 26, 34 and 47% of wild-type on chromosomes III, VIII, and VII, respectively (Figure 6).
Previously Stahl et al. [15] and Abdullah et al. [59] reported a greater loss of crossovers on larger chromosomes (VII) compared to smaller ones (III) in msh4Δ/msh5Δ mutants. These groups analyzed crossing over in wild-type, msh4Δ and msh5Δ strains in two intervals (HIS4-LEU2 and LEU2-MAT) on chromosome III (small) and two (TRP5-CYH2 and CYH2-MET13) on chromosome VII (large) in the congenic RHB strain background. They found that the crossover defect in msh4Δ and msh5Δ mutants was stronger on chromosome VII (23% and 27% of wild-type, respectively) compared to chromosome III (39% and 34% of wild type, respectively). We performed our analysis in the NHY SK1 isogenic strain. We do not have a good explanation for why our data differ from the Stahl et al. [15] and Abdullah et al. [59] studies. One possibility is that genetic mapping information from a limited number of intervals may yield a pattern due to localized recombination effects that is not seen when a larger number of intervals is examined.
Fig. 6. Chromosome size-dependent loss of the meiotic crossover buffer in msh4/5-t mutants. 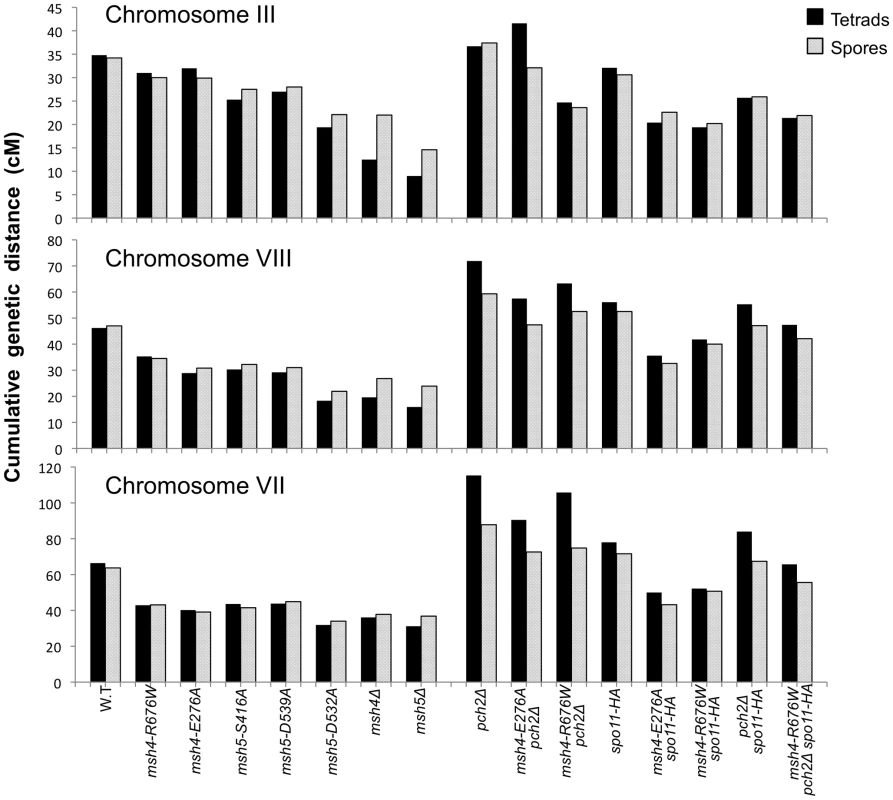
Cumulative genetic map distances for chromosomes III, VII, and VIII are shown separately for msh4/5 hypomorphs as well as their double and triple mutations with pch2Δ and spo11-HA. We then looked at crossover distribution in a msh4/5-bt mutant (msh5-D532A). This msh4/5-bt mutation conferred similar spore viability levels in the NHY and EAY strain background (65% in EAY vs 69% in NHY; Figure 4). Interestingly, the sum of genetic map distances for chromosomes III, VII, and VIII in msh5-D532A (69 cM) was similar to msh5Δ (56 cM) and msh4Δ (68 cM) (Figure 5); however, msh5-D532A showed a preferential retention of crossovers on the small chromosome III. Crossovers in this mutant were 56, 39, and 48 percent of wild-type for chromosomes III, VIII and VII, respectively (determined from tetrads; Table 2; Figure 6).
Gene conversion events were analyzed at eleven marker sites in a subset of msh4/5 mutants, (msh4-E276A, msh4-R676W, msh5-S416A, msh5-D532A, msh5-D539A). The frequency of gene conversion in these strains was similar to wild-type (Table 3). As seen previously, msh4/5Δ mutants displayed an elevated frequency of gene conversions compared to wild-type [21], [25], [60].
Tab. 3. Percentage of aberrant marker segregation in msh4, msh5 mutants in the NHY942/NHY943 strain background. 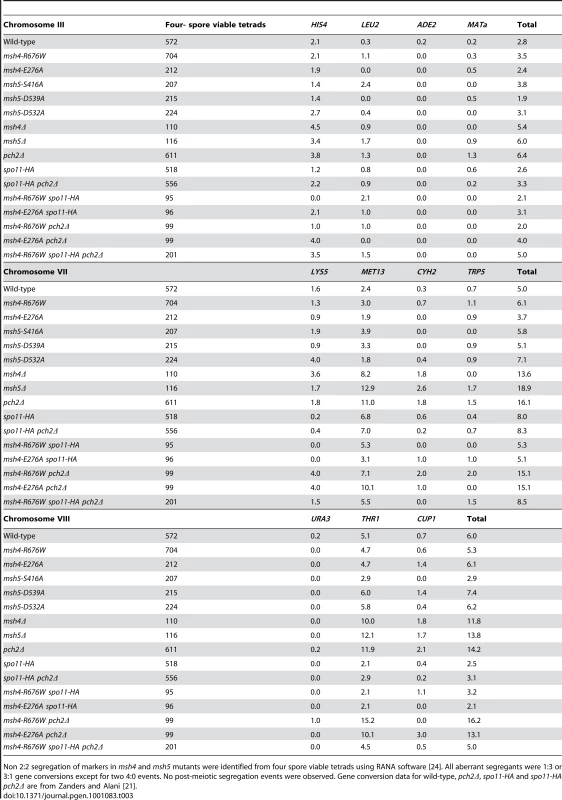
Non 2∶2 segregation of markers in msh4 and msh5 mutants were identified from four spore viable tetrads using RANA software [24]. All aberrant segregants were 1∶3 or 3∶1 gene conversions except for two 4∶0 events. No post-meiotic segregation events were observed. Gene conversion data for wild-type, pch2Δ, spo11-HA and spo11-HA pch2Δ are from Zanders and Alani [21]. Lastly, crossover interference was analyzed in a representative msh4/5-t mutant (msh4-R676W) by measuring the coefficient of coincidence (COC, ratio of observed double crossovers to those expected by chance; Table 4; [61]) and the NPD ratio (Table 5; [62]–[63]). Lack of interference yields COC and NPD values of 1 while strong interference yields values significantly less than 1. On the whole crossover interference appeared similar in wild-type and msh4-R676W. In COC analysis the msh4-R676W mutant showed a lack of interference for two intervals on chromosome III; wild-type showed a lack of interference for only one of these intervals (Table 4). For chromosomes VII and VIII, msh4-R676W and wild-type both showed crossover interference at two intervals and the absence of interference at another. NPD ratios, calculated for intervals where at least eight NPD events were expected, were determined using Stahl's “better way” calculator. This method performs a chi square test to determine if there is a significant difference between the observed PD, TT and NPD tetrad classes and those expected by random crossing over. This analysis showed the presence of interference in both wild-type and msh4-R676W in three intervals on chromosomes VII and VIII (Table 5).
Tab. 4. Analysis of crossover interference in msh4-R676W by coefficient of co-incidence. 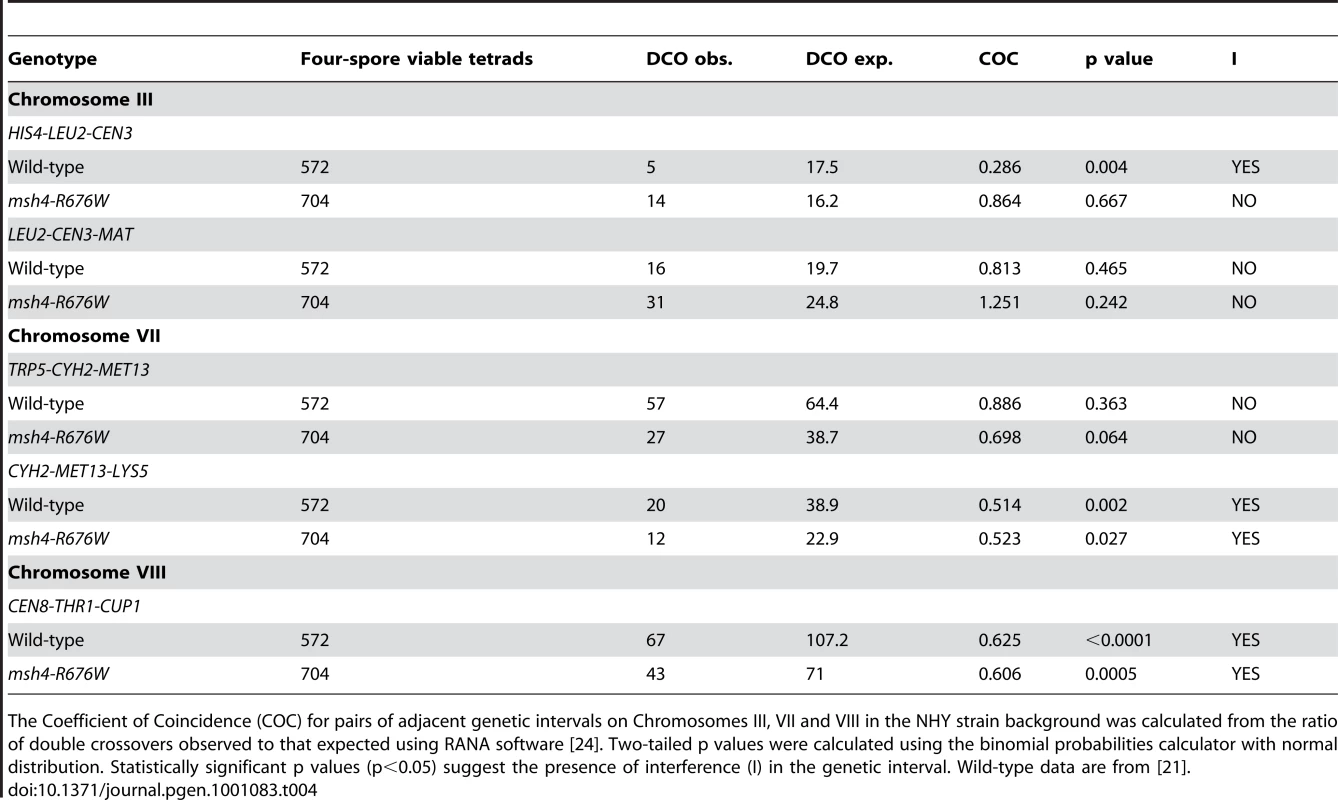
The Coefficient of Coincidence (COC) for pairs of adjacent genetic intervals on Chromosomes III, VII and VIII in the NHY strain background was calculated from the ratio of double crossovers observed to that expected using RANA software [24]. Two-tailed p values were calculated using the binomial probabilities calculator with normal distribution. Statistically significant p values (p<0.05) suggest the presence of interference (I) in the genetic interval. Wild-type data are from [21]. Tab. 5. Analysis of crossover interference in msh4-R676W by the NPD ratio. 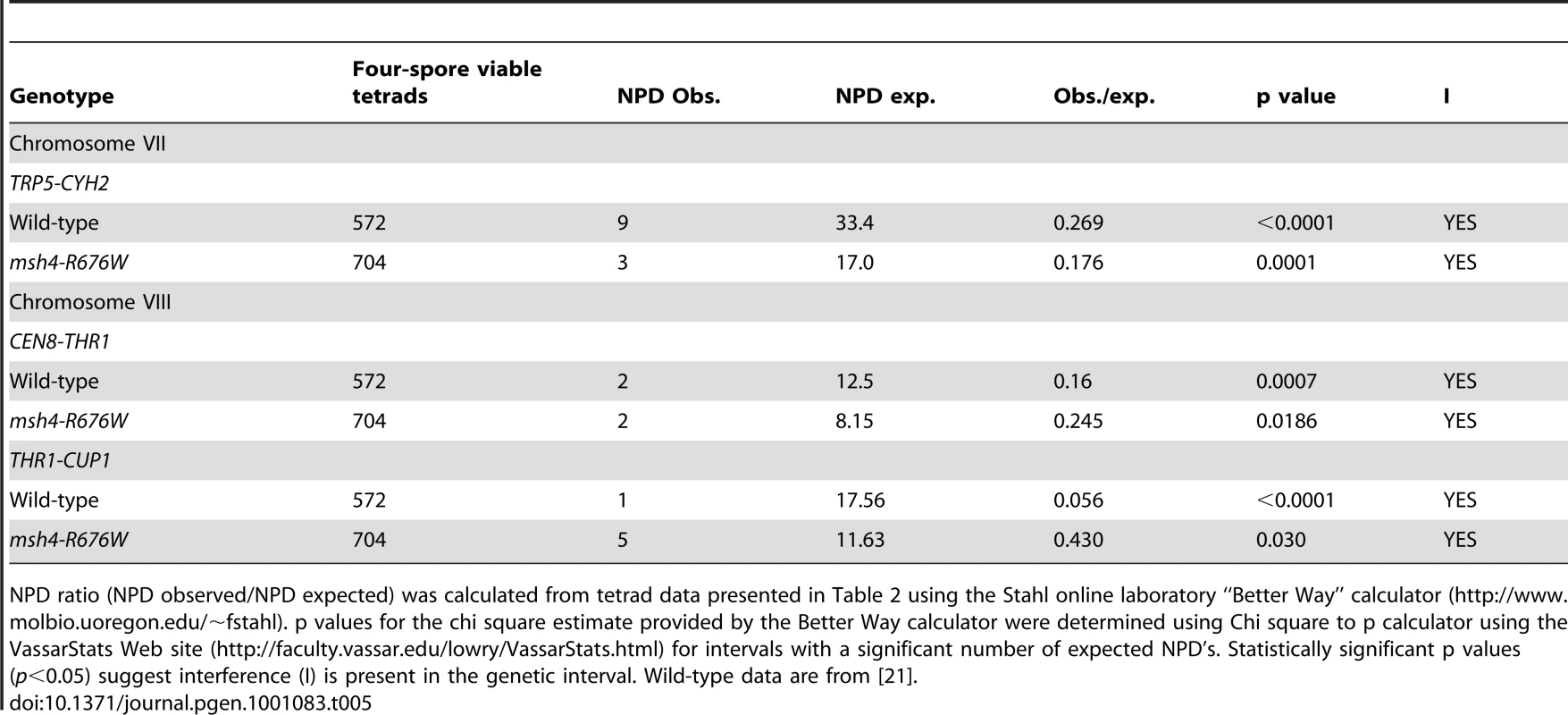
NPD ratio (NPD observed/NPD expected) was calculated from tetrad data presented in Table 2 using the Stahl online laboratory “Better Way” calculator (http://www.molbio.uoregon.edu/~fstahl). p values for the chi square estimate provided by the Better Way calculator were determined using Chi square to p calculator using the VassarStats Web site (http://faculty.vassar.edu/lowry/VassarStats.html) for intervals with a significant number of expected NPD's. Statistically significant p values (p<0.05) suggest interference (I) is present in the genetic interval. Wild-type data are from [21]. High spore viability in msh4/5-t mutants requires Pch2-mediated crossover interference
pch2Δ mutants display elevated crossing over on medium and large chromosomes, and are defective in crossover interference, yet display wild-type spore viability [21], [64]–[66]. In addition, initial genetic analyses showed that pch2Δ mutants displayed an increased ratio of crossovers to non-crossovers [21]. These observations, combined with cytological analyses indicating that Pch2 promotes domainal axis organization in meiosis [66], [67], led Zanders and Alani [21] to propose that Pch2 acts in early steps in crossover control to promote crossover interference at the crossover versus non-crossover decision. To test if msh4/5-t mutants showed increased sensitivity to early defects in crossover control, we made double and triple mutant combinations involving the msh4/5-t, spo11-HA, and pch2Δ mutations in the NHY strain background. The spo11-HA mutation was examined because strains bearing this allele display a 20% reduction in meiosis specific DSBs but show wild-type levels of crossing over and spore viability due to crossover homeostasis [20]. pch2Δ spo11-HA strains, however, display a significant loss in spore viability (73%). One explanation for this phenotype is that when DSBs become limiting, the proper distribution of crossovers becomes even more critical to ensure that every chromosome receives at least one crossover [21],[66].
As shown in Figure 4, Figure 5B, and Table 2, msh4-R676W spo11-HA and msh4-E276A spo11-HA double mutants displayed wild-type spore viability (89 and 91%, respectively) and cumulative map distances (113 and 106 cM, respectively, from tetrads). These values were similar to those seen in msh4-R676W (109 cM) and msh4-E276A (101 cM) single mutants. However, compared to msh4-R676W and msh4-E276A single mutants, msh4-R676W spo11-HA and msh4-E276A spo11-HA double mutants showed a decrease (∼30%) in crossing over in the small chromosome III that was accompanied by modest increases in crossing over in the medium and large chromosomes (Figure 6; Table 2). We do not have a good explanation for this phenotype; one possibility is that the spo11 hypomorphs confer mutant phenotypes in addition to lowering DSBs (see Discussion; [21]).
msh4-R676W pch2Δ and msh4-E276A pch2Δ double mutants also showed wild-type spore viability (93% for both, Figure 4); however the pch2Δ mutation conferred an increase in crossing over in msh4-R676W and msh4-E276A strains that appeared specific to the medium - (VIII) and large-sized (VII) chromosomes (Figure 5B, Figure 6). The cumulative map distances from tetrads in msh4-R676W pch2Δ (194 cM) and msh4-E276A pch2Δ (190 cM), were higher than wild-type (147 cM) but lower than pch2Δ (226 cM; Figure 5B). pch2Δ msh5Δ mutants were previously shown to have higher crossover frequencies than the msh5Δ mutant [21].
The wild-type spore viability profile seen in msh4/5-t spo11-HA suggested that crossover interference and homeostasis can distribute a smaller pool of crossovers to all 16 homolog pairs. In contrast, the wild-type spore viability profile seen in msh4/5-t pch2Δ can be explained by an increased number of crossovers compensating for interference defects [21]. Such explanations predict that compromising crossover interference (pch2Δ) and limiting DSB's (spo11-HA) would decrease spore viability because a random distribution of crossovers will favor large chromosomes (Figure 6; [21]). These effects are likely to be more pronounced in a msh4/5-t pch2Δ spo11-HA mutant that is predicted to be compromised for DSB formation, crossover interference, and crossing over. To test this we created the msh4-R676W pch2Δ spo11-HA triple mutant and analyzed its phenotype with respect to spore viability, crossover distribution, and chromosome III non-disjunction.
As shown in Figure 4, the msh4-R676W pch2Δ spo11-HA triple mutant displayed 55% spore viability, which was lower than spo11-HA pch2Δ (72% spore viability). The cumulative crossover level from tetrads for chromosomes III, VII and VIII in this mutant was 135 cM, which was lower than wild-type (147 cM) and pch2Δ spo11-HA (165 cM), but significantly higher than msh4-R676W (109 cM), which displayed high spore viability (Table 2; Figure 4, Figure 5B). msh4-R676W pch2Δ spo11-HA also showed a greater reduction in crossing over on chromosome III compared to pch2Δ spo11-HA mutants (Figure 6). Although crossover levels on chromosome III in msh4-R676W pch2Δ spo11-HA were similar to msh4-R676W spo11-HA, the medium (VIII) and large chromosomes (VII) in msh4-R676W pch2Δ spo11-HA showed specific increases in crossing over compared to msh4-R676W spo11-HA as predicted by the model (Figure 6, Figure S2). Consistent with this, the triple mutant displayed a spore viability profile indicating a Meiosis I disjunction defect (Figure 4). The triple mutant showed a higher frequency of non-mater two-spore viable tetrads in the triple mutant (12.7%, n = 71 two spore viable tetrads; 1.9% of total tetrads) compared to both pch2Δ spo11-HA (6.9%, n = 130; 0.96% of total tetrads) and msh4-R676W (6.8%, n = 44; 0.37% of total tetrads). Such tetrads are indicative of nondisjunction of chromosome III because the two viable spores carry both yeast mating types (MATa and MATalpha). In addition, 82% of the two spore viable tetrads in the triple mutant were sister spores compared to 68% in pch2Δ spo11-HA and 50% in msh4-R676W. These data are suggestive of non-disjunction of other chromosomes. Together this information is consistent with the triple mutant being unable to distribute at least one crossover between all homolog pairs (see Discussion).
Functional Msh4-Msh5 is required for complete Zip1 polymerization
msh4Δ and msh5Δ mutants show strong defects in Zip1 polymerization during synaptonemal complex formation [27], [29]. Our data below indicate that fully functional Msh4-Msh5 is required for complete Zip1 polymerization along homologs. Immunostaining of Msh5 and Zip1 was performed on a subset of the msh4/5-t (msh4-E276A, msh4-R676W, msh5-S416A, msh5-D539A) and msh4/5-bt (msh5-D532A) mutants in the NHY strain background four hours after induction into meiosis (Figure 7). The number and distribution of Msh5 foci on meiotic chromosomes for wild-type, msh4/5-t, and msh5-D532A mutants were similar. The average number of Msh5 foci per nucleus (n = 30) was 122 for wild-type, 120 for msh5-D532A, and 130 for msh5-D539A. However, all mutants showed a partial defect in Zip1 elongation and accumulated Zip1-specific polycomplexes. This phenotype is reminiscent of that displayed by spo16 and zip4 null mutants with the exception that spo16 and zip4 null mutants display poor spore viability [29]. One explanation for these observations is that the msh4/5 mutants present fewer crossover sites to initiate Zip1 polymerization; thus these mutants, while capable of loading Msh4-Msh5 onto meiotic chromosomes, appeared defective in steps required to implement crossing over at designated sites. Thus complete Zip1 polymerization may require feedback from Msh4-Msh5 that is delayed or does not occur in the msh4/5 mutants.
Fig. 7. msh4/5 hypomorphs are defective in Zip1 polymerization. 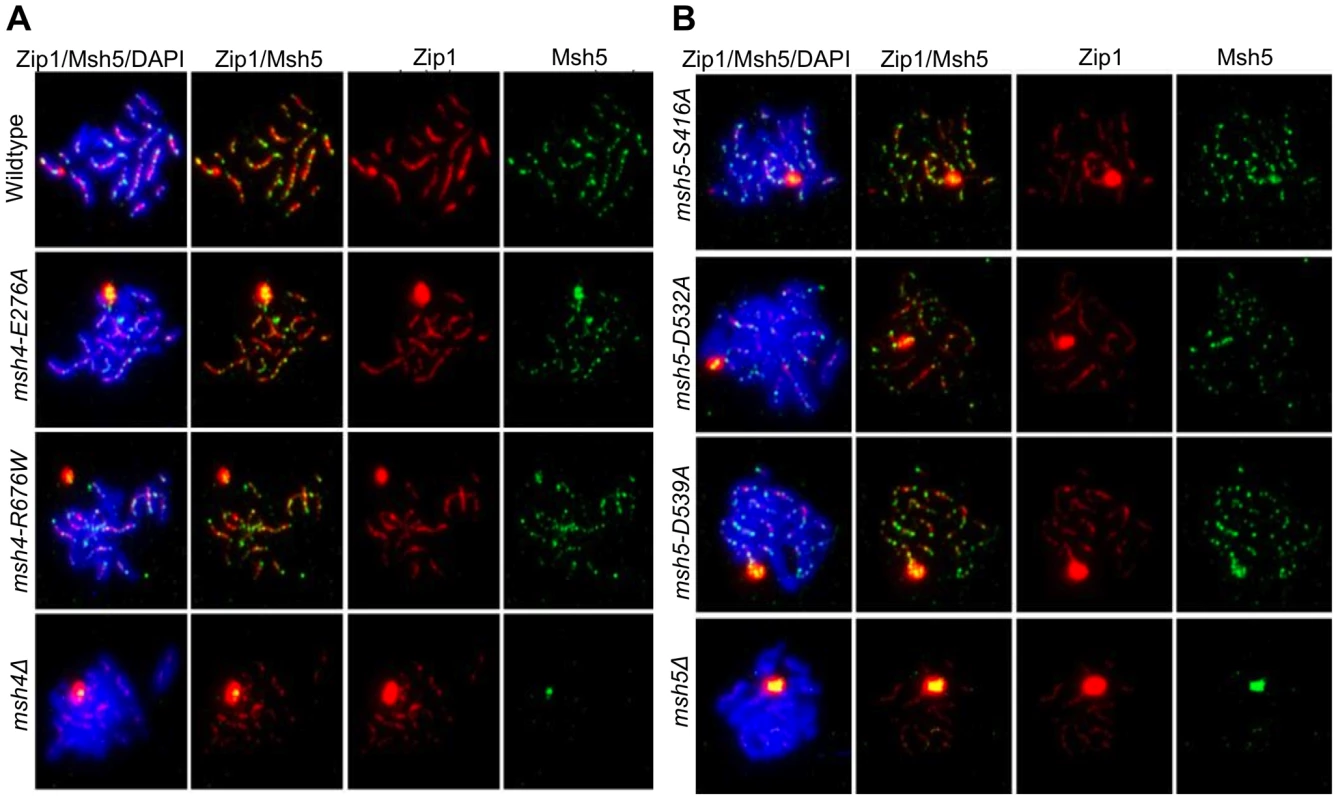
Meiotic chromosome spreads isolated from cells at 4 hr after induction into meiosis were incubated with antibodies to Zip1 and Msh5 and counterstained with DAPI. (A) Localization of Zip1 and Msh5 in wild-type, msh4-E276A, msh4-R676W and msh4Δ mutants. (B) Zip1, Msh5 localization in msh5-S416A, msh5-D532A, msh5-D539A and msh5Δ mutants. We also measured by DAPI staining the percent of cells that completed at least Meiosis I (MI/MII) for all of the strains examined by immunofluorescence. As shown in Figure S3, wild-type and one msh4/5-t threshold mutant, msh4-E276A, displayed similar timing and efficiencies of meiotic divisions. The msh4Δ, msh5Δ, three msh4/5-t mutants (msh4-R676W, msh5-S416A, msh5-D539A), and one msh4/5-bt mutant (msh5-D532A) all showed about a 1.5 to 2 hr delay relative to wild-type.
Discussion
We identified msh4 and msh5 mutants (msh4/5-t) that displayed reduced crossing over in meiosis but maintained crossover interference and wild-type spore viability. The reduction in crossing over seen in msh4/5-t mutants appeared more pronounced on large and medium-sized chromosomes that typically receive a greater proportion of Msh4/5-dependent crossovers. msh4/5-t mutants also displayed chromosome synapsis defects. These observations and the poor spore viability phenotype of the msh4-R676W pch2Δ spo11-HA triple mutant support the idea that baker's yeast form an excessive number of meiotic crossovers and that Pch2-mediated crossover interference is critical for meiotic viability when crossovers become limiting. The msh4/5-t alleles, which can be used to titrate crossover levels without reducing spore viability, provide a new tool for investigators interested in identifying factors that regulate crossover control.
Why does S. cerevisiae appear to have an excess of crossovers in meiosis?
S. cerevisiae maintains a high level of crossing over, an average of 5.6 per homolog pair [8]–[11], [20]. In most organisms that display crossover interference (C. elegans, A. thaliana, Zea mays, D. melanogaster, Mus musculus and Homo sapiens), the ratio of crossovers in meiosis to homolog pairs is less than or equal to three (reviewed in [68]). Why does S. cerevisiae enjoy such a high level of crossing over when a single crossover per homolog pair appears sufficient to promote Meiosis I disjunction [12], [15]? One possibility is that high crossover levels improve fitness by reducing mutational load through the segregation of deleterious alleles [69]. Consistent with this idea are simulation studies suggesting that meiotic crossover rates in S. cerevisiae are optimized for mutational robustness [69]. Another possibility is that excess crossovers are needed to ensure crossover formation on small chromosomes [8], [11], [21]. Consistent with the latter explanation is work in yeast showing that a small chromosome (I, 230 KB) has a higher than average recombination rate. Chromosome I also showed a frequency of non-disjunction (0.2–0.4%) that was lower than expected (5%) if it had recombined at the average rate [56], [57], [70]. The enhanced recombination rates on smaller chromosomes in S. cerevisiae are likely to result from DSBs that occur at a higher than average density and weak crossover interference [40], [57], [58], [71], [72].
Models to explain the msh4/5-t mutant phenotype
msh4/5 mutants displayed high spore viability and a higher retention of crossovers on a small chromosome (III) compared to larger chromosomes (VIII, VII and XV). We entertain two models to explain this phenotype. Both of these are based on work showing that Msh4-Msh5 is required to stabilize SEI recombination intermediates and can bind to Holliday junctions [16], [41]. In one model, msh4/5-t mutants are defective in converting all SEI and Holliday junction intermediates into crossovers with equal probability. Such a model predicts that crossover interference would not be affected in msh4/5-t mutants, and that msh4/5-t mutants would show defects in synaptonemal complex formation. Both of these phenotypes were seen in this study. This model predicts that msh4/5-t mutants would show high spore viability despite a decrease in crossing over because smaller chromosomes have a higher frequency of crossovers and the number of crossovers in yeast is much greater than the number of chromosomes. A drawback of this model is that it cannot fully explain why msh4/5 null mutants displayed more severe crossover defects on the smaller chromosome III. Such a pattern is unexpected if crossovers on small chromosomes are present at higher density and occur primarily through a non-interfering pathway [23]. It also cannot explain how msh4/5-t pch2Δ mutants make excess crossovers. We cannot rule out the possibility that the small number of intervals examined on chromosome III is not representative of the overall pattern. In the future we would like to test this model further by examining additional intervals on this chromosome as well as on another small chromosome such as chromosome I. In addition, we would like to examine the effect of the msh4/5-t mutations on early recombination intermediates such as SEIs.
We considered a second model that proposes a prioritization mechanism for the distribution of crossovers amongst chromosomes. This model is somewhat similar to that proposed by Kaback and colleagues [56], [57]. We suggest that msh4/5-t phenotypes reflect a temporal order of crossover designation that favors a crossover on every homolog pair before additional interference-dependent crossovers are made. Such a pattern can be presented within the context of a stress relief model for crossover initiation and distribution. In this model “crossover designation with accompanying interference can be explained by imposition, relief, and redistribution of compression stress and stress relief along chromosome axes” [13]. Crossover initiation on every homolog pair would lead to the release of mechanical stress along the homolog axis of every chromosome. For shorter chromosomes, interference created from stress relief at the crossover initiation site would extend to the end of the chromosome, leading to fewer interfering crossovers as was seen experimentally [57]. For large chromosomes, interference created by stress relief that accompanies obligate crossover designation would prevent additional crossovers until mechanical stresses are re-distributed. We suggest that this redistribution of stress delays additional crossover designations on larger chromosomes. In this model the msh4/5-t phenotype can be explained if mutant Msh4-Msh5 complexes can participate in initial stress relief to form an obligate crossover but are defective, perhaps due to stability issues, in subsequent crossover initiations that are subject to interference. This model could explain the synapsis defects seen in msh4/5-t mutants if the defect is specific to long chromosomes; a single synapsis initiation site on a small chromosome could be sufficient to allow polymerization along the entire chromosome. This model, however, does not account for why Msh5 focus formation appears wild-type in msh4/5 mutants. One possibility is that subsequent crossover initiations require functions that occur after Msh4-Msh5 loading onto chromosomes.
The temporal order model outlined above predicts that spore viability would be maintained in msh4/5-t mutants due to formation of the obligate crossover and that interference would appear stronger on larger chromosomes. Such an idea is consistent with previous studies in yeast showing that multiple interfering crossovers occur more frequently on large chromosomes and with models that explain the distributions of interfering crossovers seen on different sized chromosomes (e.g. [13], [15], [40], [57], [73]). While we have shown that msh4/5-t mutants maintain high spore viability and display crossover interference on large chromosomes (Figure 4; Table 4, Table 5), our data are not robust enough to test whether interference becomes stronger on these chromosomes. A caveat in this model is that msh4/5-t mutants display crossover levels on large chromosomes that are higher than wild-type in the pch2Δ mutant background. Thus msh4/5-t mutants do not appear limited in their ability to form crossovers. One way to explain this observation is that Pch2 acts as a general factor to repress recombination that increases the temporal window over which a mutant Msh4-Msh5 complex must execute crossover decisions. Alleviation of this repression results in increased crossing over in msh4/5-t pch2Δ mutants.
Crossovers in msh4-R676W pch2Δ spo11-HA triple mutants appear to be randomly distributed, thus leading to more crossing over on larger chromosomes compared to the msh4-R676W single mutant, and increased non-disjunction on a small chromosome. Previous studies have suggested that Pch2 is essential for proper meiotic axis organization following crossover designation and that crossover distribution is mediated by changes in meiotic axis organization/assembly (e.g. [13], [67], [74]). We suggest that the triple mutant phenotype can be explained in the second model if the pch2Δ mutation disrupts stress/stress relief mechanisms so that crossover designations occur without interference and no crossovers show a temporal delay. In this scenario Pch2 maintains meiotic viability when crossovers are limiting (i.e. msh4/5-t, spo11 hypomorph mutations) because it imposes a delay on additional interfering crossovers. This delay ensures that every homolog pair has received at least one crossover. One way to test this idea in yeast is to perform a genome wide analysis of crossing over in the msh4/5-t mutant versus the triple mutant [8], [11].
Mutations in Msh4 and Msh5 differentially affect function
The Msh family of mismatch repair proteins display asymmetric roles with respect to DNA binding and ATP hydrolysis. In MutS, residues in domain I of subunit A specifically stack with the mismatch while domain IV of subunit B makes non-specific contacts with the DNA backbone [35], [36]. Similarly in MutSα, domain I in Msh6 specifically interacts with the mismatch while domain IV in Msh2 makes non-specific contacts with DNA [54], [75], [76]. Msh subunits also display different affinities for ATP and ADP [77]–[79]. For example in the Msh2-Msh6 mismatch repair complex, Msh6 and Msh2 contain high affinity binding sites for ATP and ADP, respectively [80]. Such asymmetries in ATP binding by Msh subunits are thought to be important to induce coordinated conformational changes in Msh-mismatch DNA complexes that signal downstream repair factors [80]–[84].
Three observations support the presence of asymmetries in Msh4-Msh5 analogous to those seen for the Msh mismatch recognition factors. 1. Snowden et al. [85] reported that the Msh4 subunit of human Msh4-Msh5 appears to have reduced ATP binding activity. 2. We identified different spore viability phenotypes for matched sets of msh4 and msh5 mutations that map to the ATP and DNA binding domains (Figure 2B). 3. We also found that on the whole, msh5 mutations conferred more severe meiotic phenotypes than the equivalent msh4 mutations, though this could indicate different structural organizations for the two proteins rather than asymmetric functions. Msh4-Msh5 binds to both single end invasion and symmetric double Holliday junction substrates [41], [85]. Based on studies performed with Msh and Mlh mismatch repair factors, it is easy to imagine that asymmetric Msh4-Msh5 interactions with its DNA substrate will involve analogous signaling steps that activate downstream factors such as Mlh1-Mlh3. Biochemical analysis of some of the mutant complexes presented in this study can provide evidence to support or refute these ideas.
Materials and Methods
Media and yeast strains
S. cerevisiae SK1 yeast strains were grown on either yeast extract-peptone-dextrose (YPD) or synthetic complete media at 30°C [86]. When required, geneticin (Invitrogen, San Diego) and nourseothricin (Werner BioAgents, Germany) were added to media at prescribed concentrations [87], [88]. Sporulation medium was prepared as described in Argueso et al. [24]. msh4, msh5 mutants were analyzed in either the congenic EAY1108/EAY112 background (“EAY”) described in Argueso et al. [24] or the isogenic NHY942/NHY943 background (“NHY”) described in de los Santos et al. [23]. 28 msh5 and 29 msh4 point mutants were introduced in the EAY1108 background by transformation of EAY1281 and EAY2409 with integration plasmids bearing these mutations using standard techniques [89]. A smaller subset of these msh4, msh5 point mutants were made in the NHY background by transformation of EAY2844 and EAY2848 respectively. Double and triple mutants bearing different combinations of msh4, msh5, pch2Δ and spo11-HA were made in the NHY background by crossing single or double mutant strains followed by tetrad dissection. All strains used in this study are listed in Table S1.
Sequence alignment
Msh4 amino acid sequence from S. cerevisiae (YFL003C), A. thaliana (NM_117842), C. elegans (AF178755), M. musculus (BC145838), H. sapiens (NM_002440) and Msh5 amino acid sequences from S. cerevisiae (YDL154W), A. thaliana (EF471448), C. elegans (NM_070130), M. musculus (NM_013600), H. sapiens (BC002498) were aligned using ClustalW software (www.ebi.ac.uk/clustalw) and CLC free workbench. A Msh4, Msh5 consensus sequence was generated using CLC and aligned against S. cerevisiae Msh2 (YOL090W), Msh3 (YCR092C), Msh6 (YDR097C) to check if residues conserved across Msh4, Msh5 in all five species are conserved in the other Msh family members.
Mutagenesis of MSH4, MSH5 genes
The SK1 MSH4 open reading frame with 600 bp upstream sequence and 400 bp downstream sequence was amplified with pfu DNA polymerase and cloned into pRS416 with a 1.5 kb KanMX fragment inserted 90 bp downstream of the MSH4 stop codon to create the single step integrating plasmid pEAA427. The SK1 MSH5 open reading frame with 500 bp upstream sequence and 400 bp downstream sequence was similarly amplified with pfu DNA polymerase and cloned into pRS416 with a 1.5 kb KanMX fragment inserted 45 bp downstream of the stop codon to create the single step integrating plasmid pEAA424. The MSH4 and MSH5 SK1 sequences in these plasmids were confirmed by Sanger DNA sequencing.
pEAA424 and pEAA427 were mutagenized using Quick Change site directed mutagenesis method (Stratagene, La Jolla, CA) to create 28 msh5 and 29 msh4 point mutations. The entire open reading frame of MSH4, MSH5 was sequenced to ensure only the desired amino acid change was introduced. Table S1 shows a list of plasmids bearing the msh4, msh5 point mutations.
Yeast two hybrid analysis
Full length SK1 MSH4, MSH5 and point mutant derivatives were amplified by pfu DNA polymerase and cloned into pGAD424 (prey) and target pBTM116 (target) vectors kindly provided by Nancy Hollingsworth. The entire open reading frame of MSH4, MSH5 was checked by DNA sequencing to ensure that no additional mutations were created. The L40 strain [90] was co-transformed with the Prey and Target vectors and expression of the LACZ reporter gene was determined by the ortho-nitrophenyl-β-D-galactopyranoside (ONPG) assay [91].
Tetrad analysis
All msh4 and msh5 point mutations integrated into EAY1108 or NHY943 were mated to null strains bearing corresponding msh4Δ (EAY2411, EAY background; EAY2843, NHY background) and msh5Δ (EAY1280, EAY background; EAY2846, NHY background) alleles. The resulting diploids were sporulated using the zero growth mating protocol [92]. Briefly, the haploid strains were patched together on synthetic complete media for four hours and then spread on sporulation media and incubated for 2 days at 30°C. Tetrads were dissected on synthetic complete media for the EAY background and on YPD media supplemented with amino acids for the NHY background. Spore clones were replica plated onto selective media or minimal drop out plates and incubated overnight. Segregation data were analyzed using the recombination analysis software RANA to determine genetic map distances for tetrads and recombination frequencies for spores [24].
Cytological analysis of Msh5 and Zip1
Time course, DAPI, and immunostaining analyses of meiotic progression were performed as described using antibodies to Zip1 and Msh5 [29], [93]. Stable SK1 isogenic diploid strains used in the time courses were created by mating the haploid strains shown in parentheses: Wild-type (NHY942×NHY943); msh4Δ (EAY2843×EAY2844); msh4-E276A (EAY2849×EAY2843), msh4-R676W (EAY2851×EAY2843); msh5Δ (EAY2846×EAY2848): msh5-S416A (EAY2855×EAY2846); msh5-D539A (EAY2857×EAY2846); msh5-D532A (EAY2785×EAY2846).
Supporting Information
Zdroje
1. PetronczkiM
SiomosMF
NasmythK
2003 Un ménage à quatre: the molecular biology of chromosome segregation in meiosis. Cell 112 423 440
2. YuHG
KoshlandD
2005 Chromosome morphogenesis: condensin-dependent cohesin removal during meiosis. Cell 123 397 407
3. PageSL
HawleyRS
2003 Chromosome choreography: the meiotic ballet. Science 301 785 789
4. KeeneyS
GirouxCN
KlecknerN
1997 Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88 375 384
5. AllersT
LichtenM
2001 Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106 47 57
6. HunterN
KlecknerN
2001 The single-end invasion: an asymmetric intermediate at the double-strand break to double-holliday junction transition of meiotic recombination. Cell 106 59 70
7. BuhlerC
BordeV
LichtenM
2007 Mapping meiotic single-strand DNA reveals a new landscape of DNA double-strand breaks in Saccharomyces cerevisiae. PLoS Biol 5 e324 doi:10.1371/journal.pbio.0050324
8. ManceraE
BourgonR
BrozziA
HuberW
SteinmetzLM
2008 High-resolution mapping of meiotic crossovers and non-crossovers in yeast. Nature 454 479 485
9. MortimerRK
SchildD
ContopoulouCR
KansJA
1991 Genetic and physical maps of Saccharomyces cerevisiae. Methods Enzymol 194 827 863
10. CherryJM
BallC
WengS
JuvikG
SchmidtR
1997 Genetic and physical maps of Saccharomyces cerevisiae. Nature 387 67 73
11. ChenSY
TsubouchiT
RockmillB
SandlerJS
RichardsDR
2008 Global Analysis of the Meiotic Crossover Landscape. Dev Cell 15 401 415
12. HillersKJ
VilleneuveAM
2003 Chromosome-wide control of meiotic crossing over in C. elegans. Curr Biol 13 1641 1647
13. KlecknerN
ZicklerD
JonesGH
DekkerJ
PadmoreR
2004 A mechanical basis for chromosome function. Proc Natl Acad Sci USA 101 12592 12597
14. HillersKJ
2004 Crossover interference. Curr Biol 14 R1036 1037
15. StahlFW
FossHM
YoungLS
BortsRH
AbdullahMF
2004 Does crossover interference count in Saccharomyces cerevisiae? Genetics 168 35 48
16. BörnerGV
KlecknerN
HunterN
2004 Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117 29 45
17. SchwachaA
KlecknerN
1995 Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 83 783 791
18. AllersT
LichtenM
2001 Intermediates of yeast meiotic recombination contain heteroduplex DNA. Mol Cell 8 225 231
19. LaoJP
OhSD
ShinoharaM
ShinoharaA
HunterN
2008 Rad52 promotes postinvasion steps of meiotic double-strand-break repair. Mol Cell 29 517 524
20. MartiniE
DiazRL
HunterN
KeeneyS
2006 Crossover homeostasis in yeast meiosis. Cell 126 285 295
21. ZandersS
AlaniE
2009 The pch2Delta mutation in baker's yeast alters meiotic crossover levels and confers a defect in crossover interference. PLoS Genet 5 e1000571 doi:10.1371/journal.pgen.1000571
22. BarchiM
RoigI
Di GiacomoM
de RooijDG
KeeneyS
2008 ATM promotes the obligate XY crossover and both crossover control and chromosome axis integrity on autosomes. PLoS Genet 4 e1000076 doi:10.1371/journal.pgen.1000076
23. de los SantosT
HunterN
LeeC
LarkinB
LoidlJ
2003 The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics 164 81 94
24. ArguesoJL
WanatJ
GemiciZ
AlaniE
2004 Competing crossover pathways act during meiosis in Saccharomyces cerevisiae. Genetics 168 1805 1816
25. Ross-MacdonaldP
RoederGS
1994 Mutation of a meiosis-specific MutS homolog decreases crossing over but not mismatch correction. Cell 79 1069 1080
26. HollingsworthNM
PonteL
HalseyC
1995 MSH5, a novel MutS homolog, facilitates meiotic reciprocal recombination between homologs in Saccharomyces cerevisiae but not mismatch repair. Genes Dev 9 1728 1739
27. NovakJE
Ross-MacdonaldPB
RoederGS
2001 The budding yeast Msh4 protein functions in chromosome synapsis and the regulation of crossover distribution. Genetics 158 1013 1025
28. LynnA
SoucekR
BörnerGV
2007 ZMM proteins during meiosis: crossover artists at work. Chromosome Res 15 591 605
29. ShinoharaM
OhSD
HunterN
ShinoharaA
2008 Crossover assurance and crossover interference are distinctly regulated by the ZMM proteins during yeast meiosis. Nat Genet 40 299 309
30. TsubouchiT
ZhaoH
RoederGS
2006 The meiosis-specific Zip4 protein regulates crossover distribution by promoting synaptonemal complex formation together with Zip2. Dev Cell 10 809 819
31. AgarwalS
RoederGS
2000 Zip3 provides a link between recombination enzymes and synaptonemal complex proteins. Cell 102 245 255
32. ChuaPR
RoederGS
1998 Zip2, a meiosis-specific protein required for the initiation of chromosome synapsis. Cell 93 349 359
33. SymM
EngebrechtJA
RoederGS
1993 ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell 72 365 378
34. NakagawaT
OgawaH
1999 The Saccharomyces cerevisiae MER3 gene, encoding a novel helicase-like protein, is required for crossover control in meiosis. EMBO J 18 5714 5723
35. ObmolovaG
BanC
HsiehP
YangW
2000 Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature 407 703 710
36. LamersMH
PerrakisA
EnzlinJH
WinterwerpHH
de WindN
2000 The crystal structure of DNA mismatch repair protein MutS binding to a G×T mismatch. Nature 407 711 717
37. KneitzB
CohenPE
AvdievichE
ZhuL
KaneMF
2000 MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev 14 1085 1097
38. EdelmannW
CohenPE
KneitzB
WinandN
LiaM
1999 Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat Genet 21 123 127
39. de VriesSS
BaartEB
DekkerM
SiezenA
de RooijDG
1999 Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes Dev 13 523 531
40. FungJC
RockmillB
OdellM
RoederGS
2004 Imposition of crossover interference through the nonrandom distribution of synapsis initiation complexes. Cell 116 795 802
41. SnowdenT
AcharyaS
ButzC
BerardiniM
FishelR
2004 hMSH4-hMSH5 recognizes Holliday Junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell 15 437 451
42. HoffmannER
BortsRH
2004 Meiotic recombination intermediates and mismatch repair proteins. Cytogenet Genome Res 107 232 248
43. WhitbyMC
2005 Making crossovers during meiosis. Biochem Soc Trans 33 1451 1455
44. NishantKT
PlysAJ
AlaniE
2008 A mutation in the putative MLH3 endonuclease domain confers a defect in both mismatch repair and meiosis in Saccharomyces cerevisiae. Genetics 179 747 755
45. KolasNK
CohenPE
2004 Novel and diverse functions of the DNA mismatch repair family in mammalian meiosis and recombination. Cytogenet Genome Res 107 216 231
46. KolasNK
SvetlanovA
LenziML
MacalusoFP
LipkinSM
2005 Localization of MMR proteins on meiotic chromosomes in mice indicates distinct functions during prophase I. J Cell Biol 171 447 458
47. Santucci-DarmaninS
NeytonS
LespinasseF
SaunièresA
GaudrayP
2002 The DNA mismatch-repair MLH3 protein interacts with MSH4 in meiotic cells, supporting a role for this MutL homolog in mammalian meiotic recombination. Hum Mol Genet 11 1697 1706
48. SvetlanovA
CohenPE
2004 Mismatch repair proteins, meiosis, and mice: understanding the complexities of mammalian meiosis. Exp Cell Res 296 71 79
49. KneitzB
CohenPE
AvdievichE
ZhuL
KaneMF
2000 MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev 14 1085 1097
50. Santucci-DarmaninS
WalpitaD
LespinasseF
DesnuelleC
AshleyT
2000 MSH4 acts in conjunction with MLH1 during mammalian meiosis. FASEB J 14 1539 1547
51. StorlazziA
GarganoS
Ruprich-RobertG
FalqueM
DavidM
2010 Recombination proteins mediate meiotic spatial chromosome organization and pairing. Cell 141 94 106
52. PochartP
WolteringD
HollingsworthNM
1997 Conserved properties between functionally distinct MutS homologs in yeast. J Biol Chem 272 30345 30349
53. KijasAW
StudamireB
AlaniE
2003 Msh2 separation of function mutations confer defects in the initiation steps of mismatch repair. J Mol Biol 331 123 38
54. WarrenJJ
PohlhausTJ
ChangelaA
IyerRR
ModrichPL
2007 Structure of the human MutSalpha DNA lesion recognition complex. Mol Cell 26 579 592
55. AlaniE
SokolskyT
StudamireB
MiretJJ
LahueRS
1997 Genetic and biochemical analysis of Msh2p-Msh6p: role of ATP hydrolysis and Msh2p-Msh6p subunit interactions in mismatch base pair recognition. Mol Cell Biol 17 2436 2347
56. KabackDB
GuacciV
BarberD
MahonJW
1992 Chromosome size-dependent control of meiotic recombination. Science 256 228 232
57. KabackDB
BarberD
MahonJ
LambJ
YouJ
1999 Chromosome size-dependent control of meiotic reciprocal recombination in Saccharomyces cerevisiae: the role of crossover interference. Genetics 152 1475 1486
58. TurneyD
de Los SantosT
HollingsworthNM
2004 Does chromosome size affect map distance and genetic interference in budding yeast? Genetics 168 2421 2424
59. AbdullahMF
HoffmannER
CottonVE
BortsRH
2004 A role for the MutL homologue MLH2 in controlling heteroduplex formation and in regulating between two different crossover pathways in budding yeast. Cytogenet Genome Res 107 180 190
60. WanatJJ
KimKP
KoszulR
ZandersS
WeinerB
2008 Csm4, in collaboration with Ndj1, mediates telomere-led chromosome dynamics and recombination during yeast meiosis. PLoS Genet 4 e1000188 doi:10.1371/journal.pgen.1000188
61. PapazianHP
1952 The analysis of tetrad data. Genetics 37 175 188
62. SnowR
1979 Maximum likelihood estimation of linkage and interference from tetrad data. Genetics 92 231 245
63. StahlFW
2008 On the “NPD ratio” as a test for crossover interference. Genetics 179 701 704
64. San-SegundoPA
RoederGS
1999 Pch2 links chromatin silencing to meiotic checkpoint control. Cell 97 313 324
65. WuHY
BurgessSM
2006 Two distinct surveillance mechanisms monitor meiotic chromosome metabolism in budding yeast. Curr Biol 16 2473 2479
66. JoshiN
BarotA
JamisonC
BörnerGV
2009 Pch2 links chromosome axis remodeling at future crossover sites and crossover distribution during yeast meiosis. PLoS Genet 5 e1000557 doi:10.1371/journal.pgen.1000557
67. BörnerGV
BarotA
KlecknerN
2008 Yeast Pch2 promotes domainal axis organization, timely recombination progression, and arrest of defective recombinosomes during meiosis. Proc Natl Acad Sci USA 105 3327 3332
68. BerchowitzLE
CopenhaverGP
2010 Genetic Interference: Don't stand so close to me. Curr Genomics 11 91 102
69. KellerPJ
KnopM
2009 Evolution of mutational robustness in the yeast genome: a link to essential genes and meiotic recombination hotspots. PLoS Genet 5 e1000533 doi:10.1371/journal.pgen.1000533
70. KabackDB
SteensmaHY
de JongeP
1989 Enhanced meiotic recombination on the smallest chromosome of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 86 3694 3698
71. GertonJL
DeRisiJ
ShroffR
LichtenM
BrownPO
2000 Inaugural article: global mapping of meiotic recombination hotspots and coldspots in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 97 11383 11390
72. BlitzblauHG
BellGW
RodriguezJ
BellSP
HochwagenA
2007 Mapping of meiotic single-stranded DNA reveals double-stranded-break hotspots near centromeres and telomeres. Curr Biol 17 2003 2012
73. KingJS
MortimerRK
1990 A polymerization model of chiasma interference and corresponding computer simulation. Genetics 126 1127 1138
74. NabeshimaK
VilleneuveAM
HillersKJ
2004 Chromosome-wide regulation of meiotic crossover formation in Caenorhabditis elegans requires properly assembled chromosome axes. Genetics 168 1275 1292
75. DrotschmannK
YangW
BrownewellFE
KoolET
KunkelTA
2001 Asymmetric recognition of DNA local distortion. Structure-based functional studies of eukaryotic Msh2-Msh6. J Biol Chem 276 46225 46229
76. BowersJ
SokolskyT
QuachT
AlaniE
1999 A mutation in the MSH6 subunit of the Saccharomyces cerevisiae MSH2-MSH6 complex disrupts mismatch recognition. J Biol Chem 274 16115 11625
77. MartikD
BaitingerC
ModrichP
2004 Differential specificities and simultaneous occupancy of human MutSalpha nucleotide binding sites. J Biol Chem 279 28402 28410
78. BjornsonKP
ModrichP
2003 Differential and simultaneous adenosine di - and triphosphate binding by MutS. J Biol Chem 278 18557 18562
79. AntonyE
KhubchandaniS
ChenS
HingoraniMM
2006 Contribution of Msh2 and Msh6 subunits to the asymmetric ATPase and DNA mismatch binding activities of Saccharomyces cerevisiae Msh2-Msh6 mismatch repair protein. DNA Repair 5 153 162
80. MazurDJ
MendilloML
KolodnerRD
2006 Inhibition of Msh6 ATPase activity by mispaired DNA induces a Msh2(ATP)-Msh6(ATP) state capable of hydrolysis-independent movement along DNA. Mol Cell 22 39 49
81. AntonyE
HingoraniMM
2003 Mismatch recognition-coupled stabilization of Msh2-Msh6 in an ATP-bound state at the initiation of DNA repair. Biochemistry 42 7682 7693
82. BlackwellLJ
BjornsonKP
AllenDJ
ModrichP
2001 Distinct MutS DNA-binding modes that are differentially modulated by ATP binding and hydrolysis. J Biol Chem 276 34339 34347
83. MendilloML
MazurDJ
KolodnerRD
2005 Analysis of the interaction between the Saccharomyces cerevisiae MSH2-MSH6 and MLH1-PMS1 complexes with DNA using a reversible DNA end-blocking system. J Biol Chem 280 22245 22257
84. AcharyaS
FosterPL
BrooksP
FishelR
2003 The coordinated functions of the E. coli MutS and MutL proteins in mismatch repair. Mol Cell 12 233 246
85. SnowdenT
ShimKS
SchmutteC
AcharyaS
FishelR
2008 hMSH4-hMSH5 adenosine nucleotide processing and interactions with homologous recombination machinery. J Biol Chem 283 145 154
86. RoseMD
WinstonF
HieterP
1990 Methods in yeast genetics N.Y. Cold Spring Harbor Laboratory Press
87. WachA
BrachatA
PöhlmannR
PhilippsenP
1994 New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10 1793 1808
88. GoldsteinAL
McCuskerJH
1999 Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15 1541 1553
89. GietzRD
SchiestlRH
WillemsAR
WoodsRA
1995 Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11 355 360
90. VojtekAB
HollenbergSM
CooperJA
1993 Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74 205 214
91. GietzRD
WoodsRA
2002 Screening for protein-protein interactions in the yeast two-hybrid system. Methods Mol Biol 185 471 486
92. ArguesoJL
KijasAW
SarinS
HeckJ
WaaseM
2003 Systematic mutagenesis of the Saccharomyces cerevisiae MLH1 gene reveals distinct roles for Mlh1p in meiotic crossing over and in vegetative and meiotic mismatch repair. Mol Cell Biol 23 873 886
93. ShinoharaM
GasiorSL
BishopDK
ShinoharaA
2000 Tid1/Rdh54 promotes colocalization of Rad51 and Dmc1 during meiotic recombination. Proc Natl Acad Sci USA 97 10814 10819
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 8- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Mutation in the Gene Encoding Ubiquitin Ligase LRSAM1 in Patients with Charcot-Marie-Tooth Disease
- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Did Genetic Drift Drive Increases in Genome Complexity?
- The 5p15.33 Locus Is Associated with Risk of Lung Adenocarcinoma in Never-Smoking Females in Asia
- An Alpha-Catulin Homologue Controls Neuromuscular Function through Localization of the Dystrophin Complex and BK Channels in
- Epigenetically-Inherited Centromere and Neocentromere DNA Replicates Earliest in S-Phase
- Survival and Growth of Yeast without Telomere Capping by Cdc13 in the Absence of Sgs1, Exo1, and Rad9
- Tuberous Sclerosis Complex 1 Regulates dE2F1 Expression during Development and Cooperates with RBF1 to Control Proliferation and Survival
- Disease-Associated Mutations That Alter the RNA Structural Ensemble
- The Transcriptomes of Two Heritable Cell Types Illuminate the Circuit Governing Their Differentiation
- Inactivation of VCP/ter94 Suppresses Retinal Pathology Caused by Misfolded Rhodopsin in
- Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: a Meta-Analysis and Comparison with Lung Cancer and COPD
- Transcriptional Regulation by CHIP/LDB Complexes
- Conserved Role of in Ethanol Responses in Mutant Mice
- A Global Overview of the Genetic and Functional Diversity in the Pathogenicity Island
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3–Dependent Manner in
- Genetic Analysis of Baker's Yeast Msh4-Msh5 Reveals a Threshold Crossover Level for Meiotic Viability
- Genome-Wide Association Studies of Serum Magnesium, Potassium, and Sodium Concentrations Identify Six Loci Influencing Serum Magnesium Levels
- Something New: An Interview with Radoje Drmanac
- The Extinction Dynamics of Bacterial Pseudogenes
- Microtubule Actin Crosslinking Factor 1 Regulates the Balbiani Body and Animal-Vegetal Polarity of the Zebrafish Oocyte
- Consistent Association of Type 2 Diabetes Risk Variants Found in Europeans in Diverse Racial and Ethnic Groups
- Transmission of Mitochondrial DNA Diseases and Ways to Prevent Them
- Telomere Disruption Results in Non-Random Formation of Dicentric Chromosomes Involving Acrocentric Human Chromosomes
- Chromosome Axis Defects Induce a Checkpoint-Mediated Delay and Interchromosomal Effect on Crossing Over during Drosophila Meiosis
- Dynamic Chromatin Organization during Foregut Development Mediated by the Organ Selector Gene PHA-4/FoxA
- Ancient Protostome Origin of Chemosensory Ionotropic Glutamate Receptors and the Evolution of Insect Taste and Olfaction
- A Wnt-Frz/Ror-Dsh Pathway Regulates Neurite Outgrowth in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Did Genetic Drift Drive Increases in Genome Complexity?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



