-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Transmission of Mitochondrial DNA Diseases and Ways to Prevent Them
Recent reports of strong selection of mitochondrial DNA (mtDNA) during transmission in animal models of mtDNA disease, and of nuclear transfer in both animal models and humans, have important scientific implications. These are directly applicable to the genetic management of mtDNA disease. The risk that a mitochondrial disorder will be transmitted is difficult to estimate due to heteroplasmy—the existence of normal and mutant mtDNA in the same individual, tissue, or cell. In addition, the mtDNA bottleneck during oogenesis frequently results in dramatic and unpredictable inter-generational fluctuations in the proportions of mutant and wild-type mtDNA. Pre-implantation genetic diagnosis (PGD) for mtDNA disease enables embryos produced by in vitro fertilization (IVF) to be screened for mtDNA mutations. Embryos determined to be at low risk (i.e., those having low mutant mtDNA load) can be preferentially transferred to the uterus with the aim of initiating unaffected pregnancies. New evidence that some types of deleterious mtDNA mutations are eliminated within a few generations suggests that women undergoing PGD have a reasonable chance of generating embryos with a lower mutant load than their own. While nuclear transfer may become an alternative approach in future, there might be more difficulties, ethical as well as technical. This Review outlines the implications of recent advances for genetic management of these potentially devastating disorders.
Published in the journal: . PLoS Genet 6(8): e32767. doi:10.1371/journal.pgen.1001066
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1001066Summary
Recent reports of strong selection of mitochondrial DNA (mtDNA) during transmission in animal models of mtDNA disease, and of nuclear transfer in both animal models and humans, have important scientific implications. These are directly applicable to the genetic management of mtDNA disease. The risk that a mitochondrial disorder will be transmitted is difficult to estimate due to heteroplasmy—the existence of normal and mutant mtDNA in the same individual, tissue, or cell. In addition, the mtDNA bottleneck during oogenesis frequently results in dramatic and unpredictable inter-generational fluctuations in the proportions of mutant and wild-type mtDNA. Pre-implantation genetic diagnosis (PGD) for mtDNA disease enables embryos produced by in vitro fertilization (IVF) to be screened for mtDNA mutations. Embryos determined to be at low risk (i.e., those having low mutant mtDNA load) can be preferentially transferred to the uterus with the aim of initiating unaffected pregnancies. New evidence that some types of deleterious mtDNA mutations are eliminated within a few generations suggests that women undergoing PGD have a reasonable chance of generating embryos with a lower mutant load than their own. While nuclear transfer may become an alternative approach in future, there might be more difficulties, ethical as well as technical. This Review outlines the implications of recent advances for genetic management of these potentially devastating disorders.
Introduction
One in 400 people carries pathogenic mitochondrial DNA (mtDNA) mutations [1]. These may cause epilepsy, liver failure, cardiomyopathy, or sudden death; or, more commonly, milder disorders such as age-related deafness [1] and/or diabetes [2] and loss of vision [3]. Yet, management and prevention of mtDNA diseases has lagged far behind the genetics revolution [4]. Although preimplantation genetic diagnosis (PGD) has been successfully used to prevent transmission of mtDNA disease [5], [6], its use has been limited for several reasons that are developed in the following sections. Technical improvements in methods for nuclear transfer [7], [8] have aroused expectations of preventing transmission of these disorders, but is this method safe?
Dose of Mutant mtDNA Determines Severity: Implications for Prenatal Genetic Diagnosis
Chorionic villus sampling (CVS, where early placental tissue is sampled with minimal impact on the foetus) has been extremely successful in preventing recurrence of Mendelian genetic diseases, but not for maternally inherited diseases, caused by mutations in the mtDNA, because of the problem of heteroplasmy [9], [10]. Thousands of mtDNA copies are present in every nucleated cell. Normal individuals are homoplasmic (i.e., virtually all their mtDNA copies are identical), but individuals affected by mtDNA diseases are usually heteroplasmic: most of their tissues and cells have a mixture of both normal and mutant mtDNAs. There is also a threshold effect (tissues function normally unless the proportion of mutant mtDNA rises above a particular level) in most diseases. The level of this threshold varies with both tissue and mutation type, usually in the range 50 to 100% mutant mtDNA, but occasionally as low as 10% [11]. Hence, for many mtDNA mutants, disease might be prevented by selecting embryos or actively lowering the level of mutant mtDNA (for instance by using nuclear transfer). But this is not universally applicable, because some mtDNA diseases are commonly homoplasmic and lack a clear threshold [12].
Unique Inheritance of mtDNA: Heteroplasmy and the Mitochondrial Bottleneck
Heteroplasmy is one reason why the clinical severity of mtDNA disorders is highly variable and can progress with time. In mtDNA disease patients the level of mutant mtDNA commonly [13], [14] (but not always [15]) falls in blood throughout life (perhaps as a result of selection against detrimental mutant mtDNA within a rapidly dividing population of cells [13], [16]). There are a few case reports suggesting that some types of mtDNA mutant accumulate in non-dividing cells such as muscle [14], [17], [18], where mtDNA turnover is slow [19], and less subject to inter-cellular competition [20]. However, this model explains by no means all of such observations [21]. The progressive change in distribution of some human mutants parallels the dynamic of apparently neutral variants in blood and spleen in an animal model [22] and underlines our inability to define the parameters determining the characteristics that we have loosely termed “detrimental.” The scanty available evidence suggests that there is less segregation in somatic tissues between early embryo and birth than post-natally [10], [23]. However, a major component of the germline segregation during transmission of both human [24] and mouse polymorphisms probably occurs during oogenesis [23], [25], and hence during development of the mother, apparently while she was in utero herself.
Factors that affect segregation of mtDNA variants include the biological fitness of dividing cells, the mutant load, and any differences between wild-type and mutant mtDNA in the rate of replication and degradation. While accumulation of mutant mtDNA can sometimes be attributed to genetic drift [26], consistent segregation towards loss or gain of mutant mtDNA has been widely documented in human cultured cells [27]–[29]. Some mutant mtDNAs exhibit segregation in the opposite direction to that predicted on the basis of selection according to mitochondrial function [28], [30]–[33]. Moreover, biased mtDNA segregation has been demonstrated in solid tissues of mice [22]. Two mouse mtDNA variants were selected in different tissues as a result of differences in genetic background [32], [33], even though neither was associated with a marked functional defect [22], nor a detectable difference in mtDNA replication rate [22]. Because differences in production of reactive oxygen species (ROS) affect mtDNA copy number [34], they may contribute to segregation of heteroplasmic mutants.
Analysis of segregation of mtDNA mutants in tissue culture often uses “cybrid” technology, where mtDNA-free immortalized cells are fused with cytoplasm containing the mitochondria under investigation. Because such cells are aneuploid, some investigators dismiss this model as non-physiological [35]. However, it does indicate that several factors might underpin mtDNA segregation in cell lines, including cellular fitness, replication pausing, ROS production, and mitophagy (preferential breakdown and recycling of regions of the mitochondrial reticulum of organelles containing mutant mtDNAs) [29], [36]–[39]. It is now increasingly possible to test the validity of such hypotheses in whole animals [32], [33].
Genetic counseling of women who are carriers of mtDNA diseases is complex because the dose of mutant mtDNA transmitted to offspring may be determined by the so-called “mitochondrial bottleneck” [40], [41], whereby a small number of mtDNAs become the founders for the offspring. If the number of segregating units (groups of clonal mtDNAs that co-segregate) that become the mtDNA founders of the embryo is small, then large fluctuations may occur in a single generation. Hauswirth and Laipis [42]–[44] suggested that two components to this may occur at different developmental stages. Firstly, there is a massive expansion from ∼100 mtDNA genomes in the earliest stages of oocyte development or primordial germ cell (PGC) to 100,000 or so in the mature oocyte [42]. Mitochondrial DNA barely replicates during the early stages of development [45] and pre-existing mtDNA molecules segregate among the cells of the blastocyst [43], [46]–[49]. This represents a second mechanism contributing to switching in the proportion of mutant mtDNAs, since mtDNAs are progressively partitioned at each cell division, ultimately producing the very few cells that will give rise to the entire embryo (the inner cell mass) [42], [44]. Hence, both clonal proliferation of mtDNA in the developing oocyte and mtDNA segregation during early development contribute to the bottleneck.
Is the Bottleneck Determined by mtDNA Content in Germ Cell Development? Mouse Studies
Recent studies have carefully quantified mtDNA copy number of individual cells during mouse development [49], [50]–[52]. As predicted [49], [53], the number of mtDNA copies drops to ∼200 molecules in developing PGCs until embryonic day (E) 7.5-8.5 [52], corresponding to the number of segregating units inferred from postnatal analysis [47], [53]. There is, however, conflicting data suggesting that copy number does not fall to values lower than 1,000 in PGCs until E7.5 in mice [50], [51]. As well as depending on technically demanding measurements of the number of mtDNAs in single cells [50], [51], these models have assumed both that segregation in the germline is neutral [54] and that all mtDNA genomes have equal probability of replicating during a single round of cell division. Such assumptions may not be valid, since Wai et al. [52] showed that a sub-population of mtDNAs replicates during folliculogenesis in mice, replenishing the mtDNA content in oocytes and potentially explaining the shifts in mutant load between two generations (Figure 1). While this might explain the variance in mutant load that these authors found in oocytes [52], a more sophisticated analysis demonstrates that a larger set of biological data is needed to establish their claim [55], [56].
Fig. 1. Mitochondrial DNA (mtDNA) copy number and genotypic variance throughout development in germ and somatic cells of mammals. 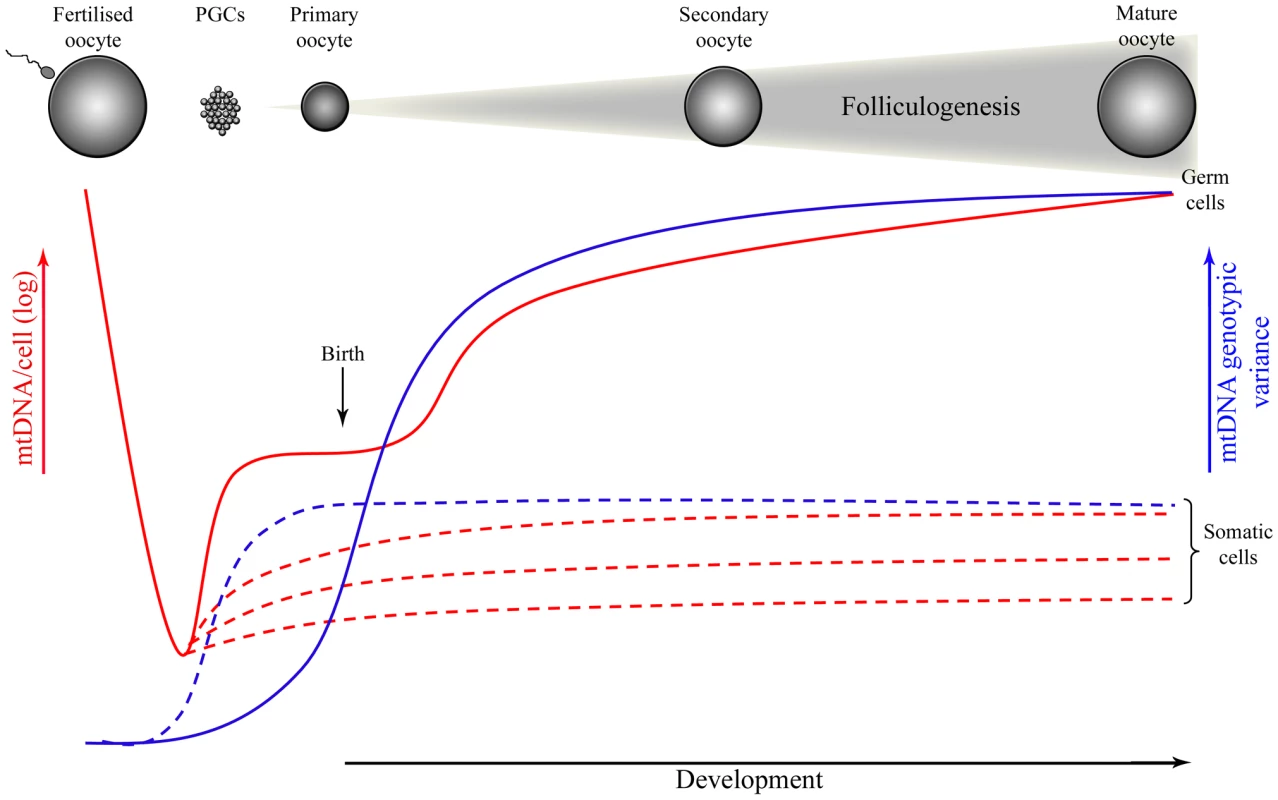
Although mtDNA genotypic variance in somatic cells increases early during development due to cellular differentiation, according to recent findings this will only occur later in germ-line development, during folliculogenesis that takes place after birth. If this is correct, then the mitochondrial genotype of the next generation would be defined only during adulthood, during the folliculogenesis that occurs every cycle of 28 days in women. In humans, although the meiotic division is initiated in the germline of the developing foetus during the last trimester of pregnancy, primary oocytes remain arrested in the first stage of the meiosis during the years between birth and puberty. In women of reproductive age, a group of oocytes is selected to grow and resume meiosis every cycle of ∼28 days. In most cases this results in the production of a single developmentally competent oocyte. It is possible that clonal expansion of a subpopulation of mtDNA during folliculogenesis in mice (between the stages of primary and mature oocyte) may correspond to the mitochondrial bottleneck [52]. If this is correct, then the segregating unit that is the physical basis of the bottleneck might be the mitochondrial nucleoid, usually containing several mtDNAs [57], rather than a single mtDNA molecule [49]. Understanding the nature of mtDNA packaging in nucleoids would then take on a new importance for biology. On the other hand, if the mitochondrial bottleneck occurs late in germline development, what is the purpose of the dramatic reduction in mtDNA copy number reported during early development? Recent studies have suggested it serves to preserve a homoplasmic population of predominantly healthy mtDNA molecules by selecting against mtDNA mutations that damage mitochondrial function (see below).
Selection against Detrimental mtDNA Mutants in the Mouse Germline
Three studies suggest that there is selection against detrimental mtDNA mutants in the mouse germline. One group developed a mouse with mtDNA rearrangements modelling Kearns-Sayre syndrome [58] in which the level of mutant mtDNAs in a mother's oocytes fell with time [59]. Like the occasional [60] mtDNA rearrangements that are maternally transmitted [61], these mice had mtDNA duplications in addition to deletions [59].
Another group investigated the transmission of randomly generated mtDNA mutations in a mouse model of mtDNA disease [62]. In this model, there is a mutation in the proof-reading domain of the mtDNA polymerase, PolgA, and this generates high levels of point mutations in the mtDNA. The homozygous founder female mice were crossed with wild type and transmitted multiple mtDNA mutations (on average 30 mutations per first generation mouse) to their offspring who were heterozygous for the PolgA mutation. Subsequent backcrossing eliminated the mutant PolgA allele and hence mtDNA mutants were passively transmitted without generating further mutations. It was thus possible to observe and compare the segregation of multiple different mtDNA mutations in a single lineage. Neutral mutations that do not alter the protein sequence undergo less selection than those that do. Purifying selection can therefore be compared with neutral drift by the relative frequency of such mutations. Clonal selection against deleterious mutations occurred in a remarkably short time frame. Indeed, many deleterious mutations were eliminated within four generations. However, selection was stronger and occurred more rapidly against mutations in genes encoding mRNAs than tRNAs. This may be linked to the apparently high frequency of pathogenic human mtDNA mutations that are identified in tRNAs [62].
A third study, focussing on two pathogenic mtDNA point mutations, again demonstrates selection in mouse [63]. These authors introduced mutant mtDNA from a well-characterised cell line into the germline using cybrid technology and a female embryonic stem cell line. Both the more severe frameshift (insertion) mutation and the milder missense mutation were initially homoplasmic, conferring a severe respiratory chain defect. However, one of the embryonic stem (ES) cell clones became heteroplasmic because a revertant of the frameshift mutation arose; a secondary deletion of the adjacent base restored the reading frame. When this line went into the germline, the mice developed a sub-clinical myopathy and cardiomyopathy but bred normally. The frameshift mutation was lost in favour of the revertant within four generations. None of the offspring had a higher level of the frameshift mutation than their mother, and studies of oocytes showed that the selection had occurred by the time oocytes were mature. These studies are consistent with other studies on mice [52] and on humans [25], [64]. The selection appears to depend on some aspect of mitochondrial function, but studies of the bottleneck have not clarified the precise mechanism or at what stage of oogenesis it is likely to have occurred. While some classic studies in humans [64], [65] and in mice [53] demonstrate that level of mutant mtDNA follows a distribution that may be random [66], others are very different [23], [30]. The latter are skewed towards virtual homoplasmy for both mutant and wild-type mtDNA in oocytes from individual women. One explanation would be that a single mtDNA passes the bottleneck, but there is no obvious mechanism for such an extreme situation. Alternatively this could arise because genetic drift can lead to fixation of neutral mutations [54]. While some investigators consider that the different distributions may be due to the specific mutation, we note that the skewed distributions have only been seen following super-ovulation. Furthermore, close examination of data suggest that the mean level of mutant mtDNA in the oocytes/offspring is not identical with that of the mother, so germline selection [59], [62], [63] is not excluded [54].
But what is the basis of the selection seen in mice and potentially in humans? Only 30% of oogonia established during fetal life develop into matured oocytes, the remainder undergoes apoptosis [67], [68]. Fan et al. [63] suggested that dysfunctional mitochondria generate high ROS levels that are the signal underlying selection against oocytes with high mtDNA mutant load by apoptosis.
A second possible mechanism for selecting against mutant mtDNA is selection at the organelle level. The number of mtDNA copies per mitochondrion in germ cells is thought to be as few as one or two molecules, in comparison to eight or so in somatic cells [57]. Thus, mutations in a few mtDNA copies can be distinguished among wild-type mtDNAs present in the same cell by the effect of mutations on mitochondrial phenotype. For instance, damaged mitochondria might be degraded by intracellular mechanisms such as autophagy or, more specifically, mitophagy [36]. Evidences of this were given by Twig et al. [69] who showed that dysfunctional mitochondria are less likely to fuse with the remaining mitochondria and are degraded by autophagy. Although this event was shown in somatic cells, autophagy is also present in germ cells and early embryos [70] and might be involved in removal of mutant mtDNA from the next generation. Another possibility for selection at the organelle level is competition between dysfunctional and normal mitochondria, where dysfunctional mitochondria might be less efficient for import and enzymatic function of the nucleus-encoded proteins that are required for mtDNA replication. This might result in an advantage of wild-type molecules to replicate over the mutant ones, thus decreasing the mutant load in germ cells and in the next generation [71]. As discussed above, Wai et al. [52] have reported that a sub-population of mtDNAs is replicated during folliculogenesis to replenish the mtDNA content in oocytes. If such a sub-population were positively selected on phenotype by an unknown mechanism, this might explain the observed pattern of selection against mtDNA mutations.
A third possible mechanism is specific to oocytes, based on a structure known as the Balbiani body or the mitochondrial cloud [72]–[74]. The Balbiani body comprises mitochondria and endoplasmic reticulum organized around Golgi elements [73]–[78] that may enable germplasm mRNAs to be specifically inherited by the PGCs in the future embryo. In the same way, a specific mitochondrial sub-population may segregate to the Balbiani bodies and ultimately populate the PGCs [73], [79]–[81], potentially explaining the pattern of selection against severe mtDNA mutations. In some non-mammalian species mitochondria with the highest membrane potentials are found in Balbiani bodies [78], [80], [81], suggesting that high-quality mitochondria and mtDNAs are selected for transmission to the PGCs of the next generation. While this is an appealing mechanism for selecting against mutant mtDNAs, there is little supporting evidence and it is still controversial, even in mouse [52]. Furthermore, the Balbiani body could not explain the progressive decrease in load of mutant mtDNA in mouse oocytes of an individual female with age.
Whatever the underlying mechanism, something occurring during early oogenesis and/or folliculogenesis seems to provide a degree of selection against mutant mtDNA molecules. Studies by Sato et al. [59] and Fan et al. [63] suggest selection occurs during adult life and, therefore, during folliculogenesis, since mutant load drops in mouse oocytes as a function of time (i.e., between two litters). On the other hand, mutations that escape this filter would then be exposed to selection at the level of the individual. Thus, several mechanisms may contribute to the bottleneck and prevent dissemination of mtDNA mutations (Figure 2).
Fig. 2. Mitochondrial DNA (mtDNA) cycle in the mouse germline. 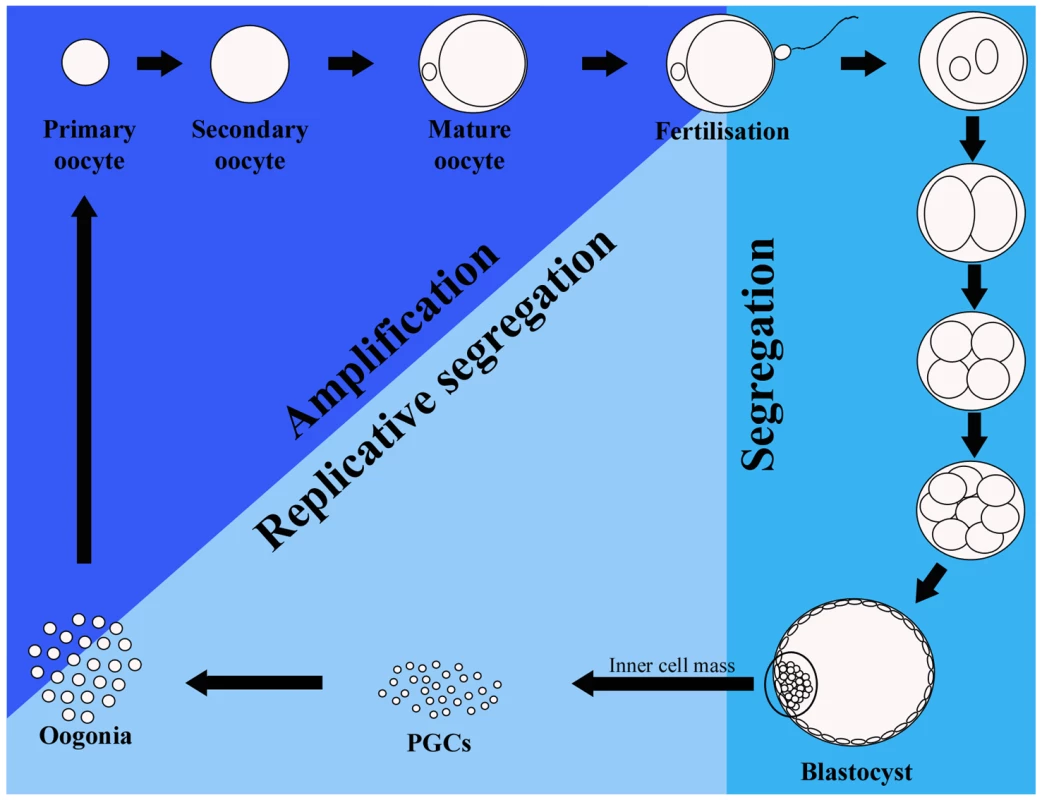
During early embryo development (“Segregation” on the diagram, representing the first seven to eight days after fertilization) the mtDNA is segregated among daughter cells without being replicated. The number of mtDNA copies thus decreases drastically, being lowest in primordial germ cells (PGCs). The next stage is marked “Replicative segregation,” which implies random replication and partitioning of mtDNAs into daughter cells. The last stage, “Amplification,” is characterized by an exponential amplification of mtDNA molecules. It has been suggested that replication of mtDNA during this stage is restricted to a sub-group of molecules leading to drastic changes in the mtDNA genotype in the mature oocyte. Yet, there seems to be during this stage a selection against mutations in the mtDNA that might occur. Mitochondrial DNA Bottlenecks in Human Germ Cell Development
Genetic management of patients with mtDNA disease depends on understanding both germline segregation and the physiological basis of the bottleneck. However, the published human data where oocytes are compared with load in maternal post mitotic tissues are minimal [54]. It is increasingly clear that a major component of this bottleneck has occurred by the time oocytes are mature in human controls [25], patients with mtDNA disease [64], [82], and mouse models [52], [53], [63]. Statistical analysis of oocytes shows that in some cases the distribution of mutant mtDNA is consistent with random drift, but does not exclude the possibility of selection in the germline at an earlier stage [66]. On the other hand, a de novo mutation in a child and in oocytes appeared to be absent from the mother's other tissues [83], suggesting that it arose within the development of her germ cells. Comparison of human and mouse data suggests potentially important differences in both the type of rearrangement that is typical [59], [60] and in the bottleneck size [55]. Hence, it may not be appropriate to extrapolate from the consistent selection against detrimental mtDNA mutants seen in the mouse [62] to humans.
Implications of Heteroplasmy for Genetic Management of Human Diseases
Oocyte donation would avoid all the problems associated with the presence of mutant mtDNA, but there is a shortage of oocyte donors. Pre-implantation genetic diagnosis for mitochondrial disease could be the best option for patients carrying high levels of mutant mtDNA [6]. This approach involves analyzing embryos produced by in vitro fertilization (IVF) and only transferring those determined to be at very low risk. Preimplantation genetic diagnosis is performed earlier in development (three days after fertilization) than CVS, and two cells are usually taken for mtDNA disease [6]. This is because analysis of one or two cells from an embryo containing 6–10 cells may be more representative of the whole conceptus [84], but not necessarily of the part that will become the foetus. Moreover, sampling two cells rather than only one provides a more confident result (the result from one cell can be compared against the other) and does not appear to impair pregnancy outcome [6], [85].
While PGD clearly has enormous promise for women with sub-clinical levels of mtDNA mutations [6], [23], it may be more complex for women carrying high mtDNA mutation loads and displaying disease symptoms [6], [86]. If such women typically transmit levels of mutant mtDNA close to their own [82], [87], they are likely to produce few if any disease free embryos. If, however, the level of mutant mtDNA in their oocytes were polarized to the two extremes as seen in neuropathy, ataxia and retinitis pigmentosa (NARP) [23], [30], they might have a reasonable chance of usable embryos. This depends to what extent the selection against detrimental mtDNA mutants that is seen in mouse germline also occurs in humans. Nevertheless, offering PGD for certain mtDNA diseases, followed by CVS to confirm that the level of mutant mtDNA in the foetus is low, would likely have advantages over CVS alone. The main drawback of CVS for mtDNA disorders is that it is not entirely certain that the level of mutant mtDNA detectable in a single CVS sample will accurately reflect that of the foetus [10]. Indeed, such data that exist suggest that there is a degree of variation of perhaps ±10% in the level of neutral [88] and pathogenic variants in placenta [83]. Moreover, certain centers are now offering PGD [5], [6], [23].
Is Nuclear Transfer the Way Forward?
Since Dolly the sheep was created by fusing an adult somatic cell with a recipient enucleated oocyte, producing in Dolly mtDNA inherited not from the somatic cell donor but the recipient oocyte [89], researchers have contemplated altering the mitochondrial population of a human embryo using nuclear transfer. It has been possible to use nuclear transplantation at the zygote stage (pro-nuclear transfer) to partially correct respiration defects and mitochondrial diseases in mice carrying a large-scale deletion of mtDNA [90].
Recently, Tachibana et al. [8] transferred nuclei at an earlier stage; spindle-chromosomal complexes were removed from mature monkey oocytes, with minimal if any adherent mtDNA, and placed into other oocytes from which the complex had been removed. This study resulted in the generation of three healthy offspring with less than 3% of nuclear donor mtDNA [8]. More recently, Craven et al. [7] transferred pro-nuclei between human zygotes resulting in minimal carry-over of nuclear donor mtDNA and compatible onward development to the blastocyst stage in vitro. Because of the current regulations and the paucity of “spare” human embryos, this study was carried out in abnormally fertilized embryos. Disappointingly, the levels of nuclear donor mtDNA were very variable between cells of the resulting embryos (ranging from less than 0.5 to 11.4%), suggesting that mtDNA segregation might be disturbed by the procedure. This may be a consequence of using genetically abnormal embryos that would not occur in bona fide treatment cycles. But it might be because they used a drug that specifically targets the microtubule-based system (nocodazole) for organizing mitochondria in the cell. Despite this, both studies [7], [8] (with their pros and cons) are of fundamental importance and hold promise for the future treatment of mtDNA diseases.
A different procedure, ooplasm donation (cytoplasm from a donor oocyte), offers an alternative [91]. Ooplasm donation has been used in humans as a treatment for poor IVF embryo development for a type of infertility that might be due to intrinsic defects of the oocyte cytoplasm. In this experimental procedure, mitochondria, cytoplasm, and associated structures from a donor oocyte are injected into a recipient unfertilised oocyte prior to IVF. Mitochondrial DNA analysis of children born following the procedure demonstrated that the contribution of donor mtDNA is small [92], but, in some cases, the proportion of donor mtDNA far exceeded the expected 10–15% [93], based on the volume of cytoplasm derived from the donor. While genetic drift might occasionally underlie such a change, experiments on bovine zygotes suggest that mitochondrial replacement can be consistently improved by centrifugation and removal of the recipient mtDNA without apparent effects on development [94], [95]. Centrifugation causes mitochondria to concentrate in one of the zygote's poles [94], [95], allowing removal of mitochondrion-enriched cytoplasm by micromanipulation. Doing this, it is possible to remove over 60% of recipient-zygote mtDNA before ooplasmic transfer [94]. Furthermore, the use of purified mitochondria as donor mtDNA [96]–[98] might decrease the mutant load to low levels, ultimately avoiding transmission of the mitochondrial disorders.
Will any of these procedures be viable alternative strategies to more conventional genetic management? Nuclear transfer sounds simple and seems effective in mice [90], monkeys [8], and in human pre-implantation embryos [7], yet there remain very many unknowns. Mitochondrial DNA encodes only a handful of proteins, the remainder of the thousand or so proteins that go to make up the mitochondrion being encoded by the nucleus. This arrangement necessitates nucleo-mitochondrial interactions, which are as yet poorly understood. In embryos derived either by nuclear transfer or ooplasm donation, the genetic material originates from three unrelated parents (two providing the nucleus and one the mtDNA). While extreme (non-physiological) mismatch between nuclear and mitochondrial DNA has clearly deleterious effects on nucleo-mitochondrial interactions [99], [100], might subtle errors in these interactions occur following nuclear transfer? The consequences of uncoupling the mitochondria and nucleus, followed by the introduction of DNA from an unrelated individual are unknown. Genetic studies of such interactions strongly suggest that major problems are unlikely [32], [33]. However, backcrossing mice so that one mtDNA was substituted for another on a standardized nuclear background can alter either physical [101] or cognitive performance [102] and even the anatomy of the brain [102]. Furthermore, studies on mice suggest that mtDNA carried-over with the nuclear DNA of the donor zygote (karyoplast) may be replicated faster than that of the recipient, perhaps depending on its proximity to the nucleus [103]. Since nuclear transfer experiments in multiple species show that donor mtDNA may persist in embryos and tissues from the offspring [104], one cannot assume that the mitochondria from the “healthy” enucleated oocyte will ultimately outnumber the mutant mitochondria in the tissues of the foetus and child. Furthermore, even in the best hands, the success rate of achieving a pregnancy per egg is low and donor oocytes are scarce.
Conclusion
In conclusion, the many ethical, scientific, and pragmatic problems have been a major impediment in the genetic management of mtDNA diseases. Recent experiments on animals suggest that nuclear transplant holds future promise. Currently, the most ethical course of action may be to weigh-up the uncertainties and use new approaches such as PGD in an attempt to help these distressed families.
Zdroje
1. ManwaringN
JonesMM
WangJJ
RochtchinaE
HowardC
2007 Population prevalence of the MELAS A3243G mutation. Mitochondrion 7 230 233
2. van den OuwelandJM
LemkesHH
RuitenbeekW
SandkuijlLA
de VijlderMF
1992 Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet 1 368 371
3. WallaceDC
SinghG
LottMT
HodgeJA
SchurrTG
1988 Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science 242 1427 1430
4. PoultonJ
TurnbullDM
2000 74th ENMC international workshop: mitochondrial diseases 19-20 november 1999, Naarden, The Netherlands. Neuromuscul Disord 10 460 462
5. ThorburnD
WiltonL
Stock-MyerS
2009 Healthy baby girl born following pre-implantation Genetic diagnosis for mitochondrial DNA m.8993t>g Mutation. Mol Genet Metab 98 5 6
6. PoultonJ
BredenoordAL
2010 174th ENMC International workshop: applying pre-implantation genetic diagnosis to mtDNA diseases: implications of scientific advances 19-21 March 2010, Naarden, The Netherlands. Neuromuscul Disord, in press
7. CravenL
TuppenHA
GreggainsGD
HarbottleSJ
MurphyJL
2010 Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 465 82 85
8. TachibanaM
SparmanM
SritanaudomchaiH
MaH
ClepperL
2009 Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 461 367 372
9. PoultonJ
MarchingtonD
1997 Prospects for DNA-based Prenatal Diagnosis of Mitochondrial Disorders. Prenat Diagn 16 1247 1256
10. BouchetC
SteffannJ
CorcosJ
MonnotS
PaquisV
2006 Prenatal diagnosis of MELAS syndrome: contribution to understanding mitochondrial DNA segregation during human embryo fetal development. J Med Genet 43 788 792
11. SacconiS
SalviatiL
NishigakiY
WalkerWF
Hernandez-RosaE
2008 A functionally dominant mitochondrial DNA mutation. Hum Mol Genet 17 1814 1820
12. BlackGC
MortenK
LabordeA
PoultonJ
1996 Leber's hereditary optic neuropathy: heteroplasmy is likely to be significant in the expression of LHON in families with the 3460 ND1 mutation. Br J Ophthalmol 80 915 917
13. RahmanS
PoultonJ
MarchingtonD
SuomalainenA
2001 Decrease of 3243 A->G mtDNA mutation from blood in MELAS syndrome: a longitudinal study. Am J Hum Genet 68 238 240
14. LarssonNG
HolmeE
KristianssonB
OldforsA
TuliniusM
1990 Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr Res 28 131 136
15. WhiteS
ShanskeS
McGillJ
MountainH
GeraghtyM
1999 Mitochondrial DNA mutations at nucleotide 8993 show a lack of tissue - or age-related variation. J Inherit Metab Dis 22 899 914
16. RajasimhaHK
ChinneryPF
SamuelsDC
2008 Selection against Pathogenic mtDNA Mutations in a Stem Cell Population Leads to the Loss of the 3243A->G Mutation in Blood. Am J Hum Genet 82 333 343
17. PoultonJ
O'RahillyS
MortenK
ClarkA
1995 Mitochondrial DNA, diabetes and pancreatic pathology in Kearns-Sayre syndrome. Diabetologia 38 868 871
18. WeberK
WilsonJN
TaylorL
BrierleyE
JohnsonMA
1997 A new mtDNA mutation showing accumulation with time and restriction to skeletal muscle. Am J Hum Genet 60 373 380
19. GrossNJ
GetzGS
RabinowitzM
1969 Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem 244 1552 1562
20. WeberK
WilsonJ
TaylorL
BrierleyE
JohnsonM
1997 A New mtDNA mutation showing accumulation with time and restriction to skeletal muscle. Am J Hum Genet 60 373 380
21. PyleA
TaylorRW
DurhamSE
DeschauerM
SchaeferAM
2007 Depletion of mitochondrial DNA in leukocytes harboring the 3243A->G mtDNA mutation. J Med Genet 44 69 74
22. JenuthJ
PetersonA
ShoubridgeE
1997 Tissue-specific selection for different mtDNA genotypes in heteroplasmic mice. Nat Genet 16 93 95
23. SteffannJ
FrydmanN
GigarelN
BurletP
RayPF
2006 Analysis of mtDNA variant segregation during early human embryonic development: a tool for successful NARP preimplantation diagnosis. J Med Genet 43 244 247
24. MarchingtonD
PoultonJ
SellerA
HoltI
1996 Do sequence variants in the major non-coding region of the mitochondrial genome influence mitochondrial mutations associated with disease. Hum Mol Genet 5 473 479
25. MarchingtonD
HartshorneG
BarlowD
PoultonJ
1997 Homopolymeric tract heteroplasmy in mtDNA from tissues and single oocytes: support for a genetic bottleneck. Am J Hum Genet 60 408 416
26. ChinneryPF
SamuelsDC
1999 Relaxed replication of mtDNA: A model with implications for the expression of disease. Am J Hum Genet 64 1158 1165
27. HayashiJ
OhtaS
KikuchiA
TakemitsuM
GotoY
1991 Introduction of disease related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc Natl Acad Sci U S A 88 10614 10618
28. YonedaM
ChomynA
MartinuzziA
HurkoO
AttardiG
1992 Marked replicative advantage of human mtDNA carrying a point mutation that causes the MELAS encephalomyopathy. Proc Natl Acad Sci U S A 89 11164 11168
29. DunbarD
MoonieP
JacobsH
HoltI
1995 Different cellular backgrounds confer a marked advantage to either mutant or wild-type mitochondrial genomes. Proc Natl Acad Sci U S A 92 6562 6566
30. BlokR
CookD
ThorburnD
DahlH
1997 Skewed segregation of the mtDNA nt 8993 (T->G) mutation in human ocytes. Am J Hum Genet 60 1495 1501
31. Vergani LRR
BrierleyCH
HannaM
HoltIJ
1999 Introduction of heteroplasmic mitochondrial DNA (mtDNA) from a patient with NARP into two human rho degrees cell lines is associated either with selection and maintenance of NARP mutant mtDNA or failure to maintain mtDNA. Hum Mol Genet 8 1751 1755
32. BattersbyBJ
Loredo-OstiJC
ShoubridgeEA
2003 Nuclear genetic control of mitochondrial DNA segregation. Nat Genet 33 183 186
33. BattersbyBJ
ShoubridgeEA
2001 Selection of a mtDNA sequence variant in hepatocytes of heteroplasmic mice is not due to differences in respiratory chain function or efficiency of replication. Hum Mol Genet 10 2469 2479
34. Moreno-LoshuertosR
Acin-PerezR
Fernandez-SilvaP
MovillaN
Perez-MartosA
2006 Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat Genet 38 1261 1268
35. LehtinenSK
HanceN
El MezianeA
JuholaMK
JuholaKM
2000 Genotypic stability, segregation and selection in heteroplasmic human cell lines containing np 3243 mutant mtDNA. Genetics 154 363 380
36. ChenH
ChanDC
2009 Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18 R169 176
37. EmmersonCF
BrownGK
PoultonJ
2001 Synthesis of mitochondrial DNA in permeabilised human cultured cells. Nucleic Acids Res 29 E1
38. HyvarinenAK
PohjoismakiJL
ReyesA
WanrooijS
YasukawaT
2007 The mitochondrial transcription termination factor mTERF modulates replication pausing in human mitochondrial DNA. Nucleic Acids Res 35 6458 6474
39. HessJF
ParisiMA
BennettJL
ClaytonDA
1991 Impairment of mitochondrial transcription termination by a point mutation associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 351 236 239
40. PoultonJ
1995 Transmission of mtDNA: Cracks in the bottleneck. Am J Hum Genet 57 224 226
41. PoultonJ
MarchingtonD
MacaulayV
1998 Is the bottleneck cracked? Am J Hum Genet 62 752 757
42. OlivoPD
Van de WalleMJ
LaipisPJ
HauswirthWW
1983 Nucleotide sequence evidence for rapid genotypic shifts in the bovine mitochondrial DNA D-loop. Nature 306 400 402
43. LaipisP
HauswirthW
O'BrianT
MichaelsG
1988 Unequal partitioning of bovine mitochondrial genotypes among siblings. Proc Natl Acad Sci U S A 85 8107 8110
44. AshleyC
LaipisP
HauswirthW
1989 Rapid sequestration of heteroplasmic bovine mitochondria. Nucleic Acids Res 17 7325 7331
45. AikenCE
Cindrova-DaviesT
JohnsonMH
2008 Variations in mouse mitochondrial DNA copy number from fertilization to birth are associated with oxidative stress. Reprod Biomed Online 17 806 813
46. PikoL
TaylorKD
1987 Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol 123 364 374
47. McConnellM
PetrieL
2004 Mitochondrial DNA turnover occurs during preimplantation development and can be modulated by environmental factors. Reprod Biomed Online 9 418 424
48. ThundathilJ
FilionF
SmithLC
2005 Molecular control of mitochondrial function in preimplantation mouse embryos. Mol Reprod Dev 71 405 413
49. CreeLM
SamuelsDC
de Sousa LopesSC
RajasimhaHK
WonnapinijP
2008 A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat Genet 40 249 254
50. CaoL
ShitaraH
HoriiT
NagaoY
ImaiH
2007 The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat Genet 39 386 390
51. CaoL
ShitaraH
SugimotoM
HayashiJ
AbeK
2009 New evidence confirms that the mitochondrial bottleneck is generated without reduction of mitochondrial DNA content in early primordial germ cells of mice. PLoS Genet 5 e1000756 doi:10.1371/journal.pgen.1000756
52. WaiT
TeoliD
ShoubridgeEA
2008 The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet 40 1484 1488
53. JenuthJ
PetersonA
FuK
ShoubridgeE
1996 Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat Genet 14 146 151
54. PoultonJ
MacaulayV
MarchingtonD
2003 Transmission, genetic counselling and prenatal diagnosis of mitochondrial DNA disease.
HoltI
Genetics of Mitochondrial Disease Oxford Oxford University Press 309 326
55. WonnapinijP
ChinneryPF
SamuelsDC
2010 Previous Estimates of Mitochondrial DNA Mutation Level Variance Did Not Account for Sampling Error: Comparing the mtDNA Genetic Bottleneck in Mice and Humans. Am J Hum Genet 86 1 11
56. SamuelsDC
WonnapinijP
CreeLM
ChinneryPF
2010 Reassessing evidence for a postnatal mitochondrial genetic bottleneck. Nat Genet 42 471 472
57. IborraFJ
KimuraH
CookPR
2004 The functional organization of mitochondrial genomes in human cells. BMC Biol 2 9
58. InoueK
NakadaK
OguraA
IsobeK
GotoY
2000 Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat Genet 26 176 181
59. SatoA
NakadaK
ShitaraH
KasaharaA
YonekawaH
2007 Deletion-mutant mtDNA increases in somatic tissues but decreases in female germ cells with age. Genetics 177 2031 2037
60. ChinneryPF
DiMauroS
ShanskeS
SchonEA
ZevianiM
2004 Risk of developing a mitochondrial DNA deletion disorder. Lancet 364 592 596
61. PoultonJ
HoltI
1994 Mitochondrial DNA: does more lead to less? Nat Genet 8 313 315
62. StewartJB
FreyerC
ElsonJL
WredenbergA
CansuZ
2008 Strong purifying selection in transmission of mammalian mitochondrial DNA. PLoS Biol 6 e10 doi:10.1371/journal.pbio.0060010
63. FanW
WaymireKG
NarulaN
LiP
RocherC
2008 A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science 319 958 962
64. MarchingtonDR
MacaulayV
HartshorneGM
BarlowD
PoultonJ
1998 Evidence from human oocytes for a genetic bottleneck in an mtDNA disease. Am J Hum Genet 63 769 775
65. Brown DTSD
MichaelEM
TurnbullDM
ChinneryPF
2001 Random genetic drift determines the level of mutant mtDNA in human primary oocytes. Am J Hum Genet 68 533 536
66. WonnapinijP
ChinneryPF
SamuelsDC
2008 The distribution of mitochondrial DNA heteroplasmy due to random genetic drift. Am J Hum Genet 83 582 593
67. TillyJL
TillyKI
1995 Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology 136 242 252
68. HusseinMR
2005 Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update 11 162 177
69. TwigG
ElorzaA
MolinaAJ
MohamedH
WikstromJD
2008 Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27 433 446
70. TsukamotoS
KumaA
MurakamiM
KishiC
YamamotoA
2008 Autophagy is essential for preimplantation development of mouse embryos. Science 321 117 120
71. StewartJB
FreyerC
ElsonJL
LarssonNG
2008 Purifying selection of mtDNA and its implications for understanding evolution and mitochondrial disease. Nat Rev Genet 9 657 662
72. ZhouRR
WangB
WangJ
SchattenH
ZhangYZ
2010 Is the mitochondrial cloud the selection machinery for preferentially transmitting wild-type mtDNA between generations? Rewinding Muller's ratchet efficiently. Curr Genet 56 101 107
73. KlocM
BilinskiS
EtkinLD
2004 The Balbiani body and germ cell determinants: 150 years later. Curr Top Dev Biol 59 1 36
74. PeplingME
WilhelmJE
O'HaraAL
GephardtGW
SpradlingAC
2007 Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc Natl Acad Sci U S A 104 187 192
75. KlocM
EtkinLD
1995 Two distinct pathways for the localization of RNAs at the vegetal cortex in Xenopus oocytes. Development 121 287 297
76. KlocM
EtkinLD
1998 Apparent continuity between the messenger transport organizer and late RNA localization pathways during oogenesis in Xenopus. Mech Dev 73 95 106
77. KlocM
LarabellC
EtkinLD
1996 Elaboration of the messenger transport organizer pathway for localization of RNA to the vegetal cortex of Xenopus oocytes. Dev Biol 180 119 130
78. ZhangYZ
OuyangYC
HouY
SchattenH
ChenDY
2008 Mitochondrial behavior during oogenesis in zebrafish: a confocal microscopy analysis. Dev Growth Differ 50 189 201
79. D'HerdeK
CallebautM
RoelsF
De PrestB
van NassauwL
1995 Homology between mitochondriogenesis in the avian and amphibian oocyte. Reprod Nutr Dev 35 305 311
80. CoxRT
SpradlingAC
2003 A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development 130 1579 1590
81. CoxRT
SpradlingAC
2006 Milton controls the early acquisition of mitochondria by Drosophila oocytes. Development 133 3371 3377
82. BrownDT
SamuelsDC
MichaelEM
TurnbullDM
ChinneryPF
2001 Random genetic drift determines the level of mutant mtDNA in human primary oocytes. Am J Hum Genet 68 533 536
83. MarchingtonD
MalikS
BanerjeeA
TurnerK
SamuelsD
2010 Information for genetic management of mtDNA disease: Sampling pathogenic mtDNA mutants in the human germline and in placenta. J Med Genet 47 257 261
84. DeanNL
BattersbyBJ
AoA
GosdenRG
TanSL
2003 Prospect of preimplantation genetic diagnosis for heritable mitochondrial DNA diseases. Mol Hum Reprod 9 631 638
85. GoossensV
De RyckeM
De VosA
StaessenC
MichielsA
2008 Diagnostic efficiency, embryonic development and clinical outcome after the biopsy of one or two blastomeres for preimplantation genetic diagnosis. Hum Reprod 23 481 492
86. BredenoordA
DondorpW
PenningsG
De Die-SmuldersC
SmeetsH
2009 Preimplantation genetic diagnosis for mitochondrial DNA disorders: ethical guidance for clinical practice. Eur J Hum Genet 17 1550 1559
87. ChinneryPF
ThorburnDR
SamuelsDC
WhiteSL
DahlHM
2000 The inheritance of mitochondrial DNA heteroplasmy: random drift, selection or both? Trends Genet 16 500 505
88. MarchingtonD
Scott-BrownM
BarlowD
JPoulton
2006 Moscaicism for mitochondrial DNA polymorphic variants in placenta has implications for the feasibility of prenatal diagnosis in mtDNA diseases. Eur J Hum Genet 14 816 823
89. CampbellKH
McWhirJ
RitchieWA
WilmutI
1996 Sheep cloned by nuclear transfer from a cultured cell line. Nature 380 64 66
90. SatoA
KonoT
NakadaK
IshikawaK
InoueS
2005 Gene therapy for progeny of mito-mice carrying pathogenic mtDNA by nuclear transplantation. Proc Natl Acad Sci U S A 102 16765 16770
91. SillsES
TakeuchiT
TuckerMJ
PalermoGD
2004 Genetic and epigenetic modifications associated with human ooplasm donation and mitochondrial heteroplasmy - considerations for interpreting studies of heritability and reproductive outcome. Med Hypotheses 62 612 617
92. BarrittJA
BrennerCA
MalterHE
CohenJ
2001 Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod 16 513 516
93. BrennerCA
KubischHM
PierceKE
2004 Role of the mitochondrial genome in assisted reproductive technologies and embryonic stem cell-based therapeutic cloning. Reprod Fertil Dev 16 743 751
94. ChiarattiMR
BressanFF
FerreiraCR
CaetanoAR
SmithLC
2010 Embryo mitochondrial DNA depletion is reversed during early embryogenesis in cattle. Biol Reprod 82 76 85
95. FerreiraCR
BurgstallerJP
PerecinF
GarciaJM
ChiarattiMR
2010 Pronounced segregation of donor mitochondria introduced by bovine ooplasmic transfer to the female germ-line. Biol Reprod 82 563 571
96. HuaS
ZhangY
LiXC
MaLB
CaoJW
2007 Effects of granulosa cell mitochondria transfer on the early development of bovine embryos in vitro. Cloning Stem Cells 9 237 246
97. El ShourbagySH
SpikingsEC
FreitasM
St JohnJC
2006 Mitochondria directly influence fertilisation outcome in the pig. Reproduction 131 233 245
98. PinkertCA
IrwinMH
JohnsonLW
MoffattRJ
1997 Mitochondria transfer into mouse ova by microinjection. Transgenic Res 6 379 383
99. McKenzieM
TrounceI
2000 Expression of Rattus norvegicus mtDNA in Mus musculus cells results in multiple respiratory chain defects. J Biol Chem 275 31514 31519
100. DeyR
BarrientosA
MoraesCT
2000 Functional constraints of nuclear-mitochondrial DNA interactions in xenomitochondrial rodent cell lines. J Biol Chem 275 31520 31527
101. NagaoY
TotsukaY
AtomiY
KanedaH
LindahlKF
1998 Decreased physical performance of congenic mice with mismatch between the nuclear and the mitochondrial genome. Genes Genet Syst 73 21 27
102. RoubertouxPL
SluyterF
CarlierM
MarcetB
Maarouf-VerayF
2003 Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat Genet 35 65 69
103. MeirellesF
SmithL
1998 Mitochondrial genotype segregation during preimplantation development in mouse heteroplasmic embryos. Genetics 148 877 883
104. BowlesE
CampbellK
JohnJS
2007 Nuclear transfer: preservation of a nuclear genome at the expense of its associated mtDNA genome(s).
JohnJS
CTDB Volume, The Mitochondrion in the Germline and Early Development San Diego, CA Elsevier
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 8- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Mutation in the Gene Encoding Ubiquitin Ligase LRSAM1 in Patients with Charcot-Marie-Tooth Disease
- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Did Genetic Drift Drive Increases in Genome Complexity?
- The 5p15.33 Locus Is Associated with Risk of Lung Adenocarcinoma in Never-Smoking Females in Asia
- An Alpha-Catulin Homologue Controls Neuromuscular Function through Localization of the Dystrophin Complex and BK Channels in
- Epigenetically-Inherited Centromere and Neocentromere DNA Replicates Earliest in S-Phase
- Survival and Growth of Yeast without Telomere Capping by Cdc13 in the Absence of Sgs1, Exo1, and Rad9
- Tuberous Sclerosis Complex 1 Regulates dE2F1 Expression during Development and Cooperates with RBF1 to Control Proliferation and Survival
- Disease-Associated Mutations That Alter the RNA Structural Ensemble
- The Transcriptomes of Two Heritable Cell Types Illuminate the Circuit Governing Their Differentiation
- Inactivation of VCP/ter94 Suppresses Retinal Pathology Caused by Misfolded Rhodopsin in
- Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: a Meta-Analysis and Comparison with Lung Cancer and COPD
- Transcriptional Regulation by CHIP/LDB Complexes
- Conserved Role of in Ethanol Responses in Mutant Mice
- A Global Overview of the Genetic and Functional Diversity in the Pathogenicity Island
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3–Dependent Manner in
- Genetic Analysis of Baker's Yeast Msh4-Msh5 Reveals a Threshold Crossover Level for Meiotic Viability
- Genome-Wide Association Studies of Serum Magnesium, Potassium, and Sodium Concentrations Identify Six Loci Influencing Serum Magnesium Levels
- Something New: An Interview with Radoje Drmanac
- The Extinction Dynamics of Bacterial Pseudogenes
- Microtubule Actin Crosslinking Factor 1 Regulates the Balbiani Body and Animal-Vegetal Polarity of the Zebrafish Oocyte
- Consistent Association of Type 2 Diabetes Risk Variants Found in Europeans in Diverse Racial and Ethnic Groups
- Transmission of Mitochondrial DNA Diseases and Ways to Prevent Them
- Telomere Disruption Results in Non-Random Formation of Dicentric Chromosomes Involving Acrocentric Human Chromosomes
- Chromosome Axis Defects Induce a Checkpoint-Mediated Delay and Interchromosomal Effect on Crossing Over during Drosophila Meiosis
- Dynamic Chromatin Organization during Foregut Development Mediated by the Organ Selector Gene PHA-4/FoxA
- Ancient Protostome Origin of Chemosensory Ionotropic Glutamate Receptors and the Evolution of Insect Taste and Olfaction
- A Wnt-Frz/Ror-Dsh Pathway Regulates Neurite Outgrowth in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Did Genetic Drift Drive Increases in Genome Complexity?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



