-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
Tuberculosis is deadly human disease caused by infection with the bacterium Mycobacterium tuberculosis. This pathogen has a complex metabolism with many genes required for the synthesis of components of its unique cell envelope. We have investigated a family of closely related genes coding for different acyl CoA carboxylase enzyme complexes with previously unexplained genetic redundancy that have been thought to have an involvement in the synthesis of these cell envelope components. We identified five functional multienzyme complexes. Of the two complexes with hitherto unknown function we chose to investigate, one specifically and to our surprise it is required for the degradation of the amino acid leucine. To our knowledge this is the first demonstration that mycobacteria have a specific pathway for leucine degradation and thus broaden the functional diversity associated with acyl CoA carboxylase coding genes.
Published in the journal: . PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004623
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004623Summary
Tuberculosis is deadly human disease caused by infection with the bacterium Mycobacterium tuberculosis. This pathogen has a complex metabolism with many genes required for the synthesis of components of its unique cell envelope. We have investigated a family of closely related genes coding for different acyl CoA carboxylase enzyme complexes with previously unexplained genetic redundancy that have been thought to have an involvement in the synthesis of these cell envelope components. We identified five functional multienzyme complexes. Of the two complexes with hitherto unknown function we chose to investigate, one specifically and to our surprise it is required for the degradation of the amino acid leucine. To our knowledge this is the first demonstration that mycobacteria have a specific pathway for leucine degradation and thus broaden the functional diversity associated with acyl CoA carboxylase coding genes.
Introduction
Biotin-dependent acyl-CoA carboxylases (YCCs) are ubiquitous multifunctional enzymes that are found in all kingdoms of life. They have key roles in the metabolism of fatty acids, amino acids, and carbohydrates and have various, more specialized functions in microorganisms [1,2]. YCC substrates are generally coenzyme A [3] esters, in which the site for carboxylation is either the α-carbon position of saturated acid esters, such as acetyl-CoA and propionyl-CoA, or the γ-carbon position of α-β unsaturated acid esters. The reaction schemes of the YCCs all have two sequential steps in common: first, the biotin post-translationally attached to a biotin carboxyl carrier protein (BCCP) is carboxylated in an ATP-dependent manner by a biotin carboxylase (BC); second, carboxybiotin attached to the BCCP is transferred to the active site of the carboxyltransferase (CT), where the transfer of the carboxyl group from biotin to the acyl-CoA substrate takes place to extend the acyl-CoA by one carboxyl group. Depending on the organism, there are large variations in how these enzymatic activities are arranged, from single multifunctional, multidomain sequences to multisubunit oligomeric assemblies that have separate subunit activities.
Human YCCs have already been established as primary drug targets against diseases such as cancer and type II diabetes, and plastid YCCs have been targeted by commercialized herbicides for several decades [2]. The BC enzymatic activity of bacterial YCCs has also been successfully used for the identification of potent inhibitors against Gram-negative bacteria [4]. However, despite a significant number of attempts, there has been no breakthrough in using YCCs from mycobacteria as drug targets [5]. One of the reasons for this lack of progress is that there is no structural and functional repository of all mycobacterial YCC complexes, which would allow the development of specific approaches against individual members.
In actinomycetes, YCCs are generally organized into oligomeric assemblies composed of two different subunits: the α-subunit comprises the BC activity and the β-subunit is responsible for the CT activity. In Mycobacterium tuberculosis and related mycobacteria, there is an unusual redundancy in both genes, with three different YCC α-subunits and six different β-subunits being encoded, named AccA1 to AccA3 and AccD1 to AccD6, respectively. Whereas all three α-subunit sequences share more than 40% sequence identity in M. tuberculosis and M. smegmatis, the β-subunit CT sequences that determine the acyl-CoA substrate specificity are more divergent and can be categorized into three groups (S1 Table): the first comprises AccD1 and AccD2; the second includes AccD4, AccD5 and AccD6; and the third is AccD3, which shares less than 20% sequence identity with other β-subunit members.
The principle substrates for two of the six M. tuberculosis YCC β-subunits, AccD6 and AccD5, have been identified as acetyl-CoA and propionyl-CoA, respectively [6,7,8]. Acetyl-CoA YCC carboxylation generates malonyl-CoA, which is a main building block in fatty-acid biosynthesis. Propionyl-CoA carboxylation leads to methylmalonyl-CoA, which is essential for the synthesis of the methyl-branched lipids of the outer mycobacterial cell wall and capsule, and is an intermediate in the methylmalonyl pathway to catabolize propionyl-CoA [9,10,11]. The mycobacterial AccD4-containing YCC complex carboxylates long-chain acyl-CoA, which is required for the biosynthesis of unusual very long-chain fatty acids such as mycolic acid [12,13]. Based on these findings, YCC redundancy in mycobacteria was generally thought of being related to the complex requirements of lipid biosynthesis pathways [5,14].
Previous molecular YCC interaction studies have been limited to the AccA3 BC subunit, which revealed oligomeric complexes with oligomeric complexes with integer multiples of a 1 : 1 α:β subunit stochiometry with CT subunits AccD4, AccD5, and AccD6 [6,15,16]. A systematic analysis of the YCC interactions in mycobacteria is still required to establish an integrative view on the overall functional portfolio of YCC complexes. Here, we have addressed this gap along with the resulting mechanistic questions by combining genetic, proteomic, lipidomic, metabolomic, biochemical and structural approaches.
Our interaction screen revealed nine binary protein/protein interactions that generate at least four different mycobacterial YCC holo complexes. From these identified assemblies, the AccD1 (Rv2502c)-AccA1 (Rv2501c) YCC complex was selected for functional and structural studies. We demonstrate that this complex encodes a 3-methylcrotonyl-CoA carboxylase (MCC) involved in leucine catabolism. Electron micrographs of the AccD1-AccA1 holo complex reveal the general architecture of an MCC. In summary, our data show that mycobacterial YCC redundancy adds unexpected functional diversity in both α-carbon and γ-carbon acyl-CoA carboxylation biochemistry, reflected by distinct YCC structural arrangements.
Results
Protein/protein interaction map of YCC complexes
To rationalize our selection of mycobacterial YCC complexes for mechanistic studies, we first identified a complete set of protein/protein interactions of mycobacterial YCC genes. We used all nine known mycobacterial YCC BT (α-subunit) and CT (β-subunit) genes to perform pull-down experiments in M. smegmatis, which has both comparable YCC gene organization and high sequence identity to their respective loci in M. tuberculosis (S1 Table). We also included the YCC gene coding for a small ε-subunit that has been shown to be involved in the formation of some YCC holo complexes and to act as a potential regulator of activity [5,6,13,16]. All ten M. smegmatis genes were tagged with C-terminal enhanced Green Fluorescent Protein (eGFP). The C-terminus of each α-subunit was selected for tagging, as it is located in the BCCP domain, which is next to the flexible linker allowing the BCCP to move between the active sites of the complex. Any interference of the eGFP tag with the BC/CT protein/protein interactions is hence expected to be minimal. The C-terminus of each β-subunit was tagged, as the N-terminus is located at the BC-CT interface [17]. Interacting protein partners of enriched eGFP-fusion proteins were identified by liquid chromatography-coupled tandem mass spectrometry (LC-MS/MS) (S2 Table). As a control, we found strong self-assembly of all gene products tested, which is in line with various data on the involvement of both BC and CT subunit oligomerization in YCC holo complex formation [5].
In total, we found nine binary protein/protein interactions, of which five were confirmed by the reverse experiment that swapped bait and prey functions of interacting protein partners (Fig. 1, S2 Table). Such reciprocal interactions between a BC α-subunit and a CT β-subunit were identified for the protein pairs AccD1-AccA1, AccD4-AccA3, AccD5-AccA3. In addition, a reciprocal interaction between the AccA3 BC α-subunit and the small AccE ε-subunit was found. When using AccE as bait, interactions could also be identified with AccD4 and AccD5, confirming that the ε-subunit gene is involved in the formation of the AccD4-AccA3 and AccD5-AccA3 holo complexes [6,12]. Reciprocal interactions for the CT pair AccD4-AccD5 was also observed, confirming previous interaction data centered on the mycobacterial AccD4 subunit [12]. These findings are suggestive of a mixed ε-subunit-mediated AccD4/AccD5/AccA3 holo complex that unfortunately has not yet been isolated at sufficient purity for further functional and structural studies [6].
Fig. 1. Mycobacterial YCC protein/protein interactions. 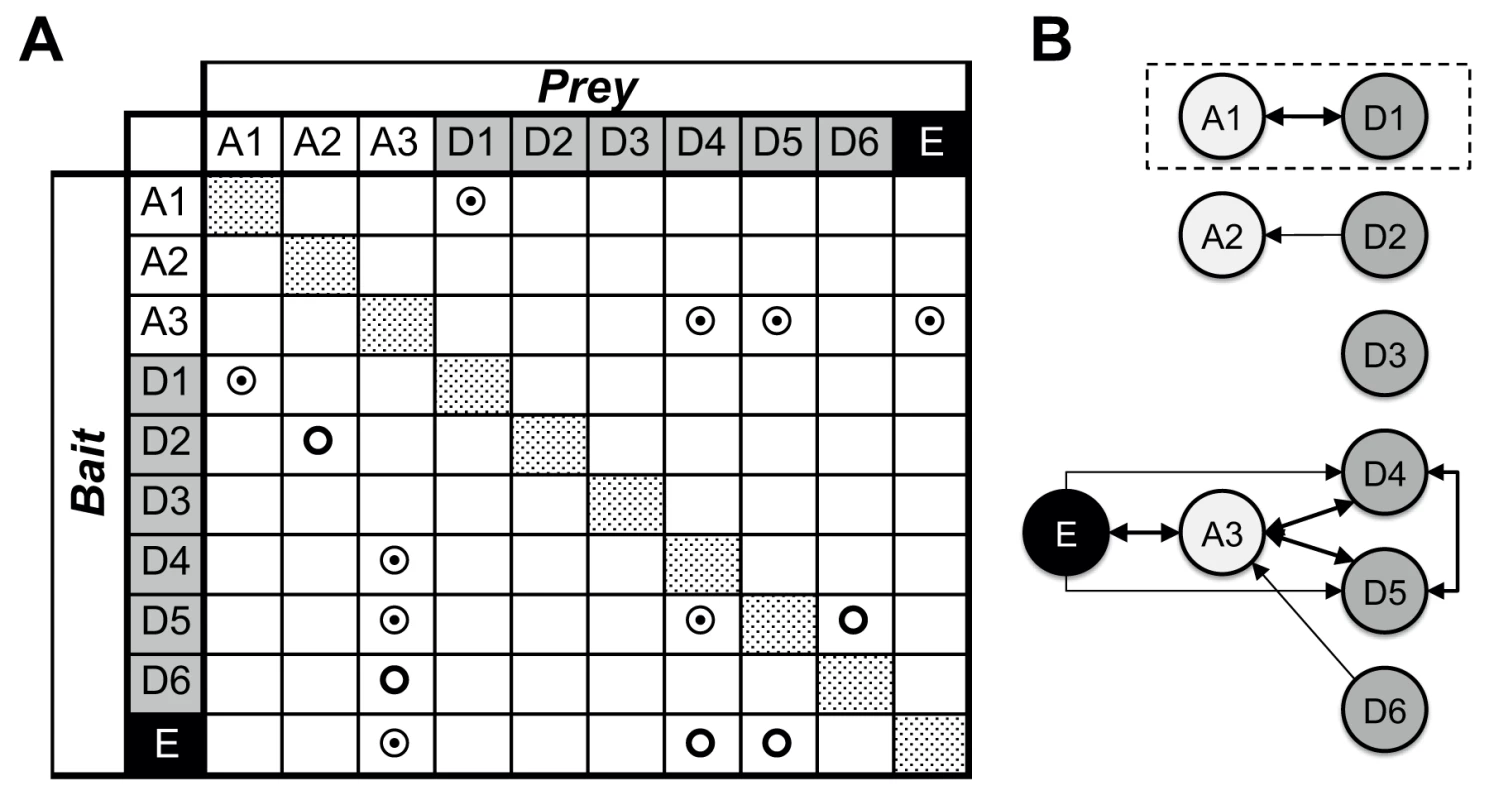
(A) Binary, hetero-dimeric protein/protein interactions are indicated by double circles when found reciprocally, by using reverse bait/prey protein pairs, and by simple circles when identified only in one of the two possible combinations. Homo-oligomeric assemblies were found for all proteins tested (dotted patterns). Mycobacterial YCC CT subunits AccD1 to AccD6 are denoted “D1” to “D6” (grey), BT subunits AccA1 to AccA3 are denoted “A1” to “A3” (no background), and the AccE ε-subunit is labeled “E” (black). (B) Identification of YCC holo complexes. Arrows point to the protein component used as prey; protein/protein assemblies, for which binary interactions were identified by the reverse use of bait/prey are indicated by thick double arrows. For further details, see S2 Table. Non-reciprocal interactions were found for the pairs AccD2-AccA2 and AccD6-AccA3, using AccA2 and AccD6 as bait, respectively. Despite a sequence identity of more than 50% between AccD6 and AccD5, no direct interaction between AccD6 and AccE could be detected (S1 Table). This suggests that the involvement of the AccE ε-subunit is limited to the formation of the AccD4-AccA3 and AccD5-AccA3 holo complexes and points to a different structural and, hence, most likely functional role for AccD6. AccD3 did not interact with any protein partner used in this analysis, raising doubts as to whether this protein indeed functions as a CT subunit in a YCC holo complex [18]. A separate functional role for AccD3 may also be inferred from the low sequence similarity of 22% or less with the other CT subunits, which share 28–52% similarity among each other (S1 Table).
Taking these pull-down and sequence comparison data together, our findings indicate that the YCCs of mycobacteria consist of at least two clusters of YCC holo complexes: two highly related complexes that do not require the ε-subunit (AccD1-AccA1, AccD2-AccA2) and another two complexes (AccD4-AccA3, AccD5-Acc3) that are consistently associated with the ε-subunit AccE. The involvement of the two remaining proteins (AccD3, AccD6) in YCC holo complexes is less clear, questioning their precise role in established YCC functions that require the presence of a BT carboxybiotin donor. By making use of the so established mycobacterial YCC interaction map, we subsequently selected the AccD1-AccA1 YCC holo complex for mechanistic studies as the formation of this complex had been verified reversibly and its functional role was unknown.
AccA1 and AccD1 form a high molecular weight protein/protein complex in vitro
To generate an efficient AccD1-AccA1 holo complex purification protocol we took advantage of the operon organization of the accD1 and accA1 genes, which are part of the citE-scoA operon (Rv2498c to Rv2504c in M. tuberculosis; MS4713 to MS4716 in M. smegmatis, respectively) (S1 Fig.). Putative ribosome-binding sites are only found in accD1 upstream of accA1 and hence we cloned the two M. tuberculosis genes as a single bicistronic sequence into an expression plasmid for heterologous co-expression in M. smegmatis to mimic their natural operon organization. A hexa-histidine tagged AccD1 version was used for the purification of the AccD1-AccA1 complex. When the cleared cell lysate was subjected to a Ni-affinity matrix, both AccD1 and AccA1 could be extracted demonstrating a direct interaction between the two subunits, and they had an apparent molecular mass of approximately 45 kDa and 65 kDa, respectively (Fig. 2A). The identity of the two proteins was verified by peptide mass fingerprinting.
Fig. 2. AccD1-AccA1 holo complex formation. 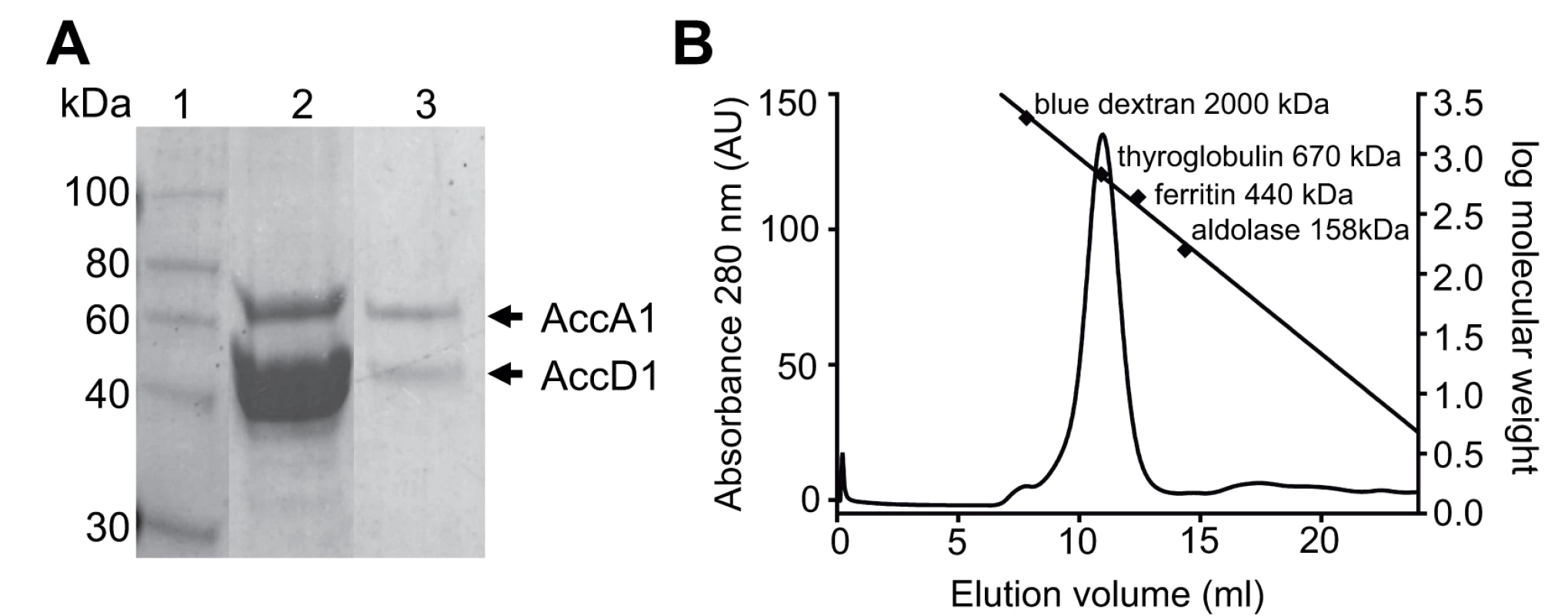
(A) SDS-PAGE showing the Ni-affinity eluate (lane 2), and after gel filtration (panel B) in lane 3; molecular markers are shown in Lane 1. (B) Size-exclusion chromatogram of purified AccD1-AccA1. The elution volume and molecular mass of the molecular weight calibration standards are indicated. The equation describing the linear regression line is f(x) = -0.16x + 4.6 and has a R2 = 0.99. Further evidence of a direct protein/protein interaction between AccD1 and AccA1 was obtained by loading the affinity-purified proteins onto a size-exclusion column. Both proteins co-eluted as a single symmetric peak, indicating that the protein complex has a defined, homogenous association state (Fig. 2B). According to calibration mass standards, the molecular mass of the protein complex is around 700 kDa, suggesting that there are either five or six AccD1-AccA1 heterodimers corresponding to a calculated molecular mass of 637 kDa and 764 kDa, respectively. The same co-purification protocol was applied to confirm the interaction and demonstrate complex formation of the AccD2-AccA2 complex, which we used as a control for subsequent functional studies (S2 Fig.).
AccD1-AccA1 is involved in the leucine degradation pathway and carboxylates 3-methylcrotonyl-CoA
To identify the function of the AccD1-AccA1 complex in mycobacteria, we generated knockout strains of M. smegmatis accD1-accA1 and their close paralogues accD2-accA2. Growth of both strains was comparable to wild type (wt) M. smegmatis in 7H9 medium supplemented with glycerol indicating that both complexes are dispensable under standard in vitro growth conditions (S3 Fig.).
We first tested a possible role for AccD1-AccA1 in lipid biosynthesis. When comparing the lipid composition in wt M. smegmatis, ΔaccD1-ΔaccA1 and ΔaccD2-ΔaccA2 strains we found no significant differences in the composition and concentration of either fatty acid methyl esters (FAMEs) analyzed by thin-layer chromatography (TLC) and gas chromatography—mass spectrometry (GC-MS) or mycolic acids analyzed by TLC and MALDI-TOF mass spectrometry (S3 Table).
We then searched for potential metabolic intermediates in de novo fatty acid and mycolic acid synthesis by [14C]acetate tracing of fatty acids and mycolic acids during the growth of wt M. smegmatis and the two mutant strains. TLC analysis of newly synthesized compounds indicated that fatty acids and all species of mycolic acids characteristic for M. smegmatis are present in all three strains. No additional radioactive lipid intermediates, such as meromycolic acid and alkyl-malonic acid, could be observed (S4 Fig.). The absence of alkyl-malonic acids was confirmed by GC-MS analysis of the cold total FAME pool [12]. Based on these findings from volatile and non-volatile lipid analysis, we concluded that neither AccD1-AccA1 nor AccD2-AccA2 complexes have a direct role in lipid metabolism.
To subsequently test for potential alternative metabolic functions of the AccD1-AccA1 complex, we performed metabolic profiling specific to CoA thioesters in wt M. smegmatis and the ΔaccD1-ΔaccA1 and ΔaccD2-ΔaccA2 deletion strains by liquid chromatography—tandem mass spectrometry (LC-MS/MS) [19]. In these CoA-specific metabolite profiles we identified a single additional peak in the ΔaccD1-ΔaccA1 extracts relative to the other two strains (Fig. 3A). Based on its mass and retention time, this peak was assigned to 3-methylcrotonyl-CoA (3-methylbut-2-enoyl-CoA) suggesting that the AccD1-AccA1 holo complex functions as a methylcrotonyl-CoA carboxylase [20].
Fig. 3. Identification and biochemical characterization of the AccD1-AccA1 substrate 3-methylcrotonyl-CoA. 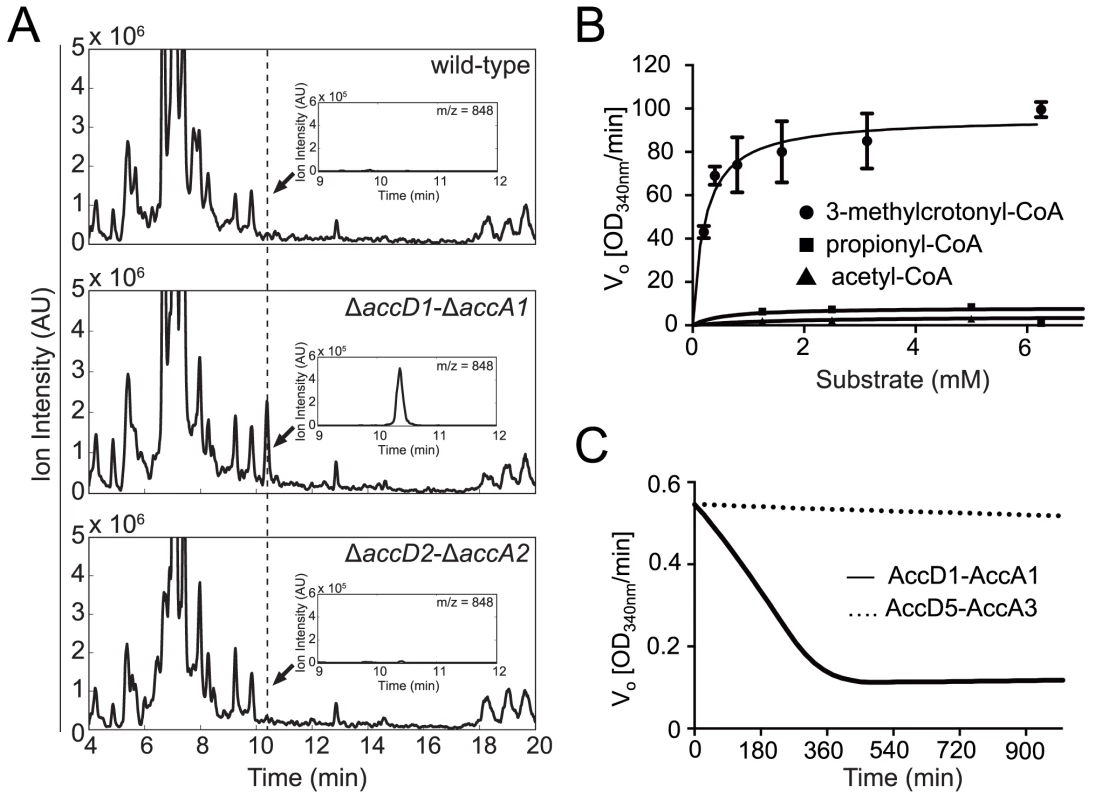
(A) Untargeted CoA-profiles of YCC knockout strains. Mass spectra of Co-A profiles of M. smegmatis wt and knockout strains. Insets show spectra at dashed line. The peak evident in the ΔaccD1-ΔaccA1 spectrum is that of 3-methylcrotonyl-CoA. (B) Steady-state kinetics of AccD1-AccA1 incubated with 3-methylcrotonyl-CoA, propionyl-CoA and acetyl-CoA. (C) Time course of the consumption of 3-methylcrotonyl-CoA by purified M. tuberculosis AccD1-AccA1 and AccD5-AccA3. To confirm this finding biochemically, we used the recombinant M. tuberculosis AccD1-AccA1 complex and 3-methylcrotonyl-CoA as the substrate in an in vitro enzymatic activity assay. As the enzyme assay measures carboxylation activity indirectly, we first determined the formation of the reaction product 3-methylglutaconyl-CoA demonstrating that the assay is useful in determining kinetic parameters (S5 Fig.). The KM of the AccD1-AccA1 complex for 3-methylcrotonyl-CoA turnover was determined as 0.22 ± 0.05 mM, and the kcat/Km as 303 ± 15 mM-1 s-1 (Fig. 3B). Only residual enzyme activity could be measured for propionyl-CoA and acetyl-CoA demonstrating the specificity of the enzyme for γ-carbon carboxylation. As an additional control, we also tested purified AccD5-AccA3 complex for 3-methylcrotonyl-CoA carboxylation but did not find any measurable activity, demonstrating that the two enzyme systems do not overlap (Fig. 3C). Our biochemical data are thus in agreement with the metabolomics findings and the growth characterization and therefore support a role for the AccD1-AccA1 complex as an MCC.
To further corroborate these findings, we quantified the metabolites of the canonical leucine-degradation pathway, in which 3-methylcrotonyl-CoA is one of the substrates (Fig. 4A, S6 Fig.). We investigated wt M. smegmatis and the two knockout strains under glycerol and leucine catabolizing growth conditions (Fig. 4B—C). When transferred to culture media containing leucine as the carbon source, 3-methylcrotonyl-CoA accumulated in the ΔaccD1-ΔaccA1 strain to levels approximately 100 times above those in wt and the ΔaccD2-ΔaccA2 strain (Fig. 4B, grey bars). The blockage of the metabolic pathway at the level of the MCC activity did not lead to the accumulation of isovaleryl-CoA upstream of 3-methylcrotonyl-CoA, as there is little difference between its levels in the wt and ΔaccD1-ΔaccA1 strains (Fig. 4B). This observation could be explained by alternative reactions consuming methylcrotonyl-CoA to form 3-hydroxyisovaleryl-CoA, the levels of which also increase in the ΔaccD1-ΔaccA1 strain (Fig. 4B). The accumulation of metabolites directly upstream in the pathway of an impaired enzymatic activity is a common phenomenon and, in general, is a valid parameter to localize enzymatic function [21][21]. Consistently, the levels of 3-methylglutaconyl-CoA and 3-hydroxy-3-methylglutaryl (HMG)-CoA, localized downstream of the MCC, were similar in all strains (Fig. 4C). Acetyl-CoA is the final product of leucine degradation and is involved in many different metabolic pathways. Its level in the ΔaccD1-ΔaccA1 strain cultivated in leucine-containing media is lower than that of the wt strain, which could be caused by impaired viability (Fig. 4C, grey bars). These metabolomics data thus further demonstrate that AccD1-AccA1 has MCC activity and is involved in leucine catabolism.
Fig. 4. Metabolite analysis upstream and downstream of 3-methylcrotonyl-CoA. 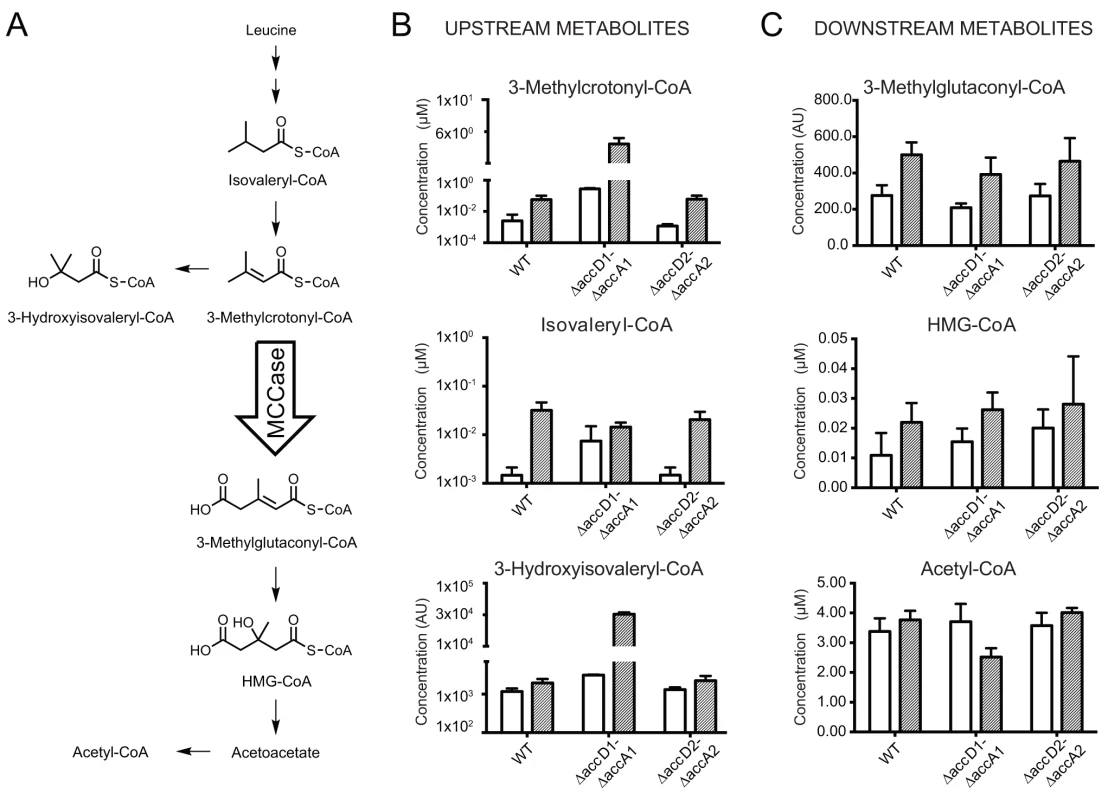
(A) The leucine degradation pathway in eubacteria, modified from pathway ko00280 of the KEGG database (www.kegg.jp/kegg-bin/show_pathway?map00280). (B) Metabolites upstream of the putative MCC activity. The metabolite 3-methylcrotonyl-CoA accumulates during leucine degradation in ΔaccD1-ΔaccA1 M. smegmatis. However, this does not lead to backlog accumulation of isovaleryl-CoA upstream of MCC catalysis during leucine degradation. An increased pool of 3-methylcrotonyl-CoA upon MCC blockage also triggers accumulates of hydroxyisovaleryl-CoA. (C) Metabolites downstream of the AccD1-AccA1 MCC activity: Both methylglutaconyl-CoA and HMG-CoA show generally higher levels during leucine-degrading conditions. The amounts are comparable between wt and ΔaccD1-ΔaccA1 strains, as one would expect for metabolites downstream of a blocked enzymatic function. For compounds that are commercially available, chemical standard metabolite concentrations in the metabolite extracts are given in μM. Otherwise, relative concentrations are indicated and the compounds are annotated based on their mass. Average concentrations and standard deviations were calculated from four independent bacterial cultures of each strain. M. smegmatis metabolizes leucine, but cannot actively grow on leucine as the sole carbon source (S7 Fig.). Therefore, we studied the growth behavior of wt M. smegmatis and the ΔaccD1-ΔaccA1 and ΔaccD2-ΔaccA2 strains in media containing isovalerate as the sole carbon source. Isovalerate is an intermediate of leucine degradation upstream of MCC, which leads to the accumulation of methylcrotonyl-CoA in ΔaccD1-ΔaccA1 comparable to leucine feeding. While growth of the ΔaccD2-ΔaccA2 strain was similar to wt, ΔaccD1-ΔaccA1 did not grow on isovalerate (Fig. 5A). These data directly illustrate the involvement of the AccD1-AccA1 complex in the catabolism of isovalerate and, hence, in the metabolic pathway of leucine degradation.
Fig. 5. Growth phenotype characterization and complementation of mycobacterial accD1 and accA1 genes. 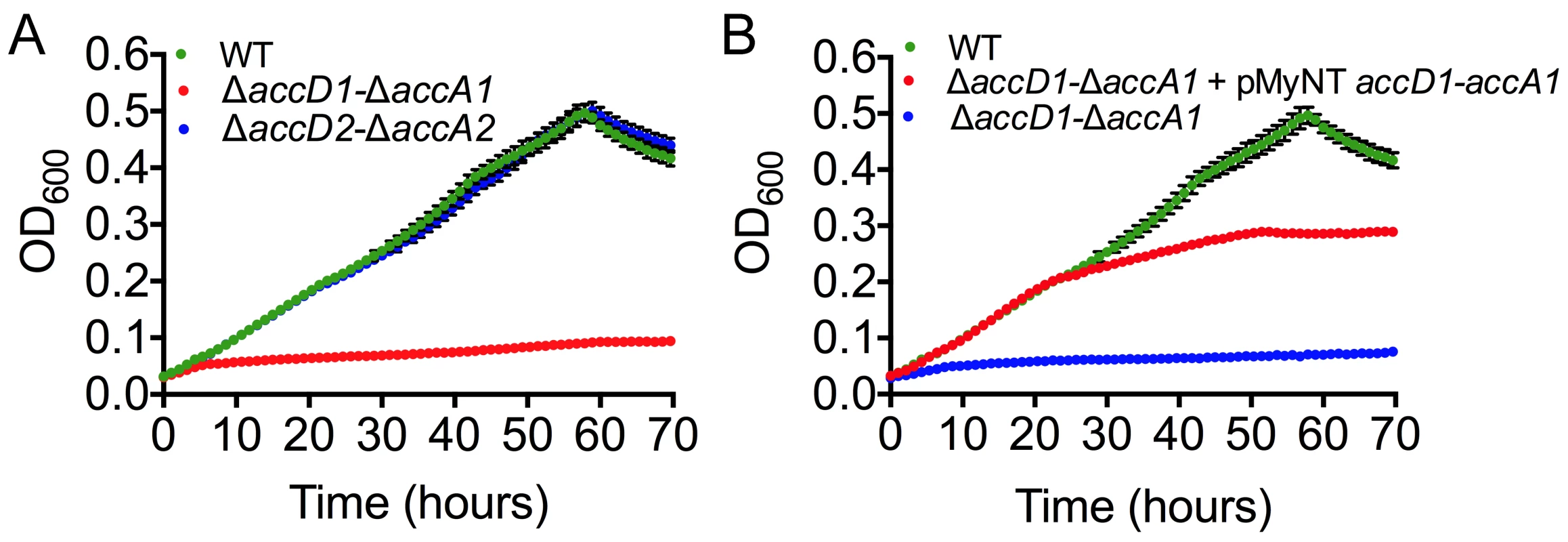
(A) Growth of M. smegmatis wt and knockout strains in minimal 7H9 media supplemented with isovalerate (IVAL). The wt is in green; ΔaccD1-ΔaccA1 is in red; ΔaccD2-ΔaccA2 (control) is in blue. Error bars are in black. (B) Rescue of the M. smegmatis growth phenotype observed in isovalerate-containing minimal media by complementing ΔaccD1-ΔaccA1 (blue line) with the M. tuberculosis homolog of AccD1-AccA1 (red line). The wt is in green. Average values for the culture density were calculated from four replicates. To demonstrate the specificity of the observed growth defect of ΔaccD1-ΔaccA1, we complemented the ΔaccD1-ΔaccA1 strain with the homologous M. tuberculosis accD1-accA1 genes. Growth of the ΔaccD1-ΔaccA1 strain on isovalerate was rescued by M. tuberculosis accD1-accA1 (Fig. 5B). These findings confirm that the MCC activity is specific to the AccD1-AccA1 complex and that its function is conserved in both M. smegmatis and M. tuberculosis.
AccD1-AccA1 forms a dodecameric 6 : 6 YCC holo complex
The YCC complex of Pseudomonas aeruginosa is currently the only structurally characterized prokaryotic complex with reported MCC substrate specificity [2]. We were hence eager to determine the structural features of the identified mycobacterial MCC and compare it to the reported quaternary assembly of the P. aeruginosa MCC. To this end, we carried out electron microscopy (EM) studies on the recombinant AccD1-AccA1 complex of M. tuberculosis. The homogeneity of the complex in electron micrographs confirms the existence of a stable assembly with a defined stoichiometry (Fig. 6A). From a total of 20 class averages (S8 Fig.), we selected four representative classes for further interpretation. A three-layered structure could be identified that is consistent with 32-point group symmetry (Fig. 6B). By using available information from related non-mycobacterial YCCs [2], we assigned the three tiers to be composed of a central MC β-subunit hexamer with internal 32 symmetry, which is flanked on either side by distal BC α-subunit trimers, leading to an overall α3–β6–α3 arrangement of the complete holo complex. The estimated length of the complex along the three-fold axis is 217 ± 10 Å, and the diameter of the inner cavity observed is 42 ± 5 Å. The width of the central β-subunit tier is 117 ± 7 Å, and that of the two peripheral α-subunit layers is 83 ± 9 Å (Fig. 6C, Table 1). Although the density distribution in particle top views suggests that the complex encloses a cavity along the three-fold axis, different top views observed in our class averages (S8 Fig.) suggest conformational variability at the particle ends. As discussed in more detail below, these structural parameters point to a distinct MCC quaternary assembly type that is different from generic YCC holo complexes that catalyze α-carbon carboxylation reactions.
Tab. 1. Dimensions of AccD1-AccA1 and YCC complexes with known high-resolution structures. 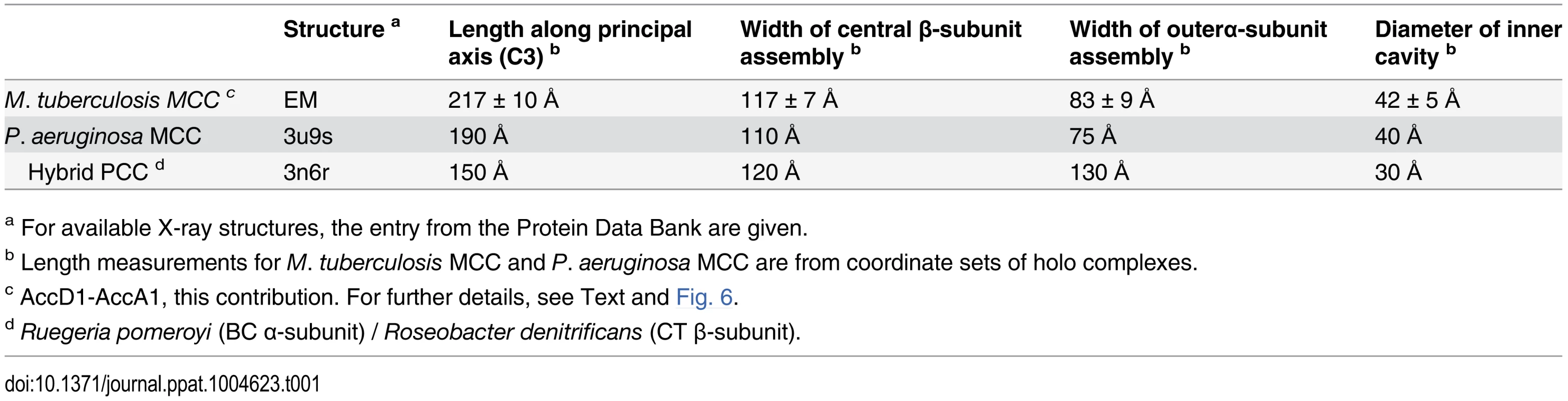
a For available X-ray structures, the entry from the Protein Data Bank are given. Fig. 6. Architecture of the Mycobacterium tuberculosis AccD1-AccA1 complex. 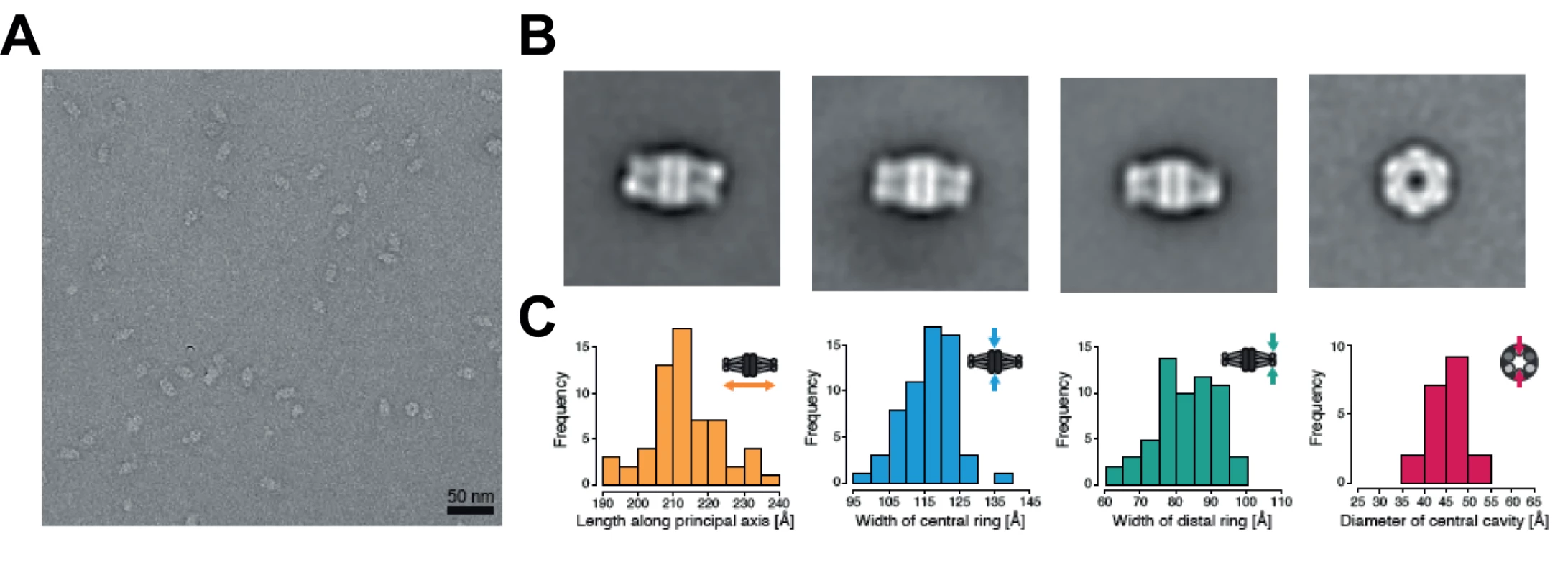
(A) Electron micrograph of negatively stained AccD1-AccA1 complex. (B) Selected class averages representing the most abundant views of the AccD1-AccA1 complex. (C) Histograms of particle dimensions. The individual dimensions are schematically indicated in relation to the particle appearance in the class averages. Discussion
To unravel functionality in the highly redundant YCC complexes of M. tuberculosis, we performed a systematic proteomics analysis by which we identified and validated nine binary protein/protein interactions and four acyl-CoA carboxylase holo complexes. Based on these data, we selected the YCC complex AccD1-AccA1 of hitherto unknown function for further mechanistic and functional characterization. We discovered by a combined genetics, metabolomics and structure-oriented approach that this complex is involved in leucine catabolism. It functions as an MCC catalyzing the γ-carboxylation of an unsaturated fatty acid CoA ester rather than the α-carboxylation of saturated fatty acid esters that are catalyzed by various other YCCs. Our and previous data suggest that these two types of reactions are associated with distinct structural requirements. To the best our knowledge, this is the first characterization of a mycobacterial MCC at the molecular level.
The importance of leucine biosynthesis in mycobacteria has been demonstrated previously by generating an M. tuberculosis leucine auxotroph with an attenuated infection profile in vivo [22]. However, very little has been published about the specific role of branched amino-acid catabolism in mycobacteria. Hence, we became interested in whether the genes accD1 and accA1 are embedded in a genetic environment that would further support an involvement in this process. In closely related mycobacteria such as M. tuberculosis, M. avium and M. smegmatis, the organization of the accD1- and accA1-containing operon covering coded genes Rv2498c to Rv2504c (citE-scoA operon) is conserved, except for Rv2503c to Rv2505c (S1 Fig.). Interestingly, this pattern of conservation extends into the neighboring pdhABC operon that includes genes Rv2495c to Rv2497c, which encode a lipoamide dehydrogenase-dependent keto acid dehydrogenase complex [23]. This complex acts upstream in branched amino-acid catabolism and one of its key functions is thought to be similar to that associated with genes from the citE-scoA operon, namely to avoid accumulation of toxic levels of branched-chain amino keto acids and to provide an alternative pool for acetyl-CoA biosynthesis. Mechanistically, lipoylation of the keto acid dehydrogenase complex by lipoamide dehydrogenase (LPD) is analogous to the requirement for the biotinylation of YCC enzymes.
Remarkably, in addition to the conserved operon organization, the expression of most genes (Rv2495c to Rv2501c) of the citE-scoA operon and the pdhABC operon are strongly correlated (for further details see Operon Correlation Browser (www.broadinstitute.org/annotation/tbdb/operon/), suggesting that the effects observed are coupled. This observation has been confirmed recently by data from M. smegmatis showing that the two operons form one regulon that is under the control of the TetR-like repressor bkaR, which is encoded next to the citE-scoA operon by Rv2506 in M. tuberculosis and MSMEG_4718 in M. smegmatis [24]. Data on the pdhABC operon show that the genes it contains crucially contribute to mycobacterial virulence [23] and it will be interesting to unravel whether the argument can also be extended to the genes involved in the citE-scoA (Rv2498c-Rv2505c) operon. Taking our data and previously published findings together, it becomes evident that the MCC-encoding genes accD1 and accA1 are embedded into a genetic environment that feeds amino-acid degradation into the production of metabolites that ultimately provide an alternative pool for acetyl-CoA. At this point, however, we cannot exclude that the physiological purpose of such an alternative pool of acetyl-CoA in M. smegmatis and M. tuberculosis may serve different purposes. Therefore, the precise physiological role of leucine catabolism in M. tuberculosis still remains to be elucidated.
Previous structural data on several YCCs have shown that, despite the different chemical requirements for α-carboxylation and γ-carboxylation, the conformation of the CT active site is conserved and deeply buried in a symmetric CT-CT interface [25,26,27,28]. However, recent structures of two holo propionyl-CoA carboxylase (PCC) and MCC complexes revealed that the overall conformations of the dodecameric complexes are substantially different despite significant global sequence similarities [2,17,29]. The observed differences are because of a swap in the arrangement of the N-terminal and C-terminal domains within the individual CT β-subunits, leading to relocation of the BCCP domain. The overall dimension estimates of the two arrangements are also substantially different and can be distinguished even at low resolution (Table 1). Mechanistically, the reasons for the divergent arrangements, however, are not understood [2].
The dodecameric arrangement of α - and β-subunits in YCCs has been observed in the AccD1-AccA1 and the related MCC of P. aeruginosa, as well as the chimeric PCC [17,29]. The AccD5-AccA3 complex is presumed to be a dodecamer based on its size estimates from gel filtration data and the β-subunit structure on its own is a hexamer similar to those -subunits in YCC dodecamers [6,25,28]. It would therefore seem that a dodecameric arrangement is common to most bacterial YCCs. However, the M. tuberculosis AccD6 has a dimeric quaternary structure [30]. The hexameric β-subunits are trimers of dimers, AccD6 being structurally equivalent to one such dimer. A dodecameric arrangement of subunits can therefore not be assumed for all YCC complexes. Interestingly, a new structure of an unusual bacterial single-chain YCC assembles into a homo-hexameric assembly with yet another unrelated overall arrangement [31], indicating that possible combinations for different arrangements may be even more diverse than previously anticipated.
Comparison of the CT β-subunit sequence of the M. tuberculosis AccD1-AccA1 complex with the sequences used for structure determination of the two known YCC holo complexes reveals 66% sequence identity with the P. aeruginosa CT subunit of the MCC complex and 33% sequence identity with the Roseobacter denitrificans CT subunit of the PCC complex. In structural terms, the set of M. tuberculosis AccD1-AccA1 class averages in comparison to simulated projections of the MCC (PDB ID 3u9s) and PCC (PDB ID 3n6r) complexes illustrates that the conformation of AccD1-AccA1 closely resembles that of the P. aeruginosa MCC complex (Fig. 6, S8 Fig.). All molecular dimensions inferred from individual AccD1-AccA1 particles in our electron micrographs are compatible with structural parameters derived from the MCC complex (PDB ID 3u9s) (Table 1). By contrast, the dimension estimates from the structure of the only available PCC complex (PDB ID 3n6r) differ significantly. Thus, our functional findings—identifying AccD1-AccA1 from mycobacteria as an MCC—are supported at the level of structure and sequence.
At present, there is no atomic structure of an MCC CT domain in the presence of 3-methylcrotonyl-CoA. However, as the structure of the known MCC holo complex contains CoA in the active site of the CT domain, it was possible to approximately localize the methylcrotonyl-CoA-binding pocket, and this points to the crucial importance of Phe191 in the P. aeruginosa MCC sequence as one of the key substrate specificity determinants (Fig. 7A) [17]. A sequence alignment with the M. tuberculosis AccD1 sequence reveals complete conservation of a 16-residue stretch “RQDEVFPDREHFGRIF” flanking the equivalent Phe157 and Phe191 in the M. tuberculosis and P. aeruginosa sequences, respectively (Fig. 7B, S9 Fig.). By contrast, this sequence segment is highly divergent in all other YCC CT subunit sequences, implying functional divergence of the AccD1-AccA1 complex. Interestingly, this also applies to the CT AccD2 subunit, which of all the other M. tuberculosis CT sequences is most closely related in overall sequence to AccD1 (Fig. 7B). Based on these and our functional metabolomics data, we predict that the mycobacterial AccD2-AccA2 complex may have the same overall architecture as the AccD1-AccA1 complex, but differs in YCC substrate specificity (Fig. 7C–D), leaving it an open question how this YCC is involved in mycobacterial metabolism.
Fig. 7. Structural conservation of the M. tuberculosis AccD1 CT active site. 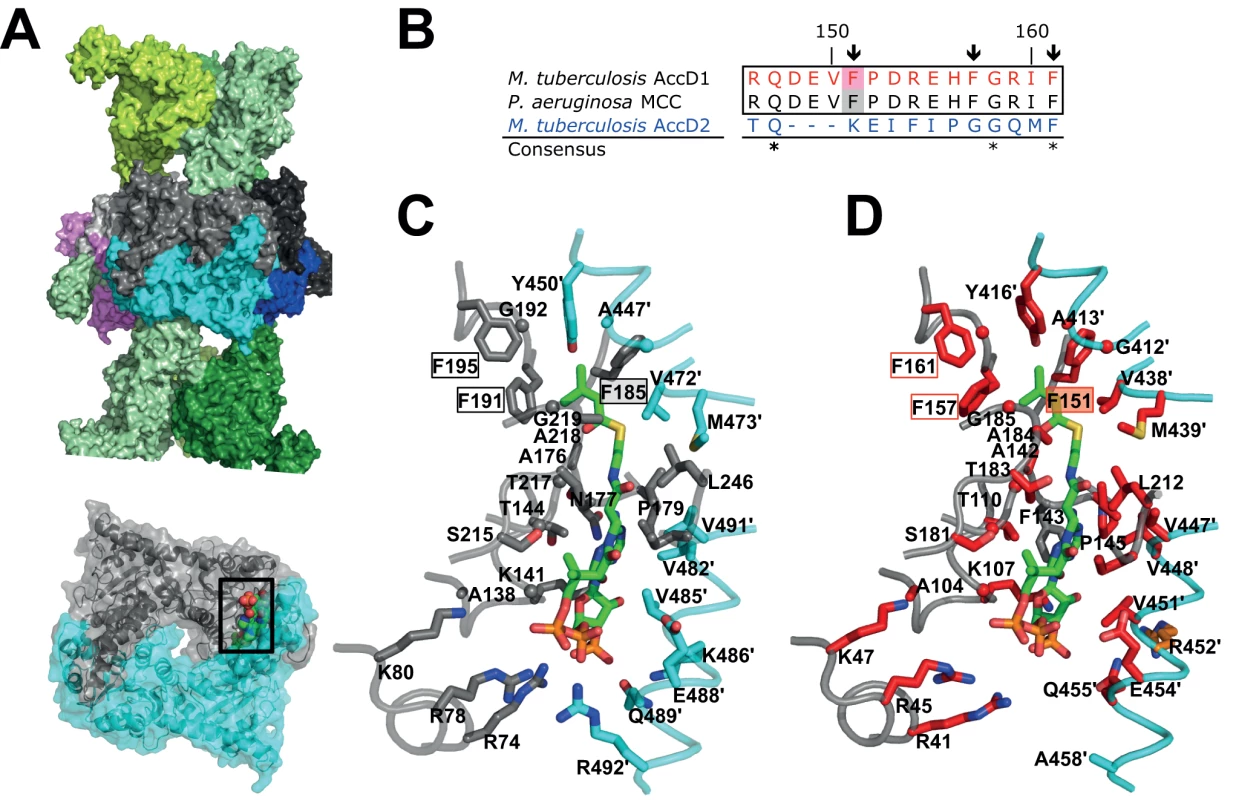
(A) Overall MCC quaternary arrangement, as determined previously for MCC from P. aeruginosa [17]. The BT subunits of the two distal trimeric tiers are shown in different green shadings. The central hexameric CT subunit assembly is shown in different colors. One dimeric CT subunit assembly in grey/cyan has been depicted in the lower panel and the CT active site formed within the dimeric interface is indicated by sphere presentation of the modeled substrate 3-methylcrotonyl-CoA with atom-specific colors (carbon, green) [17]. (B) Sequence alignment of the predicted M. tuberculosis AccD1 3-methylcrotonyl-CoA substrate-binding segment with the respective sequence segments of the P. aeruginosa MCC and M. tuberculosis AccD2 of unknown function. The residues numbers indicated refer to the AccD1 sequence. Conserved residue positions are indicated with asterisks. Residue positions that are conserved in the two upper confirmed MCC sequences (Phe151, Phe158, Phe161 in M. tuberculosis AccD1) but not in the AccD2 sequence are indicated by arrows. The complete sequence alignment is shown in S9 Fig. (C) Residues that interact with 3-methylcrotonyl-CoA in the P. aeruginosa MCC model [17], as determined with PISA [47], are shown in stick presentation and are labeled. Color codes are as in panel A. (D) Homology model of the M. tuberculosis AccD1 CT active site. Residues that are invariant in an alignment of the CT sequences from P. aeruginosa and M. tuberculosis (S9 Fig.) are in red, demonstrating a very high level of conservation of the AccD1 active site; conserved residues are in orange, non-conserved residues are in grey. Finally, we tried to devise possible structural and functional rationales to explain why the M. tuberculosis AccD1-AccA1 holo complex fits the conformation of the P. aeruginosa MCC rather than that of the only available PCC structure known to date. Attempts in modeling the AccD1 and AccA1 sequences onto the two available MCC and PCC holo complex structures, however, did not reveal any substantial energetic differences between the resulting models that could explain why one conformation would be preferred over the other. Therefore, experimental high-resolution structural data of holo YCC complexes will be essential to clarify the role of this helix and other possible factors in defining the overall arrangement of these enzymes.
Materials and Methods
Co-immunoprecipitation and mass spectrometry
All YCC genes from M. smegmatis (S1 Table) were amplified and cloned into a pKW08 mycobacterial tetracycline-inducible vector [32,33]. The amplicons were cloned upstream of the Tobacco Etch Virus (TEF) cleavage site, followed by an Enhanced Green Fluorescent Protein (eGFP) tag, and downstream of the TetR08 promoter. Cloning, expression, and a single-step protein purification protocol followed by LC-MS/MS analysis was applied as described [34]. Proteins were identified by searching the data against the M. smegmatis protein database (http://cmr.jcvi.org/tigr-scripts/CMR/CmrHomePage.cgi). The database was modified to add randomized sequences of all entries as a control of false-positive identifications during analysis. Fragmentation spectra were searched using the Andromeda search engine integrated into the MaxQuant (v1.3.0.5) platform. Computational data analysis was performed using the Perseus tool (v1.3.0.4, Cox J., Max Planck, 2012). Contaminants and random protein identification were excluded. Proteins that were identified by less than two peptides were excluded from the result list. The proteomics data have been submitted to the Proteomicsdb database (https://www.proteomicsdb.org/proteomicsdb/#projects/4170).
Creation of knockout strains
M. smegmatis YCC genes accD1, accA1, accD2 and accA2 were deleted by recombineering [35], creating the double-knockout strains ΔaccD1- ΔaccA1 and ΔaccD2- ΔaccA2. As accD1-accA1 and accD2-accA2 are each encoded in an operon, both sets of genes could be deleted using a single recombination event. To produce the double knockouts a linear target substrate had to be created. Two 500 bp or 1000 bp fragments, homologous to the up - and downstream regions of the relevant genes, were amplified by PCR using specific primer pairs and subsequently digested with specific restriction endonucleases (S4 Table). The amplicons were ligated into pYUB854 digested with KpnI-XbaI or AflII-AvrI (for the fragment of the upstream region, depending on the type of restriction sites present) and HindIII-SpeI (for the fragment of the downstream region) [36]. In this manner, constructs pEN30 and pEN43 were created (S5 Table). To obtain the linear target substrate, pEN30 was digested with KpnI and SpeI, and pEN43 with AflII and SpeI. The linear DNAs were subsequently transformed into M. smegmatis mc2155 electrocompetent cells expressing the recombinase genes on pJV53 [35] generating hygromycin-resistant recombinants. The hygromycin-resistance cassette was removed using δγ-resolvase expressed on pGH542 generating the unmarked deletion strains [37].
Genomic DNA of potential mutants was isolated [35] and Southern hybridization was used to confirm the absence of the particular gene (S10 Fig.). Genomic DNA (5 μg) for Southern blot analysis was digested with the appropriate enzymes, separated on a 0.9% agarose gel and transferred to a positively charged nylon membrane (Roche). DNA-probe labeling, hybridization and detection were performed using the digoxigenin high prime DNA labeling and detection starter kit 1 as described by the manufacturer (Roche).
Lipid analysis of M. smegmatis strains
Cells from M. smegmatis mc2 155 and the M. smegmatis knockout strains ΔaccD1-ΔaccA1 and ΔaccD2-ΔaccA2 were grown for 24h on 7H9 medium supplemented with 0.2% glycerol and 5% OADC at 37°C. Wet cell pellets were treated with mixtures of CHCl3/CH3OH (1 : 2, 1 : 1, 2 : 1) to yield total lipid extracts (TLE) that were pooled and dried under vacuum. Fatty acids and mycolic acids were isolated by treating the bacterial cells or TLE with a 5% KOH solution in methanol/toluene (8 : 2 v/v) under reflux for 8 hours. After acidification, fatty acids were extracted with diethyl ether and methylated with an ethereal solution of diazomethane. The fatty acid methyl ester (FAME) and mycolic acid methyl ester patterns of the strains were determined by analytical thin-layer chromatography (TLC) on Silica Gel 60 (Macherey-Nagel). For fatty acid and mycolic acid labeling during bacterial growth, cultures of wt and the knockout strains were grown for 24 hours and then centrifuged for 15 minutes at 3000 x g at 4°C. The bacterial pellets were suspended in fresh 7H9/glycerol medium and incubated for 30 min at 37°C before addition of 1 μCi/μl of [1–14C] acetic acid (56.6 mCi/mmole, Perkin Elmer). After 1 hour incubation with shaking (200 rpm) the bacteria were harvested by centrifugation and the cell pellet was saponified and treated as above. Radio-labeled methyl esters were resolved in CH2Cl2 as running solvent at room temperature and visualized by exposing the TLC to Fujifilm imaging plate prior to phosphorImager detection (Typhoon Trio GE Healthcare).
Metabolic sampling and untargeted CoA-profiling
Bacterial strains were pre-cultured overnight in modified 7H9 medium containing 0.2% glycerol and 0.025% tyloxapol at 37°C, 300 rpm. We defined modified 7H9 media as media containing all constituents of 7H9 with the exception of glutamic acid, as this could serve as a C-source and could affect growth data collected in selective media to which we have added a specific C-source. Preculture was inoculated into 25 mls of the same culture media to an OD600 of 0.015 and incubated at 37°C under constant shaking at 300 rpm. Metabolic samples were collected during mid-exponential growth phase (OD600 = 0.6 to 1.0). Sampling of metabolites followed the same principle as previously described [19]. In brief, a culture volume corresponding to a biomass of 3 ml bacterial culture at an OD600 of 1.0 was rapidly harvested through 0.45 μm filters by vacuum filtration and washed with 75 mM ammonium carbonate buffer (pH 6.6). The entire harvesting and washing procedure took less than 20 s. Filters were immediately submerged in 60% ethanol at 78°C for 2 min and then shock-frozen in liquid nitrogen. Samples were dried at 30°C in a SpeedVac equipped with a cooling trap at -85°C. The dried extracts were stored at -80°C until further analysis by LC-MS/MS. The samples were re-suspended in 100 μl water and 10 μl were injected for LC-MS/MS analysis. Untargeted profiling of the CoA thioesters was performed as described previously [19].
Culture media shift experiments
Bacterial strains were cultured and sampled for metabolic measurements during mid-exponential growth phase as described above. Bacteria of the remaining cultures were centrifuged at 3000 x g for 2 min, cells were washed with 15 ml of modified 7H9 medium supplemented with 0.025% tyloxapol and after centrifugation re-suspended in 15 ml of modified 7H9 supplemented with 0.025% tyloxapol and containing 0.4% leucine. Metabolic samples were collected after 1 hour of additional incubation at 37°C under constant shaking at 300 rpm.
Quantification of CoA thioesters by SRM assays
The same chromatographic separation protocol that was used for the untargeted profiling of CoA thioesters was applied for the quantification of CoA thioesters by selected reaction monitoring (SRM) using LC-MS/MS. Ion pairing-reverse phase chromatography at ultrahigh pressure using a Waters Acquity UPLC (Waters Corporation, Milford, MA, United States) with a Waters Acquity HSS T3 column with dimensions 150 mm × 2.1 mm ×1.8 μm (Waters Corporation, Milford, MA, United States) at 40°C was used. A gradient of mobile phases A (10 mM tributylamine, 15 mM acetic acid at pH 5.0, 5% (v/v) methanol) and B (2 - propanol) was applied. This yielded in the following settings: initial conditions: 0% B, 0.4 mL/min; 0.5 min: 0% B, 0.4 mL/min; 1.5 min: 12% B, 0.4 mL/min; 10 min: 27.5% B, 0.25 mL/min; 20 min: 90% B, 0.15 mL/min; 25 min: 90% B, 0.15 mL/min; 28 min: 0% B; 0.15 mL/min; 35 min: 0% B; 0.4 mL/min. The fragment specific for coenzyme A with a m/z = 408 at negative operation mode of the mass spectrometer was used for the SRM assays on a Thermo TSQ Quantum Ultra triple quadrupole instrument (Thermo Fisher Scientific, Waltham, MA, United States). The other SRM parameters were set to 140 V and 39 (arbitrary unit) for the tube lens and the collision energy, respectively. Dried metabolic samples were re-suspended in 100 μl water and 10 μl were injected for LC-MS/MS analysis. Concentrations of CoA thioesters were quantified using dilutions of analytical standards between 14 and 0.45 μM, if commercially available. Estimated purity of the compounds according to the manufacturer was taken into account for the calculations.
M. smegmatis growth assays
Wt and knockout strains of M. smegmatis were pre-cultured from frozen glycerol stocks in 7H9 ADC culture medium to mid-exponential growth phase (37°C, 300 rpm). The bacteria were centrifuged at 3000 x g for 2 min, washed with one culture volume (3 ml) of modified 7H9 medium, centrifuged again and re-suspended in culture medium containing the carbon source to be tested (3 ml). The bacteria were incubated at 37°C under constant shaking (300 rpm) for another 24 h. These cultures were used to inoculate a Nunc 48-well plate (Nunclon delta SI, 500 μl per well) with the according culture medium. A plate reader (Tecan Infinite M200) was used to incubate the plate at 37°C under constant orbital shaking and to monitor the culture density by measuring the OD600 every 10 min (25 flashes, 9 nm bandwidth). Average values for the culture density were calculated from four replicates.
The same procedure was applied for the complementation of the knockout strain. However, in addition 0.001% acetamide was added to induce protein expression for the second pre-culture and the culturing in 48-well plates. The creation of the complementation plasmid is described in a subsequent section.
Creation of expression and complementation construct
The sequence of M. tuberculosis AccD1 (Rv2502c) and AccA1 (Rv2501c) are encoded in an operon, with only a 4 base-pair intergenic region whereas M. tuberculosis AccD2 (RV0974c) and AccA2 (Rv0973c) are encoded in one operon lacking an intergenic region S1 Fig.). Co-expression of proteins encoded in operons has been a successful strategy for the production of mycobacterial protein complexes [38] and was used to express these YCC complexes. The entire coding sequence of accD1 and accA1, including the intergenic region, was amplified using primers MTE01 and MTE02 from M. tuberculosis H37Rv genomic DNA (S4 Table). The primers MTE03 and MTE04 were used to amplify the entire accD2-accA2 region. Both the amplicon of accD1-accA1 and of accD2-accA2 were directionally cloned into the mycobacterial expression vector pMyNT, between the restriction enzyme sites NcoI and HindIII, immediately downstream of a histidine tag (S5 Table). Only the AccD protein was His-tagged. The constructs were sequenced to verify insertion of the amplicon. The construct pMyNT D1A1, encoding accD1-accA1, was used in the M. smegmatis complementation experiment and for production of recombinant AccD1-AccA1 for biochemical characterization. The construct pMyNT D2A2, encoding accD2-accA2, was used to produce recombinant AccD2-AccA2.
Protein production and purification
Constructs were transformed into M. smegmatis mc2155 groEL1ΔC to improve protein yield during purification [39]. Transformation was performed as described [38]. AccD1-AccA1 and AccD2-AccA2 were produced as follows. Transformants were cultured in 7H9 medium supplemented with 0.2% glucose, 0.2% glycerol, 0.05% Tween 80, and 0.1 nM hygromycin. A starter culture of 50 ml volume was grown for 2 days at 37°C. One percent of this starter served as an inoculum for a larger 500 ml culture, which was incubated under the same conditions. Protein expression was induced with acetamide, at a final concentration of 30 mM, when cultures had reached an optical density of 3.5 measured at 600 nm. The cultures were incubated for a further 20 hours. Thereafter the cells were collected by centrifugation at 3000 x g and 4°C for 60 min. Culture pellets were resuspended in 25 ml buffer containing 50 mM Tris-HCl pH 7.2, 300 mM NaCl and complete protease inhibitor cocktail (Serva) and stored at -20°C until needed. The production of the AccD5-AccA3 complex has been reported elsewhere [39].
Cells were lysed by sonication, following the addition of 1 mg/ml DNase I (Serva) was added. Cells were sonicated using a Bandelin Sonoplus HD3200 sonicator set to pulse with an on-off cycle of 0.3 sec—0.7 sec and an amplitude of 45% for a total of 3 min. Sonication was repeated 3 times with a 3 min period between sonication events to prevent the sample from overheating. The sample was cooled on ice throughout. The lysate was centrifuged at 38,700 x g for 1 hour. The resultant cleared lysate was then filtered through a 0.22 μm filter and the filtrate loaded onto a 5 ml nickel-nitrilotriacetic acid affinity column (Qiagen). The affinity column was washed with 4 column volumes of 50 mM Tris-HCl pH 7.2, 100 mM NaCl, 20 mM imidazole and protein eluted with 50 mM Tris-HCl pH 7.2, 100 mM NaCl, 250 mM imidazole. Affinity eluates containing the protein were pooled, concentrated and loaded onto a Superose 6 10/300 GL size-exclusion column (GE Healthcare), which had been pre-equilibrated with 50 mM Tris-HCl pH 7.2, 100 mM NaCl, 1 mM dithiothreitol. Protein fractions from the size-exclusion column were collected and analyzed by electrophoresis using gels that had a 10% polyacrylamide separating gel. The identity of the proteins was confirmed by peptide-mass fingerprinting performed by the EMBL Proteomics Core Facility, Heidelberg, Germany. A sample of pure protein complex was re-loaded onto the analytical Superose column to estimate its molecular size. A high molecular weight standards kit was used to calibrate the column (Amersham).
Enzyme assay
The assay we used to measure CT activity is a spectrophotometric-coupled enzyme assay [40]. The ATP-dependent carboxylation of acyl-CoA substrates by the YCC is coupled to the ADP-dependent dephosphorylation of phosphoenolpyruvate and the subsequent NADH reduction of the lactate product, catalyzed by pyruvate kinase and lactate dehydrogenase, respectively. The latter reaction results in the consumption of NADH, which is monitored at 340 nm. Spectrophotometric data were recorded using a PowerWaveX Select spectrophotometer (Bio-Tek Instruments) and Greiner Bio-one UV-transparent, flat-bottom 96-well plates. Data were recorded using KC4 Kineticalc version 3.01 (Bio-Tek Instruments) and analyzed using GraphPad Prism 5 version 5.03 (Graphpad Software Inc.). The reaction mixture contained 7 U pyruvate kinase, 10 U lactate dehydrogenase, 0.5 mM phosphoenolpyruvate, 0.2 mM NADH, 5 mM MgCl2, 100 mM K2HPO4 pH 7.6, 3 mM ATP, 50 mM NaHCO3, 0.3 mg/ml bovine serum albumin and varying concentrations of 3-methylcrotonyl-CoA substrate between 1.5 mM and 25 mM. Both the reaction mixture and protein samples were incubated at 30°C for 2 min prior to the start of the assay. The reaction was started upon addition of purified protein to the reaction mixture and consumption of NADH then measured every 30 sec for 1 hour at 30°C. The enzyme concentration-dependent carboxylation of the different substrates had been previously assessed and the concentration of enzyme at which first-order kinetics are maintained was 3 μM. This concentration of AccD1-AccA1 was used in all experiments.
The experiment comparing AccD1-AccA1 and AccD5-AccA3 activity used the same reaction as described above, with the exception that the protein concentration was fixed at 0.6 μM and the concentration of 3-methylcrotonyl-CoA was 25 mM. Carboxylation of the substrate was followed for 15 min.
Electron microscopy
Purified AccD1-AccA1 complex at a concentration of approximately 0.1 mg/ml in 50 mM HEPES (pH 7.2), 100 mM NaCl, 1 mM DTT, 2 mM MgCl2 was applied to the carbon-side of a carbon-mica interface before it was transferred to an EM grid and stained with 2% uranyl acetate. Images were recorded under low-dose conditions at ambient temperature using a Philips CM200 FEG microscope operated at 200 kV. Images were acquired at a nominal 27,500× magnification resulting in a pixel size of 6.2 Å at the specimen level. All micrographs were recorded on a bottom-mounted GATAN 794 1 x 1K CCD Camera (GATAN Inc., Pleasanton, CA).
Semi-automatic particle selection was performed using EMAN2’s e2boxer [41]. A total of 5,837 particles were selected from 100 1K x 1K CCD images and windowed into 80 × 80 pixel particles that were subsequently low-pass filtered at 25 Å resolution and normalized. Initial translational alignment was performed with respect to the rotationally averaged total sum of the particle images. An initial reference-free classification step with the multivariate statistical analysis (MSA) procedure [42] was followed by five iterative rounds of multi-reference alignment to improve translational and rotational alignment of the particle images and subsequent MSA classification, which resulted in 20 stable classes.
Simulated projections of Pseudomonas aeruginosa MCC (PDB ID 3u9s) were generated using the SPIDER processing package [43]. To obtain a uniform distribution of orientations, projections of a simulated map at 25 Å resolution were generated in 5° angular increments. For visual comparison we aligned the individual projections to the AccD1-AccA1 class averages.
Homology modeling
Homology modeling of the AccD1-AccA1 complex was performed with MODELLER-9v7 [44]. Based on multiple sequence alignment of M. tuberculosis AccA1 and AccD1 to the α - and β-subunits of PaMCC (PDB ID 3u9s) and PCC (3n6r) using MUSCLE (https://www.ebi.ac.uk/Tools/msa/muscle/), four sets of five homology models each were built using monomeric α - and β-subunits of PaMCC (PDB ID 3u9s) or PCC (PDB ID 3n6r) as template structures for AccA1 and AccD1, respectively. The resulting models were ranked according to the DOPE statistical potential score [45]. The best ranking models were used for generation of the holo complexes by applying symmetry transformations and subsequently energy minimized in PHENIX [46].
Supporting Information
Zdroje
1. Cronan JE Jr., Waldrop GL (2002) Multi-subunit acetyl-CoA carboxylases. Prog Lipid Res 41 : 407–435. 12121720
2. Tong L (2013) Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci 70 : 863–891. doi: 10.1007/s00018-012-1096-0 22869039
3. Tong L, Harwood HJ Jr., (2006) Acetyl-coenzyme A carboxylases: versatile targets for drug discovery. J Cell Biochem 99 : 1476–1488. 16983687
4. Miller JR, Dunham S, Mochalkin I, Banotai C, Bowman M, et al. (2009) A class of selective antibacterials derived from a protein kinase inhibitor pharmacophore. Proc Natl Acad Sci U S A 106 : 1737–1742. doi: 10.1073/pnas.0811275106 19164768
5. Gago G, Diacovich L, Arabolaza A, Tsai SC, Gramajo H (2011) Fatty acid biosynthesis in actinomycetes. FEMS Microbiol Rev 35 : 475–497. doi: 10.1111/j.1574-6976.2010.00259.x 21204864
6. Gago G, Kurth D, Diacovich L, Tsai SC, Gramajo H (2006) Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J Bacteriol 188 : 477–486. 16385038
7. Daniel J, Oh TJ, Lee CM, Kolattukudy PE (2007) AccD6, a member of the Fas II locus, is a functional carboxyltransferase subunit of the acyl-coenzyme A carboxylase in Mycobacterium tuberculosis. J Bacteriol 189 : 911–917. 17114269
8. Kurth DG, Gago GM, de la Iglesia A, Bazet Lyonnet B, Lin TW, et al. (2009) ACCase 6 is the essential acetyl-CoA carboxylase involved in fatty acid and mycolic acid biosynthesis in mycobacteria. Microbiology 155 : 2664–2675. doi: 10.1099/mic.0.027714-0 19423629
9. Karakousis PC, Bishai WR, Dorman SE (2004) Mycobacterium tuberculosis cell envelope lipids and the host immune response. Cell Microbiol 6 : 105–116. 14706097
10. Camacho LR, Constant P, Raynaud C, Laneelle MA, Triccas JA, et al. (2001) Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J Biol Chem 276 : 19845–19854. 11279114
11. Savvi S, Warner DF, Kana BD, McKinney JD, Mizrahi V, et al. (2008) Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids. J Bacteriol 190 : 3886–3895. doi: 10.1128/JB.01767-07 18375549
12. Portevin D, de Sousa-D’Auria C, Montrozier H, Houssin C, Stella A, et al. (2005) The acyl-AMP ligase FadD32 and AccD4-containing acyl-CoA carboxylase are required for the synthesis of mycolic acids and essential for mycobacterial growth: identification of the carboxylation product and determination of the acyl-CoA carboxylase components. J Biol Chem 280 : 8862–8874. 15632194
13. Oh TJ, Daniel J, Kim HJ, Sirakova TD, Kolattukudy PE (2006) Identification and characterization of Rv3281 as a novel subunit of a biotin-dependent acyl-CoA Carboxylase in Mycobacterium tuberculosis H37Rv. J Biol Chem 281 : 3899–3908. 16354663
14. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393 : 537–544. 9634230
15. Haase FC, Henrikson KP, Treble DH, Allen SH (1982) The subunit structure and function of the propionyl coenzyme A carboxylase of Mycobacterium smegmatis. J Biol Chem 257 : 11994–11999. 7118926
16. Diacovich L, Peiru S, Kurth D, Rodriguez E, Podesta F, et al. (2002) Kinetic and structural analysis of a new group of Acyl-CoA carboxylases found in Streptomyces coelicolor A3(2). J Biol Chem 277 : 31228–31236. 12048195
17. Huang CS, Ge P, Zhou ZH, Tong L (2011) An unanticipated architecture of the 750-kDa alpha6beta6 holoenzyme of 3-methylcrotonyl-CoA carboxylase. Nature 481 : 219–223. doi: 10.1038/nature10691 22158123
18. Gande R, Gibson KJ, Brown AK, Krumbach K, Dover LG, et al. (2004) Acyl-CoA carboxylases (accD2 and accD3), together with a unique polyketide synthase (Cg-pks), are key to mycolic acid biosynthesis in Corynebacterianeae such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J Biol Chem 279 : 44847–44857. 15308633
19. Zimmermann M, Thormann V, Sauer U, Zamboni N (2013) Nontargeted profiling of coenzyme A thioesters in biological samples by tandem mass spectrometry. Anal Chem 85 : 8284–8290. doi: 10.1021/ac401555n 23895734
20. Massey LK, Sokatch JR, Conrad RS (1976) Branched-chain amino acid catabolism in bacteria. Bacteriol Rev 40 : 42–54. 773366
21. Fendt SM, Buescher JM, Rudroff F, Picotti P, Zamboni N, et al. (2010) Tradeoff between enzyme and metabolite efficiency maintains metabolic homeostasis upon perturbations in enzyme capacity. Mol Syst Biol 6 : 356. doi: 10.1038/msb.2010.11 20393576
22. Hondalus MK, Bardarov S, Russell R, Chan J, Jacobs WR Jr., et al. (2000) Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect Immun 68 : 2888–2898. 10768986
23. Venugopal A, Bryk R, Shi S, Rhee K, Rath P, et al. (2011) Virulence of Mycobacterium tuberculosis depends on lipoamide dehydrogenase, a member of three multienzyme complexes. Cell Host Microbe 9 : 21–31. doi: 10.1016/j.chom.2010.12.004 21238944
24. Balhana RJ, Swanston SN, Coade S, Withers M, Sikder MH, et al. (2013) bkaR is a TetR-type repressor that controls an operon associated with branched-chain keto-acid metabolism in Mycobacteria. FEMS Microbiol Lett 345 : 132–140. doi: 10.1111/1574-6968.12196 23763300
25. Holton SJ, King-Scott S, Nasser Eddine A, Kaufmann SH, Wilmanns M (2006) Structural diversity in the six-fold redundant set of acyl-CoA carboxyltransferases in Mycobacterium tuberculosis. FEBS Lett 580 : 6898–6902. 17157300
26. Zhang H, Yang Z, Shen Y, Tong L (2003) Crystal structure of the carboxyltransferase domain of acetyl-coenzyme A carboxylase. Science 299 : 2064–2067. 12663926
27. Bilder P, Lightle S, Bainbridge G, Ohren J, Finzel B, et al. (2006) The structure of the carboxyltransferase component of acetyl-coA carboxylase reveals a zinc-binding motif unique to the bacterial enzyme. Biochemistry 45 : 1712–1722. 16460018
28. Lin TW, Melgar MM, Kurth D, Swamidass SJ, Purdon J, et al. (2006) Structure-based inhibitor design of AccD5, an essential acyl-CoA carboxylase carboxyltransferase domain of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103 : 3072–3077. 16492739
29. Huang CS, Sadre-Bazzaz K, Shen Y, Deng B, Zhou ZH, et al. (2010) Crystal structure of the alpha(6)beta(6) holoenzyme of propionyl-coenzyme A carboxylase. Nature 466 : 1001–1005. doi: 10.1038/nature09302 20725044
30. Reddy MC, Breda A, Bruning JB, Sherekar M, Valluru S, et al. (2014) Structure, activity, and inhibition of the Carboxyltransferase beta-subunit of acetyl coenzyme A carboxylase (AccD6) from Mycobacterium tuberculosis. Antimicrob Agents Chemother 58 : 6122–6132. doi: 10.1128/AAC.02574-13 25092705
31. Tran TH, Hsiao YS, Jo J, Chou CY, Dietrich LE, et al. (2014) Structure and function of a single-chain, multi-domain long-chain acyl-CoA carboxylase. Nature. doi: 10.1038/nature14135 25533962
32. Williams KJ, Joyce G, Robertson BD (2010) Improved mycobacterial tetracycline inducible vectors. Plasmid 64 : 69–73. doi: 10.1016/j.plasmid.2010.04.003 20434484
33. Li MZ, Elledge SJ (2007) Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat Methods 4 : 251–256. 17293868
34. Plocinski P, Laubitz D, Cysewski D, Stodus K, Kowalska K, et al. (2014) Identification of protein partners in mycobacteria using a single-step affinity purification method. PLoS One 9: e91380. doi: 10.1371/journal.pone.0091380 24664103
35. van Kessel JC, Hatfull GF (2007) Recombineering in Mycobacterium tuberculosis. Nat Methods 4 : 147–152. 17179933
36. Bardarov S, Bardarov Jr S Jr., Pavelka Jr MS Jr., Sambandamurthy V, Larsen M, et al. (2002) Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148 : 3007–3017. 12368434
37. Piuri M, Hatfull GF (2006) A peptidoglycan hydrolase motif within the mycobacteriophage TM4 tape measure protein promotes efficient infection of stationary phase cells. Mol Microbiol 62 : 1569–1585. 17083467
38. Poulsen C, Holton S, Geerlof A, Wilmanns M, Song YH (2010) Stoichiometric protein complex formation and over-expression using the prokaryotic native operon structure. FEBS Lett 584 : 669–674. doi: 10.1016/j.febslet.2009.12.057 20085764
39. Noens EE, Williams C, Anandhakrishnan M, Poulsen C, Ehebauer MT, et al. (2011) Improved mycobacterial protein production using a Mycobacterium smegmatis groEL1DeltaC expression strain. BMC Biotechnol 11 : 27. doi: 10.1186/1472-6750-11-27 21439037
40. Guchhait RB, Polakis SE, Dimroth P, Stoll E, Moss J, et al. (1974) Acetyl coenzyme A carboxylase system of Escherichia coli. Purification and properties of the biotin carboxylase, carboxyltransferase, and carboxyl carrier protein components. J Biol Chem 249 : 6633–6645. 4154089
41. Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, et al. (2007) EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol 157 : 38–46. 16859925
42. van Heel M, Harauz G, Orlova EV, Schmidt R, Schatz M (1996) A new generation of the IMAGIC image processing system. J Struct Biol 116 : 17–24. 8742718
43. Shaikh TR, Gao H, Baxter WT, Asturias FJ, Boisset N, et al. (2008) SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat Protoc 3 : 1941–1974. doi: 10.1038/nprot.2008.156 19180078
44. Eswar N, Eramian D, Webb B, Shen MY, Sali A (2008) Protein structure modeling with MODELLER. Methods Mol Biol 426 : 145–159. doi: 10.1007/978-1-60327-058-8_8 18542861
45. Shen MY, Sali A (2006) Statistical potential for assessment and prediction of protein structures. Protein Sci 15 : 2507–2524. 17075131
46. Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66 : 213–221. doi: 10.1107/S0907444909052925 20124702
47. Krissinel E, Henrick K (2007) Inference of macromolecular assemblies from crystalline state. J Mol Biol 372 : 774–797. 17681537
48. Laval F, Laneelle MA, Deon C, Monsarrat B, Daffe M (2001) Accurate molecular mass determination of mycolic acids by MALDI-TOF mass spectrometry. Anal Chem 73 : 4537–4544. 11575804
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek 2014 Reviewer Thank YouČlánek Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water LabelingČlánek High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



