-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Livestock-Associated : The United States Experience
article has not abstract
Published in the journal: . PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004564
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004564Summary
article has not abstract
Background and Overview
Staphylococcus aureus is a gram-positive bacterium that colonizes a variety of animal species [1]. S. aureus infections in animals are most commonly reported as a cause of mastitis in dairy-producing animals (including cattle and goats) and “bumblefoot” in chickens [2], as well as being identified as a pathogen of farmed rabbits [3]. Most reports characterizing animal-associated S. aureus have demonstrated that strains affecting animals are distinct from those infecting humans, suggesting that there are host-specific lineages which only rarely cross species boundaries [4].
Livestock-associated strains may evolve on farms because of the use of antibiotics in animal husbandry. These may be used as feed additives for growth promotion in industrial livestock and poultry [5], for prevention of disease within a herd, or for treatment of an existing disease outbreak. Agricultural-use antibiotics include many classes that are relevant for human health, including tetracyclines, macrolides, penicillins, and sulfonamides, among others. Antimicrobial resistance generated during animal husbandry may then be spread to the general human population in a number of different manners: contact with contaminated meat products (via handling or ingestion); occupational contact (farmers, meat packers, butchers, etc.) and potential secondary spread into the larger community from those who are occupationally exposed; entry into and transmission via hospitals or other health care facilities; or spread via environmental routes including air, water, or manure in areas in proximity to live animal farms or crop farms where manure has been used as a fertilizer (Fig. 1).
Fig. 1. The tangled web of S. aureus in the US. 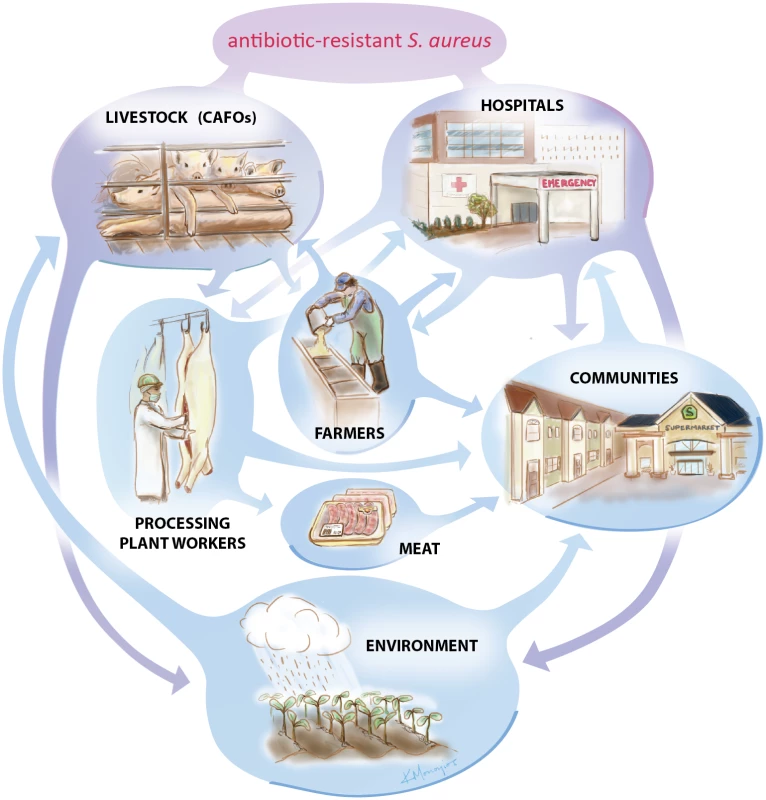
Antibiotic-resistant Staphylococcus aureus is a growing public health concern, but tracing the origins of the bacterium is complicated. Evidence suggests that antibiotic-resistant strains of S. aureus can spread in livestock operations and hospitals where antibiotics are regularly used. These antibiotic-resistant organisms can then spread into communities and the environment. More research is needed to determine exactly how these transfers occur. Image by Kalliopi Monoyios. While methicillin resistance has been the most commonly investigated phenomenon and will be the main topic of this review, resistance to any of these antibiotics can occur and can potentially be a threat to successful treatment of S. aureus infections and therefore to human health outcomes. As such, my research group and others have begun to look more broadly at any S. aureus present on farms, including those that may be susceptible to methicillin but resistant to other antibiotics.
Livestock-Associated Methicillin-Resistant Staphylococcus aureus (MRSA): An Overview
In the early part of the 21st century, a novel pig-associated strain of MRSA was identified: sequence type 398 (ST398) and related strains (collectively grouped into clonal complex 398, or CC398, reviewed in [6]). CC398 was first identified in pigs and swine workers but has since been found in other animals (including cattle, poultry, and dogs as well as humans) in a number of countries in Europe, Asia, and North and South America, as well as Australia. The discovery of this strain led to the addition of livestock-associated MRSA (LA-MRSA) to the lexicon, to complement hospital-associated (HA) and community-associated (CA) strains.
In most European countries, CC398 remains the most commonly identified type of LA-MRSA [6–9], leading to a presumption that the terms LA-MRSA and CC398 are practically interchangeable. However, while CC398 strains have been found in livestock across the globe, the epidemiology of livestock-associated S. aureus has been found to differ in other geographic areas. Several Asian studies have demonstrated that a different strain of MRSA, ST9, appears to be the prominent type of LA-MRSA in several Asian countries [10–14]. Poultry may harbor CC398 strains [15–17] but also other types unrelated to CC398, including CC5 [15, 18] or other types [17]. In the United States, the diversity of livestock-associated S. aureus appears to be higher than that identified in Europe or Asia, with reports of both CC398 as well as a variety of “human” types of S. aureus in live animals, as described below.
The epidemiology of CC398 and other strains found in both animals and humans [12] has led to a reexamination of the idea of host specificity in S. aureus. CC398 appears to be frequently shared between animals and humans and is capable of causing active symptomatic infections in both species [19, 20]. Furthermore, both CC398 and a poultry-adapted S. aureus strains of CCT5 have been phylogenetically analyzed and appear to have originated in humans, who transmitted strains to animals, in which the strains subsequently spread and evolved a variety of host adaptations [21, 22]. As such, there exist both human-associated CC398 strains as well as true livestock strains, complicating studies of origin or host association based only on knowledge of sequence type.
Epidemiology of CC398 and Other Livestock-Associated S. aureus in the US
The epidemiology of LA-SA in the US appears to be notably different than in European countries, where the bulk of LA-SA research has been carried out. While early studies on farms and of meat-identified CC398 strains in animals, farm workers, and meat products, [23, 24], contemporaneous studies also documented CC398 in populations with no obvious livestock contact [25–27]. In one Texas publication carried out in a jail setting rather than on a farm, CC398 isolates made up a significant portion (13.2%) of all methicillin-susceptible S. aureus (MSSA) identified within this population. Clearly, the association of CC398 exclusively with an agricultural reservoir did not appear to hold in the US.
While CC398 can have LA as well as human versions, other human strains of S. aureus have also been found in US livestock. Studies carried out on swine farms in the US have identified human strains within the noses of live animals [28–30] or as components of environmental samples of farm dust [31]. Several papers have found CC5 strains rather than CC398-associated types to be the dominant strain isolated from pig farms in both Iowa and Ohio [31, 32], while others have found CC398 to be the most common molecular type [23, 33]. Three studies in North Carolina examining workers on pig farms and in processing plants similarly found substantial diversity among S. aureus isolated from workers, including CC398, CC5, and CC8 strains, among others [34–36].
Transmission between Animals and Humans in the Farming Setting
Studies of individuals living in proximity to concentrated animal feeding operations (CAFOs) support the idea that nonlivestock strains may be spreading within areas proximal to farms. Two independent studies carried out in Iowa and Pennsylvania that examined the relationship between animal farms and MRSA found an increased risk of MRSA colonization or infection in those living close to farms or in areas where manure was spread on fields [37, 38]. In both studies, however, no classic LA strains were found when molecular typing was carried out on isolates collected. This suggests that either strains other than LA isolates are evolving on farms (consistent with on-farm sampling described above) or that it may be the presence of antibiotic resistance genes and antibiotic residues on farms that are moving to the subjects’ own bacterial flora and causing a shift toward antibiotic-resistant strains in these populations, or perhaps a combination of both mechanisms. Firm conclusions are difficult to make in the absence of a concerted, national-level on-farm sampling effort, which is difficult to carry out in the US because of private/corporate ownership of many farms and laws in several states that are unfriendly to farm visitors.
Human Infections with Livestock-Associated S. aureus Strains
A number of human infections with CC398 strains have been reported. Most of these have been documented in Europe [39–41]; however, CC398 infections from the US [26, 42, 43] and Canada [44] have been reported as well. Because many infection reports were published prior to the recognition of distinct lineages of CC398, it is not always clear, particularly for individuals lacking exposure to livestock, whether the CC398 strains identified are ancestral human strains, or derived livestock types. This has significance for prevention and treatment, as human-origin strains appear to be more virulent than true livestock strains but may also be less likely to be multidrug resistant (and as such, more easily treatable) [22]. Nonetheless, the majority of reported infections with CC398 strains appear to be similar in scope to community-associated S. aureus strains, causing skin and soft tissue infections and, more rarely, serious invasive infections and death.
Potential for Meat Products as a Source of LA-SA in the Community
Just as a variety of human and livestock strains have been found in live animals on farms, so have they been found in meat products sampled in the US [18, 24, 45–49]. CC398 strains have been found in pork and chicken products in the US and appear to be the dominant contaminating strains in raw turkey meat. S. aureus may be transmitted to humans from meat products by handling of contaminated products or by the cross contamination of household surfaces (such as countertops and sinks), which are then touched by family members. While antibiotic use on farms may drive selection of antibiotic-resistant strains of S. aureus that eventually end up in meat products, eliminating consumer exposure to such bacteria is not as straightforward as simply purchasing products raised in an antibiotic-free environment. In a study examining conventional versus antibiotic-free pork products, no difference was found in prevalence of MRSA between these types of samples [46]. This was a different result obtained from sampling results on conventional versus antibiotic-free farms [33], suggesting the potential for either contamination of pigs with MRSA in the lairage area prior to slaughter or contamination of meat products during processing or packaging, either via humans in the plants who may spread MRSA to meat products or from bacterial residues present from conventional products. It is currently not known what the risk is to consumers from S. aureus–contaminated meat products.
Conclusions, Significance, and Future Studies
Livestock-associated S. aureus is an emerging category of S. aureus throughout the world. Currently, the research carried out has focused more closely on carriage than on transmission and infection, but these strains appear to be less likely to cause human infections and to spread person-to-person than typical human strains [50]. However, these conclusions should be noted with caution, as few well-designed prospective studies have been conducted to answer these questions to date.
Recent research suggests that bidirectional transmission of strains of S. aureus between humans and livestock is not a rare occurrence. In addition to the movement of CC398 between animals and humans, studies have suggested that a human pandemic clone, CC97, had its origin in cattle [51]. Additionally, antibiotic resistance genes, including mecA [52, 53] and mecC [54, 55], have been suggested to have an animal origin.
Currently, we are limited in the ways we can distinguish whether any particular strain of S. aureus is a human or livestock-adapted isolate. We can use the presence of marker genes, including the loss scn and presence of tet(M), both of which are genotypes associated with livestock adaptation of CC398 lineages [22, 56] or examine the presence of a single-nucleotide polymorphism (SNP) that has also been identified in this clade [56]. However, large-scale studies validating these markers in other lineages (CC5, CC8, and more) are lacking. Additional large-scale studies in both human and animal populations are necessary in order to gather isolates that are epidemiologically well characterized. These isolates can then be analyzed in order to validate current genomic markers, as well as to identify novel ones in lineages besides CC398.
S. aureus surveillance is most commonly carried out within a human clinical or hospital setting, with far fewer research dollars devoted to analysis of carriage within communities, particularly in a rural setting, and very little research examining animal strains. As such, it is likely we are missing other spillover events of S. aureus from livestock to humans or vice versa. To track such events and facilitate both surveillance and source tracking of novel isolates, the buy-in of industry is needed. All too often, the relationship between public health and the agricultural and food industry is one of antagonism rather than assistance. Working together will mean both safer food products and well-protected workers. More attention to this type of research is needed, as we are rapidly approaching a “post-antibiotic era” [57]. The effectiveness of antimicrobial stewardship in the clinical setting may be reduced if pathogens and resistance genes from the agricultural environment are repeatedly, but silently, being introduced into the human population [58].
Zdroje
1. Weese JS (2010) Methicillin-resistant Staphylococcus aureus in animals. ILAR J 51 : 233–244. doi: 10.1093/ilar.51.3.233
2. McNamee PT, Smyth JA (2000) Bacterial chondronecrosis with osteomyelitis (‘femoral head necrosis’) of broiler chickens: a review. Avian Pathol 29 : 253–270. doi: 10.1080/030794500750047243 19184815
3. Viana D, Selva L, Segura P, Penades JR, Corpa JM (2007) Genotypic characterization of Staphylococcus aureus strains isolated from rabbit lesions. Vet Microbiol 121 : 288–298. doi: 10.1016/j.vetmic.2006.12.003 17208392
4. Shepheard MA, Fleming VM, Connor TR, Corander J, Feil EJ, et al. (2013) Historical zoonoses and other changes in host tropism of Staphylococcus aureus, identified by phylogenetic analysis of a population dataset. PLoS One 8: e62369. doi: 10.1371/journal.pone.0062369 23667472
5. Silbergeld EK, Graham J, Price LB (2008) Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health 29 : 151–169. doi: 10.1146/annurev.publhealth.29.020907.090904 18348709
6. Fluit AC (2012) Livestock-associated Staphylococcus aureus. Clin Microbiol Infect 18 : 735–744. doi: 10.1111/j.1469-0691.2012.03846.x 22512702
7. Smith TC, Pearson N (2011) The emergence of Staphylococcus aureus ST398. Vector Borne Zoonotic Dis 11 : 327–339. doi: 10.1089/vbz.2010.0072 20925523
8. Johnson AP (2011) Methicillin-resistant Staphylococcus aureus: the European landscape. J Antimicrob Chemother 66 Suppl 4: iv43–iv48. doi: 10.1093/jac/dkr076 21521706
9. Kock R, Mellmann A, Schaumburg F, Friedrich AW, Kipp F, et al. (2011) The epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in Germany. Dtsch Arztebl Int 108 : 761–767. doi: 10.3238/arztebl.2011.0761 22163252
10. Larsen J, Imanishi M, Hinjoy S, Tharavichitkul P, Duangsong K, et al. (2012) Methicillin-resistant Staphylococcus aureus ST9 in pigs in Thailand. PLoS One 7: e31245. doi: 10.1371/journal.pone.0031245 22363594
11. Neela V, Arif MZ, Nor Shamsudin M, van Belkum A, Khoon LY, et al. (2009) Prevalence of ST9 MRSA among Pigs and Pig Handlers in Malaysia. J Clin Microbiol 47 : 4138–4140. doi: 10.1128/JCM.01363-09 19812280
12. Wagenaar JA, Yue H, Pritchard J, Broekhuizen-Stins M, Huijsdens X, et al. (2009) Unexpected sequence types in livestock associated methicillin-resistant Staphylococcus aureus (MRSA): MRSA ST9 and a single locus variant of ST9 in pig farming in China. Vet Microbiol 139 : 405–409. doi: 10.1016/j.vetmic.2009.06.014 19608357
13. Patchanee P, Tadee P, Arjkumpa O, Love D, Chanachai K, et al. (2014) Occurrence and characterization of livestock associated-methicillin resistant Staphylococcus aureus in pig industries of northern Thailand. J Vet Sci 15 : 529–536. doi: 10.4142/jvs.2014.15.4.529 25530702
14. Fang HW, Chiang PH, Huang YC (2014) Livestock-associated methicillin-resistant Staphylococcus aureus ST9 in pigs and related personnel in Taiwan. PLoS One 9: e88826. doi: 10.1371/journal.pone.0088826 24551168
15. Argudin MA, Cariou N, Salandre O, Le Guennec J, Nemeghaire S, et al. (2013) Genotyping and antimicrobial resistance of Staphylococcus aureus isolates from diseased turkeys. Avian Pathol 42 : 572–580. doi: 10.1080/03079457.2013.854308 24224550
16. Wendlandt S, Kadlec K, Fessler AT, Monecke S, Ehricht R, et al. (2013) Resistance phenotypes and genotypes of methicillin-resistant Staphylococcus aureus isolates from broiler chickens at slaughter and abattoir workers. J Antimicrob Chemother 68 : 2458–2463. doi: 10.1093/jac/dkt239 23798670
17. Nemeghaire S, Roelandt S, Argudin MA, Haesebrouck F, Butaye P (2013) Characterization of methicillin-resistant Staphylococcus aureus from healthy carrier chickens. Avian Pathol 42 : 342–346. doi: 10.1080/03079457.2013.805183 23777220
18. Buyukcangaz E, Velasco V, Sherwood JS, Stepan RM, Koslofsky RJ, et al. (2013) Molecular typing of Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) isolated from animals and retail meat in North Dakota, United States. Foodborne Pathog Dis 10 : 608–617.
19. van Duijkeren E, Jansen MD, Flemming SC, de Neeling H, Wagenaar JA, et al. (2007) Methicillin-resistant Staphylococcus aureus in pigs with exudative epidermitis. Emerg Infect Dis 13 : 1408–1410. doi: 10.3201/eid1309.061268 18252124
20. Graveland H, Duim B, van Duijkeren E, Heederik D, Wagenaar JA (2011) Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Int J Med Microbiol 301 : 630–634. doi: 10.1016/j.ijmm.2011.09.004 21983338
21. Lowder BV, Guinane CM, Ben Zakour NL, Weinert LA, Conway-Morris A, et al. (2009) Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc Natl Acad Sci U S A 106 : 19545–19550. doi: 10.1073/pnas.0909285106 19884497
22. Price LB, Stegger M, Hasman H, Aziz M, Larsen J, et al. (2012) Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio 3: e00305–11. doi: 10.1128/mBio.00305-11 22354957
23. Smith TC, Male MJ, Harper AL, Kroeger JS, Tinkler GP, et al. (2009) Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS ONE 4: e4258. doi: 10.1371/journal.pone.0004258 19145257
24. Hanson BM, Dressler AE, Harper AL, Scheibel RP, Wardyn SE, et al. (2011) Prevalence of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) on retail meat in Iowa. Journal of Infection and Public Health 4 : 169–174. doi: 10.1016/j.jiph.2011.06.001 22000843
25. Bhat M, Dumortier C, Taylor BS, Miller M, Vasquez G, et al. (2009) Staphylococcus aureus ST398, New York City and Dominican Republic. Emerg Infect Dis 15 : 285–287. doi: 10.3201/eid1502.080609 19193274
26. Uhlemann AC, Porcella SF, Trivedi S, Sullivan SB, Hafer C, et al. (2012) Identification of a highly transmissible animal-independent Staphylococcus aureus ST398 clone with distinct genomic and cell adhesion properties. mBio 3: e00027–12. doi: 10.1128/mBio.00027-12 22375071
27. David MZ, Siegel J, Lowy FD, Zychowski D, Taylor A, et al. (2013) Asymptomatic carriage of sequence type 398, spa type t571 methicillin-susceptible Staphylococcus aureus in an urban jail: a newly emerging, transmissible pathogenic strain. J Clin Microbiol 51 : 2443–2447. doi: 10.1128/JCM.01057-13 23658269
28. Dressler AE, Scheibel RP, Wardyn S, Harper AL, Hanson BM, et al. (2012) Prevalence, antibiotic resistance and molecular characterisation of Staphylococcus aureus in pigs at agricultural fairs in the USA. Vet Rec 170 : 495. doi: 10.1136/vr.100570 22505242
29. Osadebe LU, Hanson B, Smith TC, Heimer R (2013) Prevalence and characteristics of Staphylococcus aureus in Connecticut swine and swine farmers. Zoonoses Public Health 60 : 234–243. doi: 10.1111/j.1863-2378.2012.01527.x 22883566
30. Gordoncillo MJ, Abdujamilova N, Perri M, Donabedian S, Zervos M, et al. (2012) Detection of methicillin-resistant Staphylococcus aureus (MRSA) in backyard pigs and their owners, Michigan, USA. Zoonoses Public Health 59 : 212–216. doi: 10.1111/j.1863-2378.2011.01437.x 21914153
31. Frana TS, Beahm AR, Hanson BM, Kinyon JM, Layman LL, et al. (2013) Isolation and characterization of methicillin-resistant Staphylococcus aureus from pork farms and visiting veterinary students. PLoS One 8: e53738. doi: 10.1371/journal.pone.0053738 23301102
32. Molla B, Byrne M, Abley M, Mathews J, Jackson CR, et al. (2012) Epidemiology and genotypic characteristics of methicillin-resistant Staphylococcus aureus strains of porcine origin. J Clin Microbiol 50 : 3687–3693. doi: 10.1128/JCM.01971-12 22972820
33. Smith TC, Gebreyes WA, Abley MJ, Harper AL, Forshey BM, et al. (2013) Methicillin-resistant Staphylococcus aureus in pigs and farm workers on conventional and antibiotic-free swine farms in the USA. PLoS One 8: e63704. doi: 10.1371/journal.pone.0063704 23667659
34. Rinsky JL, Nadimpalli M, Wing S, Hall D, Baron D, et al. (2013) Livestock-associated methicillin and multidrug resistant Staphylococcus aureus is present among industrial, not antibiotic-free livestock operation workers in North Carolina. PLoS One 8: e67641. doi: 10.1371/journal.pone.0067641 23844044
35. Neyra RC, Frisancho JA, Rinsky JL, Resnick C, Carroll KC, et al. (2014) Multidrug-Resistant and Methicillin-Resistant Staphylococcus aureus (MRSA) in Hog Slaughter and Processing Plant Workers and Their Community in North Carolina (USA). Environ Health Perspect 122 : 471–477. doi: 10.1289/ehp.1306741 24508836
36. Nadimpalli M, Rinsky JL, Wing S, Hall D, Stewart J, et al. (2014) Persistence of livestock-associated antibiotic-resistant Staphylococcus aureus among industrial hog operation workers in North Carolina over 14 days. Occup Environ Med. E-pub ahead of print. doi: 10.1136/oemed-2014-102095
37. Casey JA, Curriero FC, Cosgrove SE, Nachman KE, Schwartz BS (2013) High-density livestock operations, crop field application of manure, and risk of community-associated methicillin-resistant Staphylococcus aureus infection in Pennsylvania. JAMA Intern Med 173 : 1980–1990. doi: 10.1001/jamainternmed.2013.10408 24043228
38. Carrel M, Schweizer ML, Sarrazin MV, Smith TC, Perencevich EN (2014) Residential proximity to large numbers of swine in feeding operations is associated with increased risk of methicillin-resistant Staphylococcus aureus colonization at time of hospital admission in rural Iowa veterans. Infect Control Hosp Epidemiol 35 : 190–193. doi: 10.1086/674860 24442084
39. Rasigade JP, Laurent F, Hubert P, Vandenesch F, Etienne J (2010) Lethal necrotizing pneumonia caused by an ST398 Staphylococcus aureus strain. Emerging Infectious Diseases 16 : 1330. doi: 10.3201/eid1608.100317 20678343
40. Valentin-Domelier AS, Girard M, Bertrand X, Violette J, Francois P, et al. (2011) Methicillin-susceptible ST398 Staphylococcus aureus responsible for bloodstream infections: an emerging human-adapted subclone? PLoS ONE 6: e28369. doi: 10.1371/journal.pone.0028369 22163008
41. van Belkum A, Melles DC, Peeters JK, van Leeuwen WB, van Duijkeren E, et al. (2008) Methicillin-resistant and—susceptible Staphylococcus aureus sequence type 398 in pigs and humans. Emerg Infect Dis 14 : 479–483. doi: 10.3201/eid1403.0760 18325267
42. Mediavilla JR, Chen L, Uhlemann AC, Hanson BM, Rosenthal M, et al. (2012) Methicillin-Susceptible Staphylococcus aureus ST398, New York and New Jersey, USA. Emerging Infectious Diseases 18 : 700–702. doi: 10.3201/eid1804.111419 22469250
43. Orscheln RC, Hunstad DA, Fritz SA, Loughman JA, Mitchell K, et al. (2009) Contribution of genetically restricted, methicillin-susceptible strains to the ongoing epidemic of community-acquired Staphylococcus aureus infections. Clinical Infectious Diseases 49 : 536–542. doi: 10.1086/600881 19589082
44. Golding GR, Bryden L, Levett PN, McDonald RR, Wong A, et al. (2010) Livestock-associated methicillin-resistant Staphylococcus aureus sequence type 398 in humans, Canada. Emerg Infect Dis 16 : 587–594. doi: 10.3201/eid1604.091435 20350371
45. Waters AE, Contente-Cuomo T, Buchhagen J, Liu CM, Watson L, et al. (2011) Multidrug-Resistant Staphylococcus aureus in US Meat and Poultry. Clin Infect Dis 52 : 1227–1230. doi: 10.1093/cid/cir181 21498385
46. O’Brien AM, Hanson BM, Farina SA, Wu JY, Simmering JE, et al. (2012) MRSA in conventional and alternative retail pork products. PLoS ONE 7: e30092. doi: 10.1371/journal.pone.0030092 22276147
47. Pu S, Han F, Ge B (2008) Isolation and Characterization of Methicillin-Resistant Staphylococcus aureus from Louisiana Retail Meats. Appl Environ Microbiol 75 : 265–267. doi: 10.1128/AEM.01110-08 18978079
48. Bhargava K, Wang X, Donabedian S, Zervos M, de Rocha L, et al. (2011) Methicillin-resistant Staphylococcus aureus in retail meat, Detroit, Michigan, USA. Emerg Infect Dis 17 : 1135–1137. doi: 10.3201/eid1706.101095 21749794
49. Jackson CR, Davis JA, Barrett JB (2013) Prevalence and characterization of methicillin-resistant Staphylococcus aureus isolates from retail meat and humans in Georgia. J Clin Microbiol 51 : 1199–1207. doi: 10.1128/JCM.03166-12 23363837
50. Hetem DJ, Bootsma MC, Troelstra A, Bonten MJ (2013) Transmissibility of livestock-associated methicillin-resistant Staphylococcus aureus. Emerg Infect Dis 19 : 1797–1802. doi: 10.3201/eid1911.121085 24207050
51. Spoor LE, McAdam PR, Weinert LA, Rambaut A, Hasman H, et al. (2013) Livestock origin for a human pandemic clone of community-associated methicillin-resistant Staphylococcus aureus. MBio 4: e00356–13. doi: 10.1128/mBio.00356-13 23943757
52. Wu S, Piscitelli C, de Lencastre H, Tomasz A (1996) Tracking the evolutionary origin of the methicillin resistance gene: cloning and sequencing of a homologue of mecA from a methicillin susceptible strain of Staphylococcus sciuri. Microb Drug Resist 2 : 435–441. doi: 10.1089/mdr.1996.2.435 9158816
53. (1978) Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ 56 : 271–293. 307456
54. Garcia-Alvarez L, Holden MT, Lindsay H, Webb CR, Brown DF, et al. (2011) Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis 11 : 595–603. doi: 10.1016/S1473-3099(11)70126-8 21641281
55. Shore AC, Deasy EC, Slickers P, Brennan G, O’Connell B, et al. (2011) Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 55 : 3765–3773. doi: 10.1128/AAC.00187-11 21636525
56. Stegger M, Liu CM, Larsen J, Soldanova K, Aziz M, et al. (2013) Rapid differentiation between livestock-associated and livestock-independent Staphylococcus aureus CC398 clades. PLoS One 8: e79645. doi: 10.1371/journal.pone.0079645 24244535
57. WHO (2014) Antimicrobial resistance: global report on surveillance Geneva, Switzerland. pp. 257.
58. Smith DL, Harris AD, Johnson JA, Silbergeld EK, Morris JG Jr. (2002) Animal antibiotic use has an early but important impact on the emergence of antibiotic resistance in human commensal bacteria. Proc Natl Acad Sci U S A 99 : 6434–6439. doi: 10.1073/pnas.082188899 11972035
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek 2014 Reviewer Thank YouČlánek Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water LabelingČlánek High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



