-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
The intracellular fate of the agent of the human tuberculosis agent in phagocytes is a question of great biological relevance. Among the mycobacterial survival strategies, the escape of Mycobacterium tuberculosis from phagosomes has been subject of scientific debate for a long time. However, technically improved methods recently reinforced the occurrence of this phenomenon. Here, we focused on the host factors involved in phagosomal rupture and provide first and singular evidence of M. tuberculosis-mediated phagosomal rupture in vivo in mouse lungs and inside the granuloma. We show that partial blockage of phagosomal acidification, induced by mycobacteria, is a prerequisite for efficient vacuolar breakage by M. tuberculosis and link maturation arrest, cytosolic contact and the corresponding immune responses. From our results we conclude that vacuolar breakage induced by M. tuberculosis is not an ex vivo artifact of cell cultures, but an important process that occurs inside infected phagocytes within organs during several days that strongly determines the outcome of infection with this key pathogen.
Published in the journal: . PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004650
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004650Summary
The intracellular fate of the agent of the human tuberculosis agent in phagocytes is a question of great biological relevance. Among the mycobacterial survival strategies, the escape of Mycobacterium tuberculosis from phagosomes has been subject of scientific debate for a long time. However, technically improved methods recently reinforced the occurrence of this phenomenon. Here, we focused on the host factors involved in phagosomal rupture and provide first and singular evidence of M. tuberculosis-mediated phagosomal rupture in vivo in mouse lungs and inside the granuloma. We show that partial blockage of phagosomal acidification, induced by mycobacteria, is a prerequisite for efficient vacuolar breakage by M. tuberculosis and link maturation arrest, cytosolic contact and the corresponding immune responses. From our results we conclude that vacuolar breakage induced by M. tuberculosis is not an ex vivo artifact of cell cultures, but an important process that occurs inside infected phagocytes within organs during several days that strongly determines the outcome of infection with this key pathogen.
Introduction
The pathogenic potential of Mycobacterium tuberculosis (Mtb), the etiologic agent of human tuberculosis (TB), depends largely on the type VII secretion system ESX-1 [1,2], which is responsible for the secretion of the 6-kDa Early Secreted Antigenic Target (ESAT-6), its protein partner, the 10-kDa Culture Filtrate Protein (CFP-10), and several ESX-1 associated proteins (Esps) [3,4]. ESX-1 secretion is evolutionary conserved in most members of the M. tuberculosis complex [5], and the more distantly related tubercle bacilli of the Mycobacterium canettii clade [6,7], as well as in some non-tuberculous mycobacteria such as Mycobacterium marinum [8]. This secretion system governs numerous aspects of interaction between pathogenic mycobacteria and the host cell [1,2], including membrane-damaging activity [9–11], thought to be implicated in phagosomal escape at later stages of infection [12–16]. Although this phenomenon is a matter of debate [2,17–20], by use of a single-cell Fluorescence Resonance Energy Transfer (FRET)-based technology [21], we recently demonstrated that ESX-1-proficient Mtb and recombinant Mycobacterium bovis BCG::ESX-1 were able to induce phagosome rupture in human THP-1 macrophage (MΦ)-like cells [15]. This assay uses the ability of the surface-exposed BlaC β-lactamase of Mtb [22,23] to cleave the FRET substrate CCF-4, which consists of a cephalosporin core linking 7-hydroxycoumarin to fluorescein that has also been used for exploring effector injection and intracellular localization of Gram-negative bacteria [21,24,25]. The ESX-1-induced rupture of the phagosomal membrane, which results in the exit of mycobacterial products from the endosomal pathway and in extra-phagosomal localization of bacilli [13–16] is of relevance for the outcome of the immune control and bacterial dissemination [26–29]. Phagosomes are reported to be specialized platforms for pathogen recognition [30] and there is also growing evidence of a link between the functionality of the ESX-1 secretion system and the presence of mycobacteria-associated molecular patterns in the host cytosol. Peptidoglycans [31,32] and extracellular mycobacterial DNA [33] were reported to be sensed by the cytosolic receptors of the innate system with multiple biological consequences. Indeed, the Mtb-mediated induction of Nucleotide binding Oligomerization Domain (NOD)-Like Receptor pathways, i.e., NOD2 / Receptor-interacting protein 2 kinase (Rip2) / TANK-Binding Kinase 1 (TBK1) / Interferon regulatory factor (Irf) 5, is responsible for a significant part of type I interferon (IFN) production [31,32]. On the other hand, the signaling through the Stimulator of IFN Genes (STING) / TBK1 / Irf3 pathway [33] leads to a type I IFN signature on which depends the expression of CCL5, CXCL10 and Nitric Oxide Synthase 2 [34,35]. The formation of Nucleotide-binding domain and Leucin-rich Repeat pyrin–containing Protein-3 (NLRP-3) / ASC (Apoptosis-associated Speck-like protein containing a carboxy-terminal CARD) / caspase-1 inflammasome complex, is required in humans for the processing of the pro-IL-1β into biologically active pleïotropic immune mediatorIL-1β following Mtb infection [36,37]. Moreover, the ubiquitination of Mtb prior to its delivery to the autophagic machinery also necessitates the ESX-1-dependent translocation of extracellular Mtb DNA to the cytosol [16,33,38,39]. Thus, the events arising from mycobacterial cytoplasmic access may substantially influence both the immune control of Mtb and the inflammation-induced tissue damage.
The impact of selected components of the ESX-1 system on phagosomal rupture has recently been assessed [13,15,16], however, other potential intervening factors, including those from the host cell remain largely unexplored. Here, we have investigated the host parameters modulating the Mtb-mediated vacuolar breakage, by developing a CCF-4 FRET-based approach that can be used for the study of Mtb-infected cells by flow cytometry. This approach, which permits to combine the detection of phagosomal rupture with the analysis of numerous host cell phenotypic and functional parameters, allowed us to explore multiple phagocyte types, including those isolated from mouse airways. Our results provide first and unique evidence that Mtb-induced phagosomal rupture does occur in vivo inside the lungs and spleens of infected experimental animals and lasts over several days. Moreover, we here explore the impact of vacuolar acidification that constitutes a fundamental cellular defense mechanism [40] and demonstrate that the characteristic partial prevention of phagosomal acidification by Mtb is a prerequisite for phagosomal escape of the pathogen. Our study thus reveals novel details and presents a refined model of cellular events during infection with Mtb.
Results
ESX-1-dependent Mtb-mediated phagosomal rupture detected by FRET-based flow cytometry
To evaluate mycobacteria-mediated phagosomal rupture in different phagocyte types and different physiological contexts, we adapted the previously used microscopy-based CCF-4 FRET technique [15] for flow cytometry. The latter approach not only allows monitoring of bacteria-induced phagosomal rupture or tracking of endosome-to-cytosol antigen translocation [25,41], but also permits the simultaneous inspection of surface markers and analysis of hundreds of thousands of host cells. At first, we infected differentiated THP-1 cells at a multiplicity of infection (MOI) of 1 either with Mtb H37Rv WT or the isogenic ΔESX-1 derivative, Mtb H37Rv-ΔRD1 [10], which both display similar β-lactamase activity [15]. These THP-1 cells were then incubated with CCF-4-AM, an esterified, lipophilic form of the CCF-4 substrate that can readily enter into cells, where it is converted by endogenous cytoplasmic esterases into negatively charged CCF-4, which is retained in the cytosol and emits green fluorescence (500–550 nm) upon stimulation at 320–380 nm, due to FRET from the coumarin moiety to the fluoroscein part (S1 Fig.). In the case of Mtb-induced phagosomal rupture, cleavage of CCF-4 by the intrinsic Mtb BlaC β-lactamase leads to loss of FRET and a change of the CCF-4 emission spectrum from green to blue coumarin fluorescence (410–470 nm). As depicted in Fig. 1A, the CCF-4 emission signals of CD11b+ gated THP-1 cells, infected with wild-type (WT) Mtb H37Rv, showed a marked shift of the CCF-4 emission towards blue at 4 days post infection (dpi). In contrast, a much weaker shift of the CCF-4 spectrum was observed for Mtb ΔESX-1-infected cells, validating our experimental setup and confirming the fundamental virulence differences between the used ESX-1-proficient and ESX-1–deficient Mtb strains [10,15]. The residual blue shift in Mtb ΔESX-1-infected cells relative to non-infected cells is likely a consequence of paraformaldehyde (PFA) fixation prior to signal acquisition (S2A–B Fig.). These results were further corroborated by ratios of Mean Fluorescence Intensities (MFI) of blue vs. green signals (Fig. 1B), and blue MFI447 nm (Fig. 1C). Moreover, we also used fluorescent Mtb (DsRed-Mtb H37Rv) to infect THP-1 cells, at a weaker initial dose (MOI = 0.3), and thereby observed that the CCF-4 blue emission shift selectively occurred in cells that had engulfed the bacteria (Fig. 1D). This approach thus allowed a quantitative study of phagosomal rupture in host cells that have engulfed Mtb, and whose subtype can be identified/determined by staining of the specific surface markers. Hence, our experimental setup was adapted to be used for various cell types and physiological situations, including the detection of vacuolar rupture in rare (infected) cells that were dispersed in a large and heterogeneous cell background population.
Fig. 1. Detection of Mtb-mediated phagosome disruption by flow cytometry. 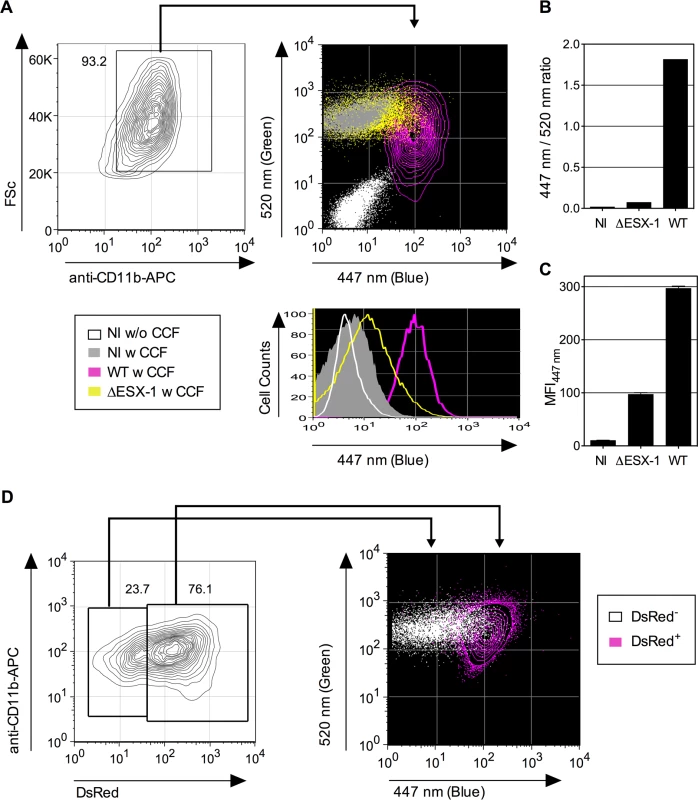
(A) Phagosomal rupture detected by CCF-4 FRET-based flow cytometry. Differentiated THP-1 cells were infected with Mtb, WT or ΔESX-1 strain (MOI = 1); NI = not infected. At 4 dpi, cells were successively stained with CCF-4 and anti-CD11b mAb, fixed, and their green (520 nm) vs. blue (447 nm) fluorescent signals were analyzed after gating on CD11b+ cells. Results are depicted as signal overlays of different groups as dot or contour blots. (B-C) Shown are ratios of MFI447 nm / MFI520 nm (B) and blue MFI447 nm (C), calculated as described in Materials and Methods. (D) Differentiated THP-1 cells were infected with DsRed-expressing Mtb H37Rv strain (MOI = 0.3). At 4 dpi, cells were stained as in A. The cells having phagocytosed DsRed-Mtb (DsRed+) were first gated for their red signal and their green vs. blue CCF-4 signals were compared to the cells in the same culture that had not engulfed DsRed-Mtb (DsRed-). The results are representative of at least 3 independent experiments. Mtb-induced phagosomal rupture in dendritic cells and macrophages, relationship with the infection rate and cell necrosis
Dendritic cells (DC) and MΦ do not play the same roles during the infection. DCs that have engulfed Mtb, are more prone to process and present pathogen-derived antigens and to prime T cells than Mtb-laden MΦ which are thought to initiate the inflammatory program and are considered as long-term Mtb reservoirs. We thus comparatively evaluated the potential of Mtb to induce phagosome rupture in bone-marrow-derived (BM)-DC and -MΦ At first, by using fluorescent DsRed WT and ΔESX-1 Mtb variants, we showed similar uptake and infectivity of both strains at the beginning of the infection (Fig. 2A). Infection of BM-DC and BM-MΦ with WT Mtb then resulted in a strong blue shift at 3 dpi and thereafter, whereas for cells infected with the ΔESX-1 Mtb strain only a minor blue shift was detected (Fig. 2B). The relatively stable CCF-4 green signal and its progressively increasing blue shift for WT Mtb resulted in a blue/green ratio of 15 in BM-DC and 10 in BM-MΦ respectively, at 6 dpi (Fig. 2C). Similar as observed for THP-1 cells (Fig. 1D), infection of BM-DC with DsRed expressing Mtb showed that cells, which had engulfed DsRed Mtb, progressively increased their CCF4 blue shift over the observation period of 3 to 5 dpi (S3 Fig.). Together, these results suggest that ESX-1-dependent, Mtb-induced phagosomal rupture does occur in DC and MΦ.
Fig. 2. Mtb-mediated phagosome disruption in different phagocyte types, relationship with infection dose and cell death. 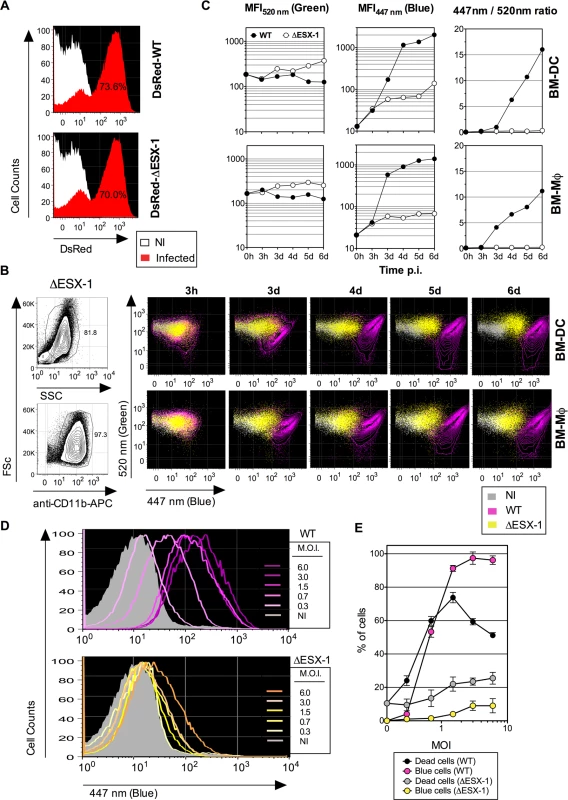
(A) Comparative infectivity of WT and ΔESX-1 Mtb. BM-DC were infected with DsRed-WT or-ΔESX-1 strain and the red fluorescence was assessed by cytometry at 1 dpi. Percentages of cells having phagocytized DsRed-mycobacteria are indicated. (B) Detection of phagosomal rupture subsequent to infection with WT or ΔESX-1 strains, as determined by green vs. blue CCF-4 signals in BM-DC or BM-MΦ, infected with untagged Mtb, WT or ΔESX-1 (MOI = 1) at different time points, as detected after exclusion of cell debris and free bacteria by FSc/SSc gating and inclusion of CD11b+ cells. (C) MFI520 nm, MFI447 nm and MFI447 nm/MFI520 nm ratios in infected BM-DC or BM-MΦ at different time points. (D) Phagosomal rupture, monitored at 4 dpi by CCF-4 staining, in BM-DC infected with different MOI of WT or ΔESX-1 Mtb. (E) Percentages of dead cells, as determined by the use of Pacific Blue Dead/Live reagents, compared to those of cells displaying a CCF-4 blue shift. Due to the emission overlap of CCF-4-Coumarin and Pacific Blue fluorochromes, the two different assays were performed in separate tubes in parallel, in cells from the same BM-DC cultures. The results are representative of 2 independent experiments. Of note, the decrease of the dead cell percentage for WT Mtb at very high MOI is likely due to generation of cell debris, not anymore measurable by cytometry. To ascertain that the absence of FRET inhibition in cells infected with the ΔESX-1 Mtb mutant was not due to other molecular reasons than the absence of the ESX-1 secretion system, we complemented the Mtb ΔESX-1 strain with the integrative cosmid p2F9, containing 32 kb of the ESX-1 encoding genomic region from Mtb H37Rv [42]. This complementation reconstituted the ability of the resulting strain to induce phagosomal rupture, and thereby validated the ΔESX-1 mutants used throughout this study (S2C Fig.).
When uncontrolled inside the host cell, Mtb infection may lead to necrosis [27,43], which could theoretically allow exchanges between phagosome and cytosol and thereby establish a contact between mycobacterial β-lactamase located within the phagosome and CCF-4 located inside the cytosol. To investigate this key question, we determined whether the cytosolic access of Mtb was a consequence of host cell necrosis. In a dose-response experiment, changes in the FRET signal for the Mtb WT strain were seen as a function of the MOI (Fig. 2D). Except for an MOI below 1, the proportions of BM-DC displaying FRET inhibition were higher than the percentages of necrotic cells (Fig. 2E). In contrast, BM-DC infected with Mtb ΔESX-1 at the same MOIs displayed much weaker CCF-4 blue shifts. These data suggest that ESX-1-mediated phagosomal rupture progressively occurs in phagocytes in an MOI-dependent manner and that the resultant presence of mycobacterial β-lactamase activity in the host cell cytosol does not arise from host cell necrosis but rather precedes cell death.
Early minor levels of phagosome disruption and their full proportionality with type I IFN production
So far, Mtb-induced phagosomal rupture has only been observed at later stages of infection, i.e, 3–5 dpi, a kinetic situation, which cannot explain the very early, ESX-1-dependent release of type I IFNs or IL-1β, that requires recognition of mycobacterial components by the host cytosolic sensors [44]. However, our highly sensitive approach allowed now detection of minor levels of FRET inhibition indicated by enhanced MFI447 nm (blue), as early as 3 hours post infection (hpi) with WT Mtb (Fig. 3A-B). The blue shift then progressively increased at 24 and 48 hpi, although it remained still low compared to values obtained for later time points (Fig. 2B-C). Comparison of these results with those from infection experiments using the Mtb ΔESX-1 deletion mutant, which overall showed much lower MFI447nm (blue) values (Fig. 3B), suggests that Mtb-mediated phagosomal rupture begins already at such early time-points, likely caused by initial ESX-1-induced pore forming activity, and progresses into stronger phagosomal disassembly over time. These findings suggest that the time during which the Mtb-infected host cell displays phagosomal rupture and Mtb cytosolic access, prior to host cell death, is longer than previously estimated [15].
Fig. 3. Early Mtb-mediated phagosomal rupture, relationship with secretion of type I IFNs. 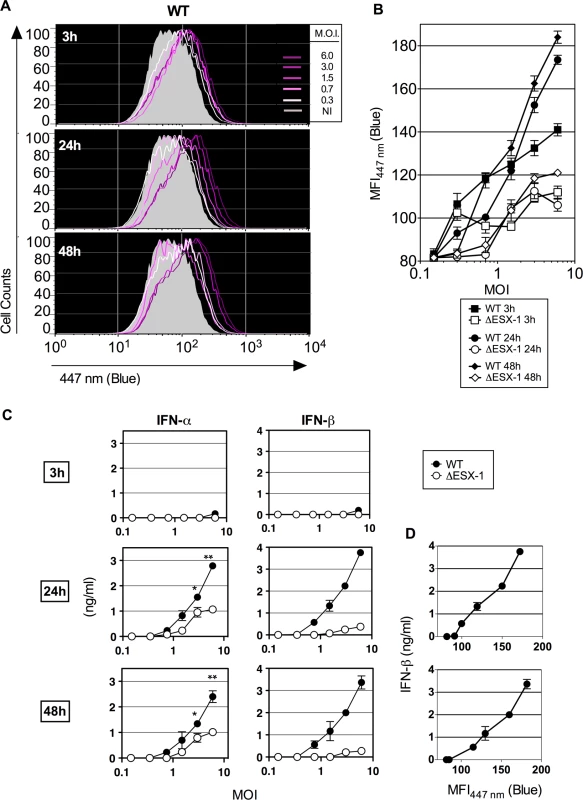
(A) BM-DC were infected with Mtb WT at different MOI and the phagosomal rupture was assessed by CCF-4 staining at early time points of 3, 24 and 48 hpi. (B) MFI447 nm of the infected cells at each time point and for different MOI of WT or ΔESX-1 strain. (C) IFN-α and-β concentrations, as quantified in the supernatants of the same infected cells by ELISA. *, ** = statistically significant, p<0.01 or p<0.001, respectively, as determined by the Student's t test. (D) Linear relationship between the amounts of IFN-β produced and Mtb-induced phagosomal rupture in BM-DC. Shown are representative data from 2 independent experiments. Considering the long Mtb replication time of ≈ 20h, such early initiation of Mtb-mediated phagosomal rupture suggests that this phenomenon does not depend on bacterial replication, but on the functions of the implicated bacterial virulence factors. The levels of phagosome disruption were entirely proportional to the amounts of secreted IFN-β (Fig. 3C). A partially ESX-1-dependent increase in the IFN-α secretion was also detected, which might be linked to the induction of Irf7 subsequent to IFN-β induction [45]. Therefore, minute levels of early phagosomal rupture are in direct correlation with the kinetics of the induction of type I IFN production. In contrast, no differences were found between ESX-1-proficient and ΔESX-1 Mtb strains when IL-1β secretion was studied (S4 Fig.), which is consistent with the inflammasome/caspase-1-independent IL-1β secretion in mice during Mtb infection [46] and which is different to the situation in humans [36].
Link between the phagosomal environment and the ability to induce phagosomal rupture
We next evaluated whether the characteristic Mtb-mediated partial inhibition of phagosome acidification was connected to the phenomenon of phagosome rupture. Given the previously established role of Natural resistance-associated macrophage protein (Nramp)-1, a phagosomal bivalent cation transporter, in phagosomal acidification and pH regulation [47–49], we evaluated its possible impact on mycobacteria-mediated phagosomal rupture. We thus used Mtb WT or ΔESX-1 strains to infect cells from the murine MΦ cell line Raw264.7, deficient in functional Nramp-1, which had been transfected with a non-functional nramp-1S (Sensitive) or a functional nramp-1R (Resistance) allele [50]. At 3 dpi, intense CCF-4 blue shifts were observed in WT Mtb-infected parental Raw264.7 cells and Raw264.7::Nramp-1S cells, whereas much less FRET inhibition was detected in Raw264.7::Nramp-1R cells (Fig. 4A-C). As assessed for various MOI, the intracellular mycobacterial load inside parental, Nramp-1S - or Nramp-1R-transfected Raw264.7 cells was comparable at 3 dpi, when the phagosomal rupture was monitored (Fig. 4D). Thus, the functional Nramp-1R seems to provide protection against Mtb-induced phagosomal rupture for the benefit of the host cell. The Nramp-1-mediated rescue of the host cells occurred at any MOI and independently of the host cell proliferation rate, which as we noticed, both influence the control of the infection (S5 Fig.). We obtained further confirmation of our results by using an nramp-1 gene silencing strategy in Raw264.7::Nramp-1R cells (Fig. 4E), which reversed the phenotype and promoted Mtb-mediated phagosomal rupture (Fig. 4F-G).
Fig. 4. Host Nramp-1 transporter counteracts the phagosomal rupture in Mtb-infected MΦ. 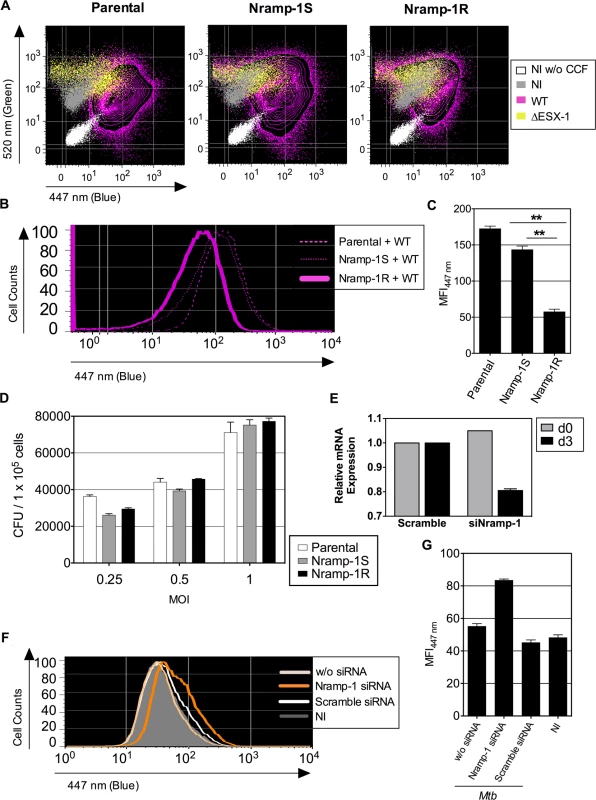
(A) Raw264.7 cells, parental or transfected with non-functional nramp-1S or functional nramp-1R allele, were infected with Mtb, WT or ΔESX-1 (MOI = 1). At 3 dpi, phagosomal rupture was monitored in CD11b+ cells. (B, C) The blue CCF-4 signal overlays (B) and MFI447 nm (C) are plotted for different Raw264.7 cell lines infected with Mtb WT. ** = statistically significant, as determined by the Student's t test, p<0.001. (D) Mycobacterial loads in different Raw264.7 cell lines, infected with various MOI of WT Mtb, as determined at 3 dpi. (E-G) Raw264.7::nramp-1R were transfected with Nramp-1-specific or scramble siRNA and the effective gene silencing was checked 3 days later by qRT-PCR (E). The siRNA-treated Raw264.7::nramp-1R cells were then infected with WT Mtb (MOI = 1) and studied for phagosomal rupture at 3 dpi. The blue CCF-4 signal (F) and the MFI447 nm (G) are plotted. The results are representative of at least 3 experiments. We further treated Raw264.7::Nramp-1R cells or, as primary phagocytes, BM-DC from Sv129 (nramp-1R) mice with bafilomycin, a specific inhibitor of vacuolar proton ATPases, prior to infection with WT Mtb H37Rv. As shown in Fig. 5A-B, the bafilomycin-mediated reduction of phagosomal acidification resulted in enhanced phagosomal rupture in both cell types. This observation provides additional evidence for a link between restriction of phagosome acidification and the strength of observed phagosomal rupture. In this FRET-based method, the β-lactamase operates on CCF-4 located in the host cytosol, where the pH remains neutral [25,41]. However, to further ascertain that the micro-environmental acidity did not affect the functionality of mycobacterial BlaC, we tested the β-lactamase enzymatic activity of Mtb at different pH levels by the use of nitrocefin, a chromogenic β-lactamase substrate. These experiments confirmed that Mtb, grown at different pH, ranging from 5 to 7, preserves entirely its β-lactamase enzymatic activity (Fig. 5C).
Fig. 5. Inhibition of phagosomal acidification intensifies phagosomal rupture in Mtb-infected phagocytes. 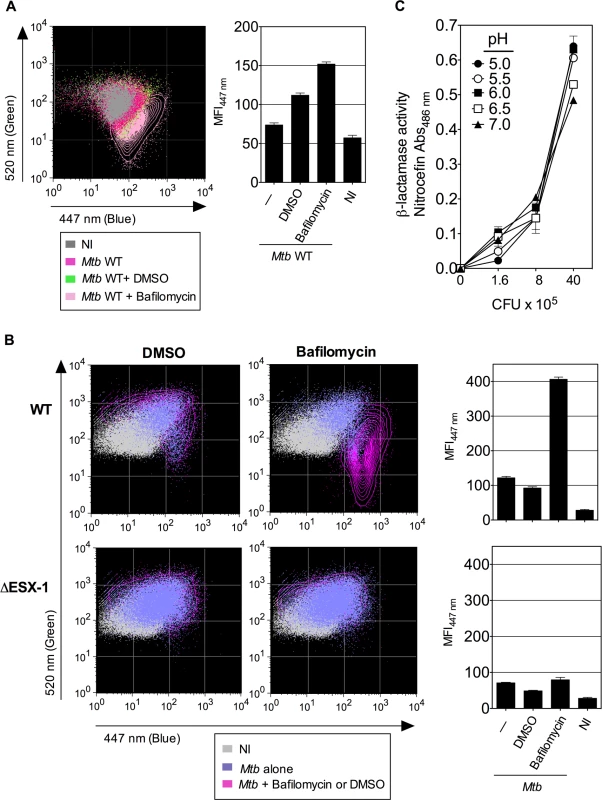
(A-B) Raw264.7::nramp-1R cells (A) or BM-DC from Sv129 nramp-1R mice (B) were treated with 20 nM of bafilomycin or DMSO 1h before infection with WT or ΔESX-1 Mtb (MOI = 1) and were assessed for phagosomal rupture at 4 dpi. (C) The intrinsic β-lactamase activity of WT Mtb, grown in Dubos broth with various pH, as measured by nitocephin, a chromogenic β-lactamase substrate. The results are representative of 2 experiments. Thus, acidification of the phagosomal lumen seems to be a critical host cell parameters, which exerts an antagonistic effect on Mtb-mediated phagosomal rupture in phagocytes. The finding that both phenomena are linked provides a new basis for elucidating the molecular key players that govern the host-pathogen interaction during Mtb infection.
ESX-1-dependent Mtb-mediated phagosome disruption in pulmonary phagocytes and in vivo in lungs and spleen of infected mice
Previous studies on vacuolar rupture and phagosomal escape of M. marinum [12,51] and Mtb [13,15,16] used infected MΦ or DC under in vitro conditions. To extend our investigations towards cells from the lung, we examined the Mtb-mediated phagosomal rupture in different phagocyte types of mouse airways. To this end, low-density cells isolated from mouse lung parenchyma were infected ex vivo at an MOI of 1 with ΔESX-1 or WT Mtb strains. CCF-4 signals obtained from monocytes/MΦ (CD11bhi CD11c-) and DC (CD11bint CD11c+) were analyzed at 4 dpi, when changes in the FRET signal were detected in lung monocytes/MΦ and DC (Fig. 6A), showing the occurrence of Mtb-mediated phagosomal rupture in the primary lung phagocytes.
Fig. 6. Mtb-mediated phagosomal rupture in different cell subsets ex vivo and in vivo. 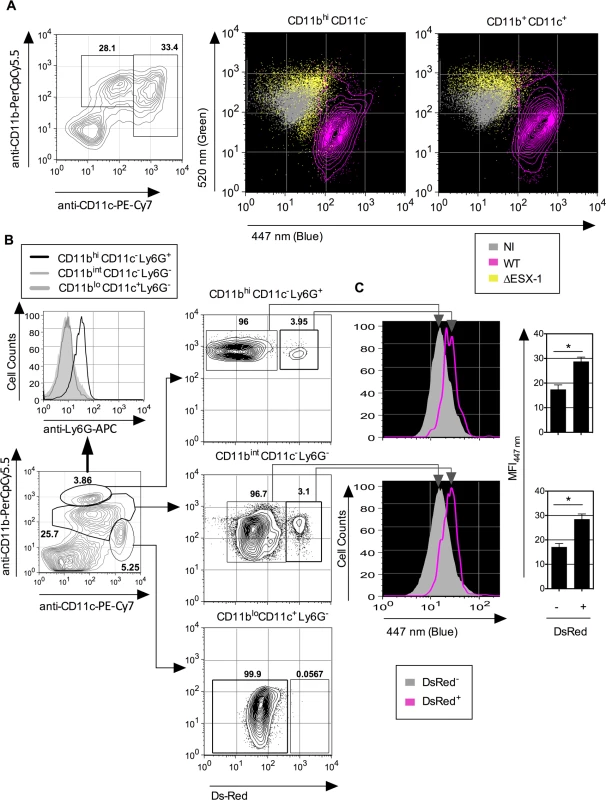
(A) Low-density cells were isolated from C57BL/6 mouse lung parenchyma and infected ex vivo with WT or ΔESX-1 Mtb (MOI = 1). Monocytes/MΦ (CD11b+ CD11c-) and DC (CD11bint CD11c+) were assessed for CCF-4 signals at 4 dpi. (B) Phagosomal rupture detected in vivo in different Mtb-infected phagocyte subsets. C57BL/6 mice (n = 3) were injected i.v. with 1 x 106 CFU of DsRed WT Mtb and at 3 weeks post infection. Alive low-density cells were isolated on Optiprep gradient from the spleen and were sequentially stained with CCF-4 and a cocktail of mAbs to distinguish neutrophils (CD11bhi CD11c- Ly6G+), MΦ/monocytes (CD11bint CD11c- Ly6G-) or DC (CD11blo CD11c+ Ly6G-). (C) Inside each innate cell subsets, the blue CCF-4 signals of the DsRed+ and DsRed- cells were compared together. The results are representative of 2 independent experiments. To assess the relevance of mycobacteria-mediated phagosomal rupture in phagocytes in vivo, in a first attempt we used T-/B-cell deficient recombination activation gene (rag) 2 knock-out mice in which infection with Mtb is more persistent and the innate cell compartments more developed than in their immunocompetent counterparts. However, flow cytometric analysis of lung - or spleen-derived MΦ/monocytes, DC and neutrophils obtained from infected (1 x 106 CFU i.v. /mouse of WT or ΔESX-1 Mtb) or uninfected rag2°/° mice displayed indistinguishable CCF-4 blue profiles (S6 Fig.). The apparent failure in the detection of phagosomal rupture in this experimental setting seems to be related to the very low frequencies of mycobacteria-infected cells within each innate cell subset and/or a possible furtive feature of the phenomenon in vivo due to possible efferocytosis [52] of the primary phagocytes, in which phagosomal rupture and certain damage signals would have been initiated.
To distinguish infected and non-infected cells, we then used fluorescent DsRed-WT Mtb (1 x 106 CFU/mouse) for intravenous (i.v.) infection of C57BL/6 mice, which allowed us to focus on the relatively few Mtb-infected phagocytes present during the initial phase of chronic infection. At 3 weeks p.i. mice were sacrificed, the spleens homogenized and resulting cells enriched and subjected to flow cytometric analysis. We have focused on the phagocytes of the spleen because this organ is particularly targeted by the i.v. route of infection. When the CCF-4 blue signal of the innate immune cells that contained DsRed Mtb was compared to the other cells inside each cell subset in the spleen (Fig. 6B), a slight increase in CCF-4 blue signal was notably detected in Mtb-containing cells in the subsets of neutrophils (CD11bhiCD11c-Ly6G+) and MΦ/monocytes (CD11bintCD11c-Ly6G-) (Fig. 6C), which suggests the occurrence of weak, albeit reproducible, levels of phagosomal rupture in these infected cells. Interestingly, no DsRed+ cells were detected inside the CD11bloCD11c+Ly6G- DC subset, which might be due to possible rapid turnover of infected DC or to their CD11b up-regulation. In this chronic infection model, it was however not possible to compare WT and ΔESX-1 Mtb strains, because of the non-persistence of the latter. To overcome this limitation we developed an alternative in vivo model whereby mice were instilled intra-nasally with cells that were infected with Mtb in vitro prior to transfer, and whose infection status in vivo could be specifically monitored. To this end, BM-DC from mice with CD45.1 hematopoietic allelic marker were infected in vitro with WT or ΔESX-1 Mtb, in conditions that allowed up to 70% of the cells to be infected (Fig. 2A), whereas control cells were left uninfected. At 16 hpi, the cells were instilled into the airways of congenic CD45.2 recipients. At different time points post-transfer, the lung low-density cells were isolated and the CCF-4 blue shift in the CD11b+ CD45.1 cell subset of the different experimental groups assessed (Figs. 7A and S7). Strikingly, at day 4 and day 6 post-transfer, in the CD11b+ CD45.1 population infected with WT Mtb, a blue shift was detected in comparison to the non-infected or ΔESX-1-infected transferred cells (Fig. 7B-C).
Fig. 7. Detection of phagosomal rupture in vivo in Mtb-infected phagocytes. 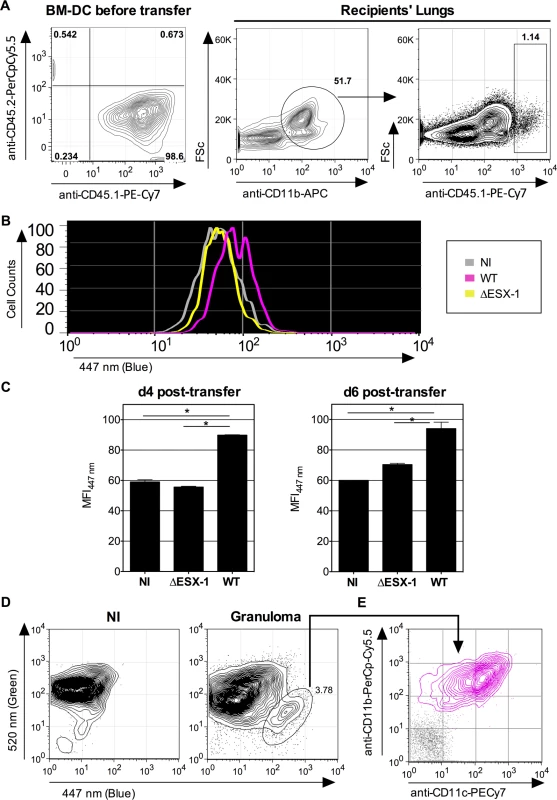
(A-B) BM-DC from CD45.1 donors were left non-infected or were infected with Mtb WT or ΔESX-1 (MOI = 1) for 16h. Of note, cultures of CD45.1 BM-DC, infected with DsRed-mycobacteria in the same conditions, showed that >70% of cells had uptaken mycobacteria like shown in Fig. 2A. Cells were recovered and transferred i.n. (2 x 106 cells/mouse) into CD45.2 congenic recipients. (B) Four days post-transfer, alive lung low-density cells from the recipients (n = 3/group) were isolated on Optiprep gradient and incubated with mAbs specific to CD11b and CD45.1, subsequent to incubation with CCF-4. The blue signals from the three experimental groups are overlaid. (C) Histograms show the comparative blue CCF-4 signals in the CD11b+ CD45.1+ cells from different groups at days 4 or 6 post transfer. * = statistically significant, as determined by the Student's t test, p<0.01. (D) Lung granuloma from C57BL/6 mice, infected via aerosol route with ≈200 CFU/mouse of WT Mtb, were removed at 6 weeks p.i. and were treated with collagenase and DNAse-I and enriched in low-density cells. In parallel to these cells, lung low-density cells from uninfected controls were assessed for CCF-4 blue switch. (E) CD11b vs. CD11c surface expression of the cells showing the increased CCF-4 shift (pink), compared to unstained negative control incubated with control Ig isotypes (gray). The results are representative of 2 independent experiments. Moreover, independent flow cytometric examination of cells extracted directly from surface lung granuloma tissue of Mtb-infected C57BL/6 mice revealed a small, distinct cell population that displayed a clear-cut blue signal and a CD11b+ CD11c+ phenotype (Fig. 7D), which points to the presence of innate cells in these lungs wherein Mtb-mediated phagosomal rupture had occurred.
Altogether, our data suggest that the Mtb-induced phagosomal rupture does indeed happen in vivo, in Mtb-infected cells in the organs of small laboratory animals. The detected phagocytes containing intracellular bacteria seem to have a life-time of several days, which however does not exclude the possibility that a portion of the total number of infected phagocytes might get eliminated by efferocytosis [52], as suggested by the relatively modest differences in blue shift observed in the in vivo settings.
Discussion
The pathogenic potential of Mtb is intimately linked to the interplay between the host defense and the persistence of the mycobacteria. The intracellular localization and cytosolic access of the bacterium has substantial consequences on the recognition of mycobacteria-associated patterns by the cytosolic receptors of the innate immunity that determine innate and adaptive immune responses and ultimately the fate of the host cell and the bacterium [27]. Subsequent to phagocytosis, in order to avoid the acidified environment generated by the phagosome-lysosome fusion, some specialized intracellular bacteria, such as S. flexneri, Listeria monocytogenes or Francisella tularensis, evolved to rapidly escape from phagosomes into the cytosol [21,53,54]. In contrast, Mtb has been described as a bacterium that resists degradation in the phagosome by inhibiting the fusion with lysosomes, a characteristic feature that seems to protect the bacilli from bactericidal mechanisms of the phagocytes and allows intracellular survival and multiplication [10,18,55–57]. However, recent reports based on in vitro infection of phagocytes also suggest that at later stages of infection ESX-1-dependent vacuolar breakage might be an important requirement for the pathogenic potential of Mtb, given that ESX-1-deficient bacilli that are unable to perforate and lyse the phagosomal membrane are—in general—attenuated [13,15,16,18,56–59].
In previous studies, Mtb-mediated phagosomal escape has only been reported at late time points like 2–5 dpi, a kinetic feature that was not reconcilable with the intracellular host immune events, like type I IFN induction, which require the early recognition of mycobacterial components by cytosolic sensors. Here, the use of highly sensitive FRET-based cytometry enabled us to highlight minor levels of cytosolic contact of Mtb and its products initiated as soon as 3 hpi, which is kinetically concordant and proportional with the amounts of IFN-β released by DC. While we cannot exclude the possibility that some of this effect may have been caused by bacterial products translocating through permeable phagosomal membranes [30], the reproducible differences observed between the WT and the ΔESX-1 Mtb strains argue for a specific, ESX-1-mediated impact. We also noted that distinct cell types might display different susceptibility to phagosomal rupture, with THP-1 cells as the most susceptible ones, followed by BM-DC/BM-MΦ, and the Raw264.7 MΦ as the least affected cell types, tested.
Our results show that the phagosomal bivalent cation transporter Nramp-1 interferes with Mtb-induced phagosomal rupture as observed at 3 dpi, i.e., a time point at which mycobacterial loads were still comparable in Mtb-infected MΦ harboring Nramp-1S (non functional) - or Nramp-1R (functional) allelic forms. In line with that, the effect of bafilomycin, reported to inhibit phagosomal acidification [60], reconstituted in Nramp-1R-proficient phagocytes the capacity of Mtb to enhance phagosomal rupture to the level of Nramp-1S phagocytes. Thus, the partial inhibition of phagosome acidification emerges as a prerequisite to mycobacterial phagosomal rupture. Plausibly, only when phagosome acidification is partially inhibited, mycobacteria may survive, use their virulence factors and induce phagosomal membrane disruption.
Although cellular models may provide important new insights into cell biological mechanisms, evaluation of the accuracy of the findings in an in vivo model, i.e. in tissues or organs is of crucial importance to emphasize their relevance. Previous electron microscopy analyses of lung innate cells isolated from TB patients or mycobacteria-infected mice have led to discrepancies with regards to intracellular location [18]. In alveolar MΦ of TB patients and in granuloma or lung homogenates of infected mice, Mtb has been detected as single bacterium or pairs of bacilli inside phagosomes [61,62], whereas Mtb has also been observed in membrane-disrupted compartments or free in the cytosol in the mouse granulomas [63,64]. Moreover, heavily infected human alveolar MΦ [62] and damaged mouse MΦ of inflammatory sites [65] contain multiple mycobacteria per phagosome. In this context, our results from carefully designed in vivo infection experiments add new elements to the discussion. Although the strength of the FRET-inhibition was found weaker under in vivo conditions (Figs. 6 and 7) than observed for the cell culture-based infection assays (Figs. 1 and 2), the reproducibility and complementarity of the results from the three distinct in vivo settings analyzed, point to biological relevance of mycobacteria-induced phagosomal rupture in the organs of Mtb-infected laboratory animals. It should be noted that in our experiment with BM-DC from mice with the CD45.1 hematopoietic allelic markers (Fig. 7), we cannot exclude that in the infected DC some minor cytosolic contact might develop already in vitro, prior to their instillation to the CD45.2 recipient mice. However, the finding that FRET inhibition remains detectable for several days after the transfer into the lungs of the CD45.2 recipients suggests that the phagocytes in which cytosolic access of Mtb progressively builds up, can survive in the host environment for some days. Together with ex vivo results from MΦ/monocytes and DC isolated from the lung parenchyma, the in vivo demonstration of cytosolic access of Mtb provides important new insights into the cellular events during infection inside the organs. Our data suggest that after infection, the concerned phagocytes may persist in the organs long enough to have a potential impact on host defense mechanisms that likely also include key cellular processes, such as autophagy, which requires Mtb ubiquitination in an ESX-1-dependent manner [16,33,38,39].
The intracellular localization of mycobacteria and mycobacteria-mediated phagosomal rupture have been subject of numerous controversies, which may be explained by the differences between the level of virulence of mycobacterial strains used, the MOI and the conditions of the mycobacterial cultures in vitro [18]. For the virulent strains, here we used WT and DsRed Mtb previously passaged in immunocompetent mice to maintain a normal degree of virulence and to remain as close to natural infection as possible. We only used mycobacterial cultures in mid-log10 growth phase to minimize bacterial mortality, and we cultured the bacteria in the presence of Tween 80 to avoid clumping, as phagocytosis of non-viable or clumped mycobacteria may lead to rapid phagosome-lysosome fusion and prevent visualization of phagosomal rupture [18]. In addition, we systematically compared the ESX-1-proficient and ESX-1-deficient mycobacterial strains and detected a relevant phagosomal rupture only with ESX-1-proficient strains.
Previous observations with numerous virulent and attenuated Mtb strains suggest that the capacity of a strain to induce phagosomal rupture in vitro is often correlated with its virulence [15,16]. Hence, the ESX-1-dependent, mycobacteria-induced phagosomal rupture emerges as a major characteristic feature of Mtb infection, which likely initiates the first damages caused by this intracellular pathogen to the host cell. Consequently, modulation of the parameters, which orchestrate this phenomenon, may constitute a promising base for vaccinal or therapeutic interventions against TB. For example, we have previously noticed that recombinant BCG and M. microti strains with a reconstituted ESX-1 secretion system showed enhanced protective efficacy [66,67]. More recently, a dedicated study identified small molecule inhibitors belonging to the benzyloxybenzylidene-hydrazine and the benzothiophene chemical classes, which interfered with ESAT-6 secretion and thereby protected host cells from Mtb-induced lysis [68]. Molecules belonging to closely related chemical scaffolds were also identified in a high content phenotypic screen as agents that interfered with the intracellular growth and the virulence of Mtb [69]. Hence, it is conceivable that future phenotypic library screening might identify novel pharmacological compounds that inhibit Mtb-mediated phagosomal rupture in the host cell. Such molecules would represent interesting anti-virulence compounds to be tested as addition to conventional treatment regimens against TB.
In conclusion, our study suggests that Mtb is not the passive pathogen that induces pathology only by the over-boarding reaction of the host immune system. We show that ESX-1-mediated phagosomal rupture contributes in a significant way to establish mycobacterial cytosolic contact, which is however only possible if the maturation / acidification of the phagosome is limited in a first process. In this direction, our study also opens new perspectives for future studies on the mycobacterial components involved in the modulation of phagosomal acidification such as the phthiocerol dimycocerosates and other mycobacterial factors, reported to intervene in this process [70,71].
The ESX-1 system might thus represent one of the final members in a chain of virulence factors that determine the pathogenicity of Mtb through the induction of phagosomal rupture, and its function might therefore have been evolutionary preserved [5,7]. As such, our work has the potential to reconcile the outcome of previous studies on mycobacterial virulence factors that interfere with vacuolar acidification [71–74] and studies on cellular localization of Mtb [13–16] and establishes Mtb-mediated phagosomal rupture as a basic biological mechanism involved in TB pathogenesis.
Materials and Methods
Animal infection model
C57BL/6 mice, rag2°/° or CD45.1 were obtained from Animal Facilities of Institut Pasteur. C57BL/6 mice were purchased from Janvier Le Genest-Saint-Isle France). CD45.2 mice were anesthetized by i.p. injection of 100 mg/kg Ketamine (Lyon, France) and 10 mg/kg Xylazine (KCP Kiel, Germany) before cell transfer by i.n. route. Mouse infection with Mtb via aerosol route was performed as previously described [75]. Granuloma were recovered from the surface lung parenchyma of infected C57BL/6 mice at 6 weeks p.i.
Ethics statement
Mouse studies were approved by the Institut Pasteur Safety Committee, in accordance with French and European guidelines and regulations (Directive 86/609/CEE and Decree 87–848 of 19 October 1987) and the Animal Experimentation Ethics Committee Ile-de-France-1 (reference number 2012–0005).
Cell cultures
THP-1 cells (our laboratory stock collection, initially originating from ATCC provided cells) were maintained in RPMI, complemented with 10% heat-inactivated FBS and were treated with 20 ng/ml of Phorbol 12-Myristate 13-Acetate for 72h to induce their differentiation into MΦ. Raw264.7 cells transfected with nramp-1S or -1R allele (kind gift of Pr J. Blackwell) [50] were treated with 8 μg/ml of the selective antibiotic puromycin.
BM-MΦ or -DC were generated from femur hematopoietic precursors, respectively by use of M-CSF or GM-CSF. Rat anti-mouse IFN-α mAb (RMMA-1), biotinylated polyclonal rabbit anti-mouse IFN-α (R&D), rat anti-mouse IFN-β (8.S.415) (LifeSpan BioSciences) and biotinylated polyclonal rabbit anti-mouse IFN-β (R&D) were used to quantify the cytokines produced in the culture supernatants by ELISA.
Mtb cultures and cell infection
Mtb H37Rv, WT, ΔESX-1 (kind gift of Pr. W. Jacobs) [10] or ΔESX::ESX-1 [42] were maintained in 7H9 medium supplemented with ADC (Difco). Seven-to-10 days before cell infection, bacteria were transferred into Dubos medium, which contains Tween 80, to avoid mycobacterial clumping. DsRed-WT or -ΔESX-1 strains were obtained by complementation with the pMRF plasmid containing a DsRed cassette, under the hsp60 promoter (kind gift of Dr. S. Cho) and were cultured in the continuous presence of 20 μg/ml of the selective antibiotic kanamycin. In in vivo experiments, we used an Mtb H37Rv strain with a plasmid containing the DsRed and hygromycin resistance genes (kind gift of Dr. O. Neyrolles). Only mycobacteria grown to mid-log10 phase were used to minimize the frequency of death bacteria.
Raw264.7 cells were infected at various MOI with Mtb in complete antibiotic-free RPMI. At 3 dpi, equal numbers of cells were lysed by addition of 0.1% Triton X-100 in PBS and the intracellular CFU was determined by plating serial dilutions of cell lysates on 7H9 Agar medium and incubation at 37°C for 3 weeks.
CCF-4 assay and flow cytometry
The principle of the β-lactamase CCF-4 FRET assay is summarized in S1 Fig.. To measure the Mtb phagosomal rupture, cells were stained during 1h at RT, with 8 μM CCF-4 (Invitrogen) in EM buffer (120 mM NaCl, 7 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 5 mM glucose and 25 mM Hepes, pH 7.3) complemented with 2.5 μM probenecid. Cells were then stained with anti-CD11c-PE-Cy7, anti-CD11b-PerCp-Cy5.5 (eBiosciences) or anti-CD11b-APC (BD) mAbs andfixed with 4% PFA overnight at 4°C. Cell mortality in the same cultures of infected cells was determined by use of Pacific Blue Dead/Live reagent (Invitrogen), which reacts with free amines both inside and outside of the plasma membrane, yielding log10 1 more intense fluorescent staining of dead cells. Anti-CD45.1-PE-Cy7 and anti-CD45.2-PerCpCy5.5 were from eBiosciences. To avoid fluorochromes with emission signals overlapping with those of CCF-4 (λem 500–550 nm and λem 410–470 nm), APC (λem 660 nm)-, PerCp-Cy5.5 (λem 696 nm) - or PE-Cy7 (λem 778 nm)-conjugated mAbs were chosen for concomitant cell surface staining. Cells were analyzed in a CyAn cytometer using Summit software (Beckman Coulter, France). At least 100,000 events per sample were acquired for in vitro assays. For in vivo detection of CCF-4 signal in CD45 congenic mouse model, 1,000,000 events per sample have been acquired. Data were analyzed with FlowJo software (Treestar, OR).
Gene silencing
siRNA transfection to cells was performed by using reverse transfection method. A pool of four Nramp-1-specific siRNAs, GGUCAAGUCUAGAGAAGUA, GAUCCUAGGCUGUCUCUUU, GGGCGACUGUGCUAGGUUU and GAAGUCAUCGGGACGGCUA, at final concentration of 50 nM, was mixed with 6 μl of lipofectamine (Invitrogen) in 500 μl of PBS in 6-well plates. After 30 min incubation at RT, 3 x 105 cells contained in 2 ml of complete RPMI were added to the mixture and incubated for 3 days at 37°C. The efficiency of gene silencing was determined by qRT-PCR before the infection. One mg of total RNA was transcribed into cDNA. Then, 4 μL of cDNA was tested by qRT-PCR with LightCycler 480 SYBR Green using GCCACTGTGCTAGGTTTGCT and AATGGTGATCAGTACACCGC primers. All experiments were run in triplicate and the Livak method [76] was applied for relative quantification with β-actin.
Mycobacterial β-lactamase activity assay
The β-lactamase activity of Mtb, grown in Dubos broth with various pH, was measured by use of the chromogenic β-lactamase substrate, nitrocefin. Briefly 1 x 106 bacteria, re-suspended in 100 μl of Dubos broth at indicated pH, were incubated in 96-well plates with 50 μl of nitrocefin, reconstituted at 0.5 mg/ml in PBS which contained 5% DMSO. Absorbance by nitrocefin at 486 nm was measured after 3 hours of incubation at 37°C.
Enrichment of innate immune cells
Lungs or spleen were removed aseptically and were digested by treatment with 400 U/ml type IV collagenase and DNase I (Roche). Following a 45 min incubation at 37°C, single-cell suspensions were prepared by use of a Gentle Macs (Miltenyi) and by passage through 100-μm nylon filters (Cell Strainer, BD Falcon). When indicated, cell suspensions were enriched in low-density cells on iodixanol gradient medium (OptiPrep, Axis-Shield), according to the manufacturer’s protocol. Notably this gradient only selects alive cells, as confirmed by blue Trypan exclusion assay. These cells were either used directly in flow cytometry analyses or were plated in 12 well culture plates in complete RPMI to be infected ex vivo with mycobacteria.
Supporting Information
Zdroje
1. Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, et al. (2007) Type VII secretion system of mycobacteria show the way. Nat Rev Microbiol 5 : 883–891. 17922044
2. Majlessi L, Prados-Rosales R, Casadevall A, Brosch R (2015) Release of mycobacterial antigens. Immunol Rev 264: (1–21; in press).
3. Bitter W, Houben EN, Bottai D, Brodin P, Brown EJ, et al. (2009) Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog 5: e1000507. doi: 10.1371/journal.ppat.1000507 19876390
4. Houben EN, Korotkov KV, Bitter W (2014) Take five—Type VII secretion systems of mycobacteria. Biochim Biophys Acta 1844 : 1707–1716.
5. Gonzalo-Asensio J, Malaga W, Pawlik A, Astarie-Dequeker C, Passemar C, et al. (2014) Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc Natl Acad Sci U S A 111 : 11491–11496. doi: 10.1073/pnas.1406693111 25049399
6. Supply P, Marceau M, Mangenot S, Roche D, Rouanet C, et al. (2013) Genomic analysis of smooth tubercle bacilli provides insights into ancestry and pathoadaptation of Mycobacterium tuberculosis. Nat Genet 45 : 172–179. doi: 10.1038/ng.2517 23291586
7. Boritsch EC, Supply P, Honore N, Seeman T, Stinear TP, et al. (2014) A glimpse into the past and predictions for the future: the molecular evolution of the tuberculosis agent. Mol Microbiol 93 : 835–852. doi: 10.1111/mmi.12720 25039682
8. Stinear TP, Seemann T, Harrison PF, Jenkin GA, Davies JK, et al. (2008) Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res 18 : 729–741. doi: 10.1101/gr.075069.107 18403782
9. de Jonge MI, Pehau-Arnaudet G, Fretz MM, Romain F, Bottai D, et al. (2007) ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol 189 : 6028–6034. 17557817
10. Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, et al. (2003) The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A 100 : 12420–12425. 14557547
11. Smith J, Manoranjan J, Pan M, Bohsali A, Xu J, et al. (2008) Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun 76 : 5478–5487. doi: 10.1128/IAI.00614-08 18852239
12. Stamm LM, Morisaki JH, Gao LY, Jeng RL, McDonald KL, et al. (2003) Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J Exp Med 198 : 1361–1368. 14597736
13. van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, et al. (2007) M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129 : 1287–1298. 17604718
14. Hagedorn M, Rohde KH, Russell DG, Soldati T (2009) Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science 323 : 1729–1733. doi: 10.1126/science.1169381 19325115
15. Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, et al. (2012) Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog 8: e1002507. doi: 10.1371/journal.ppat.1002507 22319448
16. Houben D, Demangel C, van Ingen J, Perez J, Baldeon L, et al. (2012) ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol 14 : 1287–1298. doi: 10.1111/j.1462-5822.2012.01799.x 22524898
17. Fortune SM, Rubin EJ (2007) The complex relationship between mycobacteria and macrophages: it's not all bliss. Cell Host Microbe 2 : 5–6. 18005712
18. Harriff MJ, Purdy GE, Lewinsohn DM (2012) Escape from the phagosome: The explanation for MHC-I processing of mycobacterial antigens? Front Immunol 3 : 40.: doi: 10.3389/fimmu.2012.00040 22566923
19. Molloy S (2012) BACTERIAL PATHOGENESIS TB blurs the lines. Nature Reviews Microbiology 10 : 442–442. doi: 10.1038/nrmicro2825 22699958
20. Friedrich N, Hagedorn M, Soldati-Favre D, Soldati T (2012) Prison break: pathogens' strategies to egress from host cells. Microbiol Mol Biol Rev 76 : 707–720. doi: 10.1128/MMBR.00024-12 23204363
21. Ray K, Bobard A, Danckaert A, Paz-Haftel I, Clair C, et al. (2010) Tracking the dynamic interplay between bacterial and host factors during pathogen-induced vacuole rupture in real time. Cell Microbiol 12 : 545–556. doi: 10.1111/j.1462-5822.2010.01428.x 20070313
22. Flores AR, Parsons LM, Pavelka MS Jr. (2005) Genetic analysis of the beta-lactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to beta-lactam antibiotics. Microbiology 151 : 521–532. 15699201
23. Malen H, Pathak S, Softeland T, de Souza GA, Wiker HG (2010) Definition of novel cell envelope associated proteins in Triton X-114 extracts of Mycobacterium tuberculosis H37Rv. BMC Microbiol 10 : 132. doi: 10.1186/1471-2180-10-132 20429878
24. Charpentier X, Oswald E (2004) Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J Bacteriol 186 : 5486–5495. 15292151
25. Nothelfer K, Dias Rodrigues C, Bobard A, Phalipon A, Enninga J (2011) Monitoring Shigella flexneri vacuolar escape by flow cytometry. Virulence 2 : 54–57. doi: 10.4161/viru.2.1.14666 21317555
26. Majlessi L, Brodin P, Brosch R, Rojas MJ, Khun H, et al. (2005) Influence of ESAT-6 secretion system 1 (RD1) of Mycobacterium tuberculosis on the interaction between mycobacteria and the host immune system. J Immunol 174 : 3570–3579. 15749894
27. Behar SM, Divangahi M, Remold HG (2010) Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol 8 : 668–674. doi: 10.1038/nrmicro2387 20676146
28. Behar SM, Martin CJ, Booty MG, Nishimura T, Zhao X, et al. (2011) Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol 4 : 279–287. doi: 10.1038/mi.2011.3 21307848
29. Aguilo J, Alonso H, Uranga S, Marinova D, Arbues A, et al. (2013) ESX-1-induced apoptosis is involved in cell-to-cell spread of Mycobacterium tuberculosis. Cell Microbiol. 15 : 1994–2005. doi: 10.1111/cmi.12169 23848406
30. Nakamura N, Lill JR, Phung Q, Jiang Z, Bakalarski C, et al. (2014) Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature 509 : 240–244. doi: 10.1038/nature13133 24695226
31. Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, et al. (2005) NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog 1 : 279–285. 16322770
32. Pandey AK, Yang Y, Jiang Z, Fortune SM, Coulombe F, et al. (2009) NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog 5: e1000500. doi: 10.1371/journal.ppat.1000500 19578435
33. Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS (2012) Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11 : 469–480. doi: 10.1016/j.chom.2012.03.007 22607800
34. Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R (2011) Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol 2011 : 405310. doi: 10.1155/2011/405310 21603213
35. Shi S, Blumenthal A, Hickey CM, Gandotra S, Levy D, et al. (2005) Expression of many immunologically important genes in Mycobacterium tuberculosis-infected macrophages is independent of both TLR2 and TLR4 but dependent on IFN-alphabeta receptor and STAT1. J Immunol 175 : 3318–3328. 16116224
36. Wong KW, Jacobs WR Jr. (2011) Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell Microbiol 13 : 1371–1384. doi: 10.1111/j.1462-5822.2011.01625.x 21740493
37. Dorhoi A, Nouailles G, Jorg S, Hagens K, Heinemann E, et al. (2012) Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur J Immunol 42 : 374–384. doi: 10.1002/eji.201141548 22101787
38. Watson RO, Manzanillo PS, Cox JS (2012) Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 150 : 803–815. doi: 10.1016/j.cell.2012.06.040 22901810
39. Romagnoli A, Etna MP, Giacomini E, Pardini M, Remoli ME, et al. (2012) ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy 8 : 1357–1370. doi: 10.4161/auto.20881 22885411
40. Sokolovska A, Becker CE, Ip WK, Rathinam VA, Brudner M, et al. (2013) Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat Immunol 14 : 543–553. doi: 10.1038/ni.2595 23644505
41. Cebrian I, Visentin G, Blanchard N, Jouve M, Bobard A, et al. (2011) Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell 147 : 1355–1368. doi: 10.1016/j.cell.2011.11.021 22153078
42. Brodin P, de Jonge MI, Majlessi L, Leclerc C, Nilges M, et al. (2005) Functional analysis of early secreted antigenic target-6, the dominant T-cell antigen of Mycobacterium tuberculosis, reveals key residues involved in secretion, complex formation, virulence, and immunogenicity. J Biol Chem 280 : 33953–33959. 16048998
43. Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, et al. (2009) Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol 10 : 899–906. doi: 10.1038/ni.1758 19561612
44. Stanley SA, Johndrow JE, Manzanillo P, Cox JS (2007) The Type I IFN Response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol 178 : 3143–3152. 17312162
45. Conzelmann KK (2005) Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. J Virol 79 : 5241–5248. 15827138
46. Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, et al. (2010) Caspase-1 independent IL-1beta production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol 184 : 3326–3330. doi: 10.4049/jimmunol.0904189 20200276
47. Hackam DJ, Rotstein OD, Zhang W, Gruenheid S, Gros P, et al. (1998) Host resistance to intracellular infection: mutation of natural resistance-associated macrophage protein 1 (Nramp1) impairs phagosomal acidification. J Exp Med 188 : 351–364. 9670047
48. Vidal MJ, Stahl PD (1993) The small GTP-binding proteins Rab4 and ARF are associated with released exosomes during reticulocyte maturation. Eur J Cell Biol 60 : 261–267. 8330623
49. Forbes JR, Gros P (2001) Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol 9 : 397–403. 11514223
50. Lang T, Prina E, Sibthorpe D, Blackwell JM (1997) Nramp1 transfection transfers Ity/Lsh/Bcg-related pleiotropic effects on macrophage activation: influence on antigen processing and presentation. Infect Immun 65 : 380–386. 9009286
51. Gao LY, Guo S, McLaughlin B, Morisaki H, Engel JN, et al. (2004) A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol 53 : 1677–1693. 15341647
52. Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM, et al. (2012) Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe 12 : 289–300. doi: 10.1016/j.chom.2012.06.010 22980326
53. Barel M, Charbit A (2013) Francisella tularensis intracellular survival: To eat or to die. Microbes Infect 25 : 00206–00202.
54. Cossart P (2011) Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc Natl Acad Sci U S A 108 : 19484–19491 doi: 10.1073/pnas.1112371108 22114192
55. Armstrong JA, Hart PD (1971) Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med 134 : 713–740. 15776571
56. Leake ES, Myrvik QN, Wright MJ (1984) Phagosomal membranes of Mycobacterium bovis BCG-immune alveolar macrophages are resistant to disruption by Mycobacterium tuberculosis H37Rv. Infect Immun 45 : 443–446. 6430807
57. McDonough KA, Kress Y, Bloom BR (1993) Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun 61 : 2763–2773. 8514378
58. Creasey EA, Isberg RR (2014) Maintenance of vacuole integrity by bacterial pathogens. Curr Opin Microbiol 17 : 46–52. doi: 10.1016/j.mib.2013.11.005 24581692
59. Myrvik QN, Leake ES, Wright MJ (1984) Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am Rev Respir Dis 129 : 322–328. 6421212
60. Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y (1991) Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 266 : 17707–17712. 1832676
61. Mwandumba HC, Russell DG, Nyirenda MH, Anderson J, White SA, et al. (2004) Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J Immunol 172 : 4592–4598. 15034077
62. Russell DG, Mwandumba HC, Rhoades EE (2002) Mycobacterium and the coat of many lipids. J Cell Biol 158 : 421–426. 12147678
63. Kondo E, Yasuda T, Kanai K (1982) Electron microscopic demonstration of close contact between intracellular mycobacteria and the phagosomal membrane. Jpn J Med Sci Biol 35 : 197–201. 6818395
64. Merckx JJ, Brown AL Jr., Karlson AG (1964) An electron-microscopic study of experimental infections with Acid-Fast Bacilli. Am Rev Respir Dis 89 : 485–496. 14139316
65. Moreira AL, Wang J, Tsenova-Berkova L, Hellmann W, Freedman VH, et al. (1997) Sequestration of Mycobacterium tuberculosis in tight vacuoles in vivo in lung macrophages of mice infected by the respiratory route. Infect Immun 65 : 305–308. 8975928
66. Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, et al. (2003) Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 9 : 533–539. 12692540
67. Brodin P, Majlessi L, Brosch R, Smith D, Bancroft G, et al. (2004) Enhanced protection against tuberculosis by vaccination with recombinant Mycobacterium microti vaccine that induces T cell immunity against region of difference 1 antigens. J Infect Dis 190 : 115–122. 15195250
68. Rybniker J, Chen JM, Sala C, Hartkoorn RC, Vocat A, et al. (2014) Anticytolytic screen identifies inhibitors of mycobacterial virulence protein secretion. Cell Host Microbe 16 : 538–548. doi: 10.1016/j.chom.2014.09.008 25299337
69. Christophe T, Jackson M, Jeon HK, Fenistein D, Contreras-Dominguez M, et al. (2009) High content screening identifies decaprenyl-phosphoribose 2' epimerase as a target for intracellular antimycobacterial inhibitors. PLoS Pathog 5: e1000645. doi: 10.1371/journal.ppat.1000645 19876393
70. Astarie-Dequeker C, Le Guyader L, Malaga W, Seaphanh FK, Chalut C, et al. (2009) Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog 5: e1000289. doi: 10.1371/journal.ppat.1000289 19197369
71. Brodin P, Poquet Y, Levillain F, Peguillet I, Larrouy-Maumus G, et al. (2010) High content phenotypic cell-based visual screen identifies Mycobacterium tuberculosis acyltrehalose-containing glycolipids involved in phagosome remodeling. PLoS Pathog 6: e1001100. doi: 10.1371/journal.ppat.1001100 20844580
72. MacGurn JA, Cox JS (2007) A genetic screen for Mycobacterium tuberculosis mutants defective for phagosome maturation arrest identifies components of the ESX-1 secretion system. Infect Immun 75 : 2668–2678. 17353284
73. Stewart GR, Patel J, Robertson BD, Rae A, Young DB (2005) Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathog 1 : 269–278. 16322769
74. Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S (2008) A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med 14 : 849–854. doi: 10.1038/nm.1795 18641659
75. Sayes F, Sun L, Di Luca M, Simeone R, Degaiffier N, et al. (2012) Strong immunogenicity and cross-reactivity of Mycobacterium tuberculosis ESX-5 type VII secretion-encoded PE-PPE proteins predicts vaccine potential. Cell Host Microbe 11 : 352–363. doi: 10.1016/j.chom.2012.03.003 22520463
76. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 : 402–408. 11846609
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek 2014 Reviewer Thank YouČlánek Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water LabelingČlánek High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



